ABSTRACT
Diet may play an important role in the occurrence of esophageal cancer (EC). The aim of this umbrella review was to grade the evidence for the association between dietary factors and EC risk. A protocol for this review was registered with the PROSPERO database (CRD42021283232). Publications were identified by searching PubMed, EMBASE, Web of Science, Cochrane Database of Systematic Reviews, and CINAHL databases. Only systematic reviews and meta-analyses of observational studies (cohort studies, case-cohort studies, nested case-control studies) were eligible. AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews) was used to assess the methodological quality of included systematic reviews. For each association, random-effects pooled effect size, 95% CI, number of cases, 95% prediction interval, heterogeneity, small-study effect, and excess significance bias were calculated to grade the evidence. From 882 publications, 107 full-text articles were evaluated for eligibility, and 20 systematic reviews and meta-analyses describing 32 associations between dietary factors and EC risk were included in the present umbrella review. By assessing the strength and validity of the evidence, 1 association (positively associated with alcohol intake) was supported by highly suggestive evidence and 1 (inversely associated with calcium intake) showed a suggestive level of evidence. Evidence for 7 associations was weak (positively associated with red meat and processed-meat intake; inversely associated with whole grains, fruits, green leafy vegetables, green tea, and zinc intake). The remaining 23 associations were nonsignificant. In conclusion, the findings of this umbrella review emphasize that habitually consuming calcium, whole grains, fruits, green leafy vegetables, green tea, and zinc and reducing alcohol, red meat, and processed-meat intake are associated with a lower risk of EC. Since this umbrella review included only observational study data and some of the associations were graded as weak, caution should be exercised in interpreting these relations.
Keywords: diet, esophageal cancer, evidence, meta-analyses, umbrella review
Statement of Significance: The previous umbrella review's data for associations between dietary factors and esophageal cancer risk were up to 28 February 2014, and since 2014, several relevant meta-analyses have been published, so we believe it is necessary to combine these additional results to assess the robustness of the evidence. Among 4 associations (calcium, whole grains, green tea, and zinc intake), which were not evaluated in the previous review, 1 association (inversely associated with calcium intake) was supported by suggestive evidence and 3 associations (inversely associated with whole grains, green tea, and zinc intake) showed a weak level of evidence in the present umbrella review.
Introduction
Esophageal cancer (EC) is the seventh most commonly diagnosed cancer and the sixth leading cause of cancer deaths worldwide in 2020, with approximately 604,000 new cases and 544,000 deaths (1). Since the early clinical symptoms are relatively insidious, EC is often diagnosed during its advanced stages (2). Despite improvements in the management and treatment of patients with EC, the overall outcomes for 5-y survival rate (∼10%) and 5-y post-esophagectomy survival rate (∼15–40%) remain poor (2, 3). Identifying, exploring, and intervening on potential risk factors may have an important impact on the incidence rates of EC. Recent evidence suggests that modifiable lifestyle factors, including excessive obesity, poor diet, and physical inactivity, play an important role in the occurrence and progression of this disease (2–4).
Numerous observational studies have been conducted to investigate the association between dietary factors and EC incidence. Most of these studies were usually summarized through the results of systematic reviews and meta-analyses. However, pooled estimates from meta-analyses of observational studies might not represent causal associations due to the prevalence of bias in those studies (e.g., recall bias, selection bias), subsequently reducing the strength of aggregated scientific evidence (5, 6). Therefore, the robustness of the evidence needs to be assessed before using the evidence to inform public health policy. In 2021, Papadimitriou et al. (7) conducted an umbrella review to evaluate the strength and validity of the evidence for the association between food/nutrient intake and the risk of developing or dying from 11 primary cancers, including EC. Data for that review were extracted from the World Cancer Research Fund (WCRF) Third Expert Report, and the search for EC was up to 28 February 2014 (8). Since 2014, several relevant meta-analyses have been published, and we believe it is necessary to combine these additional results to assess the robustness of the evidence. Therefore, we conducted this umbrella review of systematic reviews and meta-analyses to evaluate the evidence for the association between dietary factors and EC risk.
Methods
The protocol of this umbrella review has been registered at PROSPERO. The registration number is CRD42021283232. This umbrella review adhered to the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guideline (9).
Search strategy
A comprehensive literature search was performed to assess the association between dietary factors and EC risk. PubMed, EMBASE, Web of Science, Cochrane Database of Systematic Reviews, and CINAHL databases were searched from database inception to 4 October 2021. The following search terms were used: (diet* OR food* OR nutrition* OR eating) AND (esophageal OR esophagus) AND (Tumor* OR Neoplas* OR Cancer* OR Carcinoma* OR Malignanc*) AND (meta-analysis OR systematic review OR systematic overview). Additionally, we manually searched reference lists from original literature reports for potential complements.
Eligibility criteria
Two authors (XQ and GJ) independently screened titles and abstracts. Disagreements were resolved by consensus with another independent author (XZ). The full texts of all potentially eligible articles were then retrieved and screened by the same 2 authors, and any discrepancies were resolved by a third author (XZ).
The criteria for eligibility were as follows: 1) systematic reviews and meta-analyses of observational studies (cohort studies, case-cohort studies, nested case-control studies) among adults, 2) studies investigating the incidence of EC in different dietary categories or dietary patterns, 3) multivariate-adjusted pooled risk estimates and corresponding 95% CIs were available, and 4) published in English. Meta-analyses of randomized controlled trials were not eligible for the present umbrella review. If an article conducted separate meta-analyses of multiple eligible dietary factors, each factor was evaluated separately. If the association between dietary factors and EC incidence was assessed by both highest versus lowest intake and dose-response analysis, we included the dose-response analysis (10). Whenever more than 1 meta-analysis focused on the same association, by searching the reference lists of each meta-analysis to assess study overlap we selected the most recent one with more available information and the largest number of cases, since the number of cases was part of our criteria.
Studies were excluded based on the following criteria: 1) cross-sectional or case-control studies, 2) the interest of studies focused between dietary factors and EC was not the incidence but the other outcome (e.g., mortality), 3) studies that did not provide sufficient data for quantitative synthesis, 4) animal and/or in vitro studies, 5) conference abstracts, and 6) published in other languages.
Data extraction
Data were extracted by 2 researchers (XQ and GJ), and any discrepancies were resolved by discussion and consultation with the third author (ZY). We recorded the following data from each eligible article: 1) name of first author, 2) year of publication, 3) dietary factors, 4) number of studies included, 5) number of cases and total participants, 6) meta-analyses metric (OR; RR), and 7) effect size and corresponding 95% CI. In addition, we extracted the data of the original study from the eligible meta-analyses for further analysis: 1) name of first author, 2) year of publication, 3) number of cases and total participants, 4) effect size and its 95% CI, and 5) comparison data from (highest vs. lowest intake; dose-response analysis).
Assessment of methodological quality
The AMSTAR-2 (A Measurement Tool to Assess Systematic Reviews) (11) is a tool to assess the quality of systematic review methods that contains 16 distinct domains. Seven of these domains are considered critical. This tool was used to evaluate the methodological quality of the included systematic reviews independently by 2 authors (XQ and GJ). Any disagreements were resolved by consulting a third author (ZY). The methodological quality of the reviews was rated as high, moderate, low, or critically low.
Statistical analysis
The original studies data obtained from the eligible meta-analyses were recalculated to obtain additional information to evaluate the level of evidence for the associations (12). We used the DerSimonian and Laird random-effects model to estimate the pooled effect size and its 95% CI (13). We also calculated the effect size for the largest data study of each association. Statistical heterogeneity was checked with the I2 statistic. An I2 value below 25% or 50% indicated low or moderate heterogeneity of the data, while an I2 value above 50% or 75% indicated significant or considerable heterogeneity, respectively (14). In addition, we estimated the 95% prediction interval (PI), which further accounts for between-study heterogeneity and evaluates the uncertainty of the effect size that would be anticipated in a new study addressing the same association (15, 16). We also assessed the small-study effects, commonly known as publication bias, to determine whether such studies tended to provide greater estimates of risk than larger studies (17). An Egger P value <0.10 was considered statistical evidence for small-study effects (18). Furthermore, we applied the excess significance test to evaluate whether the observed number (O) of statistically significant studies in the meta-analysis was different from the expected number (E) (19). Excess significance bias was set at P < 0.10. The analysis was carried out using R software (version 4.1.2; R Core Team, 2021), meta package (version 5.2.0; Guido Schwarzer, 2022), and metafor package (version 3.0.2; Viechtbauer, 2021).
Grading the quality of evidence
Statistically significant (P < 0.05) associations between dietary factors and EC incidence were classified into 4 levels of evidence strength according to the grading scheme applied in previously published umbrella reviews (20–22). When P ≥ 0.05, there was no significant association.
The criteria for determining the level of evidence were as follows:
Convincing (class I): 1) statistical significance with P < 10−6, 2) number of cases >1000, 3) largest component study with a statistically significant effect (P < 0.05), 4) 95% PI excluded the null, 5) no large heterogeneity (I2 < 50%), 6) no small-study effects (P > 0.10), and 7) no excess significance bias (P > 0.10).
Highly suggestive (class II): 1) statistical significance with P < 10−6, 2) number of cases >1000, and 3) largest component study with a statistically significant effect (P < 0.05).
Suggestive (class III): 1) statistical significance with P < 10−3 and 2) number of cases >1000.
Weak (class IV): statistical significance with P < 0.05.
Nonsignificant association: P ≥ 0.05.
Results
Literature identification and selection
As shown in Figure 1, the systematic search retrieved 882 publications from 5 electronic databases. After removing duplicates, 496 records were excluded through scanning titles and abstracts. A total of 107 full-text articles were identified for further evaluation, of which 87 were excluded according to the eligibility criteria. Finally, 20 systematic reviews and meta-analyses were included in the present umbrella review (23–42). Full details of the 87 articles we excluded are provided in Supplemental Table 1.
FIGURE 1.
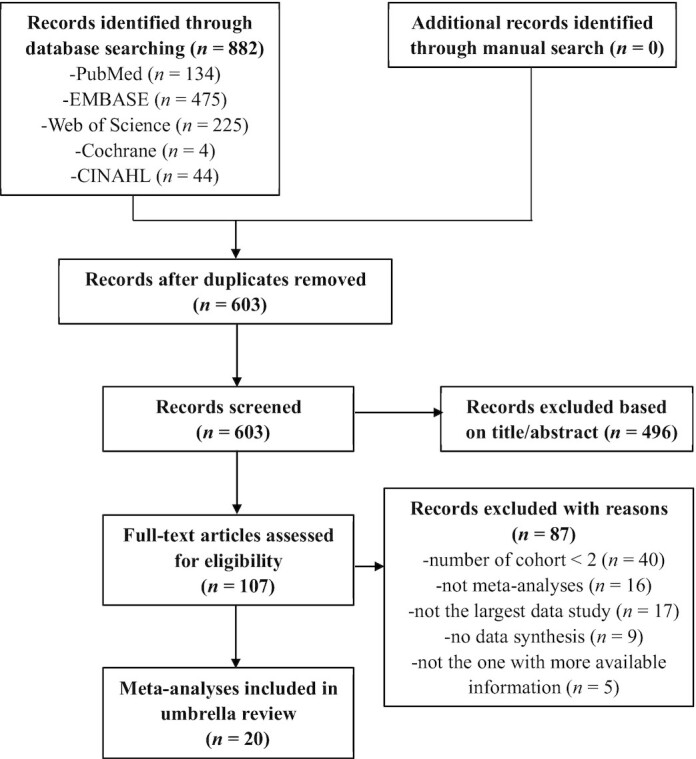
Flowchart of the literature selection process.
Description of included meta-analyses
These 20 meta-analyses describing 32 associations were published between 2013 and 2021. As shown in Table 1, 10 meta-analyses used the Newcastle-Ottawa Quality Assessment Scale (NOS) to assess the methodological quality of individual studies (24, 30, 31, 33–35, 38, 40–42); the remaining 10 meta-analyses did not conduct assessment (23, 25–29, 32, 36, 37, 39). The number of observational studies for each association ranged from 2 to 11. Of the 32 associations, 30 associations were evaluated using cohort studies alone and the remaining 2 associations were assessed using both cohort and nested case-control studies. The number of participants ranged from 7945 to 4,471,875, the number of cases ranged from 14 to 3526, and the number of cases was greater than 1000 for 15 associations.
TABLE 1.
Methodological quality of included meta-analyses that evaluate dietary factors and esophageal cancer risk1
| First author, year (reference) | Dietary factor | Studies, n | Cases, n | Participants, n | Quality assessment | AMSTAR-2 |
|---|---|---|---|---|---|---|
| Miyazaki, 2017 (23) | Alcohol cessation | 2 | 14 | 28,638 | NA | Critically low |
| Choi, 2013 (24) | Red meat | 8 | 2324 | 1,149,981 | NOS | Moderate |
| Han, 2013 (25) | Fish | 4 | 1279 | 730,702 | NA | Low |
| Salehi, 2013 (26) | Total meat | 3 | 2124 | 569,284 | NA | Low |
| White meat | 2 | 845 | 494,979 | |||
| Jiang, 2016 (27) | Poultry | 3 | 805 | 1,013,643 | NA | Low |
| Vingeliene, 2016 (28) | Citrus fruits | 8 | 1059 | 1,160,130 | NA | Low |
| Yan, 2018 (29) | Pickled vegetables | 2 | 3120 | 42,277 | NA | Critically low |
| Zhang, 2020 (30) | Whole grains | 2 | 281 | 148,584 | NOS | Moderate |
| Sakai, 2021 (31) | Fruits | 6 | 2818 | 177,648 | NOS | Moderate |
| Vingeliene, 2017 (32) | Vegetables | 8 | 3229 | 1,528,067 | NA | Low |
| Green leafy vegetables | 6 | 730 | 1,015,145 | |||
| Processed meat | 6 | 1385 | 1,320,178 | |||
| Coffee | 6 | 1074 | 1,448,033 | |||
| Alcohol | 11 | 3526 | 2,937,707 | |||
| Li, 2016 (33) | Milk | 2 | 541 | 238,516 | NOS | Moderate |
| Yi, 2020 (34) | Green tea | 4 | 473 | 708,139 | NOS | Low |
| He, 2017 (35) | Total fat | 2 | 845 | 494,978 | NOS | Low |
| Saturated fat | 2 | 845 | 494,978 | |||
| Polyunsaturated fat | 2 | 845 | 494,978 | |||
| Monounsaturated fat | 2 | 845 | 494,978 | |||
| Hong, 2016 (36) | Selenium | 2 | 111 | 7945 | NA | Low |
| Xie, 2016 (37) | Nitrate | 6 | 1106 | 687,254 | NA | Critically low |
| Nitrite | 7 | 1161 | 710,617 | |||
| Li, 2017 (38) | Calcium | 3 | 1412 | 343,431 | NOS | Moderate |
| Liu, 2017 (39) | Folate | 3 | 1257 | 521,876 | NA | Critically low |
| Ma, 2018 (40) | Iron | 6 | 1385 | 1,329,168 | NOS | High |
| Copper | 2 | 944 | 49,922 | |||
| Zinc | 2 | 944 | 49,922 | |||
| Ma, 2018 (41) | Vitamin B-6 | 42 | 267 | 4,471,830 | NOS | Moderate |
| Vitamin B-12 | 42 | 314 | 4,471,875 | |||
| Banda, 2020 (42) | Sodium | 3 | 895 | 501,798 | NOS | Moderate |
AMSTAR, A Measurement Tool to Assess Systematic Reviews; NA, not available; NOS, Newcastle-Ottawa Quality Assessment Scale.
Four studies = 2 cohort studies + 2 nested case-control studies.
The included meta-analyses provided summary estimates on the associations between dietary behaviors (1 association, the alcohol cessation) (23), food groups or food items (12 associations, including fish, poultry, red meat, processed meat, total meat, white meat, fruits, vegetables, and whole-grain intake) (24–32), beverages (4 associations, including milk, coffee, green tea, and alcohol intake) (32–34), macronutrients (4 associations, including total fat, saturated fat, polyunsaturated fat, and monounsaturated fat intake) (35), and micronutrients (11 associations, including nitrate, nitrite, folate, vitamin B-6, vitamin B-12, calcium, sodium, iron, copper, zinc, and selenium intake) (36–42) and the risk of EC.
As shown in Table 1, the assessment of methodological quality using AMSTAR-2 revealed 1 meta-analysis (40) of high quality and 7 (24, 30, 31, 33, 38, 41, 42) of moderate quality. A total of 8 (25–28, 32, 34–36) meta-analyses were of low quality, with the remaining 4 (23, 29, 37, 39) rated as critically low quality. The detailed assessments of methodological quality are shown in Supplemental Table 2.
Summary of associations
As shown in Table 2, the 32 associations included in the 20 meta-analyses were recalculated using random-effects models to assess the level of evidence. A total of 9 associations yielded nominal statistical significance at P < 0.05. Of these, only 2 reached statistical significance at P < 10−6. Six of nine associations were associated with a lower risk of EC, including higher intakes of whole grains, fruits, green leafy vegetables, green tea, calcium, and zinc; the remaining 3 associations were associated with a higher risk of EC, including higher intake of red meat, processed meat, and alcohol.
TABLE 2.
Quality of evidence of associations between dietary factors and esophageal cancer risk1
| First author (reference) | Dietary factor | Comparison | MA metric | Random effect size (95% CI) | P | I 2, % | Largest study, 95% CI | 95% prediction interval | Egger's P value | Excess significance test | Evidence class2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| O/E | P | |||||||||||
| Miyazaki, 2017 (23) | Alcohol cessation | Quit drinking vs. current drinking | RR | 0.57 (0.27, 1.17) | 0.12 | 36.1 | 0.33–2.6 | NE | NA | 1/0.7 | 0.58 | No |
| Choi, 2013 (24) | Red meat | Highest vs. lowest | RR | 1.28 (1.07, 1.53) | 0.008 | 35.5 | 1.11–1.69 | 0.93–1.75 | NA | 2/1.6 | 0.51 | IV |
| Han, 2013 (25) | Fish | Highest vs. lowest | RR | 0.84 (0.65, 1.09) | 0.19 | 36.1 | 0.72–1.26 | 0.36–1.99 | NA | 1/0.8 | 0.59 | No |
| Salehi, 2013 (26) | Total meat | Highest vs. lowest | RR | 0.92 (0.58, 1.44) | 0.7 | 63.5 | 0.62–0.86 | 0.006–148.5 | NA | 1/1 | NP5 | No |
| White meat | Highest vs lowest | RR | 0.8 (0.64, 1.0) | 0.051 | 0 | 0.65–1.09 | NE | NA | 0/0.6 | NP | No | |
| Jiang, 2016 (27) | Poultry | Highest vs. lowest | RR | 1.03 (0.62, 1.73) | 0.91 | 66.2 | 0.72–1.26 | 0.001–385 | NA | 0/1 | NP | No |
| Vingeliene, 2016 (28) | Citrus fruits | Per 100-g/d increment | RR | 0.9 (0.79, 1.02) | 0.1 | 0 | 0.78–1.25 | 0.76–1.05 | NA | 1/0.7 | 0.53 | No |
| Yan, 2018 (29) | Pickled vegetables | Highest vs. lowest | OR | 1.04 (0.93, 1.15) | 0.53 | 0 | 0.92–1.15 | NE | NA | 0/0.1 | NP | No |
| Zhang, 2020 (30) | Whole grains | Highest vs. lowest | RR | 0.54 (0.38, 0.76) | 0.0004 | 0 | 0.34–0.82 | NE | NA | 2/1.4 | 0.46 | IV |
| Sakai, 2021 (31) | Fruits | Highest vs. lowest | RR | 0.75 (0.6, 0.92) | 0.01 | 80.9 | 0.7–0.91 | 0.39–1.45 | NA | 3/2.8 | 0.62 | IV |
| Vingeliene, 2017 (32) | Vegetables | Per 100-g/d increment | RR | 0.93 (0.86, 1.01) | 0.07 | 33.7 | 0.93–1.1 | 0.77–1.13 | NA | 1/1.8 | NP | No |
| Green leafy vegetables | Per 50-g/d increment | RR | 0.86 (0.78, 0.95) | 0.004 | 0 | 0.74–0.97 | 0.74–0.99 | NA | 1/1.3 | NP | IV | |
| Processed meat | Per 50-g/d increment | RR | 1.35 (1.06, 1.74) | 0.02 | 56.5 | 0.87–1.36 | 0.66–2.78 | NA | 2/2.3 | 0.75 | IV | |
| Coffee | Per 1-cup/d increment | RR | 0.99 (0.94, 1.03) | 0.56 | 30.6 | 0.91–1.05 | 0.92–1.06 | NA | 0/0.3 | NP | No | |
| Alcohol | Per 10-g/d increment | RR | 1.21 (1.13, 1.31) | <10–6 | 95.4 | 1.35–1.6 | 0.93–1.57 | 0.004 | 8/7.8 | 0.61 | II | |
| Li, 2016 (33) | Milk | Highest vs. lowest | RR | 0.8 (0.48, 1.33) | 0.39 | 62.7 | 0.71–1.41 | NE | NA | 0/0.7 | NP | No |
| Yi, 2020 (34) | Green tea | Highest vs. lowest | RR | 0.75 (0.61, 0.93) | 0.007 | 23.4 | 0.51–1.08 | 0.48–1.19 | NA | 1/1.1 | 0.73 | IV |
| He, 2017 (35) | Total fat | Highest vs. lowest | RR | 0.93 (0.64, 1.34) | 0.69 | 0 | 0.6–1.42 | NE | NA | 0/0.1 | NP | No |
| Saturated fat | Highest vs. lowest | RR | 1.17 (0.88, 1.56) | 0.27 | 0 | 0.91–1.78 | NE | NA | 0/0.2 | NP | No | |
| Polyunsaturated fat | Highest vs. lowest | RR | 0.97 (0.77, 1.22) | 0.79 | 0 | 0.7–1.19 | NE | NA | 0/0.1 | NP | No | |
| Monounsaturated fat | Highest vs. lowest | RR | 1.28 (0.96, 1.71) | 0.1 | 0 | 0.84–1.66 | NE | NA | 0/0.4 | NP | No | |
| Hong, 2016 (36) | Selenium | Highest vs. lowest | RR | 0.99 (0.55, 1.78) | 0.96 | 32.6 | 0.41–1.4 | NE | NA | 0/0.2 | NP | No |
| Xie, 2016 (37) | Nitrate | Highest vs lowest | RR | 1.06 (0.82, 1.39) | 0.65 | 0 | 0.75–1.61 | 0.73–1.55 | NA | 0/0.3 | NP | No |
| Nitrite | Highest vs. lowest | RR | 1.1 (0.92, 1.32) | 0.31 | 11.5 | 0.84–1.68 | 0.87–1.39 | NA | 0/0.5 | NP | No | |
| Li, 2017 (38) | Calcium | Highest vs. lowest | OR | 0.67 (0.54, 0.84) | 0.0004 | 23.7 | 0.56–1.26 | 0.16–2.84 | NA | 2/1.6 | 0.53 | III |
| Liu, 2017 (39) | Folate | Highest vs. lowest | OR | 0.85 (0.64, 1.14) | 0.27 | 48.6 | 0.61–1.08 | 0.05–15.28 | NA | 0/0.7 | NP | No |
| Ma, 2018 (40) | Iron | Highest vs. lowest | OR | 1.15 (0.89, 1.47) | 0.28 | 56.7 | 0.99–2.17 | 0.56–2.34 | NA | 1/1.6 | NP | No |
| Copper | Per 1-mg/d increment | OR | 1.02 (0.64, 1.62) | 0.93 | 95.7 | 1.2–1.37 | NE | NA | 2/1.4 | 0.5 | No | |
| Zinc | Per 5-mg/d increment | OR | 0.78 (0.71, 0.86) | <0.0001 | 0 | 0.7–0.89 | NE | NA | 2/1.7 | 0.71 | IV | |
| Ma, 2018 (41) | Vitamin B-6 | Highest vs. lowest | OR | 1.02 (0.7, 1.48) | 0.93 | 51.8 | 0.76–1.32 | 0.25–4.2 | NA | 1/0.7 | 0.58 | No |
| Vitamin B-12 | Highest vs. lowest | OR | 1.02 (0.82, 1.25) | 0.89 | 0 | 0.8–1.35 | 0.64–1.61 | NA | 0/0.2 | NP | No | |
| Banda 2020 (42) | Sodium | Highest vs. lowest | OR | 1.04 (0.99, 1.08) | 0.06 | 0 | 0.98–1.07 | 0.81–1.33 | NA | 0/0.6 | NP | No |
MA, meta-analyses; NA, not applicable because number of studies (<10) too small to test for small study effects; NE, not estimated because number of studies (<3) too small to test for 95% prediction interval; NP, not pertinent because estimated number is larger than observed; O/E, observed/expected number of studies with significant results.
Evidence class—class I (convincing): statistical significance at P < 10−6, >1000 cases, the largest component study with a significant effect (P < 0.05), the 95% prediction interval excluded the null, no large heterogeneity (I2 < 50%), no small-study effects (P > 0.10), and no excess significance bias (P > 0.10); class II (highly suggestive): significance at P < 10−6, >1000 cases, the largest component study with a significant effect (P < 0.05); class III (suggestive): statistical significance at P < 10−3, >1000 cases; class IV (weak): the remaining significant associations at P < 0.05; No, nonsignificant association P ≥ 0.05.
After calculating the effect size of the largest data study for each association, 6 of the 9 associations (whole grains, fruits, green leafy vegetables, zinc, red meat, and alcohol intake) showed statistical significance. The minimum number of studies to test for 95% PI was 3. After estimating the 95% PI, all 9 associations were excluded because their 95% PI included the null or had fewer than 3 studies.
Some associations (9/32) showed large heterogeneity (I2 > 50%). According to Egger's test, there was no evidence of small-study effects. However, the minimum number of studies for assessing small-study effects was 10, and only 1 association (32) (alcohol intake) contained 11 original studies that provided statistical power for Egger's test. No excess significance bias was found for any association.
Grading the quality of evidence
Convincing evidence (class I)
After calculating the random-effects summary effect size, 95% CI, 95% PI, and assessing heterogeneity, evidence for small-study effect, and evidence for excess significance bias, no association was supported by convincing evidence.
Highly suggestive evidence (class II)
There is a positive association between higher intake of alcohol and EC risk by highly suggestive evidence. The estimated increased RR of EC was 21% for each 10 g (∼0.35 ounces)/d increment of alcohol intake (32).
Suggestive evidence (class III)
Our results supported an inverse association between higher intake of calcium and EC risk by suggestive evidence (38).
Weak evidence (class IV)
Evidence for 7 associations was weak. Five associations, including higher intake of whole grains, fruits, green leafy vegetables, green tea, and zinc, were inversely associated with the EC risk (30–32, 34, 40). The remaining 2 associations, including higher intake of red meat and processed meat, were positively associated with the EC risk (24, 32).
Nonsignificant association
A total of 23 associations were nonsignificant.
The detailed analysis results on which the evidence grading is based are shown in Table 2.
Discussion
Within single meta-analysis, statistical methods are frequently inadequate or misused, which can lead to misleading results, distortions, and bias. Therefore, in 2009, Ioannidis et al. (43) first proposed the concept of an umbrella review, which aims to provide a conclusive summary of the report, highlighting the level of evidence. Recently, the practice of establishing the level of evidence has become more important to provide summaries of information for health care decision makers (12).
We conducted this umbrella review to evaluate the robustness of the evidence from systematic reviews and meta-analyses of observational studies on the relation between dietary factors and EC risk for further applications to inform health policy or dietary guidelines. Overall, we included 20 published meta-analyses describing 32 associations. After grading the quality of evidence, we found 9 associations that were statistically significant and the strength of the evidence for associations between alcohol, fruits, green leafy vegetables, red meat, processed meat, and EC risk is consistent with previous umbrella analyses (7). In addition, calcium, whole grains, green tea, and zinc, which were not evaluated in the previous review, were identified as being inversely associated with EC risk in the present umbrella review.
Based on 11 studies including 3526 cases and 2,937,707 participants, our umbrella review supports a positive association between higher intake of alcohol and EC risk by highly suggestive evidence, which is consistent with the WCRF/American Institute for Cancer Research Continuous Update Project report (44). The mechanisms by which alcohol consumption exerts its carcinogenic effects are diverse and not fully understood. The main mechanism of carcinogenesis is related to the alcohol metabolite acetaldehyde and the production of reactive oxygen species by enzymes induced by chronic alcohol consumption (45). Finally, alcohol may cause direct damage to the esophageal epithelium and facilitate the absorption of carcinogens (46). Although the 95% PI for this association includes the null value, there were no small-study effects or excess significance bias, and the number of cases exceeded 1000, suggesting that the evidence is likely robust.
We found an inverse association between high consumption of calcium and the incidence of EC by suggestive evidence. As a ubiquitous second messenger, calcium plays a crucial role in human health (47). Calcium intake was hypothesized to reduce cancer risk by downregulating 1,25-dihydroxyvitamin D synthesis, promoting activation of the transcription factor cAMP response element-binding protein, inducing cell cycle arrest, and promoting cell differentiation and tumor cell apoptosis (48, 49). In addition, previous studies showed that a high-calcium diet induced cell differentiation and inhibited cell proliferation and carcinogenesis by promoting p120-catenin expression and p120-dependent E-cadherin–β-catenin–p120-catenin complex formation in mouse epithelial tissues (50, 51). However, this meta-analysis did not include a dose-response effect analysis due to the incomplete data on dietary calcium intake, and the average highest dietary calcium intake in Asian populations (621 mg/d) is much lower than in Americans (1284 mg/d) and Europeans (1232 mg/d), so it is difficult to define the accurate intake in grams per day for health recommendations (38).
The present umbrella review also indicated inverse associations between high consumption of whole grains, fruits, green leafy vegetables, green tea, and zinc and EC risk by weak evidence. Whole grains may influence EC risk through a variety of mechanisms. First, whole grains are an important source of dietary fiber. Inositol hexaphosphate, 1 component of food sources high in dietary fiber, has been demonstrated to inhibit the growth rate of Barrett's-associated esophageal adenocarcinoma cells by reducing cell proliferation and stimulating apoptosis in vitro (52). Second, whole grains are rich sources of antioxidants, including vitamins (such as vitamin E), trace minerals (such as selenium), phenolic acids, lignans, and phytoestrogens, which together reduce oxidative damage and stress, thereby reducing cancer risk (53). With regard to fruits and green leafy vegetables, they are rich in a variety of antioxidants, vitamins, minerals, and other bioactive substances (including vitamin C, lycopene, selenium, folate, and flavonoids) that reduce oxidative stress and inflammation, and maintain nucleotide synthesis and methylation, which play an important role in EC prevention (54–56). With respect to green tea, the main active ingredients are polyphenols, with the most common being epigallocatechin gallate and epicatechin (57). Studies have shown that epigallocatechin gallate has significant antitumor effects because it can inhibit the proliferation of cancer cells and promote their apoptosis by reducing the activity of enzymes and hindering signal transduction pathways (58). With regard to zinc, it plays an important role in immune function and transcription and controls cell proliferation, apoptosis, and various signaling pathways, but the exact biological mechanisms leading to the inverse relation between zinc intake and EC risk are not fully understood (59). Although there were no small-study effects or excess significance bias, our results included null values for the 95% PI; the heterogeneity for an association between fruit intake and EC risk was large; and the numbers of cases for whole grains, green leafy vegetables, green tea, and zinc were all less than 1000. Therefore, more caution should be exercised in interpreting these relations.
We also found positive associations between red meat, processed-meat intake, and EC risk by weak evidence. Several potential mechanisms may underlie the effects of red and processed-meat consumption on EC risk. First, cooked red meat is one of the major sources of carcinogens, such as polycyclic aromatic hydrocarbons, heterocyclic amines, nitrates, and N-nitroso compounds, which are thought to play an important role in the development of EC (60). Second, the practice of cooking red meat at high temperature may lead to the production of heterocyclic amines and polycyclic aromatic hydrocarbons, both of which are thought to increase the risk of cancer in humans (61, 62). Third, processed meat is a source of nitrate and nitrite, thereby increasing the formation of N-nitroso compounds, which are considered animal carcinogens and possible human carcinogens (63, 64).
However, several limitations of this umbrella review should be considered. First, we included studies from published meta-analyses, so individual studies may have been missed if they have not yet been assessed through meta-analyses. Second, we did not perform subgroup analyses (e.g., by sex, age group, or pathological type of EC such as squamous cell carcinoma or adenocarcinoma) due to a lack of subgroup data to grade the quality of evidence for most exposures. Third, since this umbrella review only included observational study data, which often contain recall bias and selection bias, while we can describe and assess the associations, we cannot establish causality, nor can we accurately give an individual's daily dietary intake standard. Fourth, the umbrella review's reliability depends directly on the included meta-analyses and indirectly on the original studies, and it was not possible for us to control for biases in the original study, so further prospective studies are needed to draw firm conclusions.
In conclusion, the findings of this umbrella review emphasize that habitually consuming calcium, whole grains, fruits, green leafy vegetables, green tea, and zinc and reducing alcohol, red meat, and processed-meat intake are associated with a lower risk of EC. Since this umbrella review included only observational study data and some of the associations were graded as weak, caution should be exercised in interpreting these relations.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—ZY and XQ: contributed to the umbrella review design; XQ, GJ, and XZ: conducted the literature search, extracted the data, and conducted the analyses; XQ: wrote the first draft of the manuscript; ZY is the guarantor of the paper; and all authors: contributed to writing and reviewing the manuscript and read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1 and 2 and Supplemental References are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: AMSTAR-2, A Measurement Tool to Assess Systematic Reviews; EC, esophageal cancer; PI, prediction interval; WCRF, World Cancer Research Fund.
Contributor Information
Xianpeng Qin, Department of Gastrointestinal Surgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Guiqing Jia, Department of Gastrointestinal Surgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Xiaogang Zhou, Department of Gastrointestinal Surgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Zhou Yang, Department of Gastrointestinal Surgery, Sichuan Provincial People's Hospital, University of Electronic Science and Technology of China, Chengdu, China.
Data Availability
Data described in the manuscript, code book, and analytic code will be made available upon request.
References
- 1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal Aet al. . Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2. Huang FL, Yu SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. 2018;41(3):210–15. [DOI] [PubMed] [Google Scholar]
- 3. Short MW, Burgers KG, Fry VT. Esophageal cancer. Am Fam Physician. 2017;95(1):22–8. [PubMed] [Google Scholar]
- 4. Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21(26):7933–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19(5):640–8. [DOI] [PubMed] [Google Scholar]
- 6. Dwan K, Gamble C, Williamson PR, Kirkham JJ. Systematic review of the empirical evidence of study publication bias and outcome reporting bias—an updated review. PLoS One. 2013;8(7):e66844–e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Papadimitriou N, Markozannes G, Kanellopoulou A, Critselis E, Alhardan S, Karafousia Vet al. . An umbrella review of the evidence associating diet and cancer risk at 11 anatomical sites. Nat Commun. 2021;12(1):4579–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Cancer Research Fund/American Institute for Cancer Research . Diet, nutrition, physical activity and cancer: a global perspective. 2018. Available from: https://www.aicr.org/research/third-expert-report/.
- 9. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie Det al. . Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–12. [DOI] [PubMed] [Google Scholar]
- 10. Sun H, Gong T-T, Xia Y, Wen Z-Y, Zhao L-G, Zhao Y-Het al. . Diet and ovarian cancer risk: an umbrella review of systematic reviews and meta-analyses of cohort studies. Clin Nutr. 2021;40(4):1682–90. [DOI] [PubMed] [Google Scholar]
- 11. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran Jet al. . AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:4008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid-Based Healthcare. 2015;13(3):132–40. [DOI] [PubMed] [Google Scholar]
- 13. Deeks JJ, Higgins JPT, Altman DG. (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VAeditors, Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
- 14. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 16. Graham PL, Moran JL. Robust meta-analytic conclusions mandate the provision of prediction intervals in meta-analysis summaries. J Clin Epidemiol. 2012;65(5):503–10. [DOI] [PubMed] [Google Scholar]
- 17. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau Jet al. . Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
- 18. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ioannidis JP, Trikalinos TA. An exploratory test for an excess of significant findings. Clin Trials. 2007;4(3):245–53. [DOI] [PubMed] [Google Scholar]
- 20. Kim TL, Jeong GH, Yang JW, Lee KH, Kronbichler A, van der Vliet HJet al. . Tea consumption and risk of cancer: an umbrella review and meta-analysis of observational studies. Adv Nutr. 2020;11(6):1437–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lee KH, Seong HJ, Kim G, Jeong GH, Kim JY, Park Het al. . Consumption of fish and ω-3 fatty acids and cancer risk: an umbrella review of meta-analyses of observational studies. Adv Nutr. 2020;11(5):1134–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu D, Meng X, Tian Q, Cao W, Fan X, Wu Let al. . Vitamin D and multiple health outcomes: an umbrella review of observational studies, randomized controlled trials, and Mendelian randomization studies. Adv Nutr. 2022;13(4):1044–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Miyazaki T, Kitagawa Y, Kuwano H, Kusano M, Oyama T, Muto Met al. . Decreased risk of esophageal cancer owing to cigarette and alcohol cessation in smokers and drinkers: a systematic review and meta-analysis. Esophagus. 2017;14(4):290–302. [Google Scholar]
- 24. Choi Y, Song S, Song Y, Lee JE. Consumption of red and processed meat and esophageal cancer risk: meta-analysis. World J Gastroenterol. 2013;19(7):1020–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Han YJ, Li J, Huang W, Fang Y, Xiao LN, Liao ZE. Fish consumption and risk of esophageal cancer and its subtypes: a systematic review and meta-analysis of observational studies. Eur J Clin Nutr. 2013;67(2):147–54. [DOI] [PubMed] [Google Scholar]
- 26. Salehi M, Moradi-Lakeh M, Salehi MH, Nojomi M, Kolahdooz F. Meat, fish, and esophageal cancer risk: a systematic review and dose-response meta-analysis. Nutr Rev. 2013;71(5):257–67. [DOI] [PubMed] [Google Scholar]
- 27. Jiang G, Li B, Liao X, Zhong C. Poultry and fish intake and risk of esophageal cancer: a meta-analysis of observational studies. Asia Pac J Clin Oncol. 2016;12(1):e82–91. [DOI] [PubMed] [Google Scholar]
- 28. Vingeliene S, Chan D, Aune D, Vieira A, Polemiti E, Stevens Cet al. . An update of the WCRF/AICR systematic literature review on esophageal and gastric cancers and citrus fruits intake. Cancer Causes Control. 2016;27(7):837–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yan B, Zhang L, Shao Z. Consumption of processed and pickled food and esophageal cancer risk: a systematic review and meta-analysis. Bull Cancer. 2018;105(11):992–1002. [DOI] [PubMed] [Google Scholar]
- 30. Zhang X-F, Wang X-K, Tang Y-J, Guan X-X, Guo Y, Fan J-Met al. . Association of whole grains intake and the risk of digestive tract cancer: a systematic review and meta-analysis. Nutr J. 2020;19(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sakai M, Kitagawa Y, Saeki H, Miyazaki T, Yamaji T, Nemoto Ket al. . Fruit and vegetable consumption and risk of esophageal cancer in the Asian region: a systematic review and meta-analysis. Esophagus. 2022;19(1):27–38. [DOI] [PubMed] [Google Scholar]
- 32. Vingeliene S, Chan D, Vieira AR, Polemiti E, Stevens C, Abar Let al. . An update of the WCRF/AICR systematic literature review and meta-analysis on dietary and anthropometric factors and esophageal cancer risk. Ann Oncol. 2017;28(10):2409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li BL, Jiang GX, Xue Q, Zhang H, Wang C, Zhang GXet al. . Dairy consumption and risk of esophageal squamous cell carcinoma: a meta-analysis of observational studies. Asia Pac J Clin Oncol. 2016;12(2):e269–79. [DOI] [PubMed] [Google Scholar]
- 34. Yi Y, Liang H, Jing H, Jian Z, Guang Y, Jun Zet al. . Green tea consumption and esophageal cancer risk: a meta-analysis. Nutr Cancer. 2020;72(3):513–21. [DOI] [PubMed] [Google Scholar]
- 35. He D, Huang X, Wang ZP, Chen D, Chen J, Duan CY. Dietary fat intake and risk of esophageal carcinoma: a metaanalysis of observational studies. Oncotarget. 2017;8(58):99049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hong B, Huang L, Mao N, Xiong T, Li C, Hu Let al. . Association between selenium levels and oesophageal adenocarcinoma risk: evidence from a meta-analysis. Biosci Rep. 2016;36(4):e356–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xie L, Mo M, Jia HX, Liang F, Yuan J, Zhu J. Association between dietary nitrate and nitrite intake and site-specific cancer risk: evidence from observational studies. Oncotarget. 2016;7(35):56915–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Li Q, Cui L, Tian Y, Cui H, Li L, Dou Wet al. . Protective effect of dietary calcium intake on esophageal cancer risk: a meta-analysis of observational studies. Nutrients. 2017;9(5):510–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu W, Zhou H, Zhu Y, Tie C. Associations between dietary folate intake and risks of esophageal, gastric and pancreatic cancers: an overall and dose-response meta-analysis. Oncotarget. 2017;8(49):86828–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ma J, Li Q, Fang X, Chen L, Qiang Y, Wang Jet al. . Increased total iron and zinc intake and lower heme iron intake reduce the risk of esophageal cancer: a dose-response meta-analysis. Nutr Res. 2018;59:16–28. [DOI] [PubMed] [Google Scholar]
- 41. Ma JL, Zhao Y, Guo CY, Hu HT, Zheng L, Zhao EJet al. . Dietary vitamin B intake and the risk of esophageal cancer: a meta-analysis. Cancer Manage Res. 2018;10:5395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Banda KJ, Chiu HY, Hu SH, Yeh HC, Lin KC, Huang HC. Associations of dietary carbohydrate and salt consumption with esophageal cancer risk: a systematic review and meta-analysis of observational studies. Nutr Rev. 2020;78(8):688–98. [DOI] [PubMed] [Google Scholar]
- 43. Ioannidis JP. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. Can Med Assoc J. 2009;181(8):488–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. World Cancer Research Fund/American Institute for Cancer Research . Continuous Update Project expert report 2018. Diet, nutrition, physical activity and oesophageal cancer. Available from:dietandcancerreport.org. [Google Scholar]
- 45. Toh Y, Oki E, Ohgaki K, Sakamoto Y, Ito S, Egashira Aet al. . Alcohol drinking, cigarette smoking, and the development of squamous cell carcinoma of the esophagus: molecular mechanisms of carcinogenesis. Int J Clin Oncol. 2010;15(2):135–44. [DOI] [PubMed] [Google Scholar]
- 46. Rumgay H, Murphy N, Ferrari P, Soerjomataram I. Alcohol and cancer: epidemiology and biological mechanisms. Nutrients. 2021;13(9):3173–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1(1):11–21. [DOI] [PubMed] [Google Scholar]
- 48. Lipskaia L, Lompré AM. Alteration in temporal kinetics of Ca2+ signaling and control of growth and proliferation. Biol Cell. 2004;96(1):55–68. [DOI] [PubMed] [Google Scholar]
- 49. Terrié E, Coronas V, Constantin B. Role of the calcium toolkit in cancer stem cells. Cell Calcium. 2019;80:141–51. [DOI] [PubMed] [Google Scholar]
- 50. Xie Z, Yuan Y, Jiang Y, Shrestha C, Chen Y, Liao Let al. . p120-Catenin is required for dietary calcium suppression of oral carcinogenesis in mice. J Cell Physiol. 2017;232(6):1360–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li L, Ji S, Shrestha C, Jiang Y, Liao L, Xu Fet al. . p120-catenin suppresses proliferation and tumor growth of oral squamous cell carcinoma via inhibiting nuclear phospholipase C-γ1 signaling. J Cell Physiol. 2020;235(12):9399–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McFadden DW, Riggs DR, Jackson BJ, Cunningham C. Corn-derived carbohydrate inositol hexaphosphate inhibits Barrett's adenocarcinoma growth by pro-apoptotic mechanisms. Oncol Rep. 2008;19(2):563–6. [PubMed] [Google Scholar]
- 53. Fardet A. New hypotheses for the health-protective mechanisms of whole-grain cereals: what is beyond fibre?. Nutr Res Rev. 2010;23(1):65–134. [DOI] [PubMed] [Google Scholar]
- 54. Bradbury KE, Appleby PN, Key TJ. Fruit, vegetable, and fiber intake in relation to cancer risk: findings from the European Prospective Investigation into Cancer and Nutrition (EPIC). Am J Clin Nutr. 2014;100(Suppl_1):394S–8S. [DOI] [PubMed] [Google Scholar]
- 55. Mayne ST, Risch HA, Dubrow R, Chow WH, Gammon MD, Vaughan TLet al. . Nutrient intake and risk of subtypes of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2001;10(10):1055–62. [PubMed] [Google Scholar]
- 56. Traber MG, Stevens JF. Vitamins C and E: beneficial effects from a mechanistic perspective. Free Radic Biol Med. 2011;51(5):1000–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Jankun J, Selman SH, Swiercz R, Skrzypczak-Jankun E. Why drinking green tea could prevent cancer. Nature. 1997;387:561. [DOI] [PubMed] [Google Scholar]
- 58. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu CG, Zhang L, Jiang Y, Chatterjee D, Croce CM, Huebner Ket al. . Modulation of gene expression in precancerous rat esophagus by dietary zinc deficit and replenishment. Cancer Res. 2005;65(17):7790–9. [DOI] [PubMed] [Google Scholar]
- 60. Samraj AN, Pearce OM, Läubli H, Crittenden AN, Bergfeld AK, Banda Ket al. . A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci. 2015;112(2):542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rohrmann S, Zoller D, Hermann S, Linseisen J. Intake of heterocyclic aromatic amines from meat in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Heidelberg cohort. Br J Nutr. 2007;98(6):1112–15. [DOI] [PubMed] [Google Scholar]
- 62. Turesky RJ. Mechanistic evidence for red meat and processed meat intake and cancer risk: a follow-up on the International Agency for Research on Cancer evaluation of 2015. Chimia. 2018;72(10):718–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Scanlan RA. Formation and occurrence of nitrosamines in food. Cancer Res. 1983;43(5 Suppl):2435s–40s. [PubMed] [Google Scholar]
- 64. Tricker AR, Preussmann R. Carcinogenic N-nitrosamines in the diet: occurrence, formation, mechanisms and carcinogenic potential. Mutat Res Genet Toxicol. 1991;259(3-4):277–89. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request.


