Abstract
Na+-K+-2Cl− cotransporter-1 (NKCC1) facilitates basolateral K+ and Cl− uptake, supporting their efflux across mucosal membranes of colonic epithelial cells. NKCC1 activity has also been shown to be critical for electrogenic K+ secretion induced by aldosterone, which is known to stimulate large conductance K+ (BK) channel expression in mucosal membranes. This study was aimed to 1) identify whether aldosterone enhances NKCC1 expression specifically to support BK-mediated K+ secretion and 2) to determine whether increased NKCC1 supports electrogenic Cl− secretion in parallel to K+ secretion. Dietary Na+ depletion was used to induce secondary hyperaldosteronism in rats, or aldosterone was administered ex vivo to rat distal colonic mucosae. NKCC1-dependent electrogenic K+ or Cl− secretion was measured as a function of short circuit current (ISC). qRT-PCR, western blot and immunofluorescence analyses were performed using standard techniques. Aldosterone enhanced NKCC1 and BKα expression and electrogenic K+ secretion in the distal colon, which was inhibited by either serosal bumetanide (NKCC1 inhibitor) or mucosal iberiotoxin (IbTX; BK channel blocker), but not TRAM-34 (IK channel blocker). Expression of NKCC1 and BK α proteins was enhanced in crypt cells of hyper-aldosterone rats. However, neither NKCC1-dependent Cl− secretion nor CFTR (apical Cl− channel) expression was enhanced by aldosterone. We conclude that aldosterone enhances NKCC1 to support BK-mediated K+ secretion independently of Cl− secretion in the distal colon. The regulation of NKCC1 expression/K+ secretion by aldosterone may be a therapeutic target in treating gastrointestinal disorders associated with alterations in colonic K+ transport, such as colonic pseudo-obstruction, and hyperkalemia associated with renal disease.
Keywords: short circuit current, pseudo-obstruction, hyperkalemia, end stage renal disease
INTRODUCTION
The mammalian colon plays a critical role in maintaining body K+ homeostasis, as it exhibits both active K+ absorptive and secretory processes.1,2 In the distal colon, H+,K+-ATPases mediate electroneutral active K+ absorption across mucosal membranes3,4,5,6, while both K+-Cl− cotransport (via KCC1) and the parallel function of intermediate conductance K+ (IK) channels and Cl− (CLC2) channels have been shown to mediate K+ exit across the basolateral membranes in an “electroneutral” manner.7,8 Conversely, active K+ secretion in the distal colon is “electrogenic” and mediated primarily by large conductance K+ (BK) channels in the mucosal membranes.1,9,10 The Na+,K+-ATPase and Na+-K+-2Cl− cotransporter (NKCC1) both contribute to K+ uptake across the basolateral membrane, and both transporters are required for supporting electrogenic K+ secretion in colon.5,11 However, NKCC1 is particularly important to this process, because although its activity is dependent on the Na+ gradient established by the Na+,K+-ATPase, inhibition of NKCC1 alone is sufficient to disrupt K+ secretion, while leaving Na+,K+-ATPase activity intact.11
Alterations in colonic K+ transport can have a profound impact on disease pathogenicity, either harmful or beneficial. For example, excessive colonic K+ secretion is associated with various diarrheal diseases, either secondarily as a result of large stool volume losses, as in V. cholera infection12, or as a primary driver of watery stool production, as has been suggested in colonic pseudo-obstruction (Ogilvie’s syndrome)13,14 or ulcerative colitis.15,16 On the other hand, increased colonic K+ secretion is often a critical adaptation in end stage renal disease (ESRD), providing a secondary outlet for K+ from the body, and preventing or delaying life-threatening hyperkalemia.17,18 As such, furthering our current understanding of colonic K+ transport and its regulation is of high clinical value.
Aldosterone, a mineralocorticoid hormone, is a major physiological regulator of electrolyte transporters (including K+ transporters) in the kidney and colon.11,19,20 In addition to its more prominent role in the regulation of Na+ absorption,21,22 aldosterone is also known to induce active K+ secretion by enhancing BK channel expression in the distal colon.15,23,24 Aldosterone-induced K+ secretion was shown to be dependent on NKCC1 function11 and there is evidence to suggest that aldosterone regulates NKCC1 expression, although a direct functional connection between aldosterone-stimulated NKCC1 and BK channels has not yet been established.10,25,26 Beyond this, it is unclear whether aldosterone-stimulated NKCC1 activity also supports an enhanced capacity for Cl− secretion. Cl− absorption, which is electroneutral and coupled to Na+ absorption in the distal colon, is reduced by aldosterone.27 However, aldosterone’s influence on active (i.e. stimulated) Cl− secretion – a process dependent on NKCC1 activity – is unknown.
This study was therefore designed to test the hypothesis that aldosterone directly upregulates NKCC1 in the distal colon, specifically to facilitate active BK-mediated K+ secretion, without concurrently enhancing Cl− secretion in the distal colon. Using the model of secondary hyperaldosteronism induced by dietary Na+ depletion in rats, or using exogenous aldosterone applied directly to ex vivo tissues, we provide novel evidence that aldosterone upregulates NKCC1 and BK channel expression in a coordinated manner to facilitate active K+ secretion, without concurrently enhancing Cl− secretion in the distal colon. Using the model of secondary hyperaldosteronism induced by dietary Na+ depletion in rats, our results demonstrate that aldosterone increases the expression of NKCC1 along the crypt/surface axis in the distal colon, and that coordinated NKCC1 and BK channel activity is a key feature of aldosterone-induced colonic K+ secretion. Additional studies confirmed that enhanced NKCC1 expression and activity is specific to the K+ transport pathway, as basal and cAMP-stimulated anion (Cl−) secretion were not altered by aldosterone. Regulation of NKCC1 by aldosterone may therefore be relevant to developing treatment strategies targeting pathologies associated with colonic K+ handling, or others involving aberrant NKCC1 function.
MATERIALS AND METHODS
Animals:
Male and female Sprague-Dawley rats (200 – 250g) were either purchased from Charles River laboratories or bred inhouse (generously provided by Dr. Timothy Nurkiewicz, Department of Physiology and Pharmacology, West Virginia University, Morgantown, WV). All procedures used in this study were approved by WVU’s animal care and use committee. Rats were randomly assigned to one of two groups receiving either standard chow or Na+-deficient diet (MP biomedical #0296023210) for six day to induce secondary hyperaldosteronism.28 All rats were given diet and water ad libitum. While anesthetized under 5% isoflurane, whole colon was removed and washed with ice cold Ringer’s solution containing (in mM): 140 Na+, 119.8 Cl−, 25 HCO3−, 5.2 K+, 1.2 Ca2+, 1.2 Mg2+, 2.4 HPO42-, 0.4 H2PO4−, and 10 glucose (pH 7.4) before being used for mucosal scraping or electrophysiological studies. Euthanasia was then immediately performed by cutting the diaphragm and blood was collected via cardiac puncture.
Aldosterone assay:
Serum aldosterone level was determined using an ELISA kit (Invitrogen #EIAALD). Aldosterone concentration was calculated by fitting sample absorbance values to a standard curve using non-linear regression in GraphPad Prism 8.0 software, as indicated in the kit’s instructions.
Mucosal scraping:
Distal colon sacs were made by filling with ice cold buffer containing (in mM): 15.4 NaCl, 8 HEPES/Tris (pH 7.5), 5 ethylenediaminetetraacetic acid (EDTA), 0.5 dithiothreitol (DTT), and 0.5 phenylmethylsulphonyl fluoride (PMSF) and incubated in the same solution on ice for 45 minutes. The drained sacs were opened along the mesenteric border. The distal mucosal (epithelial) layer was scraped using glass slides and immediately frozen in liquid nitrogen until use. Scraped mucosae were used for RNA isolation and protein extraction.
RNA isolation and qRT-PCR analysis: Total RNA was isolated from frozen colonic mucosa (50 mg) using Trizol method and quantitated with NanoDrop spectrometer (Thermo Sci. ND2000C). Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) was performed using One-Step kit (NEB #E3005) per the manufacturer’s protocol. In brief, custom gene-specific primers were designed using the published DNA sequence and synthesized by Thermo Scientific, and are listed in Table 1.
Table 1:
Primer sequences used in qRT-PCR analysis of RNA extracts from rat distal colon.
| Gene Target and Accession number |
Primer Sequence | Amplicon length |
|---|---|---|
|
NKCC1 (Slc12a2)
NM_001270617.1 |
F: 5’- TGGCAAGACTTCAACTCAGC - 3’ R: 5’- GGTATCAACAAGGTCAAACCTCC - 3’ |
177 |
|
ENaCγ (Scnn1g) NM_017046.2 |
F: 5’- ATGATACCTCTGACTGCGCC - 3’ R: 5’- AAAAGCGTGAAGTTCCTGGC - 3’ |
199 |
|
BKα (Kcnma1)
NM_001393699.1 |
F: 5’- ATGTCTACAGTGGGTTACGG - 3’ R: 5’- TGGGTGGTAGTTCTTTATGG - 3’ |
464 |
|
NaKα (ATP1a1) NM_012504.1 |
F: 5’- CATCAACGCAGAGGATGTCG - 3’ R: 5’- AGTGAGGAGTTATCCACCTTGC - 3’ |
117 |
|
CFTR (Cftr)
NM_031506.1 |
F: 5’- TTCTTCAGCTGGACCACACC - 3’ R: 5’- TGGAAGCTTGTTCCCTGTCC - 3’ |
106 |
|
β-actin (Actb)
NM_031144.3 |
F: 5’- AGATCAAGATCATTGCTCCTCC - 3’ R: 5’- AGTAACAGTCCGCCTAGAAGC - 3’ |
165 |
Western blot analysis:
Proteins were extracted from frozen colonic mucosa (50 mg) by homogenizing in lysis buffer (150 mM NaCl, 50 mM Tris base, 0.5% sodium deoxycholate, 0.1% SDS, 1.0 % Triton-X100) plus protease inhibitor cocktail (Thermo A32965), followed by a brief sonication (Fisher Sonic Dismembranator Model 100). Following centrifugation, the supernatant was mixed with 4x Laemmli buffer (Life Technologies NP0007) and heated to 90°C for 5 minutes. Samples were then chilled on ice before adding β–mercaptoethanol (5% w/v), and the aliquoted samples were flash frozen and stored at −80°C. Proteins (~20 μg) were resolved on 8% Tris-glycine polyacrylamide gels and transferred on to PVDF membranes. Membranes were incubated in blocking buffer (3% BSA in tris-buffered saline plus 0.1% Tween-20 (TBS-T) for 1 hour at room temperature, then incubated overnight with either rabbit anti-NKCC1 (Cell Signaling Technologies 14581, 1:1000)29 or horseradish peroxidase-conjugated rabbit anti-actin (Santa Cruz Biotechnologies SC-4778, 1:1000) at 4°C. The membranes were then washed in TBST (5 minutes x5). Blots incubated with anti-NKCC1 were incubated with secondary antibody solution containing goat anti-rabbit (Thermo 31462, 1:20,000) for 1 hour at room temperature and washed again in TBST (5 min × 5). Immune complexes were detected using enhanced chemiluminescence (West Dura, Thermo 34075). Images were captured using a G:BOX (Syngene) and band intensities were quantified by densitometry using Image J software and normalized to β–actin as a loading control.
Immunofluorescence studies:
Distal colon segments were fixed in 4% paraformaldehyde in PBS overnight at 4°C, washed in PBS, and flash frozen in isopentane cooled with liquid nitrogen before embedding in tissue freezing medium (Thermo 1437365) and storing at −80°C. Sections (8 μm) were cut and mounted on charged glass slides. Antigen retrieval was then performed by heating to 70° C in 10 mM citrate (pH 6.0) for 40 minutes using a microwave on low power. After cooling, sections were permeabilized and blocked in 5% goat serum and 0.1% Tween-20 in PBS (PBST) for 30 minutes, then incubated in blocking solution containing rabbit anti-NKCC1 (1:500) and mouse anti-BKα (Millipore MABN70, 1:100)30 antibodies overnight at 4°C. The following day, sections were washed in PBST (5 minutes × 5) and incubated for 1 hour at room temperature in blocking solution containing Alexa Fluor 488 goat anti-rabbit (Thermo A11008) and Alexa Fluor 568 goat anti-mouse (Thermo A11031). After washing again in PBST, coverslips were mounted with SlowFade Diamond mountant containing DAPI (Thermo S36973) and sealed with clear nail polish. Images were captured with a Zeiss 710 confocal microscope (Carl Zeiss, NY).
Ussing chamber studies:
Distal colon segments placed in ice cold Ringer’s solution containing indomethacin (5 μM; to inhibit prostaglandin production)31 were opened along the mesenteric border and the mucosal layers were separated, as described previously8. Briefly, the colons were placed in a dissecting dish and a shallow lateral incision was made into the mucosal layer just proximal to the most distal Peyer’s patch (~2 cm from the rectum). The distal mucosal layer (2–6 cm proximal to the rectum) was then separated from the underlying smooth muscle layers. Mucosae mounted on EasyMount sliders (1.1 cm2 aperture) placed Ussing Style chambers (Physiologic Instruments, San Diego CA) were bathed bilaterally with Ringer’s solution, bubbled with 5% CO2 balanced with oxygen, and warmed to 37°C. Tetrodotoxin (1 μM) was added to the serosal bath to inhibit residual neuronal activity31. Tissues were allowed to equilibrate until trans-epithelial potential (VTE) had stabilized (~20 minutes). Then, tissues were voltage clamped and short-circuit current (ISC) was measured by computer-operated software (Physiologic Instruments, San Diego CA). For all data presented here, positive ISC reflect anion secretion (serosal to mucosal direction) and/or cation absorption (mucosal to serosal direction). Trans-epithelial conductance (GTE) was monitored by generating a bipolar 5 mV pulse at 5 second intervals. All tissues were allowed to reach steady-state ISC (i.e., plateau) for at least 10 minutes prior to the addition of any drugs.
RESULTS
Dietary Na+ depletion induces secondary hyperaldosteronism in a sex-dependent manner.
Dietary Na+ depletion is commonly used to generate experimental hyper-aldosteronism.27,32,33 In the past, the effects of experimental hyper-aldosteronism on kidney and colon electrolyte transport have been mostly characterized in male, but not in female animals. However, in accordance with current NIH guidelines regarding biological variability related to sex, we examined the effect of dietary Na+ depletion on plasma aldosterone levels in both male and female rats. Although the serum aldosterone levels were similar in both normal male and female (244.9 ± 29.9 vs 245.9 ± 61.7 pg/mL), dietary Na+ depletion induced a 14.2-fold increase in serum aldosterone level in male rats (normal vs Na+-depleted: 244.9 ± 29.9 vs. 3,485.0 ± 949.1 pg/mL), but only a 3.4-fold increase in female rats (normal vs Na+-depleted: 245.9 ± 61.7 vs. 827.5 ± 57.3 pg/mL) (Supplemental Fig. S1A).
Because dietary Na+ depletion-induced hyperaldosteronism is known to induce epithelial Na+ channel (ENaC)-mediated Na+ absorption21,22, we also examined whether the differentially increased aldosterone levels were reflected in ENaC activity in male and female rat distal colon. Similar to the difference observed regarding aldosterone generation, Na+ depletion induced ENaC activity proportionately lower (~ 4-fold) in dietary Na+-depleted female compared to male rat distal colon, as measured by 100 μM amiloride-sensitive short circuit current (ISC; ENaC-mediated Na+ absorption) (Na+-depleted male vs female: ΔISC(AMIL) = −388.3 ± 28.3 vs −101.2 ± 31.6 μA/cm2) (Supplemental Fig. S1B). A concentration of 100 μM amiloride was selected based on similar published experiments.23,24 To assess whether a sex-specific effect of dietary Na+ depletion had occurred, a Two-way analysis of variance (ANOVA) with Tukey’s post-hoc was performed. Analysis showed significantly elevated serum aldosterone level in dietary Na+-depleted male (p < 0.01), but not in female (p = 0.82) rats; and significantly induced ENaC activity in dietary Na+-depleted male (p < 0.001), but not female (p = 0.06) rat distal colon.
Although serum aldosterone and ENaC activity were appreciably high in dietary Na+-depleted female rats, and thus the dietary effects are certainly not negligible, the response was not as robust as in the males and therefore requires separate independent study to identify the mechanistic nature of lower aldosterone generation level in Na+-depleted female rats. However, as the specific goal of this investigation is to evaluate the regulation of NKCC1 and BK-mediated K+ secretion by aldosterone, all subsequent experiments were designed to incorporate only male rats to avoid confounding effects resulting from sex differences, as well as to maintain consistency with existing literature regarding aldosterone-regulated K+ transport.
Dietary Na+ depletion stimulates electrogenic Na+ absorption and K+ secretion in rat distal colon.
Further initial studies were performed to establish that dietary Na+ depletion induces ENaC-mediated electrogenic Na+ absorption and BK channel-mediated electrogenic K+ secretion in rat distal colon, as has been previously reported.24,34 As shown in Supplemental Fig. S2A, the large, positive basal ISC measured in Na+-depleted rat distal colon indicates the presence of ENaC activity (normal vs dietary Na+-depleted: basal ISC = 28.3 ± 3.2 vs 353.8 ± 8.9 μA/cm2; p < 0.001), while inhibition by mucosal amiloride further confirmed that ENaC activity was the source of this basal ISC in dietary-Na+ depleted rat distal colon (normal vs dietary Na+-depleted: ISC post-amiloride = 17.9 ± 0.9 vs −40.0 ± 3.3 μA/cm2; p < 0.001). Negative ISC in the presence of amiloride indicates electrogenic K+ secretion (Supplemental Fig. S2A). Lower basal ISC that was less sensitive to amiloride, as well as a positive residual ISC after amiloride application, suggested that normal distal colon does not exhibit a significant amount of electrogenic Na+ absorption and K+ secretion, respectively. The positive ISC in normal rat distal colon likely represents anion (Cl−/HCO3−) secretion (Supplemental Fig. S2A). A parallel increase in ENaCγ subunit (12.5-fold), as well as BKα subunit (2.3-fold) specific mRNA abundance indicated that ENaC and BK channel expression were induced in dietary Na+-depleted rat distal colon (Supplemental Fig. S2B). These observations are consistent with the observed functional increase in both electrogenic Na+ absorption and electrogenic K+ secretion in distal colon from normal versus Na+-depleted rats. These data validated the model of secondary hyperaldosteronism by Na+ deprivation used in the experiments to follow.
Dietary Na+ depletion stimulates NKCC1 expression in rat distal colon.
qRT-PCR analyses were performed to determine whether, in parallel to BKα mRNA, the abundance of basolateral K+ transporters, Na+,K+-ATPase and NKCC1 were also increased in dietary Na+-depleted rat distal colon. NKCC1 (2.2-fold; p < 0.01), but not NaKα (catalytic subunit of Na+,K+-ATPase) specific mRNA abundance was significantly increased (Fig. 1A). Previous studies have also found that Na+,K+-ATPase subunits are not transcriptionally regulated by aldosterone, but rather an increase in activity is achieved via an increase in transporter density within the basolateral membrane35,36. A single product of predicted size (177 bp) confirmed the specific amplification of NKCC1 mRNA in the qRT-PCR reactions (Fig. 1B). Western blot analysis also showed increased NKCC1 protein expression in dietary Na+-depleted compared to normal rat distal colon (2.3-fold; p < 0.01) (Fig. 1C,D).
Figure 1. Dietary Na+ depletion induces NKCC1 expression in rat distal colon.

(A) NKCC1 and Na+, K+-ATPase (NaKα) mRNA abundance measured by qRT-PCR using total-RNA isolated from normal and dietary-Na+ depleted rat distal colon. (B) qRT-PCR-amplified NKCC1 transcript products were resolved on 1.5% agarose gel show a single product of expected size (177 bp) in total-RNA isolated from both normal and dietary Na+-depleted rat distal colon. DNA ladder and corresponding fragment sizes are indicated on the left. (C) Representative Western blot of NKCC1 and β-actin (internal control) protein expression in mucosal homogenates from normal (N) and dietary Na+-depleted (D) rat distal colon. Protein ladder is shown in the extreme left lane, and corresponding molecular weights in kDa are indicated. (D) Densitometry quantitation of NKCC1 protein normalized to β-actin. NKCC1 band intensity was measured and divided by the β-actin band intensity for each sample using FIJI (ImageJ) software. Data were then transformed so that control NKCC1/β-actin values were set equal to 1. Lines and error bars represent means ± SEM, *p<0.01, as determined by Student’s unpaired t-test.
Dietary Na+ depletion induces bumetanide-sensitive K+ secretion in rat distal colon.
Experiments were next performed to establish that basolateral K+ uptake via NKCC1 supports electrogenic K+ secretion in Na+-depleted rat distal colon. The contribution of NKCC1-supported K+ secretion was measured as a function of bumetanide-sensitive ISC in an Ussing-style recording chamber. Bumetanide (NKCC1-specific inhibitor; 200 μM) -sensitive ISC was measured in the presence of mucosal amiloride (100 μM), to eliminate interference by highly electrogenic ENaC activity. A concentration of 200 μM bumetanide was selected based on previous literature.11,24 In the presence of amiloride, positive and negative ISC values were measured in normal and dietary Na+-depleted rat distal colon, respectively (normal vs dietary Na+-depleted: ISC = 16.8 ± 0.7 vs −39.9 ± 9.8 μA/cm2; p < 0.01) (Fig. 2A,C). Application of bumetanide to the serosal bath induced a reversal from negative to positive ISC in Na+-depleted rat distal colon (basal vs bumetanide: ISC = −39.9 ± 4.4 vs 7.1 ± 1.7 μA/cm2; p < 0.01), while having no significant effect in normal distal colon (basal vs bumetanide: ISC = 16.8 ± 0.7 vs 13.0 ± 0.7 μA/cm2; p = 0.74) (Fig. 2A,C). The average change in ISC induced by bumetanide was significantly larger in Na+-depleted vs normal rat distal colon (normal vs Na+-depleted: ΔISC(BUMET) = −4.6 ± 0.8 vs 47.0 ± 3.1 μA/cm2; p < 0.01) (Fig. 2E).
Figure 2. Inhibition of NKCC1 significantly inhibits electrogenic K+ secretion in dietary Na+-depleted, but not in normal rat distal colon.
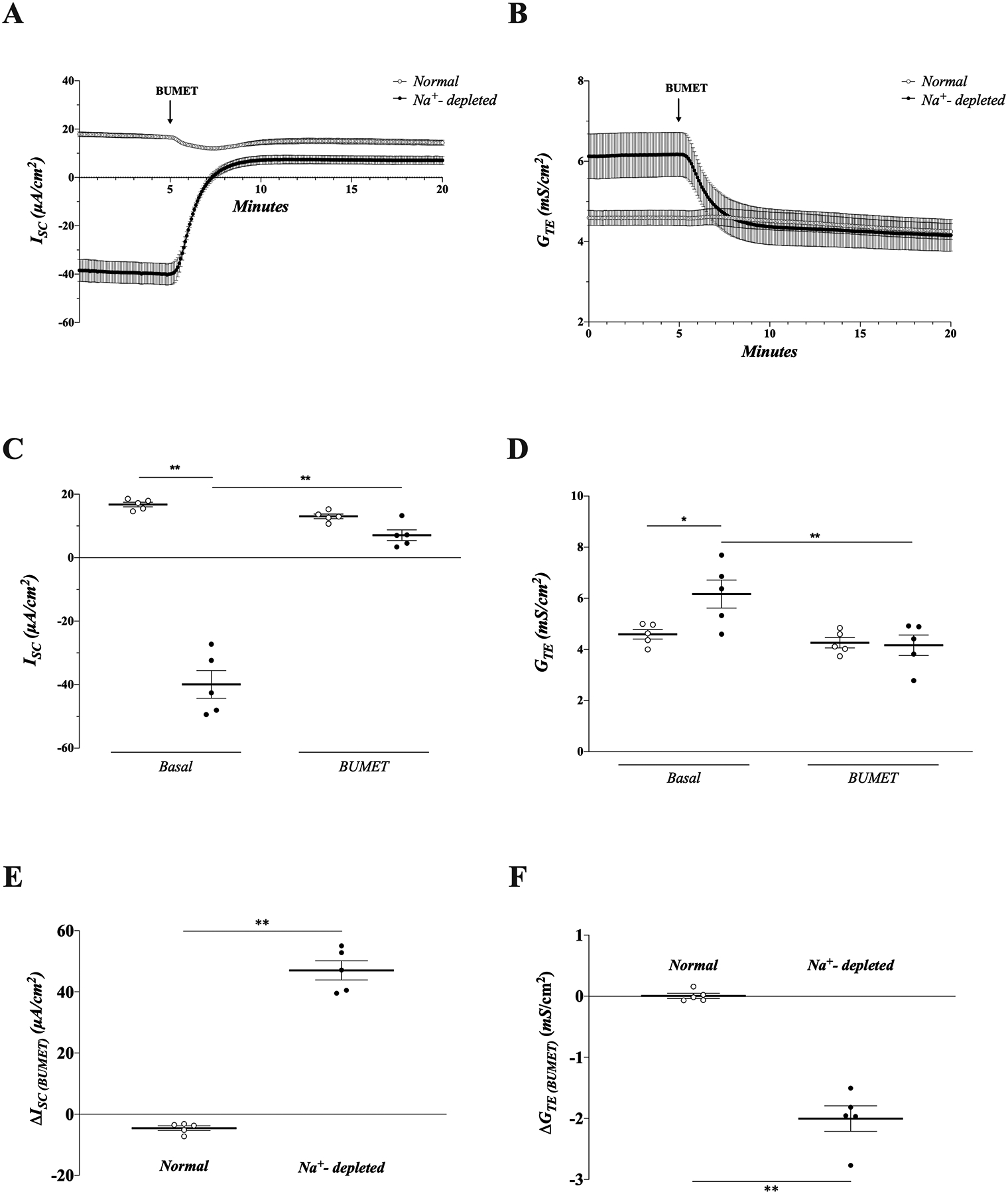
ISC (A) and GTE (B) measurements from normal (open circles) and dietary Na+-depleted (closed circles) distal colonic mucosae in the presence of 100 μM amiloride in the mucosal bath. Tissues were treated with 200 μM bumetanide (BUMET) in the serosal bath where indicated. (C-D) Group data showing ISC (C) and GTE (D) from normal and aldosterone tissues are before and after application of bumetanide, in the presence of mucosal amiloride. Average ISC and GTE values over the course of 1 minute immediately prior to bumetanide addition (basal), and 1 minute at the end of the recording period (BUMET) were calculated for each tissue. (E-F) Change in (Δ) ISC (E) and GTE (F) induced by bumetanide in normal and dietary Na+-depleted rat distal colon in the presence of mucosal amiloride. ΔISC and ΔGTE were calculated for each tissue by subtracting the basal value from the value at the end of the recording period, after BUMET addition. Lines and error bars represent means ± SEM. * p<0.05, ** p<0.01 as determined by one-way ANOVA with Tukey’s post-hoc (panels C-D) or Student’s unpaired t-test (panels E-F).
Serosal application of bumetanide also significantly inhibited GTE in dietary Na+-depleted (basal vs bumetanide: GTE = 6.2 ± 0.5 vs. 4.2 ± 0.4 mS/cm2; p < 0.01), but not normal (basal vs bumetanide: 4.6 ± 0.2 vs. 4.3 ± 0.2 mS/cm2; p = 0.26) rat distal colon (Fig. 2B,D), suggesting the positive change in ISC induced by bumetanide was due to a reduction in cation (K+) secretion rather than an increase in anion (Cl−/HCO3−) secretion or cation (Na+) absorption. The average change in GTE induced by bumetanide was also significantly greater in Na+-depleted vs normal rat distal colon (normal vs Na+-depleted: ΔGTE(BUMET) = −0.01 ± 0.04 vs −2.0 ± 0.2 mS/cm2; p < 0.01) (Fig. 2F). These observations suggest that NKCC1 functions as a major basolateral K+ loader during electrogenic K+ secretion in dietary Na+-depleted rat distal colon, while having little effect on basal secretion in normal distal colon.
Ex vivo aldosterone induces bumetanide-sensitive K+ secretion and NKCC1 expression in normal rat distal colon.
To establish that aldosterone, but not the dietary Na+ depletion per se, is critical for induced electrogenic K+ secretion and enhanced NKCC1/BKα expression, the effect of aldosterone applied ex vivo was examined in normal rat distal colon mucosae mounted in the recording chamber. Similar to dietary Na+ depletion, ex vivo aldosterone (10 nM, based upon measured serum aldosterone levels) over an 8-hour incubation period induced both ENaC activity (i.e., increased amiloride-sensitive ISC), as well as electrogenic K+ secretion (i.e., negative ISC) in presence of amiloride, which were not observed in control tissues or tissues treated with aldosterone plus 10 μM spironolactone, a mineralocorticoid antagonist (Fig. 3A,B). It should be noted that colonic mucosae remain viable for many hours under these conditions, as previously demonstrated.37 Serosal addition of bumetanide completely inhibited the aldosterone-induced electrogenic K+ secretion (basal vs bumetanide: ISC = −29.9 ± 1.3 vs 8.6± 1.9 μA/cm2; p < 0.01). Bumetanide-sensitive ISC was significantly greater in aldosterone-treated versus control tissues (control vs. aldosterone-treated: ΔISC(BUMET) = −5.7 ± 1.4 vs. 30.9 ± 1.3 μA/cm2; p < 0.001) and aldosterone alone compared to aldosterone plus spironolactone-treated tissues (aldosterone vs. aldosterone + spironolactone: ΔISC(BUMET) = −4.0 ± 1.1 vs. 30.9 ± 1.3 μA/cm2; p < 0.001) (Fig. 3C). Bumetanide-sensitive-GTE followed the same pattern (control vs. aldosterone-treated: ΔGTE(BUMET) = −0.2 ± 0.02 vs. −1.0 ± 0.04 mS/cm2; p < 0.001); (aldosterone vs. aldosterone + spironolactone : ΔGTE(BUMET) = −0.2 ± 0.01 vs. −1.0 ± 0.04 mS/cm2; p < 0.001) (Fig. 3D). qRT-PCR analyses performed on total RNA isolated from the same tissues revealed that aldosterone treatment significantly increased the NKCC1 (1.8-fold; p < 0.05) and BKα (5.9-fold; p < 0.01) specific mRNA abundance compared to control or aldosterone plus spironolactone-treated tissues (Fig. 3E). Interestingly, BKα mRNA abundance was still increased in aldosterone plus spironolactone-treated tissues compared to control (2.7-fold; p < 0.05), suggesting either that 10 μM spironolactone is insufficient to completely inhibit BKα induction by aldosterone, or that aldosterone-induced BKα expression may be partially mediated by a spironolactone-insensitive mechanism. Nonetheless, these data indicate that the increased serum aldosterone, but not the dietary Na+ depletion alone, was likely responsible for the enhanced NKCC1 and BKα expression and electrogenic K+ secretion in rat distal colon observed in earlier experiments.
Figure 3. Ex vivo aldosterone induces ENaC activity and bumetanide-sensitive K+ secretion and increases NKCC1 and BKα mRNA abundance in normal rat distal colon.

ISC (A) and transepithelial resistance (GTE; B) were continuously monitored in normal rat distal colonic mucosae mounted under voltage clamp conditions in the presence and absence of 10 nM aldosterone, or 10 nM aldosterone plus 10 μM spironolactone. Following an 8-hour incubation period, ISC and GTE were progressively increased (evidence for induced ENaC activity) in aldosterone-treated tissues (closed circles), while no change was observed in control (open circles) or aldosterone plus spironolactone-treated tissues (grey triangles). ISC and GTE data over the 8-hour incubation period are not shown here, so as to prevent the depicted data from being distorted by the lengthy time scale. ISC recordings depicted here were begun at 8 hours post-treatment. All tissues were treated with mucosal amiloride (AMIL; 100 μM), followed by serosal bumetanide (BUMET; 200 μM) where indicated. (C-D) Group data showing the bumetanide-sensitive change in Isc (ΔISC; C) and GTE (ΔGTE; D) in control, aldosterone-, and aldosterone plus spironolactone-treated tissues. Average ISC and GTE values over the course of 1 minute immediately prior to bumetanide addition, and 1 minute at the end of the recording period were calculated for each tissue. ΔISC and ΔGTE were calculated by subtracting the basal value from the value at the end of the recording period, after bumetanide application. (E) NKCC1 and BKα mRNA transcript abundance measured by qRT-PCR analysis using RNA extracted from control, aldosterone- and aldosterone plus spironolactone-treated mucosae used in the above experiments. Lines and error bars represent means ± SEM. * p<0.05 and ** p<0.01, as determined by one-way ANOVA with Tukey’s post-hoc.
Bumetanide-sensitive electrogenic K+ secretion is mediated by mucosal BK channels in dietary Na+-depleted rat distal colon.
We have previously found increased mucosal BK and IK channel activities in dietary Na+-depleted rat distal colon.37 Thus, to identify whether BK and/or IK channels mediate bumetanide-sensitive K+ exit across mucosal membranes, the effect of mucosal K+ channel blockers Ba2+ (a nonspecific K+ channel blocker), Iberiotoxin (IbTX; a selective BK channel blocker) and Tram-34 (a selective IK channel blocker) were examined. K+ channel blocker concentrations were selected based on similar experiments described in the literature.9,38 Mucosal Ba2+ (3mM) inhibited basal K+ secretion (ΔISC(Ba2+) = 19.8 ± 3.1μA/cm2) (Fig. 4A,D) and significantly attenuated the effect of bumetanide in Na+-depleted rat distal colon (no inhibitor vs. Ba2+: ΔISC(BUMET) = 47.0 ± 3.1 vs. 19.3 ± 2.5 μA/cm2; p < 0.001) (Fig. 4F). Mucosal IbTX also inhibited basal K+ secretion (ΔISC(IbTx) = 33.8 ± 4.0 μA/cm2) (Fig. 4B,D) and attenuated the effect of serosal bumetanide as well (no inhibitor vs. IbTX: ΔISC(BUMET) = 47.0 ± 3.1 vs. 15.9 ± 1.8 μA/cm2; p < 0.001) (Fig. 4F), although a much longer time period was required to achieve inhibition with IbTX, as is expected.23 Addition of mucosalTram-34 (250 nM) produced no appreciable change in basal K+ secretion (ΔISC(TRAM-34) = −1.7 ± 1.8 μA/cm2) (Fig. 4C,D) and did not alter the response to serosal bumetanide (no inhibitor vs. Tram-34: ΔISC(BUMET) = 47.0 ± 3.1 vs. 44.5 ± 4.2 μA/cm2; p > 0.98) (Fig. 4F). Together, these data suggest that NKCC1-dependent K+ secretion in Na+-depleted rat distal colon is likely mediated by mucosal membrane BK, but not IK channels.
Figure 4. Mucosal K+ channel blockers inhibit basal and bumetanide-sensitive electrogenic K+ secretion in dietary Na+-depleted rat distal colon.

ISC recordings from Na+-depleted rat distal colonic mucosae treated with mucosal Ba2+ (3 mM) (A), Iberiotoxin (IbTX; 250 nM) (B), or TRAM-34 (250 nM) (C) in the presence of 100 μM mucosal amiloride. Tissues were subsequently treated with serosal bumetanide (BUMET; 200 μM). (D-E) Group data showing change in ISC (ΔISC; D) in the presence of Ba2+, IbTX or TRAM-34, as well as the residual ISC (ISC(RES); E) after the application of each drug. Average ISC values over the course of 1 minute immediately prior to Ba2+, IbTX or TRAM-34 application, 1 minute immediately prior to the addition of bumetanide, and 1 minute at the end of the recording period were calculated for each tissue. (F) Change in ISC induced by bumetanide (ΔISC(BUMET)) in the presence IbTX, Tram-34 or Ba2+. ΔISC values were calculated by subtracting residual ISC after Ba2+, IbTX or TRAM-34 application, from the final ISC measured after bumetanide application. Data points with no inhibitor (-) (far left) are re-plotted from Fig. 4E for comparison. Lines and error bars represent means ± SEM. * p<0.05 and ** p<0.01, as determined by one-way ANOVA with Tukey’s post-hoc.
Addition of Ba2+ (non-selective K+ channel blocker) produced a similar effect to IbTX with respect to inhibition of basal K+ secretion (Fig. 4C), although IbTX-induced inhibition was significantly greater than that of Ba2+ (ΔISC(IbTX) vs. ΔISC(Ba2+) = 33.8 ± 4.0 vs. 19.8 ± 3.1 μA/cm2; p < 0.05) (Fig. 4D). However, residual ISC following IbTX or Ba2+ administration was not different (IbTX vs. Ba2+: ISC(RES) = −8.8 ± 2.4 vs. −12.9 ± 2.5 μA/cm2; p = 0.61) (Fig. 4E), suggesting this difference may have resulted from variability in the initial ISC values. In the continued presence of mucosal Ba2+, IbTX or TRAM-34, serosal bumetanide completely inhibited the electrogenic K+ secretion and unmasked the apparent presence of a small degree of anion secretion (i.e. positive ISC) (Fig. 4A–C), similar to Figures 2 and 3. It is also to be noted that only a negligible amount of IbTX-sensitive K+ secretion was present in normal compared to dietary Na+-depleted rat distal colon (normal vs dietary Na+-depleted: ΔISC(IbTX) = 3.1 ± 1.2 vs. 33.8 ± 4.0 μA/cm2; p < 0.001), supporting the earlier finding that BKα subunits expression is upregulated by dietary Na+ depletion (Supplemental Fig. S2).
Unexpectedly, serosal bumetanide appeared to inhibit residual K+ secretion even in the presence of the non-selective K+ channel blocker, Ba2+. Incomplete inhibition of apical K+ channels may have resulted from the effective (free) Ba2+ concentration being reduced by mucins or carbonate anions present in the apical bath, or from tissue architecture impeding the diffusion of drugs to the target cell membranes. Nonetheless, the inhibitory effect of bumetanide on basal K+ secretion was substantially diminished by IbTX and Ba2+ (60–70%), suggesting that coordinated uptake via NKCC1 at the basolateral membrane and exit via BK channels at the apical membrane are both essential for basal secretion of K+ induced by aldosterone in rat distal colon.
Dietary Na+ depletion enhances NKCC1 and BKα channel protein expression together in rat distal colonic crypts.
Inhibition of basal electrogenic K+ secretion by bumetanide and/or IbTX suggested that basolateral NKCC1 and mucosal BK channels may be coordinately regulated by aldosterone in dietary Na+-depleted rats. Thus, immunofluorescence studies were performed to identify whether NKCC1 and BKα channel specific proteins are localized in the basolateral and mucosal membranes of the same cell(s) in distal colon sections. As shown in Fig. 5, NKCC1-like proteins in normal distal colon were localized predominantly in the lateral membranes of lower crypt epithelia, with minimal labelling observed in surface cells (Fig. 5A,D,I,J). BKα-like proteins were diffusely localized in surface epithelial cells, with some apical membrane localization apparent within the crypt epithelium (Fig. 5B,D,I,J). In contrast, NKCC1-like proteins are localized in the lateral membranes of both upper- and lower-crypt epithelia, as well as surface cells in Na+ depleted rat distal colon (Fig. 5E,H,K,L). In addition, BKα-like proteins were concentrated on the mucosal membranes of both upper- and lower-crypt epithelia of dietary Na+-depleted rat distal colon (Fig. 5B), while remaining diffuse in surface epithelial cells. BKα, but not NKCC1 proteins were also localized in smooth muscle cells, as well as other unidentified cell types in the lamina propria of both normal and dietary Na+-depleted rat distal colon (Fig. 5B,F).
Figure 5. Immunofluorescence localization of NKCC1 and BKα channel specific proteins in normal and dietary Na+-depleted rat distal colon.
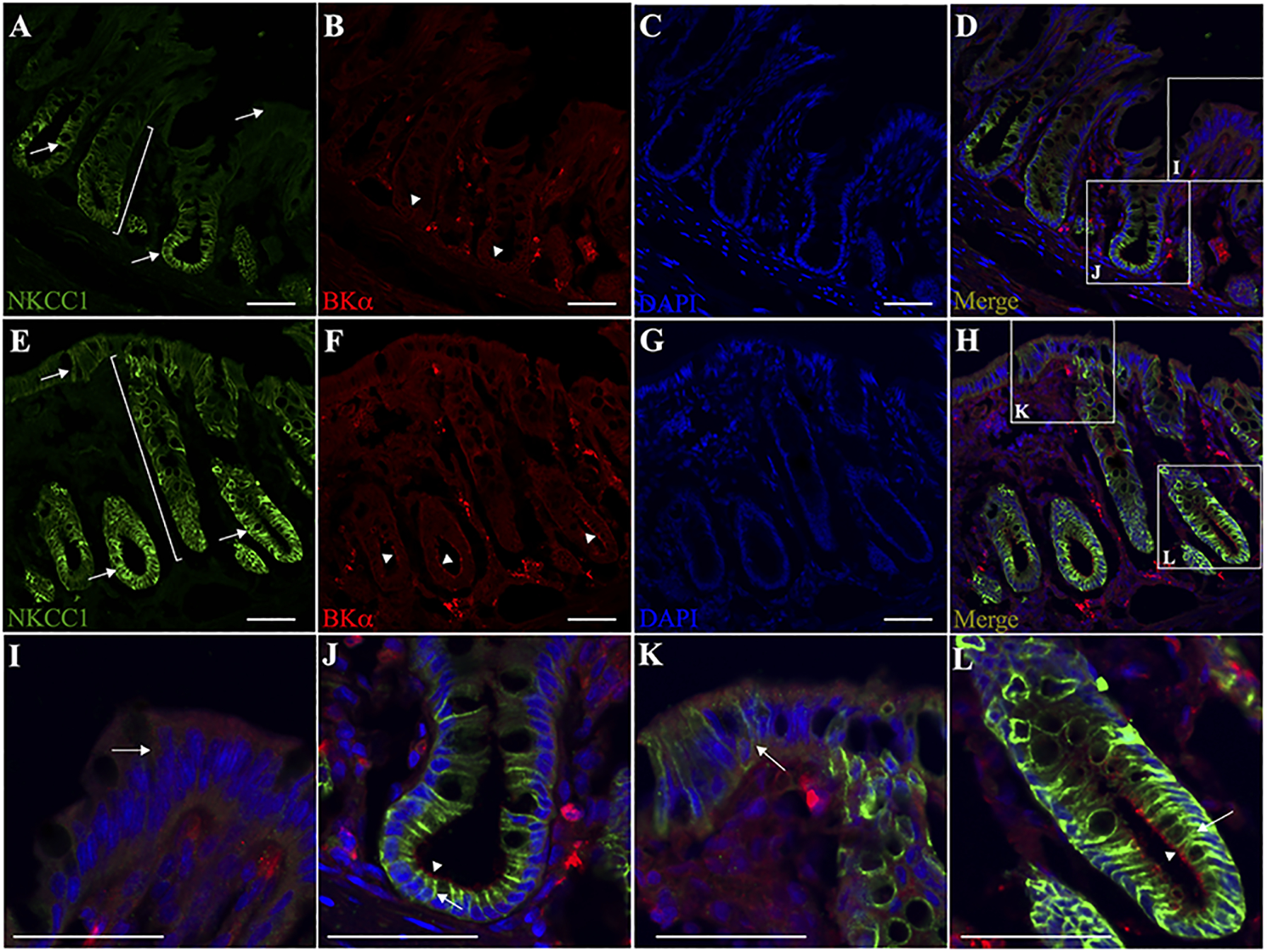
Normal (A-D) and dietary Na+-depleted (E-H) rat distal colon sections were labelled with both anti-NKCC1 (green) and anti-BKα (red) antibodies, while nuclei were labelled with DAPI (blue). Merged images are shown in panels D and H. All images were captured at 20x magnification, scale bar = 50 μm. Magnified surface cell regions are shown for normal (I) and dietary Na+-depleted (K) rat distal colon images, as indicated by the white boxes in panels D and H. Magnified crypt regions are also shown for normal (J) and dietary Na+-depleted (L) rat distal colon. Arrows indicate basolateral membrane labelling of NKCC1 in the lower crypt region of normal (A,J), and the whole crypt as well as surface cells in Na+-depleted rat distal colon sections (E,K,L). Brackets in panels A and E illustrate that NKCC1 labelling appears mostly restricted to the lower crypt in normal, while extending upward to the surface region in Na+-depleted rat distal colon. Arrowheads indicate the apparent mucosal membrane-localized BK channels in the crypts of both normal (B,J) and Na+-depleted (F,L) rat distal colon sections, while BK labelling in the surface regions was diffuse in both normal and Na+-depleted colon sections.
Merged images clearly indicate that NKCC1 and BKα-channel proteins are localized in basolateral and mucosal membranes, respectively, in epithelial cells of the crypts of normal and dietary Na+-depleted rat distal colon (Fig. 5D,H,J,L). Qualitatively, it appears that both NKCC1 and BK-channel protein expression were substantially increased in dietary Na+-depleted rat distal colon, consistent with our qRT-PCR and Western blot data (Fig.1). These observations further support the working hypothesis that the expression of basolateral NKCC1 and mucosal BKα channel proteins are coordinately regulated to produce the electrogenic K+ secretion induced by aldosterone in the distal colon, and further suggest that the crypt region may be the primary location of aldosterone-induced K+ secretion that is mediated by NKCC1 and BK channels.
Enhanced NKCC1 expression does not support enhanced Cl− secretion in dietary Na+ depleted rat distal colon.
Studies were next designed to determine whether in addition to functioning as a basolateral K+ loader, increased NKCC1 expression would also function as Cl− loader supporting active Cl− secretion in dietary Na+-depleted rat distal colon, as active Cl− secretion is known to be dependent on basolateral NKCC1 activity.39 36Cl− flux experiments have previously demonstrated that both absorption and secretion of Cl− are reduced in Na+-depleted rat distal colon27, indicating that NKCC1 up-regulation may be unrelated to basal Cl− secretion. Our ISC data support this, as in the presence of mucosal K+ channel blockers Ba2+ or IbTX, ISC remained negative (Fig. 4). However, aldosterone’s regulation of stimulated Cl− secretion in the distal colon – and its dependence on NKCC1 – is not known. Thus, the effect of forskolin (FSK; an adenylyl cyclase activator that stimulated cAMP-dependent Cl− secretion) was assessed in normal and dietary Na+-depleted rat distal colon.
When added to the serosal bath, FSK induced a robust increase in ISC (i.e., Cl− secretion) in both normal and dietary Na+-depleted rat distal colon (normal vs dietary Na+-depleted: ΔISC(FSK) = 123.6 ± 15.1 vs. 92.4 ± 22.8 μA/cm2; p = 0.28) (Fig. 6A,C). The increased ISC was accompanied by increased GTE (normal vs dietary Na+-depleted: ΔGTE(FSK) = 4.2 ± 0.6 vs. 6.2 ± 1.6 mS/cm2; p = 0.30) (Fig. 6B,D). Importantly, cAMP is also known to stimulate electrogenic K+ secretion independently of Cl− secretion in normal distal colon40, which may ostensibly “mask” the FSK-stimulated Cl− secretion under these conditions. However, when Na+-depleted rat distal colon was pre-treated for 60 minutes with mucosal IbTX, neither ΔISC(FSK) (77.7 ± 11.4 μA/cm2) nor ΔGTE(FSK) (4.0 ± 0.6 mS/cm2) were significantly different from either normal or untreated Na+-depleted tissues (p > 0.15 for both) (Fig. 6A–D). Congruent with the finding that stimulated Cl− secretion is not enhanced by aldosterone, abundance of cystic fibrosis transmembrane conductance regulator (CFTR; cAMP-activated Cl− channel) mRNA was also found to be unaffected by Na+ depletion (Fig. 6E). Thus, it is likely that the increased NKCC1 expression induced by aldosterone primarily functions to support enhanced electrogenic K+ secretion, without a concurrent increase in basal and/or stimulated Cl− secretion.
Figure 6. Enhanced NKCC1 expression does not support increased capacity for stimulated Cl− secretion.
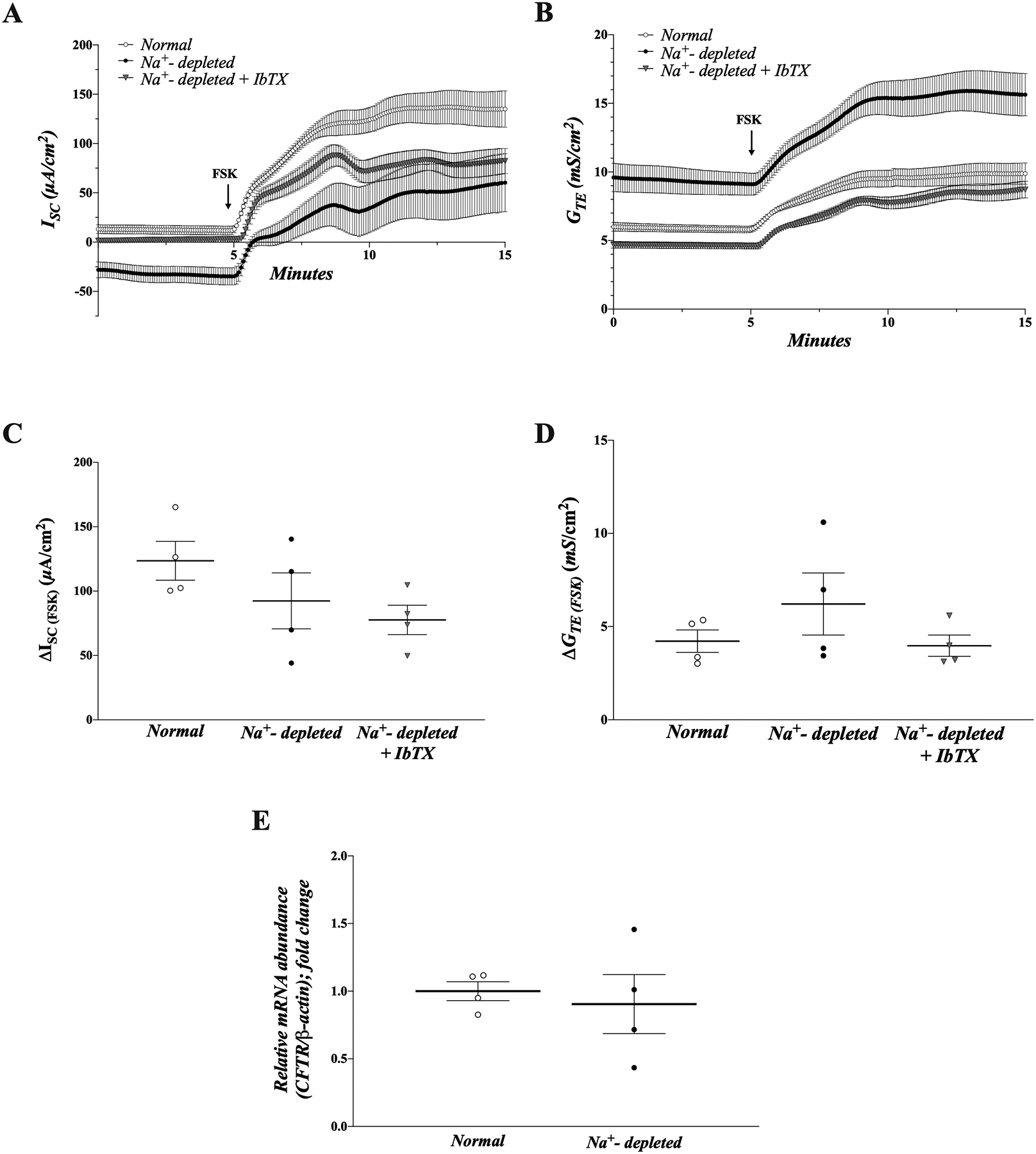
ISC (A) and GTE (B) measurements from normal (open circles) and dietary Na+ depleted (closed circles) rat distal colon, as well as dietary Na+-depleted rat distal colon pre-treated with mucosal IbTX (250 nM) (grey triangles) in the presence of mucosal 100 μM amiloride. Tissues were treated with 10 μM forskolin (FSK) in the serosal bath where indicated. (C-D) Group data showing change in (Δ) ISC (C) and GTE (D) induced by FSK in each group. Average ISC and GTE values over the course of 1 minute immediately prior to FSK addition, and 1 minute at the end of the recording period were calculated for each tissue. ΔISC and ΔGTE were calculated by subtracting the basal value from the value at the end of the recording period, after FSK addition. Lines and error bars represent means ± SEM.
DISCUSSION
This study provides novel evidence that aldosterone increases the expression of both NKCC1 and BK channels in a coordinated manner to facilitate electrogenic K+, but not Cl−secretion in rat distal colon. This conclusion is supported by the following observations: 1) both the activity (bumetanide-sensitive ISC) and expression of NKCC1 (mRNA and protein) is enhanced by aldosterone, via dietary Na+ depletion or by aldosterone application ex vivo, 2) K+ secretion induced by aldosterone is mediated by basolateral NKCC1 and mucosal BK channels working in tandem, as indicated by the attenuating effect of mucosal IbTX on bumetanide-sensitive ISC, as well as the apparent robust co-expression of BKα and NKCC1 in crypt cells of Na+-depleted rats, and 3) aldosterone-induced upregulation of NKCC1 does not also facilitate an increase in basal or FSK-stimulated Cl− secretion, suggesting its regulation by aldosterone is specific to the K+ secretory pathway. Figure 7 summarizes these observations and proposes a basic cellular model of the regulation of K+ secretion by aldosterone in the rat distal colon.
Figure 7. Cellular model of aldosterone-regulated electrogenic K+ secretion in the distal colon.
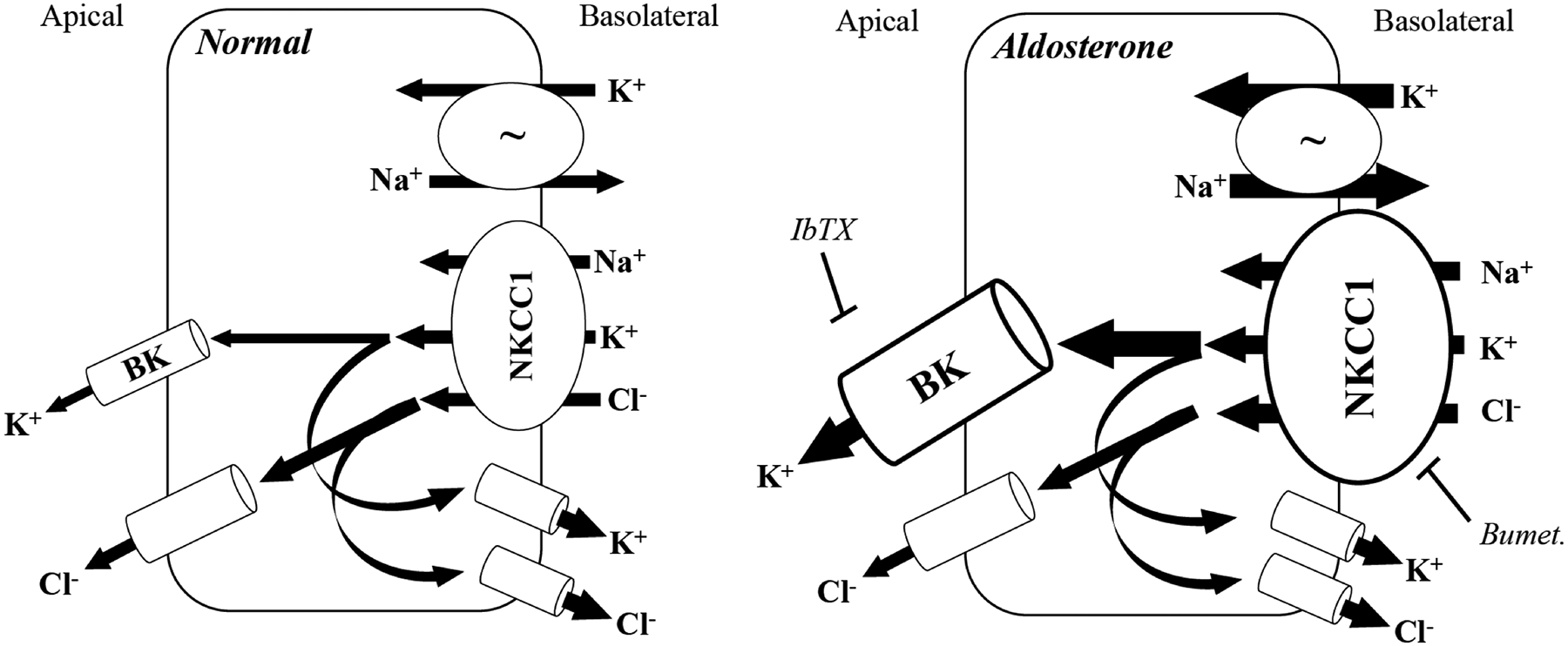
Normal distal colon secretes both K+ and Cl− across the apical membrane. Na+, K+ ATPase (~) activity drives overall transport by establishing the Na+ gradient that allows basolateral uptake of K+ and Cl− via NKCC1. Apical membrane secretion of K+ and Cl− is supported by basolateral exit of Cl− and K+ ions, respectively, to maintain the necessary electrochemical gradient. Multiple basolateral K+ and Cl− channels may be involved in this process, including intermediate conductance (IK) and voltage-gated (KV7.1) K+ channels, as well as CLC-2 Cl− channels. Aldosterone stimulates electrogenic K+ secretion by increasing basolateral Na+, K+ ATPase activity (represented by enlarged arrows) and increasing the expression and activity of BK channels and NKCC1 co-transporter (represented by enlarged image objects and arrows, respectively). Electrogenic K+ secretion is inhibited by either IbTX (apical membrane BK channels) or bumetanide (basolateral NKCC1). To support the aldosterone-induced sustained K+ secretion at the apical membrane, basolateral Cl− exit also must presumably be enhanced, although the identity of the channel(s) involved remains undetermined. Despite the increase in NKCC1 expression and activity, electrogenic Cl− secretion is not enhanced by aldosterone, suggesting that the regulation of NKCC1 by aldosterone is specific to the K+ transport pathway.
Stimulation of K+ secretion is a fundamental effect of aldosterone signaling in the colonic epithelium and occurs independently of its parallel effects on electrogenic Na+ absorption.24 While the effects of aldosterone on apical K+ channels have been described in detail, here we provide evidence that expression of the basolateral NKCC1 co-transporter is also under the control of aldosterone in the distal colon, which had not been demonstrated previously in rat. Further, we have shown that augmented NKCC1 activity contributes to the vectorial transport of K+, but not Cl−, across the colonic epithelium, suggesting a critical role for NKCC1 specifically in aldosterone-regulated K+ transport.
Short-term aldosterone treatment has been recently shown to regulate NKCC1 protein levels in several epithelial and non-epithelial cells (e.g. cochlear and smooth muscle) in vitro,25,41–43 indicating the phenomenon is not specific to intestinal epithelia. However, the role of NKCC1 in vectorial BK-mediated K+ secretion was not investigated, and the reported mechanism of NKCC1 up-regulation was suggested to be that aldosterone prevents its degradation via the proteasomal pathway, not via transcription upregulation,25,41 in contrast to our findings. This discrepancy may be due to inherent differences in the response to aldosterone between in native tissue and immortalized cell lines, or because of different environment factors associated with cell culture versus within native tissue. For example, short chain fatty acids produced by the microbiota, as well as neuronal input are both known to have a substantial impact on cellular homeostasis and electrolyte transport in vivo,44–48 but these factors are typically absent under normal cell culture conditions. Whether aldosterone also regulates the proteasomal degradation of NKCC1 in native tissue remains to be determined but is entirely possible.
A somewhat controversial issue regarding colonic K+ transport is whether crypt or surface epithelial regions (or both) participate in luminal K+ secretion,10,34,38,49 and there may be considerable variation among species. Sorensen et. al. identified the presence of BK channels and BK-mediated K+ secretion to be restricted to the crypt region in mice, and also found NKCC1 mRNA transcript to be elevated ~ 2-fold in the crypts of mice fed a high K+ diet, another dietary model of hyperaldosteronism.10 Although obtained from rats and not mice, our ISC and immunofluorescence data are in agreement with these findings, as IbTX significantly attenuated the inhibition of K+ secretion by bumetanide, while strong labelling of both NKCC1 and BKα was apparent only in the crypts of Na+-depleted rat distal colonic sections. Jakob et. al. also demonstrated that NKCC1 expression is confined primarily to the crypt region throughout the rat GI tract.50 It is therefore likely that NKCC1/BKα-mediated K+ secretion, a process enhanced by aldosterone, occurs mainly in the crypt region of the distal colonic epithelium. However, aldosterone-induced K+ secretion in surface epithelial cells has also been suggested to occur as a consequence of increased ENaC activity, favoring apical efflux of K+ through one or more voltage-gated K+ channels in response to the depolarization brought on by Na+ influx.51 Thus, luminal K+ secretion may occur in both surface and crypt regions simultaneously. Here, NKCC1 labelling was detected in surface epithelial cells of Na+-depleted animals, but clear membrane localization of BK channels was not apparent. Unfortunately, while NKCC1/BK-mediated K+ secretion was clearly enhanced by aldosterone, the precise localization of this transport pathway within the tissue – whether solely in the crypt or the surface (or both) – cannot be determined with certainty based upon these experiments.
It should be noted that the degree of hyperaldosteronism induced by dietary Na+ depletion was dependent on the sex of the animals. As described above, serum aldosterone was somewhat elevated in Na+-depleted females versus females fed a normal diet, but the effect of diet was much smaller in females compared to males. It is possible that sex hormone activity in some way modulates the response to aldosterone production or confers some resistance to the impact of Na+ depletion itself. Vivas et al using an acute model of Na+ depletion by restricting dietary Na+ and administering furosemide (a loop diuretic), found that male mice were more sensitive to the effects of Na+ depletion in terms of resultant Na+ appetite, as well as other cardiovascular and neuronal outcomes.52 However, serum aldosterone was not measured in these studies. While little work has been done to date, the mechanism(s) underlying the sex-specific effects of Na+ depletion and accompanying hyperaldosteronism warrant further investigation, as this important aspect of mineralocorticoid function remains incompletely understood.
Alterations in colonic K+ transport play an important role in various pathologies.1,5,53 For example, active colonic K+ secretion via mucosal BK channels becomes imperative in patients with ESRD, providing an alternative outlet for K+ and thus preventing life-threatening hyperkalemia.17,18 On the other hand, excessive K+ secretion is a hallmark of colonic pseudo-obstruction, contributing to diarrhea and hypokalemia in severe cases,13,14 with at least one documented case of a patient who was successfully treated with spironolactone, a mineralocorticoid antagonist, to reduce fecal K+ excretion and hypokalemia associated with diarrhea.14 Another report demonstrated that spironolactone reduced deleterious fecal K+ losses and significantly increased stool Na+:K+ ratio in cholera patients, although overall stool rate was not affected.12 Accordingly, there is clinical value in furthering our understanding of mineralocorticoid regulation of colonic K+ transport because, as development of new interventions may prove useful in treating conditions like those described above.
In conclusion, here we provide evidence that aldosterone stimulates the expression of basolateral NKCC1, supporting luminal K+, but not Cl− secretion in rat distal colon. Establishing that NKCC1 is an aldosterone-regulated component to the K+ secretory pathway in the colonic epithelium may aid in the development of novel therapeutic strategies centered around the regulation of K+ transport, such as colonic pseudo-obstruction or ESRD.
Supplementary Material
Acknowledgements:
This study was supported by the National Institute of Health NIDDK R01DK104791 and DK112085 grants to VMR.
Abbreviations:
- ISC
short circuit current
- GTE
transepithelial conductance
- IbTX
iberiotoxin
- BK
large conductance K+ channel
- IK
intermediate conductance K+ channel
- Bumet
bumetanide
- ESRD
end stage renal disease
- ENaC
epithelial Na+ channel
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
REFERENCES
- 1.Sorensen MV, Matos JE, Praetorius HA, Leipziger J Colonic potassium handling. Pflugers Arch Eur J Physiol. 2010;459(5):645–656. doi: 10.1007/s00424-009-0781-9 [DOI] [PubMed] [Google Scholar]
- 2.Archampong EQ, Harris J, Clark CG. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. 1972:880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Foster S, Hayslett JP, Binder J, Emily S, Hayslew JP, Henry J. Mechanism of active potassium absorption and secretion in the rat colon. 1984. [DOI] [PubMed]
- 4.Kliger AS, Binder HJ, Bastl C, Hayslett JP. Demonstration of active potassium transport in the mammalian colon. J Clin Invest. 1981;67(4):1189–1196. doi: 10.1172/JCI110134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rajendran VM, Sandle GI. Colonic Potassium Absorption and Secretion in Health and Disease. Compr Physiol. 2018;8(4):1513–1536. doi: 10.1002/cphy.c170030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajendran VM, Singh SK, Geibel J, Binder HJ. Differential localization of colonic H+-K+-ATPase isoforms in surface and crypt cells. Am J Physiol - Gastrointest Liver Physiol. 1998;274(2 37–2):424–429. doi: 10.1152/ajpgi.1998.274.2.g424 [DOI] [PubMed] [Google Scholar]
- 7.Sunuwar L, Asraf H, Donowitz M, Sekler I, Hershfinkel M. The Zn2+-sensing receptor, ZnR/GPR39, upregulates colonocytic Cl− absorption, via basolateral KCC1, and reduces fluid loss. Biochim Biophys Acta - Biomembr. 2017;1863(4):947–960. doi: 10.1016/j.bbadis.2017.01.009.The [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rehman S, Narayanan K, Nickerson AJ, et al. Parallel intermediate conductance K+ and Cl- channel activity mediates electroneutral K+ exit across basolateral membranes in rat distal colon. Am J Physiol - Gastrointest Liver Physiol. 2020;319(2):G142–G150. doi: 10.1152/ajpgi.00011.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sausbier M, Matos JE, Sausbier U, et al. Distal colonic K+ secretion occurs via BK channels. J Am Soc Nephrol. 2006;17(5):1275–1282. doi: 10.1681/ASN.2005101111 [DOI] [PubMed] [Google Scholar]
- 10.Sørensen MV, Strandsby AB, Larsen CK, Praetorius HA, Leipziger J. The secretory KCa1.1 channel localises to crypts of distal mouse colon: Functional and molecular evidence. Pflugers Arch Eur J Physiol. 2011;462(5):745–752. doi: 10.1007/s00424-011-1000-z [DOI] [PubMed] [Google Scholar]
- 11.Sweiry JH, Binder HJ. Characterization of aldosterone-induced potassium secretion in rat distal colon. J Clin Invest. 1989;83(3):844–851. doi: 10.1172/JCI113967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guerrant RL, Chen LC, Rohde JE. Effect of spironolactone on stool electrolyte losses during human cholera. Gut. 1972;13(3):197–200. doi: 10.1136/gut.13.3.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Dinter TG, Fuerst FC, Richardson CT, et al. Stimulated active potassium secretion in a patient with colonic pseudo-obstruction: A new mechanism of secretory diarrhea. Gastroenterology. 2005;129(4):1268–1273. doi: 10.1053/j.gastro.2005.07.029 [DOI] [PubMed] [Google Scholar]
- 14.Ram P, Goyal A, Lu M, Sloan J, McElhaugh W. Use of Aldosterone Antagonist to Treat Diarrhea and Hypokalemia of Ogilvie’s Syndrome. Case Rep Gastrointest Med. 2016;2016:1–3. doi: 10.1155/2016/1207240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanthesh BM, Sandle GI, Rajendran VM. Enhanced K+ secretion in dextran sulfate-induced colitis reflects upregulation of large conductance apical K+ channels (BK; Kcnma1). AJP Cell Physiol. 2013;305(9):C972–C980. doi: 10.1152/ajpcell.00165.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sandle GI, Perry MD, Mathialahan T, et al. Altered cryptal expression of luminal potassium (BK) channels in ulcerative colitis. J Pathol. 2007;212(1):66–73. doi: 10.1002/path.2159 [DOI] [PubMed] [Google Scholar]
- 17.Mathialahan T, Sandle GI. Dietary potassium and laxatives as regulators of colonic potassium secretion in end-stage renal disease. Nephrol Dial Transplant. 2003;18(2):341–347. doi: 10.1093/ndt/18.2.341 [DOI] [PubMed] [Google Scholar]
- 18.Mathialahan T, Maclennan KA, Sandle LN, Verbeke C, Sandle GI. Enhanced large intestinal potassium permeability in end-stage renal disease. J Pathol. 2005;206(1):46–51. doi: 10.1002/path.1750 [DOI] [PubMed] [Google Scholar]
- 19.Tumamian SG, Binder HJ. Regulation of active sodium and potassium transport in the distal colon of the rat. J Clin Invest. 1989;84(6):1924–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foster ES, Jones WJ, Hayslett JP, Binder HJ. Role of Aldosterone and Dietary Potassium in Potassium Adaptation in the Distal Colon of the Rat. Gastroenterology. 1985;88(1):41–46. doi: 10.1016/S0016-5085(85)80130-X [DOI] [PubMed] [Google Scholar]
- 21.Amasheh S, Epple HJ, Mankertz J, et al. Differential Regulation of ENaC by Aldosterone in Rat Early and Late Distal Colon. Ann N Y Acad Sci. 2006;915(1):92–94. doi:https://doi-org.wvu.idm.oclc.org/10.1111/j.1749-6632.2000.tb05227.x [DOI] [PubMed] [Google Scholar]
- 22.Greig ER, Baker EH, Mathialahan T, Boot-Handford RP, Sandle GI. Segmental variability of ENaC subunit expression in rat colon during dietary sodium depletion. Pflugers Arch Eur J Physiol. 2002;444(4):476–483. doi: 10.1007/s00424-002-0828-7 [DOI] [PubMed] [Google Scholar]
- 23.Sørensen MV, Matos JE, Sausbier M, et al. Aldosterone increases KCa1.1 (BK) channel-mediated colonic K+ secretion. J Physiol. 2008;586(Pt 17):4251–4264. doi: 10.1113/jphysiol.2008.156968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rechkemmer G, Halm DANR. Aldosterone stimulates K secretion across mammalian colon independent of Na absorption. Proc Natl Acad Sci. 1989;86(January):397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazard P, Ding B, Chittam HK, et al. Aldosterone up-regulates voltage-gated potassium currents and NKCC1 protein membrane fractions. Sci Rep. 2020;10(1):1–14. doi: 10.1038/s41598-020-72450-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang C, Kawabe H, Rotin D. The ubiquitin ligase Nedd4L regulates the Na/K/2Cl co-transporter NKCC1/SLC12A2 in the colon. J Biol Chem. 2017;292(8):3137–3145. doi: 10.1074/jbc.M116.770065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Halevy J, Budinger ME, Hayslett JP, Binder HJ. Role of Aldosterone in the Regulation of Sodium and Chloride Transport in the Distal Colon of Sodium-Depleted Rats. Gastroenterology. 1986;91(5):1227–1233. doi: 10.1016/S0016-5085(86)80021-X [DOI] [PubMed] [Google Scholar]
- 28.Martin RS, Jones WJ, Hayslett JP. Animal model to study the effect of adrenal hormones on epithelial function. Kidney Int. 1983;24(3):386–391. doi: 10.1038/ki.1983.171 [DOI] [PubMed] [Google Scholar]
- 29.Hu D, Yu ZL, Zhang Y, et al. Bumetanide treatment during early development rescues maternal separation-induced susceptibility to stress. Sci Rep. 2017;7(1):1–16. doi: 10.1038/s41598-017-12183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho Mhatre V. and Kelsey C. Martin J-AL. Immunolocalization of the Ca2+ -Activated K+ Channel Slo1 in Axons and Nerve Terminals of Mammalian Brain and Cultured Neurons. Bone. 2012;23(1):1–7. doi: 10.1002/cne.20931.Immunolocalization [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clarke LL. A guide to Ussing chamber studies of mouse intestine. AJP Gastrointest Liver Physiol. 2009;296(6):G1151–G1166. doi: 10.1152/ajpgi.90649.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edmonds CJ, Edmonds CJ Willis CL. THE EFFECT OF DIETARY SODIUM AND POTASSIUM INTAKE ON POTASSIUM SECRETION AND KINETICS IN RAT DISTAL COLON. J Physiol. 2019;53(9):1689–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayslett JP, Myketey N, Binder HJ, Aronson PS. Mechanism of increased potassium secretion in potassium loading and sodium deprivation. Am J Physiol - Ren Fluid Electrolyte Physiol. 1980;8(4):378–382. doi: 10.1152/ajprenal.1980.239.4.f378 [DOI] [PubMed] [Google Scholar]
- 34.Lomax B, Mcnicholas M, Lombes M, Sandle I, Mcnicholas M. Aldosterone-induced apical Na+ and K+ conductances are located primarily in surface cells in rat distal colon. 1994;29:G71–G82. [DOI] [PubMed] [Google Scholar]
- 35.Escoubet B, Coureau C, Bonvalet JP, Farman N. Noncoordinate regulation of epithelial na channel and Na pump subunit mRNAs in kidney and colon by aldosterone. Am J Physiol - Cell Physiol. 1997;272(5 41–5). doi: 10.1152/ajpcell.1997.272.5.c1482 [DOI] [PubMed] [Google Scholar]
- 36.Hayslett JP, Binder HJ. Mechanism of potassium adaptation. Am J Physiol - Ren Fluid Electrolyte Physiol. 1982;12(2). doi: 10.1152/ajprenal.1982.243.2.f103 [DOI] [PubMed] [Google Scholar]
- 37.Singh SK, O’Hara B, Talukder JR, Rajendran VM. Aldosterone induces active K+ secretion by enhancing mucosal expression of Kcnn4c and Kcnma1 channels in rat distal colon. Am J Physiol - Cell Physiol. 2012;302(9):1353–1360. doi: 10.1152/ajpcell.00216.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Halm ST, Liao T, Halm DR. Distinct K+ conductive pathways are required for Cl- and K+ secretion across distal colonic epithelium. Am J Physiol - Cell Physiol. 2006;291(4):636–648. doi: 10.1152/ajpcell.00557.2005 [DOI] [PubMed] [Google Scholar]
- 39.Payne JA, Xu JC, Haas M, Lytle CY, Ward D, Forbush B 3rd. Primary structure, functional expression, and chromosomal localization of the bumetanide-sensitive Na-K-Cl cotransporter in human colon. J Biol Chem. 1995;270(30):17977–17985. doi: 10.1074/jbc.270.30.17977 [DOI] [PubMed] [Google Scholar]
- 40.Sandle GI, Rajendran VM. Cyclic AMP-induced K+ secretion occurs independently of Cl- secretion in rat distal colon. Am J Physiol - Cell Physiol. 2012;303(3):328–333. doi: 10.1152/ajpcell.00099.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ding B, Frisina RD, Zhu X, Sakai Y, Sokolowski B, Walton JP. Direct control of Na(+)-K(+)-2Cl(-)-cotransport protein (NKCC1) expression with aldosterone. Am J Physiol Cell Physiol. 2014;306(1):C66–75. doi: 10.1152/ajpcell.00096.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tadros SF, Frisina ST, Mapes F, Frisina DR, Frisina RD. Higher serum aldosterone correlates with lower hearing thresholds: a possible protective hormone against presbycusis. Hear Res. 2005;209(1–2):10–18. doi: 10.1016/j.heares.2005.05.009 [DOI] [PubMed] [Google Scholar]
- 43.Jiang G, Cobbs S, Klein JD, O’Neill WC. Aldosterone regulates the Na-K-2Cl cotransporter in vascular smooth muscle. Hypertens (Dallas, Tex 1979). 2003;41(5):1131–1135. doi: 10.1161/01.HYP.0000066128.04083.CA [DOI] [PubMed] [Google Scholar]
- 44.Gross ER, Gershon MD, Margolis KG, Gertsberg ZV., Cowles RA. Neuronal serotonin regulates growth of the intestinal mucosa in mice. Gastroenterology. 2012;143(2):408–417.e2. doi: 10.1053/j.gastro.2012.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inagaki A, Hayashi M, Andharia N, Matsuda H. Involvement of butyrate in electrogenic K + secretion in rat rectal colon. Pflugers Arch Eur J Physiol. 2019;471(2):313–327. doi: 10.1007/s00424-018-2208-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cooke HJ. Role of the “little brain” in the gut in water and electrolyte homeostasis 1. FASEB J. 1989;3(2):127–138. doi: 10.1096/fasebj.3.2.2464517 [DOI] [PubMed] [Google Scholar]
- 47.Neunlist M, Toumi F, Oreschkova T, et al. Human ENS regulates the intestinal epithelial barrier permeability and a tight junction-associated protein ZO-1 via VIPergic pathways. Am J Physiol - Gastrointest Liver Physiol. 2003;285(5 48–5):1028–1036. doi: 10.1152/ajpgi.00066.2003 [DOI] [PubMed] [Google Scholar]
- 48.Cuff MA, Shirazi-Beechey SP. The importance of butyrate transport to the regulation of gene expression in the colonic epithelium. Biochem Soc Trans. 2004;32(6):1100–1102. doi: 10.1042/BST0321100 [DOI] [PubMed] [Google Scholar]
- 49.Hay-Schmidt A, Grunnet M, Abrahamse SL, Knaus HG, Klaerke DA. Localization of Ca2+-activated big-conductance K+ channels in rabbit distal colon. Pflugers Arch Eur J Physiol. 2003;446(1):61–68. doi: 10.1007/s00424-002-0983-x [DOI] [PubMed] [Google Scholar]
- 50.Jakab RL, Collaco AM, Ameen NA. Physiological relevance of cell-specific distribution patterns of CFTR, NKCC1, NBCe1, and NHE3 along the crypt-villus axis in the intestine. Am J Physiol - Gastrointest Liver Physiol. 2011;300(1):82–98. doi: 10.1152/ajpgi.00245.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grotjohann I, Gitter AH, Köckerling A, Bertog M, Schulzke JD, Fromm M. Localization of cAMP- and aldosterone-induced K+ secretion in rat distal colon by conductance scanning. J Physiol. 1998;507(2):561–570. doi: 10.1111/j.1469-7793.1998.561bt.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vivas L, Dadam FM, Caeiro XE. Sex differences in body fluid homeostasis: Sex chromosome complement influences on bradycardic baroreflex response and sodium depletion induced neural activity. Physiol Behav. 2015;152(Pt B):416–421. doi: 10.1016/j.physbeh.2015.08.010 [DOI] [PubMed] [Google Scholar]
- 53.Heitzmann D, Warth R. Physiology and pathophysiology of potassium channels in gastrointestinal epithelia. Physiol Rev. 2008;88(3):1119–1182. doi: 10.1152/physrev.00020.2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


