Abstract
The predominant histological subtype of breast mucinous carcinoma in older women is type B (hypercellular type), and, in younger women, it is type A (hypocellular type). The characteristics of mucinous carcinomas of the same histological subtype may differ between older and younger women. This study aims to systematically clarify the pathological/immunohistochemical features of mucinous carcinomas. A total of 21 surgical cases of mucinous carcinoma (type A/B: 9/12 cases) in the older group (≥65 years) and 16 cases (type A/B: 14/2 cases) in the younger group (≤55 years) (n = 37) were included. Gross cystic disease fluid protein-15 (GCDFP-15) and eight other markers were used for immunostaining. The GCDFP-15-positive rate in the older group was high regardless of the histological subtype (type A, 77.8%; type B, 91.7%). The GCDFP-15 positivity in the older group was significantly higher than that in the younger group (p < 0.001 for Allred score). Among type A, GCDFP-15 positivity was significantly higher in the older group than in the younger group (p = 0.042 for the Allred score and p = 0.007 for the positivity rate). The present results suggest that GCDFP-15 expression characterizes mucinous carcinomas in older women.
Keywords: breast, mucinous carcinoma, gross cystic disease fluid protein-15 (GCDFP-15), older, apocrine
1. Introduction
Mucinous carcinoma is an invasive breast cancer histologically characterized by clusters of tumor cells suspended in extracellular mucin. Pure mucinous carcinoma is more common in older individuals and is generally associated with an excellent prognosis. Of all breast cancers, 2–4% are mucinous carcinomas, while in older women mucinous carcinomas account for more than 10% of breast cancer cases [1,2,3,4]. Histologically, pure mucinous carcinoma is classified as either type A (hypocellular: tubular, ribbon-like, and small papillary clusters with a large amount of extracellular mucin) or type B (hypercellular: large epithelial clumps or sheets with a small amount of extracellular mucin) [4,5]. Type B lesions are frequently positive for neuroendocrine markers (e.g., synaptophysin, chromogranin A, CD56) [6,7], and the surrounding tissues often have ductal carcinoma in situ (DCIS) components with a neuroendocrine tendency, which are considered precursor lesions [8].
In our previous study, approximately 16% of patients aged 85 years and older had mucinous carcinomas, and 11% had apocrine carcinomas [3]. In older patients with mucinous carcinomas, type B was predominant, as previously mentioned, and most type B lesions were positive for the apocrine marker gross cystic disease fluid protein-15 (GCDFP-15) [3,7]. Eosinophilic cytoplasm is also a characteristic cytological feature of type B lesions, which may reflect an apocrine character. Many lobular carcinomas in older patients are pleomorphic lobular carcinomas, which are referred to as apocrine-type lobular carcinomas. Type B mucinous carcinomas may also be considered apocrine-type mucinous carcinomas. For apocrine differentiated carcinomas, treatments targeting the androgen receptor (AR) have recently attracted attention due to their characteristic AR expression.
Mucinous carcinomas are generally considered hormone receptor-positive and human epidermal growth factor receptor 2 (HER2)-negative. They are the so-called luminal A-type cancers that have a favorable prognosis and a uniform clinical response [9,10]. However, genetic analysis has shown that type B mucinous carcinoma and neuroendocrine cancers have a common spectrum and a worse prognosis than type A [2]. Thus, the optimal clinical response may vary according to type A or B [11].
Type A and B mucinous carcinomas of the breast are common in younger and older women, respectively. As the hormonal environment of women varies greatly with age, even mucinous carcinomas of the same histological type may differ in their biological properties between older and younger individuals. In our previous study of mucinous carcinoma in older patients, there were special type A carcinomas with cytological features of type B carcinomas [7]. Therefore, this study aims to systematically clarify the clinicopathological features of mucinous carcinomas in older women by comparing the classical factors, such as type A/B, estrogen receptor (ER), progesterone receptor (PgR), HER2, nuclear grade, and Ki-67 score, as well as the expression of neuroendocrine and apocrine markers, with those from young to middle-aged women.
2. Materials and Methods
2.1. Subjects and Classification of Mucinous Carcinoma
The histological classification was based on the WHO classification [4]. Of the patients with surgical specimens diagnosed as pure-type mucinous carcinoma of the female breast between 2004 and 2017, 40 patients were aged 65 years and older (older group), and 16 were patients aged 55 years and younger (younger group).
Capella et al. reported that type A (hypocellular variant) has a mucus component of 60–90% while type B (hypercellular variant) has a mucus component of 33–75% [5]. In our study, to classify types A and B, specimens were evaluated by two separate pathologists. Specimen scores were obtained by scoring the cell component ratio using five levels (1: <20%, 2: 20–39%, 3: 40–59%, 4: 60–79%, 5: ≥80%), and cell cluster size was obtained by using three levels (1: small, 2: intermediate, and 3: large) and adding them [5]. Specimens with scores 2–3 and 6–8 were concordant, and they were classified as type A and type B, respectively. Cases with scores of 4 and 5 were evaluated as borderline, and when the outcome was discordant, the two pathologists reviewed the glass slides and decided together.
According to the histological type, there were 9 cases of type A and 31 cases of type B in the older group and 14 cases of type A and 2 cases of type B in the younger group (Fisher‘s exact test, p < 0.001). In the histological/immunohistochemical examination, 12 out of 31 cases of type B in the older group were available.
2.2. Clinicopathological Analysis
Pathological staging was based on UICC [12]. Nuclear grading was assessed according to the nuclear grading classification in the “Japanese Classification of Breast Cancer”, which is routinely used in Japan and has been confirmed to reflect the prognosis of Japanese breast cancer patients [13,14]. Briefly, the sum of nuclear atypia (1, mild; 2, moderate; 3, severe) and mitotic counts per 10 high-power fields (1, <5; 2, 5–10; 3, >10) was classified into a nuclear grade (I, 2 or 3; II, 4; III, 5 or 6).
2.3. Immunohistochemical Procedures and Evaluations
Immunohistochemical analyses for GCDFP-15, chromogranin A (CGA), synaptophysin (SYP), CD56, AR, ER, PgR, HER2, and Ki-67 were applied to the representative slides of formalin-fixed and paraffin-embedded tissues (Table 1). After antigen retrieval (none or heat-treatment for 40 min at pH6 or pH9), the slides were incubated for 30 min with the primary antibodies GCDFP-15, CGA, SYP, CD56, AR, ER, PgR, and Ki-67. After the endogenous peroxidase was quenched with 3% H2O2 in distilled water, the slides were incubated with secondary antibodies and detected using Histofine Simple Stain MAX-PO (MULTI) (Nichirei Biosciences Inc., Tokyo, Japan) and DAB substrate kits (Nichirei). HER2 immunohistochemical staining was performed according to the kit’s protocol (SV2-61γ, monoclonal: Nichirei).
Table 1.
Experimental conditions for immunohistochemistry of breast mucinous carcinoma.
| Primary Antibody | Primary Ab (Clone Name) |
Dilution | Antigen Retrieval Method | Intracellular Localization | Positive Thresholds | Supplier |
|---|---|---|---|---|---|---|
| GCDFP-15 | M (D6) | 1:700 | None | Cp | AS ≥ 4 | SIGNET |
| CGA | P | RtoU | None | Cp | AS ≥ 3 | Nichirei |
| SYP | M (27G12) | RtoU | None | Cp | AS ≥ 4 | Nichirei |
| CD56 | M (MRQ-42) | RtoU | 40 min, pH 9 | Cm | AS ≥ 3 | Nichirei |
| AR | M (AR27) | 1:25 | 40 min, pH 9 | N | AS ≥ 3 | Novocastra |
| ER | M (SPI) | RtoU | 40 min, pH 9 | N | AS ≥ 3 | Nichirei |
| PgR | M (A9621A) | RtoU | 40 min, pH 9 | N | AS ≥ 3 | Nichirei |
| HER2 | M (SV2-61γ) | Kit | None | Cm | HS ≥ 3+ | Nichirei |
| Ki-67 | M (MIB-1) | 1:200 | 40 min, pH 6 | N | LI ≥ 5% | Dako |
AR: androgen receptor; CGA: chromogranin A; ER: estrogen receptor; GCDFP-15: gross cystic disease fluid protein-15; HER2: human epidermal growth receptor 2; PgR: progesterone receptor; SYP: synaptophysin; Ab, antibody; M, monoclonal; P, polyclonal; RtoU, ready to use; Cp, cytoplasm; Cm, cell membrane; N, nucleus; AS, Allred score (total score); HS, HER2 score; LI, labeling index; SIGNET, SIGNET Lab, Inc., Dedham, USA; Nichirei, Nichirei Biosciences Inc., Tokyo, Japan; Novocastra, Novocastra Lab, Ltd., Sheffield UK; Dako Japan Inc., Tokyo, Japan.
To assess the staining, the percentage of immunoreactive cancerous cells was independently estimated in the nucleus (AR, ER, PgR, and Ki-67), cytoplasm (GCDFP-15, CGA, SYP, and CD56), and cytoplasmic membrane (HER2). We used the classification score proposed by Allred et al. for ER/PgR estimation in 1998 [15,16]. A positive case was defined as having an Allred score of 3 or more for CGA, CD56, AR, ER, and PgR or having a score of 4 or more for GCDFP-15 and SYP. In terms of HER2, a score of 3 was considered positive [17]. The Ki-67 score was defined as low when Ki-67-positive cells were <5% and high when Ki-67-positive cells were ≥5%. A high Ki-67 score was considered positive (Table 1).
2.4. Statistical Analyses
The Wilcoxon rank sum test was used to compare the Allred scores for each factor between the two groups. The Kruskal–Wallis test was used to compare the Allred score for each factor among the three groups. The Dunn test was used for pair-by-pair comparisons if the Kruskal–Wallis test was significant. Fisher’s exact test was used for contingency tables. The level of significance was set at p < 0.05. SPSS Statistics version 25 (IBM, Japan, Ltd., Tokyo, Japan) was used for statistical calculations.
3. Results
3.1. Clinicopathological Features
The mean age of the patients was 81.7 ± 6.8 years (range, 67–92 years) for the older group and 44.6 ± 8.6 years (range, 28–55 years) for the younger group. The T category that accounted for 50% or more of the cases was T2 for the older group and T1 for the younger group. The older group tended to have larger tumor sizes than the younger group. There were no N2 and N3 N-stage cases, and there was no significant difference between the two groups. All patients were negative for distant metastases. The TNM stage that accounted for 50% or more of the cases was stage II for the older group and stage I for the younger group. Nuclear grading showed no significant differences (Table 2). All patients were free from recurrence.
Table 2.
Clinicopathological summary of breast mucinous carcinoma.
| Older Group (≥65 y/o) |
Younger Group (<55 y/o) |
Fisher’s Exact Test (p-Value) | |
|---|---|---|---|
| Number of cases | 21 | 16 | |
| Age, mean ± SD (range) |
81.7 ± 6.81 (67–92) |
44.6 ± 8.63 (28–55) |
|
| T category (%) | 0.733 | ||
| T0 | 0 (0%) | 0 (0%) | |
| T1 | 7 (33.3%) | 8 (50.0%) | |
| T2 | 11 (52.4%) | 6 (37.5%) | |
| T3 | 3 (14.3%) | 2 (12.5%) | |
| T4 | 0 (0%) | 0 (0%) | |
| N stage | 1.000 | ||
| N0 | 18 (85.7%) | 14 (87.5%) | |
| N1 | 3 (14.3%) | 2 (12.5%) | |
| N2, N3 | 0 (0%) | 0 (0%) | |
| M category | |||
| M0 | 21 (100%) | 16 (100%) | |
| M1 | 0 (0%) | 0 (0%) | |
| TNM stage | 0.364 | ||
| Stage 0 | 0 (0%) | 0 (0%) | |
| Stage I | 7 (33.3%) | 8 (50%) | |
| Stage II | 13 (61.9%) | 6 (37.5%) | |
| Stage III | 1(4.8%) | 2 (12.5%) | |
| Stage IV | 0 (0%) | 0 (0%) | |
| Nuclear grade | 0.832 | ||
| Grade I | 4 (19.0%) | 4 (25.0%) | |
| Grade II | 10 (47.6%) | 8 (50.0%) | |
| Grade III | 7 (33.3%) | 4 (25.0%) |
SD, standard deviation.
3.2. Immunohistochemical Study
Typical histological images of each type are shown in Figure 1A,B. Typical microscopic images of GCDFP-15 immunostaining from the different age groups and carcinoma types are shown in Figure 1C–F. Additional positive immunostaining images are shown in Figure 2. The Allred scores are statistically analyzed in Table 3, Table 4 and Table 5. When the cases were divided into four groups according to age group and carcinoma type, there were only two type B cases in the younger group. Thus, the remaining three groups were compared, as shown in Table 4 and Table 5.
Figure 1.
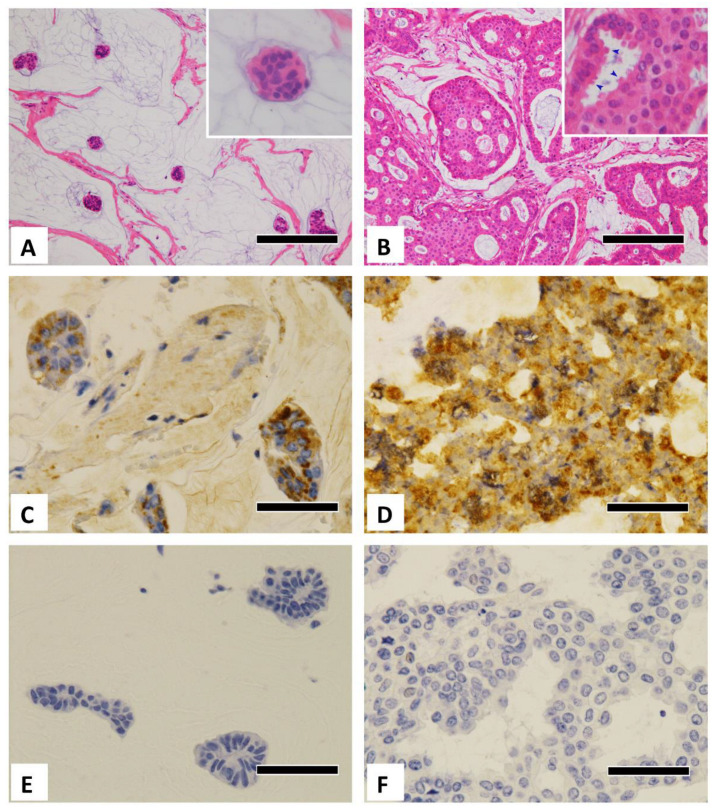
Microscopic pictures of breast mucinous carcinoma. (A) Histological image of a type A mucinous carcinoma. Note tubular and papillary small clusters with a large amount of extracellular mucin (HE stain). The inset shows a magnified image. (B) Histological image of a type B mucinous carcinoma. Large epithelial clumps or sheets composed of tumor cells with eosinophilic cytoplasm and a small amount of extracellular mucin (HE staining). The magnified image inset shows tumor cells with apocrine snouts (arrowheads) and abundant eosinophilic cytoplasm. (C) Older, type A (immunohistochemical pictures of GCDFP-15 positivity). (D) Older, type B (immunohistochemical pictures of GCDFP-15 positivity). (E) Younger, type A (immunohistochemical pictures of GCDFP-15 negativity). (F) Younger, type B (immunohistochemical pictures of GCDFP-15 negativity). GCDFP-15: gross cystic disease fluid protein-15. Scale bar = 200 μm (A,B), 50 μm (C–F).
Figure 2.
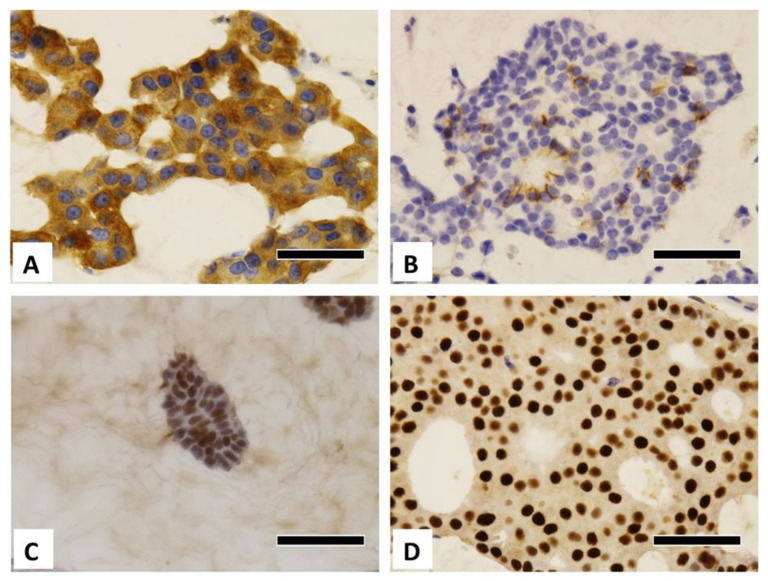
Immunohistochemical pictures of breast mucinous carcinoma. (A) SYP-positive cancer (older, type B), (B) CD56-positive cancer (older, type B), (C) AR-positive cancer (younger, type A), (D) AR-positive cancer (older, type B). AR, androgen receptor; SYP, synaptophysin. Scale bar = 50 μm.
Table 3.
Comparisons of immunohistochemical features of breast mucinous carcinoma by age group and type.
| Median Score (Range) | Median Score (Range) | |||||
|---|---|---|---|---|---|---|
| Antibodies | Older | Younger | p-Value (Older vs. Younger) | Type A | Type B | p-Value (Type A vs. B) |
| Number of cases | 21 | 16 | 23 | 14 | ||
| GCDFP-15 | 5 (0–8) | 0 (0–5) | <0.001 | 3 (0–8) | 5.5 (0–8) | 0.014 |
| CGA | 0 (0–6) | 2.5 (0–7) | 0.046 | 2 (0–6) | 0 (2–8) | 0.394 |
| SYP | 4 (0–8) | 2 (0–8) | 0.059 | 3 (0–8) | 6 (0–8) | 0.186 |
| CD56 | 0 (0–7) | 0 (0–3) | 0.201 | 0 (0–6) | 1 (0–7) | 0.237 |
| AR | 6 (3–8) | 6 (2–7) | 0.250 | 6 (4–8) | 6 (2–8) | 0.652 |
| ER | 8 (6–8) | 7.5 (4–8) | 0.906 | 7 (4–8) | 8 (7–8) | 0.032 |
| PgR | 6 (2–8) | 7 (0–8) | 0.376 | 6 (2–8) | 5.5 (0–8) | 0.525 |
| HER2 | 0 (0–2) | 0 (0–3) | 1.000 | 0 (0–3) | 0 (0–2) | 0.904 |
| Ki-67 | 1.5 (1–30) | 1.5 (0–15) | 0.874 | 1.5 (0–10) | 1.75 (1–30) | 0.190 |
p-values are calculated by the Wilcoxon rank sum test. AR: androgen receptor; CGA: chromogranin A; ER: estrogen receptor; GCDFP-15: gross cystic disease fluid protein-15; HER2: human epidermal growth receptor 2; PgR: progesterone receptor; SYP: synaptophysin.
Table 4.
Immunohistochemical features of breast mucinous carcinomas among four subgroups.
| Median Score (Range) | ||||||
|---|---|---|---|---|---|---|
| Antibodies | Older Type A (1) |
Older Type B (2) |
Younger Type A (3) |
Younger Type B |
p-Value (1) (2) (3) (KW Test) |
p-Value (1) vs. (2)/(1) vs. (3) (Dunn Test) |
| Number of cases | 9 | 12 | 14 | 2 | ||
| GCDFP-15 | 5 (0–8) | 6 (3–8) | 0 (0–5) | 0, 0 | <0.001 | 1.000/0.042 |
| CGA | 0 (0–6) | 0 (0–6) | 2 (0–6) | 3, 7 | 0.124 | n.a. |
| SYP | 4 (0–8) | 5 (0–8) | 2 (0–5) | 6, 8 | 0.024 | 1.000/0.093 |
| CD56 | 0 (0–6) | 1 (0–7) | 0 (0–6) | 0, 3 | 0.545 | n.a. |
| AR | 7 (4–8) | 6 (3–8) | 6 (4–7) | 7, 2 | 0.447 | n.a. |
| ER | 7 (6–8) | 8 (7–8) | 7.5 (4–8) | 7, 8 | 0.077 | n.a. |
| PgR | 6 (2–8) | 5.5 (2–8) | 7 (2–8) | 0, 8 | 0.793 | n.a. |
| HER2 | 0 (0–1) | 0 (0–2) | 0 (0–3) | 0, 0 | 0.738 | n.a. |
| Ki-67 | 1.5 (1–5) | 1.5 (1–30) | 1.25 (0–10) | 5, 15 | 0.248 | n.a. |
p-values are calculated by the Kruskal–Wallis test and Dunn test. AR: androgen receptor; CGA: chromogranin A; ER: estrogen receptor; GCDFP-15: gross cystic disease fluid protein-15; HER2: human epidermal growth receptor 2; KW test: Kruskal–Wallis test; PgR: progesterone receptor; SYP: synaptophysin; n.a.: not applicable.
Table 5.
Immunohistochemical features of carcinomas among four subgroups.
| Antibodies | Older Type A (1) |
Older Type B (2) |
Younger Type A (3) |
Younger Type B |
p-Value (1) vs. (2)/(1) vs. (3) |
|---|---|---|---|---|---|
| n = 9 +/− |
n = 12 +/− |
n = 14 +/− |
n = 2 +/− |
||
| GCDFP-15 | 7/2 | 11/1 | 2/12 | 0/2 | 0.553/0.007 |
| CGA | 4/5 | 3/9 | 6/8 | 2/0 | 0.397/1.000 |
| SYP | 6/3 | 7/5 | 3/11 | 2/0 | 1.000/0.077 |
| CD56 | 3/6 | 5/7 | 2/12 | 1/1 | 1.000/0.343 |
| AR | 8/0 | 10/0 | 14/0 | 1/1 | 1.000/1.000 |
| ER | 9/0 | 12/0 | 14/0 | 2/0 | 1.000/1.000 |
| PgR | 8/1 | 10/2 | 12/2 | 1/1 | 1.000/1.000 |
| HER2 | 0/9 | 0/12 | 1/13 | 0/2 | 1.000/1.000 |
| Ki-67 | 1/8 | 3/9 | 4/10 | 2/0 | 0.603/0.611 |
p-values were calculated using Fisher’s exact test. AR: androgen receptor; CGA: chromogranin A; ER: estrogen receptor; GCDFP-15: gross cystic disease fluid protein-15; HER2: human epidermal growth receptor 2; PgR: progesterone receptor; SYP: synaptophysin.
3.2.1. GCDFP-15
The Allred scores were significantly higher in the older group than in the younger group (p < 0.001), and they were significantly higher in type B carcinoma than in type A (p = 0.014) (Table 3). They were also significantly different among the three groups (p < 0.001), as shown in Table 4. No significant difference was observed between the older type A and older type B groups (p = 1.000), whereas a significant difference was observed between the older type A and younger type A groups (p = 0.042). In the dichotomous positive/negative comparison, GCDFP-15-positive expression was seen in 18 of 21 cases in the older group (85.7%) and 2 of 16 cases in the younger group (12.5%), yielding significant differences (p < 0.001). In the older group, 7 of 9 type A cases (77.8%) and 11 of 12 type B cases (91.7%) were positive for GCDFP-15, indicating a high positivity rate regardless of the carcinoma type (p = 0.553, Table 5. Figure 1C–F). Among type A, the positivity rate was significantly higher in the older group than in the younger group (p = 0.007).
3.2.2. Neuroendocrine Markers
The Allred score for CGA was lower in the older group than in the younger group (p = 0.046) (Table 3), however, other CGA tests showed no significant differences (Table 4 and Table 5).
The Allred scores for SYP were insignificantly higher in the older group than in the younger group (p = 0.059) (Table 3). The difference was significant among the three groups (p = 0.024) (Table 4), however, no significant difference was obtained by pair-by-pair comparisons. The dichotomous analyses for SYP did not yield significant differences (Table 5).
CD56 did not differ significantly in any comparison (Table 3, Table 4 and Table 5).
3.2.3. Steroid Hormone Receptors
There were no significant differences in the AR Allred scores between the older and younger patients (p = 0.250) or between type A and type B (p = 0.652) (Table 3) and in any further analyses (Table 4 and Table 5).
The ER Allred scores did not significantly differ between older and younger patients (p = 0.906) but were significantly higher in type B carcinoma than in type A (p = 0.032) (Table 3). No significant differences were found in further studies (Table 4 and Table 5). The PgR Allred scores did not differ significantly in any studies (Table 3, Table 4 and Table 5).
3.2.4. HER2 and Ki-67 Immunostaining
There were no significant differences in the expression of HER2 and Ki-67 in any comparison (Table 3, Table 4 and Table 5).
3.2.5. Comparison between GCDFP-15 Expression and Other Factors
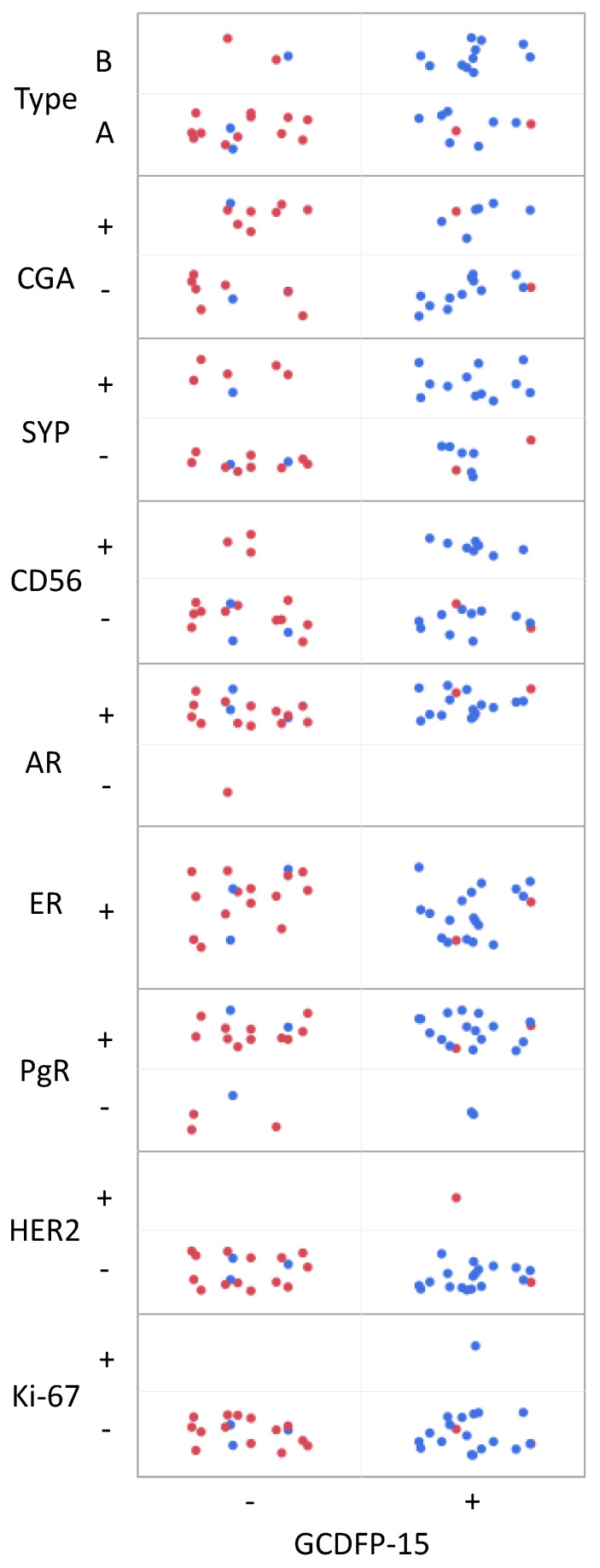
Figure 3 shows the relationships between GCDFP-15 expression and the type of mucinous carcinoma or the expression of other immunohistochemical markers considering age. Most of the mucinous carcinomas in older patients were GCDFP-15-positive irrespective of other factors, whereas those in younger patients exhibited opposite results.
Figure 3.
The relationships between GCDFP-15 expression and type of mucinous carcinoma or the expression of other immunohistochemical markers considering age (older patients in blue and younger patients in red). AR: androgen receptor; CGA: chromogranin A; ER: estrogen receptor; GCDFP-15: gross cystic disease fluid protein-15; HER2: human epidermal growth receptor 2; PgR: progesterone receptor; SYP: synaptophysin.
4. Discussion
Our results showed that GCDFP-15 expression clearly characterizes mucinous carcinoma in older patients regardless of the mucinous carcinoma subtype or the other immunohistochemical markers. The neuroendocrine character was not necessarily characteristic of mucinous carcinoma of type B or in older patients.
4.1. Apocrine Markers (GCDFP-15/AR)
We previously reported, in older patients, a high rate of mucinous and apocrine cancers and higher rates of GCDFP-15 and AR-positive cancers [3]. In the present study, mucinous carcinomas in older patients were mostly positive for both GCDFP-15 and AR and showed abundant eosinophilic cytoplasm or apocrine snouts, suggesting their apocrine-like characteristics (Figure 1). Although apocrine metaplasia in mucinous carcinomas is described in the WHO classification [18], we clearly showed for the first time that GCDFP-15 positivity was more prevalent in older patients. So far, mucinous carcinoma in older patients has been characterized by type B morphology or neuroendocrine features; however, our results demonstrated that GCDFP-15 expression most clearly characterizes the mucinous carcinoma of older patients. Pleomorphic invasive lobular carcinoma, often GCDFP-15/AR-positive and regarded as an apocrine-type invasive lobular carcinoma, is frequent in older women [18,19,20,21]. As for tumors in the other organs, about 5% of lung adenocarcinoma were reported to be positive for GCDFP-15, and most of them occurred in older individuals [22,23]. Of note, GCDFP-15 has been reportedly positive for a mucin-rich variant of salivary duct carcinoma [24] and endocrine mucin-producing sweat gland carcinoma [25], both of which commonly affects older patients, suggesting a similar phenomenon in the other organs.
Interestingly, the type A mucinous carcinomas of older individuals also exhibited apocrine-like immunohistochemical characteristics (GCDFP-15 positivity and AR positivity). The relationship between type B carcinomas and neuroendocrine characteristics is well known. However, the apocrine-like characteristic was not limited to type B carcinomas, as de Andrade Natal reported [6] but rather was found in either type of mucinous carcinoma in older patients. Conversely, type A carcinomas in older patients might differ in their biological characteristics from type A carcinomas in younger patients. We conclude that the biologically essential features of mucinous carcinomas in older patients are apocrine-like immunohistochemical features (GCDFP-15/AR positivity), rather than neuroendocrine features. Of note, apocrine differentiation is generally characterized by GCDFP-15 positivity, AR positivity, ER negativity, and PgR negativity [18]. Almost all intraductal and invasive apocrine carcinomas are positive for GCDFP-15/AR and negative for ER and PgR [26]. Most mucinous carcinomas are ER-positive and PgR-positive, regardless of patient age and histological type, and thus they are not entirely apocrine-differentiated carcinomas— they partially have apocrine character. Interestingly, GCDFP-15 tended to be negative in younger patients despite AR positivity. These points are discussed further below.
4.2. GCDFP-15 and AR/PgR Expression
The expression of GCDFP-15 is induced by AR activation caused by the binding of androgens, such as testosterone or dihydrotestosterone, to AR [27]. In our study, there was no significant difference in the expression of AR between older and younger patients. Frequent expression of AR in mucinous carcinomas was previously reported; de Andrade Natal et al. reported that AR positivity was seen in 5 of 16 cases (31.6%) of type A breast mucinous carcinomas and 13 of 23 cases (56.5%) of type B breast mucinous carcinomas [6]. Cho et al. reported that the rate of AR positivity was 21.7% in breast mucinous carcinomas, of which 47.8% of all patients were 50 years old or older [28]. AR positivity is generally higher in luminal cancers (ER/PgR-positive cancers), and it may be reasonable that mucinous cancers with higher ER/PgR positivity have higher AR positivity. However, it is worth noting that, unlike in older patients, GCDFP-15 positivity was low in younger patients despite high AR positivity. As AR is structurally similar to PgR, progesterone has the ability to bind AR and inhibits its action [29,30]. AR action may be inhibited in younger patients due to their higher blood progesterone levels. The reduced GCDFP-15 expression in younger women might be a result of progesterone binding to the AR. Blood androgen levels decrease with age, albeit at a slow rate, whereas progesterone levels decrease sharply after menopause [31,32,33,34]. In older individuals, the androgen/progesterone ratio is higher than that in younger individuals. AR is less inhibited in the older groups, and this may maintain the GCDFP-15 expression. Consequently, GCDFP-15 positivity may have been higher in older patients than in younger patients. GCDFP-15 can be an indicator of normal androgen-AR signaling, as PgR is for ER; then, it may be revealed to work as a predictor of AR-targeting therapy in the future.
4.3. Expression of Other Immunohistochemical Markers
Previous reports showed a neuroendocrine feature in type B mucinous carcinomas [6,7,35]. Our results showed that SYP positivity in older women tended to be higher than that in younger individuals (Table 3), and that it was different among three groups (p = 0.024) (Table 4). In contrast, the CGA positivity was significantly higher in younger women than in older women and was not significantly different between type A and type B (Table 3) or in any other comparisons (Table 4 and Table 5). CD56 did not significantly differ in any comparison. Both SYP and CGA are good neuroendocrine markers with high sensitivity and specificity; however, the results of these neuroendocrine markers were inconsistent. Neuroendocrine features can also be examined by an electron microscope. Previous studies on mucinous carcinoma showed controversial results regarding the presence of neuroendocrine granules, suggesting “pseudo” neuroendocrine differentiation [36,37]. Thus, further studies are warranted to elucidate this neuroendocrine marker discrepancy.
Our immunohistochemical findings for ER, PgR, HER2, and Ki-67 suggested mucinous carcinomas were nearly all luminal A. A summary of previous reports is presented in Table 6. In all studies, more than 90% were positive for ER, and more than 80% were positive for PgR in most studies. The HER2 positivity rate was also low [1,6,38,39]. Our results were almost consistent with those reports regarding ER, PgR, and HER2. It is difficult to compare the results of Ki-67 as the Ki-67 index threshold is not universally standardized.
Table 6.
Reported immunohistochemical property of breast mucinous carcinoma.
| Study | Group | Number of Cases | Mean Age | ER | PgR | HER2 | Ki-67 | Ki-67 Threshold |
|---|---|---|---|---|---|---|---|---|
| Our study | Older (67–92 y/o) | 21 | 81.7 | 100% | 85.7% | 0% | 19% | 5% |
| Younger (28–55 y/o) | 16 | 44.6 | 100% | 81.3% | 6.3% | 37.5% | 5% | |
| Li et al. [1] | 50–89 y/o | 2730 | n.a. | 96% | 83% | n.a. | n.a. | n.a. |
| 30–49 y/o | 516 | n.a. | 91% | 81% | n.a. | n.a. | n.a. | |
| Di Saverio et al. [38] | 25–85 y/o | 11422 | 68.3 | 94.1% | 81.5% | n.a. | n.a. | n.a. |
| de Andrade Natal et al. [6] | Type A | 17 | 57.0 | 100% | 52.9% | 5.9% | 0% | 14% |
| Type B | 23 | 66.0 | 95.7% | 73.9% | 4.3% | 21.7% | 14% | |
| Lacroix-Triki et al. [39] | 35 | n.a. | 100% | 85.7% | 2.9% | 8.6% | 10% |
ER, estrogen receptor; HER2, human epidermal growth receptor 2; PgR, progesterone receptor; n.a., not available.
4.4. Limitations of This Study
The small sample size of our study necessitates studies with larger sample sizes to validate our results.
5. Conclusions
Our results showed that mucinous carcinomas in older patients are more clearly characterized by GCDFP-15 expression than type B or neuroendocrine differentiation, which has been considered to characterize them.
Acknowledgments
The authors are grateful to Tetuo Mikami, Kayo Tsuburaya, and Maho Yokoyama from the Department of Pathology, Kazutoshi Shibuya, and the staff of the Department of Surgical Pathology, Toho University Faculty of Medicine, for their valuable assistance. We also thank the staff of the Department of Pathology, Tokyo Metropolitan Geriatric Hospital, for supporting this study.
Author Contributions
Conceptualization, N.H.; methodology, M.K., M.S. and N.H.; validation, M.K., M.N.M. and M.S.; investigation, M.K., T.A. and N.H.; resources, T.A.; data curation, M.K.; writing—original draft preparation, M.K., M.S. and N.H.; writing—review and editing, Y.S., M.S. and N.H.; visualization, M.K., M.S. and N.H.; supervision, M.S. and N.H.; project administration, M.S. and N.H.; funding acquisition, N.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Tokyo Metropolitan Geriatric Hospital Institutional Research Board (R16-19, 26 August 2016) and the Toho University Faculty of Medicine Ethics Committee (A19061_A16078_27132, 13 December 2019).
Informed Consent Statement
Informed opt-out consent was obtained from all subjects involved in this study.
Data Availability Statement
No applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by JSPS/MEXT KAKENHI grant number 16K08660.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Li C.I., Uribe D.J., Daling J.R. Clinical Characteristics of Different Histologic Types of Breast Cancer. Br. J. Cancer. 2005;93:1046–1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Komaki K., Sakamoto G., Sugano H., Morimoto T., Monden Y. Mucinous Carcinoma of the Breast in Japan. A Prognostic Analysis Based on Morphologic Features. Cancer. 1988;61:989–996. doi: 10.1002/1097-0142(19880301)61:5<989::AID-CNCR2820610522>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 3.Honma N., Sakamoto G., Akiyama F., Esaki Y., Sawabe M., Arai T., Hosoi T., Harada N., Younes M., Takubo K. Breast Carcinoma in Women Over the Age of 85: Distinct Histological Pattern and Androgen, Oestrogen, and Progesterone Receptor Status. Histopathology. 2003;42:120–127. doi: 10.1046/j.1365-2559.2003.01542.x. [DOI] [PubMed] [Google Scholar]
- 4.Wen H.Y., Desmedt C., Reis-Filho J.S., Schmitt F. Breast Tumours (WHO Classification of Tumours) 5th ed. International Agency for Research on Cancer; Lyon, France: 2019. Mucinous carcinoma; pp. 123–125. [Google Scholar]
- 5.Capella C., Eusebi V., Mann B., Azzopardi J.G. Endocrine Differentiation in Mucoid Carcinoma of the Breast. Histopathology. 1980;4:613–630. doi: 10.1111/j.1365-2559.1980.tb02957.x. [DOI] [PubMed] [Google Scholar]
- 6.de Andrade Natal R., Derchain S.F., Pavanello M., Paiva G.R., Sarian L.O., Vassallo J. Expression of Unusual Immunohistochemical Markers in Mucinous Breast Carcinoma. Acta Histochem. 2017;119:327–336. doi: 10.1016/j.acthis.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Shirahata H., Honma N., Kotani T., Imaizumi M., Hamashima Y., Esaki Y., Kinoshita M., Suzuki A., Sakurai U., Arai T. Cytological Characteristics of Mucinous Carcinoma of the Breast in the Elderly with Bibliographical Considerations (in Japanese with English Abstract) J. Jpn. Soc. Clin. Cytol. 2017;56:75–84. doi: 10.5795/jjscc.56.75. [DOI] [Google Scholar]
- 8.Kryvenko O.N., Chitale D.A., Yoon J., Arias-Stella J., Meier F.A., Lee M.W. Precursor Lesions of Mucinous Carcinoma of the Breast: Analysis of 130 Cases. Am. J. Surg. Pathol. 2013;37:1076–1084. doi: 10.1097/PAS.0b013e31828de420. [DOI] [PubMed] [Google Scholar]
- 9.Corben A.D., Brogi E. Mucinous carcinoma. In: Hoda S.A., Brogi E., Koemer F.C., Rosen P.P., editors. Rosen’s Breast Pathology. 4th ed. Lippincott Willams & Wilkins; Philadelphia, PA, USA: 2014. pp. 611–644. [Google Scholar]
- 10.Budzik M.P., Fudalej M.M., Badowska-Kozakiewicz A.M. Histopathological Analysis of Mucinous Breast Cancer Subtypes and Comparison with Invasive Carcinoma of no Special Type. Sci. Rep. 2021;11:5770. doi: 10.1038/s41598-021-85309-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weigelt B., Geyer F.C., Horlings H.M., Kreike B., Halfwerk H., Reis-Filho J.S. Mucinous and Neuroendocrine Breast Carcinomas are Transcriptionally Distinct from Invasive Ductal Carcinomas of no Special Type. Mod. Pathol. 2009;22:1401–1414. doi: 10.1038/modpathol.2009.112. [DOI] [PubMed] [Google Scholar]
- 12.Brierley J.D., Gospodarowicz M.K., Wittekind C. Breast Tumours. In: Brierley J.D., Gospodarowicz M.K., Wittekind C., editors. TNM Classification of Malignant Tumours. 8th ed. Wiley-Blackwell; Hoboken, NJ, USA: 2017. pp. 151–158. [Google Scholar]
- 13.Tsuda H., Akiyama F., Kurosumi M., Sakamoto G., Watanabe T. Establishment of Histological Criteria for High-Risk Node-Negative Breast Carcinoma for a Multi-Institutional Randomized Clinical Trial of Adjuvant Therapy. Japan National Surgical Adjuvant Study of Breast Cancer (NSAS-BC) Pathology Section. Jpn. J. Clin. Oncol. 1998;28:486–491. doi: 10.1093/jjco/28.8.486. [DOI] [PubMed] [Google Scholar]
- 14.Otsuki Y., Shimizu S., Suwa K., Yoshida M., Kanzaki M., Kobayashi H. Which is the Better Pathological Prognostic Factor, the Nottingham Histological Grade Or the Japanese Nuclear Grade? A Large Scale Study with a Long-Term Follow-Up. Jpn. J. Clin. Oncol. 2007;37:266–274. doi: 10.1093/jjco/hym026. [DOI] [PubMed] [Google Scholar]
- 15.Fitzgibbons P.L., Dillon D.A., Alsabeh R., Berman M.A., Hayes D.F., Hicks D.G., Hughes K.S., Nofech-Mozes S. Template for Reporting Results of Biomarker Testing of Specimens from Patients with Carcinoma of the Breast. Arch. Pathol. Lab. Med. 2014;138:595–601. doi: 10.5858/arpa.2013-0566-CP. [DOI] [PubMed] [Google Scholar]
- 16.Soares M., Madeira S., Correia J., Peleteiro M., Cardoso F., Ferreira F. Molecular Based Subtyping of Feline Mammary Carcinomas and Clinicopathological Characterization. Breast. 2016;27:44–51. doi: 10.1016/j.breast.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 17.Tsuda H., Akiyama F., Terasaki H., Hasegawa T., Kurosumi M., Shimadzu M., Yamamori S., Sakamoto G. Detection of HER-2/Neu (C-Erb B-2) DNA Amplification in Primary Breast Carcinoma. Interobserver Reproducibility and Correlation with Immunohistochemical HER-2 Overexpression. Cancer. 2001;92:2965–2974. doi: 10.1002/1097-0142(20011215)92:12<2965::AID-CNCR10156>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 18.Provenzano E., Gatalica Z., Vranic S. Breast Tumours (WHO Classification of Tumours) 5th ed. International Agency for Research on Cancer; Lyon, France: 2019. Carcinoma with apocrine differentiation; pp. 131–133. [Google Scholar]
- 19.Kasashima S., Kawashima A., Zen Y., Ozaki S., Kobayashi M., Tsujibata A., Minato H. Expression of Aberrant Mucins in Lobular Carcinoma with Histiocytoid Feature of the Breast. Virchows Arch. 2007;450:397–403. doi: 10.1007/s00428-007-0381-z. [DOI] [PubMed] [Google Scholar]
- 20.Shimizu S., Kitamura H., Ito T., Nakamura T., Fujisawa J., Matsukawa H. Histiocytoid Breast Carcinoma: Histological, Immunohistochemical, Ultrastructural, Cytological and Clinicopathological Studies. Pathol. Int. 1998;48:549–556. doi: 10.1111/j.1440-1827.1998.tb03948.x. [DOI] [PubMed] [Google Scholar]
- 21.Tan P.H., Harada O., Thike A.A., Tse G.M. Histiocytoid Breast Carcinoma: An Enigmatic Lobular Entity. J. Clin. Pathol. 2011;64:654–659. doi: 10.1136/jcp.2011.088930. [DOI] [PubMed] [Google Scholar]
- 22.Striebel J.M., Dacic S., Yousem S.A. Gross Cystic Disease Fluid Protein-(GCDFP-15): Expression in Primary Lung Adenocarcinoma. Am. J. Surg. Pathol. 2008;32:426–432. doi: 10.1097/PAS.0b013e318157a5a6. [DOI] [PubMed] [Google Scholar]
- 23.Wang L.J., Greaves W.O., Sabo E., Noble L., Tavares R., Ng T., DeLellis R.A., Resnick M.B. GCDFP-15 Positive and TTF-1 Negative Primary Lung Neoplasms: A Tissue Microarray Study of 381 Primary Lung Tumors. Appl. Immunohistochem. Mol. Morphol. 2009;17:505–511. doi: 10.1097/PAI.0b013e3181a8e809. [DOI] [PubMed] [Google Scholar]
- 24.Simpson R.H.W., Prasad A.R., Lewis J.E., Skálová A., David L. Mucin-Rich Variant of Salivary Duct Carcinoma: A Clinicopathologic and Immunohistochemical Study of Four Cases. Am. J. Surg. Pathol. 2003;27:1070–1079. doi: 10.1097/00000478-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Ravi P.Y., Walsh N.M., Archibald C., Pasternak S. Endocrine Mucin-Producing Sweat Gland Carcinoma: Emerging Evidence of Multicentric Cutaneous Origin and Occasional Concurrence with Analogous Breast Tumors. Am. J. Dermatopathol. 2022;44:321–326. doi: 10.1097/DAD.0000000000002132. [DOI] [PubMed] [Google Scholar]
- 26.Brogi E. Apocrine carcinoma. In: Hoda S., Brogi E., Koerner F.C., Rosen P.P., editors. Rosen’s Breast Pathology. 4th ed. Wolters Kluwer Health, Inc.; Philadelphia, PA, USA: 2014. pp. 645–666. [Google Scholar]
- 27.Loos S., Schulz K.D., Hackenberg R. Regulation of GCDFP-15 Expression in Human Mammary Cancer Cells. Int. J. Mol. Med. 1999;4:135–140. doi: 10.3892/ijmm.4.2.135. [DOI] [PubMed] [Google Scholar]
- 28.Cho L., Hsu Y. Expression of Androgen, Estrogen and Progesterone Receptors in Mucinous Carcinoma of the Breast. Kaohsiung J. Med. Sci. 2008;24:227–232. doi: 10.1016/S1607-551X(08)70146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raudrant D., Rabe T. Progestogens with Antiandrogenic Properties. Drugs. 2003;63:463–492. doi: 10.2165/00003495-200363050-00003. [DOI] [PubMed] [Google Scholar]
- 30.Bardin C.W., Brown T., Isomaa V.V., Jänne O.A. Progestins can Mimic, Inhibit and Potentiate the Actions of Androgens. Pharmacol. Ther. 1983;23:443–459. doi: 10.1016/0163-7258(83)90023-2. [DOI] [PubMed] [Google Scholar]
- 31.Hankinson S.E., Eliassen A.H. Endogenous Estrogen, Testosterone and Progesterone Levels in Relation to Breast Cancer Risk. J. Steroid Biochem. Mol. Biol. 2007;106:24–30. doi: 10.1016/j.jsbmb.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manjer J., Johansson R., Berglund G., Janzon L., Kaaks R., Agren A., Lenner P. Postmenopausal Breast Cancer Risk in Relation to Sex Steroid Hormones, Prolactin and SHBG (Sweden) Cancer Causes Control. 2003;14:599–607. doi: 10.1023/A:1025671317220. [DOI] [PubMed] [Google Scholar]
- 33.Missmer S.A., Eliassen A.H., Barbieri R.L., Hankinson S.E. Endogenous Estrogen, Androgen, and Progesterone Concentrations and Breast Cancer Risk among Postmenopausal Women. J. Natl. Cancer Inst. 2004;96:1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 34.Sieri S., Krogh V., Bolelli G., Abagnato C.A., Grioni S., Pala V., Evangelista A., Allemani C., Micheli A., Tagliabue G., et al. Sex Hormone Levels, Breast Cancer Risk, and Cancer Receptor Status in Postmenopausal Women: The ORDET Cohort. Cancer Epidemiol. Biomark. Prev. 2009;18:169–176. doi: 10.1158/1055-9965.EPI-08-0808. [DOI] [PubMed] [Google Scholar]
- 35.Scopsi L., Andreola S., Pilotti S., Bufalino R., Baldini M.T., Testori A., Rilke F. Mucinous Carcinoma of the Breast. A Clinicopathologic, Histochemical, and Immunocytochemical Study with Special Reference to Neuroendocrine Differentiation. Am. J. Surg. Pathol. 1994;18:702–711. doi: 10.1097/00000478-199407000-00006. [DOI] [PubMed] [Google Scholar]
- 36.Dickersin G.R., Maluf H.M., Koerner F.C. Solid Papillary Carcinoma of Breast: An Ultrastructural Study. Ultrastruct. Pathol. 1997;21:153–161. doi: 10.3109/01913129709021314. [DOI] [PubMed] [Google Scholar]
- 37.Ng W. Mammary Mucinous Carcinoma with Marked Cytoplasmic Hyalinization. A Report of 2 Cases with Emphasis on Fine Needle Aspiration Cytologic Findings. Acta Cytol. 2003;47:1045–1049. doi: 10.1159/000326644. [DOI] [PubMed] [Google Scholar]
- 38.Di Saverio S., Gutierrez J., Avisar E. A Retrospective Review with Long Term Follow Up of 11.400 Cases of Pure Mucinous Breast Carcinoma. Breast Cancer Res. Treat. 2008;111:541–547. doi: 10.1007/s10549-007-9809-z. [DOI] [PubMed] [Google Scholar]
- 39.Lacroix-Triki M., Suarez P.H., MacKay A., Lambros M.B., Natrajan R., Savage K., Geyer F.C., Weigelt B., Ashworth A., Reis-Filho J.S. Mucinous Carcinoma of the Breast is Genomically Distinct from Invasive Ductal Carcinomas of no Special Type. J. Pathol. 2010;222:282–298. doi: 10.1002/path.2763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No applicable.