Abstract
Severe acute behavioral and emotional problems represent one of the most serious treatment-related adverse effects for children and adolescents who have cancer. The critical and severe nature of these symptoms often makes necessary the use of psychotropic drugs. A working group composed of experts in multiple disciplines had the task of creating an agreement regarding a management plan for severe acute behavioral and emotional problems (SABEPs) in children and adolescents treated for cancer. To obtain global information on the use of psychotropic drugs in pediatric oncology, the working group first developed and mailed a 15-item questionnaire to many Italian pediatric oncology centers. Overall, an evident lack of knowledge and education regarding the use of psychotropic medications for the treatment of SABEPs was found. Thus, by referring to an adapted version of the Delphi method of consensus and standard methods for the elaboration of clinical questions (PICOs), the working group elaborated evidence-based recommendations for psychotropic drugs in the pediatric oncology setting. Furthermore, based on a thorough multivariate analysis of needs and difficulties, a comprehensive management flow was developed to optimize therapeutic interventions, which allows more accurate and efficient matching of the acute needs of patients while guiding treatment options.
Keywords: pediatric oncology, psychotropic drugs, antidepressant, mental health
1. Introduction
Each year, in Italy, approximately 1400 children (0–14 years of age) and 800 adolescents (aged 15–19 years) are diagnosed with cancer [1,2]. Regardless of the enormous progress in treatment and enhanced overall survival, children and adolescents often face a high burden of disease, which is related both to the type of tumor, as well as the related treatment regimen, which frequently influence their physical, mental, social, behavioral, and emotional health [3,4]. Consequently, controlling efficient bio-psycho-social symptoms is the foundation of an elevated level of oncological care.
Among the clinically most serious treatment-related adverse effects for children and adolescents who have cancer are severe acute behavioral and emotional problems (SABEPs), including delirium, anxiety, and major depressive disorders [5,6,7]. SABEPs can be highly distressing not only for the (young) patients but also for their caregivers and the medical staff, representing a global health priority [5,6,7].
Furthermore, insufficient recognition or lack of adequate treatment of the symptoms of these disorders may cause or exacerbate possible complications, interfere with efficient treatment, increase the number of days spent in the hospital, and result in adverse effects and compromise short- and long-term overall outcomes [8,9,10]. Not least, these problems possibly interfere with the oncological treatment and may put children and adolescents at serious risk [11,12,13].
Thus, the early recognition and identification of acute behavioral toxicity might provide the pediatric oncologist with a timely chance to intervene (i.e., adjusting or changing the therapeutic regimen) and prevent further worsening of the extreme distress of the patients. The reader is directed to Appendix A (Figure A1) for a clinical presentation of SABEPs in a pediatric oncology setting.
While non-pharmacological interventions would constitute the preferred approach, the acute and severe nature often makes the use of psychotropic drugs necessary [14,15].
However, the lack of guidance, especially concerning the use of these medications, represents a significant issue for successful interventions. To date, no randomized trials, guidelines, or expert consensus exist in the pediatric oncology setting regarding the use of psychotropic treatment in managing severe acute behavioral and emotional problems [16]. Furthermore, the existing literature consists of studies with small sample sizes or case series and mainly considers the administration of psychotropic drugs in a non-acute setting [16,17,18].
Given the lack of evidence-based studies and consensus guidelines that assist the pediatric oncologist, as a rule, they use the medications with which they are most familiar. However, these may not offer the best option or even refrain from treatment.
In this complex scenario, an interdisciplinary working group of experts was formed with the overall aim of developing consensus recommendations regarding the use of psychotropic medication for SABEPs in the pediatric oncology setting and suggest solutions for the main problems encountered by pediatric oncologists. This paper describes and summarizes the results of the different rounds of consultation of the working group, which led to the development of a comprehensive management plan for the treatment of SABEPs and evidence-based recommendations for psychotropic drugs in the pediatric oncology setting.
2. Methods
The study started because of the many problems encountered in a clinical setting and reported in a non-formal way. As a consequence, a working group on the use of psychotropic drugs in pediatric oncology was formed to present a comprehensive management plan for the treatment of SABEPs, with a specific focus on the use of psychotropic medications for instantaneous and short-term therapies. The premise of the working group’s efforts was that the early recognition of patient-specific risk could help choose the most suitable patient-specific treatment for pediatric cancer patients [19,20].
2.1. Working Group and Study Design
An adapted version of the Delphi method of consensus was used. An intersociety working group (Italian Society for Psycho-oncology—SIPO and the Italian Association for Hematology and Pediatric Oncology—AIEOP) was created and it was enriched with experts from multiple disciplines. Experts included specialists in pediatric oncology, pediatric and adult psycho-oncology, neurology, pediatric neuroscience, psychiatry, (neuro)psychology, and neuropharmacology and were selected based on their expertise and geographic representation. The working group had the task of creating a consensus regarding a management plan for SABEPs in children and adolescents treated for cancer [14]. This group met in a first round of consultation and decided that a questionnaire was needed to better understand the entity of the problem.
Thus, a 15-item questionnaire (adapted from the questionnaire used by Kersun and Kazak (2006)) was developed and sent out to 16 pediatric oncology centers. The participating centers covered the entire national territory (i.e., Emilia-Romagna, Lombardia, Marche, Piemonte, Liguria, Campania, and Sicilia) and reflected typical regional variability in center size and patient characteristics. Pediatric oncologists and child mental health specialists were asked to complete the questionnaire enquiring about their opinions and current practice of prescribing psychotropic medications for the treatment of severe acute behavioral and emotional problems, such as depression, anxiety, delirium, and psychosis, in children and adolescents suffering from cancer [21]. Details about the questionnaire and outcomes are included in the Supplementary Materials S1.
After careful analysis of the literature and the existing guidelines, as well as direct input from the questionnaire, a second round of consultation was performed, and clinical questions—reported and discussed below—emerged. Thus, the experts in the working group were asked to answer the clinical questions, providing detailed and updated information. A final round of consultation was then performed to develop the recommendations reported in this study and to bring attention to possible barriers and problems related to this complex topic.
2.2. Development of Relevant Clinical Questions to Guide Evidence-Based Recommendations
The working group used standard methods for the elaboration of clinical questions (PICO, PICOS [22]), which then led to the development of evidence-based recommendations for the use of psychotropic drugs in the pediatric oncology setting [23,24,25]. The target population for which the recommendations were developed includes children (aged 0–14), adolescents (aged 15–19), and, occasionally, young adults exposed to a pediatric treatment regimen.
2.3. State of the Art
A review of the literature was performed to provide an overview of the current state of the art, including the presence of barriers and problems.
3. Clinical Questions
In the pediatric oncology setting, delirium, psychosis, depression, and anxiety commonly require consultation and psychopharmacological medication [26,27]. Perhaps, in an ideal world, pediatric psychopharmacology would have a range of treatments to offer for these problems, based on extensive data, indicating the safest and most effective drugs while counting on a solid consensus on what to use, when, and for whom.
However, we do not live in such an ideal world. Instead, we must deal with prevalent, often extremely acute, or sometimes chronic mental health problems in these young patients, causing substantial functional impairment. As emerged from discussions within the working group and confirmed by our questionnaires, this scenario is further complicated by the absence of reliable data and therapeutic knowledge. Treating SABEPs in our less-than-perfect world, what to use, when, and for whom should be based first on urgency and the importance of symptoms.
Currently, there are no clear indications approved by the Food and Drug Administration (FDA) [28] or European Medicines Agency (EMA) [29] for psychopharmacological medications in medically ill children with delirium, depression, or anxiety [30,31,32,33].
Furthermore, a small group of patients may come with preexisting behavioral problems, such as hyperactivity attention disorders, which are treated with psychostimulants and other compounds [34,35,36]. As for the other psychotropic medications, pediatric oncologists need to pay attention when selecting the appropriate therapy, especially in relation to possible interactions with the cancer treatment proposed, avoiding that the combination of these treatments creates SABEPs [37].
Thus, after careful analysis of the literature and the existing guidelines, as well as direct input from the questionnaire, we formulated several clinical questions that may help to inform policy and recommendations. One of the indications we used was the needed number to treat (NNT), which in the pediatric setting and, more importantly, in specific clinical realities, such as that of pediatric oncology, has an added value because it gives a clear idea of the benefit of treatment to the individual patients [38].
The NNT provided a way to assess the impact of therapy by estimating the number of patients that need to be treated to have an effect on one patient. Especially in this vulnerable patient group, this concept might be helpful, as we know that not everyone is helped by just medicine but might need concomitant psychotherapeutic interventions as well [19,39]. In other words, some children might benefit, while some might have no benefit, but we must avoid that some are harmed at all costs. In this acute setting, an NNT of two or three indicates that treatment should be considered quite effective [40].
The formulation of the following questions—to which we provide a conditional reply—was guided by data from the literature and the results of our questionnaire.
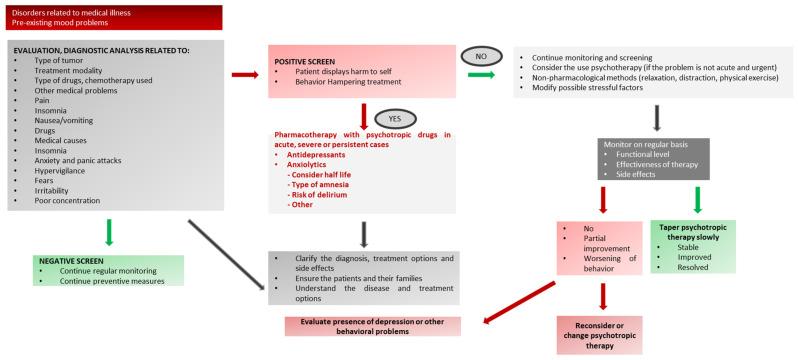
Furthermore, to assist pediatric oncologists and other healthcare professionals in identifying symptoms of delirium, mood disorders, and anxiety disorders in this patient population, we created specific clinical management workflows (Appendix B, Appendix C, Appendix D, Appendix E).
Q1: What is the risk of suffering from cancer-related severe and acute behavioral and emotional problems in children and adolescents: is the treatment phase critical?
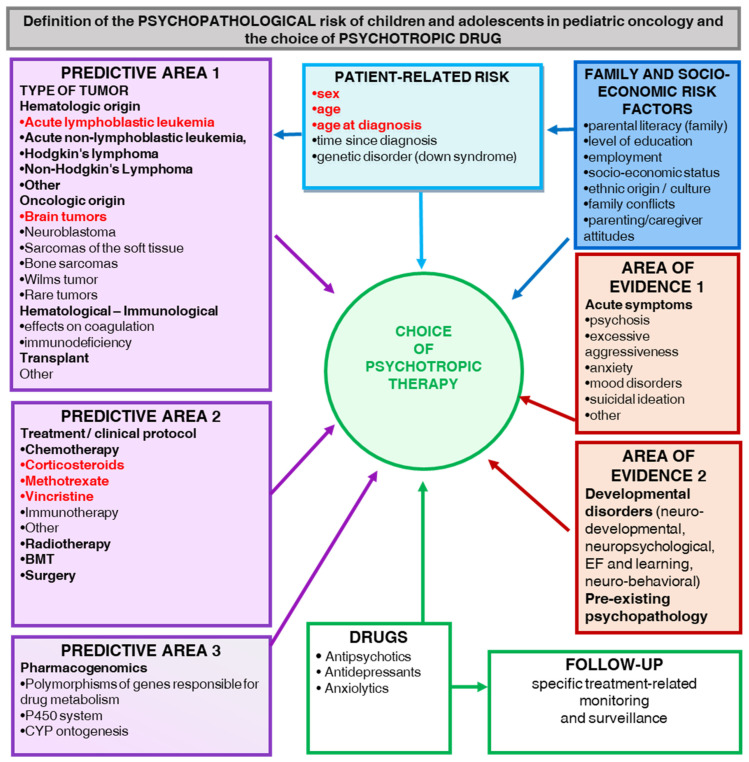
Some evidence suggests that the early phases of treatment are associated with an increased risk for SABEPs, including chemotherapy, radiotherapy, surgery, and bone or stem cell transplantation [41]. Furthermore, there is evidence that children and adolescents treated with corticosteroids have an increased risk for SABEPs [42,43,44,45,46]. Moreover, children and adolescents treated with higher doses of cranial radiation showed an enhanced risk of developing SABEPs [47,48]. Thus, to assist pediatric oncologists and other healthcare professionals in defining the psychopathological risk of children and adolescents in pediatric oncology and the choice of psychotropic drugs, a specific risk-based management flow was created (Appendix E).
Q2: What is the influence of age at treatment on the risk of SABEPs in children and adolescents with cancer?
Evidence suggests that patients younger than six years or older than 11 years are more at risk for SABEPs [49,50,51]. Adolescents and young adults (up to 21-years-old) treated with pediatric protocols for hematologic malignancies were particularly vulnerable to suffering from psychological problems [52,53,54]. In particular, more than one-third of these patients experience depression or anxiety [55]. Furthermore, anxiety was two times as common for patients in treatment when compared to early survivors [56,57].
Q3: What should be done when severe acute symptoms are found?
First, a consensus exists on the necessity to screen children and adolescents consistently for symptoms of distress based on the severity and the acute nature of behavioral and emotional symptoms; the patient should then be directed to the most appropriate treatment. See Appendix A and Appendix D for symptom-specific decision flowcharts.
Q4: What is the best available research evidence on preventing and treating delirium in pediatric oncology patients?
The pharmacological approach to prevent and/or treat delirium may be driven by different goals:
(1) prevention through controlling risk factors.
(2) management of delirium-related symptoms (i.e., psychosis or agitation).
(3) resolution of the underlying cause of delirium or modulation of the neurochemical cascade [58,59]. However, to date, a clear strategy to prevent and manage delirium in oncopediatric patients has yet to be adequately studied.
In general, the treatment of delirium requires an accurate diagnosis, continued monitoring and re-evaluation, therapeutic intervention for hyperactive manifestations that may cause the patient damage, avoidance of risk factors that may worsen delirium severity, and, if possible, diagnosis and treatment of the underlying etiology [24,26,60,61,62,63,64] (Table 1). Haloperidol is the most frequently prescribed antipsychotic for the treatment of delirium [65,66,67,68,69]. The successful treatment of hyperactive delirium in pediatric intensive care unit patients with haloperidol was reported by Schieveld and colleagues [70].
Table 1.
Antipsychotic drugs used and recommended in the Italian pediatric haemato-oncology setting.
| Antipsychotic Drug | Age | Recommended Dose |
|---|---|---|
| Chlorpromazine (approved for ≥ 18) * |
1 year and 5 months | 3 drops 3 times/die Up to 6 drops, 3 times/die |
| Promazine (approved for ≥ 18) * |
>6 years old | 1 drop twice a day |
| Haloperidol (approved for ≥ 18) * |
pre-puberty post-puberty |
0.5–8 mg/die 1–16 mg/die |
| Risperidone (approved for ≥ 18) * |
>10 years old | 1 mg then in steps 0.75 for 3 days and 0.5 for the following range 0.25–6 mg/kg |
| Olanzapine Quetiapine Aripiprazole (approved for ≥ 18) * |
>6 years old | At low dose 2.5–20 mg/kg |
* Note: the approval of the drugs refers to the EMA.
On the other hand, atypical antipsychotics such as risperidone, olanzapine, and ziprasidone may be prescribed as alternatives to haloperidol for the treatment of delirium [66,71,72,73]. These drugs act on dopamine receptors, as well as on serotonin, acetylcholine, and norepinephrine neurotransmission [74,75].
All antipsychotics have potentially serious side effects, including extrapyramidal movement disorders, malignant hyperthermia, dysregulated metabolism, laryngeal spasm, constipation, urinary retention, and xerostomia [76]. Although atypical antipsychotics are associated with fewer side effects [77], the lack of well-designed randomized trials on the efficacy of either typical or atypical antipsychotics for the treatment or prevention of delirium in critically ill patients still represents a serious gap [78].
Q5: What is the best available research evidence on preventing and treating depression and anxiety symptoms in oncopediatric patients?
While the evidence base in child psychiatry is already small, data on the use of these medications in pediatric oncology are even smaller. Therefore, we concisely review the most up-to-date data about antidepressant efficacy, tolerability, and safety in pediatric patients [79] and suggest their applicability in pediatric oncology, indicating medications from which to choose, and how to start and stop the antidepressant medication.
We briefly consider benefits, risks, the black-box warning, and the significance of off-label prescribing (Discussed below) (Table 2 and Table 3).
Table 2.
Antidepressant drugs used and recommended in the Italian pediatric haemato-oncology setting.
| Antidepressive Drug | Age | Recommended Dose | Side Effects |
|---|---|---|---|
| Fluoxetine Fluvoxamine (approved for ≥ 18) * |
>5 years old | 10–20 mg/die 50–200 mg/die |
Headaches, gastrointestinal side effects, feeling jittery, disinhibited, activated impulsivity, agitation, and suicidality |
| Citalopram (approved for ≥ 18) * |
>6 years old, especially in adolescent patients | 10–40 mg/die | Headaches, gastrointestinal side effects, feeling jittery, disinhibited, activated impulsivity, agitation, and suicidality |
| Escitalopram (approved for ≥ 18) * |
pre-puberty post-puberty |
5–20 mg/die 1–16 mg/die |
Headaches, gastrointestinal side effects, feeling jittery, disinhibited, activated impulsivity, agitation, and suicidality |
| Sertraline (approved for ≥ 18) * |
6–17 years old | 12.5–200 mg/die | Headaches, gastrointestinal side effects, feeling jittery, disinhibited, activated impulsivity, agitation, and suicidality |
| Nortriptyline Desipramine Amitriptyline (approved for ≥ 18) * |
>6 years old >12 years old >12 years old |
1–3 mg/kg/die 25–100 mg/die 10–200 mg/die |
Cardiovascular and anticholinergic side effects. May be lethal in overdose. |
| Mirtazapine (approved for ≥ 18) * |
>6 years old | 7.5–45 mg/die | Somnolence, QTc prolongation, weight gain, and agranulocytosis |
* Note: the approval of the drugs refers to the EMA.
Table 3.
Anxiolytic drugs used and recommended in the Italian pediatric haemato-oncology setting.
| Anxiolytic Drug | Age | Recommended Dose | Side Effects |
|---|---|---|---|
| Lorazepam (approved for ≥ 18) * |
>10 years old | 0.5–2 mg | Sedation, fatigue, dizziness, anticholinergic side effect, and paradoxical activation (in younger children) |
| Delorazepam (approved for ≥ 18) * |
>3 years old | 10 drops/day | / |
| Alprazolam (approved for ≥ 18) * |
>14 years old | 1 mg × 3 times/die Cp 0.25 2 times/die |
/ |
| Clonazepam (approved for ≥ 18) * |
>6 years old | 0.5 mg/die | Sedation, confusion, and paradoxical activation, particularly in youth with central nervous system dysfunctions |
* Note: the approval of the drugs refers to the EMA.
Antidepressants are the first line in the treatment of major depressive disorders and anxiety disorders, as well as post-traumatic stress disorders, which are all common in children and adolescents, with a collective prevalence ranging from 15% to 30% [80,81,82].
The preferred and often most efficient treatment modality in these age groups is a combination of psychotherapy and psychopharmacology, depending on the nature of the symptoms [83]. While this holds for the pediatric population in general, children and adolescents treated for cancer often suffer from acute and severe manifestations, especially anxiety-related disorders for which immediate interventions are warranted.
While antidepressants provide substantial benefits in the short and long term, delayed therapeutic onset, as well as intolerance, limited effectiveness, and relapse issues, may limit the efficacy of these drugs [62,63,64]. Although antidepressants induce chemical changes within the brain a few hours after their administration, these do not correspond to a clinical change [65,66,67], which usually takes days and weeks to achieve [66]. In other words, despite the immediate increase in monoamine levels following antidepressant treatment, their positive effects on mood only occur weeks later [68,69]. This delay in efficacy represents a difficult clinical problem to overcome [70]. In fact, not achieving remission from depressive symptoms increases the risk of a more chronic and debilitating course of illness [71,72]. On the other hand, starting early at the first manifestation of severe mood-related problems provides a way to reduce the onset of longer-term problems later on [68,73,74,75].
4. Recommendations and Critical Issues
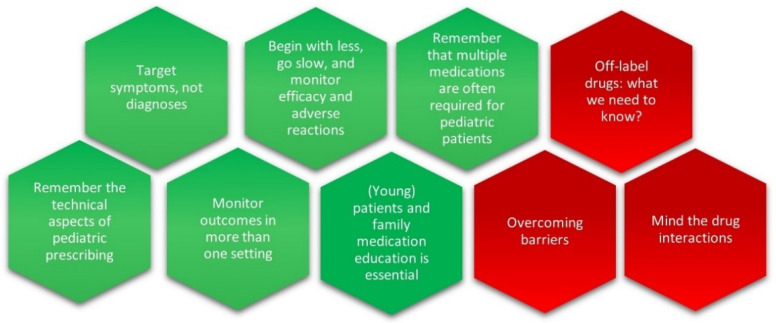
As emerged from our questionnaire, many pediatric oncologists report a lack of training and expressed a need to improve their knowledge and facilitate access to information and resources that allow them to better assist their patients in need of acute help for the severe psychological and psychiatric manifestations that cause unnecessary suffering. After a careful analysis of the literature and guided by the real-world attitudes of pediatric oncologists and healthcare professionals and prescribing practices, we recommend six key points for physicians to keep in mind when prescribing psychotropic drugs for young patients suffering from SABEPs. In addition, we draw pediatric oncologists’ attention to three critical issues to be considered (Figure 1).
Figure 1.
Recommendations are in green and critical issues are in red.
4.1. Recommendations
The recommendations are intended primarily for pediatric oncologists and other healthcare professionals caring for children and adolescents with cancer.
Target symptoms, not diagnoses. Diagnosis can be difficult in children, especially in the case of comorbidity. Although a working diagnosis is helpful to frame expectations and communicate with young patients and their caregivers, treatment should target key symptoms.
Remember the technical aspects of pediatric prescribing. The process of consent is mandatory. The participation of young/adolescent patients and their caregivers in the decision-making process related to their (mental) health and treatment with off-label prescribed drugs needs utmost attention. The Medicine Act of 1968 and European legislation make provisions for physicians to use drugs in an off-label or out-of-license capacity or to use unlicensed medicines. However, individual prescribers need to ensure adequate information to support the efficacy, safety, quality, and intended use of a drug before prescribing it. It is recognized that unlicensed applications are often necessary for pediatric practices.
Begin with less, go slow, and monitor efficacy and adverse reactions. In out-patient care, the dosage usually commences lower in mg/kg per day terms than in adults. Evidence suggests that various antidepressants and second-generation antipsychotics may offer a tool to treat severe acute problems but present a variety of safety profiles that should be monitored very closely to detect and manage possible side effects in a timely way. Thus, the dose should be gradually increased as needed and interrupted at a dose that adequately controls symptoms with minimum adverse reactions. Finally, the time necessary to produce a clinical effect is to be considered.
Keep in mind that multiple medications are often required for oncopediatric patients. Although monotherapy is ideal, childhood-onset illness can be severe and may require psychosocial interventions in combination with more than one treatment. It is important to distinguish between co-pharmacy, which refers to the use of different medications for different disorders or symptoms, and polypharmacy, which is the use of multiple drugs to manage the same problem. Since children often show multiple co-occurring conditions, co-pharmacy is common.
Monitor outcomes in more than one setting. The expression of symptoms may be different across settings (e.g., hospital, home, and school). This is particularly important in the case of symptomatic treatments (e.g., delirium). In this context, current innovative digital technologies, by integrating diverse sources of information, offer a unique opportunity to create predictive models of individual vulnerability [76].
(Young) patients and family medication education is essential. The first experiences with treatments are critical for long-term outcomes and adherence to the therapy. Delirium, anxiety, and major depression are the most severe and prevalent complications observed in critically ill children and are the result of their medical condition, its treatment, or both, and cause a temporary severe and acute dysfunction at the cognitive and behavioral level [77,78,79]. Interruption of treatment or subsiding of the disease often puts an end to the episode but is often not a desirable option [80]. Thus, patients and their caregivers should not only be educated regarding the therapies and the possible side effects but should also be encouraged to ask for changes to their treatment regimens if necessary.
4.2. Critical Issues
Finally, three critical issues need to be considered by pediatric oncologists when prescribing psychotropic drugs for young patients suffering from SABEPs: prescription of off-label drugs, drug–drug interactions, and the gaps that still need to be filled for the safer use of psychotropic drugs in a pediatric oncology setting.
4.2.1. Off-Label Prescribing
Off-label drug prescribing means prescribing drugs for an indication or using a dosage that has not been approved by regulatory agencies, such as the FDA or EMA. Since these regulatory agencies do not regulate the practice of medicine, the use of off-label drugs has become common [18]. Importantly, the term “off-label” does not imply improper, illegal, or contraindicated use [81]. On the contrary, the lack of labeling for a specific disorder, symptom, age group, or dosage does not automatically mean that the use of the drug is inappropriate for that particular use. Instead, it suggests that the inclusion of a specific drug on the label has not been approved by the EMA and/or FDA [82,83,84,85]. Thus, the responsibilities for the prescription of that therapy rely on the prescriber only.
Previous studies reported that, in the United States, up to 60% of the prescribed drugs are off-label, of which a considerable proportion are prescribed to children and adolescents [81,86,87]. This is mostly due to the lack of research [88].
Clinicians are called to balance beneficence (treat and enhance the quality of life) with nonmaleficence (protect patients from unsafe or ineffective treatments). In general, the therapeutic decision should be driven by the best interest of the patient based on the most convincing available evidence [87,89].
Since pharmaceutical companies are not allowed to advertise their medications for off-label use, doctors should prescribe drugs only for indications approved by the FDA and EFSA labeling systems [63,72].
However, we live in an era of global exchange of medical information, which allows healthcare professionals to weigh the risks and benefits of off-label drug use and provide the best possible care for their patients [90,91,92,93,94,95].
Overall, if there is evidence supporting the use of an off-label drug for a specific indication in a particular patient, informed-consent forms should be obtained/signed, and risks, benefits, and alternatives should be explained and discussed with caregivers and, where possible, patients [81,96]. For example, palliative care—consisting mostly of off-label drugs—has long been recognized as a necessary part of the treatment in the pediatric oncology setting, to assist in managing the high symptom burden of these ‘young’ patients [97].
4.2.2. Drug–Drug Interaction
Given the complexity of the mechanisms of action of drugs used in pediatric oncology [98] and the potential interactions they may have with psychotropic drugs, in this section, we underline some drug interactions to watch out for.
First, it should be remembered that, while treatment with chemotherapeutic drugs follows lengthy treatment regimens, the treatment of SEBAPs is generally short-lasting. Therefore, the risk of pharmacodynamic interactions in acute treatment, although rare, may occur when both drugs (i.e., psychotropic and chemotherapeutic drugs) act on the same pharmacological target. Therefore, the mechanisms of action and pharmacological targets of the drugs need to be considered when they are co-administered.
More likely is the risk of pharmacokinetic interactions if the acutely administered drug is metabolized by the same enzymes as the cancer treatment.
Particular emphasis should be placed on compounds that act as inducers, inhibitors, or substrates of the cytochrome P450 system. Most antineoplastic drugs undergo biotransformation by CYP 450 3A4 [99,100] and, when co-administered with antidepressants that exert inhibitory properties on this CYP isoform, such as fluoxetine, paroxetine, sertraline, and fluvoxamine, may impair the antineoplastic drugs’ efficacy and/or induce higher toxicity. On the other hand, among the antidepressant drugs, escitalopram, citalopram, mirtazapine, venlafaxine, and milnacipran represent alternatives with a higher safety profile for oncologic patients because of their weak CYP 450 inhibitory potential [101].
Consequently, while fluoxetine is the most widely approved SSRI for the treatment of depression by the FDA and EMA, the interactions of fluoxetine with other drugs constitute an important drawback for its use in pediatric oncology patients [102,103,104]. Sertraline, citalopram, and escitalopram are characterized by substantially fewer drug interactions and are therefore preferred [59,105,106].
Citalopram, especially, is secure and effective [107], whereas the use of monoamine oxidase inhibitors should be avoided because of their adverse interactions with other substances [108].
4.2.3. Barriers to Mental Health Services in Pediatric Oncology
To date, there are still some gaps that need to be filled for the safer use of psychotropic drugs in a pediatric oncology setting:
(1) Lack of information related to psychiatric manifestations in the pediatric oncology setting.
(2) Limitations of the acute setting that influence the timely and comprehensive evaluation of emotional-behavioral problems.
(3) Lack of education and training of the medical and mental health staff regarding the identification and management of pediatric psychiatric manifestations.
5. Conclusions and Considerations
Overall, a major lack of information exists regarding the use of psychotropic medication for acute severe problems in the pediatric oncology setting. Furthermore, a major difficulty surrounded the appropriate identification of symptoms displayed by children and adolescents with cancer. Beyond symptoms that cluster together, many factors affect the pattern and magnitude of the emotional, cognitive, and behavioral response of the child or adolescent both to disease and treatment, including the age of onset, the duration of treatment, the type of treatment, sex, and genetic background of the child/adolescent, as well as the psychosocial setting of the patient and their family. Furthermore, future studies should consider some characteristics of healthcare professionals, such as age, work experience, sex, and geographic context, as potentially influencing prescribing practices.
Growing evidence stresses the fact that (early) experiences are incorporated into the developing brain. When young children and adolescents are exposed to major adversity, maturing brain circuits can be harmed, and the risk for problems in learning, behavior, and mental health is significantly enhanced [109,110]. Therefore, policy and practice in our centers should focus more on protecting children and adolescents from the neurodevelopmental consequences of the adversities posed by their disease and its treatment. Psychopharmacological treatment of acute and severe problems may help alleviate the damaging effects of significant and repetitive adversity.
In the context of the social, biological, and psychological dimensions of care for pediatric cancer patients, pediatric oncologists, in the absence of developmental psychiatrists, need to be able to distinguish and manage possible acute and severe neuropsychiatric conditions. Especially children and adolescents treated for acute lymphoblastic leukemia and those diagnosed and treated for a brain tumor display a heightened association with significant comorbid psychopathology and exhibit the most extensive behavioral and emotional complications [111].
Here, we describe an approach to managing severe and acute behavioral and emotional problems in pediatric oncology that is useful for physicians considering the complexity of the illness and the psychopathological problems and side effects of the disease and its treatment. Together with pain, comorbid psychiatric disorders represent a significant source of distress, often impairing functioning. The pharmacological treatment of these disorders can feel challenging because patients are typically young and rather sick. However, a full recovery from their problems is often possible even in the most severely ill children and adolescents if we act in a timely way.
Our efforts have the purpose of heightening the collective awareness of this type of devastating concurrent problem and hopefully result in intensifying the endeavors to improve our understanding of the long-term consequences of these problems if left untreated, as well as lead to exploring potentially promising protective treatment regimens. The recommendations of this working group do not represent a formal consensus statement but, as stated before, are the result of several cycles of continuous consultation before reaching an agreement among all members and co-authors.
Finally, further interdisciplinary multi-center research is necessary to investigate the effects of SABEPs on long-term outcomes related to the burden of disease and mental health. Additional efforts should be dedicated to studying the effects of the short-term use of psychotropic drugs while being treated for a tumor, as a possible preventive or protective intervention. Incorporating a framework of standardized screening and developing individual-based interventions, which may include psychotropic drugs when the problem is severe and acute, is an essential investment because it may attenuate long-term problems. In the meantime, our short-term efforts should focus on advocacy and education. Due to the small numbers, children and adolescents with different types of cancers are grouped into broad categories that are not clinically meaningful from a mental health point of view.
Demonstrating a clear causal relationship between emotional and behavioral problems and global or specific neurodevelopmental outcomes for treatments that include multiple drugs exerting cytotoxicity through several different pathways may appear as an almost impossible challenge. However, the treatment of acute behavioral manifestations of toxicity should be considered a critical problem and would benefit from treatment recommendations that integrate different fields of knowledge, such as neurodevelopmental physiology, biochemistry, pediatric pharmacology, and genetics. The brain of children and adolescents suffering from cancer undergoes a series of events that can be defined as a “multiple hit” model, where the type of cancer, its treatment, being diagnosed and hospitalized for it, and exposure to repeated painful procedures all interact with the genetic make-up and resilience or sensitivity to stress of the patients, where the sensitivity to environmental influences determines vulnerable phenotypes [109,112,113].
Finally, the translation of research-based knowledge into clinical care guidelines or recommendations is becoming increasingly important to direct our attention to translating knowledge from research to the development of intervention-based approaches, which should be designed to avoid, prevent, or ameliorate adverse outcomes. These interventions should include social and behavioral, but, when necessary, in acute stages, neuro-pharmacological approaches.
In particular, the results of our questionnaire highlight the need to increase the amount and dissemination of information, education, and training related to the management of acute mental health-related problems presented by children and adolescents suffering from cancer. This, then, may enable the pediatric oncologist to act in a timely and the best possible way, thereby avoiding unnecessary suffering. More importantly, we should evaluate new ways and methods to increase communication and collaboration among pediatric oncologists and mental health specialists, who are called to collaborate to improve survivorship care and the quality of life of young patients.
Given the young age of pediatric cancer patients and survivors, as well as their potential longevity, delayed consequences of therapy are likely to have a greater impact on their lives and families than the acute complications of treatment. The timely provision of interventions to remediate or prevent complications can help monitor neurodevelopment and offer a significant step in reducing any possible risk.
In sum, this comprehensive and updated review on the use of psychotropic drugs for the treatment of SABEPs in children and adolescents treated for cancer underlines the need for close collaboration among pediatric oncologists, child mental health professionals, and other experts. In the presence of risk and uncertainty in the realm of care for pediatric cancer patients, asking the ‘right questions at the right time’ is fundamental.
We think and anticipate that by providing a practical tool, we might assist pediatric oncologists and mental health professionals, as well as pediatric patients and their caregivers, in the decision-making process related to what, when, who, and for whom to prescribe psychotropic drugs in the pediatric oncology setting.
Acknowledgments
We would like to thank all the experts who participated in the different phases of the project.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/children9121878/s1, Supplementary Materials S1: A questionnaire was sent out to Italian pediatric oncology centers.
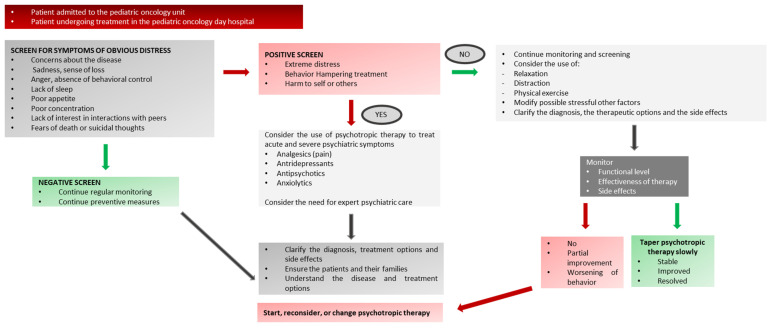
Appendix A
Figure A1.
Clinical Presentation. * Note: the use of psychotropic drugs in children is almost exclusively off-label (see Table 1, Table 2 and Table 3 for dose/age).
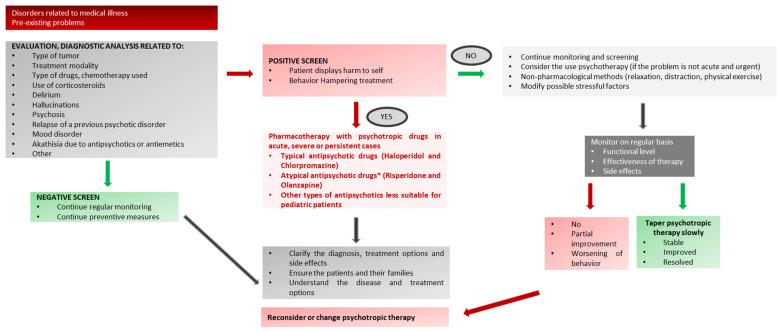
Appendix B
Figure A2.
Psychotic Symptoms and Delirium in Pediatric Oncology.* Note: the use of psychotropic drugs in children is almost exclusively off-label (see Table 1 for dose/age).
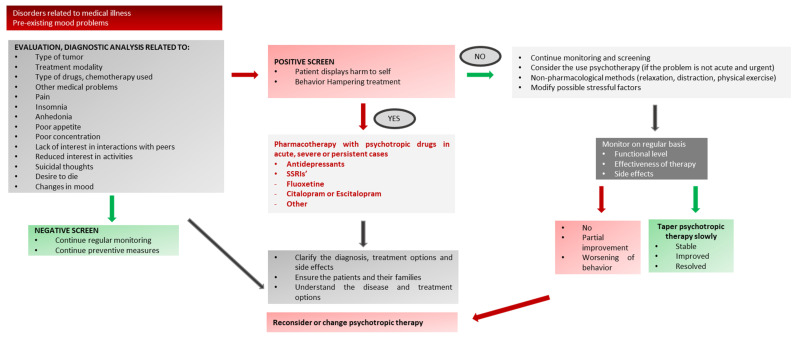
Appendix C
Figure A3.
Depressive Symptoms or Mood Disorders in Pediatric Oncology. * Note: the use of anxiolytic drugs in children is almost exclusively off-label (see Table 2 for dose/age).
Appendix D
Figure A4.
Anxiety-Related Symptoms in Pediatric Oncology. * Note: the use of anxiolytic drugs in children is almost exclusively off-label (see Table 2 and Table 3 for dose/age).
Appendix E
Figure A5.
Definition of the Psychopathological Risk of Children and Adolescents in Pediatric Oncology and the Choice of Psychotropic Drugs.
Author Contributions
Conceptualization: J.M.C.B., D.C., M.B. and D.S.; Methodology, J.M.C.B., D.C. and C.A.C.; Data collection: J.M.C.B., A.P., C.C. and V.R.; Data curation and formal analysis: J.M.C.B., D.S., M.C. and M.B. Members of the intersociety work group (SIPO-AIEOP): J.M.C.B., C.C., A.P., E.B., M.B., C.A.C., C.F.-S., S.D.G., M.J., G.P., R.T., F.T. and D.S.; Writing—original draft preparation, J.M.C.B. and M.B.; Writing—review and editing, J.M.C.B., V.R., L.P., C.A.C., R.T., F.T., M.C. and D.S.; Visualization, J.M.C.B. and A.P.; Supervision, J.M.C.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zecca M., Ferrari A., Quarello P., Rabusin M., Balduzzi A., Buldini B., Rostagno E., Prete A., Favre C., Massimino M., et al. Childhood Cancer in Italy: Background, Goals, and Achievements of the Italian Paediatric Hematology Oncology Association (AIEOP) Tumori. 2021;107:370–375. doi: 10.1177/03008916211007934. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari A., Quarello P., Mascarin M., Milano G.M., Tornesello A., Bertolotti M., Spinelli M., Ballotta P., Read Borghi M., Maule M., et al. Evolving Services for Adolescents with Cancer in Italy: Access to Pediatric Oncology Centers and Dedicated Projects. J. Adolesc. Young Adult Oncol. 2020;9:196–201. doi: 10.1089/jayao.2019.0134. [DOI] [PubMed] [Google Scholar]
- 3.Zahed G., Koohi F. Emotional and Behavioral Disorders in Pediatric Cancer Patients. Iran. J. Child Neurol. 2020;14:113–121. [PMC free article] [PubMed] [Google Scholar]
- 4.Barrera M., Alexander S., Atenafu E.G., Chung J., Hancock K., Solomon A., Desjardins L., Shama W., Mills D. Psychosocial Screening and Mental Health in Pediatric Cancer: A Randomized Controlled Trial. Health Psychol. 2020;39:381–390. doi: 10.1037/hea0000825. [DOI] [PubMed] [Google Scholar]
- 5.Ogundele M.O. Behavioural and Emotional Disorders in Childhood: A Brief Overview for Paediatricians. World J. Clin. Pediatr. 2018;7:9–26. doi: 10.5409/wjcp.v7.i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dosa N.P., Boeing N.M., Ms N., Kanter R.K. Excess Risk of Severe Acute Illness in Children With Chronic Health Conditions. Pediatrics. 2001;107:499–504. doi: 10.1542/peds.107.3.499. [DOI] [PubMed] [Google Scholar]
- 7.Arango C. Present and Future of Developmental Neuropsychopharmacology. Eur. Neuropsychopharmacol. 2015;25:703–712. doi: 10.1016/j.euroneuro.2014.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Wilson J.E., Mart M.F., Cunningham C., Shehabi Y., Girard T.D., MacLullich A.M.J., Slooter A.J.C., Ely E.W. Delirium. Nat. Rev. Dis. Primers. 2020;6:1–26. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chao C.-C., Chen S.-H., Wang C.-Y., Wu Y.-C., Yeh C.-H. Psychosocial Adjustment among Pediatric Cancer Patients and Their Parents. Psychiatry Clin. Neurosci. 2003;57:75–81. doi: 10.1046/j.1440-1819.2003.01082.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz B.P., Abrams A.N. Psychiatric Aspects of Pediatric Cancer. Child Adolesc. Psychiatr. Clin. 2010;19:401–421. doi: 10.1016/j.chc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Adjustment to Cancer: Anxiety and Distress (PDQ®)–Health Professional Version-NCI. [(accessed on 5 October 2022)]; Available online: https://www.cancer.gov/about-cancer/coping/feelings/anxiety-distress-hp-pdq.
- 12.Yi J.C., Syrjala K.L. Anxiety and Depression in Cancer Survivors. Med. Clin. North Am. 2017;101:1099–1113. doi: 10.1016/j.mcna.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jeong E., Park J., Lee J. Diagnostic Test Accuracy of the 4AT for Delirium Detection: A Systematic Review and Meta-Analysis. Int. J. Env. Res. Public Health. 2020;17:7515. doi: 10.3390/ijerph17207515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Del Giovane C., Cortese S., Cipriani A. Combining Pharmacological and Nonpharmacological Interventions in Network Meta-Analysis in Psychiatry. JAMA Psychiatry. 2019;76:867–868. doi: 10.1001/jamapsychiatry.2019.0574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clerici C.A., Ferrari A., Simonetti F., Massimino M. Psychotherapy and Psychopharmacology for Mental Health in Adolescents with Cancer: What Integration Is Possible? Expert Rev. Anticancer Ther. 2011;11:1623–1625. doi: 10.1586/era.11.158. [DOI] [PubMed] [Google Scholar]
- 16.Mavrides N., Pao M. Updates in Pediatric Psycho-Oncology. Int. Rev. Psychiatry. 2014;26:63–73. doi: 10.3109/09540261.2013.870537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pao M., Ballard E.D., Rosenstein D.L., Wiener L., Wayne A.S. Psychotropic Medication Use in Pediatric Patients With Cancer. Arch. Pediatrics Adolesc. Med. 2006;160:818–822. doi: 10.1001/archpedi.160.8.818. [DOI] [PubMed] [Google Scholar]
- 18.Nordenmalm S., Tomasi P., Pallidis C. More Medicines for Children: Impact of the EU Paediatric Regulation. Arch. Dis. Child. 2018;103:557–564. doi: 10.1136/archdischild-2017-313309. [DOI] [PubMed] [Google Scholar]
- 19.Marchak J.G., Christen S., Mulder R.L., Baust K., Blom J.M.C., Brinkman T.M., Elens I., Harju E., Kadan-Lottick N.S., Khor J.W.T., et al. Recommendations for the Surveillance of Mental Health Problems in Childhood, Adolescent, and Young Adult Cancer Survivors: A Report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2022;23:e184–e196. doi: 10.1016/S1470-2045(21)00750-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blom J.M.C., Benatti C. Comprehensive Pain Management Using Opioids for Children and Adolescents: Still a Wild Goose to Chase? Children. 2022;9:347. doi: 10.3390/children9030347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kersun L.S., Kazak A.E. Prescribing Practices of Selective Serotonin Reuptake Inhibitors (SSRIs) among Pediatric Oncologists: A Single Institution Experience. Pediatric Blood Cancer. 2006;47:339–342. doi: 10.1002/pbc.20484. [DOI] [PubMed] [Google Scholar]
- 22.Methley A.M., Campbell S., Chew-Graham C., McNally R., Cheraghi-Sohi S. PICO, PICOS and SPIDER: A Comparison Study of Specificity and Sensitivity in Three Search Tools for Qualitative Systematic Reviews. BMC Health Serv. Res. 2014;14:579. doi: 10.1186/s12913-014-0579-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peled O., Lavan O., Stein J., Vinograd I., Yahel A., Valevski A., Weizman A., Kimmel-Tamir E., Apter A., Fennig S., et al. Psychopharmacology in the Pediatric Oncology and Bone Marrow Transplant Units: Antipsychotic Medications Palliate Symptoms in Children with Cancer. J. Child Adolesc. Psychopharmacol. 2020;30:486–494. doi: 10.1089/cap.2019.0164. [DOI] [PubMed] [Google Scholar]
- 24.Turkel S.B., Hanft A. The Pharmacologic Management of Delirium in Children and Adolescents. Paediatr. Drugs. 2014;16:267–274. doi: 10.1007/s40272-014-0078-0. [DOI] [PubMed] [Google Scholar]
- 25.Shonkoff J.P., Radner J.M., Foote N. Expanding the Evidence Base to Drive More Productive Early Childhood Investment. Lancet. 2017;389:14–16. doi: 10.1016/S0140-6736(16)31702-0. [DOI] [PubMed] [Google Scholar]
- 26.Hatherill S., Flisher A.J. Delirium in Children and Adolescents: A Systematic Review of the Literature. J. Psychosom. Res. 2010;68:337–344. doi: 10.1016/j.jpsychores.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 27.Silver G., Traube C., Kearney J., Kelly D., Yoon M.J., Nash Moyal W., Gangopadhyay M., Shao H., Ward M.J. Detecting Pediatric Delirium: Development of a Rapid Observational Assessment Tool. Intensive Care Med. 2012;38:1025–1031. doi: 10.1007/s00134-012-2518-z. [DOI] [PubMed] [Google Scholar]
- 28.Commissioner of the U.S. Food and Drug Administration [(accessed on 5 October 2022)]; Available online: https://www.fda.gov/home.
- 29.EMA European Medicines Agency [(accessed on 5 October 2022)]. Available online: https://www.ema.europa.eu/en.
- 30.Muriel A.C., McCulloch R., Hammel J.F. Depression, Anxiety, and Delirium. In: Goldman A., Hain R., Liben S., editors. Oxford Textbook of Palliative Care for Children. Oxford University Press; Oxford, UK: 2012. [Google Scholar]
- 31.McDonnell G.A., Salley C.G., Barnett M., DeRosa A.P., Werk R.S., Hourani A., Hoekstra A.B., Ford J.S. Anxiety Among Adolescent Survivors of Pediatric Cancer. J. Adolesc. Health. 2017;61:409–423. doi: 10.1016/j.jadohealth.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yardeni M., Abebe Campino G., Hasson-Ohayon I., Basel D., Hertz-Palmor N., Bursztyn S., Weisman H., Pessach I.M., Toren A., Gothelf D. Trajectories and Risk Factors for Anxiety and Depression in Children and Adolescents with Cancer: A 1-Year Follow-Up. Cancer Med. 2021;10:5653–5660. doi: 10.1002/cam4.4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bachmann C.J., Aagaard L., Burcu M., Glaeske G., Kalverdijk L.J., Petersen I., Schuiling-Veninga C.C.M., Wijlaars L., Zito J.M., Hoffmann F. Trends and Patterns of Antidepressant Use in Children and Adolescents from Five Western Countries, 2005–2012. Eur. Neuropsychopharmacol. 2016;26:411–419. doi: 10.1016/j.euroneuro.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Mora D.C., Kristoffersen A.E., Overvåg G., Jong M.C., Mentink M., Liu J., Stub T. Safety of Complementary and Alternative Medicine (CAM) Treatment among Children and Young Adults Who Suffer from Adverse Effects of Conventional Cancer Treatment: A Systematic Review. Integr. Cancer Ther. 2022;21:15347354221105564. doi: 10.1177/15347354221105563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huber B.M., Rodondi P.-Y. Interest and Need for Continuing Medical Education in Pediatric Complementary and Integrative Medicine: A Cross-Sectional Survey from Switzerland. BMC Complementary Med. Ther. 2022;22:106. doi: 10.1186/s12906-022-03581-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mechler K., Banaschewski T., Hohmann S., Häge A. Evidence-Based Pharmacological Treatment Options for ADHD in Children and Adolescents. Pharmacol. Ther. 2022;230:107940. doi: 10.1016/j.pharmthera.2021.107940. [DOI] [PubMed] [Google Scholar]
- 37.Lüthi E., Diezi M., Danon N., Dubois J., Pasquier J., Burnand B., Rodondi P.-Y. Complementary and Alternative Medicine Use by Pediatric Oncology Patients before, during, and after Treatment. BMC Complement Med. Ther. 2021;21:96. doi: 10.1186/s12906-021-03271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan H., Goddard K., Howard A.F. Utility of the Number Needed to Treat in Paediatric Haematological Cancer Randomised Controlled Treatment Trials: A Systematic Review. BMJ Open. 2019;9:e022839. doi: 10.1136/bmjopen-2018-022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colliva C., Cellini M., Porta F.D., Ferrari M., Bergamini B.M., Guerra A., Giuseppe S.D., Pinto A., Capasso R., Caprino D., et al. Psychosocial Assessment of Families Caring for a Child with Acute Lymphoblastic Leukemia, Epilepsy or Asthma: Psychosocial Risk as Network of Interacting Symptoms. PLoS ONE. 2020;15:e0230194. doi: 10.1371/journal.pone.0230194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karyotaki E., Smit Y., Holdt Henningsen K., Huibers M.J.H., Robays J., de Beurs D., Cuijpers P. Combining Pharmacotherapy and Psychotherapy or Monotherapy for Major Depression? A Meta-Analysis on the Long-Term Effects. J. Affect. Disord. 2016;194:144–152. doi: 10.1016/j.jad.2016.01.036. [DOI] [PubMed] [Google Scholar]
- 41.Smith V., Foster J. High-Risk Neuroblastoma Treatment Review. Children. 2018;5:114. doi: 10.3390/children5090114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ismail M.F., Lavelle C., Cassidy E.M. Steroid-Induced Mental Disorders in Cancer Patients: A Systematic Review. Future Oncol. 2017;13:2719–2731. doi: 10.2217/fon-2017-0306. [DOI] [PubMed] [Google Scholar]
- 43.Bodum K.S., Hjerrild B.E., Dalsgaard S., Rubak S.L.M. Behavioural Side Effects of Inhaled Corticosteroids among Children and Adolescents with Asthma. Respir. Res. 2022;23:192. doi: 10.1186/s12931-022-02112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mrakotsky C.M., Silverman L.B., Dahlberg S.E., Alyman M.C.A., Sands S.A., Queally J.T., Miller T.P., Cranston A., Neuberg D.S., Sallan S.E., et al. Neurobehavioral Side Effects of Corticosteroids During Active Treatment for Acute Lymphoblastic Leukemia in Children Are Age-Dependent: Report from Dana-Farber Cancer Institute ALL Consortium Protocol 00-01. Pediatr. Blood Cancer. 2011;57:492–498. doi: 10.1002/pbc.23060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kenna H.A., Poon A.W., de los Angeles C.P., Koran L.M. Psychiatric Complications of Treatment with Corticosteroids: Review with Case Report. Psychiatry Clin. Neurosci. 2011;65:549–560. doi: 10.1111/j.1440-1819.2011.02260.x. [DOI] [PubMed] [Google Scholar]
- 46.Stuart F.A., Segal T.Y., Keady S. Adverse Psychological Effects of Corticosteroids in Children and Adolescents. Arch. Dis. Child. 2005;90:500–506. doi: 10.1136/adc.2003.041541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeNunzio N.J., Yock T.I. Modern Radiotherapy for Pediatric Brain Tumors. Cancers. 2020;12:1533. doi: 10.3390/cancers12061533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laver J.H., Barredo J.C., Amylon M., Schwenn M., Kurtzberg J., Camitta B.M., Pullen J., Link M.P., Borowitz M., Ravindranath Y., et al. Effects of Cranial Radiation in Children with High Risk T Cell Acute Lymphoblastic Leukemia: A Pediatric Oncology Group Report. Leukemia. 2000;14:369–373. doi: 10.1038/sj.leu.2401693. [DOI] [PubMed] [Google Scholar]
- 49.Sun H., Yang Y., Zhang J., Liu T., Wang H., Garg S., Zhang B. Fear of Cancer Recurrence, Anxiety and Depressive Symptoms in Adolescent and Young Adult Cancer Patients. Neuropsychiatr. Dis. Treat. 2019;15:857–865. doi: 10.2147/NDT.S202432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spencer R., Nilsson M., Wright A., Pirl W., Prigerson H. Anxiety Disorders in Advanced Cancer Patients: Correlates and Predictors of End of Life Outcomes. Cancer. 2010;116:1810–1819. doi: 10.1002/cncr.24954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friend A.J., Feltbower R.G., Hughes E.J., Dye K.P., Glaser A.W. Mental Health of Long-Term Survivors of Childhood and Young Adult Cancer: A Systematic Review. Int. J. Cancer. 2018;143:1279–1286. doi: 10.1002/ijc.31337. [DOI] [PubMed] [Google Scholar]
- 52.Janssen S.H.M., van der Graaf W.T.A., van der Meer D.J., Manten-Horst E., Husson O. Adolescent and Young Adult (AYA) Cancer Survivorship Practices: An Overview. Cancers. 2021;13:4847. doi: 10.3390/cancers13194847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dozzo M., Carobolante F., Donisi P.M., Scattolin A., Maino E., Sancetta R., Viero P., Bassan R. Burkitt Lymphoma in Adolescents and Young Adults: Management Challenges. Adolesc. Health Med. Ther. 2016;8:11–29. doi: 10.2147/AHMT.S94170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wood W.A., Lee S.J. Malignant Hematologic Diseases in Adolescents and Young Adults. Blood. 2011;117:5803–5815. doi: 10.1182/blood-2010-12-283093. [DOI] [PubMed] [Google Scholar]
- 55.Bandelow B., Michaelis S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015;17:327–335. doi: 10.31887/DCNS.2015.17.3/bbandelow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Greer J.A., Solis J.M., Temel J.S., Lennes I.T., Prigerson H.G., Maciejewski P.K., Pirl W.F. Anxiety Disorders in Long-Term Survivors of Adult Cancers. Psychosomatics. 2011;52:417–423. doi: 10.1016/j.psym.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Naser A.Y., Hameed A.N., Mustafa N., Alwafi H., Dahmash E.Z., Alyami H.S., Khalil H. Depression and Anxiety in Patients With Cancer: A Cross-Sectional Study. Front. Psychol. 2021;12:585534. doi: 10.3389/fpsyg.2021.585534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith H.A.B., Fuchs D.C., Pandharipande P.P., Barr F.E., Ely E.W. Delirium: An Emerging Frontier in Management of Critically Ill Children. Crit. Care Clin. 2009;25:593–614. doi: 10.1016/j.ccc.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nibras S., Kentor R., Masood Y., Price K., Schneider N.M., Tenenbaum R.B., Calarge C. Psychological and Psychiatric Comorbidities in Youth with Serious Physical Illness. Children. 2022;9:1051. doi: 10.3390/children9071051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Traube C., Gerber L.M., Mauer E.A., Small K., Broglie L., Chopra Y.R., Duncan C.N., Ebens C.L., Fitzgerald J.C., Freedman J.L., et al. Delirium in Children Undergoing Hematopoietic Cell Transplantation: A Multi-Institutional Point Prevalence Study. Front. Oncol. 2021;11:627726. doi: 10.3389/fonc.2021.627726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Piovani D., Clavenna A., Bonati M. Prescription Prevalence of Psychotropic Drugs in Children and Adolescents: An Analysis of International Data. Eur. J. Clin. Pharmacol. 2019;75:1333–1346. doi: 10.1007/s00228-019-02711-3. [DOI] [PubMed] [Google Scholar]
- 62.Moret C. Combination/Augmentation Strategies for Improving the Treatment of Depression. Neuropsychiatr. Dis. Treat. 2005;1:301–309. [PMC free article] [PubMed] [Google Scholar]
- 63.Kurian B.T., Greer T.L., Trivedi M.H. Strategies to Enhance the Therapeutic Efficacy of Antidepressants: Targeting Residual Symptoms. Expert Rev. Neurother. 2009;9:975–984. doi: 10.1586/ern.09.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Demyttenaere K., Frank E., Castle D., Cindik-Herbrüggen E. Integrating Patients’ Expectations into the Management of Their Depression: Report of a Symposium at the European College of Neuropsychopharmacology Congress. Adv. Ther. 2019;36:73–90. doi: 10.1007/s12325-019-01038-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Godlewska B.R., Harmer C.J. Cognitive Neuropsychological Theory of Antidepressant Action: A Modern-Day Approach to Depression and Its Treatment. Psychopharmacology. 2021;238:1265–1278. doi: 10.1007/s00213-019-05448-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harmer C.J., Duman R.S., Cowen P.J. How Do Antidepressants Work? New Perspectives for Refining Future Treatment Approaches. Lancet Psychiatry. 2017;4:409–418. doi: 10.1016/S2215-0366(17)30015-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moncrieff J., Cooper R.E., Stockmann T., Amendola S., Hengartner M.P., Horowitz M.A. The Serotonin Theory of Depression: A Systematic Umbrella Review of the Evidence. Mol. Psychiatry. 2022:1–14. doi: 10.1038/s41380-022-01661-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Malhi G.S., Bell E., Morris G., Hamilton A. The Delay in Response to Antidepressant Therapy: A Window of Opportunity? Aust. N. Z. J. Psychiatry. 2020;54:127–129. doi: 10.1177/0004867419900313. [DOI] [PubMed] [Google Scholar]
- 69.Brigitta B. Pathophysiology of Depression and Mechanisms of Treatment. Dialogues Clin. Neurosci. 2002;4:7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Blackburn T.P. Depressive Disorders: Treatment Failures and Poor Prognosis over the Last 50 Years. Pharmacol. Res. Perspect. 2019;7:e00472. doi: 10.1002/prp2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Saragoussi D., Touya M., Haro J.M., Jönsson B., Knapp M., Botrel B., Florea I., Loft H., Rive B. Factors Associated with Failure to Achieve Remission and with Relapse after Remission in Patients with Major Depressive Disorder in the PERFORM Study. Neuropsychiatr. Dis. Treat. 2017;13:2151–2165. doi: 10.2147/NDT.S136343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dunlop B.W., Binder E.B., Cubells J.F., Goodman M.M., Kelley M.E., Kinkead B., Kutner M., Nemeroff C.B., Newport D.J., Owens M.J., et al. Predictors of Remission in Depression to Individual and Combined Treatments (PReDICT): Study Protocol for a Randomized Controlled Trial. Trials. 2012;13:106. doi: 10.1186/1745-6215-13-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O’Donovan C., Alda M. Depression Preceding Diagnosis of Bipolar Disorder. Front. Psychiatry. 2020;11:500. doi: 10.3389/fpsyt.2020.00500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Allen J.P., Chango J., Szwedo D., Schad M. Long-Term Sequelae of Sub-Clinical Depressive Symptoms in Early Adolescence. Dev. Psychopathol. 2014;26:171–180. doi: 10.1017/S095457941300093X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang P.S., Berglund P.A., Olfson M., Kessler R.C. Delays in Initial Treatment Contact after First Onset of a Mental Disorder. Health Serv. Res. 2004;39:393–416. doi: 10.1111/j.1475-6773.2004.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blom J.M.C., Colliva C., Benatti C., Tascedda F., Pani L. Digital Phenotyping and Dynamic Monitoring of Adolescents Treated for Cancer to Guide Intervention: Embracing a New Era. Front. Oncol. 2021;11:673581. doi: 10.3389/fonc.2021.673581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta R.D., Roth A.J. Psychiatric Considerations in the Oncology Setting. CA A Cancer J. Clin. 2015;65:299–314. doi: 10.3322/caac.21285. [DOI] [PubMed] [Google Scholar]
- 78.Pralong A., Perrar K.M., Kremeike K., Rosendahl C., Voltz R. Depression, anxiety, delirium and desire to die in palliative care: Recommendations of the S3 guideline on palliative care for patients with incurable cancer. Nervenarzt. 2020;91:391–397. doi: 10.1007/s00115-020-00896-y. [DOI] [PubMed] [Google Scholar]
- 79.Wasan A.D., Artamonov M., Nedeljkovic S.S. Delirium, Depression, and Anxiety in the Treatment of Cancer Pain. Tech. Reg. Anesth. Pain Manag. 2005;9:139–144. doi: 10.1053/j.trap.2005.06.006. [DOI] [Google Scholar]
- 80.Keks N., Schwartz D., Hope J. Stopping and Switching Antipsychotic Drugs. Aust. Prescr. 2019;42:152–157. doi: 10.18773/austprescr.2019.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Frattarelli D.A., Galinkin J.L., Green T.P., Johnson T.D., Neville K.A., Paul I.M., Van Den Anker J.N. American Academy of Pediatrics Committee on Drugs Off-Label Use of Drugs in Children. Pediatrics. 2014;133:563–567. doi: 10.1542/peds.2013-4060. [DOI] [PubMed] [Google Scholar]
- 82.Giurin M.S., Trojniak M.P., Arbo A., Carrozzi M., Abbracciavento G., Monasta L., Zanus C. Safety of Off-Label Pharmacological Treatment in Pediatric Neuropsychiatric Disorders: A Global Perspective From an Observational Study at an Italian Third Level Children’s Hospital. Front. Pharmacol. 2022;13:837692. doi: 10.3389/fphar.2022.837692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.van der Zanden T.M., Smeets N.J.L., de Hoop-Sommen M., Schwerzel M.F.T., Huang H.J., Barten L.J.C., van der Heijden J.E.M., Freriksen J.J.M., Horstink A.A.L., Holsappel I.H.G., et al. Off-Label, but on-Evidence? A Review of the Level of Evidence for Pediatric Pharmacotherapy. Clin. Pharmacol. Ther. 2022;112:1243–1253. doi: 10.1002/cpt.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.van der Zanden T.M., Mooij M.G., Vet N.J., Neubert A., Rascher W., Lagler F.B., Male C., Grytli H., Halvorsen T., de Hoog M., et al. Benefit-Risk Assessment of Off-Label Drug Use in Children: The Bravo Framework. Clin. Pharmacol. Ther. 2021;110:952–965. doi: 10.1002/cpt.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Feka A., Di Paolo E.R., Pauchard J.-Y., Mariguesa A., Gehri M., Sadeghipour F. Off-Label Use of Psychotropic Drugs in a Swiss Paediatric Service: Similar Results from Two Different Cohort Studies. Swiss Med. Wkly. 2022;152:w30124. doi: 10.4414/smw.2022.w30124. [DOI] [PubMed] [Google Scholar]
- 86.Allen H.C., Garbe M.C., Lees J., Aziz N., Chaaban H., Miller J.L., Johnson P., DeLeon S. Off-Label Medication Use in Children, More Common than We Think: A Systematic Review of the Literature. J. Okla. State Med. Assoc. 2018;111:776–783. [PMC free article] [PubMed] [Google Scholar]
- 87.Pandolfini C., Bonati M. A Literature Review on Off-Label Drug Use in Children. Eur. J. Pediatr. 2005;164:552–558. doi: 10.1007/s00431-005-1698-8. [DOI] [PubMed] [Google Scholar]
- 88.Sharma A.N., Arango C., Coghill D., Gringras P., Nutt D.J., Pratt P., Young A.H., Hollis C. BAP Position Statement: Off-Label Prescribing of Psychotropic Medication to Children and Adolescents. J. Psychopharmacol. 2016;30:416–421. doi: 10.1177/0269881116636107. [DOI] [PubMed] [Google Scholar]
- 89.Bonati M., Jacqz-Aigrain E., Choonara I. Licensed Medicines, off-Label Use or Evidence-Based. Which Is Most Important? Arch. Dis. Child. 2017;102:53–54. doi: 10.1136/archdischild-2016-311527. [DOI] [PubMed] [Google Scholar]
- 90.Braüner J.V., Johansen L.M., Roesbjerg T., Pagsberg A.K. Off-Label Prescription of Psychopharmacological Drugs in Child and Adolescent Psychiatry. J. Clin. Psychopharmacol. 2016;36:500–507. doi: 10.1097/JCP.0000000000000559. [DOI] [PubMed] [Google Scholar]
- 91.Persico A.M., Arango C., Buitelaar J.K., Correll C.U., Glennon J.C., Hoekstra P.J., Moreno C., Vitiello B., Vorstman J., Zuddas A., et al. Unmet Needs in Paediatric Psychopharmacology: Present Scenario and Future Perspectives. Eur. Neuropsychopharmacol. 2015;25:1513–1531. doi: 10.1016/j.euroneuro.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 92.Zuddas A., Zanni R., Usala T. Second Generation Antipsychotics (SGAs) for Non-Psychotic Disorders in Children and Adolescents: A Review of the Randomized Controlled Studies. Eur. Neuropsychopharmacol. 2011;21:600–620. doi: 10.1016/j.euroneuro.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 93.Carton L., Cottencin O., Lapeyre-Mestre M., Geoffroy P.A., Favre J., Simon N., Bordet R., Rolland B. Off-Label Prescribing of Antipsychotics in Adults, Children and Elderly Individuals: A Systematic Review of Recent Prescription Trends. Curr. Pharm. Des. 2015;21:3280–3297. doi: 10.2174/1381612821666150619092903. [DOI] [PubMed] [Google Scholar]
- 94.Ventola C.L. Off-Label Drug Information: Regulation, Distribution, Evaluation, and Related Controversies. P T. 2009;34:428–440. [PMC free article] [PubMed] [Google Scholar]
- 95.Fraguas D., Correll C.U., Merchán-Naranjo J., Rapado-Castro M., Parellada M., Moreno C., Arango C. Efficacy and Safety of Second-Generation Antipsychotics in Children and Adolescents with Psychotic and Bipolar Spectrum Disorders: Comprehensive Review of Prospective Head-to-Head and Placebo-Controlled Comparisons. Eur. Neuropsychopharmacol. 2011;21:621–645. doi: 10.1016/j.euroneuro.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 96.O’Brien P.L., Cummings N., Mark T.L. Off-Label Prescribing of Psychotropic Medication, 2005-2013: An Examination of Potential Influences. Psychiatr. Serv. 2017;68:549–558. doi: 10.1176/appi.ps.201500482. [DOI] [PubMed] [Google Scholar]
- 97.Snaman J., McCarthy S., Wiener L., Wolfe J. Pediatric Palliative Care in Oncology. JCO. 2020;38:954–962. doi: 10.1200/JCO.18.02331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Drugs Approved for Childhood Cancers-NCI. [(accessed on 5 October 2022)]; Available online: https://www.cancer.gov/about-cancer/treatment/drugs/childhood-cancer-fda-approved-drugs.
- 99.Zanger U.M., Schwab M. Cytochrome P450 Enzymes in Drug Metabolism: Regulation of Gene Expression, Enzyme Activities, and Impact of Genetic Variation. Pharmacol. Ther. 2013;138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 100.Miguel C., Albuquerque E. Drug Interaction in Psycho-Oncology: Antidepressants and Antineoplastics. Pharmacology. 2011;88:333–339. doi: 10.1159/000334738. [DOI] [PubMed] [Google Scholar]
- 101.Hakkola J., Hukkanen J., Turpeinen M., Pelkonen O. Inhibition and Induction of CYP Enzymes in Humans: An Update. Arch. Toxicol. 2020;94:3671–3722. doi: 10.1007/s00204-020-02936-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cheer S.M., Goa K.L. Fluoxetine: A Review of Its Therapeutic Potential in the Treatment of Depression Associated with Physical Illness. Drugs. 2001;61:81–110. doi: 10.2165/00003495-200161010-00010. [DOI] [PubMed] [Google Scholar]
- 103.Rossi A., Barraco A., Donda P. Fluoxetine: A Review on Evidence Based Medicine. Ann. Gen. Hosp. Psychiatry. 2004;3:2. doi: 10.1186/1475-2832-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shuster J.L., Stern T.A., Greenberg D.B. Pros and Cons of Fluoxetine for the Depressed Cancer Patient. Oncology. 1992;6 [PubMed] [Google Scholar]
- 105.Zhou X., Teng T., Zhang Y., Del Giovane C., Furukawa T.A., Weisz J.R., Li X., Cuijpers P., Coghill D., Xiang Y., et al. Comparative Efficacy and Acceptability of Antidepressants, Psychotherapies, and Their Combination for Acute Treatment of Children and Adolescents with Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet Psychiatry. 2020;7:581–601. doi: 10.1016/S2215-0366(20)30137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.van Poelgeest E.P., Pronk A.C., Rhebergen D., van der Velde N. Depression, Antidepressants and Fall Risk: Therapeutic Dilemmas—a Clinical Review. Eur. Geriatr. Med. 2021;12:585–596. doi: 10.1007/s41999-021-00475-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.DeJong M., Fombonne E. Citalopram to Treat Depression in Pediatric Oncology. J. Child Adolesc. Psychopharmacol. 2007;17:371–377. doi: 10.1089/cap.2006.0076. [DOI] [PubMed] [Google Scholar]
- 108.Sub Laban T., Saadabadi A. StatPearls. StatPearls Publishing; Treasure Island (FL), CA, USA: 2022. Monoamine Oxidase Inhibitors (MAOI) [PubMed] [Google Scholar]
- 109.Bick J., Nelson C.A. Early Adverse Experiences and the Developing Brain. Neuropsychopharmacology. 2016;41:177–196. doi: 10.1038/npp.2015.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wade M., Wright L., Finegold K.E. The Effects of Early Life Adversity on Children’s Mental Health and Cognitive Functioning. Transl. Psychiatry. 2022;12:244. doi: 10.1038/s41398-022-02001-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Madhusoodanan S., Ting M.B., Farah T., Ugur U. Psychiatric Aspects of Brain Tumors: A Review. World J. Psychiatry. 2015;5:273–285. doi: 10.5498/wjp.v5.i3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shonkoff J.P., Boyce W.T., Levitt P., Martinez F.D., McEwen B. Leveraging the Biology of Adversity and Resilience to Transform Pediatric Practice. Pediatrics. 2021;147:e20193845. doi: 10.1542/peds.2019-3845. [DOI] [PubMed] [Google Scholar]
- 113.Sutherland J.V., Bailar J.C. The Multihit Model of Carcinogenesis: Etiologic Implications for Colon Cancer. J. Chronic Dis. 1984;37:465–480. doi: 10.1016/0021-9681(84)90030-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.