Abstract
This year marks the 50th anniversary of Baird’s rules of aromaticity — a set of perturbational molecular orbital theory analyses that has garnered considerable attention in the past ten years in light of its many real-world applications in photochemistry.
Aromaticity, an ever-evolving concept first developed to explain the properties of ‘benzene-like’ compounds, has since expanded to describe a myriad of organic and inorganic species, in two and three dimensions, and in the ground and excited states. Hückel’s early investigations into the stability of planar conjugated annulenes led to the now widely known [4n+2] π-electron rule of aromaticity. A couple of decades later, Baird reported his theoretical analysis for cyclic polyenes showing that, in their lowest triplet state, [4n] rings were instead aromatic and [4n+2] rings antiaromatic (Fig. 1a).
Fig. 1 |. Baird’s rule is a reversal of the Hückel electron-counting rule for aromaticity and antiaromaticity in the S1 and T1 ππ* states.
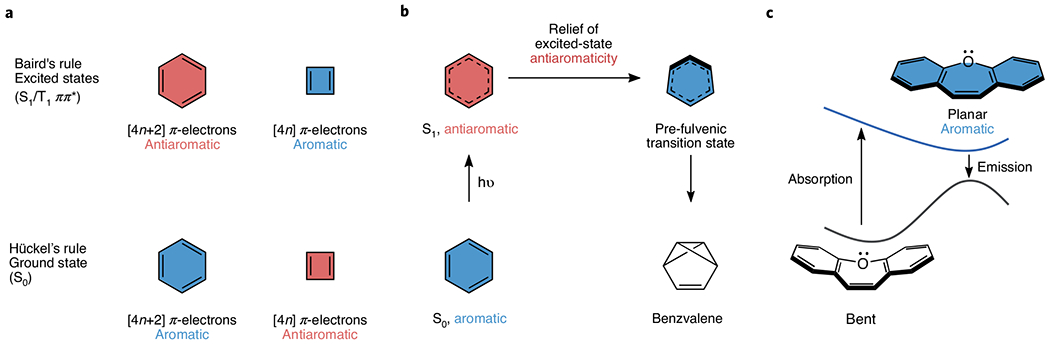
a, Ground- and excited-state aromaticity and antiaromaticity for benzene and cyclobutadiene. b, Photorearrangement of benzene to fulvene and benzvalene. c, Dibenz[b,f] oxepin displays a large Stokes shift due to excited-state aromaticity.
This reversal of electron-counting rules for aromaticity and antiaromaticity in the lowest ππ* excited states — which arose from a set of perturbational molecular orbital theory analyses1 — didn’t immediately attract all that much excitement in the community. In the first four decades after its publication, Baird’s 1972 paper1 had garnered just shy of 150 citations. Yet, references to this theory have more than tripled in the ten years since. What sparked this increased interest?
It wasn’t that manifestations of Baird’s rules were lacking during those four first decades — it was relating observation to theory that was often missed. Photorearrangement of benzene to fulvene and benzvalene (Fig. 1b)2, for example, has been known since the mid-1960s; Baird1, and later Aihara3, had even linked the photoreactivity of benzene to its excited-state antiaromatic character at the time. These conclusions were overlooked for many years, however.
The first direct spectroscopic evidence for the reversal of excited-state aromaticity and antiaromaticity in the lowest excited state compared to the ground state had come in 19934. Wan and Krogh5 showed that dibenz[b,f]oxepin (Fig. 1c) displayed large Stokes shifts resulting from planarization of the 8π-electron core in the S1 state. They commented that: “The driving force for this geometry change is the attainment of a cyclically conjugated system of 8 electrons in the central ring, which is believed to have inherent stability on the excited-state surface.” This experiment formed the basis for many recent designs of fluorophores by use of Baird’s rules. Reversal of excited-state aromaticity and antiaromaticity was also evident in many of the experiments performed by Wan and co-workers during the 1980s and 1990s, including a pioneering 1985 paper that showed that photolysis of fluoren-9-ol generated exceptionally stable 4π cationic systems, which was insightfully interpreted as a consequence of excited-state aromaticity5.
The community’s interest in Baird’s rules first took a turn in 1998 when Schleyer and co-workers6 published an important paper demonstrating that magnetic shieldings for open-shell species — while not accessible by NMR experiments — could be readily computed, and that the results obtained for neutral and charged [4n] π-electron annulenes supported Baird’s theory. Triplet state [4n] π-electron annulenes exhibited negative nucleus-independent chemical shifts values, downfield chemical shifts and prominent diamagnetic susceptibility exaltations in line with their expected aromatic character. Quoting the authors: “These open-shell calculations of magnetic properties may be ‘unphysical’ but they are instructive and useful for many purposes.” And indeed, they were.
Although the conclusions of the 1998 paper were derived by calculation, the simplicity of the nucleus-independent chemical shifts method and its apparent connection to real NMR measurements made the findings valuable to a wide community of chemists. It spawned theoretical and experimental interest in Baird’s rules. Between 1998 and 2008, Baird’s rules were extended to the lowest singlet excited-state of annulenes and then expanded significantly to include many organic and inorganic species.
In 2014, an influential Review published by Ottosson, Kilsa and co-workers7 proved to be a tipping point that propelled the field of excited-state aromaticity and antiaromaticity research towards real-world applications. The 64-page opus amassed an enormous collection of examples demonstrating the significance of Baird’s rules for excited-state properties, synthesis and photoreactions. It provided important reinterpretations for hundreds of experiments and highlighted the tremendous potential of Baird’s rules for photophysics and photochemistry.
Then in 2015, Ottosson and Papadakis8 described the reactivity of benzene with reference to the Scottish horror fiction: Strange Case of Dr Jekyll and Mr Hyde. They related the ground state to the mild-mannered Dr Jekyll and its excited state to the turbulent Mr Hyde8. This vivid picture influenced the work of many, including our own9. Benzene — perhaps the most well-known aromatic compound — was also the archetype of excited-state antiaromaticity!
Now Baird’s theory is realizing many of its promised impacts, including for developments of synthetic methods10, light-active molecules and materials11,12, and fluorophores13. Alabugin and Ottosson showed that synthetic routes to a stereoelectronically disfavoured cycloaromatization reaction could be achieved by twisting a benzannelated double bond via antiaromaticity relief of a triplet-state benzene ring10. The smallest red-light emitter resulting from excited-state antiaromaticity relief and ring bond length elongation of a benzene core was just reported11. Using time-resolved electron diffraction, Hada and co-workers12 captured dynamic structural changes induced by reversal of excited-state aromaticity for stacked columns of cyclooctatetraenes. It was shown that aggregation-induced emission — a phenomenon with tremendously attractive biomedical engineering applications — can arise from large conformation changes resulting from reversal of excited-state aromaticity13.
Using time-resolved spectroscopy, Kim and Osuka14 reported the first direct experimental evidence for aromaticity and antiaromaticity reversal in the lowest triplet state. Reversed spectroscopic signatures were recorded for a pair of aromatic and antiaromatic bis-rhodium hexaphyrins, which interconverted to antiaromatic and aromatic, respectively, in their triplet states. Synergy between experiment and theory was critical to forming the conclusions of this landmark paper; experiments provided evidence, but theory endorsed the interpretation.
Yet, Baird’s rules must be applied with care. Aromatic rings with S1 and T1 states of nπ* character can preserve aromaticity and are not subject to Baird’s rules. Some Craig-type Möbius aromatic organometallic rings can be aromatic in both the S0 and T1 states due to metal-to-ring excitation15. The photophysics of annulenes are often assumed to occur from the lowest singlet or triplet states but can actually involve higher excited states. Polycyclic compounds can retain Hückel aromaticity by altering ring current pathways in the S1 and T1 states versus the S0 state16.
Beyond their implications and limitations, the story of Baird’s rules is an excellent example of experiments guided by theory.
Footnotes
Competing interests
The authors declare no competing interests.
References
- 1.Baird NC J. Am. Chem. Soc 94, 4941–4948 (1972). [Google Scholar]
- 2.Slanina T et al. J. Am. Chem. Soc 142, 10942–10954 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aihara J-I Bull. Chem. Soc. Jpn 51, 1788–1792 (1788). [Google Scholar]
- 4.Shukla D & Wan P J. Am. Chem. Soc 115, 2990–2991 (1993). [Google Scholar]
- 5.Wan P & Krogh E J. Chem. Soc. Chem. Commun 1207–1208 (1985). [Google Scholar]
- 6.Gogonea V, Schleyer Pv. R. & Schreiner PR Angew. Chem. Int. Ed 37, 1945–1948 (1998). [Google Scholar]
- 7.Rosenberg M et al. Chem. Rev 114, 5379–5425 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Papadakis R & Ottosson H Chem. Soc. Rev 44, 6472–6493 (2015). [DOI] [PubMed] [Google Scholar]
- 9.Karas LJ, Wu C-H & Wu JI J. Am. Chem. Soc 143, 17970–17974 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mohamed RK et al. J. Am. Chem. Soc 137, 15441–15450 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Kim H et al. Nat. Commun 12, 5409 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hada M et al. J. Am. Chem. Soc 139, 15792–15800 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Zhao Z et al. Nat. Commun 10, 2952 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sung YM et al. Nat. Chem 7, 418–422 (2015). [DOI] [PubMed] [Google Scholar]
- 15.Chen D et al. Commun. Chem 1, 18 (2018). [Google Scholar]
- 16.Zeng W et al. Chem. Sci 12, 6159–6171 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


