Abstract
In the past decade, interest in nanoparticles for clinical indications has been steadily gaining traction. Most recently, Lipid Nanoparticles (LNP) have been used successfully to construct the SARS-CoV-2 mRNA vaccines for rapid pandemic response. Similarly, silica is another nanomaterial which holds much potential to create nanomedicines against pathogens of interest. One major advantage of silica-based nanoparticles is its crystalline and highly ordered structure, which can be specifically tuned to achieve the desired properties needed for clinical applications. Increasingly, clinical research has shown the potential of silica nanoparticles not only as an antiviral, but also its ability as a delivery system for antiviral small molecules and vaccines against viruses. Silica has an excellent biosafety profile and has been tested in several early phase clinical trials since 2012, demonstrating good tolerability and minimal reported side effects. In this review, we discuss the clinical development of silica nanoparticles to date and identify the gaps and potential pitfalls in its path to clinical translation.
1. Introduction
In the last decade, nanotechnology has greatly revolutionized medicine. The most recent breakthrough was the use of lipid-nanoparticles (LNP) to deliver the SARS-CoV-2 mRNA vaccines, which have since shown to be highly effective against severe COVID-19 and played a huge role in the pandemic since its rollout globally (Dagan et al., 2021). In general, nanoparticles do not have direct antiviral effects but instead mainly function as drug delivery vehicles – improving drug therapeutic effects (Kozhikhova et al., 2018). Firstly, protection offered by the nanoparticles reduces degradation by host enzymes, greatly increasing drug bioavailability. Secondly, these nanoparticles can offer controlled drug release, extending the therapeutic window. Lastly, by functionalizing the nanoparticle surfaces with ligand targeting small molecules; drug delivery may be targeted to sites of infection – further increasing drug concentration for greater localized therapeutic effects (Allen and Cullis, 2013; Sung and Kim, 2020). The majority of these drugs are based on nanoparticle chemistries which are either polymeric or liposomal (Zhang et al., 2008). Several of such drugs have already been developed and used clinically in the management of infections. One example is Ambisome, which consists of amphotericin B encapsulated in 100 nm or smaller liposomes for the treatment of invasive fungal infections (Takemoto and Kanazawa, 2017).
Besides LNP, silica nanoparticles are another class of nanoparticles which can perform similar functions such as drug delivery. However, compared to LNP, silica has the added advantage of being structurally tunable; its physical characteristics such as its size (Li et al., 2017) and internal nanonetwork structure (Jadhav et al., 2015) can be precisely tuned in order to fulfil specific applications such as controlled release of its payload (Prabhakar et al., 2016). These physical characteristics have been demonstrated to allow silica nanoparticles to deliver combinations of antiviral drugs, such as for the treatment of HIV in animal models (Zang et al., 2022). Silica nanoparticles have also been used to create vaccines. Having the capability to perform sustained release of its payload, silica nanoparticles may be fashioned into virus-like particles with gradual releases of viral antigens in the host cells to induce a prolonged immunogenic response (Hou et al., 2022). There have been pre-clinical studies which have also shown the ability of silica to deliver nucleic acids (Wang et al., 2018). Not surprisingly, of late there has been interest to develop silica based mRNA vaccines for the prevention of COVID-19 (Theobald, 2020). It is expected that lessons learnt from the development of such silica-based mRNA vaccines could also be applied for the development of vaccines for other infectious diseases. The physical structure of silica can also be tweaked to create antiviral silica nanoparticles, which have the ability to interact with surface viral proteins and consequently causing interference in the viral infection and/or replication cycle (Szunerits et al., 2015).
Furthermore, silica has an excellent safety profile and its ultrasmall solid form (≤10 nm in size) has gained U.S. FDA approval as an Investigational New Drug (IND) for oncologic imaging applications since 2011 (Selvarajan et al., 2020; Benezra et al., 2011). Silicon is a naturally occurring dietary supplement, having important functions in bone and skin metabolism (Jugdaohsingh, 2007). The content of silicon in the human body ranges from 6 to 7 g, almost twice as much as iron, and is involved in many other important biological processes (Putko and Kwaśny, 2019). Even in its nanoparticulate form, it has good biocompatibility, degrading to nontoxic silicic acid and is subsequently excreted from the body through the urinary system (Chen et al., 2018).
2. Structure of silica
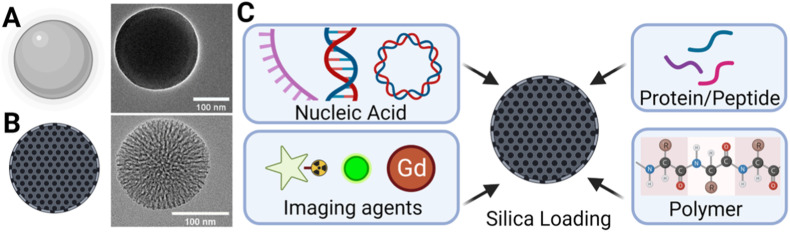
Silica nanoparticles are highly ordered, crystalline particles made of silicon dioxide (SiO2). The surface consists of siloxane structures and silanol groups which are easily conjugatable to many biological molecules to suit the intended application (Guzman and Gates, 2003; Bagheri et al., 2018). There are 2 main types of silica: solid silica nanoparticles (SSN) (Fig. 1 A) and mesoporous silica nanoparticles (MSN) (Fig. 1B). The latter contains pores of sizes ranging from 2 to 50 nm and possess an internal nanonetwork of highly ordered channels linking these pores to one another (Kresge et al., 1992; Beck et al., 1992). MSNs have high surface areas to volume ratio, allowing the loading of various compounds for therapeutic and/or imaging applications (Tarn et al., 2013) (Fig. 1C). The size of silica affects its indications. Smaller sizes consisting of diameters of ≤10 nm are rapidly excreted renally and via the hepatobiliary system within 72 h of intravenous administration (Phillips et al., 2014), hence making smaller silica particles suitable for applications such as imaging, where rapid clearance of dye encapsulated particles are needed. Larger silica particle sizes, particularly within diameters of 50–300 nm may be used for drug delivery applications as these sizes have demonstrated facile cellular endocytosis without significant cytotoxicity (SlowingVivero-Escoto et al., 2008). Finally, silica particles of diameters ≥300 nm have a large internal capacity and have been have been demonstrated to perform oral drug delivery in clincal trials (Jammaer et al., 2009; Tan et al., 2014). These particles may also be used to perform the function of sequestration as shown by Baek et al., where 300 × 1300 nm sized MSN was used for gastrointestinal enzyme sequestration for blood sugar control (Baek et al., 2022). An important characteristic of silica particles of such sizes are that it has shown no increased gut absorption compared to smaller sizes with the same surface chemistry in animal models (Yoshida et al., 2014). In the proceeding sections of this review, we discuss silica nanoparticles’ clinical safety, antiviral effects, its role as a carrier for antiviral drugs and its potential as vaccine adjuvant or carrier.
Fig. 1.
Silica Nanoparticles: (A) Solid silica nanoparticles (SSN)* inset showing Transmission Electron Microscopy (TEM) image#, (B) Mesoporous silica nanoparticles (MSN)* inset showing TEM image# and (C) Loading of MSN with various components for delivery to fulfil different biomedical applications*. (*Figure created with biorender.com. #Häffne et al., ACS Nano, 15, 4, 2021; licensed under a Creative Commons Attribution (CC BY) license.).
3. Safety of silica nanoparticles in human
The safety of silica depends on the physical configuration (diameter, shape, pore size, charge, etc.), route and dosage (Shi et al., 2016). In its macro form, U.S. FDA considers silica ‘generally recognized as safe (GRAS)’ as a food additive for oral consumption up to 1500 mg/day (Watermann and Brieger, 2017). As a nanoparticle, its ultrasmall solid form (≤10 nm in diameter) has IND status since 2011. Though its other physical forms – SSN ≥10 nm in diameter and MSN of any size, have yet to receive regulatory approval, there have been several completed clinical trials in the past decade which have used silica in its various forms and administered via various routes (oral, intravenous, intradermal, topical). These trials (Table 1 ) did not report any severe adverse reactions to the various forms of silica, highlighting the safety and tolerability of silica.
Table 1.
Current status of in-human silica studies.
| Study | Design/Route | Application and Mechanism | Reported adverse events/major findings |
|---|---|---|---|
| Core-Shell silica (Zanoni et al., 2021) (6 nm) | Non-randomized (Phase I/IIa) | Imaging of Sentinel Lymph Nodes using silica shells with fluorescent dye, coated with poly(ethylene glycol) (PEG) and integrin-targeting, cyclic arginine-glycine–aspartic acid–tyrosine peptides (cRGDY) | Route: Intradermal |
| “Cornell-dots” | No adverse events observed | ||
| Core-Shell silica (Phillips et al., 2014) (<10 nm) | Microdosing | Imaging of melanoma using silica shell with fluorescent dye, coated with PEG and cRGDY with 124Iodine | Route: Intravenous |
| “Cornell-dots” | No toxic or adverse events related to the nanoparticles observed | ||
| Silica-gold-iron nanoparticles (Kharlamov et al., 2015) (90–150 nm) | Multi-center, observational, open-label, three arms | Atherosclerotic plaque reduction using plasmonic photothermal therapy | Route: Intraarterial/ intraarterial implant |
| No target lesion major cardiac adverse events | |||
| Gold-silica nanoshells (Rastinehad et al., 2019) (150 nm) | NR | Ablation of prostate tumors using photothermal ablative therapy | Route: Intravenous |
| “Auroshell” | No serious adverse events during the procedure and at 90 d | ||
| Mesoporous silica-lipid nanoparticles (Meola et al., 2021) (19 & 50 nm) | Randomized, cross-over, double-blinded (Phase I/II) | Enhanced delivery of simvastatin (SIM) using mesoporous silica | Route: Oral |
| No serious adverse events reported during the study | |||
| Mesoporous rod shaped silica (Baek et al., 2022) (300 × 1300 nm) | Open-label, single-arm, multicenter | Improvement of blood sugar control using silica to sequester gastrointestinal enzymes | Route: Oral |
| “SiPore15” | No severe adverse events after 12 weeks. Most adverse events were mild and were gastrointestinal in origin | ||
| Mesoporous silica (Bukara et al., 2016) (500 nm (Jammaer et al., 2009)) | Open-label, randomized, two-way cross-over | Enhanced delivery of fenofibrate using mesoporous silica compared to micronized fenofibrate | Route: Oral |
| No serious adverse events reported and no relevant differences in clinical laboratory tests between the subjects in the treatment and the reference groups | |||
| Porous silica-lipid nanoparticles (Tan et al., 2014) (15.20 μm with 3–20 nm pores) | Randomized, double-blinded, one-period single oral dose (Phase I) | Enhanced delivery of ibuprofen using silica to improve solubility compared to commercial tablet | Route: Oral |
| Negligible acute side effects related to the administration of both the investigational and reference formulations. | |||
| Colloidal silica (Tieroshyn et al., 2020) (Size NR) | Randomized, double-blind, placebo-controlled, 4-center (Phase II) | Silica as an anti-diarrheal agent compared to placebo | Route: Oral |
| “Carbowhite” | Carbowhite was well tolerated and no adverse effects were reported | ||
| Silica Shells (van Zuuren et al., 2021) (Size NR) | Multi-centered randomized, double blind, vehicle controlled parallel group (Phase II) – Recently FDA Approved (Randles-Friedman, Iwaniuk) | Prolonged release of benzoyl peroxide using silica shells | Route: Topical |
| 52-week long-term safety study, side effects mostly mild | |||
| Silicic acid anhydride (Zschocke et al., 2008) (Size NR) | Randomized, open‐label, comparator‐controlled | Comparison of silica vs Topical acyclovir for the treatment of recurrent herpes labialis | Route: Topical |
| No serious adverse events |
There have also been several clinical trials using intravenously administered SSN for both imaging and therapeutic applications. The physical form in terms of particle size varied widely from 10 nm (Phillips et al., 2014) to 150 nm in diameter (Kharlamov et al., 2015; Rastinehad et al., 2019). Despite these variations, there were no severe adverse events attributable to the use of the silica nanoparticles in these studies (Phillips et al., 2014; Kharlamov et al., 2015; Rastinehad et al., 2019). The first in-human study using intravenous SSN was conducted in 2012, where 10 nm core-shell silica nanoparticles containing iodine tracers were administered for PET imaging. Using whole body imaging, it was found that nanoparticle clearance occurred within 72 h via both the renal and hepatobiliary systems (Phillips et al., 2014). Rastinehad et al. have also utilized 150 nm gold shell – silica core nanoparticles for the photothermal ablation of prostate cancer. These nanoparticles were intravenously infused into patients and allowed to preferentially accumulate within the tumor tissues. A laser was then used to perform tumor directed nanoparticle enhanced ablation. There were no severe adverse events reported during or post procedure (Rastinehad et al., 2019). In another study, Kharlamov et al. performed a study using similiar gold shell – silica core nanoparticles in the range of 90–150 nm for thermal ablation of coronary atherosclerotic plaques. The nanoparticles were introduced directly at the plaque site and no severe adverse events were attributed to the nanoparticle therapy were reported during the follow-up period of 90 days, suggesting these nanoparticles are well tolerated in cardiac and vascular tissues (Kharlamov et al., 2015).
The tolerability and toxicity of orally administered silica has been demonstrated in several clinical trials. Silica as small as 19 nm (Meola et al., 2021) and as large as 15.20 μm (Tan et al., 2014) were evaluated with no serious safety concerns identified. Unlike the trials in the previous section where SSN was administered intravenously, these trials studied silica in its MSN configuration. The most common side effects were mild gastrointestinal adverse events. Bukara et al. conducted a study to evaluate the oral toxicity of 500 nm sized MSN. For safety monitoring,12-lead Electrocardiogram, hematology, serum biochemistry and urinalysis tests were performed for all subjects on day 0 (pre-dose), day 1 (post-dose) and day 5–7 post dose. These tests did not show any abnormalities attributable to the consumption of silica (Bukara et al., 2016). The longest duration trial was a 12-weeks long study by Baek et al. (2022), which confirmed the longer term safety of silica. Additionally, Hagman et al. have shown that oral dosages as high as 9 g/day orally were well tolerated (Hagman et al., 2020) for up to 12 weeks.
Other than oral and intravenous silica nanoparticles, a smaller number of studies have also demonstrated the safety of silica administered via other routes. For example, Zanoni et al. administered SSN intradermally using 6 nm sized core-shell silica loaded with Cy5.5 fluorescent dye for the imaging of sentinel lymph nodes in head and neck melanoma patients (Zanoni et al., 2021). No severe adverse events were reported up to 2 weeks post study. In another study, the safety of silica as a topical agent was evaluated as part of a 52-week long phase III study. The study utilized a product containing benzyol peroxide encapsulated in silica particles for the treatment of rosacea involving 733 participants. The product was applied once a day with no safety concern reported (Leyden, 2014; van Zuuren et al., 2021). This product has recently been given U.S. FDA approval under the brand name of Epsolay® (Randles-Friedman, Iwaniuk). These clinical trials highlighted that silica is generally safe when administered via different routes.
Additionally, there are other Phase I clinical trials which are targeted to complete in 2024 {NCT03465618, NCT02106598 and NCT04167969} (Janjua et al., 2021). Though no results have been reported so far, these trials are using nanoparticles based on Cornell dots, which have been shown to have excellent human safety records (Phillips et al., 2014; Zanoni et al., 2021).
4. The potential of silica nanoparticles as antivirals
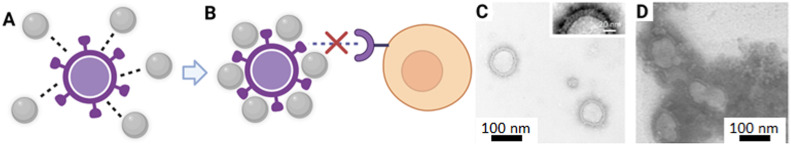
Nanoparticles have been observed to interfere with viral-host interactions though close interactions with viruses causing them to be adsorbed on viral surfaces (Fig. 2 A). This leads to local transformations of the viral surface, causing agglutination of viral surface proteins, preventing virus penetration into host cells (Fig. 2B). Using Transmission Electron Microscopy (TEM), this phenomena has been demonstrated in vitro by Osminkina et al. where porous silica nanoparticles formed an agglomeration complex after incubation with the H1N1 Influenza virus (Fig. 2C and D) (Osminkina et al., 2022). This physical interaction between viruses and silica nanoparticles, exerts an antiviral action by preventing the direct interaction between viruses and cells, preventing infection (de Souza e Silva et al., 2016). Conversely, if the size of the virions is significantly smaller than the silica nanoparticles, the virions may instead agglomerate around silica. This not only interferes with the viral infection process, but also has the added benefit of reducing virus load such as HIV and RSV during an infection (Osminkina et al., 2014). In another study, AbouAitah et al., have demonstrated in vitro that MSNs have a viral inhibitory effect. They showed that when MSN was incubated with H5N1 avian influenza virus infected MDCK cells for 24 h, the presence of the MSNs resulted in 30% viral inhibition as compared to controls without MSN. It was postulated that the underlying inhibitory mechanism was due to the negative charges from the MSN surfaces which lead to an attraction of the positive amino acids of glycoproteins in the viral envelope. The resulting charge transfer resulted in protein alterations which inhibited the viral infection of the MDCK cells. In the same study, when MSN was loaded with shikimic acid (SH) or quercetin (QR) antiviral prodrugs, a synergistic effect was produced which further reduced the lethality of the H5N1 virus in vitro (AbouAitah et al., 2020).
Fig. 2.
Antiviral mechanism effects of MSNs: (A) Schematic diagram of silica nanoparticle virion deactivation via surface charge interactions*, (B) Inactivated virion unable to interact with cellular target to initiate infection*, (C) Transmission electron microscopy (TEM) of Influenza virons before incubation with silica nanoparticles# and (D) TEM image of Influenza virions after inactivation by silica nanoparticles, arrows indicate the virions#. (*Created with biorender.com. #Osminkina et al., Bioact Mater, 7, 39–46, 2022; licensed under a Creative Commons Attribution (CC BY-NC-ND license.).
Silica nanoparticles have also shown antiviral properties in vivo. G2-S16, which is a silica-based dendrimer, was shown to have anti-HIV-1 activity in the early stages of viral replication, likely due to G2-interaction with the HIV gp120 protein, inhibiting the cell-to-cell transmission. Evaluation in h-BLT mouse models was subsequently done by incorporating GS2-S16 into a vaginal gel. A challenge study was then done by exposing the mice to R5-HIV-1JR-CSF virus, with five of six topically G2-S16-treated h-BLT tested negative for the presence of HIV-1-RNA in plasma (Sepúlveda-Crespo et al., 2015). Besides spherical nanoparticles, nanoscale silicate platelets, which have superior surface area to volume ratio, have been shown to have potent effects against flaviviruses when surface functionalized with anionic sodium dodecyl sulfate (SDS). This construct was observed to interfere with the infectivity of Japanese encephalitis (JEV) and dengue viruses (DEN) at non-cytotoxic concentrations. Interestingly, when the negative surface charge was removed using surface modification, there was no appreciable antiviral activity in vivo. Thus, these electrostatic interactions are likely highly responsible for the reduction in lethality of JEV and DEN infection in mouse challenge models. When administered to C57BL/6 mice 6 h after JEV infection, treatment with these platelets (80 μg/ml per treatment) which were modified with 30% SDS, prevented infection in 60–80% of mice inoculated with JEV. This protective effect however, was not observed when administration was delayed by 1 day after JEV infection, suggesting that the mechanism of inhibition targets the early phases of the viral life cycle and that such antivirals may have to be administered as early as possible to be effective (Liang et al., 2014). The use of such a nanoparticle as an anti-viral may also have a role as prophylactic treatment for individuals at high risk of developing severe illness or complications.
Lastly, silica also demonstrated potential anti-viral effects in humans. Zschocke et al. have conducted a randomized open-label comparator-controlled clinical trial, where silica gel was evaluated against the standard of care, acyclovir cream, in the treatment of recurrent herpes labials. The trial examined symptoms (tautness, tingling, itching, burning sensation and pain), lesion stage, efficacy, tolerability, and duration until the onset of improvement. Silica gel was reported to work more quickly with improvement in all symptoms noted within 1.1–2 h, while subjects using acyclovir cream reported improvement in 5.7–8.3 h (Zschocke et al., 2008).
5. Silica nanoparticles as antiviral drug carrier
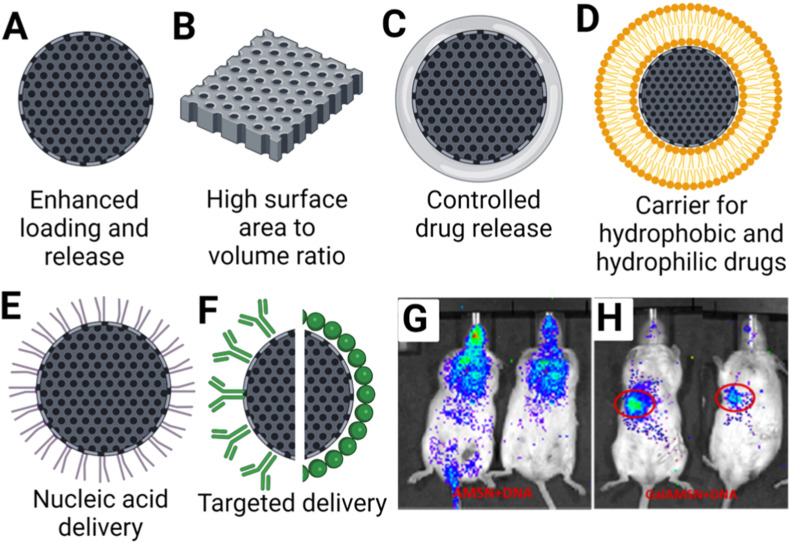
Preclinical development of small molecules fails at times to translate in part because of factors such as poor solubility, bioavailability and incompatible pharmacokinetic profiles. Mesoporous silica (MSN) can fill the translational gap as it has several desirable properties as a drug delivery system namely its stability, high loading capacity and ability to perform controlled release (Manzano and Vallet‐Regí, 2020). MSN can be modified to other configurations according to the desired therapeutic profile (Fig. 3 ). Its classical spherical configuration (Fig. 3A) has extremely high loading capacity and has been shown in some cases to even exceed 35% of its own weight (Mamaeva et al., 2013). A high surface area platelet configuration has also been demonstrated by Liang et al. with antiviral effects (Liang et al., 2014) (Fig. 3B). Polymer coated MSNs can achieve different rates of drug delivery to suit the required pharmacokinetic profiles for antiviral drug activity (Fig. 3C). One such compound with poor aqueous solubility is ML336, which is a Quinazolinone-based inhibitor against Venezuelan Equine Encephalitis Virus (VEEV) (Chung et al., 2013). LaBauve et al. described a lipid coated MSN (LC-MSN) (Fig. 3D) which is able to contain ML336. The large surface area of the MSN core promotes hydrophobic drug loading while the liposome coating retains the drug and enables enhanced circulation time and biocompatibility, providing an ideal ML336 delivery platform. ML336-loaded LC-MSNs significantly inhibited VEEV in vitro in a dose-dependent manner compared to unloaded LC-MSNs controls. Moreover, cell-based studies suggested that additional release of ML336 occurs after endocytosis, thus further extending its therapeutic duration. Further in vivo mouse safety studies showed LC-MSNs dosed at 0.11 g LC-MSNs/kg/day for four days was non-toxic. ML336-loaded LC-MSNs showed significant reduction of brain viral titer in VEEV infected mice compared to PBS controls (LaBauve et al., 2018). Overall, these results highlight the potential of silica-based nanoparticles such as, LC-MSNs as drug delivery vehicles for poorly soluble antiviral compounds.
Fig. 3.
Variations of MSN/Silica for drug delivery: (A) Mesoporous silica (MSN)*, (B) Silica platelets*, (C) Hydrogel coated silica*, (D) Liposomal silica*, (E) Polymer coated silica*, (F) Antibody/Antigen modified silica*, (G) Non-specific distribution of Amine MSN (AMSN)# and (H) Targeted delivery to mice liver using galactose modified AMSN (Gal-MSN)#. (*Created with biorender.com. #Reprinted (adapted) with permission from Mukherjee et al., ACS Appl. Bio Mater, 3, 11, 2020; copyright (2020) American Chemical Society.).
Besides small molecules, antiviral nucleic acid therapies also hold tremendous potential in the treatment of viral infections. MSN can be tuned to load negatively charged nucleic acids (C Silva et al., 2015). Furthermore, MSNs hydrophobic outer shell prevents interaction of the host degradation enzymes with the nucleic acid payload, increasing its bioavailability (Cha et al., 2017). Positively charged polyethylenimine coating is a common configuration for MSN delivery of nucleic acid (Fig. 3E). Mukherjee et al. have demonstrated the feasibility of this approach to effectively deliver small-hairpin DNA (shDNA) to cells for Hepatitis C virus (HCV) therapy. The shDNA targets the conserved 5′-untranslated region (UTR) in the RNA of HCV to inhibit its replication. In vitro infection studies in HCV-JFH1 cell culture demonstrated a significant (94%) reduction of viral RNA levels upon delivery of the shDNA using MSN. To improve shDNA delivery in vivo, the MSNs were surface functionalized with galactose (Gal-MSN) (Fig. 3F) to trigger targeted hepatic delivery. Biodistribution studies in in vivo models indeed confirmed that the localization of Gal-MSN accumulating preferentially in the liver as compared to other organs in comparison with MSN without galactose (Fig. 3G and H) (Mukherjee et al., 2020).
MSN has also been tested in early-stage clinical trials to evaluate its capability to perform drug delivery in humans. Tan et al. conducted a Phase I clinical trial in which Ibuprofen (IBU) was loaded into silica based lipoceramic nanoparticles (Lipoceramic-IBU) for more effective delivery (Tan et al., 2014). Lipoceramic-IBU was compared head-to-head with a reference commercial tablet (i.e., Nurofen®). It showed a significantly higher solubility which resulted in 1.95-fold greater drug bioavailaiblity than Nurofen® after the 6 h of dosing. Subjects who received the test Lipoceramic-IBU formulation showed a relatively rapid, early absorption phase (t max = 1.2 ± 0.9 h) with prolonged plasma concentrations of IBU in comparison to the reference treatment (t max = 2.0 ± 1.0 h). Throughout the 6 h study period, Lipoceramic-IBU treatment group displayed higher plasma levels of IBU as compared to the reference treatment. This proof of concept clinical trial thus illustrated the feasibility of the silica based nanoparticles to enhance the oral pharmacokinetics (PK) of IBU, a weak acid BCS Class II drug with poor solubility (Tan et al., 2014). The results from this study is particularly relevant for viral therapy formulations as Ibuprofen is a Nonsteroidal Anti-inflammatory (NSAID) Drug and NSAIDs have been shown to exhibit antiviral properties (Terrier et al., 2021). Findings from these proof-of-concept studies can serve as the basis for silica to be used for the delivery of other poorly soluble antivirals to improve PK of drug delivery and eventual efficacy.
6. Silica nanoparticles as future vaccines platform
Antiviral small molecules exert their therapeutic effects when administered early in the infective process. For instance, Paxlovid, an antiviral directed against SARS-CoV-2, has a short treatment window opportunity of only 5 days (Pepperrell et al., 2022), but early diagnosis of many acute viral illnesses is highly challenging as the symptoms are often non-specific (McClain et al., 2021). Therefore, for many acute viral infections, vaccines will continue to play an important part in disease prevention and control. As seen in the ongoing COVID-19 pandemic, nanotechnology has played a crucial role in the rapid development and deployment of the SARS-CoV-2 vaccines for pandemic control. Thus, silica nanoparticles with its many advantageous properties, have great potential to further advance the field of mRNA vaccine platform.
Traditionally, non-live attenuated vaccines rely on adjuvants to improve immunogenicity. Aluminum hydroxide (alum) is the classical adjuvant of choice, functioning as an antigen depot as well as a mild inflammatory agent, causing the recruitment of leukocytes necessary for the generation of an immune response (Shaw et al., 2008). Skrastina et al. have done a head-to-head in vivo comparison using silica nanoparticles and alum in the development of a Hepatitis B core protein (HBc) vaccine. Despite loading with approximately 55% less HBc than alum, silica nanoparticles were able to achieve the same typical IgG2a/IgG1 ratios as Alum in BALB/c mice when administered intraperitoneally. When silica was used with monophosphoryl lipid A (MPL) a T-cell adjuvant, a similar enhancement of the HBc-specific T-cell induction was seen compared with the Alum using the same amount of MPL (Skrastina et al., 2014). These findings suggest the potential of silica as a vaccine adjuvant and its different physical configurations are currently being actively investigated for the development of other vaccines (Ferreira Soares et al., 2020).
Second generation mRNA vaccine development can also benefit from the more efficient mRNA release characteristics of MSNs. For instance, Dendritic Mesoporous Silica Nanoparticles (DMSNs) have been shown to perform complete release of its mRNA cargo over 48 h (Wang et al., 2018). This is in comparison to the lower LNP mRNA release efficiency, which may account for the lack of longevity of immunogenicity associated with the current LNP based mRNA vaccines. In one study, mRNA release from LNPs was noted to be as low as 1–2% over a 1-hr duration (Gilleron et al., 2013). From the dose escalation studies of the mRNA-1273 SARS-CoV-2 vaccines, it was observed that higher dose groups experienced more adverse effects after both the first and second dose, where reactogenicity was reported to be most pronounced in subjects who received the highest dose of 250 μg (Jackson et al., 2020). It has been postulated that the reactogenicity could be attributable to the lipid components within the LNP carriers which are highly inflammatory (Ndeupen et al., 2021; Ghasemiyeh and Mohammadi-Samani, 2018). A nanoparticle with more efficient mRNA delivery may thus trigger the required immune responses using a smaller dose of mRNA, resulting in less adverse effects. Indeed, some groups have started investigating silica as a delivery platform for mRNA vaccines against COVID-19 (Mehta et al., 2020).
7. Current limitations and future directions
In the past decade, there have been several clinical trials which have used silica-based nanoparticles for various indications. These trials have thus far demonstrated the excellent safety of silica nanoparticles despite different physical configurations of silica nanoparticles used. Though there has been an early in-human study which showed silica gel was equivalent to acyclovir in the topical treatment for herpes labialis (Zschocke et al., 2008), similar subsequent in-human studies have not been attempted and development in this area had remained slow before the COVID-19 pandemic. However, with the widespread use of the latest LNP based mRNA vaccines against SARS-CoV-2, interest in silica-based nanoparticle vaccines has been invigorated (Rajput et al., 2022).
Nonetheless, before more silica-based nanomedicines therapeutics against viruses can be translated for clinical use, the long-term effects of silica nanoparticle exposure need to be defined. Mohammadpour et al. have recently explored long term effects of repeated intravenous administration of silica nanoparticles in mouse models in a year-long study. They found no significant changes in post necropsy examination of internal organs and organ-to-body weight ratio. However, liver inflammation and histocyte with neutrophil aggregations in the spleen were noted – which may suggest ongoing or resolving injury processes. Most of these observations were seen when large, non-porous solid silica nanoparticles were administered, but no significant chronic toxicity was observed for smaller sized non-porous particles or mesoporous particles (Mohammadpour et al., 2020). As seen in pre-clinical studies, the mesoporous form of silica has higher drug loading capacity and nucleic acid delivery capabilities, yet current clinical studies have only evaluated MSN administered orally. Though many studies have suggested that MSN are more well tolerated intravenously than solid silica nanoparticles, the maximum limits have yet to be established. (Yu et al., 2012), (Lu et al., 2010) (Fu et al., 2013). Future studies will need to examine how physical parameters such as porosity and size can be optimized to improve drug delivery and efficacy – whilst balancing for biodistribution, elimination dynamics and potential long-term side effects when administered intravenously.
The scalable synthesis of silica nanoparticles, such as the large scale synthesis of LNP using microfluidics technology (Shepherd et al., 2021), is another area needing urgent further developmental research. As seen with the deployment of mRNA vaccines for the COVID-19 pandemic, the ability for rapid scale-up manufacturing was key for the mass deployment and administration of LNP based mRNA vaccines during the COVID-19 pandemic (Khurana et al., 2021). Presently, the silica nanoparticle platform still faces several challenges in producing nanoparticles at large scale. Some of these challenges include achieving narrow size distributions, consistent pore structures and stability in colloidal suspensions (Jafari et al., 2019). As discussed in the earlier sections, the characteristics of silica are highly dependent on its structure. Thus, scaled-up synthesis optimization to maintain tight control over these characteristics would need to be a major priority should silica-based technology continue to show promise in clinical translation. Recent work such as that demonstrated by Kim et al. have shown how controlled synthesis of MSN up to 50 nm in diameter can be achieved at room temperature on a large scale basis (Kim et al., 2019). With the increasing emphasis of nanotechnologies in medicine, it is expected that the development of more of such approaches will follow in the coming years.
Another concern with silica synthesis is that many available synthesis methodologies involve the use of reagents such as surfactants which may also have potential toxic concerns. These reagents can remain in-situ after processing and precautionary steps must be taken during purification (He et al., 2009). Though it has been demonstrated that silica synthesis is possible without surfactants (Baù et al., 2009; Xu et al., 2022), large-scale production of silica using a surfactant-free approach has not yet been demonstrated. Continued improvements in the synthesis pathway will be imperative for the eventual safe development of silica for clinical uses. Close collaboration between clinicians, scientists and engineers will be key to success of silica-based nanomedicines.
8. Conclusion
Silica and its various physical configurations are well-tolerated and safe as a nano construct when administered orally, intradermally, intravenously and topically. Silica has several desirable characteristics such as its antiviral properties, efficient carrier and release efficiency for antiviral therapeutics and vaccines applications. Data from preclinical studies thus far hold promising results for its clinical applications with several clinical trials currently undergoing evaluation for translation. It is evident that silica nanoparticles will continue to generate intense interest in biomedical advances not just against viruses but also against other pathogens of interest.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
No data was used for the research described in the article.
References
- AbouAitah K., Swiderska-Sroda A., Kandeil A., Salman A.M.M., Wojnarowicz J., Ali M.A., Opalinska A., et al. Virucidal action against avian influenza H5N1 virus and immunomodulatory effects of nanoformulations consisting of mesoporous silica nanoparticles loaded with natural prodrugs. Int. J. Nanomed. 2020;15:5181–5202. doi: 10.2147/ijn.S247692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen T.M., Cullis P.R. Liposomal drug delivery systems: from concept to clinical applications. Adv. Drug Deliv. Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- Baek J., Robert-Nicoud G., Herrera Hidalgo C., Borg M.L., Iqbal M.N., Berlin R., Lindgren M., et al. Engineered mesoporous silica reduces long-term blood glucose, HbA1c, and improves metabolic parameters in prediabetics. Nanomedicine. 2022;17(1):9–22. doi: 10.2217/nnm-2021-0235. [DOI] [PubMed] [Google Scholar]
- Bagheri E., Ansari L., Abnous K., Taghdisi S.M., Charbgoo F., Ramezani M., Alibolandi M. Silica based hybrid materials for drug delivery and bioimaging. J. Contr. Release. 2018;277:57–76. doi: 10.1016/j.jconrel.2018.03.014. [DOI] [PubMed] [Google Scholar]
- Baù L., Bártová B., Arduini M., Mancin F. Surfactant-free synthesis of mesoporous and hollow silica nanoparticles with an inorganic template. Chem. Commun. 2009;(48):7584–7586. doi: 10.1039/B917561J. [DOI] [PubMed] [Google Scholar]
- Beck J.S., Vartuli J.C., Roth W.J., Leonowicz M.E., Kresge C.T., Schmitt K.D., Chu C.T.W., et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Soc. 1992;114(27):10834–10843. [Google Scholar]
- Benezra M., Penate-Medina O., Zanzonico P.B., Schaer D., Ow H., Burns A., DeStanchina E., et al. Multimodal silica nanoparticles are effective cancer-targeted probes in a model of human melanoma. J. Clin. Invest. 2011;121(7):2768–2780. doi: 10.1172/jci45600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukara K., Schueller L., Rosier J., Martens M.A., Daems T., Verheyden L., Eelen S., et al. Ordered mesoporous silica to enhance the bioavailability of poorly water-soluble drugs: proof of concept in man. Eur. J. Pharm. Biopharm. 2016;108:220–225. doi: 10.1016/j.ejpb.2016.08.020. [DOI] [PubMed] [Google Scholar]
- C Silva A., Lopes C M., M Sousa Lobo J., Helena Amaral M. Nucleic acids delivery systems: a challenge for pharmaceutical technologists. Curr. Drug Metabol. 2015;16(1):3–16. doi: 10.2174/1389200216666150401110211. [DOI] [PubMed] [Google Scholar]
- Cha W., Fan R., Miao Y., Zhou Y., Qin C., Shan X., Wan X., et al. Mesoporous silica nanoparticles as carriers for intracellular delivery of nucleic acids and subsequent therapeutic applications. Molecules. 2017;22(5):782. doi: 10.3390/molecules22050782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F., Hableel G., Zhao E.R., Jokerst J.V. Multifunctional nanomedicine with silica: role of silica in nanoparticles for theranostic, imaging, and drug monitoring. J. Colloid Interface Sci. 2018;521:261–279. doi: 10.1016/j.jcis.2018.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung D., Schroeder C.E., Sotsky J., Yao T., Roy S., Smith R.A., Tower N.A., et al. ML336: development of quinazolinone-based inhibitors against Venezuelan equine encephalitis virus (VEEV) Probe Rep. NIH Mol. Lib. Prog. 2013 [Internet] [PubMed] [Google Scholar]
- Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza e Silva J.M., Hanchuk T.D.M., Santos M.I., Kobarg J., Bajgelman M.C., Cardoso M.B. Viral inhibition mechanism mediated by surface-modified silica nanoparticles. ACS Appl. Mater. Interfaces. 2016;8(26):16564–16572. doi: 10.1021/acsami.6b03342. [DOI] [PubMed] [Google Scholar]
- Ferreira Soares D.C., Soares L.M., Miranda de Goes A., Melo E.M., Branco de Barros A.L., Alves Santos Bicalho T.C., Leao N.M., et al. Mesoporous SBA-16 silica nanoparticles as a potential vaccine adjuvant against Paracoccidioides brasiliensis. Microporous Mesoporous Mater. 2020;291 doi: 10.1016/j.micromeso.2019.109676. [DOI] [Google Scholar]
- Fu C., Liu T., Li L., Liu H., Chen D., Tang F. The absorption, distribution, excretion and toxicity of mesoporous silica nanoparticles in mice following different exposure routes. Biomaterials. 2013;34(10):2565–2575. doi: 10.1016/j.biomaterials.2012.12.043. [DOI] [PubMed] [Google Scholar]
- Ghasemiyeh P., Mohammadi-Samani S. Solid lipid nanoparticles and nanostructured lipid carriers as novel drug delivery systems: applications, advantages and disadvantages. Res. pharmaceut. sci. 2018;13(4):288. doi: 10.4103/1735-5362.235156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleron J., Querbes W., Zeigerer A., Borodovsky A., Marsico G., Schubert U., Manygoats K., et al. Image-based analysis of lipid nanoparticle–mediated siRNA delivery, intracellular trafficking and endosomal escape. Nat. Biotechnol. 2013;31(7):638–646. doi: 10.1038/nbt.2612. [DOI] [PubMed] [Google Scholar]
- Guzman J., Gates B.C. Supported molecular catalysts: metal complexes and clusters on oxides and zeolites. Dalton Trans. 2003;(17):3303–3318. [Google Scholar]
- Hagman E., Elimam A., Kupferschmidt N., Ekbom K., Rössner S., Iqbal M.N., Johnston E., et al. Oral intake of mesoporous silica is safe and well tolerated in male humans. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0240030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Q., Zhang Z., Gao Y., Shi J., Li Y. Intracellular localization and cytotoxicity of spherical mesoporous silica nano‐and microparticles. Small. 2009;5(23):2722–2729. doi: 10.1002/smll.200900923. [DOI] [PubMed] [Google Scholar]
- Hou F., Teng Z., Ru J., Liu H., Li J., Zhang Y., Sun S., et al. Flower-like mesoporous silica nanoparticles as an antigen delivery platform to promote systemic immune response. Nanomed. Nanotechnol. Biol. Med. 2022;42 doi: 10.1016/j.nano.2022.102541. [DOI] [PubMed] [Google Scholar]
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., et al. An mRNA vaccine against SARS-CoV-2 — Preliminary report. N. Engl. J. Med. 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav K.S., Dumbare P.S., Pande V.V. Mesoporous silica nanoparticles (MSN): a nanonetwork and hierarchical structure in drug delivery. J. Nano Res. 2015;2(5):1–8. [Google Scholar]
- Jafari S., Derakhshankhah H., Alaei L., Fattahi A., Varnamkhasti B.S., Saboury A.A. Mesoporous silica nanoparticles for therapeutic/diagnostic applications. Biomed. Pharmacother. 2019;109:1100–1111. doi: 10.1016/j.biopha.2018.10.167. [DOI] [PubMed] [Google Scholar]
- Jammaer J., Aerts A., D'Haen J., Seo J.W., Martens J.A. Convenient synthesis of ordered mesoporous silica at room temperature and quasi-neutral pH. J. Mater. Chem. 2009;19(44):8290–8293. [Google Scholar]
- Janjua T.I., Cao Y., Yu C., Popat A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021;6(12):1072–1074. doi: 10.1038/s41578-021-00385-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jugdaohsingh R. Silicon and bone health. J. Nutr. Health Aging. 2007;11(2):99–110. [PMC free article] [PubMed] [Google Scholar]
- Kharlamov A.N., Tyurnina A.E., Veselova V.S., Kovtun O.P., Shur V.Y., Gabinsky J.L. Silica–gold nanoparticles for atheroprotective management of plaques: results of the NANOM-FIM trial. Nanoscale. 2015;7(17):8003–8015. doi: 10.1039/c5nr01050k. [DOI] [PubMed] [Google Scholar]
- Khurana A., Allawadhi P., Khurana I., Allwadhi S., Weiskirchen R., Banothu A.K., Chhabra D., et al. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today. 2021;38 doi: 10.1016/j.nantod.2021.101142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Yoon S., Lee J.H. Facile large-scale synthesis of mesoporous silica nanoparticles at room temperature in a monophasic system with fine size control. Microporous Mesoporous Mater. 2019;288 doi: 10.1016/j.micromeso.2019.109595. [DOI] [Google Scholar]
- Kozhikhova K.V., Ivantsova M.N., Tokareva M.I., Shulepov I.D., Tretiyakov A.V., Shaidarov L.V., Rusinov V.L., et al. Preparation of chitosan-coated liposomes as a novel carrier system for the antiviral drug Triazavirin. Pharmaceut. Dev. Technol. 2018;23(4):334–342. doi: 10.1080/10837450.2016.1242624. [DOI] [PubMed] [Google Scholar]
- Kresge aCT., Leonowicz M.E., Roth W.J., Vartuli J.C., Beck J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature. 1992;359(6397):710–712. [Google Scholar]
- LaBauve A.E., Rinker T.E., Noureddine A., Serda R.E., Howe J.Y., Sherman M.B., Rasley A., et al. Lipid-coated mesoporous silica nanoparticles for the delivery of the ML336 antiviral to inhibit encephalitic alphavirus infection. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32033-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden J.J. Randomized, phase 2, dose-ranging study in the treatment of rosacea with encapsulated benzoyl peroxide gel. J. Drugs Dermatol. JDD. 2014;13(6):685–688. [PubMed] [Google Scholar]
- Li Y., Li N., Pan W., Yu Z., Yang L., Tang B. Hollow mesoporous silica nanoparticles with tunable structures for controlled drug delivery. ACS Appl. Mater. Interfaces. 2017;9(3):2123–2129. doi: 10.1021/acsami.6b13876. [DOI] [PubMed] [Google Scholar]
- Liang J.-J., Wei J.-C., Lee Y.-L., Hsu S-h, Lin J.-J., Lin Y.-L. Surfactant-modified nanoclay exhibits an antiviral activity with high potency and broad spectrum. J. Virol. 2014;88(8):4218–4228. doi: 10.1128/JVI.03256-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Liong M., Li Z., Zink J.I., Tamanoi F. Biocompatibility, biodistribution, and drug‐delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small. 2010;6(16):1794–1805. doi: 10.1002/smll.201000538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamaeva V., Sahlgren C., Lindén M. Mesoporous silica nanoparticles in medicine—recent advances. Adv. Drug Deliv. Rev. 2013;65(5):689–702. doi: 10.1016/j.addr.2012.07.018. [DOI] [PubMed] [Google Scholar]
- Manzano M., Vallet‐Regí M. Mesoporous silica nanoparticles for drug delivery. Adv. Funct. Mater. 2020;30(2) [Google Scholar]
- McClain M.T., Constantine F.J., Nicholson B.P., Nichols M., Burke T.W., Henao R., Jones D.C., et al. A blood-based host gene expression assay for early detection of respiratory viral infection: an index-cluster prospective cohort study. Lancet Infect. Dis. 2021;21(3):396–404. doi: 10.1016/S1473-3099(20)30486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta M., Prasher P., Sharma M., Shastri M.D., Khurana N., Vyas M., Dureja H., et al. Advanced drug delivery systems can assist in targeting coronavirus disease (COVID-19) Hypothesis. 2020;144 doi: 10.1016/j.mehy.2020.110254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meola T.R., Abuhelwa A.Y., Joyce P., Clifton P., Prestidge C.A. A safety, tolerability, and pharmacokinetic study of a novel simvastatin silica-lipid hybrid formulation in healthy male participants. Drug Deliv. Transl. Res. 2021;11(3):1261–1272. doi: 10.1007/s13346-020-00853-x. [DOI] [PubMed] [Google Scholar]
- Mohammadpour R., Cheney D.L., Grunberger J.W., Yazdimamaghani M., Jedrzkiewicz J., Isaacson K.J., Dobrovolskaia M.A., et al. One-year chronic toxicity evaluation of single dose intravenously administered silica nanoparticles in mice and their Ex vivo human hemocompatibility. J. Contr. Release. 2020;324:471–481. doi: 10.1016/j.jconrel.2020.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee M.B., Mullick R., Reddy B.U., Das S., Raichur A.M. Galactose functionalized mesoporous silica nanoparticles as delivery vehicle in the treatment of hepatitis C infection. ACS Appl. Bio Mater. 2020;3(11):7598–7610. doi: 10.1021/acsabm.0c00814. [DOI] [PubMed] [Google Scholar]
- Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyártó B.Z. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24(12) doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osminkina L.A., Timoshenko V.Y., Shilovsky I.P., Kornilaeva G.V., Shevchenko S.N., Gongalsky M.B., Tamarov K.P., et al. Porous silicon nanoparticles as scavengers of hazardous viruses. J. Nanoparticle Res. 2014;16(6):2430. doi: 10.1007/s11051-014-2430-2. [DOI] [Google Scholar]
- Osminkina L.A., Agafilushkina S.N., Kropotkina E.A., Saushkin N.Y., Bozhev I.V., Abramchuk S.S., Samsonova J.V., et al. Antiviral adsorption activity of porous silicon nanoparticles against different pathogenic human viruses. Bioact. Mater. 2022;7:39–46. doi: 10.1016/j.bioactmat.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepperrell T., Ellis L., Wang J., Hill A., editors. Barriers to Worldwide Access for Paxlovid, a New Treatment for COVID-19. Oxford University Press US; 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips E., Penate-Medina O., Zanzonico P.B., Carvajal R.D., Mohan P., Ye Y., Humm J., et al. Clinical translation of an ultrasmall inorganic optical-PET imaging nanoparticle probe. Sci. Transl. Med. 2014;6(260) doi: 10.1126/scitranslmed.3009524. 260ra149-260ra149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar N., Zhang J., Desai D., Casals E., Gulin-Sarfraz T., Näreoja T., Westermarck J., et al. Stimuli-responsive hybrid nanocarriers developed by controllable integration of hyperbranched PEI with mesoporous silica nanoparticles for sustained intracellular siRNA delivery. Int. J. Nanomed. 2016;11:6591–6608. doi: 10.2147/ijn.S120611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putko P., Kwaśny M. Determination of the silicon content in dietary supplements and in water. J. Elementol. 2019;24(3) [Google Scholar]
- Rajput S., Vadia N., Mahajan M. In: Advanced Functional Porous Materials: from Macro to Nano Scale Lengths. Uthaman A., Thomas S., Li T., Maria H., editors. Springer International Publishing; Cham: 2022. Role of mesoporous silica nanoparticles as drug carriers: evaluation of diverse mesoporous material nanoparticles as potential host for various applications; pp. 205–234. [Google Scholar]
- Randles-Friedman Z, Iwaniuk S. Galderma Launches EPSOLAY Cream, a Medical Advance to Treat Bumps and Blemishes of Rosacea Galderma Launches EPSOLAY® Cream, a Medical Advance to Treat Bumps and Blemishes of Rosacea Tweet.
- Rastinehad A.R., Anastos H., Wajswol E., Winoker J.S., Sfakianos J.P., Doppalapudi S.K., Carrick M.R., et al. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc. Natl. Acad. Sci. USA. 2019;116(37):18590–18596. doi: 10.1073/pnas.1906929116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvarajan V., Obuobi S., Ee P.L.R. Silica nanoparticles—a versatile tool for the treatment of bacterial infections. Front. Chem. 2020;8:602. doi: 10.3389/fchem.2020.00602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepúlveda-Crespo D., Serramía M.J., Tager A.M., Vrbanac V., Gómez R., De La Mata F.J., Jiménez J.L., et al. Prevention vaginally of HIV-1 transmission in humanized BLT mice and mode of antiviral action of polyanionic carbosilane dendrimer G2-S16. Nanomed. Nanotechnol. Biol. Med. 2015;11(6):1299–1308. doi: 10.1016/j.nano.2015.04.013. [DOI] [PubMed] [Google Scholar]
- Shaw A.R., Feinberg M.B. In: Clinical Immunology. third ed. Rich R.R., Fleisher T.A., Shearer W.T., Schroeder H.W., Frew A.J., Weyand C.M., editors. Mosby; Edinburgh: 2008. 92 - vaccines; pp. 1353–1382. [Google Scholar]
- Shepherd S.J., Warzecha C.C., Yadavali S., El-Mayta R., Alameh M.-G., Wang L., Weissman D., et al. Scalable mRNA and siRNA lipid nanoparticle production using a parallelized microfluidic device. Nano Lett. 2021;21(13):5671–5680. doi: 10.1021/acs.nanolett.1c01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Miller M.L., Di Pasqua A.J. Biocompatibility of mesoporous silica nanoparticles? Comments Mod. Chem. 2016;36(2):61–80. doi: 10.1080/02603594.2015.1088439. [DOI] [Google Scholar]
- Skrastina D., Petrovskis I., Lieknina I., Bogans J., Renhofa R., Ose V., Dishlers A., et al. Silica nanoparticles as the adjuvant for the immunisation of mice using hepatitis B core virus-like particles. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0114006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slowing, Vivero-Escoto J.L., Wu C.-W., Lin V.S.Y. Mesoporous silica nanoparticles as controlled release drug delivery and gene transfection carriers. Adv. Drug Deliv. Rev. 2008;60(11):1278–1288. doi: 10.1016/j.addr.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Sung Y.K., Kim S.W. Recent advances in polymeric drug delivery systems. Biomater. Res. 2020;24(1):1–12. doi: 10.1186/s40824-020-00190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szunerits S., Barras A., Khanal M., Pagneux Q., Boukherroub R. Nanostructures for the inhibition of viral infections. Molecules. 2015;20(8):14051–14081. doi: 10.3390/molecules200814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K., Kanazawa K. AmBisome: relationship between the pharmacokinetic characteristics acquired by liposomal formulation and safety/efficacy. J. Liposome Res. 2017;27(3):186–194. doi: 10.1080/08982104.2016.1205087. [DOI] [PubMed] [Google Scholar]
- Tan A., Eskandar N.G., Rao S., Prestidge C.A. First in man bioavailability and tolerability studies of a silica–lipid hybrid (Lipoceramic) formulation: a Phase I study with ibuprofen. Drug Deliv. Transl. Res. 2014;4(3):212–221. doi: 10.1007/s13346-013-0172-9. [DOI] [PubMed] [Google Scholar]
- Tarn D., Ashley C.E., Xue M., Carnes E.C., Zink J.I., Brinker C.J. Mesoporous silica nanoparticle nanocarriers: biofunctionality and biocompatibility. Accounts Chem. Res. 2013;46(3):792–801. doi: 10.1021/ar3000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier O., Dilly S., Pizzorno A., Chalupska D., Humpolickova J., Bouřa E., Berenbaum F., et al. Antiviral properties of the NSAID drug naproxen targeting the nucleoprotein of SARS-CoV-2 coronavirus. Molecules. 2021;26(9):2593. doi: 10.3390/molecules26092593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald N. Emerging vaccine delivery systems for COVID-19: functionalised silica nanoparticles offer a potentially safe and effective alternative delivery system for DNA/RNA vaccines and may be useful in the hunt for a COVID-19 vaccine. Drug Discov. Today. 2020;25(9):1556. doi: 10.1016/j.drudis.2020.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tieroshyn V., Moroz L., Prishliak O., Shostakovich-Koretska L., Kruglova O., Gordienko L. Colloidal silicon dioxide in tablet form (Carbowhite) efficacy in patients with acute diarrhea: results of randomized, double-blind, Placebo-controlled, multi-center study. Sci. Rep. 2020;10(1):6344. doi: 10.1038/s41598-020-62386-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuuren E.J., Arents B.W.M., van der Linden M., Vermeulen S., Fedorowicz Z., Tan J. Rosacea: new concepts in classification and treatment. Am. J. Clin. Dermatol. 2021;22(4):457–465. doi: 10.1007/s40257-021-00595-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Song H., Yu M., Xu C., Liu Y., Tang J., Yang Y., et al. Room temperature synthesis of dendritic mesoporous silica nanoparticles with small sizes and enhanced mRNA delivery performance. J. Mater. Chem. B. 2018;6(24):4089–4095. doi: 10.1039/c8tb00544c. [DOI] [PubMed] [Google Scholar]
- Watermann A., Brieger J. Mesoporous silica nanoparticles as drug delivery vehicles in cancer. Nanomaterials. 2017;7(7):189. doi: 10.3390/nano7070189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Lei C., Wang Y., Yu C. Dendritic mesoporous nanoparticles: structure, synthesis and properties. Angew. Chem. Int. Ed. 2022;61(12) doi: 10.1002/anie.202112752. [DOI] [PubMed] [Google Scholar]
- Yoshida T., Yoshioka Y., Takahashi H., Misato K., Mori T., Hirai T., Nagano K., et al. Intestinal absorption and biological effects of orally administered amorphous silica particles. Nanoscale Res. Lett. 2014;9(1):532. doi: 10.1186/1556-276X-9-532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T., Hubbard D., Ray A., Ghandehari H. In vivo biodistribution and pharmacokinetics of silica nanoparticles as a function of geometry, porosity and surface characteristics. J. Contr. Release. 2012;163(1):46–54. doi: 10.1016/j.jconrel.2012.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang H., Fofana J., Xu F., Nodder S.B., Gummuluru S., Reinhard B.M. Characterizing lipid‐coated mesoporous silica nanoparticles as CD169‐binding delivery system for Rilpivirine and Cabotegravir. Adv. NanoBiomed. Res. 2022 doi: 10.1002/anbr.202100157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanoni D.K., Stambuk H.E., Madajewski B., Montero P.H., Matsuura D., Busam K.J., Ma K., et al. Use of ultrasmall core-shell fluorescent silica nanoparticles for image-guided sentinel lymph node biopsy in head and neck melanoma: a nonrandomized clinical trial. JAMA Netw. Open. 2021;4(3) doi: 10.1001/jamanetworkopen.2021.1936. e211936-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Gu F.X., Chan J.M., Wang A.Z., Langer R.S., Farokhzad O.C. Nanoparticles in medicine: therapeutic applications and developments. Clin. Pharmacol. Ther. 2008;83(5):761–769. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- Zschocke I., Reich C., Zielke A., Reitmeier N., Reich K. Silica gel is as effective as acyclovir cream in patients with recurrent herpes labialis: results of a randomized, open‐label trial. J. Dermatol. Treat. 2008;19(3):176–181. doi: 10.1080/09546630701593457. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.