Abstract
The plasma membrane of eukaryotic cells is composed of a large number of lipid species that are laterally segregated into functional domains as well as asymmetrically distributed between the outer and inner leaflets. Additionally, the spatial distribution and organization of these lipids dramatically change in response to various cellular states, such as cell division, differentiation, and apoptosis. Division of one cell into two daughter cells is one of the most fundamental requirements for the sustenance of growth in all living organisms. The successful completion of cytokinesis, the final stage of cell division, is critically dependent on the spatial distribution and organization of specific lipids. In this review, we discuss the properties of various lipid species associated with cytokinesis and the mechanisms involved in their polarization, including forward trafficking, endocytic recycling, local synthesis, and cortical flow models. The differences in lipid species requirements and distribution in mitotic vs. male meiotic cells will be discussed. We will concentrate on sphingolipids and phosphatidylinositols because their transbilayer organization and movement may be linked via the cytoskeleton and thus critically regulate various steps of cytokinesis.
Keywords: cytokinesis, sphingolipids, phosphatidylinositol phosphates, lipids, lipid polarization, membrane traffic, male meiotic cytokinesis, multivesicular endosomes, multivesicular bodies, forward trafficking, endocytic recycling, cortical flow, transbilayer coupling, rab GTPase, membrane curvature, membrane bending, cholesterol, phosphatidylethanolamine, triacylglycerols, phosphatidic acid, phosphatidyl serine, phosphatidylinositol, very long chain fatty acids, very long chain polyunsaturated fatty acids, PUFA, disease, sphingolipidoses, lowe syndrome, cataract, aging, cancer, sphingomyelin, ceramide phosphoethanolamine
1. Introduction
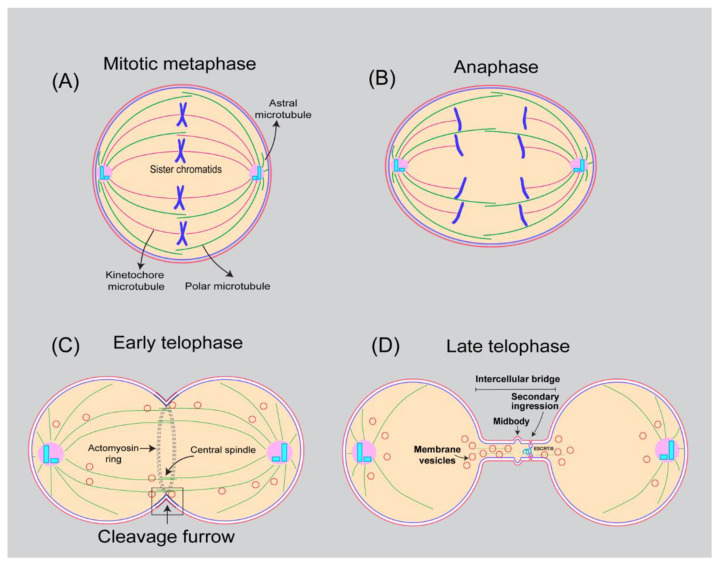
Division of one cell into two daughter cells is among the basic and essential functions of cells for their proliferation and growth. Animal cell membranes undergo extensive remodulation in preparation for cell division. At the onset of mitosis, cells shift their shape from being flat at interphase to round at metaphase and elongated ovoid shape at anaphase (Figure 1) [1,2]. Deep invagination of the plasma membrane around the equatorial region with the help of an actomyosin contractile ring in the elongated cell creates a cleavage furrow during cytokinesis, the final stage of cell division (Figure 1). Cleavage furrow ingression, stabilization, and cutting of the membrane (abscission) in the intercellular bridge eventually leads to the separation of nascent daughter cells (Figure 1) [3,4,5,6,7,8]. Precise execution of cytokinesis is essential for genome stability and viability. Since cytokinesis occurs immediately after chromosomal segregation, failure of cytokinesis has been associated with polyploidy, chromosomal instability as well as aneuploidy, features characteristic of many types of cancers [9].
Figure 1.
Schematic representation of mitotic cells undergoing division. (A) At metaphase, cells acquire round shape, chromosomes attached to kinetochore microtubules become aligned at the equatorial plane. (B) At anaphase, cells elongate, sister chromatids separate, and the daughter chromosomes move towards the poles. (C) Once daughter chromosomes reach poles and form two nuclei, the cytoplasm between the two nuclei is divided and physically separated by a process known as cytokinesis. During early stages of telophase, the mitotic spindle determines the site of cleavage furrow, by recruiting two master regulators Centralspindlin complex and Chromosomal Passenger Complex (CPC). These multisubunit complexes activate downstream signaling proteins including Rho-GEF (Ect-2), Rho GTPase and cytoskeletal regulators to promote assembly of actomyosin contractile ring (composed of unbranched actin and myosin-II) positioned midway between segregated chromosomes. Plasma membrane at the cytokinetic furrow is anchored to the contractile ring via Anillin and Septins and anionic phospholipid PI(4,5)P2. Constriction of the ring with concomitant membrane addition via both endocytosis and forward trafficking, generates the cleavage furrow. (D) Cleavage furrow ingression and stabilization results in the formation of intercellular bridge with an electron dense structure in the middle called midbody. The final steps of cytokinesis known as abscission involve endocytic trafficking, clearance of PIP2 on furrow membrane, microtubule severing, F-actin clearance, fusion of endosomes with the plasma membrane leading to secondary ingression. In a final step, there is assembly of the endosomal sorting complex required for transport (ESCRT III) helical filaments downstream of Cep55 and ALIX-TSG101 activation, resulting in membrane cutting that will physically separate the cytoplasm between two daughter cells.
A growing number of studies suggest that successful assembly of the actomyosin ring, cleavage furrow ingression and abscission at the midbody critically depends on lipid trafficking and polarization [4,10]. Lipids with unique biophysical properties have been shown to be enriched at the cleavage furrow, intercellular bridge and midbodies [11,12]. However, the mechanisms by which specific lipids accumulate at the target site and the mechanisms by which they promote cytokinesis remain an active area of research in the field of cytokinesis. Abnormal lipid metabolism affects cytokinesis and has been linked to several human diseases (Box 1). In this review, we will discuss physical and chemical properties of various lipids in promoting high membrane curvature and their distinct distribution during cytokinesis. We will address differences in lipid composition and distribution in mitotic and male meiotic cells. Finally, we will discuss various mechanisms involved in lipid polarization during cytokinesis. We will specifically focus on the role of sphingolipids and phosphatidylinositols in the regulation of cytokinesis.
Box 1. Diseases with cytokinetic failures due to abnormal lipid metabolism.
Although cytokinetic failures are normal and important for certain physiological functions such as differentiation of hepatocytes, cardiomyocytes, vascular smooth muscle, megakaryocytes, and germ cell differentiation, cytokinetic failures in certain tissues induce pathological states such as cancer, certain blood diseases, female infertility, Lowe syndrome, neurofibromatosis type II, sphingolipidoses, and age-related macular degeneration [13]. Here we briefly discuss sphingolipidoses, Lowe syndrome, cataract in age-related diseases and cancer due to their direct association with lipid metabolism.
Sphingolipidoses:
Sphingolipidoses are a group of inherited lysosomal storage disorders caused by deficiency of lysosomal enzymes, such as β-galactosylcerebrosidase in Krabbe disease, β-glucocerebrosidase in Gaucher disease, Globotriaosylceramidase in Fabry disease and acid sphingomyelinase in Niemann Pick type A&B [14,15]. Patients deficient in these enzymes show significant accumulation of its immediate sphingolipid precursor such as β-galactosylceramide and its corresponding metabolic deacylated lyso-form, galactosylsphingosine (psychosine) in Krabbe disease. The metabolic origin of psychosine was unknown until recently; Yedda Li et. al. have shown that psychosine was generated through the catabolic deacylation of galactosylceramide by acid ceramidase [16]. One of the characteristic features of Krabbe disease is the presence of multinucleated cells (globoid cells) in the white matter of the brain. Interestingly, psychosine was shown to induce cytokinetic defects and the formation multinucleated giant cells in several mouse and human cell lines in vitro [17]. Cytokinetic defects were also observed when cultured primary human umbilical vein endothelial cells were treated with quasi-pathological concentrations of beta-glucosylsphingosine, a lysosphingolipid that accumulates in Gaucher’s disease [18]. These studies suggested that lysosphingolipids are potent inhibitors of cytokinesis. Although molecular mechanisms are not fully understood, psychosine was shown to accumulate in membrane microdomains and disrupt lipid rafts [19], disperse intracellular membrane vesicles, prevent their accumulation at the cleavage furrow [20], and activate G-protein coupled receptors, T cell death-associated gene 8 (TDAG-8), and inhibit forskolin-driven accumulation of cAMP [17].
Lowe syndrome:
Oculocerebrorenal dystrophy, also known as Lowe syndrome, is caused by mutation in the OCRL gene that encodes for inositol 5-phosphatase. In Drosophila, OCRL was shown to mediate PI(4,5)P2 homeostasis on endosomal membranes and is required for cytokinesis [21,22]. In the absence of OCRL, PI(4,5)P2 was shown to accumulate on endosomal membranes, and as a result, several cleavage furrow-specific proteins are mislocalized to endosomes instead of cleavage furrow, leading to cytokinetic failure [21]. OCRL was shown to be required for remodelling of PI(4,5)P2 and F-actin disassembly at the intercellular bridge during cytokinetic abscission [23]. Although cytokinesis failures have not been linked to Lowe syndrome pathologies, cytokinetic abscission defects are observed in Lowe syndrome patient-derived cell lines [23].
Cataract, senescence, and premature aging:
Cytokinesis defects result in binucleation and polyploidy, leading to cellular senescence. It was shown that the late onset of cataracts in elderly people is linked to cytokinesis and premature cellular senescence [24,25]. Further, mutations in ESCRT complex subunits CHMP4B and VPS4 are associated with the early onset of cataract in vitro [26,27,28]. Interestingly, Phosphatidylinositol-4-phosphate 3-kinase (PIK3C2A)-null patients show congenital syndromic features resembling premature aging, early onset of cataract and secondary glaucoma [29]. Federico Gulluni et al. recently demonstrated that PIK3C2A localizes to midbody by binding to γ-tubulin and PI(4,5)P2. At the midbody, PIK3C2A generates PI(3,4)P2 using PI(4)P as a substrate and recruits ESCRT-II/VPS36, which recruit ESCRT-III subunit CHMP4B to the midbody in an ALIX independent pathway to mediate abscission. PI(3,4)P2 is required for VPS36 localization to the midbody [10].
Cancers:
Cancer is caused by abnormal cells that divide uncontrollably and have the ability to spread to other parts of the body. For offspring with the correct complement of chromosomes, chromosome segregation must be tightly coordinated with cytokinesis. Failure in cytokinesis results in multinucleated polypoid and aneuploid cells that have been historically observed in tumors [9,13]. Although much less is known, dysregulated lipid metabolism is commonly found in cancer cells, suggesting cancer cells require lipids for their rapid divisions [30]. Diacylglycerol (DAG), Sphingosine 1-phosphate (S1P), and ceramides are important signaling second messengers that have been implicated in cancers [31,32,33]. In response to growth factors, sphingosine kinase (SphK)-mediated production of S1P promotes cell proliferation in quiescent Swiss 3T3 fibroblasts via activation of dual signal transduction pathways, including the release of calcium from internal stores independent of Inositol triphosphate and activation of phospholipase D [34,35]. As opposed to S1P, ceramide, the backbone of all sphingolipids and precursor to S1P, acts as a growth arrest signal and promotes apoptosis in response to cytokines [36]. Thus, the dynamic balance between S1P and ceramide plays an important role in cell fate determination [37]. In addition, the acyl chain composition of phosphatidylinositols has been linked to p53 mutations in cancer cells [38]. Although altered lipid metabolism is intimately associated with cancer development, and cytokinetic failures, not much is known about the interrelationships between lipids, cytokinesis, and the development of cancer.
2. Lipid Shapes, Asymmetric Distribution, Lateral Organization, and Polarization during Cytokinesis
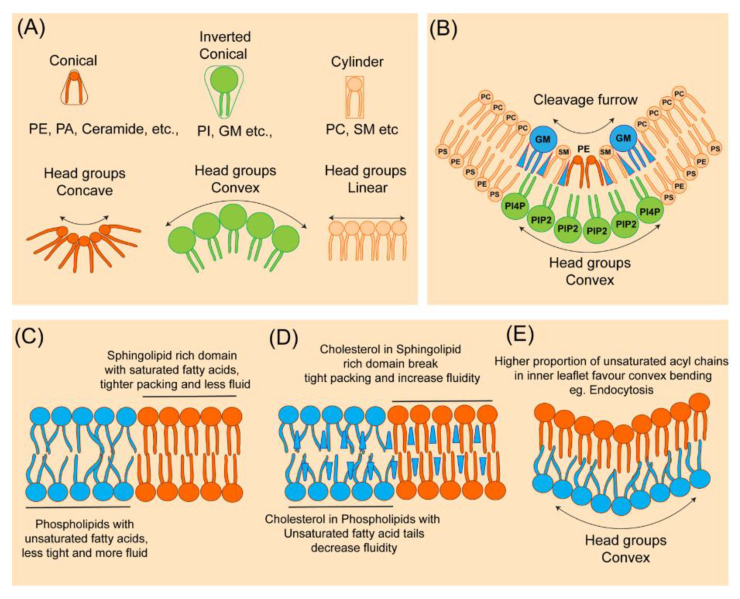
Induction of the highest plasma membrane curvature at the site of cytokinesis requires remodeling of protein and lipid composition. Biophysical and chemical properties of lipids play essential roles in mediating such high membrane curvature. Lipid head group chemical composition, acyl chain length, and saturation dictate membrane bending plasticity (Figure 2). Several lipids, including cholesterol [39,40], phosphatidylinositol phosphates (PIPs) [41,42], gangliosides GM1 [40] sphingomyelin (SM) [43], and phosphatidylethanolamine (PE) [44], have been shown to be enriched at the cytokinetic furrow (Figure 2). Further, lipidomic analysis of purified midbodies from cultured HeLa cells and neuroepithelial cells from cerebrospinal fluid showed accumulation of several sphingolipids, phosphatidylserine (PS), phosphatidic acid (PA), PE, ether PE, ether phosphatidylcholine (PC), and triacylglycerols (TAG) with unique acyl chain composition [11,12].
Figure 2.
Chemical and physical properties of lipids and their influence on membrane curvature during cytokinesis. (A) Lipid shape is based on the cross-sectional area of the head group relative to their fatty acid acyl chains. (B) Representative lipid composition at the cleavage furrow of a typical mitotic cell. (C,D) Lipids with saturated fatty acids tend to pack more tightly than unsaturated fatty acid-containing lipids, and as a result, they are less flexible and rigid (red). However, when cholesterol is present, it can prevent the tight packing of saturated fatty acid-containing lipids and thus increase fluidity. In contrast, cholesterol increases the packing density of unsaturated fatty acids and thus reduces fluidity in these lipid domains. (E) Unsaturated fatty acid-containing lipids in the inner leaflet of the plasma membrane favor convex bending, for instance in the case of endocytosis.
Lipids with small head groups relative to their acyl chains, such as PE, can have a conical shape that allows the head groups of PE to come close together to favor concave bending of the membrane leaflet (Figure 2). Conversely, lipids with larger head groups relative to their acyl chains, such as PIPs, can have an inverted conical shape that confers convex membrane bending property to the leaflet (Figure 2). Lipids that have similar head and tail cross sectional area are cylindrical in shape and therefore do not influence membrane curvature (ex. PC and PS) [45,46]. However, successful bilayer bending may require both concave and convex bending lipids to be present on the opposite leaflets. For instance, at the cytokinetic furrows in mammalian cells, the concave bending lipid PE was shown to be enriched at the outer leaflet of PM; in contrast, PI(4, 5)P2, a convex bending lipid, localized to the inner leaflet (Figure 2). Besides lipid composition, the successful development of essential membrane curvature is also influenced by protein composition, such as the shape of transmembrane proteins, protein–protein crowding [47], and by proteins that insert their amphipathic/hydrophobic helix (ADP ribosylation factors (Arf), Bin/Amphiphysin/Rvs (BAR) domain, etc.,) into the membrane [48,49,50]. Finally, the cytoskeleton is important in generating the force required to achieve high membrane curvature [51,52]. BAR domain proteins are important regulators of membrane curvature; they act as connecting links between the membrane and dynamic actin. BAR domain proteins are crescent-shaped, contain positively charged residues at the concave side that allow preferential binding to anionic phospholipids [53]. The F-BAR domain protein CDC15 plays important roles in the assembly of the contractile ring in the fission yeast Schizosaccharomyces pombe [54]. CDC15-mediated contractile ring assembly involves a sequence of events including dephosphorylation of the hyperphosphorylated state at interphase, binding to formin CDC12, localization to the site of cytokinesis, recruitment of Myo1 (class II myosin heavy chain), and activation of the Arp2/3 complex to promote actin cable network formation [53,55,56,57,58,59,60,61,62,63,64,65].
It is well known that several lipids are asymmetrically distributed between the inner and outer leaflets of biological membranes [66,67]. In mammalian cells, amino phospholipids such as PE and PS and negatively charged phospholipids such as phosphatidylinositols (PIs) are localized in the inner leaflet, whereas choline containing phospholipids such as PC and SM and glycosphingolipids (GSLs) are enriched in the outer leaflet of the plasma membrane. In contrast to mammalian cell plasma membranes, amino phospholipids PS and PE in insect plasma membranes are symmetrically distributed between inner and outer leaflets [68]. However, transbilayer and lateral organization of phosphatidylinositol phosphates and sphingolipids is conserved in all eukaryotic cells. The acyl chain composition is also asymmetrically distributed between the two leaflets; for instance, it was shown that the cytoplasmic leaflet is two-fold more unsaturated than the exoplasmic leaflet [69]. In addition to transbilayer asymmetry, lipids and proteins in the PM are segregated in the lateral dimension, whose behavior is best explained by three mutually non-exclusive models, including the picket-fence, lipid raft, and protein island models. Please see Box 2 for more details on each of these models. In the following subsections, we will discuss the role of individual lipids in cytokinesis in detail.
Box 2. Models of plasma membrane organization.
Our understanding of plasma membrane structure continues to evolve as newer imaging methods, such as single molecule imaging, become available. As a result, significant modifications are added to the textbook fluid mosaic model of the plasma membrane. Currently, plasma membrane structure and function are best explained by three mesoscale organizing principles, including actin-based partitioning of membrane proteins and associated lipids (the picket-fence model), the formation of liquid-ordered domains due to molecular affinities between lipids and proteins (the lipid raft model) and existence of proteins as dynamic molecular complexes (the protein island model).
Picket fence model: Single molecule tracking studies have shown that diffusion of molecules in the plasma membrane of living cells is 5–50-fold slower than that in the artificial membranes [70,71,72,73]. Based on the experimental evidence, it was proposed that the plasma membrane is divided into unspecific membrane compartments (40–300 nm) where molecules are temporarily confined. Such molecular confinement is dependent on the actin cytoskeleton, as depolymerization of actin increases the confinement zones. These observations were interpreted as transmembrane proteins with their immediate lipid environment (pickets) being anchored to an actin-based membrane skeleton (fence) located parallel to the plasma membrane in the cytosol. Molecules show unrestricted diffusion within the confinement zone but undergo hop-diffusion when they enter the neighboring zone. Since the borders of confinement zones are unspecific, they cover the entire plasma membranes without affecting protein distribution, and the lipid rafts can coexist, although their dynamics are likely affected by diffusion barriers [70,74].
Lipid raft model: Lipid rafts are small dynamic nanoscale (2–20 nm) assemblies composed of cholesterol, sphingolipids, and proteins that can be stabilized to grow into mesoscale (2–300 nm) functional domains [70,74,75,76]. Lipid rafts are also known as liquid ordered domains (lo domains) or detergent resistant membranes (DRMs). According to this model, at any given time, about 35 percent of all membrane proteins are localized to lipid rafts, and the rest of the 65% can move freely according to Singer and Nicolson’s fluid mosaic model [77,78]. Lipid raft dynamics can also be influenced by actin cytoskeleton via transbilayer coupling involving long acyl chain interdigitation and in the cytoplasm, phosphatidylserine head group to cytoskeleton interactions via adaptor proteins [79,80,81,82,83]. An emerging concept called “active emulsions” suggests that lipid raft formation and growth into mesoscale domains require transbilayer coupling, cortical actomyosin contraction, and lateral molecular interactions [81,83]. Thus, the active emulsion concept connects the lipid raft with the cortical actin and cortical flows to mediate the formation of mesoscale nonequilibrium lateral membrane organization [81,83].
Protein Island model: Based on the electron and super-resolution microscopy analyses, it was proposed that all the plasma membrane proteins are segregated according to their nature and function into distinct domains called protein islands. These domains can be classified as raft or non-raft depending on their interactions with the lipid molecules; additionally, transmembrane proteins can interact with the actin cytoskeleton for dynamic movement. Molecules undergo hop-diffusion when they translocate from one protein island to another via shared borders. The protein-free and low cholesterol membrane domains separate these islands [84,85].
3. Phosphatidylethanolamine (PE)
In mammalian cells, PE is normally localized to the inner leaflet of the PM; however, during cytokinesis, it gets redistributed to the outer leaflet of the cleavage furrow. Immobilization of cell surface PE by PE binding peptide or reduction of PE levels by genetic ablation of PE synthesis in CHO cells specifically blocked disassembly of the contractile ring after furrow ingression, leading to the formation of a long cytoplasmic bridge between daughter cells. This phenotype was rescued by the addition of PE or its precursor ethanolamine, suggesting an important role for transbilayer PE redistribution in the disassembly of the contractile ring [44]. Immobilization of PE at the outer leaflet of the plasma membrane led to PI(4,5)P2 over accumulation in the intercellular bridge, prevented actin disassembly, and inhibited abscission [41].
The acyl chain composition of PE is also important in cytokinesis. The ether-linked PE species with longer and more unsaturated fatty acids accumulated in the midbody lipidome of mouse neuroepithelial cells. In contrast, phosphatidylcholine species were composed of shorter and more saturated acyl chains [11]. In Arabidopsis thaliana, depletion of very long chain fatty acids (VLCF) by mutation in a microsomal elongase gene significantly reduced VLCFA-containing PE species and induced cytokinetic defects by affecting endomembrane dynamics [86]. Thus, PE redistribution and its acyl chain composition may play an important role in cytokinesis via regulation of PI(4,5)P2 levels [41].
4. Phosphatidylinositol Phosphates (PIPs)
Phosphatidylinositols (PI) are glycerophospholipids with a myo-inositol head group. PIs are initially synthesized on the membranes of the endoplasmic reticulum and subsequently transported to other cellular membranes. The cytosolically exposed hydrophilic myo-inositol head group on the target membranes gets modified by selective PI-kinases or PI-phosphatases to generate unique PIP domains in response to various cellular cues [87]. Phosphorylation of the myo-inositol head group generates seven forms of phosphatidylinositol phosphates (PIPs) including three monophosphates (PI3P, PI4P, and PI5P), three bisphosphates [PI(4,5)P2, PI(3,4)P2, and PI(3,5)P2] and one triphosphate, PI(3,4,5)P3 [88,89]. Importantly, specific PI functions are achieved through the recruitment of specific PI-binding proteins that are selective for one or a few PIs (e.g., Anillin’s PH domain binds to PI(4,5)P2 or FYVE-CENT binds to PI(3)P). PIPs are uniquely distributed in the subcellular membrane compartments. PI(4)P is localized to PM, endosomes, and the trans Golgi. PI(3)P is found in early endosomes, whereas PI(5)P is found on the PM, endosomes, nuclear envelope. PI(4,5)P2 localized to PM, recycling endosomes, and lysosomes; PI(3,4)P2 to PM and early endosomes; PI(3,5)P2 to late endosomes and lysosomes; PI(3,4,5)P3 is localized to the PM and endocytic compartments [90]. To date, several PIs have been implicated in cell division and cytokinesis, including, PI, PI3P, PI4P, PI(4,5)P2, PI(3,4)P2, and PI(3,4,5)P3 [3,4,10,88,89].
PI(4,5)P2 is the most extensively studied lipid in animal cell cytokinesis, owing to its obvious enrichment in cytokinetic furrows in various cell types and organisms, as well as its broad implications in lipid–lipid and lipid–protein interactions and signaling [21,23,41,42,43,91,92,93,94,95]. Disruption of PI(4,5)P2 levels is associated with cytokinetic defects in several model organisms and mammalian cells [40,96,97,98,99]. PI(4,5)P2 normally localizes uniformly to the inner leaflet of the plasma membrane in mammalian cells during prophase; however, during cytokinesis, PI(4,5)P2 is specifically enriched at the inner leaflet of the cleavage furrow [41,42]. However, PI(4,5)P2 accumulation at the furrow is not universal because its enrichment was not observed in Drosophila spermatocytes [94,99] and Dictyostelium [100], suggesting the existence of alternative mechanisms in different cell types or organisms. PI(4,5)P2 is required for adhesion of plasma membrane to the contractile ring [42]. In yeast, PI(4,5)P2 is required for localization of RhoA, a small GTPase required for assembly of the contractile ring [101,102]. Many cytoskeletal elements and regulatory proteins, including RhoA [43,102,103,104,105,106], Ect2 RhoGEF [103,105], Anillin [107], Syndapin [94], Septins [108,109,110,111], MgcRacGAP [112] and subunits of the vesicle-tethering complex Exocyst [113], bind to PI(4,5)P2. Furthermore, PI(4,5)P2 regulates cytokinesis via exocytosis, endocytosis, and endocytic recycling [6,114,115,116,117,118,119,120,121,122,123,124,125]. Recently, it was shown that a phosphatidylinositol-4-phosphate 3-kinase catalytic subunit type 2A (PI3KC2A) localizes to the midbody via binding to PI(4,5)P2 and γ-tubulin and produces PI(3,4)P2 from PI(4)P. The ESCRT II component VPS36 binds to PI(3,4)P2 and recruits, ESCRT III component CHMP4B in an ALIX independent pathway to mediate abscission in eye lens cells [10]. Besides PI(4,5)P2 production, its turnover and clearance also influence cytokinesis [41,92,99]. Several excellent reviews have focused on PIs and their role in cytokinesis and will not be discussed extensively here [3,4,88,89,126,127,128,129].
In mitotically dividing cells, PI(3) kinase and its product PI(3,4,5)P3 localized to polar regions; in contrast, PTEN, a PI(3,4,5)P3 phosphatase, localized to concentrates at the cleavage furrow [100]. Similarly, PI4P 5-kinase and its product PI(4,5)P2 accumulate at the furrow, thus effectively enriching PI(4,5)P2, and depleting PI(3,4,5)P3 at the furrow [42,130]. Interestingly, we have shown that a PI(3,4,5)P3 specific probe tGPH (GFP fused to pleckstrin homology domain of Grp1/Steppke under tubulin promoter) was enriched at the cleavage furrow in dividing Drosophila spermatocytes indicating PI(3,4,5)P3 localization to this region. However, tGPH enrichment at the furrow was observed only in intact cysts, i.e., spermatocytes encased by cyst cell membrane, and acute loss of cyst cell membranes and concomitant reduction in PI(3,4,5)P3 accumulation at the furrow did not prevent ingression of cleavage furrow during cytokinesis in spermatocytes [131]. The significance of such PI(3,4,5)P3 enrichment at the furrow is currently unknown and warrants future investigation. A study on plasma membrane expansion during cellularization in Drosophila embryos found that PI (3,4,5)P3 inhibited PI(4,5)P2-dependent actomyosin contractility, promoting actomyosin network disassembly [132]. The PI(3,4,5)P3 probe, Steppke, localized to actomyosin networks and reduced tissue tension by inhibiting the actomyosin activity at the adherens junction during dorsal closure in Drosophila embryos [133]. Thus, PI(4,5)P2 and PI(3,4,5)P3 are likely to play distinct and antagonistic roles in the regulation of actomyosin ring contractility during cytokinesis.
5. Sphingolipids
Sphingolipids are essential for cytokinesis, as disruption of sphingolipid biosynthesis either by genetic ablation or pharmacological methods induces cytokinetic defects [12,134,135,136]. It was shown that inhibition of sphingolipid synthesis reduces cell surface area by 45% and disrupts the actin cytoskeleton in Swiss 3T3 cells, and these effects are reversed by the addition of ganglioside GM3 [135]. Inactivation of glucosyl ceramide synthase (GCS) either by RNA interference (RNAi) or by 1-phenyl-2-palmitoyl-amino-3-morpholino-1-propanol (PPMP) caused failure of cleavage furrow ingression [134] due to accumulation of C16, C20, and C22 ceramides. Ceramides added exogenously to cultured cells caused cytokinetic defects. It was shown that inhibition of GCS results in mislocalization of cytoskeletal proteins, including actin and ERMs (ezrin, radixin, and moesin) that connect the PM with the actin cortex [134]. PPMP was also shown to induce cytokinetic defects in Giardia lamblia [136]. Long-chain sphingolipids, including dihydroceramide (C22 and C24), ceramide (C24), and hexosylceramides (C16 and C24) species, were found in the midbody lipidome of cultured HeLa cells, and RNAi knockdown of various sphingolipid metabolic enzymes, including biosynthetic and catabolic enzymes, resulted in cytokinetic defects [12]. Sphingolipids were shown to be essential for cytokinesis in Trypanosoma brucei. Depletion of serine palmitoyl transferase subunit 2 (SPT2) by RNAi or its inhibition with myriocin affected cell cycle progression but not vesicular trafficking or lipid raft formation [137]. Very long acyl chain (C24) glycosphingolipids (GSLs) such as glucosyl-ceramides were shown to be essential for cell plate formation during cytokinesis in plants [138].
Sphingolipids such as SM and gangliosides in combination with cholesterol were shown to form liquid ordered domains (Lo domains) on the outer leaflet of the PM [75]. Ganglioside GM1 and the cholesterol-rich domains were shown to be enriched in cleavage furrows of dividing sea urchin eggs [40]. Accumulation of GM1 and the cholesterol-rich domain at the furrow is dependent on the onset of anaphase, myosin light chain phosphorylation, actin, and microtubules. Saturated fatty acids but not unsaturated fatty acid-containing lipids were found to accumulate at the furrow. Membrane-associated proteins such as Src (non-receptor tyrosine kinase) and PLC γ (phospholipase C) are enriched in Lo domains, and their activation via tyrosine phosphorylation is essential for cytokinesis [40]. PLC γ activation leading to Ca2+ release is essential for cytokinesis [98]. Inositol 1,4,5-triphosphate (IP3) receptor-mediated calcium release is required for furrowing in spermatocytes and sea urchin eggs [99,139].
Sphingomyelin (SM), a major sphingolipid, is normally present uniformly on the outer leaflet of PM, and, during cytokinesis, it specifically gets enriched at the cleavage furrow. Interestingly, SM at the outer leaflet regulates PI(4,5)P2 accumulation in the inner leaflet of the furrow [43]. Depletion of SM at the outer leaflet using SMase treatment specifically disrupts PI(4,5)P2 but not cholesterol accumulation [43]. Cleavage furrow-specific enrichment of SM is dependent on cholesterol but not on PI(4,5)P2. Although it is unclear how SM regulates PI(4,5)P2 enrichment at the furrow, it has been proposed that SM could directly or indirectly interact with PI(4,5)P2 to limit its diffusion as well as locally activate PI(4)P 5-kinase for confined PI(4,5)P2 synthesis [43]. Proteins embedded in the cytoplasmic leaflet of the cleavage furrow indeed show slower diffusional rates than the outer leaflet, and this process is regulated by septins in mammalian cells [140,141,142]. Thus, sphingolipids regulate cytokinesis via the formation of Lo domains, transbilayer coupling, signal transduction, and cytoskeletal reorganization.
6. Cholesterol
Cholesterol was shown to accumulate at the cleavage furrow in sea urchin eggs [40], more specifically at the outer leaflet of the cleavage furrow, whereas it remained uniformly distributed in the inner leaflet [43]. Sterol rich membrane domains have been identified in growing tip and septum of S. pombe, and their integrity is essential for proper cytokinesis [143,144]. Optimal sterol levels are important for normal cytokinesis; in fission yeast, it was shown that increasing ergosterol levels delays formin cdc12-dependent assembly of F-actin and disrupts division plane positioning [144,145]. In human tissue culture cells, cholesterol depletion results in polyploidy [39]. Lo domain-associated proteins flotillins were shown to form large, stable domains in the plasma membrane and accumulate at a higher density in the cleavage furrow of hematopoietic cells [146]. Cholesterol was shown to mediate local endocytosis in the ICB via the formation of midbody tubules, and that depletion of cholesterol prevents the formation of such midbody tubules and induces cytokinetic defects [147]. Caveolae are cup shaped 50 to 100 nm plasma membrane invaginations enriched in cholesterol and sphingolipids [148,149,150,151]. Caveolae were shown to be enriched at the cleavage furrow, and intercellular bridge, and midbody in mammalian cells and early zebrafish embryos [152,153,154]. Functionally, caveolae at the intercellular bridge were shown to buffer membrane tension and limit contractibility to promote ESCRT-III assembly and cytokinetic abscission [152].
7. Triacylglycerols (TAG)
Budding yeast quadruple mutants are1Δ, are2Δ, dga1Δ, and Iro1Δ, that cannot synthesize triacylglycerols show abnormal cytokinesis and septation defects [155]. TAGs with short chain acyl chains (16:1/12:0/18:1) have been shown to be specifically enriched in purified midbodies of HeLa cells and neuroepithelial cells [11,12]. RNAi knockdown of TAG metabolic enzyme DGAT2 significantly increased cytokinetic defects in HeLa cells [12]. Although TAG accumulation at the midbodies is intriguing, its specific mechanism of action remains unknown [8].
8. Phosphatidic Acid
Phosphatidic acid (PA) is yet another phospholipid enriched in purified midbodies from HeLa cells, and RNAi knockdown of its metabolic enzyme ABHD5 resulted in cytokinetic defects [12]. However, the mechanism by which PA regulates cytokinesis remains an open question. Phosphatidic acid is a conical shaped lipid that is localized to the cytosolic leaflet of the PM and imparts concave membrane bending properties. Phosphatidic acids are important signaling molecules, involved in vesicular trafficking, and are also precursors for the synthesis of most of the glycerophospholipids, such as phosphatidylserine and phosphatidylinositols [156,157,158]. PA has been shown to stimulate PI(4)P 5-kinase via Arf6 to promote PI(4,5)P2 synthesis [159,160,161]. PA can also recruit sphingosine kinase to the plasma membrane, where it catalyzes the synthesis of sphingosine-1-phosphate, an important signaling molecule [157]. Inhibition of sphingosine kinase 1 compromises PKC activity and cytokinesis [162]. Phosphorylation of nonconventional PKC is required for completion of cytokinesis, and inhibition of it results in sustained RhoA activation and delayed contractile ring disassembly [163,164]. The Drosophila sphingosine-1-phosphate lyase gene (Sply) and sphingosine kinase 2 were shown to be important for normal muscle development and reproductive organ function [165,166], but no direct role in cytokinesis has been demonstrated.
9. Phosphatidylserine (PS)
PS is synthesized in the endoplasmic reticulum and trafficked via the Golgi to the plasma membrane, where it is mostly localized to the inner leaflet. In animal cells, PS can be flipped from the inner leaflet to the outer leaflet, where it acts as ‘eat me’ signal that promotes phagocytosis to engulf the cells. Apoptotic cells activate scramblases that quickly expose PS to the outer leaflet [167]. Relatively little is known about the role of PS in cytokinesis, although its specific enrichment was observed in the purified midbody lipidome of HeLa cells, particularly unsaturated long-chain PS [12]. In fission yeast, genetic ablation, or overexpression of Phosphatidylserine synthase (pps1), induced cell morphology and cytokinetic defects, suggesting a dose-dependent role for PS in cytokinesis [168]. Recently, it was shown that PS with very long-chain fatty acids are required for cytokinesis in Arabidopsis roots, and it was suggested that PS may help mediate cargo vesicle trafficking during cell plate formation [169]. Anionic phospholipids like PS and PA likely mediate important functions in cytokinesis by binding to various peripheral membrane proteins that have lipid binding domains such as BAR domain proteins, Annexin repeats, and C2 domain containing proteins (Table 1). Future studies are required to demonstrate the direct involvement of PS in animal cell cytokinesis.
Table 1.
Membrane/Lipid binding proteins involved in cytokinesis.
| Protein Name/Protein Complex | Localization | Predicted/Known Lipid Binding |
Mechanism in Cytokinesis | Ref. |
|---|---|---|---|---|
| ADP-ribosylation factor like protein 2–3 (ARL2, ARL3) | Midbody | N-terminal amphipathic helix/Anionic PLs. | Small GTPase, regulate microtubule dynamics | [170,171] |
| ADP-ribosylation factors (Arf1, Arf3, Arf6) | PM, Golgi, midbody, and endosomes | N-myristoylated | Small GTPases involved in Intra cellular vesicular trafficking, required during furrow ingression and abscission | [172,173] |
| Alix | Endosomes, midbody | Lysobisphosphatidic acid | Recruits ESCRT machinery to abscission site at midbody | [174,175] |
| ANCHR (abscission/NoCut checkpoint regulator; ZFYVE19) | Midbody | PIPs | Regulates the abscission checkpoint via retention of ESCRT component VPS4 at the midbody ring | [176] |
| Anillin | Contractile ring | PI(4,5)P2 | Scaffolds contractile ring at the cell equator | [177,178] |
| Annexin A2; Annexin11 | Cleavage furrow (A2), midbody (A11) | PIPs | AnnexinA2: connects equatorial cortex to central spindle and helps in localization of RhoGEF Ect2; Annexin A11, required for MKLP1 and Aurora B localization to the midbody | [179,180] |
| ARHGAP19 | Cleavage furrow | Anionic PLs | Controls cytokinesis in T lymphocytes by acting as GAP for RhoA | [181] |
| Armadillo protein p007/Plakophilin-4 (PKP4) | Midzone, midbody | Unknown | Interact with RhoA and Ect2 and regulates Rho signaling | [182] |
| Capping protein (CAPZB) | Cleavage furrow | Anionic PLs | Required for midbody maturation, regulates actin dynamics | [183,184] |
| Chronophin/PDXP | Localizes to PM, Cleavage furrow, midbody | Unknown | Regulates cofilin dependent actin dynamics | [185] |
| Citron Rho-interacting kinase (CIT) | Cleavage furrow and midbody | Unknown | Regulates midbody formation | [186] |
| Cofilin-1 | Cleavage furrow and midbody | PI(4,5,)P2 | Regulate actin filament severing | [187,188] |
| ESCRT complex | Midbody | Anionic PLs | Involved in membrane cut during abscission | [189,190,191] |
| Exocyst complex Sec3, Sec5, Sec6, Sec8, Sec10, Sec15, Exo70 and Exo84 |
Endosomes, Early, late cleavage furrow and midbody | Sec3 and Exo70 interact PI(4,5)P2 | Tethering of secretory vesicles to plasma membrane | [192,193,194,195] |
| Ezrin, Radixin, Moesin (ERM) proteins | Cholesterol dependent localization to cleavage furrow | PI(4,5,)P2 | Regulate membrane to cytoskeleton interaction | [196,197] |
| F-BAR domain/Cdc15 | Cell middle/contractile ring | Anionic PLs | Interacts with formin Cdc12 and promote contractile ring formation | [65] |
| Gin4 (Nim1 protein kinase) | PIPs | Septin assembly | [198] | |
| Golgi phosphoprotein 3 (GOLPH3) |
Golgi, cleavage furrow | PI4(P) effector | Required for localization of Rab11-positive PI(4)P enriched vesicles at the cleavage furrow | [199] |
| GPCRs | Spindle pole, midzone, midbody | Multi-pass membrane proteins | Affects actin cytoskeleton, knockdown causes defects | [200,201,202] |
| Integrin beta-1 | Rab21 endosomes | Single pass membrane protein | Rab21 mediated integrin trafficking to and from the cleavage furrow | [203] |
| IPIP27 | Endomembranes | PIPs | Scaffolds OCRL and couples it to endocytic BAR domain proteins (SH3PX1 or Pacsin2); involved in PI(4,5,)P2 homeostasis | [204] |
| MICAL (1 and 3) | Intercellular bridge | Unknown | MICAL-L1 and MICAL3 mediate targeting of Rab11-FIP3/Rab35 and Rab8 positive endosomes respectively to the ICB; regulate F-actin levels at midbody | [3] |
| MIT domain containing protein 1 (MITD1) | Late endosomes and midbody | PI(4,5,)P2 | Interacts with ESCRT-III components and mediates abscission | [205] |
| Mso1 (Mint1) and Sec1 (Munc18) | Cell division site | Interact membrane via SNAREs | Vesicle fusion and cargo delivery; CR constriction, disassembly, and membrane closure defects | [206] |
| Myosin 19 (MYO19) | Mitochondria outer membrane | PA, PIP, PIP2 and PIP3 | Involved in mitochondrial segregation via actin-based motor activity | [207,208] |
| Nonmuscle myosin-II | Cleavage furrow/ Contractile ring |
Anionic phospholipids | Directly binds to membranes independent of F-actin | [209] |
| OCRL | Endosomes/intercellular bridge | PI(4,5)P2 | Rab35 dependent localization to ICB, hydrolyze PI(4,5)P2 which in turn help in actin clearance. | [23] |
| Opy1 | Dual PH domain containing protein | PI(4,5)P2 | Endogenous PI(4,5)P2 sensor binds to its3 | [210] |
| P190RhoGAP (ARHGAP35 and ARHGAP5) | Cleavage furrow | Anionic PLs | Regulates RhoA activity | [211,212] |
| P50RhoGAP (ARHGAP1) | Midbody | Anionic PLs | Promote actin clearance in the intercellular bridge | [213] |
| PDZD-8, TEX-2, OCRL and UNC-26/synaptojanin | ER/intracellular membrane compartments | PI(4,5)P2 | Endosomal PI(4,5)P2 homeostasis | [214] |
| PI3K-C2α | Midbody | PI(3,4)P2 | VPS36 binds to PI(3,4)P2 at the midbody and recruits CHAMP4B to mediate abscission | [10] |
| PI3K-III kinase complex (VPS15, VPS34, Beclin1, UVRAG and BIF-1 | Localizes to endosomes and midbody | PI, PIP, N-myristoylation (Vps15) | Phosphorylates PI to PI(3)P; required for abscission | [215,216] |
| Pkd2/polycystins | PM/cell equatorial plane | Transmembrane protein | Membrane stretch activated Ca+ influx | [217] |
| PRIP (phospholipase C (PLC)-related catalytically inactive protein) | Cleavage furrow | PI(4,5)P2 | regulates phosphoinositide metabolism at cleavage furrow | [218] |
| Prostate androgen regulated protein (PAR) | Centrosomes, spindle midzone, midbody | Single pass membrane protein | Form complex with Aurora A, Survivin, Aurora B and INCENP and crease Aurora B kinase activity | [219] |
| Protein kinase C epsilon (PRKCE) | Late cytokinetic furrow | DAG | Required for RhoA inactivation and actomyosin clearance during abscission | [164] |
| PTEN | Cleavage furrow | PI(3,4,5)P2 | Contributes to PI(4,5)P2 production at the furrow. | [93,100] |
| PTEN/dPLCXD | Endosomes | PI(4,5)P2 | Novel PI(4,5)P2 phosphatase, overexpression rescues OCRL loss of function phenotypes | [220] |
| Rab1 | Golgi, cleavage furrow | S-geranylgeranylations | Interact with GOLPH3 and regulates vesicular trafficking | [221] |
| Rab10 | Cleavage furrow midbody | S-geranylgeranylations | Unknown, possibly involved in delivery of Sorcin protein to cleavage site which is involved in regulating calcium homeostasis and Polo-like kinase-1 | [222] |
| Rab14 | Cleavage furrow/midbody | S-geranylgeranylations | Regulate actin clearance at the midbody. | [223,224] |
| Rab21 | Early endosomes, cleavage furrow, midbody | S-geranylgeranylations | Integrin bet-1 trafficking during late steps of cytokinesis | [203] |
| Rab24 | Mitotic spindle, cleavage furrow and intercellular bridge | S-geranylgeranylations | Affects kinetochore-microtubule attachment | [225,226] |
| Rab35 | Recycling endosomes, cleavage furrow, mid body | PIP2, S-geranylgeranylations | PIP2 and F-acting remodeling during late steps of cytokinesis | [23,92,173] |
| Rab7 | Late endosomes, multivesicular bodies | Small GTPase, S-geranylgeranylation | Membrane delivery to cleavage furrow | [131] |
| Rab8 | Midbody | S-geranylgeranylations | Required for abscission | [227] |
| RacGTPase-activating protein-1 (MgcRacGAP) | Cleavage furrow and midbody | Anionic phospholipids | Regulates Rho GTPase | [112] |
| RalA and RalB | RalA (cleavage furrow) RalB midbody | Polybasic motif and S-geranylgeranylation | RalA (exocyst targeting to cleavage furrow); RalB (exocyst targeting to midbody during abscission) | [228,229,230] |
| Ras-related protein Rab11A and Rab11 family interacting protein 3 and 4 | Recycling endosomes, Cleavage furrow midbody |
Rab11 (small GTPase) S-geranylgeranylation | Endocytic traffic during late steps of cytokinesis, | [231,232] |
| Rho GTPase-activating protein 35 (ARHGAP35) | Cleavage furrow | Anionic phospholipids | Regulates actomyosin contractility | [211,212,233] |
| Rho GTPases (RhoA, RhoB, RhoC, Cdc42 and Rac1) | Cleavage furrow | Polybasic motif, S-geranylgeranylation (all), S-palmitoylation (Cdc42, RhoB) and S-farnesylation (RhoB) | Acting cytoskeleton organization, Rho is involved in cleavage furrow formation and ingression | [234] |
| Rho-associated protein kinases 1 and 2 | Midbody | PIP2, PIP3, sphingosine, arachidonic acid, PC | Rho effector kinases. Regulates contraction of actomyosin ring by phosphorylation of myosin light chain | [235,236] |
| RhoGEF ECT2 | Cleavage furrow, midbody | Phosphoinositides | RhoA activation and cleavage furrow formation | [105] |
| Septins (SEPT1-12, SEPT14) | Cleavage furrow, midbody | PI(4,5,)P2 | GTPase, form filaments, rings during cleavage furrow ingression and abscission at midbody; act as diffusional barrier | [237,238] |
| Serine/threonine-protein kinase N2 (PKN2) | Cleavage furrow and midbody | C2 domain, anionic PLs, arachidonic acid | Function as Rho/Rac effector, required for abscission | [239,240] |
| Serologically defined colon cancer antigen (SDCCAG3) | Endosomes, midbody | unknown | Regulate Arf-mediated vesicular trafficking/signaling | [241] |
| SNARE (t-SNAREs include Sso, Sec9,) (v-SNARE Snc1/2) |
Target SNAREs(t-SNARE/syntaxin) Vesicular SNARE (v-SNARE/VAMP) |
Transmembrane proteins | Required for vesicle fusion at the target membranes | [3,242] |
| Sorting nexins (SNX9, SNX18, SNX33) |
PX and BAR domain | PIP, PIP2, PIP3 | Required for endocytosis dependent and independent roles in cytokinesis | [243] |
| Spartin (SPG20) | Lipid droplet and endosomes | unknown | Localizes to midbody by interacting with ESCRT-III protein Ist1 | [244] |
| Spastin (SPAST) | ER, endosomes, midbody | unknown | Involved in microtubule severing during abscission | [245] |
| Spectrin beta chain | PH domain | PIP2, PIP3, PS | Lateral membrane biogenesis in concert with Ankyrin-G | [246] |
| STAM-Binding protein | Endosomes, cleavage furrow, midbody | unknown | Regulation of ubiquitylation at central spindle | [247] |
| Stomatin | Plasma membrane | Cholesterol | Change in lipid species including ether lipids and PC | [248,249] |
| Supervillin | Cleavage furrow midbody | Interact with lipid raft proteins | Links plasma membrane to the cytoskeletion | [250,251,252] |
| Ubiquitin carboxy-terminal hydrolase 8 (USP8) | Endosomes, midbody | Farnesylation ? | Regulation of ubiquitylation at central spindle | [247] |
| UNC119a | Dynamic localization; spindle midzone and midbody | Interact with N-myristoylated proteins | Has role in Fyn signaling and Rab11 dependent phosphorylation at midbody | [253] |
| Ync13/UNC-13/Munc13 | Plasma membrane, cell tip, division site | PS, PIP2 | Coordinates exocytosis, endocytosis, and cell wall integrity | [254] |
| YPP1 and Efr3 | Plasma membrane | Basic residues in Efr3 binds to anionic PLs. | Scaffolds Stt4 (PI4KIIIalpha) | [255,256,257] |
| Zinc-finger FYVE domain-containing protein 26 (ZFYVE26) | midbody | PI(3)P | Interact with ESCRT-III subunits to mediate abscission | [258] |
10. Sphingolipid Acyl Chain Composition in Male Meiotic Cytokinesis
Cytokinesis in somatic cells results in the complete physical separation of cytoplasm between the daughter cells. In contrast, cytokinesis in spermatocytes is incomplete, where all the sister cells are interconnected via cytoplasmic bridges [259]. The presence of sphingolipids (ceramides, sphingomyelin, and glycosphingolipids) with very long chain polyunsaturated fatty acids (VLC-PUFA) is one of the characteristic features of mammalian spermatozoa/testes [260,261,262,263,264,265]. In general, sphingolipids are required for cytokinesis in both somatic cells and male meiotic cells; however, VLC-PUFAs containing sphingolipids are specifically associated with male meiotic cells [263]. Glycosphingolipid deficiency leads to male meiotic cytokinesis defects [266] and is required for intercellular bridge stability [267]. Ceramide synthase 3 (CerS3) was discovered to be required for the synthesis of sphingolipids containing very long chain polyunsaturated fatty acids [266,267]. A Drosophila member of the very long chain fatty acid synthase (Elovl) gene, Bond, was shown to be important for cytokinesis in spermatocytes [119]. Bond mutants show reduced cleavage furrow ingression with frequent contractile ring detachment from the cortex, constriction, and collapse of the contractile ring to one side of the cell, leading to cleavage furrow regression [268]. Lipids containing very long chain fatty acids have been proposed to promote membrane deformation and stable contractile ring and plasma membrane interaction during cytokinesis [268]. Drosophila desaturase gene family members exhibit a sex-specific expression pattern [269]. Sphingolipid delta-4 desaturase (Des1/ifc1) catalyzes the final step of de novo ceramide synthesis by introducing a double bond in the sphingoid base and is essential for spermatogenesis but not for oogenesis [270,271]. P-element insertion mutants of des-1 were shown to be defective in central spindle assembly during male meiotic cytokinesis. During anaphase and telophase, DES-1 localizes to mitochondria along the spindle apparatus. Before the biochemical identification of this protein as a sphingolipid desaturase enzyme, it was proposed that DES-1 could act as an anchoring mechanism where it links membrane-bound cellular compartments to the cytoskeletal components [270].
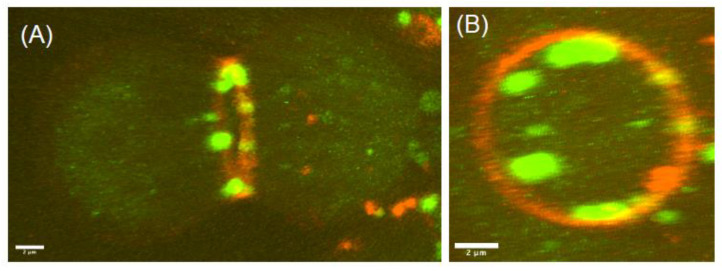
The small regulatory subunit of serine palmitoyltransferase (ssSPT), Ghiberti (also known as frodo), which is part of the Serine Palmitoyltransferase (SPT) enzyme complex, has been shown to be required for male meiotic cytokinesis [119,272]. ghi mutants display normal actomyosin ring assembly, but the ring fails to constrict to completion, leading to furrow regression and cytokinetic defects [119]. Loss of Ghi affects the d16/d14 (sphingoid base acyl chain length) ratio, with a net shift towards increased accumulation of sphingolipids with a d16 sphingoid base, suggesting a crucial role for sphingoid base acyl chain length in male meiotic cytokinesis [272]. Further, Drosophila males had more unsaturated fatty acids in their sphingolipids compared to females [272]. However, the molecular mechanisms by which these specific sphingolipids regulate cytokinesis were unclear until recently. Recent studies, including ours, showed that de novo biosynthesis of ceramide posphoethanolamine (CPE), a sphingomyelin analogue in Drosophila, is essential for male fertility [273,274]. We have shown that testis specific CPEs show increased unsaturation both in the sphingoid base and fatty acid acyl chains (e.g., d14:1/C24:1, and d14:2/C24:0). The ethanolamine head group of CPE is also essential for cytokinesis, as expression of human sphingomyelin synthase that produced SM in Drosophila spermatocytes did not rescue cytokinetic defects [131]. Live cell imaging analysis using the mushroom-derived CPE-binding protein pleurotolysin A2 (PlyA2) showed that CPE is endocytosed from the plasma membrane and delivered to the cleavage furrow via the endocytic pathway, and this CPE trafficking is essential for male meiotic cytokinesis (Figure 3) [131].
Figure 3.
Multivesicular endosomes release their intraluminal vesicles in the cytosol closure to the cleavage. (A) Horizontal view of a spermatocyte undergoing cytokinesis, showing EYFP Rab7positive endosomes (green) docked onto the mRFP-Anillin ring (red). (B) A cross sectional view of a spermatocyte in (A), showing EYPF-Rab7 positive endosomes (green) docked onto the mRFP-Anillin ring (red).
Why do male germ cells have high amounts of unsaturated fatty acid-containing lipids, and why are they important in male meiotic cytokinesis? Although exact mechanisms are currently unknown, the answer could lie in their widely known roles in signaling and modulation of membrane biophysical properties. Unsaturated fatty acid-containing lipids introduce more disorder in the membranes; thereby, they make membranes more fluid, reduce membrane thickness, increase lipid packing defects, reduce membrane rigidity/make membrane more flexible, affect membrane protein conformations, and increase sensitivity to oxidation (Figure 2) [275,276]. Sphingolipids with unsaturated fatty acids were found to be excluded from liquid-ordered domains/lipid rafts in rat spermatocyte membranes [277]. Sphingolipids with unsaturated fatty acids may thus increase membrane flexibility or curvature and or lipid–protein interactions during male meiotic cytokinesis and this requirement may be different in the case of somatic cell cytokinesis. For instance, membrane bending is not only important at the ingressing cleavage furrow but also in other cellular processes such as endocytosis and the maturation of multivesicular endosomes. It was shown that when polyunsaturated fatty acids (PUFA) containing glycerophospholipids (GPL) were present at the convex leaflet of a bent membrane, they reduced the rigidity [278] and facilitated endocytosis [279]. Indeed, it was shown that PUFA-GPLs are asymmetrically distributed in mammalian cell plasma membranes and the endocytic system, where the cytoplasmic leaflet is two-fold more unsaturated than the exoplasmic leaflet and that the outer PM leaflet is more packed and rigid [69]. However, in contrast to glycerophospholipids, sphingolipids such as sphingomyelin or glycosphingolipids are normally localized to the outer leaflet of the plasma membrane [69]. Thus, unsaturated fatty acids in the outer leaflet localized sphingolipid could have a unique convex membrane bending property, as in the case of multivesicular endosome maturation. Indeed, the sphingolipid ceramide was shown to be required for the formation of intraluminal vesicles in multivesicular endosomes via an ESCRT (endosomal sorting complex required for transport) independent mechanism [280] and the absence of DES1 significantly reduced the MVB intraluminal vesicle density [281]. In concurrence, our recent study showed that CPE is enriched in intraluminal vesicles of multivesicular endosomes, which in turn localize to cleavage furrow [131]. Together, these studies suggest that sphingolipids with unsaturated fatty acids, as opposed to saturated fatty acids, are excluded from liquid-ordered domains, reduce membrane rigidity, and facilitate endocytic membrane traffic during male meiosis cytokinesis.
11. Membrane Trafficking and Lipid Polarization at the Cytokinetic Furrow
A typical animal cell requires a total surface area increase of about 26% for successful division, and meiotic cells require about 60% due to two rounds of rapid successive divisions [192,282,283]. Such a dramatic increase in cell surface area would require multiple mechanisms of membrane trafficking and lipid remodulation, including (I) expansion of plasma membrane reserves present in the form of microvilli or other membrane projections (II) secretion of newly synthesized membranes via forward trafficking (III) membrane recycling via the endocytic pathway, and (IV) lateral movement of lipid domains and local synthesis [114,116,284]. In the following sections, we will discuss more about each of these mechanisms, with a specific emphasis on phosphoinositides and sphingolipids.
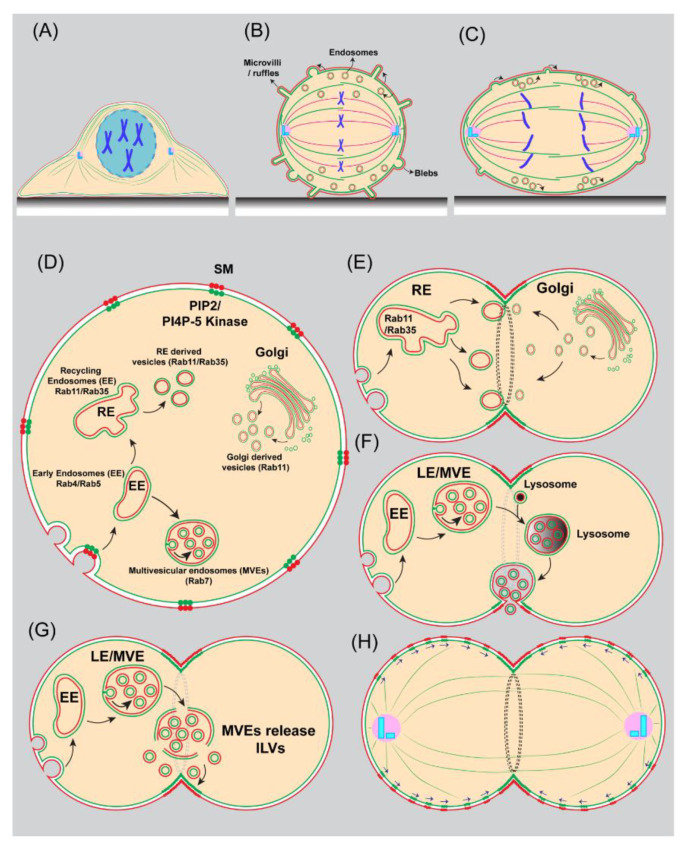
12. Regulation of Plasma Membrane Area during Mitosis
Most of the cultured animal cells entering mitosis dramatically change their shape from being flat at the interphase to round/spherical at the metaphase [284,285,286]. In addition to the shape, cells also significantly increase their volume and hydrostatic pressure from prophase to prometaphase [285]. By altering size and shape, cells provide a suitable environment for spindle formation and ensure proper segregation of chromosomes and organelles to daughter cells [285,286,287]. Mitotic cell rounding accompanies a significant reduction in cell surface area [284]. To accommodate the apparent change in cell area, the plasma membrane folds into microvilli, blebs, and ruffles (Figure 4) [288,289,290]. In addition, membranes are redistributed into endocytic vesicles that could potentially fuse with the PM at the later stages of cell division, including anaphase and telophase [291]. Using live cell imaging techniques, Boucrot, E. et al. discovered that the cell surface area decreases significantly during metaphase [284]. The decrease in cell surface area from prophase to metaphase coincides with increased endocytosis and reduced recycling. Starting at anaphase, membrane recycling resumes, resulting in the recovery of the cell surface. During this time, recovery of total cell surface occurs even in the absence of a functional Golgi [284]. We recently discovered that during male meiosis cytokinesis, CPE lipids in the plasma membrane are endocytosed and delivered to the cleavage furrow [131]. Together, these observations suggest that expansion of plasma membrane projections and endocytic recycling contribute to an increase in cell surface area during anaphase and cytokinesis. However, changes in cell shape and size have not been extensively studied in the context of crowded tissue environments and therefore warrant future investigations [285].
Figure 4.
Mechanisms for membrane traffic and lipid polarization during cytokinesis. (A–C) Regulation of plasma membrane area in a dividing mammalian cell. (A) At the interphase, cultured, adherent mammalian cells appear flat; (B) at the onset of mitosis, cells lose their attachment to the substrate and begin to round up and reduce their surface area. To accommodate this change in surface area, the plasma membrane folds into blebs, microvilli, and ruffles and becomes endocytosed. (C) During anaphase, cell surface area increases, which correlates with the fusion of recycling endosomes with the plasma membrane and the expansion of membrane projections. (D) An eukaryotic cell in prophase showing endomembrane compartments, including early endosomes (EE), recycling endosomes (RE), multivesicular endosomes (MVEs), the Golgi apparatus, and vesicles derived from these compartments. The plasma membrane bilayer is shown with an outer leaflet (red) and an inner leaflet (green). Circular dots on the plasma membrane correspond to lipid domains, including sphingomyelin-rich domains (red dots) and Phosphatidylinositol-4, 5-bisphosphate (PIP2) and its biosynthetic enzyme PI(4)P 5-kinase (green dots). (E) A diagram depicting membrane trafficking from recycling endosomes and the Golgi apparatus to the cleavage furrow. (F) Cartoon showing membrane addition via lysosome exocytosis at the cleavage furrow in mammalian cells. Late endosomes (LE), Multivesicular endosomes (MVE). (G) Schematic representation of membrane trafficking via multivesicular endosomes that release their intraluminal vesicles in the cytosol near cleavage furrow in Drosophila spermatocytes. (H) Illustration of lateral movement of transbilayer-coupled lipid domains towards the cleavage furrow via a cortical flow mechanism.
13. Forward Trafficking
Vesicle transport from the ER to the Golgi and then to the cleavage furrow was shown to be essential for cytokinesis in multiple animal model organisms, including Xenopus [292], Caenorhabditis elegans embryos [293], Drosophila embryos [294,295], and mammalian cells [124], and to the cell plate in plants [296,297]. Several lines of evidence from the studies conducted on Drosophila spermatocytes undergoing meiotic divisions have demonstrated crucial roles for Golgi-derived vesicular trafficking in cytokinesis [130,192,199,298,299,300,301,302,303,304,305]. They include essential functions of the small GTPase Rab1, the vesicular coat component COP-I, the tethering machineries (conserved oligomeric complex subunits Cog5 (FWS) and Cog7, and the transport particle (TRAPPII), and the endoplasmic reticulum-golgi trafficking regulator Zw10 of the Rod-Zwiltch-Zw10 (RZZ) complex and exocyst components Exo8 and Exo84, syntaxin 5, and PI(4)P binding protein Golgi phosphoprotein 3 (GOLPH3) [192,199,221,298,300,303,306,307,308]. Given that PI is a lipid that cannot diffuse freely across the cytosol from the ER, it is likely that the supply of PI from the ER is mediated by two processes, namely vesicular transport and via PI transfer proteins (PITPs) [309]. Importantly, phosphatidylinositol transfer protein (PITP) (Gio/Vib) and the PI4-kinase (PI4K), four-wheel drive (FWD), were shown to be important for cytokinesis in spermatocytes [130,301,310]. PITP is localized to the ER, cleavage furrow, and spindle envelope and is required for the fusion of Golgi-derived vesicles to the cleavage furrow [310]. PITP mutants show normal central spindle assembly, but furrow ingression is delayed, and they display an apparent loss of contact between the contractile ring and plasma membrane, leading to the regression of the furrow [301,310]. FWD localizes to the Golgi, and its activity is required for accumulation of PI4P on Golgi membranes as well as the midzone during the late steps of cytokinesis. FWD also physically interacts with Rab11 and helps in its recruitment to Golgi membranes, where it becomes associated with organelles containing PI4P. Thus, PI4Kβ has both catalytic and non-catalytic functions in promoting Rab11 localization during cytokinesis [282]. Similarly, in mammalian cells, PI4Kβ binds and recruits Rab11 to the Golgi where Rab11 plays a role in post-Golgi secretory trafficking [311,312]. Wild-type activity of PI4K is required for the appearance of tyrosine phosphorylation epitopes at the furrow, and normal organization of actin filaments in the contractile ring, and formation of intercellular bridge [130]. PI4P is a substrate for the synthesis of PI(4,5)P2, a major phosphoinositide, by PI4P 5-kinase, which is found in the cytokinetic furrow and intercellular bridges and whose enzymatic activity is required for cytokinesis [41,95]. PITP was shown to regulate asymmetric division in Drosophila neuroblasts by promoting the synthesis of a plasma membrane-specific pool of PI4P by PI4KIIIα. Further, PI4P in turn was shown to bind to non-muscle myosin II regulatory light chain (Sqh) in vitro, suggesting a mechanism for anchoring of myosin to the cell cortex in neuroblasts [313]. A study in S. pombe revealed that scaffolding protein efr3-mediated PI4-kinase PM attachment is required for central positioning of contractile rings, without which contractile rings slide to one end in a myosin-V-dependent manner [255,256]. GOLPH3 is an effector of PI4P that was shown to accumulate at the Golgi and cleavage furrow. Binding of GOLPH3 to PI4P is essential for localization of PI4P-enriched and Rab11-positive vesicles at the cleavage furrow [199]. GOLPH3 interacts with non-muscle myosin II regulatory light chain (Sqh) and Centralspindlin complex subunit Pavarotti (a kinesin-like protein) to regulate furrow ingression [306].
Relatively little is known about the role of other lipids in forward trafficking and cytokinesis. Very long chain sphingolipids have been shown in plant cells to be required for the fusion of Golgi-derived vesicles to the cell plate during cytokinesis [138]. Sphingolipids with very long chain fatty acids (C24, C26) at the trans Golgi were shown to regulate PI4P homeostasis by recruitment of phosphoinositide specific phospholipase C (PI-PLC) and depletion of PI4P at the trans Golgi during sorting of auxin efflux carrier PIN2 in plants [314]. Collectively, these studies suggest a crucial role for lipid trafficking, especially PI, PI(4)P and very long chain sphingolipids, via directed secretion in cytokinesis.
14. Endocytosis and Recycling
It was once believed that endocytosis was inactive during cell division. However, subsequent studies demonstrated that endocytosis is active and has an important role during cytokinesis [284,315,316,317,318,319,320]. At the onset of mitosis, during prophase, and metaphase mother cells round up, leading to a substantial reduction in cell surface area [2,284]. Live cell imaging in HeLa/BSC1 cells showed that continued endocytosis and a considerable reduction in membrane recycling back to the PM are responsible for this cell rounding. Reactivation of endosome fusion with the PM during anaphase and telophase rapidly recovers the cell surface area. Late endosomes and recycling endosomes both participate in membrane redeposition. This modulation of endosomal recycling is essential for cell division, as inhibition of endocytosis or recycling impairs cytokinesis [284]. Similar endosome fusion with the PM during cytokinesis has been found in other cell lines and organisms [294,319,321,322].
Pioneering studies conducted on Drosophila cellularization in embryos revealed an important role for Rab11-positive recycling endosomes in cytokinesis [323,324] and were also observed subsequently in mammalian cells undergoing mitosis [232]. Several studies examining cytokinesis identified Rab11 family interacting proteins 3 (FIP3) and 4 (FIP4) and Arf6 as Rab11 interacting proteins [231,232,325]. Rab11-FIP3 endosomes play a critical role in inducing secondary ingression in the late steps of cytokinesis [213,232,326,327]. It was shown that FIP3-endosomes are required for the delivery of protein cargoes SCAMP2/3 and p50RhoGAP to the intercellular bridge. p50RhoGAP inactivates Rac/Rho GTPases to promote cortical actin depolymerization in the intercellular bridge before secondary ingression during abscission [213]. siRNA screens for Rab GTPases involved in cytokinesis identified Rab35 in addition to Rab11 as an important regulator of cytokinesis both in Drosophila and mammalian cells [92]. Rab35 has been shown to reduce PI(4,5)P2 levels at the intercellular bridge via direct interaction with PI(4,5)P2 phosphatase (OCRL) to promote localized cortical actin remodeling required for intercellular bridge stability and abscission [23]. The formation of an actin-free zone in the intercellular bridge is thought to be important for vesicle fusion and/or the promotion of increased membrane dynamics [6,7]. Sagona AP, et al. identified a third class of endosomes that are enriched in phosphatidylinositol-3-phosphate and accumulate at the intercellular bridge [258]. These endosomes contain centrosomal protein FYVE-CENT and its binding partner TTC19, which in turn binds to CHMP4B, a subunit of the endosomal sorting complex required for transport (ESCRT) III, which is involved in the final abscission step of cytokinesis [258]. It was suggested that endosomal transport and fusion with the PM during cytokinesis have two major roles: addition of new membranes or specific lipids to the furrow/intercellular bridge, and fast delivery of regulatory proteins involved in the reorganization of the cytoskeleton and PM that is essential for successful abscission [7]. Besides membrane addition at the cleavage furrow, new membrane addition at the cell poles was also reported during anaphase and was dependent on actin and astral microtubules. The lipid composition of the polar region was shown to be distinct and was devoid of GM1 [328].
Recently, we have shown that the sphingolipid CPE is enriched in Rab7 (which marks late endosomes) and Rab11-positive endosomes that are specifically localized to the cleavage furrow during male meiosis cytokinesis. Further, localization of CPE-enriched endosomes to the cleavage furrow is dependent on the wild-type function of Rab11. The absence of CPE or interfering with the functions of Rab7, Rab11, or Rab35 significantly increases cytokinetic defects, suggesting that specific lipid traffic via the endocytic pathway is essential for cytokinesis. However, unlike Rab7 and Rab11 endosomes, Rab35 did not localize to the endosomal compartment in Drosophila spermatocytes; instead, it localized to mitochondrial membranes [131], suggesting that Rab35 may function differently in spermatocytes.
Membrane trafficking via the late endosome was also shown to be important for cytokinesis in mammalian cells [284]. The presence of a large number of multivesicular endosomes (MVEs) (named lytic endosomes in the study) in close proximity to the secondary ingression site was easily observable in the original EM images of the intercellular bridge [326], suggesting a conserved role of MVEs in cytokinesis. In HeLa cells, clusters of lysosomes were shown to localize to the intercellular bridge, and these clusters were regulated by calcium-binding protein-7 and PI4PIIIβ kinase [329]. Recently, these lysosome clusters were shown to fuse with the cleavage furrow and release their intraluminal contents outside of the cell as exosomes, and this process is important for cytokinesis (Figure 4) [330]. However, in Drosophila spermatocytes, fusion of MVEs with the cleavage furrow was not observed; instead, it was shown that MVEs release their intraluminal vesicles in the cytosol in the vicinity of the growing furrow, suggesting membrane delivery to the furrow membranes via small pockets (Figure 4) [131]. Together, these studies suggest that Rab11-positive recycling endosomes and late endosome mediated membrane trafficking is conserved between mammalian cells and Drosophila spermatocytes. However, the specific mechanism by which Rab35 mediates membrane trafficking during male meiotic cytokinesis in Drosophila spermatocytes remains to be investigated in the future.
15. Local Synthesis and Cortical Flow
PIPs are low-abundance, cytoplasmic leaflet-exposed phospholipids, yet they regulate several cellular processes [90,126]. The synthesis of different PIP species is tightly regulated both spatially and temporally by the action of kinases, phosphatases, and phospholipases that localize to different subcellular compartments [90,331]. Emoto et al. demonstrated that PI(4,5)P2 and its biosynthetic enzyme PI(4)P 5-kinase are found in the cleavage furrow and that overexpression of kinase-deficient PIP5K causes cytokinesis defects in CHO cells [41]. The PI(4)P 5-kinase homologue, Its3, was found to concentrate on the septum of dividing cells in fission yeast S. pombe, and mutants of Its3 delayed cytokinesis [95]. In Drosophila cells, PI(4)P 5-kinase, Skittles, and PTEN, a PI(3,4,5)P3 phosphatase, both produce PI(4,5)P2 and localize to the cleavage furrow [93]. The enrichment of PI(4,5)P2 in furrow membranes and the localization of PI4(P) 5-kinase suggest that PI(4,5)P2 local synthesis is important during cytokinesis [40,42,92,144]. However, relatively little is known about how PI(4)P 5-kinase localization to the cleavage furrow is regulated. PI(4,5)P2 has been linked to detergent-resistant membranes in several studies [332,333,334]. It was shown that during interphase, PI(4)P 5-kinase localized to the inner leaflet immediately below the sphingomyelin rich lipid domains in the outer leaflet of the PM [43]. During cytokinesis, sphingomyelin-rich lipid domains accumulate at the cleavage furrow, which in turn coincides with the localization of PI(4)P 5-kinase to the cleavage furrow. When sphingomyelin-rich domains in the outer leaflet are depleted, PI(4,5)P2 accumulation in the inner leaflet is disrupted [43]. Microvilli formation in epithelial cells was shown to depend on clustering of sphingomyelin that co-clusters the transmembrane protein podocalyxin-1, which in turn recruits ezrin/radixin/moesin (ERM) binding phosphoprotein-50 (EBP-50) and PI(4)P 5-kinase [335]. The addition of lysogalactosylceramide/galactosylsphingosine (psychosine) to Namalwa cells induced cytokinetic defects via inhibition of SM-rich lipid domain formation and suppression of PI(4, 5)P2 production [336]. It is unclear how these interleaflet-coupled domains rich in sphingomyelin, PI(4,5)P2, and PI(4)P 5-kinase concentrate at the cleavage furrow. Anchored picket fence model, combined with a lipid raft and cortical flow mechanism involving the actin cytoskeleton, may explain this phenomenon, given that sphingolipids are not synthesized locally at the cleavage furrow (Figure 4) (please see Box 2 for more details on current plasma membrane models). A thin and dynamic network of filaments just under the plasma membrane is known as the cell cortex. Flow of cytoplasm in the cell cortex causes mechanical compression of filaments in the middle and aids in the formation of a contractile ring during cytokinesis [337,338]. During cytokinesis, cortical flow is directed from low tension to high tension, i.e., from the poles to the equatorial region, and this cortical flow is dependent on non-muscle myosin II activity [339,340,341]. Several studies have shown that sphingolipid-rich domains are regulated by cortical actin [79,342,343]. Lateral organization of lipids into nanoscale cholesterol-dependent domains requires long-chain saturated fatty acid-containing lipids in one of the two leaflets. Immobilization of long-chain saturated fatty acid-containing lipids in one leaflet affects the corresponding lipids in the opposite leaflet. Immobilization of inner leaflet lipids occurs upon association with the cortical actin cytoskeleton, leading to transbilayer coupling and the formation of nanoscale cholesterol-dependent lipid clusters resembling lipid rafts [81]. The acyl chains of the outer leaflet sphingolipid GM1 interdigitate with the acyl chains of the anionic phospholipid PS in the inner leaflet, and this interaction is enhanced by cholesterol [79]. Further interaction of the cortical cytoskeleton with the anionic phospholipid PS in the inner leaflet is required for the organization of sphingolipid rich domains in the outer leaflet [79]. When transbilayer interactions are coupled with active contractile flows (via interaction of inner leaflet lipid head groups and transmembrane proteins with the dynamic actomyosin), they create nanoscale clusters which in turn facilitate lateral lipid–lipid interactions, leading to the formation of active emulsions and mesoscale liquid ordered domains [80,83,344,345,346,347]. In addition to acyl chain saturation, lipid headgroup chemical and geometric features (such as lipid shapes) may influence lipid–lipid and lipid–protein interactions [69,348,349], and thus likely have an impact on mesoscale organization. Since the cytokinetic furrow is intimately connected with the juxtamembrane contractile cortex, it is likely that lipid organization and polarization in this area arises from a combination of the picket-fence model, the active emulsion model of lipid rafts, and cortical flows (Box 2).
In addition to the cortical cytoskeleton directing the movement of specific lipid domains and associated proteins to the cleavage furrow, anionic phospholipids in the inner leaflet of these lipid domains, such as PI(4,5)P2, may also direct cortical cytoskeleton organization via activation of specific signaling cascades. Consistent with this hypothesis, it was shown that cytoskeletal elements including microfilaments, myosin II, and microtubules are required for the formation of a cholesterol- and GM1-rich membrane band at the cleavage furrow in sea urchin eggs [40,350]. Additionally, these lipid domains contained signaling proteins Src and PLCγ, which are tyrosine phosphorylated during cytokinesis [40]. A systematic RNAi screen identified several GPCRs and their effectors involved in cytokinesis [200]. PI 4-kinase, which produces a precursor for PI(4,5)P2 biosynthesis, on the other hand, is required for tyrosine phosphorylation and normal actin filament organization in the contractile ring [130]. The cortical association of Moesin, an actin/membrane linker protein, is dependent on local production of PI(4,5)P2 in Drosophila [93]. Psychosine treatment of the myelomonocyte cell line U937 altered actin filament organization and caused cytokinetic defects [351]. Psychosine-mediated induction of cytokinetic defects also strictly depended on the G-protein-coupled receptor TDAG8 (T-cell death-associated gene 8) [17]. Thus, interplay between lipid domains, the cytoskeleton, and signaling proteins is crucial for the successful completion of cytokinesis.
16. Conclusions and Perspectives
Eukaryotic cells invest a significant amount of genetic information and energy to produce and degrade thousands of lipid species, whose complexity and biological roles are only beginning to be explored. Lipid composition dramatically changes between each cell type and within the same cell type depending on the different cellular states in which they are examined, for instance when they are dividing or differentiating [67,352]. In fact, cellular diversity is dependent on lipid diversity, which actively remodulates metabolic programs by regulating signaling receptors [352]. Although we now have a broad idea about the major types of lipid species associated with cell division and cytokinesis, we know relatively little about how these lipids regulate cytokinesis, especially lipids other than phosphoinositides. Even within phosphoinositides, we know little about their fatty acid acyl chain composition and how they affect cytokinesis. The lack of reliable probes to detect dynamic changes in lipid composition is one of the major limitations in studying these lipids. There are almost no in vivo tools available to study dynamic changes in acyl chain composition.
Midbody lipidomes revealed a number of novel lipids, including ceramides, ether phospholipids, phosphatidic acid, and triacylglycerol; however, their roles in cytokinesis remain unknown [12]. Sphingolipid-specific probes developed from pore-forming toxins such as Lysenin (binds to sphingomyelin), Pleurotolysin A2 (binds to CPE) and bacterial toxins such as Cholera toxin, Shiga toxins (binds to glycosphingolipids) have allowed analysis of dynamic changes of these lipids in the exoplasmic leaflet of cultured cells [352,353,354]. However, applications of these toxins are often limited to in vitro experiments. Hence, future studies focused on developing probes that could lead to better detection of sphingolipids in vivo would advance our understanding of the role of these enigmatic lipids in cytokinesis.
Long chain PUFAs as components of phospholipids and sphingolipids have been detected in the spermatozoa of most mammals, including humans and birds. Unsaturated fatty acid-containing lipids provide the spermatozoan plasma membrane with the fluidity and flexibility that it needs to participate in membrane fusion events during fertilization, although they make the plasma membrane more susceptible to oxidation [355]. Studies conducted in mice and Drosophila have shown that very long chain unsaturated fatty acid containing sphingolipids are essential for male meiotic cytokinesis [267,356]. Our recent study suggested the importance of endocytic trafficking of unsaturated fatty acid containing CPE via multivesicular endosomes and release of their intraluminal vesicles in the vicinity of furrow membranes [131]. However, several questions remain unanswered, including how ILVs released by MVBs get inserted at the cytokinetic furrow; do these endosomes have roles other than membrane addition at the furrow; do they deliver specific cargoes or mediate signaling; and finally, do similar mechanisms exist in mammalian male germ cells. Although the docking of lysosomes at the cytokinetic furrow of mammalian cancer cells resembles that of MVBs docking in Drosophila male meiotic cells, they fundamentally differ in the release of ILVs. Lysosomes release their ILVs outside the cell as exosomes when they fuse; in contrast, in our study, we find that MVBs release their ILVs in the cytosol proximal to ingressing membranes at the furrow [131,329,330]. These differences could be partly explained by the fact that male meiotic cells are unique in their lipid composition and polarization relative to somatic cells [267,356]. However, future studies are needed to shed light on mechanisms and their significance in cytokinesis of these cell types.
There is overwhelming evidence showing polarization of specific lipids at the cytokinetic furrow, and intercellular bridges [3,8]. However, we know very little about how lipids in the plasma membrane that are distributed uniformly in the prophase become concentrated and remain at the cytokinetic furrow during early to late telophase. Further, at the end of cytokinesis, the mechanisms by which these lipids achieve uniform distribution remain unknown, particularly when considering sphingolipids. Mechanisms involving transbilayer lipid domain coupling and their interactions with the cytoskeleton and lipid kinases is another area that needs future investigation. Furthermore, the role of the cortical flow model in lateral movement of lipid domains to the cleavage furrow via interaction with the cytoskeleton must be investigated. Especially due to a recent study conducted in C. elegans embryos warranted that, the cortical flows play only a minor role in actomyosin ring assembly and cytokinesis [357]. Several studies have now highlighted the importance of directed membrane secretion via the Golgi and membrane recycling via the endocytic pathway in the early and late steps of cytokinesis [3]. However, we lack much information on the specific cargos they carry and their role in cytokinesis. Future studies must therefore focus more on the identification of specific cargoes and their role in cytokinesis.
Lastly, sphingolipids and cholesterol-rich domains are known to co-cluster several transmembrane receptor proteins that mediate signaling [358]. Enrichment of these lipid domains at the cytokinetic furrow clearly suggests involvement of active signaling at the cytokinetic furrow and intercellular bridges. Although a few studies have found increased accumulation of tyrosine-phosphorylated proteins such as Src and PLCγ, their exact role in cytokinesis remains unknown [40]. Similarly, few GPCRs have been implicated in cytokinesis; however, mechanistic insights are still lacking [200]. Exploring the role of various signaling cascades in cytokinesis could refine mechanisms in cytokinesis, which in turn would pave the path for therapeutic interventions where cytokinesis plays an important role, such as polyploidy in certain cancers, and neurological diseases (Box 1).
Abbreviations
| Plasma membrane | (PM) |
| Phospholipids | (PLs) |
| Phosphatidylinositol phosphates | (PIPs) |
| Phosphatidylinositol 4, 5 bisphosphate | (PIP2) |
| Phosphatidylinositol 3, 4, 5 triphosphate | (PIP3) |
| Pleckstrin homology domain | (PH) |
| Endosomal sorting complex required for transport | (ESCRT) |
| Ras related protein | (Rab) |
| Ras homologues | (Rho) |
| Oculocerebrorenal Syndrome of Lowe | (OCRL) |
| Epithelial cell transforming sequence 2 | (Ect2) |
| Phosphatase and tensin homologue | (PTEN) |
| GTPase-activating protein | (GAP) |
| G protein coupled receptor | (GPCR) |
| Charged multivesicular body protein | (CHMP) |
| Apoptosis-linked gene 2 interacting protein X | (Alix) |
| Uncoordinated | (UNC) |
| Spastic paraplegia 20 | (SPG20) |
| Inositol polyphosphate phosphatase interacting protein 27 | (IPIP27) |
| Bin-Amphiphysin-Rvs | (BAR) |
| Microtubule associated monooxygenase, Calponin and LIM domain containing | (MICAL) |
| RhoA GTPase activating protein | (ARHGAP) |
| Mitotic kinase like protein | (MLKP) |
| Ubiquitin specific protease 8 | (USP8) |
| Signal-transducing adaptor molecule | (STAM) |
| PDZ domain containing 8 | (PDZD8) |
| Soluble N-ethylmale-imide-sensitive factor attachment protein receptor | (SNARE) |
| UV radiation resistance associated | (UVRAG) |
| Ras like proteins | (RAL) |
| Microtubule-interacting and trafficking | (MIT) domain |
| Diacylglycerol | (DAG) |
| Phosphatidic acid | (PA) |
| Intercellular bridge | (ICB) |
| Vacuolar protein sorting | (VPS) |
| phosphatidylcholine | (PC) |
| Guanosine 5′-triphosphate | (GTP) |
| Phospholipase C X-domain containing protein | (PLCXD) |
| Endoplasmic reticulum | (ER) |
| Non-receptor tyrosine-protein kinase | (Fyn) |
| Guanine nucleotide exchange factors Ezrin/radixin/moesin Secretory carrier associated membrane protein Tetratricopeptide repeat domain 19 Monosialotetrahexosylganglioside Sphingomyelin Ceramide phosphoethanolamine Phospholipase C |
(GEFs) (ERM) (SCAMP) TTC19 GM SM CPE PLC |
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was funded by the intramural division of the National Cancer Institute, National Institutes of Health (Division of Health and Human Services). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lancaster O.M., Baum B. Shaping up to divide: Coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Biol. 2014;34:109–115. doi: 10.1016/j.semcdb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Taubenberger A.V., Baum B., Matthews H.K. The Mechanics of Mitotic Cell Rounding. Front. Cell Dev. Biol. 2020;8:687. doi: 10.3389/fcell.2020.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fremont S., Echard A. Membrane Traffic in the Late Steps of Cytokinesis. Curr. Biol. 2018;28:R458–R470. doi: 10.1016/j.cub.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 4.Gulluni F., Martini M., Hirsch E. Cytokinetic Abscission: Phosphoinositides and ESCRTs Direct the Final Cut. J. Cell. Biochem. 2017;118:3561–3568. doi: 10.1002/jcb.26066. [DOI] [PubMed] [Google Scholar]
- 5.Pollard T.D., O’Shaughnessy B. Molecular Mechanism of Cytokinesis. Annu. Rev. Biochem. 2019;88:661–689. doi: 10.1146/annurev-biochem-062917-012530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schiel J.A., Childs C., Prekeris R. Endocytic transport and cytokinesis: From regulation of the cytoskeleton to midbody inheritance. Trends. Cell. Biol. 2013;23:319–327. doi: 10.1016/j.tcb.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiel J.A., Prekeris R. Membrane dynamics during cytokinesis. Curr. Opin. Cell Biol. 2013;25:92–98. doi: 10.1016/j.ceb.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Storck E.M., Ozbalci C., Eggert U.S. Lipid Cell Biology: A Focus on Lipids in Cell Division. Annu. Rev. Biochem. 2018;87:839–869. doi: 10.1146/annurev-biochem-062917-012448. [DOI] [PubMed] [Google Scholar]
- 9.Lens S.M.A., Medema R.H. Cytokinesis defects and cancer. Nat. Rev. Cancer. 2019;19:32–45. doi: 10.1038/s41568-018-0084-6. [DOI] [PubMed] [Google Scholar]
- 10.Gulluni F., Prever L., Li H., Krafcikova P., Corrado I., Lo W.T., Margaria J.P., Chen A., de Santis M.C., Cnudde S.J., et al. PI(3,4)P2-mediated cytokinetic abscission prevents early senescence and cataract formation. Science. 2021;374:eabk0410. doi: 10.1126/science.abk0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arai Y., Sampaio J.L., Wilsch-Brauninger M., Ettinger A.W., Haffner C., Huttner W.B. Lipidome of midbody released from neural stem and progenitor cells during mammalian cortical neurogenesis. Front. Cell. Neurosci. 2015;9:325. doi: 10.3389/fncel.2015.00325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atilla-Gokcumen G.E., Muro E., Relat-Goberna J., Sasse S., Bedigian A., Coughlin M.L., Garcia-Manyes S., Eggert U.S. Dividing cells regulate their lipid composition and localization. Cell. 2014;156:428–439. doi: 10.1016/j.cell.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lacroix B., Maddox A.S. Cytokinesis, ploidy and aneuploidy. J. Pathol. 2012;226:338–351. doi: 10.1002/path.3013. [DOI] [PubMed] [Google Scholar]
- 14.Leal A.F., Suarez D.A., Echeverri-Pena O.Y., Albarracin S.L., Almeciga-Diaz C.J., Espejo-Mojica A.J. Sphingolipids and their role in health and disease in the central nervous system. Adv. Biol. Regul. 2022;85:100900. doi: 10.1016/j.jbior.2022.100900. [DOI] [PubMed] [Google Scholar]
- 15.Spassieva S., Bieberich E. Lysosphingolipids and sphingolipidoses: Psychosine in Krabbe’s disease. J. Neurosci. Res. 2016;94:974–981. doi: 10.1002/jnr.23888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y., Xu Y., Benitez B.A., Nagree M.S., Dearborn J.T., Jiang X., Guzman M.A., Woloszynek J.C., Giaramita A., Yip B.K., et al. Genetic ablation of acid ceramidase in Krabbe disease confirms the psychosine hypothesis and identifies a new therapeutic target. Proc. Natl. Acad. Sci. USA. 2019;116:20097–20103. doi: 10.1073/pnas.1912108116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Im D.S., Heise C.E., Nguyen T., O’Dowd B.F., Lynch K.R. Identification of a molecular target of psychosine and its role in globoid cell formation. J. Cell Biol. 2001;153:429–434. doi: 10.1083/jcb.153.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith N.J., Fuller M., Saville J.T., Cox T.M. Reduced cerebral vascularization in experimental neuronopathic Gaucher disease. J. Pathol. 2018;244:120–128. doi: 10.1002/path.4992. [DOI] [PubMed] [Google Scholar]
- 19.White A.B., Givogri M.I., Lopez-Rosas A., Cao H., van Breemen R., Thinakaran G., Bongarzone E.R. Psychosine accumulates in membrane microdomains in the brain of krabbe patients, disrupting the raft architecture. J. Neurosci. 2009;29:6068–6077. doi: 10.1523/JNEUROSCI.5597-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kanazawa T., Takematsu H., Yamamoto A., Yamamoto H., Kozutsumi Y. Wheat germ agglutinin stains dispersed post-golgi vesicles after treatment with the cytokinesis inhibitor psychosine. J. Cell. Physiol. 2008;215:517–525. doi: 10.1002/jcp.21328. [DOI] [PubMed] [Google Scholar]
- 21.Ben El Kadhi K., Roubinet C., Solinet S., Emery G., Carreno S. The inositol 5-phosphatase dOCRL controls PI(4,5)P2 homeostasis and is necessary for cytokinesis. Curr. Biol. 2011;21:1074–1079. doi: 10.1016/j.cub.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 22.Cauvin C., Rosendale M., Gupta-Rossi N., Rocancourt M., Larraufie P., Salomon R., Perrais D., Echard A. Rab35 GTPase Triggers Switch-like Recruitment of the Lowe Syndrome Lipid Phosphatase OCRL on Newborn Endosomes. Curr. Biol. 2016;26:120–128. doi: 10.1016/j.cub.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 23.Dambournet D., Machicoane M., Chesneau L., Sachse M., Rocancourt M., El Marjou A., Formstecher E., Salomon R., Goud B., Echard A. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat. Cell. Biol. 2011;13:981–988. doi: 10.1038/ncb2279. [DOI] [PubMed] [Google Scholar]
- 24.Matsuyama M., Tanaka H., Inoko A., Goto H., Yonemura S., Kobori K., Hayashi Y., Kondo E., Itohara S., Izawa I., et al. Defect of mitotic vimentin phosphorylation causes microophthalmia and cataract via aneuploidy and senescence in lens epithelial cells. J. Biol. Chem. 2013;288:35626–35635. doi: 10.1074/jbc.M113.514737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sreekumar P.G., Hinton D.R., Kannan R. The Emerging Role of Senescence in Ocular Disease. Oxid. Med. Cell. Longev. 2020;2020:2583601. doi: 10.1155/2020/2583601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodger C., Flex E., Allison R.J., Sanchis-Juan A., Hasenahuer M.A., Cecchetti S., French C.E., Edgar J.R., Carpentieri G., Ciolfi A., et al. De Novo VPS4A Mutations Cause Multisystem Disease with Abnormal Neurodevelopment. Am. J. Hum. Genet. 2020;107:1129–1148. doi: 10.1016/j.ajhg.2020.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seu K.G., Trump L.R., Emberesh S., Lorsbach R.B., Johnson C., Meznarich J., Underhill H.R., Chou S.T., Sakthivel H., Nassar N.N., et al. VPS4A Mutations in Humans Cause Syndromic Congenital Dyserythropoietic Anemia due to Cytokinesis and Trafficking Defects. Am. J. Hum. Genet. 2020;107:1149–1156. doi: 10.1016/j.ajhg.2020.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Y., Bennett T.M., Shiels A. A charged multivesicular body protein (CHMP4B) is required for lens growth and differentiation. Differentiation. 2019;109:16–27. doi: 10.1016/j.diff.2019.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tiosano D., Baris H.N., Chen A., Hitzert M.M., Schueler M., Gulluni F., Wiesener A., Bergua A., Mory A., Copeland B., et al. Mutations in PIK3C2A cause syndromic short stature, skeletal abnormalities, and cataracts associated with ciliary dysfunction. PLoS Genet. 2019;15:e1008088. doi: 10.1371/journal.pgen.1008088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beloribi-Djefaflia S., Vasseur S., Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. doi: 10.1038/oncsis.2015.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eichmann T.O., Lass A. DAG tales: The multiple faces of diacylglycerol--stereochemistry, metabolism, and signaling. Cell. Mol. Life. Sci. 2015;72:3931–3952. doi: 10.1007/s00018-015-1982-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kunkel G.T., Maceyka M., Milstien S., Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat. Rev. Drug Discov. 2013;12:688–702. doi: 10.1038/nrd4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogretmen B. Sphingolipid metabolism in cancer signalling and therapy. Nat. Rev. Cancer. 2018;18:33–50. doi: 10.1038/nrc.2017.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Olivera A., Spiegel S. Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature. 1993;365:557–560. doi: 10.1038/365557a0. [DOI] [PubMed] [Google Scholar]
- 35.Zhang H., Desai N.N., Olivera A., Seki T., Brooker G., Spiegel S. Sphingosine-1-phosphate, a novel lipid, involved in cellular proliferation. J. Cell Biol. 1991;114:155–167. doi: 10.1083/jcb.114.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Obeid L.M., Linardic C.M., Karolak L.A., Hannun Y.A. Programmed cell death induced by ceramide. Science. 1993;259:1769–1771. doi: 10.1126/science.8456305. [DOI] [PubMed] [Google Scholar]
- 37.Cuvillier O., Pirianov G., Kleuser B., Vanek P.G., Coso O.A., Gutkind S., Spiegel S. Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature. 1996;381:800–803. doi: 10.1038/381800a0. [DOI] [PubMed] [Google Scholar]
- 38.Naguib A., Bencze G., Engle D.D., Chio I.I., Herzka T., Watrud K., Bencze S., Tuveson D.A., Pappin D.J., Trotman L.C. p53 mutations change phosphatidylinositol acyl chain composition. Cell Rep. 2015;10:8–19. doi: 10.1016/j.celrep.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fernandez C., Lobo Md Mdel V., Gomez-Coronado D., Lasuncion M.A. Cholesterol is essential for mitosis progression and its deficiency induces polyploid cell formation. Exp. Cell. Res. 2004;300:109–120. doi: 10.1016/j.yexcr.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 40.Ng M.M., Chang F., Burgess D.R. Movement of membrane domains and requirement of membrane signaling molecules for cytokinesis. Dev. Cell. 2005;9:781–790. doi: 10.1016/j.devcel.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 41.Emoto K., Inadome H., Kanaho Y., Narumiya S., Umeda M. Local change in phospholipid composition at the cleavage furrow is essential for completion of cytokinesis. J. Biol. Chem. 2005;280:37901–37907. doi: 10.1074/jbc.M504282200. [DOI] [PubMed] [Google Scholar]
- 42.Field S.J., Madson N., Kerr M.L., Galbraith K.A., Kennedy C.E., Tahiliani M., Wilkins A., Cantley L.C. PtdIns(4,5)P2 functions at the cleavage furrow during cytokinesis. Curr. Biol. 2005;15:1407–1412. doi: 10.1016/j.cub.2005.06.059. [DOI] [PubMed] [Google Scholar]
- 43.Abe M., Makino A., Hullin-Matsuda F., Kamijo K., Ohno-Iwashita Y., Hanada K., Mizuno H., Miyawaki A., Kobayashi T. A role for sphingomyelin-rich lipid domains in the accumulation of phosphatidylinositol-4,5-bisphosphate to the cleavage furrow during cytokinesis. Mol. Cell. Biol. 2012;32:1396–1407. doi: 10.1128/MCB.06113-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Emoto K., Umeda M. An essential role for a membrane lipid in cytokinesis. Regulation of contractile ring disassembly by redistribution of phosphatidylethanolamine. J. Cell Biol. 2000;149:1215–1224. doi: 10.1083/jcb.149.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cooke I.R., Deserno M. Coupling between lipid shape and membrane curvature. Biophys. J. 2006;91:487–495. doi: 10.1529/biophysj.105.078683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahon H.T., Boucrot E. Membrane curvature at a glance. J. Cell Sci. 2015;128:1065–1070. doi: 10.1242/jcs.114454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stachowiak J.C., Schmid E.M., Ryan C.J., Ann H.S., Sasaki D.Y., Sherman M.B., Geissler P.L., Fletcher D.A., Hayden C.C. Membrane bending by protein-protein crowding. Nat. Cell. Biol. 2012;14:944–949. doi: 10.1038/ncb2561. [DOI] [PubMed] [Google Scholar]
- 48.Campelo F., McMahon H.T., Kozlov M.M. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 2008;95:2325–2339. doi: 10.1529/biophysj.108.133173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Drin G., Antonny B. Amphipathic helices and membrane curvature. FEBS. Lett. 2010;584:1840–1847. doi: 10.1016/j.febslet.2009.10.022. [DOI] [PubMed] [Google Scholar]
- 50.Rao Y., Haucke V. Membrane shaping by the Bin/amphiphysin/Rvs (BAR) domain protein superfamily. Cell. Mol. Life Sci. 2011;68:3983–3993. doi: 10.1007/s00018-011-0768-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jarsch I.K., Daste F., Gallop J.L. Membrane curvature in cell biology: An integration of molecular mechanisms. J. Cell Biol. 2016;214:375–387. doi: 10.1083/jcb.201604003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kozlov M.M., Campelo F., Liska N., Chernomordik L.V., Marrink S.J., McMahon H.T. Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 2014;29:53–60. doi: 10.1016/j.ceb.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salzer U., Kostan J., Djinovic-Carugo K. Deciphering the BAR code of membrane modulators. Cell. Mol. Life Sci. 2017;74:2413–2438. doi: 10.1007/s00018-017-2478-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carnahan R.H., Gould K.L. The PCH family protein, Cdc15p, recruits two F-actin nucleation pathways to coordinate cytokinetic actin ring formation in Schizosaccharomyces pombe. J. Cell Biol. 2003;162:851–862. doi: 10.1083/jcb.200305012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Y., McDonald N.A., Naegele S.M., Gould K.L., Wu J.Q. The F-BAR Domain of Rga7 Relies on a Cooperative Mechanism of Membrane Binding with a Partner Protein during Fission Yeast Cytokinesis. Cell Rep. 2019;26:2540–2548. doi: 10.1016/j.celrep.2019.01.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mangione M.C., Chen J.S., Gould K.L. Cdk1 phosphorylation of fission yeast paxillin inhibits its cytokinetic ring localization. Mol. Biol. Cell. 2021;32:1534–1544. doi: 10.1091/mbc.E20-12-0807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mangione M.C., Snider C.E., Gould K.L. The intrinsically disordered region of the cytokinetic F-BAR protein Cdc15 performs a unique essential function in maintenance of cytokinetic ring integrity. Mol. Biol. Cell. 2019;30:2790–2801. doi: 10.1091/mbc.E19-06-0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McDonald N.A., Takizawa Y., Feoktistova A., Xu P., Ohi M.D., Vander Kooi C.W., Gould K.L. The Tubulation Activity of a Fission Yeast F-BAR Protein Is Dispensable for Its Function in Cytokinesis. Cell Rep. 2016;14:534–546. doi: 10.1016/j.celrep.2015.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McDonald N.A., Vander Kooi C.W., Ohi M.D., Gould K.L. Oligomerization but Not Membrane Bending Underlies the Function of Certain F-BAR Proteins in Cell Motility and Cytokinesis. Dev. Cell. 2015;35:725–736. doi: 10.1016/j.devcel.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Roberts-Galbraith R.H., Ohi M.D., Ballif B.A., Chen J.S., McLeod I., McDonald W.H., Gygi S.P., Yates J.R., 3rd, Gould K.L. Dephosphorylation of F-BAR protein Cdc15 modulates its conformation and stimulates its scaffolding activity at the cell division site. Mol. Cell. 2010;39:86–99. doi: 10.1016/j.molcel.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sherlekar A., Rikhy R. Syndapin promotes pseudocleavage furrow formation by actin organization in the syncytial Drosophila embryo. Mol. Biol. Cell. 2016;27:2064–2079. doi: 10.1091/mbc.E15-09-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Snider C.E., Bhattacharjee R., Igarashi M.G., Gould K.L. Fission yeast paxillin contains two Cdc15 binding motifs for robust recruitment to the cytokinetic ring. Mol. Biol. Cell. 2022;33:br4. doi: 10.1091/mbc.E21-11-0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Snider C.E., Chandra M., McDonald N.A., Willet A.H., Collier S.E., Ohi M.D., Jackson L.P., Gould K.L. Opposite Surfaces of the Cdc15 F-BAR Domain Create a Membrane Platform That Coordinates Cytoskeletal and Signaling Components for Cytokinesis. Cell Rep. 2020;33:108526. doi: 10.1016/j.celrep.2020.108526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Willet A.H., Bohnert K.A., Gould K.L. Cdk1-dependent phosphoinhibition of a formin-F-BAR interaction opposes cytokinetic contractile ring formation. Mol. Biol. Cell. 2018;29:713–721. doi: 10.1091/mbc.E17-11-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Willet A.H., McDonald N.A., Bohnert K.A., Baird M.A., Allen J.R., Davidson M.W., Gould K.L. The F-BAR Cdc15 promotes contractile ring formation through the direct recruitment of the formin Cdc12. J. Cell Biol. 2015;208:391–399. doi: 10.1083/jcb.201411097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Clarke R.J., Hossain K.R., Cao K. Physiological roles of transverse lipid asymmetry of animal membranes. Biochim. Biophys. Acta Biomembr. 2020;1862:183382. doi: 10.1016/j.bbamem.2020.183382. [DOI] [PubMed] [Google Scholar]
- 67.van Meer G., Voelker D.R., Feigenson G.W. Membrane lipids: Where they are and how they behave. Nat. Rev. Mol. Cell. Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shiomi A., Nagao K., Yokota N., Tsuchiya M., Kato U., Juni N., Hara Y., Mori M.X., Mori Y., Ui-Tei K., et al. Extreme deformability of insect cell membranes is governed by phospholipid scrambling. Cell Rep. 2021;35:109219. doi: 10.1016/j.celrep.2021.109219. [DOI] [PubMed] [Google Scholar]
- 69.Lorent J.H., Levental K.R., Ganesan L., Rivera-Longsworth G., Sezgin E., Doktorova M., Lyman E., Levental I. Plasma membranes are asymmetric in lipid unsaturation, packing and protein shape. Nat. Chem. Biol. 2020;16:644–652. doi: 10.1038/s41589-020-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kusumi A., Fujiwara T.K., Chadda R., Xie M., Tsunoyama T.A., Kalay Z., Kasai R.S., Suzuki K.G. Dynamic organizing principles of the plasma membrane that regulate signal transduction: Commemorating the fortieth anniversary of Singer and Nicolson’s fluid-mosaic model. Annu. Rev. Cell. Dev. Biol. 2012;28:215–250. doi: 10.1146/annurev-cellbio-100809-151736. [DOI] [PubMed] [Google Scholar]
- 71.Kusumi A., Ike H., Nakada C., Murase K., Fujiwara T. Single-molecule tracking of membrane molecules: Plasma membrane compartmentalization and dynamic assembly of raft-philic signaling molecules. Semin. Immunol. 2005;17:3–21. doi: 10.1016/j.smim.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 72.Kusumi A., Koyama-Honda I., Suzuki K. Molecular dynamics and interactions for creation of stimulation-induced stabilized rafts from small unstable steady-state rafts. Traffic. 2004;5:213–230. doi: 10.1111/j.1600-0854.2004.0178.x. [DOI] [PubMed] [Google Scholar]
- 73.Murase K., Fujiwara T., Umemura Y., Suzuki K., Iino R., Yamashita H., Saito M., Murakoshi H., Ritchie K., Kusumi A. Ultrafine membrane compartments for molecular diffusion as revealed by single molecule techniques. Biophys. J. 2004;86:4075–4093. doi: 10.1529/biophysj.103.035717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobson K., Liu P., Lagerholm B.C. The Lateral Organization and Mobility of Plasma Membrane Components. Cell. 2019;177:806–819. doi: 10.1016/j.cell.2019.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lingwood D., Simons K. Lipid rafts as a membrane-organizing principle. Science. 2010;327:46–50. doi: 10.1126/science.1174621. [DOI] [PubMed] [Google Scholar]
- 76.Simons K., Toomre D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell. Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 77.Levental I., Lingwood D., Grzybek M., Coskun U., Simons K. Palmitoylation regulates raft affinity for the majority of integral raft proteins. Proc. Natl. Acad. Sci. USA. 2010;107:22050–22054. doi: 10.1073/pnas.1016184107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Singer S.J., Nicolson G.L. The fluid mosaic model of the structure of cell membranes. Science. 1972;175:720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- 79.Arumugam S., Schmieder S., Pezeshkian W., Becken U., Wunder C., Chinnapen D., Ipsen J.H., Kenworthy A.K., Lencer W., Mayor S., et al. Ceramide structure dictates glycosphingolipid nanodomain assembly and function. Nat. Commun. 2021;12:3675. doi: 10.1038/s41467-021-23961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gowrishankar K., Ghosh S., Saha S.C.R., Mayor S., Rao M. Active remodeling of cortical actin regulates spatiotemporal organization of cell surface molecules. Cell. 2012;149:1353–1367. doi: 10.1016/j.cell.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 81.Raghupathy R., Anilkumar A.A., Polley A., Singh P.P., Yadav M., Johnson C., Suryawanshi S., Saikam V., Sawant S.D., Panda A., et al. Transbilayer lipid interactions mediate nanoclustering of lipid-anchored proteins. Cell. 2015;161:581–594. doi: 10.1016/j.cell.2015.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sezgin E., Levental I., Mayor S., Eggeling C. The mystery of membrane organization: Composition, regulation and roles of lipid rafts. Nat. Rev. Mol. Cell. Biol. 2017;18:361–374. doi: 10.1038/nrm.2017.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saha S., Das A., Patra C., Anilkumar A.A., Sil P., Mayor S., Rao M. Active emulsions in living cell membranes driven by contractile stresses and transbilayer coupling. Proc. Natl. Acad. Sci. USA. 2022;119:e2123056119. doi: 10.1073/pnas.2123056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lillemeier B.F., Mortelmaier M.A., Forstner M.B., Huppa J.B., Groves J.T., Davis M.M. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat. Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lillemeier B.F., Pfeiffer J.R., Surviladze Z., Wilson B.S., Davis M.M. Plasma membrane-associated proteins are clustered into islands attached to the cytoskeleton. Proc. Natl. Acad. Sci. USA. 2006;103:18992–18997. doi: 10.1073/pnas.0609009103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bach L., Gissot L., Marion J., Tellier F., Moreau P., Satiat-Jeunemaitre B., Palauqui J.C., Napier J.A., Faure J.D. Very-long-chain fatty acids are required for cell plate formation during cytokinesis in Arabidopsis thaliana. J. Cell Sci. 2011;124:3223–3234. doi: 10.1242/jcs.074575. [DOI] [PubMed] [Google Scholar]
- 87.Wang T., Montell C. A phosphoinositide synthase required for a sustained light response. J. Neurosci. 2006;26:12816–12825. doi: 10.1523/JNEUROSCI.3673-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cauvin C., Echard A. Phosphoinositides: Lipids with informative heads and mastermind functions in cell division. Biochim. Biophys. Acta. 2015;1851:832–843. doi: 10.1016/j.bbalip.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 89.Echard A. Phosphoinositides and cytokinesis: The “PIP” of the iceberg. Cytoskeleton. 2012;69:893–912. doi: 10.1002/cm.21067. [DOI] [PubMed] [Google Scholar]
- 90.Dickson E.J., Hille B. Understanding phosphoinositides: Rare, dynamic, and essential membrane phospholipids. Biochem. J. 2019;476:1–23. doi: 10.1042/BCJ20180022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kotak S., Busso C., Gonczy P. NuMA interacts with phosphoinositides and links the mitotic spindle with the plasma membrane. EMBO. J. 2014;33:1815–1830. doi: 10.15252/embj.201488147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kouranti I., Sachse M., Arouche N., Goud B., Echard A. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 2006;16:1719–1725. doi: 10.1016/j.cub.2006.07.020. [DOI] [PubMed] [Google Scholar]
- 93.Roubinet C., Decelle B., Chicanne G., Dorn J.F., Payrastre B., Payre F., Carreno S. Molecular networks linked by Moesin drive remodeling of the cell cortex during mitosis. J. Cell Biol. 2011;195:99–112. doi: 10.1083/jcb.201106048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Takeda T., Robinson I.M., Savoian M.M., Griffiths J.R., Whetton A.D., McMahon H.T., Glover D.M. Drosophila F-BAR protein Syndapin contributes to coupling the plasma membrane and contractile ring in cytokinesis. Open. Biol. 2013;3:130081. doi: 10.1098/rsob.130081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang Y., Sugiura R., Lu Y., Asami M., Maeda T., Itoh T., Takenawa T., Shuntoh H., Kuno T. Phosphatidylinositol 4-phosphate 5-kinase Its3 and calcineurin Ppb1 coordinately regulate cytokinesis in fission yeast. J. Biol. Chem. 2000;275:35600–35606. doi: 10.1074/jbc.M005575200. [DOI] [PubMed] [Google Scholar]
- 96.Na H.K., Chang C.C., Trosko J.E. Growth suppression of a tumorigenic rat liver cell line by the anticancer agent, ET-18-O-CH(3), is mediated by inhibition of cytokinesis. Cancer Chemother. Pharmacol. 2003;51:209–215. doi: 10.1007/s00280-003-0577-0. [DOI] [PubMed] [Google Scholar]
- 97.Naito Y., Okada M., Yagisawa H. Phospholipase C isoforms are localized at the cleavage furrow during cytokinesis. J. Biochem. 2006;140:785–791. doi: 10.1093/jb/mvj209. [DOI] [PubMed] [Google Scholar]
- 98.Saul D., Fabian L., Forer A., Brill J.A. Continuous phosphatidylinositol metabolism is required for cleavage of crane fly spermatocytes. J. Cell Sci. 2004;117:3887–3896. doi: 10.1242/jcs.01236. [DOI] [PubMed] [Google Scholar]
- 99.Wong R., Hadjiyanni I., Wei H.C., Polevoy G., McBride R., Sem K.P., Brill J.A. PIP2 hydrolysis and calcium release are required for cytokinesis in Drosophila spermatocytes. Curr. Biol. 2005;15:1401–1406. doi: 10.1016/j.cub.2005.06.060. [DOI] [PubMed] [Google Scholar]
- 100.Janetopoulos C., Borleis J., Vazquez F., Iijima M., Devreotes P. Temporal and spatial regulation of phosphoinositide signaling mediates cytokinesis. Dev. Cell. 2005;8:467–477. doi: 10.1016/j.devcel.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 101.Yonemura S., Hirao-Minakuchi K., Nishimura Y. Rho localization in cells and tissues. Exp. Cell. Res. 2004;295:300–314. doi: 10.1016/j.yexcr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 102.Yoshida S., Bartolini S., Pellman D. Mechanisms for concentrating Rho1 during cytokinesis. Genes. Dev. 2009;23:810–823. doi: 10.1101/gad.1785209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Frenette P., Haines E., Loloyan M., Kinal M., Pakarian P., Piekny A. An anillin-Ect2 complex stabilizes central spindle microtubules at the cortex during cytokinesis. PLoS ONE. 2012;7:e34888. doi: 10.1371/journal.pone.0034888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Heo W.D., Inoue T., Park W.S., Kim M.L., Park B.O., Wandless T.J., Meyer T. PI(3,4,5)P3 and PI(4,5)P2 lipids target proteins with polybasic clusters to the plasma membrane. Science. 2006;314:1458–1461. doi: 10.1126/science.1134389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Su K.C., Takaki T., Petronczki M. Targeting of the RhoGEF Ect2 to the equatorial membrane controls cleavage furrow formation during cytokinesis. Dev. Cell. 2011;21:1104–1115. doi: 10.1016/j.devcel.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Williams C.L. The polybasic region of Ras and Rho family small GTPases: A regulator of protein interactions and membrane association and a site of nuclear localization signal sequences. Cell. Signal. 2003;15:1071–1080. doi: 10.1016/S0898-6568(03)00098-6. [DOI] [PubMed] [Google Scholar]
- 107.Liu J., Fairn G.D., Ceccarelli D.F., Sicheri F., Wilde A. Cleavage furrow organization requires PIP(2)-mediated recruitment of anillin. Curr. Biol. 2012;22:64–69. doi: 10.1016/j.cub.2011.11.040. [DOI] [PubMed] [Google Scholar]
- 108.Bertin A., McMurray M.A., Thai L., Garcia G., 3rd, Votin V., Grob P., Allyn T., Thorner J., Nogales E. Phosphatidylinositol-4,5-bisphosphate promotes budding yeast septin filament assembly and organization. J. Mol. Biol. 2010;404:711–731. doi: 10.1016/j.jmb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Casamayor A., Snyder M. Molecular dissection of a yeast septin: Distinct domains are required for septin interaction, localization, and function. Mol. Cell. Biol. 2003;23:2762–2777. doi: 10.1128/MCB.23.8.2762-2777.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tanaka-Takiguchi Y., Kinoshita M., Takiguchi K. Septin-mediated uniform bracing of phospholipid membranes. Curr. Biol. 2009;19:140–145. doi: 10.1016/j.cub.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 111.Zhang J., Kong C., Xie H., McPherson P.S., Grinstein S., Trimble W.S. Phosphatidylinositol polyphosphate binding to the mammalian septin H5 is modulated by GTP. Curr. Biol. 1999;9:1458–1467. doi: 10.1016/S0960-9822(00)80115-3. [DOI] [PubMed] [Google Scholar]
- 112.Lekomtsev S., Su K.C., Pye V.E., Blight K., Sundaramoorthy S., Takaki T., Collinson L.M., Cherepanov P., Divecha N., Petronczki M. Centralspindlin links the mitotic spindle to the plasma membrane during cytokinesis. Nature. 2012;492:276–279. doi: 10.1038/nature11773. [DOI] [PubMed] [Google Scholar]
- 113.He B., Xi F., Zhang X., Zhang J., Guo W. Exo70 interacts with phospholipids and mediates the targeting of the exocyst to the plasma membrane. EMBO. J. 2007;26:4053–4065. doi: 10.1038/sj.emboj.7601834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Albertson R., Riggs B., Sullivan W. Membrane traffic: A driving force in cytokinesis. Trends. Cell. Biol. 2005;15:92–101. doi: 10.1016/j.tcb.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 115.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443:651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 116.Echard A. Membrane traffic and polarization of lipid domains during cytokinesis. Biochem. Soc. Trans. 2008;36:395–399. doi: 10.1042/BST0360395. [DOI] [PubMed] [Google Scholar]
- 117.Echard A., Hickson G.R., Foley E., O’Farrell P.H. Terminal cytokinesis events uncovered after an RNAi screen. Curr. Biol. 2004;14:1685–1693. doi: 10.1016/j.cub.2004.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Eggert U.S., Kiger A.A., Richter C., Perlman Z.E., Perrimon N., Mitchison T.J., Field C.M. Parallel chemical genetic and genome-wide RNAi screens identify cytokinesis inhibitors and targets. PLoS. Biol. 2004;2:e379. doi: 10.1371/journal.pbio.0020379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giansanti M.G., Farkas R.M., Bonaccorsi S., Lindsley D.L., Wakimoto B.T., Fuller M.T., Gatti M. Genetic dissection of meiotic cytokinesis in Drosophila males. Mol. Biol. Cell. 2004;15:2509–2522. doi: 10.1091/mbc.e03-08-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mayinger P. Phosphoinositides and vesicular membrane traffic. Biochim. Biophys. Acta. 2012;1821:1104–1113. doi: 10.1016/j.bbalip.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Montagnac G., Echard A., Chavrier P. Endocytic traffic in animal cell cytokinesis. Curr. Opin. Cell Biol. 2008;20:454–461. doi: 10.1016/j.ceb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 122.Neto H., Collins L.L., Gould G.W. Vesicle trafficking and membrane remodelling in cytokinesis. Biochem. J. 2011;437:13–24. doi: 10.1042/BJ20110153. [DOI] [PubMed] [Google Scholar]
- 123.Prekeris R., Gould G.W. Breaking up is hard to do—Membrane traffic in cytokinesis. J. Cell Sci. 2008;121:1569–1576. doi: 10.1242/jcs.018770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Skop A.R., Liu H., Yates J., 3rd, Meyer B.J., Heald R. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 2004;305:61–66. doi: 10.1126/science.1097931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vicinanza M., D’Angelo G., Di Campli A., De Matteis M.A. Function and dysfunction of the PI system in membrane trafficking. EMBO. J. 2008;27:2457–2470. doi: 10.1038/emboj.2008.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brill J.A., Wong R., Wilde A. Phosphoinositide function in cytokinesis. Curr. Biol. 2011;21:R930–R934. doi: 10.1016/j.cub.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 127.Janetopoulos C., Devreotes P. Phosphoinositide signaling plays a key role in cytokinesis. J. Cell Biol. 2006;174:485–490. doi: 10.1083/jcb.200603156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Logan M.R., Mandato C.A. Regulation of the actin cytoskeleton by PIP2 in cytokinesis. Biol. Cell. 2006;98:377–388. doi: 10.1042/BC20050081. [DOI] [PubMed] [Google Scholar]
- 129.Nezis I.P., Sagona A.P., Schink K.O., Stenmark H. Divide and ProsPer: The emerging role of PtdIns3P in cytokinesis. Trends. Cell. Biol. 2010;20:642–649. doi: 10.1016/j.tcb.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 130.Brill J.A., Hime G.R., Scharer-Schuksz M., Fuller M.T. A phospholipid kinase regulates actin organization and intercellular bridge formation during germline cytokinesis. Development. 2000;127:3855–3864. doi: 10.1242/dev.127.17.3855. [DOI] [PubMed] [Google Scholar]
- 131.Kunduri G., Le S.H., Baena V., Vijaykrishna N., Harned A., Nagashima K., Blankenberg D., Yoshihiro I., Narayan K., Bamba T., et al. Delivery of ceramide phosphoethanolamine lipids to the cleavage furrow through the endocytic pathway is essential for male meiotic cytokinesis. PLoS Biol. 2022;20:e3001599. doi: 10.1371/journal.pbio.3001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Reversi A., Loeser E., Subramanian D., Schultz C., De Renzis S. Plasma membrane phosphoinositide balance regulates cell shape during Drosophila embryo morphogenesis. J. Cell Biol. 2014;205:395–408. doi: 10.1083/jcb.201309079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.West J.J., Zulueta-Coarasa T., Maier J.A., Lee D.M., Bruce A.E.E., Fernandez-Gonzalez R., Harris T.J.C. An Actomyosin-Arf-GEF Negative Feedback Loop for Tissue Elongation under Stress. Curr. Biol. 2017;27:2260–2270. doi: 10.1016/j.cub.2017.06.038. [DOI] [PubMed] [Google Scholar]
- 134.Atilla-Gokcumen G.E., Bedigian A.V., Sasse S., Eggert U.S. Inhibition of glycosphingolipid biosynthesis induces cytokinesis failure. J. Am. Chem. Soc. 2011;133:10010–10013. doi: 10.1021/ja202804b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Meivar-Levy I., Sabanay H., Bershadsky A.D., Futerman A.H. The role of sphingolipids in the maintenance of fibroblast morphology. The inhibition of protrusional activity, cell spreading, and cytokinesis induced by fumonisin B1 can be reversed by ganglioside GM3. J. Biol. Chem. 1997;272:1558–1564. doi: 10.1074/jbc.272.3.1558. [DOI] [PubMed] [Google Scholar]
- 136.Sonda S., Stefanic S., Hehl A.B. A sphingolipid inhibitor induces a cytokinesis arrest and blocks stage differentiation in Giardia lamblia. Antimicrob. Agents Chemother. 2008;52:563–569. doi: 10.1128/AAC.01105-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fridberg A., Olson C.L., Nakayasu E.S., Tyler K.M., Almeida I.C., Engman D.M. Sphingolipid synthesis is necessary for kinetoplast segregation and cytokinesis in Trypanosoma brucei. J. Cell Sci. 2008;121:522–535. doi: 10.1242/jcs.016741. [DOI] [PubMed] [Google Scholar]
- 138.Molino D., Van der Giessen E., Gissot L., Hematy K., Marion J., Barthelemy J., Bellec Y., Vernhettes S., Satiat-Jeunemaitre B., Galli T., et al. Inhibition of very long acyl chain sphingolipid synthesis modifies membrane dynamics during plant cytokinesis. Biochim. Biophys. Acta. 2014;1842:1422–1430. doi: 10.1016/j.bbalip.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 139.Shuster C.B., Burgess D.R. Parameters that specify the timing of cytokinesis. J. Cell Biol. 1999;146:981–992. doi: 10.1083/jcb.146.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Schmidt K., Nichols B.J. A barrier to lateral diffusion in the cleavage furrow of dividing mammalian cells. Curr. Biol. 2004;14:1002–1006. doi: 10.1016/j.cub.2004.05.044. [DOI] [PubMed] [Google Scholar]
- 141.Caudron F., Barral Y. Septins and the lateral compartmentalization of eukaryotic membranes. Dev. Cell. 2009;16:493–506. doi: 10.1016/j.devcel.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 142.Dobbelaere J., Barral Y. Spatial coordination of cytokinetic events by compartmentalization of the cell cortex. Science. 2004;305:393–396. doi: 10.1126/science.1099892. [DOI] [PubMed] [Google Scholar]
- 143.Takeda T., Kawate T., Chang F. Organization of a sterol-rich membrane domain by cdc15p during cytokinesis in fission yeast. Nat. Cell. Biol. 2004;6:1142–1144. doi: 10.1038/ncb1189. [DOI] [PubMed] [Google Scholar]
- 144.Wachtler V., Rajagopalan S., Balasubramanian M.K. Sterol-rich plasma membrane domains in the fission yeast Schizosaccharomyces pombe. J. Cell Sci. 2003;116:867–874. doi: 10.1242/jcs.00299. [DOI] [PubMed] [Google Scholar]
- 145.Arbizzani F., Rincon S.A., Paoletti A. Increasing ergosterol levels delays formin-dependent assembly of F-actin cables and disrupts division plane positioning in fission yeast. J. Cell Sci. 2019;132:227447. doi: 10.1242/jcs.227447. [DOI] [PubMed] [Google Scholar]
- 146.Rajendran L., Masilamani M., Solomon S., Tikkanen R., Stuermer C.A., Plattner H., Illges H. Asymmetric localization of flotillins/reggies in preassembled platforms confers inherent polarity to hematopoietic cells. Proc. Natl. Acad. Sci. USA. 2003;100:8241–8246. doi: 10.1073/pnas.1331629100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kettle E., Page S.L., Morgan G.P., Malladi C.S., Wong C.L., Boadle R.A., Marsh B.J., Robinson P.J., Chircop M. A Cholesterol-Dependent Endocytic Mechanism Generates Midbody Tubules During Cytokinesis. Traffic. 2015;16:1174–1192. doi: 10.1111/tra.12328. [DOI] [PubMed] [Google Scholar]
- 148.Ortegren U., Karlsson M., Blazic N., Blomqvist M., Nystrom F.H., Gustavsson J., Fredman P., Stralfors P. Lipids and glycosphingolipids in caveolae and surrounding plasma membrane of primary rat adipocytes. Eur. J. Biochem. 2004;271:2028–2036. doi: 10.1111/j.1432-1033.2004.04117.x. [DOI] [PubMed] [Google Scholar]
- 149.Parton R.G. Caveolae: Structure, Function, and Relationship to Disease. Annu. Rev. Cell. Dev. Biol. 2018;34:111–136. doi: 10.1146/annurev-cellbio-100617-062737. [DOI] [PubMed] [Google Scholar]
- 150.Parton R.G., McMahon K.A., Wu Y. Caveolae: Formation, dynamics, and function. Curr. Opin. Cell Biol. 2020;65:8–16. doi: 10.1016/j.ceb.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 151.Parton R.G., Tillu V., McMahon K.A., Collins B.M. Key phases in the formation of caveolae. Curr. Opin. Cell Biol. 2021;71:7–14. doi: 10.1016/j.ceb.2021.01.009. [DOI] [PubMed] [Google Scholar]
- 152.Andrade V., Bai J., Gupta-Rossi N., Jimenez A.J., Delevoye C., Lamaze C., Echard A. Caveolae promote successful abscission by controlling intercellular bridge tension during cytokinesis. Sci. Adv. 2022;8:eabm5095. doi: 10.1126/sciadv.abm5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Feng B., Schwarz H., Jesuthasan S. Furrow-specific endocytosis during cytokinesis of zebrafish blastomeres. Exp. Cell. Res. 2002;279:14–20. doi: 10.1006/excr.2002.5579. [DOI] [PubMed] [Google Scholar]
- 154.Kogo H., Fujimoto T. Concentration of caveolin-1 in the cleavage furrow as revealed by time-lapse analysis. Biochem. Biophys. Res. Commun. 2000;268:82–87. doi: 10.1006/bbrc.1999.2058. [DOI] [PubMed] [Google Scholar]
- 155.Yang P.L., Hsu T.H., Wang C.W., Chen R.H. Lipid droplets maintain lipid homeostasis during anaphase for efficient cell separation in budding yeast. Mol. Biol. Cell. 2016;27:2368–2380. doi: 10.1091/mbc.e16-02-0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kunduri G., Yuan C., Parthibane V., Nyswaner K.M., Kanwar R., Nagashima K., Britt S.G., Mehta N., Kotu V., Porterfield M., et al. Phosphatidic acid phospholipase A1 mediates ER-Golgi transit of a family of G protein-coupled receptors. J. Cell Biol. 2014;206:79–95. doi: 10.1083/jcb.201405020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tanguy E., Wang Q., Moine H., Vitale N. Phosphatidic Acid: From Pleiotropic Functions to Neuronal Pathology. Front. Cell. Neurosci. 2019;13:2. doi: 10.3389/fncel.2019.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Thakur R., Naik A., Panda A., Raghu P. Regulation of Membrane Turnover by Phosphatidic Acid: Cellular Functions and Disease Implications. Front. Cell Dev. Biol. 2019;7:83. doi: 10.3389/fcell.2019.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Honda A., Nogami M., Yokozeki T., Yamazaki M., Nakamura H., Watanabe H., Kawamoto K., Nakayama K., Morris A.J., Frohman M.A., et al. Phosphatidylinositol 4-phosphate 5-kinase alpha is a downstream effector of the small G protein ARF6 in membrane ruffle formation. Cell. 1999;99:521–532. doi: 10.1016/S0092-8674(00)81540-8. [DOI] [PubMed] [Google Scholar]
- 160.Jenkins G.H., Fisette P.L., Anderson R.A. Type I phosphatidylinositol 4-phosphate 5-kinase isoforms are specifically stimulated by phosphatidic acid. J. Biol. Chem. 1994;269:11547–11554. doi: 10.1016/S0021-9258(19)78159-9. [DOI] [PubMed] [Google Scholar]
- 161.Moritz A., De Graan P.N., Gispen W.H., Wirtz K.W. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J. Biol. Chem. 1992;267:7207–7210. doi: 10.1016/S0021-9258(18)42504-5. [DOI] [PubMed] [Google Scholar]
- 162.Kotelevets N., Fabbro D., Huwiler A., Zangemeister-Wittke U. Targeting sphingosine kinase 1 in carcinoma cells decreases proliferation and survival by compromising PKC activity and cytokinesis. PLoS ONE. 2012;7:e39209. doi: 10.1371/journal.pone.0039209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brownlow N., Pike T., Crossland V., Claus J., Parker P. Regulation of the cytokinesis cleavage furrow by PKCepsilon. Biochem. Soc. Trans. 2014;42:1534–1537. doi: 10.1042/BST20140240. [DOI] [PubMed] [Google Scholar]
- 164.Saurin A.T., Durgan J., Cameron A.J., Faisal A., Marber M.S., Parker P.J. The regulated assembly of a PKCepsilon complex controls the completion of cytokinesis. Nat. Cell. Biol. 2008;10:891–901. doi: 10.1038/ncb1749. [DOI] [PubMed] [Google Scholar]
- 165.Herr D.R., Fyrst H., Creason M.B., Phan V.H., Saba J.D., Harris G.L. Characterization of the Drosophila sphingosine kinases and requirement for Sk2 in normal reproductive function. J. Biol. Chem. 2004;279:12685–12694. doi: 10.1074/jbc.M310647200. [DOI] [PubMed] [Google Scholar]
- 166.Herr D.R., Fyrst H., Phan V., Heinecke K., Georges R., Harris G.L., Saba J.D. Sply regulation of sphingolipid signaling molecules is essential for Drosophila development. Development. 2003;130:2443–2453. doi: 10.1242/dev.00456. [DOI] [PubMed] [Google Scholar]
- 167.Segawa K., Nagata S. An Apoptotic ‘Eat Me’ Signal: Phosphatidylserine Exposure. Trends Cell. Biol. 2015;25:639–650. doi: 10.1016/j.tcb.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 168.Matsuo Y., Fisher E., Patton-Vogt J., Marcus S. Functional characterization of the fission yeast phosphatidylserine synthase gene, pps1, reveals novel cellular functions for phosphatidylserine. Eukaryot. Cell. 2007;6:2092–2101. doi: 10.1128/EC.00300-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yamaoka Y., Shin S., Lee Y., Ito M., Lee Y., Nishida I. Phosphatidylserine Is Required for the Normal Progression of Cell Plate Formation in Arabidopsis Root Meristems. Plant. Cell. Physiol. 2021;62:1396–1408. doi: 10.1093/pcp/pcab086. [DOI] [PubMed] [Google Scholar]
- 170.Zhou C., Cunningham L., Marcus A.I., Li Y., Kahn R.A. Arl2 and Arl3 regulate different microtubule-dependent processes. Mol. Biol. Cell. 2006;17:2476–2487. doi: 10.1091/mbc.e05-10-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kapoor S., Fansa E.K., Mobitz S., Ismail S.A., Winter R., Wittinghofer A., Weise K. Effect of the N-Terminal Helix and Nucleotide Loading on the Membrane and Effector Binding of Arl2/3. Biophys J. 2015;109:1619–1629. doi: 10.1016/j.bpj.2015.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Altan-Bonnet N., Phair R.D., Polishchuk R.S., Weigert R., Lippincott-Schwartz J. A role for Arf1 in mitotic Golgi disassembly, chromosome segregation, and cytokinesis. Proc. Natl. Acad. Sci. USA. 2003;100:13314–13319. doi: 10.1073/pnas.2234055100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Chesneau L., Dambournet D., Machicoane M., Kouranti I., Fukuda M., Goud B., Echard A. An ARF6/Rab35 GTPase cascade for endocytic recycling and successful cytokinesis. Curr. Biol. 2012;22:147–153. doi: 10.1016/j.cub.2011.11.058. [DOI] [PubMed] [Google Scholar]
- 174.Matsuo H., Chevallier J., Mayran N., Le Blanc I., Ferguson C., Faure J., Blanc N.S., Matile S., Dubochet J., Sadoul R., et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science. 2004;303:531–534. doi: 10.1126/science.1092425. [DOI] [PubMed] [Google Scholar]
- 175.Morita E., Sandrin V., Chung H.Y., Morham S.G., Gygi S.P., Rodesch C.K., Sundquist W.I. Human ESCRT and ALIX proteins interact with proteins of the midbody and function in cytokinesis. EMBO J. 2007;26:4215–4227. doi: 10.1038/sj.emboj.7601850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Thoresen S.B., Campsteijn C., Vietri M., Schink K.O., Liestol K., Andersen J.S., Raiborg C., Stenmark H. ANCHR mediates Aurora-B-dependent abscission checkpoint control through retention of VPS4. Nat. Cell Biol. 2014;16:550–560. doi: 10.1038/ncb2959. [DOI] [PubMed] [Google Scholar]
- 177.Field C.M., Alberts B.M. Anillin, a contractile ring protein that cycles from the nucleus to the cell cortex. J. Cell Biol. 1995;131:165–178. doi: 10.1083/jcb.131.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Sun L., Guan R., Lee I.J., Liu Y., Chen M., Wang J., Wu J.Q., Chen Z. Mechanistic insights into the anchorage of the contractile ring by anillin and Mid1. Dev. Cell. 2015;33:413–426. doi: 10.1016/j.devcel.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Benaud C., Le Dez G., Mironov S., Galli F., Reboutier D., Prigent C. Annexin A2 is required for the early steps of cytokinesis. EMBO Rep. 2015;16:481–489. doi: 10.15252/embr.201440015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Tomas A., Futter C., Moss S.E. Annexin 11 is required for midbody formation and completion of the terminal phase of cytokinesis. J. Cell Biol. 2004;165:813–822. doi: 10.1083/jcb.200311054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.David M.D., Petit D., Bertoglio J. The RhoGAP ARHGAP19 controls cytokinesis and chromosome segregation in T lymphocytes. J. Cell Sci. 2014;127:400–410. doi: 10.1242/jcs.135079. [DOI] [PubMed] [Google Scholar]
- 182.Wolf A., Keil R., Gotzl O., Mun A., Schwarze K., Lederer M., Huttelmaier S., Hatzfeld M. The armadillo protein p0071 regulates Rho signalling during cytokinesis. Nat. Cell Biol. 2006;8:1432–1440. doi: 10.1038/ncb1504. [DOI] [PubMed] [Google Scholar]
- 183.Edwards M., Zwolak A., Schafer D.A., Sept D., Dominguez R., Cooper J.A. Capping protein regulators fine-tune actin assembly dynamics. Nat. Rev. Mol. Cell Biol. 2014;15:677–689. doi: 10.1038/nrm3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Terry S.J., Dona F., Osenberg P., Carlton J.G., Eggert U.S. Capping protein regulates actin dynamics during cytokinetic midbody maturation. Proc. Natl. Acad. Sci. USA. 2018;115:2138–2143. doi: 10.1073/pnas.1722281115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Gohla A., Birkenfeld J., Bokoch G.M. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat. Cell Biol. 2005;7:21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- 186.Bassi Z.I., Audusseau M., Riparbelli M.G., Callaini G., D’Avino P.P. Citron kinase controls a molecular network required for midbody formation in cytokinesis. Proc. Natl. Acad. Sci. USA. 2013;110:9782–9787. doi: 10.1073/pnas.1301328110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chen Q., Pollard T.D. Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J. Cell Biol. 2011;195:485–498. doi: 10.1083/jcb.201103067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Kusano K., Abe H., Obinata T. Detection of a sequence involved in actin-binding and phosphoinositide-binding in the N-terminal side of cofilin. Mol. Cell Biochem. 1999;190:133–141. doi: 10.1023/A:1006962210692. [DOI] [PubMed] [Google Scholar]
- 189.Gatta A.T., Carlton J.G. The ESCRT-machinery: Closing holes and expanding roles. Curr. Opin. Cell Biol. 2019;59:121–132. doi: 10.1016/j.ceb.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 190.Hurley J.H. ESCRTs are everywhere. EMBO J. 2015;34:2398–2407. doi: 10.15252/embj.201592484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Vietri M., Radulovic M., Stenmark H. The many functions of ESCRTs. Nat. Rev. Mol. Cell Biol. 2020;21:25–42. doi: 10.1038/s41580-019-0177-4. [DOI] [PubMed] [Google Scholar]
- 192.Giansanti M.G., Vanderleest T.E., Jewett C.E., Sechi S., Frappaolo A., Fabian L., Robinett C.C., Brill J.A., Loerke D., Fuller M.T., et al. Exocyst-Dependent Membrane Addition Is Required for Anaphase Cell Elongation and Cytokinesis in Drosophila. PLoS Genet. 2015;11:e1005632. doi: 10.1371/journal.pgen.1005632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Heider M.R., Munson M. Exorcising the exocyst complex. Traffic. 2012;13:898–907. doi: 10.1111/j.1600-0854.2012.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martin-Urdiroz M., Deeks M.J., Horton C.G., Dawe H.R., Jourdain I. The Exocyst Complex in Health and Disease. Front. Cell Dev. Biol. 2016;4:24. doi: 10.3389/fcell.2016.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mei K., Guo W. Exocytosis: A New Exocyst Movie. Curr. Biol. 2019;29:R30–R32. doi: 10.1016/j.cub.2018.11.022. [DOI] [PubMed] [Google Scholar]
- 196.Hiruma S., Kamasaki T., Otomo K., Nemoto T., Uehara R. Dynamics and function of ERM proteins during cytokinesis in human cells. FEBS Lett. 2017;591:3296–3309. doi: 10.1002/1873-3468.12844. [DOI] [PubMed] [Google Scholar]
- 197.Kunda P., Rodrigues N.T., Moeendarbary E., Liu T., I Ivetic, A.; Charras, G.; Baum, B. PP1-mediated moesin dephosphorylation couples polar relaxation to mitotic exit. Curr. Biol. 2012;22:231–236. doi: 10.1016/j.cub.2011.12.016. [DOI] [PubMed] [Google Scholar]
- 198.Au Yong J.Y., Wang Y.M., Wang Y. The Nim1 kinase Gin4 has distinct domains crucial for septin assembly, phospholipid binding and mitotic exit. J. Cell Sci. 2016;129:2744–2756. doi: 10.1242/jcs.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sechi S., Colotti G., Belloni G., Mattei V., Frappaolo A., Raffa G.D., Fuller M.T., Giansanti M.G. GOLPH3 is essential for contractile ring formation and Rab11 localization to the cleavage site during cytokinesis in Drosophila melanogaster. PLoS Genet. 2014;10:e1004305. doi: 10.1371/journal.pgen.1004305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Zhang X., Bedigian A.V., Wang W., Eggert U.S. G protein-coupled receptors participate in cytokinesis. Cytoskeleton. 2012;69:810–818. doi: 10.1002/cm.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhang X., Eggert U.S. Non-traditional roles of G protein-coupled receptors in basic cell biology. Mol. Biosyst. 2013;9:586–595. doi: 10.1039/C2MB25429H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhang X., Wang W., Bedigian A.V., Coughlin M.L., Mitchison T.J., Eggert U.S. Dopamine receptor D3 regulates endocytic sorting by a Prazosin-sensitive interaction with the coatomer COPI. Proc. Natl. Acad. Sci. USA. 2012;109:12485–12490. doi: 10.1073/pnas.1207821109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pellinen T., Tuomi S., Arjonen A., Wolf M., Edgren H., Meyer H., Grosse R., Kitzing T., Rantala J.K., Kallioniemi O., et al. Integrin trafficking regulated by Rab21 is necessary for cytokinesis. Dev. Cell. 2008;15:371–385. doi: 10.1016/j.devcel.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 204.Carim S.C., Ben El Kadhi K., Yan G., Sweeney S.T., Hickson G.R., Carreno S., Lowe M. IPIP27 Coordinates PtdIns(4,5)P2 Homeostasis for Successful Cytokinesis. Curr. Biol. 2019;29:775–789. doi: 10.1016/j.cub.2019.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hadders M.A., Agromayor M., Obita T., Perisic O., Caballe A., Kloc M., Lamers M.H., Williams R.L., Martin-Serrano J. ESCRT-III binding protein MITD1 is involved in cytokinesis and has an unanticipated PLD fold that binds membranes. Proc. Natl. Acad. Sci. USA. 2012;109:17424–17429. doi: 10.1073/pnas.1206839109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Gerien K.S., Zhang S., Russell A.C., Zhu Y.H., Purde V., Wu J.Q. Roles of Mso1 and the SM protein Sec1 in efficient vesicle fusion during fission yeast cytokinesis. Mol. Biol. Cell. 2020;31:1570–1583. doi: 10.1091/mbc.E20-01-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rohn J.L., Patel J.V., Neumann B., Bulkescher J., McHedlishvili N., McMullan R.C., Quintero O.A., Ellenberg J., Baum B. Myo19 ensures symmetric partitioning of mitochondria and coupling of mitochondrial segregation to cell division. Curr. Biol. 2014;24:2598–2605. doi: 10.1016/j.cub.2014.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hawthorne J.L., Mehta P.R., Singh P.P., Wong N.Q., Quintero O.A. Positively charged residues within the MYO19 MyMOMA domain are essential for proper localization of MYO19 to the mitochondrial outer membrane. Cytoskeleton. 2016;73:286–299. doi: 10.1002/cm.21305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Liu X., Shu S., Billington N., Williamson C.D., Yu S., Brzeska H., Donaldson J.G., Sellers J.R., Korn E.D. Mammalian Nonmuscle Myosin II Binds to Anionic Phospholipids with Concomitant Dissociation of the Regulatory Light Chain. J. Biol. Chem. 2016;291:24828–24837. doi: 10.1074/jbc.M116.739185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Snider C.E., Willet A.H., Brown H.T., Chen J.S., Evers J.M., Gould K.L. Fission yeast Opy1 is an endogenous PI(4,5)P2 sensor that binds to the phosphatidylinositol 4-phosphate 5-kinase Its3. J. Cell Sci. 2020;133 doi: 10.1242/jcs.247973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Su L., Agati J.M., Parsons S.J. p190RhoGAP is cell cycle regulated and affects cytokinesis. J. Cell Biol. 2003;163:571–582. doi: 10.1083/jcb.200308007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Heraud C., Pinault M., Lagree V., Moreau V. p190RhoGAPs, the ARHGAP35- and ARHGAP5-Encoded Proteins, in Health and Disease. Cells. 2019;8 doi: 10.3390/cells8040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Schiel J.A., Simon G.C., Zaharris C., Weisz J., Castle D., Wu C.C., Prekeris R. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat. Cell Biol. 2012;14:1068–1078. doi: 10.1038/ncb2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Jeyasimman D., Ercan B., Dharmawan D., Naito T., Sun J., Saheki Y. PDZD-8 and TEX-2 regulate endosomal PI(4,5)P2 homeostasis via lipid transport to promote embryogenesis in C. elegans. Nat. Commun. 2021;12:6065. doi: 10.1038/s41467-021-26177-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Thoresen S.B., Pedersen N.M., Liestol K., Stenmark H. A phosphatidylinositol 3-kinase class III sub-complex containing VPS15, VPS34, Beclin 1, UVRAG and BIF-1 regulates cytokinesis and degradative endocytic traffic. Exp. Cell Res. 2010;316:3368–3378. doi: 10.1016/j.yexcr.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 216.Rostislavleva K., Soler N., Ohashi Y., Zhang L., Pardon E., Burke J.E., Masson G.R., Johnson C., Steyaert J., Ktistakis N.T., et al. Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science. 2015;350:aac7365. doi: 10.1126/science.aac7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Poddar A., Hsu Y.Y., Zhang F., Shamma A., Kreais Z., Muller C., Malla M., Ray A., Liu A.P., Chen Q. Membrane stretching activates calcium permeability of a putative channel Pkd2 during fission yeast cytokinesis. Mol. Biol. Cell. 2022;33:ar134. doi: 10.1091/mbc.E22-07-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Asano S., Ikura Y., Nishimoto M., Yamawaki Y., Hamao K., Kamijo K., Hirata M., Kanematsu T. Phospholipase C-related catalytically inactive protein regulates cytokinesis by protecting phosphatidylinositol 4,5-bisphosphate from metabolism in the cleavage furrow. Sci. Rep. 2019;9:12729. doi: 10.1038/s41598-019-49156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Platica M., Ionescu A., Ivan E., Holland J.F., Mandeli J., Platica O. PAR, a protein involved in the cell cycle, is functionally related to chromosomal passenger proteins. Int. J. Oncol. 2011;38:777–785. doi: 10.3892/ijo.2011.900. [DOI] [PubMed] [Google Scholar]
- 220.Mondin V.E., Ben El Kadhi K., Cauvin C., Jackson-Crawford A., Belanger E., Decelle B., Salomon R., Lowe M., Echard A., Carreno S. PTEN reduces endosomal PtdIns(4,5)P2 in a phosphatase-independent manner via a PLC pathway. J. Cell Biol. 2019;218:2198–2214. doi: 10.1083/jcb.201805155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Sechi S., Frappaolo A., Fraschini R., Capalbo L., Gottardo M., Belloni G., Glover D.M., Wainman A., Giansanti M.G. Rab1 interacts with GOLPH3 and controls Golgi structure and contractile ring constriction during cytokinesis in Drosophila melanogaster. Open Biol. 2017;7 doi: 10.1098/rsob.160257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Lalioti V.S., Ilari A., O’Connell D.J., Poser E., Sandoval I.V., Colotti G. Sorcin links calcium signaling to vesicle trafficking, regulates Polo-like kinase 1 and is necessary for mitosis. PLoS ONE. 2014;9:e85438. doi: 10.1371/journal.pone.0085438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gibieza P., Peterman E., Hoffman H.K., Van Engeleburg S., Skeberdis V.A., Prekeris R. Rab14/MACF2 complex regulates endosomal targeting during cytokinesis. Mol. Biol. Cell. 2021;32:554–566. doi: 10.1091/mbc.E20-09-0607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kelly E.E., Horgan C.P., Adams C., Patzer T.M., Ni Shuilleabhain D.M., Norman J.C., McCaffrey M.W. Class I Rab11-family interacting proteins are binding targets for the Rab14 GTPase. Biol. Cell. 2009;102:51–62. doi: 10.1042/BC20090068. [DOI] [PubMed] [Google Scholar]
- 225.Militello R.D., Munafo D.B., Beron W., Lopez L.A., Monier S., Goud B., Colombo M.I. Rab24 is required for normal cell division. Traffic. 2013;14:502–518. doi: 10.1111/tra.12057. [DOI] [PubMed] [Google Scholar]
- 226.Qiu D., Li S., Guo L., Yuan R., Ou X. Rab24 functions in meiotic apparatus assembly and maturational progression in mouse oocyte. Cell Cycle. 2019;18:2893–2901. doi: 10.1080/15384101.2019.1660115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Kaplan A., Reiner O. Linking cytoplasmic dynein and transport of Rab8 vesicles to the midbody during cytokinesis by the doublecortin domain-containing 5 protein. J. Cell Sci. 2011;124:3989–4000. doi: 10.1242/jcs.085407. [DOI] [PubMed] [Google Scholar]
- 228.Cascone I., Selimoglu R., Ozdemir C., Del Nery E., Yeaman C., White M., Camonis J. Distinct roles of RalA and RalB in the progression of cytokinesis are supported by distinct RalGEFs. EMBO J. 2008;27:2375–2387. doi: 10.1038/emboj.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chen X.W., Inoue M., Hsu S.C., Saltiel A.R. RalA-exocyst-dependent recycling endosome trafficking is required for the completion of cytokinesis. J. Biol. Chem. 2006;281:38609–38616. doi: 10.1074/jbc.M512847200. [DOI] [PubMed] [Google Scholar]
- 230.Holly R.M., Mavor L.M., Zuo Z., Blankenship J.T. A rapid, membrane-dependent pathway directs furrow formation through RalA in the early Drosophila embryo. Development. 2015;142:2316–2328. doi: 10.1242/jcs.176560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Fielding A.B., Schonteich E., Matheson J., Wilson G., Yu X., Hickson G.R., Srivastava S., Baldwin S.A., Prekeris R., Gould G.W. Rab11-FIP3 and FIP4 interact with Arf6 and the exocyst to control membrane traffic in cytokinesis. EMBO J. 2005;24:3389–3399. doi: 10.1038/sj.emboj.7600803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Wilson G.M., Fielding A.B., Simon G.C., Yu X., Andrews P.D., Hames R.S., Frey A.M., Peden A.A., Gould G.W., Prekeris R. The FIP3-Rab11 protein complex regulates recycling endosome targeting to the cleavage furrow during late cytokinesis. Mol. Biol. Cell. 2005;16:849–860. doi: 10.1091/mbc.e04-10-0927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Levay M., Settleman J., Ligeti E. Regulation of the substrate preference of p190RhoGAP by protein kinase C-mediated phosphorylation of a phospholipid binding site. Biochemistry. 2009;48:8615–8623. doi: 10.1021/bi900667y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hodge R.G., Ridley A.J. Regulating Rho GTPases and their regulators. Nat. Rev. Mol. Cell Biol. 2016;17:496–510. doi: 10.1038/nrm.2016.67. [DOI] [PubMed] [Google Scholar]
- 235.Kosako H., Yoshida T., Matsumura F., Ishizaki T., Narumiya S., Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–6064. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 236.Lowery D.M., Clauser K.R., Hjerrild M., Lim D., Alexander J., Kishi K., Ong S.E., Gammeltoft S., Carr S.A., Yaffe M.B. Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 2007;26:2262–2273. doi: 10.1038/sj.emboj.7601683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Arbizzani F., Mavrakis M., Hoya M., Ribas J.C., Brasselet S., Paoletti A., Rincon S.A. Septin filament compaction into rings requires the anillin Mid2 and contractile ring constriction. Cell Rep. 2022;39:110722. doi: 10.1016/j.celrep.2022.110722. [DOI] [PubMed] [Google Scholar]
- 238.Karasmanis E.P., Hwang D., Nakos K., Bowen J.R., Angelis D., Spiliotis E.T. A Septin Double Ring Controls the Spatiotemporal Organization of the ESCRT Machinery in Cytokinetic Abscission. Curr. Biol. 2019;29:2174–2182. doi: 10.1016/j.cub.2019.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Schmidt A., Durgan J., Magalhaes A., Hall A. Rho GTPases regulate PRK2/PKN2 to control entry into mitosis and exit from cytokinesis. EMBO J. 2007;26:1624–1636. doi: 10.1038/sj.emboj.7601637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yoshinaga C., Mukai H., Toshimori M., Miyamoto M., Ono Y. Mutational analysis of the regulatory mechanism of PKN: The regulatory region of PKN contains an arachidonic acid-sensitive autoinhibitory domain. J. Biochem. 1999;126:475–484. doi: 10.1093/oxfordjournals.jbchem.a022476. [DOI] [PubMed] [Google Scholar]
- 241.Hagemann N., Ackermann N., Christmann J., Brier S., Yu F., Erdmann K.S. The serologically defined colon cancer antigen-3 interacts with the protein tyrosine phosphatase PTPN13 and is involved in the regulation of cytokinesis. Oncogene. 2013;32:4602–4613. doi: 10.1038/onc.2012.485. [DOI] [PubMed] [Google Scholar]
- 242.Neto H., Kaupisch A., Collins L.L., Gould G.W. Syntaxin 16 is a master recruitment factor for cytokinesis. Mol. Biol. Cell. 2013;24:3663–3674. doi: 10.1091/mbc.e13-06-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Ma M.P., Chircop M. SNX9, SNX18 and SNX33 are required for progression through and completion of mitosis. J. Cell Sci. 2012;125:4372–4382. doi: 10.1242/jcs.105981. [DOI] [PubMed] [Google Scholar]
- 244.Renvoise B., Parker R.L., Yang D., Bakowska J.C., Hurley J.H., Blackstone C. SPG20 protein spartin is recruited to midbodies by ESCRT-III protein Ist1 and participates in cytokinesis. Mol. Biol. Cell. 2010;21:3293–3303. doi: 10.1091/mbc.e09-10-0879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Connell J.W., Lindon C., Luzio J.P., Reid E. Spastin couples microtubule severing to membrane traffic in completion of cytokinesis and secretion. Traffic. 2009;10:42–56. doi: 10.1111/j.1600-0854.2008.00847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kizhatil K., Yoon W., Mohler P.J., Davis L.H., Hoffman J.A., Bennett V. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J. Biol. Chem. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 247.Mukai A., Mizuno E., Kobayashi K., Matsumoto M., Nakayama K.I., Kitamura N., Komada M. Dynamic regulation of ubiquitylation and deubiquitylation at the central spindle during cytokinesis. J. Cell Sci. 2008;121:1325–1333. doi: 10.1242/jcs.027417. [DOI] [PubMed] [Google Scholar]
- 248.Dona F., Ozbalci C., Paquola A., Ferrentino F., Terry S.J., Storck E.M., Wang G., Eggert U.S. Removal of Stomatin, a Membrane-Associated Cell Division Protein, Results in Specific Cellular Lipid Changes. J. Am. Chem. Soc. 2022;144:18069–18074. doi: 10.1021/jacs.2c07907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rungaldier S., Umlauf E., Mairhofer M., Salzer U., Thiele C., Prohaska R. Structure-function analysis of human stomatin: A mutation study. PLoS ONE. 2017;12:e0178646. doi: 10.1371/journal.pone.0178646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Hasegawa H., Hyodo T., Asano E., Ito S., Maeda M., Kuribayashi H., Natsume A., Wakabayashi T., Hamaguchi M., Senga T. The role of PLK1-phosphorylated SVIL in myosin II activation and cytokinetic furrowing. J. Cell Sci. 2013;126:3627–3637. doi: 10.1242/jcs.124818. [DOI] [PubMed] [Google Scholar]
- 251.Smith T.C., Fang Z., Luna E.J. Novel interactors and a role for supervillin in early cytokinesis. Cytoskeleton. 2010;67:346–364. doi: 10.1002/cm.20449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Smith T.C., Fridy P.C., Li Y., Basil S., Arjun S., Friesen R.M., Leszyk J., Chait B.T., Rout M.P., Luna E.J. Supervillin binding to myosin II and synergism with anillin are required for cytokinesis. Mol. Biol. Cell. 2013;24:3603–3619. doi: 10.1091/mbc.e12-10-0714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lee Y., Chung S., Baek I.K., Lee T.H., Paik S.Y., Lee J. UNC119a bridges the transmission of Fyn signals to Rab11, leading to the completion of cytokinesis. Cell Cycle. 2013;12:1303–1315. doi: 10.4161/cc.24404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Zhu Y.H., Hyun J., Pan Y.Z., Hopper J.E., Rizo J., Wu J.Q. Roles of the fission yeast UNC-13/Munc13 protein Ync13 in late stages of cytokinesis. Mol. Biol. Cell. 2018;29:2259–2279. doi: 10.1091/mbc.E18-04-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Snider C.E., Willet A.H., Brown H.T., Gould K.L. Analysis of the contribution of phosphoinositides to medial septation in fission yeast highlights the importance of PI(4,5)P2 for medial contractile ring anchoring. Mol. Biol. Cell. 2018;29:2148–2155. doi: 10.1091/mbc.E18-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Snider C.E., Willet A.H., Chen J.S., Arpag G., Zanic M., Gould K.L. Phosphoinositide-mediated ring anchoring resists perpendicular forces to promote medial cytokinesis. J. Cell. Biol. 2017;216:3041–3050. doi: 10.1083/jcb.201705070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wu X., Chi R.J., Baskin J.M., Lucast L., Burd C.G., De Camilli P., Reinisch K.M. Structural insights into assembly and regulation of the plasma membrane phosphatidylinositol 4-kinase complex. Dev. Cell. 2014;28:19–29. doi: 10.1016/j.devcel.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Sagona A.P., Nezis I.P., Pedersen N.M., Liestol K., Poulton J., Rusten T.E., Skotheim R.I., Raiborg C., Stenmark H. PtdIns(3)P controls cytokinesis through KIF13A-mediated recruitment of FYVE-CENT to the midbody. Nat. Cell Biol. 2010;12:362–371. doi: 10.1038/ncb2036. [DOI] [PubMed] [Google Scholar]
- 259.Frappaolo A., Piergentili R., Giansanti M.G. Microtubule and Actin Cytoskeletal Dynamics in Male Meiotic Cells of Drosophila melanogaster. Cells. 2022;11 doi: 10.3390/cells11040695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Aveldano M.I., Robinson B.S., Johnson D.W., Poulos A. Long and very long chain polyunsaturated fatty acids of the n-6 series in rat seminiferous tubules. Active desaturation of 24:4n-6 to 24:5n-6 and concomitant formation of odd and even chain tetraenoic and pentaenoic fatty acids up to C32. J. Biol. Chem. 1993;268:11663–11669. doi: 10.1016/S0021-9258(19)50251-4. [DOI] [PubMed] [Google Scholar]
- 261.Furland N.E., Oresti G.M., Antollini S.S., Venturino A., Maldonado E.N., Aveldano M.I. Very long-chain polyunsaturated fatty acids are the major acyl groups of sphingomyelins and ceramides in the head of mammalian spermatozoa. J. Biol. Chem. 2007;282:18151–18161. doi: 10.1074/jbc.M700709200. [DOI] [PubMed] [Google Scholar]
- 262.Furland N.E., Zanetti S.R., Oresti G.M., Maldonado E.N., Aveldano M.I. Ceramides and sphingomyelins with high proportions of very long-chain polyunsaturated fatty acids in mammalian germ cells. J. Biol. Chem. 2007;282:18141–18150. doi: 10.1074/jbc.M700708200. [DOI] [PubMed] [Google Scholar]
- 263.Sandhoff R. Very long chain sphingolipids: Tissue expression, function and synthesis. FEBS Lett. 2010;584:1907–1913. doi: 10.1016/j.febslet.2009.12.032. [DOI] [PubMed] [Google Scholar]
- 264.Sandhoff R., Geyer R., Jennemann R., Paret C., Kiss E., Yamashita T., Gorgas K., Sijmonsma T.P., Iwamori M., Finaz C., et al. Novel class of glycosphingolipids involved in male fertility. J. Biol. Chem. 2005;280:27310–27318. doi: 10.1074/jbc.M502775200. [DOI] [PubMed] [Google Scholar]
- 265.Santiago Valtierra F.X., Penalva D.A., Luquez J.M., Furland N.E., Vasquez C., Reyes J.G., Aveldano M.I., Oresti G.M. Elovl4 and Fa2h expression during rat spermatogenesis: A link to the very-long-chain PUFAs typical of germ cell sphingolipids. J. Lipid Res. 2018;59:1175–1189. doi: 10.1194/jlr.M081885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Rabionet M., van der Spoel A.C., Chuang C.C., von Tumpling-Radosta B., Litjens M., Bouwmeester D., Hellbusch C.C., Korner C., Wiegandt H., Gorgas K., et al. Male germ cells require polyenoic sphingolipids with complex glycosylation for completion of meiosis: A link to ceramide synthase-3. J. Biol. Chem. 2008;283:13357–13369. doi: 10.1074/jbc.M800870200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rabionet M., Bayerle A., Jennemann R., Heid H., Fuchser J., Marsching C., Porubsky S., Bolenz C., Guillou F., Grone H.J., et al. Male meiotic cytokinesis requires ceramide synthase 3-dependent sphingolipids with unique membrane anchors. Hum. Mol. Genet. 2015;24:4792–4808. doi: 10.1093/hmg/ddv204. [DOI] [PubMed] [Google Scholar]
- 268.Szafer-Glusman E., Giansanti M.G., Nishihama R., Bolival B., Pringle J., Gatti M., Fuller M.T. A role for very-long-chain fatty acids in furrow ingression during cytokinesis in Drosophila spermatocytes. Curr. Biol. 2008;18:1426–1431. doi: 10.1016/j.cub.2008.08.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Keays M.C., Barker D., Wicker-Thomas C., Ritchie M.G. Signatures of selection and sex-specific expression variation of a novel duplicate during the evolution of the Drosophila desaturase gene family. Mol. Ecol. 2011;20:3617–3630. doi: 10.1111/j.1365-294X.2011.05208.x. [DOI] [PubMed] [Google Scholar]
- 270.Endo K., Akiyama T., Kobayashi S., Okada M. Degenerative spermatocyte, a novel gene encoding a transmembrane protein required for the initiation of meiosis in Drosophila spermatogenesis. Mol. Gen. Genet. 1996;253:157–165. doi: 10.1007/s004380050308. [DOI] [PubMed] [Google Scholar]
- 271.Ternes P., Franke S., Zahringer U., Sperling P., Heinz E. Identification and characterization of a sphingolipid delta 4-desaturase family. J. Biol. Chem. 2002;277:25512–25518. doi: 10.1074/jbc.M202947200. [DOI] [PubMed] [Google Scholar]
- 272.Guan X.L., Cestra G., Shui G., Kuhrs A., Schittenhelm R.B., Hafen E., van der Goot F.G., Robinett C.C., Gatti M., Gonzalez-Gaitan M., et al. Biochemical membrane lipidomics during Drosophila development. Dev. Cell. 2013;24:98–111. doi: 10.1016/j.devcel.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 273.Kunduri G., Turner-Evans D., Konya Y., Izumi Y., Nagashima K., Lockett S., Holthuis J., Bamba T., Acharya U., Acharya J.K. Defective cortex glia plasma membrane structure underlies light-induced epilepsy in cpes mutants. Proc. Natl. Acad. Sci. USA. 2018;115:E8919–E8928. doi: 10.1073/pnas.1808463115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Xiupeng Chen J.L., Gao Z., Yang Y., Kuang W., Dong Y., Chua G.H., Huang X., Jiang B., Tian H., Wang Y., et al. Endogenous ceramide phosphoethanolamine modulates circadian rhythm via neural-glial coupling in Drosophila. Natl. Sci. Rev. 2022 doi: 10.1093/nsr/nwac148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Harayama T., Shimizu T. Roles of polyunsaturated fatty acids, from mediators to membranes. J. Lipid Res. 2020;61:1150–1160. doi: 10.1194/jlr.R120000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Rao R.P., Yuan C., Allegood J.C., Rawat S.S., Edwards M.B., Wang X., Merrill A.H., Jr., Acharya U., Acharya J.K. Ceramide transfer protein function is essential for normal oxidative stress response and lifespan. Proc. Natl. Acad. Sci. USA. 2007;104:11364–11369. doi: 10.1073/pnas.0705049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Santiago Valtierra F.X., Mateos M.V., Aveldano M.I., Oresti G.M. Sphingomyelins and ceramides with VLCPUFAs are excluded from low-density raft-like domains in differentiating spermatogenic cells. J. Lipid Res. 2017;58:529–542. doi: 10.1194/jlr.M072595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Tiberti M.L., Antonny B., Gautier R. The transbilayer distribution of polyunsaturated phospholipids determines their facilitating effect on membrane deformation. Soft. Matter. 2020;16:1722–1730. doi: 10.1039/C9SM02107H. [DOI] [PubMed] [Google Scholar]
- 279.Pinot M., Vanni S., Pagnotta S., Lacas-Gervais S., Payet L.A., Ferreira T., Gautier R., Goud B., Antonny B., Barelli H. Lipid cell biology. Polyunsaturated phospholipids facilitate membrane deformation and fission by endocytic proteins. Science. 2014;345:693–697. doi: 10.1126/science.1255288. [DOI] [PubMed] [Google Scholar]
- 280.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brugger B., Simons M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 281.Wu C.Y., Jhang J.G., Lin W.S., Chuang P.H., Lin C.W., Chu L.A., Chiang A.S., Ho H.C., Chan C.C., Huang S.Y. Dihydroceramide desaturase promotes the formation of intraluminal vesicles and inhibits autophagy to increase exosome production. iScience. 2021;24:103437. doi: 10.1016/j.isci.2021.103437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Polevoy G., Wei H.C., Wong R., Szentpetery Z., Kim Y.J., Goldbach P., Steinbach S.K., Balla T., Brill J.A. Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. J. Cell Biol. 2009;187:847–858. doi: 10.1083/jcb.200908107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Tanaka M., Fujimoto K., Yumura S. Regulation of the Total Cell Surface Area in Dividing Dictyostelium Cells. Front Cell Dev. Biol. 2020;8:238. doi: 10.3389/fcell.2020.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Boucrot E., Kirchhausen T. Endosomal recycling controls plasma membrane area during mitosis. Proc. Natl. Acad. Sci. USA. 2007;104:7939–7944. doi: 10.1073/pnas.0702511104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Champion L., Linder M.I., Kutay U. Cellular Reorganization during Mitotic Entry. Trends Cell Biol. 2017;27:26–41. doi: 10.1016/j.tcb.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 286.Ramkumar N., Baum B. Coupling changes in cell shape to chromosome segregation. Nat. Rev. Mol. Cell Biol. 2016;17:511–521. doi: 10.1038/nrm.2016.75. [DOI] [PubMed] [Google Scholar]
- 287.Lancaster O.M., Le Berre M., Dimitracopoulos A., Bonazzi D., Zlotek-Zlotkiewicz E., Picone R., Duke T., Piel M., Baum B. Mitotic rounding alters cell geometry to ensure efficient bipolar spindle formation. Dev. Cell. 2013;25:270–283. doi: 10.1016/j.devcel.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 288.Erickson C.A., Trinkaus J.P. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp. Cell Res. 1976;99:375–384. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- 289.Knutton S., Sumner M.C., Pasternak C.A. Role of microvilli in surface changes of synchronized P815Y mastocytoma cells. J. Cell Biol. 1975;66:568–576. doi: 10.1083/jcb.66.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Porter K., Prescott D., Frye J. Changes in surface morphology of Chinese hamster ovary cells during the cell cycle. J. Cell Biol. 1973;57:815–836. doi: 10.1083/jcb.57.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Bluemink J.G., van Maurik P.A., Tertoolen L.G., van der Saag P.T., de Laat S.W. Ultrastructural aspects of rapid plasma membrane growth in mitotic neuroblastoma cells. Eur. J. Cell Biol. 1983;32:7–16. [PubMed] [Google Scholar]
- 292.Danilchik M.V., Bedrick S.D., Brown E.E., Ray K. Furrow microtubules and localized exocytosis in cleaving Xenopus laevis embryos. J. Cell Sci. 2003;116:273–283. doi: 10.1242/jcs.00217. [DOI] [PubMed] [Google Scholar]
- 293.Skop A.R., Bergmann D., Mohler W.A., White J.G. Completion of cytokinesis in C. elegans requires a brefeldin A-sensitive membrane accumulation at the cleavage furrow apex. Curr. Biol. 2001;11:735–746. doi: 10.1016/S0960-9822(01)00231-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lecuit T., Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J. Cell Biol. 2000;150:849–860. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Sisson J.C., Field C., Ventura R., Royou A., Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J. Cell Biol. 2000;151:905–918. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Buschmann H., Muller S. Update on plant cytokinesis: Rule and divide. Curr. Opin. Plant Biol. 2019;52:97–105. doi: 10.1016/j.pbi.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 297.Sinclair R., Hsu G., Davis D., Chang M., Rosquete M., Iwasa J.H., Drakakaki G. Plant cytokinesis and the construction of new cell wall. FEBS Lett. 2022;596:2243–2255. doi: 10.1002/1873-3468.14426. [DOI] [PubMed] [Google Scholar]
- 298.Belloni G., Sechi S., Riparbelli M.G., Fuller M.T., Callaini G., Giansanti M.G. Mutations in Cog7 affect Golgi structure, meiotic cytokinesis and sperm development during Drosophila spermatogenesis. J. Cell Sci. 2012;125:5441–5452. doi: 10.1242/jcs.108878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Dyer N., Rebollo E., Dominguez P., Elkhatib N., Chavrier P., Daviet L., Gonzalez C., Gonzalez-Gaitan M. Spermatocyte cytokinesis requires rapid membrane addition mediated by ARF6 on central spindle recycling endosomes. Development. 2007;134:4437–4447. doi: 10.1242/dev.010983. [DOI] [PubMed] [Google Scholar]
- 300.Farkas R.M., Giansanti M.G., Gatti M., Fuller M.T. The Drosophila Cog5 homologue is required for cytokinesis, cell elongation, and assembly of specialized Golgi architecture during spermatogenesis. Mol. Biol. Cell. 2003;14:190–200. doi: 10.1091/mbc.e02-06-0343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Gatt M.K., Glover D.M. The Drosophila phosphatidylinositol transfer protein encoded by vibrator is essential to maintain cleavage-furrow ingression in cytokinesis. J. Cell Sci. 2006;119:2225–2235. doi: 10.1242/jcs.02933. [DOI] [PubMed] [Google Scholar]
- 302.Giansanti M.G., Belloni G., Gatti M. Rab11 is required for membrane trafficking and actomyosin ring constriction in meiotic cytokinesis of Drosophila males. Mol. Biol. Cell. 2007;18:5034–5047. doi: 10.1091/mbc.e07-05-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Robinett C.C., Giansanti M.G., Gatti M., Fuller M.T. TRAPPII is required for cleavage furrow ingression and localization of Rab11 in dividing male meiotic cells of Drosophila. J. Cell Sci. 2009;122:4526–4534. doi: 10.1242/jcs.054536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Sechi S., Frappaolo A., Belloni G., Colotti G., Giansanti M.G. The multiple cellular functions of the oncoprotein Golgi phosphoprotein 3. Oncotarget. 2015;6:3493–3506. doi: 10.18632/oncotarget.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Xu H., Brill J.A., Hsien J., McBride R., Boulianne G.L., Trimble W.S. Syntaxin 5 is required for cytokinesis and spermatid differentiation in Drosophila. Dev. Biol. 2002;251:294–306. doi: 10.1006/dbio.2002.0830. [DOI] [PubMed] [Google Scholar]
- 306.Sechi S., Frappaolo A., Karimpour-Ghahnavieh A., Fraschini R., Giansanti M.G. A novel coordinated function of Myosin II with GOLPH3 controls centralspindlin localization during cytokinesis in Drosophila. J. Cell Sci. 2020;133 doi: 10.1242/jcs.252965. [DOI] [PubMed] [Google Scholar]
- 307.Kitazawa D., Yamaguchi M., Mori H., Inoue Y.H. COPI-mediated membrane trafficking is required for cytokinesis in Drosophila male meiotic divisions. J. Cell Sci. 2012;125:3649–3660. doi: 10.1242/jcs.103317. [DOI] [PubMed] [Google Scholar]
- 308.Wainman A., Giansanti M.G., Goldberg M.L., Gatti M. The Drosophila RZZ complex - roles in membrane trafficking and cytokinesis. J. Cell Sci. 2012;125:4014–4025. doi: 10.1242/jcs.099820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Balakrishnan S.S., Basu U., Raghu P. Phosphoinositide signalling in Drosophila. Biochim. Biophys. Acta. 2015;1851:770–784. doi: 10.1016/j.bbalip.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 310.Giansanti M.G., Bonaccorsi S., Kurek R., Farkas R.M., Dimitri P., Fuller M.T., Gatti M. The class I PITP giotto is required for Drosophila cytokinesis. Curr. Biol. 2006;16:195–201. doi: 10.1016/j.cub.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 311.Chen W., Feng Y., Chen D., Wandinger-Ness A. Rab11 is required for trans-golgi network-to-plasma membrane transport and a preferential target for GDP dissociation inhibitor. Mol. Biol. Cell. 1998;9:3241–3257. doi: 10.1091/mbc.9.11.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.de Graaf P., Zwart W.T., van Dijken R.A., Deneka M., Schulz T.K., Geijsen N., Coffer P.J., Gadella B.M., Verkleij A.J., van der Sluijs P., et al. Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol. Biol. Cell. 2004;15:2038–2047. doi: 10.1091/mbc.e03-12-0862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Koe C.T., Tan Y.S., Lonnfors M., Hur S.K., Low C.S.L., Zhang Y., Kanchanawong P., Bankaitis V.A., Wang H. Vibrator and PI4KIIIalpha govern neuroblast polarity by anchoring non-muscle myosin II. Elife. 2018;7 doi: 10.7554/eLife.33555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Ito Y., Esnay N., Platre M.P., Wattelet-Boyer V., Noack L.C., Fougere L., Menzel W., Claverol S., Fouillen L., Moreau P., et al. Sphingolipids mediate polar sorting of PIN2 through phosphoinositide consumption at the trans-Golgi network. Nat. Commun. 2021;12:4267. doi: 10.1038/s41467-021-24548-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Goss J.W., Toomre D.K. Both daughter cells traffic and exocytose membrane at the cleavage furrow during mammalian cytokinesis. J. Cell Biol. 2008;181:1047–1054. doi: 10.1083/jcb.200712137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Gromley A., Yeaman C., Rosa J., Redick S., Chen C.T., Mirabelle S., Guha M., Sillibourne J., Doxsey S.J. Centriolin anchoring of exocyst and SNARE complexes at the midbody is required for secretory-vesicle-mediated abscission. Cell. 2005;123:75–87. doi: 10.1016/j.cell.2005.07.027. [DOI] [PubMed] [Google Scholar]
- 317.Monzo P., Gauthier N.C., Keslair F., Loubat A., Field C.M., Le Marchand-Brustel Y., Cormont M. Clues to CD2-associated protein involvement in cytokinesis. Mol. Biol. Cell. 2005;16:2891–2902. doi: 10.1091/mbc.e04-09-0773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Schweitzer J.K., Burke E.E., Goodson H.V., D’Souza-Schorey C. Endocytosis resumes during late mitosis and is required for cytokinesis. J. Biol. Chem. 2005;280:41628–41635. doi: 10.1074/jbc.M504497200. [DOI] [PubMed] [Google Scholar]
- 319.Shuster C.B., Burgess D.R. Targeted new membrane addition in the cleavage furrow is a late, separate event in cytokinesis. Proc. Natl. Acad. Sci USA. 2002;99:3633–3638. doi: 10.1073/pnas.052342699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Tacheva-Grigorova S.K., Santos A.J., Boucrot E., Kirchhausen T. Clathrin-mediated endocytosis persists during unperturbed mitosis. Cell Rep. 2013;4:659–668. doi: 10.1016/j.celrep.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Li W.M., Webb S.E., Chan C.M., Miller A.L. Multiple roles of the furrow deepening Ca2+ transient during cytokinesis in zebrafish embryos. Dev. Biol. 2008;316:228–248. doi: 10.1016/j.ydbio.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 322.Li W.M., Webb S.E., Lee K.W., Miller A.L. Recruitment and SNARE-mediated fusion of vesicles in furrow membrane remodeling during cytokinesis in zebrafish embryos. Exp. Cell Res. 2006;312:3260–3275. doi: 10.1016/j.yexcr.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 323.Pelissier A., Chauvin J.P., Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr. Biol. 2003;13:1848–1857. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 324.Riggs B., Rothwell W., Mische S., Hickson G.R., Matheson J., Hays T.S., Gould G.W., Sullivan W. Actin cytoskeleton remodeling during early Drosophila furrow formation requires recycling endosomal components Nuclear-fallout and Rab11. J. Cell Biol. 2003;163:143–154. doi: 10.1083/jcb.200305115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Hickson G.R., Matheson J., Riggs B., Maier V.H., Fielding A.B., Prekeris R., Sullivan W., Barr F.A., Gould G.W. Arfophilins are dual Arf/Rab 11 binding proteins that regulate recycling endosome distribution and are related to Drosophila nuclear fallout. Mol. Biol. Cell. 2003;14:2908–2920. doi: 10.1091/mbc.e03-03-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Schiel J.A., Park K., Morphew M.K., Reid E., Hoenger A., Prekeris R. Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J. Cell Sci. 2011;124:1411–1424. doi: 10.1242/jcs.081448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Simon G.C., Schonteich E., Wu C.C., Piekny A., Ekiert D., Yu X., Gould G.W., Glotzer M., Prekeris R. Sequential Cyk-4 binding to ECT2 and FIP3 regulates cleavage furrow ingression and abscission during cytokinesis. EMBO J. 2008;27:1791–1803. doi: 10.1038/emboj.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Gudejko H.F., Alford L.M., Burgess D.R. Polar expansion during cytokinesis. Cytoskeleton. 2012;69:1000–1009. doi: 10.1002/cm.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Rajamanoharan D., McCue H.V., Burgoyne R.D., Haynes L.P. Modulation of phosphatidylinositol 4-phosphate levels by CaBP7 controls cytokinesis in mammalian cells. Mol. Biol. Cell. 2015;26:1428–1439. doi: 10.1091/mbc.E14-07-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nugues C., Rajamanoharan D., Burgoyne R.D., Haynes L.P., Helassa N. Lysosome exocytosis is required for mitosis in mammalian cells. Biochem. Biophys. Res. Commun. 2022;626:211–219. doi: 10.1016/j.bbrc.2022.08.024. [DOI] [PubMed] [Google Scholar]
- 331.van den Bout I., Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: Regulation and cellular functions. J. Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 332.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 333.Hope H.R., Pike L.J. Phosphoinositides and phosphoinositide-utilizing enzymes in detergent-insoluble lipid domains. Mol. Biol. Cell. 1996;7:843–851. doi: 10.1091/mbc.7.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Laux T., Fukami K., Thelen M., Golub T., Frey D., Caroni P. GAP43, MARCKS, and CAP23 modulate PI(4,5)P(2) at plasmalemmal rafts, and regulate cell cortex actin dynamics through a common mechanism. J. Cell Biol. 2000;149:1455–1472. doi: 10.1083/jcb.149.7.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Ikenouchi J., Hirata M., Yonemura S., Umeda M. Sphingomyelin clustering is essential for the formation of microvilli. J. Cell Sci. 2013;126:3585–3592. doi: 10.1242/jcs.122325. [DOI] [PubMed] [Google Scholar]
- 336.Watanabe H., Okahara K., Naito-Matsui Y., Abe M., Go S., Inokuchi J., Okazaki T., Kobayashi T., Kozutsumi Y., Oka S., et al. Psychosine-triggered endomitosis is modulated by membrane sphingolipids through regulation of phosphoinositide 4,5-bisphosphate production at the cleavage furrow. Mol. Biol. Cell. 2016;27:2037–2050. doi: 10.1091/mbc.E15-08-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Reymann A.C., Staniscia F., Erzberger A., Salbreux G., Grill S.W. Cortical flow aligns actin filaments to form a furrow. Elife. 2016;5:e17807. doi: 10.7554/eLife.17807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Sokac A.M., Biel N., De Renzis S. Membrane-actin interactions in morphogenesis: Lessons learned from Drosophila cellularization. Semin. Cell Dev. Biol. 1016. [DOI] [PMC free article] [PubMed]
- 339.Benink H.A., Mandato C.A., Bement W.M. Analysis of cortical flow models in vivo. Mol. Biol. Cell. 2000;11:2553–2563. doi: 10.1091/mbc.11.8.2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Canman J.C., Bement W.M. Microtubules suppress actomyosin-based cortical flow in Xenopus oocytes. Pt 16J. Cell Sci. 1997;110 (Pt 16):1907–1917. doi: 10.1242/jcs.110.16.1907. [DOI] [PubMed] [Google Scholar]
- 341.Mandato C.A., Benink H.A., Bement W.M. Microtubule-actomyosin interactions in cortical flow and cytokinesis. Cell Motil. Cytoskelet. 2000;45:87–92. doi: 10.1002/(SICI)1097-0169(200002)45:2<87::AID-CM1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 342.Frisz J.F., Lou K., Klitzing H.A., Hanafin W.P., Lizunov V., Wilson R.L., Carpenter K.J., Kim R., Hutcheon I.D., Zimmerberg J., et al. Direct chemical evidence for sphingolipid domains in the plasma membranes of fibroblasts. Proc. Natl. Acad. Sci. USA. 2013;110:E613–E622. doi: 10.1073/pnas.1216585110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Fujita A., Cheng J., Fujimoto T. Segregation of GM1 and GM3 clusters in the cell membrane depends on the intact actin cytoskeleton. Biochim. Biophys. Acta. 2009;1791:388–396. doi: 10.1016/j.bbalip.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 344.Chaudhuri A., Bhattacharya B., Gowrishankar K., Mayor S., Rao M. Spatiotemporal regulation of chemical reactions by active cytoskeletal remodeling. Proc. Natl. Acad. Sci. USA. 2011;108:14825–14830. doi: 10.1073/pnas.1100007108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Goswami D., Gowrishankar K., Bilgrami S., Ghosh S., Raghupathy R., Chadda R., Vishwakarma R., Rao M., Mayor S. Nanoclusters of GPI-anchored proteins are formed by cortical actin-driven activity. Cell. 2008;135:1085–1097. doi: 10.1016/j.cell.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Koster D.V., Husain K., Iljazi E., Bhat A., Bieling P., Mullins R.D., Rao M., Mayor S. Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proc. Natl. Acad. Sci. USA. 2016;113:E1645–E1654. doi: 10.1073/pnas.1514030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Rao M., Mayor S. Active organization of membrane constituents in living cells. Curr. Opin. Cell Biol. 2014;29:126–132. doi: 10.1016/j.ceb.2014.05.007. [DOI] [PubMed] [Google Scholar]
- 348.Ingolfsson H.I., Melo M.N., van Eerden F.J., Arnarez C., Lopez C.A., Wassenaar T.A., Periole X., de Vries A.H., Tieleman D.P., Marrink S.J. Lipid organization of the plasma membrane. J. Am. Chem. Soc. 2014;136:14554–14559. doi: 10.1021/ja507832e. [DOI] [PubMed] [Google Scholar]
- 349.Valentine M.L., Waterland M.K., Fathizadeh A., Elber R., Baiz C.R. Interfacial Dynamics in Lipid Membranes: The Effects of Headgroup Structures. J. Phys. Chem. B. 2021;125:1343–1350. doi: 10.1021/acs.jpcb.0c08755. [DOI] [PubMed] [Google Scholar]
- 350.Kraft M.L. Sphingolipid Organization in the Plasma Membrane and the Mechanisms That Influence It. Front Cell Dev. Biol. 2016;4:154. doi: 10.3389/fcell.2016.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Kanazawa T., Nakamura S., Momoi M., Yamaji T., Takematsu H., Yano H., Sabe H., Yamamoto A., Kawasaki T., Kozutsumi Y. Inhibition of cytokinesis by a lipid metabolite, psychosine. J. Cell Biol. 2000;149:943–950. doi: 10.1083/jcb.149.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Capolupo L., Khven I., Lederer A.R., Mazzeo L., Glousker G., Ho S., Russo F., Montoya J.P., Bhandari D.R., Bowman A.P., et al. Sphingolipids control dermal fibroblast heterogeneity. Science. 2022;376:eabh1623. doi: 10.1126/science.abh1623. [DOI] [PubMed] [Google Scholar]
- 353.Pina D.G., Johannes L. Cholera and Shiga toxin B-subunits: Thermodynamic and structural considerations for function and biomedical applications. Toxicon. 2005;45:389–393. doi: 10.1016/j.toxicon.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 354.Yamaji-Hasegawa A., Hullin-Matsuda F., Greimel P., Kobayashi T. Pore-forming toxins: Properties, diversity, and uses as tools to image sphingomyelin and ceramide phosphoethanolamine. Biochim. Biophys. Acta. 2016;1858:576–592. doi: 10.1016/j.bbamem.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 355.Wathes D.C., Abayasekara D.R., Aitken R.J. Polyunsaturated fatty acids in male and female reproduction. Biol. Reprod. 2007;77:190–201. doi: 10.1095/biolreprod.107.060558. [DOI] [PubMed] [Google Scholar]
- 356.Govind Kunduri S.-H.L., Baena V., Vijaykrishna N., Harned A., Nagashima K., Blankenberg D., Yoshihiro I., Narayan K., Bamba T., Acharya U., et al. Endosomes deliver ceramide phosphoethanolamine with unique acyl chain anchors to the cleavage furrow during male meiotic cytokinesis. bioRxiv. 2022 doi: 10.1101/2022.03.14.484204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Leite J., Chan F.Y., Osorio D.S., Saramago J., Sobral A.F., Silva A.M., Gassmann R., Carvalho A.X. Equatorial Non-muscle Myosin II and Plastin Cooperate to Align and Compact F-actin Bundles in the Cytokinetic Ring. Front. Cell Dev. Biol. 2020;8:573393. doi: 10.3389/fcell.2020.573393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Mollinedo F., Gajate C. Lipid rafts as signaling hubs in cancer cell survival/death and invasion: Implications in tumor progression and therapy: Thematic Review Series: Biology of Lipid Rafts. J. Lipid Res. 2020;61:611–635. doi: 10.1194/jlr.TR119000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.