Abstract
Lawsonia intracellularis is a recently identified bacterial pathogen which causes disease in a broad range of animals. Invasion of intestinal epithelial cells and the resultant hyperplasia of infected cells are central processes in disease pathogenesis. In this study, we aimed to establish whether immunocompetent mice were susceptible to infection and whether gamma interferon (IFN-γ) contributed to the pathogenesis of infection. Wild-type 129-Sv-Ev mice (129 mice) and IFN-γ receptor knockout mice based on the 129 background (IFN-γR−) were challenged orally with approximately 5.5 × 107 L. intracellularis cells. Both 129 and IFN-γR− mice became infected, although the extent of infection (as determined by the proportion of infected crypts) was substantially lower in 129 mice than in IFN-γR− mice. Despite these differences, infected crypts showed characteristics typical of proliferative enteropathies of other animals, i.e., intracellular colonization of epithelial cells by L. intracellularis with resultant epithelial hyperplasia. Infection in 129 mice was cleared between days 21 and 28 postchallenge, whereas infection in IFN-γR− mice was evident in 100% of animals from day 21 onward. Additionally, in IFN-γR− mice the infection was so extensive that fatalities resulted. IFN-γ therefore plays a significant role in limiting intracellular infection and increased cellular proliferation associated with L. intracellularis. L. intracellularis infection is generally associated with modest cellular infiltration; therefore, further comparative examinations will be necessary to determine pathogenicity factors and define the role of IFN-γ in controlling this infection.
Lawsonia intracellularis is a recently identified intracellular pathogen which is phylogenetically unrelated to other pathogens (10, 26). It is an obligate intracellular enteropathogen and is the etiological agent of infectious intestinal hyperplasias for which several synonyms have been applied including proliferative enteropathy (PE), intestinal adenomatosis, and ileitis. L. intracellularis causes a disease complex which is primarily recognized in pigs (20, 36); however, as diagnostic methods improve, L. intracellularis is emerging as a cause of intestinal hyperplasia in an increasing range of mammalian and avian species (reviewed in reference 20). For example, L. intracellularis has most recently been reported as a cause of fatal enteritis in captive macaques (19). Previously it was also suggested to be a possible agent of ulcerative colitis in humans, although the etiological role was not established (31). On the basis of 16S rRNA gene sequences, L. intracellularis is most closely related to sulfate-reducing bacteria of the genus Desulfovibrio (7, 10, 30) and to Bilophila wadsworthia (20). Although the latter and some species of the former are intestinal inhabitants and occasional pathogens, there are no other apparent similarities to L. intracellularis.
L. intracellularis preferentially invades intestinal epithelial crypt cells, where the bacteria reside and replicate within the cytoplasm. Infection with most enteroinvasive bacteria commonly involves infiltration of inflammatory cells (polymorphonuclear leukocytes or monocytes), leading to foci of infection, epithelial necrosis and often dissemination to other sites (e.g., the liver). Although it also targets the intestinal epithelium, L. intracellularis is significantly different from the other enteroinvasive bacteria and exhibits unique pathological characteristics (20). The major recognized pathological consequence of infection is hyperplasia of infected crypts with negligible evidence of an inflammatory response (20). The organism is almost exclusively associated with the intestinal epithelium, exhibiting no detectable dissemination to other organs.
Intestinal epithelial hyperplasia is known to occur as a major pathological consequence of infection with only a small number of bacterial pathogens. In addition to L. intracellularis, these bacteria include Helicobacter species and Citrobacter rodentium, both of which are relatively well characterized in comparison to L. intracellularis (22, 27). Although the pathological characteristics of L. intracellularis infection are well documented (see e.g., references 20 and 36), it is apparent that this bacterium is a unique enteropathogen for which pathogenic mechanisms remain speculative. Currently we have only rudimentary knowledge of either the host or bacterial factors which contribute to proliferative enteropathies caused by L. intracellularis.
Gamma interferon (IFN-γ) is central to the progression of infection by intracellular bacteria and other intracellular pathogens, as demonstrated by a variety of approaches. For instance, transgenic mice with specific deletions in IFN-γ or IFN-γ receptor (knockouts) or treated with antibodies specific for IFN-γ demonstrate increased susceptibility to challenge with, for example, Salmonella enterica serovar Typhimurium (11, 29), Shigella spp. (47), or Listeria monocytogenes (3). Since Lawsonia infection is relatively noninflammatory and induces increased epithelial proliferation, the cytokine regulatory networks and cascades are likely to differ from those induced by invasive inflammatory bacteria. Similarly, the roles of host cellular defenses are likely to differ.
In order to examine the possible role of IFN-γ in Lawsonia infection, we established an infection system in mice using bacteria cultured in vitro and examined infection and hyperplasia in IFN-γ receptor-deficient and isogenic wild-type mice.
MATERIALS AND METHODS
Bacteria and preparation of inoculum.
L. intracellularis isolate LR189/5/83 was originally obtained from a pig with porcine proliferative enteropathy in the United Kingdom (21). Bacteria were isolated from homogenized intestinal tissue and grown in cell culture in IEC-18 intestinal epithelial cells by methods described previously (21). Following isolation and serial passage in the laboratory for up to nine passages, this strain can reproduce disease in experimentally inoculated pigs (42, 43). From pure, frozen stocks of bacteria, L. intracellularis was cultured to a final number of 11 passages; cells were then lysed and bacterial suspensions were prepared in SPG (sucrose-potassium-glutamate) buffer with 5% fetal calf serum. To enumerate L. intracellularis, infected-cell lysate was serially diluted for infection of IEC-18 cells on 13-mm coverslips (carried out in triplicate). Numbers of infectious units (IU; equating to viable bacteria) in this suspension were estimated as a product of the number of infected cells, dilution, and an estimated number of 50 bacteria per infected cell (based on previous observations). Detection of L. intracellularis in infected monolayers was carried out by our routine immunodetection methods (21) using a monoclonal antibody specific for an L. intracellularis surface antigen (27). The purity of bacterial suspensions (including freedom from mycoplasmas and Chlamydia) was confirmed as described previously (21). Aliquots of suspension containing approximately 2.2 × 108 L. intracellularis IU · ml−1 were stored at −70°C and thawed immediately prior to inoculation of animals.
Mice and experimental design.
All procedures were conducted in accordance with the guidelines of the United Kingdom Home Office Animals (Scientific Procedures) Act of 1986 and approved by an Ethical Review Committee. Mouse strains challenged were of the 129-Sv-Ev genotype (wild type, designated 129) and IFN-γ receptor knockout mice based on this background (IFN-γR−) (16). Cohorts of mice were regularly screened for pathogens of laboratory rodents and were maintained under specific-pathogen-free conditions. At the time of challenge the animals were of mixed genders and aged 47 to 62 days old. The mice were dosed with 0.25 ml of L. intracellularis suspension (four mice per challenge group, with each mouse receiving approximately 5.5 × 107 IU) or with 0.25 ml of SPG buffer (control group) by oral gavage with a ball-ended 21-gauge needle. The animals were given access to water and feed ad libitum and were monitored daily for health status. At each required time point postinfection (14, 21, 28, and 35 days), groups of four mice inoculated with L. intracellularis and one control mouse were removed for examination. Following euthanasia by CO2 asphyxiation, the animals were weighed and a postmortem examination was carried out as described below.
Monitoring of infection and pathological examinations.
Multiple regions of small and large intestine were removed at postmortem, sectioned longitudinally, and examined for evidence of intestinal thickening or hemorrhage. Samples were either fixed in 10% neutral buffered formalin or mounted in OCT compound (Merck) and snap-frozen in a slurry of dry ice and isopentane. The formalin-fixed tissue was trimmed, dehydrated through graded alcohol concentrations, and embedded in paraffin wax, and 4-μm-thick sections were cut. These were stained with hematoxylin and eosin for routine histopathological examination. For immunohistochemistry, 4-μm sections were cut onto treated slides (BioBond; Biocell) and rehydrated through graded alcohol concentrations, and labeling was carried out using an indirect immunoperoxidase technique. Samples comprising mesenteric lymph nodes, spleen, and liver were also taken, mounted, and snap-frozen as above. Frozen material was cut as 6-μm sections on a cryostat, mounted, and air dried for 1 to 2 h at room temperature. Slides were fixed in acetone for 10 min, air dried twice, and then used immediately for immunohistochemical detection of Lawsonia.
Rabbit polyclonal serum 1080/76 (raised against a field isolate of L. intracellularis) was used as primary antibody, and detection was performed using anti-rabbit immunoglobulin-peroxidase conjugate (Vectastain Elite ABC kit; Vector Labs) as specified by the manufacturer. Following this, sections were counterstained with haematoxylin and then mounted in DePex (Merck). Tissues were examined histologically for features typical of L. intracellularis infection, i.e., intestinal epithelial hyperplasia and intracellular L. intracellularis. The relative proportion of infected crypts in the ileum and colon was determined to establish the extent of infection; the proportion of infected (and hence hyperplastic) crypts was counted over 10 random fields under a magnification of ×250.
RESULTS
Clinical and pathological determinations.
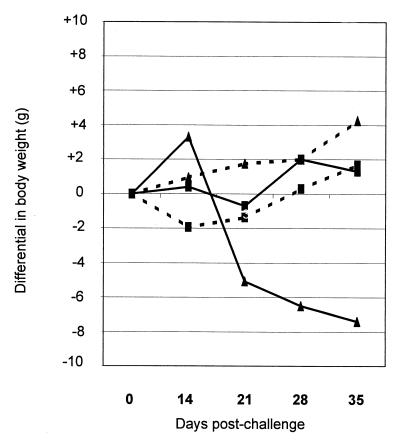
Weight loss or failure to gain weight is characteristic of natural PE; however, this is a variable feature of experimental challenge of pigs with L. intracellularis (28, 42). This was borne out in our own experimental challenges of mice, where one of the two series had far greater clinical impact on mice than the other did although the proportions of infected mice were similar. The differences cannot be ascribed to differences in the ages or genders of the mice or in the L. intracellularis inocula, which were similar in each series. The reasons for the variations in disease in these replicates, whether in mice, pigs, or hamsters, are unclear. Over the course of these challenges, the weights of infected 129 mice were lower than those of the control (sham-inoculated) mice on days 14, 21, and 28 postchallenge; however, these differences represented less than 10% of mouse body weight and were not significant. In IFN-γR− mice there were substantial differences in weights, and the results of one of two challenge experiments are presented in Fig. 1. The mean weights of challenged IFN-γR− mice were significantly lower after day 14 postchallenge, with the reduction in the average weight for the group (compared to uninoculated control) representing 29, 35, and 44% of body weight on days 21, 28, and 35, respectively.
FIG. 1.
Weight differentials in 129 (■) and IFN-γR− (▴) mice following oral inoculation with 5.5 × 107 IU of L. intracellularis ( ) or carrier buffer (–––). Zero on the y axis represents the mean weight of mice at inoculation. Values represent the means obtained from four mice at each time point except on day 35 in the IFN-γR− mice, when only three mice were weighed; see the text for details. Figures on the x axis represent days postchallenge.
) or carrier buffer (–––). Zero on the y axis represents the mean weight of mice at inoculation. Values represent the means obtained from four mice at each time point except on day 35 in the IFN-γR− mice, when only three mice were weighed; see the text for details. Figures on the x axis represent days postchallenge.
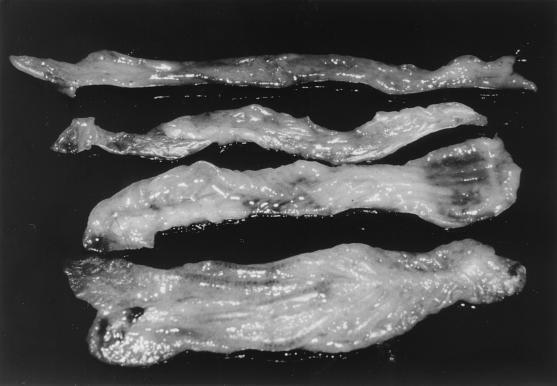
At some time points, gross pathological examination of mice demonstrated significantly enlarged intestines (a feature typical of PE in other animals) in a at least one of the mice per group. In the affected animals, intestinal enlargement was evident in both ileum and colon, with the latter being more profoundly enlarged. Figure 2 shows typical examples of enlarged intestines in both wild-type 129 and IFN-γR− mice. Mucosal hemorrhage in the intestine is an occasional feature of some components of the PE disease complex (hemorrhagic enteropathy), and this was observed in the intestine from one of the IFN-γR− mice (not shown).
FIG. 2.
Mouse colons obtained on day 28 postchallenge, showing characteristics typical of L. intracellularis infection. From top to bottom: 129 mouse sham challenged; IFN-γR− mouse sham challenged; 129 mouse challenged with L. intracellularis; IFN-γR− mouse challenged with L. intracellularis.
Characteristics of L. intracellularis infection in mice.
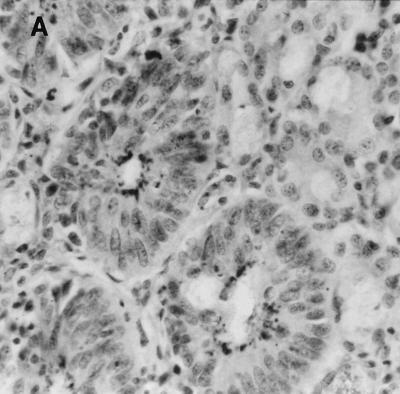
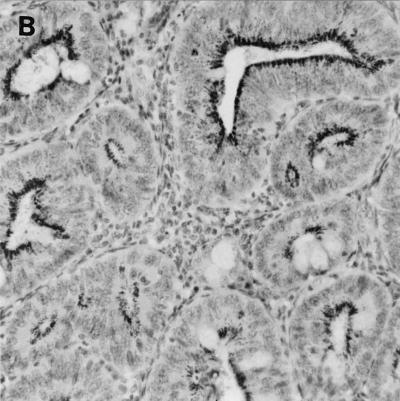
Specific detection of L. intracellularis was carried out on intestinal sections by immunohistochemistry, the accepted standard for confirmation of infection by this bacterium. Characteristic intracellular bacteria and associated epithelial hyperplasia were detected in ileum and colon specimens in all mice exhibiting intestinal enlargement (Fig. 3). L. intracellularis was detected in both wild-type 129 and IFN-γR− mice, although the extent of infection was greater in IFN-γR− mice. Lawsonia was not detected in the mesenteric lymph nodes, livers, or spleens of any of the mice examined (including IFN-γR− mice which died as a result of extensive Lawsonia infection in the intestine [see below]).
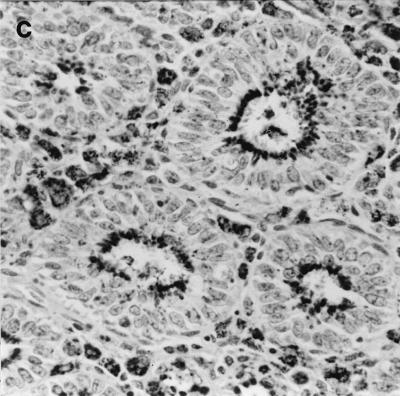
FIG. 3.
Immunochemistry of colonic tissue for L. intracellularis antigen, counterstained with hematoxylin. Dark-staining areas represent L. intracellularis organisms within epithelial cells. Infected crypts show epithelial hyperplasia. Original magnification, ×250. (A) Wild-type 129 mouse on day 28 post-challenge; (B) IFN-γR− mouse on day 28 postchallenge; C, IFN-γR− mouse that died (day 32 postchallenge).
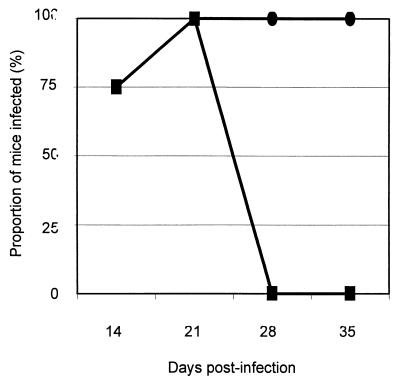
Immunohistological detection was used to determine the proportion of mice infected with L. intracellularis (strain designation LR189/5/83) at each time point postchallenge (Fig. 4). Both wild-type and IFN-γR− mice became infected and exhibited intracellular bacteria and hyperplastic crypts. In wild-type mice, infection was detected from the first sample time point (day 14) and on day 21 postchallenge. After this point, these intracellular bacteria were not detectable by immunohistochemistry. Similar results (not shown) were obtained in previous experimental challenges of 129 and IFN-γR− mice. In these preceding experimental challenges, bacteria were not observed in either the colon or ileum on day 7 postinfection; therefore, this and ethical considerations led to omission of this time point from this series of experiments. By comparison, the C57BL/6 strain of mice (results not shown) exhibited similar susceptibility to infection to that of 129 wild-type mice. The observed pattern of infection in mice mimicked that observed in experimentally infected pigs (18, 24, 42, 43), where intracellular infection becomes demonstrable on days 10 to 14 postchallenge and resolves 2 weeks or more later.
FIG. 4.
Proportions of wild-type 129 (■) and IFN-γR− (●) mice infected over the duration of the challenge study as determined by immunohistochemical examination of ileal and colonic mucosa.
The extent of infection in the ileum and colon was determined by counting the proportion of infected (and hence hyperplastic) crypts over 10 random fields under a magnification of ×250. In wild-type 129 mice, levels of infection in the ileum and colon were low, showing, respectively, 1.3 and 3.5% of crypts infected on day 14 postchallenge. IFN-γR− mice showed a higher susceptibility to infection and a progressive increase in infected crypts in both ileum and colon to day 28 postchallenge. The proportion of infected colonic crypts on days 14, 21, 28, and 35 were 10, 60, 69, and 40%, whereas the corresponding values in the ileum were 10, 5, 44, and 0%, respectively. The proportion of infected crypts reached 100% in mice which died as a result of challenge. An example of this extensive infection of IFN-γR− mice is shown in Fig. 2.
Three of the four IFN-γR− mice from the group for the final time point (scheduled for day 35) died or were euthanized in extremis between days 31 and 32 postchallenge, representing a fatality rate of 75%. These animals were excessively anorexic, showing a reduction in body weight to approximately 50% of that of controls. Immunohistological examination of the colon and ileum from these mice indicated extensive epithelial infection with L. intracellularis, with nearly 100% of epithelial cells affected in 100% of crypts. Even in the mice that died, there was no evidence of bacterial dissemination to the liver or spleen.
DISCUSSION
In this study we have demonstrated unequivocally that immunocompetent, wild-type 129-Sv-Ev mice are susceptible to infection with L. intracellularis under experimental conditions although infection was detectable only at a low level, as indicated by the proportion of infected crypts. Previously it has been shown that pigs (18, 24, 42, 43) and hamsters (17, 44) are both susceptible under experimental conditions but that mice, rats, and chickens are highly refractory (1). In the present study, not only were inoculated mice successfully colonized by L. intracellularis but also infection led to clinical and pathological features characteristic of PE in other animals, i.e., intracellular bacteria, epithelial hyperplasia, and (in some mice) anorexia. This extends further the range of animals susceptible to enteric disease caused by this bacterium. The range of animals in which natural infection and associated disease has been detected has broadened over the last decade and now includes pigs, hamsters, ferrets, foxes, deer, rabbits, horses, emus, ostriches, macaques, and others (reviewed in reference 20). Although to our knowledge natural infection has not been detected in either wild or laboratory mice, the former represent a potential reservoir; hence, our findings have further implications for the ecology and epidemiology of PE, particularly in pigs, where the environment is likely to be cohabited by rodents.
Although pigs have been considered the major susceptible animal, it is becoming increasingly apparent that L. intracellularis has a broad host range which is potentially wider than currently known. Despite evidence of infection in primates, there is currently no direct evidence that L. intracellularis can infect humans, although one report (31) has suggested a link with human disease. In view of the known broad host range of L. intracellularis and the wide spectrum in clinical presentation (20, 36), we should remain alert to the possibility that the range of susceptible species may broaden further and may incorporate humans.
In the mice examined, the principal site of infection, as demonstrated by gross pathology, histology, and immunohistochemistry, was the colon rather than the ileum. The main site of infection (and hence hyperplasia) differs among the animals in which natural disease has been reported (2, 4–6, 8, 15, 20, 23, 30, 41, 48). This may reflect physiological or immunological differences between the animals. Additional factors may be the differing commensal populations or expression of particular bacterial receptors in those sites. Which, if any, of these possibilities are involved remains to be determined; however, it has been shown that commensal populations are required for successful infection with L. intracellularis (25), a factor which does not exclude any of the above possibilities. In none of the mice (even those which died or were euthanized in extremis) was Lawsonia detectable in sites other than the intestinal epithelium. This confirms the absolute specificity of the tropism of L. intracellularis for intestinal epithelial cells, particularly immature (crypt) cells, even in animals in which Th1 cell-mediated responses are diminished.
The general pattern of infection in wild-type mice resembled that seen in experimentally infected pigs in previous studies (18, 24, 42, 43). Epithelial infection becomes detectable on around day 14 postchallenge, remains detectable for a variable period thereafter, and then resolves spontaneously. The presence of detectable intraepithelial L. intracellularis coincides with the development of a primary serological response to major surface antigens (S. C. Mitchell, N. MacIntyre, J. R. Thomson, S. Rhind, and D. G. E. Smith, unpublished data). Resolution of infection presumably coincides with developing immune (or other defense or repair) responses; however, further detailed examinations of immune responses are necessary to corroborate this.
Histologically, disease in experimentally challenged mice closely corresponds to disease in other animals, showing intracellular bacteria and epithelial hyperplasia in infected crypts. These studies also suggest that infection is accompanied by some cellular infiltration in mice, at least in the 129-Sv-Ev genetic background (results not shown). Although it is generally accepted that uncomplicated proliferative enteropathy in pigs is not accompanied by extensive leukocyte infiltration, such responses have been observed in other animals.
It is possible that other strains of mice may respond in a different manner from that of 129-Sv-Ev mice; however, the contribution of mouse genotype (other than IFN-γ receptor) to immune responses and susceptibility was not an objective of this study since they are governed by multiple genetic factors. Preliminary data, however, do show that wild-type mice of the 129 and C57BL/6 (results not shown) backgrounds are both colonized intracellularly by L. intracellularis with resultant hyperplasia. Our results with C57BL/6 mice conflict with a previous report (1) which failed to demonstrate any susceptibility of these mice to infection with this bacterium. These differences in apparent susceptibility between these two studies cannot be ascribed to differences in inocula, which were numerically similar. A study of the influence of inoculum, bacterial strain, and mouse genotype on susceptibility and a detailed examination of immune responses have yet to be conducted.
IFN-γ, as well as other effectors, is elicited in the early phase in pathogenesis of other enteroinvasive bacteria (3, 11, 29, 32, 47) and is important in both the progression and control of epithelial infection. Given the important role of IFN-γ in other intracellular bacterial infections, we sought to establish whether IFN-γ played a role in the control of this intracellular infection. Mice in which IFN-γ receptor was deleted (IFN-γR−) demonstrated a markedly increased susceptibility to experimental L. intracellularis challenge. Rates of infection were higher in IFN-γR− mice than in wild-type mice: whereas in wild-type mice there was no evidence of infection by day 28 postchallenge, all IFN-γR− mice in each group demonstrated infection for the 35-day duration of the study. IFN-γR− mice also showed a far greater proportion of infected crypts (and consequently hyperplasia) compared to wild-type mice. Additionally, fatalities resulting from Lawsonia infection occurred around day 31 postchallenge in 75% of IFN-γR− mice in one challenge series. Despite the differences in pathological presentation between L. intracellularis and other enteroinvasive bacteria, it thus appears that IFN-γ is important in the control of L. intracellularis infection as well as of infections caused by other intracellular pathogens. IFN-γ has multiple possible roles in limiting and controlling intracellular infections and may contribute to the control of Lawsonia infection by exerting effects directly upon epithelial cells, by immune-mediated mechanisms, or by a combination of means. The possible roles for IFN-γ in control of L. intracellularis infection remain to be pursued.
From the current investigation, it was evident that there was a marked increase in crypt hyperplasia in L. intracellularis-infected IFN-γR− mice compared to wild-type mice. The absence of a functional IFN-γ receptor in the IFN-γR− mice therefore led to enhanced epithelial proliferation during L. intracellularis infection. The involvement of IFN-γ in regulating epithelial cell differentiation and proliferation has been noted previously (37, 46). Despite the demonstration of the involvement of IFN-γ in suppression of proliferation, its role in regulating hyperplasia caused by bacteria is complex. In an investigation of murine colonic hyperplasia (13), infection of IFN-γR− mice with Citrobacter rodentium failed to produce hyperplasia. Additionally, in the IFN-γR− mouse background, massive lymphocyte infiltration, which is typical of infection in the wild-type background, did not occur. IFN-γ and lymphocyte infiltration are also involved in Helicobacter infection (12, 35, 38, 45). Infection with L. intracellularis, in contrast, is not noted to correspond to such extensive inflammatory infiltration, and in IFN-γR− mice infection resulted in amplification of hyperplasia rather than abrogation. This indicates that there is an apparent dichotomy in the potential roles of IFN-γ in hyperplasia induced by these bacterial infections. We are continuing to examine in detail the lymphocyte responses in situ in both mice and pigs infected with L. intracellularis and their contribution to control and pathology.
Characterization of Lawsonia-induced hyperplasia will provide interesting insights since differences in the apparent roles of IFN-γ and lymphocytes may be reflected in other mechanistic distinctions. For instance, increased epithelial cell proliferation in infections caused by Helicobacter spp. and C. rodentium is associated with epidermal growth factor-like (33, 34) factors and keratinocyte growth factor (13, 14), respectively. We have yet to determine whether these or other similar growth factors are involved in Lawsonia infection. Similarly, direct involvement of bacterial effectors has been demonstrated to induce hyperplasia by both Helicobacter spp. (45) and C. rodentium (13, 14). Investigations of L. intracellularis to date have not detected sequences homologous to intimin (D. G. E. Smith, B. W. Wren, and J. Hannigan, unpublished data), which is essential for pathological changes associated with murine colonic hyperplasia (9, 39, 40), although we have yet to examine this bacterium for determinants homologous to those conferred on the Helicobacter pathogenicity island. It appears, therefore, that although there is functional convergence there is no paradigm for bacterially induced hyperplasia since the mechanisms underlying L. intracellularis-induced epithelial hyperplasia differ from those in Helicobacter and C. rodentium infection. The bacterial and host factors involved in L. intracellularis infection remain to be ascertained; however, further comparative examinations of bacterial and host factors involved will be beneficial to the advancement of understanding and control of growth disturbances.
In this study we have established that mice are susceptible to infection with L. intracellularis, at least under the experimental conditions employed. We have thus developed an in vivo model in which some of the characteristics of intestinal hyperplasia caused by this bacterium can be further analyzed. Through application of this experimental infection system, we have initiated a definition of host factors which contribute to both the pathology and control of disease caused by L. intracellularis. This bacterium is one of a range of diverse prokaryotic enteropathogens which have evolved life-styles which involve invasion of the intestinal epithelium; however, it is unique among these in inducing epithelial hyperplasia as its major pathological consequence without substantial leukocyte involvement. Induction of epithelial hyperplasia appears to be essential to L. intracellularis since bacterial proliferation and host cell replication are interdependent (22), a feature which represents an unusual evolutionary adaptation significantly different from that of other enteroinvasive bacterial pathogens.
This investigation is the first to establish categorically that mice are susceptible to infection with this bacterium and that, as in other intracellular bacterial infections, IFN-γ is crucial in the control of L. intracellularis infection, although the mechanism by which this control is exerted has yet to be defined. This bacterium is also unique among the small group of enteropathogenic bacteria which induce epithelial hyperplasia, and our investigations also make it apparent that no single mechanism is responsible for this kind of pathological alteration. It is clear that host mechanisms in these infections are complex and require further elucidation. Since there are apparently a multiplicity of routes by which disturbances in cellular growth and differentiation may arise, L. intracellularis represents another organism useful in the examinations of epithelial hyperplasia and its control. Our establishment of infection in the mouse offers a valuable system to continue investigations of immune and other regulatory mechanisms in this and other intestinal hyperplastic conditions.
ACKNOWLEDGMENTS
S.C.M. is a recipient of a postgraduate studentship from the Medical Research Council (MRC) of the United Kingdom. Funding for this work was provided by the Biotechnology and Biological Sciences Research Council (BBSRC).
We thank J. Hannigan, N. MacIntyre, and the staff of the Experimental Pathogens Unit for their excellent technical support.
REFERENCES
- 1.Collins A M, Love R J, Jasni S, McOrist S. Attempted infection of mice, rats and chickens by porcine strains of Lawsonia intracellularis. Aust Vet J. 1999;77:120–122. doi: 10.1111/j.1751-0813.1999.tb11680.x. [DOI] [PubMed] [Google Scholar]
- 2.Cooper D M, Swanson D L, Barns S M, Gebhart C G. Comparison of the 16S ribosomal DNA sequence from the intracellular agents of proliferative enteritis in a hamster, deer, and ostrich with the sequence of a porcine isolate of Lawsonia intracellularis. Int Syst Bacteriol. 1997;47:635–639. doi: 10.1099/00207713-47-3-635. [DOI] [PubMed] [Google Scholar]
- 3.DiTirro J, Rhoades E R, Roberts A D, Burke J M, Mukasa A, Cooper A M, Frank A A, Born W K, Orme I M. Disruption of the cellular inflammatory response to Listeria monocytogenes infection in mice with disruptions in targeted genes. Infect Immun. 1998;66:2284–2289. doi: 10.1128/iai.66.5.2284-2289.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drolet R, Larochelle D, Gebhart C J. Proliferative enteritis associated with Lawsonia intracellularis (Ileal symbiont intracellularis) in white-tailed deer. J Vet Diagn Investig. 1996;8:250–253. doi: 10.1177/104063879600800219. [DOI] [PubMed] [Google Scholar]
- 5.Eriksen K, Landsverk T, Bratberg B. Morphology and immunoperoxidase studies of intestinal adenomatosis in the blue fox, Alopex lagopus. J Comp Pathol. 1990;102:265–278. doi: 10.1016/s0021-9975(08)80016-3. [DOI] [PubMed] [Google Scholar]
- 6.Fox J G, Lawson G H K. Campylobacter-like omega intracellular antigen in proliferative colitis of ferrets. Lab Anim Sci. 1988;38:34–36. [PubMed] [Google Scholar]
- 7.Fox J G, Dewhirst F E, Fraser G J, Paster B J, Shames B, Murphy J C. Intracellular Campylobacter-like organism from ferrets and hamsters with proliferative bowel disease is a Desulfovibrio sp. J Clin Microbiol. 1994;32:1229–1237. doi: 10.1128/jcm.32.5.1229-1237.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frank N, Fishman C E, Gebhart C J, Levy M. Lawsonia intracellularis proliferative enteropathy in a weanling foal. Equine Vet J. 1998;30:549–552. doi: 10.1111/j.2042-3306.1998.tb04533.x. [DOI] [PubMed] [Google Scholar]
- 9.Frankel G, Phillips A D, Novakova M, Field H, Candy D C A, Schauer D B, Douce G, Dougan G. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin response to intimin and EspB. Infect Immun. 1996;64:5315–5325. doi: 10.1128/iai.64.12.5315-5325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gebhart C J, Barns S M, McOrist S, Lin G F, Lawson G H K. Ileal symbiont intracellularis, an obligate intracellular bacterium of porcine intestines showing a relationship to Desulfovibrio species. Int J Syst Bacteriol. 1993;43:533–538. doi: 10.1099/00207713-43-3-533. [DOI] [PubMed] [Google Scholar]
- 11.Gulig P A, Doyle T J, Clare-Salzler M J, Maiese R L, Matsui H. Systemic infection of mice by wild-type but not Spv−Salmonella typhimurium is enhanced by neutralization of gamma interferon and tumor necrosis factor alpha. Infect Immun. 1997;65:5191–5197. doi: 10.1128/iai.65.12.5191-5197.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeberle H A, Kubin M, Bamford K B, Garofalo R, Graham D Y, El-Zaatari F, Karttunen R, Crowe S E, Reyes V E, Ernst P B. Differential stimulation of interleukin-12 (IL-12) and IL-10 by live and killed Helicobacter pylori in vitro and association of IL-12 production with gamma-interferon-producing T cells in the human gastric mucosa. Infect Immun. 1997;65:4229–4235. doi: 10.1128/iai.65.10.4229-4235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higgins L M, Frankel G, Connerton I, Goncalves N S, Dougan G, MacDonald T T. Role of bacterial intimin in colonic hyperplasia and inflammation. Science. 1999;285:588–591. doi: 10.1126/science.285.5427.588. [DOI] [PubMed] [Google Scholar]
- 14.Higgins L M, Frankel G, Douce G, Dougan G, MacDonald T T. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hotchkiss C E, Shames B, Perkins S E, Fox J G. Proliferative enteropathy of rabbits: the intracellular Campylobacter-like organisms is closely related to Lawsonia intracellularis. Lab Anim Sci. 1996;46:623–627. [PubMed] [Google Scholar]
- 16.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune-response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 17.Jasni S, McOrist S, Lawson G H K. Reproduction of proliferative enteritis in hamsters with a pure culture of porcine ileal symbiont intracellularis. Vet Microbiol. 1994;41:1–9. doi: 10.1016/0378-1135(94)90130-9. [DOI] [PubMed] [Google Scholar]
- 18.Joens L A, Nibbelink S, Glock R D. Induction of gross and microscopic lesions of porcine proliferative enteritis by Lawsonia intracellularis. Am J Vet Res. 1997;58:1125–1131. [PubMed] [Google Scholar]
- 19.Klein E C, Gebhart C J, Duhamel G E. Fatal outbreaks of proliferative enteritis caused by Lawsonia intracellularis in young colony-raised rhesus macaques. J Med Primatol. 1999;28:11–18. doi: 10.1111/j.1600-0684.1999.tb00084.x. [DOI] [PubMed] [Google Scholar]
- 20.Lawson G H K, Gebhart C J. Proliferative enteropathy. J Comp Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- 21.Lawson G H K, McOrist S, Jasni S, Mackie R A. Intracellular bacteria of porcine proliferative enteropathy—cultivation and maintenance in vitro. J Clin Microbiol. 1993;31:1136–1142. doi: 10.1128/jcm.31.5.1136-1142.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lawson G H K, Mackie R A, Smith D G E, McOrist S. Infection of cultured rat enterocytes by Ileal symbiont intracellularis depends on host-cell function and actin polymerization. Vet Microbiol. 1995;45:339–350. doi: 10.1016/0378-1135(94)00142-j. [DOI] [PubMed] [Google Scholar]
- 23.Lemarchand T X, Tully T N, Shane S M, Duncan D E. Intracellular Campylobacter-like organisms associated with rectal prolapse and proliferative enteroproctitis in emus (Dromaius novaehollandiae) Vet Pathol. 1997;34:152–156. doi: 10.1177/030098589703400209. [DOI] [PubMed] [Google Scholar]
- 24.McOrist S, Jasni S, Mackie R A, MacIntyre N, Neef N, Lawson G H K. Reproduction of porcine proliferative enteropathy with pure cultures of Ileal symbiont intracellularis. Infect Immun. 1993;61:4286–4292. doi: 10.1128/iai.61.10.4286-4292.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McOrist S, Mackie R A, Neef N, Aitken I, Lawson G H K. Synergism of Ileal symbiont intracellularis and gut bacteria in the reproduction of porcine proliferative enteropathy. Vet Rec. 1994;134:331–332. doi: 10.1136/vr.134.13.331. [DOI] [PubMed] [Google Scholar]
- 26.McOrist S, Gebhart C J, Boid R, Barns S M. Characterization of Lawsonia intracellularis gen. nov., sp. nov., the obligately intracellular bacterium of porcine proliferative enteropathy. Int J Syst Bacteriol. 1995;45:820–825. doi: 10.1099/00207713-45-4-820. [DOI] [PubMed] [Google Scholar]
- 27.McOrist S, Mackie R A, Lawson G H K, Smith D G E. In vitro interactions of Lawsonia intracellularis with cultured enterocytes. Vet Microbiol. 1997;54:385–392. doi: 10.1016/s0378-1135(96)01283-7. [DOI] [PubMed] [Google Scholar]
- 28.McOrist S, Smith S H, Green L E. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579–581. doi: 10.1136/vr.140.22.579. [DOI] [PubMed] [Google Scholar]
- 29.Muotiala A, Makela P H. The role of IFN-γ in murine Salmonella-typhimurium infection. Microb Pathog. 1990;8:135–141. doi: 10.1016/0882-4010(90)90077-4. [DOI] [PubMed] [Google Scholar]
- 30.Peace T A, Brock K V, Stills H F. Comparative-analysis of the 16S ribosomal RNA gene sequence of the putative agent of proliferative ileitis of hamsters. Int J Syst Bacteriol. 1994;44:832–835. doi: 10.1099/00207713-44-4-832. [DOI] [PubMed] [Google Scholar]
- 31.Pitcher M C L, Goddard M, McOrist S, Cummings J H. Ulcerative colitis and porcine proliferative enteropathy—a common bacterial etiology. Gastroenterology. 1995;108(Suppl. 4):A894. [Google Scholar]
- 32.Ramarathinam L, Niesel D W, Klimpel G R. Salmonella typhimurium induces IFN-gamma production in murine splenocytes—role of natural-killer-cells and macrophages. J Immunol. 1993;150:3973–3981. [PubMed] [Google Scholar]
- 33.Romano M, Ricci V, Memoli A, Tuccillo C, Di Popolo A, Sommi P, del Vecchio Blanco C, Zarrilli R. Helicobacter pylori up-regulates cyclooxygenase-2 mRNA expression and prostaglandin E2 synthesis in MKN-28 gastric mucosal cells in vitro. J Biol Chem. 1998;273:28560–28563. doi: 10.1074/jbc.273.44.28560. [DOI] [PubMed] [Google Scholar]
- 34.Romano M, Ricci V, Di Popolo A, Sommi P, del Vecchio Blanco C, Bruni C B, Ventura U, Cover T L, Blaser M J, Coffey R J, Zarrilli R. Helicobacter pylori up-regulates the expression of epidermal growth factor-related peptides but inhibits their proliferative effect in MKN28 gastric mucosal cells. J Clin Investig. 1998;101:1604–1613. doi: 10.1172/JCI1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roth K A, Kapadia S B, Martin S M, Lorenz R G. Cellular immune responses are essential for the development of Helicobacter felis-associated gastric pathology. J Immunol. 1999;163:1490–1497. [PubMed] [Google Scholar]
- 36.Rowland A C, Lawson G H K. Porcine proliferative enteropathies. In: Leman A D, Straw B E, Mengeling W L, D'Allaire S, Taylor D J, editors. Diseases of swine. 7th ed. Ames, Iowa: Iowa State University Press; 1992. pp. 560–569. [Google Scholar]
- 37.Ruemmele F M, Gurbindo C, Mansour A M, Marchand R, Levy E, Seidman E G. Effects of interferon gamma on growth, apoptosis, and MHC class II expression of immature rat intestinal crypt (IEC-6) cells. J Cell Physiol. 1998;176:120–126. doi: 10.1002/(SICI)1097-4652(199807)176:1<120::AID-JCP14>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 38.Sawai N, Kita M, Kodama T, Tanahashi T, Yamaoka Y, Tagawa Y I, Ikawura Y, Imanishi J. Role of gamma interferon in Helicobacter pylori-induced gastric inflammatory responses in a mouse model. Infect Immun. 1999;67:279–285. doi: 10.1128/iai.67.1.279-285.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schauer D B, Falkow S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmisible murine colonic hyperplasia. Infect Immun. 1993;61:4654–4661. doi: 10.1128/iai.61.11.4654-4661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schauer D B, Falkow S. Attaching and effacing locus of a Citrobacter freundii biotype that causes transmissible murine colonic hyperplasia. Infect Immun. 1993;61:2486–2492. doi: 10.1128/iai.61.6.2486-2492.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoeb T R, Fox J G. Enterocecocolitis associated with intraepithelial Campylobacter-like bacteria in rabbits (Oryctolagus cuniculus) Vet Pathol. 1990;27:73–80. doi: 10.1177/030098589002700201. [DOI] [PubMed] [Google Scholar]
- 42.Smith S H. Epidemiological features of porcine proliferative enteropathy. Ph.D. thesis. Edinburgh, Scotland: University of Edinburgh; 1998. [Google Scholar]
- 43.Smith S H, McOrist S. Development of persistent intestinal infection and excretion of Lawsonia intracellularis by piglets. Res Vet Sci. 1997;62:6–10. doi: 10.1016/s0034-5288(97)90171-5. [DOI] [PubMed] [Google Scholar]
- 44.Stills H F. Isolation of an intracellular bacterium from hamsters (Mesocricetus auratus) with proliferative ileitis and reproduction of the disease with a pure culture. Infect Immun. 1991;59:3227–3236. doi: 10.1128/iai.59.9.3227-3236.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Doorn N E M, Van Rees E P, Namavar F, Ghiara P, Vandenbroucke-Grauls C M J E, DeGraff J. The inflammatory response in CD1 mice shortly after infection with a CagA+/VacA+ Helicobacter pylori strain. Clin Exp Immunol. 1999;115:421–427. doi: 10.1046/j.1365-2249.1999.00789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Veldman R J, Klappe K, Hoekstra D, Kok J W. Interferon-γ-induced differentiation and apoptosis of HT29 cells: dissociation of (glucosyl)ceramide signalling. Biochem Biophys Res Commun. 1998;247:802–808. doi: 10.1006/bbrc.1998.8896. [DOI] [PubMed] [Google Scholar]
- 47.Way S S, Borczuk A C, Dominitz R, Goldberg M B. An essential role for gamma interferon in innate resistance to Shigella flexneri infection. Infect Immun. 1998;66:1342–1348. doi: 10.1128/iai.66.4.1342-1348.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Williams N M, Harrison L R, Gebhart C G. Proliferative enteropathy in a foal caused by a Lawsonia intracellularis-like bacterium. J Vet Diagn Investig. 1996;8:254–256. doi: 10.1177/104063879600800220. [DOI] [PubMed] [Google Scholar]