ABSTRACT
The impact of gut microbiota–targeted interventions on the incidence, duration, and severity of respiratory tract infections (RTIs) in nonelderly adults, and factors moderating any such effects, are unclear. This systematic review and meta-analysis aimed to determine the effects of orally ingested probiotics, prebiotics, and synbiotics compared with placebo on RTI incidence, duration, and severity in nonelderly adults, and to identify potential sources of heterogeneity. Studies were identified by searching CENTRAL, PubMed, Scopus, and Web of Science up to December 2021. English-language, peer-reviewed publications of randomized, placebo-controlled studies that tested an orally ingested probiotic, prebiotic, or synbiotic intervention of any dose for ≥1 wk in adults aged 18–65 y were included. Results were synthesized using intention-to-treat and per-protocol random-effects meta-analysis. Heterogeneity was explored by subgroup meta-analysis and meta-regression. Risk of bias was assessed using the Cochrane risk-of-bias assessment tool for randomized trials version 2 (RoB2). Forty-two manuscripts reporting effects of probiotics (n = 38), prebiotics (n = 2), synbiotics (n = 1) or multiple -biotic types (n = 1) were identified (n = 9179 subjects). Probiotics reduced the risk of experiencing ≥1 RTI (relative risk = 0.91; 95% CI: 0.84, 0.98; P = 0.01), and total days (rate ratio = 0.77; 95% CI: 0.71, 0.83; P < 0.001), duration (Hedges’ g = −0.23; 95% CI: −0.39, −0.08; P = 0.004), and severity (Hedges’ g = −0.16; 95% CI: −0.29, −0.03; P = 0.02) of RTIs. Effects were relatively consistent across different strain combinations, doses, and durations, although reductions in RTI duration were larger with fermented dairy as the delivery matrix, and beneficial effects of probiotics were not observed in physically active populations. Overall risk of bias was rated as “some concerns” for most studies. In conclusion, orally ingested probiotics, relative to placebo, modestly reduce the incidence, duration, and severity of RTIs in nonelderly adults. Physical activity and delivery matrix may moderate some of these effects. Whether prebiotic and synbiotic interventions confer similar protection remains unclear due to few relevant studies. This trial was registered at https://www.crd.york.ac.uk/prospero/ as CRD42020220213.
Keywords: respiratory infection, respiratory illness, common cold, influenza, coronavirus, gut microbiome, fermentable fiber, dietary supplement
Statement of Significance: This systematic review and meta-analysis extends previous meta-analyses relying primarily on studies of pediatric populations by demonstrating that orally ingested probiotics also reduce the incidence, duration, and severity of respiratory tract infections (RTIs) in nonelderly adult populations. Further, this analysis identifies physical activity level and probiotic delivery matrix as potential effect moderators and highlights the need for research regarding effects of prebiotics and synbiotics on RTI incidence, duration, and severity in adult populations.
Introduction
Respiratory tract infections (RTIs), defined as respiratory illnesses that culminate in infections of the sinuses, throat, airways, and lungs, are common and present major worldwide public health issues (1). RTI symptoms often impair quality of life and productivity, and exist on a spectrum from nuisance, such as sore throat, cough, nasal obstruction, and headache, to potentially life-threatening complications, such as pneumonia, myocarditis, otitis media, and glomerulonephritis (2). RTIs are a leading cause for seeking outpatient medical care, generate a high number of hospital admissions, increase medical costs, contribute to overwhelmed health care systems, and are responsible for >2 million deaths annually (1, 3, 4). Despite this high prevalence and disease burden, current therapies are limited and often palliative rather than preventative, especially for the most common RTIs. Although vaccines are available to prevent certain types of RTIs, developing new vaccines can be a lengthy and costly process that frequently results in products with variable effectiveness (4, 5). Hence, identifying novel and cost-effective strategies for reducing the incidence, duration, and severity of a broad-spectrum of RTIs is of interest.
Interventions targeting the gut microbiota may provide one such strategy. This complex and dynamic community plays a critical role in several physiological functions, which includes protecting against pathogens and regulating host immune function (6). Approaches to modulating the composition and function of the gut microbiota, if only transiently, include consumption of probiotics, prebiotics, and synbiotics. Probiotics are defined as live organisms that, when administered in adequate amounts, confer a health benefit on the host and are found in dietary supplements and some fermented food products (7). Prebiotics are substrates that are selectively utilized by host microorganisms conferring a health benefit (8). Established prebiotics include the fermentable saccharides inulin, fructo-oligosaccharides, oligofructose, and galacto-oligosaccharides. Other oligo- and polysaccharides are also fermented by beneficial gut microbes. While those compounds may not currently meet the definition of a prebiotic, microbial metabolism of those saccharides can result in the production of the same health-promoting and immune-modulating compounds produced during utilization of prebiotics (8). Synbiotics are a mixture of live microorganisms and substrate(s) selectively utilized by host microorganisms that confer a health benefit on the host (9). Synbiotics include combinations of probiotics and prebiotics that work independently or live microbes and substrates selectively utilized by those microbes. Evidence from preclinical and clinical studies indicates that health benefits of these gut microbiota–targeted interventions may include modification of local and systemic immune function (7–10). However, immunomodulatory effects may vary according to the probiotic strains or prebiotic substrates used, have not been observed in every population studied, and do not always result in observable effects on infection and illness in vivo (11).
Interest in using prebiotics, synbiotics, and probiotics, in particular, for reducing RTI burden (i.e., incidence, duration, and severity) is reflected by a growing evidence base summarized within several recent narrative reviews, systematic reviews, and meta-analyses (11–21). The latter have collectively reported that probiotics lower the odds or risk of experiencing RTIs by 11% to 47% (15, 16, 18), synbiotics reduce RTI incidence by 16% (13), and prebiotics reduce RTI incidence by 27% (12). However, several knowledge gaps remain. First, the majority of studies included in these meta-analyses involve pediatric populations. To what extent findings are generalizable to adults and their more developed immune systems is unclear. Other systematic reviews and meta-analyses have focused only on physically active and athletic populations (14, 19). Whether findings are applicable to less active adult populations is unclear given that exercise itself modulates RTI risk (22). In addition, the effects of probiotics, prebiotics, and synbiotics on RTI risk may differ based on the product type and duration of use. However, few meta-analyses have empirically tested those factors as potential sources of heterogeneity in study results. Finally, few meta-analyses have considered the duration and severity of RTI as outcomes, which are relevant to understanding the full potential impact of probiotic, prebiotic, and synbiotic interventions on disease burden. This has recently been evidenced by the global experience with coronavirus disease 2019 (COVID-19) wherein disease severity ranges from no symptoms to terminal illness, and symptom duration can last from days to months (23).
The objective of this systematic review and meta-analysis was to address these gaps by determining the effects of orally ingested probiotics, prebiotics, and synbiotics on the incidence, duration, and severity of RTIs in nonelderly adults. Secondary objectives were to explore potential sources of heterogeneity in those effects resulting from the physical activity level of the population studied and dose, duration, type, and form of treatment.
Methods
The systematic review protocol was registered on the PROSPERO International Register of Systematic Reviews (National Institute for Health Research, University of York, UK; https://www.crd.york.ac.uk/prospero/) on 11 December 2020 (CRD42020220213) and was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Search strategy
Initial searches of the electronic databases CENTRAL (Cochrane Central Register of Controlled Trials), PubMed, Scopus, and Web of Science were conducted on 9 November 2020 for all articles published up to that date. Searches used terms and Medical Subject Headings (MeSH) designed specifically for both interventions and outcomes, and included a validated randomized controlled trials filter for each database except for CENTRAL (Supplemental Tables 1–4). Reference lists of other relevant systematic reviews were hand-searched to identify any articles not captured in the initial search. All databases except for CENTRAL were searched again on 4 December 2021 using the same strategy used for the initial searches.
Eligibility criteria
English-language, published, randomized-controlled trials examining effects of orally ingested probiotics, prebiotics, or synbiotics on the incidence, duration, or severity of RTIs in human adults were included. For the purposes of this review, probiotic was defined as any live population of unicellular microorganisms characterized to at least the species level being studied for a health benefit. Prebiotic was defined as any nondigestible saccharide fermented by the gut microbiota, and synbiotic was defined as any combination of probiotic(s) and prebiotic(s). Abstracts, conference proceedings, clinical trial registrations, and other gray literature were excluded. Additional exclusion criteria included the following: animal or in vitro studies; mean or median age of the study population not between 18 and 65 y; studies of hospitalized patients or cohorts with an immunodeficiency, autoimmune or other immune system disorder, or taking immune-modulating therapies; intervention periods <1 wk total duration; unmatched intervention and placebo, defined as the intervention or placebo containing added bioactive ingredients not found in the other product; nonrandomized or observational studies; and incidence, duration, or severity of RTIs not measured or reported.
Selection process and data collection
Citations and corresponding abstracts identified through each database search were uploaded into Covidence systematic review software (Veritas Health Innovation) and duplicate entries were removed using the software's automated system. The title and abstract of each entry were screened by 2 reviewers blinded to the other's responses. Any differences in voting were adjudicated by a third reviewer. Entries were eliminated only if 2 reviewers voted to exclude that entry. Full texts of each entry that passed screening were retrieved and screened by 2 reviewers blinded to the other's responses. Reasons for any exclusions were noted and any differences were adjudicated by a third reviewer.
Data extraction and risk-of-bias assessment were then conducted for all articles deemed eligible for inclusion. The same reviewer completed both the data extraction and risk-of-bias assessment for an article, and each article underwent data extraction and risk-of-bias assessment by 2 reviewers who were blinded to the other's responses. A third reviewer compared the data extraction and risk-of-bias assessments and determined consensus.
Risk-of-bias assessment used the Cochrane risk-of-bias assessment tool for randomized trials version 2 (RoB2) (24). The RoB2 assesses risk of bias resulting from the randomization process (domain 1), due to deviations from intended interventions (domain 2), due to missing outcome data (domain 3), in measurement of the outcome (domain 4), and in selection of the reported result (domain 5). Risk of bias for each domain was determined by answering the domain-specific signaling questions, recorded as “low,” “high,” or “some concerns,” and used to determine overall bias (24). As the primary aim of this review was to assess effects of assignment to the intervention, signaling questions were structured based on an intention-to-treat (ITT) effect. If needed, clinical trial registrations or other publications from the same study were sought to complete risk-of-bias assessments.
Data extraction was completed using a modified version of the standard template provided within the Covidence software. Descriptive information extracted from all articles deemed eligible for inclusion included funding source(s), study first author, year of publication, general description of study setting and population including eligibility criteria, number randomized, and number completing the study. Information extracted for each intervention included intervention type (pro-, pre-, or synbiotic), number of -biotics used (single or multiple), strain of probiotic, brand name and type of -biotic, treatment dose and duration, form of intervention (capsule, powder, beverage, fermented dairy product), and information on any other ingredients used in the intervention or placebo products. Outcome data extracted included incidence of RTI (defined as number of subjects with ≥1 RTI, total number of RTIs, or odds/risk ratio of either outcome), RTI duration (total days of illness, days per event, days per person), and RTI severity as defined by the study authors. Although the number of missed days of school, work, or athletic training due to RTI was also extracted, too few studies reported those data to conduct a meta-analysis. Attempts were made to contact corresponding authors by e-mail when relevant outcomes were not reported or not reported in sufficient detail for inclusion in the meta-analysis. Manual measurements were used to extract outcome data presented only in figure format by digitally measuring the location of means and error bars and converting those values using a height-to-unit ratio determined from digital measurement of the y-axis units (25).
Meta-analysis and publication bias
All analyses were completed using Comprehensive Meta Analysis version 3.3.070 software (Biostat). Outcomes included risk ratio for the number of participants experiencing ≥1 RTI during the intervention period, Hedges’ g effect size for mean duration and mean severity of each RTI episode, and the rate ratio for total days of illness with an RTI. Risk ratios were calculated as the proportion with ≥1 RTI in the intervention group relative to the proportion with ≥1 RTI in the placebo group. Rate ratios were calculated using the incidence rate in the intervention group (total days of RTI illness/number of person-years) relative to the incidence rate in the placebo group. Hedges’ g effect sizes were calculated using the means and SDs reported for each group, and interpreted as small (0.20), medium (0.50), or large (0.80) effects (26).
All outcomes were analyzed using the DerSimonian and Laird inverse variance method for random-effects meta-analysis and are presented as effect measure (95% CI). Risk ratios and rate ratios were analyzed using both ITT (primary analysis) and per-protocol (secondary analysis) analyses. For ITT analyses, all subjects who were randomly assigned were included in the calculation of risk or rate ratio. For per-protocol analyses, all subjects completing the study were included in the risk and rate ratio calculations. Duration and severity of RTI were analyzed using per-protocol analyses only. Heterogeneity was assessed using the I2 statistic from a fixed-effects model. I2 statistics were interpreted based on Cochrane handbook recommendations, wherein 30–60%, 50–90%, and 75–100% may suggest moderate, substantial, and considerable heterogeneity, respectively (27). Publication bias was detected by visually inspecting funnel plots for asymmetry and using Egger's test (28).
Subgroup meta-analyses and meta-regressions were undertaken for all outcomes to assess potential sources of heterogeneity. These analyses were largely defined a priori, although several were developed a posteriori based on characteristics of identified studies (use of fermented dairy and population physical activity level). Subgroups included number of strains used in a probiotic intervention (single or multi-strain), genus and species of probiotic, whether the probiotic was delivered in a fermented dairy product, and physical activity level of the study population. Random-effects meta-analysis for each subgroup was conducted whenever 3 or more studies were available for inclusion in the analysis. Random-effects meta-regression was conducted using the DerSimonian and Laird method to compare effects within subgroups and to determine associations between study outcomes and daily dose of probiotic, duration of intervention, and the total dose of probiotic (dose × duration).
Two studies included only 1 placebo group but multiple treatment groups (29, 30). Prior to calculating risk and rate ratios, the number of events and number of participants in the placebo group were divided by the number of intervention groups. Prior to calculating effect sizes, the number of participants in the placebo group was divided by the number of intervention groups with no adjustments made to the mean or SD in the placebo group. Each intervention group along with the reduced placebo group was treated as a separate study in the meta-analysis (27). Although this approach does not fully overcome unit-of-analysis error resulting from correlated measurements, it does allow these studies to be included in subgroup analyses. Several studies used multiple intervention forms, doses, or durations within a single intervention group (31–34) or reported data for different types of RTIs separately but not combined (35, 36), which affected whether and how these studies were included in the analyses. Any assumptions or decisions made regarding treatment of these studies in individual analyses are described in the table legends. Finally, data from the 4 crossover studies identified in the search could not be included in the meta-analyses due to insufficient data (37–40).
Results
Study selection and characteristics
The study screening and selection process is shown in Figure 1. Of 17,409 records screened, 39 studies reporting results in 42 separate manuscripts met the inclusion criteria (Tables 1 and 2). Of the studies included, 35 used a parallel-group design and 4 used a crossover design. Publication year ranged from 2001 to 2021. Studies were conducted in 16 countries, with continental percentages as follows: 53%, Europe; 17%, North America; 14%, Asia; 14%, Australia/Oceania; and 2%, South America. Populations included healthy adults (n = 19), healthy physically active adults (n = 15), and adults with chronic illness (n = 5). Within the 39 studies, 39 probiotic (n = 8046 subjects), 4 prebiotic (n = 499 subjects), and 5 synbiotic (n = 634 subjects) interventions were tested. Intervention durations ranged from 3 to 52 wk. Intervention dose ranged from 40 million to 100 billion CFU/d of probiotic, 2.5 to 5.6 g/d of prebiotic, and for synbiotics, 5 to 10 billion CFU/d of probiotic in addition to 2.5 to 3 g/d of prebiotic. The probiotic interventions were delivered as single strains of Bifidobacterium (n = 7), Enterococcus (n = 2), Lactobacillus (n = 19), and Lactococcus (n = 1) or as multiple-strain products (n = 10). Prebiotic interventions consisted of galacto-oligosaccharides (n = 1), oat β-glucan (n = 1), and xylo-oligosaccharides (n = 1). The synbiotics used multi- or single-strain formulations containing Bifidobacterium and/or Lactobacillus, and galacto-oligosaccharides, fructo-oligosaccharides, or xylo-oligosaccharides. Interventions were provided in the forms of capsules, tablets, and powder, or delivered in fermented dairy (n = 10) and nonfermented foods and beverages.
FIGURE 1.
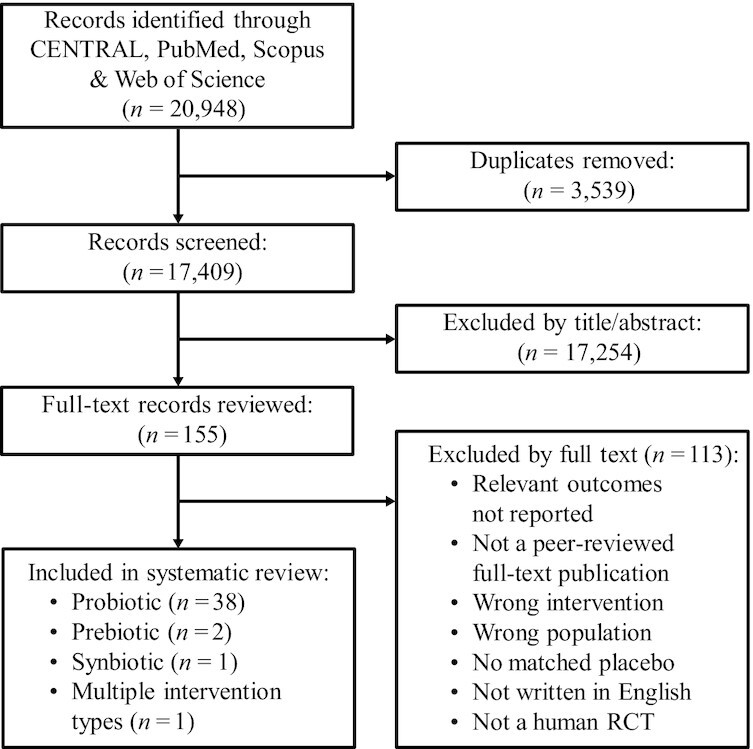
Flow diagram for screening and selection of studies assessing effects of orally ingested probiotics, prebiotics, or synbiotics on the incidence, duration, or severity of respiratory tract infections in nonelderly adults. RCT, randomized controlled trial.
TABLE 1.
Characteristics of included studies examining effects of probiotics on the incidence, duration, or severity of respiratory tract infections in nonelderly adults1
| Study, year; country (reference) | Study design | n (% Female), age,2 y | Population | Intervention (CFU/d); form | Duration, wk |
|---|---|---|---|---|---|
| Ahrén et al., 2021; Sweden (41) | RCT, parallel group | PRO: 448 (63), 41 ± 13PLA: 450 (65), 41 ± 14 | Healthy adults with reoccurring colds | PRO: Lactiplantibacillus plantarum HEAL9 DSM 15312 (1 × 109), Lacticaseibacillus paracasei 8700:2 DSM 13434 (1 × 109); powderPLA: maltodextrin, powder | 12 |
| Altadill et al., 2021; Malaysia (42); Chong et al., 2019; Malaysia (43) | RCT, parallel group | PRO: 56 (70), 30 (21–58)PLA: 53 (72), 28 (21–63) | Healthy adults | PRO: Lactobacillus plantarum DR7 (1 × 109); powderPLA: maltodextrin; powder | 12 |
| Berggren et al., 2011; Sweden (44) | RCT, parallel group | PRO: 137 (66), 46 ± NRPLA: 135 (67), 43 ± NR | Healthy adults | PRO: Lactobacillus plantarum (1 × 109); powderPLA: maltodextrin; powder | 12 |
| Childs et al., 2014; United Kingdom (40) | RCT, crossover | 44 (50), 43 ± 12 | Healthy adults | PRO: Bifidobacterium animalis subsp. lactis Bi-07 (1 × 109); powderPLA: maltodextrin; powder | 3 |
| Cox et al., 2010; Australia (39) | RCT, crossover | 20 (0), 27.3 ± 6.4 | Highly trained distance runners | PRO: Lactoacillus fermentum VRI-003 (1.2 × 1010); capsulePLA: microcrystalline cellulose; capsule | 4 |
| de Vrese et al., 2005 and 2006; Germany (34, 45) | RCT, parallel group | PRO: 225 (NR), 37 ± 12PLA: 229 (NR), 38 ± 14 | Healthy adults | PRO: Lactobacillus gasseri PA 16/8 (5 × 107), Bifidobacterium longum SP 07/3 (4 × 107), Bifidobacterium bifidum MF 20/5 (5 × 106); tabletPLA: multivitamin; tablet | 12–22 |
| Gleeson et al., 2011; United Kingdom (46) | RCT, parallel group | PRO: 32 (NR), 32 ± 14PLA: 26 (NR), 25 ± 9 | Healthy recreational/elite endurance athletes | PRO: Lactobacillus caseiShirota (1.3 × 1010); fermented beveragePLA: fermented milk; beverage | 16 |
| Gleeson et al., 2012; United Kingdom (47) | RCT, parallel group | PRO: 27 (NR), 25 ± 5PLA: 27 (NR), 24 ± 4 | Healthy, highly active adults | PRO: Lactobacillus salivarius (2 × 1010); powderPLA: maltodextrin, magnesium stearate; powder | 16 |
| Gleeson et al., 2016; United Kingdom (48) | RCT, parallel group | PRO: 126 (42), 20 ± 2PLA: 117 (41), 21 ± 2 | Healthy, endurance-trained, with normal hematology | PRO: Lactobacillus caseiShirota (1.3 × 1010); fermented beveragePLA: fermented milk; beverage | 20 |
| Guillemard et al., 2010; Germany (35) | RCT, parallel group | PRO: 478 (96), 32 ± 9PLA: 484 (97), 32 ± 9 | Healthy adult shift workers | PRO: Lactobacillus casei, Streptococcus thermophiles and Lactobacillus delbrueckii (2 × 109); fermented beveragePLA: nonfermented, acidified, sweetened, flavored dairy drink without active components; beverage | 12 |
| Habermann et al., 2001; Germany (32) | RCT, parallel group | PRO: 70 (NR), 47 ± 11PLA: 66 (NR), 47 ± 11 | Confirmed medical history of chronic recurrent bronchitis | PRO: Enterococcus faecalis group D (11.25–33.75 × 107); dropletPLA: isotonic saline with starch; liquid | 26 |
| Habermann et al., 2002; Germany (33) | RCT, parallel group | PRO: 78 (NR), 40 ± 12PLA: 79 (NR), 42 ± 13 | Chronic recurrent hypertrophic sinusitis | PRO: Enterococcus faecalis (11.25–33.75 × 107); dropletPLA: starch suspension diluted in saline; liquid | 26 |
| Haywood et al., 2014; New Zealand (38) | RCT, crossover | 30 (0), 25 ± 4 | Elite rugby athletes | PRO: Lactobacillus gasseri (2.6 × 109), Bifidobacterium bifidum (0.2 × 109), Bifidobacterium longum (0.2 × 109); capsulePLA: corn flour; capsule | 4 |
| Hor et al., 2018; Malaysia (49) | RCT, parallel group | PRO: 72 (51), 44 ± 15PLA: 65 (58), 45 ± 17 | Healthy adults | PRO: Lactobacillus casei LCZ (1 × 109); powderPLA: brown rice powder and maltodextrin; powder | 52 |
| Jespersen et al., 2015; Denmark (36) | RCT, parallel group | PRO: 538 (NR), 32 ± NRPLA: 528 (NR), 31 ± NR | Healthy adults | PRO: Lactobacillus paracaseiL. casei 431 (1 × 109); acidified beveragePLA: acidified milk; beverage | 6 |
| Kalima et al., 2016; Finland (50) | RCT, parallel group | PRO: 121 (NR), NRPLA: 103 (NR), NRPRO: 63 (NR), NRPLA: 73 (NR), NR | Military | PRO: Bifidobacterium animalis ssp. lactis BB12 (16 × 109), Lactobacillus rhamnosus GG (4 × 1010); tabletPLA: xylitol, sorbitol, microfibrous cellulose, magnesium stearate, and citrus flavor; tablet | 1221 |
| Kekkonen et al., 2007; Finland (31) | RCT, parallel group | PRO: 61 (NR), 40 ± NRPLA: 58 (NR), 40 ± NR | Healthy marathon runners | PRO: Lactobacillus rhamnosus GG (1 to 4 × 1010); beveragePLA: milk-based fruit drink; beverage | 12 |
| Kumpu et al., 2015; United States (51); Tapiovaara et al., 2016; Finland (52) | RCT, parallel group | PRO: 19 (63), 24 ± 11PLA: 20 (65), 22 ± 9 | Healthy adults | PRO: Lactobacillus rhamnosus GG (1 × 109); beveragePLA: fruit juice; beverage | 6 |
| Langkamp-Henken et al., 2015; United States (29) | RCT, parallel group | PRO1: 142 (60), 20 ± 1PRO2: 148 (66), 20 ± 1PRO3: 145 (62), 20 ± 1PLA: 147 (67), 20 ± 1 | Healthy university students under academic stress | PRO1: Bifidobacterium bifidum R0071 (3 × 109); capsulePRO2: Bifidobacterium longum ssp. infantis R0033 (3 × 109); capsulePRO3: Lactobacillus helveticus R0052 (3 × 109); capsulePLA: magnesium stearate and potato starch; capsule | 6 |
| Meng et al., 2016; United States (37) | RCT, crossover | 30 (63), 28 ± 1.2 | Healthy adults | PRO: Bifidobacterium animalis subsp. lactis BB-12 (1 × 1010); fermented yogurt smoothiePLA: yogurt; yogurt smoothie | 4 |
| Michael et al., 2020; Bulgaria (53) | RCT, parallel group | PRO: 110 (60), 45 ± 10PLA: 110 (61), 47 ± 10 | Adults classified as overweight or obese | PRO: Lactobacillus acidophilus CUL60 (NCIMB 30157), Lactobacillus acidophilus CUL21 (NCIMB 30156), Lactobacillus plantarum CUL66 (NCIMB 30280) Bifidobacterium bifidum CUL20 (NCIMB 30153) and Bifidobacterium animalis subsp. lactis CUL34 (NCIMB 30172) (5 × 1010); capsulePLA: microcrystalline cellulose; capsule | 26 |
| Michalickova et al., 2016; Serbia (54) | RCT, parallel group | PRO: 20 (25), 23 ± 3PLA: 19 (26), 23 ± 3 | Elite athletes | PRO: Lactobacillus helveticus (2 × 1010); capsulePLA: Maltodextrin capsule | 14 |
| Nishihira et al., 2016; Japan (55) | RCT, parallel group | PRO: 94 (83), 50 ± 13PLA: 94 (82), 50 ± 12 | Healthy adults | PRO: Lactobacillus gasseri SBT2055 LG2055 (1 × 109), fermented drinkable yogurtPLA: lactic acid bacteria starter (Streptococcus thermophiles), skim milk powder, flavoring, polysaccharide from soybeans, pectin, sucralose; drinkable yogurt | 16 |
| Pumpa et al., 2019; Australia (56) | RCT, parallel group | PRO: 9 (NR), 27 ± 3PLA: 10 (NR), 27 ± 3 | Elite athletes | PRO: Lactobacillus rhamnosus, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus plantarum, Lactobacillus fermentum, Bifiodbacteriumlactis, Bifidobacterium bifidum, Streptococcus thermophilus, Saccharomyces bouladri (6 × 109); capsulePLA: cellulose; capsule | 17 |
| Rizzardini et al., 2012; Italy (57) | RCT, parallel group | PRO: 53 (47), 29 ± 11PLA: 48 (56), 31 ± 11PRO: 56 (55), 37 ± 14PLA: 54 (65), 35 ± 14 | Healthy adults | PRO: Bifidobacterium animalis ssp. lactis BB-12 (1 × 109); capsulePLA: NR; capsulePRO: Lactobacillus casei 431 (1 × 109); acidified beveragePLA: acidified dairy drink; beverage | 6 |
| Schröder et al., 2015; Germany (58) | RCT, parallel group | PRO: 79 (0), 42 ± 10PLA: 80 (0), 42 ± 10 | Healthy adults involved in physically active or sedentary work | PRO: Lactobacillus reuteri DSM 17938 (5 × 108); tabletPLA: isomalt, xylitol, palm oil, lemon-lime flavoring, citric acid; tablet | 13 |
| Shida et al., 2017; Japan (59) | RCT, parallel group | PRO: 50 (0), 41 ± 5PLA: 50 (0), 41 ± 6 | Healthy adults working within office buildings | PRO: Lactobacillus caseiShirota YIT 9029 (1 × 1011); fermented beveragePLA: milk; 1 beverage | 12 |
| Smith et al., 2013; United States (60) | RCT, parallel group | PRO: 101 (82), NRPLA: 97 (72), NR | Healthy university students | PRO: Bifidobacterium animalis ssp. lactis BB-12 (1 × 109), Lactobacillus (1 × 109); powderPLA: strawberry-flavored powder; powder | 12 |
| Strasser et al., 2016; Austria (61) | RCT, parallel group | PRO: 14 (43), 26 ± 3PLA: 15 (67), 27 ± 3 | Healthy, trained athletes | PRO: Bifidobacterium bifidum W23, Bifidobacterium lactis W51, Enterococcus faecium W54, Lactobacillus acidophilus W22, Lactobacillus brevis W63, and Lactococcus lactis W58 (1 × 1010); powderPLA: cornstarch, maltodextrin, vegetable protein, MgSO4, MnSO4, and KCl; powder | 12 |
| Sugimura et al., 2015; Japan (62) | RCT, parallel group | PRO: 106 (58), 45 ± 8PLA: 107 (56), 45 ± 8 | Healthy adults | PRO: Lactococcus lactis JCM5805 (1 × 1011); fermented yogurt beveragePLA: milk, powdered skim milk, milk peptide, granulated sugar, pectin, lactic acid, flavoring agent and water; yogurt beverage | 10 |
| Tiollier et al., 2007; France (63) | RCT, parallel group | PRO: 24 (0), 21 ± 1PLA: 23 (0), 21 ± 2 | Military cadets | PRO: Lactobacillus casei DN-114 001 (NR); fermented beveragePLA: nonfermented milk; beverage | 3.5 |
| Turner et al., 2017; United States (64) | RCT, parallel group | PRO: 58 (67), 22 ± 6PLA: 57 (57), 23 ± 7 | Healthy adults susceptible to rhinovirus type 39 | PRO: Bifidobacterium animalis (2 × 109); powderPLA: sucrose; powder | 4.5 |
| Vaisberg et al., 2019; Brazil (65) | RCT, parallel group | PRO: 20 (0), 40 ± 9PLA: 22 (0), 40 ± 10 | Amateur marathon runners | PRO: Lactobacillus casei strain Shirota (40 × 109); fermented beveragePLA: unfermented milk; beverage | 4 |
| West et al., 2011; Australia (66) | RCT, parallel group | PRO: 18 (100), 36 ± 9PLA: 17 (100), 36 ± 10PRO: 29 (0), 35 ± 10PLA 33 (0), 36 ± 9 | Female cyclists and triathletesMale cyclists and triathletes | PRO: Lactobacillus fermentum (1 × 109); capsulePLA: microcrystalline cellulose; capsule | 11 |
| West et al., 2014; Australia (30) | RCT, parallel group | PRO1: 161 (50); 36 ± 12PRO2: 155 (46); 36 ± 11PLA: 149 (48); 37 ± 11 | Healthy active adults | PRO1: Bifidobacterium animalis subsp. lactis Bl-04 (2 × 10 (2 × 109); powderPRO2: Lactobacillus acidophilus NCFM and Bifidobacterium animalis subsp. lactis Bi-07 (1 × 1010); powderPLA: sucrose; powder | 21.5 |
| Zhang et al., 2018; China (67) | RCT, parallel group | PRO: 67 (51), 34 ± 6PLA: 67 (51) 33 ± 6 | Healthy adults with ≥4 RTIs per year | PRO: Lactobacillus paracasei (4.5 × 109), Lactobacillus casei 431 (4.5 × 109), Lactobacillus fermentium PCC (4.5 × 108); fermented beveragePLA: starter culture and milk; beverage | 12 |
NR, not reported; PLA, placebo; PRO, probiotic; RCT, randomized controlled trial.
Values are means ± SDs or means (range).
TABLE 2.
Characteristics of included studies examining effects of prebiotics and synbiotics on the incidence, duration, or severity of respiratory tract infections in nonelderly adults1
| Study, year; country (reference) | Study design | n (% female), age,2 y | Population | Intervention; form | Duration, wk |
|---|---|---|---|---|---|
| Prebiotic | |||||
| Childs et al., 2014; United Kingdom (40) | RCT, crossover | 44 (50), 43 ± 12 | Healthy adults | PRE: xylo-oligosaccharide (8 g/d); powderPLA: maltodextrin; powder | 3 |
| Hughes et al., 2011; United States (68) | RCT, parallel group | PRE1: 140 (50), 20 ± 1PRE2:139 (51),20 ± 1PLA: 140 (51),20 ± 1.2 | Healthy university students under academic stress and ≥1 cold in past year | PRE1: GOS (2.5 g/d); powderPRE2: GOS (5 g/d); powderPLA: sucrose, silicone dioxide; NR | 8 |
| Nieman et al., 2008; United States (69) | RCT, parallel group | PRE: 19 (0), 22 ± 4PLA: 17 (0),25 ± 9 | Healthy active cyclists | PRE: B-glucan (5.6 g); beveragePLA: cornstarch, sports drink; beverage | 2.5 |
| Synbiotic | |||||
| Childs et al., 2014; United Kingdom (40) | RCT, crossover | 44 (50), 43 ± 12 | Healthy adults | SYN: Bifidobacterium animalis subsp. lactis Bi-07 (1 × 109 CFU/d) and xylo-oligosaccharide (8 g/d); powderPLA: maltodextrin, powder | 3 |
| Pregliasco et al., 2008; Italy (70) | RCT, parallel group | SYN: 114 (NR), 39 ± 16PLA: 105 (NR), 35 ± 20SYN: 74 (NR), 38 ± 19PLA: 68 (NR),38 ± 19SYN1: 76 (NR), 42 ± 15SYN2: 78 (NR), 45 ± 16PLA: 75 (NR),42 ± 19 | Healthy adults | SYN: Lactobacillus plantarum probial LP 02-LMG P-21020 (10 × 109 CFU/d), Lactobacillus rhamnosus probial LR 04-DSM 16605 (10 × 109 CFU/d), Bifidobacterium lactis probial BS 01-LMG P-21384 (10 × 109 CFU/d), FOS (3 g/d); powderPLA: glucose, maltodextrin; powderSYN: Lactobacillus plantarum probial LP 02-LMG P-21020 (10 × 109 CFU/d), Lactobacillus rhamnosus probial LR 04-DSM 16605 (10 × 109 CFU/d), Bifidobacterium lactis probial BS 01-LMG P-21384 (10 × 109 CFU/d), FOS (3 g/d); powderPLA: glucose, maltodextrin; powderSYN1: Lactobacillus plantarum probial LP 01-LMG P-21021 (5 × 109 CFU/d), Lactobacillus plantarum probial LP 02-LMG P-21020 (5 × 109 CFU/d), Lactobacillus rhamnosus probial LR 04-DSM 16605 (5 × 109 CFU) Lactobacillus rhamnosus probial LR 05-DSM 19739 (5 × 109 CFU/d), Bifidobacterium lactis probial BS 01-LMG P-21384 (5 × 109 CFU/d), GOS (2.5 g/d); powderSYN2: Lactobacillus plantarum probial LP 01-LMG P-21021 (5 × 109 CFU/d), Lactobacillus plantarum probial LP 02-LMG P-21020 (5 × 109 CFU/d), Lactobacillus rhamnosus probial LR 04-DSM 16605 (5 × 109 CFU/d), Lactobacillus rhamnosus probial LR 05-DSM 19739 (5 × 109 CFU/d), Bifidobacturium lactis probial BS 01-LMG P-21384 (5 × 109 CFU/d), FOS (3 g/d); powderPLA: glucose, maltodextrin; powder | 12 |
FOS, fructo-oligosaccharide; GOS, galacto-oligosaccharide; NR, not reported; PLA, placebo; PRE, prebiotic; RCT, randomized controlled trial; SYN, synbiotic.
Values are means ± SDs or means (range).
Effects of orally ingested probiotics on incidence, duration, and severity of RTI
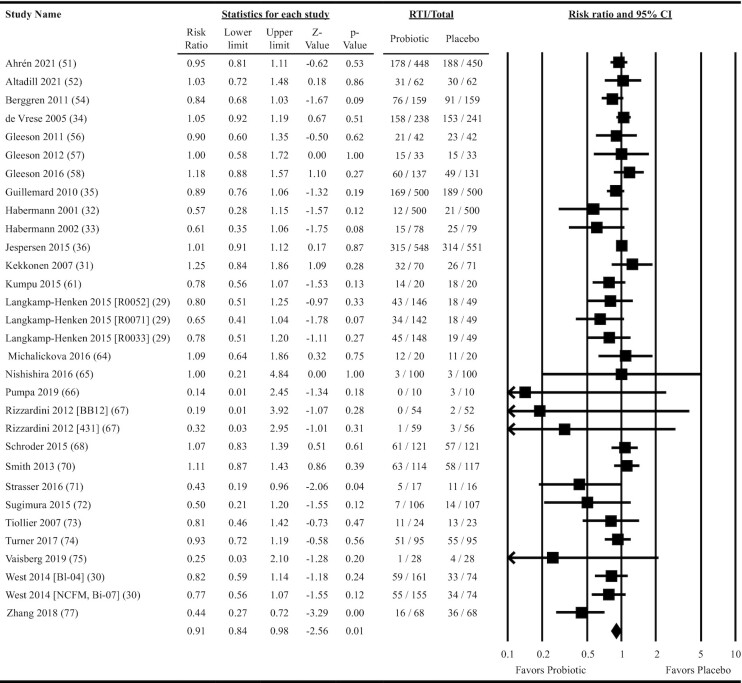
Twenty-seven studies providing data on 31 probiotic versus placebo comparisons were included in meta-analyses determining the effects of probiotics on RTI incidence. Overall, oral ingestion of probiotics reduced the risk of experiencing 1 or more RTI by 9% (risk ratio = 0.91; 95% CI: 0.84, 0.98; P = 0.01: ITT analysis; Figure 2). Heterogeneity in the fixed-effects model was moderate (I2 = 33.4, P = 0.04). Subgroup analyses revealed a statistically significant risk reduction for single-strain Bifidobacterium interventions, but this reduction was not statistically different from single-strain Lactobacillus interventions in the meta-regression (ITT analysis; Figure 3 and Supplemental Table 5). Subgroup analyses also revealed a risk reduction for populations not classified as physically active, although the risk reduction did not differ from that in physically active populations in the meta-regression (Figure 3). Heterogeneity within the subgroup fixed-effects models varied, ranging from none to substantial (I2 = 0–63.4, P = 0.01–0.61; Supplemental Table 5). The overall risk reduction was slightly greater in the per-protocol analysis (risk ratio = 0.90; 95% CI: 0.84, 0.96; P = 0.002; Supplemental Figure 1) and analyses were statistically significant for several subgroups including both single- and multi-strain interventions, single-strain Bifidobacterium and B. animalis subsp. lactis interventions, and both fermented dairy and other intervention delivery forms (Figure 3 and Supplemental Table 6). However, no significant differences based on these subgroups were observed in the per-protocol meta-regression analyses (Figure 3 and Supplemental Table 6). Both the funnel plot (Supplemental Figure 2) and Egger's test (P < 0.001) provided evidence of publication bias (ITT analysis).
FIGURE 2.
Forest plot for the effects of orally ingested probiotics versus placebo on the risk of experiencing 1 or more respiratory tract infections in nonelderly adults. Intention-to-treat random effects meta-analysis using DerSimonian and Laird inverse variance method. Data extracted for Jespersen et al. (36) and Guillemard et al. (35) reflect the incidence of upper respiratory tract infections. Lower and upper limits are 95% CIs. Individual study effect estimates (squares; sized by study weight) and pooled effects (diamond) are plotted. Heterogeneity from the fixed-effects model: I2 = 33.4, P = 0.04. RTI, number of individuals experiencing ≥1 respiratory tract infection; Total, number randomized.
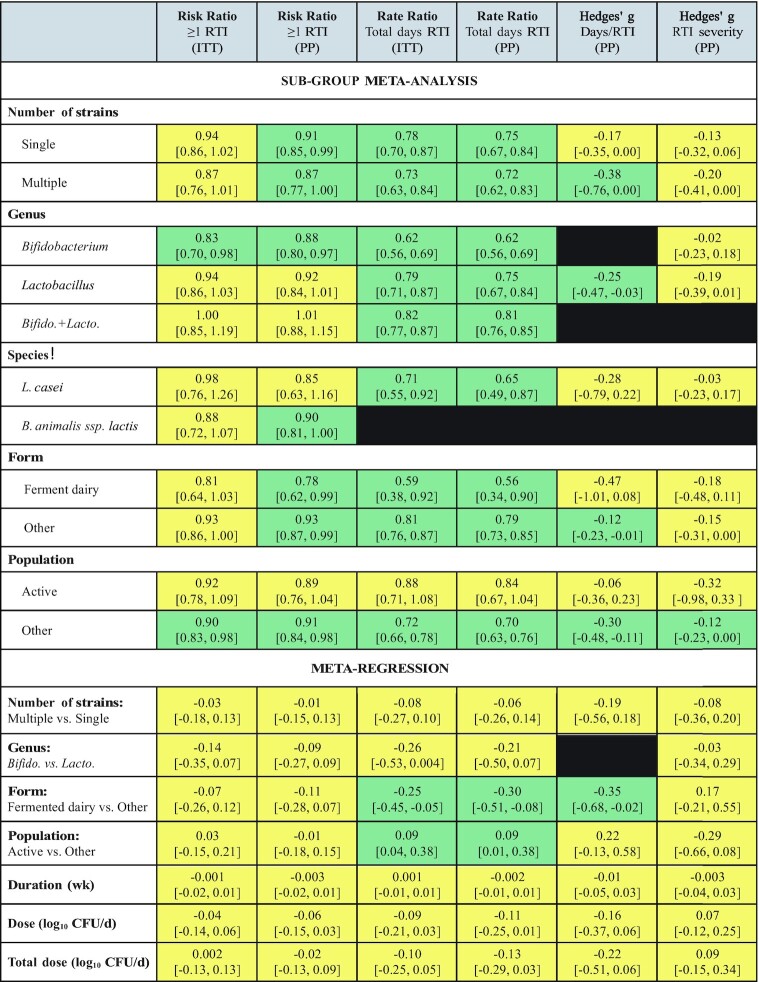
FIGURE 3.
Summary of subgroup meta-analyses and meta-regressions for effects of orally ingested probiotics on incidence, duration, and severity of RTIs in nonelderly adults. Subgroup results are presented as effect size [95% CI]. Meta-regression results are presented as β [95% CI]. Results shaded in green are statistically significant (P < 0.05) and those in yellow are not statistically significant (P ≥ 0.05). Black shading indicates insufficient data for analysis. Subgroups were analyzed using random-effects meta-analysis using the DerSimonian and Laird inverse variance method. Meta-regression used the DerSimonian and Laird inverse variance method. See Supplemental Tables 5–10 for P values and measures of heterogeneity. Bifido., Bifidobacterium; ITT, intention-to-treat analysis; Lacto., Lactobacillus; PP, per-protocol analysis; RTI, respiratory tract infection.
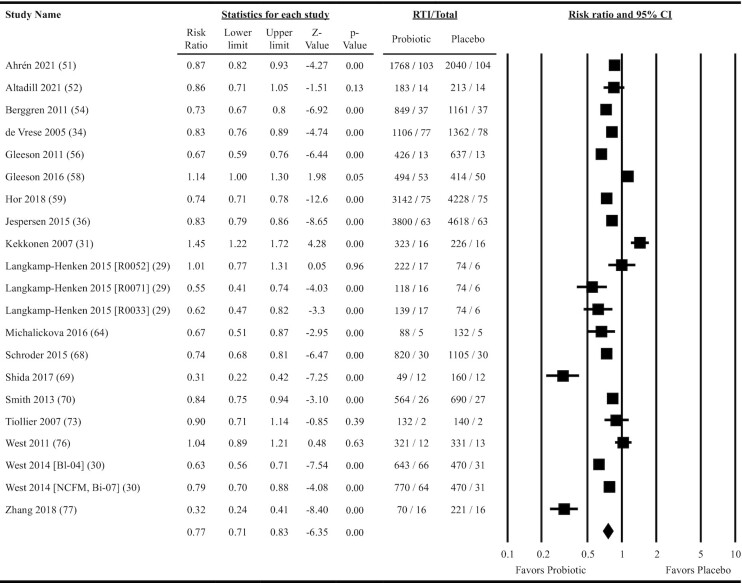
Eighteen studies providing data on 21 probiotic versus placebo comparisons were included in meta-analyses determining the effects of probiotics on total days of illness due to RTI. Overall, oral ingestion of probiotics reduced the rate ratio by 23% (rate ratio: 0.77; 95% CI: 0.71, 0.83; P = 0.001; ITT analysis; Figure 4). Heterogeneity in the fixed-effects model was considerable (I2 = 91.4, P < 0.001). Reductions in the rate ratio were significant across all subgroups except in physically active populations (ITT analysis; Figure 3 and Supplemental Table 7). Meta-regression indicated that delivering probiotics in the form of fermented dairy lowered the rate ratio to a greater extent than other forms of delivery and that the rate reduction in populations not considered physically active was greater than that in physically active populations (Figure 3 and Supplemental Table 7). Heterogeneity within the subgroup fixed-effects models was considerable for most subgroups analyzed (I2 ≥ 89.8, P < 0.001; Supplemental Table 7). Per-protocol analyses were largely consistent with the ITT analyses (overall rate ratio = 0.74; 95% CI: 0.68, 0.81; P < 0.001; Supplemental Figure 3, Figure 3, and Supplemental Table 8). Neither the funnel plot (Supplemental Figure 4) nor Egger's test (P = 0.26) suggested publication bias.
FIGURE 4.
Forest plot for the effects of orally ingested probiotics versus placebo on the total days of illness due to RTIs in nonelderly adults. Intention-to-treat random-effects meta-analysis using DerSimonian and Laird inverse variance method. Lower and upper limits are the 95% CIs. Individual study effect estimates (squares; sized by study weight) and pooled effects (diamond) are plotted. Heterogeneity from the fixed-effects model: I2 = 91.4, P < 0.001. RTI, total days of respiratory tract infection; Total, total person years of exposure.
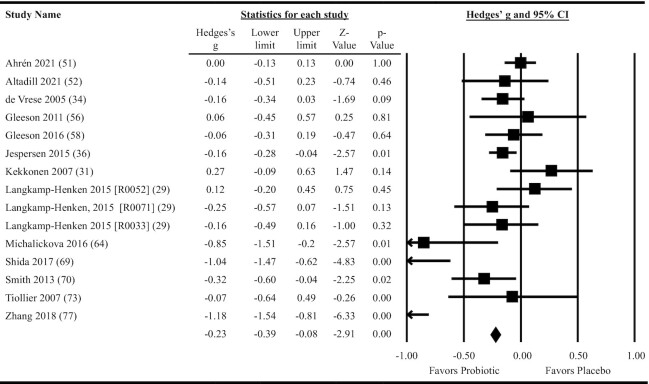
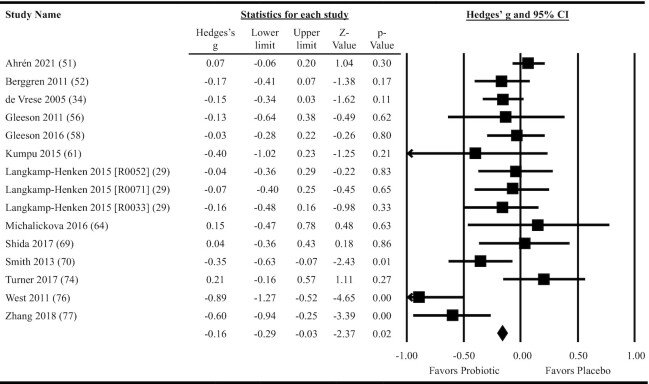
Thirteen studies providing data on 15 probiotic versus placebo comparisons were included in meta-analyses determining the effects of probiotics on the duration of individual RTI episodes. Overall, oral ingestion of probiotics reduced the duration of RTI episodes (Hedges’ g: –0.23; 95% CI: –0.39, –0.08; P = 0.004; per-protocol analysis; Figure 5). Heterogeneity in the fixed-effects model was considerable (I2 = 79.4, P < 0.001). When the 10 studies that provided results as days per RTI episode were meta-analyzed, the reduction in duration amounted to 1.0 day/RTI episode (95% CI: 0.2, 1.7; P = 0.02). Subgroup analyses were statistically significant for multiple-strain interventions, single-strain Lactobacillus interventions, and for populations not considered physically active (Figure 3 and Supplemental Table 9). Meta-regression indicated a greater reduction in effect size for interventions delivered in fermented dairy products compared with other forms of delivery, but no differences in effect size between single- and multi-strain interventions or based on population physical activity level (Figure 3 and Supplemental Table 9). Heterogeneity within the subgroup fixed-effects models ranged from moderate to considerable across all subgroups analyzed (I2 = 49.6–91.8, P ≤ 0.06; Supplemental Table 9). Neither the funnel plot (Supplemental Figure 5) nor Egger's test (P = 0.10) suggested publication bias.
FIGURE 5.
Forest plot for the effects of orally ingested probiotics versus placebo on the duration of respiratory tract infection episodes in nonelderly adults. Per-protocol random-effects meta-analysis using DerSimonian and Laird inverse variance method. Lower and upper limits are the 95% CIs. Individual study effect estimates (squares; sized by study weight) and pooled effects (diamond) are plotted. Heterogeneity from the fixed-effects model: I2 = 79.4, P < 0.001.
Thirteen studies providing data on 15 probiotic versus placebo comparisons were included in meta-analyses determining the effects of probiotics on RTI severity. Overall, oral ingestion of probiotics reduced RTI severity (Hedges’ g: –0.16; 95% CI: –0.29, –0.03; P = 0.02; Figure 6). Heterogeneity in the fixed-effects model was substantial (I2 = 65.1, P < 0.001). Subgroup analyses were statistically significant only for populations not considered physically active, but the reduction in effect size was not statistically different from physically active populations in the meta-regression (Figure 3 and Supplemental Table 10). Heterogeneity within the subgroup fixed-effects models ranged from none to considerable across all subgroups analyzed (I2 = 0–80.8, P = 0.0001–0.88; Supplemental Table 10). Neither the funnel plot (Supplemental Figure 6) nor Egger's test (P = 0.09) suggested publication bias.
FIGURE 6.
Forest plot for the effects of orally ingested probiotics versus placebo on the severity of respiratory tract infections in nonelderly adults. Per-protocol random-effects meta-analysis using DerSimonian and Laird inverse variance method. Lower and upper limits are the 95% CIs. Individual study effect estimates (squares; sized by study weight) and pooled effects (diamond) are plotted. Heterogeneity from the fixed-effects model: I2 = 65.1, P < 0.001.
Three crossover studies were identified by the systematic review but did not provide sufficient data to be included in the meta-analyses. These studies did not find any effects of orally ingested probiotics on the incidence of RTIs (37, 39, 40), but 2 did report that the probiotic used reduced the duration of RTIs (37, 39). Oral ingestion of probiotics was reported to have either no effect on RTI severity (37) or demonstrated a tendency to lower severity (39).
Effects of orally ingested prebiotics on incidence, duration, and severity of RTIs
Only 3 studies providing 4 comparisons of orally ingested prebiotics versus placebo were identified in the search (Table 2). Given the small sample size, heterogeneous interventions, and differences in outcomes reported, meta-analysis was not conducted. Hughes et al. (68), using a dose–response design, reported cold or flu duration was reduced by 40% following consumption of 5.0 g/d, but not 2.5 g/d, galacto-oligosaccharides in subjects with a BMI (in kg/m2) of 18.5–24.9. Both doses reduced RTI severity, but in the 5.0-g/d intervention, that effect was only observed in individuals experiencing lower stress levels. Nieman et al. (69) found no effect of β-glucan supplementation on incidence or duration of RTI. Similarly, Childs et al. (40) did not find any effect of xylo-oligosaccharides on the incidence of RTI.
Effects of orally ingested synbiotics on incidence, duration, and severity of RTIs
Only 2 studies providing 5 comparisons of orally ingested synbiotic versus placebo comparisons were identified in the search (Table 2). Meta-analysis was conducted using the total number of RTIs and total days of illness with RTI reported for the 4 synbiotic interventions studied by Pregliasco et al. (70). The synbiotic interventions reduced the total number of RTIs (rate ratio = 0.66; 95% CI: 0.53, 0.80; P < 0.001; fixed-effects I2 = 0, P = 0.57) and the total days of illness (rate ratio = 0.65; 95% CI: 0.55, 0.78; P < 0.001; fixed-effects I2 = 78.1, P = 0.01) in ITT analyses. Results were similar in the per-protocol analysis for both total number of RTIs (rate ratio = 0.64; 95% CI: 0.52, 0.79; P < 0.002; fixed-effects I2 = 0, P = 0.64) and total days of illness (rate ratio = 0.64; 95% CI: 0.52, 0.78; P < 0.001; fixed-effects I2 = 82.3, P = 0.003).
Risk of bias
The overall risk of bias for a majority of the included studies was rated as “some concerns” (Figure 7 and Supplemental Figure 7). That overall rating was frequently due to not enough information being provided to determine if results were analyzed in accordance with a prespecified analysis plan (domain 5).
FIGURE 7.
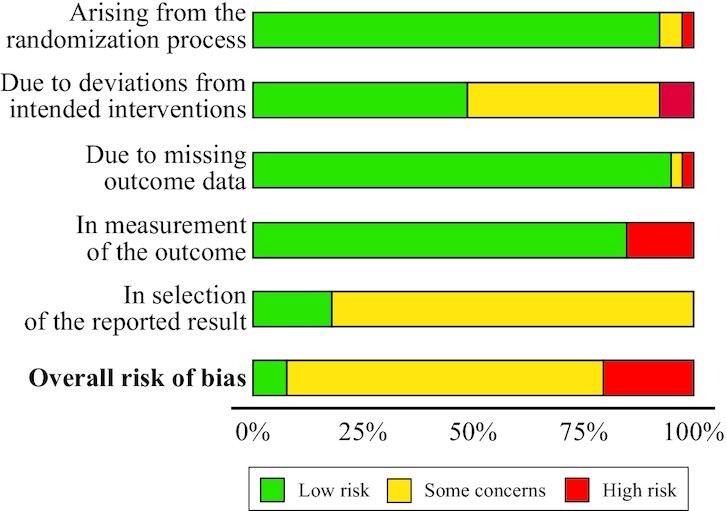
Risk-of-bias assessment for all studies identified in the systematic review. Phrases not in bold font are sources of bias. Assessed using the Cochrane risk-of-bias assessment tool version 2.0. Plot produced using robvis (McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Syn Meth 2020;1–7; https://mcguinlu.shinyapps.io/robvis/).
Discussion
This systematic review identified 39 studies conducted in nonelderly adults that assessed effects of at least 1 orally ingested probiotic intervention on the incidence, duration, or severity of RTIs, but far fewer studies assessing effects of orally ingested prebiotics or synbiotics on the same outcomes and in the same population. The main finding was that orally ingested probiotic interventions, relative to matched placebo, modestly reduced the incidence, duration, and severity of RTIs in nonelderly adults. Meta-regression indicated that those benefits did not significantly differ by the number of strains, genus, or dose of probiotic administered, or the duration of treatment. However, the physical activity level of the population studied and whether fermented dairy products were used as the delivery matrix did influence effects of probiotic interventions on the total days of illness due to RTI. Whether orally ingested prebiotic and synbiotic interventions confer similar protection against RTIs in nonelderly adults remains unclear.
The finding that probiotics reduced RTI burden is consistent with recent meta-analyses (15, 16, 18, 20). Herein, that reduction was attributable to both a slight 9% decrease in the risk of experiencing 1 or more RTI (Figure 2) and a small (Hedges’ g = –0.23; Figure 5) ∼1 d/RTI episode reduction in the mean duration of individual RTI episodes, resulting in a 23% lower daily rate of RTI illness during the intervention period (Figure 4). These effect sizes are at the lower end of what have been reported in recent meta-analyses on the topic, which have ranged from an 11% to 47% reduction in the risk or odds of experiencing at least 1 RTI (15, 16, 18) and a 0.8- to 2.7-d reduction in the duration of individual RTI episodes (15, 16, 20). Discrepancies in effect sizes are likely attributable to differences in the inclusion and exclusion criteria used for each systematic review. Specifically, Wang et al. (18) included only studies conducted in children, Hao et al. (15) and Li et al. (16) focused on upper-RTI rather than all RTIs while including few studies conducted in nonelderly adult populations, and King et al. (20) restricted their review to only studies using Lactobacillus or Bifidobacterium while including studies in children and non-RTI outcomes. Findings from this meta-analysis therefore extend that evidence base by revealing that orally ingested probiotic interventions provide modest reductions in the incidence, duration, and severity of RTIs across a large selection of studies using different probiotic types and conducted within nonelderly adult populations.
Statistical heterogeneity across probiotic studies was moderate to considerable for overall analyses and ranged from none to considerable for subgroup analyses, with greater heterogeneity seen with RTI duration and severity than incidence. The number of studies included in this analysis provided an opportunity to examine population- and intervention-related factors that may contribute to that heterogeneity. Notably, subgroup analyses suggested that, in aggregate, orally ingested probiotics may not be effective, or may be less effective, for reducing RTI incidence, duration, and severity in physically active populations (Figure 3). Those findings are generally consistent with results of a recent meta-analysis that reported that probiotics did not reduce RTI incidence or duration, but did reduce RTI severity, in physically active adults (14). Findings also align with recent systematic reviews that have described inconsistent effects of orally ingested probiotics on markers of immune function and measures of RTI burden in athletes, inconsistencies that may be attributable to differences in the intervention strains and training loads of the study populations (19, 71). Reasons why probiotics may be less effective for reducing RTI burden, or have less consistent effects, in physically active populations are unclear. However, exercise with adequate rest and recovery may favorably modulate the gut microbiota and support immune function (72, 73). Physically active adults generally also have other lifestyle behaviors that may reduce RTI risk such as high-quality diets (74). Possibly, probiotics do not provide any additional immune benefit in this context. On the other hand, high levels of exercise without adequate rest and recovery may compromise immune function and increase RTI risk, although this is controversial (72). Given the relatively small number of studies, the range of population physical activity levels (e.g., recreationally active to elite athletes in training), and the variety of different interventions studied, more research is warranted to better define if, when, and which oral ingestion of probiotics may confer benefit for reducing RTI burden in physically active adults.
In addition to population-based differences, effects of probiotics on RTI burden may be genus-, species-, or strain-specific and vary by the dose or form of administration (7). Herein, dose and duration of orally ingested probiotics were not associated with reductions in RTI incidence, duration, or severity, and while subgroup analyses suggested potential genus-level differences, those differences were not statistically significant in the meta-regressions (Figure 3). Too few strains were tested in multiple studies to provide adequate power to detect strain-level differences, and whether single or multiple strains were used in the intervention largely did not impact results. However, analyses did suggest that using fermented dairy products as an oral delivery matrix for probiotics may result in greater reductions in days of illness due to RTI relative to other forms of oral delivery. One reason may relate to the fermentation process itself. Specifically, lactic acid bacteria used in dairy fermentations and as probiotics may increase the bioavailability of immunomodulatory nutrients in milk (75). Additionally, lactic acid bacteria used in fermentation and added to probiotic supplements metabolize nutrients within milk, producing a variety of bioactive immunomodulatory compounds during and after the fermentation process, such as lactic acid, SCFAs, various peptides and amino acids, polysaccharides, vitamins, and antimicrobial compounds (76, 77). Collectively, these findings suggest that effects of orally ingested probiotics on reducing RTI incidence, duration, and severity in nonelderly adults are relatively consistent across the different intervention compositions, doses, and durations tested, and might be enhanced using fermented dairy as a delivery matrix. However, results should not be interpreted as indicating that all products are equally effective and are limited by small numbers of studies within certain subgroups, most notably a lack of multiple studies using the same strain or strain combinations. Further, results should be interpreted cautiously given the substantial-to-considerable level of heterogeneity observed in many of the analyses, evidence of publication bias for RTI incidence, and that few studies were considered to have a low overall risk of bias.
Few studies identified for inclusion in this systematic review tested effects of prebiotics or synbiotics. This is somewhat surprising given evidence that probiotics, prebiotics, and synbiotics improve markers of immune function or RTI-related outcomes in cell models and in studies of animals, infants, and children (9, 10). Most salient to the present study are 2 recent meta-analyses. In one, both orally ingested prebiotic and synbiotic interventions were reported to lower the odds of experiencing RTIs by ∼25% and increase NK cell cytotoxicity ex vivo (12). In another, orally ingested synbiotics were found to lower the incidence of RTIs by 16% (13). However, both meta-analyses were largely based on studies in pediatric populations. As with probiotics, it cannot be assumed that any effects of prebiotics or synbiotics on RTI burden will generalize to all populations, nor should it be assumed that all compounds and formulations will have the same effects as was evidenced by the few studies included in this review (40, 68–70). The effects of orally ingested prebiotics and synbiotics on RTI burden in nonelderly adults therefore remain a significant knowledge gap.
Strengths of this systematic review and meta-analysis include the comprehensive search strategy, extensive subgroup and meta-regression analyses to identify sources of heterogeneity, and inclusion of both ITT and per-protocol analyses. However, the analyses generally did not extend to examining interactions between effect modifiers (e.g., population type and genus of probiotic strain), assess confounding in meta-regression analyses (e.g., more favorable effects of fermented dairy may be an artifact of the strains used), or consider whether investigators analytically verified the composition of the intervention and placebo products. This analysis also included several limitations resulting from the available literature. These limitations included a lack of relevant studies on orally ingested prebiotics and synbiotics, some concerns regarding various sources of bias among most of the studies included, and evidence of publication bias for certain outcomes. Additionally, not all included studies reported data for all outcomes or reported all outcomes in a format that could be used for meta-analysis. Finally, few strains were tested in multiple studies, making it difficult to identify strain-specific effects.
In conclusion, this systematic review and meta-analysis extends previous meta-analyses relying primarily on studies of pediatric populations, by demonstrating that orally ingested probiotics also reduce the incidence, duration, and severity of RTIs in nonelderly adult populations. Effect sizes were generally modest and, despite heterogeneity in results between studies, were relatively consistent across the different intervention compositions, doses, and durations. Whether these observations apply to orally ingested prebiotics or synbiotics should be an objective of future research, as should further elucidation of sources of heterogeneity in study results and analyses of the cost-benefit ratio of orally ingested probiotics in relation to RTIs. Further, replication of positive findings for individual probiotic strains and strain combinations in high-quality randomized controlled trials is necessary in order to conclusively identify the most effective strains and dosing strategies.
Supplementary Material
Acknowledgements
The authors acknowledge the US Department of Defense Tri-Service Microbiome Consortium for establishing the collaborative infrastructure that inspired and facilitated this work. The authors acknowledge Dr. Chad Porter, Dr. Sandra Isidean, Dr. Michael Goodson, and Jason Soares for assistance in conceptualizing the systematic review, and Dr. Andrew Young for editorial review. The authors’ responsibilities were as follows—JPK: designed the research; JPK and ES: designed the search strategy; ES: conducted the literature search; JLC, AH-M, SDS, JTA, RTA, HSF, ASB, and JPK: screened abstracts; JLC, AH-M, SDS, JTA, RTA, and JPK: screened full texts; JLC, AH-M, JTA, SDS, and JPK: extracted data and conducted risk-of-bias assessments; JPK: analyzed the data; JLC and JPK: wrote the manuscript; JPK: has final primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
Supported in part by the US Army Medical Research and Development Command and appointment to the US Army Research Institute of Environmental Medicine administered by the Oak Ridge Institute for Science and Education (to JLC, JTA, and SDS) through an interagency agreement between the US Department of Energy and the US Army Medical Research and Development Command.
Author disclosures: The authors report no conflicts of interest. The sponsor had no role in the study design, data analysis, or interpretation of results.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or reflecting the views of the Army or the Department of Defense. Any citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations. Approved for public release; distribution is unlimited.
Supplemental Figures 1–7 and Supplemental Tables 1–10 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ITT, intention-to-treat; RoB2, Cochrane risk-of-bias assessment tool for randomized trials version 2; RTI, respiratory tract infection.
Contributor Information
Julie L Coleman, US Army Research Institute of Environmental Medicine, Natick, MA, USA; Oak Ride Institute of Science and Education, Belcamp, MD, USA.
Adrienne Hatch-McChesney, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
Stephanie D Small, US Army Research Institute of Environmental Medicine, Natick, MA, USA; Oak Ride Institute of Science and Education, Belcamp, MD, USA.
Jillian T Allen, US Army Research Institute of Environmental Medicine, Natick, MA, USA; Oak Ride Institute of Science and Education, Belcamp, MD, USA.
Elaine Sullo, The George Washington University, Washington, DC, USA.
Richard T Agans, Naval Medical Research Unit Dayton, Dayton, OH, USA; PARSONS Government Services, San Antonio, TX, USA.
Heather S Fagnant, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
Asma S Bukhari, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
J Philip Karl, US Army Research Institute of Environmental Medicine, Natick, MA, USA.
Data Availability
Data will be made available upon reasonable request.
References
- 1. GBD 2016 Causes of Death Collaborators . Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet North Am Ed. 2017;390(10100):1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jin X, Ren J, Li R, Gao Y, Zhang H, Li Jet al. . Global burden of upper respiratory infections in 204 countries and territories, from 1990 to 2019. EClinicalMedicine. 2021;37:100986. doi: 10.1016/j.eclinm.2021.100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cherry DK, Burt CW, Woodwell DA. National Ambulatory Medical Care Survey: 2001 summary. Adv Data. 2003(337):1–44. [PubMed] [Google Scholar]
- 4. Fendrick AM, Monto AS, Nightengale B, Sarnes M. The economic burden of non-influenza-related viral respiratory tract infection in the United States. Arch Intern Med. 2003;163(4):487–94. [DOI] [PubMed] [Google Scholar]
- 5. Flannery B, Kondor RJG, Chung JR, Gaglani M, Reis M, Zimmerman RKet al. . Spread of antigenically drifted influenza A(H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. J Infect Dis. 2020;221(1):8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thursby E, Juge N. Introduction to the human gut microbiota. Biochem J. 2017;474(11):1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot Bet al. . Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. 2014;11(8):506–14. [DOI] [PubMed] [Google Scholar]
- 8. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJet al. . Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. [DOI] [PubMed] [Google Scholar]
- 9. Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke Ket al. . The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17(11):687–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16(10):605–16. [DOI] [PubMed] [Google Scholar]
- 11. Karl JPC. Gut microbiota-targeted interventions for reducing the incidence, duration, and severity of respiratory tract infections in healthy non-elderly adults. Mil Med. 2021;186(3-4):e310–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Williams LM, Stoodley IL, Berthon BS, Wood LG. The effects of prebiotics, synbiotics, and short-chain fatty acids on respiratory tract infections and immune function: a systematic review and meta-analysis. Adv Nutr. 2022;13(1):167–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chan CKY, Tao J, Chan OS, Li HB, Pang H. Preventing respiratory tract infections by synbiotic interventions: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2020;11(4):979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Łagowska K, Bajerska J. Effects of probiotic supplementation on respiratory infection and immune function in athletes: systematic review and meta-analysis of randomized controlled trials. J Athl Train. 2021;56(11):1213–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hao Q, Dong BR, Wu T. Probiotics for preventing acute upper respiratory tract infections. Cochrane Database Syst Rev. 2015;(2):CD006895. doi: 10.1002/14651858.CD006895.pub3. [DOI] [PubMed] [Google Scholar]
- 16. Li L, Hong K, Sun Q, Xiao H, Lai L, Ming Met al. . Probiotics for preventing upper respiratory tract infections in adults: a systematic review and meta-analysis of randomized controlled trials. Evid-Based Complement Altern Med. 2020;2020:1. doi: 10.1155/2020/8734140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Emre IE, Eroğlu Y, Kara A, Dinleyici EC, Özen M. The effect of probiotics on prevention of upper respiratory tract infections in the paediatric community—a systematic review. Benef Microbes. 2020;11(3):201–11. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Li X, Ge T, Xiao Y, Liao Y, Cui Yet al. . Probiotics for prevention and treatment of respiratory tract infections in children: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2016;95(31):e4509. doi: 10.1097/md.0000000000004509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Möller GB, da Cunha Goulart MJV, Nicoletto BB, Alves FD, Schneider CD. Supplementation of probiotics and its effects on physically active individuals and athletes: systematic review. Int J Sport Nutr Exercise Metab. 2019;29(5):481–92. [DOI] [PubMed] [Google Scholar]
- 20. King S, Glanville J, Sanders ME, Fitzgerald A, Varley D. Effectiveness of probiotics on the duration of illness in healthy children and adults who develop common acute respiratory infectious conditions: a systematic review and meta-analysis. Br J Nutr. 2014;112(1):41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Agans RT, Giles GE, Goodson MS, Karl JP, Leyh S, Mumy KLet al. . Evaluation of probiotics for warfighter health and performance. Front Nutr. 2020;7:70. doi: 10.3389/fnut.2020.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martin SA, Pence BD, Woods JA. Exercise and respiratory tract viral infections. Exerc Sport Sci Rev. 2009;37(4):157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parasher A. COVID-19: current understanding of its pathophysiology, clinical presentation and treatment. Postgrad Med J. 2021;97(1147):312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron Iet al. . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 25. Tsafnat G, Glasziou P, Choong MK, Dunn A, Galgani F, Coiera E. Systematic review automation technologies. Syst Rev. 2014;3(1):74. doi: 10.1186/2046-4053-3-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Durlak JA. How to select, calculate, and interpret effect sizes. J Pediatr Psychol. 2009;34(9):917–28. [DOI] [PubMed] [Google Scholar]
- 27. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJet al. . Cochrane handbook for systematic reviews of interventions. Available from:www.training.cochrane.org/handbook.
- 28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langkamp-Henken B, Rowe CC, Ford AL, Christman MC, Nieves C Jr, Khouri Let al. . Bifidobacterium bifidum R0071 results in a greater proportion of healthy days and a lower percentage of academically stressed students reporting a day of cold/flu: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2015;113(3):426–34. [DOI] [PubMed] [Google Scholar]
- 30. West NP, Horn PL, Pyne DB, Gebski VJ, Lahtinen SJ, Fricker PAet al. . Probiotic supplementation for respiratory and gastrointestinal illness symptoms in healthy physically active individuals. Clin Nutr. 2014;33(4):581–7. [DOI] [PubMed] [Google Scholar]
- 31. Kekkonen RA, Vasankari TJ, Vuorimaa T, Haahtela T, Julkunen I, Korpela R. The effect of probiotics on respiratory infections and gastrointestinal symptoms during training in marathon runners. Int J Sport Nutr Exercise Metab. 2007;17(4):352–63. [DOI] [PubMed] [Google Scholar]
- 32. Habermann W, Zimmermann K, Skarabis H, Kunze R, Rusch V. [The effect of a bacterial immunostimulant (human Enterococcus faecalis bacteria) on the occurrence of relapse in patients with chronic recurrent bronchitis]. Arzneimittelforschung. 2001;51(11):931–7. [DOI] [PubMed] [Google Scholar]
- 33. Habermann W, Zimmermann K, Skarabis H, Kunze R, Rusch V. [Reduction of acute recurrence in patients with chronic recurrent hypertrophic sinusitis by treatment with a bacterial immunostimulant (Enterococcus faecalis bacteriae of human origin)]. Arzneimittelforschung. 2002;52(8):622–7. [DOI] [PubMed] [Google Scholar]
- 34. de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue Cet al. . Effect of Lactobacillus gasseri PA 16/8, Bifidobacterium longum SP 07/3, B. bifidum MF 20/5 on common cold episodes: a double blind, randomized, controlled trial. Clin Nutr. 2005;24(4):481–91. [DOI] [PubMed] [Google Scholar]
- 35. Guillemard E, Tanguy J, Flavigny A, de la Motte S, Schrezenmeir J. Effects of consumption of a fermented dairy product containing the probiotic Lactobacillus casei DN-114 001 on common respiratory and gastrointestinal infections in shift workers in a randomized controlled trial. J Am Coll Nutr. 2010;29(5):455–68. [DOI] [PubMed] [Google Scholar]
- 36. Jespersen L, Tarnow I, Eskesen D, Morberg CM, Michelsen B, Bügel Set al. . Effect of Lactobacillus paracaseisubsp.paracasei, L. casei 431 on immune response to influenza vaccination and upper respiratory tract infections in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Am J Clin Nutr. 2015;101(6):1188–96. [DOI] [PubMed] [Google Scholar]
- 37. Meng H, Lee Y, Ba Z, Peng J, Lin J, Boyer ASet al. . Consumption of Bifidobacterium animalis subsp. lactis BB-12 impacts upper respiratory tract infection and the function of NK and T cells in healthy adults. Mol Nutr Food Res. 2016;60(5):1161–71. [DOI] [PubMed] [Google Scholar]
- 38. Haywood BA, Black KE, Baker D, McGarvey J, Healey P, Brown RC. Probiotic supplementation reduces the duration and incidence of infections but not severity in elite rugby union players. J Sci Med Sport. 2014;17(4):356–60. [DOI] [PubMed] [Google Scholar]
- 39. Cox AJ, Pyne DB, Saunders PU, Fricker PA. Oral administration of the probiotic Lactobacillus fermentum VRI-003 and mucosal immunity in endurance athletes. Br J Sports Med. 2010;44(4):222–6. [DOI] [PubMed] [Google Scholar]
- 40. Childs CE, Röytiö H, Alhoniemi E, Fekete AA, Forssten SD, Hudjec Net al. . Xylo-oligosaccharides alone or in synbiotic combination with Bifidobacterium animalis subsp. lactis induce bifidogenesis and modulate markers of immune function in healthy adults: a double-blind, placebo-controlled, randomised, factorial cross-over study. Br J Nutr. 2014;111(11):1945–56. [DOI] [PubMed] [Google Scholar]
- 41. Ahrén IL, Hillman M, Nordström EA, Larsson N, Niskanen TM. Fewer community-acquired colds with daily consumption of Lactiplantibacillus plantarum HEAL9 and Lacticaseibacillus paracasei 8700:2. A randomized, placebo-controlled clinical trial. J Nutr. 2021;151(1):214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altadill T, Espadaler-Mazo J, Liong MT. Effects of a lactobacilli probiotic on reducing duration of URTI and fever, and use of URTI-associated medicine: a re-analysis of a randomized, placebo-controlled study. Microorganisms. 2021;9(3):528. doi: 10.3390/microorganisms9030528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chong HX, Yusoff NAA, Hor YY, Lew LC, Jaafar MH, Choi SBet al. . Lactobacillus plantarum DR7 improved upper respiratory tract infections via enhancing immune and inflammatory parameters: a randomized, double-blind, placebo-controlled study. J Dairy Sci. 2019;102(6):4783–97. [DOI] [PubMed] [Google Scholar]
- 44. Berggren A, Lazou Ahrén I, Larsson N, Önning G. Randomised, double-blind and placebo-controlled study using new probiotic lactobacilli for strengthening the body immune defence against viral infections. Eur J Nutr. 2011;50(3):203–10. [DOI] [PubMed] [Google Scholar]
- 45. de Vrese M, Winkler P, Rautenberg P, Harder T, Noah C, Laue Cet al. . Probiotic bacteria reduced duration and severity but not the incidence of common cold episodes in a double blind, randomized, controlled trial. Vaccine. 2006;24(44-46):6670–4. [DOI] [PubMed] [Google Scholar]
- 46. Gleeson M, Bishop NC, Oliveira M, Tauler P. Daily probiotic's (Lactobacillus casei Shirota) reduction of infection incidence in athletes. Int J Sport Nutr Exercise Metab. 2011;21(1):55–64. [DOI] [PubMed] [Google Scholar]
- 47. Gleeson M, Bishop NC, Oliveira M, McCauley T, Tauler P, Lawrence C. Effects of a Lactobacillus salivarius probiotic intervention on infection, cold symptom duration and severity, and mucosal immunity in endurance athletes. Int J Sport Nutr Exercise Metab. 2012;22(4):235–42. [DOI] [PubMed] [Google Scholar]
- 48. Gleeson M, Bishop NC, Struszczak L. Effects of Lactobacillus casei Shirota ingestion on common cold infection and herpes virus antibodies in endurance athletes: a placebo-controlled, randomized trial. Eur J Appl Physiol. 2016;116(8):1555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hor Y-Y, Lew L-C, Lau AS-Y, Ong J-S, Chuah L-O, Lee Y-Yet al. . Probiotic Lactobacillus caseiZhang (LCZ) alleviates respiratory, gastrointestinal & RBC abnormality via immuno-modulatory, anti-inflammatory & anti-oxidative actions. J Funct Foods. 2018;44:235–45. [Google Scholar]
- 50. Kalima K, Lehtoranta L, He L, Pitkäniemi J, Lundell R, Julkunen Iet al. . Probiotics and respiratory and gastrointestinal tract infections in Finnish military conscripts—a randomised placebo-controlled double-blinded study. Benef Microbes. 2016;7(4):463–71. [DOI] [PubMed] [Google Scholar]
- 51. Kumpu M, Kekkonen RA, Korpela R, Tynkkynen S, Järvenpää S, Kautiainen Het al. . Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo-controlled pilot trial. Benef Microbes. 2015;6(5):631–9. [DOI] [PubMed] [Google Scholar]
- 52. Tapiovaara L, Kumpu M, Mäkivuokko H, Waris M, Korpela R, Pitkäranta Aet al. . Human rhinovirus in experimental infection after peroral Lactobacillus rhamnosus GG consumption, a pilot study. Int Forum Allergy Rhinology. 2016;6(8):848–53. [DOI] [PubMed] [Google Scholar]
- 53. Michael DR, Jack AA, Masetti G, Davies TS, Loxley KE, Kerry-Smith Jet al. . A randomised controlled study shows supplementation of overweight and obese adults with lactobacilli and bifidobacteria reduces bodyweight and improves well-being. Sci Rep. 2020;10(1):4183. doi: 10.1038/s41598-020-60991-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Michalickova D, Minic R, Dikic N, Andjelkovic M, Kostic-Vucicevic M, Stojmenovic Tet al. . Appl Physiol Nutr Metab. 2016;41(7):782–9. [DOI] [PubMed] [Google Scholar]
- 55. Nishihira J, Moriya T, Sakai F, Kabuki T, Kawasaki Y, Nishimura M. Lactobacillus gasseri SBT2055 stimulates immunoglobulin production and innate immunity after influenza vaccination in healthy adult volunteers: a randomized, double-blind, placebo-controlled, parallel-group study. Funct Foods Health Dis. 2016;6(9):544. doi: 10.31989/ffhd.v6i9.284. [Google Scholar]
- 56. Pumpa KL, McKune AJ, Harnett J. A novel role of probiotics in improving host defence of elite rugby union athlete: a double blind randomised controlled trial. J Sci Med Sport. 2019;22(8):876–81. [DOI] [PubMed] [Google Scholar]
- 57. Rizzardini G, Eskesen D, Calder PC, Capetti A, Jespersen L, Clerici M. Evaluation of the immune benefits of two probiotic strains Bifidobacterium animalis ssp. lactis, BB-12® and Lactobacillus paracasei ssp. paracasei, L. casei 431® in an influenza vaccination model: a randomised, double-blind, placebo-controlled study. Br J Nutr. 2012;107(6):876–84.. doi: 10.1017/s000711451100420x. [DOI] [PubMed] [Google Scholar]
- 58. Schröder C, Schmidt S, Garbe E, Röhmel J, Giersiepen K. Effects of the regular intake of the probiotic Lactobacillus reuteri(DSM 17938) on respiratory and gastrointestinal infections in a workplace setting: a double-blind randomized placebo-controlled trial. BMC Nutr. 2015;1(1):3. doi: 10.1186/2055-0928-1-3. [Google Scholar]
- 59. Shida K, Sato T, Iizuka R, Hoshi R, Watanabe O, Igarashi Tet al. . Daily intake of fermented milk with Lactobacillus casei strain Shirota reduces the incidence and duration of upper respiratory tract infections in healthy middle-aged office workers. Eur J Nutr. 2017;56(1):45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Smith TJ, Rigassio-Radler D, Denmark R, Haley T, Touger-Decker R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br J Nutr. 2013;109(11):1999–2007. [DOI] [PubMed] [Google Scholar]
- 61. Strasser B, Geiger D, Schauer M, Gostner JM, Gatterer H, Burtscher Met al. . Probiotic supplements beneficially affect tryptophan-kynurenine metabolism and reduce the incidence of upper respiratory tract infections in trained athletes: a randomized, double-blinded, placebo-controlled trial. Nutrients. 2016;8(11):752. doi: 10.3390/nu8110752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sugimura T, Takahashi H, Jounai K, Ohshio K, Kanayama M, Tazumi Ket al. . Effects of oral intake of plasmacytoid dendritic cells-stimulative lactic acid bacterial strain on pathogenesis of influenza-like illness and immunological response to influenza virus. Br J Nutr. 2015;114(5):727–33. [DOI] [PubMed] [Google Scholar]
- 63. Tiollier E, Chennaoui M, Gomez-Merino D, Drogou C, Filaire E, Guezennec CY. Effect of a probiotics supplementation on respiratory infections and immune and hormonal parameters during intense military training. Mil Med. 2007;172(9):1006–11. [DOI] [PubMed] [Google Scholar]
- 64. Turner RB, Woodfolk JA, Borish L, Steinke JW, Patrie JT, Muehling LMet al. . Effect of probiotic on innate inflammatory response and viral shedding in experimental rhinovirus infection—a randomised controlled trial. Benef Microbes. 2017;8(2):207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vaisberg M, Paixão V, Almeida EB, Santos JMB, Foster R, Rossi Met al. . Daily intake of fermented milk containing Lactobacillus caseiShirota (Lcs) modulates systemic and upper airways immune/inflammatory responses in marathon runners. Nutrients. 2019;11(7):1678. doi: 10.3390/nu11071678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. West NP, Pyne DB, Cripps AW, Hopkins WG, Eskesen DC, Jairath Aet al. . Lactobacillus fermentum (PCC®) supplementation and gastrointestinal and respiratory-tract illness symptoms: a randomised control trial in athletes. Nutr J. 2011;10(1):30. doi: 10.1186/1475-2891-10-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zhang H, Yeh C, Jin Z, Ding L, Liu BY, Zhang Let al. . Prospective study of probiotic supplementation results in immune stimulation and improvement of upper respiratory infection rate. Synth Syst Biotechnol. 2018;3(2):113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hughes C, Davoodi-Semiromi Y, Colee JC, Culpepper T, Dahl WJ, Mai Vet al. . Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: a randomized, double-blind, controlled trial in healthy university students. Am J Clin Nutr. 2011;93(6):1305–11. [DOI] [PubMed] [Google Scholar]
- 69. Nieman DC, Henson DA, McMahon M, Wrieden JL, Davis JM, Murphy EAet al. . Beta-glucan, immune function, and upper respiratory tract infections in athletes. Med Sci Sports Exerc. 2008;40(8):1463–71. [DOI] [PubMed] [Google Scholar]
- 70. Pregliasco F, Anselmi G, Fonte L, Giussani F, Schieppati S, Soletti L. A new chance of preventing winter diseases by the administration of synbiotic formulations. J Clin Gastroenterol. 2008;42(Suppl 3):S224–33. [DOI] [PubMed] [Google Scholar]
- 71. Jäger R, Mohr AE, Carpenter KC, Kerksick CM, Purpura M, Moussa Aet al. . International Society of Sports Nutrition position stand: probiotics. J Int Soc Sports Nutr. 2019;16(1):62. doi: 10.1186/s12970-019-0329-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Simpson RJ, Campbell JP, Gleeson M, Krüger K, Nieman DC, Pyne DBet al. . Can exercise affect immune function to increase susceptibility to infection?. Exerc Immunol Rev. 2020;26:8–22. [PubMed] [Google Scholar]
- 73. Walsh NP, Oliver SJ. Exercise, immune function and respiratory infection: an update on the influence of training and environmental stress. Immunol Cell Biol. 2016;94(2):132–39. [DOI] [PubMed] [Google Scholar]
- 74. Joo J, Williamson SA, Vazquez AI, Fernandez JR, Bray MS. The influence of 15-week exercise training on dietary patterns among young adults. Int J Obesity. 2019;43(9):1681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. García-Burgos M, Moreno-Fernández J, Alférez MJM, Díaz-Castro J, López-Aliaga I. New perspectives in fermented dairy products and their health relevance. J Funct Foods. 2020;72:104059. doi: https://doi.org/10.1016/j.jff.2020.104059. [Google Scholar]
- 76. Marco ML, Sanders ME, Gänzle M, Arrieta MC, Cotter PD, De Vuyst Let al. . The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on fermented foods. Nat Rev Gastroenterol Hepatol. 2021;18(3):196–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marco ML, Heeney D, Binda S, Cifelli CJ, Cotter PD, Foligné Bet al. . Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. 2017;44:94–102.. doi: 10.1016/j.copbio.2016.11.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available upon reasonable request.