Abstract
Streptococcus gallolyticus (S. gallolyticus) has been linked to the development of infections in adults; however, in neonates S. gallolyticus sepsis is very rare and resembles Group B Streptococcal infections. In this case report, we present the case of a full-term neonate who developed early-onset sepsis due to S. gallolyticus. A systematic review of the literature was also conducted. The neonate had good APGAR scores at 1′ and 5′. At 5 h postnatally, the neonate developed poor feeding and respiratory distress. She received oxygen in a head box, and a complete blood count and biochemistry, blood, CSF and body surface cultures were obtained. Empiric intravenous antibiotics (ampicillin and tobramycin) were initiated, and she was transferred to a tertiary NICU for further treatment. The neonate was mechanically ventilated and received dopamine and colloid fluids for circulatory support. A cardiology consultation revealed pulmonary hypertension on day one. S. gallolyticus was isolated in the blood culture. Central nervous system ultrasonography, brainstem auditory evoked potentials, and a second cardiology evaluation were normal on day three. Clinical and laboratory improvement was noted on day three, and the baby was discharged after a 12-day hospitalization. Follow-up visits were scheduled for reevaluation.
Keywords: Streprococcus gallolyticus, neonatal sepsis
1. Introduction
Streptococcus gallolyticus (S. gallolyticus) is a gram-positive coccus, which belongs to group D Streptococci, frequently found as part of the human intestinal flora, previously known as Streptococcus bovis (S. bovis) [1]. S. gallolyticus has been linked with infective endocarditis and meningitis in adults. Colon cancer is recognized as a risk factor for the development of bacteriemia, resulting in the aforementioned infections [2]. However, S. gallolyticus is a rare cause of neonatal infection, both in full- and pre-term neonates, and its clinical presentation resembles that of group B Streptococcal (GBS) infections [1]. We present the case of a full-term female neonate who developed early-onset sepsis due to S. gallolyticus, complicated with pulmonary hypertension. We also report all cases of neonatal infection caused by this infectious organism found in the literature.
2. Case Presentation
A female neonate was born at 38+1 weeks of gestation via cesarian section (CS) due to failure to progress by a primiparous 32-year-old mother with good antenatal follow-up. Rupture of membranes occurred 10 h prior to the CS. Pregnancy history was significant for intrahepatic cholestasis of pregnancy, gestational diabetes mellitus treated with diet, and vaginal bleeding at 30 weeks, which was treated conservatively. Vaginal swab culture at 35+3 weeks was negative for pathogenic bacteria.
The neonate cried immediately at birth, and APGAR scores were 9 and 10, at 1′ and 5′, respectively. Birth weight was 3.250 g (65th centile for gestational age, maternal age and somatometric measurements). Skin-to-skin with the mother was performed, the neonate’s physical examination was normal, and the neonate was kept in the mother’s room, as rooming-in is a standard practice in our institution. At the age of 5 h, the neonate developed grunting and poor feeding and was transferred to the neonatal unit of our department for clinical evaluation. Clinical signs of respiratory distress, including tachypnoea (respiratory rate, RR: 75/min) and grunting, were present; heart rate (HR), blood pressure (BP), capillary refilling time (CRT) and temperature were 220/min, 83/41 mmHg (Mean arterial pressure 53 mmHg), 3 s and 39 °C respectively. Oxygen was administered in a head box (FiO2 35%), and complete blood count (CBC), blood biochemistry, and blood, cerebrospinal fluid (CSF), as well as body surface cultures, were obtained. Initial laboratory results were significant for neutropenia (neutrophils 1469/μL). C-Reactive Protein was not elevated (CRP = 0.6 mg/L), and CSF values were within normal limits. Empiric intravenous antibiotics were administered (ampicillin and tobramycin), and the neonate was transferred to a tertiary neonatal intensive care unit (NICU) for further treatment.
On admission to the NICU, the neonate was febrile (38.3 °C), in respiratory distress, with a grade I-II/VI heart murmur, RR 85/min, SpO2 100%, HR 190/min, BP 78/33 (49) mmHg and CRT 4 s. Within a few hours, the neonate’s clinical condition deteriorated rapidly, requiring mechanical ventilation. Sepsis work-up was performed again, and the antibiotic treatment was changed to ampicillin, teicoplanin and cefotaxime for broader coverage. Inotropes (dopamine) and colloid fluids for circulatory support were also initiated. Consultation by a pediatric cardiology specialist revealed patent ductus arteriosus (PDA) of 3 mm, with the left-to-right flow, increased pulmonary mean artery pressure (50 mmHg), normal left ventricular contractility (Ejection Fraction—EF > 65%) while on dopamine treatment, and a small atrial septal defect (ASD).
On the second day of life (DOL), CRP reached peak value (CRP: 41.3 mg/L), and with the application of biochemical testing (Vitek 2), S. gallolyticus was isolated in the initial blood culture, sensitive to benzylpenicillin, ampicillin, cefotaxime, levofloxacin, linezolid, teicoplanin, vancomycin, and resistant to tetracycline and clindamycin; further, identification was not performed in the laboratory. According to the antimicrobial susceptibility testing, the neonate’s treatment was changed to ampicillin and cefotaxime. A second cardiology evaluation revealed that ductus arteriosus was constricted, mean pulmonary artery pressure returned to normal, and inotropes support was no longer required. Central nervous system ultrasonography and brainstem auditory evoked potentials study were normal.
Clinical and laboratory improvement was noted from the third DOL. The baby was extubated on the fifth DOL and remained in oxygen for two more days before she was completely weaned from it. Intravenous antibiotic treatment stopped on the tenth DOL. The baby was discharged after a 12-day hospitalization. Follow-up cardiological study was scheduled at three to four months of age, and brainstem auditory evoked potentials study for reevaluation at three months of age; both were performed at the age of three months and were completely normal. Therefore, the infant was discharged without any sequelae.
3. Literature Review
3.1. Materials and Methods
Our systematic literature review was conducted until 27 October 2022 and followed the protocol proposed by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). We performed a Pubmed and Scopus search using the following keywords: “neonate”, “newborn”, “infant”, “Streptococcus bovis”, “Streptococcus gallolyticus”. An additional manual electronic search was carried out to identify reports not found in our initial search. We used predetermined inclusion and exclusion criteria to find all relevant articles. Reports describing cases of neonatal infection by S. gallolyticus were eligible to be included in our review, and there was no restriction on publication year. Reports referring to cases of S. gallolyticus infection in any age group other than neonates, reports not including identification of S. bovis to the subspecies level, reviews, as well as studies not published in English were excluded from our study. Two authors (KK, MT) independently screened titles and abstracts of the retrieved studies for possible inclusion in the review and then reviewed the articles in depth. Any disagreement was resolved by a third author (RS).
3.2. Results
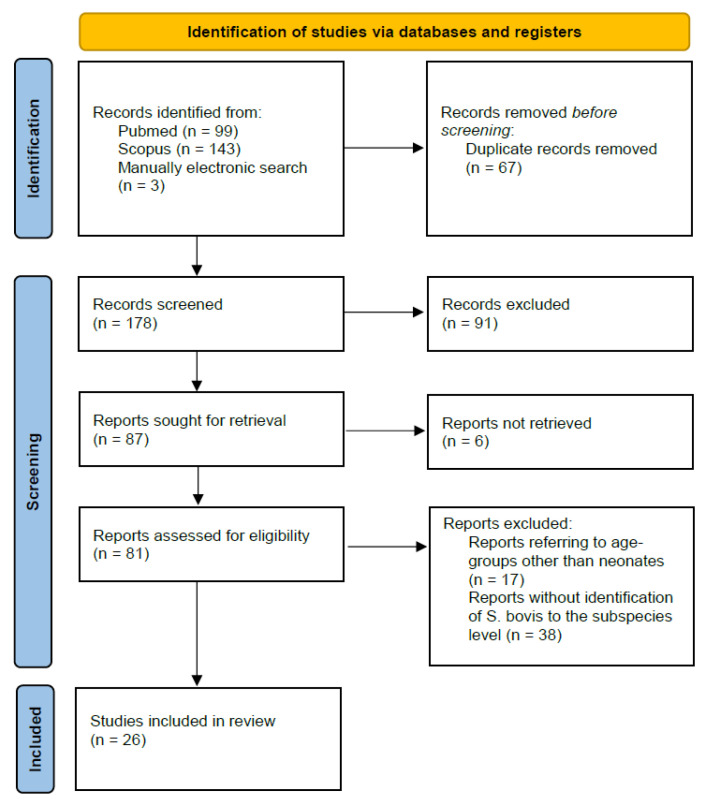
Our systematic search retrieved 245 articles. Duplicates were removed, and the remaining 178 studies were screened for eligibility. Ninety-one records were excluded during the process of title and/or abstract evaluation, and six reports were not accessible in full text to the authors; therefore, eighty-one studies were assessed in full text for possible inclusion in our review. Finally, 26 reports fulfilled the selection criteria and were thus included in our study. The study selection process is depicted in the respective flowchart (Figure 1).
Figure 1.
Flowchart of the selection process.
A total of 26 articles reported 66 cases [2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. For reasons of completeness, we decided to include in our review three cases of neonatal infection caused by S. bovis biotype II, since, according to the bibliography, neonatal infections caused by S. gallolyticus species (i.e., S. bovis biotype II/2) are much more common than neonatal infections caused by S. infantarius (i.e., S. bovis biotype II/1). We analyzed these 66 cases and the present case. Characteristics of the cases describing neonatal infection caused by S. gallolyticus and S. bovis biotype II are summarized in Table 1 and Table 2.
Table 1.
Characteristics of cases of early-onset sepsis caused by S. gallolyticus or S. bovis biotype II.
| First Author, Publication Year | GA | Birth Weight (Grams) | Mode of Delivery | Length of Rupture of Membranes | Maternal Vaginal Cultures | Maternal Peripartum Antibiotics | Symptoms Onset Time (Days) | Clinical Signs and Symptoms | Site of Isolation | Diagnosis | Microorganism | Complications | Definitive Antibiotic Treatment | Patient Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gavin, 2003 [5] | Term | 3.925 | Vaginal delivery | <18 h | GBS (-) | N/A | 3 | Fever, irritability, poor oral intake, decreased urine output | Blood, CSF | Biochemical tests (RapID STR, Vitek 2, API 20 Strep, CFA profile) | S. bovis biotype II/2 | Meningitis, Seizure-like episodes | Penicillin G for 14 days | Full recovery |
| Khan, 2009 [7] | N/A | N/A | N/A | N/A | N/A | N/A | 3 | Apnoeic episodes, lethargy | Blood, CSF | Biochemical tests (API 20 Strep kit) | S. gallolyticus subsp. pasteurianus | Meningitis | Penicillin and gentamicin for 14 days | Recovery |
| Klatte, 2012 [9] | Term | N/A | Vaginal delivery | 3 h | N/A | N/A | 2 | Poor feeding, lethargy, seizure activity | Blood, CSF | Βiochemical tests (Vitek 2), molecular tests (16S rRNA sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | Ampicillin for 16 days, cefotaxime for 6 days | Recovery |
| Thatrimontrichai, 2012 [11] | 39 weeks | 3.188 | Vaginal delivery | <18 h | Gamma Streptococci not in group D (after onset of neonatal symptoms) | N/A | 2 | Fever, lethargy, poor feeding, slightly bulging anterior fontanel | CSF, maternal urine | N/A | S. gallolyticus subsp. pasteurianus | Meningitis, right IVH grade I | Cefotaxime for 14 days and gentamicin for 5 days | Recovery |
| Beneteau, 2015 [16] | 10 preterm and 5 term neonates * | 2.160 (1.860–3.430) for neonates with symptoms onset <4 days, 2.240 (1.480–3.570) for neonates with symptoms onset >4 and ≤28 days | N/A | N/A | N/A | N/A | 3 neonates ≤ 4 days, 12 neonates >4 and ≤28 days | Fever, hypothermia, digestive signs, respiratory signs, irritability, neurologic signs, sepsis | Blood and/or CSF | N/A | S. gallolyticus pasteurianus in 8 cases and S. gallolyticus gallolyticus in 2 cases ** | Meningitis | Amoxicillin and/or 3rd generation cephalosporin and aminoglycocide for 10–25 days | All neonates survived |
| Nguyen, 2019 [20] | 39+1 weeks | 4.195 | Vaginal delivery | 12 h | GBS (-) | N/A | 1 | Respiratory distress | Blood | N/A | S. gallolyticus subsp. pasteurianus | Meningitis, endocarditis | Cefepime for 28 days and gentamicin for 14 days | Recovery without sequelae |
| 40+1 weeks | 3.250 | Vaginal delivery | 4 h | GBS unknown | No | 1 | Poor respiratory effort, irritability | Blood | N/A | S. gallolyticus subsp. pasteurianus | Septic shock, PPHN | Cefepime and clindamycin for 14 days | Recovery without sequelae | |
| Sim, 2021 [21] | 39 weeks | 3.374 | Vaginal delivery | <18 h | GBS (-) | No | 1 | Respiratory distress | Blood, CSF | Βiochemical tests (MALDI-TOF) | S. gallolyticus | Meningitis | Vancomycin | Recovery without sequelae |
| Chen, 2021 [22] | 35+1 weeks | N/A | Cesarean section | N/A | N/A | N/A | 3 | Apnea, desaturation | Blood | Βiochemical tests (MALDI-TOF), molecular tests (16S rRNA and sodA gene sequencing, PCR-RFLP assays of groESL gene) | S. gallolyticus subsp. pasteurianus | None | Ampicillin and cefotaxime for 14 days | Recovery without sequelae |
| 37+3 weeks | N/A | Cesarean section | N/A | GBS (+) | No | 2 | Tachypnea, desaturation, poor activity, fever | Blood, CSF | Βiochemical tests (MALDI-TOF), molecular tests (16S rRNA and sodA gene sequencing, PCR-RFLP assays of groESL gene) | S. gallolyticus subsp. pasteurianus | Meningitis | Ampicillin and cefotaxime for 14 days | Recovery without sequelae | |
| Geetha, 2021 [23] | 36+6 weeks | 3.776 | Vaginal delivery | <18 h | GBS (-) | No | 1 | Respiratory distress | Blood | Biochemical tests (MALDI-TOF, Vitek) | S. gallolyticus subsp. pasteurianus | Liver abscess | Cefotaxime for 5 weeks, co-amoxiclav for 3 weeks | Recovery without sequelae |
| Srour, 2022 [24] | 36+4 weeks | 3.720 | Vaginal delivery | <18 h | GBS (-) | N/A | 1 | Intermittent cyanosis, hypothermia, tachypnea | Blood, CSF | N/A | S. gallolyticus | Meningitis, ventriculitis, seizure-like activity | Penicillin G for 21 days | Recovery without sequelae |
| Williams, 2022 [25] | 26 weeks | 950 | Vaginal delivery | PPROM 12 days | GBS (-) | IV ampicillin for 2 days and pos amoxicillin for 5 days and a single dose of azithromycin | 1 | Poor respiratory effort, metabolic and respiratory acidosis | Blood | Βiochemical tests (MALDI-TOF) | S. gallolyticus | Septic shock | Ampicillin and gentamicin (empirical treatment) | Deceased neonate |
| Orbea, 2022 [26] | N/A (9 neonates) *** | N/A | N/A | N/A | N/A | N/A | Median age 24 days (1–74 days) | Fever, irritability, difficulty feeding, lethargy, respiratory distress, apnea, seizure-like activity, emesis, diarrhea | Blood and/or CSF | Biochemical tests (MALDI-TOF, Vitek) | S. gallolyticus, S. gallolyticus subsp. pasteurianus **** | Meningitis (11 infants), bilateral grade III IVH (1neonate), ventriculitis and subdural collection and/or subarachnoid debris (3 neonates) ***** | Antibiotic therapy for a median of 14 days (9–28 days) | One deceased neonate, one neonate with neurologic complications |
| Sasi, 2022 [27] | 37 weeks | 3.125 | Vaginal delivery | N/A | N/A | N/A | 1 | Tachycardia, tachypnea, poor sucking | Maternal blood cultures, placental tissue culture, no growth in neonatal blood culture | Biochemical tests (MALDI-TOF) | S. gallolyticus subsp. gallolyticus | None | Ampicillin and amikacin for 5 days | Recovery without sequelae |
| 38 weeks | 2.670 | Vaginal delivery | 3 h | N/A | N/A | 1 | Tachycardia, tachypnea | Maternal blood cultures, no growth in neonatal blood culture | Biochemical tests (MALDI-TOF) | S. gallolyticus subsp. gallolyticus | None | Ampicillin and amikacin for 7 days | Recovery without sequelae | |
| 39 weeks | 3.620 | Cesarean section | N/A | N/A | N/A | 1 | Respiratory distress | Maternal blood and urine cultures, no growth in neonatal blood culture | Biochemical tests (MALDI-TOF) | S. gallolyticus subsp. gallolyticus | Meningitis | Ampicillin and amikacin for 10 days | Recovery without sequelae | |
| 41 weeks | 4.170 | Cesarean section | N/A | N/A | N/A | 1 | Fever, tachycardia, respiratory distress | Maternal blood cultures, placental tissue culture, no growth in neonatal blood culture | Biochemical tests (MALDI-TOF) | S. gallolyticus subsp. gallolyticus | Pneumonia | Ampicillin and amikacin for 5 days | Recovery without sequelae | |
| This case | 38+1 weeks | 3.250 | Cesarean section | 10 h | GBS (-) | No | 1 | Respiratory distress, poor feeding, fever | Blood | Biochemical tests (Vitek 2) | S. gallolyticus | PPHN | Ampicillin and cefotaxime for 10 days | Recovery |
Abbreviations: CFA, cellular fatty acid; CSF, cerebrospinal fluid; GBS, group B streptococci; IV, intravenous; IVH, intraventricular hemorrhage; MALDI-TOF, matrix-assisted laser desorption ionization-time of flight mass spectrometry; N/A, not available; PFGE, pulsed-field gel electrophoresis; PCR, polymerase chain reaction; PPHN, persistent pulmonary hypertension of the newborn; RFLP, restriction fragment length polymorphism. * This study included 23 infants, 15 of whom were neonates. ** Subspecies of S. bovis were identified in 10 cases. Not specified if the data of S. gallolyticus identification refers to neonates or to infants. *** This study included 15 infants, 9 of whom were neonates. **** S. bovis was not classified into subspecies in one case. Not specified if the data of S. gallolyticus identification refers to neonates or to infants. ***** Not specified if cases of meningitis refer to neonates or to infants.
Table 2.
Characteristics of cases of late-onset sepsis caused by S. gallolyticus or S. bovis biotype II.
| First Author, Publication Year | GA | Birth Weight (Grams) | Mode of Delivery | Length of Rupture of Membranes | Maternal Vaginal Cultures | Maternal Peripartum Antibiotics | Symptoms Onset Time (Days) | Clinical Signs and Symptoms | Site of Isolation | Diagnosis | Microorganism | Complications | Definitive Antibiotic Treatment | Patient Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cheung, 2000 [3] | 32 weeks | 2.340 | Vaginal delivery | N/A | N/A | Prophylactic clindamycin | 28 | Lethargy, possible seizure activity, apnea | Blood, CSF | Biochemical tests (API 20 strep, cellular fatty acid profile) | S. bovis biotype II | Meningitis | Ampicillin and gentamicin changed to penicillin for 18 days | Recovery without sequelae |
| Koh, 2002 [4] | 34 weeks | 1.675 | Cesarean section | N/A | N/A | N/A | 19 | Lethargy, apnea | Blood, CSF | Biochemical tests (API 20 strep test) | S. bovis biotype II | Meningitis | Penicillin G for 14 days | Recovery without sequelae |
| Onoyama, 2009 [6] | Term | 3.192 | Vaginal delivery | <18 h | GBS (-) | N/A | 4 | Fever, poor activity | Blood, CSF | Biochemical tests (API 20 Strep test), molecular tests (16S rRNA and sodA gene sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | Cefotaxime for 14 days | Recovery without sequelae |
| Floret, 2010 [8] | 5 preterm | N/A | 3 vaginal deliveries, 2 cesarean sections | N/A | N/A | N/A | Median age 18 days (13–56) | Abdominal distention, diarrhea, signs of umbilical inflammation | Blood | Molecular tests (sodA gene sequencing, 16S rRNA gene sequencing, PFGE) | S. gallolyticus subsp. pasteurianus | N/A | Cefotaxime for 10 days | All neonates survived |
| Klatte, 2012 [9] | Term | N/A | Vaginal delivery | N/A | N/A | N/A | 12 | Congestion, increased work of breathing, lethargy | CSF | Βiochemical tests (Vitek 2), molecular tests (16S rRNA sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | Cefotaxime for 16 days | Recovery |
| Term | N/A | Vaginal delivery | N/A | N/A | N/A | 4 | Fever, seizure-like activity | Blood, CSF | Βiochemical tests (Vitek 2), molecular tests (16S rRNA sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | Ampicillin for 14 days | Recovery | |
| Post-term | N/A | Vaginal delivery | 13 h | N/A | N/A | 4 | Fever, rhinorrhea, fussiness | Blood, CSF | Βiochemical tests (Vitek 2), molecular tests (16S rRNA sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | Cefotaxime for 14 days | Recovery | |
| Nagamatsu, 2012 [10] | Term | 3.092 | Vaginal delivery | N/A | N/A | N/A | 8 | Superior tonic eye deviation, fever, irritability, decreased oral intake | CSF | Βiochemical tests (API Rapid ID 32 Strep, VITEK 2), molecular tests (16S rRNA gene sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis, seizures | Ampicillin for 20 days and panipenem/ betamipron for 14 days | Recovery without sequelae |
| Tarakci, 2014 [12] | 30 weeks | 1.300 | Cesarean section | N/A | N/A | N/A | 37 | Apnea, lethargy, cyanosis, superficial respiration | Blood | N/A | S. gallolyticus subsp. pasteurianus | None | Meropenem for 14 days | Recovery |
| Hede, 2015 [13] | 32 weeks | 1.474 | Cesarean section | N/A | GBS unknown | No | 24 | Intermittent tachypnea | Blood | Βiochemical tests (Vitek 2), molecular tests (16S rRNA sequencing, PFGE) | S. gallolyticus subsp. pasteurianus | None | Ampicillin for 10 days | Survived |
| 32 weeks | 2.120 | Cesarean section | N/A | GBS unknown | No | 24 | Pale color, loose stools, respiratory distress, hypoxemia, hypothermia | Blood, CSF | Βiochemical tests (Vitek 2), molecular tests (16S rRNA sequencing, PFGE) | S. gallolyticus subsp. pasteurianus | Meningitis, acute respiratory failure, septic shock, seizures, grade III IVH | Ampicillin for 14 days | Survived | |
| Park, 2015 [14] | 38+4 weeks | 3.600 | Vaginal delivery | N/A | N/A | N/A | 27 | Fever, lethargy, moaning sounds | Blood, CSF, urine | Μolecular tests (16S rRNA gene sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis, bilateral reduction in visual evoked potentials, subdural effusion and seizures | Ampicillin and cefotaxime for 21 days, ampicillin and cefotaxime for 31 days due to CNS complications | Discharge, improvement of visual evoked potential in follow-up |
| Kennedy, 2015 [15] | 37 weeks | 3.050 | Vaginal delivery | 6 h | GBS (-) | N/A | 4 | Lethargy, irritability, decreased urine output, poor feeding, fever | Blood, CSF | Βiochemical tests (RapID STR), molecular tests (16S rRNA gene sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | Ampicillin for 14 days | Recovery without sequelae |
| Beneteau, 2015 [16] | 10 preterm and 5 term neonates * | 2.160 (1.860–3.430) for neonates with symptoms onset <4 days, 2.240 (1.480–3.570) for neonates with symptoms onset >4 and ≤28 days | N/A | N/A | N/A | N/A | 3 neonates ≤ 4 days, 12 neonates >4 and ≤28 days | Fever, hypothermia, digestive signs, respiratory signs, irritability, neurologic signs, sepsis | Blood and/or CSF | N/A | S. gallolyticus pasteurianus in 8 cases and S. gallolyticus gallolyticus in 2 cases ** | Meningitis | Amoxicillin and/or 3rd generation cephalosporin and aminoglycocide for 10–25 days | All neonates survived |
| Parain, 2016 [17] | Term | N/A | N/A | N/A | N/A | N/A | 28 | Altered consciousness, hypertonic limbs, bulging fontanel | CSF | N/A | S. gallolyticus | Meningitis, hydrocephalus with tetraventricular dilatation requiring external ventricular drainage, recurrence of hydrocephalus requiring ventriculoperitoneal bypass | Cefotaxime for 17 days and amikacin for 2 days | Recovery with neurologic complications (neuromotor delay with poor spontaneous motor mobilization and hypertonia of the limbs at 9 months) |
| Saegeman, 2016 [2] | 30 weeks | N/A | Vaginal delivery | N/A | N/A | N/A | 7 | Hemodynamic instability | Blood | Βiochemical tests (MALDI-TOF mass spectrometry), molecular tests (PFGE, 16S rRNA gene sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis | N/A | Recovery |
| 32 weeks | N/A | N/A | N/A | N/A | N/A | 34 | Septic shock, respiratory failure, pulmonary hemorrhage | Blood | Βiochemical tests (MALDI-TOF mass spectrometry), molecular tests (PFGE, 16S rRNA gene sequencing) | S. gallolyticus subsp. pasteurianus | Septic shock | N/A | Deceased neonate | |
| Yamamura, 2018 [18] | Term | 3.680 | Vaginal delivery | N/A | N/A | N/A | 27 | Fever, lethargy, irritability | Blood, CSF | Βiochemical tests (API 20 Strep test), molecular tests (16s rRNA gene sequencing) | S. gallolyticus subsp. pasteurianus | Meningitis, ventriculitis | Ampicillin for 21 days | Recovery without sequelae |
| Mettananda, 2018 [19] | 34 weeks | 1.680 | Cesarean section | N/A | N/A | N/A | 25 | Fever, poor sucking, reduced activity | Blood | Βiochemical tests | S. bovis biotype II | Meningitis | Penicillin G and cefotaxime for 21 days | Recovery without sequelae |
| Sim, 2021 [21] | 39 weeks | 3.268 | Vaginal delivery | <18 h | GBS (-) | No | 4 | Fever, respiratory distress | Blood, CSF | Βiochemical tests (MALDI-TOF) | S. gallolyticus | Meningitis | Ampicillin | Recovery without sequelae |
| 39 weeks | 3.194 | Vaginal delivery | <18 h | GBS (-) | No | 23 | Fever, lethargy | Blood | Βiochemical tests (MALDI-TOF) | S. gallolyticus | None | Ampicillin | Recovery without sequelae | |
| 34 weeks | 1.812 | Cesarean section | <18 h | GBS (-) | No | 15 | Fever, poor feeding | Blood | Βiochemical tests (MALDI-TOF) | S. gallolyticus | None | Clindamycin | Recovery without sequelae | |
| Chen, 2021 [22] | 37+3 weeks | N/A | Cesarean section | N/A | GBS (+) | No | 5 | Fever, tachypnea | CSF | Βiochemical tests (MALDI-TOF), molecular tests (16S rRNA and sodA gene sequencing, PCR-RFLP assays of groESL gene) | S. gallolyticus subsp. pasteurianus | Meningitis | Ampicillin and cefotaxime for 14 days | Recovery without sequelae |
| Orbea, 2022 [26] | N/A (9 neonates) *** | N/A | N/A | N/A | N/A | N/A | Median age 24 days (1–74 days) | Fever, irritability, difficulty feeding, lethargy, respiratory distress, apnea, seizure-like activity, emesis, diarrhea | Blood and/or CSF | Biochemical tests (MALDI-TOF, Vitek) | S. gallolyticus, S. gallolyticus subsp. pasteurianus **** | Meningitis (11 infants), bilateral grade III IVH (1neonate), ventriculitis and subdural collection and/or subarachnoid debris (3 neonates) ***** | Antibiotic therapy for a median of 14 days (9–28 days) | One deceased neonate, one neonate with neurologic complications |
Abbreviations: CSF, cerebrospinal fluid; GBS, group B streptococci; IV, intravenous; IVH, intraventricular hemorrhage; MALDI-TOF, matrix-assisted laser desorption ionization-time of flight mass spectrometry; N/A, not available; PFGE, pulsed-field gel electrophoresis; PCR, polymerase chain reaction; RFLP, restriction fragment length polymorphism. * This study included 23 infants, 15 of whom were neonates. ** Subspecies of S. bovis were identified in 10 cases. Not specified if the data of S. gallolyticus identification refers to neonates or to infants. *** This study included 15 infants, 9 of whom were neonates. **** S. bovis was not classified into subspecies in one case. Not specified if the data of S. gallolyticus identification refers to neonates or to infants. ***** Not specified if cases of meningitis refer to neonates or to infants.
Preterm and term newborns were equally represented among the cases included in this review. Twenty-eight cases (49.5%) referred to preterm neonates (GA 26–36+6), and twenty-eight (49.5%) to term neonates. One neonate was post-term, while gestational age was not reported in 10 cases. Regarding the type of delivery, there were 14 cesarean sections (35%), 26 vaginal deliveries (65%), and, in 27 cases, the delivery type was not recorded. Data regarding the length of rupture of membranes were only available in 17 cases; among them, there was only 1 case of prolonged rupture of membranes (preterm premature rupture of the membranes, PPROM). Similarly, details concerning maternal perineal and vaginal cultures were provided in only 18 cases; the majority was GBS negative (12/18, 67%), 3 cases were of unknown GBS status (3/18, 16.5%), 2 cases were GBS positive (2/18, 11%) and 1 case (1/18, 5.5%) was positive for gamma Streptococci that did not belong to Group D. Peripartum antibiotics were used in only 2 cases; one was for premature spontaneous labor at 32 weeks and one for PPROM. However, in most cases (54 out of 67), relevant information was not reported. The time of onset of symptoms was specified in 43 cases. There were 17 cases (39.5%) with early-onset sepsis (data reported in Table 1) and the remaining (60.5%) referred to late-onset sepsis (data reported in Table 2). Among the 67 cases reported, respiratory distress, poor feeding, fever and lethargy were the symptoms most frequently described, followed by seizures, apnea, and symptoms from the gastrointestinal tract. Meningitis was by far the most common complication, being reported in 40 out of 53 cases (75%) (in 14 cases, complications were not listed). Bacteremia complicated with meningitis was reported in 47% (8/17 cases) of early-onset sepsis and in 61.5% (16/26 cases) of late-onset sepsis. Complications from the nervous system also included five cases of ventriculitis (7%), two cases of grade III intraventricular hemorrhage (IVH) (3%), three cases of subdural effusion (4.5%) and one case of hydrocephalus requiring ventriculoperitoneal bypass (1.5%). Regarding the respiratory tract, two cases with persistent pulmonary hypertension were described (3%) (including the present case), one case with pulmonary hemorrhage (1.5%) and one case with pneumonia (1.5%). In almost half of the cases (32 out of 67 cases), S. gallolyticus was identified to the subspecies level; in 28 cases (87.5%), S. gallolyticus subspecies pasteurianus was isolated, and in 4 cases (12.5%) S. gallolyticus subspecies gallolyticus. Penicillin, or a penicillin-derivative, alone or in combination with another agent (usually cephalosporin or aminoglycoside) was the definitive treatment of choice in 41 out of 56 cases (73%). Among 67 cases, only three neonates (4.5%) died.
4. Discussion
This is a case of early-onset neonatal sepsis due to S. gallolyticus, with rapid clinical deterioration leading to mechanical ventilation and administration of inotropes, complicated by the development of pulmonary hypertension, with finally benign clinical course and discharge after a 12-day hospitalization.
S. gallolyticus belongs to group D Streptococci in the S. bovis-S. equinus complex. The taxonomy and nomenclature of group D Streptococci were modified in 2003 [28]. S. bovis, based on its ability or inability to ferment mannitol, was designated biotype I (mannitol fermentation-positive) or biotype II (mannitol-fermentation-negative). Biotype II was further divided into biotype II/1 and biotype II/2 depending on further biochemical characteristics. These strains are now known as S. gallolyticus subsp. gallolyticus (biotype I), S. infantarius (biotype II/1) and S. gallolyticus subsp. pasteurianus (biotype II/2). Proper identification is usually accomplished with a combination of various biochemical tests (e.g., API 20 Strep and Rapid ID 32 Strep test system, Vitek 2, matrix-assisted laser desorption ionization-time of flight mass spectrometry-MALDI TOF) with molecular tests (e.g., 16S rRNA and sodA gene sequencing, pulsed-field gel electrophoresis—PFGE) [1]. Based on our literature search, biochemical testing is initially applied to identify bacteria at the species level. However, enzymatic reaction interpretation is not always definite, and this could lead to the misidentification of the isolated strain. Therefore, to minimize the risk of misidentification, various phenotypic tests should be used to verify the results. Moreover, whenever available, genotypic identification of the isolated pathogen with the utilization of molecular tests should be preferred in order to confirm the identity of the isolated strains and to further identify them to the subspecies level. Gene sequencing and PFGE are the methods more widely used to identify the isolated microorganism [29,30] reliably.
In adults, the most common source of infection includes the gastrointestinal and hepatobiliary tracts. S. gallolyticus subsp. gallolyticus is mainly associated with colonic neoplasia and endocarditis in adults, while S. infantarius is linked to non-colonic cancers [31]. Due to the epidemiological correlation between S. gallolyticus infection and colon cancer, it is strongly recommended that all adults with S. gallolyticus bacteremia are evaluated with colonoscopy [27]. No gastrointestinal disease was known in the mother of the present case; a colonoscopy was scheduled after discharge from the maternity hospital. Recent studies also suggest that contact with infected animals or contaminated food promotes fecal colonization of healthy adults with S. gallolyticus, indicating a potential zoonotic origin [32].
The pathophysiology of neonatal infection caused by S. gallolyticus species remains unclear. Based on our review of the literature, S. gallolyticus subsp. pasteurianus is the most common pathogen among S. gallolyticus species in neonates. Similarly to GBS, it is believed that colonization of the vagina and vertical transmission to the fetus via an ascending route, especially in case of prolonged rupture of membranes, or transmission to the fetus during parturition, could lead to neonatal colonization and probably infection [33]. In the present case, the exact contagious route was not clarified since no pathogens were isolated in the vaginal swab at 35 weeks of gestation, and there was no prolonged rupture of membranes. Fikar and Levy reported a case of neonatal meningitis caused by S. bovis, whose mother had positive vaginal and rectal swabs for the same microorganism [34]. Additionally, Binghuai et al. described a case of intrauterine infection complicated by postpartum bacteremia caused by S. gallolyticus subsp. pasteurianus in a mother whose infant tested positive for the same infectious agent in ear and throat swabs [35]. This neonate remained asymptomatic, contrary to the cases reported by Sasi et al. [27]. In their study, four out of five neonates born to mothers with intrauterine infection and postpartum bacteremia caused by S. gallolyticus subsp. gallolyticus had symptoms compatible with sepsis and/ or meningitis. However, no microorganism was isolated from neonatal blood and/ or CSF cultures. Abnormalities of the immune system may further predispose neonates to infections, including those caused by S. gallolyticus, as described by Koh et al. and Mettananda et al. [4,19]. Moreover, as stated by Saegeman et al. [2] and Floret et al. [8], nosocomial transmission may play a role in some cases of neonatal infections by S. gallolyticus species; this underlines the importance of adherence of medical staff with health protocols and raises awareness of healthcare-associated infections.
A benign clinical course is usually reported for S. gallolyticus infections in neonates. Based on our search of the literature, the clinical presentation of S. gallolyticus neonatal infection cannot be easily distinguished from GBS infection, with signs and symptoms including sepsis and metabolic acidosis, respiratory distress, apnea and poor overall activity being prominent. Meningitis, which may present with seizures, constitutes a common complication, especially of late-onset sepsis, though it seems to have a favorable outcome. Despite the increased frequency of concomitant bacteremia and meningitis, the neonate of the present case did not have any signs or laboratory evidence of meningitis during its clinical course. Additional complications of the central nervous system described in the literature include ventriculitis, [18,24,26] hydrocephalus [17] and intraventricular hemorrhage (IVH) (two cases with grade III IVH, though one refers to an extremely preterm neonate and hence IVH could be either a complication of the infection or due to underlying prematurity) [13,26]. Complications from the respiratory tract seem to be rare: two cases with pulmonary hypertension have been described (including the present case), [20] one case of pulmonary hemorrhage (referring to a preterm neonate) and one case of pneumonia [27]. Even though only a couple of cases of persistent pulmonary hypertension (PPHN) secondary to S. gallolyticus infection have been described thus far, PPHN has been clearly described in the literature as a complication of neonatal sepsis [36]. Release in the bloodstream of bacterial endotoxins, together with inflammatory mediators (including endothelin-1 and thromboxane, which constitute potent vasoconstrictors) and various cytokines, seem to contribute to the pathophysiology of PPHN associated with neonatal sepsis. [37,38] Additionally, although S. gallolyticus has been clearly linked with cases of endocarditis in adult patients, to our knowledge, only one case has been reported in the neonatal population; this patient recovered without sequelae [20]. The overall prognosis is encouraging, with three neonatal deaths by S. gallolyticus being recorded in the literature out of 67 cases of neonatal infection (including one extremely premature and extremely low birth weight neonate) and 2 cases complicated by severe neurologic sequelae [2,17,25,26]. Postmortem examination findings of the deceased neonates were not described in the literature. Procalcitonin constitutes a promising diagnostic marker of neonatal sepsis, being able to discriminate cases of infection versus non-infectious inflammatory disorders; it could be thus speculated that immunohistochemical techniques, with anti-procalcitonin antibodies, among others, could contribute to the postmortem diagnosis of neonatal sepsis, in cases of diagnostic ambiguity [39,40].
S. gallolyticus, in the majority of cases, is sensitive to penicillin and to penicillin derivatives, ref. [5,22] and this was also the case for the neonatal infection caused by S. gallolyticus hereby presented. However, we should mention two reports by Khan et al. [7] and Klatte et al. [9] referring to two cases of neonatal meningitis caused by S. pasteurianus relatively resistant to penicillin. Therefore, de-escalation of antibacterial therapy after accurate identification of the causative agent and antimicrobial susceptibility testing is legitimate to minimize the risk of multi-drug resistant pathogens. According to our search of the literature, the duration of therapy is variable depending on the clinical course, ranging from a minimum of 5 days [27] to a maximum of approximately 50 days (one case with liver abscess formation [23] and one case with late central nervous system complications) [14].
There are some limitations in our systematic review. In many cases, documentation regarding perinatal history was incomplete, including type of delivery, duration of rupture of membranes, maternal perineal and vaginal swabs and use of prophylactic peripartum antibiotics; consequently, the impact of these perinatal parameters on the pathogenesis of neonatal infection by S. gallolyticus cannot be precisely estimated. Additionally, there may be cases of infection by S. gallolyticus that have not been captured. Specifically, there are cases of neonatal infection by S. bovis in the literature that have not been identified at the subspecies level or cases that may have been misclassified as other Streptococcus species, mainly before the currently available biochemical and molecular techniques. Therefore, the true prevalence of S. gallolyticus infection and its subspecies remains unknown.
5. Conclusions
In conclusion, the occurrence of S. gallolyticus infection in the neonate of the present case report indicates that S. gallolyticus may be considered a novel and emerging pathogen in the field of neonatal sepsis. Increased clinical suspicion may be warranted in sepsis cases resembling GBS infections, even in the presence of negative GBS vaginal swab cultures. Precise identification of the pathogens is necessary to detect possible epidemiological changes. More studies are needed to clarify the epidemiology, pathogenesis and possible sequelae of neonatal infections caused by S. gallolyticus species.
Author Contributions
All authors contributed to the writing of this manuscript. Z.I., K.K. and M.T. conceived and developed the idea and wrote the initial manuscript. H.B., E.T., S.O., A.P., M.D. and C.P. collected the data and the bibliography. T.B., R.S. and N.I. made the revisions. N.I. and R.S. verified the underlying data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dekker J.P., Lau A.F. An Update on the Streptococcus bovis Group: Classification, Identification, and Disease Associations. J. Clin. Microbiol. 2016;54:1694–1699. doi: 10.1128/JCM.02977-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saegeman V., Cossey V., Loens K., Schuermans A., Glaser P. Streptococcus gallolyticus subsp. Pasteurianus Infection in A Neonatal Intensive Care Unit. Pediatr. Infect. Dis. J. 2016;35:1272–1275. doi: 10.1097/INF.0000000000001290. [DOI] [PubMed] [Google Scholar]
- 3.Cheung M., Pelot M., Nadarajah R., Kohl S. Neonate with Late Onset Streptococcus bovis Meningitis: Case Report and Review of the Literature. Pediatr. Infect. Dis. J. 2000;19:891–893. doi: 10.1097/00006454-200009000-00019. [DOI] [PubMed] [Google Scholar]
- 4.Koh T.H., Ho S. Streptococcus bovis Meningitis in a Neonate with Ivemark Syndrome. Scand. J. Infect. Dis. 2002;34:63–64. doi: 10.1080/00365540110076958. [DOI] [PubMed] [Google Scholar]
- 5.Gavin P.J., Thomson R.B., Horng S.J., Yogev R. Neonatal sepsis caused by Streptococcus bovis variant (biotype 11/2): Report of a case and review. J. Clin. Microbiol. 2003;41:3433–3435. doi: 10.1128/JCM.41.7.3433-3435.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Onoyama S., Ogata R., Wada A., Saito M., Okada K., Harada T. Neonatal bacterial meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J. Med. Microbiol. 2009;58:1252–1254. doi: 10.1099/jmm.0.006551-0. [DOI] [PubMed] [Google Scholar]
- 7.Khan A. Relative Penicillin Resistance in Streptococcus bovis. A Case of Neonatal Meningitis. J. Paediatr. Child Health. 2009;45:474–475. doi: 10.1111/j.1440-1754.2009.01541.x. [DOI] [PubMed] [Google Scholar]
- 8.Floret N., Bailly P., Thouverez M., Blanchot C., Alez-Martin D., Menget A., Thiriez G., Hoen B., Talon D., Bertrand X. A cluster of bloodstream infections caused by Streptococcus gallolyticus subspecies pasteurianus that involved 5 preterm neonates in a university hospital during a 2-month period. Infect. Control Hosp. Epidemiol. 2010;31:194–196. doi: 10.1086/650380. [DOI] [PubMed] [Google Scholar]
- 9.Klatte J.M., Clarridge J.E., Bratcher D., Selvarangan R. A longitudinal case series description of meningitis due to Streptococcus gallolyticus subsp. pasteurianus in infants. J. Clin. Microbiol. 2012;50:57–60. doi: 10.1128/JCM.05635-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagamatsu M., Takagi T., Ohyanagi T., Yamazaki S., Nobuoka S., Takemura H., Akita H., Miyai M., Ohkusu K. Neonatal meningitis caused by Streptococcus gallolyticus subsp. pasteurianus. J. Infect. Chemother. 2012;18:265–268. doi: 10.1007/s10156-011-0320-4. [DOI] [PubMed] [Google Scholar]
- 11.Thatrimontrichai A., Chanvitan P., Janjindamai W., Dissaneevate S., Maneenil G. Early onset neonatal bacterial meningitis caused by streptococcus gallolyticus subsp. pasteurianus. Southeast Asian J. Trop Med. Public Health. 2012;43:145–151. [PubMed] [Google Scholar]
- 12.Tarakçi N., Daǎi H.T., Uǧur A.R., Tuncer I., Taştekin A. Late-onset Streptococcus pasteurianus sepsis in a preterm baby in a neonatal intensive care unit. Turk. Arch. Pediatr. 2014;49:157–159. doi: 10.5152/tpa.2014.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hede S.V., Olarte L., Chandramohan L., Kaplan S.L., Hulten G. Streptococcus gallolyticus subsp. pasteurianus infection in twin infants. J. Clin. Microbiol. 2015;53:1419–1422. doi: 10.1128/JCM.02725-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park J.W., Eun S.H., Kim E.C., Seong M.W., Kim Y.K. Neonatal invasive Streptococcus gallolyticus subsp. pasteurianus infection with delayed central nervous system complications. Korean J. Pediatr. 2015;58:33–36. doi: 10.3345/kjp.2015.58.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy G.J., Kavanagh K.L., Kashimawo L.A., Cripe P.J., Steele R.W. An Unlikely Cause of Neonatal Sepsis. Clin. Pediatr. 2015;54:1017–1020. doi: 10.1177/0009922815591894. [DOI] [PubMed] [Google Scholar]
- 16.Beneteau A., Levy C., Foucaud P., Béchet S., Cohen R., Raymond J., Dommergues M.-A. Childhood meningitis caused by Streptococcus bovis group: Clinical and biologic data during a 12-year period in France. J. Pediatr. Infect. Dis. 2015;34:136–139. doi: 10.1097/INF.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 17.Parain D., De La Villeon G., Pinto Cardoso G., Abily Donval L., Marret S., Pinquier D. Neonatal Meningoencephalitis Caused by Streptococcus gallolyticus. Pediatr. Infect. Dis. J. 2016;35:597. doi: 10.1097/INF.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 18.Yamamura Y., Mihara Y., Nakatani K., Nishiguchi T., Ikebe T. Unexpected Ventriculitis Complication of Neonatal Meningitis Caused by Streptococcus gallolyticus subsp. pasteurianus: A Case Report. Jpn. J. Infect. Dis. 2018;71:68–71. doi: 10.7883/yoken.JJID.2017.053. [DOI] [PubMed] [Google Scholar]
- 19.Mettananda S., Kamalanathan P., Namalie K.D. Streptococcus bovis—Unusual etiology of meningitis in a neonate with Down syndrome: A case report. J. Med. Case Rep. 2018;12:93. doi: 10.1186/s13256-018-1634-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen M.T., Idriss S., Guzman E., De Oliveira E.R. Neonatal meningitis, endocarditis, and pneumonitis due to Streptococcus gallolyticus subsp. pasteurianus: A case report. BMC Pediatr. 2019;19:1–5. doi: 10.1186/s12887-019-1645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sim J.Y., Wang L.-W., Chow J.C., Hsu W.-Y., Chen Y.-C., Chang Y.-H., Chou Y., Chen W.-Y., Tang H.-J., Chang T.-H. Streptococcus Gallolyticus—A potentially neglected pathogen causing neonatal sepsis not covered by routine group B streptococcus screening. J. Microbiol. Immunol. Infect. 2021;54:1190–1192. doi: 10.1016/j.jmii.2021.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Chen W.-C., Lee P.-I., Lin H.-C., Chang L.-Y., Lee T.-F., Chen J.-M., Hsueh P.-R. Clustering of Streptococcus gallolyticus subspecies pasteurianus bacteremia and meningitis in neonates. J. Microbiol. Immunol. Infect. 2021;54:1078–1085. doi: 10.1016/j.jmii.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Geetha O., Cherie C., Natalie T.W.H., Merchant K., Chien C.M., Chandran S. Streptococcus gallolyticus subspecies pasteurianus causing early onset neonatal sepsis complicated by solitary liver abscess in a preterm infant. Access Microbiol. 2021;3:200. doi: 10.1099/acmi.0.000200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Srour N., Dasnadi S., Korulla A., Shah P.J. Early-onset neonatal ventriculomeningitis due to Streptococcus gallolyticus: A case report. Pediatr. Neonatol. 2022;63:430–431. doi: 10.1016/j.pedneo.2021.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Williams C., Sakaria R.P., Pourcyrous M. Early-Onset Fulminant Sepsis in a Preterm Neonate due to Streptococcus gallolyticus: A Case Report and Literature Review. AJP Rep. 2022;12:E117–E122. doi: 10.1055/a-1762-0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orbea M., Desai N., Foster C. Invasive Streptococcus gallolyticus Infections in Infants At Texas Children’s Hospital: A 9-Year Retrospective Review. Pediatr. Infect. Dis. J. 2022;41:e494–e497. doi: 10.1097/INF.0000000000003682. [DOI] [PubMed] [Google Scholar]
- 27.Sasi S., Abid FBen Wilson G.J., Zaqout A., Nair A.P., Chitrambika P. Intrauterine infection and postpartum bacteremia due to Streptococcus gallolyticus subsp. gallolyticus: An emerging concern. IDCases. 2022;29:e01562. doi: 10.1016/j.idcr.2022.e01562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlegel L., Grimont F., Ageron E., Grimont P.A.D., Bouvet A. Reappraisal of the taxonomy of the Streptococcus bovis/Streptococcus equinus complex and related species: Description of Streptococcus gallolyticus subsp. gallolyticus subsp. nov., S. gallolyticus subsp. macedonicus subsp. nov. and S. gallolyticus subsp. Int. J. Syst. Evol. Microbiol. 2003;53:631–645. doi: 10.1099/ijs.0.02361-0. [DOI] [PubMed] [Google Scholar]
- 29.Beck M., Frodl R., Funke G. Comprehensive study of strains previously designated Streptococcus bovis consecutively isolated from human blood cultures and emended description of Streptococcus gallolyticus and Streptococcus infantarius subsp. coli. J. Clin. Microbiol. 2008;46:2966–2972. doi: 10.1128/JCM.00078-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Funke G., Funke-Kissling P. Performance of the new VITEK 2 GP card for identification of medically relevant gram-positive cocci in a routine clinical laboratory. J. Clin. Microbiol. 2005;43:84–88. doi: 10.1128/JCM.43.1.84-88.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romero B., Morosini M.-I., Loza E., Rodríguez-Baños M., Navas E., Cantón R., del Campo R. Reidentification of Streptococcus bovis isolates causing bacteremia according to the new taxonomy criteria: Still an issue? J. Clin. Microbiol. 2011;49:3228–3233. doi: 10.1128/JCM.00524-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dumke J., Vollmer T., Akkermann O., Knabbe C., Dreier J. Case-control study: Determination of potential risk factors for the colonization of healthy volunteers with Streptococcus gallolyticus subsp. gallolyticus. PLoS ONE. 2017;12:e0176515. doi: 10.1371/journal.pone.0176515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simonsen K.A., Anderson-berry A.L., Delair S.F., Davies D.H. Early-Onset Neonatal Sepsis. Clin. Microbiol. Rev. 2014;27:21–47. doi: 10.1128/CMR.00031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fikar C.R., Levy J. Streptococcus bovis meningitis in a neonate. Am. J. Dis. Child. 1979;133:1149–1150. doi: 10.1001/archpedi.1979.02130110057009. [DOI] [PubMed] [Google Scholar]
- 35.Binghuai L., Wenjun S., Xinxin L. Intrauterine infection and post-partum bacteraemia due to Streptococcus gallolyticus subsp. pasteurianus. Pt 10J. Med. Microbiol. 2013;62:1617–1619. doi: 10.1099/jmm.0.054106-0. [DOI] [PubMed] [Google Scholar]
- 36.Lakshminrusimha S., Keszler M. Persistent pulmonary hypertension of the newborn. Neoreviews. 2015;16:680–692. doi: 10.1542/neo.16-12-e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deshpande S., Suryawanshi P., Holkar S., Singh Y., Yengkhom R., Klimek J., Gupta S. Pulmonary hypertension in late onset neonatal sepsis using functional echocardiography: A prospective study. J. Ultrasound. 2022;25:233–239. doi: 10.1007/s40477-021-00590-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohsen A.A., Amin A. Risk factors and outcomes of persistent pulmonary hypertension of the newborn in neonatal intensive care unit of al-minya university hospital in Egypt. J. Clin. Neonatol. 2013;2:78–82. doi: 10.4103/2249-4847.116406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.La Russa R., Maiese A., Viola R.V., De Matteis A., Pinchi E., Frati P., Fineschi V. Searching for highly sensitive and specific biomarkers for sepsis: State-of-the-art in post-mortem diagnosis of sepsis through immunohistochemical analysis. Int. J. Immunopathol. Pharmacol. 2019;33:2058738419855226. doi: 10.1177/2058738419855226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.MMaiese A., Del Nonno F., Dell’Aquila M., Moauro M., Baiocchini A., Mastracchio A., Bolino G. Postmortem diagnosis of sepsis: A preliminary immunohistochemical study with an anti-procalcitonin antibody. Leg Med. 2017;28:1–5. doi: 10.1016/j.legalmed.2017.07.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.