Abstract
Simple Summary
Cancer is considered as a large group of diseases involving abnormal cell growth, which results in an alarming rise in the mortality rate at the worldwide level. Presently, the main point of interest in pharmaceutical research is the development of the novel and efficient anticancer drugs based on natural sources. In this regard, plants, and some microbial species, due to their composition, ecology, phytochemical, and ethnopharmacological properties, play a significant role. Accordingly, a series of plant-derived bioactive compounds are in the clinical development phase against cancer. The present review highlights the significance of several medicinal plants, plant extracts, and their bioactive compounds for their potential anticancer activities. The presented results can be useful for researchers in developing novel anticancer drugs, public health specialists, and for the public in general.
Abstract
Cancer is one of the major deadly diseases globally. The alarming rise in the mortality rate due to this disease attracks attention towards discovering potent anticancer agents to overcome its mortality rate. The discovery of novel and effective anticancer agents from natural sources has been the main point of interest in pharmaceutical research because of attractive natural therapeutic agents with an immense chemical diversity in species of animals, plants, and microorganisms. More than 60% of contemporary anticancer drugs, in one form or another, have originated from natural sources. Plants and microbial species are chosen based on their composition, ecology, phytochemical, and ethnopharmacological properties. Plants and their derivatives have played a significant role in producing effective anticancer agents. Some plant derivatives include vincristine, vinblastine, irinotecan, topotecan, etoposide, podophyllotoxin, and paclitaxel. Based on their particular activity, a number of other plant-derived bioactive compounds are in the clinical development phase against cancer, such as gimatecan, elomotecan, etc. Additionally, the conjugation of natural compounds with anti-cancerous drugs, or some polymeric carriers particularly targeted to epitopes on the site of interest to tumors, can generate effective targeted treatment therapies. Cognizance from such pharmaceutical research studies would yield alternative drug development strategies through natural sources which could be economical, more reliable, and safe to use.
Keywords: natural products, anticancer drugs, medicinal plants, medicinal mushrooms
1. Introduction
Cancer is the anomalous growth of cells in the body; it is the leading cause of death and is also known as the biggest public health burden [1]. Cancer cells can also attack and damage the body’s normal cells [2]. Millions of people have died due to four common types of cancers every year, including breast, lung, prostate, and rectum/colon cancer with an unknown etiology. The present tenet indicates a conspicuous difference between cancer chemotherapy and chemoprevention. Cancer chemotherapy is the control of the developed disease, while cancer chemoprevention is the phenomenon of a carcinogenesis intervention by blocking the agents of the induction of the neoplastic process or averting the processing of transformed cells to the malignant phenotype using suppressing agents. Cancer chemoprevention may also implicate the reversal of the progression of cancer cells [3].
The investigation of anticancer agents through natural sources dates back to about 1550 BC. However, the scientific exploration of this research is very recent and originated in the 1950s with the generation of majorly found plant-derived anticancer agents, including vinca alkaloid analogs, camptothecin derivatives, podophyllotoxin derivatives, and taxol semi-synthetic analogs which are clinically helpful anticancer therapeutic drugs (Figure 1) [4,5]. Over 180,000 microbial-derived anticancer agents, 16,000 marine-derived organisms, and 114,000 plant-derived compounds were screened by the US National Cancer Institute (NCI) for their anti-cancerous activity from the 1960s to the 1980s [6]. Plant-based drug development also provided a platform for synthesizing efficient and safe anti-tumor drugs through the complete cognizance of a synergistic relation between numerous components of anti-tumor herbs [7,8]. According to the WHOs estimation, approximately 80% of African and Asian countries rely on traditional medicines for fundamental health care. A neoteric study shows that approximately more than 60% of patients use herbs or vitamins as cancer therapy [9,10]. Herbal remedies are among the most favored form of traditional medicine and are tremendously profit-making at the international commercial level. By the 2050s, the worldwide herbal medicine market is expected to hit USD 5 trillion [11].
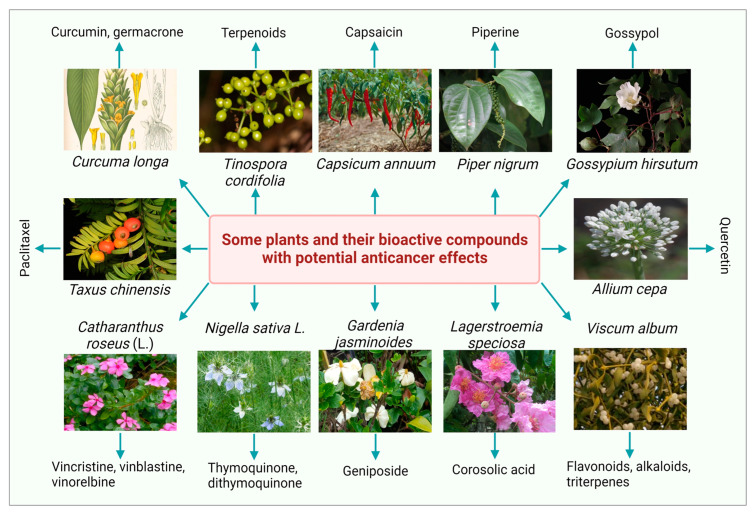
Figure 1.
Some medicinal plants and their bioactive compounds having potential anticancer properties.
Natural products provide a sustainable source with a considerable efficacy to treat and overcome several disorders and fatal diseases, including cancer. In the last time period, the role of the bioactive compound and natural products, as a source of anticancer drugs, has been marked within a collaborative, integrated, and multidisciplinary approach. Plants have long been known for having medicinal effects since aeon [12,13,14,15,16]. More than 50% of modern clinical drugs are of a natural source origin and have the capability to treat cancer cells [17]. A neoteric study shows that approximately more than 60% of patients use herbs or vitamins as cancer therapy. The ability of natural sources as anticancer agents were identified in the 1950s by the US National Cancer Institute and contributed to finding new naturally existing anti-tumor agents [18]. Plant-based drug development needs a specific production strategy with optimized environmental conditions and nutrient availability. In 1998, Sohn et al. estimated that an extraction from 10,000 kg of the bark of yew trees is required to produce 1 kg of taxol. The production of 25 kg of taxol required 38,000 yew trees for the treatment of 12,000 cancer patients [19]. The plant collection for finding anticancer agents ended in 1982, but in 1986, the generation of new screening strategies led to the amelioration of plants and the collection of other organisms mainly focused on the sub-tropical and tropical zones of the world. Hartwell listed more than 3000 plants in his review against cancer treatment [20]. Various anti-cancerous drugs are available to treat cancer, but they also exhibit toxic effects that limit their use [18,21]. Because of the severe side effects of radiotherapy and chemotherapy and the high mortality rate, recent research revolves around the need to design appropriate chemotherapy for cancer treatment without side effects [21,22]. Biodiversity has been determined to be a significant source of remarkable anticancer agents until now [23,24,25,26,27,28].
A significant investigation is devoted to finding more effective treatments with minimum undesirable toxic effects. However, many anti-tumor agents exhibit a restricted therapeutic window due to a lack of specificity of against cancer cells [29,30]. The ultimate objective of a cancer treatment is the generation of safe and effective drugs that can particularly kill malignant cancer cells or make them benign cancer cells without killing normal cells [31]. This review aims to highlight the significance of several medicinal plants, plant extracts, and their bioactive compounds with potential anticancer activities to facilitate the researchers to develop novel anticancer drugs providing some considerable benefits, such as highly selective and significant anticancer activity, less to no toxicity, low side effects, being economic and eco-friendly, as well as providing a cancer preventing role via an increasing immunity [32,33,34].
2. Methodology
A descriptive review was conducted in order to evaluate the anticancer potential of several natural plants and their bioactive compounds. The articles documented on anticancer, chemopreventive, and cytotoxic effects with experimental investigations (such as in vitro, in vivo, and clinical trials) were critically evaluated. The bibliographic material was collected using the Google Scholar, ScienceDirect, Web of Science, and PubMed databases. Several search terms such as “natural plants”, “medicinal plants”, “bioactive compounds”, “phytoconstituents”, “phytochemicals”, “plant extracts”, “anticancer agents”, “plant-derived therapeutics”, “cytoxicity analysis”, “antiproliferative”, and “apoptotic effects” were used to collect literature in a variety of combination forms. The generated articles were screened in order to fulfill the desired selection criteria by evaluating their titles and further abstract summaries. The irrelevant articles, like those with any other language except English, or articles with insignificant outcomes, aiming to evaluate natural plant products or their bioactive compounds as effective anticancer agents treating different types of cancers, were excluded from the selected data. Moreover, all of the selected publications were exclusively read to gather effective literature based on their potential anticancer effects indicated by experimental investigations.
3. Plant-Derived Bioactive Compounds as Anti-Cancerous Agents
Over the last decade, several researchers have investigated the ethnopharmacological and ethnomedicinal properties of numerous plant-derived bioactive compounds and, recently, their antimicrobial and antibiofilm activities [35]. Several in vitro and in vivo experimental investigations revealed the therapeutic significance of numerous phytochemicals (Table 1). Some photos of the most studied plants with a significant anticancer potential with their bioactive compounds are presented in Figure 1. The most common plant-derived anti-cancerous agents include vinca alkaloids and their derivatives, camptothecin and its derivatives, podophyllotoxin and its semi-synthetic analogs, and terpenes.
Table 1.
Plant-derived bioactive compounds as anti-cancerous agents along with their modes of action.
| Plant Source | Bioactive Compound/ Phytochemical |
Cancer Cells Type | Study Type | Mode of Action | Ref |
|---|---|---|---|---|---|
| Aconitum sinomontanum | Lappaconitine | Liver | In vitro | Downregulation of Bcl-2 and upregulation of P53 and Bax expression | [36] |
| Alliaria petilata | Benzyl isothiocyanate | Colon | In vitro | MAPK and PKC pathways inhibition | [37] |
| Allium cepa | Quercetin | Thyroid | In vitro | Pro—NAG-1/GDF15 pathways upregulation | [38] |
| Artemisia annua | Artemisinin | Breast | In vitro | G2/M (cell cycle) arrest, autophagy, antiproliferative, apoptosis | [39] |
| Camellia sinensis | Catechins | Prostate | In vitro | Increased expression of cytochrome c and decreased expression of B-cell lymphoma-2 induced apoptosis | [40] |
| Cannabis sativa | Cannabinoids | Liver | In vivo | Anti-apoptotic | [41] |
| Capsicum annuum | Capsaicin | Breast | In vitro, in vivo |
NF-kB inactivation mediated by the FBI-1 downregulation | [42] |
| Capsicum frutescens | Capsaicin | Pancreatic | In vitro, in vivo |
β-catenin/TCF-1 signaling inhibition-mediated apoptosis | [43] |
| Carica papaya | Benzyl isothiocyanate | Pancreatic | In vitro, in vivo |
FOXO/PI3K/AKT pathways-mediated tumor apoptosis | [44] |
| Chamaemelum nobile | Phenolic compounds | Breast | In vitro | Mitochondrial pathway activation-induced apoptosis | [45] |
| Citrus limon | Hesperidin | Breast | In vitro | NF-kB and Akt downregulation-mediated PD-L1 expression inhibition | [46] |
| Prostate | In vitro | ROS-induced apoptosis | [47] | ||
| Crocus sativus | Safranal | Prostate | In vitro, in vivo |
Downregulation of NF-kB and AKT signaling pathways | [48] |
| Cucumis sativus | Cucurbitacin B | Neuroblastoma | In vitro | MAPKs- and JAK2/STAT3-mediated apoptosis | [49] |
| Cucurbitacin B (in combination with gefitinib) | Colorectal | In vitro | JAK/STAT and EGFR-induced apoptosis | [50] | |
| Denrobium chrysotoxum | Erianin | Breast | In vitro | PI3K/Akt pathway activation | [51] |
| Eclipta alba | Luteolin | Breast | In vitro, in vivo |
Intrinsic apoptotic pathway activation | [52] |
| Galanthus nivalis | Gallic acid | Colon | In vitro, in vivo |
EGFR and SRC phosphorylation inhibition | [53] |
| Liver | In vitro | Wnt/β-catenin pathway suppression | [54] | ||
| Glycyrrhiza glabra | Licochalcone A | Lung | In vitro | JNK suppression, P38 and ERK activation | [55] |
| Gossypium hirsutum | Gossypol | Skin | In vitro | Mitochondrial apoptosis | [56] |
| Cervical | In vitro, in vivo |
FAK pathway inhibition and TGF-β1-mediated EMT reversal | [57] | ||
| Colon | In vitro | Downregulation of FAS, CLAUDIN1, GAPDH, ELK1, ZFAND5, IL2, and IL8 expression | [58] | ||
| Lagerstroemia speciosa | Corosolic acid | Bladder | In vitro, in vivo |
SQSTM1/P62, UBB, and NBR upregulation | [59] |
| Liver | Ex vivo, in vitro, in vivo |
YAP/CDK19/O-GlcNAcylation inactivation | [60] | ||
| Colon | In vitro, in vivo |
HER2 and HER3 heterodimerization inhibition | [61] | ||
| Mortonia greggii | Pristimerin | Lung | In vitro | MMP2 and integrin β1 expression downregulation | [62] |
| Myrica nagi | Myricetin | Lung | In vitro | FAK-ERK pathway inhibition | [63] |
| Nelumbo nucifera | Hyperoside, rutin | Colon | In vitro | Mitochondrial pathway activation-induced apoptosis | [64] |
| Panax ginseng | Ginsenosides | Breast | In vitro, in vivo |
VEGF-R2 pathway inhibition correlated with anti-angiogenesis | [65] |
| ROS generation, mitochondrial dysfunction, apoptosis | [66] | ||||
| Papaver somniferum | Noscapine | Colon | In vitro | AKT/PI3K/mTOR pathway inhibition | [67] |
| Perovskia abrotanoides | Tanshinones | Hella cell lines | In vitro | Antiproliferative, apoptosis | [68] |
| Piper longum | Piperlongumine | Prostate | In vitro | DNA damage-mediated proliferation inhibition | [69] |
| Lung | In vitro, in vivo |
mTOR/AKT/PI3K pathway inhibition-induced apoptosis | [70] | ||
| Piper nigrum | Piperine | Colon | In vitro | Wnt/β-catenin pathway suppression | [71] |
| Polygonum cuspidatum | Pterostilbene | Colon | In vitro, in vivo |
DNA repairing by Top1/Tdp1 pathway | [72] |
| Pongamiopsis pervilleana | Epipervilline | Ovarian | In vitro | Antiproliferative | [73] |
| Pueraria radix | Puerarin | Prostate | In vitro | Keap1/Nrf2/Are pathway inhibition | [74] |
| Quercus alba | Quercetin | Prostate | In vitro | ROS modulation, AKT/NF-kB pathway activation | [75] |
| Reseda luteola | Luteolin | Lung | In vitro | FAK-Src signaling inhibition | [76] |
| Rheum palmatum | Emodin | Lung | In vitro | HAS2-HACD44/RHAMM signaling pathway suppression | [77] |
| Ruta graveolens | Psoralens | Breast, colon, and prostate | In vitro | Inhibits proliferation of cancer cells | [78] |
| Salvia involucrate | Hispidulin | Lung | In vitro, in vivo |
ER stress activation-induced apoptosis | [79] |
| Solanum lycopersicum | Lycopene | Cervical | In vitro | Bcl-2 downregulation and Bax upregulation | [80] |
| Lung | In vitro, in vivo |
Increase in RARβ protein expression | [81] | ||
| Brain | In vitro | Caspases activation | [82] | ||
| Lycopene (in combination with quinacrine) | Breast | In vitro | Wnt-TCF signaling inhibition | [83] | |
| Sophora flavescens | Matrine | Liver | In vitro, in vivo |
HOXD3 and circ-0027345 downregulation and miR-345-5p upregulation | [84] |
| Spinacia oleracea | Kaempferol | Pancreatic | In vitro | ROS-induced Akt/mTOR signaling inactivation | [85] |
| Breast | In vitro | Upregulation of caspase-3 and -9 and H2AX expression | [86] | ||
| Trianthema portulacastrum | Ecdysterone | Breast | In vivo | β-catenin/Wnt signaling inhibition inducing pro-apoptotic and antiproliferative effects | [87] |
| Vitis vinifera | Resveratrol | Breast | In vitro | Cell cycle inhibition, apoptosis | [88] |
| Osteosarcoma | In vitro, in vivo |
STAT3 pathway and cell cycle inhibition | [89] | ||
| Colorectal | In vitro | NF-kB pathway inhibition, apoptosis | [90] | ||
| Zingiber officinale | Gingerol | Lung | In vitro, in vivo |
A549 cells death by iron accumulation, USP14 expression inhibition | [91] |
| Breast | In vitro | ROS generation, activation of p53 expression mediated apoptosis, | [92] |
3.1. Vinca Alkaloids and Their Derivatives
The use of plants as anticancer agents was established with two alkaloids’ isolation, vincristine, and vinblastine, using Catharanthus roseus and Madagascar periwinkle [93]. These drugs have been clinically used in oncology for about 50 years. They perform their function by blocking the polymerization phenomenon of tubulin molecules, averting the mitotic spindle formation, and resulting in apoptosis or metaphase arrest [94]. Several anticancer drugs, such as vincristine, vinblastine, vinorelbine, vinflunine, veratridine, and berbamine, are plant-derived natural alkaloids (Figure 2).
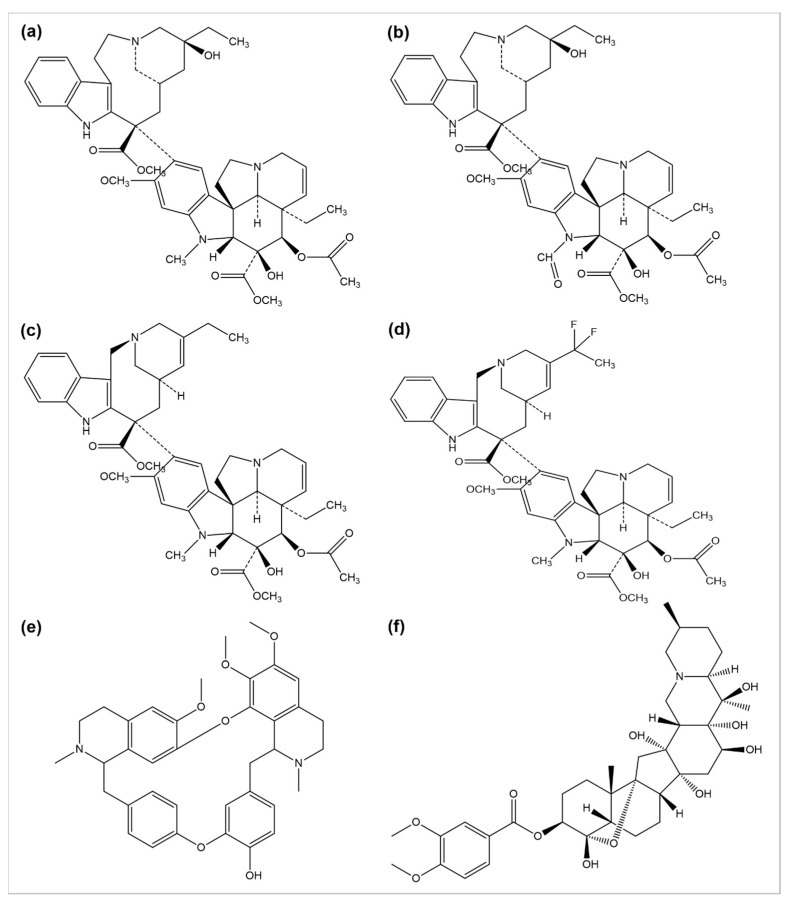
Figure 2.
Chemical structures of (a) vinblastine, (b) vincristine, (c) vinorelbine, (d) vinflunine, (e) berbamine, and (f) veratridine.
A number of semi-synthetic analogs of these two alkaloid drugs have been produced. Vindestine was produced by the replacement of the C acetyl group with an amino group in vinblastine [95], primarily applied for the treatment of acute lymphocytic leukemia (ALL) and rarely prescribed for chronic myelocytic leukemia (CML), breast cancer, non-small cell lungs cancer (NSCLC), colorectal cancer, and renal cancer treatment. Vinorelbine (also known as navelbine) is another semi-synthetic analog of vinblastine synthesized by shortening one carbon from the indole ring linking the bridge to piperidine nitrogen, resulting in a water elimination from the piperidine ring, and was approved in 1989 in France for the treatment of NSCLC under the brand name Navelbine. Vinflunine, a dihydrofluoro semi-synthetic analog of vinorelbine, is used as the second line of treatment in metastatic urothelial cancer. It was approved in 2009 by the European medical agency [96,97]. Alike other semi-synthetic analogs of vinca alkaloids, vinflunine also attaches to tubulin molecules resulting in the inhibition of microtubule polymerization and the formation of tubulins para crystals [98,99,100,101].
Cao et al. investigated the anticancer effects of 13 isoquinoline alkaloids extracted from Hylomecon japonica on MCF-7 breast cancer cells. Among these 13 alkaloids, 6,10-dimethoxydihydrochelerythrine, 6S/R-acroleinyl-dihydrochelerythrine, 9-methoxy-10-hydroxy-norchelerythrine, 10-methoxy boconoline, 6-methoxydihydrosanguinarine, dihydrosanguinaline, and 6-acetaldehyde-dihydrochelerythrine exhibited a significant inhibitory potential with an IC50 of ˂20 μM on MCF-7 cells [102]. Freeling et al. determined the tumor suppression potential of the plant-based alkaloid veratridine (VTD). VTD activates the expression of UBXN2A (an anti-tumor protein) by deactivating a dominant protein, mortalin, involved in the development of cancer [103]. Liu et al. evaluated the antiproliferative and anti-migratory effect of the alkaloid berbamine. Berbamine suppressed the growth of negative breast cancer cells by regulating the PI3K/Akt/mTOR and PI3K/Akt/MDM2/p53 pathways [104]. Esnaashari et al. investigated the synergistic effect of the alkaloid doxorubicin (DOX) with noscapine-loaded polymeric nanoparticles (NOS-NPs) for breast cancer treatment. The anticancer potential of NOS-NPs combined with DOX and alone was evaluated against 4T1 breast cancer cells (in vitro) and mice (in vivo). The NOS-NPs, in combination with DOX, significantly showed a 68.50% inhibition against the growth of breast cancer. The DOX and NOS-NPs alone exhibited a 32 to 55.10% inhibition, respectively [105].
3.2. Camptothecin and Its Derivatives
Camptotheca acuminata plant species are a source of the anticancer agent camptothecin (CPT), a quinoline alkaloid that acts by inhibiting the activity of topoisomerase-I, causing DNA damage and, ultimately, cell death [106]. Because of its severe toxicity and low aqueous solubility, it was terminated from clinical trials. Several CPT derivatives are developed and approved for clinical use to combat these limitations. Some of the CPT derivatives are irinotecan, belotecan, and topotecan, which actively inhibit DNA topoisomerase–I, an enzyme involved in DNA replication and transcription (Figure 3a,b,d) [107]. 9-aminocamptothecin (9-AC) is another CPT semi-synthetic derivative that exhibited a sound activity effect pre-clinical analysis but has not shown clinically effective anticancer activity hitherto.
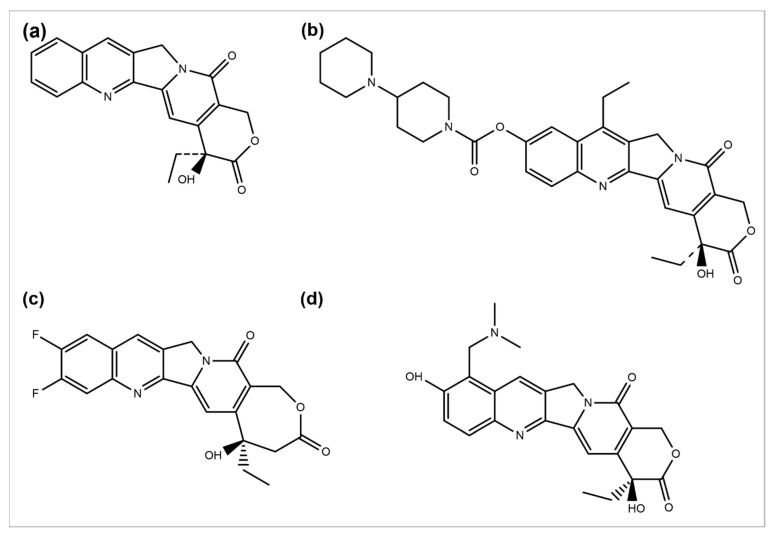
Figure 3.
Chemical structures of (a) camptothecin, (b) irinotecan, (c) diflomotecan, and (d) topotecan.
In 1993, 9-AC entered in phase-I trials and revealed the dose-dependent phenomenon of myelosuppression as a major toxic effect of the respective drug. Subsequently, in phase-II trials, the drug was found to be active against malignant and ovarian lymphoma and inactive against colon or lung cancer. Consequently, in 1999, it was terminated from any further development [108]. However, some phase-I or II trials have been reviewed to predict its efficacy, safety, and tolerability separately or in combination with some other analogs [109]. Several drugs such as diflomotecan (for advanced solid tumors treatment at phase I) (Figure 2c) [110], gimatecan (for advanced solid tumors treatment at phase I) [111], (for recurrent ovarian, peritoneal, or fallopian tumor treatment at phase II) [112], elomotecan (for advanced solid tumors treatment at phase I) [113], and EZN-2208 (for advanced malignancies treatment) [114] have been reported as clinical trial-based studies.
3.3. Podophyllotoxin and Its Semi-Synthetic Analogs
Podophyllum peltatum plant is an important source of the anticancer compound Podophyllotoxin and has two key analogs, Teniposide and Etoposide (Figure 4) [115], which are useful in the treatment of different types of cancer acts by inhibiting the function of the topoisomerase II enzyme [5]. The above two analogs combat some problems and issues, such as a metabolic inactivation, poor water solubility, and acquired drug resistance. The improved efficacy and potency led to the development of some semi-synthetic derivatives, including azatoxin, NK-611, Top-53, tafluposide, GL-331, and etoposide phosphate, either as clinical drugs or new trial candidates for cancer treatment [116].
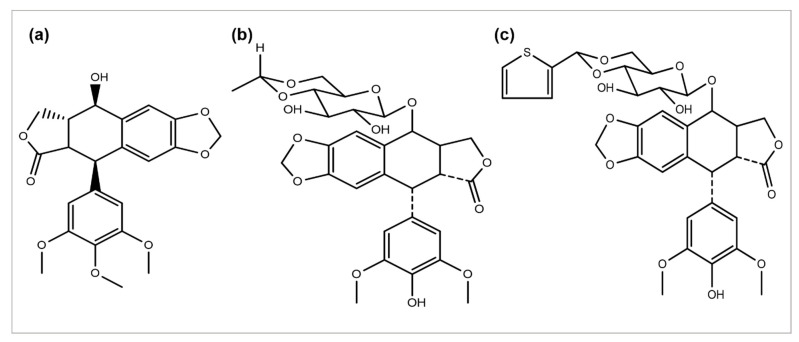
Figure 4.
Chemical structures of (a) podophyllotoxin, (b) etoposide, and (c) teniposide.
3.4. Taxane Diterpenoids
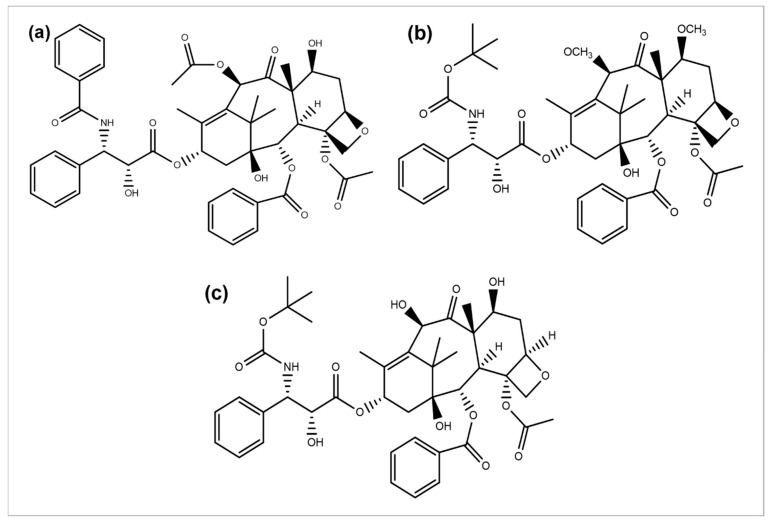
Paclitaxel discovery from the bark extract of the Yew tree further provided evidence for a successful drug discovery through natural products. Taxol was the first compound discovered for a microtubule synthesis promotion. It has been known to be used in treating several types of cancers, particularly breast, ovarian, and NSCLC [117]. A wide range of its derivatives has been produced (Figure 5). Docetaxel was the first to be clinically used with significant clinical activity against different tumors [118,119]. Both of the authorized taxane drugs, paclitaxel and docetaxel, still have limitations of use, and the researchers are trying to overcome their side effects by synthesizing the modified derivatives. Alterations in their structures has led to the discovery of new agents with a diminished toxicity, enhanced solubility, and refined cytotoxicity. The restricted ability of docetaxel and paclitaxel to cross the blood–brain barrier is concluded to be generated by the P-glycoprotein efflux pump tremendously expressed in the BBB [120,121,122]. In 2010, another FDA-approved taxane derivative, Cabazitaxel, was established in combination with prednisone for treating hormone-refractory prostate and prostate cancers. Cabazitaxel suppresses the proliferation of cancer cells by stabilizing tubulin and inhibiting the depolymerization of microtubules [123]. Nanoparticle formulations are also being applied to obtain better results. Abraxane is the albumin-bound nanoparticle-based formulation of paclitaxel free of any solvent, which acts as a mitotic inhibitor, and shows that it can have dramatically improved effects. New taxanes are also being developed to improve the therapeutic effect and pharmacology and replace docetaxel and paclitaxel which are currently used for the treatment of NSCLC [124].
Figure 5.
Chemical structures of (a) paclitaxel, (b) cabazitaxel, and (c) docetaxel.
4. Some Other Plant-Derived Anticancer Agents
Omacetaxine mepesuccinate is an alkaloid anticancer agent extracted from Cephalotaxus harringtonia and approved by the FDA; it is a translation process inhibitor. Omacetaxine inhibits the translation of proteins by inhibiting the elongation of the protein synthesis process by interfering the A-site and averting the correct amino acid positioning to aminoacyl tRNA [125]. Ingenol mebutate is found in the plant sap of Euphorbia peplus, and in January 2012, it was approved by the US FDA and EMA in gel form for the acid keratosis treatment. This compound is an ester of angelic acid and diterpene [126]. Betulinic acid is a secondary metabolite of Betula species from the Betulaceae family, occurring in natural form as pentacyclic triterpenoid. It was isolated from the Zizyphus plant species, like Mauritiana oenoplia and Mauritiana rugose [127,128], exhibiting numerous biological activities such as anti-oxidant, antibacterial, anti-inflammatory, anti-retroviral, and anti-HIV properties [129,130]. Dai et al. conducted the in vivo and in vitro studies to determine the anticancer potential of Taxus chinensis var. mairei (TC) against lung cancer. The aqueous extract of TC showed a significant anticancer effect by degrading CD47 with less toxicity [131]. Wu et al. investigated the antitumor efficacy of a polysaccharide (CPTC-2) isolated from Taxus chinensis var. mairei against gastric cell lines (SGC-7901). The outcomes obtained by the flow cytometry and MTS assay revealed significant antitumor activity in a dose-dependent way [132].
Flavopiridol is a synthetic compound having an identical structural similarity with rohitukine extracted from an Indian indigenous plant, Dysoxylum binectariferum [133]. It is known for targeting the cyclin-dependent kinase activity, including the cyclin T complex or CDK9, suppressing the regulation of Mc1-1 and anti-apoptotic proteins, and inducing changes in the permeability of mitochondria [134,135,136]. It is also known as the first potential inhibitor of cyclin-dependent kinases to gain clinical trials [137]. Curcuma longa plant, also known as turmeric, is derived from the polyphenol Curcumin and has a wide range of therapeutic properties, including anti-inflammatory, analgesic, antiseptic, and antioxidant activity [138]. Turmeric plants contain some compounds called curcuminoids containing curcumin, bisdemethoxycurcumin, and demethoxicurcumin. Of all curcuminoids, curcumin has a significant therapeutic effect [139]. It has also been found to have anticancer activity by affecting the biological pathways connected with oncogene expression, mutagenesis, metastasis, apoptosis, and the regulation of the cell cycle [139].
5. Mushrooms as a Source of Anticancer Agents
Mushrooms carrying medicinal properties have an established history as a traditional medicine. Mushroom-derived therapeutic substances can function in human bodies and are progressively being grown [140]. Numerous traditionally utilized mushrooms from the genera of Trametes, Ganoderma, Auricularia, Tremella, and Flammulina are known to have remarkable medicinal properties [141]. Many medicinal properties of mushrooms have dragged researchers’ attention to explore the finding of new mushrooms and their important metabolites, bearing profound anticancer and antioxidant properties [142]. In 1975, Lucas and his fellows first described the anticancer activity of higher Basidiomycetes [141]. The considerable physiological properties and pharmacological efficacy of medicinal mushrooms are of a homeostasis maintenance, the enhancement of the immune response (bioregulation), the biorhythm regulation, the prevention and cure of different diseases, and the amelioration of human health from life-threatening diseases such as heart diseases, cancer, and cerebral stroke, etc. Mushrooms are also known to have antibacterial, anti-mycotic, antiviral, anti-tumor, anti-inflammatory, antithrombotic, antidiabetic, hypotensive, and hypolipidemic activities [143]. Rutckeviski et al. investigated the synergistic effect of Agaricus bisporus extract β-(1→6)-d-glucan in combination with doxorubicin against breast cancer cells (MDA-MB-231). The outcomes exhibited a synergistic effect of doxorubicin and A. bisporus, which decreased the 31% viability of the tumor cells. Moreover, a β-(1→6)-d-glucan treatment combined with doxorubicin enhanced the sensitivity of MDA-MB-231 to doxorubicin [144].
Yoon et al. investigated the anti-tumor effect of the derivatives of adenosine isolated from Cordyceps militaris against ovarian cancer cells. The outcomes revealed autophagic death, mediated by adenosine derivatives in ovarian cancer cells by the ENT1-AMPK-mTOR pathway [145]. Cen et al. evaluated the anticancer potential of Ganoderma lucidum against ovarian cancer. The obtained results exhibited the reactive-induced species-induced activation of the ERK pathway [146]. Thimmaraju et al. studied the anti-tumor effect of polysaccharide (HUP-2) isolated from Hypsizygus ulmarius. HUP-2 was isolated using the hot water extraction method. HUP-2 revealed considerable cytotoxicity and inhibition against PC3 prostate cells [147]. Fekry et al. determined the anticancer potential of Pleurotus ostreatus (selenium enriched mushroom) in colon cancer. The outcomes showed significant anticancer activity by enhancing the production of IL-6 and IL-10, reducing the production of TNF-α and the targeting of the Raf-1 pathway [148]. Meng et al. studied the antitumor effect of water-soluble polysaccharide obtained from Boletus edulis (BE) mushroom against breast cancer cells (Ca761, MDA-MB-231) using an MTT assay. The obtained outcomes revealed that BE can significantly induce mitochondrial apoptosis and proliferation inhibition [149]. Another study revealed the potential anticancer effect of the silver nanoparticles (Ag NPs) prepared by Boletus edulis and Coriolus versicolor mushrooms against colorectal, breast, and hepatocellular carcinoma cells (HT-29, MCF-7, and HUH-7 cells, respectively). The outcomes showed significant anticancer activity by inhibiting proliferation and ROS-generated apoptosis [150].
Some examples of significant medicinal mushrooms with anti-tumor properties are given in Table 2.
Table 2.
Some mushroom species, along with their anti-tumor activities.
| Mushroom Specie | Extract/Bioactive Compound | Cancer Type | Study Type | Outcomes | Ref |
|---|---|---|---|---|---|
| Boletus edulis | Polysaccharide | Breast | In vitro (Ca761, MDA-MB231 cells) | Proliferation inhibition and mitochondrial apoptosis. | [149] |
| Antitumor protein | Non-small cell lung cancer | In vivo and in vitro (A549 cells) | Cell cycle arrest at G1 phase and apoptosis. | [151,152] | |
|
Boletus edulis,
Coriolus versicolor |
Silver nanoparticles | Colorectal, breast and hepatocellular carcinoma | In vitro (HT-29, MCF-7, HUH-7 cells) | Cell viability inhibition by proliferation inhibition and ROS-generated apoptosis. | [150] |
| Cantharellus cibarius, Coprinus comatus, Lactarius deliciosus, Lycoperdon perlatum | Ethanol and water extracts | Glioblastoma | In vitro (LN-18, U87MG cells) | Proliferation inhibition, cell cycle arrest at G1 and G2/M phase induced apoptosis, metallo-proteinases inactivation. | [153] |
| Innotus obliquus | Hot water extract | Breast | In vivo (mice) | Anticancer activity by innate immunity activation | [154] |
| Ganoderma lucidum | Spore oil | Breast | In vitro (MDA-MB231 cells), in vivo (mice) | Mitochondrial apoptosis | [155] |
| Pleurotus highking | Purified fraction III | Breast | In vitro (HCC-1937, MDA-MB-231 cells) | Akt signaling suppression induced proliferation and migration inhibition | [156] |
| Lignosus tigris | Cold water extract | Breast | In vitro (MCF-7 cells), in vivo (mice) | Proliferation inhibition, signaling pathways induced apoptosis, and tumor growth inhibition. | [157] |
| Sarcodon imbricatus | Water extract | Breast | In vitro, in vivo (MCF-7, 4T1 cells) | Tumor growth inhibition by inhibiting migration and invasion of tumor cells and immunomodulatory activity. | [158] |
| Taiwanofungus camphoratus | Mycelia broth | Adenocarci-noma | In vitro (A549 cells) |
Caspase-3 and ROS-induced apoptosis. | [159] |
| Ganoderma neojaponicum | Butanol, chloroform, hexane, and water extracts | Colon | In silico (HT29 and CT 116 cells), in vitro | Proliferation inhibition by apoptosis induction. | [160] |
| Hexagonia glabra | Water, ethyl acetate, and ethanol extract | Cervical cancer | In vitro (CaSki, HeLa, and SiHa cells) | Apoptosis induced by cell cycle arrest at G2/M and increased expression of caspase-3 and -9. | [161] |
| Calocyba indica | Ethanol extract | Pancreatic | In vitro (MIAPaCa2 and PANC-1 cells) | Modulation of p53 and caspase-3 and -9-induced apoptosis and growth inhibition. | [162] |
| Fomitopsis pinicola | Ethanol extract | Prostate cancer | In vivo (Mouse model-PCa cells) | Reduction in tumor growth. | [163] |
| Ganoderma lucidum | Ethanol extract | Hepatocarci-noma | In vitro (SK-Hep1 and QGY7703), in vivo (mice) | Ras/Raf/MEK/ERK pathways inhibition-mediated anticancer activity. | [164] |
| Water extract | Glioblastoma | In vitro (GBM8901 and U87MG cells) | Proliferation suppression, cell cycle arrest at S-phase, mitochondrial apoptosis. | [165] | |
| Ganoderma tsugae | Ethanol extract | Endometrial | In vitro (KLE, AN3 CA, and HEC-1-A cells) | Proliferation inhibition, G1/S phase cell arrest, Akt signaling pathway inhibition, mitochondrial apoptosis. | [166] |
| Heterobasidion annosum | Methanol extract | Colon | In vivo (mice) | Proliferation suppression by Akt signaling pathway. | [167] |
| Termitomyces clypeatus | Water soluble extract | Astrocytoma | In vitro (HepG2, U373MG, Y-79, MDA-MB-468, U937, HL-60, OAW-42, A549 cells), in vivo (Swiss albino mice) | Anti-tumor effect | [168] |
| Antrodia cinnamonea | Crude ethanol extract | Bladder | In vitro (tsgh-8301, RT4, T24 cells) |
Cell death was mediated by anti-migratory activity and down-regulation of cyclin B1 and CDC2 | [169] |
Several researchers have reported the anti-tumor potentials of different mushroom species and reduced the adverse effects like anemia, nausea, insomnia, drug resistance, and bone marrow suppression after radiation and chemotherapy [170]. Additionally, some potential mushroom species have been evaluated in clinical trials (Table 3) [171].
Table 3.
List of some significant mushroom species evaluated in clinical trials [171].
| Mushroom Specie | Bioactive Compound | Cancer Type | Phase of Study | Status of Study | Identifier Number |
|---|---|---|---|---|---|
| Agaricus bisporus | Polysaccharides, lectin | Prostate | 1b | Completed | NCT00779168 |
| Agaricus bisporus | Polysaccharides, lectin | Breast cancer and cancer survivors | 1 | Completed | NCT00709020 |
| Agaricus blazei Murill | Agaricus polysaccharides | Multiple myeloma | 2 | Completed | NCT00970021 |
| Lentinula edodes | Genistein combined polysaccharide | Prostate | - | Completed | NCT00269555 |
| Lentinula edode | Arabinoxylan extract combined with L. edode | Hepatocellular carcinoma | - | Completed | NCT01018381 |
| Grifola frondosa | Polysaccharides | Breast carcinoma, lung neoplasms | 1 | Completed | NCT02603016 |
| Omphalotus illudens | Semi-synthetic derivative of illudin-S | Thyroid | 2 | Completed | NCT00124527 |
| Omphalotus illudens | Semi-synthetic derivative of illudin-S | Recurrent epithelial ovarian cancer | 2 | Completed | NCT00019552 |
| Trametes versicolor | PSK, Krestin, PSP | Breast | 1 | Completed | NCT00680667 |
A medicinally reported mushroom, Agaricus blazei, has been revealed to possess significant anti-tumor activity by an enlarged number of T-regulatory and plasmacytoid dendritic cells and an enhanced level of human leukocyte, immunoglobulin, and killer-immunoglobulin receptor genes [172]. Misgiati et al. isolated ergosterol using n-hexane extracts prepared with Agaricus blazei Murill mushroom. The anticancer activity of the ergosterol using an MTT assay was identified against MCF-7 cells. The outcomes showed significant anticancer activity with an IC50 of 43.10 µg/mL by inhibiting the cell cycle and inducing apoptosis [173]. Sun et al. extracted an RNA-protein complex (FA-2-b-B) using Agaricus blazei Murill to evaluate the anticancer potential against chronic myeloid leukemia. The outcomes exhibited that the FA-2-b-B protein complex has a significant proapoptotic and antiproliferative effect, suggesting that the intake of Agaricus blazei Murill may provide an effective alternative approach for the management and treatment of chronic myeloid leukemia [174].
Jeitler et al. reported a considerable decrease in anorexia over time after 6 cycles of Agaricus sylvaticus combined with chemotherapy; simultaneously, some side effects such as anorexia, vomiting, diarrhea, nausea, and constipation were observed in the placebo group [175]. Coriolus versicolor was used for the treatment of hepatocellular carcinoma patients that are mostly inoperable. No difference was observed with the Coriolus versicolor treatment compared to the placebo group, possessing an improved quality of life compared to the placebo on the treatment. This study suggested using a supplementation for palliative care [176].
The Agaricus bisporus (white button mushroom) powder was used for the treatment of prostate cancer. A continuous rise in the levels of a prostate-specific antigen (PSA) was observed in patients with prostate cancer; an escalation in the PSA level may direct the recurrence of the disease. The results showed a therapy-induced drop in myeloid-derived suppressor cells, and patients with partial and complete responses exhibited increased baseline interleukin-15 levels compared to the non-respondents [177]. Torkelson et al. conducted a phase-I trial using Trametes versicolor against immune-compromised patients of breast cancer. They reported an improved immune status with the enhanced activity of natural killer cells, lymphocyte counts, and a dose-dependent increase in CD8+ T-cells and CD19+ B-cells. It can be suggested that a Trametes versicolor treatment can be used to improve the immunity levels in the immune-compromised patients of breast cancer [178].
6. Conclusions
The extracts or bioactive compounds from plants and fungi exhibit several mechanisms with anti-tumor effects. The mushroom and plant-derived bioactive compounds may regulate or activate the immune system, by disturbing the immune cell’s maturation, differentiation, and proliferation mechanisms, thus inhibiting the growth of cancer cells. Plant-derived compounds may not directly be used as drugs, but they encouraged the researchers to design and develop novel anticancer agents. A thorough understanding of the mechanisms of action of plant and mushroom-derived bioactive compounds with anticancer properties is essentially required for cancer treatment, providing cancer patients with an improved quality of life. However, the clinical studies of numerous plant and mushroom-derived bioactive compounds reveal significant anticancer potentials with immunomodulation and the reduced side effects of conventional treatments. Consequently, more clinical studies must be conducted, particularly by applying a prime methodology, standard preparations, large sample sizes, and long-lasting follow-ups. Future studies should also focus on establishing the preventive or defensive aspects of plants and mushrooms for reducing the development of cancers, by including them in one’s daily life for a healthy diet.
Author Contributions
Conceptualization, writing—original draft preparation, and resources, S.T.A.; supervision and writing—original draft preparation, U.A.; supervision and writing—review and editing, K.I.; conceptualization and validation, A.M.; visualization and review and editing, S.R.A.S. and S.Z.H.; writing—original draft preparation and resources, D.A.-A.; visualization and review and editing, H.D., Z.H.-M., F.R.I., A.S., D.M. and K.Z.; visualization, validation, and funding acquisition, V.H.; visualization and review and editing, A.A.; visualization, review and editing and validation, C.A. and S.I. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This review was supported by the project “Increasing the impact of excellence research on the capacity for innovation and technology transfer within USAMVB Timișoara” code 6PFE, submitted in the competition Program 1—Development of the national system of research—development, Subprogram 1.2—Institutional performance, Institutional development projects—Development projects of excellence in RDI.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Tan G., Gyllenhaal C., Soejarto D. Biodiversity as a source of anticancer drugs. Curr. Drug Targets. 2006;7:265–277. doi: 10.2174/138945006776054942. [DOI] [PubMed] [Google Scholar]
- 2.De Mesquita M.L., de Paula J.E., Pessoa C., de Moraes M.O., Costa-Lotufo L.V., Grougnet R., Michel S., Tillequin F., Espindola L.S. Cytotoxic activity of Brazilian Cerrado plants used in traditional medicine against cancer cell lines. J. Ethnopharmacol. 2009;123:439–445. doi: 10.1016/j.jep.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 3.Hong W.K., Sporn M.B. Recent advances in chemoprevention of cancer. Science. 1997;278:1073–1077. doi: 10.1126/science.278.5340.1073. [DOI] [PubMed] [Google Scholar]
- 4.Chandra S. Endophytic fungi: Novel sources of anticancer lead molecules. Appl. Microbiol. Biotechnol. 2012;95:47–59. doi: 10.1007/s00253-012-4128-7. [DOI] [PubMed] [Google Scholar]
- 5.Srivastava V., Negi A.S., Kumar J., Gupta M., Khanuja S.P. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005;13:5892–5908. doi: 10.1016/j.bmc.2005.05.066. [DOI] [PubMed] [Google Scholar]
- 6.Newman D.J., Cragg G.M. Natural Products. Springer; Berlin/Heidelberg, Germany: 2005. The discovery of anticancer drugs from natural sources; pp. 129–168. [Google Scholar]
- 7.Larkin T. Herbs are often more toxic than magical. FDA Consumers. 1983;17:4–10. [Google Scholar]
- 8.Saxe T. Toxicity of medicinal herbal preparations. Am. Fam. Physician. 1987;35:135–142. doi: 10.1016/0378-8741(88)90243-7. [DOI] [PubMed] [Google Scholar]
- 9.Madhuri S., Pandey G. Some dietary agricultural plants with anticancer properties. Plant Arch. 2008;8:13–16. [Google Scholar]
- 10.Sivalokanathan S., Ilayaraja M., Balasubramanian M. Efficacy of Terminalia arjuna (Roxb.) on N-nitrosodiethylamine induced hepatocellular carcinoma in rats. Indian J. Exp. Biol. 2005;43:264–267. [PubMed] [Google Scholar]
- 11.Chaudhary A., Singh N. Contribution of world health organization in the global acceptance of Ayurveda. J. Ayurveda Integr. Med. 2011;2:176–186. doi: 10.4103/0975-9476.90769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anand U., Jacobo-Herrera N., Altemimi A., Lakhssassi N. A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites. 2019;9:258. doi: 10.3390/metabo9110258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semeniuc C.A., Socaciu M.-I., Socaci S.A., Mureșan V., Fogarasi M., Rotar A.M. Chemometric comparison and classification of some essential oils extracted from plants belonging to Apiaceae and Lamiaceae families based on their chemical composition and biological activities. Molecules. 2018;23:2261. doi: 10.3390/molecules23092261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pengelly A., Bone K. The Constituents of Medicinal Plants: An Introduction to the Chemistry and Therapeutics of Herbal Medicine. Routledge; Abingdon, UK: 2020. [DOI] [Google Scholar]
- 15.Ahmad I., Mehmood Z., Mohammad F. Screening of some Indian medicinal plants for their antimicrobial properties. J. Ethnopharmacol. 1998;62:183–193. doi: 10.1016/S0378-8741(98)00055-5. [DOI] [PubMed] [Google Scholar]
- 16.Singla R.K., De R., Efferth T., Mezzetti B., Sahab Uddin M.d., Sanusi, Ntie-Kang F., Wang D., Schultz F., Kharat K.R., et al. The International Natural Product Sciences Taskforce (INPST) and the power of Twitter networking exemplified through #INPST Hashtag Analysis. Phytomedicine. 2023;108:154520. doi: 10.1016/j.phymed.2022.154520. [DOI] [PubMed] [Google Scholar]
- 17.Rosangkima G., Prasad S. Antitumour activity of some plants from Meghalaya and Mizoram against murine ascites Dalton's lymphoma. Indian J. Exp. Biol. 2004;42:981–988. [PubMed] [Google Scholar]
- 18.Cragg G.M., Newman D.J. Plants as a source of anticancer agents. J. Ethnopharmacol. 2005;100:72–79. doi: 10.1016/j.jep.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Sohn H., Okos M.R. Paclitaxel (taxol): From nutt to drug. J. Microbiol. Biotechnol. 1998;8:427–440. [Google Scholar]
- 20.Kaur R., Kapoor K., Kaur H. Plants as a source of anticancer agents. J. Nat. Prod. Plant Resour. 2011;1:119–124. [Google Scholar]
- 21.Fouché G., Cragg G., Pillay P., Kolesnikova N., Maharaj V., Senabe J. In vitro anticancer screening of South African plants. J. Ethnopharmacol. 2008;119:455–461. doi: 10.1016/j.jep.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Georgaki S., Skopeliti M., Tsiatas M., Nicolaou K.A., Ioannou K., Husband A., Bamias A., Dimopoulos M.A., Constantinou A.I., Tsitsilonis O.E. Phenoxodiol, an anticancer isoflavene, induces immunomodulatory effects in vitro and in vivo. J. Cell Mol. Med. 2009;13:3929–3938. doi: 10.1111/j.1582-4934.2009.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kinghorn A., Farnsworth N., Beecher C., Soejarto D., Cordell G., Pezzuto J., Wall M., Wani M., Brown D., O'neill M.M., et al. Novel strategies for plant-derived anticancer agents. Int. J. Pharmacogn. 1995;33:48–58. doi: 10.3109/13880209509067087. [DOI] [Google Scholar]
- 24.Kinghorn A.D., Su B.-N., Jang D.S., Chang L.C., Lee D., Gu J.-Q., Carcache-Blanco E.J., Pawlus A.D., Lee S.K., Park E.J. Natural inhibitors of carcinogenesis. Planta Med. 2004;70:691–705. doi: 10.1055/s-2004-827198. [DOI] [PubMed] [Google Scholar]
- 25.Powis G. Anticancer Drugs: Antimetabolite Metabolism and Natural Anticancer Agents. 1st ed. Pergamon; Oxford, UK: 1994. Section 140. [Google Scholar]
- 26.Yun T.K. Update from Asia: Asian studies on cancer chemoprevention. Ann. N. Y. Acad. Sci. 1999;889:157–192. doi: 10.1111/j.1749-6632.1999.tb08734.x. [DOI] [PubMed] [Google Scholar]
- 27.Kamath K., Park K. Mucosal adhesive preparations. In: Swarbrick J., Boylan J.C., editors. Encyclopedia of Pharmaceutical Technology. Volume 10. Marcel Dekker Inc.; New York, NY, USA: 1988. p. 133. [Google Scholar]
- 28.Sagini K., Urbanelli L., Buratta S., Leonardi L., Emiliani C. Nanovesicles from plants as edible carriers of bioactive compounds. AgroLife Sci. J. 2017;6:167–171. [Google Scholar]
- 29.Shengquan L., Ngong H.S. Design of low-molecular-weight prodrugs for targeted delivery of anticancer agents; Proceedings of the 3rd International Conference and Exhibition on Pharmaceutics & Novel Drug Delivery Systems; Chicago, IL, USA. 8–10 April 2013. [Google Scholar]
- 30.Kratz F., Müller I.A., Ryppa C., Warnecke A. Prodrug strategies in anticancer chemotherapy. ChemMedChem. 2008;3:20–53. doi: 10.1002/cmdc.200700159. [DOI] [PubMed] [Google Scholar]
- 31.Sporn M.B., Liby K.T. Cancer chemoprevention: Scientific promise, clinical uncertainty. Nat. Clin. Pract. Oncol. 2005;2:518–525. doi: 10.1038/ncponc0319. [DOI] [PubMed] [Google Scholar]
- 32.Duffy R., Wade C., Chang R. Discovery of anticancer drugs from antimalarial natural products: A MEDLINE literature review. Drug Discov. Today. 2012;17:942–953. doi: 10.1016/j.drudis.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Pereira D.M., Valentao P., Correia-da-Silva G., Teixeira N., Andrade P.B. Plant secondary metabolites in cancer chemotherapy: Where are we? Curr. Pharm. Biotechnol. 2012;13:632–650. doi: 10.2174/138920112799857530. [DOI] [PubMed] [Google Scholar]
- 34.Singh S., Awasthi M., Pandey V.P., Dwivedi U.N. Natural products as anticancerous therapeutic molecules with special reference to enzymatic targets topoisomerase, COX, LOX and aromatase. Curr. Protein Pept. Sci. 2018;19:238–274. doi: 10.2174/1389203718666170106102223. [DOI] [PubMed] [Google Scholar]
- 35.Ertas Onmaz N., Demirezen Yilmaz D., Imre K., Morar A., Gungor C., Yilmaz S., Gundog D.A., Dishan A., Herman V., Gungor G. Green synthesis of gold nanoflowers using Rosmarinus officinalis and Helichrysum italicum extracts: Comparative studies of their antimicrobial and antibiofilm activities. Antibiotics. 2022;11:1466. doi: 10.3390/antibiotics11111466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song N., Ma J., Zhang X., Qu D., Hui L., Sang C., Li H. Lappaconitine hydrochloride induces apoptosis and S phase cell cycle arrest through MAPK signaling pathway in human liver cancer HepG2 cells. Pharmacogn. Mag. 2021;17:334–341. doi: 10.4103/pm.pm_251_20. [DOI] [Google Scholar]
- 37.Lai K.-C., Huang A.-C., Hsu S.-C., Kuo C.-L., Yang J.-S., Wu S.-H., Chung J.-G. Benzyl isothiocyanate (BITC) inhibits migration and invasion of human colon cancer HT29 cells by inhibiting matrix metalloproteinase-2/-9 and urokinase plasminogen (uPA) through PKC and MAPK signaling pathway. J. Agric. Food Chem. 2010;58:2935–2942. doi: 10.1021/jf9036694. [DOI] [PubMed] [Google Scholar]
- 38.Hong Y., Lee J., Moon H., Ryu C.H., Seok J., Jung Y.-S., Ryu J., Baek S.J. Quercetin induces anticancer activity by upregulating pro-NAG-1/GDF15 in differentiated thyroid cancer cells. Cancers. 2021;13:3022. doi: 10.3390/cancers13123022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guan X., Guan Y. Artemisinin induces selective and potent anticancer effects in drug resistant breast cancer cells by inducing cellular apoptosis and autophagy and G2/M cell cycle arrest. JBUON. 2020;25:1330–1336. [PubMed] [Google Scholar]
- 40.Tsai Y.-J., Chen B.-H. Preparation of catechin extracts and nanoemulsions from green tea leaf waste and their inhibition effect on prostate cancer cell PC-3. Int. J. Nanomed. 2016;11:1907–1926. doi: 10.2147/IJN.S103759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussein N.A.E.M., El-Toukhy M.A.E.-F., Kazem A.H., Ali M.E.-S., Ahmad M.A.E.-R., Ghazy H.M.R., El-Din A.M.G. Protective and therapeutic effects of cannabis plant extract on liver cancer induced by dimethylnitrosamine in mice. Alex. J. Med. 2014;50:241–251. doi: 10.1016/j.ajme.2014.02.003. [DOI] [Google Scholar]
- 42.Chen M., Xiao C., Jiang W., Yang W., Qin Q., Tan Q., Lian B., Liang Z., Wei C. Capsaicin inhibits proliferation and induces apoptosis in breast cancer by down-regulating FBI-1-mediated NF-κB pathway. Drug Des. Dev. Ther. 2021;15:125–140. doi: 10.2147/DDDT.S269901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pramanik K.C., Fofaria N.M., Gupta P., Ranjan A., Kim S.-H., Srivastava S.K. Inhibition of β-catenin signaling suppresses pancreatic tumor growth by disrupting nuclear β-catenin/TCF-1 complex: Critical role of STAT-3. Oncotarget. 2015;6:11561–11574. doi: 10.18632/oncotarget.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boreddy S.R., Pramanik K.C., Srivastava S.K. Pancreatic tumor suppression by benzyl isothiocyanate is associated with inhibition of PI3K/AKT/FOXO pathway. Clin. Cancer Res. 2011;17:1784–1795. doi: 10.1158/1078-0432.CCR-10-1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kandelous H.M., Salimi M., Khori V., Rastkari N., Amanzadeh A., Salimi M. Mitochondrial apoptosis induced by Chamaemelum nobile extract in breast cancer cells. Iranian J. Pharm. Res. 2016;15:197–204. [PMC free article] [PubMed] [Google Scholar]
- 46.Kongtawelert P., Wudtiwai B., Shwe T.H., Pothacharoen P., Phitak T. Inhibitory effect of hesperidin on the expression of programmed death ligand (PD-L1) in breast cancer. Molecules. 2020;25:252. doi: 10.3390/molecules25020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ning L., Zhao W., Gao H., Wu Y. Hesperidin induces anticancer effects on human prostate cancer cells via ROS-mediated necrosis like cell death. J. BUON. 2020;25:2629–2634. [PubMed] [Google Scholar]
- 48.Jiang X., Li Y., Feng J.-L., Nik Nabil W.N., Wu R., Lu Y., Liu H., Xi Z.-C., Xu H.-X. Safrana l prevents prostate cancer recurrence by blocking the Re-activation of quiescent cancer cells via downregulation of S-phase kinase-associated protein 2. Front. Cell Dev. Biol. 2020;8:598620. doi: 10.3389/fcell.2020.598620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zheng Q., Liu Y., Liu W., Ma F., Zhou Y., Chen M., Chang J., Wang Y., Yang G., He G. Cucurbitacin B inhibits growth and induces apoptosis through the JAK2/STAT3 and MAPK pathways in SH-SY5Y human neuroblastoma cells. Mol. Med. Rep. 2014;10:89–94. doi: 10.3892/mmr.2014.2175. [DOI] [PubMed] [Google Scholar]
- 50.Yar Saglam A., Alp E., Elmazoglu Z., Menevse S. Treatment with cucurbitacin B alone and in combination with gefitinib induces cell cycle inhibition and apoptosis via EGFR and JAK/STAT pathway in human colorectal cancer cell lines. Hum. Exp. Toxicol. 2016;35:526–543. doi: 10.1177/0960327115595686. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y., Fang R., Shao J., Cai Z. Erianin induces triple-negative breast cancer cells apoptosis by activating PI3K/Akt pathway. Biosci. Rep. 2021;41:BSR20210093. doi: 10.1042/BSR20210093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arya R.K., Singh A., Yadav N.K., Cheruvu S.H., Hossain Z., Meena S., Maheshwari S., Singh A.K., Shahab U., Sharma C. Anti-breast tumor activity of Eclipta extract in-vitro and in-vivo: Novel evidence of endoplasmic reticulum specific localization of Hsp60 during apoptosis. Sci. Rep. 2015;5:18457. doi: 10.1038/srep18457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lin X., Wang G., Liu P., Han L., Wang T., Chen K., Gao Y. Gallic acid suppresses colon cancer proliferation by inhibiting SRC and EGFR phosphorylation. Exp. Ther. Med. 2021;21:638. doi: 10.3892/etm.2021.10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi C.-J., Zheng Y.-B., Pan F.-F., Zhang F.-W., Zhuang P., Fu W.-M. Gallic acid suppressed tumorigenesis by an LncRNA MALAT1-Wnt/β-catenin axis in hepatocellular carcinoma. Front. Pharmacol. 2021;12:708967. doi: 10.3389/fphar.2021.708967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo W., Sun R., Chen X., Li J., Jiang J., He Y., Shi S., Wen H. ERK activation-mediated autophagy induction resists licochalcone A-induced anticancer activities in lung cancer cells in vitro. OncoTargets Ther. 2020;13:13437–13450. doi: 10.2147/OTT.S278268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Haasler L., Kondadi A.K., Tsigaras T., von Montfort C., Graf P., Stahl W., Brenneisen P. The BH3 mimetic (±) gossypol induces ROS-independent apoptosis and mitochondrial dysfunction in human A375 melanoma cells in vitro. Arch. Toxicol. 2021;95:1349–1365. doi: 10.1007/s00204-021-02987-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsieh Y.-S., Chu S.-C., Huang S.-C., Kao S.-H., Lin M.-S., Chen P.-N. Gossypol reduces metastasis and epithelial-mesenchymal transition by targeting protease in human cervical cancer. Am. J. Chin. Med. 2021;49:181–198. doi: 10.1142/S0192415X21500105. [DOI] [PubMed] [Google Scholar]
- 58.Cao H., Sethumadhavan K., Cao F., Wang T.T. Gossypol decreased cell viability and down-regulated the expression of a number of genes in human colon cancer cells. Sci. Rep. 2021;11:5922. doi: 10.1038/s41598-021-84970-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cui A., Li X., Ma X., Wang X., Liu C., Song Z., Pan F., Xia Y., Li C. Transcriptome and proteome analysis reveals corosolic acid inhibiting bladder cancer via targeting cell cycle and inducing mitophagy in vitro and in vivo. Res. Sq. 2021. preprint . [DOI] [PubMed]
- 60.Zhang C., Niu Y., Wang Z., Xu X., Li Y., Ma L., Wang J., Yu Y. Corosolic acid inhibits cancer progression by decreasing the level of CDK19-mediated O-GlcNAcylation in liver cancer cells. Cell Death Dis. 2021;12:889. doi: 10.1038/s41419-021-04164-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang B.Y., Zhang L., Chen Y.M., Qiao X., Zhao S.L., Li P., Liu J.F., Wen X., Yang J. Corosolic acid inhibits colorectal cancer cells growth as a novel HER2/HER3 heterodimerization inhibitor. Br. J. Pharmacol. 2021;178:1475–1491. doi: 10.1111/bph.15372. [DOI] [PubMed] [Google Scholar]
- 62.Li J., Guo Q., Lei X., Zhang L., Su C., Liu Y., Zhou W., Chen H., Wang H., Wang F. Pristimerin induces apoptosis and inhibits proliferation, migration in H1299 lung cancer cells. J. Cancer. 2020;11:6348–6355. doi: 10.7150/jca.44431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kang H.R., Moon J.Y., Ediriweera M.K., Song Y.W., Cho M., Kasiviswanathan D., Cho S.K. Dietary flavonoid myricetin inhibits invasion and migration of radioresistant lung cancer cells (A549-IR) by suppressing MMP-2 and MMP-9 expressions through inhibition of the FAK-ERK signaling pathway. Food Sci. Nutr. 2020;8:2059–2067. doi: 10.1002/fsn3.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guon T.E., Chung H.S. Hyperoside and rutin of Nelumbo nucifera induce mitochondrial apoptosis through a caspase-dependent mechanism in HT-29 human colon cancer cells. Oncol. Lett. 2016;11:2463–2470. doi: 10.3892/ol.2016.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim J.S., Yoo J.-M., Park J.E., Kim J., Kim S.-G., Seok Y.M., Son J.-H., Kim H.J. Anti-angiogenic effect of mountain ginseng in vitro and in vivo: Comparison with farm-cultivated ginseng. Mol. Med. Rep. 2021;24:615. doi: 10.3892/mmr.2021.12254. [DOI] [PubMed] [Google Scholar]
- 66.Kim J., Yoo J.-M., Kim J.S., Kim S.-G., Park J.E., Seok Y.M., Son J.-H., Kim H.J. Anticancer effect of mountain ginseng on human breast cancer: Comparison with farm-cultivated ginseng. Evid. Based Complement. Alternat. Med. 2020;2020:2584783. doi: 10.1155/2020/2584783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tian X., Liu M., Huang X., Zhu Q., Liu W., Chen W., Zou Y., Cai Y., Huang S., Chen A. Noscapine induces apoptosis in human colon cancer cells by regulating mitochondrial damage and Warburg effect via PTEN/PI3K/mTOR signaling pathway. OncoTargets Ther. 2020;13:5419–5428. doi: 10.2147/OTT.S232137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Geryani M.A., Mahdian D., Mousavi S.H., Hosseini A. Ctotoxic and apoptogenic effects of Perovskia abrotanoides flower extract on MCF-7 and HeLa cell lines. Avicenna J. Phytomed. 2016;6:410–417. [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang D.-F., Yang Z.-C., Chen J.-Q., Jin X.-X., Qiu Y.-D., Chen X.-J., Shi H.-Y., Liu Z.-G., Wang M.-S., Liang G. Piperlongumine inhibits migration and proliferation of castration-resistant prostate cancer cells via triggering persistent DNA damage. BMC Complement. Med. Ther. 2021;21:195. doi: 10.1186/s12906-021-03369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang F., Mao Y., You Q., Hua D., Cai D. Piperlongumine induces apoptosis and autophagy in human lung cancer cells through inhibition of PI3K/Akt/mTOR pathway. Int. J. Immunopathol. Pharmacol. 2015;28:362–373. doi: 10.1177/0394632015598849. [DOI] [PubMed] [Google Scholar]
- 71.De Almeida G.C., Oliveira L.F., Predes D., Fokoue H.H., Kuster R.M., Oliveira F.L., Mendes F.A., Abreu J.G. Piperine suppresses the Wnt/β-catenin pathway and has anticancer effects on colorectal cancer cells. Sci. Rep. 2020;10:1161. doi: 10.1038/s41598-020-68574-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y., Li Y., Sun C., Chen X., Han L., Wang T., Liu J., Chen X., Zhao D. Effect of pterostilbene, a natural derivative of resveratrol, in the treatment of colorectal cancer through Top1/Tdp1-mediated DNA repair pathway. Cancers. 2021;13:4002. doi: 10.3390/cancers13164002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harinantenaina L., Brodie P.J., Slebodnick C., Callmander M.W., Rakotobe E., Randrianasolo S., Randrianaivo R., Rasamison V.E., TenDyke K., Shen Y. Antiproliferative compounds from Pongamiopsis pervilleana from the Madagascar dry forest. J. Nat. Prod. 2010;73:1559–1562. doi: 10.1021/np100430r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J., Xiong C., Xu P., Luo Q., Zhang R. Puerarin induces apoptosis in prostate cancer cells via inactivation of the Keap1/Nrf2/ARE signaling pathway. Bioengineered. 2021;12:402–413. doi: 10.1080/21655979.2020.1868733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ward A.B., Mir H., Kapur N., Gales D.N., Carriere P.P., Singh S. Quercetin inhibits prostate cancer by attenuating cell survival and inhibiting anti-apoptotic pathways. World J. Surg. Oncol. 2018;16:108. doi: 10.1186/s12957-018-1400-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Masraksa W., Tanasawet S., Hutamekalin P., Wongtawatchai T., Sukketsiri W. Luteolin attenuates migration and invasion of lung cancer cells via suppressing focal adhesion kinase and non-receptor tyrosine kinase signaling pathway. Nutr. Res. Pract. 2020;14:127–133. doi: 10.4162/nrp.2020.14.2.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li M., Jin S., Cao Y., Xu J., Zhu S., Li Z. Emodin regulates cell cycle of non-small lung cancer (NSCLC) cells through hyaluronan synthase 2 (HA2)-HA-CD44/receptor for hyaluronic acid-mediated motility (RHAMM) interaction-dependent signaling pathway. Cancer Cell Int. 2021;21:19. doi: 10.1186/s12935-020-01711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fadlalla K., Watson A., Yehualaeshet T., Turner T., Samuel T. Ruta graveolens extract induces DNA damage pathways and blocks Akt activation to inhibit cancer cell proliferation and survival. Anticancer Res. 2011;31:233–241. [PMC free article] [PubMed] [Google Scholar]
- 79.Lv L., Zhang W., Li T., Jiang L., Lu X., Lin J. Hispidulin exhibits potent anticancer activity in vitro and in vivo through activating ER stress in non-small-cell lung cancer cells. Oncol. Rep. 2020;43:1995–2003. doi: 10.3892/or.2020.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Aktepe O.H., Şahin T.K., Güner G., Arik Z., Yalçin Ş. Lycopene sensitizes the cervical cancer cells to cisplatin via targeting nuclear factorkappaB (NF- B) pathway. Turk. J. Med. Sci. 2021;51:368–374. doi: 10.3906/sag-2005-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cheng J., Miller B., Balbuena E., Eroglu A. Lycopene protects against smoking-induced lung cancer by inducing base excision repair. Antioxidants. 2020;9:643. doi: 10.3390/antiox9070643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Czarnik-Kwaśniak J., Kwaśniak K., Kwasek P., Świerzowska E., Strojewska A., Tabarkiewicz J. The influence of lycopene, [6]-gingerol, and silymarin on the apoptosis on U-118MG glioblastoma cells in vitro model. Nutrients. 2019;12:96. doi: 10.3390/nu12010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Preet R., Mohapatra P., Das D., Satapathy S.R., Choudhuri T., Wyatt M.D., Kundu C.N. Lycopene synergistically enhances quinacrine action to inhibit Wnt-TCF signaling in breast cancer cells through APC. Carcinogenesis. 2013;34:277–286. doi: 10.1093/carcin/bgs351. [DOI] [PubMed] [Google Scholar]
- 84.Lin S., Zhuang J., Zhu L., Jiang Z. Matrine inhibits cell growth, migration, invasion and promotes autophagy in hepatocellular carcinoma by regulation of circ_0027345/miR-345-5p/HOXD3 axis. Cancer Cell Int. 2020;20:246. doi: 10.1186/s12935-020-01293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang F., Wang L., Qu C., Chen L., Geng Y., Cheng C., Yu S., Wang D., Yang L., Meng Z. Kaempferol induces ROS-dependent apoptosis in pancreatic cancer cells via TGM2-mediated Akt/mTOR signaling. BMC Cancer. 2021;21:396. doi: 10.1186/s12885-021-08158-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu L., Xue L. Kaempferol suppresses proliferation and induces cell cycle arrest, apoptosis, and DNA damage in breast cancer cells. Oncol. Res. 2019;27:629–634. doi: 10.3727/096504018X15228018559434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bishayee A., Mandal A. Trianthema portulacastrum Linn. exerts chemoprevention of 7, 12-dimethylbenz (a) anthracene-induced mammary tumorigenesis in rats. Mutat. Res. 2014;768:107–118. doi: 10.1016/j.mrfmmm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 88.Wu H., Chen L., Zhu F., Han X., Sun L., Chen K. The cytotoxicity effect of resveratrol: Cell cycle arrest and induced apoptosis of breast cancer 4T1 cells. Toxins. 2019;11:731. doi: 10.3390/toxins11120731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng L., Jiang D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS ONE. 2018;13:e0205918. doi: 10.1371/journal.pone.0205918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Buhrmann C., Shayan P., Goel A., Shakibaei M. Resveratrol regulates colorectal cancer cell invasion by modulation of focal adhesion molecules. Nutrients. 2017;9:1073. doi: 10.3390/nu9101073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tsai Y., Xia C., Sun Z. The inhibitory effect of 6-gingerol on ubiquitin-specific peptidase 14 enhances autophagy-dependent ferroptosis and anti-tumor in vivo and in vitro. Front. Pharmacol. 2020;11:598555. doi: 10.3389/fphar.2020.598555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sp N., Kang D.Y., Lee J.-M., Bae S.W., Jang K.-J. Potential anti-tumor effects of 6-gingerol in p53-dependent mitochondrial apoptosis and inhibition of tumor sphere formation in breast cancer cells. Int. J. Mol. Sci. 2021;22:4660. doi: 10.3390/ijms22094660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verma A., Singh R. Induced dwarf mutant in Catharanthus roseus with enhanced antibacterial activity. Indian J. Pharm. Sci. 2010;72:655–657. doi: 10.4103/0250-474X.78541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jordan M.A., Thrower D., Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Res. 1991;51:2212–2222. [PubMed] [Google Scholar]
- 95.Jordan M.A., Wilson L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer. 2004;4:253–265. doi: 10.1038/nrc1317. [DOI] [PubMed] [Google Scholar]
- 96.Mamtani R., Vaughn D.J. Vinflunine in the treatment of advanced bladder cancer. Expert Rev. Anticancer Ther. 2011;11:13–20. doi: 10.1586/era.10.196. [DOI] [PubMed] [Google Scholar]
- 97.Bachner M., De Santis M. Vinflunine in the treatment of bladder cancer. Ther. Clin. Risk Manag. 2008;4:1243–1253. doi: 10.2147/TCRM.S3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bennouna J., Delord J.-P., Campone M., Nguyen L. Vinflunine: A new microtubule inhibitor agent. Clin. Cancer Res. 2008;14:1625–1632. doi: 10.1158/1078-0432.CCR-07-2219. [DOI] [PubMed] [Google Scholar]
- 99.Kruczynski A., Barret J.-M., Etiévant C., Colpaert F., Fahy J., Hill B.T. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochem. Pharmacol. 1998;55:635–648. doi: 10.1016/S0006-2952(97)00505-4. [DOI] [PubMed] [Google Scholar]
- 100.Pourroy B., Carré M., Honoré S., Bourgarel-Rey V., Kruczynski A., Briand C., Braguer D. Low concentrations of vinflunine induce apoptosis in human SK-N-SH neuroblastoma cells through a postmitotic G1 arrest and a mitochondrial pathway. Mol. Pharmacol. 2004;66:580–591. doi: 10.1124/mol.66.3. [DOI] [PubMed] [Google Scholar]
- 101.Simoens C., Vermorken J.B., Korst A.E., Pauwels B., De Pooter C.M., Pattyn G.G., Lambrechts H.A., Breillout F., Lardon F. Cell cycle effects of vinflunine, the most recent promising Vinca alkaloid, and its interaction with radiation, in vitro. Cancer Chemother. Pharmacol. 2006;58:210–218. doi: 10.1007/s00280-005-0147-8. [DOI] [PubMed] [Google Scholar]
- 102.Cao Z., Zhu S., Xue Z., Zhang F., Zhang L., Zhang Y., Guo Y., Zhan G., Zhang X., Guo Z. Isoquinoline alkaloids from Hylomecon japonica and their potential anti-breast cancer activities. Phytochemistry. 2022;202:113321. doi: 10.1016/j.phytochem.2022.113321. [DOI] [PubMed] [Google Scholar]
- 103.Freeling J.L., Scholl J.L., Eikanger M., Knoblich C., Potts R.A., Anderson D.J., Rower J.E., Farjoo M.H., Zhao H., Pillatzki A. Pre-clinical safety and therapeutic efficacy of a plant-based alkaloid in a human colon cancer xenograft model. Cell Death Discov. 2022;8:135. doi: 10.1038/s41420-022-00936-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu L., Yan J., Cao Y., Yan Y., Shen X., Yu B., Tao L., Wang S. Proliferation, migration and invasion of triple negative breast cancer cells are suppressed by berbamine via the PI3K/Akt/MDM2/p53 and PI3K/Akt/mTOR signaling pathways. Oncol. Lett. 2021;21:70. doi: 10.3892/ol.2020.12331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Esnaashari S.S., Muhammadnejad S., Amanpour S., Amani A. A combinational approach towards treatment of breast cancer: An analysis of noscapine-loaded polymeric nanoparticles and doxorubicin. AAPS PharmSciTech. 2020;21:166. doi: 10.1208/s12249-020-01710-3. [DOI] [PubMed] [Google Scholar]
- 106.Hsiang Y.-H., Hertzberg R., Hecht S., Liu L. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J. Biol. Chem. 1985;260:14873–14878. doi: 10.1016/S0021-9258(17)38654-4. [DOI] [PubMed] [Google Scholar]
- 107.Oberlies N.H., Kroll D.J. Camptothecin and taxol: Historic achievements in natural products research. J. Nat. Prod. 2004;67:129–135. doi: 10.1021/np030498t. [DOI] [PubMed] [Google Scholar]
- 108.Pazdur R., Diaz-Canton E., Ballard W.P., Bradof J.E., Graham S., Arbuck S.G., Abbruzzese J.L., Winn R. Phase II trial of 9-aminocamptothecin administered as a 72-hour continuous infusion in metastatic colorectal carcinoma. J. Clin. Oncol. 1997;15:2905–2909. doi: 10.1200/JCO.1997.15.8.2905. [DOI] [PubMed] [Google Scholar]
- 109.Farray D., Ahluwalia M.S., Snyder J., Barnett G.H., Cohen B.H., Suh J.H., Peereboom D.M. Pre-irradiation 9-amino [20s] camptothecin (9-AC) in patients with newly diagnosed glioblastoma multiforme. Invest. New Drugs. 2006;24:177–180. doi: 10.1007/s10637-005-2464-5. [DOI] [PubMed] [Google Scholar]
- 110.Scott L., Soepenberg O., Verweij J., de Jonge M., Th Planting A., McGovern D., Principe P., Obach R., Twelves C. A multicentre phase I and pharmacokinetic study of BN80915 (diflomotecan) administered daily as a 20-min intravenous infusion for 5 days every 3 weeks to patients with advanced solid tumours. Ann. Oncol. 2007;18:569–575. doi: 10.1093/annonc/mdl439. [DOI] [PubMed] [Google Scholar]
- 111.Zhu A.X., Ready N., Clark J.W., Safran H., Amato A., Salem N., Pace S., He X., Zvereva N., Lynch T.J. Phase I and pharmacokinetic study of gimatecan given orally once a week for 3 of 4 weeks in patients with advanced solid tumors. Clin. Cancer Res. 2009;15:374–381. doi: 10.1158/1078-0432.CCR-08-1024. [DOI] [PubMed] [Google Scholar]
- 112.Pecorelli S., Ray-Coquard I., Tredan O., Colombo N., Parma G., Tisi G., Katsaros D., Lhomme C., Lissoni A., Vermorken J. Phase II of oral gimatecan in patients with recurrent epithelial ovarian, fallopian tube or peritoneal cancer, previously treated with platinum and taxanes. Ann. Oncol. 2009;21:759–765. doi: 10.1093/annonc/mdp514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Trocóniz I.F., Cendrós J.-M., Soto E., Pruñonosa J., Perez-Mayoral A., Peraire C., Principe P., Delavault P., Cvitkovic F., Lesimple T. Population pharmacokinetic/pharmacodynamic modeling of drug-induced adverse effects of a novel homocamptothecin analog, elomotecan (BN80927), in a Phase I dose finding study in patients with advanced solid tumors. Cancer Chemother. Pharmacol. 2012;70:239–250. doi: 10.1007/s00280-012-1906-y. [DOI] [PubMed] [Google Scholar]
- 114.Kurzrock R., Goel S., Wheler J., Hong D., Fu S., Rezai K., Morgan-Linnell S.K., Urien S., Mani S., Chaudhary I. Safety, pharmacokinetics, and activity of EZN-2208, a novel conjugate of polyethylene glycol and SN38, in patients with advanced malignancies. Cancer. 2012;118:6144–6151. doi: 10.1002/cncr.27647. [DOI] [PubMed] [Google Scholar]
- 115.Imbert T. Discovery of podophyllotoxins. Biochimie. 1998;80:207–222. doi: 10.1016/S0300-9084(98)80004-7. [DOI] [PubMed] [Google Scholar]
- 116.Sargent J.M., Elgie A.W., Williamson C.J., Hill B.T. Ex vivo effects of the dual topoisomerase inhibitor tafluposide (F 11782) on cells isolated from fresh tumor samples taken from patients with cancer. Anti Cancer Drugs. 2003;14:467–473. doi: 10.1097/00001813-200307000-00013. [DOI] [PubMed] [Google Scholar]
- 117.Kinghorn A.D., Seo E.-K. Plants as sources of drugs. In: Fuller G., McKeon T.A., Bills D.D., editors. Agricultural Materials as Renewable Resources. Volume 647. ACS Publications; Washington, DC, USA: 1996. pp. 179–193. [Google Scholar]
- 118.Gelmon K. The taxoids: Paclitaxel and docetaxel. The Lancet. 1994;344:1267–1272. doi: 10.1016/S0140-6736(94)90754-4. [DOI] [PubMed] [Google Scholar]
- 119.Bissery M.-C., Nohynek G., Sanderink G.-J., Lavelle F. Docetaxel (Taxotere): A review of preclinical and clinical experience. Part I: Preclinical experience. Anticancer Drugs. 1995;6:339–355, 363–368. doi: 10.1097/00001813-199506000-00001. [DOI] [PubMed] [Google Scholar]
- 120.Beaulieu E., Demeule M., Ghitescu L., Béliveau R. P-glycoprotein is strongly expressed in the luminal membranes of the endothelium of blood vessels in the brain. Biochem. J. 1997;326:539–544. doi: 10.1042/bj3260539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fellner S., Bauer B., Miller D.S., Schaffrik M., Fankhänel M., Spruß T., Bernhardt G., Graeff C., Färber L., Gschaidmeier H. Transport of paclitaxel (Taxol) across the blood-brain barrier in vitro and in vivo. J. Clin. Invest. 2002;110:1309–1318. doi: 10.1172/JCI0215451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kemper E.M., van Zandbergen A.E., Cleypool C., Mos H.A., Boogerd W., Beijnen J.H., van Tellingen O. Increased penetration of paclitaxel into the brain by inhibition of P-Glycoprotein. Clin. Cancer Res. 2003;9:2849–2855. [PubMed] [Google Scholar]
- 123.Paller C.J., Antonarakis E.S. Cabazitaxel: A novel second-line treatment for metastatic castration-resistant prostate cancer. Drug Des. Dev. Ther. 2011;5:117–124. doi: 10.2147/DDDT.S13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bao R., Chan P. Novel compounds in the treatment of lung cancer: Current and developing therapeutic agents. J. Exp. Pharmacol. 2011;3:21–34. doi: 10.2147/JEP.S7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wetzler M., Segal D. Omacetaxine as an anticancer therapeutic: What is old is new again. Curr. Pharm. Des. 2011;17:59–64. doi: 10.2174/138161211795049778. [DOI] [PubMed] [Google Scholar]
- 126.Fallen R.S., Gooderham M. Ingenol mebutate: An introduction. Ski. Ther. Lett. 2012;17:1–3. [PubMed] [Google Scholar]
- 127.Pisha E., Chai H., Lee I.-S., Chagwedera T.E., Farnsworth N.R., Cordell G.A., Beecher C.W., Fong H.H., Kinghorn A.D., Brown D.M. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995;1:1046–1051. doi: 10.1038/nm1095-1046. [DOI] [PubMed] [Google Scholar]
- 128.Shoeb M. Anti-cancer agents from medicinal plants. Bangladesh J. Pharmacol. 2006;1:35–41. doi: 10.3329/bjp.v1i2.486. [DOI] [Google Scholar]
- 129.Onwuchekwa C., Oluwole F. Anti-gastric ulcer and antiinflammatory properties of betulinic acid in male albino rats. Sci. World J. 2010;5:15–17. [PubMed] [Google Scholar]
- 130.Tzakos A.G., Kontogianni V.G., Tsoumani M., Kyriakou E., Hwa J., Rodrigues F.A., Tselepis A.D. Exploration of the antiplatelet activity profile of betulinic acid on human platelets. J. Agric. Food Chem. 2012;60:6977–6983. doi: 10.1021/jf3006728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Dai S., Liu Y., Zhao F., Wang H., Shao T., Xu Z., Shou L., Chen S., Shu Q. Aqueous extract of Taxus chinensis var. mairei targeting CD47 enhanced antitumor effects in non-small cell lung cancer. Biomed. Pharmacother. 2022;154:113628. doi: 10.1016/j.biopha.2022.113628. [DOI] [PubMed] [Google Scholar]
- 132.Wu M., Zhang F., Yu Z., Lin J., Yang L. Chemical characterization and in vitro antitumor activity of a single-component polysaccharide from Taxus chinensis var. mairei. Carbohydr. Polym. 2015;133:294–301. doi: 10.1016/j.carbpol.2015.06.107. [DOI] [PubMed] [Google Scholar]
- 133.Naik R.G., Kattige S., Bhat S., Alreja B., De Souza N., Rupp R. An antiinflammatory cum immunomodulatory piperidinylbenzopyranone from Dysoxylum binectariferum: Isolation, structure and total synthesis. Tetrahedron. 1988;44:2081–2086. doi: 10.1016/S0040-4020(01)90352-7. [DOI] [Google Scholar]
- 134.Chao S.-H., Price D.H. Flavopiridol inactivates P-TEFb and blocks most RNA polymerase II transcription in vivo. J. Biol. Chem. 2001;276:31793–31799. doi: 10.1074/jbc.M102306200. [DOI] [PubMed] [Google Scholar]
- 135.de Azevedo W.F., Canduri F., da Silveira N.J.F. Structural basis for inhibition of cyclin-dependent kinase 9 by flavopiridol. Biochem. Biophys. Res. Commun. 2002;293:566–571. doi: 10.1016/S0006-291X(02)00266-8. [DOI] [PubMed] [Google Scholar]
- 136.Hussain S.-R.A., Lucas D.M., Johnson A.J., Lin T.S., Bakaletz A.P., Dang V.X., Viatchenko-Karpinski S., Ruppert A.S., Byrd J.C., Kuppusamy P. Flavopiridol causes early mitochondrial damage in chronic lymphocytic leukemia cells with impaired oxygen consumption and mobilization of intracellular calcium. Blood. 2008;111:3190–3199. doi: 10.1182/blood-2007-10-115733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shapiro G.I. Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin. Cancer Res. 2004;10:4270–4275. doi: 10.1158/1078-0432.CCR-040020. [DOI] [PubMed] [Google Scholar]
- 138.Akram M., Shahab-Uddin A.A., Usmanghani K., Hannan A., Mohiuddin E., Asif M. Curcuma longa and curcumin: A review article. Rom. J. Biol. Plant Biol. 2010;55:65–70. [Google Scholar]
- 139.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: A review of anticancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zaidman B.-Z., Yassin M., Mahajna J., Wasser S.P. Medicinal mushroom modulators of molecular targets as cancer therapeutics. Appl. Microbiol. Biotechnol. 2005;67:453–468. doi: 10.1007/s00253-004-1787-z. [DOI] [PubMed] [Google Scholar]
- 141.Wasser S. Medicinal mushrooms as a source of anti-tumor and immunomodulating polysaccharides. Appl. Microbiol. Biotechnol. 2002;60:258–274. doi: 10.1007/s00253-002-1076-7. [DOI] [PubMed] [Google Scholar]
- 142.Jong S., Birmingham J. Medicinal benefits of the mushroom Ganoderma. Adv. Appl. Microbiol. 1992;37:101–134. doi: 10.1016/s0065-2164(08)70253-3. [DOI] [PubMed] [Google Scholar]
- 143.Wasser S.P., Weis A.L. Medicinal properties of substances occurring in higher basidiomycetes mushrooms: Current perspectives. Int. J. Med. Mushrooms. 1999;1:31–62. doi: 10.1615/IntJMedMushrooms.v1.i1.30. [DOI] [PubMed] [Google Scholar]
- 144.Rutckeviski R., Corso C.R., Román-Ochoa Y., Cipriani T.R., Centa A., Smiderle F.R. Agaricus bisporus β-(1→ 6)-d-glucan induces M1 phenotype on macrophages and increases sensitivity to doxorubicin of triple negative breast cancer cells. Carbohidr. Polym. 2022;278:118917. doi: 10.1016/j.carbpol.2021.118917. [DOI] [PubMed] [Google Scholar]
- 145.Yoon S.Y., Lindroth A.M., Kwon S., Park S.J., Park Y.J. Adenosine derivatives from Cordyceps exert anti-tumor effects against ovarian cancer cells through ENT1-mediated transport, induction of AMPK signaling, and consequent autophagic cell death. Biomed. Pharmacother. 2022;153:113491. doi: 10.1016/j.biopha.2022.113491. [DOI] [PubMed] [Google Scholar]
- 146.Cen K., Chen M., He M., Li Z., Song Y., Liu P., Jiang Q., Xu S., Jia Y., Shen P. Sporoderm-broken spores of Ganoderma lucidum sensitizes ovarian cancer to cisplatin by ROS/ERK signaling and attenuates chemotherapy-related toxicity. Front. Pharmacol. 2022;13:826716. doi: 10.3389/fphar.2022.826716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Thimmaraju A., Govindan S. Novel studies of characterization, antioxidant, anticoagulant and anticancer activity of purified polysaccharide from Hypsizygus ulmarius mushroom. Bioact. Carbohydr. Diet. Fibre. 2022;27:100308. doi: 10.1016/j.bcdf.2022.100308. [DOI] [Google Scholar]
- 148.Fekry T., Salem M.F., Abd-Elaziz A.A., Muawia S., Naguib Y.M., Khalil H. Anticancer properties of selenium-enriched oyster culinary-medicinal mushroom, Pleurotus ostreatus (Agaricomycetes), in colon cancer in vitro. Int. J. Med. Mushrooms. 2022;24:1–20. doi: 10.1615/IntJMedMushrooms.2022045181. [DOI] [PubMed] [Google Scholar]
- 149.Meng T., Yu S.-S., Ji H.-Y., Xu X.-M., Liu A.-J. A novel acid polysaccharide from Boletus edulis: Extraction, characteristics and antitumor activities in vitro. Glycoconj. J. 2021;38:13–24. doi: 10.1007/s10719-021-09972-0. [DOI] [PubMed] [Google Scholar]
- 150.Kaplan Ö., Tosun N.G., Özgür A., Tayhan S.E., Bilgin S., Türkekul İ., Gökce İ. Microwave-assisted green synthesis of silver nanoparticles using crude extracts of Boletus edulis and Coriolus versicolor: Characterization, anticancer, antimicrobial and wound healing activities. J. Drug Deliv. Sci. Technol. 2021;64:102641. doi: 10.1016/j.jddst.2021.102641. [DOI] [Google Scholar]
- 151.Zhang Y., Zhou R., Liu F., Ng T.B. Purification and characterization of a novel protein with activity against non-small-cell lung cancer in vitro and in vivo from the edible mushroom Boletus edulis. Int. J. Biol. Macromol. 2021;174:77–88. doi: 10.1016/j.ijbiomac.2021.01.149. [DOI] [PubMed] [Google Scholar]
- 152.Fogarasi M., Socaciu M.-I., Sălăgean C.-D., Ranga F., Fărcaș A.C., Socaci S.A., Socaciu C., Țibulcă D., Fogarasi S., Semeniuc C.A. Comparison of different extraction solvents for characterization of antioxidant potential and polyphenolic composition in Boletus edulis and Cantharellus cibarius mushrooms from Romania. Molecules. 2021;26:7508. doi: 10.3390/molecules26247508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Nowakowski P., Markiewicz-Żukowska R., Gromkowska-Kępka K., Naliwajko S.K., Moskwa J., Bielecka J., Grabia M., Borawska M., Socha K. Mushrooms as potential therapeutic agents in the treatment of cancer: Evaluation of anti-glioma effects of Coprinus comatus, Cantharellus cibarius, Lycoperdon perlatum and Lactarius deliciosus extracts. Biomed. Pharmacother. 2021;133:111090. doi: 10.1016/j.biopha.2020.111090. [DOI] [PubMed] [Google Scholar]
- 154.Fang J., Gao S., Islam R., Teramoto Y., Maeda H. Extracts of Phellinus linteus, bamboo (Sasa senanensis) leaf and chaga mushroom (Inonotus obliquus) exhibit anti-tumor activity through activating innate immunity. Nutrients. 2020;12:2279. doi: 10.3390/nu12082279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jiao C., Chen W., Tan X., Liang H., Li J., Yun H., He C., Chen J., Ma X., Xie Y. Ganoderma lucidum spore oil induces apoptosis of breast cancer cells in vitro and in vivo by activating caspase-3 and caspase-9. J. Ethnopharmacol. 2020;247:112256. doi: 10.1016/j.jep.2019.112256. [DOI] [PubMed] [Google Scholar]
- 156.Haque M.A., Reza A.A., Nasrin M.S., Rahman M.A. Pleurotus highking mushrooms potentiate antiproliferative and antimigratory activity against triple-negative breast cancer cells by suppressing Akt signaling. Integr. Cancer Ther. 2020;19:1534735420969809. doi: 10.1177/1534735420969809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kong B.H., Teoh K.H., Tan N.H., Tan C.S., Ng S.T., Fung S.Y. Proteins from Lignosus tigris with selective apoptotic cytotoxicity towards MCF7 cell line and suppresses MCF7-xenograft tumor growth. PeerJ. 2020;8:e9650. doi: 10.7717/peerj.9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tan X., Chen W., Jiao C., Liang H., Yun H., He C., Chen J., Ma X., Xie Y. Anti-tumor and immunomodulatory activity of the aqueous extract of Sarcodon imbricatus in vitro and in vivo. Food Funct. 2020;11:1110–1121. doi: 10.1039/C9FO01230C. [DOI] [PubMed] [Google Scholar]
- 159.Wang W., Wang L.-Y., Yang H.-R., Zhang Y.-P., Zhang H.-N., Fan H., Zhao X.-L., Zhang J., Jia W. Antitumor effect of By-1 from spent broth from submerged cultures of stout camphor medicinal mushroom, Taiwanofungus camphoratus (higher Basidiomycetes), on A549 adenocarcinoma cells. Int. J. Med. Mushrooms. 2017;19:225–232. doi: 10.1615/IntJMedMushrooms.v19.i3.40. [DOI] [PubMed] [Google Scholar]
- 160.Lau M.F., Chua K.H., Sabaratnam V., Kuppusamy U.R. In vitro and in silico anticancer evaluation of a medicinal mushroom, Ganoderma neo-japonicum Imazeki, against human colonic carcinoma cells. Biotechnol. Appl. Biochem. 2021;68:902–917. doi: 10.1002/bab.2013. [DOI] [PubMed] [Google Scholar]
- 161.Ghosh S.K., Sanyal T., Bera T. Anticancer activity of solvent extracts of Hexogonia glabra against cervical cancer cell lines. Asian Pac. J. Cancer Prev. 2020;21:1977–1986. doi: 10.31557/APJCP.2020.21.7.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ghosh S., Sanyal T. Antiproliferative and apoptotic effect of ethanolic extract of Calocybe indica on PANC-1 and MIAPaCa2 cell lines of pancreatic cancer. Exp. Oncol. 2020;42:178–182. doi: 10.32471/exp-oncology. [DOI] [PubMed] [Google Scholar]
- 163.Kao C.H., Greenwood D.R., Jamieson S.M., Coe M.E., Murray P.M., Ferguson L.R., Bishop K.S. Anticancer characteristics of Fomitopsis pinicola extract in a xenograft mouse model—A preliminary study. Nutr. Cancer. 2020;72:645–652. doi: 10.1080/01635581.2019.1648693. [DOI] [PubMed] [Google Scholar]
- 164.Zhu L., Wu M., Li P., Zhou Y., Zhong J., Zhang Z., Li Y., Yao W., Xu J. High-pressure supercritical CO2 extracts of Ganoderma lucidum fruiting body and their anti-hepatoma effect associated with the Ras/Raf/MEK/ERK signaling pathway. Front. Pharmacol. 2020;11:602702. doi: 10.3389/fphar.2020.602702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cheng A.-Y., Chien Y.-C., Lee H.-C., Hsieh Y.-H., Yu Y.-L. Water-extracted Ganoderma lucidum induces apoptosis and S-phase arrest via cyclin-CDK2 pathway in glioblastoma cells. Molecules. 2020;25:3585. doi: 10.3390/molecules25163585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tsai Y.T., Kuo P.H., Kuo H.P., Hsu C.Y., Lee Y.J., Kuo C.L., Liu J.Y., Lee S.L., Kao M.C. Ganoderma tsugae suppresses the proliferation of endometrial carcinoma cells via Akt signaling pathway. Environ. Toxicol. 2021;36:320–327. doi: 10.1002/tox.23037. [DOI] [PubMed] [Google Scholar]
- 167.Sadowska A., Zapora E., Sawicka D., Niemirowicz-Laskowska K., Surażyński A., Sułkowska-Ziaja K., Kała K., Stocki M., Wołkowycki M., Bakier S. Heterobasidion annosum induces apoptosis in DLD-1 cells and decreases colon cancer growth in in vivo model. Int. J. Mol. Sci. 2020;21:3447. doi: 10.3390/ijms21103447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Mondal A., Banerjee D., Majumder R., Maity T.K., Khowala S. Evaluation of in vitro antioxidant, anticancer and in vivo antitumour activity of Termitomyces clypeatus MTCC 5091. Pharm. Biol. 2016;54:2536–2546. doi: 10.3109/13880209.2016.1168854. [DOI] [PubMed] [Google Scholar]
- 169.Peng C.-C., Chen K.-C., Peng R.Y., Chyau C.-C., Su C.-H., Hsieh-Li H.M. Antrodia camphorata extract induces replicative senescence in superficial TCC, and inhibits the absolute migration capability in invasive bladder carcinoma cells. J. Ethnopharmacol. 2007;109:93–103. doi: 10.1016/j.jep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 170.Jayachandran M., Xiao J., Xu B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017;18:1934. doi: 10.3390/ijms18091934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Park H.-J. Current uses of mushrooms in cancer treatment and their anticancer mechanisms. Int. J. Mol. Sci. 2022;23:10502. doi: 10.3390/ijms231810502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hetland G., Tangen J.-M., Mahmood F., Mirlashari M.R., Nissen-Meyer L.S.H., Nentwich I., Therkelsen S.P., Tjønnfjord G.E., Johnson E. Antitumor, anti-inflammatory and antiallergic effects of Agaricus blazei mushroom extract and the related medicinal basidiomycetes mushrooms, Hericium erinaceus and Grifola frondosa: A review of preclinical and clinical studies. Nutrients. 2020;12:1339. doi: 10.3390/nu12051339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Misgiati M., Widyawaruyanti A., Raharjo S.J., Sukardiman S. Ergosterol isolated from Agaricus blazei Murill n-hexane extracts as potential anticancer MCF-7 activity. Pharmacogn. J. 2021;13:418–426. doi: 10.5530/pj.2021.13.53. [DOI] [Google Scholar]
- 174.Sun Y., Cheng M., Dong L., Yang K., Ma Z., Yu S., Yan P., Bai K., Zhu X., Zhang Q. Agaricus blazei extract (FA-2-b-β) induces apoptosis in chronic myeloid leukemia cells. Oncol. Lett. 2020;20:270. doi: 10.3892/ol.2020.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jeitler M., Michalsen A., Frings D., Hübner M., Fischer M., Koppold-Liebscher D.A., Murthy V., Kessler C.S. Significance of medicinal mushrooms in integrative oncology: A narrative review. Front. Pharmacol. 2020;11:580656. doi: 10.3389/fphar.2020.580656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Chay W.Y., Tham C.K., Toh H.C., Lim H.Y., Tan C.K., Lim C., Wang W.-W., Choo S.-P. Coriolus versicolor (Yunzhi) use as therapy in advanced hepatocellular carcinoma patients with poor liver function or who are unfit for standard therapy. J. Altern. Complement. Med. 2017;23:648–652. doi: 10.1089/acm.2016.0136. [DOI] [PubMed] [Google Scholar]
- 177.Twardowski P., Kanaya N., Frankel P., Synold T., Ruel C., Pal S.K., Junqueira M., Prajapati M., Moore T., Tryon P. A phase I trial of mushroom powder in patients with biochemically recurrent prostate cancer: Roles of cytokines and myeloid-derived suppressor cells for Agaricus bisporus–induced prostate-specific antigen responses. Cancer. 2015;121:2942–2950. doi: 10.1002/cncr.29421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Torkelson C.J., Sweet E., Martzen M.R., Sasagawa M., Wenner C.A., Gay J., Putiri A., Standish L.J. Phase 1 clinical trial of Trametes versicolor in women with breast cancer. ISRN Oncol. 2012;2012:251632. doi: 10.5402/2012/251632. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.