ABSTRACT
Reported breast milk lipid concentrations may vary with geographical region, postnatal age, and year of sample collection. In this review, we summarized data on the concentrations of total fat, total phospholipids, cholesterol, and fatty acids in human milk worldwide and their variation according to lactation stage, study area, and sample collection year. A systematic literature search was performed using the PubMed, Embase, Web of Science, and Medline databases for English-language papers and Wanfang and China National Knowledge Infrastructure databases for Chinese-language papers. A total of 186 studies evaluating the human milk lipid profiles were included. According to random-effects models based on worldwide data, the summarized means (95% CIs) as percentages of total fat were 42.2% (41.1%, 43.3%) for SFAs, 36.6% (35.6%, 37.5%) for MUFAs, and 21.0% (19.3%, 22.7%) for PUFAs. However, the study heterogeneity was high for most types of fatty acids (I2 > 99%). Human milk from Western countries had higher concentrations of MUFAs and 18:1n–9 (ω-9), but lower concentrations of PUFAs, 18:2n–6, 20:4n–6, 18:3n–3, 20:5n–3, 22:6n–3, and total n–6 PUFA compared with those from non-Western countries (P < 0.001–0.011). Significant lactation stage differences were observed for total fat and some individual fatty acids. The concentrations of SFAs and 16:0 were significantly negatively correlated with sampling year (P < 0.001–0.028). In contrast, a significant positive correlation between the concentrations of 18:2n–6 and 18:3n–3 and sampling year was observed (P < 0.001–0.035). Our results suggest that the pooling of data on human milk lipid profiles in different studies should be done with caution due to the high between-study heterogeneity. The concentration of lipids, including total fat, cholesterol, and specific fatty acids, differs in human milk according to lactation stage, geographical region, and year of sample collection.
Keywords: human milk, lipids, fat, fatty acids, breastfeeding, infants
Statement of Significance: This review study compiled the published concentrations of total fat, phospholipids, cholesterol, and individual fatty acids in human milk and investigated their variation with respect to geographical area, lactation stage, and year of sample collection. We found that the pooling of data on human milk lipid profiles in different studies should be done with caution owing to the high heterogeneity across studies. The concentration of lipids, including total fat, cholesterol, and specific fatty acids, differs in human milk according to lactation stage, geographical region, and year of sample collection.
Introduction
Lipids are present in human milk in the form of fat globules, which mainly consist of triglycerides surrounded by a structural membrane composed of phospholipids, cholesterol, proteins, and glycoproteins. Fat from human milk provides ∼50–60% of the energy intake of young infants, as well as providing essential fatty acids (FAs) and fat-soluble vitamins (1). Triacylglycerols make up 98–99% of the total fat content of human milk and infant formulae. Their properties depend on the length and degree of unsaturation of the FAs esterified to the glycerol backbone (2). The most widely studied FAs in human milk are the long-chain PUFAs. Although some epidemiologic studies have found that children exposed to higher PUFA concentrations in breast milk exhibited better mental development (3, 4), others showed that extremely high concentrations of some subtypes or total PUFAs in colostrum were associated with poor motor and cognitive scores (5), increased risks of developing allergic rhinitis and eczema (6), and other negative outcomes (e.g., sensitization, reduced lung function, and fat mass growth) (6, 7). Therefore, information on lipid profiles can provide guidance for defining optimal nutrient intakes for infants and can serve as the basis for the development of infant formulae.
Many individual studies worldwide have investigated the FAs, total fat, phospholipid, and cholesterol contents of human milk. There have also been some pooled data analyses of lipids in human milk, but these have only focused on specific FAs, like EPA and DHA (8, 9), total fat (10), or phospholipids (11). The composition of human milk changes dynamically with feeding, time of day, and lactation period. It also varies between individual mothers and between women of different ethnicities, and it is modulated by the maternal diet (12). Fat is one of the most variable nutrients in human milk. However, a consolidated lipid profile, based on multiple studies and reflecting geographical differences and changes through the progression of lactation and over different decades, is not yet available.
We conducted a systematic review and meta-analysis of studies of breast milk lipid content (fats, phospholipids, cholesterol, and FAs) to determine the lipid concentrations around the world and also applied meta-regression to evaluate whether lactation period, year of sample collection, and geographical location should be considered when analyzing the lipid content of breast milk.
Methods
Literature search and inclusion and exclusion criteria
A systematic literature search, up to March 2021, was performed using the PubMed, Embase, Web of Science, and Medline databases for English-language papers and Wanfang and China National Knowledge Infrastructure databases for Chinese-language papers. The following search terms were used: (fatty acid* OR lipid* OR fat OR phospholipid* OR cholesterol* OR triacylglyceride) AND (human milk OR breastmilk OR breast-milk OR breast milk). Further information was retrieved through a manual search of references from recent reviews (including meta-analyses) and relevant published original studies, as well as through searches of Google Scholar (https://scholar.google.com.hk/). The protocol has been registered on the INPLASY website, and the registration number is INPLASY202240079.
Study selection
Three reviewers (HC, MS, and JZ) independently extracted the data. Discrepancies were resolved by group discussions. Studies were eligible for inclusion if they reported the total fat, phospholipid, cholesterol, and/or FA composition of human milk. Studies were included in this meta-analysis if they 1) analyzed 24-h milk samples or single samples from healthy mothers, with data reported as g/100 g or g/100 mL for total fat, phospholipids, and cholesterol, and percentage of total FAs for fatty acids; 2) contained data presented as means or medians, with the SEM, SD, range, 95% CI, and/or interquartile range; 3) measured FA concentrations by high-performance liquid chromatography, gas chromatography, or gas–liquid chromatography; and 4) were written in English or Chinese. Studies were excluded if they 1) used donor human milk samples that underwent additional processing; 2) had samples with an unidentified type or lactation stage; 3) pooled samples from multiple mothers or lactation stages; 4) were maternal dietary restriction studies or studies of mothers with diseases, such as gestational diabetes, preeclampsia, or HIV; or 5) were reviews or reported data measured using a human milk analyzer. For multiple reports from the same study, we included the most recent and/or most complete study. References from the retrieved articles were manually screened for additional eligible studies.
Data extraction and standardization
Human milk lipid profile data including the concentrations of total fat; phospholipids; cholesterol; the SFAs 6:0, 8:0, 10:0, 12:0, 13:0, 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 22:0, and 24:0; the MUFAs 16:1n–7, 18:1n–9, 20:1n–9, and 22:1n–9; and the PUFAs 18:2n–6 [linoleic acid (LA)], 18:3n–6, 20:2n–6, 20:3n–6, 20:4n–6 [arachidonic acid (ARA)], 18:3n–3 [α-linolenic acid (ALA)], 20:3n–3, 20:5n–3 (EPA), 22:6n–3 (DHA), total n–6, and total n–3 were extracted. When data were reported as the mean ± SEM, mean (95% CI), or median (interquartile range), we standardized them to the same units (mean ± SD) for further analysis. Following Wan et al. (13), we used a formula to transform medians to means and full ranges or interquartile ranges to SDs, whereas 95% CIs were transformed to SDs according to the formula recommended by the Cochrane Handbook (14). For studies with repeated observations from the same participants, the overall means and SDs incorporating all time points were calculated first and subsequently used for further analysis as recommended by the Cochrane Handbook (14).
Statistical analyses
Data analyses were performed using 2 methods. First, the inverse-variance weighting method was applied using the “rma.uni” function of the Metafor package of the statistical software R Studio (version 1.1.383, 2009–17), which has been previously validated (15). For total fat, phospholipid, cholesterol, and individual FA concentrations, the inverse-variance weighted means and 95% CIs were calculated. Heterogeneity between studies was assessed using the I2 and τ2 parameters (14). Fixed-effects models were used to pool data when heterogeneity was low or moderate (I2 < 50%), and random-effects models were performed when heterogeneity was high (I2 ≥ 50%). A multivariate meta-regression model was applied to investigate the associations of lipid concentrations with lactation stage (colostrum: 0–6 d postpartum, transitional: 7–14 d postpartum, mature milk: ≥15 d postpartum or overall mean of repeated measures across different lactation stages), year of sample collection, and geographical region. For studies that did not report the year of sample collection, the publication year was used instead. Logarithmic or exponential transformation was performed based on the characteristics of the data so that the lower limits of 95% CIs of the predicted values would not be negative. For the geographical region, 2 groups of countries were included in the analysis. One group (“Western”) includes studies from North America, Europe, Australia, and New Zealand. The second (“non-Western”) includes studies from all other countries (from South America, Asia, and Africa). We also performed subgroup analysis based on lactation stages (colostrum, transitional, or mature milk) to identify potential sources of heterogeneity, and the group differences were estimated. In addition, the overall SD values were calculated based on the SDs of all eligible studies using the equations of “combining groups” recommended by the Cochrane Handbook (14) and the study of Zhang et al. (16) as follows:
Combined SD2 = [(A1 + A2 + A3 + … + Ai) − (M1·n1 + M2·n2 + M3·n3 + … + Mi·ni)2/(n1 + n2 + n3 + …+ ni)] / (N − 1);
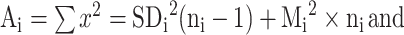
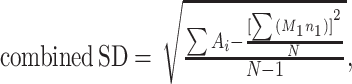
where ni and Mi are the number of subjects and mean value of study i, respectively, and SDi is the standard deviation of study i.
Results
The search yielded 17,526 reports (Supplemental Figure 1). After reviewing the abstracts and titles, we selected 269 articles for full-text review. Of these, 183 published studies met our inclusion criteria (5, 12, 17–197), and 3 unpublished studies in China were also included. The detailed information of these studies is presented in Supplemental Table 1.
The lipid subtype concentrations and FA composition in human milk
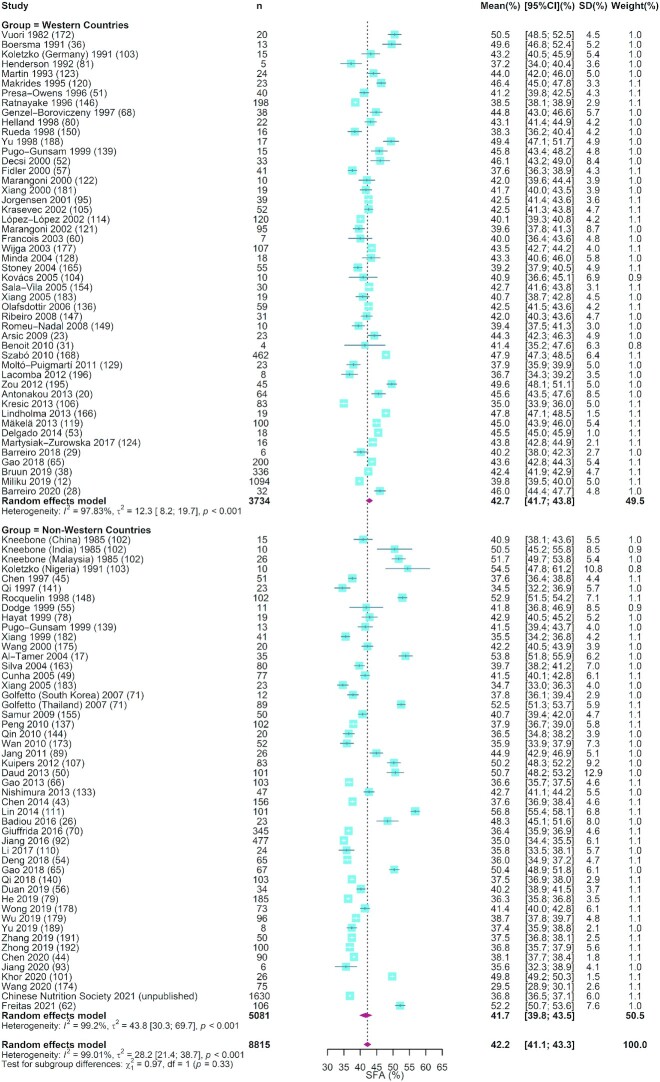
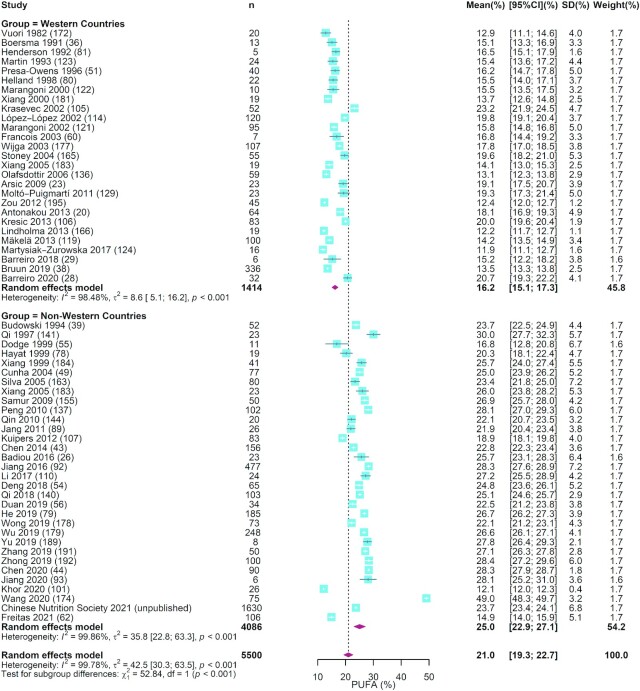
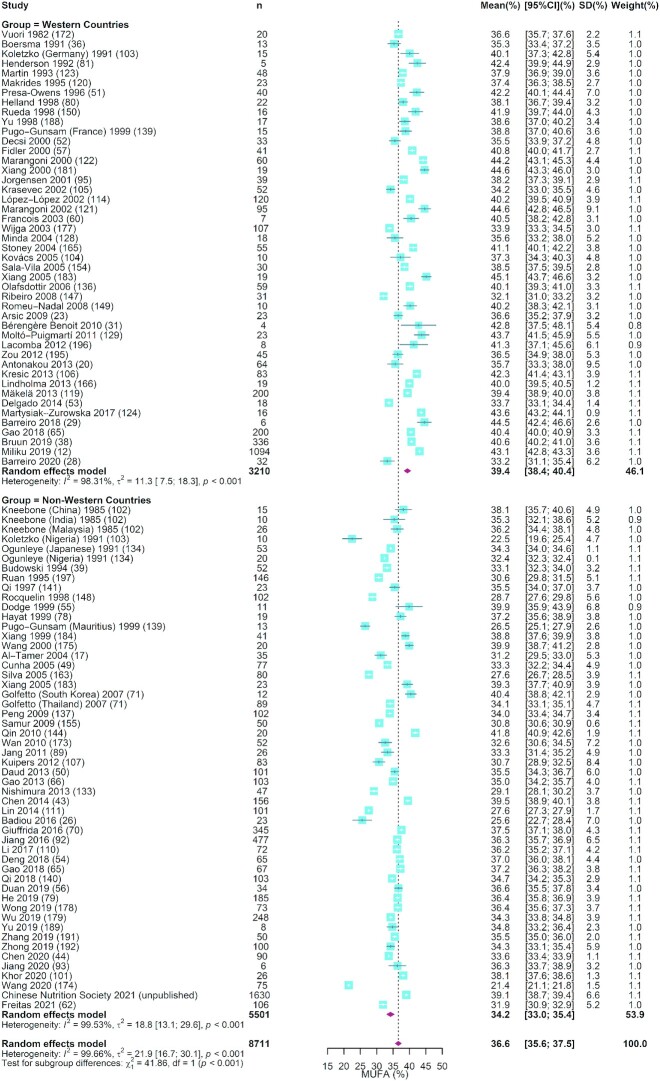
As shown in Table 1, our analysis of 34 studies that measured the total fat in 14,984 human milk samples showed that the mean (95% CI) for total fat concentration was 3.40 g/100 mL (3.13, 3.66 g/100 mL). Eleven studies with a total of 14,216 samples reported the concentration of phospholipids in human milk. The summarized mean (95% CI) was 34.1 mg/100 mL (26.5, 41.6 mg/100 mL). For SFAs, MUFAs, and PUFAs, the mean (95% CI) values were 42.2% (41.1%, 43.3%), 36.6% (35.6%, 37.5%), and 21.0% (19.3%, 22.7%), respectively (Table 1 and Figures 1 –3). In terms of the individual FAs, 18:1n–9 was the most abundant (32.7%; 95% CI: 31.7%, 33.7%), followed by 16:0 (22.1%; 95% CI: 21.5%, 22.7%) and then 18:2n–6 (15.3%; 95% CI: 14.4%, 16.2%), which together made up ∼70% of the total FA content. The corresponding estimates for 20:4n–6, 20:5n–3, and 22:6n–3 were 0.57% (0.53%, 0.60%), 0.13% (0.10%, 0.15%), and 0.42% (0.38%, 0.46%), respectively. The forest plots for these FAs are shown in Supplemental Figures 2–8. The studies included in most of the estimates had high I2 values (97.0%–100%), indicating a high level of heterogeneity between studies (Table 1).
TABLE 1.
Subtypes of lipids and the fatty acids composition in human milk around the world
| Variable | n 1 | K2 | Mean (95% CI) | SD | I 2 , % | Study regions | ||
|---|---|---|---|---|---|---|---|---|
| β | SE | P value3 | ||||||
| Total fat, g/100 mL | 34 | 14,984 | 3.40 (3.13, 3.66) | 0.90 | 97.0 | 0.429 | 0.265 | 0.105 |
| Phospholipid, mg/100 mL | 11 | 14,216 | 34.1 (26.5, 41.6) | 9.94 | 99.0 | –14.9 | 12.7 | 0.243 |
| Cholesterol, mg/100 mL | 10 | 1198 | 16.0 (12.0, 20.0) | 6.17 | 99.3 | 2.35 | 5.38 | 0.662 |
| SFA, % | 90 | 8815 | 42.2 (41.1, 43.3) | 7.60 | 99.0 | –0.279 | 1.10 | 0.799 |
| 6:0 | 20 | 1411 | 0.11 (0.06, 0.17) | 0.45 | 99.6 | –0.094 | 0.066 | 0.157 |
| 8:0 | 53 | 4482 | 0.20 (0.15, 0.24) | 0.21 | 99.4 | 0.030 | 0.052 | 0.567 |
| 10:0 | 84 | 9942 | 1.29 (1.17, 1.40) | 0.86 | 99.2 | 0.063 | 0.110 | 0.569 |
| 12:0 | 102 | 10,800 | 5.34 (4.95, 5.72) | 2.88 | 98.9 | 0.586 | 0.381 | 0.124 |
| 13:0 | 24 | 2261 | 0.09 (0.02, 0.16) | 0.14 | 98.9 | 0.130 | 0.092 | 0.158 |
| 14:0 | 108 | 11,006 | 6.47 (6.13, 6.81) | 2.98 | 98.2 | 0.153 | 0.345 | 0.658 |
| 15:0 | 61 | 5307 | 0.25 (0.23, 0.28) | 0.16 | 99.3 | –0.111 | 0.022 | <0.001 |
| 16:0 | 113 | 11,164 | 22.1 (21.5, 22.7) | 4.74 | 99.0 | 0.400 | 0.584 | 0.494 |
| 17:0 | 63 | 8538 | 0.31 (0.29, 0.33) | 0.34 | 99.1 | –0.032 | 0.023 | 0.162 |
| 18:0 | 113 | 11,150 | 6.34 (6.13, 6.54) | 2.03 | 98.5 | –0.957 | 0.185 | <0.001 |
| 20:0 | 82 | 7933 | 0.23 (0.21, 0.26) | 0.27 | 99.2 | 0.011 | 0.024 | 0.647 |
| 22:0 | 64 | 6447 | 0.13 (0.11, 0.15) | 0.17 | 98.5 | 0.041 | 0.023 | 0.078 |
| 24:0 | 55 | 6295 | 0.12 (0.10, 0.14) | 0.11 | 98.6 | 0.045 | 0.020 | 0.027 |
| MUFA, % | 90 | 8711 | 36.6 (35.6, 37.5) | 6.48 | 99.7 | –5.56 | 0.805 | <0.001 |
| 16:1n–7 | 52 | 6822 | 2.30 (2.11, 2.48) | 0.93 | 98.9 | 0.059 | 0.194 | 0.760 |
| 18:1n–9 | 72 | 7473 | 32.7 (31.7, 33.7) | 5.83 | 99.5 | –4.55 | 0.983 | <0.001 |
| 20:1n–9 | 60 | 5757 | 0.53 (0.47, 0.59) | 0.37 | 99.3 | 0.009 | 0.051 | 0.868 |
| 22:1n–9 | 40 | 5526 | 0.18 (0.13, 0.22) | 0.67 | 98.3 | 0.093 | 0.045 | 0.039 |
| PUFA, % | 58 | 5500 | 21.0 (19.3, 22.7) | 7.76 | 99.8 | 8.22 | 1.45 | <0.001 |
| 18:2n–6 | 113 | 12,433 | 15.3 (14.4, 16.2) | 6.84 | 99.7 | 4.56 | 0.796 | <0.001 |
| 18:3n–6 | 85 | 9672 | 0.17 (0.14, 0.21) | 0.22 | 99.4 | 0.040 | 0.039 | 0.312 |
| 20:2n–6 | 73 | 6367 | 0.48 (0.43, 0.53) | 0.36 | 99.2 | 0.137 | 0.033 | <0.001 |
| 20:3n–6 | 99 | 10,702 | 0.44 (0.41, 0.47) | 0.23 | 98.9 | 0.038 | 0.026 | 0.154 |
| 20:4n–6 | 117 | 12,485 | 0.57 (0.53, 0.60) | 0.30 | 99.2 | 0.116 | 0.032 | <0.001 |
| 18:3n–3 | 118 | 12,596 | 1.07 (0.98, 1.17) | 1.00 | 99.6 | 0.326 | 0.098 | 0.001 |
| 20:3n–3 | 46 | 5579 | 0.11 (0.07, 0.15) | 0.14 | 98.9 | 0.082 | 0.042 | 0.051 |
| 20:5n–3 | 95 | 10,288 | 0.13 (0.10, 0.15) | 0.62 | 98.3 | 0.075 | 0.029 | 0.011 |
| 22:6n–3 | 115 | 12,204 | 0.42 (0.38, 0.46) | 0.32 | 99.7 | 0.203 | 0.036 | <0.001 |
| Total n–6 | 75 | 9045 | 17.5 (16.1, 18.9) | 7.22 | 100.0 | 6.18 | 1.39 | <0.001 |
| Total n–3 | 75 | 8237 | 1.93 (1.63, 2.23) | 2.29 | 99.7 | 0.219 | 0.351 | 0.532 |
n: total number of studies.
K: total sample size.
P: derived from meta-regression after adjustment for sample size, lactation stages, and year of sample collection.
FIGURE 1.
The forest plot of meta-analysis of studies assessing the concentration of SFAs in human milk. The blue squares represent the weighted mean difference in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The red diamonds indicate the summary weighted mean difference. n, sample size.
FIGURE 3.
The forest plot of meta-analysis of studies assessing the concentration of PUFAs in human milk. The blue squares represent the weighted mean difference in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The red diamonds indicate the summary weighted mean difference. n, sample size.
Variations in lipid subtype concentrations and FA composition in human milk across regions, lactation stages, and year of sample collection
Meta-regression analysis showed that the study heterogeneity could be attributed to differences across regions, lactation stages, and year of sample collection. As shown in Table 1, in comparison with nursing women in non-Western countries, those in Western countries had higher concentrations of 15:0, MUFAs, and 18:1n–9 but lower concentrations of PUFAs, 18:2n–6, 20:4n–6, 18:3n–3, 20:5n–3, 22:6n–3, and total n–6 PUFA in human milk (P < 0.001–0.011). In contrast, no significant regional difference was detected for SFAs (P = 0.799) and most other subtypes of FAs. The summarized mean values for SFAs, MUFAs, PUFAs, 16:0, 18:1n–9, 18:2n–6, 20:4n–6, 18:3n–3, 22:5n–3, 22:6n–3, and total n–6 PUFA in Western and non-Western countries were 42.7% and 41.7% (Figure 1), 39.4% and 34.2% (Figure 2), 16.2% and 25.0% (Figure 3), 22.1% and 22.1% (Supplemental Figure 2), 34.7% and 30.8% (Supplemental Figure 3), 12.8% and 18.1% (Supplemental Figure 4), 0.51% and 0.63% (Supplemental Figure 5), 0.88% and 1.29% (Supplemental Figure 6), 0.09% and 0.17% (Supplemental Figure 7), 0.33% and 0.53% (Supplemental Figure 8), and 14.6% and 21.0% (Supplemental Figure 9), respectively.
FIGURE 2.
The forest plot of meta-analysis of studies assessing the concentration of MUFAs in human milk. The blue squares represent the weighted mean difference in each study, with square size reflecting the study-specific weight and the 95% CI represented by horizontal bars. The red diamonds indicate the summary weighted mean difference. n, sample size.
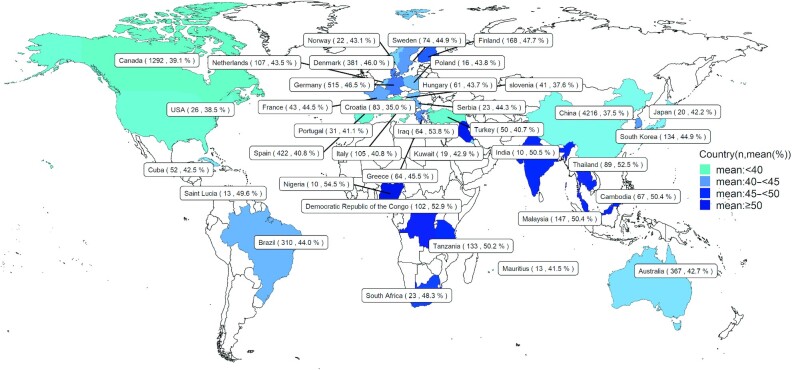
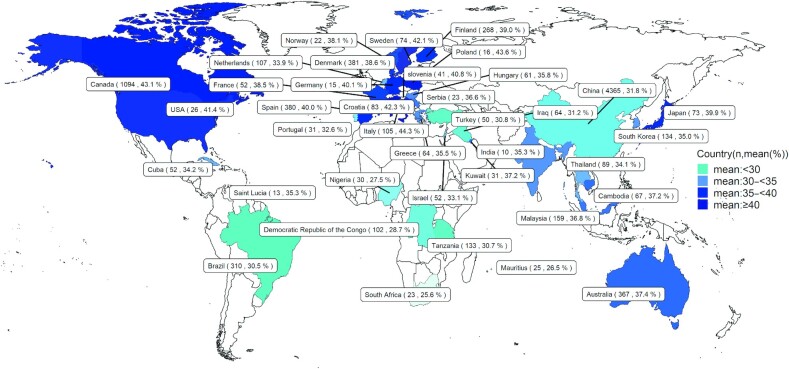
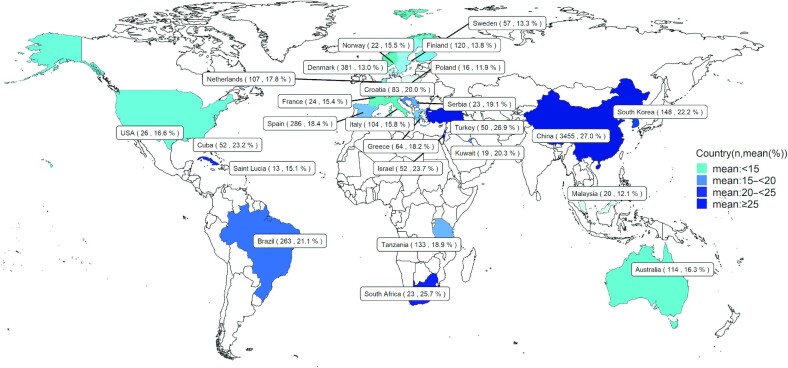
In different regions around the world, the summarized mean values for SFAs, MUFAs, and PUFAs ranged from 35.0% to 54.5% (Figure 4), 25.6% to 44.3% (Figure 5), and 11.9% to 27.0% (Figure 6), respectively. The means for 16:0, 18:1n–9, 18:2n–6, 20:4n–6, 18:3n–3, 22:5n–3, and 22:6n–3 in different regions are also listed in Supplemental Figures 10–16.
FIGURE 4.
World map for the total sample size and concentration of SFAs in human milk across different countries. n, sample size.
FIGURE 5.
World map for the total sample size and concentration of MUFAs in human milk across countries. n, sample size.
FIGURE 6.
World map for the total sample size and concentration of PUFAs in human milk across countries. n, sample size.
Table 2 lists the changes in lipid subtype concentrations and FA composition in human milk across different lactation stages. The total fat concentration in colostrum (2.34 g/100 mL; 95% CI: 2.07, 2.61 g/100 mL) was significantly lower than in transitional (3.22 g/100 mL; 95% CI: 2.91, 3.54 g/100 mL) and mature milk (3.61 g/100 mL; 95% CI: 3.37, 3.86 g/100 mL) (P < 0.001). Based on a limited number of studies, the cholesterol concentration in human milk significantly decreased across successive stages of lactation (P < 0.001). Palmitic acid (16:0) is the predominant SFA in human milk. The summarized mean (95% CI) values for 16:0 were 23.6% (22.8%, 24.5%), 21.5% (20.6%, 22.5%), and 21.5% (20.9%, 22.2%) for the colostrum, transitional, and mature milk stages (P < 0.001), respectively. The corresponding values for 20:4n–6 and 22:6n–3 were 0.78% (0.74%, 0.83%) and 0.59% (0.52%, 0.65%) in colostrum, respectively; 0.63% (0.57%, 0.69%) and 0.48% (0.42%, 0.55%) in transition milk, respectively; and 0.50% (0.47%, 0.53%) and 0.37% (0.33%, 0.40%) in mature milk, respectively (P < 0.001). No significant changes in the total SFA, MUFA, PUFA, 15:0, 17:0, 18:1n–9, 18:2n–6, 18:3n–3, or 20:5n–3 concentrations were observed between different lactation stages.
TABLE 2.
Differences in lipid subtype concentrations and fatty acid compositions across lactation stages of women throughout the world
| Variable | Colostrum | Transition | Mature | P value3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n 1 | K2 | Mean (95% CI) | SD | I 2 , % | n 1 | K2 | Mean (95% CI) | SD | I 2 , % | n 1 | K2 | Mean (95% CI) | SD | I 2 , % | ||
| Total fat, g/100 mL | 13 | 533 | 2.34 (2.07, 2.61) | 1.20 | 89.6 | 13 | 555 | 3.22 (2.91, 3.54) | 1.14 | 96.6 | 30 | 14,523 | 3.61 (3.37, 3.86) | 0.90 | 96.5 | <0.001 |
| Phospholipid, mg/100 mL | 8 | 395 | 43.1 (30.4, 55.7) | 23.8 | 99.1 | 8 | 383 | 40.2 (28.0, 52.5) | 26.0 | 98.7 | 10 | 13,475 | 30.7 (24.4, 37.0) | 7.79 | 98.6 | 0.134 |
| Cholesterol, mg/100 mL | 2 | 48 | 30.9 (23.7, 38.0) | 9.57 | 65.2 | 1 | 11 | 19.7 (15.6, 23.8) | 7.00 | NA4 | 9 | 1162 | 14.0 (11.8, 16.1) | 5.55 | 99.1 | <0.001 |
| SFA, % | 44 | 2406 | 40.0 (38.6, 41.5) | 6.54 | 98.4 | 25 | 1524 | 40.6 (38.6, 42.5) | 6.57 | 99.0 | 86 | 7754 | 42.2 (41.0, 43.3) | 7.73 | 99.1 | 0.059 |
| 6:0 | 9 | 461 | 0.06 (0.02, 0.10) | 0.06 | 99.7 | 6 | 292 | 0.10 (0.03, 0.16) | 0.07 | 99.7 | 19 | 1252 | 0.13 (0.07, 0.20) | 0.47 | 99.6 | 0.159 |
| 8:0 | 23 | 1061 | 0.08 (0.06, 0.10) | 0.13 | 98.2 | 13 | 596 | 0.22(0.14, 0.30) | 0.13 | 98.9 | 59 | 3833 | 0.21 (0.17, 0.26) | 0.22 | 99.5 | <0.001 |
| 10:0 | 37 | 2081 | 0.69 (0.57, 0.82) | 0.50 | 99.7 | 23 | 1477 | 1.29 (1.07, 1.51) | 0.64 | 99.5 | 94 | 8938 | 1.37 (1.25, 1.48) | 0.86 | 99.4 | <0.001 |
| 12:0 | 46 | 2376 | 3.15 (2.79, 3.50) | 1.99 | 98.3 | 29 | 1572 | 5.31 (4.66, 5.97) | 2.61 | 99.1 | 108 | 9531 | 5.70 (5.28, 6.11) | 2.81 | 99.2 | <0.001 |
| 13:0 | 11 | 565 | 0.05 (0.02, 0.08) | 0.06 | 98.3 | 7 | 292 | 0.09 (0.03, 0.14) | 0.09 | 99.0 | 32 | 1927 | 0.10 (0.03, 0.17) | 0.15 | 99.3 | 0.345 |
| 14:0 | 53 | 2582 | 5.29 (4.97, 5.61) | 2.01 | 96.2 | 32 | 1616 | 6.31(5.70, 6.92) | 2.31 | 96.3 | 113 | 9653 | 6.56 (6.16, 6.95) | 3.05 | 98.6 | <0.001 |
| 15:0 | 28 | 1317 | 0.23 (0.18, 0.27) | 0.18 | 99.2 | 16 | 781 | 0.21 (0.17, 0.24) | 0.13 | 98.3 | 72 | 4465 | 0.25 (0.23, 0.28) | 0.16 | 99.3 | 0.136 |
| 16:0 | 53 | 2589 | 23.6 (22.8, 24.5) | 3.79 | 99.5 | 34 | 1636 | 21.5 (20.6, 22.5) | 4.62 | 99.3 | 117 | 9838 | 21.5 (20.9, 22.2) | 4.82 | 99.3 | <0.001 |
| 17:0 | 30 | 2608 | 0.30 (0.27, 0.34) | 0.51 | 99.0 | 19 | 1304 | 0.29 (0.25, 0.32) | 0.36 | 96.7 | 70 | 5772 | 0.31 (0.28, 0.33) | 0.15 | 98.8 | 0.656 |
| 18:0 | 55 | 2638 | 6.27 (5.97, 6.56) | 2.20 | 97.7 | 34 | 1633 | 6.21 (5.74, 6.68) | 1.82 | 99.2 | 118 | 9783 | 6.36 (6.14, 6.58) | 2.07 | 98.7 | 0.810 |
| 20:0 | 37 | 1572 | 0.23 (0.20, 0.26) | 0.15 | 98.0 | 22 | 929 | 0.25 (0.19, 0.31) | 0.62 | 99.1 | 88 | 6798 | 0.23 (0.21, 0.26) | 0.17 | 99.1 | 0.847 |
| 22:0 | 31 | 1385 | 0.14 (0.11, 0.17) | 0.13 | 98.1 | 16 | 576 | 0.11 (0.09, 0.13) | 0.06 | 98.1 | 72 | 5685 | 0.12 (0.10, 0.14) | 0.17 | 98.8 | 0.221 |
| 24:0 | 30 | 1405 | 0.21 (0.17, 0.24) | 0.17 | 97.4 | 17 | 600 | 0.13 (0.10, 0.15) | 0.08 | 97.1 | 64 | 5489 | 0.10 (0.08, 0.11) | 0.09 | 98.8 | <0.001 |
| MUFA, % | 44 | 2348 | 37.8 (36.4, 39.1) | 7.17 | 99.5 | 23 | 1435 | 35.8 (34.0, 37.6) | 6.16 | 99.7 | 86 | 7404 | 36.3 (35.3, 37.4) | 6.46 | 99.7 | 0.143 |
| 16:1n–7 | 24 | 1094 | 1.86 (1.68, 2.05) | 0.76 | 96.4 | 17 | 614 | 2.09 (1.87, 2.31) | 0.67 | 96.8 | 59 | 6432 | 2.3 (2.11, 2.49) | 0.92 | 98.9 | 0.005 |
| 18:1n–9 | 35 | 1859 | 33.7 (32.3, 35.1) | 7.05 | 99.5 | 25 | 1274 | 32.5 (30.8, 34.1) | 7.41 | 99.7 | 75 | 6892 | 32.6 (31.5, 33.7) | 5.84 | 99.6 | 0.416 |
| 20:1n–9 | 30 | 2541 | 0.80 (0.69, 0.91) | 0.39 | 99.8 | 22 | 1219 | 0.51 (0.42, 0.60) | 0.29 | 99.1 | 64 | 4378 | 0.46 (0.41, 0.50) | 0.28 | 99.2 | <0.001 |
| 22:1n–9 | 22 | 1456 | 0.25 (0.20, 0.29) | 0.32 | 98.5 | 16 | 1063 | 0.19 (0.13, 0.24) | 0.42 | 97.9 | 49 | 4984 | 0.18 (0.12, 0.23) | 0.69 | 99.7 | 0.131 |
| PUFA, % | 29 | 1848 | 22.5 (19.9, 25.2) | 8.71 | 99.8 | 17 | 1281 | 24.5 (20.7, 28.3) | 8.43 | 99.9 | 53 | 4512 | 21.2 (19.3, 23.1) | 8.18 | 99.8 | 0.287 |
| 18:2n–6 | 52 | 3395 | 15.3 (13.6, 17.0) | 7.19 | 99.7 | 27 | 1482 | 18.0 (15.5, 20.5) | 7.83 | 99.8 | 117 | 10,214 | 15.7 (14.7, 16.7) | 7.15 | 99.7 | 0.193 |
| 18:3n–6 | 44 | 3369 | 0.12 (0.08, 0.15) | 0.16 | 99.4 | 19 | 788 | 0.13 (0.09, 0.18) | 0.10 | 98.6 | 85 | 7593 | 0.19 (0.15, 0.23) | 0.24 | 99.3 | 0.017 |
| 20:2n–6 | 41 | 2043 | 0.90 (0.82, 0.99) | 0.4 | 98.8 | 24 | 792 | 0.59 (0.53, 0.65) | 0.23 | 97.6 | 80 | 5570 | 0.37 (0.34, 0.40) | 0.19 | 99.4 | <0.001 |
| 20:3n–6 | 51 | 3674 | 0.62 (0.58, 0.66) | 0.24 | 97.2 | 29 | 1619 | 0.50 (0.46, 0.54) | 0.21 | 97.9 | 102 | 8241 | 0.37 (0.35, 0.40) | 0.18 | 97.9 | <0.001 |
| 20:4n–6 | 56 | 3576 | 0.78 (0.74, 0.83) | 0.29 | 97.9 | 28 | 1478 | 0.63 (0.57, 0.69) | 0.24 | 98.7 | 119 | 10,139 | 0.50 (0.47, 0.53) | 0.25 | 98.6 | <0.001 |
| 18:3n–3 | 53 | 3463 | 0.95 (0.81, 1.10) | 0.96 | 99.7 | 28 | 1493 | 1.18 (0.90, 1.47) | 2.17 | 99.7 | 121 | 10,367 | 1.11 (1.00, 1.21) | 1.05 | 99.6 | 0.169 |
| 20:3n–3 | 23 | 1281 | 0.15 (0.10, 0.20) | 0.14 | 99.3 | 10 | 809 | 0.20 (0.03, 0.37) | 0.22 | 99.2 | 51 | 4949 | 0.10 (0.06, 0.14) | 0.12 | 99.2 | 0.171 |
| 20:5n–3 | 47 | 3174 | 0.14 (0.08, 0.20) | 0.44 | 99.9 | 23 | 1167 | 0.16 (0.06, 0.26) | 0.87 | 99.8 | 97 | 8366 | 0.12 (0.10, 0.15) | 0.67 | 98.9 | 0.677 |
| 22:6n–3 | 54 | 3368 | 0.59 (0.52, 0.65) | 0.3 | 98.2 | 31 | 1522 | 0.48 (0.42, 0.55) | 0.27 | 97.9 | 118 | 10,083 | 0.37 (0.33, 0.40) | 0.31 | 99.7 | <0.001 |
| Total n–6 | 37 | 2975 | 17.8 (15.6, 20.1) | 8.52 | 99.8 | 21 | 1402 | 19.8 (16.6, 22.9) | 8.00 | 99.9 | 69 | 6821 | 17.8 (16.2, 19.3) | 7.51 | 100.0 | 0.512 |
| Total n–3 | 40 | 3064 | 1.97 (1.70, 2.24) | 0.87 | 99.9 | 23 | 1502 | 1.85 (1.48, 2.21) | 2.41 | 99.9 | 68 | 5933 | 1.88 (1.55, 2.21) | 2.63 | 99.8 | 0.846 |
n: total number of studies.
K: total sample size.
P: for subgroup difference.
NA: not applicable.
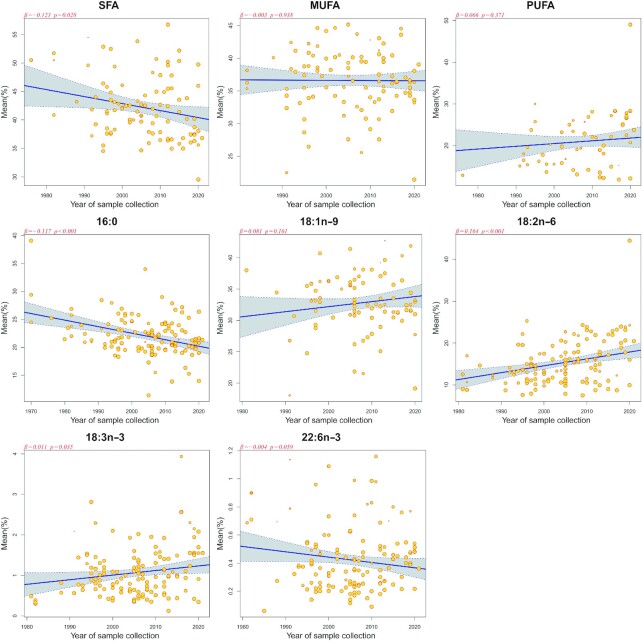
There were significant inverse associations between the concentrations of SFAs and 16:0 and year of sample collection (P < 0.001–0.028), that is, they were lower in more recent studies (Figure 7). Similar associations were detected for 15:0 and 17:0 (Supplemental Figure 18). In contrast, a significant positive correlation was observed between the concentrations of 18:2n–6 and 18:3n–3 and year of sample collection (P < 0.001–0.035). No significant change in concentration across sample collection year was detected for most of the other subtypes of fatty acids (Supplemental Figures 17–20).
FIGURE 7.
The meta-regression analysis on the relation between selected fatty acids concentration in human milk and year of sample collection. β and P values were derived from meta-regression analysis after adjustment for sample size, study regions, and lactation stages. Each circle represents a study, and the size of the circle represents the weight of the study.
Discussion
This study evaluated human milk lipid profiles worldwide and assessed whether the study region, lactation stage, and year of sample collection influenced the pooled estimates. The results showed substantial heterogeneity among the included studies for most of the lipid profiles. Meta-regression indicated that the study region, lactation stage, and year of sample collection significantly contributed to the variation in lipid profiles.
Several systematic reviews have previously assessed specific FAs such as EPA (20:5n–3) and DHA (22:6n–3) (8, 9), total fat (10), or phospholipids (11) in human milk. Using the mean and SD of 84 mean values from 65 studies, Brenna et al. (9) reported that the mean ± SD concentration of DHA in breast milk was 0.32% ± 0.22% and that of ARA (20:4n–6) was 0.47% ± 0.13%. Floris et al. (8) applied the inverse-variance weighted method to pooled data and found that the mean ± SEM concentrations of DHA and ARA were 0.51% ± 0.04% and 0.77% ± 0.04%, respectively. In the present data, the mean values for those 2 FAs were 0.43% and 0.58%, respectively. Different methods for pooling data are one of the reasons for study differences. To our knowledge, ours is the most comprehensive study thus far to evaluate the composition of human milk lipids and to determine the associations between these estimates and study regions, lactation stages, and sampling year. Yuhas et al. (190) compared the FA contents of human milk across 9 countries; the ARA concentrations were reported to range between 0.36% and 0.49%, with the lowest concentration in the United Kingdom and the highest in China. In agreement with that study, we also observed that region was a significant determinant of the variations in FA content. The stage of lactation is another important factor affecting the concentration of fat in human milk. Herein, the concentrations of total fat, cholesterol, and some individual FAs (16:0, 20:4n–6, and 22:6n–3) in mature milk were significantly lower than those in colostrum, which is consistent with the pooled analysis reported by Floris et al. (8).
FAs in breast milk are derived from 3 sources: the diet, adipose tissue, and endogenous synthesis. A recent review by Keikha et al. (198) reported that maternal dietary intake, particularly the intake of FAs, was related to the FA composition of breast milk (199, 200). Dietary sources of LA (18:2n–6) and ALA (18:3n–3) include flaxseeds and flaxseed oil, walnuts and walnut oil, soybeans and soybean oil, pumpkin seeds, rapeseed (canola) oil, and olive oil (201). The principal sources of dietary EPA and DHA are oily fish, fish oil, and certain types of seafood (202). Rich sources of dietary SFAs include butter, animal fat, and dairy products. A systematic review that analyzed FA intake data from 40 countries showed that total fat intake contributed 11.1–46.2% of energy intake, SFAs contributed 2.9–20.9%, and PUFAs contributed 2.8–11.3% (203). In a systematic analysis including 266 country-specific nutrition surveys, Micha et al. (204) reported that country-specific n–6 consumption contributed 1.2–12.5% of energy intake; meanwhile, the total consumption amounts of other fat sources were 97–440 mg/d for dietary cholesterol, 5–3886 mg/d for seafood-derived n–3, and <100–5542 mg/d for plant-derived n–3. In many places, lactating women traditionally consume considerable quantities of plant oils (e.g., in China), seafood (e.g., in Japan), or dairy products or animal foods (e.g., in Western countries), leading to a relatively high concentration of PUFAs in human milk. According to the traditional practices of postpartum care, known as zuoyuezi in China, mothers are encouraged to increase their intake of meat and eggs (205). The dietary practices in different postpartum periods may contribute to the variations of lipid concentrations in breast milk.
Supplement use is another source of EPA and DHA in lactating women. Using a stable-isotope method, Fidler et al. (58) observed that 2 wk of DHA supplementation resulted in an almost 2-fold increase in human milk DHA concentration (0.37% compared with 0.21%, P = 0.003). The total ARA concentration in human milk was found to increase with increasing ARA supplementation, from 0.4% (no ARA) to 0.49% (200 mg/d of ARA) and up to 0.56% (400 mg/d of ARA) of the total FA content at the end of the 8-wk intervention period (206). Other double-blind placebo-controlled randomized trials have also found that the concentrations of ARA and DHA in human milk are sensitive to maternal supplementation (207–209). Therefore, supplement use in different countries or lactation stages may be another reason for the lipid profile differences in breast milk. However, no information on supplement use was reported in most of the included studies in this review, so we cannot evaluate its influence.
In addition to the maternal diet, the concentrations of DHA and ARA in breast milk also depend on their level of biosynthesis from precursors. Previous intervention studies in human adults have demonstrated that the consumption of an additional 3–40 g/d ALA for 3.2–42 wk induces a –27% to 250% change in the EPA concentration and a 0% to 21% change in the DHA concentration (210). Stable-isotope studies have estimated various conversion rates of ALA into EPA, ranging from 0.2% to 8% (211). Overall, the most common dietary SFAs are 16:0 and stearic acid (18:0); however, these SFAs can also be synthesized endogenously (212).
De novo lipogenesis (DNL) is a complex and highly regulated process for the endogenous synthesis of trigly-cerides and other lipids from dietary starch, sugar, and protein. Palmitic acid (16:0) is the major FA product of DNL and can be elongated to stearic acid (18:0), which can then be desaturated to form palmitoleic acid (16:1n–7) or converted to oleic acid (18:1n–9) (213). Many transcription factors, such as liver X receptor, sterol regulatory element-binding protein 1c, and carbohydrate response element-binding protein exert significant control over the DNL of FAs (214). In obese individuals, hepatic lipogenesis was found to be elevated, which may contribute to their excessive fat mass (215). Large between-country differences in obesity prevalence have also been reported, from <10% to ≥25% or higher (216). Differences in carbohydrate and protein intake, weight status, and genetic background (e.g., single-nucleotide polymorphisms of the FADS1/FADS2 and ChREBP genes) also modulate FA metabolism, leading to differences in FA concentrations (217). This is another reason for the regional variation of n–3 FA concentration. However, no study has evaluated whether the concentration of DHA and ARA biosynthesis in nursing mothers differs across lactation periods. Further studies are needed to address this issue.
Another finding of the present study was that some types of FAs varied significantly across year of sample collection. Whether the longitudinal change in human milk FA content is related to changes in the diet of nursing mothers over time has not been thoroughly investigated. Odd-chain FAs, including 15:0 and 17:0, which cannot be synthesized in humans, are considered valid biomarkers of dairy fat intake (218). Dairy product consumption by adolescents in developed countries is reported to have declined over time (219). Consistent with that finding, we also observed a significant negative correlation between sampling year and the concentration of 15:0 and 17:0 in human milk. However, a study of Korean adults indicated that the per-capita intake of milk and dairy products (e.g., yogurt) did not significantly change from 1998 to 2010 (220). In our study, the human milk concentrations of 18:2n–6 and 18:3n–3 were also positively correlated with sample collection year. This agreed with the study by Micha et al. (204), which found that n–6, seafood-derived n–3, and plant-derived n–3 fat intakes each increased between 1990 and 2010 globally.
This study has several important strengths. First, multiple databases were systematically queried to ensure the identification of all related published studies and thus minimize the potential for publication bias and misclassification. Second, this meta-analysis had a large sample size, including 186 studies of lipid profiles in >20,000 human milk samples. Third, an investigation of the regional, lactation-stage, and longitudinal variations in the lipid content of human milk was made for the first time, to our knowledge. Fourth, 2 methods of data pooling were used, which enabled the calculation of means and CIs and also individual variation (SD values). However, several limitations of this study merit careful consideration. First, although we conducted analyses for total fat, phospholipids, cholesterol, and most subtypes of FAs, more specific subtypes of fat (e.g., trans fat) were not included because of the limited number of publications. Second, although we selected the studies under strict criteria, the between-study heterogeneity was still high. Except for dietary information or supplementation use, other information that may influence the lipid content of human milk such as sampling protocol, methods for FAs derivatization, and laboratory settings was also not collected (221). Thus, residual confounding in the primary studies cannot be ruled out, and the pooled data should be interpreted cautiously because of the high between-study heterogeneity.
In conclusion, this review provided a compilation of the published values of human milk lipid concentrations and investigated their variations with respect to study region, lactation stage, and year of sample collection. Our results suggest that the pooling of data on human milk lipid profiles in different studies should be done with caution due to the high between-study heterogeneity.
Supplementary Material
Acknowledgements
We thank Yan Li, Fengyan Chen, Zhen Hong, Xuanrui Zhang, Xiaoping Lin, Jiapeng Huang, and Zhaochang Huang, for their help in data checking.
The authors’ responsibilities were as follows—YS and YY: contributed to the study conception and design; ZZ, YW, JZ, HC, and MS: conducted the study selection, provided data extraction, and checked the data and conducted the analysis; ZZ and YW: drafted the manuscript, which was critically revised for important intellectual content by XY, YC, HZ, and XX; and all authors: reviewed and approved the final version of the manuscript.
Notes
This work was funded by the Yihai Kerry Group and National Nutrition Science Research Grant (CNS-NNSRG2022-147).
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–20 and Supplemental Table 1 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
ZZ and YW contributed equally to the article.
Abbreviations used: ALA, α-linolenic acid; ARA, arachidonic acid; DNL, de novo lipogenesis; FA, fatty acid; LA, linoleic acid.
Contributor Information
Zheqing Zhang, Department of Nutrition and Food Hygiene, Guangdong Provincial Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University, Guangzhou, China.
Yingyao Wang, Chinese Nutrition Society, Beijing, China; CNS Academy of Nutrition and Health (Beijing Zhongyinghui Nutrition and Health Research Institute), Beijing, China.
Xiaoguang Yang, National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
Yiyong Cheng, Institute of Health & Environmental Medicine, Tianjin, China.
Hong Zhang, Wilmar (Shanghai) Biotechnology Research & Development Center Co., Ltd., Shanghai, China.
Xuebing Xu, Wilmar (Shanghai) Biotechnology Research & Development Center Co., Ltd., Shanghai, China.
Jin Zhou, CNS Academy of Nutrition and Health (Beijing Zhongyinghui Nutrition and Health Research Institute), Beijing, China.
Hengying Chen, Department of Nutrition and Food Hygiene, Guangdong Provincial Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University, Guangzhou, China.
Mengyang Su, Department of Nutrition and Food Hygiene, Guangdong Provincial Key Laboratory of Tropical Disease Research, School of Public Health, Southern Medical University, Guangzhou, China.
Yuexin Yang, Chinese Nutrition Society, Beijing, China; National Institute for Nutrition and Health, Chinese Center for Disease Control and Prevention, Beijing, China.
Yixiang Su, Guangdong Provincial Key Laboratory of Food, Department of Nutrition and Food Hygiene, School of Public Health, Sun Yat-Sen University, Guangzhou, China.
References
- 1. Koletzko B. Human milk lipids. Ann Nutr Metab. 2016;69(Suppl 2):28–40. [DOI] [PubMed] [Google Scholar]
- 2. Mazzocchi A, D'Oria V, De Cosmi V, Bettocchi S, Milani GP, Silano Met al. The role of lipids in human milk and infant formulae. Nutrients. 2018;10(5):567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zielinska MA, Hamulka J, Grabowicz-Chadrzynska I, Brys J, Wesolowska A. Association between breastmilk LC PUFA, carotenoids and psychomotor development of exclusively breastfed infants. Int J Environ Res Public Health. 2019;16(7):1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guxens M, Mendez MA, Molto-Puigmarti C, Julvez J, Garcia-Esteban R, Forns Jet al. Long-chain polyunsaturated fatty acids in colostrum, and infant mental development. Pediatrics. 2011;128(4):e880–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernard JY, Armand M, Garcia C, Forhan A, De Agostini M, Charles MAet al. The association between linoleic acid levels in colostrum and child cognition at 2 and 3 y in the EDEN cohort. Pediatr Res. 2015;77(6):829–35. [DOI] [PubMed] [Google Scholar]
- 6. Waidyatillake NT, Stoney R, Thien F, Lodge CJ, Simpson JA, Allen KJet al. Breast milk polyunsaturated fatty acids: associations with adolescent allergic disease and lung function. Allergy. 2017;72(8):1193–201. [DOI] [PubMed] [Google Scholar]
- 7. Much D, Brunner S, Vollhardt C, Schmid D, Sedlmeier EM, Bruderl Met al. Breast milk fatty acid profile in relation to infant growth and body composition: results from the INFAT study. Pediatr Res. 2013;74(2):230–7. [DOI] [PubMed] [Google Scholar]
- 8. Floris LM, Stahl B, Abrahamse-Berkeveld M, Teller IC. Human milk fatty acid profile across lactational stages after term and preterm delivery: a pooled data analysis. Prostaglandins Leukot Essent Fatty Acids. 2020;156:102023. [DOI] [PubMed] [Google Scholar]
- 9. Brenna JT, Varamini B, Jensen RG, Diersen-Schade DA, Boettcher JA, Arterburn LM. Docosahexaenoic and arachidonic acid concentrations in human breast milk worldwide. Am J Clin Nutr. 2007;85(6):1457–64. [DOI] [PubMed] [Google Scholar]
- 10. Gidrewicz DA, Fenton TR. A systematic review and meta-analysis of the nutrient content of preterm and term breast milk. BMC Pediatr. 2014;14(1):216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cilla A, Diego Quintaes K, Barbera R, Alegria A. Phospholipids in human milk and infant formulas: benefits and needs for correct infant nutrition. Crit Rev Food Sci Nutr. 2016;56(11):1880–92. [DOI] [PubMed] [Google Scholar]
- 12. Miliku K, Duan QL, Moraes TJ, Becker AB, Mandhane PJ, Turvey SEet al. Human milk fatty acid composition is associated with dietary, genetic, sociodemographic, and environmental factors in the CHILD cohort study. Am J Clin Nutr. 2019;110(6):1370–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Method. 2014;14(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.3. (updated February 2022). Cochrane, 2022. Available from: www.training.cochrane.org/handbook [Google Scholar]
- 15. Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. [Google Scholar]
- 16. Zhang ZQ, Ho SC, Chen ZQ, Zhang CX, Chen YM. Reference values of bone mineral density and prevalence of osteoporosis in Chinese adults. Osteoporos Int. 2014;25(2):497–507. [DOI] [PubMed] [Google Scholar]
- 17. Al-Tamer YY, Mahmood AA. Fatty-acid composition of the colostrum and serum of fullterm and preterm delivering Iraqi mothers. Eur J Clin Nutr. 2004;58(8):1119–24. [DOI] [PubMed] [Google Scholar]
- 18. Álvarez-Sala A, Garcia-Llatas G, Barberá R, Lagarda MJ. Determination of cholesterol in human milk: an alternative to chromatographic methods. Nutr Hosp. 2015;32(4):1535–40. [DOI] [PubMed] [Google Scholar]
- 19. Anderson DM, Williams FH, Merkatz RB, Schulman PK, Kerr DS, Pittard WB. Length of gestation and nutritional composition of human milk. Am J Clin Nutr. 1983;37(5):810–14. [DOI] [PubMed] [Google Scholar]
- 20. Antonakou A, Skenderi KP, Chiou A, Anastasiou CA, Bakoula C, Matalas AL. Breast milk fat concentration and fatty acid pattern during the first six months in exclusively breastfeeding Greek women. Eur J Nutr. 2013;52(3):963–73. [DOI] [PubMed] [Google Scholar]
- 21. Armand M, Bernard JY, Forhan A, Heude B, Charles MA. Maternal nutritional determinants of colostrum fatty acids in the EDEN mother-child cohort. Clin Nutr. 2018;37(6):2127–36. [DOI] [PubMed] [Google Scholar]
- 22. Arnold J, Leslie G, Chen S. Protein, lactose and fat concentration of breast milk of mothers of term and premature neonates. Aust Paediatr J. 1987;23(5):299–300. [DOI] [PubMed] [Google Scholar]
- 23. Arsić A, Prekajski N, Vucic V, Tepsic J, Popovic T, Vrvic Met al. Milk in human nutrition: comparison of fatty acid profiles. Acta Vet. 2009;59:5–6. [Google Scholar]
- 24. Aydin İ, Turan Ö, Aydin FN, Koç E, Hirfanoğlu İ M, Akyol Met al. Comparing the fatty acid levels of preterm and term breast milk in Turkish women. Turkish J Med Sci. 2014;44(2):305–10. [PubMed] [Google Scholar]
- 25. Babiszewska M. Effects of energy and essential fatty acids content in breast milk on infant's head dimensions. Am J Hum Biol. 2020;32(6):e23418. [DOI] [PubMed] [Google Scholar]
- 26. Badiou S, Tuaillon E, Viljoen J, Escudié JB, Cristol JP, Newell MLet al. Association between breast milk fatty acids and HIV-1 transmission through breastfeeding. Prostaglandins Leukot Essent Fatty Acids. 2016;105:35–42. [DOI] [PubMed] [Google Scholar]
- 27. Bahrami G, Rahimi Z. Fatty acid composition of human milk in western Iran. Eur J Clin Nutr. 2005;59(4):494–7. [DOI] [PubMed] [Google Scholar]
- 28. Barreiro R, Regal P, López-Racamonde O, Cepeda A, Fente C. Evolution of breast milk fatty acids in Spanish mothers after one year of uninterrupted lactation. Prostaglandins Leukot Essent Fatty Acids. 2020;159:102141. [DOI] [PubMed] [Google Scholar]
- 29. Barreiro R, Regal P, López-Racamonde O, Cepeda A, Fente CA. Comparison of the fatty acid profile of Spanish infant formulas and Galician women breast milk. J Physiol Biochem. 2018;74(1):127–38. [DOI] [PubMed] [Google Scholar]
- 30. Beggio M, Cruz-Hernandez C. Quantification of total cholesterol in human milk by gas chromatography. J Sep Sci. 2018;41(8):1805–11. [DOI] [PubMed] [Google Scholar]
- 31. Benoit B, Fauquant C, Daira P, Peretti N, Guichardant M, Michalski MC. Phospholipid species and minor sterols in French human milks. Food Chem. 2010;120(3):684–91. [Google Scholar]
- 32. Birberg-Thornberg U, Karlsson T, Gustafsson PA, Duchen K. Nutrition and theory of mind—the role of polyunsaturated fatty acids (PUFA) in the development of theory of mind. Prostaglandins Leukot Essent Fatty Acids. 2006;75(1):33–41. [DOI] [PubMed] [Google Scholar]
- 33. Bitman J, Freed LM, Neville MC, Wood DL, Hamosh P, Hamosh M. Lipid composition of prepartum human mammary secretion and postpartum milk. J Pediatr Gastroenterol Nutr. 1986;5(4):608–15. [DOI] [PubMed] [Google Scholar]
- 34. Bitman J, Wrenn TR, Weyant JR, Wood DL. Effect of dietary fat and cholesterol on uptake of oleic acid and triolein by everted sacs of bovine small intestine. J Dairy Sci. 1982;65(7):1148–54. [DOI] [PubMed] [Google Scholar]
- 35. Boediman D, Ismail D, Iman S, I, Ismadi SD. Composition of breast milk beyond one year. J Trop Pediatr Environ Child Health. 1979;25(4):107–10. [DOI] [PubMed] [Google Scholar]
- 36. Boersma ER, Offringa PJ, Muskiet FA, Chase WM, Simmons IJ. Vitamin E, lipid fractions, and fatty acid composition of colostrum, transitional milk, and mature milk: an international comparative study. Am J Clin Nutr. 1991;53(5):1197–204. [DOI] [PubMed] [Google Scholar]
- 37. Borschel MW, Elkin RG, Kirksey A, Story JA, Galal O, Harrison GGet al. Fatty acid composition of mature human milk of Egyptian and American women. Am J Clin Nutr. 1986;44(3):330–5. [DOI] [PubMed] [Google Scholar]
- 38. Bruun S, van Rossem L, Lauritzen L. Content of n-3 LC-PUFA in breast milk four months postpartum is associated with infancy blood pressure in boys and infancy blood lipid profile in girls. Nutrients. 2019;11(2):235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Budowski P, Druckmann H, Kaplan B, Merlob P. Mature milk from Israeli mothers is rich in polyunsaturated fatty acids. World Rev Nutr Diet. 1994;75:105–8. [DOI] [PubMed] [Google Scholar]
- 40. Butte NF, Garza C, Johnson CA, Smith EO, Nichols BL. Longitudinal changes in milk composition of mothers delivering preterm and term infants. Early Hum Dev. 1984;9(2):153–62. [DOI] [PubMed] [Google Scholar]
- 41. Bzikowska-Jura A, Czerwonogrodzka-Senczyna A. The concentration of omega-3 fatty acids in human milk is related to their habitual but not current intake. Nutrients. 2019;11(7):1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cannon AM, Gridneva Z, Hepworth AR, Lai CT, Tie WJ, Khan Set al. The relationship of human milk leptin and macronutrients with gastric emptying in term breastfed infants. Pediatr Res. 2017;82(1):72–8. [DOI] [PubMed] [Google Scholar]
- 43. Chen A, Zhang W, Jiang M, He J, Wu S, Huang Qet al. Analysis of fatty acid composition in human milk from five regions in China. J Clin Pediatr. 2014;32(1):48–54. [Google Scholar]
- 44. Chen YJ, Zhou XH, Han B, Li SM, Xu T, Yi HXet al. Composition analysis of fatty acids and stereo-distribution of triglycerides in human milk from three regions of China. Food Res Int. 2020;133:109196. [DOI] [PubMed] [Google Scholar]
- 45. Chen ZY, Kwan KY, Tong KK, Ratnayake WM, Li HQ, Leung SS. Breast milk fatty acid composition: a comparative study between Hong Kong and Chongqing Chinese. Lipids. 1997;32(10):1061–7. [DOI] [PubMed] [Google Scholar]
- 46. Chisaguano AM, Lozano B, Molto-Puigmarti C. Elaidic acid, vaccenic acid and rumenic acid (c9,t11-CLA) determination in human plasma phospholipids and human milk by fast gas chromatography. Anal Methods. 2013;5(5):1264–72. [Google Scholar]
- 47. Clark RM, Ferris AM, Fey M, Brown PB, Hundrieser KE, Jensen RG. Changes in the lipids of human milk from 2 to 16 weeks postpartum. J Pediatr Gastroenterol Nutr. 1982;1(3):311–16. [DOI] [PubMed] [Google Scholar]
- 48. Claumarchirant L, Cilla A, Matencio E, Sanchez-Siles LM, Castro-Gomez P, Fontecha Jet al. Addition of milk fat globule membrane as an ingredient of infant formulas for resembling the polar lipids of human milk. Int Dairy J. 2016;61:228–38. [Google Scholar]
- 49. Da Cunha J, Macedo da Costa TH, Ito MK. Influences of maternal dietary intake and suckling on breast milk lipid and fatty acid composition in low-income women from Brasilia, Brazil. Early Hum Dev. 2005;81(3):303–11. [DOI] [PubMed] [Google Scholar]
- 50. Daud AZ, Mohd-Esa N, Azlan A, Chan YM. The trans fatty acid content in human milk and its association with maternal diet among lactating mothers in Malaysia. Asia Pac J Clin Nutr. 2013;22(3):431–42. [DOI] [PubMed] [Google Scholar]
- 51. De la Presa-Owens S, López-Sabater MC, Rivero-Urgell M. Fatty acid composition of human milk in Spain. J Pediatr Gastroenterol Nutr. 1996;22(2):180–5. [DOI] [PubMed] [Google Scholar]
- 52. Decsi T, Oláh S, Molnár S, Burus I. Fatty acid composition of human milk in Hungary. Acta Paediatr. 2000;89(11):1394–5. [DOI] [PubMed] [Google Scholar]
- 53. Delgado FJ, Cava R, Delgado J, Ramirez R. Tocopherols, fatty acids and cytokines content of holder pasteurised and high-pressure processed human milk. Dairy Sci Technol. 2014;94(2):145–56. [Google Scholar]
- 54. Deng L, Zou Q, Liu B, Ye W, Zhuo C, Chen Let al. Fatty acid positional distribution in colostrum and mature milk of women living in Inner Mongolia, North Jiangsu and Guangxi of China. Food Function. 2018;9(8):4234–45. [DOI] [PubMed] [Google Scholar]
- 55. Dodge ML, Wander RC, Xia Y, Butler JA, Whanger PD. Glutathione peroxidase activity modulates fatty acid profiles of plasma and breast milk in Chinese women. J Trace Elem Med Biol. 1999;12(4):221–30. [DOI] [PubMed] [Google Scholar]
- 56. Duan B, Shin JA, Qin Y, Kwon JI, Lee KT. A study on the relationship of fat content in human milk on carotenoids content and fatty acid compositions in Korea. Nutrients. 2019;11(9):2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Fidler N, Salobir K, Stibilj V. Fatty acid composition of human milk in different regions of Slovenia. Ann Nutr Metab. 2000;44(5–6):187–93. [DOI] [PubMed] [Google Scholar]
- 58. Fidler N, Sauerwald T, Pohl A, Demmelmair H, Koletzko B. Docosahexaenoic acid transfer into human milk after dietary supplementation: a randomized clinical trial. J Lipid Res. 2000;41(9):1376–83. [PubMed] [Google Scholar]
- 59. Finley DA, Lönnerdal B, Dewey KG, Grivetti LE. Breast milk composition: fat content and fatty acid composition in vegetarians and non-vegetarians. Am J Clin Nutr. 1985;41(4):787–800. [DOI] [PubMed] [Google Scholar]
- 60. Francois CA, Connor SL, Bolewicz LC, Connor WE. Supplementing lactating women with flaxseed oil does not increase docosahexaenoic acid in their milk. Am J Clin Nutr. 2003;77(1):226–33. [DOI] [PubMed] [Google Scholar]
- 61. Francois CA, Connor SL, Wander RC, Connor WE. Acute effects of dietary fatty acids on the fatty acids of human milk. Am J Clin Nutr. 1998;67(2):301–8. [DOI] [PubMed] [Google Scholar]
- 62. Freitas RF, Macedo MS, Lessa ADC, Pinto N, Teixeira RA. Relationship between the diet quality index in nursing mothers and the fatty acid profile of mature breast milk. Rev Paul Pediatr. 2021;39:e2019089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Friesen R, Innis SM. Trans fatty acids in human milk in Canada declined with the introduction of trans fat food labeling. J Nutr. 2006;136(10):2558–61. [DOI] [PubMed] [Google Scholar]
- 64. Gao C, Liu G, McPhee AJ, Miller J, Gibson RA. A simple system for measuring the level of free fatty acids in human milk collected as dried milk spot. Prostaglandins Leukot Essent Fatty Acids. 2020;158:102035. [DOI] [PubMed] [Google Scholar]
- 65. Gao C, Liu G, Whitfield KC, Kroeun H, Green TJ, Gibson RAet al. Comparison of human milk fatty acid composition of women from Cambodia and Australia. J Hum Lact. 2018;34(3):585–91. [DOI] [PubMed] [Google Scholar]
- 66. Gao YX, Zhang J, Wang C, Li L, Man Q, Song Pet al. The fatty acid composition of colostrum in three geographic regions of China. Asia Pac J Clin Nutr. 2013;22(2):276–82. [DOI] [PubMed] [Google Scholar]
- 67. Garza C, Johnson CA, Smith EO, Nichols BL. Changes in the nutrient composition of human milk during gradual weaning. Am J Clin Nutr. 1983;37(1):61–5. [DOI] [PubMed] [Google Scholar]
- 68. Genzel-Boroviczény O, Wahle J, Koletzko B. Fatty acid composition of human milk during the 1st month after term and preterm delivery. Eur J Pediatr. 1997;156(2):142–7. [DOI] [PubMed] [Google Scholar]
- 69. Gibson RA, Kneebone GM. Fatty acid composition of human colostrum and mature breast milk. Am J Clin Nutr. 1981;34(2):252–7. [DOI] [PubMed] [Google Scholar]
- 70. Giuffrida F, Cruz-Hernandez C, Bertschy E, Fontannaz P, Masserey Elmelegy I, Tavazzi Iet al. Temporal changes of human breast milk lipids of Chinese mothers. Nutrients. 2016;8(11):715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Golfetto I, McGready R, Ghebremeskel K, Min Y, Dubowitz L, Nosten Fet al. Fatty acid composition of milk of refugee Karen and urban Korean mothers: is the level of DHA in breast milk of Western women compromised by high intake of saturated fat and linoleic acid?. Nutr Health. 2007;18(4):319–32. [DOI] [PubMed] [Google Scholar]
- 72. Gómez-Cortés P, Martínez Marín AL, de la Fuente MA. Detailed fatty acid profile of serum lipid classes in lactating women and their relationship with milk fat. Eur J Lipid Sci Technol. 2017;119(4):1600095. [Google Scholar]
- 73. Granot E, Ishay-Gigi K, Malaach L, Flidel-Rimon O. Is there a difference in breast milk fatty acid composition of mothers of preterm and term infants?. J Matern Fetal Neonatal Med. 2016;29(5):832–5. [DOI] [PubMed] [Google Scholar]
- 74. Groh-Wargo S, Valentic J, Khaira S, Super DM, Collin M. Human milk analysis using mid-infrared spectroscopy. Nutr Clin Pract. 2016;31(2):266–72. [DOI] [PubMed] [Google Scholar]
- 75. Grote V, Verduci E, Scaglioni S, Vecchi F, Contarini G, Giovannini Met al. Breast milk composition and infant nutrient intakes during the first 12 months of life. Eur J Clin Nutr. 2016;70(2):250–6. [DOI] [PubMed] [Google Scholar]
- 76. Hahn-Holbrook J, Fish A, Glynn LM. Human milk omega-3 fatty acid composition is associated with infant temperament. Nutrients. 2019;11(12):2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hansen IB, Clausen J, Somers K, Patel AK. The fatty acid composition of serum, breastmilk and foetal brain in two different environments. Correlation of data from Ugandan and Danish populations with the topographic distribution of some disease entities. Acta Neurol Scand. 1970;46(3):301–12. [DOI] [PubMed] [Google Scholar]
- 78. Hayat L, Sughayer MA, Afzal M. Fatty acid composition of human milk in Kuwaiti mothers. Comp Biochem Physiol B Biochem Mol Biol. 1999;124(3):261–7. [DOI] [PubMed] [Google Scholar]
- 79. He G, Li G, Zhou B, Xiao H, Chu X, Ye X. Study on the fatty acid composition and distribution of breast milk in different lactation periods in Shanghai and Zhejiang Province. J Chin Inst Food Sci Technol. 2019;19(4):249–57. [Google Scholar]
- 80. Helland IB, Saarem K, Saugstad OD, Drevon CA. Fatty acid composition in maternal milk and plasma during supplementation with cod liver oil. Eur J Clin Nutr. 1998;52(11):839–45. [DOI] [PubMed] [Google Scholar]
- 81. Henderson RA, Jensen RG, Lammi-Keefe CJ, Ferris AM, Dardick KR. Effect of fish oil on the fatty acid composition of human milk and maternal and infant erythrocytes. Lipids. 1992;27(11):863–9. [DOI] [PubMed] [Google Scholar]
- 82. Hibberd CM, Brooke OG, Carter ND, Haug M, Harzer G. Variation in the composition of breast milk during the first 5 weeks of lactation: implications for the feeding of preterm infants. Arch Dis Child. 1982;57(9):658–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Huang HL, Chuang LT, Li HH, Lin CP, Glew RH. Docosahexaenoic acid in maternal and neonatal plasma phospholipids and milk lipids of Taiwanese women in Kinmen: fatty acid composition of maternal blood, neonatal blood and breast milk. Lipids Health Dis. 2013;12(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ingvordsen Lindahl IE, Artegoitia VM, Downey E, O'Mahony JA, O'Shea CA, Ryan CA, Kelly ALet al. Quantification of human milk phospholipids: the effect of gestational and lactational age on phospholipid composition. Nutrients. 2019;11(2):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Innis SM, Kuhnlein HV. Long-chain n-3 fatty acids in breast milk of Inuit women consuming traditional foods. Early Hum Dev. 1988;18(2–3):185–9. [DOI] [PubMed] [Google Scholar]
- 86. Innis SM, Nelson CM, Rioux MF, King DJ. Development of visual acuity in relation to plasma and erythrocyte omega-6 and omega-3 fatty acids in healthy term gestation infants. Am J Clin Nutr. 1994;60(3):347–52. [DOI] [PubMed] [Google Scholar]
- 87. Iranpour R, Kelishadi R, Babaie S, Khosravi-Darani K, Farajian S. Comparison of long chain polyunsaturated fatty acid content in human milk in preterm and term deliveries and its correlation with mothers' diet. J Res Med Sci. 2013;18(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- 88. Jacobson JL, Jacobson SW, Muckle G, Kaplan-Estrin M, Ayotte P, Dewailly E. Beneficial effects of a polyunsaturated fatty acid on infant development: evidence from the Inuit of Arctic Quebec. J Pediatr. 2008;152(3):356–64.e1. [DOI] [PubMed] [Google Scholar]
- 89. Jang SH, Lee BS, Park JH, Chung EJ, Um YS, Lee-Kim YCet al. Serial changes of fatty acids in preterm breast milk of Korean women. J Hum Lact. 2011;27(3):279–85. [DOI] [PubMed] [Google Scholar]
- 90. Jansson L, Akesson B, Holmberg L. Vitamin E and fatty acid composition of human milk. Am J Clin Nutr. 1981;34(1):8–13. [DOI] [PubMed] [Google Scholar]
- 91. Jiang HY, Chen HG, Wang YH, Wang XL, Huang YX, Yao Qet al. Analysis of nutrient content in the milk of Nanning lactating mothers. J Guangxi Med Univ. 2005;5:42–4. [Google Scholar]
- 92. Jiang J, Wu K, Yu Z, Ren Y, Zhao Y, Jiang Yet al. Changes in fatty acid composition of human milk over lactation stages and relationship with dietary intake in Chinese women. Food Function. 2016;7(7):3154–62. [DOI] [PubMed] [Google Scholar]
- 93. Jiang W, Zhang X, Cheng J, Song J, Jin Q, Wei Wet al. Variation of fat globule size and fatty acids in human milk in the first 30 days of lactation. Int Dairy J. 2020;100:104567. [Google Scholar]
- 94. Jirapinyo P, Densupsoontorn N, Wiraboonchai D, Vissavavejam U, Tangtrakulvachira T, Chungsomprasong Pet al. Fatty acid composition in breast milk from 4 regions of Thailand. J Med Assoc Thai. 2008;91(12):1833–7. [PubMed] [Google Scholar]
- 95. Jørgensen MH, Hernell O, Hughes E, Michaelsen KF. Is there a relation between docosahexaenoic acid concentration in mothers' milk and visual development in term infants?. J Pediatr Gastroenterol Nutr. 2001;32(3):293–6. [DOI] [PubMed] [Google Scholar]
- 96. Jørgensen MH, Hernell O, Lund P, Hølmer G, Michaelsen KF. Visual acuity and erythrocyte docosahexaenoic acid status in breast-fed and formula-fed term infants during the first four months of life. Lipids. 1996;31(1):99–105. [DOI] [PubMed] [Google Scholar]
- 97. Kallio MJ, Siimes MA, Perheentupa J, Salmenperä L, Miettinen TA. Cholesterol and its precursors in human milk during prolonged exclusive breast-feeding. Am J Clin Nutr. 1989;50(4):782–5. [DOI] [PubMed] [Google Scholar]
- 98. Kamelska AM, Pietrzak-Fiećko R, Bryl K. Variation of the cholesterol content in breast milk during 10 days collection at early stages of lactation. Acta Biochim Pol. 2012;59(2):243–7. [PubMed] [Google Scholar]
- 99. Kelishadi R, Hadi B, Iranpour R, Khosravi-Darani K, Mirmoghtadaee P, Farajian Set al. A study on lipid content and fatty acid of breast milk and its association with mother's diet composition. J Res Med Sci. 2012;17(9):824–7. [PMC free article] [PubMed] [Google Scholar]
- 100. Khan S, Hepworth AR, Prime DK, Lai CT, Trengove NJ, Hartmann PE. Variation in fat, lactose, and protein composition in breast milk over 24 hours: associations with infant feeding patterns. J Hum Lact. 2013;29(1):81–9. [DOI] [PubMed] [Google Scholar]
- 101. Khor GL, Tan SS, Stoutjesdijk E, Ng KWT, Khouw I, Bragt Met al. Temporal changes in breast milk fatty acids contents: a case study of Malay breastfeeding women. Nutrients. 2020;13(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Kneebone GM, Kneebone R, Gibson RA. Fatty acid composition of breast milk from three racial groups from Penang, Malaysia. Am J Clin Nutr. 1985;41(4):765–9. [DOI] [PubMed] [Google Scholar]
- 103. Koletzko B, Thiel I, Abiodun PO. Fatty acid composition of mature human milk in Nigeria. Z Ernahrungswiss. 1991;30(4):289–97. [DOI] [PubMed] [Google Scholar]
- 104. Kovács A, Funke S, Marosvölgyi T, Burus I, Decsi T. Fatty acids in early human milk after preterm and full-term delivery. J Pediatr Gastroenterol Nutr. 2005;41(4):454–9. [DOI] [PubMed] [Google Scholar]
- 105. Krasevec JM, Jones PJ, Cabrera-Hernandez A, Mayer DL, Connor WE. Maternal and infant essential fatty acid status in Havana, Cuba. Am J Clin Nutr. 2002;76(4):834–44. [DOI] [PubMed] [Google Scholar]
- 106. Krešić G, Dujmović M, Mandić ML, Delaš I. Relationship between Mediterranean diet and breast milk fatty acid profile: a study in breastfeeding women in Croatia. Dairy Sci Technol. 2013;93(3):287–301. [Google Scholar]
- 107. Kuipers RS, Luxwolda MF, Dijck-Brouwer DA, Muskiet FA. Fatty acid compositions of preterm and term colostrum, transitional and mature milks in a sub-Saharan population with high fish intakes. Prostaglandins Leukot Essent Fatty Acids. 2012;86(4–5):201–7. [DOI] [PubMed] [Google Scholar]
- 108. Lattka E, Rzehak P, Szabó É, Jakobik V, Weck M, Weyermann Met al. Genetic variants in the FADS gene cluster are associated with arachidonic acid concentrations of human breast milk at 1.5 and 6 mo postpartum and influence the course of milk dodecanoic, tetracosenoic, and trans-9-octadecenoic acid concentrations over the duration of lactation. Am J Clin Nutr. 2011;93(2):382–91. [DOI] [PubMed] [Google Scholar]
- 109. Lemons JA, Moye L, Hall D, Simmons M. Differences in the composition of preterm and term human milk during early lactation. Pediatr Res. 1982;16(2):113–17. [DOI] [PubMed] [Google Scholar]
- 110. Li H, Guo QY, Liu P, Zhang YC, Dai ZY, Gao Yet al. Changes in fatty acid levels in breast milk at different times. Chin J Food Nutr. 2017;23(5):54–8. [Google Scholar]
- 111. Lin Q, Li GB, Ge P, Xu RX. Analysis of fatty acid content of breast milk and study of factors influencing it. Chin J Prev Med. 2014;15(7):663–7. [Google Scholar]
- 112. Liu J, Zhang KP, Guo QH. A study on the protein and fatty acid content of breast milk in Huhehaote. Food Res Dev. 2016;37(17):39–42. [Google Scholar]
- 113. Liyanage C, Hettiarachchi M, Mangalajeewa P, Malawipathirana S. Adequacy of vitamin A and fat in the breast milk of lactating women in south Sri Lanka. Public Health Nutr. 2008;11(7):747–50. [DOI] [PubMed] [Google Scholar]
- 114. López-López A, López-Sabater MC, Campoy-Folgoso C, Rivero-Urgell M, Castellote-Bargalló AI. Fatty acid and sn-2 fatty acid composition in human milk from Granada (Spain) and in infant formulas. Eur J Clin Nutr. 2002;56(12):1242–54. [DOI] [PubMed] [Google Scholar]
- 115. Lubetzky R, Sever O, Mimouni FB, Mandel D. Human milk macronutrients content: effect of advanced maternal age. Breastfeed Med. 2015;10(9):433–6. [DOI] [PubMed] [Google Scholar]
- 116. Luukkainen P, Salo MK, Nikkari T. Changes in the fatty acid composition of preterm and term human milk from 1 week to 6 months of lactation. J Pediatr Gastroenterol Nutr. 1994;18(3):355–60. [DOI] [PubMed] [Google Scholar]
- 117. Lyu ZM, Ma HQ. Study of maternal diet and fat content in breast milk. Hebei Med. 2004;8:684–6. [Google Scholar]
- 118. Ma L, MacGibbon AKH, Jan Mohamed HJB, Loy S, Rowan A, McJarrow Pet al. Determination of phospholipid concentrations in breast milk and serum using a high performance liquid chromatography–mass spectrometry–multiple reaction monitoring method. Int Dairy J. 2017;71:50–9. [Google Scholar]
- 119. Mäkelä J, Linderborg K, Niinikoski H, Yang B, Lagström H. Breast milk fatty acid composition differs between overweight and normal weight women: the STEPS study. Eur J Nutr. 2013;52(2):727–35. [DOI] [PubMed] [Google Scholar]
- 120. Makrides M, Simmer K, Neumann M, Gibson R. Changes in the polyunsaturated fatty acids of breast milk from mothers of full-term infants over 30 wk of lactation. Am J Clin Nutr. 1995;61(6):1231–3. [DOI] [PubMed] [Google Scholar]
- 121. Marangoni F, Agostoni C, Lammardo AM, Bonvissuto M, Giovannini M, Galli Cet al. Polyunsaturated fatty acids in maternal plasma and in breast milk. Prostaglandins Leukot Essent Fatty Acids. 2002;66(5–6):535–40. [DOI] [PubMed] [Google Scholar]
- 122. Marangoni F, Agostoni C, Lammardo AM, Giovannini M, Galli C, Riva E. Polyunsaturated fatty acid concentrations in human hindmilk are stable throughout 12-months of lactation and provide a sustained intake to the infant during exclusive breastfeeding: an Italian study. Br J Nutr. 2000;84(1):103–9. [PubMed] [Google Scholar]
- 123. Martin JC, Bougnoux P, Fignon A, Theret V, Antoine JM, Lamisse Fet al. Dependence of human milk essential fatty acids on adipose stores during lactation. Am J Clin Nutr. 1993;58(5):653–9. [DOI] [PubMed] [Google Scholar]
- 124. Martysiak-Żurowska D, Puta M, Barczak N, Dąbrowska J, Malinowska-Pańczyk E, Kiełbratowska Bet al. Effect of high pressure and sub-zero temperature on total antioxidant capacity and the content of vitamin C, fatty acids and secondary products of lipid oxidation in human milk. Pol J Food Nutr Sci. 2017;67(2):117–22. [Google Scholar]
- 125. Maurage C, Guesnet P, Pinault M, Rochette de Lempdes J, Durand G, Antoine Jet al. Effect of two types of fish oil supplementation on plasma and erythrocyte phospholipids in formula-fed term infants. Neonatology. 1998;74(6):416–29. [DOI] [PubMed] [Google Scholar]
- 126. McJarrow P, Radwan H. Human milk oligosaccharide, phospholipid, and ganglioside concentrations in breast milk from United Arab Emirates mothers: results from the MISC cohort. Nutrients. 2019;11(10):2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mellies MJ, Burton K, Larsen R, Fixler D, Glueck CJ. Cholesterol, phytosterols, and polyunsaturated/saturated fatty acid ratios during the first 12 months of lactation. Am J Clin Nutr. 1979;32(12):2383–9. [DOI] [PubMed] [Google Scholar]
- 128. Minda H, Kovács A, Funke S, Szász M, Burus I, Molnár Set al. Changes of fatty acid composition of human milk during the first month of lactation: a day-to-day approach in the first week. Ann Nutr Metab. 2004;48(3):202–9. [DOI] [PubMed] [Google Scholar]
- 129. Moltó-Puigmartí C, Castellote AI, Carbonell-Estrany X, López-Sabater MC. Differences in fat content and fatty acid proportions among colostrum, transitional, and mature milk from women delivering very preterm, preterm, and term infants. Clin Nutr. 2011;30(1):116–23. [DOI] [PubMed] [Google Scholar]
- 130. Morales E, García-Esteban R, Guxens M, Guerra S, Mendez M, Moltó-Puigmartí Cet al. Effects of prolonged breastfeeding and colostrum fatty acids on allergic manifestations and infections in infancy. Clin Exp Allergy. 2012;42(6):918–28. [DOI] [PubMed] [Google Scholar]
- 131. Nagra SA. Longitudinal study in biochemical composition of human milk during first year of lactation. J Trop Pediatr. 1989;35(3):126–8. [DOI] [PubMed] [Google Scholar]
- 132. Narang AP, Bains HS, Kansal S, Singh D. Serial composition of human milk in preterm and term mothers. Indian J Clin Biochem. 2006;21(1):89–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Nishimura RY, Castro GS, Jordão AA Jr, Sartorelli DS. Breast milk fatty acid composition of women living far from the coastal area in Brazil. J Pediatr (Rio J). 2013;89(3):263–8. [DOI] [PubMed] [Google Scholar]
- 134. Ogunleye A, Fakoya AT, Niizeki S, Tojo H, Sasajima I, Kobayashi Met al. Fatty acid composition of breast milk from Nigerian and Japanese women. J Nutr Sci Vitaminol (Tokyo). 1991;37(4):435–42. [DOI] [PubMed] [Google Scholar]
- 135. Okolo SN, VanderJagt TJ, Vu T, VanderJagt TA, VanderJagt DJ, Okonji Met al. The fatty acid composition of human milk in northern Nigeria. J Hum Lact. 2000;16(1):28–35. [DOI] [PubMed] [Google Scholar]
- 136. Olafsdottir AS, Thorsdottir I, Wagner KH, Elmadfa I. Polyunsaturated fatty acids in the diet and breast milk of lactating Icelandic women with traditional fish and cod liver oil consumption. Ann Nutr Metab. 2006;50(3):270–6. [DOI] [PubMed] [Google Scholar]
- 137. Peng Y, Zhou T, Wang Q, Liu P, Zhang T, Zetterström Ret al. Fatty acid composition of diet, cord blood and breast milk in Chinese mothers with different dietary habits. Prostaglandins Leukot Essent Fatty Acids. 2009;81(5–6):325–30. [DOI] [PubMed] [Google Scholar]
- 138. Luna P, Juárez M, de la Fuente MA. Fatty acid and conjugated linoleic acid isomer profiles in human milk fat. Eur J Lipid Sci Technol. 2007;109(12):1160–6. [Google Scholar]
- 139. Pugo-Gunsam P, Guesnet P, Subratty AH, Rajcoomar DA, Maurage C, Couet C. Fatty acid composition of white adipose tissue and breast milk of Mauritian and French mothers and erythrocyte phospholipids of their full-term breast-fed infants. Br J Nutr. 1999;82(4):263–71. [PubMed] [Google Scholar]
- 140. Qi C, Sun J, Xia Y, Yu R, Wei W, Xiang Jet al. Fatty acid profile and the sn-2 position distribution in triacylglycerols of breast milk during different lactation stages. J Agric Food Chem. 2018;66(12):3118–26. [DOI] [PubMed] [Google Scholar]
- 141. Qi QF, Wu SM, Zhang W. Dynamics of fatty acid content in breast milk. Acta Nutrimenta Sinica. 1997;3:70–7. [Google Scholar]
- 142. Qian J, Chen T, Lu W, Wu S, Zhu J. Breast milk macro- and micronutrient composition in lactating mothers from suburban and urban Shanghai. J Paediatr Child Health. 2010;46(3):115–20. [DOI] [PubMed] [Google Scholar]
- 143. Qian J, Wu S, Zhang W, Cao L, Yang H, Ao L. Survey on the composition of breast milk in Shanghai. Shanghai Med J. 2002;7:396–8. [Google Scholar]
- 144. Qin XL, Yang B, Wang YH. Study on the fatty acid composition of human colostrum and the distribution of fatty acids at sn-2 position. Technol Ind Food. 2010;31(5):81–8. [Google Scholar]
- 145. Quinn EA, Diki Bista K, Childs G. Milk at altitude: human milk macronutrient composition in a high-altitude adapted population of Tibetans. Am J Phys Anthropol. 2016;159(2):233–43. [DOI] [PubMed] [Google Scholar]
- 146. Ratnayake WM, Chen ZY. Trans, n-3, and n-6 fatty acids in Canadian human milk. Lipids. 1996;31(1):S279–82. [DOI] [PubMed] [Google Scholar]
- 147. Ribeiro M, Balcao V, Guimaraes H, Rocha G, Moutinho C, Matos Cet al. Fatty acid profile of human milk of Portuguese lactating women: prospective study from the 1st to the 16th week of lactation. Ann Nutr Metab. 2008;53(1):50–6. [DOI] [PubMed] [Google Scholar]
- 148. Rocquelin G, Tapsoba S, Dop MC, Mbemba F, Traissac P, Martin-Prével Y. Lipid content and essential fatty acid (EFA) composition of mature Congolese breast milk are influenced by mothers' nutritional status: impact on infants' EFA supply. Eur J Clin Nutr. 1998;52(3):164–71. [DOI] [PubMed] [Google Scholar]
- 149. Romeu-Nadal M, Castellote AI, Lopez-Sabater MC. Effect of cold storage on vitamins C and E and fatty acids in human milk. Food Chem. 2008;106(1):65–70. [Google Scholar]
- 150. Rueda R, Ramírez M, García-Salmerón JL, Maldonado J, Gil A. Gestational age and origin of human milk influence total lipid and fatty acid contents. Ann Nutr Metab. 1998;42(1):12–22. [DOI] [PubMed] [Google Scholar]
- 151. Saarela T, Kokkonen J, Koivisto M. Macronutrient and energy contents of human milk fractions during the first six months of lactation. Acta Paediatr. 2005;94(9):1176–81. [DOI] [PubMed] [Google Scholar]
- 152. Sala-Vila A, Campoy C, Castellote AI, Garrido FJ, Rivero M, Rodríguez-Palmero Met al. Influence of dietary source of docosahexaenoic and arachidonic acids on their incorporation into membrane phospholipids of red blood cells in term infants. Prostaglandins Leukot Essent Fatty Acids. 2006;74(2):143–8. [DOI] [PubMed] [Google Scholar]
- 153. Sala-Vila A, Castellote AI, Campoy C, Rivero M, Rodriguez-Palmero M, López-Sabater MC. The source of long-chain PUFA in formula supplements does not affect the fatty acid composition of plasma lipids in full-term infants. J Nutr. 2004;134(4):868–73. [DOI] [PubMed] [Google Scholar]
- 154. Sala-Vila A, Castellote AI, Rodriguez-Palmero M, Campoy C, López-Sabater MC. Lipid composition in human breast milk from Granada (Spain): changes during lactation. Nutrition. 2005;21(4):467–73. [DOI] [PubMed] [Google Scholar]
- 155. Samur G, Topcu A, Turan S. Trans fatty acids and fatty acid composition of mature breast milk in Turkish women and their association with maternal diet's. Lipids. 2009;44(5):405–13. [DOI] [PubMed] [Google Scholar]
- 156. Sánchez-Hernández S, Esteban-Muñoz A, Giménez-Martínez R, Aguilar-Cordero MJ, Miralles-Buraglia B, Olalla-Herrera M. A comparison of changes in the fatty acid profile of human milk of Spanish lactating women during the first month of lactation using gas chromatography-mass spectrometry. A comparison with infant formulas. Nutrients. 2019;11(12):3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Sann L, Bienvenu F, Lahet C, Bienvenu J, Bethenod M. Comparison of the composition of breast milk from mothers of term and preterm infants. Acta Paediatr. 1981;70(1):115–16. [DOI] [PubMed] [Google Scholar]
- 158. Saphier O, Blumenfeld J, Silberstein T, Tzor T, Burg A. Fatty acid composition of breastmilk of Israeli mothers. Indian Pediatr. 2013;50(11):1044–6. [DOI] [PubMed] [Google Scholar]
- 159. Scholtens S, Wijga AH, Smit HA, Brunekreef B, de Jongste JC, Gerritsen Jet al. Long-chain polyunsaturated fatty acids in breast milk and early weight gain in breast-fed infants. Br J Nutr. 2009;101(1):116–21. [DOI] [PubMed] [Google Scholar]
- 160. Scopesi F, Zunin P, Mazzella M, Testa M, Boggia R, Evangelisti Fet al. 7-ketocholesterol in human and adapted milk formulas. Clin Nutr. 2002;21(5):379–84. [DOI] [PubMed] [Google Scholar]
- 161. Shehadeh N, Aslih N, Shihab S, Werman MJ, Sheinman R, Shamir R. Human milk beyond one year post-partum: lower content of protein, calcium, and saturated very long-chain fatty acids. J Pediatr. 2006;148(1):122–4. [DOI] [PubMed] [Google Scholar]
- 162. Shi YD, Sun GQ, Zhang ZG, Deng X, Kang XH, Liu ZDet al. The chemical composition of human milk from Inner Mongolia of China. Food Chem. 2011;127(3):1193–8. [DOI] [PubMed] [Google Scholar]
- 163. Silva MHL, Silva MTC, Brandao SCC, Gomes JC, Peternelli LA, Franceschini SD. Fatty acid composition of mature breast milk in Brazilian women. Food Chem. 2005;93(2):297–303. [Google Scholar]
- 164. Song S, Liu TT, Liang X, Liu ZY, Yishake D, Lu XTet al. Profiling of phospholipid molecular species in human breast milk of Chinese mothers and comprehensive analysis of phospholipidomic characteristics at different lactation stages. Food Chem. 2021;348:129091. [DOI] [PubMed] [Google Scholar]
- 165. Stoney RM, Woods RK, Hosking CS, Hill DJ, Abramson MJ, Thien FC. Maternal breast milk long-chain n-3 fatty acids are associated with increased risk of atopy in breastfed infants. Clin Exp Allergy. 2004;34(2):194–200. [DOI] [PubMed] [Google Scholar]
- 166. Storck Lindholm E, Strandvik B, Altman D, Möller A, Palme Kilander C. Different fatty acid pattern in breast milk of obese compared to normal-weight mothers. Prostaglandins Leukot Essent Fatty Acids. 2013;88(3):211–17. [DOI] [PubMed] [Google Scholar]
- 167. Su M-Y, Jia H-X, Chen W-L, Qi X-Y, Liu C-P, Liu Z-M. Macronutrient and micronutrient composition of breast milk from women of different ages and dietary habits in Shanghai area. Int Dairy J. 2018;85:27–34. [Google Scholar]
- 168. Szabó E, Boehm G, Beermann C, Weyermann M, Brenner H, Rothenbacher Det al. Fatty acid profile comparisons in human milk sampled from the same mothers at the sixth week and the sixth month of lactation. J Pediatr Gastroenterol Nutr. 2010;50(3):316–20. [DOI] [PubMed] [Google Scholar]
- 169. Thakkar SK, Giuffrida F, Cristina CH, De Castro CA, Mukherjee R, Tran LAet al. Dynamics of human milk nutrient composition of women from Singapore with a special focus on lipids. Am J Hum Biol. 2013;25(6):770–9. [DOI] [PubMed] [Google Scholar]
- 170. Underwood BA, Hepner R, Abdullah H. Protein, lipid, and fatty acids of human milk from Pakistani women during prolonged periods of lactation. Am J Clin Nutr. 1970;23(4):400–7. [DOI] [PubMed] [Google Scholar]
- 171. VanderJagt DJ, Arndt CD, Okolo SN, Huang YS, Chuang LT, Glew RH. Fatty acid composition of the milk lipids of Fulani women and the serum phospholipids of their exclusively breast-fed infants. Early Hum Dev. 2000;60(2):73–87. [DOI] [PubMed] [Google Scholar]
- 172. Vuori E, Kiuru K, Mäkinen SM, Väyrynen P, Kara R, Kuitunen P. Maternal diet and fatty acid pattern of breast milk. Acta Paediatr. 1982;71(6):959–63. [DOI] [PubMed] [Google Scholar]
- 173. Wan ZX, Wang XL, Xu L, Geng Q, Zhang Y. Lipid content and fatty acids composition of mature human milk in rural North China. Br J Nutr. 2010;103(6):913–16. [DOI] [PubMed] [Google Scholar]
- 174. Wang L, Li X, Hussain M, Liu L, Zhang Y, Zhang H. Effect of lactation stages and dietary intake on the fatty acid composition of human milk (a study in northeast China). Int Dairy J. 2020;101:104580. [Google Scholar]
- 175. Wang L, Shimizu Y, Kaneko S, Hanaka S, Abe T, Shimasaki Het al. Comparison of the fatty acid composition of total lipids and phospholipids in breast milk from Japanese women. Pediatr Int. 2000;42(1):14–20. [DOI] [PubMed] [Google Scholar]
- 176. Wei W. Phospholipid composition and fat globule structure I: comparison of human milk fat from different gestational ages, lactation stages, and infant formulas. J Agric Food Chem. 2019;67(50):13922–8. [DOI] [PubMed] [Google Scholar]
- 177. Wijga A, Houwelingen ACv, Smit HA, Kerkhof M, Vos AP, Neijens HJet al. Fatty acids in breast milk of allergic and non-allergic mothers: the PIAMA birth cohort study. Pediatr Allergy Immunol. 2003;14(3):156–62. [DOI] [PubMed] [Google Scholar]
- 178. Wong VW, Ng YF, Chan SM, Su YX, Kwok KW, Chan HMet al. Positive relationship between consumption of specific fish type and n-3 PUFA in milk of Hong Kong lactating mothers. Br J Nutr. 2019;121(12):1431–40. [DOI] [PubMed] [Google Scholar]
- 179. Wu K, Gao R, Tian F, Mao Y, Wang B, Zhou Let al. Fatty acid positional distribution (sn-2 fatty acids) and phospholipid composition in Chinese breast milk from colostrum to mature stage. Br J Nutr. 2019;121(1):65–73. [DOI] [PubMed] [Google Scholar]
- 180. Wu TC, Lau BH, Chen PH, Wu LT, Tang RB. Fatty acid composition of Taiwanese human milk. J Chin Med Assoc. 2010;73(11):581–8. [DOI] [PubMed] [Google Scholar]
- 181. Xiang M, Alfvén G, Blennow M, Trygg M, Zetterström R. Long-chain polyunsaturated fatty acids in human milk and brain growth during early infancy. Acta Paediatr. 2000;89(2):142–7. [DOI] [PubMed] [Google Scholar]
- 182. Xiang M, Harbige LS, Zetterström R. Long-chain polyunsaturated fatty acids in Chinese and Swedish mothers: diet, breast milk and infant growth. Acta Paediatr. 2005;94(11):1543–9. [DOI] [PubMed] [Google Scholar]
- 183. Xiang M, Harbige LS, Zetterstrom R. Breast milk levels of zinc and omega-6 polyunsaturated fatty acids and growth of healthy Chinese infants. Acta Paediatr. 2007;96(3):387–90. [DOI] [PubMed] [Google Scholar]
- 184. Xiang M, Lei S, Li T, Zetterström R. Composition of long chain polyunsaturated fatty acids in human milk and growth of young infants in rural areas of northern China. Acta Paediatr. 1999;88(2):126–31. [DOI] [PubMed] [Google Scholar]
- 185. Xu L, Du Y, Ma J, Sheng Q. A survey on the amino acid and fatty acid content of breast milk in a region of Hebei Province. Food Sci Technol. 2008;4:231–3. [Google Scholar]
- 186. Yang L, Li J, Hu R, Xu L, Li Y, Sheng W. Correlation between fatty acid fractions of human milk and neonatal breast milk jaundice. Chin J Contemp Pediatr. 2020;22(12):1256–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Yang Z, Jiang R, Li H, Wang J, Duan Y, Pang Xet al. Human milk cholesterol is associated with lactation stage and maternal plasma cholesterol in Chinese populations. Pediatr Res. 2022;; 91(4):970–6. [DOI] [PubMed] [Google Scholar]
- 188. Yu G, Duchén K, Björkstén B. Fatty acid composition in colostrum and mature milk from non-atopic and atopic mothers during the first 6 months of lactation. Acta Paediatr. 1998;87(7):729–36. [DOI] [PubMed] [Google Scholar]
- 189. Yu J, Yuan T, Zhang X, Jin Q, Wei W. Quantification of nervonic acid in human milk in the first 30 days of lactation: influence of lactation stages and comparison with infant formulae. Nutrients. 2019;11(8):1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Yuhas R, Pramuk K, Lien EL. Human milk fatty acid composition from nine countries varies most in DHA. Lipids. 2006;41(9):851–8. [DOI] [PubMed] [Google Scholar]
- 191. Zhang Y, Wang L, Zhang HD, Li XD, Leng YB, Gong YNet al. Analysis of the differences in fatty acid composition of breastmilk, cow's milk and goat's milk. Technol Ind Food. 2019;40(4):21–6. [Google Scholar]
- 192. Zhong YB, Chen LJ, Zhao J Y, Dong XY, Li JF, Liu Bet al. Changes in fatty acids in breast milk during lactation and factors influencing their polyunsaturated fatty acids. Food Sci. 2019;40(4):237–43. [Google Scholar]
- 193. Zhu M, Yang Z, Ren Y, Duan Y, Gao H, Liu Bet al. Comparison of macronutrient contents in human milk measured using mid-infrared human milk analyser in a field study vs. chemical reference methods. Matern Child Nutr. 2017;13(1): 10.1111/mcn.12248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Zhuang M, Huang L. Analysis of DHA and AA content in breast milk in Zhoushan area. Chin J Child Health Care. 2006;(5):514–5. [Google Scholar]
- 195. Zou XQ, Guo Z, Huang JH, Jin QZ, Cheong LZ, Wang XGet al. Human milk fat globules from different stages of lactation: a lipid composition analysis and microstructure characterization. J Agric Food Chem. 2012;60(29):7158–67. [DOI] [PubMed] [Google Scholar]
- 196. Lacomba R, Cilla A, Alegría A, Barberá R, Silvestre D, Lagarda MJ. Stability of fatty acids and tocopherols during cold storage of human milk. Int Dairy J. 2012;27(1–2):22–6. [Google Scholar]
- 197. Ruan C, Liu X, Man H, Ma X, Lu G, Duan Get al. Milk composition in women from five different regions of China: the great diversity of milk fatty acids. J Nutr. 1995;125(12):2993–8. [DOI] [PubMed] [Google Scholar]
- 198. Keikha M, Bahreynian M, Saleki M, Kelishadi R. Macro- and micronutrients of human milk composition: are they related to maternal diet? A comprehensive systematic review. Breastfeed Med. 2017;12(9):517–27. [DOI] [PubMed] [Google Scholar]
- 199. Dror DK, Allen LH. Overview of nutrients in human milk. Adv Nutr. 2018;9(Suppl 1):278S–94S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Bravi F, Wiens F, Decarli A, Dal Pont A, Agostoni C, Ferraroni M. Impact of maternal nutrition on breast-milk composition: a systematic review. Am J Clin Nutr. 2016;104(3):646–62. [DOI] [PubMed] [Google Scholar]
- 201. Tur JA, Bibiloni MM, Sureda A, Pons A. Dietary sources of omega 3 fatty acids: public health risks and benefits. Br J Nutr. 2012;107(Suppl 2):S23–52. [DOI] [PubMed] [Google Scholar]
- 202. Abedi E, Sahari MA. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci Nutr. 2014;2(5):443–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Harika RK, Eilander A, Alssema M, Osendarp SJ, Zock PL. Intake of fatty acids in general populations worldwide does not meet dietary recommendations to prevent coronary heart disease: a systematic review of data from 40 countries. Ann Nutr Metab. 2013;63(3):229–38. [DOI] [PubMed] [Google Scholar]
- 204. Micha R, Khatibzadeh S, Shi P, Fahimi S, Lim S, Andrews KGet al. Global, regional, and national consumption levels of dietary fats and oils in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys. BMJ. 2014;348:g2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Cheung NF. Chinese zuo yuezi (sitting in for the first month of the postnatal period) in Scotland. Midwifery. 1997;13(2):55–65. [DOI] [PubMed] [Google Scholar]
- 206. Weseler AR, Dirix CE, Bruins MJ, Hornstra G. Dietary arachidonic acid dose-dependently increases the arachidonic acid concentration in human milk. J Nutr. 2008;138(11):2190–7. [DOI] [PubMed] [Google Scholar]
- 207. van Goor SA, Dijck-Brouwer DA, Hadders-Algra M, Doornbos B, Erwich JJ, Schaafsma Aet al. Human milk arachidonic acid and docosahexaenoic acid contents increase following supplementation during pregnancy and lactation. Prostaglandins Leukot Essent Fatty Acids. 2009;80(1):65–9. [DOI] [PubMed] [Google Scholar]
- 208. Jensen CL, Maude M, Anderson RE, Heird WC. Effect of docosahexaenoic acid supplementation of lactating women on the fatty acid composition of breast milk lipids and maternal and infant plasma phospholipids. Am J Clin Nutr. 2000;71(1):292s–9s. [DOI] [PubMed] [Google Scholar]
- 209. Schaefer E, Demmelmair H, Horak J, Holdt L, Grote V, Maar Ket al. Multiple micronutrients, lutein, and docosahexaenoic acid supplementation during lactation: a randomized controlled trial. Nutrients. 2020;12(12):3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Barcelo-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n-3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n-3 fatty acid. Am J Clin Nutr. 2008;88(3):801–9. [DOI] [PubMed] [Google Scholar]
- 211. Burdge G. Alpha-linolenic acid metabolism in men and women: nutritional and biological implications. Curr Opin Clin Nutr Metab Care. 2004;7(2):137–44. [DOI] [PubMed] [Google Scholar]
- 212. Iggman D, Riserus U. Role of different dietary saturated fatty acids for cardiometabolic risk. Clin Lipidol. 2011;6(2):209–23. [Google Scholar]
- 213. Song Z, Xiaoli AM, Yang F. Regulation and metabolic significance of de novo lipogenesis in adipose tissues. Nutrients. 2018;10(10):1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45(3):199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Ameer F, Scandiuzzi L, Hasnain S, Kalbacher H, Zaidi N. De novo lipogenesis in health and disease. Metabolism. 2014;63(7):895–902. [DOI] [PubMed] [Google Scholar]
- 216. NCD Risk Ractor Collaboration . Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet. 2017;390(10113):2627–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Minihane AM. Impact of genotype on EPA and DHA status and responsiveness to increased intakes. Nutrients. 2016;8(3):123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Pranger IG, Joustra ML, Corpeleijn E, Muskiet FAJ, Kema IP, Elferink Set al. Fatty acids as biomarkers of total dairy and dairy fat intakes: a systematic review and meta-analysis. Nutr Rev. 2019;77(1):46–63. [DOI] [PubMed] [Google Scholar]
- 219. Dror DK, Allen LH. Dairy product intake in children and adolescents in developed countries: trends, nutritional contribution, and a review of association with health outcomes. Nutr Rev. 2014;72(2):68–81. [DOI] [PubMed] [Google Scholar]
- 220. Lim H, Kim SY, Wang Y, Lee SJ, Oh K, Sohn CYet al. Preservation of a traditional Korean dietary pattern and emergence of a fruit and dairy dietary pattern among adults in South Korea: secular transitions in dietary patterns of a prospective study from 1998 to 2010. Nutr Res. 2014;34(9):760–70. [DOI] [PubMed] [Google Scholar]
- 221. Brenna JT, Plourde M, Stark KD, Jones PJ, Lin YH. Best practices for the design, laboratory analysis, and reporting of trials involving fatty acids. Am J Clin Nutr. 2018;108(2):211–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.