ABSTRACT
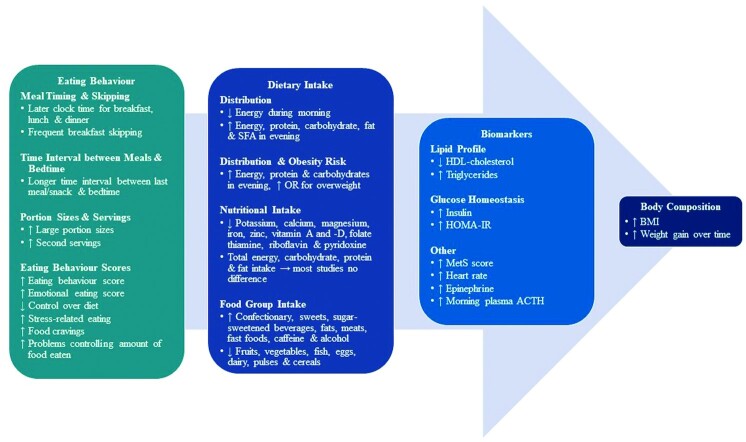
The timing and nutritional composition of food intake are important zeitgebers for the biological clocks in humans. Thus, eating at an inappropriate time (e.g., during the night) may have a desynchronizing effect on the biological clocks and, in the long term, may result in adverse health outcomes (e.g., weight gain, obesity, and poor metabolic function). Being a very late or early chronotype not only determines preferred sleep and wake times but may also influence subsequent mealtimes, which may affect the circadian timing system. In recent years, an increased number of studies have examined the relation between chronotype and health outcomes, with a main focus on absolute food intake and metabolic markers and, to a lesser extent, on dietary intake distribution and eating behavior. Therefore, this review aimed to systematically determine whether chronotype indirectly affects eating behaviors, dietary intake (timing, choice, nutrients), and biomarkers leading to body composition outcomes in healthy adults. A systematic literature search on electronic databases (PubMed, CINAHL, MEDLINE, SCOPUS, Cochrane library) was performed (International Prospective Register of Systematic Reviews number: CRD42020219754). Only studies that included healthy adults (aged >18 y), classified according to chronotype and body composition profiles, using outcomes of dietary intake, eating behavior, and/or biomarkers, were considered. Of 4404 articles, 24 met the inclusion criteria. The results revealed that late [evening type (ET)] compared with early [morning type (MT)] chronotypes were more likely to be overweight/obese with poorer metabolic health. Both MT and ET had similar energy and macronutrient intakes, consuming food during their preferred sleep–wake timing: later for ET than MT. Most of the energy and macronutrient intakes were distributed toward nighttime for ET and exacerbated by unhealthy eating behaviors and unfavorable dietary intakes. These findings from our systematic review give further insight why higher rates of overweight/obesity and unhealthier metabolic biomarkers are more likely to occur in ET.
Keywords: morning type, evening type, circadian, meal timing, nutritional intake, eating habits
Statement of significance: This systematic review exemplifies differences in food choice, timing and distribution during the day, nutritional quality, and eating behaviors between chronotypes. To our knowledge, this is the first systematic review that comprehensively compares not only dietary patterns and food composition but also eating behavior and metabolic outcome markers between morning and evening types. Our findings highlight that it might be important for long-term metabolic health to include someone's chronotype when tailoring meal and food plans for healthy cohorts but also for patients.
Introduction
Most organisms, including humans, have evolved an internal timekeeping system that generates circadian rhythms of metabolism, gene expression, and behaviors (1–5). The circadian rhythms of clocks in each cell are controlled by the central clock located in the suprachiasmatic nucleus (SCN) in the hypothalamus of the brain (6). In turn, the SCN is entrained to the earth's 24-h light/dark cycle (7) as it receives external light input via the eyes and optic nerve and synchronizes the downstream peripheral cell and tissue clocks (8, 9). Environmental light is the primary zeitgeber (time cue) for the central circadian clock, but other external cues, such as food intake, including the timing and composition of food intake, are capable of setting the rhythms in the peripheral clocks as well as the clock-controlled genes in the body tissues and organs (5, 10, 11). These clock genes in turn influence the timing of digestion, nutrient uptake and metabolism, metabolite and hormonal regulation, food intake, behavior, and appetite (5, 11). Timing of food intake, as well as composition of food intake (particularly macronutrients), is therefore an important zeitgeber for the circadian timing system (12).
Humans are physiologically suited to spend about two-thirds of their 24-h day awake, being active and eating and storing energy. They usually spend one-third of their time asleep, being in a fasting state at nighttime (13). During the day, ingested food provides energy to support metabolic processes, whereas during the night, when sleep usually occurs, stored energy is mobilized to maintain homeostasis (14, 15). Thus, eating at an inappropriate time can have a desynchronizing effect on the biological circadian clocks, resulting in adverse health outcomes, including weight gain, obesity, and poor metabolic health outcomes (16–18). Studies not considering different chronotypes have shown that a higher energy intake during the biological night (the normal resting and fasting cycles) results in enhanced fat storage and ultimately obesity (19, 20). This is further supported by McHill et al. (20), who showed that obese individuals typically consume most of their energy an hour closer to the melatonin secretion onset time (circadian phase marker, which usually occurs 2–4 h before sleep onset) in comparison to lean individuals. In addition, eating later in the day is associated with an increased risk for type 2 diabetes mellitus (21), as well as metabolic alterations, including impairment of lipid profiles, daily cortisol concentrations, and glucose tolerance (22–26).
Evidence from shift work studies has further accentuated that incorrect timing of food intake in combination with other dietary factors, such as poor food choices, eating behaviors, and meal and snack frequency, plays a role in the adverse health outcomes seen in individuals (27–29). An eating pattern that is high in energy-dense foods, such as sugar-sweetened beverages, fast foods, and fatty foods, and low in micronutrient-rich foods, such as fruit, vegetables, and fiber, is associated with weight gain (30) and an increased risk of metabolic syndrome and diabetes (31, 32). Furthermore, disinhibited or restrained eating behaviors are known to affect energy intake by influencing the types and amounts of foods eaten, the timing of food intake, and the eating occasion or where food intake occurs (33). This ultimately leads to increases in BMI and body fat percentage (34), as well as subsequent detrimental metabolic health outcomes such as poor glycemic control (35). These findings can be explained by the various metabolic processes and hormones involved in energy expenditure that are governed in precise timed relations to each other across a 24-h day (14, 15).
The altered timing of food intake, poor food choices, and behaviors are influenced by various other factors, such as work schedules and social events, but likely also by individual chronotypes. The term chronotype (36) is widely used to describe the preferred sleep–wake timing of an individual relative to the light/dark cycle that influences the timing of their diurnal preferences and the modulation of physiologic functions and behavior. Intrinsic sleep–wake time preferences in humans can be classified as early [morning type (MT)], intermediate [intermediate type (IT)], or late [evening type (ET)] chronotypes (37–39). The MTs habitually prefer an early bedtime and early morning rise time (37–39). On the other hand, ETs prefer a later bedtime and a late morning rise time (37–39). Morning and evening types have also been shown to exhibit genetic differences in allele frequencies (40, 41) and different intrinsic period length of the circadian clocks (42), as well as different phase angles of entrainment (e.g., between circadian phase of the melatonin rhythms and sleep–wake times) (43). Chronotype may therefore drive not only sleep and wake time (43) but also the timing of food intake (fasting or eating).
Assessment of a person's chronotype can thus be used as a proxy for the phase of entrainment between the external 24-h cycle and the internal circadian phase of sleep and wakefulness. Hence, some of the assessment instruments [e.g., the Munich Chronotype Questionnaire (MCTQ)] use midsleep as proxy for chronotype (which is the midpoint of the sleep episode after habitual sleep-onset and wakeup times on free and workdays). Such differences in sleep–wake timing consequently lead to differences in food intake (44). However, not only the shift in mealtimes seems to be different between chronotypes, but also nutrient and food choices, behaviors, and consequently biomarkers may also be important (44–46). There appears to be a difference in inherent eating patterns displayed between MTs and ETs (44, 47), although the number of studies is limited. One study, for example, has shown that normal-weight MTs consume more energy earlier during the day, whereas normal-weight ETs consume food later during the day (44), and another study has found no association between chronotype and BMI (47). One study has found that ETs have a poorer lipid profile in comparison with MTs (44), but this has not been extensively studied yet in a healthy population. Furthermore, the ETs tend to display unhealthy eating behaviors, leading to less control over their dietary intake, which may favor a dietary pattern that results in weight gain and obesity (45, 46), although the effect on body composition has not been explored.
A limited number of systematic reviews have been conducted regarding chronotype and diet (48–51). Most of these reviews had a specific focus on disease conditions (48, 50) or included unhealthy (type 2 diabetes mellitus) individuals, specific populations (e.g., post–bariatric surgery), or nightshift workers (49) or investigated eating patterns including behavior related to temporal eating patterns (meal frequency and skipping) and energy intake (51). The number of studies investigating the potential link between different chronotypes and the diet has grown in the past 10 y. This systematic review identified as a gap that the associations with individual dietary aspects and health outcomes have not been explored extensively, nor does a comprehensive framework exist that presents the dietary components beyond energy intakes together with eating behaviors as a whole. Therefore, the aim of this systematic review was to determine whether chronotype indirectly affects eating behavior, dietary intake (timing, choice, nutrients), and biomarkers leading to body composition outcomes in healthy adults.
Methods
Study design
This review was designed as a systematic review without meta-analysis. It was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (52). The main research question that was aimed to be answered was the following: “Is body composition, dietary intake, eating behavior, and biomarker outcomes in healthy adults dependent on chronotype?” The systematic review protocol was registered prospectively in the International Prospective Register of Systematic Reviews (CRD42020219754) and can be accessed at https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=219754.
Search strategy and eligibility criteria
A systematic literature search was conducted in May 2020, followed by a rerun in November–December 2020. The following electronic databases were searched: PubMed, CINAHL, MEDLINE, SCOPUS, and Cochrane library. The search was limited to articles published in English, published within the past 10 y reflecting the surge of chronotype research, and including studies with participants older than 18 y. The search strategy was based on the following categorical keywords and their synonyms: adults, chronotype, body composition, dietary intake, eating behavior, and biomarkers (see Supplemental Table 1 for the full list of search terms). All relevant study designs except for conference proceedings, editorial letters, review articles, and pharmacologic studies were included. Studies that determined BMI and used this anthropometrical measurement as a comparator were included. Studies that recruited adults aged <18 y, pregnant and lactating women, nightshift workers (these individuals already exhibit altered sleep–wakefulness and fasting–feeding cycles due to work obligations and not necessarily because of their chronotype), and participants with diagnosed acute, preexisting, and chronic conditions that may influence sleep–wake timings (e.g., eating disorders, bariatric patients, mental illness, sleep disorders, diabetes) were excluded.
Study selection
In order to answer the research question (see above) the Population, Intervention, Comparison, Outcomes, and Study (PICOS) criteria (52) were used from primarily retrieved publications:
Population:adults
Intervention: chronotype assessment
Comparisons: body composition measures including BMI (in kg/m2) and body fat percentage categories, waist circumference, and weight change
Outcomes: dietary intake, eating behavior, and biomarkers
Study design: all relevant designs except for conference proceedings, editorial letters, review articles, and pharmacologic studies (see Supplemental Table 1)
All records retrieved from the databases were exported using the Endnote X9 citation management software (Clarivate Analytics) (53). Duplicates were removed using Endnote, and the remaining references were exported into Rayyan QCRI (54). Two authors (CvdM, RK) independently screened the titles, abstracts, and full text for eligibility using the PICOS criteria before final inclusion in the review (CvdM, RK). In the case of conflicting decisions, a third reviewer (MM) participated. Studies were included if participants were classified according to chronotypes and their body composition profiles were compared. Studies were included if they reported at least 1 variable from the following 4 outcomes:
Dietary intake: diet composition (energy, macro- and micronutrients); food groups or food and drink categories (e.g., fruit and vegetables, sugar, fiber, alcohol, starch, meat, and dairy), and portion sizes
Eating occasions: meal timing, frequency, or skipping
Eating behavior: dietary restraint (conscious restriction of food intake to control body weight and shape), disinhibition (loss of control of food intake that leads to overconsumption), binge eating, and perceived hunger
Biomarkers: glucose, insulin, lipid profiles, and blood pressure and genetic profiles (such as genotyping of the PERIOD3 clock gene)
Studies were excluded if they did not include at least 1 of the predefined outcomes, were not designed to compare body composition profiles, or did not analyze nightshift workers separately from day workers.
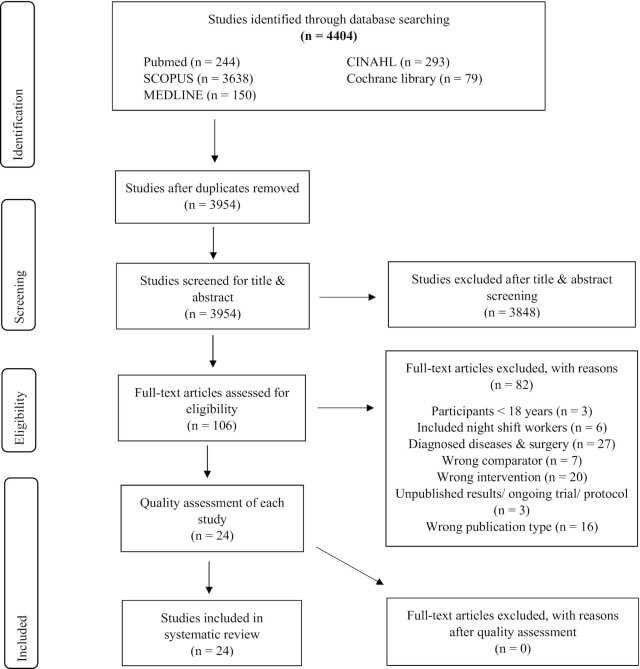
Detailed reasons for exclusion of studies are reported in the PRISMA guidelines, and a PRISMA flow diagram outlines the study selection for this review (Figure 1) (52).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram for search strategy and study selection.
Data extraction
Data were extracted by CvdM in table format with the following variables: authors, publication year, country, study design, number of participants, type of participants, age, body composition, method of chronotype classification, and distribution of chronotype. The study had the following outcomes: dietary intake, eating behaviors, and biomarkers. The information in the tables was organized into the specific outcome categories and then presented according to the differences between chronotypes. Additional analysis, such as correlation analysis, was also reported next to each outcome category. A second researcher (RK) reviewed the extracted data for accuracy by using the full-text articles.
The quality of each study was assessed using the appropriate Joanna Briggs Institute (JBI) checklists for analytical cross-sectional studies, cohort studies, and randomized controlled trials (55). Each study was assessed independently by 2 authors (CvdM, RK) using the appropriate checklist for each study design assessing issues of bias, data collection, analysis, and reporting. Studies were allocated a score according to the number of JBI checklist criteria that were met (55) (Supplemental Table 2, Supplemental Table 3, and Supplemental Table 4).
Only statistically significant differences between chronotypes derived from the included articles and statistically significant associations (correlations), including significant linear relations (P-trend analyses), are reported in the text, but all P values and additional analysis are reported in the tables. If the mean differences (absolute or in percentage; e.g., calories of energy intake per chronotype group) were not directly available for this systematic review, they were calculated based on information from the tables or figures in the original journal papers (marked with ‡ in the text).
Results
A total of 4404 articles were initially identified, of which 339 were duplicates. After screening the remaining ones (title and abstract), 106 full-text articles were assessed by applying the eligibility criteria. Finally, 24 full-text articles were eligible for inclusion in this review. The main reasons for exclusion of studies were that participants with acute, preexisting, and chronic diseases were included (n = 27) and/or incorrect intervention (n = 20) and/or incorrect publication type (n = 16) was used (Figure 1), based on predefined exclusion criteria.
Study and participant characteristics
The sample size of the 24 studies varied between 44 and 3304 participants (men and women), of which 3 studies recruited women only (56–58). Most of the included studies (n = 20) had a cross-sectional design (56, 58–76). The remaining studies included 1 randomized controlled trail (57), 2 cohort studies (77, 78), and 1 population-based study (79) (Table 1).
TABLE 1.
Study and Participant Characteristics1
| Author, year, country | Study population, N | Sex, n (%) | Age, y | Chronotype assessment method | Chronotype distribution, n (%) | |||
|---|---|---|---|---|---|---|---|---|
| Women | Men | MT | IT | ET | ||||
| Xiao et al., 2019 (77)United States | Middle- to older-aged adults 872 | 443 (51) | 429 (49) | MT: 62.3 ± 6.0 ET: 63.8 ± 5.7 | MCTQ | 4362 | — | 4363 |
|
Sato-Mito et al., 2011 (56) Japan |
Dietetic students 3304 | 3304 (100) | — |
Range: 18–20 18.1 ± 0.3 |
Midpoint of sleep quintiles | 534 (16)4 | 2169 (66)5-7 | 601 (18)8 |
|
Vera et al., 2018 (71) Spain |
Overweight and obese adults 2126 | 1722 (81) | 404 (19) | 40.5 ± 12.4 | MEQ | 1098 (51.6) | — | 1028 (48.4) |
|
Najem et al., 2020 (76) Lebanon |
Adult university students 644 | 453 (70.3) | 190 (29.5) | 20.2 ± 1.8 | MEQ | 56 (8.7) | 452 (70.2) | 132 (20.5) |
|
Lázár et al., 2012 (73) United Kingdom |
Healthy adults675 | 262 (38.8) | 413 (61.2) |
Range: 20–35 26.1 ± 4.0 |
MEQ and the total score and the single question from the MCTQ referring to self-assessed chronotype | 208 (31) | 227 (34) | 228 (34) |
|
Yoshizaki et al., 2018 (59) Japan |
Nurses 2559 Nurses: day workers 1095 |
1095 (100) | — | Range: 20–59 41.2 ± 9.4 | MEQ | 336 (31)9 | 359 (33)10 | 400 (37)11 |
|
Silva et al., 2016 (60) Brazil |
University students 204 |
112 (55) | 92 (45) | Range: 18–39 21.6 ± 3.9 | MSFsc | — | ||
|
Lai and Say, 2013 (61) Malaysia |
Tertiary students 1118 |
632 (56.5) | 486 (43.5) | Range: 18–27 20.1 ± 1.53 | MEQ | — | 2 (0.2) | 1116 (99.8) |
|
Muñoz, 2020 (57) Spain |
Overweight and obese adults 200 Chrono-group 102 |
102 (100) | — | Range: 18–65 | MEQ | 61 (60) | — | 41 (40) |
|
Lucassen et al., 2013 (62) United States |
Obese men and premenopausal women 119 |
92 (77.3) | 27 (22.7) | Range: 18–50 | MEQ | 80 (67) | — | 39 (33) |
|
Mota et al., 2016 (63) Brazil |
Healthy university medical residents 72 |
52 (72.2) | 20 (27.8) | 29.2 ± 2.0 | MEQ | 26 (36) | 36 (50) | 10 (14) |
|
Zerón-Rugerio et al., 2019 (64) Spain |
University students 534 |
137 (25.7) | 397 (74.3) | Range: 18–25 21.5 ± 3.0 | MEQ | 91 (17.0) | 333 (62.4) | 110 (20.6) |
|
Maukonen et al., 2019 (78) Finland |
Adults1097 | 619 (56.4) | 478 (43.6) | Range: 25–74 | MEQ | 552 (50.3) | 433 (39.5) | 112 (10.2) |
|
Maukonen et al., 2017 (79) Finland |
Adults 1854 |
1003 (54.1) | 851 (45.9) |
Range: 25–74 MT: 53.4 ± 0.4 IT: 48.4 ± 0.5 ET: 43.9 ± 0.9 |
MEQ | 904 (49) | 726 (39) | 224 (12) |
|
Maukonen et al., 2016 (65) Finland |
Adults 4421 |
2408 (54.5) | 2013 (45.5) | Range: 25–74 | MEQ | 1655 (37) | 1529 (35) | 1237 (28) |
| Teixeira et al., 2018 (66)Brazil |
Undergraduate students 721 |
488 (67.7) | 233 (32.3) | >18 | MEQ | 151 (21) | 446 (62) | 124 (17) |
|
Li et al., 2018 (74) China |
Undergraduate students 788 |
517 (65.6) | 271 (34.4) | 19.8 ± 1.1 | MEQ | 172 (21.8) | 495 (62.8) | 121 (15.45) |
|
De Amicis et al., 2020 (67) Italy |
Adults 416 |
289 (69.5) | 127 (30.5) | 50 ± 13 | MEQ | 135 (32.5) | 243 (58.1) | 38 (9.1) |
|
Culnan et al., 2013 (72) United States |
University undergraduates 135 |
79 (58) | 56 (40.9) | 18.25 ± 0.56 | MEQ | 7 (5) | 65 (48) | 64 (47) |
|
Baron et al., 2011 (75) United States |
Adult, volunteers 52 |
25 (48) | 27 (52) | Range: 18–71 31 ± 12 | Midpoint of sleep | — | 28 (54)12 | 23 (44)13 |
|
Baron et al., 2013 (68) United States |
Adults 52 |
25 (48) | 27 (52) | Range: 18–71 31 ± 12 | Midpoint of sleep | — | 28 (54)12 | 23 (44)13 |
|
Beaulieu et al., 2020 (69) England |
Adults 44 |
28 (63.6) | 16 (36.4) | Range: 18–25 | MEQ | 22 (50) | — | 22 (50) |
|
Muscogiuri et al., 2020 (70) Italy, Naples |
Middle-aged adults 172 |
123 (71.5) | 49 (28.5) | 51.8 ± 15.7 | MEQ | 100 (58.1) | 50 (29.2) | 22 (12.8) |
|
Zerón-Rugerio et al., 2020 (58) Mexico |
Undergraduate students 133 |
133 (100) | — | Range: 18–25 | Median splits of the time in which each participant went to bed and woke up | 34 (25.6)14 | 66 (49.6)15,16 | 33 (24.8)17 |
ET, evening type; IT, intermediate type; MCTQ, Munich Chronotype Questionnaire; MEQ, Morning–Eveningness Questionnaire; MSFsc, midsleep corrected for sleep duration on free days; MT, morning type.
Early chronotype was defined as a chronotype earlier than the median (03:04 h).
Late chronotype was defined as a chronotype later than the median (03:04 h).
Based on earliest midpoint of sleep quintiles.
Based on midpoint of sleep quintile 2.
Based on midpoint of sleep quintile 3.
Based on midpoint of sleep quintile 4.
Based on latest midpoint of sleep quintiles.
Based on MEQ score tertile 1: 34–53.
Based on MEQ score tertile 2: 54–59.
Based on MEQ score tertile 3: 60–76.
Based on normal sleep timing (midpoint 04:08 h).
Based on late sleep timing (midpoint of sleep 07:15 h).
Based on wakeup time <07:52 h and early bedtime <23:48 h and defined as early bedtime/early rise (EE).
Based on early bedtime (<23:48 h) and late rise (wakeup time ≥07:12 h) and defined as early bedtime/late rise (EL).
Based on late bedtime (≥23:48 h) and wakeup time (<07:52 h) and defined as late bedtime/early rise (LE).
Based on late bedtime (≥23:48 h) and late rise (wakeup time ≥07:12 h) and defined as late bedtime/late rise (LL).
The included studies assessed chronotypes using 1 of 2 validated questionnaires: the Morningness–Eveningness Questionnaire (MEQ) (39) or the MCTQ (80). Some of the studies used a mixed methodology by calculating midsleep from rest–activity recordings or using sleep and wake timings (from sleep logs) to create MT and ET categories. Most of the studies (n = 18) included in this review determined chronotype using the MEQ (57–59, 61–63, 65–67, 69, 70, 72–74, 76, 78, 79), 1 study used the MCTQ (77), 4 studies used calculations of the midpoint of sleep from rest–activity recordings (56, 60, 68, 75), and 1 used sleep and wake timings to create 4 categories (58) (Table 1). Therefore, there was some heterogeneity among the classification of the different chronotypes.
Most studies (n = 15) used the MEQ cutoff values to classify chronotypes (57, 61–64, 66, 67, 69, 70, 72–74, 76, 78, 79). However, Xiao et al. (77) and Vera et al. (71) classified participants as ET and MT based on the median chronotype score instead of using the cutoff values from the MEQ (i.e., IT = scores 42–58, MT = scores >58, or ET = scores <42) or the short version of the MEQ (see supplement) and the MCTQ (i.e., midsleep time <3 = MT; midsleep time 3–5 = IT; midsleep >5 = ET). Two studies used tertiles of chronotype scores using the MEQ (59, 65). For a detailed overview of the chronotype assessment methods used in each publication and thresholds of scores, see Supplemental Table 5 as well as Table 1.
Differences between chronotype and body composition or biomarkers
From the 24 included studies, 21 found significant differences between body composition outcomes and the different chronotypes (Table 2).
TABLE 2.
Differences between Chronotype and Body Composition or Biomarkers1
| Reference | Body composition distribution | Differences between types | |||
|---|---|---|---|---|---|
| MT | IT | ET | P value (ET vs. IT/MT) and other analysis | ||
| BMI, weight, and height | |||||
| Xiao et al., 2019 (77) |
Normal BMI: Women: 65.5% Men: 34.5% Overweight BMI: Women: 40.1% Men: 59.9% Obese BMI: Women: 52.8% Men: 47.2% |
—2 | — | Overweight (3.1 ± 1.0 h) and obese (3.2 ± 1.1 h) participants had on average a later chronotype in comparison with those with a normal BMI (2.9 ± 1.0 h)3 | P < 0.007 |
| — | — | Overweight (3.5 ± 0.9)/obese (3.6 ± 1.0) had a later midpoint of time in bed during weekends (3.6 ± 1.0) h in comparison with those with a normal BMI (3.3 ± 1.0)3 | P < 0.001 | ||
| Sato-Mito et al., 2011 (56) |
Height: 158 ± 5.3 cm Weight: 52.2 ± 7.6 kg BMI: 20.9 ± 2.8 |
MT421.2 ± 3.1 Underweight:14.2%Normal: 77.3%Overweight: 8.4% | IT5,6,720.9 ± 2.8Underweight: 14.6% Normal: 78.9%Overweight: 6.6%520.8 ± 2.5Underweight: 13.8%Normal: 79.4% Overweight: 6.9%620.9 ± 2.7Underweight: 15.3%Normal: 77.8%Overweight: 6.9%7 | ET820.9 ± 2.7 Underweight: 14.0%Normal: 79.0%Overweight and obese: 7.0% | P-trend = 0.30 |
| Vera et al., 2018 (71) | BMI: 31.3 ± 5.41 | 40.0 ± 0.16 | — | 31.3 ± 0.16ET showed a linear association toward higher BMI | P = 0.16P-trend = 0.02 |
| Najem et al., 2020 (76) | BMI: 22.3 ± 3.61 Range: 15.6–38.6 | — | — | — | Other analysis:No correlation (r= 0.025 , P = 0.54) between BMI and ME scores |
| Lázár et al., 2012 (73) | BMI: 23.7 ± 2.8 Range: 18–30 | — | — | — | — |
| Yoshizaki et al., 2018 (59) | BMI day workers:21.2 ± 2.7 | 21.2 ± 2.79 | 21.2 ± 2.610 | 21.1 ± 2.811 | P-trend = 0.33 |
| Silva et al., 2016 (60) | BMI: 22.8 ± 3.2Overweight and obese: n = 47 (23%) | — | — | — | — |
| Lai et al., 2013 (74) | BMI:Underweight: n = 270Normal: n = 585Overweight: n = 181Obese: n = 82 | — | — | — | Other analysis: BMI not correlated (r = 0.04; P = 0.15) with MES scores |
| Muñoz, 2020 (57) | Range: BMI >25Chrono group: 30.37 ± 2.56 | BMI changes: –3.4 ± 1.0 | — | BMI changes: –2.9 ± 0.6 | P = 0.219 |
| Lucassen et al., 2013 (62) | BMI: 38.5 ± 6.4Range: 30–55 | 38.2 ± 6.3 | — | 39.1 ± 6.6 | P = 0.47Other analysis:Scores toward ET were associated with an increase in BMI (P = 0.05, R2 = 0.06)Effect size: 10-unit change in chronotype score was associated with a change of 1.2 in BMI |
| Mota et al., 2016 (63) |
BMI: 22.9 ± 3.4 BMI ≥25: 33.4% |
Chronotype scores not associated with BMI (β-coefficient = −0.01) | P = 0.98 | ||
| Zerón-Rugerio et al., 2019 (64) | BMI: n = 21.7 (3.1%)Underweight: n = 54 (10.1%)Normal weight: n = 413 (77.3%) Overweight: n = 56 (10.5%)Obese: n = 11 (2.1%) | — | — | ET had a higher BMI (β-coefficient = –0.03) | P = 0.04 |
| Maukonen et al., 2019 (78) | BMI: MT: 26.5 (0.2)IT: 26.6 (0.2)ET: 26.7 (0.4) | No increase in BMI over 7-y follow-up period | — | Mean increase in BMI over 7- year follow-up period: 0.4 (0.2) | P = 0.23 |
| Proportion of subjects with BMI increases of ≥5% over the 7-y follow-up period: 22% | — | Higher proportion of subjects (33%) with BMI increases of ≥5% over the 7-y follow-up period | P > 0.05 | ||
| Obese at end of the follow-up: 17% of subjects | — | Obese at end of the follow-up: 26% of subjects | P = 0.061 | ||
| Increase in BMI of MT women: −0.1 | — | ET women had a greater increase in BMI (0.7) than MT women | P = 0.024 | ||
| Maukonen et al., 2017 (79) | — | 27.1 ± 0.2 | 26.7 ± 0.2 | 27.6 ± 0.3 | P = 0.44 |
| Not associated with chronotype score | P-trend = 0.66 | ||||
| Maukonen et al., 2016 (65) | BMI:MT: 27.2 (SE 0.13)IT: 27.1 (SE 0.09)ET: 26.9 (SE 0.16) | No difference in both sexes | P > 0.05 | ||
| Chronotype score was positively associated with BMI in menMTs were associated with a higher BMI in men (β-coefficient = 0.05) | — | — | P = 0.04 | ||
| Teixeira et al., 2018 (66) | — | 22.6 ± 3.2 | 22.3 ± 3.8 | 22.2 ± 3.6 | P = 0.71 |
| Overweight: n = 14.6 (22%) | Overweight: n = 18.8 (84%) | Overweight: n = 20.2 (25%) | P = 0.41 | ||
| Li et al., 2018 (74) | Weight:Underweight: n = 158 (20.1%)Normal: n = 585 (74.2%)Overweight: n = 32 (4.1%)Obese: n = 13 (1.6%) | — | — | — | Other analysis:Positive correlation between chronotype and BMI. MT was associated with a higher BMI (r = 0.51, P < 0.01) |
| De Amicis et al., 2020 (67) | — | 29.7 ± 5.6 | 29.1 ± 6.1 | 29.4 ± 6.1 | P > 0.05 |
| Culnan et al., 2013 (72) | Weight—baseline: 139 ± 28.8 kgWeight—follow-up: 143 ± 29.5 kgBMI—baseline: 22.0 ± 3.26BMI—follow-up: 22.9 ± 3.41 | Baseline: Chronotype not associated with weight (unstandardized β = –1.70) | P > 0.05 | ||
| Baseline: Chronotype not associated with BMI (unstandardized β = – 0.26) | P > 0.05 | ||||
| — | — | 8-wk follow-up: increase in BMI of 0.50 BMI points (unstandardized β = 0.50; 95% CI: 0.04, 0.95) | P = 0.03 | ||
| Baron et al., 2011 (75) | BMI:IT12: 23.7 ± 3.2ET13: 26.0 ± 6.9 | — | 2 of 27 ITs12 reported BMI ≥30 | 6 of 22 ETs13 reported BMI ≥30 | P = 0.15Other analysis:BMI positively correlated with ET13 (P < 0.01) |
| Baron et al., 2013 (68) | BMI:IT12: 23.7 ± 3.2ET13: 26.0 ± 6.9 | — | — | — | Other analysis:BMI moderately positive correlated with midpoint of sleep (r = 0.35, P < 0.05) |
| Beaulieu et al., 2020 (69) | BMI: 24.5 ± 3.2 | 24.1 ± 2.7 | — | 24.9 ± 3.6 | P = 0.01Other analysis:Inverse relation between MEQ score and BMI (ET showing a lower BMI r = −0.37, P = 0.01) |
| Weight: 72.9 ± 11.4 kg | 73.4 ± 10.3 kg | — | 72.4 ± 12.7 kg | P > 0.05 | |
| Muscogiuri et al., 2020 (70) | BMI: (32.1 ± 6.3)Normal BMI: 18 (10.5%)Overweight BMI: 47 (27.3%)Obesity: Class I: 58 (33.7%)Class II: 29 (16.9%)Class III: 20 (11.6) | 31.4 ± 5.8Normal BMI: 10 (10.0%)Overweight BMI: 33 (33.0%)Obesity:Class I: 32 (32.0%)Class II: 15 (15.0%)Class III:10 (10.0%) | 33.1 ± 7.3Normal BMI: 7 (14.0%)Overweight BMI: 9 (18.0%)Obesity:Class I: 15 (30.0%)Class II: 11 (22.0%)Class III: 8 (16.0%) | 32.6 ± 5.5Normal BMI:1 (4.5%)Overweight BMI: 5 (22.7%)Obesity:Class I: 11 (50.0%)Class II: 3 (13.6%)Class III: 2 (9.1%) | P = 0.27Other analysis:Chronotype was inversely correlated to BMI (r = −0.16, P = 0.04). MTs were associated with a lower BMI |
| Weight | 82.9 ± 19.0 kg | 88.1 ± 20.6 kg | 83.7 ± 12.5 kg | P = 0.29 | |
| Zerón-Rugerio et al., 2020 (58) | BMI: 23.7 ± 4.0 | 25.4 ± 4.014 | 23.8 ± 4.51523.0 ± 3.016 | 22.5 ± 3.817 | P = 0.02P-trend = 0.002 |
| Associated with increased BMI 2.315 | — | — | P < 0.05 | ||
| Body fat percentage, abdominal, visceral, and subcutaneous adipose tissue | |||||
| Vera et al., 2018 (71) | BF%: 37.2 ± 6.71 | BF%: 37.0 (0.19) | — | BF%: 37.0 (0.19) | P = 0.85P-trend = 0.54 |
| Muñoz et al., 2020 (57) | — | BF% changes between baseline and end point: – 4.2 ± 2.3 | — | BF% changes between baseline and end point: – 3.2 ± 2.1 | P = 0.28 |
| Maukonen et al., 2016 (65) | — | BF%: 35.2 (0.23) | BF%: 35.2 (0.15) | BF%: 35.3 (0.24) | P = 0.92 |
| Teixeira et al., 2018 (66) | — | Inadequate abdominal fat: n = 17.9 (27%) | Inadequate abdominal fat: n = 23.5 (105%) | Inadequate abdominal fat: n = 25.8 (32%) | P = 0.24 |
| De Amicis et al., 2020 (67) | — | SAT: 2.6 ± 1.3 cm | SAT: 2.5 ± 1.1 cm | SAT: 2.5 ± 1.3 cm | P > 0.05 |
| VAT: 5.1 ± 2.3 cmLower abdominal VAT for every 1 point of rMEQ scoreMTs were associated with lower VAT of −0.06 (–0.11, –0.01) cm | VAT: 5.1 ± 2.5 cm | VAT: 5.2 ± 2.9 cm | P > 0.05P < 0.05 | ||
| Beaulieu et al., 2020 (69) | BF%: 27.7 ± 8.3 | BF%: 27.3 ± 8.4 | — | BF%: 28.2 ± 8.4 | P > 0.05 |
| Zerón-Rugerio et al., 2020 (58) | — | Fat mass, %: 32.2 ± 7.414 | Fat mass, %: 31.5 ± 7.81530.5 ± 5.316 | Fat mass, %: 29.5 ± 6.417 | P = 0.39P-trend = 0.08 |
| Waist circumference | |||||
| Silva et al., (60) | Abdominal obesity: 31 (15%) | — | — | — | — |
| Muñoz et al., 2020 (57) | — | Changes between end point and baseline: –9.8 ± 2.7 cm | — | Changes between end point and baseline: –8.8 ± 3.6 cm | P = 0.44 |
| Lucassen et al., 2013 (62) | — | 113 ± 13.6 cm | — | 115 ± 11.5 cm | P = 0.51 |
| Mota et al., 2016 (63) | WC >94 cm in males and >30 cm in females: 33.3% | Chronotype scores were not associated with WC (β-coefficient = 0.09) | P = 0.41 | ||
| Maukonen et al., 2019 (78) | MT: 89.8 (SE 0.5) cmIT: 90.8 (SE 0.6) cmET: 92.3 (SE 1.1) cm | Mean increase: 2.2 cm for both types over the 7-y follow-up period | P = 1.00 | ||
| Proportion of subjects whose WC increased by ≥5% over 7-y follow-up period: 33% | — | Proportion of subjects whose WC increased by ≥5% over 7-y follow-up period: 39% | P > 0.05 | ||
| Maukonen et al., 2016 (65) | MT: 86 (SE 0.42) cmIT: 86.5 (SE 0.27) cmET: 86.9 (SE 0.43) cm | No difference in both sexes | P > 0.05 | ||
| Teixeira et al., 2018 (66) | — | 78.3 ± 8.3 cm | 79.0 ± 11.3 cm | 79.0 ± 11.6 cm | P = 0.75 |
| De Amicis et al., 2020 (67) | — | 98.4 ± 13.2 cmWC decreases by –0.19 as rMEQ score increasesMT associated with a lower WC | 97.8 ± 14.5 cm | 99.6 ± 13.5 cm | P > 0.05P < 0.01 |
| Beaulieu et al., 2020 (69) | 84.3 ± 7.9 cm | 84.2 ± 6.2 | — | 84.3 ± 7.9 cm | P > 0.05 |
| Muscogiuri et al., 2020 (70) | — | 103 ± 16.4 cm | 103 ± 17.3 cm | 105 ± 11.8 cm | P = 0.89Other analysis:Chronotype not correlated with WC (r = −0.04, P = 0.57) |
| Zerón-Rugerio et al., 2020 (58) | — | 98.4 ± 6.9 cm14 | 76.2 ± 9.7 cm1574.9 ± 8.4 cm16 | 72.8 ± 7.4 cm17 | P = 0.06P-trend = 0.01 |
| Associated with increased WC of 5.2 cm14 | P-trend < 0.05 | ||||
| Hip circumference | |||||
| Beaulieu et al., 2020 (69) | 98.4 ± 6.9 cm | 99.2 ± 4.8 cm | — | 97.6 ± 8.6 cm | P > 0.05 |
| Zerón-Rugerio et al., 2020 (58) | — | 99.5 ± 7.7 cm14 | 97.3 ± 10.7 cm1596.3 ± 6.8 cm16 | 95.2 ± 7.3 cm17 | P = 0.19P-trend = 0.03 |
| Waist-to-hip ratio | |||||
| Beaulieu et al., 2020 (69) | 0.86 ± 0.06 | 0.85 ± 0.07 | — | 0.86 ± 0.06 | P > 0.05 |
| Neck circumference | |||||
| Lucassen et al., 2013 (62) | — | 38.8 ± 3.8 cm | — | 39.6 ± 3.8 cm | P = 0.34Other analysis:Scores toward eveningness were associated with a larger NC (P = 0.03)Effect size: a 10-unit change in chronotype score was associated with a change of 0.6 cm in NC |
| Weight loss/gain | |||||
| Muñoz et al., 2020 (57) | — | Total weight loss, %: 10.2 ± 2.6 | — | Total weight loss, %: 9.6 ± 1.8 | P = 0.52 |
| Mota et al., 2016 (63) | — | — | — | Chronotype scores (MT, IT, ET) not associated with weight gain after the beginning of residency (β-coefficient = −0.10) | P = 0.48 |
| Maukonen et al., 2019 (78) | — | Mean weight gain: 0.6 kg | — | Mean weight gain: 1.4 kg | P = 0.35 |
| — | Proportion of subjects who gained weight of ≥5% over the 7-y follow-up period: 22% | — | Proportion of subjects who gained weight of ≥5% over the 7-y follow-up period: 37% | P > 0.05 | |
| — | Weight gain in MT women over the 7-y follow-up period: 0.3 kg | — | Weight gain in ET women over the 7-y follow-up period: 2.4 kg | P = 0.02 | |
| Culnan et al.,2013 (72) | — | — | — | 8-wk follow-up: weight gain of 2.35 pounds (1.07 kg) (unstandardized β = 2.35 pounds; 95% CI: –1.62, 4.87) | P = 0.07 |
| Biomarkers | |||||
| Vera et al., 2018 (71) | Fasting glucose: glucose oxidase methodTriglycerides and HDL cholesterol: commercial kits Arterial pressure: mercury sphygmomanometerMetS score: IDF criteria; summing MetS components Fasting insulin: solid-phase, 2-site chemiluminescent immunometric assay Insulin resistance: (HOMA-IR; fasting glucose × fasting insulin/22.5)Blood samples via standard procedures: DNA isolation and genotyping and GRS | Triglyceride concentrations: 101 ± 1.71 mg/dLMetS scores: 2.06 ± 0.04 HDL cholesterol concentrations: 57.1 ± 0.46 mg/dLInsulin concentrations: 7.40 ± 0.22 μUI/mLHOMA-IR concentrations: 1.61 ± 0.05 Not reported | — | Triglyceride concentrations: 105 ± 1.79 mg/dLMetS scores: 2.16 ± 0.04HDL cholesterol concentrations: 55.6 ± 0.48 mg/dLInsulin concentrations: 7.62 ± 0.23 μUI/mLHOMA-IR concentrations: 1.68 ± 0.06 Higher evening genetic risk score | P = 0.01P = 0.01P = 0.03P-trend < 0.001P-trend = 0.002P = 0.04 |
| Lázár et al., 2012 (73) | Genotyping of the PER3 VNTR was performed according to standard procedure | Frequency of PER35/5 genotype: 15.4% | — | Frequency of PER35/5 genotype: 7.5% | _ |
| Genotype: effect on diurnal preference measured by MEQ (F2, 619 = 4.43) | P = 0.01 | ||||
| Genotype: marginal effect on diurnal preference measured by MCTQ | P = 0.06 | ||||
| The main effect of genotype was significant for MEQ (F2, 636 = 5.97) | P = 0.003 | ||||
| The main effect of genotype was significant for the self-assessment question from the MCTQ (F2, 642 = 4.12) | P = 0.02 | ||||
| Lucassen et al., 2013 (62) | 24-h urinary epinephrine concentrations 3 (2–5) μg/24 h | — | 24-h urinary epinephrine concentrations: 4 (3–7) μg/24 h; 0–30% higher | P = 0.04 | |
| HDL cholesterol: 48 (42–58) mg/dL | — | HDL cholesterol: 49 (41–52) mg/dL | P = 0.51 | ||
| Resting heart rates: 68.4 ± 10.1 beats/min | — | Resting heart rates: 74.0 ± 10.1 beats/min | P = 0.01 | ||
| Plasma ACTH: 17 (12–24) pg/mL | — | Plasma ACTH: 21 (16–32) pg/mL | P = 0.02 | ||
| 24-h urinary norepinephrine: 39 (28–56) μg/24h | — | 24-h urinary norepinephrine: 45 (37–61) μg/24 h | P = 0.05 | ||
Values are reported as mean ± SD unless stated otherwise. BMI is reported in kg/m2 with the following categories: underweight, <18.5; normal, <18.5 to <25; overweight and obese, ≥25. OR (95% CI), P-trend refers to the continuous association between the MEQ or MCTQ score and exposures of interest. ACTH, adrenocorticotropic hormone; BF%, body fat percentage; ET, evening type; GRS, genetic risk score; IDF, International Diabetes Federation; IT, intermediate type; MCTQ, Munich Chronotype Questionnaire; MEQ, Morning–Eveningness Questionnaire; MES, Morningness-Eveningness Scale; MetS, metabolic syndrome; MT, morning type; NC, neck circumference; PER3, PERIOD3 clock gene; rMEQ, reduced Morning-Eveningness Questionnaire; SAT, subcutaneous adipose tissue; VAT, visceral adipose tissue; VNTR, variable number tandem repeat ; WC, waist circumference.
Early chronotype was defined as midsleep earlier than the median midsleep (03:04 h).
Later chronotype was defined as midsleep later than the median midsleep (03:04 h).
Based on earliest midpoint of sleep quintiles.
Based on midpoint of sleep quintile 2.
Based on midpoint of sleep quintile 3.
Based on midpoint of sleep quintile 4.
Based on latest midpoint of sleep quintiles.
Based on MEQ score tertile 1: 34–53.
Based on MEQ score tertile 2: 54–59.
Based on MEQ score tertile 3: 60–76.
Based on normal sleep timing (midpoint 04:08 h).
Based on late sleep timing (midpoint of sleep 07:15 h).
Based on wakeup time <07:52 h and early bedtime <23:48 h and defined as early bedtime/early rise (EE).
Based on early bedtime (<23:48 h) and late rise (wakeup time ≥07:12 h) and defined as early bedtime/late rise (EL).
Based on late bedtime (≥23:48 h) and wakeup time (<07:52 h) and defined as late bedtime/early rise (LE).
Based on late bedtime (≥23:48 h) and late rise (wakeup time ≥07:12 h) and defined as late bedtime/late rise (LL).
BMI, weight, and height
In comparison with other chronotypes, 2 studies reported a higher BMI in ET (64, 77), 1 study reported that ET compared with MT women had a greater increase in BMI (0.7 compared with −0.1, P = 0.024) (78), 1 study reported a linear relation toward a higher BMI in ET (MT: 30.99; ET: 31.3) (71), and 2 studies reported a correlation between ET and a higher BMI, ranging between 26.0 and 32.6 (70, 75). Three studies showed that being an ET was associated with an increase in BMI points of 0.50–1.2‡ (62, 72, 78). In contrast to these findings, 4 studies reported a higher BMI in MT than other chronotypes (58, 65, 69, 74).
Weight loss/gain
Four studies reported on weight gain/loss over time between ET and MT (57, 63, 67, 78), of which only 1 study by Maukonen et al. (78) reported that ET compared with MT women had a greater mean weight gain (+2.4 kg compared with +0.3 kg, P = 0.016) over a 7-y follow-up period.
Biomarkers
Only 4 studies investigated chronotype differences/associations with biomarkers (57, 62, 71, 73) (Table 2). Investigating the differences in lipid profile and glucose homeostasis, Vera et al. (71) found that ETs compared with MTs had higher concentrations of triglycerides (105 ± 1.79 mg/dL compared with 101 ± 1.71 mg/dL, P = 0.009) and lower HDL (55.6 ± 0.48 mg/dL compared with 57.1 ± 0.46 mg/dL, P = 0.026). The ETs had higher fasting blood insulin (7.62 ± 0.23 μUI/mL compared with 7.40 ± 0.22 μUI/mL, P < 0.001) and HOMA-IR scores (1.68 ± 0.06 compared with 1.61 ± 0.05) than MTs. Vera et al. (71) also calculated the metabolic syndrome score, which was higher for ETs compared with MTs (2.16 ± 0.04 compared with 2.06 ± 0.04, P = 0.011). Lucassen et al. (62) investigated resting heart rate, epinephrine, and morning plasma adrenocorticotropic hormone concentrations and found this to be higher in ETs (P = 0.007, P = 0.039, and P = 0.019, respectively) compared with ITs/MTs.
Differences between chronotype and dietary intake
Total daily energy intake
Fifteen studies reported total energy intake among chronotypes (56–59, 62, 63, 65, 66, 68, 69, 71, 75, 77–79) (Table 3). Only 1 study (63) found that chronotype scores (toward ETs) were negatively associated with energy intake/day, thus ET consuming significantly more energy than MTs (P= 0.02). However, across the other 14 studies, there were no differences in energy intakes between chronotypes ( 56–59, 62, 65, 66, 68, 69, 71, 75, 77–79). Furthermore, Teixeira et al. (66) found that if ETs also skipped breakfast, they would have a higher total energy intake per day.
TABLE 3.
Differences between Chronotype and Dietary Intake1
| Reference | Method of assessment | Differences between types | |||
|---|---|---|---|---|---|
| MT | IT | ET | P value (ET vs. IT/MT) and other analysis | ||
| Total daily energy intake | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | 2114.5 ± 634 kcal/d2 | — | 2147.4 ± 588 kcal/d3 | P-trend = 0.33 |
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | 1836 ± 20 kcal/d4 | 1776 ± 16 kcal/d51803 ± 17 kcal/d61814 ± 17 kcal/d7 | 1768 ± 18 kcal/d8 | P-trend = 0.10 |
| Vera et al., 2018 (71) | Single 24-h recalls | 1972.8 ± 23.8 kcal/d | — | 1918.6 ± 24.68 kcal/d | P = 0.12P-trend = 0.94 |
| Yoshizaki et al., 2018 (59) | A semiquantitative FFQ | 1854 ± 29 kcal/d9 | 1853 ± 27 kcal/d10 | 1825 ± 26 kcal/d11 | P-trend = 0.47 |
| Lucassen et al., 2013 (62) | 3-d food recall diary | Working day: 2129 ± 631 kcalNonworking day: 2383 ± 928 kcal | — | Working day: 2276 ± 815 kcalNonworking day: 2378 ± 883 kcal | P = 0.37P = 0.92 |
| Mota et al., 2016 (63) | 3-d self-administered food diary | — | — | Chronotype scores (toward ET) were negatively associated with daily energy intake; kcal/kg/dETs were associated with higher intake (β coefficient = −0.28) | P = 0.02 |
| Maukonen et al., 2019 (78) | 48-h dietary recalls over 2 previous consecutive days | 7709 (SEM 97) kJ | — | 7679 (SEM 215) kJ | P = 1.00 |
| Maukonen et al., 2017 (79) | 48-h dietary recalls | 7808 (SEM 170) kJ on weekdays | 7960 (SEM 171) kJ on weekdays | 7881 (SEM 210) kJ on weekdays | P = 1.00 |
| 7841 (SEM 283) kJ on weekends | 7871 (SEM 283) kJ on weekends | 7992 (SEM 367) kJ on weekends | P = 1.00 | ||
| Maukonen et al., 2016 (65) | FFQ; Baltic Sea diet score | Men: 11,597 (SEM 130) kJ/dWomen: 9489 (SEM 103) kJ/d | Men: 11,676 (SEM 90) kJ/dWomen: 9433 (SEM 64) kJ/d | Men: 11,776 (SE 159) kJ/dWomen: 9389 (SE 105) kJ/d | P-trend = 0.43P-trend = 0.54 |
| Teixeira et al., 2018 (66) | 24-h recall | 1552.8 [1233.4–2090.6] kcal/d | 1734.2 [1356.3–2218.3] kcal/d | 1692.9 [1333.8–2197.9] kcal/d | P = 0.07 |
| — | — | Breakfast skippers were negatively associated with energy intake (kcal/d)ET breakfast skippers had higher intake β = −0.25 | P < 0.001 | ||
| Baron et al., 2011 (75) | 7-d food logs | — | 1905 ± 526 kcal/d12 | 2153 ± 524 kcal/d13248 kcal/d5 | P = 0.10 |
| Baron et al., 2013 (68) | 7-d food logs | — | 1905 ± 526 kcal/d12 | 2153 ± 524 kcal/d13 | p > 0.05 |
| Beaulieu et al., 2020 (69) | 24-h dietary record tool (myfood24) | 1843 ± 681 kcal/d | — | 1737 ± 659 kcal/d | p > 0.05 |
| Zerón-Rugerio et al., 2020 (58) | 6-d food logs | 1517 ± 404 kcal/d14 | 1596 ± 425 kcal/d151555 ± 412 kcal/d16 | 1676 ± 420 kcal/d17 | P = 0.45 |
| Total daily carbohydrate intake | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | Carbohydrate: 240.5 ± 79.0 g/d2 | — | Carbohydrate: 244.9 ± 72.3 g/d3 | P-trend = 0.27 |
| Sugar: 103 ± 46.6 g/d2 | Sugar: 109 ± 43.9 g/d3 | P-trend = 0.02 | |||
| Fiber:19.9 ± 8.1 g/d2 | Fiber: 19.8 ± 7.7 g/d3 | P-trend = 0.96 | |||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | 56.3 ± 0.3 E%4 | 55.9 ± 0.2 E%555.5 ± 0.3 E%655.4 ± 0.2 E%7 | 55.1 ± 0.3 E%8 | P-trend < 0.01 |
| Vera et al., 2018 (71) | Single 24-h recalls | 205 ± 3.07 g/d | — | 194 ± 3.18 g/d | P = 0.02P-trend = 0.67 |
| Yoshizaki et al., 2018 (59) | A semiquantitative FFQ | 235 ± 4.30 g/d9 | 237 ± 4.0 g/d10 | 230 ± 3.9 g/d11 | P-trend = 0.50 |
| Lucassen et al., 2013 (62) | 3-d food recall diary | No significant differences in total intakes before and after 20:00 h | P = 0.84 | ||
| Mota et al., 2016 (63) | 3-d self-administered food diary | — | — | Chronotype scores were negatively associated with carbohydrate (g/kg/d)ETs had a higher intake (β = –0.26) | P = 0.03 |
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Weekdays: 48.6 (0.6) E% | Weekdays: 48.1 (0.6) E% | Weekdays: 48.8 (0.7) E% | P = 1.00 |
| Weekends: 49.6 (0.8) E% | Weekends: 48.8 (0.8) E% | Weekends: 47.8 (1.0) E% | P = 0.09 | ||
| ME score was positively associated with carbohydrate intakes on weekendsMTs were associated with higher intake on weekends | — | — | P-trend = 0.04 | ||
| Fiber: 2.5 (0.1) E% on weekdays | Fiber: 2.4 (0.1) E% on weekdays | Fiber: 2.5 (0.1) E% on weekdays | P = 1.00 | ||
| Fiber: 2.5 (0.1) E% on weekends | Fiber: 2.4 (0.1) E% on weekends | Fiber: 2.4 (0.1) E% on weekends | P = 1.00 | ||
| Fiber: ME score was positively associated with fiber intakes on weekendsMTs were associated with higher intake | — | — | P-trend = 0.04 | ||
| Sucrose: 9.5 (0.4) E% on weekdays | Sucrose: 9.4 (0.4) E% on weekdays | Sucrose: 10.1 (0.5) E% on weekdays | P = 0.46 | ||
| — | — | Sucrose: Intakes increased with lower ME scores (ET) on weekdays | P-trend = 0.02 | ||
| Sucrose: 10.3 (0.5) E% on weekends | Sucrose: 10.0 (0.5) E% on weekends | Sucrose: 9.7 (0.7) E% on weekends | P = 0.91 | ||
| Teixeira et al., (66) | 24-h food recall | Carbohydrate: 198.6 [155.6–275.1] g/d | Carbohydrate: 226.4 [169.2–295.5] | Carbohydrate: 225.3 [169.9–293.2] | P = 0.10 |
| — | — | Breakfast skippers were negatively associated with carbohydrate intake (g/d)ET breakfast skippers had higher intake (β-coefficient = – 0.19) | P < 0.05 | ||
| Fiber: 16.0 [10.2–21.8] g/d | Fiber: 15.8 [10.9–22.1] g/d | Fiber: 15.6 [10.6–21.1] g/d | P = 0.93 | ||
| Baron et al., 2011 (75) | 7-d food logs | — | 49 ± 7.9 E%12 | 49 ± 7.8 E%13 | P > 0.05 |
| Baron et al., 2013 (68) | 7-d food logs | — | 237 ± 81 g/d1249 ± 7.9 E%12 | 260 ± 72 g/d1349 ± 7.8 E%13 | P > 0.05 |
| Total daily protein intake | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | 87.4 ± 27.1 g/d2 | — | 87.7 ± 27.6 g/d3 | P-trend = 0.97 |
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | 13.5 ± 0.1 E%4 | 13.6 ± 0.1 E%513.5 ± 0.1 E%613.3 ± 0.1 E%7 | 13.2 ± 0.1 E%8 | P-trend < 0.01 |
| Vera et al., 2018 (71) | Single 24-h recalls | 83.01 ± 1.11 g/d | — | 82.34 ± 1.15 g/d | P = 0.68P-trend = 0.94 |
| Yoshizaki et al., 2018 (59) | A semiquantitative FFQ | 66.0 ± 1.2 g/d9 | 64.1 ± 1.1 g/d10 | 63.4 ± 1.0 g/d11 | P-trend = 0.08 |
| Lucassen et al., 2013 (62) | 3-d food recall diary | No significant difference in total intakes before and after 20:00 h | P = 0.89 | ||
| Mota et al., 2016 (63) | 3-d self-administered food diary | — | — | Chronotype score was negatively associated with protein intake (g/kg/d)ETs had a higher intake (β-coefficient = −0.23) | P = 0.04 |
| Maukonen et al., 2017 (79) | 48-h dietary recalls | 17.3 (0.3) E% on weekdays | 17.4 (0.3) E% on weekdays | 16.4 E% (0.3) on weekdays | P = 0.02 |
| Teixeira et., 2018 (66) | 24-h food recall | 71.9 [55.0–97.2] g/d | 79.3 [60.0–100.2] g/d | 75.6 [57.3–105.8] g/d | P = 0.16 |
| Baron et al., 2011 (75) | 7-d food logs | — | 14 ± 2.7 E%12 | 15 ± 2.0 E%13 | P > 0.05 |
| Baron et al., 2013 (68) | 7-d food logs | — | 69 ± 21 g/d (14%)12 | 84 ± 26 g/d (15%)13 | P > 0.05 |
| Total daily fat intake | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | Fat: 84.4 ± 31.6 g/d2 | — | Fat: 85.6 ± 30.0 g/d3 | P-trend = 0.43 |
| Saturated fat: 28.2 ± 11.3 g/d2 | — | Saturated fat: 28.8 ± 11.3 g/d3 | P-trend = 0.50 | ||
| Polyunsaturated fat: 18.5 ± 7.7 g/d2 | — | Polyunsaturated fat: 18.5 ± 7.3 g/d3 | P-trend = 0.85 | ||
| Monounsaturated fat: 30.4 ± 12.3 g/d2 | — | Monounsaturated fat: 31.0 ± 11.3 g/d3 | P-trend = 0.24 | ||
| Cholesterol: 304.3 ± 139.6 g/d2 | — | Cholesterol: 308.0 ± 147.9 g/d3 | P-trend = 0.73 | ||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Fat: 28.9 ± 0.2 E%4 | Fat: 29.3 ± 0.2 E%529.7 ± 0.2 E%629.9 ± 0.21E%7 | Fat: 30.1 ± 0.2 E%8 | P-trend < 0.01 |
| Cholesterol: 168 ± 3 mg/1000 kcal4 | Cholesterol: 169 ± 2 mg/1000 kcal5165 ± 2 mg/1000 kcal6161 ± 2 mg/1000 kcal7 | Cholesterol: 162 ± 3 mg/1000 kcal8 | P-trend < 0.05 | ||
| Vera et al., 2018 (71) | Single 24-h recalls | 93.79 ± 1.500 g/d | — | 93.03 ± 1.54 g/d | P-trend = 0.73P-trend = 0.49 |
| Yoshizaki et al., 2018 (59) | A semiquantitative FFQ | 65.8 ± 1.3 g/d9 | 66.0 ± 1.2 g/d10 | 66.3 ± 1.10 g/d11 | P-trend = 0.88 |
| Lucassen et al., 2013 (62) | 3-d food recall diary | No significant differences in total intakes before and after 20:00 h | P = 0.14 | ||
| Mota et al., 2016 (63) | 3-d self-administered food diary | — | — | Chronotype score was negatively associated with cholesterol intake (mg/d)ETs had a higher intake (β-coefficient = −0.24) | P = 0.04 |
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Fat: 31.7 (0.6) E% on weekdays | Fat: 32.1 (0.6) E% on weekdays | Fat: 32.3 (0.7) E% on weekdays | P = 0.81 |
| Fat: 31.1 (0.7) E% on weekends | Fat: 32.0 (0.7) E% on weekends | Fat: 33.1 (0.9) E% on weekends | P = 0.05 | ||
| Fat: Inversely associatedHigher intake on weekends | P-trend < 0.05 | ||||
| SFAs: 11.6 (0.3) E% on weekdays | SFAs: 11.9 (0.3) E% on weekdays | SFAs: 11.8 (0.3) E% on weekdays | P = 1.00 | ||
| SFAs: 11.2 (0.4) E% on weekends | SFAs: 11.7 (0.4) on weekends | SFAs: 12.2 (0.5) E% on weekends | P = 0.06 | ||
| ME score was inversely associated on weekendsETs were associated with higher intake of SFAs | P-trend < 0.05 | ||||
| Maukonen et al., 2016 (65) | FFQ; Baltic Sea diet score | Fat: 32 E% in men | Fat: 32 E% in men | Fat: 32 E% in men | P-trend = 0.67 |
| Fat: 30 E% in women | Fat: 31 E% in women | Fat: higher intake of 31 E% in women | P-trend = 0.02 | ||
| Teixeira et al., 2018 (66) | 24-h food recall | Fat: 45.2 [33.9–69.2] g/d | Fat: 53.7 [38.6–69.8] g/d | Fat: 53.6 [34.7–74.5] g/d | P = 0.10 |
| Breakfast skippers were negatively associated with fat intake (g/d)ET breakfast skippers had higher intake (β-coefficient = –0.18) | P < 0.05 | ||||
| Cholesterol: 180.5 [102.7–278.9] mg/d | Cholesterol: 208.0 [142.1–296.6] mg/d | Cholesterol: 193.5 [127.6–297] mg/d | P = 0.18 | ||
| Baron et al., 2011 (75) | 7-d food logs | — | 38 ± 7.2 E%12 | 35 ± 7.7 E%13 | P > 0.05 |
| Baron et al., 2013 (68) | 7-dfood logs | — | 78 ± 23 g/d (38%)12 | 82 ± 24 g/d (35%)13 | P > 0.05 |
| Total daily micronutrient intake | |||||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Potassium: 1094 ± 12 mg/1000 kcal4 | Potassium: 1101 ± 10 g/1000 kcal51084 ± 11 mg/1000 kcal61083 ± 10 mg/1000 kcal7 | Potassium: 1046 ± 11 mg/1000 kcal8 | P-trend < 0.05 |
| Magnesium: 120 ± 1 mg/1000 kcal4 | Magnesium: 121 ± 1 mg/1000 kcal5120 ± 1 mg/1000 kcal6119 ± 1 mg/1000 kcal7 | Magnesium: 115 ± 1 mg/1000 kcal8 | P-trend < 0.05 | ||
| Iron: 3.73 ± 0.04 mg/1000 kcal4 | Iron: 3.72 ± 0.03 mg/1000 kcal53.70 ± 0.03 mg/1000 kcal63.70 ± 0.03 mg/1000 kcal7 | Iron: 3.59 ± 0.04 mg/1000 kcal8 | P-trend < 0.05 | ||
| Zinc: 4.12 ± 0.02 mg/1000 kcal4 | Zinc: 4.14 ± 0.02 mg/1000 kcal54.11 ± 0.02 mg/1000 kcal64.07 ± 0.02mg/1000 kcal7 | Zinc: 4.04 ± 0.02 mg/1000 kcal8 | P-trend < 0.05 | ||
Vitamin A: 308 ± 10 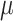 g/1000 kcal4 g/1000 kcal4
|
Vitamin A: 294 ± 9 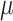 g/1000 kcal5287 ± 9 g/1000 kcal5287 ± 9 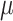 g/1000 kcal6297 ± 9 g/1000 kcal6297 ± 9 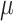 g/1000 kcal7 g/1000 kcal7
|
Vitamin A: 271 ± 10 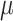 g/1000 kcal8 g/1000 kcal8
|
P-trend < 0.05 | ||
Vitamin D: 3.7 ± 0.1 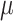 g/1000 kcal4 g/1000 kcal4
|
Vitamin D: 3.7 ± 0.1 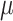 g/1000 kcal53.6 ± 0.1 g/1000 kcal53.6 ± 0.1 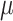 g/1000 kcal63.5 ± 0.1 g/1000 kcal63.5 ± 0.1 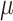 g/1000 kcal7 g/1000 kcal7
|
Vitamin D: 3.4 ± 0.1 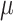 g/1000 kcal8 g/1000 kcal8
|
P-trend < 0.01 | ||
| Pyridoxine: 0.53 ± 0.01 mg/1000 kcal4 | Pyridoxine: 0.54 ± 0.01 mg/1000 kcal50.53 ± 0.01 mg/1000 kcal60.52 ± 0.01 mg/1000 kcal7 | Pyridoxine: 0.51 ± 0.01 mg/1000 kcal8 | P-trend < 0.01 | ||
| Riboflavin: 0.70 ± 0.01 mg/1000 kcal4 | Riboflavin: 0.69 ± 0.01 mg/1000 kcal50.69 ± 0.01 mg/1000 kcal60.69 ± 0.01 mg/1000 kcal7 | Riboflavin: 0.67 ± 0.01 mg/1000 kcal8 | P-trend < 0.01 | ||
| Thiamine: 0.41 ± 0.003 mg/1000 kcal4 | Thiamine: 0.42 ± 0.003 mg/1000 kcal50.41 ± 0.003 mg/1000 kcal60.41 ± 0.003 mg/1000 kcal7 | Thiamine: 0.40 ± 0.004 mg/1000 kcal8 | P-trend < 0.01 | ||
Folate: 156 ± 2 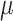 g/1000 kcal4 g/1000 kcal4
|
Folate: 155 ± 2 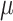 g/1000 kcal5153 ± 2 g/1000 kcal5153 ± 2 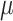 g/1000 kcal6155 ± 2 g/1000 kcal6155 ± 2 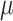 g/1000 kcal7 g/1000 kcal7
|
Folate: 145 ± 2 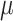 g/1000 kcal8 g/1000 kcal8
|
P-trend < 0.01 | ||
| Calcium: 275 ± 4 mg/1000 kcal4 | Calcium: 273 ± 4 mg/1000 kcal5269 ± 4 mg/1000 kcal6266 ± 4 mg/1000 kcal7 | Calcium: 251 ± 4 mg/1000 kcal8 | P-trend < 0.001 | ||
| Total daily intake of food groups | |||||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Alcohol: 0.19 ± 0.05 E%4 | Alcohol: 0.13 ± 0.04 E%50.24 ± 0.05 E%60.29 ± 0.04 E%7 | Alcohol: 0.44 ± 0.05 E%8 | P-trend < 0.01 |
| Rice: 171.4 ± 2.9 g/1000 kcal4 | Rice: 167.7 ± 2.5 g/1000 kcal5158.0 ± 2.6 g/1000 kcal6153.6 ± 2.5 g/1000 kcal7 | Rice: 150.0 ± 2.8 g/1000 kcal8 | P-trend < 0.001 | ||
| Vegetables: 126.7 ± 3.1 g/1000 kcal4 | Vegetables: 127.5 ± 2.6 g/1000 kcal5121.9 ± 2.8 g/1000 kcal6121.3 ± 2.6 g/1000 kcal7 | Vegetables: 109.8 ± 2.9 g/1000 kcal8 | P-trend < 0.001 | ||
| Pulses: 26.6± 0.8 g/1000 kcal4 | Pulses: 25.8 ± 0.7 g/1000 kcal525.7 ± 0.7 g/1000 kcal624.7 ± 0.7 g/1000 kcal7 | Pulses: 22.5 ± 0.8 g/1000 kcal8 | P-trend < 0.001 | ||
| Eggs: 19.3 ± 0.6 g/1000 kcal4 | Eggs: 19.4 ± 0.5 g/1000 kcal518.1 ± 0.5 g/1000 kcal617.1 ± 0.5 g/1000 kcal7 | Eggs: 17.4 ± 0.6 g/1000 kcal8 | P-trend < 0.001 | ||
| Noodles: 28.8 ± 1.4 g/1000 kcal4 | Noodles: 33.6 ± 1.2 g/1000 kcal536.5 ± 1.2 g/1000 kcal638.5 ± 1.2 g/1000 kcal7 | Noodles: 46.4 ± 1.3 g/1000 kcal8 | P-trend < 0.001 | ||
| Dairy: 77.4 ± 3.1 g/1000 kcal4 | Dairy: 76.5 ± 2.6 g/1000 kcal574.3 ± 2.8 g/1000 kcal671.4 ± 2.6 g/1000 kcal7 | Dairy: 65.6 ± 2.9 g/1000 kcal8 | P-trend < 0.05 | ||
| Confections: 42.5 ± 1.0 g/1000 kcal4 | Confections: 41.8 ± 0.8 g/1000 kcal544.8 ± 0.9 g/1000 kcal646.8 ± 0.9 g/1000 kcal7 | Confections: 46.7 ± 1.0 g/1000 kcal8 | P-trend < 0.05 | ||
| Meat: 33.1 ± 0.7 g/1000 kcal4 | Meat: 34.0 ± 0.6 g/1000 kcal534.8 ± 0.6 g/1000 kcal633.4 ± 0.6 g/1000 kcal7 | Meat: 35.7 ± 0.7 g/1000 kcal8 | P-trend < 0.05 | ||
| Vera et al., 2018 (71) | Single 24-h recalls | — | — | Lower intake of cereals | P < 0.05Other analysis:ETs have 1.3 times higher odds for alcohol (OR: 1.52; 95% CI: 1.25, 1.86; P < 0.001) |
| Najem et al., 2020 (76) | Yale Food Addiction Scale (YFAS) | — | — | — | Other analysis:ME score negatively correlated with number of units of caffeine-containing beverages/dETs were associated with higher intake of units of caffeine beverages/d (r = – 0.14, P = 0.00) |
| Lázár et al., 2012 (73) | Medical Questionnaire | Alcohol: reported lower intake | — | — | P < 0.001 |
| Daily caffeine intake was associated with diurnal preference; MTs reported lower intake | — | — | P < 0.05 | ||
| Yoshizaki et al., 2018 (59) | A semiquantitative FFQ | Potatoes and starches intake: higher intake of 36.4 ± 1.7 g/d9 | Potatoes and starches: 32.7 ± 1.6 g/d10 | Potatoes and starches: 30.9 ± 1.5 g/d11 | P-trend = 0.04 |
| Green/yellow vegetables: higher intake of 76.2 ± 2.2 g/d9 | Green/yellow vegetables: 67.1 ± 2.1 g/d10 | Green/yellow vegetables: 65.4 ± 2.0 g/d11 | P-trend < 0.001Other analysis:MTs9 were associated with a higher intake (β = 0.15, P < 0.001) | ||
| White vegetables: higher intake of 123 ± 3.7 g/d9 | White vegetables: 112 ± 3.4 g/d10 | White vegetables: 112 ± 3.3 g/d11 | P-trend = 0.01Other analysis:White vegetables: associated with high chronotype scoreMTs9 were associated with a higher intake (β = 0.11, P < 0.001) | ||
| Fruit: higher intake of 81.9 ± 3.8 g/d9 | Fruit: 72.7 ± 3.5 g/d10 | Fruit: 59.9 ± 3.4 g/d11 | P-trend < 0.001Other analysis:Fruit: associated with high chronotype scoreMTs9 were associated with a higher intake (β = 0.11, P < 0.001) | ||
| Algae: higher intake of 4.6 ± 0.2 g/d9 | Algae: 4.3 ± 0.2 g/d10 | Algae: 4.1 ± 0.2 g/d11 | P-trend = 0.02Other analysis:Algae: associated with high chronotype scoreMTs9 were associated with a higher intake (β = 0.10, P < 0.001) | ||
| Confectioneries/savory snacks: 80.7 ± 2.9 g/d9 | Confectioneries/savory snacks: 89.2 ± 2.7 g/d10 | Confectioneries/savory snacks: 94.9 ± 2.6 g/d11 | P-trend = 0.001Other analysis:Confectioneries/savory snacks: negatively associated with high chronotype scoreETs11 were associated with a higher intake (β = –0.10, P < 0.001) | ||
| Sugar-sweetened beverages: 42.7 ± 5.4 g/d9 | Sugar-sweetened beverages: 43.8 ± 5.0 g/d10 | Sugar-sweetened beverages: 60.8 ± 4.9 g/d11 | P-trend = 0.01Other analysis:Sugar-sweetened beverages: negatively associated with high chronotype scoreETs11 were associated with a higher intake (β = –0.13, P < 0.001) | ||
| Silva et al., 2016 (60) | FFQ | — | — | Meat: ET associated with a higher intake (β = 0.21) | P = 0.003 |
| Mota et al., 2016 (63) | 3-d self-administered food diary | — | — | Chronotype score was negatively associated with:Intake of sweets (servings/d)ETs had a higher intake (β-coefficient = −0.27)Vegetable intake (servings/d)ETs had a higher intake (β-coefficient = −0.26) |
P = 0.03 P = 0.04 |
| Chronotype score was positively associated with oil and fat intake (servings/d)MTs had a higher intake (β-coefficient = 0.27) | — | — | P = 0.03 | ||
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Alcohol: 4.6 (1.5) g on weekdays | Alcohol: 4.3 (1.5) g on weekdays | Alcohol: 9.7 (1.9) g on weekdaysAlcohol: Intakes increased with lower ME scores (ET) on weekdays | P = 0.57P-trend = 0.04 |
| Maukonen et al., 2016 (65) | FFQ; Baltic Sea diet score | Cereals: women 85 g/d and men 89 g/d | Cereals: women: 79 g/d and men: 84 g/d | Cereals: women 74 g/d and men 78 g/d | P-trend < 0.001 |
| Fish: men 55 g/d | Fish: men 53 g/d | Fish: Men consumed less, 49 g/d | P-trend < 0.05 | ||
| Alcohol: women 3.6 g/d and men 10.6 g/d | Alcohol: women: 4.4 g/d and men: 11.8 g/d | Alcohol: Consumed more. Women 5.1 g/d and men 13.3 g/d | Women: P-trend < 0.001Men: P-trend = 0.003 | ||
| Li, Wu et al., 2018 (74) | Sugary beverage consumption: number of bottles or tins consumed per day last week | — | — | — | Other analysis: Negative direct effects were found between chronotype and sugary beverage consumptionMTs had lower intake (β = − 0.15, SE = 0.03, P < 0.01) |
| Culnan et al., 2013 (72) | Gray–Donald Eating Patterns Questionnaire | Alcohol: Baseline: No difference in alcohol intake | P > 0.05 | ||
| Caffeine: No difference between chronotypes at baseline | P > 0.05 | ||||
| — | — | At follow-up: More ETs reported drinking alcohol [χ2(1, n = 54) = 5.94] | P < 0.05 | ||
| At follow-up: ETs not more likely to change alcohol drinking status throughout study [χ2(1, n = 54) = 3.19] | P > 0.05 | ||||
| Baron et al., 2011 (75) | 7-d food logs | — | Fruit and vegetables: 3.4 ± 1.8 servings/d12 | Fruit and vegetables: lower intake of 1.9 ± 1.1 servings/d13 | P < 0.01Other analysis:Fruit and vegetable intakes were negatively correlated with sleep timing (r = –0.49, P < 0.01)ITs12 were associated with higher intakes |
| Fast-food meals: 3.0 ± 1.8 servings/wk12 | Fast-food meals/wk: higher intake of 5.2 ± 3.8 servings/wk13 | P < 0.05 | |||
| Full-calorie sodas: 1.3 ± 2.5 servings/wk12 | Full-calorie sodas: higher intake of 4.5 ± 6.5 servings/wk13 | P < 0.05 | |||
| — | Caffeinated drinks: 7.3 ± 6.5 servings/wk12 | Caffeinated drinks: trend for higher intake: 13.0 ± 12.6 servings/wk13 | P < 0.10 | ||
| Muscogiuri et al., 2020 (70) | PREDIMED questionnaire | — | — | — | Other analysis:Food intake negatively associated with chronotype scoreETs associated with a higher OR for:Red/processed meat <1/d (OR: 1.05; 95% CI: 1.02, 1.08; P < 0.001); butter, cream, margarine <1/d (OR: 1.05; 95% CI: 1.02, 1.08;; P = 0.001)Commercial sweets/confectionary ≤2/wk (OR: 1.04; 95% CI: 1.01, 1.06; P = 0.007)Soda drinks <1/d (OR: 1.04; 95% CI: 1.01, 1.07; P = 0.001)Food intake positively associated with chronotype score. MTs were associated with a higher OR for:EVOO >4 tbs (OR: 1.03; 95% CI: 1.00, 1.06; P = 0.01)Vegetables ≥2 servings/d (OR: 1.05; 95% CI: 1.02, 1.07; P < 0.001)Fruit ≥3 servings/d (OR: 1.07; 95% CI: 1.04, 1.10; P < 0.001)Fish/seafood ≥3/wk (OR: 1.037; 95% CI: 1.00, 1.06; P = 0.02)Poultry more than red meats (OR: 1.05; 95% CI: 1.03, 1.08; P < 0.001)Tree nuts ≥3/wk (OR: 1.03; 95% CI: 1.00, 1.06; P = 0.01)Wine (glasses) ≥7/wk (OR: 1.05; 95% CI: 1.01, 1.09; P = 0.004) Most predictive factor of chronotype score among single contributing PREDIMED food items and score:Both MTs (R2 = 0.18, P < 0.001) |
| and ETs (R2 = 0.23, P = 0.02) most influenced by PREDIMED score and IT most influenced by butter, cream, and margarine <1/d (R2 = 0.09, P = 0.04) | |||||
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Alcohol: 1.8 (0.7) g after 20:00 on weekdays | Alcohol: 1.9 (0.7) g after 20:00 on weekdays | Alcohol: 4.0 (0.9) g after 20:00 on weekdays | P = 0.09 |
| — | — | Alcohol: Intake increased with lower ME score values (ET) after 20:00 on weekdays | P-trend < 0.05 | ||
| Daily energy distribution | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | — | — | — | Other analysis:Higher energy intake in morning window (within 2 h after getting out of bed) associated with lower OR for overweight/obese in MT2 (OR: 0.32; 95% CI: 0.16, 0.66; P-trend = 0.0006) |
| — | — | — | Other analysis:Higher energy intake at nighttime window (within 2 h before bedtime), associated with higher OR for overweight/obese in ETs3 (OR: 4.94; 95% CI: 1.61, 15.1; P-trend = 0.01) | ||
| Muñoz et al., 2020 (57) | Hypocaloric dietary treatment according to the Spanish Federation of Nutrition, Food and Dietetics guidelines | Breakfast 30%, midmorning 10%, lunch 35%, midafternoon 5%, and dinner 20% | — | Breakfast 20%, midmorning 5%, lunch 35%, midafternoon 10%, and dinner 30% | — |
| Maukonen et al., 2017 (79) | 48-h dietary recalls | 99% of MTs had energy intake >0 kJ on weekday mornings by 10:00 | — | 80% of ETs had energy intake >0 kJ on weekday mornings by 10:00 | — |
| — | — | Weekday mornings: 350 kJ (4% TEI) lower energy intake as compared with MTs | P < 0.001 | ||
| — | — | Weekend mornings by 10:00: 380 kJ lower energy than MTs | P = 0.004 | ||
| 81% of MTs had energy intake >0 kJ on weekday evenings by 20:00 | — | 94% of ETs had energy intake >0 kJ on weekday evenings by 20:00 | — | ||
| — | — | Weekday evenings by 20:00: 430 kJ (6% TEI) more energy than MTs | P < 0.001 | ||
| — | — | Weekend evenings by 20:00: 590 kJ (7% TEI) more energy than MTs | P < 0.001 | ||
| — | — | Cumulative energy intake of ET: Weekdays: lower from the beginning of the day until 22:00 Weekends: lower from the beginning of the day until 01:00 | — | ||
| Weekends: 3 peaks of energy intake of the same height at 08:00, 12:00, and 17:00 | — |
Weekdays: energy intake peaks on weekdays are an hour later than MTs Weekends: 6 peaks of energy intakeHighest peak at 19:00 |
— | ||
| Sucrose: 12.5 (1.2) E% after 20:00 on weekdays | Sucrose: 13.4 (1.2) E% after 20:00 on weekdays | Sucrose: 1.1 E% units more after 20:00 on weekdays 13.6 (1.5) E% | P < 0.05 | ||
| Sucrose: 10.2 (1.9) E% after 20:00 on weekends | Sucrose: 13.8 (1.9) after 20:00 on weekends | Sucrose: 3.1 E% units more by 20:00 on weekends 13.3 (2.5) E% | P < 0.05 | ||
| Maukonen et al., 2019 (78) (78) | 48-h dietary recalls covering 2 previous consecutive days | 1596 (41%) kJ in the morning | — | 340 kJ less energy in the morning—1252 (90%) kJ | P < 0.01 |
| 953 (43%) kJ in the evening | — | 450 kJ more in the evening—1402 (97%) kJ | P < 0.001 | ||
| — | — | — | Other analysis:% TEI in the morning and obesity risk had a significant interaction between % TEI in the morning and chronotype on increase in weight (≥5%) (P = 0.025) and increase in BMI (≥5%) (P = 0.012) | ||
| Baron et al., 2011 (75) | 7-d food logs | — | Caloric intake after 20:00: 376 ± 237 kcal/d12 | Caloric intake after 20:00: 754 ± 373 kcal/d13 | P < 0.001Other analysis:ETs were associated with more calories consumed after 20:00 (β = 0.45, r2Δ = 0.18, P = 0.001)12 |
| — | Caloric intake at breakfast: 355 ± 133 kcal/d12 | Caloric intake at breakfast: 285 ± 143 kcal/d13 | P > 0.05 | ||
| — | Caloric intake at lunch: 528 ± 188 kcal/d12 | Caloric intake at lunch: 503 ± 378 kcal/d13 | P > 0.05 | ||
| — | Caloric intake for snacks: 405 ± 284 kcal/d12 | Caloric intake for snacks: 536 ± 323 kcal/d13 | P > 0.05 | ||
| — | Caloric intake at dinner: 630 ± 198 kcal/d12 | Caloric intake at dinner: 825 ± 352 kcal/d13 | P < 0.05 | ||
| — | Caloric intake after dinner: 150 ± 151 kcal/d12 | Caloric intake after dinner: 208 ± 166 kcal/d13 | P > 0.05 | ||
| — | — | Cumulative energy intake across the day; 1-h incrementsFewer calories at 9:0013 | P < 0.001 | ||
| — | — | Fewer calories at 10:00, 11:00, and 12:0013 | P = 0.001 | ||
| — | — | Afternoon: intake increased steeply, and caloric intake matched and began to exceed normal sleepers around average dinner time13 | — | ||
| — | ITs reached a plateau as early as 21:0012 | Caloric intake of late sleepers continued to rise after 23:0013 | — | ||
| Lucassen et al., 2013 (62) | 3-d food recall | Working days: 299 ± 354 kcal after 20:00 | — | Consumed more calories after 20:00 on working days 677 ± 460 kcal | P < 0.001 |
| Nonworking days: 327 ± 354 kcal after 20:00 | — | Consumed more calories after 20:00 on nonworking days 537 ± 480 kcal/d | P = 0.03 | ||
| Zerón-Rugerio et al., 2020 (58) | 6-d food logs and Quality Index Food Consumption Pattern | Breakfast: 24.8 (10.4) % of kcal14 | Breakfast: 26.9 (10.4) % of kcal1526.5 (6.9) % of kcal16 | Breakfast: 22.8 (8.3) % of kcal17 | P = 0.26 |
| Lunch: 31.3 (7.5) % of kcal14 | Lunch: 29.5 (10.2) % of kcal1533.7 (10.5) % of kcal16 | Lunch: 30.9 (9.6) % of kcal17 | P = 0.36 | ||
| Dinner: 18.0 (10.4) % of kcal14 | Dinner:18.6 (9.8) % of kcal1520.7 (9.1) % of kcal16 | Dinner: 23.5 (11.3) % of kcal17 | P-trend = 0.02 | ||
| Daily carbohydrate distribution | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | — | — | — | Other analysis:In MTs,2 the highest quintile of % carbohydrate intake in the morning (within 2 h after getting out of bed) is associated with 80% decrease in risk for being overweight/obese (OR: 0.2; 95% CI: 0.10, 0.42; P-trend < 0.0001)In ETs,3 the highest quintile of % carbohydrate intake during the evening (within 2 h before bedtime) is associated with an increase in OR for being overweight/obese (OR: 4.48; 95% CI: 1.64, 12.2; P-trend = 0.01) |
| In ETs,3 the highest quintile of % sugar intake at night (within 2 h before bedtime) is associated with a 3-fold increase in OR for being overweight/obese (OR: 3.11; 95% CI: 1.17, 8.22; P-trend = 0.02) In MTs,2 the highest quintile of % sugar intake during the morning (within 2 h after getting out of bed) (OR: 0.23; 95% CI: 0.11, 0.49; (P-trend = 0.0003), % fiber (OR: 0.31; 95% CI: 0.15, 0.65; P-trend = 0.0008) was associated with a decrease in OR for being overweight/obese | |||||
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Intake by 10:00 on weekdays: 52.8 (1.3) E% | Intake by 10:00 on weekdays: 50.5 (1.3) E% | Intake by 10:00 on weekdays: 47.1 (1.6) E% | P < 0.001 P-trend < 0.001 |
| Intake after 20:00: 48.8 (2.0) E% on weekdays | Intake after 20:00: 51.3 (2.0) E% on weekdays | Intake after 20:00: 51.2 (2.4) E% on weekdays | P-trend = 0.01 | ||
| — | — | CHO intakes increased with lower ME score values (ET) after 20:00 on weekdays | P-trend < 0.05 | ||
| Intake by 10:00 on weekends: 52.6 (2.6) E% | Intake by 10:00 on weekends: 48.3 (2.4) E% | Intake by 10:00 on weekends: 48.5 (3.1) E% | P-trend = 0.003 | ||
| Intake after 20:00: 46.3 (3.4) on weekends | Intake after 20:00: 50.3 (3.4) on weekends | Intake after 20:00: 49.8 (4.4) on weekends | P = 1.00 | ||
| Baron et al., 2013 (68) | 7-d food logs | — | After 20:00: 47 ± 31 g (19%)12 | After 20:00: higher intake 87 ± 39 g (33%)13 | P < 0.01Other analysis:After 20:00: Moderate positive correlation with midpoint of sleepETs13 were associated with higher intake (r = 0.52, P < 0.001) |
| Daily protein distribution | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | — | — | — | In MTs,2 the highest % protein intake during the morning (within 2 h after getting out of bed) was associated with a 61% decrease in OR for being overweight/obese (OR: 0.39; 95% CI: 0.19, 0.81; P-trend = 0.03) In ETs,3 the highest % protein intake consumed at night (2 h before bedtime) is associated with 3.7-fold increase in OR for being overweight/obese (OR: 3.74; 95% CI: 1.33, 10.5; P-trend = 0.02) |
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Protein intake after 20:00 on weekdays: 12.4 (0.8) E% | Protein intake after 20:00 on weekdays: 13.1 (0.8) E% | Protein intake after 20:00 on weekdays: 13.4 (0.9) E% | P-trend = 0.04 |
| — | — | Protein intakes increased with lower ME score values (ET) after 20:00 on weekdays | P-trend < 0.05 | ||
| Protein intake after 20:00 on weekends: 11.6 (1.3) E% | Protein intake after 20:00 on weekends: 12.7 (1.3) E% | Protein intake after 20:00 on weekends: 14.2 (1.7) E% | P = 0.25 | ||
| Intake by 10:00 on weekdays: 14.8 (0.9) E% | Intake by 10:00 on weekends: 13.6 (0.9) E% | Intake by 10:00 on weekdays: 13.6 (0.9) E% | P < 0.001P-trend < 0.001 | ||
| Intake by 10:00 on weekends: 14.8 (0.9) E% | Intake by 10:00 on weekends: 13.6 (0.9) E% | Intake by 10:00 on weekends: 11.4 (1.2) E% | P < 0.003 P-trend < 0.001 | ||
| Baron et al., 2013 (68) | 7-d food logs | — | After 20:00: 15 ± 12 g (21%)12 | More protein at dinner13 | P < 0.01 |
| After 20:00: 32 ± 16 g (37%)13 | P > 0.01Other analysis:After 20:00: Moderate positive correlation with midpoint of sleepETs5 were associated with higher intake (r = 0.53 P < 0.001) | ||||
| Daily fat distribution | |||||
| Xiao et al., 2019 (77) | 24-Hour Dietary Assessment Tool | No association between timing of fat intake and BMI2,3 | Other analysis:No association between total fat intake during the morning (within 2 h after getting out of bed) (P-trend = 0.47), cholesterol (P-trend = 0.35), saturated fat (P-trend = 0.90), and monounsaturated fat (P-trend = 0.42) and OR of being overweight/obese in MTs2No association between total fat intake during night (2 h before bedtime) (P-trend = 0.30), cholesterol (P-trend = 0.06), saturated fat (P-trend = 0.34), monounsaturated fat (P-trend = 0.31), and polyunsaturated fat (P-trend = 0.08) and OR of being overweight/obese in ETs3 | ||
| Maukonen et al., 2017 (79) | 48-h dietary recalls | Fat: 23.8 (1.0) E% by 10:00 on weekdays | Fat: 23.3 (1.0) E% by 10:00 on weekdays | Fat: 19.6 (1.2) E% by 10:00 on weekdays | P < 0.001 P-trend = 0.002 |
| Fat: 22.6 (1.6) E% by 10:00 on weekends | Fat: 20.3 (1.5) E% by 10:00 on weekends | Fat: 18.8 (2.0) E% by 10:00 on weekends | P-trend = 0.001 | ||
| Fat: 21.5 (1.2) E% after 20:00 on weekdays | Fat: 23.4 (1.2) E% after 20:00 on weekdays | Fat: 26.1 E% after 20:00 on weekdays [26.1 (1.5) E%] | P = 0.0025P-trend < 0.001 | ||
| Fat: 17.3 (2.0) E% after 20:00 on weekends | Fat: 20.0 (2.0) E% after 20:00 on weekends | Fat: on weekends after 20:00 [26.0 (2.6) E%] | P < 0.001 | ||
| SFAs: 9.0 (0.5) E% by 10:00 on weekdays | SFAs: 9.5 (0.5) E% by 10:00 on weekdays | SFAs: 7.3 (0.6) E% by 10:00 on weekdays | P = 0.002 P-trend = 0.02 | ||
| SFAs: 8.3 (0.7) E% by 10:00 on weekends | SFAs: 7.1 (0.7) E% by 10:00 on weekends | SFAs: 6.4 (0.9) E% by 10:00 on weekends | P < 0.05 | ||
| SFAs: 8.8 (0.6) E% after 20:00 on weekdays | SFAs: 9.7 (0.6) E% after 20:00 on weekdays | SFAs: 10.3 (0.7) E% after 20:00 on weekdays | P < 0.03 | ||
| SFAs: 6.8 (1.0) E% after 20:00 on weekends | SFAs: 7.9 (1.0) E% after 20:00 on weekends | SFAs: on weekends after 20:00 [10.3 (1.2) E%] | P < 0.003 | ||
| Baron et al., 2013 (68) | 7-d food logs | — | After 20:00: 16 ± 12 g (19%)12 | After 20:00: 30 ± 17 g (35%)13 | P < 0.05 |
| — | 4 h before sleep: 11 ± 9 g (16%)12 | Consumed less fat in the 4 h before sleep 10 ± 12 g (12%)13 | P < 0.01Other analysis:After 20:00: Moderate positive correlation with midpoint of sleepETs13 were associated with higher intake (r = 0.48, P < 0.01) | ||
| Adherence to guidelines | |||||
| Najem et al., 2020 (76) | YFAS | — | — | Chronotype scores were negatively correlated with YFAS scores (r = – 0.10)ETs were associated with a higher YFAS score | P = 0.10 |
| Zeron-Rugerio et al., 2019 (64) | MD Quality Index for Children and Adolescents | — | — | Lower adherence to the MD (β = 0.019) | P = 0.06 |
| Maukonen et al., 2016 (65) | FFQ and Baltic Sea diet score | — | — | Lower adherence to the Baltic Sea diet score | P-trend < 0.05 |
| De Amicis et al., 2020 (67) | 14-item adherence to traditional MD questionnaire | Higher adherence (7 ± 2) | — | Lower adherence (6 ± 2) | P < 0.05 |
| Culnan et al., 2013 (72) | Gray–Donald Eating Patterns Questionnaire | — | Junk food consumption did not vary by chronotype at baseline | P > 0.05 | |
| After 8-wk, chronotype was not associated with change in scores on the Junk Food subscale | P > 0.05 | ||||
| Muscogiuri et al., 2020 (70) | PREDIMED (Prevención con Dieta Mediterránea) questionnaire | PREDIMED score: 8.8 ± 1.9 | PREDIMED score: 7.0 ± 1.5 | PREDIMED score: 5.1 ± 1.8 (lowest score) | P < 0.001Other analysis:Chronotype score was positively associated to PREDIMED scoreMTs were associated with a higher PREDIMED score (r = 0.59, P < 0.001) |
| Low adherence to MD: 3 (3.0%) subjects | Low adherence to MD: 6 (12.0%) subjects | Low adherence to MD: 12 (54.5%) subjects | P < 0.001 | ||
| Average adherence to MD: 58 (58.0%) subjects | Average adherence to MD: 42 (84.0%) | Average adherence to MD: 10 (45.5%) | P = 0.001 | ||
| High adherence to MD: 9 (39.0%) subjects | High adherence to MD: 2 (4.0%) subjects | High adherence to MD: 0 (0%) | P < 0.001 | ||
| Zerón-Rugerio et al., 2020 (58) | 6-d food logs and Quality Index Food Consumption Pattern | Diet quality: 57.9 ± 6.814 | Diet quality: 60.7 ± 8.11564.0 ± 9.816 | Diet quality:67.3 ± 9.417 | P < 0.001 or P-trend < 0.001 |
Values reported as mean ± SD unless stated otherwise. P-trend refers to the continuous association between the Morning–Eveningness Questionnaire (MEQ) or Munich Chronotype Questionnaire (MCTQ) score and exposures of interest. CHO, carbohydrate; E%, Percentage of energy intake; ET, evening type; EVOO, Extra-virgin olive oil; IT, intermediate type; PREDIMED, Prevención con Dieta Mediterránea; MD, Mediterranean diet; ME, morning-eveningness; MT, morning type; NS, XXX; TEI, Total energy intake; YFAS, Yale Food Addiction Scale.
Earlier chronotype was defined as a chronotype earlier than the median (03:04 h).
Later chronotype was defined as a chronotype later than the median (03:04 h).
Based on earliest midpoint of sleep quintiles.
Based on midpoint of sleep quintile 2.
Based on midpoint of sleep quintile 3.
Based on midpoint of sleep quintile 4.
Based on latest midpoint of sleep quintiles.
Based on MEQ score tertile 1: 34–53.
Based on MEQ score tertile 2: 54–59.
Based on MEQ score tertile 3: 60–76.
Based on normal sleep timing (midpoint 04:08 h).
Based on late sleep timing (midpoint of sleep 07:15 h).
Based on wakeup time <07:52 h and early bedtime <23:48 h and defined as early bedtime/early rise (EE).
Based on early bedtime (<23:48 h) and late rise (wakeup time ≥07:12 h) and defined as early bedtime/late rise (EL).
Based on late bedtime (≥23:48 h) and wakeup time (<07:52 h) and defined as late bedtime/early rise (LE).
Based on late bedtime (≥23:48 h) and late rise (wakeup time ≥07:12 h) and defined as late bedtime/late rise (LL).
Energy distribution
Calculated across studies, ETs consumed an overall mean intake of 6–90 kcal‡ less during the morning/at breakfast/by 10:00 (clock hour) (58, 75, 78, 79) and a total mean intake of 102–378 kcal‡ more energy during the evening/at dinner/after 20:00 than MTs (58, 62, 75, 78, 79) (Table 3). Xiao et al. (77) showed a significant linear association between energy distribution and being an ET and the likelihood of being overweight or obese. The MTs consumed more energy in the morning (within 2 h after waking up) and were less likely to be overweight or obese (OR: 0.32; 95% CI: 0.16, 0.66; P = 0.0006). The ETs consumed more energy in the evening (within 2 h before bedtime) and were 4.94 times more likely to be overweight or obese than MTs (OR: 4.94; 95% CI: 1.61, 15.1; P-trend = 0.01) (77).
Total daily carbohydrate, protein, and fat intake
Eight (57, 59, 62, 66, 68, 71, 77, 79) of 11 studies (56, 57, 59, 62, 63, 65, 66, 68, 71, 75, 79) reported macronutrient intakes but found no differences/associations between chronotypes. Three studies (56, 71, 79) reported significantly higher carbohydrate intakes in MTs (230 g/d‡) compared with ETs (217 g/d‡), 2 studies (63, 66) reported that ETs had a higher carbohydrate intake, and 1 study reported this was found only in ETs who also skipped breakfast regularly (P < 0.05) (66). Maukonen et al. (79) also found that sucrose intakes increased for ETs on weekdays (P = 0.020) in comparison with other chronotypes.
Two studies reported that MTs had a significantly higher daily intake of 4 g/d‡ of total protein (56, 63) than ITs and ETs. Another study reported a higher intake of 3 g/d only over the weekend in MTs in comparison with ITs and ETs (79).
Two studies reported that ETs compared with MTs were significantly associated with a higher total fat intake of 1 g/d‡ (56, 65), although Maukonen et al. (65) reported this linear association in ET women (compared with MT and IT women) only (31 of energy intake (E%) compared with 30 E%; P-trend = 0.018). Teixeira et al. (66) reported a higher total fat intake in ETs who regularly skip breakfast, whereas Maukonen et al. (79) reported an inverse association between total fat intake over weekends and ETs in comparison with MTs and ITs (P < 0.05).
Daily carbohydrate, protein, and fat distribution
Only 3 studies investigated the distribution of macronutrient intakes between chronotypes throughout the day (68, 77, 79). Both carbohydrate and protein intakes after 20:00 were higher in ETs than in MTs (68, 79). Maukonen et al. (79) found that ETs consumed more fat after 20:00 on both weekdays (10 g more‡) and weekends (19 g‡ more) compared with other types. They also showed that ETs compared with other types consumed more SFAs on both weekdays (3.4 g‡) and weekends (8 g‡) after 20:00 (79). Similarly, Baron et al. (68) found that being an ET compared with an IT was associated with a higher fat intake (14 g‡) after 20:00 (68). Interestingly in the morning, ETs compared with other types consumed 3.5 g‡ less fat on weekends by 10:00 (79) and 1 g less‡ fat in the 4 h before habitual bedtime (68).
Xiao et al. (77) also demonstrated that MTs who consumed more carbohydrates and protein during the morning (within 2 h after getting out of bed) were 80% (P-trend < 0.0001) and 61% (P-trend = 0.03) less likely to be overweight or obese than ETs (77). Conversely, ETs who consumed more carbohydrates and protein in the evening (2 h before bedtime) were respectively 4.5 (OR: 4.48; 95% CI: 1.64, 12.2; P-trend = 0.009) and 3.7 times (OR: 3.74; 95% CI: 1.33, 10.5; P-trend = 0.02) more likely to be overweight or obese (77) (Table 3).
Total daily micronutrient intake
Only 1 study by Sato-Mito et al. (56) reported on micronutrient intakes. Being an ET was associated with significantly lower potassium, calcium, magnesium, iron, zinc, vitamin A, thiamine, riboflavin, pyridoxine, folate, and vitamin D intakes compared with being an MT (P-trend < 0.05, Table 3).
Total daily food group intake
Thirteen of the included studies reported on total food group intakes (56, 59, 60, 63, 65, 70–76, 79). The ETs consumed larger quantities of energy-dense foods such as confectionary and sweets (56, 59, 63, 70, 79), sugar-sweetened beverages (59, 70, 74, 75), butter, cream, margarine (70), cholesterol-rich foods (63), meat (56, 60, 70), fast foods (75), caffeine (73, 76), and alcohol (56, 65, 71–73, 79) in comparison with other chronotypes. Four studies reported that ETs consumed fewer healthy foods such as fish (6 g/d less, P-trend < 0.05) (79), cereals (65, 71), and vegetables (17 g/1000 kcal‡, P < 0.001) (56) in comparison with other chronotypes. Similarly, Baron et al. (75) reported that ETs consumed only 1.9 servings/wk of fruit and vegetables compared with 3.4 servings/wk in ITs (75). The MTs consumed more nutrient-dense foods such rice and potatoes (56, 59), fiber (79), vegetables (56, 59), pulses (56), eggs (56), dairy (56), fruit and algae (P-trend < 0.05) (59), and wine (70) than ETs.
Differences between chronotype and eating behavior
The eating behaviors most investigated among chronotypes were meal timing (clock hours for meals), meal skipping, and portion sizes (Table 4).
TABLE 4.
Differences between Chronotype and Eating Behavior1
| Reference | Method of assessment | Differences between types | P value (ET vs. IT/MT) and other analysis | ||
|---|---|---|---|---|---|
| MT | IT | ET | |||
| Meal timing | |||||
| Xiao et al., 2019 (77) | Automated Self-Administered 24-Hour Dietary Assessment Tool (ASA24) | Breakfast: 7.6 ± 1.0 h2 | — | 8.6 ± 0.9 h3 | P-trend < 0.001 |
| Lunch: 12.6 ± 1.1 h2 | — | 12.8 ± 1.3 h3 | P < 0.004 | ||
| Dinner: 18.2 ± 0.8 h2 | — | 18.5 ± 1.1 h3 | P-trend < 0.001 | ||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Breakfast: 6:35 ± 0:02 h:min4 | Breakfast: 7:01 ± 0:02 h:min57:23 ± 0:02 h:min67:52 ± 0:02 h:min7 | Breakfast: 9:19 ± 0:02 h:min8 | P-trend < 0.0001P < 0.001 |
| Lunch: 12:20 ± 0:02 h:min4 | Lunch: 12:20 ± 0:02 h:min512:22 ± 0:02 h:min612:23 ± 0:02 h:min7 | Lunch: 12:42 ± 0:02 h:min8 | P < 0.0001P < 0.001 | ||
| Dinner: 18:51 ± 0:06 h:min4 | Dinner: 18:55 ± 0:05 h:min519:05 ± 0:05 h:min619:17 ± 0:05 h:min7 | Dinner: 19:19 ± 0:05 h:min8 | P < 0.0001P < 0.01 | ||
| Vera et al., 2018 (71) | Single 24-h recalls | Breakfast: 8.34 ± 0.03 h | — | Breakfast: 8.65 ± 0.035 h | P < 0.05 |
| Lunch: 14.55 ± 0.02 h | — | Lunch: 14.59 ± 0.02 h | |||
| Dinner: 21.08 ± 0.06 h | — | Dinner: 21.39 ± 0.67 h | |||
| Midpoint of food intake: 14.80 ± 0.02 h | — | Later midpoint of food intake: 15.06 ± 0.02 h | P < 0.001 | ||
| Silva et al., 2016 (60) | Preliminary questionnaire | — | — | — | Other analysis:Weak positive correlations between MSF score and breakfast time (r = 0.24, P < 0.001); ETs were associated with a later breakfast time Weak positive correlations between MSF score and lunch time; ETs were associated with later lunch times (r = 0.19, P < 0.01) |
| Lucassen et al., 2013 (62) | 3-d food records | Breakfast on working days: 1 h and 20 min earlier than ETs: 7:17 ± 1:31 h:min | — | Breakfast on working days: 8:38 ± 1:52 h:min | P < 0.001 |
| Teixeira et al., 2018 (66) | 24-h food recall (24h-FR) and questionnaire | Breakfast: 07:20 h:min | Breakfast: 07:45 h:min | Breakfast: 08:00 h:min | P < 0.001 |
| Lunch: 12:13 h:min | Lunch: 12:39 h:min | Lunch: 12:39 h:min | P = 0.02 | ||
| Baron et al., 2011 (75) | 7-d food logs | — | Breakfast: 9:07 h12 | Breakfast: 11:53 h13 | P < 0.001 |
| — | Lunch: 13:07 h12 | Lunch: 14:36 h13 | P < 0.01 | ||
| — | Dinner: 19:07 h12 | Dinner: 20:13 h13 | P < 0.05 | ||
| — | Last meal/snack of the day: 20:25 h12 | Last meal/snack of the day: 22:17 h13 | P < 0.001 | ||
| Time interval between meals and bedtime | |||||
| Xiao et al., 2019 (77) | Automated Self-Administered 24-Hour Dietary Assessment Tool (ASA24) | Duration between dinner and bedtime: 258 ± 58.6 min2 | — | Duration between dinner and bedtime: 313 ± 70.7 min3 | P < 0.001 |
| Baron et al., 2011 (75) | 7-d food logs | — | Time between breakfast and lunch: 4:01 h:min12 | Time between breakfast and lunch: 3:15 h:min13 | P < 0.01 |
| — | Time interval between last meal or snack and sleep onset: 3:53 h:min12 | Time interval between last meal or snack and sleep onset: 5:19 h:min13 | P < 0.05 | ||
| Zerón-Rugerio et al., 2020 (58) | 6-d food logs and Quality Index Food Consumption Pattern | — | — | Had on average the shortest time between dinner and the midpoint of sleep [5.8 (0.9) h]17 | P > 0.001 |
| Eating duration | |||||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Breakfast: 17.38 ± 0.21 min:s4 | Breakfast: 17.11 ± 0.17 min:s517.19 ± 0.19 min:s616.38 ± 0.20 min:s7 | Breakfast: 19.03 ± 0.18 min:s8 | P-trend = 0.0002P < 0.01 |
| Lunch: 21.50 ± 0.23 min:s4 | Lunch: 22.07 ± 0.19 min:s523.21 ± 0.20 min:s622.41 ± 0.22 min:s7 | Lunch: 25.29 ± 0.19 min:s8 | P < 0.0001P < 0.001 | ||
| Dinner: 28.45 ± 0.31 min:s4 | Dinner: 29.26 ± 0.26 min:s530.20 ± 0.28 min:s629.36 ± 0.30 min:s7 | Dinner: 32.29 ± 0.26 min:s8 | P-trend < 0.0001P < 0.001 | ||
| Skipped meals | |||||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Less skipped breakfast 0.66 ± 0.07 per week4 | Breakfast: 0.57 ± 0.06 times/wk50.91 ± 0.06 times/wk61.05 ± 0.06 times/wk7 | More skipped breakfast: 1.91 ± 0.07 per week8 | P-trend < 0.001 |
| Lunch: 0.15 ± 0.03 times/wk4 | Lunch: 0.16 ± 0.03 times/wk0.20 ± 0.03 times/wk60.22 ± 0.03 times/wk7 | Lunch: 0.29 ± 0.03 times/wk8 | P-trend = 0.0002 | ||
| Dinner: 0.26 ± 0.05 times/wk4 | Dinner: 0.29 ± 0.04 times/wk50.29 ± 0.04 times/wk60.33 ± 0.04 times/wk7 | Dinner: 0.42 ± 0.04 times/wk8 | P-trend = 0.01 | ||
| Silva et al., 2016 (60) | Preliminary questionnaire | MSF: 5.28 | — | MSF: 6.19 | P = 0.02Breakfast skippers had higher MSF values (toward ETs) |
| Teixeira et al., 2018 (66) | 24-h food recall (24h-FR) and questionnaire | Frequency of breakfast skippers: 10.0 (15%) | Frequency of breakfast skippers: 14.1 (63%) | Frequency of breakfast skippers: 21.8 (27%) | P = 0.02 |
| 1.7 times more likely to skip breakfast (OR: 1.7; 95% CI: 1.1, 2.9) | P = 0.02 | ||||
| Breakfast skippers (ETs) were associated with later lunch time (β = −0.23, r2 = 0.06) | P < 0.01 | ||||
| Breakfast skippers (ETs) were associated with later dinner time (β = −0.17, r2 = 0.04) | P < 0.04 | ||||
| TV watching during meals | |||||
| Sato-Mito et al., 2011 (56) | Dietary history questionnaire | Frequency per week during breakfast: 3.27 ± 0.084 | Frequency per week during breakfast: 3.52 ± 0.0753.50 ± 0.0763.59 ± 0.087 | Frequency/wk during breakfast: 3.55 ± 0.078 | P-trend = 0.03P < 0.05 |
| Frequency per week during lunch: 1.25 ± 0.084 | Frequency per week during lunch: 1.50 ± 0.0751.89 ± 0.0862.14 ± 0.087 | Frequency/wk during lunch: 3.24 ± 0.078 | P-trend < 0.0001P < 0.001 | ||
| Frequency per week during dinner: 3.63 ± 0.074 | Frequency/wk during dinner:3.87 ± 0.0653.87 ± 0.0763.97 ± 0.077 | Frequency/wk during dinner: 3.90 ± 0.068 | P-trend = 0.02P < 0.05 | ||
| Vera et al., 2018 (71) | Barriers to Weight-Loss Checklist, Emotional Eating Questionnaire, and a 24-h recall | — | — | — | P = 0.07Other analysis:1.2 times more likely (OR: 1.23; 95% CI: 0.99, 1.52) |
| Eating behavior score and subcategories | |||||
| Vera et al., 2018 (71) | Barriers to Weight-Loss Checklist, Emotional Eating Questionnaire, and a 24-h recall | Total eating behavior score: 0.01 ± 0.25 | — | Total eating behavior score: 1.93 ± 0.26 | P < 0.001 |
| Lázár et al., 2012 (73) | English version of the Dutch Eating Behavior Questionnaire | Reported better restrained eating | — | — | P < 0.044 |
| Reported better external eating behavior | — | — | P < 0.001 | ||
| Beaulieu et al., 2020 (69) | TFEQ | Chronotype score not associated with TFEQ | P ≥ 0.117 | ||
| Stress-related eating, control over intake, food cravings | |||||
| Vera et al., 2018 (71) | Barriers to Weight-Loss checklist, Emotional Eating Questionnaire, and single 24-h recall | Lower emotional eating score: 11.85 ± 0.19 | — | Higher emotional eating score: 12.40 ± 0.19 | P = 0.046 |
| — | — | Other analysis:Prone to stress-related eating (OR: 1.27; 95% CI: 1.04, 1.55; P = 0.02) | |||
| — | — | Problems controlling amounts of certain types of food (OR: 1.31; 95% CI: 1.08, 1,58; P = 0.01) | |||
| — | — | Feel less in control over diet when tired (OR: 1.33; 95% CI: 1.10, 1,60; P = 0.003) | |||
| — | — | Experience specific food cravings (OR: 1.20; 95% CI: 0.99, 1.45; P = 0.06) | |||
| Lázár et al., 2012 (73) | English version of the Dutch Eating Behavior Questionnaire | — | — | Emotional eating was associated with diurnal preference | P < 0.05 |
| Lai et al., 2013 (61) | Craving of high-calorie foods questionnaire | — | — | — | Other analysis:High-calorie food craving was not correlated with ME score (r = 0.003; P = 0.92) |
| Portion sizes, number of servings, number of eating occasions | |||||
| Vera et al., 2018 (71) | Barriers to Weight-Loss checklist, Emotional Eating Questionnaire, and single 24-h recall | — | — | — | Other analysis:1.4 times more likely to have larger portion sizes portion sizes (OR: 1.44; 95% CI: 1.18, 1.77; P < 0.001)1.3 times more likely to have second servings (OR: 1.27; 95% CI: 1.04, 1.56; P < 0.019) |
| Lucassen et al., 2013 (62) | Eat more frequently during working days (4.9 ± 1.5 occasions) | — | Eat less frequently during working days (4.4 ± 1.5 occasions) | P = 0.18 | |
| Number of eating occasions during nonworking days (4.2 ± 1.2 occasions) | — | Eat less frequently during nonworking days (4.3 ± 1.6 occasions) | P = 0.57 | ||
| Portion sizes during working days (461 ± 177 kcal) | — | Portion sizes during working (545± 219 kcal) | P = 0.07 | ||
| Portion sizes during nonworking days (599 ± 273 kcal) | — | Portion sizes during nonworking days (622 ± 380 kcal) | P = 0.75 | ||
| Lower food intake after 20:00 on working days: 299 ± 354 kcal (50% fewer calories in fewer eating occasions than ETs) | — | Higher food intake after 20:00 on working days: 677 ± 460 kcal | P < 0.001 | ||
| Food intake after 20:00 on nonworking days: 327 ± 354 kcal | — | Food intake after 20:00 on nonworking days: 537 ± 480 kcal | P = 0.028 | ||
| Teixeira et al., 2018 (66) | 24-h food recall (24h-FR) and questionnaire | 4.7 ± 1.2 meals/d | 4.5 ± 1.1 meals/d | 4.6 ± 1.1 meals/d | P = 0.44 |
| Junk, energy rich and high- fat foods | |||||
| Vera et al., 2018 (71) | Barriers to Weight-Loss Checklist, Emotional Eating Questionnaire, and single 24-h recall | — | — | — | Other analysis:1.4 times more likely to have energy-rich foods (OR: 1.44; 95% CI: 1.16, 1.78; P = 0.001) |
| Beaulieu et al., 2020 (69) | Appetite ratings and food reward (validated diurnal Leeds Food Preference Questionnaire) were measured in response to a standardized test meal | No differences between chronotypes with regards to liking for high-fat relative to low-fat foods | P = 0.66Other analysis:No interaction between chronotype and meal timing (AM vs. PM) with regards to liking for high-fat relative to low-fat foods | ||
| No differences between chronotypes with regards to wanting/desire for high-fat relative to low-fat foods | P > 0.05Other analysis:No relation between MEQ score and liking for high-fat food (r = −0.15, P > 0.05)Inverse association between MEQ score and desire for high-fat foodETs were associated with greater wanting/desire (P = 0.01, r = − 0.42) | ||||
| No differences between chronotype with regards to desire for high-fat relative to low-fat foods | P > 0.05Other analysis:No interaction between chronotype and meal timing regarding desire for high-fat relative to low-fat foods | ||||
| Other | |||||
| Vera et al., 2018 (71) | Barriers to Weight-Loss Checklist, Emotional Eating Questionnaire, and single 24-h recall | — | — | — | Other analysis:1.3 times more likely to eat in places other than the kitchen/dining room (OR: 1.32; 95% CI: 0.99, 1.52; P = 0.02) |
| — | — | — | 1.3 times more likely to eat directly from packets and containers (OR: 1.31; 95% CI: 1.06, 1.62; P = 0.01) | ||
| Beaulieu et al., 2020 (69) | Appetite ratings and food reward (validated diurnal Leeds Food Preference Questionnaire) were measured in response to a standardized test meal | No differences in ratings of sweetness or pleasantness between the different meal timings (AM vs. PM) and chronotype | P ≥ 0.157Other analysis:No interactions between meal timing (AM vs. PM) and chronotype with regards to ratings of sweetness or pleasantness | ||
| No differences between chronotype and prospective test meal consumption | Other analysis:No interaction between meal timing (AM vs. PM) and chronotype with regards to prospective test meal consumption | ||||
| No differences between chronotype and savory perception of test meal and no interaction between meal timing (AM vs. PM) and chronotype with regards to savory perception | P ≥ 0.26Other analysis:No interactions among meal timing (AM vs. PM), appetite ratings, chronotype, and time point for test meal | ||||
| No differences between chronotype and appetite ratings | P > 0.05 | ||||
| No interactions among meal timing (AM vs. PM), chronotype, appetite ratings, and time point (of test meal session) | Other analysis:No interaction between meal timing (AM vs. PM), chronotype, and perceived test meal fillingness | ||||
| Perceived test meal fillingness was higher | — | — | P ≤ 0.04 | ||
Values reported as mean ± SD unless stated otherwise. P-trend refers to the continuous association between the Morning–Eveningness Questionnaire (MEQ) or Munich Chronotype Questionnaire (MCTQ) score and exposures of interest. ET, evening type; IT, intermediate type; MSFsc, midsleep corrected for sleep duration on free days; MT, morning type; MSF, mid-sleep time on free days.
Earlier chronotype was defined as a chronotype earlier than the median (03:04 h).
Later chronotype was defined as a chronotype later than the median (03:04 h).
Based on earliest midpoint of sleep quintiles.
Based on midpoint of sleep quintile 2.
Based on midpoint of sleep quintile 3.
Based on midpoint of sleep quintile 4.
Based on latest midpoint of sleep quintiles.
Based on MEQ score tertile 1: 34–53.
Based on normal sleep timing (midpoint 04:08 h).
Based on late sleep timing (midpoint of sleep 07:15 h).
Based on late bedtime (≥23:48 h) and late rise (wakeup time ≥07:12 h) and defined as late bedtime/late rise (LL).
Meal timing, skipping, and intervals between meals and bedtime
Compared with other types, ETs were more likely to display undesirable eating behavior, for example, reporting later clock times for main meals (56, 60, 62, 66, 71, 75, 77). As to be expected, the ETs had later clock times for breakfast than MTs (56, 60, 62, 66, 71, 75, 77) or even skipped breakfast altogether (56, 60, 66). Based on their individual preferences, ETs also had later habitual clock times for lunch (56, 60, 66, 71, 75, 77) and dinner (56, 71, 75, 77) than MTs. Across the studies, ETs had breakfast from 14 min to 2 h 44 min‡ later, lunch from 2 min to 1 h and 23 min‡ later, and dinner from 3 min to 1 h ‡ later (56, 62, 66, 71, 77). Interestingly, ETs had a longer time interval between the last meal or snack of the day (dinner) and bedtime (average of 316 min‡) compared with ITs/MTs (average of 231 min‡) (75, 77).
Portion sizes, number of servings, and eating occasions
ETs were 1.4 times (OR: 1.44; 95% CI: 1.16, 1.78; P = 0.001) more likely to consume larger portion sizes and 1.3 times (OR: 1.27; 95% CI: 1.04, 1.56; P < 0.019) more likely to have second servings (71), as well as to watch television while eating (56), compared with MTs and ITs. There were no differences or associations between the number of eating occasions per day (62, 66).
Eating behavior scores
Four studies (61, 69, 71, 73) investigated the associations and differences between chronotypes and the Three-Factor Eating Questionnaire (TFEQ) scores (restraint, which is the conscious restriction of food intake to control body weight and shape; disinhibition, which is the loss of control of food intake that leads to overconsumption of food; and hunger, which is feelings and subjective perceptions of hunger that lead to food intake) (81) and their subcategories (flexible and rigid control; habitual, emotional, and situational disinhibition; internal and external locus for hunger). A higher score indicates a higher level of these eating behaviors. Vera et al. (71) observed that ETs had a higher total eating behavior score (1.93 ± 0.26) (higher scores = more deleterious eating behaviors) and emotional eating score (12.40 ± 0.19) (<12, nonemotional; ≥12, emotional) than the MTs with scores of 0.01 ± 0.25 and 11.85 ± 0.19, respectively (P < 0.001 and P < 0.046, respectively). The ETs also felt less in control over their diet (OR: 1.33; 95% CI: 1.10, 1.60; P = 0.003), experienced more stress-related eating (OR: 1.27; 95% CI: 1.04, 1.55; P = 0.019) and food cravings (OR: 1.20; 95% CI: 0.99, 1.45; P = 0.063), and had greater problems controlling the amount of food consumed (OR: 1.31; 95% CI: 1.03, 1.58; P = 0.006) (71).
Discussion
The aim of this project was to systematically review the existing evidence that chronotype affects body composition and biomarker outcomes by also considering behavioral factors such dietary intake and eating habits/behavior in healthy adults. In this systematic review, we consistently found that ETs compared with MTs were more likely to be overweight/obese. This finding may be linked to their irregular eating patterns and unhealthy eating behaviors that could lead to circadian misalignment. Both MTs and ETs had very similar dietary intakes (energy and macronutrients), but clear differences were apparent regarding the distribution of food intake throughout the 24-h day, skipping and timing of meals, and diet quality (micronutrients, food groups, and types), which may lead to body composition changes. Furthermore, ETs displayed a higher risk of metabolic disease (see Figure 2 depicting the main outcomes ).
FIGURE 2.
Main outcomes—late chronotypes (evening types) in comparison to early chronotypes (morning types). ACTH, adrenocorticotropic hormone; MetS, metabolic syndrome.
Most of the studies that explored meal timing found that individuals tend to consume food based on preferences according to their chronotype (48, 49, 51, 71). In ETs, most of their energy and macronutrient intake were distributed toward the biological night (82), and clock times for meals were later than those of MTs. The mechanisms of this chronotype–body composition relation are yet to be fully explained; however, it may be hypothesized and in part supported from data of this systematic review that several interconnected mechanisms, including mistimed food intake, lower diet quality, and eating behaviors that favor weight gain and metabolic alterations, have an influence.
Meal skipping, especially breakfast skipping, was also prevalent in ETs. These irregular eating patterns, including later timing of food intake and skipping of meals, seen in ETs may be explained by their later preferred sleep and wake timing (44, 56, 75, 83, 84) and are often in conflict with work time obligations or social demands (85). Those extreme ETs may experience significant misalignment between their internal circadian rhythms and their work hours as well as social demands. For example, during the week, ETs have to wake up early for work and subsequently go to sleep earlier, in contrast with their internal timing, but during the weekends, they stay up longer and wake up later. This difference between sleep and wake times during the week and the weekend has been termed “social jetlag” (86) and may result in adverse health outcomes such as greater risks for obesity and adverse metabolic health outcomes (87).
Such results are exacerbated by too short sleep durations during the week, as often occurs in ETs, because they are more prone to accumulate sleep debt throughout their workweek and consequently attempt to resolve this by altering their sleep schedules over the weekend, resulting in a higher social jetlag and altered circadian rhythms (86, 88). Forced early wake times (for school or work), as often found in ETs, especially in teenagers and young adults, may then lead to redistribution or “catchup” of the skipped meals to later in the day, because “normal” breakfast times would still be closer to their biological night, which is supported similar to the findings from this review. This may support the popular breakfast skipping theory, which poses that those individuals who omit breakfast tend to be hungrier later in the day, leading to an overcompensation of energy intake, especially during the evening (89). This occurs despite ETs and MTs still consuming the same amount of food within 24 h (46, 85). According to Manoogian and Panda (12), the external cues of feeding and fasting can affect metabolic processes. If these cues are disrupted, it can lead to increased risk of disease. Since ETs eat closer to their bedtime, and they wake up late, their fasting period is shortened, which may be more detrimental to their health, potentially delaying digestion. In time-restricted eating (TRE) studies, it was demonstrated that longer fasting periods are more beneficial for health than shorter ones (12). However, this review found that ET had a longer time interval between the last snack/meal of the day and sleep onset in comparison with IT (75).
Metabolically, ingested calories are optimally used during the morning, possibly due to the higher thermic effect of food in comparison with the evening. When healthy individuals omit breakfast, they are in a fasted state at the beginning of their biological day (90). Consequently, the overnight fasting period is prolonged and an increase in postprandial insulin concentrations and fat oxidation is seen (91). Ultimately, low-grade inflammation and impaired glucose metabolism may result in the development of metabolic inflexibility and weight gain (91). This review found that MTs were more likely to have more regular eating patterns, and this has been linked by another researcher to higher postprandial thermogenesis and lower fasting and total LDL cholesterol (92). This highlights the endogenous circadian control of metabolic responses and the importance of meal timing. Glucose tolerance changes during the day, peaking during the daylight hours when food is normally eaten, and troughs during the night when fasting occurs. Therefore, if ETs shift their eating to later in the day than MTs, they may develop poor glycemic control (93). In comparison, this review found that ETs were at higher risk of adverse metabolic health, as shown by their lower HDL cholesterol and higher triglyceride concentrations, higher metabolic syndrome scores (71), urinary epinephrine concentrations (62, 71), insulin concentrations and HOMA-IR (73), similar to the findings of Lotti et al. (48). Other studies have also linked hormonal alterations and altered glucose metabolism, in conjunction with misaligned eating, as further mechanisms by which ETs are more likely to become overweight/obese and are also at higher risk for preclinical states of diabetes (45, 94–96).
These findings align well with previous studies (not chronotype focused) that have linked aspects of mistimed food intake in humans such as breakfast skipping, late lunch eating, and higher energy intake at dinner with indicators of obesity (97–101).
Energy requirements and the oxidation of macronutrients vary across the 24-h light/dark cycle (102); consequently, the timing of food intake has different effects on energy utilization and as a result may change weight-loss effectiveness and body composition (103, 104). In this systematic review, only 1 study found that consuming a higher amount of energy and proportion of carbohydrates and protein in the morning and during the early part of the day seemed to be protective against developing obesity in MTs. Consuming more energy, protein, and carbohydrates toward the evening in ETs was found to favor weight gain and obesity (77). This reinforced previous studies that showed the detrimental effect of late eating (103). Generally, it seems that the timing of eating in alignment with one's chronotype could be an important and beneficial factor when considering body composition outcomes (57). When participants of the latter study were following chronotype-adjusted diets, they had greater weight-loss success compared with the traditional, hypocaloric control diet. The weight loss between MTs and ETs was similar, suggesting that this may be an effective approach for any chronotype (57). Besides chronotype, other factors also target the circadian metabolic functions (including effective weight loss and reducing fat mass in obese adults), for example, TRE, which has also been widely known for its beneficial effects on cardiometabolic health (including lipid profiles and glucose concentrations) in humans (105).
Eating behaviors may alter energy intake by influencing the types and amounts of foods eaten, timing of food intake, and where food intake occurs, ultimately affecting body weight (33). The ETs often displayed unhealthy eating behaviors such as consuming larger portion sizes, second servings, experiencing more food cravings, emotional- and stress-related eating, and presenting TFEQ scores that reflect unhealthy eating behaviors (71, 73). Studies have found that emotional eaters consumed snacks more often than nonemotional eaters. This suggests that there is a link between diet and body weight that may be mediated in part by dieting behavior. In comparison, MTs showed greater control of their eating behaviors (73). MTs had higher restraint scores (73), which have been linked by other researchers to a higher consumption of “healthy food” such as green vegetables and fewer energy-dense foods such as fats (106), which was also seen in this review.
Other studies showed that insufficient sleep (shorter sleep duration) and being an ET impaired the appetite-regulating hormone leptin (107), as well as increased insulin concentrations (108), which may lead to insulin resistance. Therefore, chronic misalignment of the circadian clocks leads to elevated leptin concentrations during the day and night, possibly due to oversecretion and leptin resistance, which is a vicious circle because, in turn, it can contribute again to overeating (108).
These often unhealthy eating behaviors among ETs are further exaggerated by the higher intake of unhealthy, stimulating, and energy-dense foods (56, 59, 63, 70–77, 79). Exploring food group intake differences between chronotypes gives an idea of the micronutrient intakes. The fruit and vegetables intakes of ETs found in this review are far below the World Health Organization guidelines of 5 servings per day (109) and may account for the lower micronutrient intake reported by Sato-Mito et al. (56). The ETs also consumed more caffeine and alcohol. The stimulating effects of these items may account for the higher intake (110). In comparison, MTs reported healthier, nutrient-dense food choices (56, 59, 79). They also displayed more control over their eating behavior, which may account for the higher prevalence of a normal BMI in MTs.
Strengths and Limitations
To our knowledge, this is the most comprehensive review of different dietary elements, including nutrients and energy, but also taking into account food intake, eating patterns, and timing and composition of meals that compare extreme chronotypes with different body compositions. This systematic review has several strengths: first, we included not only dietary intake (energy and macronutrient intakes) and body composition profiles but also eating and behavioral aspects and biomarkers as outcomes for different chronotypes. Second, the formatting of the data tables to compare the different chronotypes allowed a more comprehensive review of the literature, rather than only listing the specific outcomes. Another strength of this systematic review is that it did not include studies that recruited participants with acute, preexisting, and chronic diseases, which may have influenced chronotype.
One limitation of this systematic review was that included studies varied considerably in their classification method of chronotypes, the statistical analysis approach, and study design, which made comparisons between chronotypes challenging. Another limitation was that not all the included studies were designed to assess differences between body composition groups as their main comparator.
Recommendations for Research
Further studies are needed to explore interindividual and personalized optimal meal timing and the distribution of macronutrients at eating occasions with regard to weight management. Such optimization could be alleviated by assessment of the person's chronotype. In the same vein, the pathogenesis of obesity is not yet fully understood despite decades of research dedicated to the investigation of the underlying mechanisms and the development of successful interventions and treatments. Thus, it is crucial to add emerging evidence that consideration of extreme chronotypes and circadian misalignment is a contributing factor. More research is required to establish whether food intake that is in “misalignment with one's chronotype” is an important factor to consider (and to potentially adress) in weight management. It should also be explored whether food intake should be adapted to chronotype-related wake and sleep–wake timing or whether food intake should simply be prioritized to “day” hours and limited during “night” hours.
In conclusion, this systematic review showed that ETs were more likely to be overweight/obese and have poorer metabolic health in comparison with MTs (and ITs). It also highlighted key areas for clarification; first, this review found limited evidence of detailed assessment of diet quality, micronutrient intakes, food choices, and quantities consumed between chronotypes, as well as an inadequate focus on timing of intake or investigating both in conjunction with eating and other eating behaviors. Such data could inform strategies (e.g., eating in alignment with internal body clocks, improvement of sleep timing and quality, adjusting mealtimes to improve the eating and fasting windows, e.g., TRE) around healthy weight management in the future. This systematic review supports the assumptions that chronotype have an impact on body composition through interconnected mechanisms, including mistimed food intake, eating behaviors and food choices that favor weight gain, and metabolic alterations.
Supplementary Material
Acknowledgements
The authors’ responsibilities were as follows—CvdM and RK: designed the research and performed the quality assessment; CvdM: conducted the systematic literature search, performed the data extraction, and wrote the first draft of the paper; CvdM, RK, and MM: were involved in data collection; and all authors: contributed to writing and reviewing the manuscript and read and approved the final manuscript.
Notes
CvdM holds a doctoral scholarship funded by Massey University, New Zealand. MM was supported by the VELUX Foundation Switzerland. This research received no external funding.
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–5 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ET, evening type; IT, intermediate type; JBI, Joanna Briggs Institute; MCTQ, Munich Chronotype Questionnaire; MEQ, Morningness–Eveningness Questionnaire; MT, morning type; PICOS, Population, Intervention, Comparison, Outcomes, and Study; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; SCN, suprachiasmatic nuclei; TFEQ, Three-Factor Eating Questionnaire; TRE, time-restricted eating.
Contributor Information
Carlien van der Merwe, School of Sport, Exercise and Nutrition, Massey University East Precinct, Albany, Auckland, New Zealand.
Mirjam Münch, Centre for Chronobiology, Transfaculty Research Platform Molecular and Cognitive Neurosciences, Psychiatric Hospital of the University of Basel, Basel, Switzerland; Research Centre for Hauora and Health, Massey University, Wellington, New Zealand.
Rozanne Kruger, School of Sport, Exercise and Nutrition, Massey University East Precinct, Albany, Auckland, New Zealand.
References
- 1. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest. 2011;121(6):2133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Garaulet M, Madrid JA. Chronobiological aspects of nutrition, metabolic syndrome and obesity. Adv Drug Deliv Rev. 2010;62(9–10):967–78. [DOI] [PubMed] [Google Scholar]
- 4. Caroline CKo, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15(Suppl 2):R271–77. [DOI] [PubMed] [Google Scholar]
- 5. Bass J. Circadian topology of metabolism. Nature. 2012;491(7424):348–56. [DOI] [PubMed] [Google Scholar]
- 6. Moore RY, Lenn NJ. A retinohypothalamic projection in the rat. J Comp Neurol. 1972;146(1):1–14. [DOI] [PubMed] [Google Scholar]
- 7. King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MPet al. Positional cloning of the mouse circadian clock gene. Cell. 1997;89(4):641–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DWet al. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284(5423):2177–81. [DOI] [PubMed] [Google Scholar]
- 9. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72(1):517–49. [DOI] [PubMed] [Google Scholar]
- 10. Youngstedt SD, Kline CE, Elliott JA, Zielinski MR, Devlin TM, Moore TA. Circadian phase-shifting effects of bright light, exercise, and bright light+exercise. J Circadian Rhythms. 2016;14:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang PT, Turek FW. Timing of meals: when is as critical as what and how much. Am J Physiol Endocrinol Metab. 2017;312(5):E369– E80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Manoogian EN, Panda S. Circadian rhythms, time-restricted feeding, and healthy aging. Ageing Res Rev. 2017;39:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Buxton OM, L'Hermite-Balériaux M, Hirschfeld U, Van Cauter E. Acute and delayed effects of exercise on human melatonin secretion. J Biol Rhythms. 1997;12(6):568–74. [DOI] [PubMed] [Google Scholar]
- 14. Cipolla-Neto J, Amaral FG, Afeche SC, Tan DX, Reiter RJ. Melatonin, energy metabolism, and obesity: a review. J Pineal Res. 2014;56(4):371–81. [DOI] [PubMed] [Google Scholar]
- 15. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salgado-Delgado R, Angeles-Castellanos M, Saderi N, Buijs RM, Escobar C. Food intake during the normal activity phase prevents obesity and circadian desynchrony in a rat model of night work. Endocrinology. 2010;151(3):1019–29. [DOI] [PubMed] [Google Scholar]
- 17. Challet E. The circadian regulation of food intake. Nat Rev Endocrinol. 2019;15(7):393–405. [DOI] [PubMed] [Google Scholar]
- 18. Nelson RJ, Chbeir S. Dark matters: effects of light at night on metabolism. Proc Nutr Soc. 2018;77(3):223–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fong M, Caterson ID, Madigan CD. Are large dinners associated with excess weight, and does eating a smaller dinner achieve greater weight loss? A systematic review and meta-analysis. Br J Nutr. 2017;118(8):616–28. [DOI] [PubMed] [Google Scholar]
- 20. McHill AW, Phillips AJ, Czeisler CA, Keating L, Yee K, Barger LKet al. Later circadian timing of food intake is associated with increased body fat. Am J Clin Nutr. 2017;106(5):1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St-Onge MP, Ard J, Baskin ML, Chiuve SE, Johnson HM, Kris-Etherton Pet al. Meal timing and frequency: implications for cardiovascular disease prevention: a scientific statement from the American Heart Association. Circulation. 2017;135(9):e96–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Scheer F, Hu K, Evoniuk H, Kelly EE, Malhotra A, Hilton MFet al. Impact of the human circadian system, exercise, and their interaction on cardiovascular function. Proc Natl Acad Sci. 2010;107(47):20541–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grimaldi D, Carter JR, Van Cauter E, Leproult R. Adverse impact of sleep restriction and circadian misalignment on autonomic function in healthy young adults. Hypertension. 2016;68(1):243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Morris CJ, Purvis TE, Hu K, Scheer FAJL. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc Nat Acad Sci U S A. 2016;113:E1402–E11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Depner CM, Melanson EL, McHill AW, Wright KP. Mistimed food intake and sleep alters 24-hour time-of-day patterns of the human plasma proteome. Proc Nat Acad Sci U S A. 2018;115(23):E5390–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collado MC, Engen PA, Bandín C, Cabrera-Rubio R, Voigt RM, Green SJet al. Timing of food intake impacts daily rhythms of human salivary microbiota: a randomized, crossover study. FASEB J. 2018;32(4):2060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roenneberg T, Kantermann T, Juda M, Vetter C, Allebrandt KV. Light and the human circadian clock. In: A Kramer, M MerrowCircadian clocks. Heidelberg Berlin: Springer; 2013. p. 311–31. [DOI] [PubMed] [Google Scholar]
- 28. Antunes L, Levandovski R, Dantas G, Hidalgo MP. Obesity and shift work: chronobiological aspects. Nutr Res Rev. 2010;23(1):155–68. [DOI] [PubMed] [Google Scholar]
- 29. Morikawa Y, Miura K, Sasaki S, Yoshita K, Yoneyama S, Sakurai Met al. Evaluation of the effects of shift work on nutrient intake: a cross-sectional study. J Occup Health. 2008:50:270–278. [DOI] [PubMed] [Google Scholar]
- 30. Rouhani HR, Haghighatdoost F, Surkan PJ, Azadbakht L. Associations between dietary energy density and obesity: a systematic review and meta-analysis of observational studies. Nutrition. 2016;32(10):1037–47. [DOI] [PubMed] [Google Scholar]
- 31. Wang J, Luben R, Khaw K-T, Bingham S, Wareham NJ, Forouhi NG. Dietary energy density predicts the risk of incident type 2 diabetes: the European Prospective Investigation of Cancer (EPIC)-Norfolk study. Diabetes Care. 2008;31(11):2120–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Esmaillzadeh A, Azadbakht L. Dietary energy density and the metabolic syndrome among Iranian women. Eur J Clin Nutr. 2011;65(5):598–605. [DOI] [PubMed] [Google Scholar]
- 33. French SA, Epstein LH, Jeffery RW, Blundell JE, Wardle J. Eating behavior dimensions. Associations with energy intake and body weight. A review. Appetite. 2012;59(2):541–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kruger R, De Bray JG, Beck KL, Conlon CA, Stonehouse W. Exploring the relationship between body composition and eating behavior using the Three Factor Eating Questionnaire (TFEQ) in young New Zealand women. Nutrients. 2016;8(7):386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martyn-Nemeth P, Quinn L, Hacker E, Park H, Kujath AS. Diabetes distress may adversely affect the eating styles of women with type 1 diabetes. Acta Diabetol. 2014;51(4):683–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roenneberg T, Wirz-Justice A, Merrow M. Life between clocks: daily temporal patterns of human chronotypes. J Biol Rhythms. 2003;18(1):80–90. [DOI] [PubMed] [Google Scholar]
- 37. Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: a comprehensive review. Chronobiol Int. 2012;29(9):1153–75. [DOI] [PubMed] [Google Scholar]
- 38. Ehret CF. The sense of time: evidence for its molecular basis in the eukaryoticgene-action system. Adv Biol Med Phys. 1974;15:47–77. [DOI] [PubMed] [Google Scholar]
- 39. Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4:97–110. [PubMed] [Google Scholar]
- 40. Chang A-M, Duffy JF, Buxton OM, Lane JM, Aeschbach D, Anderson Cet al. Chronotype genetic variant in PER2 is associated with intrinsic circadian period in humans. Sci Rep. 2019;9(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Archer SN, Robilliard DL, Skene DJ, Smits M, Williams A, Arendt Jet al. A length polymorphism in the circadian clock gene per3 is linked to delayed sleep phase syndrome and extreme diurnal preference. Sleep. 2003;26(4):413–15. [DOI] [PubMed] [Google Scholar]
- 42. Brown SA, Kunz D, Dumas A, Westermark PO, Vanselow K, Tilmann-Wahnschaffe Aet al. Molecular insights into human daily behavior. Proc Natl Acad Sci. 2008;105(5):1602–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Duffy JF, Rimmer DW, Czeisler CA. Association of intrinsic circadian period with morningness–eveningness, usual wake time, and circadian phase. Behav Neurosci. 2001;115(4):895–99. [DOI] [PubMed] [Google Scholar]
- 44. Muñoz JSG, Cañavate R, Hernández CM, Cara-Salmerón V, Morante JJH. The association among chronotype, timing of food intake and food preferences depends on body mass status. Eur J Clin Nutr. 2017;71(6):736–42. [DOI] [PubMed] [Google Scholar]
- 45. Schubert E, Randler C. Association between chronotype and the constructs of the Three-Factor-Eating-Questionnaire. Appetite. 2008;51(3):501–505. [DOI] [PubMed] [Google Scholar]
- 46. Meule A, Roeser K, Randler C, Kübler A. Skipping breakfast: morningness-eveningness preference is differentially related to state and trait food cravings. Eat Weight Disord. 2012;17(4):e304–e8. [DOI] [PubMed] [Google Scholar]
- 47. Kanerva N, Kronholm E, Partonen T, Ovaskainen ML, Kaartinen NE, Konttinen Het al. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29(7):920–27. [DOI] [PubMed] [Google Scholar]
- 48. Lotti S, Pagliai G, Colombini B, Sofi F, Dinu M. Chronotype differences in energy intake, cardiometabolic risk parameters, cancer, and depression: a systematic review with meta-analysis of observational studies. Adv Nutr. 2022;13(1):269–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mazri FH, Manaf ZA, Shahar S, Mat Ludin AF. The association between chronotype and dietary pattern among adults: a scoping review. Int J Environ Res Public Health. 2020;17(1):68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Almoosawi S, Vingeliene S, Gachon F, Voortman T, Palla L, Johnston JDet al. Chronotype: implications for epidemiologic studies on chrono-nutrition and cardiometabolic health. Adv Nutr. 2019;10(1):30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Phoi YY, Rogers M, Bonham MP, Dorrian J, Coates AM. A scoping review of chronotype and temporal patterns of eating of adults: tools used, findings, and future directions. Nutr Res Rev. 2021:112–135..35: [DOI] [PubMed] [Google Scholar]
- 52. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–41. [DOI] [PubMed] [Google Scholar]
- 53. The Endnote Team, Endnote. Clarivate; Philadelphia, PA2013.Endnote 20 [Google Scholar]
- 54. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. 2016.5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Internet: E Aromataris, Z MunnJBI Manual for Evidence Synthesis. 2020; JBI; 10.46658/JBIMES-20-01https://synthesismanual.jbi.global. [DOI] [Google Scholar]
- 56. Sato-Mito N, Sasaki S, Murakami K, Okubo H, Takahashi Y, Shibata Set al. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Med. 2011;12(3):289–94. [DOI] [PubMed] [Google Scholar]
- 57. Muñoz JG, Gallego MG, Soler ID, Ortega MB, Cáceres CM, Morante JH. Effect of a chronotype-adjusted diet on weight loss effectiveness: a randomized clinical trial. Clin Nutr. 2020;39(4):1041–48. [DOI] [PubMed] [Google Scholar]
- 58. Zerón-Rugerio MF, Longo-Silva G, Hernáez Á, Ortega-Regules AE, Cambras T, Izquierdo-Pulido M. The elapsed time between dinner and the midpoint of sleep is associated with adiposity in young women. Nutrients. 2020;12(2):410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Yoshizaki T, Komatsu T, Tada Y, Hida A, Kawano Y, Togo F. Association of habitual dietary intake with morningness-eveningness and rotating shift work in Japanese female nurses. Chronobiol Int. 2018;35(3):392–404. [DOI] [PubMed] [Google Scholar]
- 60. Silva CM, Mota MC, Miranda MT, Paim SL, Waterhouse J, Crispim CA. Chronotype, social jetlag and sleep debt are associated with dietary intake among Brazilian undergraduate students. Chronobiol Int. 2016;33(6):740–48. [DOI] [PubMed] [Google Scholar]
- 61. Lai P-P, Say Y-H. Associated factors of sleep quality and behavior among students of two tertiary institutions in northern Malaysia. Med J Malaysia. 2013;68(3):196–203. [PubMed] [Google Scholar]
- 62. Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge Let al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PLoS One. 2013;8(3):e56519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mota MC, Waterhouse J, De-Souza DA, Rossato LT, Silva CM, Araujo MBJet al. Association between chronotype, food intake and physical activity in medical residents. Chronobiol Int. 2016;33(6):730–39. [DOI] [PubMed] [Google Scholar]
- 64. Zerón-Rugerio MF, Cambras T, Izquierdo-Pulido M. Social jet lag associates negatively with the adherence to the Mediterranean diet and body mass index among young adults. Nutrients. 2019;11(8):1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Maukonen M, Kanerva N, Partonen T, Kronholm E, Konttinen H, Wennman Het al. The associations between chronotype, a healthy diet and obesity. Chronobiol Int. 2016;33(8):972–81. [DOI] [PubMed] [Google Scholar]
- 66. Teixeira GP, Mota MC, Crispim CA. Eveningness is associated with skipping breakfast and poor nutritional intake in Brazilian undergraduate students. Chronobiol Int. 2018;35(3):358–67. [DOI] [PubMed] [Google Scholar]
- 67. De Amicis R, Galasso L, Leone A, Vignati L, De Carlo G, Foppiani Aet al. Is abdominal fat distribution associated with chronotype in adults independently of lifestyle factors?. Nutrients. 2020;12(3):592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baron KG, Reid KJ, Horn LV, Zee PC. Contribution of evening macronutrient intake to total caloric intake and body mass index. Appetite. 2013;60(1):246–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Beaulieu K, Oustric P, Alkahtani S, Alhussain M, Pedersen H, Quist JSet al. Impact of meal timing and chronotype on food reward and appetite control in young adults. Nutrients, 2020;12(5):1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Laudisio Det al. Chronotype and adherence to the Mediterranean diet in obesity: results from the Opera Prevention Project. Nutrients. 2020;12(5):1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vera B, Dashti HS, Gómez-Abellán P, Hernández-Martínez AM, Esteban A, Scheer FAet al. 1–11. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Rep. 2018;8(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Culnan E, Kloss JD, Grandner M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol Int. 2013;30(5):682–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Lázár AS, Slak A, Lo JC-Y, Santhi N, Von Schantz M, Archer SNet al. Sleep, diurnal preference, health, and psychological well-being: a prospective single-allelic-variation study. Chronobiol Int. 2012;29(2):131–46. [DOI] [PubMed] [Google Scholar]
- 74. Li W, Wu M, Yuan F, Zhang H. Sugary beverage consumption mediates the relationship between late chronotype, sleep duration, and weight increase among undergraduates: a cross-sectional study. Environ Health Prev Med. 2018;23(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity. 2011;19(7):1374–81. [DOI] [PubMed] [Google Scholar]
- 76. Najem J, Saber M, Aoun C, El Osta N, Papazian T, Rabbaa Khabbaz L. Prevalence of food addiction and association with stress, sleep quality and chronotype: a cross-sectional survey among university students. Clin Nutr. 2020;39(2):533–39. [DOI] [PubMed] [Google Scholar]
- 77. Xiao Q, Garaulet M, Scheer F. Meal timing and obesity: interactions with macronutrient intake and chronotype. Int J Obes. 2019;43(9):1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Maukonen M, Kanerva N, Partonen T, Mannisto S. Chronotype and energy intake timing in relation to changes in anthropometrics: a 7-year follow-up study in adults. Chronobiol Int. 2019;36(1):27–41. [DOI] [PubMed] [Google Scholar]
- 79. Maukonen M, Kanerva N, Partonen T, Kronholm E, Tapanainen H, Kontto Jet al. Chronotype differences in timing of energy and macronutrient intakes: a population-based study in adults. Obesity. 2017;25(3):608–15. [DOI] [PubMed] [Google Scholar]
- 80. Roenneberg T, Kuehnle T, Juda M, Kantermann T, Allebrandt K, Gordijn Met al. Epidemiology of the human circadian clock. Sleep Med Rev. 2007;11(6):429–38. [DOI] [PubMed] [Google Scholar]
- 81. Stunkard AJ, Messick S. The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res. 1985;29(1):71–83. [DOI] [PubMed] [Google Scholar]
- 82. Qin LQ, Li J, Wang Y, Wang J, Xu JY, Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73(19):2467–75. [DOI] [PubMed] [Google Scholar]
- 83. Kanerva N, Kronholm E, Partonen T, Ovaskainen ML, Kaartinen NE, Konttinen Het al. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int. 2012;29(7):920–27. [DOI] [PubMed] [Google Scholar]
- 84. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int. 2014;31(1):64–71. [DOI] [PubMed] [Google Scholar]
- 85. Dashti HS, Scheer FA, Jacques PF, Lamon-Fava S, Ordovás JM. Short sleep duration and dietary intake: epidemiologic evidence, mechanisms, and health implications. Adv Nutr. 2015;6(6):648–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1–2):497–509. [DOI] [PubMed] [Google Scholar]
- 87. Covassin N, Singh P, Somers VK. Keeping up with the clock. Hypertension. 2016;68(5):1081–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol. 2012;22(10):939–43. [DOI] [PubMed] [Google Scholar]
- 89. Stanton JL Jr, Keast DR. Serum cholesterol, fat intake, and breakfast consumption in the United States adult population. J Am Coll Nutr. 1989;8(6):567–72. [DOI] [PubMed] [Google Scholar]
- 90. Yoshida C, Shikata N, Seki S, Koyama N, Noguchi Y. Early nocturnal meal skipping alters the peripheral clock and increases lipogenesis in mice. Nutr Metab. 2012;9(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nas A, Mirza N, Hägele F, Kahlhöfer J, Keller J, Rising Ret al. Impact of breakfast skipping compared with dinner skipping on regulation of energy balance and metabolic risk. Am J Clin Nutr. 2017;105(6):1351–61. [DOI] [PubMed] [Google Scholar]
- 92. Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci. 2009;106(11):4453–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Henry CJ, Kaur B, Quek RYC. Chrononutrition in the management of diabetes. Nutr Diabetes. 2020;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Hogben AL, Ellis J, Archer SN, von Schantz M. Conscientiousness is a predictor of diurnal preference. Chronobiol Int. 2007;24(6):1249–54. [DOI] [PubMed] [Google Scholar]
- 95. Broms U, Kaprio J, Hublin C, Partinen M, Madden PA, Koskenvuo M. Evening types are more often current smokers and nicotine-dependent—a study of Finnish adult twins. Addiction. 2011;106(1):170–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Deyoung C, Hasher L, Djikic M, Criger B, Peterson J. Morning people are stable people: circadian rhythm and the higher-order factors of the big five. Personality Individual Differences. 2007;43(2):267–76. [Google Scholar]
- 97. Horikawa C, Kodama S, Yachi Y, Heianza Y, Hirasawa R, Ibe Yet al. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: a meta-analysis. Prev Med. 2011;53(4–5):260–67. [DOI] [PubMed] [Google Scholar]
- 98. Aronoff NJ, Geliebter A, Zammit G. Gender and bodymass index as related to the night-eating syndrome in obese outpatients. J Am Diet Assoc. 2001;101(1):102–4. [DOI] [PubMed] [Google Scholar]
- 99. Bo S, Musso G, Beccuti G, Fadda M, Fedele D, Gambino Ret al. Consuming more of daily caloric intake at dinner predisposes to obesity: a 6-year population-based prospective cohort study. PLoS One. 2014;9(9):e108467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Kutsuma A, Nakajima K, Suwa K. Potential association between breakfast skipping and concomitant late-night-dinner eating with metabolic syndrome and proteinuria in the Japanese population. Scientifica. 2014;2014:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wang JB, Patterson RE, Ang A, Emond JA, Shetty N, Arab L. Timing of energy intake during the day is associated with the risk of obesity in adults. J Hum Nutr Diet. 2014;27(2):255–62. [DOI] [PubMed] [Google Scholar]
- 102. Rácz B, Dušková M, Stárka L, Hainer V, Kunešová M. Links between the circadian rhythm, obesity and the microbiome. Physiol Res. 2018;67(Suppl 3):S409–20. [DOI] [PubMed] [Google Scholar]
- 103. Garaulet M, Gómez-Abellán P, Alburquerque-Béjar JJ, Lee Y, Ordovás JM, Scheer FAJL. Timing of food intake predicts weight loss effectiveness. Int J Obes. 2013;37(4):604–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Jakubowicz D, Wainstein J, Landau Z, Raz I, Ahren B, Chapnik Net al. Influences of breakfast on clock gene expression and postprandial glycemia in healthy individuals and individuals with diabetes: a randomized clinical trial. Diabetes Care. 2017;40(11):1573–79. [DOI] [PubMed] [Google Scholar]
- 105. Adafer R, Messaadi W, Meddahi M, Patey A, Haderbache A, Bayen Set al. Food timing, circadian rhythm and chrononutrition: a systematic review of time-restricted eating's effects on human health. Nutrients. 2020;12(12):3770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Fleurbaix Laventie Ville Sante Study Group . The Three-Factor Eating Questionnaire-r18 is able to distinguish among different eating patterns in a general population. J Nutr. 2004;134(9):2372–80. [DOI] [PubMed] [Google Scholar]
- 107. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Nguyen J, Wright KP Jr. Influence of weeks of circadian misalignment on leptin levels. Nat Sci Sleep. 2010;2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. WHO . Healthy Diet. 2022. Internet: acessed (September, 15, 2022)https://www.who.int/news-room/fact-sheets/detail/healthy-diet.
- 110. Peuhkuri K, Sihvola N, Korpela R. Dietary factors and fluctuating levels of melatonin. Food Nutr Res. 2012;56(1):17252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.