Abstract
The human granulocytic ehrlichiosis (HGE) agent, which replicates in neutrophils, was found not to induce superoxide anion (O2−) generation or extracellular release by human peripheral blood neutrophils, as measured by a luminol-dependent chemiluminescence assay or a cytochrome c reduction assay, respectively. Furthermore, the HGE agent completely prevented O2− release by neutrophils upon stimulation with phorbol myristate acetate (PMA), formylmethionyl-leucyl-phenylalanine, or Escherichia coli. The inhibition was HGE agent dose dependent, required ehrlichial contact with the host cells, and was reversible upon removal of the extracellular HGE agent bound to the host cells prior to PMA stimulation. Structural integrity of or new protein synthesis by the HGE agent was not required for the inhibition; carbohydrate but not surface protein of the HGE agent was required. The HGE agent did not prevent O2− generation in human peripheral blood monocytes derived from the same individual. This neutrophil-specific prevention of O2− generation by the HGE agent would be critical in survival of the HGE agent. This is the first demonstration of the rapid inhibition of preexisting NADPH oxidase in human neutrophils by the HGE agent.
An emerging tick-borne zoonosis caused by the human granulocytic ehrlichiosis (HGE) agent was first described in 1994 (5) and has been increasingly recognized in the United States and several European countries. HGE is a disease characterized by systemic illness such as fever, chills, headache, malaise, and/or myalgia. Laboratory tests may reveal thrombocytopenia, leukopenia, elevated C-reactive protein levels, and elevated liver enzyme activities. The HGE agent is a unique obligatory intracellular bacterium that replicates in neutrophils. Neutrophils have a strong ability to kill invading microorganisms through activation of the NADPH oxidase, which rapidly generates superoxide anion (O2−) and subsequent formation of other bactericidal reactive oxygen intermediates (hydrogen peroxide, myeloperoxide, hypochlorous acid, hydroxyl radical, or longer-lived N-chloroamines) (1, 7, 9). Recently Banerjee et al. (4) reported that after 5 days of infection with the HGE agent, HL-60 cells (a human promyelocytic leukemia cell line) exhibited a lack of luminol-dependent chemiluminescence (LDCL) response to phorbol myristate acetate (PMA) and down regulation of mRNA of gp91phox, one component of the NADPH complex. However, for the HGE agent to survive in neutrophils, this inhibition must occur immediately rather than 5 days after establishment of infection. Although use of neutrophils is more difficult than use of cell lines, we determined whether human peripheral blood neutrophils produce O2− upon exposure to the HGE agent. Since we found that the HGE agent does not induce O2− generation, we determined whether this is a generalized and/or active inhibition and whether both intracellular and extracellular O2− generation is prevented. We further examined what type of ehrlichial factors and interactions between the HGE agent and neutrophils are required for this inhibition. Such data will provide new insights into a unique capability of the HGE agent to overcome the neutrophil's powerful microbicidal mechanisms.
(Part of this study was presented elsewhere [J. Mott and Y. Rikihisa, Abstr. 99th Annu. Meet. Am. Soc. Microbiol., abstr. D/B-128, p. 234, 1999].)
MATERIALS AND METHODS
Ehrlichia culture.
HGE agent, HZ strain, was cultured in the HL-60 human promyelocytic leukemia cell line in RPMI 1640 medium as previously described (19). When ∼75% of the HL-60 cells were infected as determined by Diff-Quik (Baxter Scientific Products, Obetz, Ohio) staining, cells were harvested for experiments.
Preparation of neutrophils and mononuclear cells.
Human buffy coat (∼50 ml) from healthy donors was centrifuged at 1,500 × g for 5 min. Following centrifugation, the plasma was removed, and 10 ml of buffy coat was placed in a 50-ml centrifuge tube containing 10 ml of Histopaque 1077 overlaying 15 ml of Histopaque 1119 (Sigma Chemical Co., St. Louis, Mo.). Following centrifugation at 2,200 × g for 25 min, the interface between Histopaque 1077 and Histopaque 1119 was collected and added to 0.83% NH4Cl for 5 min at room temperature to lyse any remaining red blood cells. Neutrophils were centrifuged at 750 × g for 5 min and washed twice with Hanks' balanced salt solution (HBSS) without phenol red and sodium bicarbonate (Sigma). By Diff-Quik staining, cells were determined to be ∼97% neutrophils. The viability of the neutrophil preparations was determined before and after each experiment by trypan blue exclusion and was found to be ∼99%. Mononuclear cells in the interface overlaying Histopaque 1077 from the same donor were also collected.
Ferricytochrome c reduction assay.
Extracellular O2− was measured on the basis of the superoxide dismutase (SOD)-inhibitable reduction of ferricytochrome c as described by Johnston (12). Briefly, 2 × 106 neutrophils per 50 μl of HBSS containing 2 mg of dextrose per ml (HBSSd) were added to the wells of a 24-well plate containing 300 μl of reaction mixture with 80 μM ferricytochrome c type III from horse heart (Sigma) and with or without SOD (50 μg/ml) from bovine erythrocytes (Sigma). Freshly prepared host cell-free intact HGE agent from 2 × 106 infected HL-60 cells, or HL-60 cell lysate (obtained by sonication of 2 × 106 uninfected cells) as a control, in 50 μl of HBSSd was added to neutrophils in triplicate wells and incubated for 30 min at 37°C in 5% CO2–95% air. Dose dependency was determined by adding the host cell-free HGE agent in 1×, 10×, and 100× dilutions. To examine the effect of the recombinant major outer membrane 44-kDa protein (rP44) (27), rP44 (2.5 μg/ml) was added in place of host cell-free HGE agent. Lysed HGE agent was prepared by strong sonication (setting 7 for 10 s) of host cell-free HGE agent derived from 2 × 106 infected HL-60 cells in 50 μl of HBSSd. Mixtures were then stimulated by adding PMA (0.5 μg/ml, final concentration; Sigma) and incubated for 2 h at 37°C in 5% CO2–95% air. Alternatively, the mixtures were stimulated with 1 μM N-formyl-Met-Leu-Phe (fMLP; Sigma) or 2 × 106 Escherichia coli strain INVαF′ cells in 50 μl of HBSSd. The A550 of the supernatants was measured in a model DU-70 spectrophotometer (Beckman Instruments, Inc., Fullerton, Calif.). The average of A550 of three wells of cytochrome c blanks was subtracted from individual A550 of sample wells. The nanomoles of cytochrome c reduced was calculated from the A550 using the extinction coefficient ΔE = 21.0 × 103 m−1 cm−1. To consider individual human variations, all experiments were independently repeated more than three times on different days using neutrophils derived from different donors and freshly prepared host cell-free HGE agent. Donor cells were never mixed, and each donor's neutrophil assay included positive and negative controls to ensure the quality of both neutrophil and HGE agent preparation.
Time course of LDCL.
Neutrophils were suspended in HBSSd at 2 × 106 cells/ml in Clinicon luminometer cuvettes and incubated at 37°C for 5, 10, 20, or 30 min in the presence or absence of host cell-free HGE agent derived from 2 × 106 infected HL-60 cells. Alternatively, host cell-free HGE agent was added 1 min after PMA addition. PMA (0.5 μg/ml, final concentration), fMLP (1 μM), or 2 × 106 E. coli cells in 50 μl of HBSSd was added. LDCL was monitored with a model 1251 luminometer (LKB Wallace, San Francisco, Calif.) with constant shaking at 37°C.
Two-compartment assays.
To wells of 12-well Transwell culture plates containing 160 μM ferricytochrome c in HBSSd, 2 × 106 neutrophils/50 μl was added. Host cell-free HGE agent in 50 μl of HBSSd was added either to Transwell inserts with a porous bottom (Costar, Cambridge, Mass.) or to the wells and incubated for 30 min at 37°C. PMA was added to the inserts, and the plates were incubated for 2 h as previously described. As controls, host cell-free HGE agent and PMA were incubated for 30 min at 37°C in a microcentrifuge tube prior to addition to Transwells, or neutrophils were incubated with PMA only in the Transwells.
Pronase treatment of neutrophils preincubated with HGE agent or neuraminidase treatment.
Neutrophils were incubated for 30 min at 37°C in HBSSd in the presence or absence of host cell-free HGE agent in HBSSd. Following incubation, pronase E (2 mg/ml; Sigma) was added and incubated for 30 min at 37°C to remove uninternalized host cell-bound HGE agent. Cells were then washed by centrifugation at 1,500 × g for 5 min. For neuraminidase treatment, neutrophils were incubated with or without neuraminidase type X (Sigma) at 1 U/ml for 2 h at 37°C in 0.5 ml of 0.15 M NaCl–5 mM CaCl2 (pH 6.0) and washed in RPMI 1640 medium.
Treatment of host cell-free HGE agent.
Host cell-free HGE agent was incubated with 20 mM sodium periodate (Sigma) in 50 mM sodium acetate buffer (pH 4.5) at room temperature for 1 h in the dark as described by Woodward et al. (25). Following a brief rinse with 50 mM sodium acetate, ehrlichiae were incubated with 50 mM sodium borohydride (Sigma) in phosphate-buffered saline at room temperature for 30 min. Ehrlichiae were then centrifuged and resuspended in HBSSd. For trypsin treatment, host cell-free HGE agent was incubated in a 0.25% trypsin (Sigma) in HBSSd for 15 min at room temperature, and ehrlichiae were washed twice in HBSSd.
Effects of oxytetracycline or cycloheximide on inhibition of O2− generation by the HGE agent.
Host cell-free HGE agent was treated with 10 μg of oxytetracycline/ml for 30 min at 37°C in 5% CO2–95% air and added to neutrophils in HBSSd containing 10 μg of oxytetracycline/ml. Neutrophils were incubated in the presence of cycloheximide (2 μg/ml) for 30 min at 37°C prior to addition of host cell-free HGE agent and/or PMA. Reagents were present throughout the ferricytochrome c reduction assay.
RESULTS
Upon exposure to neutrophils, the HGE agent did not induce extracellular O2− release and prevented O2− release in response to PMA, E. coli, or fMLP.
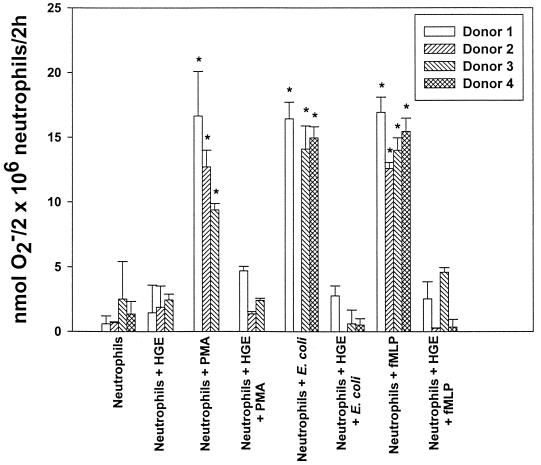
The normally dormant NADPH oxidase can be activated by either receptor-dependent mechanisms (fMLP, various bacteria, Fc receptor cross-linking, complement factor C5a, or zymosan) or receptor-independent mechanisms (PMA [a protein kinase C {PKC} activator] or long-chained unsaturated fatty acids) (1, 20). In vitro, the HGE agent did not induce extracellular O2− release by human peripheral blood neutrophils when measured by the ferricytochrome c reduction assay (Table 1). PMA, E. coli, and fMLP all induced significant levels of O2− release, an effect that was inhibitable by addition of nonpermeable SOD to the incubation medium (Table 1; Fig. 1). Host cell-free HGE agent added 30 min prior to the addition of PMA, E. coli, or fMLP almost completely blocked O2− release for the 2-h incubation period (Fig. 1; Table 1); therefore, the inhibition does not appear to be a delay in enzyme activation. Since the HGE agent was cultivated in HL-60 cells, neutrophils were also incubated with uninfected HL-60 cell lysate to ensure that neutrophils were not inhibited by HL-60 cell components. A level of O2− release similar to that without HL-60 cell lysate was detected in response to PMA. The responses of neutrophils from several donors were similar (Fig. 1). The viability of the human neutrophil preparations as determined at the end of incubation period (2 to 3 h) for each experiment was ∼99%.
TABLE 1.
Inhibition of O2− release by human neutrophils (donor 5) in response to PMA by the HGE agent
| Treatmenta | Reduction of ferricytochrome c (nmol of O2−/106 neutrophils/2 h)b
|
|
|---|---|---|
| −SOD | +SOD | |
| Medium control | 2.4 ± 0.5 | 1.5 ± 0.0 |
| PMA | 13.8 ± 2.0* | 0.8 ± 1.3 |
| HL-60 lysate | 1.3 ± 0.6 | 2.5 ± 0.6 |
| HL-60 lysate + PMA | 11.1 ± 0.5* | 0.0 ± 0.0 |
| HGE agent | 0.0 ± 0.0 | 0.0 ± 0.0 |
| HGE agent + PMA | 1.0 ± 0.9 | 0.0 ± 0.0 |
Neutrophils from donor 5 were incubated for 30 min with medium, uninfected HL-60 lysate, or host cell-free HGE agent prior to PMA stimulation in the presence or absence of SOD.
Mean ± standard deviation (n = 3). Results are representative of three independent experiments. ∗, P < 0.01 compared to neutrophil-alone (no PMA) control (Student's t test).
FIG. 1.
The HGE agent blocks O2− release induced by PMA, E. coli, or fMLP. Neutrophils were incubated with or without PMA, E. coli, or fMLP in the presence or absence of the HGE agent. O2−release was determined by measuring the reduction of ferricytochrome c after 2 h of stimulation. Bars represent means ± standard deviations (n = 3). ∗, P < 0.01 compared to neutrophil-alone control. (Student's t test).
The HGE agent prevented total O2− generation by neutrophils in response to PMA and E. coli.
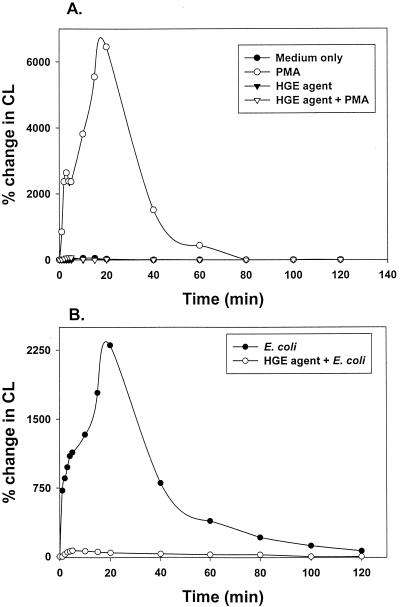
Various bacteria are known to alter the site of O2− generation. For example, Neisseria gonorrhoeae does not prevent O2− generation but prevents extracellular release of O2−, thus allowing extracellular neisseriae to survive (16). On the other hand, Salmonella enterica serovar Typhimurium does not prevent O2− generation but blocks release of O2− in salmonella-containing vacuoles, so that intracellular salmonellae can survive (23). Luminol is a small membrane-permeable molecule that can be used to measure total (intra- and extracellular) O2− production (8). We found that the HGE agent completely prevented total O2− production by neutrophils in response to PMA and E. coli (Fig. 2) for the entire 2-h stimulation period. This result also supports our observation in the ferricytochrome c reduction assay that the inhibition is not simply a delay in enzyme activation but rather complete inhibition. At least 30 min of preincubation with the HGE agent was required for complete cessation of total O2− production in response to PMA. Addition of the HGE agent 10 or 20 min prior to PMA stimulation partially reduced and delayed the total O2− production by neutrophils in response to PMA (data not shown).
FIG. 2.
Time course of inhibition of total (extra- and intracellular) O2−generation induced by PMA or E. coli by the HGE agent as measured by LDCL assay. Neutrophils were stimulated with PMA (A) or E. coli (B) in the presence or absence of the HGE agent. Results are representative of three independent experiments. CL, chemiluminescence.
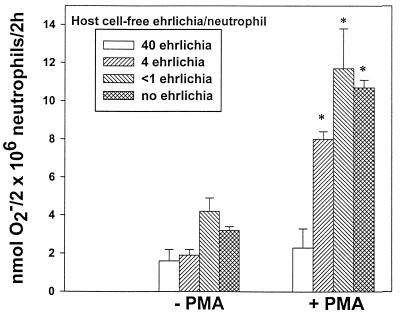
Inhibitory effects of the HGE agent on O2− release were dose dependent.
O2− release in response to PMA was completely prevented with approximately 40 bacteria per neutrophil (Fig. 3). Use of <1 organism per neutrophil resulted in no inhibition.
FIG. 3.
Inhibition of O2− release by the HGE agent is dose dependent. Neutrophils were incubated with decreasing numbers of host cell-free HGE agent with or without PMA. O2− secretion was determined by measuring the reduction of ferricytochrome c. Bars represent means ± standard deviations (n = 3). Results are representative of three independent experiments. ∗, P < 0.01 compared to neutrophil-alone (no PMA) control (Student's t test).
Contact was required.
Neutrophils incubated with the HGE agent in an insert separated by a porous membrane from neutrophils in Transwell plates produced levels of extracellular O2− nearly identical to those produced by neutrophils without host cell-free HGE agent stimulated with PMA (Table 2). In contrast, when ehrlichiae were added directly to wells containing neutrophils (as for Fig. 1 but in a Transwell), the PMA-induced O2− release was blocked. Host cell-free HGE agent incubated for 30 min with PMA prior to addition to the Transwell did not block the O2− release (data not shown), indicating that PMA was not directly inactivated by ehrlichial organisms. These results suggest that inhibition of the NADPH oxidase system by the HGE agent requires contact between host cell and ehrlichia and that soluble factors released by HGE agent (if any) likely are not involved.
TABLE 2.
Ehrlichial contact, but not internalization, and ehrlichial carbohydrate, but not protein, are required for inhibition of O2− release by neutrophils in response to PMA
| Treatmenta | Reduction of ferricytochrome c (nmol of O2−/106 neutrophils)b |
|---|---|
| Donor 6 (Transwell)c | |
| PMA | 13.9 ± 0.3* |
| HGE agent (separated from neutrophils by membrane) + PMA | 12.6 ± 0.4* |
| HGE agent (in contact with neutrophils) + PMA | 0.4 ± 0.3 |
| Donor 7 | |
| Medium control | 0.6 ± 0.6 |
| PMA | 16.7 ± 3.4* |
| HGE agent + PMA | 4.7 ± 0.4 |
| Trypsin-treated HGE agent + PMAd | 4.5 ± 1.2 |
| Periodate-treated HGE agent + PMAd | 14.4 ± 0.3* |
| Pronase treatment of neutrophilse preincubated with the HGE agent + PMA | 18.7 ± 1.8* |
| Pronase treatment of neutrophils + PMA | 16.8 ± 0.1* |
| Donor 8 | |
| Medium control | 0.1 ± 0.1 |
| PMA | 13.3 ± 0.3* |
| HGE agent lysate + PMAf | 3.9 ± 0.2 |
Neutrophils were pretreated with the HGE agent with or without reagent for 30 min prior to PMA stimulation.
Mean ± standard deviation (n = 3). Results are representative of more than three independent experiments. ∗, P < 0.01 compared to neutrophil-alone (no PMA) control (Student's t test).
Neutrophils in one chamber were incubated without or with the HGE agent added to the same or another chamber separated by a Nuclepore polycarbonate porous membrane, using two-compartment Transwell plates. PMA was added to all wells.
Host cell-free HGE agent was treated with 0.25% trypsin for 15 min at room temperature or with 20 mM sodium periodate for 1 h prior to addition to cytochrome c reduction assay mixtures and PMA stimulation.
Neutrophils were incubated with host cell-free HGE agent for 30 min at 37°C, incubated with pronase E (2 mg/ml) for 30 min at 37°C to remove uninternalized bacteria, then added to cytochrome c reduction assay mixtures, and stimulated with PMA.
Neutrophils were incubated for 30 min at 37°C with lysed HGE agent prior to PMA stimulation.
Inhibition of O2− release in response to PMA was reversible upon pronase treatment.
When neutrophils preincubated with the HGE agent for 30 min were further incubated with pronase to remove bound extracellular HGE agent, neutrophils regained responsiveness to subsequent PMA stimulation. Pronase treatment alone, in the absence of the HGE agent, had no influence on O2− release in response to PMA (Table 2). This result suggests that for inhibition of O2− release to occur, the HGE agent must remain associated with the neutrophil and that the HGE agent does not permanently alter the cellular components or cell signaling pathways of the host cell involved in O2− release.
Neuraminidase treatment did not prevent O2− release inhibition by the HGE agent.
A recent study by Goodman et al. (10) has shown that treatment of HL-60 cells with neuraminidase prevents both binding and infection by the HGE agent. To examine whether an external sialic acid on the neutrophil surface is required for inhibition of O2− release by the HGE agent, neutrophils were pretreated with neuraminidase prior to incubation with the HGE agent. Preincubation of neutrophils with neuraminidase alone induced extracellular O2− release that was further enhanced by the addition of PMA (Fig. 4). Neutrophils preincubated with buffer alone showed no stimulatory or inhibitory effects with or without PMA. The HGE agent completely inhibited O2− release by neuraminidase-treated neutrophils in the presence or absence of PMA. Unlike the case for HL-60 cells (10), treatment of neutrophils with neuraminidase did not inhibit infection by the HGE agent. Following 16 h of incubation, distinct morulae (microcolonies of ehrlichiae) were present in 6.2% ± 0.5% and 4.1% ± 0.5% (n = 3) of nontreated and treated neutrophils, respectively. The percentage of morula-positive cells, though small, is the percentage normally seen in patient blood (26). These results indicate that HGE agent infection and HGE agent-dependent inhibition of O2− release are not dependent on an external sialic acid on the neutrophil surface.
FIG. 4.
HGE agent inhibition of superoxide release in response to PMA is not abrogated by pretreatment of neutrophils with neuraminidase type X. Neutrophils were treated with neuraminidase type X for 2 h prior to incubation with host cell-free HGE agent. PMA was then added to stimulate release of O2−. O2− production was determined by measuring the reduction of ferricytochrome c. Bars represent means ± standard deviations (n = 3). Results are representative of three independent experiments. ∗, P < 0.01 compared to neutrophil-alone control (Student's t test).
Ehrlichial carbohydrate but not protein was required for O2− inhibition by the HGE agent.
Sodium periodate treatment inactivated the ability of the HGE agent to inhibit O2− release in response to PMA, whereas the HGE agent retained the ability to block O2− release after trypsin treatment (Table 2). The trypsin treatment was sufficient for ehrlichiae to lose infectivity. These results suggest that a carbohydrate moiety but not surface protein of the HGE agent is required for inhibition of the O2− release by PMA. The major surface 44-kDa protein of the HGE agent was previously cloned and expressed, and the recombinant protein was designated rP44 (27). Although rP44 was shown to interact and induce proinflammatory cytokine gene expression in peripheral blood leukocytes (14), it did not inhibit the PMA-induced O2− release (data not shown), suggesting that the 44-kDa major outer membrane protein of the HGE agent is not responsible for the inhibition of O2− release.
Ehrlichial new protein synthesis or intact structure was not required.
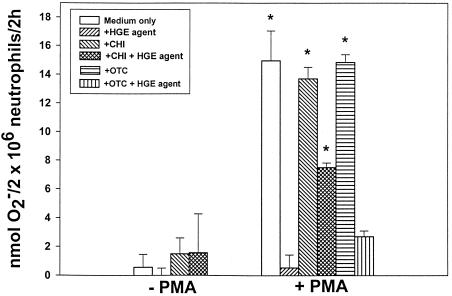
To examine whether the HGE agent requires new protein synthesis to inhibit extracellular O2− generation by the host cell, we preincubated host cell-free HGE agent in the presence of oxytetracycline, a bacteriostatic antibiotic that inhibits new protein synthesis by acting on the 30S ribosome (6). Preincubation of bacteria with oxytetracycline did not reverse the inhibitory effects on O2− production by the HGE agent in PMA-stimulated neutrophils (Fig. 5). Oxytetracycline alone had no effect on O2− production in PMA-stimulated neutrophils. This result suggests that the HGE agent does not require new or ongoing protein synthesis to inhibit O2− production. Furthermore, lysed HGE agent had the same inhibitory effect as intact viable HGE agent, indicating that structural integrity or viability of ehrlichiae is not required for inhibition (Table 2).
FIG. 5.
HGE agent inhibition of O2− secretion in response to PMA is partially blocked by cycloheximide but not oxytetracycline treatment. Neutrophils were incubated with cycloheximide (CHI) 30 min prior to addition of the HGE agent, or the HGE agent was incubated with oxytetracycline (OTC) for 30 min prior to addition of neutrophils. Mixtures were incubated with or without PMA stimulation in the presence of cycloheximide or oxytetracycline, and O2− release was determined by measuring the reduction of ferricytochrome c after 2 h of PMA stimulation. Bars represent means ± standard deviations (n = 3). Results are representative of three independent experiments. ∗, P < 0.01 compared to neutrophil-alone control (Student's t test).
Protein synthesis by neutrophils was involved in inhibition of O2− release by the HGE agent.
Cycloheximide inhibits eukaryotic but not prokaryotic protein synthesis (3). In the presence of cycloheximide, HGE agent inhibition was partially abrogated following stimulation by PMA (Fig. 5). Cycloheximide alone did not inhibit O2− release in response to PMA. This result indicates that de novo protein synthesis by the host cell is required for complete inhibition of O2− release induced by the HGE agent, suggesting that a host protein that is rapidly turned over is involved in this inhibition.
Human mononuclear cell O2− generation in response to PMA was not prevented by the HGE agent.
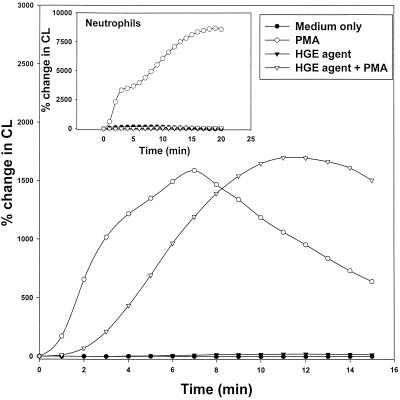
Preincubation of mononuclear cells with the HGE agent reproducibly delayed O2− generation in response to PMA stimulation by several minutes, but a significant level of LDCL was detected (Fig. 6). Neutrophils from the same donor, when incubated with the HGE agent, did not respond to PMA stimulation (Fig. 6, inset), indicating that inhibition of O2− generation by the HGE agent is neutrophil specific.
FIG. 6.
Lack of inhibition of PMA-induced total (extra- and intracellular) O2− generation in mononuclear cells by the HGE agent as measured by LDCL. Human peripheral blood mononuclear cells were stimulated with PMA in the presence or absence of the HGE agent. The neutrophil response from the same donor to the same stimulus is shown in the inset. Results are representative of three independent experiments. CL, chemiluminescence.
DISCUSSION
The HGE agent survives and replicates exclusively within inclusions in granulocytes, the primary effector cells of the host's antimicrobial defense. Neutrophils are the most powerful generators of O2−; upon contact with most bacteria, parasites, or fungi, O2− production is rapidly induced. In this study, we demonstrated that the HGE agent subverts the ability of human neutrophils to generate O2− in response to both soluble stimuli (PMA or fMLP) and powerful particulate stimuli (E. coli) which activate the NADPH oxidase through different signaling pathways (13). These results suggest that the HGE agent suppresses the neutrophil respiratory burst by interfering with an event downstream of PKC at the stage of the NADPH oxidase assembly. Many pathogenic microorganisms are known to reduce O2− generation in neutrophils or monocytes. However, as far as we know, none of these microorganisms can prevent O2− generation as thoroughly or as universally as the HGE agent does. For example, Yersinia enterocolitica inhibits O2− generation in human granulocytes induced by fMLP but not PMA (24). fMLP-induced O2− secretion by human neutrophils is inhibited by an acid phosphatase released from Coxiella burnetii (2). Pseudomonas aeruginosa hemolytic phospholipase C suppresses PMA-induced but not fMLP-induced secretion of O2− (22). Infection of human monocytes with Legionella pneumophila reduces PMA-induced but not zymosan-induced O2− secretion (11). Chlamydia trachomatis partially (30 to 65%) inhibits fMLP- or PMA-induced O2− secretion by human neutrophils (21).
The microbial factors utilized by the HGE agent for inhibition of O2− secretion are different from any other known mechanisms. For example, L. pneumophila-induced inhibition of O2− secretion requires intracellular multiplication of the organism (11). However, proliferation of the HGE agent was not required to inhibit O2− secretion. Inhibition of human neutrophil O2− secretion by C. trachomatis requires viable organisms (21). However, viability of the HGE agent was not required. Carbohydrate but not protein of the HGE agent was required for the inhibition, whereas most known microbial factors are proteins (2, 16, 22, 23). The nature of this carbohydrate remains to be studied. Although ehrlichiae are gram-negative bacteria, this carbohydrate does not seem to be lipopolysaccharide, which primes, rather than inhibits, the NADPH oxidase enzyme for O2− generation, and lipopolysaccharide has not been demonstrated in Ehrlichia spp. The requirement of carbohydrate for inhibition of O2− generation was opposite the requirement for induction of proinflammatory cytokine gene expression or inhibition of apoptosis by the HGE agent (14, 26), since the latter two require a protein component of the HGE agent but not the carbohydrate moiety (14, 26). These results indicate that the HGE agent contains both protein and carbohydrate surface components which interact with host cells and induce several divergent cellular responses for its survival.
Ehrlichial inhibition of O2− secretion required contact between the HGE agent and neutrophils. Our results demonstrated a dose-dependent inhibition of O2− production following PMA stimulation of neutrophils that may reflect a need for a critical number of ehrlichia-host cell receptor occupancies to counteract the O2− production signaling pathway activated by PMA. The fact that the inhibition is reversible upon removal of the extracellular HGE agent by pronase treatment suggests that the host cell receptor transducing a signal for the inhibition is a protein. The fact that O2− production by neutrophils but not monocytes is specifically prevented suggests that the receptor is present or active on neutrophils but not on monocytes. The result also suggests that the HGE agent does not infect monocytes because it is killed by reactive oxygen intermediates generated by monocytes.
Our study revealed that complete inhibition of O2− release by the HGE agent requires 30 min, indicating that HGE agent binding and subsequent signaling are slower than PKC-activated production of O2−. A host protein synthesized during this time period may be required for complete inhibition, since incubation of neutrophils with the protein synthesis inhibitor cycloheximide partially abrogated the inhibition of O2− release by the HGE agent. This is similar to results for ehrlichial apoptosis inhibition (26). The requirement for protein synthesis suggests that the protein(s) involved in inhibition of O2− release or apoptosis is not present in sufficient concentrations or may have a short half-life. Since previous studies have revealed that at 37°C, internalization of Ehrlichia risticii into P388D1 cells, a murine macrophage cell line, occurs within 3 to 4 h of exposure (17), the inhibition by the HGE agent most likely occurs upon contact with the host cell prior to internalization.
Neutrophils also can kill invading microorganisms by oxygen-independent mechanisms, such as fusion of the phagosomes containing bacteria with granules containing both antimicrobial peptides (e.g., defensins or lysozymes) and lysosomal hydrolytic enzymes or through sequestering vital nutrients (e.g., iron) (7). We previously found that the inclusion compartments of the HGE agent are unique in lacking both endosomal and lysosomal properties (15). Therefore, the HGE agent has the ability to block oxygen-dependent and -independent microbicidal mechanisms of neutrophils.
ACKNOWLEDGMENTS
This work was supported by grants R01 AI30010 and F32 AI09968 from the National Institutes of Health.
We thank Ning Zhi for providing the purified rP44 protein and Hyung-Yong Kim for assistance in isolating fresh human neutrophils.
REFERENCES
- 1.Babior B. NADPH oxidase: an update. Blood. 1999;93:1464–1476. [PubMed] [Google Scholar]
- 2.Baca O G, Roman M J, Glew R H, Christner R F, Buhler J E, Aragon A S. Acid phosphatase activity in Coxiella burnetii: a possible virulence factor. Infect Immun. 1993;61:4232–4239. doi: 10.1128/iai.61.10.4232-4239.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baliga B S, Pronczuk A W, Munro H N. Mechanism of cycloheximide inhibition of protein synthesis in a cell-free system prepared from rat liver. J Biol Chem. 1969;244:4480–4489. [PubMed] [Google Scholar]
- 4.Banerjee R, Anguita J, Roos D, Fikrig E. Cutting edge: infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J Immunol. 2000;164:3946–3949. doi: 10.4049/jimmunol.164.8.3946. [DOI] [PubMed] [Google Scholar]
- 5.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chopra I, Hawkey P M, Hinton M. Tetracyclines, molecular and clinical aspects. J Antimicrob Chemother. 1992;29:245–277. doi: 10.1093/jac/29.3.245. [DOI] [PubMed] [Google Scholar]
- 7.Cohen M S. Molecular events in the activation of human neutrophils for microbial killing. Clin Infect Dis. 1994;18:170–179. doi: 10.1093/clinids/18.supplement_2.s170. [DOI] [PubMed] [Google Scholar]
- 8.Dahlgren C, Briheim G, Stendahl O. Measurement of luminol-dependent leukocyte chemiluminescence originated from intracellular and extracellular events. In: Kricka L J, Stanley P E, Thorpe G H G, Whitehead T P, editors. Annals of Applied Bioluminescence Chemiluminescence: Proceedings Academic Press, N.Y. 1984. pp. 335–338. [Google Scholar]
- 9.DeLeo F R, Allen L-A H, Apicella M, Nauseef W N. NADPH oxidase activation and assembly during phagocytosis. J Immunol. 1999;163:6732–6740. [PubMed] [Google Scholar]
- 10.Goodman J L, Nelson C M, Klein M B, Hayes S F, Weston B W. Leukocyte infection by the granulocytic ehrlichiosis agent is linked to expression of a selectin ligand. J Clin Investig. 1999;103:407–412. doi: 10.1172/JCI4230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacob T, Escallier J C, Sanguedolce M V, Chicheportiche C, Bongrand P, Capo C, Mege J L. Legionella pneumophila inhibits superoxide generation in human monocytes via the down-modulation of a and b protein kinase C isotypes. J Leukoc Biol. 1994;55:310–312. doi: 10.1002/jlb.55.3.310. [DOI] [PubMed] [Google Scholar]
- 12.Johnston R B. Secretion of superoxide anion. In: Adams D O, Edelson PO, Koren H, editors. Methods for studying mononuclear phagocytes. New York, N.Y: Academic Press; 1981. pp. 489–497. [Google Scholar]
- 13.Karlsson A, Markfjall M, Stromberg N, Dahlgren C. Escherichia coli-induced activation of neutrophil NADPH-oxidase: lipopolysaccharide and formylated peptides act synergistically to induce release of reactive oxygen metabolites. Infect Immun. 1995;63:4604–4612. doi: 10.1128/iai.63.12.4606-4612.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H-Y, Rikihisa Y. Expression of interleukin-1β, tumor necrosis factor-alpha, and interleukin-6 in human peripheral blood leukocytes exposed to viable human granulocytic ehrlichiosis agent or recombinant major surface antigen P44. Infect Immun. 2000;68:3394–3402. doi: 10.1128/iai.68.6.3394-3402.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mott J, Barnewall R, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naids F L, Rest R F. Stimulation of human neutrophil oxidative metabolism by nonopsonized Neisseria gonorrhoeae. Infect Immun. 1991;59:4383–4390. doi: 10.1128/iai.59.12.4383-4390.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park J, Rikihisa Y. Inhibition of Ehrlichia risticii growth in murine peritoneal macrophage by gamma interferon, calcium ionophore, and concanavalin A. Infect Immun. 1991;59:3418–3423. doi: 10.1128/iai.59.10.3418-3423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rikihisa Y, Zhi N, Wormser G P, Wen B, Horowitz H M, Hechemy K E. Ultrastructural and antigenic characterization of granulocytic ehrlichia directly isolated and cultivated from a patient in New York State. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 20.Steinbeck M J, Robinson J M, Karnovsky M J. Activation of the neutrophil NADPH-oxidase by free fatty acids requires the ionized carboxyl group and partitioning into membrane lipid. J Leukoc Biol. 1991;49:360–368. doi: 10.1002/jlb.49.4.360. [DOI] [PubMed] [Google Scholar]
- 21.Tauber A I, Pavlotsky N, Lin J S, Rice P A. Inhibition of human neutrophil NADPH oxidase by Chlamydia serovars E, K, and L2. Infect Immun. 1989;57:1108–1112. doi: 10.1128/iai.57.4.1108-1112.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terada L S, Johansen K A, Nowbar S, Vasil A I, Vasil M L. Pseudomonas aeruginosa hemolytic phospholipase C suppresses neutrophil respiratory burst activity. Infect Immun. 1999;67:2371–2376. doi: 10.1128/iai.67.5.2371-2376.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vazquez-Torres A, Xu Y, Jones-Carson J, Holden D W, Lucia S M, Dinauer M C, Mastroeni P, Fang F C. Salmonella pathogenicity island 2-dependent evasion of the phagocyte NADPH oxidase. Science. 2000;287:1655–1658. doi: 10.1126/science.287.5458.1655. [DOI] [PubMed] [Google Scholar]
- 24.Visser L G, Seijmonsbergen E, Nibbering P H, van den Broek P J, van Furth R. Yops of Yersinia enterocolitica inhibit receptor-dependent superoxide anion production by human granulocytes. Infect Immun. 1999;67:1245–1250. doi: 10.1128/iai.67.3.1245-1250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodward M P, Young W W, Jr, Bloodgood R A. Detection of monoclonal antibodies specific for carbohydrate epitopes using periodate oxidation. J Immunol Methods. 1985;78:143–153. doi: 10.1016/0022-1759(85)90337-0. [DOI] [PubMed] [Google Scholar]
- 26.Yoshiie K, Kim H-Y, Mott J, Rikihisa Y. Intracellular infection by human granulocytic ehrlichiosis agent inhibits human neutrophil apoptosis. Infect Immun. 2000;68:1125–1133. doi: 10.1128/iai.68.3.1125-1133.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhi N, Ohashi N, Rikihisa Y, Horowitz H W, Wormser G P, Hechemy K. Cloning and expression of the 44-kilodalton major outer membrane protein gene of the human granulocytic ehrlichiosis agent and application of the recombinant protein to serodiagnosis. J Clin Microbiol. 1998;36:1666–1673. doi: 10.1128/jcm.36.6.1666-1673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]