Abstract
Conventional tools for elucidating gene function are relatively scarce in Candida albicans, the most prevalent human fungal pathogen. To this end, we developed a convenient system to control gene expression in C. albicans by the tetracycline-regulatable (TR) promoters. When the sea pansy Renilla reniformis luciferase gene (RLUC1) was placed under the control of this system, doxycycline (DOX) inhibited the luciferase activity almost completely. In the absence of DOX, the RLUC1 gene was induced to express luciferase at a level 400- to 1,000-fold higher than that in the presence of DOX. The same results were obtained in hypha-forming cells. The replacement of N-myristoyltransferase or translation elongation factor 3 promoters with TR promoters conferred a DOX-dependent growth defect in culture media. Furthermore, all the mice infected with these mutants, which are still virulent, survived following DOX administration. Consistently, we observed that the number of these mutant cells recovered from the mouse kidneys was significantly reduced following DOX administration. Thus, this system is useful for investigating gene functions, since this system is able to function in both in vitro and in vivo settings.
Candida albicans is the most important opportunistic fungal pathogen of humans. In recent years, the incidence of candidiasis has been increasing, particularly among patients with immune systems compromised by human immunodeficiency virus infection, organ transplantation, and/or chemotherapy for cancer (18). Current therapies for treating systemic fungal infection have limited effectiveness and have created problems of adverse reactions and drug resistance (3, 19). Therefore, the search for novel antifungal drugs has been carried out.
The C. albicans genome-sequencing project has recently begun (11), and many novel genes are being identified. Therefore, there should be increasing demands to assess gene function by genetic approaches. To date, it has been difficult to apply conventional approaches to C. albicans because of its diploid genome and because of difficulty in generating haploid strains. Integrative transformation of C. albicans through homologous recombination has made gene disruption possible (1, 10). Such studies are impossible, however, if the ablated genes are essential for cell growth. To overcome these issues, the development of convenient genetic tools, such as a gene expression system, is needed.
The tetracycline-regulatable (TR) expression system is a popular gene expression system among eukaryotic cells (6, 7, 16, 17). This system consists of two components: one is a TR transactivator, a fusion protein of the Escherichia coli tetracyline repressor protein (TetR) and the activation domain of transcriptional activators, such as VP16, Gal4p, and Hap4p (7, 16). The other is a TR promoter, comprising a minimal promoter element with a tetracycline operator sequence (tetO). The system is based on the molecular mechanism of tetR in association with tetO, which is well characterized in E. coli as a tetracyline-resistant gene expression machinery on the Tn10 transposon (8). In the absence of tetracycline, tetR can specifically bind tetO as a dimer. However, their dissociation is rapid in the presence of tetracycline since tetR dimerization is inhibited by this small compound possessing a high binding constant with tetR (6). Therefore, gene expression under this system can be actively expressed in the absence of tetracycline by the binding of the TR transactivator to tetO, and it can be repressed by adding tetracycline, which inhibits such binding. Compared with the alternative systems for controlling gene expression in eukaryotes, the TR expression system has distinct advantages; it is highly specific, nontoxic, and noneukaryotic and is consequently expected to have no pleiotropic effect on host cell genes (6, 7). When applied to pathogenic fungi, it could also function in cells infecting an animal host (17).
Here we report the establishment of a TR expression system in C. albicans. This system enables tight repression and highfold induction of gene expression in both in vivo and in vitro settings. Our results suggest that this system is a powerful tool for functional analyses of C. albicans genes.
MATERIALS AND METHODS
Strains and growth media.
E. coli DH5α was used as the host strain for all plasmid preparations, and was grown in Luria-Bertani medium. All of the C. albicans strains used in this study (Table 1) were cultivated at 30°C in YEPD (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone, and 2% [wt/vol] glucose). YEPGlcNAc (1% [wt/vol] yeast extract, 2% [wt/vol] Bacto Peptone, and 2% [wt/vol] N-acetylglucosamine) or RPMI 1640 (Sigma) was used for induction of hyphal formation. YNB (0.67% [wt/vol] yeast nitrogen base, 2% [wt/vol] glucose, and supplement for auxotrophic requirement) was used for selective medium after transformation (9).
TABLE 1.
Strains used in this study
| Strain | Parent strain | Genotype | Source or reference |
|---|---|---|---|
| CAF2 | SC5314 | URA3/ura3::imm434 | 4 |
| CAI8 | CAF2 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 | 4 |
| THE1 | CAI8 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 | This study |
| 97RL | THE1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 ade2::hisG/ade2::ADE2-URA3-97t-RLUC1 | This study |
| 98RL | THE1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 ade2::hisG/ade2::ADE2-URA3-98t-RLUC1 | This study |
| 99RL | THE1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 ade2::hisG/ade2::ADE2-URA3-99t-RLUC1 | This study |
| HUTEF3 | THE1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 TEF3/tef3::hisG-URA3-hisG | This study |
| HTEF3 | HUTEF3 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 TEF3/tef3::hisG | This study |
| 97ATEF3 | HTEF3 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 tef3::hisG/tef3::97t-TEF3-URA3 | This study |
| 98ATEF3 | HTEF3 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 tef3::hisG/tef3::98t-TEF3-URA3 | This study |
| 99ATEF3 | HTEF3 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 tef3::hisG/tef3::99t-TEF3-URA3 | This study |
| HUNMT1 | THE1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 NMT1/nmt1::hisG-URA3-hisG | This study |
| HNMT1 | HUNMT1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 NMT1/nmt1::hisG | This study |
| 97ANMT1 | HNMT1 | ′ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 nmt1::hisG/nmt1::97t-NMT1-URA3 | This study |
| 98ANMT1 | HNMT1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 nmt1::hisG/nmt1::98t-NMT1-URA3 | This study |
| 99ANMT1 | HNMT1 | ade2::hisG/ade2::hisG ura3::imm434/ura3::imm434 ENO1/eno1::ENO1-tetR-ScHAP4AD-3×HA-ADE2 nmt1::hisG/nmt1::99t-NMT1-URA3 | This study |
Plasmid construction.
Primers and linkers used in this study are listed in Table 2. Plasmid pCAITHE5 (Fig. 1B), which harbors the gene encoding the fusion transactivator, tetR-ScHAP4AD (16), was constructed as follows. tetR-ScHAP4AD was introduced into PstI/XhoI sites of pCRW3 (21) after we replaced all four CUG codons with other leucine codons by PCR-mediated mutagenesis (Table 2). This mutagenesis was completed by connecting six fragments. The N-terminal portion of tetR-ScHAP4AD was generated by annealing synthetic oligonucleotides TETRFL and TETRRL. The other five fragments were amplified by PCR using primer pairs TETRF-TETRR, HAP401F-HAP401R, HAP402F-HAP402R, HAP403F-HAP403R, or HAP404F-HAP404R, and then they were digested with appropriated endonucleases (Fig. 1A). To express the gene in C. albicans, the CaENO1 promoter region (nucleotides [nt] −528 to −6), which was amplified by PCR with the primers PCAENO1F and PCAENO1R, was introduced into the ClaI/PstI site of pCRW3. To confirm the expression of tetR-ScHAP4AD, the triplet of the hemagglutinin (3×HA) epitope, which was generated by annealing HAFL and HARL, was introduced into the C-terminal of tetR-ScHAP4AD (XhoI/ApaI site).
TABLE 2.
Linkers and primers used in this study
| Primer or linker | Designed region | Sequence (5′-3′)a |
|---|---|---|
| Primer | ||
| TETRF | tetR C′-terminal sense | TTTTTAAGCTT CTTAATGAGGTCGG |
| TETRR | tetR C′-terminal antisense | TTTTTTCCGCGGCCACTTTCACATTTAAGTTGTTT |
| HAP401F | ScHAP4AD sense | TTTTCTGCAGTTCCGCGGTAACGAAAATAATGATCTCTGG |
| HAP401R | ScHAP4AD antisense | TTTTACATGTTATCGTCATTGTCCACTTGTT |
| HAP402F | ScHAP4AD sense | TTTTACATGTCTTTATTGAATTTGCCAATTTTG |
| HAP402R | ScHAP4AD antisense | TTTTGGATCCTGAAGAAACAAATAGGTTTC |
| HAP403F | ScHAP4AD sense | TTTTTTTGATCAGGATGAAAGCGCTG |
| HAP403R | ScHAP4AD antisense | TTTTTTAGTACTTTGGTGTAGTCGTCATC |
| HAP404F | ScHAP4AD sense | TTTTAGTACTTAAATCCAAAAAAATTTCTACGTCG |
| HAP404R | ScHAP4AD | TTTTCTCGAGAAAATACTTGTACCTTTAAAAAATC |
| PCAENO1F | CaENO1 5′-flanking sense | TTTTATCGATGGGATCAAGATTTGTTTACAGG |
| PCAENO1R | CaENO1 5′-flanking antisense | TTTCTGCAGAATATTCCTGAATTATCAATTGATG |
| TADH101 | ScADH1 3′-flanking sense | TTTTCCCGGGTGGACTTCTTCGCCAGAGG |
| HOP101 | ScHOP1 5′-flanking antisense | TTTTCTGCAGACTAGTTTTCTGAGATAAAGCTGTTTTT |
| HOP102SAL | ScHOP1 5′-flanking antisense | TTTTGTCGACTTTTCTGAGAATAAAGCTGTTTTT |
| TEFAF | CaTEF3 5′-flanking sense | TTTTGAATTCGGTACCTCATCGGATTTTGATTCAATGC |
| TEFAR | CaTEF3 5′-flanking antisense | TTTTCCCGGGTCGACGTTCCTGTATATTAGTTGCC |
| TEFBF | CaTEF3 N′-terminal sense | TTTTACTAGTTGGAAAATGTCTGCTGCTAGT |
| TEFBRN | CaTEF3 N′-terminal antisense | TTTTGCGGCCGCAGAAGCAATGGCCAATAAAG |
| TEFDBR | CaTEF3 3′-flanking antisense | TTTTGGATGCTCTTCTTCTTCATCATCCTCC |
| NMTAF | CaNMT1 5′-flanking sense | TTTTGGATCCTATAGCCAACTACTCCATAAATT |
| NMTAR | CaNMT1 5′-flanking antisense | TTTTGTCGACTTGATCGTTAATTGTTTTTAC |
| NMTBF | CaNMT1 N′-terminal sense | TTTTACTAGTTAGAATATGTCGGGAGATAAC |
| NMTBRN | CaNMT1 N′-terminal antisense | TTTTGCGGCCGCCAATCTTTTCTCCATCCC |
| NMT3SPH | CaNMT1 3′-franking antisense | CTACTAGTCATAGCATGCAAATGGGATGAGATTG |
| Linker | ||
| TETRFL | tetR N′-terminal sense | GATGTCTAGATTAGATAAAAGTAAAGTGATTAACAGCGCATTAG |
| TETRRL | tetR N′-terminal antisense | AGCTCTAATGCGCTGTTAATCACTTTACTTTTATCTAATCTAGACATCTGCA |
| HAFL | Triple HA epitope sense | TCGAGCAGCTAGCTACCCATACGATGTTCCGGATTACGCTTACCCTTACGAT GTGCCGGATTACGCTTACCCATACGATGTACCGGATTACGCTAG |
| HARL | Triple HA epitope antisense | CTCAATCACACAGCTAGCGTAATCCGGTACATCGTATGGGTAAGCGTAATC CGGCACATCGTAAGGGTAAGCGTAATCCGGAACATCGTATGGGTA |
Italic letters indicate the restriction enzyme site. Underlined italic letters indicate replaced codons for CTG.
FIG. 1.
Schematic representation of plasmid constructions and the TR promoters 97t, 98t, and 99t. (A) Schematic representation of the construction of the tetR-ScHAP4AD fragment. The hatched box shows the fragment generated by annealing synthetic oligonucleotides. The gray boxes show the fragments amplified by PCR. The asterisks indicate the mutation points. HindIII+, HindIII site was disrupted by connecting fragments. (B) Restriction map of plasmid pCAITHE5 which harbors the gene for the TR transactivator tetR-ScHAP4AD. (C) Schematic representation of the TR promoters 97t, 98t, and 99t (16, 17). tADH is the termination sequence of ScADH1. Hatched boxes show derivatives of the ScHOP1 promoter. (D) Restriction enzyme map of the reporter plasmids p97RLU, p98RLU, and p99RLU, in which the RLUC1 gene is connected with TR promoters. (E) Restriction enzyme map of p97CAU, p98CAU, and p99CAU, in which the URA3 gene and TR promoters are located on the multicloning site on pBluescript SK II(+). These plasmids are utilized to prepare fragments that are used for replacing the endogenous promoter with TR promoters.
Reporter plasmids p97RLU, p98RLU, and p99RLU (Fig. 1D) were constructed by introducing the URA3 gene, the ADE2 gene, the TR promoter, and the RLUC1 gene into pUC18. The URA3 gene was excised as a 1.3-kb fragment from pCA1U (14). The ADE2 gene and the RLUC1 gene were prepared from pCRW3 as a 2.4-kb fragment and as a 1-kb fragment, respectively. The TR promoters 97t, 98t, and 99t consist of 350 bp of Saccharomyces cerevisiae ADH1 terminator (tADH), tetO, and S. cerevisiae HOP1 promoter derivatives (Fig. 1C; references 16 and 17) generated by PCR with primers TADH101 and HOP102SAL.
Two plasmids, pTEFD11 and pNMTD1, for disrupting TEF3 or NMT1 were generated as follows. The TEF3 and NMT1 genes were amplified by PCR with primer pairs TEFAF-TEFDBR or NMTAF-3SPH. These genes were cloned once into pCR2.1 (Invitrogen), and then their internal-regions, the 2.8-kb NspV fragment of TEF3 and the 1.1-kb MunI fragment of NMT1, were respectively replaced with the hisG-URA3-hisG module, which was excised from pCA1U (14).
Three plasmids, p97CAU, p98CAU, and p99CAU, were generated by introducing a 1.3-kb fragment from pCA1U containing the URA3 gene and 0.6-kb PCR-generated SmaI/SpeI fragments containing TR promoters (97t, 98t, or 99t) into pBluescript SK II(+). The SmaI/SpeI fragments were amplified by PCR with the primers TADH101 and HOP101 from p97CGH, p98CGH, and p99CGH (17).
Six plasmids, p97TEF3, p98TEF3, p99TEF3, p97NMT1, p98NMT1, and p99NMT1, were constructed by introducing the 5′-flanking region (region A) and the 5′ portion of the coding region (region B) of TEF3 or NMT1 into the KpnI/SalI site (for region A's) and into the SpeI/NotI site (for region B's) of p97CAU, p98CAU, and p99CAU (Fig. 1E). Region A (nt −726 to −369) or region B (nt −6 to 367) of TEF3 was also amplified by PCR with primer pairs TEFAF-TEFAR or TEFBF-TEFBRN, respectively. Region A (nt −113 to −17) or region B (nt −6 to 411) of NMT1 was amplified by PCR with primer pairs NMTAF-NMTAR or NMTBF-NMTBRN, respectively.
Generation of test strains and transactivator-expressing strain.
Yeast transformations were carried out by the lithium acetate method (9). In all generated strains, the correct integrations of the prepared fragments on the target locus were confirmed by Southern blot and PCR analyses (data not shown).
The gene-encoding TR transactivator, TetR-ScHAP4AD, was introduced into ENO1 locus in CAI8 (4) by transforming with pCAITHE5 that was linearized with AccI. Strain THE1 was obtained.
Reporter strains 97RL, 98RL, and 99RL that contain the RLUC1 gene on their ADE2 loci were obtained by transforming THE1 with p97RLU, p98RLU, and p99RLU after linearization with EcoT22I.
A scheme for creating TEF3- or NMT1-controllable strains is shown in Fig. 2. Gene disruptions to generate the heterozygous strains were carried out according to the ura blaster method (1). THE1 was transformed with the NotI/SpeI fragment of pTEFD11 or the XhoI/SpeI fragment of pNMTD1, yielding strains HUTEF3 and HUNMT1, respectively. Subsequently, both HUTEF3 and HUNMT1 cells were plated onto YNB plates containing 5-fluoroorotic acid (Wako), yielding their uracil auxotrophs, HTEF3 and HNMT1. To replace the endogenous promoters with the TR promoter in HTEF3 and HNMT1, the plasmids p97TEF3, p98TEF3, p99TEF3, p97NMT1, p98NMT1, and p99NMT1 were digested with KpnI/SacII and used to transform HTEF3 or HNMT1, yielding strains 97ATEF3, 98ATEF3, 99ATEF3, 97ANMT1, 98ANMT1, and 99ANMT1.
FIG. 2.
Schematic representation of construction of controllable strains. TG, target gene; step 1, disruption of one allele of TG by the ura blaster method (1). Step 2, replacement of endogenous promoters on another allele with TR promoters (Fig. 1C) by homologous recombination. Fragments used for this step were prepared from the plasmids generated by introducing the 5′-flanking region and 5′ portion of the open reading frame of the target gene into p97CAU1, p98CAU1, or p99CAU1 (see Fig. 1E).
Western blot analysis.
Cells were harvested after cultivation for 14 h in YEPD containing 0 or 20 μg of doxycycline (DOX) per ml. After the cells were disrupted with glass beads in phosphate-buffered saline (PBS) containing 1 mM phenylmethylsulfonyl fluoride (PMSF), soluble fractions were prepared by centrifugation at 12,000 × g for 10 min. The protein concentration of each supernatant was determined using a bicinchoninic acid protein assay kit (Pierce). Ten micrograms of each sample was separated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis on a 4 to 20% polyacrylamide gel, and the separated protein fractions were blotted onto a polyvinylidene difluoride membrane filter. The filter was blocked for 1 h at room temperature in PBS containing 5% (wt/vol) skim milk (Difco). The filter was incubated with an anti-HA mouse monoclonal antibody (Roche Diagnostics) diluted 1:1,000 for 1 h at 37°C. Then the filter was incubated with a horseradish peroxidase-conjugated anti-mouse immunoglobulin G antibody (Amersham-Pharmacia) diluted 1:5,000 for 30 min at 37°C. According to the manufacturer's instructions, signals were visualized on an X-ray film (Kodak) using enhanced chemiluminescence reagent (Amersham-Pharmacia).
Northern blot analysis.
Approximately 107 cells were inoculated in YEPD and cultivated with or without DOX (20 μg/ml) for 2 h. Cells were then harvested, and their total RNAs were prepared using Sepasol solution (Nacalai Tesque) according to the manufacturer's instructions. Ten micrograms of total RNA was separated on an agarose gel, and the separated RNA fractions were transferred onto a nylon membrane (Hybond-N; Amersham-Pharmacia). The probe DNAs used for hybridization were amplified by PCR with primer pair TEFBF-TEFBRN (for TEF3) or NMT1BF-NMT1BRN (for NMT1). These DNAs were radiolabeled by the random-priming method using [α-32P]dCTP. For normalization, a probe for exon 2 of the ACT1 gene was used. The hybridization was carried out in hybridization buffer (50% [vol/vol] formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% [wt/vol] SDS, 0.25% [wt/vol] skim milk, and 50 mM sodium phosphate [pH 6.5]) at 42°C for 16 h. After the membrane was washed with 0.2× SSC at 55°C for 1 h, the signals were visualized by autoradiography.
Luciferase assay.
After cultivation in the absence or presence of DOX (20 μg/ml) until the mid-log phase, approximately 2 × 108 cells were harvested and resuspended in RLUC buffer (500 mM NaCl, 100 mM K2HPO4 [pH 6.7], 1 mM sodium EDTA, 0.6 mM sodium azide, 1 mM PMSF, and 0.02% [wt/vol] bovine serum albumin) (13). After disruption of the cells, soluble fractions were prepared by centrifugation and mixed with the reaction buffer (RLUC buffer containing 0.5 μM coelentrazine [Molecular Probes, Inc.]). The light emission level of the mixture was measured at 480 nm for 30 s using a luminometer (Wallac). Relative light units (RLUs) represent light emitted per 30 s per microgram of protein.
Systemic infection of mice and quantification of C. albicans in infected tissue.
Male CD-1 mice (4 weeks, 21.5 g) were fed food and water ad libitum throughout the course of experiment. In the DOX-treated group, mice were administered with DOX (2 mg/ml) dissolved in 5% sucrose solution as drinking water from 2 days before the inoculation of C. albicans cells. In this dose regimen, each mouse drank approximately 5 ml of sucrose solution every day. Results show that the concentrations of DOX in serum, liver, and kidney were maintained at more than 2 μg/ml of serum, 8 μg/g of liver, and 10 μg/g of kidney, respectively (17). Precultured cells were suspended in saline and were counted using a hemocytometer. The cell suspension (0.2 ml) was inoculated into the mice intravenously. In each survival experiment, 106 or 107 of C. albicans cells were inoculated into five or seven mice, respectively. The numbers of surviving mice after the infection were counted. For each strain, mice were sacrificed 5 or 96 h after inoculation of 107 of C. albicans cells, and their kidneys were removed and homogenized. The homogenates were plated on YEPD plates containing penicillin G (200 U/ml) and streptomycin (200 μg/ml). After 36 h of incubation at 30°C, the numbers of C. albicans colonies were counted.
RESULTS
Generation of strain expressing a TR transactivator.
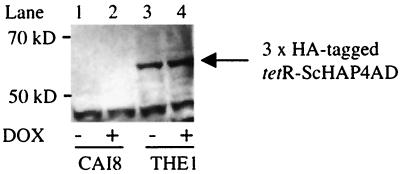
We introduced a TR transactivator into C. albicans. As the partner of tetR, we used S. cerevisiae HAP4AD (the activation domain of Hap4p: amino acid positions 330 to 554), which is well characterized as the minimal region for transcriptional activation (5) and is known to be functionable in S. cerevisiae (16). To ensure that tetR-ScHAP4AD functions as a transactivator, all four CUG codons in the gene were replaced with leucine codons (Table 2) to avoid loss of function due to abnormal usage of the CUG codon as serine in C. albicans (20). To express tetR-ScHAP4AD in C. albicans, the gene was introduced under the control of the CaENO1 promoter (12). After transforming CAI8 (4) with AccI-linearized pCAITHE5 (Fig. 1B), a TR-transactivator-expressing strain, THE1, was obtained. To confirm the expression, we performed Western blot analysis using an antibody against the HA epitope that was introduced into the C terminus of tetR-ScHAP4AD. As shown in Fig. 3, the signal at 55 kDa was specifically detected in THE1, indicating that tetR-ScHAP4AD was expressed in this strain. Furthermore, the expression of tetR-ScHAP4AD in THE1 was not affected following the addition of DOX (lanes 3 and 4).
FIG. 3.
Detection of expression of tetR-ScHAP4AD. The 3×HA-tagged tetR-ScHAP4AD was expressed in CAI8 cells (lanes 1 and 2) and THE1 cells (lanes 3 and 4) as an approximately 55-kDa protein (arrow).
DOX-regulated expression of sea pansy Renilla reniformis luciferase gene (RLUC1) in C. albicans.
As a reporter gene, the RLUC1 gene (13, 21) was used to characterize transcriptional regulation of the TR expression system in C. albicans. As TR promoters, we used the tetO-ScHOP1 promoter derivatives, 97t, 98t, and 99t (Fig. 1C), because they have varying activity levels and are almost completely repressed by DOX in S. cerevisiae and C. glabrata (16, 17). In these promoters, the 350-bp fragment from the ScADH1 terminator (tADH) is located upstream of tetO, eliminating readthrough from the promoter-like sequence located upstream of the TR promoters. Reporter plasmids, p97RLU, p98RLU, and p99RLU (Fig. 1D), were introduced into the ADE2 locus in THE1, yielding the reporter strains 97RL, 98RL, and 99RL, respectively. When we compared the luciferase activity of each strain in the absence of DOX, each strain showed an apparent luciferase activity at various levels; the highest was observed in 99RL (Table 3). On the other hand, in the presence of DOX (20 μg/ml), the luciferase activity levels were dramatically decreased. These results suggest that the fusion transactivator, tetR-ScHAP4AD, can promote transcription via TR promoters in C. albicans and that DOX is able to inhibit this transcription machinery.
TABLE 3.
DOX-regulated luciferase activity in C. albicans
| Strain | Luciferase activity (mean RLU/mg of total protein ± SD)a of:
|
|||
|---|---|---|---|---|
| Yeast form
|
Hyphal form
|
|||
| −DOX | +DOX | −DOX | +DOX | |
| 97RL | 504.3 ± 30.3 | 0.8 ± 0.2 | 936.8 ± 51.8 | 0.5 ± 0.2 |
| 98RL | 811.1 ± 23.3 | 0.7 ± 0.1 | 958.3 ± 69.6 | 1.1 ± 0.1 |
| 99RL | 1,003.7 ± 178.0 | 2.7 ± 0.5 | 1,508.7 ± 36.8 | 2.6 ± 0.4 |
| THE1 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 | 0.1 ± 0.0 |
Values are means ± the standard deviations (three independent samples per group) for luciferase activity.
Control of RLUC1 gene by TR promoters in hypha-forming cells.
C. albicans is known to form both yeast and hyphal shapes. It is presumed that a dynamic alternation of the gene expression profile is involved in such morphological changes. Therefore, we examined whether or not the gene expression driven by this system is stable in hypha-forming cells. The luciferase activity of hypha-forming 97RL, 98RL, and 99RL cells was measured in the absence or presence of DOX (20 μg/ml). The hyphal formation was induced by N-acetylglucosamine (GlcNAc) and higher temperature (37°C). Although the luciferase activities of hypha-forming cells were higher than those of yeast cells, all reporter strains exhibited a DOX-dependent repression of the luciferase activities (Table 3). Similar results were obtained when RPMI 1640 was used as a hyphal formation inducer (data not shown). These results strongly suggest that this TR expression system can be fully applicable in controlling gene expression in C. albicans in hypha-forming cells as well as in yeast cells.
Control of TEF3 and NMT1 genes by TR promoter.
We applied the TR expression system to control the expression of a particular endogenous gene in C. albicans. As target genes, we chose the TEF3 and NMT1 genes, which are essential to cell growth (2, 15, 22, 23). We generated three controllable strains for each target gene (97ATEF3, 98ATEF3, 99ATEF3, 97ANMT1, 98ANMT1, and 99ANMT1). In these strains, one allele of the target gene was already disrupted by a hisG sequence, and the promoter region of another allele was replaced with TR promoters (Fig. 2 and see also Materials and Methods). Since the TEF3 gene and the NMT1 gene are required for viability, it was expected that the repression of these genes would confer DOX-dependent growth defect upon the cells. As shown in Fig. 4A and B, DOX did not affect the growth of the parent strain, CAF2. In contrast, all six controllable strains (97ATEF3, 98ATEF3, 99ATEF3, 97ANMT1, 98ANMT1, and 99ANMT1) showed severe growth defects on YEPD plates containing DOX (20 μg/ml), whereas all of them could grow similar to CAF2 in the absence of DOX. These results suggest that each transcriptional activity of TR promoters could be sufficient to support cell growth and that the DOX-mediated repression of TEF3 or NMT1 transcription hampered cell growth. To confirm these results, we investigated the transcriptional levels of TEF3 or NMT1 using the activated (in the absence of DOX) or repressed (in the presence of DOX) TR promoters by Northern blot analysis. As shown by the results of the luciferase reporter assay, the TR promoters provided various expression levels in the absence of DOX; 99t showed the highest transcriptional activity. On the other hand, in the presence of DOX (20 μg/ml), the mRNA level of the TEF3 or NMT1 transcript from the TR promoters markedly decreased within 2 h after addition of DOX, whereas DOX did not significantly affect the level of ACT1 mRNA (Fig. 4C and D). From these results, we conclude that the TR expression system could control endogenous gene expression in C. albicans.
FIG. 4.
Control of the TEF3 or NMT1 gene expression in C. albicans cells. (A) Effect of DOX on the growth of the TEF3-controllable strains 97ATEF3, 98ATEF3, and 99ATEF3. Cells of each strain were plated on YEPD agar (left) and YEPD agar containing DOX (20 μg/ml) (right) and then incubated for 36 h at 30°C. (B) Effect of DOX on the growth of the NMT1-controllable strains 97ANMT1, 98ANMT1, and 99ANMT1. Cells of each strain were plated on YEPD agar (left) and YEPD agar containing DOX (20 μg/ml) (right) and then incubated for 36 h at 30°C. (C) Northern blot analysis result of TEF3. Total RNA was prepared from cells cultured with (lanes 1, 3, 5, and 7) or without DOX (20 μg/ml; lanes 2, 4, 6, and 8) for 2 h. Fractionated RNA (10 μg) obtained by agarose gel electrophoresis was blotted onto Hybond-N, and the membrane was incubated with probes for the TEF3 and ACT1 genes. The signal obtained from ACT1 was used to normalize RNA signals. (D) Northern blot analysis result of NMT. Total RNA was prepared from the cells cultured with (lanes 1, 3, 5, and 7) or without DOX (20 μg/ml; lanes 2, 4, 6, and 8) for 2 h. Fractionated RNA (10 μg) obtained by agarose gel electrophoresis was blotted onto Hybond-N, and the membrane was incubated with probes for the NMT1 and ACT1 genes. The signal obtained from ACT1 was used to normalize RNA signals.
Feasibility of TR system in C. albicans cells infecting mice.
We investigated whether or not this system can control gene expression in C. albicans infecting mice. The controllable strains, 99ATEF3 and 97ANMT1, were chosen as test strains using a mouse systemic candidiasis model, because it was estimated that the mRNA level of TEF3 in 99ATEF3 or that of NMT1 in 97ANMT1 was likely to be the same as that of the endogenous TEF3 or NMT1 in CAF2 (Fig. 4C and D). DOX was administered to mice in their drinking water from 2 days before the infection. It had been confirmed that this regimen can maintain sufficient DOX concentration in tissues for the C. glabrata TR expression system to function in mice (17). All mice infected with CAF2 cells died without significant differences in the death rate between groups of mice (Fig. 5), suggesting that the course of infection of C. albicans cells is not affected by DOX. On the other hand, all DOX-treated mice infected with 99ATEF3 or 97ANMT1 cells survived, although 99ATEF3 or 97ANMT1 cells were still virulent: DOX-untreated mice succumbed to infection with 99ATEF3 or 97ANMT1 cells even at a low infection dose (106 cells) (Fig. 5). In addition, we investigated the number of C. albicans cells in mouse kidneys infected with 99ATEF3 and 97ANMT1. Although these cells were equally recovered from both DOX-treated and DOX-untreated mice 5 h after infection, the number of recovered cells dramatically decreased by 96 h after infection (Table 4). These results strongly suggest that DOX could repress the expression of a C. albicans gene in mice.
FIG. 5.
Survival rate of DOX-treated (closed symbols) or DOX-untreated (open symbols) mice that were inoculated with 99ATEF3, 97ANMT1, and CAF2. Mice were intravenously infected with 106 cells (circles) or 107 cells (squares). The percent survival shows the ratio of the number of surviving mice to total number of mice (n = 5, 106 cells infected; and n = 7, 107 cells infected). Experiments were performed twice with the same results.
TABLE 4.
Number of C. albicans cells recovered from mouse kidneys treated or not treated with DOXa
| Strain | Mean no. of C. albicans cells ± SD recovered from:
|
||
|---|---|---|---|
| Mice treated with DOX at:
|
Untreated mice at 5 h | ||
| 5 h | 96 h | ||
| 99ATEF3 | 5.19 ± 0.23 | 0b | 4.93 ± 0.08 |
| 97ANMT1 | 4.92 ± 0.11 | 2.65 ± 0.43 | 5.37 ± 0.21 |
Values are means ± standard deviation (three independent samples per group) for log the CFU.
No C. albicans cells were detected.
DISCUSSION
Studies on particular gene functions, particularly essentiality, have been difficult in C. albicans since the application of conventional genetic approaches has been hindered by its diploidy. In this study, we showed that the TR expression system can control gene expression in C. albicans with several advantages of this system over other gene expression systems in C. albicans. First, the activities of the TR promoters 97t, 98t, and 99t can drive gene expression at different levels in the absence of DOX. Second, a specific component of culture media, such as the carbon source, is not required to repress gene expressions. These expressions can be repressed by simply adding DOX into the media without affecting the cell growth. We have shown that 50 μg of DOX per ml does not affect the growth of C. albicans in YEPD, YEPGlcNAc or RPMI 1640, and the MIC for CAF2 cells cultured in YEPD or YNB was more than 200 μg of DOX per ml (data not shown). The TR expression system can be applicable at least in hyphal-formation-inducing media such as YEPGlcNAc and RPMI 1640, which contains 0.9% [wt/vol] glucose, because TR promoters may have similar activity levels as we observed in YEPD (Table 3). Moreover, this system is applicable in C. albicans cells infecting mice since DOX-treated mice infected with the controllable strains survived, and the number of viable cells in them was markedly reduced (Fig. 5 and Table 4). Thus, we could demonstrate that this system would be easily applicable to studying gene essentiality in various culture settings including in mice in vivo.
Nevertheless, some limitations, described as follows, are anticipated. A previous study reported that the transcripts from the ENO1 promoter are induced by as much as 6- to 13-fold in glucose medium (12). The activity of TR promoters may be reduced by a gluconeogenic carbon source such as ethanol, since the expression level of tetR-ScHAP4AD, which strongly affects the activity of TR promoters, depends on the activity of ENO1 promoters. In the mouse systemic candidiasis model, the virulence levels of the controllable strains, 99ATEF3 and 97ANMT1, were slightly reduced compared with that of CAF2. These reductions in virulence may be caused by altering the level or the timing of expression of the tetR-ScHAP4 protein, whereas expression level of the TEF3 gene in 99ATEF3 and that of the NMT1 gene in 97ANMT1 were judged to be comparable to those in CAF2 under a cultivation with YEPD. It should be also noted that targeted integration of pCAITHE5 on the ENO1 locus may inactivate the adjacent allele, resulting in a haplo-insufficiency that causes reduction of virulence. In spite of these limitations, the essentiality of in vivo for the target genes was clearly elucidated by comparing the survival rate or the number of recovered C. albicans cells from the kidneys of DOX-treated and DOX-untreated mice. Thus, this expression system could also be a powerful tool for functional analyses of C. albicans genes and may enable us to isolate of novel virulence factors and to understand the mechanism of fungal infection.
ACKNOWLEDGMENTS
We thank W. Fonzi (Georgetown University) for the gift of CAI8 and S. Miwa F. Ford and P. Hartmann for critically reading the manuscript.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Di Domenico B J, Lupisella J, Sandbaken M, Chakraburtty K. Isolation and sequence analysis of the gene encoding translation elongation factor 3 from Candida albicans. Yeast. 1992;8:337–352. doi: 10.1002/yea.320080502. [DOI] [PubMed] [Google Scholar]
- 3.Gallis H A, Drew R H, Pickard W W. Amphotericin B: 30 years of clinical experience. Rev Infect Dis. 1990;12:308–329. doi: 10.1093/clinids/12.2.308. [DOI] [PubMed] [Google Scholar]
- 4.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Forsburg S L, Guarente L. Identification and characterization of HAP4: a third component of the CCAAT-bound HAP2/HAP3 heteromer. Genes Dev. 1989;3:1166–1178. doi: 10.1101/gad.3.8.1166. [DOI] [PubMed] [Google Scholar]
- 6.Gossen M, Bonin A L, Bujard H. Control of gene activity in higher eukaryotic cells by prokaryotic regulatory elements. Trends Biochem Sci. 1993;18:471–475. doi: 10.1016/0968-0004(93)90009-c. [DOI] [PubMed] [Google Scholar]
- 7.Gossen M A, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillen W, Wissmann A. Protein-nucleic acid interaction. Vol. 10. London, United Kingdom: Macmillan Press; 1989. Topics in molecular and structural biology; pp. 143–162. [Google Scholar]
- 9.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurtz M B, Kelly R, Kirsch D R. The genetics of Candida. Boca Raton, Fla: CRC Press; 1990. pp. 21–74. [Google Scholar]
- 11.Magee P T, Scherer S. Genome mapping and gene discovery in Candida albicans. ASM News. 1998;64:505–511. [Google Scholar]
- 12.Mason A B, Buckley H R, Gorman J A. Molecular cloning and characterization of the Candida albicans enolase gene. J Bacteriol. 1993;175:2632–2639. doi: 10.1128/jb.175.9.2632-2639.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matthews J C, Hori K, Cormier M J. Purification and properties of Renilla reniformis luciferase. Biochemistry. 1977;16:85–91. doi: 10.1021/bi00620a014. [DOI] [PubMed] [Google Scholar]
- 14.Mio T, Adachi-Shimizu M, Tachibana Y, Tabuchi H, Inoue S B, Yabe T, Yamada-Okabe T, Arisawa M, Watanabe T, Yamada-Okabe H. Cloning of the Candida albicans homolog of Saccharomyces cerevisiae GSC1/FKS1 and its involvement in β-1,3-glucan synthesis. J Bacteriol. 1997;179:4096–4105. doi: 10.1128/jb.179.13.4096-4105.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Myers K K, Fonzi W A, Sypherd P S. Isolation and sequence analysis of the gene for translation elongation factor 3 from Candida albicans. Nucleic Acids Res. 1992;20:1705–1710. doi: 10.1093/nar/20.7.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagahashi S, Nakayama H, Hamada K, Yang H, Arisawa M, Kitada K. Regulation by tetracycline of gene expression in Saccharomyces cerevisiae. Mol Gen Genet. 1997;255:372–375. doi: 10.1007/s004380050508. [DOI] [PubMed] [Google Scholar]
- 17.Nakayama H, Izuta M, Nagahashi S, Sihta E Y, Sato Y, Yamazaki T, Arisawa M, Kitada K. A controllable gene-expression system for the pathogenic fungus Candida glabrata. Microbiology. 1998;144:2407–2415. doi: 10.1099/00221287-144-9-2407. [DOI] [PubMed] [Google Scholar]
- 18.Odds F C. Candida and candidosis: a review and bibliography. 2nd ed. London, United Kingdom: Bailliere Tindall; 1988. [Google Scholar]
- 19.Rex J H, Rinaldi M G, Pfaller M A. Resistance of Candida species to fluconazole. Antimicrob Agents Chemother. 1995;39:1–8. doi: 10.1128/aac.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santos M A, Tuite M F. The CUG codon is decoded in vivo as serine and not leucine in Candida albicans. Nucleic Acids Res. 1995;23:1481–1486. doi: 10.1093/nar/23.9.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srikantha T, Klapach A, Lorenz W W, Tsai L K, Laughlin L A, Gorman J A, Soll D R. The sea pansy Renilla reniformis luciferase serves as a sensitive bioluminescent reporter for differential gene expression in Candida albicans. J Bacteriol. 1996;178:121–129. doi: 10.1128/jb.178.1.121-129.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weinberg R A, McWherter C A, Freeman S K, Wood D C, Gordon J I, Lee S C. Genetic studies reveal that myristoyl CoA: protein N-myristoyltransferase is an essential enzyme in Candida albicans. Mol Microbiol. 1995;16:241–250. doi: 10.1111/j.1365-2958.1995.tb02296.x. [DOI] [PubMed] [Google Scholar]
- 23.Wiegand R C, Carr C, Minnerly J C, Pauley A M, Carron C P, Langner C A, Duronio R J, Gordon J I. The Candida albicans myristoyl-CoA: protein N-myristoyltransferase gene. Isolation and expression in Saccharomyces cerevisiae and Escherichia coli. J Biol Chem. 1992;267:8591–8598. [PubMed] [Google Scholar]