Abstract
Viruses can escape T-cell surveillance by infecting macrophages and thereby induce apoptosis of noninfected T cells. This ability had not been demonstrated for bacteria. We investigated whether infection of macrophages with the important human pathogen Chlamydia trachomatis can induce T-cell apoptosis. Because Chlamydia-Mycoplasma coinfection is a frequent event, the ability of Mycoplasma fermentans-infected macrophages to induce T-cell apoptosis was also studied. Infected macrophages were cocultivated with autologous T cells in different activation states. Propidium iodide-based fluorescence-activated cell sorter analysis demonstrated that macrophages infected with viable chlamydiae induced T-cell death. Apoptosis was identified as the mode of death induction by using a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay. Induction of T-cell death was macrophage dependent. Incubation of T cells with infectious chlamydiae in the absence of macrophages did not lead to T-cell apoptosis. UV irradiation of chlamydiae diminished the ability to induce death. T-cell death was induced by a cell-free supernatant of infected macrophages. Not only phytohemagglutinin-preactivated T cells but also non-mitogen-preactivated T cells were susceptible to C. trachomatis-induced apoptosis. In contrast, M. fermentans infection of macrophages did not induce T-cell death. Coinfection had no additional effect. In summary, intracellular chlamydial infection of macrophages can induce T-cell apoptosis. Apoptosis induction by chlamydiae possibly explains how persistently infected macrophages escape T-cell surveillance and why the Chlamydia-specific T-cell response is diminished during persistent chlamydial infection.
The progress of apoptosis-related research has prompted great interest in the mechanisms of cell death (41). Apoptosis (programmed cell death) occurs when a normally functioning cell receives a variety of different cell signals (25, 27). Microbe-induced apoptosis was first identified for viral infections and has subsequently been reported in cases of infections with any of a large number of pathogenic bacteria and parasites. The ability of pathogens to induce apoptosis may play a role in the initiation of the infection, the survival of the pathogens, and their escape from the host immune response (4, 49, 57). Infection of cells not only changes the susceptibility of host cells to apoptosis-inducing mechanisms but also can mediate apoptosis of noninfected cells. It was demonstrated that human immunodeficiency virus infection of macrophages leads to upregulation of Fas ligand on infected host cells and, consequently, to the induction of apoptosis of noninfected T cells (5, 35). The induction of apoptosis of cytotoxic T cells is an attractive model to explain the persistence of intracellular pathogens in host cells. However, this mechanism had not been demonstrated for bacterial infections.
Chlamydia trachomatis is an obligate intracellular bacterial pathogen known to be the etiological agent responsible for several diseases, such as blinding trachoma, nongonococcal urethritis, and reactive arthritis (8, 51). Using molecular biology methods, we and others have frequently detected C. trachomatis nucleic acids in synovial tissues of patients with reactive arthritis or undifferentiated oligoarthritis (9, 15, 22, 32, 43, 46). It was further shown that synovial chlamydiae are viable and metabolically active (14). They reside predominantly within macrophages below the synovial lining (9, 32). There is evidence that antibiotic treatment of Chlamydia-induced arthritis can modify the course of the disease (7, 26). The eradication of chlamydiae in the synovial tissue seems to be a reasonable goal in amelioration of the synovitis. Therefore, for the improvement of treatment, it is vital to understand how chlamydia can persist in the synovial membrane.
Other bacteria recently linked with apoptosis induction are members of the genus Mycoplasma (36, 39). Paddenberg et al. showed that Mycoplasma-infected fibroblasts undergo apoptosis after treatment with cycloheximide (36). They have demonstrated that some Mycoplasma strains possess an endonuclease. This nuclease can cleave DNA in a manner similar to the endogenous endonucleases that become activated as apoptosis progresses. The activities of both nucleases lead to DNA ladder formation, the hallmark of apoptosis. Mycoplasma fermentans has become an object of interest as a human pathogen. It probably serves as a cofactor in AIDS (10, 39). M. fermentans is a facultative intracellular gram-negative bacterium which can survive in macrophages (28, 40).
We have developed a human macrophage model to study persistent chlamydial infection (13, 24). Macrophages are the primary host cells for C. trachomatis in synovial tissue (32). Despite the presence of a detectable T-cell response in joints, C. trachomatis-infected macrophages are not eliminated (9). Su and Caldwell have reported that adding infected macrophages to T cells in a mouse model can induce T-cell death (45). We hypothesized that Chlamydia-induced T-cell death can be mediated by the induction of apoptosis. We used the in vitro macrophage model for persistent chlamydial infection, added autologous T cells, and assayed this cell system for T-cell apoptosis. In addition, we investigated whether infection with the facultative intracellular bacterium M. fermentans can also induce T-cell apoptosis. The rationale for studying M. fermentans was that it can persistently infect macrophages, and coinfection with both bacteria occurs in vivo and in vitro (19, 28, 30, 40, 50). A large percentage of Chlamydia strains used for experimental studies were coinfected with Mycoplasma (23, 30). It was therefore possible that previous observations were attributable to Mycoplasma coinfection.
MATERIALS AND METHODS
Organisms.
C. trachomatis elementary bodies (EBs) were prepared as previously described (42). In brief, infectious EBs of C. trachomatis serovar K (UW/31/Cx; Washington Research Foundation, Seattle, Wash.) were grown in HEp-2 cells. Serovar K was chosen because of its potential to induce arthritis. Chlamydial EBs were purified from the cells by urografin (Schering AG, Berlin, Germany) density gradient centrifugation. Purified EBs were resuspended in 1 ml of sucrose phosphate buffer (0.01 M sodium phosphate, 0.25 M sucrose, 5 mM l-glutamic acid, pH 7.2 [all chemicals were supplied by Sigma, Diesenhofen, Germany]). EBs were stored in small aliquots at −80°C. Inclusion-forming units were quantitated by titration on HEp-2 cells and subsequent indirect immunoperoxidase assay. To visualize inclusion bodies in HEp-2 cells, cells were incubated with serum from a C. trachomatis antibody-positive patient and then with a peroxidase-conjugated goat anti-human immunoglobulin G antibody (Sigma). The antichlamydial activity of the patient serum was tested by a commercially available enzyme-linked immunosorbent assay (r-ELISA; Medac, Hamburg, Germany). The titer for immunoglobulin G antibodies was 1:200,000. After addition of the substrate 4-chloro-1-naphthol, inclusion bodies appeared as black dots and were counted by light microscopy. The method was controlled by application of a fluorescein isothiocyanate (FITC)-labeled, mouse-derived anti-major outer membrane protein (anti-MOMP) antibody (Boehringer, Mannheim, Germany). Incubation of C. trachomatis-infected HEp-2 cells with this antibody and detection by fluorescence microscopy led to similar results. C. trachomatis cultures were routinely screened for Mycoplasma infection by culture and PCR techniques followed by restriction enzyme digestion as described previously (23). The protocol used for purification of C. trachomatis after coinfection with M. fermentans was recently published (23).
M. fermentans strain PG 18 was kindly provided by G. Gerlach, Department of Microbiology, Veterinary School Hannover, Hannover, Germany. Bacteria were expanded in glass tubes containing mycoplasma broth for 4 days at 37°C in an atmosphere of 5% CO2 and tested for viability by a microtiter dilution method as described elsewhere (1). Mycoplasma suspension was serially diluted in sterile microtiter plates (Nunc, Wiesbaden, Germany). After dilution, a 10-μl volume from each well of the dilution assay was inoculated on the surface of mycoplasma agar plates. Plates were incubated at 37°C in 5% CO2 until colonies could be detected by inverted light microscopy at 40× magnification, usually after 7 to 10 days of culture. The numbers of CFU per milliliter of the original Mycoplasma suspension were calculated by multiplying the microtiter dilution by the average number of colonies counted.
Purification and stimulation of PBMCs and monocytes.
Peripheral blood mononuclear cells (PBMCs) were isolated from heparinized buffy coats obtained from normal blood donors of our blood bank by Ficoll-Hypaque density gradient centrifugation. For each experiment a different blood donor was used. The PBMCs were suspended in RPMI 1640 medium containing 10% fetal calf serum (FCS). One aliquot was distributed on six-well plates (107 cells per well) for monocyte isolation. After 2 h, nonadherent cells were removed by vigorous washing in Hanks' balanced salt solution (Sigma). About 10 to 15% of the inoculated mononuclear cells remained adherent, of which 80 to 85% were CD11b antigen positive. The other aliquot was stimulated with phytohemagglutinin (PHA; 10 μg/ml) for 6 days in the presence of recombinant interleukin-2 (rIL-2; 60 IU/ml; kindly provided by C. Fiehn, Medizinische Poliklinik, University of Heidelberg, Heidelberg, Germany) to stimulate and expand T cells. T cells were resuspended in RPMI 1640 containing 10% FCS, 0.1% streptomycin, and 0.1% gentamycin. They were cultured in 50-ml tissue culture flasks (Greiner, Nördlingen, Germany) in an incubator at 37°C and 5% CO2. After 6 days of culture, the proportion of T cells was determined by fluorescence-activated cell sorter (FACS) analysis with FITC-conjugated anti-CD3 monoclonal antibody (Dako, Hamburg, Germany). The proportion of CD3+ T cells was between 85 and 91%.
Infection of cells.
After purification of the macrophages and determination of their infectivity, they were infected with C. trachomatis EBs or M. fermentans at different multiplicities of infection (MOIs) or CFUs in antibiotic-free RPMI medium containing 10% FCS. Infection was performed in the wells originally used for isolation. M. fermentans was used at 1.5 × 104 or 1.5 × 105 CFU/ml, resulting in an MOI of 0.05 or 0.5, respectively. A direct cytotoxic effect on macrophages was observed upon inoculation of more than 1.5 × 105 CFU/ml. Before cocultivation with T cells, aliquots of Mycoplasma-infected cultures were plated on agar plates to test whether these microorganisms would proliferate. In several experiments, UV-irradiated bacteria were used. For UV irradiation, C. trachomatis EBs were plated in petri dishes and placed under a UV source. To ensure that UV irradiation was efficient, HEp-2 cells were reinfected with UV-irradiated EBs and analyzed by an indirect immunoperoxidase assay. Only aliquots of UV-irradiated EBs, which could not form inclusion bodies in HEp-2 cells, were used.
Coculture assay.
Before coculture, infected macrophages were washed three times with Hanks' balanced salt solution to eliminate extracellular bacteria. PHA-activated T cells or nonpreactivated PBMCs were added to the infected macrophages at a ratio of 1:1. Coculture assays were performed in antibiotic-free RPMI medium containing 10% FCS and 60 IU of rIL-2/ml. In control experiments, T cells were incubated with bacteria in the absence of macrophages. Infectious bacteria remained in the wells for the entire coculture period. Two consecutive Ficoll separations of lymphocytes from the same individual, at days 1 and 7, were performed for assays involving nonpreactivated lymphocytes. On day 1, only macrophages for bacterial infection were harvested; lymphocytes were isolated on day 7. Both populations were cocultivated after the second separation. To induce apoptosis, T cells were coincubated with infected macrophages or cell-free supernatants of infected macrophages. Cell-free supernatants were derived from macrophage cultures after 6 days of culture. The macrophages in the six-well culture plates were centrifuged at 800 × g for 10 min. Two milliliters of supernatant from each well was cautiously removed, transferred to a sterile tube, and centrifuged again. One milliliter of cell-free supernatant was added to 5 × 105 autologous PHA-activated T cells per well in a 24-well plate.
Flow cytometry.
Flow cytometry analysis with propidium iodide (PI) was applied for quantification of viable cells in coculture assays or of T cells incubated with cell-free supernatant. PI staining and FACS analysis were performed as described elsewhere (33, 35). In brief, cells were resuspended in phosphate-buffered saline (PBS) and fixed in 75% ethanol for 1 h at 4°C. They were then washed and resuspended in PBS containing PI (50 μg/ml; Sigma) and RNase A (250 μg/ml of type I-A; Sigma). The PI fluorescence of viable cells was determined by histogram analysis on a logarithmic scale, using the Cellquest software on a FACScan instrument (Becton Dickinson, Heidelberg, Germany). We restricted our analysis to viable cells with at least diploid DNA content due to the limitations of the method in clearly distinguishing between necrotic and apoptotic cells. By side- and forward-scatterplot analysis, cell debris and clumps were excluded. The gate for viable cells was set according to the PI content of control populations. Control populations included viable T cells and apoptotic T cells. T-cell apoptosis was induced according to the method of Seki et al. by irradiation of T cells with 2,000 cGy using a 137Cs source irradiator (Division of Molecular Pharmacology, Medical School Hannover) (44). Cells were resuspended in RPMI medium and cultured for an additional 24 h at 37°C and 5% CO2 before undergoing PI staining. Cells with less than diploid DNA content were excluded from the analysis; only viable nonapoptotic or nonnecrotic cells were counted. Analysis of unfixed cells from coculture experiments with an FITC-labeled anti-CD3 antibody revealed that 86 to 93% of the analyzed cells were T cells. Macrophages comprised a minor population of PI-stained cells. Only a few macrophages were resuspended as assessed by light microscopy analysis of the emptied plates.
To discriminate between T cells and macrophages and to analyze CD4+ and CD8+ T cells separately, double staining with FITC-labeled anti-CD3, anti-CD4, or anti-CD8 antibodies and PI was performed according to the method of Telford et al. (47) (Dako, Hamburg, Germany). In brief, 10 μl of antibody was added. Cells were washed with cold PBS and resuspended in 1 part of cold 50% FCS in PBS. Under conditions of constant mixing, 3 parts of cold 70% ethanol was slowly added. After incubation at 4°C for 1 h and washing in PBS containing 2% bovine serum albumin, 500 μl of PI solution and 500 μl of RNase A solution were added.
TUNEL assay.
To reveal apoptotic cells, DNA fragmentation was detected by terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) as described elsewhere (11). Cocultured cells were removed from six-well plates, and 5 × 104 cells were mounted on slides fixed with 4% paraformaldehyde in 0.1 M PBS (pH 7.4) for 20 min at 4°C and then with 70% ethanol for 30 min at room temperature. After being washed with 0.05% Tween 20 in Tris-buffered saline, the slides were incubated with digoxigenin-labeled dUTP (1.7 nmol; Boehringer, Mannheim, Germany) and TdT (12.5 U; Boehringer Mannheim) in TdT buffer (200 nM potassium cacodylate, 25 nM Tris-HCl, bovine serum albumin [1.25 mg/ml; pH 6.6], 5 nM cobalt chloride solution) for 70 min at 37°C. After another washing, incorporated dUTP was revealed by a peroxidase-labeled antidigoxigenin antibody followed by incubation with benzidine. Apoptotic nuclei appeared as brown dots. Cytoplasm was visualized by staining with haemalaun (Sigma). For quantification, at least 200 cells per slide were counted by two investigators. The results obtained by the two investigators were comparable.
Statistics.
For all statistical analyses, SPSS software, version 8.0 for Windows, was used. In most instances, values for the different tissue conditions were presented as percentages (transformed values) of the noninfected controls. Means ± standard deviations of the transformed values were given. To test whether the reduction in number of viable cells or the frequencies of apoptotic cells (nontransformed values) for different stimuli were statistically significant, a one-way analysis of variance (ANOVA) for normally distributed results with equal variances was performed. Post hoc results were corrected for multiple testing by using the Bonferroni test. For normally distributed results with unequal variances, a post hoc Tamhane test was performed. P values of <0.05 were considered statistically significant; a P value of <0.01 was considered highly significant.
RESULTS
Infection of macrophages with C. trachomatis induced apoptosis of PHA-preactivated T cells.
A C. trachomatis serovar K strain was used to address the question of whether infection of macrophages with C. trachomatis induces T-cell apoptosis. T cells were preactivated with PHA and, after 6 days of individual culture were cocultivated with autologous C. trachomatis-infected macrophages. Cocultured cells were tested for viability by PI-mediated FACS analysis (Table 1). Infection of autologous macrophages with C. trachomatis at an MOI of greater than 5 resulted in a significant reduction in the number of viable cells compared to noninfected cultures. Induction of death was dependent on the MOI of C. trachomatis. Addition of C. trachomatis EBs at an MOI of 5 without macrophages to PHA-preactivated T cells did not cause significant cell death. T-cell death was macrophage dependent. As another control, T cells were restimulated with PHA. This did not lead to a significant reduction in number of viable cells, showing that cell death was not due to restimulation or a shortage of the nutrient supply.
TABLE 1.
Cell viability in cocultures of PHA-preactivated T cells and C. trachomatis-infected macrophages
| Cultivation of PHA-stimulated T cells plus: | % of viable cellsa | Pb |
|---|---|---|
| Noninfected macrophages | 100 | |
| Noninfected macrophages plus PHA | 93 ± 19 | 1.0 |
| C. trachomatis (MOI = 5) without macrophages | 90 ± 7 | 0.24 |
| Macrophages infected with C. trachomatis at an MOI of: | ||
| 1 | 86 ± 5 | 0.27 |
| 5 | 67 ± 10 | 0.007 |
| 10 | 69 ± 11 | 0.006 |
Values, quantified by FACS analysis of PI-stained cells after 4 days of cocultivation, are means ± standard deviations of data from four independent experiments, each performed in duplicate. Percentages represent the viability of cells under the different culture conditions compared to that of cells in noninfected cultures.
P values of the different culture conditions versus cultures with noninfected macrophages, as determined by one-way ANOVA accounting for multiple comparisons by using the Bonferroni test. P in boldface are significant.
FACS analysis of C. trachomatis-infected cultures stained with PI revealed an increase of cells with a subdiploid DNA content characteristic of apoptosis. Staining of T cells with anti-CD3 antibody in parallel experiments demonstrated that the majority of cells with subdiploid DNA content were T cells. A characteristic experiment is depicted in Fig. 1. Only 17 to 37% of all analyzed cells were CD3−, and their viability after infection with C. trachomatis remained unchanged. In contrast, the viability of CD3+ T cells was reduced significantly by C. trachomatis infection.
FIG. 1.
Flow cytometry analysis of viable T cells cocultivated with C. trachomatis-infected macrophages and stained with PI and anti-CD3 antibody (FITC labeled). Staining was performed after 6 days of coculture. PHA-preactivated T cells were incubated with noninfected macrophages (A, B, and C) or with macrophages infected with C. trachomatis at an MOI of 5 (D, E, and F) at a ratio of 1:1. (A and D) Dot blot analysis of double-stained cells was used to gate CD3+ and CD3− cells. The PI contents of gated cells were subsequently determined by histogram analysis. (B and E) PI fluorescence of CD3+ T cells (gate R1 [B] or gate R3 [E]). (C and F) PI fluorescence of CD3− cells (gate R2 [C] or gate R4 [F]). M1 markers indicate viable cells with at least a diploid DNA content. Percentages in panels E and F indicate the reduction in numbers of viable cells in comparison to the cultures of T cells with noninfected macrophages in the depicted experiment. In total, five experiments were performed; the mean viability ± standard deviation of CD3− cells in C. trachomatis-infected cultures (MOI = 5) compared to that of noninfected cultures was 103% ± 12%, while that of CD3+ cells in C. trachomatis-infected cultures (MOI = 5) compared to noninfected cultures was 62% ± 6%, P = 0.004, as determined by one-way ANOVA (accounting for multiple comparisons by using the Bonferroni test).
T-cell subset analysis by application of anti-CD4 and anti-CD8 antibodies revealed that the decrease in number of viable CD8+ T cells was more pronounced than the death of CD4+ T cells (n = 6 experiments). The CD8+/CD4+ T-cell ratio changed from 1:1.3 to 1:1.8 when T cells were cultured with C. trachomatis-infected macrophages. However, this decrease was statistically not significant.
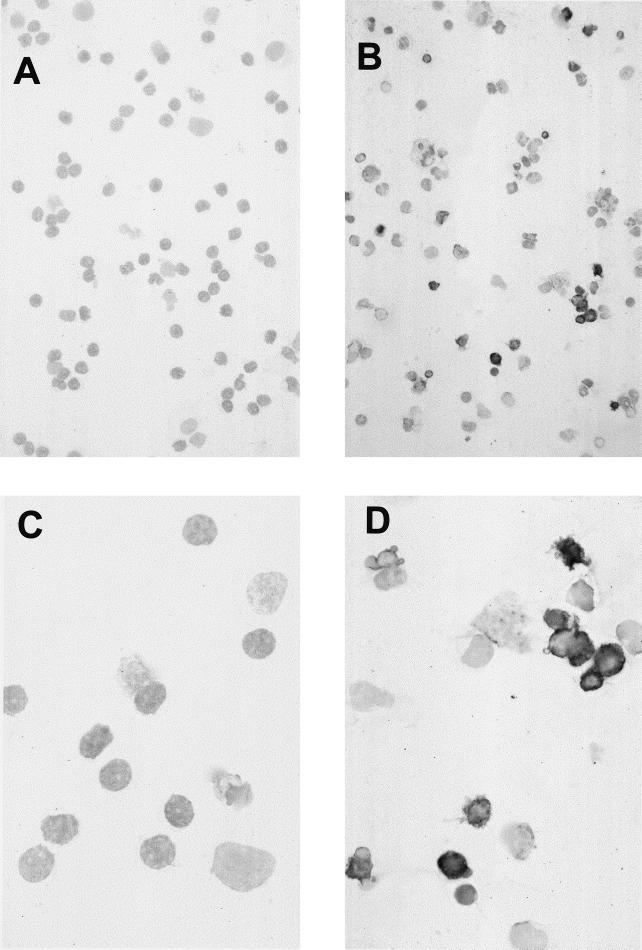
To test whether the reduction of viability was caused by apoptosis, the TUNEL technique was applied (Fig. 2). This approach confirmed that apoptosis was the molecular death mechanism. For quantification, at least 200 cells each of C. trachomatis-infected and noninfected cultures were counted. A significant increase in number of apoptotic cells was observed after chlamydial infection. Cultures with uninfected macrophages contained 28% ± 7% (mean ± standard deviation) apoptotic cells; in infected cultures (MOI = 5), 56% ± 11% apoptotic cells were present (n = 3; P = 0.001).
FIG. 2.
Detection of apoptotic cells by TUNEL assay: light microscopy analysis of PHA-preactivated T cells cocultivated with C. trachomatis-infected macrophages (MOI = 5). TUNEL staining was performed after 6 days of coculture. (A and C) Specificity controls; assays were performed without the addition of TdT; (B and D) TdT was added. Apoptotic nuclei appeared only after the addition of the enzyme. In the figure, apoptotic cells can be distinguished by their black staining. Cytoplasm was visualized by staining with haemalaun. The mean percentage ± standard deviation of apoptotic cells in these assays after coculture with C. trachomatis-infected macrophages (MOI = 5) was 56% ± 11% (n = 3 experiments); the frequency of apoptotic cells after coculture with noninfected macrophages was 28% ± 7% (n = 3 experiments; P = 0.001). Magnifications, ×30 (A and B) and ×75 (C and D).
T-cell apoptosis was induced by cell-free supernatant of C. trachomatis-infected macrophages.
To investigate whether humoral mechanisms or cell-cell contact between C. trachomatis-infected macrophages and T cells was necessary for apoptosis induction, supernatants from infected and noninfected macrophages were removed after 6 days of culture. PHA-preactivated autologous T cells were incubated with supernatant for 6 days, and viability was determined by FACS analysis of PI-stained cells. In addition, coculture of T cells and macrophages was performed (Table 2).
TABLE 2.
Induction of T-cell death by supernatant and cell-cell contact of C. trachomatis-infected macrophages
| Cultivation of PHA-activated T cells plus: | % of viable cellsa (Pb) after induction with:
|
|
|---|---|---|
| Supernatant | Cells | |
| Noninfected macrophages | 100 | 100 |
| Macrophages infected with C. trachomatis at an MOI of: | ||
| 1 | 87 ± 9 (0.005) | 84 ± 12 (0.0001) |
| 5 | 78 ± 8 (0.0001) | 61 ± 9 (0.0001) |
Percentages represent the viability of PI-stained cells under different culture conditions compared to the viability of noninfected cultures. Values are means ± standard deviations (six independent experiments, four performed in duplicate and two performed in triplicate). Supernatants were derived from macrophages after 6 days of culture prior to the addition of T cells. FACS analysis for PI-stained cells was performed after 6 days of culture.
All values were highly significantly different (P < 0.01) from value for preactivated T cells cocultivated with supernatant from uninfected macrophages or from value for preactivated T cells cocultivated with noninfected macrophages, as determined by one-way ANOVA.
Significant T-cell death in comparison to noninfected cultures was induced by cell-free supernatant of infected macrophages. Apoptosis induction was dependent on the MOI of C. trachomatis. The results were consistent with the data presented in Table 1. Due to the larger number of experiments, the decrease of viability was statistically significant for macrophages infected with C. trachomatis at an MOI of 1. Apoptosis was induced by a humoral death mechanism. However, in comparison to cell-cell contact, apoptosis induction by supernatant was less efficient (P = 0.001).
Non-mitogen-preactivated T cells were also susceptible to C. trachomatis-induced apoptosis.
The susceptibility of T cells to apoptosis is dependent on their functional state (2, 25). Having demonstrated that activated T cells undergo C. trachomatis-induced apoptosis, we investigated the susceptibility of nonprestimulated peripheral T cells. Blood from the same individual was drawn twice: first for the isolation and infection of macrophages, and 6 days later for isolation of nonactivated peripheral blood lymphocytes. After 6 days of coculture, the number of apoptotic cells was quantified by microscopic analysis of TUNEL assays. A twofold increase in number of apoptotic cells was observed when PHA-preactivated and nonactivated peripheral T cells were cultured with C. trachomatis-infected macrophages (Fig. 3). Under these culture conditions, including the addition of rIL-2, the susceptibility of non-mitogen-preactivated T cells to C. trachomatis-induced apoptosis was similar to that of PHA-preactivated T cells.
FIG. 3.
Apoptosis of nonpreactivated T cells induced by macrophages (MO) infected with C. trachomatis at an MOI of 5. After 6 days of coculture, the number of apoptotic cells was quantified by microscopic analysis of TUNEL assays (n = 3 experiments). Mean percentages of apoptotic cells ± standard deviations were as follows: for cultures of nonpreactivated T cells, 15% ± 3% (noninfected) and 35% ± 5% (C. trachomatis infected); for cultures of PHA-preactivated T cells, 28% ± 7% (non-infected) and 56% ± 11% (C. trachomatis infected). P values of the indicated comparisons were determined by one-way ANOVA accounting for multiple comparisons by using the Tamhane test. This analysis revealed that for both T-cell populations a highly significant increase in numbers of apoptotic cells occurred in the presence of C. trachomatis-infected macrophages (P = 0.001). Abbreviations: MO, macrophage; TC, T cell; Ctr., C. trachomatis.
Infection of macrophages with M. fermentans did not induce apoptosis of activated T cells.
M. fermentans is a facultative intracellular bacterium which can be detected in peripheral blood monocytes and has recently been linked with apoptosis induction (28, 39, 40). Experiments were performed to investigate whether Mycoplasma infection also primes macrophages for apoptosis induction. We infected macrophages with M. fermentans at an MOI of 0.05 or 0.5 and, after 6 days of individual culture, cocultured them with PHA-preactivated T cells. These MOIs were chosen because infection with higher concentrations of M. fermentans led to macrophage death. At these MOIs, 75% of all macrophages were viable after 6 days of individual culture.
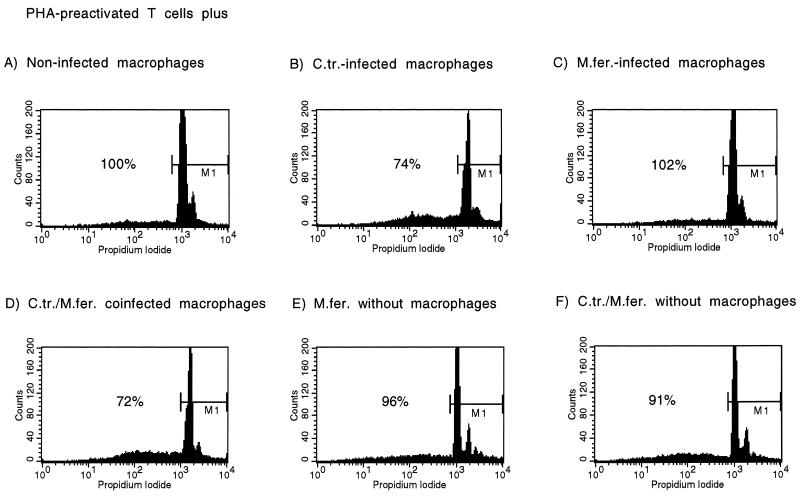
FACS analysis of PI-stained cells showed that there was no decrease in numbers of viable cells when preactivated T cells were incubated with M. fermentans alone or with M. fermentans-infected macrophages for either MOI (n = 4) (one typical experiment is shown in Fig. 4). In contrast, infection of macrophages in parallel with C. trachomatis at an MOI of 5 led to a significant reduction in the number of viable cells. Because Mycoplasma-Chlamydia coinfection occurred in vivo and in vitro, we investigated whether coinfection might have a synergistic effect on apoptosis induction (19, 30, 50). FACS analysis of preactivated T cells cocultured with doubly infected macrophages did not show an increase in apoptosis beyond that induced by infection with C. trachomatis alone. Mycoplasma coinfection had no effect on Chlamydia-induced macrophage-dependent T-cell apoptosis.
FIG. 4.
Analysis of viable cells in coculture experiments with M. fermentans-infected macrophages by flow cytometric analysis of PI-stained cells. PHA-preactivated T cells were cocultivated with macrophages. Staining was performed after 6 days of coculture. Histograms of PI-stained cells are depicted. Gated cells (marker M1) have at least a diploid DNA content. The percentages refer to the proportion of viable cells in comparison to that of the cultures of T cells with noninfected macrophages of this characteristic experiment. A total of four experiments were performed. P values refer to the different culture conditions in comparison to cultures with noninfected macrophages as determined by one-way ANOVA accounting for multiple comparisons by using the Bonferroni test. T cells were cocultivated with noninfected macrophages (A), C. trachomatis-infected (MOI = 5) macrophages (72% ± 8%; P = 0.001) (B), M. fermentans-infected (1.5 × 104 CFU/ml, MOI = 0.05) macrophages (105% ± 11%; P = 1) (C), macrophages infected with C. trachomatis (MOI = 5) and M. fermentans (1.5 × 104 CFU/ml, MOI = 0.05) 76% ± 5%; P = 0.005) (D), M. fermentans (1.5 × 104 CFU/ml, MOI = 0.05) and C. trachomatis EBs (MOI = 5) without macrophages (95% ± 8%; P = 1) (F).
UV attenuation of C. trachomatis EBs decreased the ability to induce apoptosis.
To test whether infection of macrophages with viable chlamydia was important for death induction, macrophages were incubated with UV-attenuated EBs coinfected with M. fermentans. Viability was quantified by FACS analysis of PI-stained cells (Table 3). Irradiation of bacteria with UV light resulted in minor, nonsignificant decreases in numbers of viable cells (4% after 3 days and 14% after 6 days). A significant increase in number of dead cells was seen when activated T cells were exposed to infected macrophages.
TABLE 3.
Viability of cells in coculture of PHA-preactivated T cells and macrophages incubated with UV-attenuated C. trachomatis and M. fermentans
| Cultivation of PHA-activated T cells plus: | Cell viability (%)a
|
|
|---|---|---|
| Day 3 | Day 6 | |
| Noninfected macrophages | 100 | 100 |
| Infected macrophages | 73 ± 17b | 53 ± 10b |
| Macrophages + UV-irradiated bacteria | 96 ± 9 | 86 ± 28 |
Percentage of viable cells, quantified by FACS analysis of PI-stained cells, under different culture conditions compared to that of noninfected cultures. Values are means ± standard deviations of four (day 3) or five (day 6) independent experiments performed in duplicate.
Highly significantly different (P < 0.01) from value for preactivated T cells cocultivated with uninfected macrophages and from value for preactivated T cells cocultivated with UV-irradiated bacteria, as determined by one-way ANOVA.
DISCUSSION
In this article we report that intracellular bacterial infection of macrophages can induce T-cell apoptosis. Macrophages not only can stimulate T cells but also can induce cell death (3, 29, 31, 53). This ability was recognized for certain viral infections, like that of human immunodeficiency virus, but is now newly demonstrated for bacteria. However, infection with the facultative intracellular bacterium M. fermentans failed to induce T-cell apoptosis. Coinfection of C. trachomatis and M. fermentans had no additional effect. The ability to induce apoptosis is not shared by M. fermentans. It remains to be elucidated which intracellular bacteria have this property.
For apoptosis induction, Chlamydia viability was essential. UV irradiation of Chlamydia strongly diminished apoptosis induction. This underlines the fact that macrophages have to be infected with viable bacteria. For many chlamydial diseases, the key feature is the persistent infection of human cells (8, 51). Especially with regard to Chlamydia-induced arthritis, we and others have demonstrated that chlamydiae persist in viable, aberrant forms in macrophages (13, 24). During cultivation in human macrophages, intracellular chlamydiae change and, after 1 week, strongly resemble these aberrant forms in terms of protein synthesis and morphological features (8, 13, 24, 32). It is possible that persistently infected macrophages have the ability to induce T-cell apoptosis in vivo.
The susceptibilities of PHA-preactivated and nonpreactivated peripheral T cells to apoptosis induction were not different. PHA stimulation allowed the study of properties of preactivated T cells. T cells in this activation state are sensitive to apoptosis induction. Nonstimulated peripheral T-cell populations contain a large number of naive cells and are more resistant to apoptosis induction (26). Surprisingly, naive T cells were rendered susceptible to Chlamydia-induced apoptosis by coculture with Chlamydia-infected macrophages and addition of rIL-2. In the inflamed synovia, high concentrations of different cytokines are present. C. trachomatis-induced apoptosis might be harmful to T cells present in the inflamed synovial tissue regardless of their activation state. Apoptosis induction of T cells could prevent the evolution of a T-cell response sufficient to resolve the persistent chlamydial infection in the joint.
Several observations in animal studies indicate that T cells are essential for the eradication of C. trachomatis infection (21, 38, 48, 55). By inducing murine pulmonary and genital infections with a mouse biovar of C. trachomatis, the mouse pneumonitis agent, it was demonstrated that resolution mostly depends on the presence of CD4+ Th-1-type T cells (38). However, observations of human chlamydial infection indicate that host T-cell immunity is impaired during an ongoing infection (6, 16, 17, 54). Bailey et al. showed, by measuring the T-cell immune response to chlamydial antigens with a lymphocyte proliferation assay, that patients who recovered from trachoma exhibited greater T-cell proliferation than patients with active cases of trachoma (6). Wilkinson et al. observed that patients had only minor synovial T-cell responses to chlamydial antigens despite having PCR-detectable levels of C. trachomatis in their joints (54). In contrast, patients with Chlamydia-induced arthritis who were negative for synovial chlamydial DNA by PCR testing demonstrated a high-level synovial T-cell response to chlamydial antigens. The insufficient T-cell response against C. trachomatis, which can contribute to the persistence of chlamydiae in the joints, may be caused by induction of T-cell apoptosis. However, the mechanism we have eluciated so far is not dependent on the recognition of chlamydial antigens by T cells. Because the T cells used in the present study were derived from normal donors, only a minority of them were Chlamydia specific. We do not know yet whether preferentially Chlamydia-specific T cells are eliminated by this mechanism.
Macrophages induce T-cell apoptosis by Fas-Fas ligand interaction, tumor necrosis factor alpha (TNF-α) production, or secretion of reactive oxygen species (3, 31, 35, 53, 56). Experiments showed that CD4+ T cells are preferentially eliminated by Fas ligand whereas CD8+ T cells may be more susceptible to TNF-α signaling (56). Mainly CD4+ T cells were isolated from the synovial fluid of patients with Chlamydia-induced arthritis; only a few activated CD8+ T cells were identified (16, 18). Ingalls et al. showed that infection of macrophages with C. trachomatis stimulates TNF-α production (20). We have revealed that T-cell apoptosis can be induced by a cell-free supernatant, demonstrating that humoral death mechanisms are involved in C. trachomatis-induced apoptosis. However, the higher efficacy of cell-cell contact between T cells and infected macrophages might be due to sustained production of cytokines (macrophages were allowed to produce TNF-α or oxygen species for an additional 6 days) or to a contribution of Fas-Fas ligand interaction, raising the possibility that the execution of T-cell death is the result of different apoptosis pathways. In our experiments we did not observe a significant preference for apoptosis of CD8+ T cells.
A recent report by Perfettini et al. strongly supports the important role for TNF-α in Chlamydia-induced apoptosis (37). They observed that apoptosis of noninfected cells—mainly epithelial—was induced by genital infection of mice with the mouse pneumonitis strain of C. trachomatis. By applying anti-TNF-α antibodies in vivo, they significantly diminished apoptosis induction. The elucidation of the molecular apoptosis mechanism may have therapeutic implications for Chlamydia-induced arthritis, because antibodies to or receptor ligands for TNF-α and Fas are available (52). The therapeutic goal would be to prevent T-cell apoptosis by inhibiting macrophage-derived death signals.
Our report increases the current knowledge of Chlamydia-related apoptosis by showing that (i) apoptosis induction occurs in human cells after infection with a human-pathogenic C. trachomatis strain, (ii) T cells are affected, and (iii) macrophages play a central role (12, 34, 37). We propose a new mechanism for the persistence of C. trachomatis in human macrophages: the induction of T-cell death by infected macrophages probably adds to the insufficient ability of the host immune system to eliminate infected macrophages.
ACKNOWLEDGMENTS
Our work was supported by grants from the Deutsche Forschungsgemeinschaft (KU1182/I-I and 1-3), the Bundesministerium für Bildung und Forschung (01VM9305 and 01 G/9950), the European Community (BIOMED BMH4-CT-98-3605), and the Medical School Hannover (HILF 19311022).
We appreciate the laboratory work of A. Garbe, C. Schmidt, and A. Liese and thank V. Kaever, K. Rifai, S. Bischof, and C. Fiehn for providing technical support and reagents.
REFERENCES
- 1.Albers A C, Fletcher R D. Simple method for quantification of viable mycoplasmas. Appl Environ Microbiol. 1982;43:958–960. doi: 10.1128/aem.43.4.958-960.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderson M R, Tough T W, Davis-Smith T, Braddy S, Falk B, Schooley K A, Goodwin R G, Smith C A, Ramsdell F, Lynch D A. Fas ligand mediates activation-induced cell death in human T lymphocytes. J Exp Med. 1995;181:71–77. doi: 10.1084/jem.181.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aliprantis A O, Diez-Roux G, Mulder L C F, Zychlinski A, Lang R A. Do macrophages kill through apoptosis? Immunol Today. 1996;17:573–576. doi: 10.1016/s0167-5699(96)10071-2. [DOI] [PubMed] [Google Scholar]
- 4.Ameisen J C, Estaquier J, Idziorek T. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol Rev. 1994;142:9–51. doi: 10.1111/j.1600-065x.1994.tb00882.x. [DOI] [PubMed] [Google Scholar]
- 5.Badley A D, McElhinny J A, Leibson P J, Lynch D H, Alderson M R, Paya C V. Upregulation of Fas ligand expression by human immunodeficiency virus in human macrophages mediates apoptosis of uninfected T lymphocytes. J Virol. 1996;70:199–206. doi: 10.1128/jvi.70.1.199-206.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bailey R L, Holland M J, Whittle H C, Mabey D C W. Subjects recovering from human ocular chlamydial infection have enhanced lymphoproliferative responses to chlamydial antigens compared with those of persistently diseased controls. Infect Immun. 1995;63:389–392. doi: 10.1128/iai.63.2.389-392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bardin T, Enel C, Cornelis F, Salski C, Jorgensen C, Ward R, Lathrop G M. Antibiotic treatment of venereal disease and Reiter's syndrome in a Greenland population. Arthritis Rheum. 1992;35:190–194. doi: 10.1002/art.1780350210. [DOI] [PubMed] [Google Scholar]
- 8.Beatty W L, Morrison R P, Byrne G I. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol Rev. 1994;58:686–699. doi: 10.1128/mr.58.4.686-699.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beutler A M, Schumacher H R, Whittum-Hudson J A, Salameh W A, Hudson A P. Case report: in situ hybridization for detection of inapparent infection with Chlamydia trachomatis in synovial tissue of a patient with Reiter's syndrome. Am J Med Sci. 1995;310:206–213. doi: 10.1097/00000441-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Blanchard A, Montagnier L. AIDS-associated mycoplasmas. Annu Rev Microbiol. 1994;48:687–712. doi: 10.1146/annurev.mi.48.100194.003351. [DOI] [PubMed] [Google Scholar]
- 11.Bode U, Wonigeit K, Pabst R, Westermann J. The fate of activated T cells migrating through the body: rescue from apoptosis in the tissue of origin. Eur J Immunol. 1997;27:2087–2093. doi: 10.1002/eji.1830270837. [DOI] [PubMed] [Google Scholar]
- 12.Fan T, Lu H, He H, Shi L, McClarty G A, Nance D M, Greenberg A H, Zhong G. Inhibition of apoptosis in chlamydia-infected cells: blockade of mitochondrial cytochrome c release and caspase activation. J Exp Med. 1998;187:487–496. doi: 10.1084/jem.187.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gérard H C, Köhler L, Branigan P J, Zeidler H, Schumacher H R, Hudson A P. Viability and gene expression in Chlamydia trachomatis during persistent infection of cultured human monocytes. Med Microbiol Immunol. 1998;187:115–120. doi: 10.1007/s004300050082. [DOI] [PubMed] [Google Scholar]
- 14.Gérard H C, Branigan P J, Schumacher H R, Hudson A P. Synovial Chlamydia trachomatis in patients with reactive arthritis/Reiter's syndrome are viable but show aberrant gene expression. J Rheumatol. 1998;25:734–743. [PubMed] [Google Scholar]
- 15.Hammer M, Nettelnbreker E, Hopf S, Schmitz E, Pörschke K, Zeidler H. Chlamydial rRNA in the joints of patients with Chlamydia-induced arthritis and undifferentiated arthritis. Clin Exp Rheumatol. 1992;10:63–66. [PubMed] [Google Scholar]
- 16.Hassell A B, Pilling D, Reynolds D, Life P F, Bacon P A, Gaston S H. MHC restriction of synovial fluid lymphocyte responses to the triggering organism in reactive arthritis. Absence of class I response. Clin Exp Immunol. 1992;88:442–447. doi: 10.1111/j.1365-2249.1992.tb06469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hassell A B, Life P F, Viner N J, Gaston S H. A longitudinal study of peripheral blood mononuclear cell proliferative responses to bacterial antigens in reactive arthritis. Br J Rheumatol. 1994;33:210–214. doi: 10.1093/rheumatology/33.3.210. [DOI] [PubMed] [Google Scholar]
- 18.Hermann E, Yu D T Y, Meyer zum Büschenfelde K H, Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993;342:646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- 19.Huniche B S, Jensen L T, Birkelund S, Christiansen G. Mycoplasma contamination of Chlamydia pneumoniae isolates. Scand J Infect Dis. 1998;30:181–187. doi: 10.1080/003655498750003609. [DOI] [PubMed] [Google Scholar]
- 20.Ingalls R R, Rice P A, Qureshi N, Takayama K, Shin Lin J, Golenbock D T. The inflammatory cytokine response to Chlamydia trachomatis infection is endotoxin mediated. Infect Immun. 1995;63:3125–3130. doi: 10.1128/iai.63.8.3125-3130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johannson M, Schön K, Ward M, Lycke N. Genital tract infection with Chlamydia trachomatis fails to induce protective immunity in gamma interferon receptor-deficient mice despite a strong local immunoglobulin A response. Infect Immun. 1997;65:1032–1044. doi: 10.1128/iai.65.3.1032-1044.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keat A, Thomas B, Dixey J, Osborn M, Sonnex C, Taylor-Robinson D. Chlamydia trachomatis and reactive arthritis: the missing link. Lancet. 1987;i:72–74. doi: 10.1016/s0140-6736(87)91910-6. [DOI] [PubMed] [Google Scholar]
- 23.Koehler L, Krauße-Opatz B, Dollmann P, Zeidler H, Kuipers J G. Contamination of Chlamydia with Mycoplasma and biological implication. Arthritis Rheum. 1999;42(Suppl.):S336. [Google Scholar]
- 24.Koehler L, Nettelnbreker E, Hudson A P, Ott N, Gérard H C, Branigan P J, Schumacher H R, Drommer W, Zeidler H. Ultrastructural and molecular analyses of the persistence of Chlamydia trachomatis serovar K in human peripheral blood monocytes. Microbiol Pathog. 1997;22:133–142. doi: 10.1006/mpat.1996.0103. [DOI] [PubMed] [Google Scholar]
- 25.Kroemer G. The pharmacology of T cell apoptosis. Adv Immunol. 1995;58:211–296. doi: 10.1016/s0065-2776(08)60621-5. [DOI] [PubMed] [Google Scholar]
- 26.Lauhio A, Leiresalo-Repo M, Lahdevirta J, Saikku P, Repo H. Double-blind, placebo-controlled study of three-month treatment with lymecycline in reactive arthritis. Arthritis Rheum. 1991;34:6–12. doi: 10.1002/art.1780340103. [DOI] [PubMed] [Google Scholar]
- 27.Majno G, Joris I. Apoptosis, oncosis and necrosis: an overview of cell death. Am J Pathol. 1995;146:3–15. [PMC free article] [PubMed] [Google Scholar]
- 28.Marshall A J, Miles R J, Richards L. The phagocytosis of mycoplasmas. J Med Microbiol. 1995;43:239–250. doi: 10.1099/00222615-43-4-239. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh K R, Drachman D B. Induction of apoptosis in activated T cell blasts by suppressive macrophages: a possible immunotherapeutic approach for treatment of autoimmune disease. Cell Immunol. 1999;193:24–35. doi: 10.1006/cimm.1998.1445. [DOI] [PubMed] [Google Scholar]
- 30.Messmer T O, Black C M, Thacker W L. Mycoplasma contamination of chlamydiae isolated from clinical specimens. APMIS. 1994;102:793–796. doi: 10.1111/j.1699-0463.1994.tb05236.x. [DOI] [PubMed] [Google Scholar]
- 31.Mix E, Zettl U K, Zielasek J, Hartung H P, Gold R. Apoptosis induction by macrophage-derived reactive oxygen species in myelin-specific T cells requires cell-cell contact. J Neuroimmunol. 1999;95:152–156. doi: 10.1016/s0165-5728(99)00006-5. [DOI] [PubMed] [Google Scholar]
- 32.Nanagara R, Li F, Beutler A, Hudson A P, Schumacher H R. Alteration of Chlamydia trachomatis biologic behavior in synovial membranes. Suppression of surface antigen production in reactive arthritis and Reiter's syndrome. Arthritis Rheum. 1995;38:1410–1417. doi: 10.1002/art.1780381008. [DOI] [PubMed] [Google Scholar]
- 33.Nicoletti I, Migliorati G, Pagliacci M C, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–279. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 34.Ojicius D M, Souque P, Perfettini J-L, Dautry-Varsat A. Apoptosis of epithelial cells and macrophages due to infection with the obligate intracellular pathogen Chlamydia psittaci. J Immunol. 1998;161:4220–4226. [PubMed] [Google Scholar]
- 35.Oyaizu N, Adachi Y, Hashimoto F, McCloskey T W, Hosaka N, Kayagaki N, Yagita H, Pahwa S. Monocytes express Fas ligand upon CD4 cross-linking and induce CD4+ T cells apoptosis. A possible mechanism of bystander cell death in HIV infection. J Immunol. 1997;158:2456–2463. [PubMed] [Google Scholar]
- 36.Paddenberg R, Wulf S, Weber A, Heimann P, Beck L A, Mannherz H G. Internucleosomal DNA fragmentation in cultured cells under conditions reported to induce apoptosis may be caused by mycoplasma endonucleases. Eur J Cell Biol. 1996;69:105–119. [PubMed] [Google Scholar]
- 37.Perfettini J-L, Darville T, Gachelin G, Souque P, Huerre M, Dautry-Varsat A, Ojcius D M. Effect of Chlamydia trachomatis infection and subsequent tumor necrosis factor alpha secretion on apoptosis in the murine genital tract. Infect Immun. 2000;68:2237–2244. doi: 10.1128/iai.68.4.2237-2244.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perry L L, Feilzer K, Caldwell H D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-γ-dependent and -independent pathways. J Immunol. 1997;158:3344–3352. [PubMed] [Google Scholar]
- 39.Rawadi G, Roman-Roman S, Castedo M, Dutilleul V, Susin S, Marchetti P, Geuskens M, Kroemer G. Effects of Mycoplasma fermentans on the myelomonocytic lineage. Different molecular entities with cytokine-inducing and cytocidal potential. J Immunol. 1996;156:670–678. [PubMed] [Google Scholar]
- 40.Razin S, Yogev D, Naot Y. Molecular biology and pathogenicity of mycoplasmas. Microbiol Mol Biol Rev. 1998;62:1094–1156. doi: 10.1128/mmbr.62.4.1094-1156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revillard J P, Adorini L, Goldman M, Kabelitz D, Waldman H. Apoptosis: potential for disease therapies. Immunol Today. 1998;19:291–293. doi: 10.1016/s0167-5699(98)01279-1. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz E, Nettelnbreker E, Zeidler H, Hammer M, Manor E, Wollenhaupt J. Intracellular persistence of chlamydial major outer membrane protein, lipopolysaccharide and rRNA after non-productive infection of human monocytes with Chlamydia trachomatis serovar K. J Med Microbiol. 1993;38:278–285. doi: 10.1099/00222615-38-4-278. [DOI] [PubMed] [Google Scholar]
- 43.Schnarr S, Jendro M C, Putschky N, Zeidler H, Hammer M, Wollenhaupt J. High frequency of chlamydia and borrelia in patients with unclassified oligoarthritis: result of a prospective study. Arthritis Rheum. 1997;40(Suppl.):S142. doi: 10.1002/1529-0131(200111)44:11<2679::aid-art447>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 44.Seki H, Kanegane H, Iwai K, Konno A, Ohta K, Yachie A, Taniguchi N, Miyawaki T. Ionizing radiation induces apoptotic cell death in human TcR-γ/δ+ T and natural killer cells without detectable p53 protein. Eur J Immunol. 1994;24:2914–2917. doi: 10.1002/eji.1830241150. [DOI] [PubMed] [Google Scholar]
- 45.Su H, Caldwell H D. Kinetics of chlamydial antigen processing and presentation to T cells by paraformaldehyde-fixed murine bone marrow-derived macrophages. Infect Immun. 1995;63:946–953. doi: 10.1128/iai.63.3.946-953.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taylor-Robinson D, Gibroy C B, Thomas B J, Keat A C S. Detection of Chlamydia trachomatis in the joints of reactive arthritis by polymerase chain reaction. Lancet. 1992;340:81–82. doi: 10.1016/0140-6736(92)90399-n. [DOI] [PubMed] [Google Scholar]
- 47.Telford W G, King L E, Fraker P J. Rapid quantification of apoptosis in pure and heterogeneous cell populations using flow cytometry. J Immunol Methods. 1994;172:1–16. doi: 10.1016/0022-1759(94)90373-5. [DOI] [PubMed] [Google Scholar]
- 48.Thoma-Uszynski S, Simnacher U, Marre R, Essig A. Clearance of Chlamydia trachomatis-induced polyserositis in SCID mice requires both CD4+ and CD8+ cells. Microbiol Immunol. 1998;187:71–78. doi: 10.1007/s004300050076. [DOI] [PubMed] [Google Scholar]
- 49.Thompson C B. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 50.Verkooyen R P, Sijmons M, Fries E, Van Belkum A, Verbrugh H A. Widely used, commercially available Chlamydia pneumoniae antigen contaminated with mycoplasma. J Med Microbiol. 1997;46:419–424. doi: 10.1099/00222615-46-5-419. [DOI] [PubMed] [Google Scholar]
- 51.Ward M E. The immunobiology and immunopathology of chlamydial infections. APMIS. 1995;103:769–796. doi: 10.1111/j.1699-0463.1995.tb01436.x. [DOI] [PubMed] [Google Scholar]
- 52.Weinblatt M E, Kremer J M, Bankhurst A D, Bulpitt K J, Fleischmann R M, Fox R I, Jackson C G, Lange M, Bruge D J. A trial of etanercept, a recombinant tumor necrosis factor receptor:Fc fusion protein, in patients with rheumatoid arthritis receiving methotrexate. N Engl J Med. 1999;340:253–259. doi: 10.1056/NEJM199901283400401. [DOI] [PubMed] [Google Scholar]
- 53.Wesch D, Marx S, Kabelitz D. Monocyte-dependent death of freshly isolated T lymphocytes: induction by phorbolester and mitogens and differential effects of catalase. J Immunol. 1998;161:1248–1256. [PubMed] [Google Scholar]
- 54.Wilkinson N Z, Kingsley G H, Sieper J, Braun J, Ward M E. Lack of correlation between the detection of Chlamydia trachomatis DNA in synovial fluid from patients with a range of rheumatic diseases and the presence of an antichlamydial immune response. Arthritis Rheum. 1998;41:845–854. doi: 10.1002/1529-0131(199805)41:5<845::AID-ART11>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 55.Williams D M, Grubbs B G, Pack E, Kelly K, Rank R G. Humoral and cellular immunity in secondary infection due to murine Chlamydia trachomatis. Infect Immun. 1997;65:2876–2882. doi: 10.1128/iai.65.7.2876-2882.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng L, Fisher G, Miller R E, Peschon J, Lynch D H, Lenardo M J. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377:348–351. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 57.Zychlinski A, Sansonetti P. Perspectives series: host/pathogen interactions. Apoptosis in bacterial pathogenesis. J Clin Investig. 1997;100:493–495. doi: 10.1172/JCI119557. [DOI] [PMC free article] [PubMed] [Google Scholar]