Abstract
A number of studies have investigated the potential on-specific effects of some routinely administered vaccines (e.g., influenza, pneumococcal) on COVID-19 related outcomes, with contrasting results. In order to elucidate this discrepancy, we conducted a systematic review and meta-analysis to assess the association between seasonal influenza vaccination and pneumococcal vaccination with SARS-CoV-2 infection and its clinical outcomes. PubMed and medRxiv databases were searched up to April 2022. A random effects model was used in the meta-analysis to pool odds ratio (OR) and adjusted estimates with 95% confidence intervals (CIs). Heterogeneity was quantitatively assessed using the Cochran’s Q and the I2 index. Subgroup analysis, sensitivity analysis and assessment of publication bias were performed for all outcomes. In total, 38 observational studies were included in the meta-analysis and there was substantial heterogeneity. Influenza and pneumococcal vaccination were associated with lower risk of SARS-CoV-2 infection (OR: 0.80, 95% CI: 0.75–0.86 and OR: 0.70, 95% CI: 0.57–0.88, respectively). Regarding influenza vaccination, it seems that the majority of studies did not properly adjust for all potential confounders, so when the analysis was limited to studies that adjusted for age, gender, comorbidities and socioeconomic indices, the association diminished. This is not the case regarding pneumococcal vaccination, for which even after adjustment for such factors the association persisted. Regarding harder endpoints such as ICU admission and death, current data do not support the association. Possible explanations are discussed, including trained immunity, inadequate matching for socioeconomic indices and possible coinfection.
Keywords: influenza vaccine, pneumococcal vaccine, SARS-CoV-2, systematic review, meta-analysis
1. Introduction
In 2020 in Wuhan, China, cases of pneumonia were reported [1], caused by SARS-CoV-2, which is a coronavirus (CoV), and which lead to a pandemic outbreak. The coronaviruses belong in the Nidovirales order and in the Coronaviridae family, which has four genera divisions, the alpha, beta, gamma, and delta coronaviruses. All coronaviruses contain very large genomes, are enveloped, and are non-segmented positive-sense ribonucleic acid (RNA) viruses [2]. Coronaviruses in humans mainly cause respiratory diseases, and there are many viruses in this family, with a large spectrum of diseases from the less-severe common cold to serious and highly fatal diseases such as the Severe Acute Respiratory Syndrome (SARS) and the Middle East Respiratory Syndrome (MERS). SARS-CoV-2 is a betacoronavirus in the same subgenus as SARS-CoV-2 and shares RNA with it as well as with the MERS-CoV [3]. The novel coronavirus that we are facing can cause severe disease, a syndrome termed COVID-19. As of 1 April 2022 there are a total of 486,761,597 confirmed cases and 6,142,735 confirmed deaths worldwide [4].
Seasonal influenza is another acute respiratory infection caused by the influenza viruses. Influenza viruses are part of the Orthomyxoviridae family, in which there are three main genera: A, B and C [5]. They are RNA viruses with segmented, negative-strand genomes. The diseases caused by influenza range from mild to more severe and even include death in high-risk groups [6]. As one can see, both diseases are respiratory viral diseases, they co-exist at the same period, and they can be clinically indistinguishable (fever, cough, nasal congestion or rhinorrhea, myalgia etc.) [7].
Another respiratory infection is pneumonia. The main causes of pneumonia are viruses, bacteria and fungi. One of the most common bacteria which cause pneumonia (mainly in children) is Streptococcus pneumonia [8]. Some studies have shown the co-infection of COVID-19 and influenza or COVID-19 and pneumonia [9].
One step taken towards the end of the pandemic was vaccination. There are ten vaccines that have been approved by the World Health Organization (WHO) [10]. Many non-SARS-CoV-2 vaccines have been tested for preventing SARS-CoV-2 infection or having a protective effect with regard to the severity of the disease. The booster Bacillus Calmette-Guérin (BCG) vaccine was suggested, by many studies and even by a meta-analysis, to have beneficial effects on preventing COVID-19 infection [11,12,13]. Some preliminary studies suggest some protection against SARS-CoV-2 to be conferred from vaccination to other pathogens such as influenza [14], while others have produced contrasting results [15]. Additionally, some researchers suggest that influenza vaccination would help to lighten the burden on health systems and would decrease the transmission probability of COVID-19 [16]. Moreover, some studies have shown a bacteria-virus synergy that could potentially influence the COVID-19 related outcomes [17]. Finally, Venkatakrishnan and coworkers went even further, suggesting that the mutational profile of the SARS-CoV-2 Omicron variation could have been acquired by pattern switching between the SARS-CoV-2 and the viruses that coinfect the same host cells [18]. Due to the aforementioned reasons, we conducted a systematic review and meta-analysis to assess the overall association between influenza and pneumococcal vaccination and SARS-CoV-2 infection and to investigate the potential factors that influence the contradictory results.
2. Materials and Methods
A comprehensive search was performed to identify papers that investigated influenza and pneumococcal vaccination and SARS-CoV-2 infection and severity of the disease. PubMed [19] and medRxiv [20] were investigated for relevant research articles.
The search terms for influenza vaccination were: (“Influenza Vaccines” OR “influenza vaccine” OR “flu vaccine” OR “anti-flu vaccine”) AND (“SARS-CoV-2” OR “covid-19” OR “2019-ncov” OR “2019nconv” OR “sars-cov2” OR “COVID-19”) in PubMed and “influenza vaccine” (abstract or title, match all words) in medRxiv. For pneumococcal vaccination, the terms used were: (“pneumococcal Vaccines” OR “pneumococcal vaccine”) AND (“SARS-CoV-2” OR “COVID-19” OR “2019-ncov” OR “2019nconv” OR “sars-cov2” OR “COVID-19”) in PubMed and “pneumococcal vaccine” (abstract or title, match all words) in medRxiv. To avoid selection bias, no filter was applied in language, year, method, etc. This systematic review was performed according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [21], the STROBE Statement checklist [22], and other general guidelines [23]. The search was performed up to 30 April 2022.
The information that was extracted from the selected studies was author, title, year of publication, country in which it was conducted, study design, study population, sample size, gender, comorbidities, COVID-19 outcome, the effect size related to relevant outcomes (e.g., OR, HR, RR), and other potentially relevant information. Since the risk for COVID-19 varies largely according to risk factors, studies not including adjusted odds ratio (OR), adjusted hazard ratio (HR) or adjusted relative risk (RR) as their effect size were excluded. We requested that the effect sizes were adjusted mainly for gender, age, race, comorbidities, smoking status, etc.
The main inclusion criteria were (a) vaccination period for influenza or pneumococcal from autumn 2019 until autumn 2021; (b) confirmed COVID-19 infection with either real-time PCR or serologic test, or according to WHO definition or laboratory confirmation (not specified); (c) participants who were adults; and (d) availability of an adjusted estimate of OR, HR or RR (see above).
In order to assess the association between the two vaccines (influenza and pneumococcal) and COVID-19 outcome (infection, death, hospitalization, etc.), a random effects meta-analysis was performed for each outcome, using the adjusted OR, or adjusted RR/HR, combined with their 95% confidence interval (CI) as effect size [24].The between studies heterogeneity of the pooled estimates was quantified by means of the chi-square-based Cochran’s Q statistic and the consistency index (I2) [25]. To assess publication bias, the rank correlation method of Begg and Mazumdar [26], and the fixed effects regression method of Egger [27] were applied. Subgroup analyses were performed to investigate the effect of various study-level characteristics. Sensitivity analyses were performed by omitting one study at a time to assess studies with a notable impact and examine the robustness of the overall effect.
For all statistical analyses performed, the statistical software package STATA 13 [28] was used and results with p-value < 0.05 were considered statistically significant.
3. Results
3.1. Study Selection and Study Characteristics
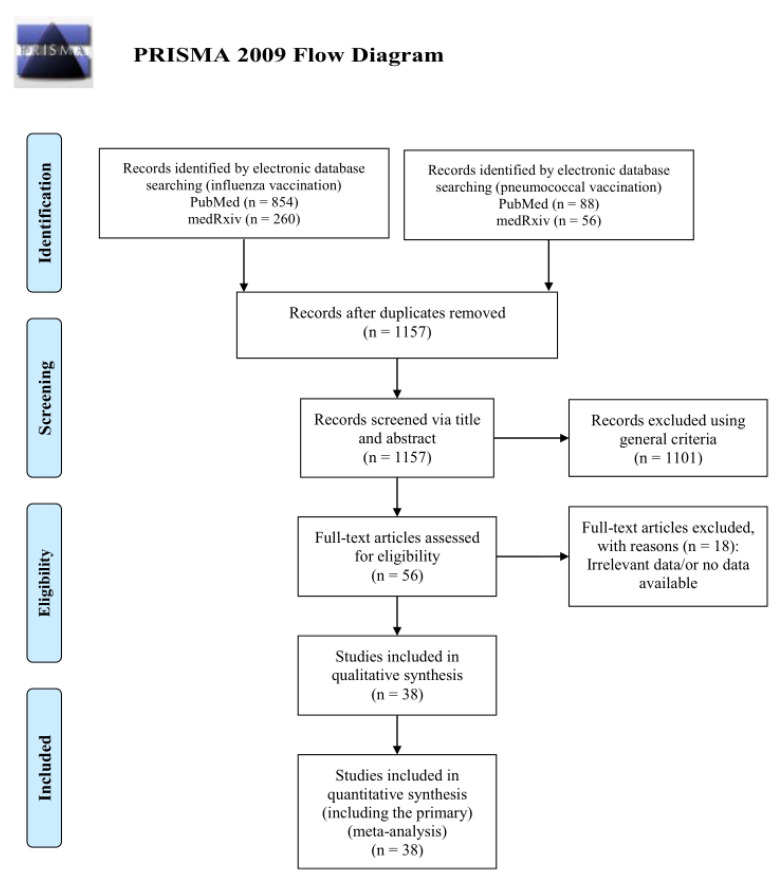
The literature search from the two databases identified a total of 1114 studies related to the influenza vaccination and 144 records for the pneumococcal vaccination search. After reading the titles and excluding duplicates, 1020 and 137 unique publications remained for influenza and pneumococcal vaccination, respectively. The simultaneous titles and abstracts review led to a further rejection of 1101 publications. The remaining 56 articles were read in full copy, as well as the citations of the retrieved articles. Finally, 38 publications met all the inclusion criteria for the quantitative analysis and were included in the final systematic review and meta-analysis. The PRISMA flow diagram of the study selection procedure is shown in Figure 1.
Figure 1.
The PRISMA flow diagram for the included studies.
Among the included publications, 22 studies focused on the association between influenza vaccination and the risk of SARS-CoV-2 infection and 10 studies on the association between pneumococcal vaccination and the risk of SARS-CoV-2 infection. These studies included a total of 55,917,587 individuals (COVID-19 patients and non-COVID patients).
Out of the 38 publications included in the meta-analysis, 19 publications assessed the association of the influenza vaccination and COVID-19 clinical outcomes. The different outcomes included: (a) need for hospitalization involving 15 studies with 55,881,321 individuals (13,123,605 vaccinated and 42,758,116 unvaccinated patients); (b) the administration of mechanical ventilation/invasive respiratory oxygen support described in four studies encompassing 255,735 patients; (c) intensive care unit admission. This outcome was assessed in 11 studies involving 446,924 vaccinated and not vaccinated patients and (d) mortality, which was assessed in 18 studies involving 407,409 vaccinated and not vaccinated COVID-19 patients. Moreover, four studies assessed the association between pneumococcal vaccination and hospitalization, including a total of 4310 patients, of which 1333 were vaccinated and 2977 were not vaccinated. Finally, three studies examined the association between pneumococcal vaccination and intensive care unit admission involving 1231 patients (921 non-vaccinated Vs 310 vaccinated). The baseline characteristics of the included studies are listed in Table 1 and Table S1 (influenza vaccination and SARS-CoV-2 infection), Table 2 and Table S2 (influenza vaccination and clinical outcomes), and Table 3 and Table S3 (pneumococcal vaccination and SARS-CoV-2 infection and clinical outcomes). Most studies enrolled individuals from the general population. Among them, five studies had health workers as a population study [29,30,31,32,33], one study involved only pregnant women [34], another study included only transplant recipients [35], and four studies were performed in older adults (age > 65) [36,37,38,39]. The laboratory method (rt-PCR) used to diagnose the disease was common in most of the included studies. The adjustment variables varied across studies with age, gender and comorbidities being the most common ones, reflecting the known COVID-19 risk factors. Studies which did not contain adjusted estimates (OR or RR/HR) as well as ecological studies were excluded from the meta-analysis and are summarized in Table S4.
Table 1.
Baseline characteristics of the 21 publications, involving 22 studies that assessed the association between influenza vaccination and SARS-CoV-2 infection.
| First Author’s Name | Year of Publication | Country | Identification of COVID-19 | Sample Size | Effect Size | Adjusted Estimate |
|---|---|---|---|---|---|---|
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 6061 | OR | 0.85 (95% CI: 0.74–0.98) |
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 619 | OR | 0.87 (95% CI: 0.59–1.28) |
| I. Martínez-Baz et al. [29] | 2020 | Spain | rt-PCR or Antibody Rapid test | 9745 | OR | 1.07(95% CI: 0.92–1.24) |
| P. Ragni et al. [40] | 2020 | Italy | rt-PCR | 17,608 | OR | 0.89 (95% CI: 0.80–0.99) |
| M. Belingheri et al. [31] | 2020 | Italy | rt-PCR | 3520 | OR | 0.41 (95% CI: 0.07–2.39) |
| A. Vila-Córcoles et al. [41] | 2020 | Spain | rt-PCR | 79,083 | HR | 1.02 (95% CI: 0.79–1.32) |
| I. Green et al. [42] | 2020 | Israel | rt-PCR | 19,089 | OR | 0.79 (95% CI: 0.67–0.98) |
| E. Kissling et al. [15] | 2021 | Europe | rt-PCR | 2147 | OR | 0.93 (95% CI: 0.66–1.32) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 27,201 | OR | 0.76 (95% CI: 0.68–0.86) |
| B. Erismis et al. [33] | 2021 | Turkey | Not specified | 203 | RR | 0.83 (95% CI: 0.75–0.93) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 2558 | HR | 0.74 (95% CI: 0.54–0.89) |
| M. Kowalska et al. [45] | 2021 | Poland | IgG antibodies | 5376 | OR | 0.68 (95% CI: 0.55–0.83) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 188 | OR | 1.70 (95% CI: 0.97–3.25) |
| K. Huang et al. [37] | 2021 | USA | Not specified | 55,667.997 | OR | 0.76 (95% CI: 0.75–0.77) |
| J. P. King et al. [47] | 2021 | USA | rt-PCR | 1356 | OR | 0.83 (95% CI: 0.63–1.10) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 12,791 | RR | 0.85 (95% CI: 0.75–0.96) |
| S. Caratozzolo et al. [36] | 2020 | Italy | rt-PCR/or infection according to WHO definition | 848 | OR | 0.47 (95% CI: 0.29–0.74) |
| M. N. Rivas et al. [32] | 2021 | USA | IgG antibodies | 6087 | OR | 1.84 (95% CI: 0.57–11.3) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 13,220 | OR | 0.79 (95% CI: 0.62–1.00) |
| P. A. Debisarun et al. [50] | 2020 | Netherlands | rt-PCR | 6856 | RR | 0.61 (95% CI: 0.46–0.82) |
| Y. Xiang et al. [51] | 2020 | UK | Laboratory confirmed | 30,835 | OR | 0.60 (95% CI: 0.53–0.68) |
| M. Alkathlan et al. [52] | 2021 | Saudi Arabia | Laboratory confirmed | 424 | OR | 0.83 (95% CI: 0.51–1.35) |
OR = adjusted odds ratio, HR = hazard odds ratio, RR = relative risk, rt-PCR = Reverse transcription polymerase chain reaction.
Table 2.
Baseline characteristics of the 17 publications involving studies that assessed the association between influenza vaccination and COVID-19 clinical outcomes: (15 studies for hospitalization, four for Mechanical Ventilation/invasive respiratory support, 11 for Intensive care and 18 for mortality).
| First Author’s Name | Year of Publication | Country | Identification of COVID-19 | Sample Size | Effect Size | Adjusted Estimate |
|---|---|---|---|---|---|---|
| Hospitalization | ||||||
| P. D. Pedote et al. [53] | 2021 | Italy | rt-PCR | 662 | OR | 1.20 (95% CI: 0.70–1.90) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 741 | OR | 1.03 (95% CI: 0.66–1.62) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 959 | RR | 1.10 (95% CI: 0.83–1.50) |
| M. Gobbato et al. [54] | 2020 | Italy | Laboratory confirmed | 3010 | OR | 0.78 (95% CI: 0.61–1.01) |
| M-J. Yang et al. [55] | 2020 | USA | Laboratory confirmed | 2005 | OR | 0.41 (95% CI: 0.28–0.59) |
| S. Greco et al. [56] | 2021 | Italy | rt-PCR | 952 | OR | 1.44 (95% CI: 1.01–2.05) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 151 | HR | 0.48 (95% CI: 0.36–0.77) |
| K. Huang et al. [37] | 2021 | USA | Not specified | 55,667,977 | OR | 0.76 (95% CI: 0.75–0.77) |
| M. Massari et al. [57] | 2021 | Italy | rt-PCR | 115,945 | RR | 0.87 (95% CI: 0.86–0.88) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | OR | 0.58 (95% CI: 0.46–0.73) |
| P. Ragni et al. [40] | 2020 | Italy | rt-PCR | 4485 | HR | 1.00 (95% CI: 0.84–1.19) |
| C. R. Wilcox et al. [58] | 2021 | UK | rt-PCR | 6921 | HR | 0.85 (95% CI: 0.75–0.97) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 1434 | OR | 1.29 (95% CI: 0.72–2.31) |
| I. Ilic et al. [59] | 2020 | Serbia | rt-PCR | 107 | OR | 1.31 (95% CI: 0.54–3.17) |
| S. M. Taghioff et al. [60] | 2021 | Netherlands | Not specified | 74,754 | OR | 1.07 (95% CI: 0.96–1.18) |
| Mechanical Ventilation/Invansive Respiratory Support | ||||||
| G. Fink et al. [61] | 2020 | Brazil | rt-PCR | 39,745 | OR | 0.83 (95% CI: 0.77–0.89) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | OR | 0.45 (95% CI: 0.27–0.78) |
| A. E. Demkina et al. [62] | 2020 | Russia | Clinically confirmed/ rt-PCR |
214,751 | OR | 0.74 (95% CI: 0.54–1.01) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 21 | OR | 0.42 (95% CI: 0.02–9.96) |
| Intensive Care | ||||||
| M-J. Yang et al. [55] | 2021 | USA | rt-PCR | 2005 | OR | 0.31 (95% CI: 0.07–0.85) |
| M. Massari et al. [57] | 2021 | Italy | rt-PCR | 111,740 | RR | 1.01 (95% CI: 0.99–1.04) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 99 | OR | 1.26 (95% CI: 0.74–2.21) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | OR | 0.64 (95% CI: 0.41–1.00) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 959 | RR | 1.10 (95% CI: 0.56–2.20) |
| G. Fink et al. [61] | 2020 | Brazil | rt-PCR | 39,156 | OR | 0.93 (95% CI: 0.87–0.99) |
| A. E. Demkina et al. [62] | 2020 | Russia | Clinically confirmed/ rt-PCR |
214,751 | OR | 0.76 (95% CI: 0.59–0.97) |
| M. Candelli et al. [63] | 2021 | Italy | rt-PCR | 602 | OR | 0.73 (95% CI: 0.35–1.56) |
| M. L. de la Cruz Contyet al. [34] | 2021 | Spain | rt-PCR | 206 | OR | 1.92 (95% CI: 0.36–10.3) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 1434 | OR | 0.65 (95% CI: 0.22–1.79) |
| S. M. Taghioff et al. [60] | 2021 | Netherlands | Not specified | 74,754 | OR | 1.18 (95% CI: 1.00–1.39) |
| Mortality | ||||||
| S. Greco et al. [56] | 2021 | Italy | rt-PCR | 952 | OR | 1.06 (95% CI: 0.60–1.88) |
| P. D. Pedote et al. [53] | 2021 | Italy | rt-PCR | 662 | OR | 1.60 (95% CI: 0.80–3.20) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 97 | OR | 1.33 (95% CI: 0.77–2.31) |
| M. Massari et al. [57] | 2021 | Italy | rt-PCR | 115,945 | RR | 1.04 (95% CI: 1.01–1.06) |
| J. M. Fernadez. Ibánez et al. [64] | 2021 | Spain | rt-PCR | 410 | OR | 1.55 (95% CI: 0.96–2.48) |
| A. Bozek et al. [44] | 2021 | Poland | rt-PCR | 2558 | HR | 0.74 (95% CI: 0.03–20.8) |
| M. Candelli et al. [63] | 2021 | Italy | rt-PCR | 602 | OR | 0.20 (95% CI: 0.08–0.51) |
| A. Conlon et al. [43] | 2021 | USA | rt-PCR | 1218 | HR | 0.94 (95% CI: 0.61–1.47) |
| P. Ragni et al. [40] | 2020 | Italy | rt-PCR | 4872 | HR | 1.14 (95% CI: 0.95–1.37) |
| C. R Wilcox et al. [58] | 2021 | UK | rt-PCR | 6368 | OR | 0.76 (95% CI: 0.64–0.90) |
| G. Fink et al. [61] | 2020 | Brazil | rt-PCR | 53,752 | OR | 0.84 (95% CI: 0.78–0.91) |
| A. E. Demkina et al. [62] | 2020 | Russia | Clinically confirmed/ rt-PCR |
117,346 | HR | 0.78 (95% CI: 0.63–0.95) |
| Y. Azzi et al. [35] | 2020 | USA | rt-PCR | 229 | OR | 0.88 (95% CI: 0.70–0.96) |
| M. Gobbato et al. [54] | 2020 | Italy | Laboratory confirmed | 3010 | OR | 0.78 (95% CI: 0.61–1.01) |
| J. G. Zein et al. [49] | 2020 | USA | Not specified | 14,654 | OR | 0.98 (95% CI: 0.39–2.43) |
| D. Giannoglou et al. [65] | 2020 | Greece | Not specified | 512 | OR | 0.38 (95% CI: 0.17–0.81) |
| E. Ortiz-Prado et al. [66] | 2020 | Ecuador | rt-PCR | 9468 | RR | 1.40 (95% CI: 0.46–4.28) |
| S. M. Taghioff et al. [57] | 2021 | Netherlands | Not specified | 74,754 | OR | 0.89 (95% CI: 0.77–1.03) |
Table 3.
Baseline characteristics of (a) six publications, involving 10 studies, that assessed the association between pneumococcal vaccination and SARS-CoV-2 infection, (b) four studies that assessed the association between pneumococcal vaccination and the risk of hospitalization, and (c) three studies that assessed the association between pneumococcal vaccination and the risk of requiring intensive care.
| First Author’s Name | Year of Publication | Country | Identification of COVID-19 | Sample Size | Effect Size | Adjusted Estimate |
|---|---|---|---|---|---|---|
| Infection | ||||||
| A. Vila-Córcoles et al. [41] | 2020 | Spain | rt-PCR | 79,083 | HR | 1.02 (95% CI0.78–1.33) |
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 6061 | OR | 0.61 (95% CI0.41–0.91) |
| M. Noale et al. [38] | 2020 | Italy | rt-PCR | 619 | OR | 0.56 (95% CI0.33–0.95) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 4693 | RR | 0.72 (95% CI0.56–0.92) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 4636 | RR | 1.06 (95% CI0.81–137) |
| M. N. Rivas et al. [32] | 2021 | USA | IgG antibodies | 6083 | OR | 0.99 (95% CI0.71–1.36) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 188 | OR | 0.40 (95% CI0.17–1.01) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 187 | OR | 0.70 (95% CI: 0.28–2.10) |
| M. Fernández-Prada et al. [46] | 2021 | Spain | rt-PCR | 188 | OR | 0.20 (95% CI: 0.06–1.18) |
| Y. Xiang et al. [51] | 2020 | UK | Laboratory confirmed | 30,835 | OR | 0.5 (95% CI: 0.31–0.82) |
| Hospitalization | ||||||
| M. Gobbato et al. [54] | 2020 | Italy | Laboratory confirmed | 3010 | OR | 1.53 (95% CI:1.91–1.97) |
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 741 | OR | 0.96 (95% CI: 0.53–1.78) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 277 | RR | 1.30 (95% CI: 0.86–2.10) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 282 | RR | 1.20 (95% CI: 0.71–2.20) |
| Intensive Care Unit | ||||||
| R. Pastorino et al. [39] | 2021 | Italy | rt-PCR | 741 | OR | 0.75 (95% CI: 0.35–1.61) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 254 | RR | 1.40 (95% CI: 0.48–3.90) |
| C. Pawlowski et al. [48] | 2021 | USA | rt-PCR | 236 | RR | 1.10 (95% CI: 0.46–2.40) |
3.2. The Association between Influenza Vaccination and SARS-CoV-2 Infection
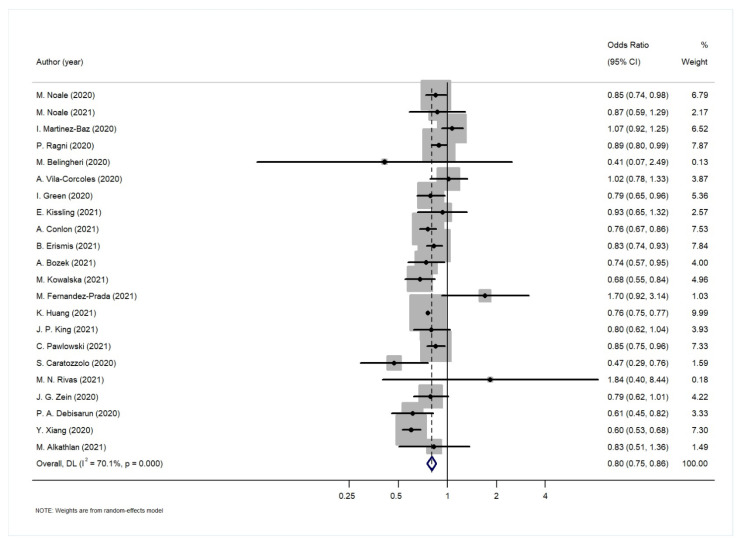
The results of the random effects analysis are summarized in Table 4. The association of the influenza vaccination and SARS-CoV-2 infection is presented graphically in the forest plot in Figure 2. Influenza vaccination is shown to be associated with lower risk of SARS-CoV-2 infection (random effects model: pooled estimate 0.80, 95% CI: 0.75–0.86). The results of the statistical tests for publication bias (Begg’s, Egger’s test) are shown in Table 4. Both tests showed no evidence of publication bias for the pooled estimates of the association between influenza vaccination and SARS-CoV-19 infection (p-value > 0.05). In the leave-one-out sensitivity analyses regarding the association between influenza vaccination and SARS-CoV-2 infection, the results showed that no individual study influenced the overall effect estimate.
Table 4.
Effect sizes, heterogeneity statistics and results for publication bias on the association between influenza vaccination, SARS-CoV-2 infection as well as clinical outcomes.
| Outcomes | No. of Studies | Adjusted Estimates (95% CI) | p-Value | I2 Value (%) | Bias (p-Value) |
|---|---|---|---|---|---|
| SARS-CoV-2 infection | 22 | 0.80 (0.75–0.86) | <0.01 | 70.1 | Begg’s test: 0.82 Egger’s test: 0.19 |
| Hospitalization | 15 | 0.88 (0.81–0.95) | <0.01 | 95.1 | Begg’s test: 0.55 Egger’s test: 0.62 |
| Mechanical Ventilation/invasive respiratory support | 4 | 0.73 (0.58–0.92) | 0.01 | 42.4 | Begg’s test: 0.73 Egger’s test: 0.21 |
| Intensive care unit admission | 11 | 0.96 (0.88–1.06) | 0.40 | 86.0 | Begg’s test: 0.76 Egger’s test: 0.14 |
| Mortality | 18 | 0.90 (0.81–1.01) | 0.07 | 78.2 | Begg’s test: 0.82 Egger’s test: 0.07 |
Figure 2.
Forest plot for the association between influenza vaccination and SARS-Cov-2 infection.
The between-studies heterogeneity was substantial with I2 = 70.1%. In order to investigate the high degree of heterogeneity, we performed a subgroup analysis investigating the potential effect of study-level characteristics (study design, study population, effect size, adjustment factors, method for assessment of COVID-19, and continent). However, none of these factors seemed to play an important role, since in all cases the results of the subgroup analysis are quite close to the overall estimate (Table 5). It is worth noting, however, that the association was diminished when the analysis was restricted to the six studies that adjusted for age, gender, comorbidities and some indices of socioeconomic status (such as residence, income or education).
Table 5.
Subgroup analysis of the association between influenza vaccination and SARS-CoV-2 infection.
| Subgroup Variables | No. of Studies | Adjusted Estimate (95% CI) | p-Value | I2 Value (%) |
|---|---|---|---|---|
| Study Design | ||||
| cross sectional study | 5 | 0.76 (0.75–0.77) | <0.01 | 0.00 |
| retrospective cohort study | 10 | 0.80 (0.75–0.86) | <0.01 | 41.5 |
| prospective cohort study | 4 | 0.80 (0.56–1.08) | 0.14 | 90.9 |
| case-control study | 3 | 0.99 (0.76–1.23) | 0.93 | 51.7 |
| Study Population | ||||
| adults-general population | 13 | 0.80 (0.74–0.87) | <0.01 | 68.4 |
| older adults | 3 | 0.72 (0.56–0.92) | 0.01 | 53.8 |
| health workers | 6 | 0.84 (0.68–1.04) | 0.12 | 66.6 |
| Effect Size | ||||
| OR | 17 | 0.80 (0.74–0.87) | <0.01 | 72.5 |
| RR/HR | 5 | 0.82 (0.73–0.92) | <0.01 | 46.4 |
| Adjustment Factors | ||||
| age/gender/age and gender | 5 | 0.86 (0.62–1.21) | 0.40 | 63.5 |
| age, gender and comorbidities | 9 | 0.82 (0.73–0.92) | <0.01 | 78.4 |
| age, gender, comorbidities, and socioeconomic status, education or residence | 6 | 0.87 (0.68–1.11) | 0.26 | 81.8 |
| COVID-19 test | ||||
| rt-PCR | 15 | 0.84 (0.77–0.92) | <0.01 | 57.7 |
| IgG/IgM antibodies | 2 | 0.83 (0.38–1.82) | 0.64 | 37.9 |
| Laboratory confirmed (not specified) | 5 | 0.74 (0.66–0.83) | <0.01 | 74.9 |
| Continent | ||||
| Europe | 13 | 0.81 (0.70–0.93) | <0.01 | 79.7 |
| N. America | 6 | 0.76 (0.75–0.77) | <0.01 | 0.00 |
| Asia | 3 | 0.83 (0.77–0.90) | <0.01 | 0.00 |
Moreover, two studies (Debisarum et al. [50], Erismis et al. [33]) were scrutinized further, as in their analysis they used only age and gender as adjustment variables. However, when both were excluded from the meta-analysis and the overall meta-analysis was repeated, the results did not differ from the overall estimate: OR 0.81 (95% CI: 0.75–0.87).
3.3. The Association between Influenza Vaccination and COVID19 Clinical Outcomes
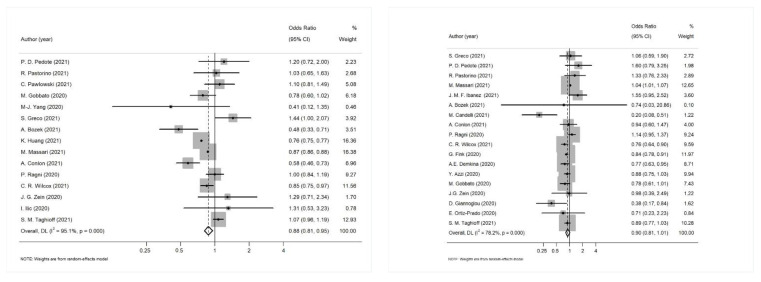
The results of the meta-analysis that was conducted separately for each clinical outcome of interest are shown in Table 4. The results suggest a potential association between influenza vaccination and hospitalization (OR: 0.88, 95% CI: 0.81–0.96) and mechanical ventilation/invasive respiratory support (OR: 0.73, 95% CI: 0.58–0.92), but not with ICU admission (OR: 0.96, 95% CI: 0.88–1.06) and mortality (OR: 0.90, 95% CI: 0.81–1.01). Both Begg’s and Egger’s tests showed no evidence of publication bias for the pooled estimates of the association between influenza vaccination and COVID-19 related clinical outcomes (all p-values > 0.05). The summary of the publication bias tests is presented in Table 4. Figure 3 represents the forest plot for the association between influenza vaccination and COVID-19 related hospitalization and between influenza vaccination and mortality.
Figure 3.
(Left): Forest plot for the association between influenza vaccination and COVID-19 related hospitalization; (Right): Forest plot for the association between influenza vaccination and mortality.
In the sensitivity analyses that followed, the pooled estimates were consistent when any single study was omitted. A subgroup analysis was performed to explain the between studies heterogeneity for the association of influenza vaccination and COVID-19 clinical outcomes (Table 6). Study design was not used as a variable in this subgroup analysis, as the majority of the studies were cohort studies with the exception of one cross-sectional study in the hospitalization outcome and two cross-sectional study investigating the mortality outcome. Study population was also not used, as the majority of the studies involved adults from the general population. Moreover, due to the limited studies reporting adjusted measures of the effect of the association between influenza vaccination and the need for mechanical ventilation, a subgroup analysis could not be performed in this case.
Table 6.
Subgroup analysis of the association between influenza vaccination and COVID-19 clinical outcomes.
| Subgroup Variables | No. of Studies | Adjusted Estimate (95% CI) | p-Value | I2 Value (%) |
|---|---|---|---|---|
| Hospitalization | ||||
| Effect Size | ||||
| OR | 10 | 0.92 (0.78–1.02) | 0.40 | 86.6 |
| RR/HR | 5 | 0.87 (0.71–0.99) | 0.03 | 71.0 |
| Adjusted Factors | ||||
| age/gender/age and gender | 1 | 1.44 (1.00–2.07) | 0.05 | 89.8 |
| age, gender and comorbidities | 7 | 0.85 (0.71–1.03) | 0.49 | 66.3 |
| COVID-19 test | ||||
| rt-PCR | 10 | 0.89 (0.78–1.02) | 0.09 | 74.6 |
| Laboratory confirmed/ (not specified) |
5 | 0.88 (0.67–1.12) | 0.30 | 90.0 |
| Continent | ||||
| Europe | 10 | 0.93 (0.83–1.04) | 0.21 | 75.7 |
| N. America | 5 | 0.80 (0.63–1.02) | 0.07 | 73.1 |
| Intensive Care Unit | ||||
| Effect Size | ||||
| OR | 9 | 0.91 (0.76–1.08) | 0.26 | 55.9 |
| HR | 2 | 1.01 (0.99–1.04) | 0.43 | 0.0 |
| COVID-19 test | ||||
| rt-PCR | 9 | 0.93 (0.84–1.03) | 0.14 | 58.5 |
| Laboratory confirmed/ (not specified) |
2 | 1.12 (0.80–1.57) | 0.52 | 14.1 |
| Adjusted Factors | ||||
| age, gender and comorbidities | 4 | 0.87 (0.65–1.17) | 0.35 | 17.6 |
| Continent | ||||
| Europe | 5 | 1.05 (0.95–1.16) | 0.32 | 20.6 |
| N. America | 4 | 0.69 (0.47–1.02) | 0.06 | 10.3 |
| S. America | 1 | 0.93 (0.87–0.99) | 0.02 | - |
| Asia | 1 | 0.76 (0.59–0.98) | 0.03 | - |
| Mortality | ||||
| Effect Size | ||||
| OR | 12 | 0.87 (0.76–0.99) | 0.03 | 60.5 |
| HR | 6 | 0.99 (0.86–1.12) | 0.82 | 46.3 |
| COVID-19 test | ||||
| rt-PCR | 14 | 0.93 (0.82–1.06) | 0.27 | 80.3 |
| Laboratory confirmed/ (not specified) |
4 | 0.81 (0.65–1.00) | 0.05 | 37.1 |
| Adjusted Factors | ||||
| age/gender/age and gender | 3 | 1.07 (0.75–1.53) | 0.70 | 59.2 |
| age, gender and comorbidities | 8 | 0.82 (0.61–1.10) | 0.19 | 75.3 |
| Continent | ||||
| Europe | 12 | 0.94 (0.80–1.09) | 0.40 | 75.3 |
| N. America | 3 | 0.89 (0.76–1.03) | 0.12 | 0.0 |
| S. America | 2 | 0.84 (0.78–0.91) | <0.01 | 0.0 |
| Asia | 1 | 0.78 (0.63–0.95) | 0.02 | - |
3.4. The Association between Pneumococcal Vaccination with SARS-CoV-2 Infection and Its Clinical Outcomes
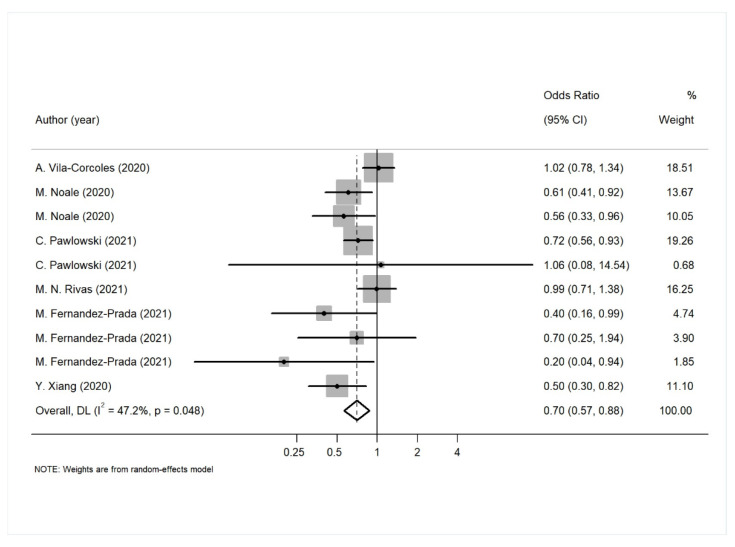
A meta-analysis of the association between pneumococcal vaccination with SARS CoV-2 infection, the need of hospitalization, and ICU admission was performed. As a result, pneumococcal vaccination was shown to be associated with a lower risk of COVID-19 infection (Figure 4, Table 7). The majority of the studies here (seven out of 10) provided estimates adjusted for age, gender, comorbidities and some indices of socioeconomic status (such as residence, income or education). The overall effect did not change, even when we restricted the analysis in the sample (OR: 0.61, 95% CI: 0.44–0.85).
Figure 4.
Forest plot for the association between pneumococcal vaccination and SARS-CoV-2 infection.
Table 7.
Effect sizes, heterogeneity statistics and results for publication bias of the association between pneumococcal vaccination and SARS-CoV-2 infection and outcomes.
| Outcomes | No. of Studies | Adjusted Estimates (95% CI) | p-Value | I2 value (%) | Bias (p-Value) |
|---|---|---|---|---|---|
| SARS-CoV-2 infection | 10 | 0.70 (0.57–0.88) | <0.01 | 47.2 | Begg’s test: 0.37 Egger’s test: 0.11 |
| Hospitalization | 4 | 1.47 (1.30–1.67) | <0.01 | 11.4 | Begg’s test: 0.09 Egger’s test: 0.05 |
| Intensive care unit admission | 3 | 0.99 (0.60- 1.64) | 0.96 | 0.0 | Begg’s test: 0.30 Egger’s test: 0.30 |
The results of the meta-analysis regarding pneumococcal vaccination and the need for hospitalization showed that the vaccine seems to be associated with a higher risk of COVID-19 hospitalization. However, only four studies investigated the particular association, and additionally there was evidence for publication bias in this analysis, making the results questionable. Finally, pneumococcal vaccination was not associated with the need of intensive care, but as before, only three studies investigated this association.
4. Discussion
Influenza and COVID-19 are different respiratory viral diseases that can be clinically indistinguishable and that can co-exist in the same period. Some preliminary studies suggest some protection against SARS-CoV-2 to be conferred from vaccination for other pathogens such as influenza [14], while others have produced a contrasting result [15]. There is also a limited number of studies that examined the association of the pneumococcal vaccine with the risk of developing SARS-CoV-2 infection and disease severity or risk of death on COVID-19 patients. In order to elucidate this discrepancy, we conducted a systematic review and meta-analysis to assess the association between seasonal influenza vaccination, pneumococcal vaccination and SARS-CoV-2 infection as well as with clinical outcomes related to COVID-19 morbidity and mortality. Our study followed all the available guidelines and ultimately included 38 publications that have evaluated the association of interest (24 studies on SARS-CoV-2 infection). Overall, our results indicate that influenza vaccination seems to be associated with lower risk for COVID-19 infection, as well as against hospitalization and mechanical ventilation, but no significant effect was found against “harder” endpoints like ICU admission and mortality. Specifically, the results of the meta-analysis showed that people vaccinated between autumn 2019 to autumn 2021 had up to a 20% lower risk of COVID-19 infection. Similarly, if vaccinated individuals were infected by SARS-CoV-2, the risk of hospitalization would be reduced by 12% and the need for mechanical ventilation by 27%. As far as the pneumococcal vaccination is concerned, the results show that it has a protective effect against SARS-CoV-2 infection, while it does not seem to have the same effect on COVID-19 hospitalization or ICU admission, although these analyses included a small number of studies.
During the last year, two systematic reviews and meta-analyses were published on the same topic, but the included studies were fewer than the ones included in our meta-analysis [67,68]. In the study of Wang et al. [67] a total of 9, 3, 2, and 3 studies were included for the association of influenza vaccination and SARS-CoV-2 infection, hospitalization, ICU admission and mortality, respectively. The authors stated in their results that “the association between influenza vaccination and COVID-19 clinical outcomes was not statistically significant by random effects model while the results by fixed effects model was somehow significant”. They attribute this result to “the substantial heterogeneity between the small number of studies and participants involved in each outcome”. In their meta-analysis, Zeynali Bujani et al. [68] included nine studies for the association of influenza vaccine and COVID-19, among which were two studies excluded from our meta-analysis. The particular studies of Jedi et al. [69] and Caban-Marntinez et al. [70], were excluded, as they reported effect sizes based on crude counts. Such data clearly violated our inclusion criteria, as we attempted to minimize the potential confounding by using only adjusted estimates. In addition, the study of Skowronski et al. [71] is a retrospective analysis from Canada that involved specimens collected during the 2010–2011 to 2016–2017 seasons, when specimens were tested for both influenza and non-influenza respiratory viruses (NIRVs), including some seasonal coronaviruses but not specific and exclusively SARS-CoV-2.
We performed the meta-analysis following strict criteria, and we included all the available data and investigated every study level covariate that may have influenced the outcome. Nevertheless, the between studies heterogeneity was high and the attempts to explain it using study-level covariates did not reveal anything of importance. In any case, heterogeneity is expected for many reasons that include the design of the study, the studied population, the sampling method, as well as the testing and vaccination policies in each country. In our analysis we included studies in which the seasonal influenza vaccination was administered between 2019 and 2021. The type of influenza vaccination and the exact date when the vaccines were administered was not specified in all studies, and as influenza varies across years, the results should be interpreted with caution regarding different influenza seasons. The testing policy implemented in each country also varied in the included studies, using different diagnostic tests. Most of the studies used molecular diagnosis with rt-PCR, and a few used antibody testing, whereas some studies did not specify the laboratory method used. Furthermore, we must consider that national influenza immunization policies can vary significantly from country to country. These differences arise from insufficient information on the relevance of influenza infection from a clinical, social and economic point of view [72].
Our findings suggest that both vaccines are associated with a lower risk of SARS-CoV-2 infection, but not with “harder” endpoints such as ICU admission and death. The influenza vaccine was additionally found to be associated with decreased risk for hospitalization and the need for mechanical ventilation. This association is counter-intuitive at first sight and hence needs to be explained. It is worth mentioning that the BCG vaccine, a vaccine primarily used against tuberculosis (TB),which is mandatory in several countries [33], seems also to have a protective effect since a meta-analysis of data from 160 countries has shown that when the BCG vaccine coverage was over 70% there was a reduction in the COVID-19 infections [12]. In addition, some studies have found that mandatory BCG vaccination is associated with a flattening of the curve in the spread of COVID-19 [73], and that differences in mortality produced by COVID-19 across countries are correlated with a country’s BCG vaccination policy [74,75]. However, these data come from ecological studies and thus need to be interpreted with caution.
Cross-reactivity is unlikely to exist between SARS-CoV-2 and influenza viruses, and thus other potential explanations should be considered. There is, for instance, some evidence that vaccines developed for a specific pathogen may have a wider role in protection against unrelated pathogens. Such cases include Bacillus Calmette-Guérin (BCG), measles and influenza [76]. The underlying mechanisms for such non-specific actions remain poorly understood, but a plausible suggested mechanism includes the induction of innate immune response following live attenuated vaccination in a way that is independent of memory cells (T or B cells). This type of immunity, termed “trained innate immunity”, may confer non-specific protection against different pathogens by inducing the upregulation of recognition receptors (such as toll-like receptors) and the secretion of proinflammatory cytokines (such as TNF-alpha and IL-6) in peripheral blood leucocytes and functional changes in natural killer cells [50,77].
Nevertheless, other more plausible explanations should not be ruled out. The studies included in this meta-analysis are heterogeneous in many respects including their design, the study population and the inclusion criteria. In terms of their design, all included studies were observational, so the risk of possible confounding should be considered. In such cases, when some important risk factors do exist (such as age, gender, and comorbidities), it would be unwise to perform the meta-analysis including crude estimates. However, even though we included only adjusted estimates, or studies with matched groups, the factors for which the adjustment were performed were not the same across studies. For instance, most studies adjusted for age and gender, others for several comorbidities (and even in this case, not for the same comorbidities), and some others for additional factors such as area of residence or socioeconomic status. In the subgroup analysis we tried to investigate this effect, but we were unable to find systematic differences across studies.
Socioeconomic factors, such as educational level, household size and income have been reported to have a significant effect on the probability of being vaccinated against influenza [78], whereas the same factors have been consistently shown to play a significant role in COVID-19 morbidity and mortality [79,80,81]. It is possible therefore to speculate that individuals who get vaccinated may pay more attention to their health status and lifestyle, and subsequently, they might have been more compliant to the various non-pharmaceutical intervention suggested for COVID-19 prevention, such as social distancing, wearing masks, hand hygiene practices and use of protective equipment, leading to a reduced potential risk of infection. Following the same rationale, since vaccinated individuals are under-represented in the lower socioeconomic categories (e.g., those who cannot work from home, those who need to use public transportation, or live in crowded households and so on), then these factors might explain the observed increased risk of getting COVID-19. This interpretation is also consistent with the results suggesting that vaccinated individuals are at increased risk of SARS-CoV-2 infection, but not at risk of ICU admission or death, since in these cases the comparison was made among those that were already infected, and the supposed confounding effect has been potentially removed.
Interestingly, when the analysis was restricted to the studies that adjusted for age, gender, comorbidities and some indices of socioeconomic status (such as area of residence, income or education), the association of influenza vaccination with the risk of SARS-CoV-2 infection was diminished. Thus, future and more carefully designed studies that will adjust more thoroughly for socioeconomic indices should be pursued to investigate this hypothesis. However, this observation does not seem to hold regarding the association of pneumococcal vaccination with SARS-CoV-2 infection, since the statistically significant association persists even after restricting the analysis in the studies that adjusted for the same factors. We need to mention that even though the studies regarding pneumococcal vaccination were fewer in total, the majority of them provided estimates adjusted for socioeconomic indices, which is in contrast to what is the case for studies of the influenza vaccine.
Most respiratory viral diseases may have as a complication bacterial coinfections, or secondary infections (super-infections), which can worsen the clinical outcome increasing thus morbidity and mortality. However, it has been argued that the proportion of COVID-19 patients with bacterial coinfections and/or super-infections may be lower compared to what we have seen in patients suffering from influenza [82,83,84]. Nevertheless, since vaccination is beneficial per se, it has been suggested that pneumococcal vaccination can, to some extent, provide additional protection to COVID-19 patients, reducing the morbidity and mortality [85]. Animal studies have also shown that an initial SARS-CoV-2 infection can increase susceptibility and pathogenicity to bacterial coinfection [86]. The limited number of available studies regarding the association of pneumococcal vaccination with hospitalization, ICU admission and death did not allow us to test this hypothesis, but as we said the association of vaccination with SARS-CoV-2 infection remained significant even after adjustment for gender, age, comorbidities and socioeconomic indices, and this should be investigated in future studies.
5. Conclusions
Overall, we conducted a systematic review and meta-analysis to assess the association between seasonal influenza vaccination and pneumococcal vaccination with SARS-CoV-2 infection and its clinical outcomes. Influenza and pneumococcal vaccination were found to be associated with a lower risk of SARS-CoV-2 infection. Regarding influenza vaccination, it seems that the majority of studies did not properly adjust for all potential confounders, so when the analysis was limited to studies that adjusted for age, gender, comorbidities and socioeconomic indices, the association diminished. This is not the case regarding the pneumococcal vaccination, for which the association persisted, even after adjustment for such factors. Regarding harder endpoints such as ICU admission and death, current data do not support the association. Possible explanations were discussed, including trained immunity, inadequate matching for socioeconomic indices and possible coinfection. Future more carefully conducted studies, at least regarding matching for socio-economic factors, should be undertaken.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics12123086/s1, Table S1. Baseline characteristics of the 21 publications, involving 22 studies that assessed the association between influenza vaccination and SARS-CoV-2 infection [15,29,31,32,33,36,37,38,40,42,43,44,45,46,47,48,49,50,51,52,60]. Table S2. Baseline characteristics of the 17 publications involving studies that assessed the association between influenza vaccination and COVID-19 clinical outcomes: (15 studies for hospitalization, 4 for Mechanical Ventilation/ invasive respiratory support, 11 for Intensive care and 18 for mortality) [34,35,37,39,40,43,44,48,49,53,54,55,56,57,58,59,60,61,62,63,64,65,66]. Table S3. Baseline characteristics of (a) 6 publications, involving 10 studies, that assessed the association between pneumococcal vaccination and SARS-CoV-2 infection, (b) 4 studies that assessed the association between pneumococcal vaccination and the risk of hospitalization and (c) 3 studies that assessed the association between pneumococcal vaccination and the risk of intensive care [32,38,39,41,46,48,51,54]. Table S4. Baseline characteristics of the studies excluded from the meta-analysis that assessed the association between influenza vaccination and SARS-CoV-2 infection or its outcome [30,36,69,70,87,88,89,90,91,92,93,94,95,96,97,98,99,100].
Author Contributions
P.G.B. conceived the study; G.V.K. and K.E.V. participated in data collection and performed the analysis; P.G.B., G.V.K. and K.E.V. participated in the interpretation of the results; All authors participated in drafting the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available in the tables.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maier H.J., Bickerton E., Britton P. Preface. Coronaviruses. Methods Mol. Biol. 2015;1282 doi: 10.1007/978-1-4939-2438-7. [DOI] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.WHO Origin of SARS-CoV-2. 2019. [(accessed on 20 May 2022)]. Available online: https://apps.who.int/iris/bitstream/handle/10665/332197/WHO-2019-nCoV-FAQ-Virus_origin-2020.1-eng.pdf.
- 5.Bouvier N.M., Palese P. The biology of influenza viruses. Vaccine. 2008;26((Suppl. 4)):D49–D53. doi: 10.1016/j.vaccine.2008.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Influenza (Seasonal) 2018. [(accessed on 6 May 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
- 7.Jiang C., Yao X., Zhao Y., Wu J., Huang P., Pan C., Liu S., Pan C. Comparative review of respiratory diseases caused by coronaviruses and influenza A viruses during epidemic season. Microbes Infect. 2020;22:236–244. doi: 10.1016/j.micinf.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO Pneumonia. [(accessed on 11 November 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia.
- 9.Xing Q.-S., Li G.-J., Xing Y.-H., Chen T., Li W.-J., Ni W., Deng K., Gao R.-Q., Chen C.-Z., Gao Y., et al. Precautions are Needed for COVID-19 Patients with Coinfection of Common Respiratory Pathogens. medRxiv. 2020 doi: 10.2139/ssrn.3550013. [DOI] [Google Scholar]
- 10.Basta N., Moodie E. COVID-19 Vaccine Tracker. [(accessed on 2 March 2022)]. Available online: https://covid19.trackvaccines.org/
- 11.Amirlak L., Haddad R., Hardy J.D., Khaled N.S., Chung M.H., Amirlak B. Effectiveness of booster BCG vaccination in preventing COVID-19 infection. Hum. Vaccines Immunother. 2021;7:1956228. doi: 10.1080/21645515.2021.1956228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Joy M., Malavika B., Asirvatham E.S., Sudarsanam T.D., Jeyaseelan L. Is BCG associated with reduced incidence of COVID-19? A meta-regression of global data from 160 countries. Clin. Epidemiol. Glob. Health. 2021;9:202–203. doi: 10.1016/j.cegh.2020.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolgikh S. Hospitalization Data Supports Correlation of Lower COVID-19 Severity vs. Universal BCG Immunization in the Early Phase of the Pandemic. medRxiv. 2021 doi: 10.1101/2021.07.21.21260913. [DOI] [Google Scholar]
- 14.Mosaddeghi P., Shahabinezhad F., Dorvash M., Goodarzi M., Negahdaripour M. Harnessing the non-specific immunogenic effects of available vaccines to combat COVID-19. Hum. Vaccines Immunother. 2021;17:1650–1661. doi: 10.1080/21645515.2020.1833577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kissling E., Hooiveld M., Brytting M., Vilcu A.M., de Lange M., Martínez-Baz I., Sigerson D., Enkirch T., Belhillil S., Meijer A., et al. Absence of association between 2019–2020 influenza vaccination and COVID-19: Results of the European I-MOVE-COVID-19 primary care project, March–August 2020. Influenza Other Respir. Viruses. 2021;15:429–438. doi: 10.1111/irv.12839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Q., Tang B., Bragazzi N.L., Xiao Y., Wu J. Modeling the impact of mass influenza vaccination and public health interventions on COVID-19 epidemics with limited detection capability. Math. Biosci. 2020;325:108378. doi: 10.1016/j.mbs.2020.108378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marotz C., Belda-Ferre P., Ali F., Das P., Huang S., Cantrell K., Jiang L., Martino C., Diner R.E., Rahman G. SARS-CoV-2 detection status associates with bacterial community composition in patients and the hospital environment. Microbiome. 2021;9:132. doi: 10.1186/s40168-021-01083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venkatakrishnan A., Anand P., Lenehan P.J., Suratekar R., Raghunathan B., Niesen M.J., Soundararajan V. Omicron variant of SARS-CoV-2 harbors a unique insertion mutation of putative viral or human genomic origin. J. Autoimmun. 2021;126:102779. [Google Scholar]
- 19.PubMed. [(accessed on 16 April 2022)];2005 Available online: https://pubmed.ncbi.nlm.nih.gov/
- 20.medRxiv. [(accessed on 26 April 2022)]. Available online: https://www.medrxiv.org/
- 21.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morton S.C., Costlow M.R., Graff J.S., Dubois R.W. Standards and guidelines for observational studies: Quality is in the eye of the beholder. J. Clin. Epidemiol. 2016;71:3–10. doi: 10.1016/j.jclinepi.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Forero D.A., Lopez-Leon S., González-Giraldo Y., Bagos P.G. Ten simple rules for carrying out and writing meta-analyses. PLoS Comput. Biol. 2019;15:e1006922. doi: 10.1371/journal.pcbi.1006922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DerSimonian R., Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. doi: 10.2307/2533446. [DOI] [PubMed] [Google Scholar]
- 27.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.StataCorp . Stata 13 Base Reference Manual. Stata Press; College Station, TX, USA: 2013. [Google Scholar]
- 29.Martinez-Baz I., Trobajo-Sanmartin C., Arregui I., Navascues A., Adelantado M., Indurain J., Fresan U., Ezpeleta C., Castilla J. Influenza Vaccination and Risk of SARS-CoV-2 Infection in a Cohort of Health Workers. Vaccines. 2020;8:611. doi: 10.3390/vaccines8040611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massoudi N., Mohit B. A Case-Control Study of the 2019 Influenza Vaccine and Incidence of COVID-19 among Healthcare Workers. J. Clin. Immunol. 2021;41:324–334. doi: 10.1007/s10875-020-00925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belingheri M., Paladino M.E., Latocca R., De Vito G., Riva M.A. Association between seasonal flu vaccination and COVID-19 among healthcare workers. Occup. Med. 2020;70:665–671. doi: 10.1093/occmed/kqaa197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rivas M.N., Ebinger J.E., Wu M., Sun N., Braun J., Sobhani K., Van Eyk J.E., Cheng S., Arditi M. BCG vaccination history associates with decreased SARS-CoV-2 seroprevalence across a diverse cohort of health care workers. J. Clin. Investig. 2021;131:JCI145157. doi: 10.1172/JCI145157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erismis B., Karabela S.N., Eksi F., Karandere F., Dogan B., Okay F., Filiz M., Kocoglu H., Issever H., Hursitoglu M., et al. Annual influenza vaccination effect on the susceptibility to COVID-19 infection. Cent. Eur. J. Public Health. 2021;29:14–17. doi: 10.21101/cejph.a6573. [DOI] [PubMed] [Google Scholar]
- 34.de la Cruz Conty M.L., Encinas Pardilla M.B., Garcia Sanchez M., Gonzalez Rodriguez L., Muner-Hernando M.L., Royuela Vicente A., Pintado Recarte P., Martinez Varea A., Martinez Diago C., Cruz Melguizo S., et al. Impact of Recommended Maternal Vaccination Programs on the Clinical Presentation of SARS-CoV-2 Infection: A Prospective Observational Study. Vaccines. 2021;9:31. doi: 10.3390/vaccines9010031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azzi Y., Parides M., Alani O., Loarte-Campos P., Bartash R., Forest S., Colovai A., Ajaimy M., Liriano-Ward L., Pynadath C., et al. COVID-19 infection in kidney transplant recipients at the epicenter of pandemics. Kidney Int. 2020;98:1559–1567. doi: 10.1016/j.kint.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caratozzolo S., Zucchelli A., Turla M., Cotelli M.S., Fascendini S., Zanni M., Bianchetti A., Psy M.P., Rozzini R., Boffelli S., et al. The impact of COVID-19 on health status of home-dwelling elderly patients with dementia in East Lombardy, Italy: Results from COVIDEM network. Aging Clin. Exp. Res. 2020;32:2133–2140. doi: 10.1007/s40520-020-01676-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang K., Lin S.W., Sheng W.H., Wang C.C. Influenza vaccination and the risk of COVID-19 infection and severe illness in older adults in the United States. Sci. Rep. 2021;11:11025. doi: 10.1038/s41598-021-90068-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Noale M., Trevisan C., Maggi S., Antonelli Incalzi R., Pedone C., Di Bari M., Adorni F., Jesuthasan N., Sojic A., Galli M., et al. The Association between Influenza and Pneumococcal Vaccinations and SARS-Cov-2 Infection: Data from the EPICOVID19 Web-Based Survey. Vaccines. 2020;8:471. doi: 10.3390/vaccines8030471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pastorino R., Villani L., La Milia D.I., Ieraci R., Chini F., Volpe E., Barca A., Fusco D., Laurenti P., Ricciardi W., et al. Influenza and pneumococcal vaccinations are not associated to COVID-19 outcomes among patients admitted to a university hospital. Vaccine. 2021;39:3493–3497. doi: 10.1016/j.vaccine.2021.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ragni P., Marino M., Formisano D., Bisaccia E., Scaltriti S., Bedeschi E., Grilli R. Association between Exposure to Influenza Vaccination and COVID-19 Diagnosis and Outcomes. Vaccines. 2020;8:675. doi: 10.3390/vaccines8040675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vila-Córcoles A., Ochoa-Gondar O., Satué-Gracia E.M., Torrente-Fraga C., Gomez-Bertomeu F., Vila-Rovira A., Hospital-Guardiola I., De Diego-Cabanes C., Bejarano-Romero F., Basora-Gallisà J. Influence of prior comorbidities and chronic medications use on the risk of COVID-19 in adults: A population-based cohort study in Tarragona, Spain. BMJ Open. 2020;10:e041577. doi: 10.1136/bmjopen-2020-041577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green I., Ashkenazi S., Merzon E., Vinker S., Golan-Cohen A. The association of previous influenza vaccination and coronavirus disease-2019. Hum. Vaccines Immunother. 2021;17:2169–2175. doi: 10.1080/21645515.2020.1852010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Conlon A., Ashur C., Washer L., Eagle K.A., Bowman M.A.H. Impact of the influenza vaccine on COVID-19 infection rates and severity. Am. J. Infect. Control. 2021;49:694–700. doi: 10.1016/j.ajic.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bozek A., Kozłowska R., Galuszka B., Grzanka A. Impact of influenza vaccination on the risk of SARS-CoV-2 infection in a middle-aged group of people. Hum. Vaccin. Immunother. 2021;17:3126–3130. doi: 10.1080/21645515.2021.1913961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kowalska M., Niewiadomska E., Barański K., Kaleta-Pilarska A., Brożek G., Zejda J.E. Association between Influenza Vaccination and Positive SARS-CoV-2 IgG and IgM Tests in the General Population of Katowice Region, Poland. Vaccines. 2021;9:415. doi: 10.3390/vaccines9050415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fernández-Prada M., García-González P., García-Morán A., Ruiz-Álvarez I., Ramas-Diez C., Calvo-Rodríguez C. Personal and vaccination history as factors associated with SARS-CoV-2 infection. Med. Clin. 2021;157:226–233. doi: 10.1016/j.medcli.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King J.P., McLean H.Q., Belongia E.A. Risk of symptomatic severe acute respiratory syndrome coronavirus 2 infection not associated with influenza vaccination in the 2019–2020 season. Influ. Other Respir. Viruses. 2021;15:697–700. doi: 10.1111/irv.12880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pawlowski C., Puranik A., Bandi H., Venkatakrishnan A.J., Agarwal V., Kennedy R., O’Horo J.C., Gores G.J., Williams A.W., Halamka J., et al. Exploratory analysis of immunization records highlights decreased SARS-CoV-2 rates in individuals with recent non-COVID-19 vaccinations. Sci. Rep. 2021;11:4741. doi: 10.1038/s41598-021-83641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zein G.J., Whelan G., Erzurum S.C. Safety of influenza vaccine during COVID-19. J. Clin. Transl. Sci. 2021;5 doi: 10.1017/cts.2020.543. [DOI] [Google Scholar]
- 50.Debisarun P.A., Struycken P., Domínguez-Andrés J., Moorlag S.J.C.F.M., Taks E., Gössling K.L., Ostermann P.N., Müller L., Schaal H., ten Oever J., et al. The effect of influenza vaccination on trained immunity: Impact on COVID-19. medRxiv. 2020 [Google Scholar]
- 51.Xiang Y., Wong K.C.-Y., So H.-C. Exploring Drugs and Vaccines Associated with Altered Risks and Severity of COVID-19: A UK Biobank Cohort Study of All ATC Level-4 Drug Categories Reveals Repositioning Opportunities. Pharmaceutics. 2021;13:1514. doi: 10.3390/pharmaceutics13091514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alkathlan M., Khalil R., Alhemaidani M.F., Alaed G.H., Almutairi S.M., A Almalki H., Alghofaili R.H., Al-Wutayd O. Trends, Uptake, and Predictors of Influenza Vaccination among Healthcare Practitioners during the COVID-19 Pandemic Flu Season (2020) and the Following Season (2021) in Saudi Arabia. J. Multidiscip. Healthc. 2021;14:2527–2536. doi: 10.2147/JMDH.S330029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pedote P.D., Termite S., Gigliobianco A., Lopalco P.L., Bianchi F.P. Influenza Vaccination and Health Outcomes in COVID-19 Patients: A Retrospective Cohort Study. Vaccines. 2021;9:358. doi: 10.3390/vaccines9040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gobbato M., Calagnan E., Burba I., Rizzi L., Grassetti L., Del Zotto S., Maso L.D., Serraiono D., Tonutti G. Clinical, demographical characteristics and hospitalisation of 3010 patients with COVID-19 in Friuli Venezia Giulia Region (Northern Italy). A multivariate, population-based, statistical analysis. Epidemiol. Prev. 2020;44:226–234. doi: 10.19191/EP20.5-6.S2.122. [DOI] [PubMed] [Google Scholar]
- 55.Yang M.-J., Rooks B.J., Le T.-T.T., Santiago I.O., Diamond J., Dorsey N.L., Mainous A.G. Influenza Vaccination and Hospitalizations among COVID-19 Infected Adults. J. Am. Board Fam. Med. 2021;34:S179–S182. doi: 10.3122/jabfm.2021.S1.200528. [DOI] [PubMed] [Google Scholar]
- 56.Greco S., Bella A., Bonsi B., Fabbri N., Califano A., Morrone S., Chessa P., Pistolesi C., Zuliani G., De Motoli F., et al. SARS-CoV-2 infection and H1N1 vaccination: Does a relationship between the two factors really exist? A retrospective analysis of a territorial cohort in Ferrara, Italy. Eur. Rev. Med. Pharmacol. Sci. 2021;25:2795–2801. doi: 10.26355/eurrev_202103_25441. [DOI] [PubMed] [Google Scholar]
- 57.Massari M., Spila-Alegiani S., Fabiani M., Belleudi V., Trifirò G., Kirchmayer U., Poggi F.R., Mancuso P., Menniti-Ippolito F., Gini R., et al. Association of Influenza Vaccination and Prognosis in Patients Testing Positive to SARS-CoV-2 Swab Test: A Large-Scale Italian Multi-Database Cohort Study. Vaccines. 2021;9:716. doi: 10.3390/vaccines9070716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcox C.R., Islam N., Dambha-Miller H. Association between influenza vaccination and hospitalisation or all-cause mortality in people with COVID-19: A retrospective cohort study. BMJ Open Respir. Res. 2021;8:e000857. doi: 10.1136/bmjresp-2020-000857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ilic I., Zdravkovic M., Timcic S., Stojanovic D.U., Bojic M., Loncar G. Pneumonia in medical professionals during COVID-19 outbreak in cardiovascular hospital. Int. J. Infect. Dis. 2021;103:188–193. doi: 10.1016/j.ijid.2020.11.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Taghioff S.M., Slavin B.R., Holton T., Singh D. Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 74,754 patients. PLoS ONE. 2021;16:e0255541. doi: 10.1371/journal.pone.0255541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fink G., Orlova-Fink N., Schindler T., Grisi S., Ferrer A.P.S., Daubenberger C., Brentani A. Inactivated trivalent influenza vaccination is associated with lower mortality among patients with COVID-19 in Brazil. BMJ Evid.-Based Med. 2021;26:192–193. doi: 10.1136/bmjebm-2020-111549. [DOI] [PubMed] [Google Scholar]
- 62.Demkina A.E., Morozov S.P., Vladzymyrskyy A.V., Kljashtorny V.G., Guseva O.I., Pugachev P.S., Artemova O.R., Reshetnikov R.V., Gombolevskiy V.A., Ryabinina M.N. Risk factors for outcomes of COVID-19 patients: An observational study of 795,572 patients in Russia. medRxiv. 2020 [Google Scholar]
- 63.Candelli M., Pignataro G., Torelli E., Gullì A., Nista E.C., Petrucci M., Saviano A., Marchesini D., Covino M., Ojetti V., et al. Effect of influenza vaccine on COVID-19 mortality: A retrospective study. Intern. Emerg. Med. 2021;16:1849–1855. doi: 10.1007/s11739-021-02702-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibáñez J.M.F., Ballesteros M.D.C.M., Anguita M.J.F., Andúgar M.G., Arias A., Barberá-Farré J.R. Influence of influenza vaccine and comorbidity on the evolution of hospitalized COVID-19 patients. Med. Clin. 2022;158:603–607. doi: 10.1016/j.medcli.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giannoglou D., Meimeti E., Provatopoulou X., Stathopoulos K., Roukas I.K., Galanis P. Predictors of mortality in hospitalized COVID-19 patients in Athens, Greece. medRxiv. 2020 [Google Scholar]
- 66.Ortiz-Prado E., Simbaña-Rivera K., Barreno L.G., Diaz A.M., Barreto A., Moyano C., Arcos V., Vásconez-González E., Paz C., Simbaña-Guaycha F., et al. Epidemiological, socio-demographic and clinical features of the early phase of the COVID-19 epidemic in Ecuador. PLOS Negl. Trop. Dis. 2021;15:e0008958. doi: 10.1371/journal.pntd.0008958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang R., Liu M., Liu J. The Association between Influenza Vaccination and COVID-19 and Its Outcomes: A Systematic Review and Meta-Analysis of Observational Studies. Vaccines. 2021;9:529. doi: 10.3390/vaccines9050529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bujani M.Z., Behnampour M., Rahimi N., Safari T., Feizabad A.K., Sarbazi A.H., Baniasadi M., Rezaei N., Moghaddam A.A. The Effect of Influenza Vaccination on COVID-19 Morbidity, Severity and Mortality: Systematic Review and Meta-Analysis. Malays. J. Med. Sci. 2021;28:20–31. doi: 10.21315/mjms2021.28.6.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jehi L., Ji X., Milinovich A., Erzurum S., Rubin B.P., Gordon S., Young J.B., Kattan M.W. Individualizing Risk Prediction for Positive Coronavirus Disease 2019 Testing: Results From 11,672 Patients. Chest. 2020;158:1364–1375. doi: 10.1016/j.chest.2020.05.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caban-Martinez A.J., Schaefer-Solle N., Santiago K., Louzado-Feliciano P., Brotons A., Gonzalez M., Issenberg S.B., Kobetz E. Epidemiology of SARS-CoV-2 antibodies among firefighters/paramedics of a US fire department: A cross-sectional study. Occup. Environ. Med. 2020;77:857–861. doi: 10.1136/oemed-2020-106676. [DOI] [PubMed] [Google Scholar]
- 71.Skowronski D.M., Zou M., Clarke Q., Chambers C., Dickinson A.J., Sabaiduc S., Olsha R., Gubbay J.B., Drews S.J., Charest H., et al. Influenza Vaccine Does Not Increase the Risk of Coronavirus or Other Noninfluenza Respiratory Viruses: Retrospective Analysis from Canada, 2010–2011 to 2016–2017. Clin. Infect. Dis. 2020;71:2285–2288. doi: 10.1093/cid/ciaa626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Principi N., Camilloni B., Esposito S. ESCMID Vaccine Study Group Influenza immunization policies: Which could be the main reasons for differences among countries? Hum. Vaccines Immunother. 2018;14:684–692. doi: 10.1080/21645515.2017.1405188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Berg M.K., Yu Q., Salvador C.E., Melani I., Kitayama S. Mandated Bacillus Calmette-Guérin (BCG) vaccination predicts flattened curves for the spread of COVID-19. Sci. Adv. 2020;6:eabc1463. doi: 10.1126/sciadv.abc1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller A., Reandelar M.J., Fasciglione K., Roumenova V., Li Y., Otazu G.H. Correlation between universal BCG vaccination policy and reduced mortality for COVID-19. medrXiv. 2020 [Google Scholar]
- 75.Singh S., Khera D., Chugh A., Khasbage S., Khera P.S., Chugh V.K. BCG vaccination impact on mortality and recovery rates in COVID-19: A meta-analysis. Monaldi Arch. Chest Dis. 2021 doi: 10.4081/monaldi.2021.1875. [DOI] [PubMed] [Google Scholar]
- 76.Covián C., Fernández-Fierro A., Retamal-Díaz A., Díaz F.E., Vasquez A., Lay M.K., Riedel C., Gonzalez P.A., Bueno S.M., Kalergis A.M. BCG-Induced Cross-Protection and Development of Trained Immunity: Implication for Vaccine Design. Front. Immunol. 2019;10:2806. doi: 10.3389/fimmu.2019.02806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Long B.R., Michaelsson J., Loo C., Ballan W.M., Vu B.-A.N., Hecht F.M., Lanier L.L., Chapman J.M., Nixon D. Elevated Frequency of Gamma Interferon-Producing NK Cells in Healthy Adults Vaccinated against Influenza Virus. Clin. Vaccine Immunol. 2008;15:120–130. doi: 10.1128/CVI.00357-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Endrich M.M., Blank P.R., Szucs T.D. Influenza vaccination uptake and socioeconomic determinants in 11 European countries. Vaccine. 2009;27:4018–4024. doi: 10.1016/j.vaccine.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 79.Rollston R., Galea S. COVID-19 and the Social Determinants of Health. Am. J. Health Promot. 2020;34:687–689. doi: 10.1177/0890117120930536b. [DOI] [PubMed] [Google Scholar]
- 80.Patel J., Nielsen F., Badiani A., Assi S., Unadkat V., Patel B., Ravindrane R., Wardle H. Poverty, inequality and COVID-19: The forgotten vulnerable. Public Health. 2020;183:110–111. doi: 10.1016/j.puhe.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wachtler B., Michalski N., Nowossadeck E., Diercke M., Wahrendorf M., Santos-Hövener C., Lampert T., Hoebel J. Socioeconomic inequalities in the risk of SARS-CoV-2 infection–First results from an analysis of surveillance data from Germany. J. Health Monit. 2020;5:18. doi: 10.25646/7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: A systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feldman C., Anderson R. The role of co-infections and secondary infections in patients with COVID-19. Pneumonia. 2021;13:5. doi: 10.1186/s41479-021-00083-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph C., Togawa Y., Shindo N. Bacterial and viral infections associated with influenza. Influ. Other Respir. Viruses. 2013;7:105–113. doi: 10.1111/irv.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Im H., Ser J., Sim U., Cho H. Promising Expectations for Pneumococcal Vaccination during COVID-19. Vaccines. 2021;9:1507. doi: 10.3390/vaccines9121507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Smith A.P., Williams E.P., Plunkett T.R., Selvaraj M., Lane L.C., Zalduondo L., Xue Y., Vogel P., Channappanavar R., Jonsson C.B., et al. Time-Dependent Increase in Susceptibility and Severity of Secondary Bacterial Infection during SARS-CoV-2 Infection. bioRxiv. 2022 doi: 10.3389/fimmu.2022.894534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bersanelli M., Giannarelli D., De Giorgi U., Pignata S., Di Maio M., Verzoni E., Clemente A., Guadalupi V., Signorelli D., Tiseo M., et al. Symptomatic COVID-19 in advanced-cancer patients treated with immune-checkpoint inhibitors: Prospective analysis from a multicentre observational trial by FICOG. Ther. Adv. Med. Oncol. 2020;12 doi: 10.1177/1758835920968463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveira L.M.d., Tiyo B.T., da Silva L.T., Fonseca L.A.M., Rocha R.C., Santos V.A.d., Ceneviva C., Bedin A.A., de Almeida A., Duarte A.J.d., et al. Prevalence of anti-SARS-CoV-2 antibodies in outpatients of a large public university hospital in Sao Paulo, Brazil. Rev. Inst. Med. Trop. São Paulo. 2020;62 doi: 10.1590/s1678-9946202062091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kindgen-Milles D., Brandenburger T., Braun J.F.W., Cleff C., Moussazadeh K., Mrosewski I., Timm J., Wetzchewald D. Prevalence of SARS-COV-2 positivity in 516 German intensive care and emergency physicians studied by seroprevalence of antibodies National Covid Survey Germany (NAT-COV-SURV) PLoS ONE. 2021;16:e0248813. doi: 10.1371/journal.pone.0248813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murillo-Zamora E., Trujillo X., Huerta M., Ríos-Silva M., Mendoza-Cano O. Male gender and kidney illness are associated with an increased risk of severe laboratory-confirmed coronavirus disease. BMC Infect. Dis. 2020;20:1–8. doi: 10.1186/s12879-020-05408-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Angulo-Zamudio U.A., Martínez-Villa F.M., Leon-Sicairos N., Flores-Villaseñor H., Velazquez-Roman J., Campos-Romero A., Alcántar-Fernández J., Urrea F., Muro-Amador S., Medina-Serrano J., et al. Analysis of Epidemiological and Clinical Characteristics of COVID-19 in Northwest Mexico and the Relationship Between the Influenza Vaccine and the Survival of Infected Patients. Front. Public Health. 2021;9:570098. doi: 10.3389/fpubh.2021.570098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alamdari N.M., Afaghi S., Rahimi F.S., Tarki F.E., Tavana S., Zali A., Fathi M., Besharat S., Bagheri L., Pourmotahari F., et al. Mortality Risk Factors among Hospitalized COVID-19 Patients in a Major Referral Center in Iran. Tohoku J. Exp. Med. 2020;252:73–84. doi: 10.1620/tjem.252.73. [DOI] [PubMed] [Google Scholar]
- 93.Sánchez-García C., Salinas-Aguirre J.E., Rodríguez-Muñoz L., Rodríguez-Sánchez R., Díaz-Castaño A., Bernal-Gómez R. History of influenza immunization in COVID-19 patients: Impact on mortality. Gac. Med. Mex. 2021;157:102–106. doi: 10.24875/GMM.M21000527. [DOI] [PubMed] [Google Scholar]
- 94.Poblador-Plou B., Carmona-Pírez J., Ioakeim-Skoufa I., Poncel-Falcó A., Bliek-Bueno K., Pozo M.C.-D., Gimeno-Feliú L.A., González-Rubio F., Aza-Pascual-Salcedo M., Bandrés-Liso A.C., et al. Baseline Chronic Comorbidity and Mortality in Laboratory-Confirmed COVID-19 Cases: Results from the PRECOVID Study in Spain. Int. J. Environ. Res. Public Health. 2020;17:5171. doi: 10.3390/ijerph17145171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sardinha D.M., Lobato D.D.C., Ferreira A.L.D.S., Lima K.V.B., Guimarães R.J.D.P.S.E., Lima L.N.G.C. Analysis of 472,688 Severe Cases of COVID-19 in Brazil Showed Lower Mortality in Those Vaccinated against Influenza. World J. Vaccines. 2021;11:28–32. doi: 10.4236/wjv.2021.113004. [DOI] [Google Scholar]
- 96.Marín-Hernández D., Schwartz R.E., Nixon D.F. Epidemiological evidence for association between higher influenza vaccine uptake in the elderly and lower COVID-19 deaths in Italy. J. Med. Virol. 2020;93:64–65. doi: 10.1002/jmv.26120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zanettini C., Omar M., Dinalankara W., Imada E.L., Colantuoni E., Parmigiani G., Marchionni L. Influenza Vaccination and COVID19 Mortality in the USA. medRxiv. 2020 doi: 10.3390/vaccines9050427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cocco P., Meloni F., Coratza A., Schirru D., Campagna M., De Matteis S. Vaccination against seasonal influenza and socio-economic and environmental factors as determinants of the geographic variation of COVID-19 incidence and mortality in the Italian elderly. Prev. Med. 2021;143:106351. doi: 10.1016/j.ypmed.2020.106351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arokiaraj M.C. Considering Interim Interventions to Control COVID-19 Associated Morbidity and Mortality—Perspectives. Front. Public Health. 2020;8:444. doi: 10.3389/fpubh.2020.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Amato M., Werba J.P., Frigerio B., Coggi D., Sansaro D., Ravani A., Ferrante P., Veglia F., Tremoli E., Baldassarre D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines. 2020;8:535. doi: 10.3390/vaccines8030535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in the tables.