Abstract
The adhesion of K21a, K26, K36, and K50 capsulated Klebsiella strains to ileocecal (HCT-8) and bladder (T24) epithelial cell lines was significantly lower than that of their corresponding spontaneous noncapsulated variants K21a/3, K26/1, K36/3, and K50/3, respectively. Internalization of the bacteria by both epithelial cell lines was also significantly reduced. Similarly, a capsule-switched derivative, K2(K36), that exhibited a morphologically larger K36 capsule and formed more capsular material invaded the ileocecal epithelial cell line poorly compared to the corresponding K2 parent strain. None of the capsulated strains exhibited significant mannose-sensitive type 1 fimbriae, whereas two of the noncapsulated variants K21a/3 and K50/3 exhibited potent mannose-sensitive hemagglutinating activity. Although hemagglutinating activity that could be attributed to mannose-resistant Klebsiella type 3 fimbriae was weak in all strains, in several cases the encapsulated parent strains exhibited lower titers than their corresponding noncapsulated variants. Although the level of adhesion to the ileocecal cells is not different from adhesion to bladder cells, bacterial internalization by bladder cells was significantly lower than internalization by ileocecal cells, suggesting that bladder cells lack components required for the internalization of Klebsiella.
Klebsiella pneumoniae is an opportunistic pathogen involved in outbreaks of nosocomial infections, such as bacteremia and sepsis, mainly in immunocompromised individuals (36). Due to the emergence of multidrug resistance among Klebsiella strains, the search for new approaches for the prevention or treatment of Klebsiella infections is now under intensive investigation (34). To be successful, these efforts will require a better understanding of the infectious process.
Multiple Klebsiella components (e.g., fimbriae, siderophores, O antigens, and capsular antigens) have been considered to be potential virulence factors (34). Of these factors, capsular antigens are probably considered the major determinants of pathogenicity (4, 9, 17, 21). As a consequence, new therapeutic approaches have been targeted against the capsule. A polysaccharide-based vaccine has been tested (5). Based on the structural variability of its capsular polysaccharides (CPS), Klebsiella has been classified into 77 serotypes (27), which differ markedly in pathogenicity potential and epidemiological relevance (3, 28, 29, 35). Epidemiological findings showed that over 70% of all cases of Klebsiella bacteremia were caused by only 25 of the 77 different serotypes (6). In clinical studies, this vaccine was proven to be safe and immunogenic (2, 16). As a potential alternative, it has been suggested that agents which reduce capsule formation might be used to enhance phagocytosis and serum killing (10–12).
The primary contribution of CPS to the pathogenicity of Klebsiella appears to be by rendering the bacteria resistant to phagocytosis by polymorphonuclear leukocytes and resistant to killing by serum (30, 33, 38, 39, 41). There are other capsule-associated activities, however, which might play important roles in pathogenicity. For instance, it has been shown that Klebsiella strains expressing CPS containing dimannose or dirhamnose repeat sequences are recognized by the mannose receptor of macrophages which bind, ingest, and kill the bacteria (25). Although K. pneumoniae is considered to be an extracellular pathogen, recent studies have demonstrated its ability to be internalized by epithelial cells (20, 24). Recently, the capsule was shown to interfere with the expression of Klebsiella adhesins which mediate binding of the bacteria to nonphagocytic cells (19, 22). Because adhesion is important for the internalization process, we sought to determine the role of capsule in the internalization process of K. pneumoniae, using capsulated strains, their noncapsulated variants, and a capsule-switched recombinant strain.
MATERIALS AND METHODS
Bacterial strains.
All Klebsiella strains used in this study belonged to the species K. pneumoniae. K. pneumoniae NTCC strains K26 (9146), K36 (9156), and K50 (9170) were from the strain collection of the Department of Medical Microbiology and Virology in Kiel. The parent K2 and K21a encapsulated strains were previously described (26). Spontaneous noncapsulated variants of serotypes K2, K21a, K26, K50, and K36 were obtained from nonmucoid segments of colonies of capsulated parent strains as described elsewhere (22, 37). Likewise, a revertant capsulated K21a variant was obtained from mucoid segments in colonies of the noncapsulated K21a strain. The capsule-switched derivative K2(K36) was constructed as described below. In most experiments, the bacteria were grown in Luria broth. In the experiments used to obtain the capsule-switched derivative, M9 medium was solidified by adding 1% Bacto Agar. Amino acids or antibiotics were added to allow selection for their auxotrophic, antibiotic-resistant, capsule-switched derivatives as described previously (26).
Cell lines and culture conditions.
Human bladder epithelial cell line T24, human ileocecal epithelial cell line HCT-8, and embryonic intestinal epithelial cell line INT 407 were cultivated as described by Oelschlaeger and Tall (24). T24 cells were cultivated in McCoy's 5A medium with 10% fetal calf serum (FCS) and subcultivated at a ratio of 1:8 twice a week. Human ileocecal epithelial cell line HCT-8 was cultivated in RPMI 1640 medium with 1 mM pyruvate, 2 mM glutamine, and 10% FCS, and the INT 407 embryonic ileocecal cells were cultivated in minimal essential medium containing 10% fetal bovine serum, 2 mM glutamine, and 0.1 mM nonessential amino acids. Additional human bladder epithelial cells (cell line RT112), provided by the group of T. F. Meyer, Max-Planck-Institut für Biologie, Abteilung Infektionsbiologie, Tübingen, Germany, were used. The cells were cultivated in Waymouth's medium MB 742/1 with 2 mM glutamine and 10% fetal bovine serum.
Genetic manipulation and construction of the capsule-switched derivative.
The capsule-switched derivative was constructed as described previously (26). Briefly, we took advantage of the close proximity of the histidine (his) locus and the CPS gene cluster, using conversion to his as a marker for the transfer of the CPS genes from the K36 prototrophic (his+) donor strain to the his auxotrophic (his) K2 recipient strain. The donor strain was also auxotrophic for arginine (arg) and spontaneously nalidixic acid resistant (Nalr). The conjugative plasmid R68.45, which elicits gene-mobilizing activity, was transferred to the donor strain from Pseudomonas aeruginosa PAO25(R68.45) as described previously (26). The CPS genes were transferred by conjugation from the Nalr his+ donor strain K36 to the his K2 recipient strain. his+ Nals colonies were then selected and tested for expression of the heterologous CPS by the capsule-swelling method using homologous sera.
Determination, visualization, and analysis of CPS by nuclear magnetic resonance spectroscopy.
For analysis of CPS, samples were deuterium exchanged several times by freeze-drying solutions of the polysaccharide in D2O and were then examined in 99.99% D2O (0.45 ml) containing a trace of acetone for use as an internal reference (δ 2.23 for 1H and 31.07 ppm for 13C). Spectra were recorded at 60°C on a Bruker AMX-400 spectrometer equipped with an X32 computer using standard Bruker software. The relative molecular weights of polysaccharides were approximated by molecular sieve chromatography as described previously (15).
For visualization, we stabilized the capsule using anti-K36 serum and employed an immunoelectron microscopic technique described previously (26). Zwittergent extracts of the strains were analyzed for CPS content by the uronic acid assay of Blumenkranz and Asboe-Hansen (1) as described elsewhere (11).
Bacteriocin typing.
A modification of the scrape-and-point method was used as described elsewhere (32). Briefly, eight bacteriocin-producing strains were spot inoculated onto tryptic soy agar. After growth for 16 h at 32°C and subsequent inactivation by chloroform vapors, the plates were overlaid with soft tryptic soy agar containing the strains to be tested. After incubation for 8 h at 37°C, susceptibility of the test strains to particular bacteriocins was indicated by a zone of growth inhibition around the spots.
PFGE.
Pulsed-field gel electrophoresis (PFGE) of the isolates was performed by a modification of the method described by Weller et al. (40). Briefly, strains were grown in brain heart infusion broth (BHI) at 37°C with shaking. After harvesting and washing in TEN buffer (0.1 M Tris, 0.15 M NaCl, 0.1 M EDTA [pH 7.5]), the bacteria were adjusted to 2 × 109 cells/ml. One milliliter of the bacterial suspension was mixed with 1 ml of 1% Incert Agarose and poured into a gel plug mold. Plugs were incubated for at 37°C in EC buffer (6 mM Tris, 1 mM NaCl, 100 mM EDTA, 0.5% Brij, 0.2% deoxycholate, sodium 0.5% lauroylsarcosine [pH 7.5]) supplemented with lysozyme (1 mg/ml) and RNase (80 μg/ml). After 16 h of incubation, the EC buffer was replaced by ES buffer (0.5 M EDTA, sodium 1% lauroylsarcosine [pH 9.3]) containing proteinase K (1 mg/ml). After overnight incubation at 50°C, the plug was washed in TE buffer (10 mM Tris, 0.1 mM EDTA). The plug was incubated for further 3 h at 37°C after addition of 100 μl of a solution of phenylmethylsulfonyl fluoride (174 mg/ml) in TE buffer. Finally, the plug was washed four times with TE buffer and stored at 4°C until use.
Digestion of DNA was performed by overnight incubation of the plug in the presence of 40 U of the restriction endonuclease XbaI at 37°C. After extensive washing in TE buffer, the plug was subjected to PFGE in an 1% agarose gel containing 1% ethidium bromide. Electrophoresis was performed in 0.5× TBE using a CHEF-DR III system (Bio-Rad Laboratories). The running conditions were 6 V/cm, 120°C, 60 to 90 s, and 22 h. The gels were photographed under UV light at 254 nm using a video documentation system.
Hemagglutination assay.
The expression of type 1 (mannose-sensitive hemagglutination [MSHA]) and type 3 (mannose-resistant Klebsiella hemagglutination [MR/K-HA]) pili was examined as described previously (31). After four passages (either serial 48-h passages in static BHI for the detection of MSHA or passage in nutrient broth for detection of MR/K-HA), the bacteria were allowed to grow in Luria broth for 2 h before being harvested and washed three times with phosphate-buffered saline. Then 50 μl of bacterial suspension (3 × 109 cells/ml) was mixed with 50 μl of guinea pig erythrocytes (5 × 108/ml) for determination of MSHA or with tanned ox erythrocytes for testing MR/K-HA. Hemagglutination activity was determined as the minimum bacterial density (expressed as CFU per milliliter) required to agglutinate erythrocytes. Agglutination was observed after 3 min of gentle shaking at room temperature and after another 10 min at 4°C.
Internalization and adhesion assays.
Internalization assays were performed as described earlier (24). Epithelial cells were grown in 24-well cell culture clusters to confluent monolayers (7 × 104 cells per well) in RPMI 1640 medium supplemented with 10% FCS. Mid-log-phase bacteria (2 × 106; A600 = 0.4 to 0.6) in 0.1 ml of buffer were then added to each well (approximately 30 bacteria per epithelial cell). After centrifugation at 200 × g for 5 min, internalization was allowed to occur for 2 h at 37°C in an atmosphere of 94% air–6% CO2. Before a second 2-h incubation period under the same conditions but with fresh medium containing 100 μg of gentamicin per ml, monolayers were washed once with Earle's balanced salts solution. Under these conditions, all extracellular (i.e., not internalized) bacteria were killed by the added gentamicin. The monolayers were then washed twice with Earle's balanced salt solution and lysed with 0.1% Triton X-100 to determine the viable counts of released intracellular bacteria. Invasion ability was expressed as the percentage of inoculum that survived gentamicin treatment. All assays were conducted in duplicate and were repeated independently at least five times.
To determine the level of bacterial adhesion, mid-log-phase bacteria were suspended in FCS-free RPMI 1640 buffer and added to 24-well plates containing epithelial cells that had been prewashed three times with the same buffer. The plates were then centrifuged at 200 × g for 5 min and further incubated for 30 min at 37°C. The plates were then washed three times to remove nonadherent bacteria, and the epithelial cells were lysed to enumerate adherent bacteria as described above. The lack of internalization in FCS-free medium after 30 min was demonstrated by performing a gentamicin killing assay parallel to the adhesion assay. No bacterial growth could be detected during the 30-min incubation period in the FCS-free medium.
Assay of intracellular survival and replication.
Following the invasion period as described above, a medium containing 10 rather than 100 μg of gentamicin per ml was added to the infected ileocecal intestinal cells. The transfected cells were incubated at 37°C in 5% CO2 and lysed at indicated times up to 72 h later by determination of viable bacteria. The results were recorded as percentage of the original inoculum.
Statistical analysis.
The significance of differences between the biological activities of the tested bacteria was evaluated by the nonparametric analysis of variance test of Kruskal-Wallis followed by Dunn's posttest.
RESULTS
Genetic, phenotypic, and serological characterization of the parent strains, noncapsulated variants, and capsule-switched derivative.
PFGE of DNA was performed to confirm the genetic identity of parents and their capsule-switched derivative. PFGE patterns of the DNA of the parental K2 strain and the capsule-switched derivative K2(K36) were indistinguishable (data not shown). API 20E biochemical reactions, bacteriocin sensitivity patterns (data not shown), and MSHA and MR/K-HA activities of the recombinant strain were identical to those of the K2 recipient strain (Table 1). In contrast, immunoserotyping and nuclear magnetic resonance spectroscopy of CPS showed that the recombinant K2(K36) strain expressed a capsule structurally identical to that of the donor K36 strain.
TABLE 1.
MSHA and MR/K-HA by capsulated strains, noncapsulated variants, and capsule-switched derivative strainsa
| Strain | Minimal no. of bacteria (CFU/ml) causing:
|
|
|---|---|---|
| MSHA | MR/K-HA | |
| K21a | 1.5 × 109 | 3.0 × 109 |
| K21a/3 (CPS−) | 2.25 × 106 | 3.0 × 109 |
| KPTA 46 (CPS−) | 3.5 × 107 | 3.0 × 109 |
| KPTA 47 (CPS−) | 2.25 × 106 | 3.0 × 109 |
| KPTA 55 (CPS+, revertant) | 1.5 × 109 | 3.0 × 109 |
| K26 | 3.0 × 109 | 7.5 × 108 |
| K26/1 (CPS−) | 3.0 × 109 | 4.0 × 108 |
| KPTA 50 (CPS−) | 3.0 × 109 | 7.5 × 108 |
| KPTA 51 (CPS−) | 3.0 × 109 | 7.0 × 107 |
| K36 | 1.5 × 109 | 1.5 × 109 |
| K36/3 (CPS−) | 1.5 × 109 | 4.0 × 108 |
| KPTA 40 (CPS−) | 1.5 × 109 | 4.0 × 108 |
| KPTA 41 (CPS−) | 1.5 × 109 | 3.0 × 109 |
| K50 | 4.0 × 108 | 7.5 × 108 |
| K50/3 (CPS−) | 2.25 × 106 | 4.0 × 108 |
| KPTA43 (CPS−) | 9.0 × 106 | 4.0 × 108 |
| KPTA 44 (CPS−) | 2.25 × 106 | 7.0 × 108 |
| K2 | 3.0 × 109 | 3.0 × 109 |
| KPTA 38 (CPS−) | 3.0 × 109 | 3.0 × 109 |
| KPTA 52 (CPS−) | 3.0 × 109 | 3.0 × 109 |
| KPTA 53 (CPS−) | 3.0 × 109 | 3.0 × 109 |
| K2(K36) | 3.0 × 109 | 3.0 × 109 |
MSHA and MR/K-HA activities were determined using bacteria harvested after 48 h of growth (after four passages) in BHI (for MSHA) or in nutrient broth (for MR/K-HA) or using bacteria harvested after 2 h of growth in LB inoculated with passage 4 bacteria. Values for 48- and 2-h incubations were the same. CPS−, noncapsulated variant.
Identity of the noncapsulated variants to their capsulated parent strains was assayed by their biochemical reactions (API 20E), bacteriocin sensitivity patterns, and PFGE. The results showed that in all cases the noncapsulated variants were identical to their parent strains (data not shown). Likewise, the PFGE and biochemical properties of the K21a revertant phase variant strain KPTA 55 were the same as for the corresponding parent capsulated strain (KPTA 20) as well as the noncapsulated variant K21a/3 from which it was derived (data not shown).
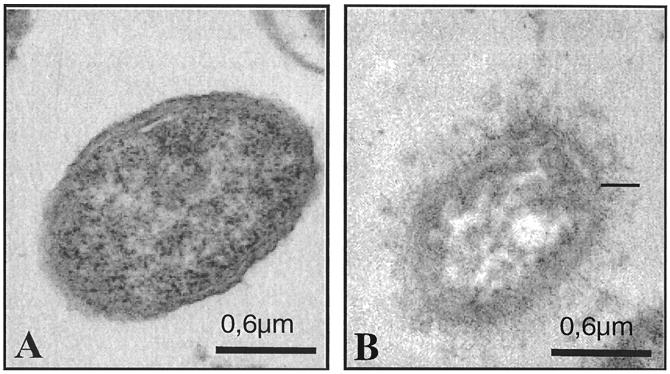
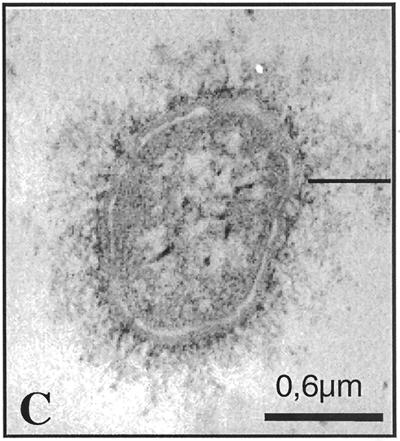
The amounts of capsular material produced by the parent strains K21a, K26, K36, K50, and K2 ranged between 28 and 62 μg of glucuronic acid/109 bacteria. The noncapsulated strains exhibited up to 2.2 μg of glucuronic acid/109 bacteria, but had negative quellung reactions with anticapsular antibodies, indicating that these variants are noncapsulated. The capsule-switched strain K2(K36) produced 92 μg of glucuronic acid/109 bacteria. Electron microscopic analysis of antibody-stabilized capsular material also indicated that the K2(K36) recombinant expressed a capsule larger than that of the K36 parent strain (Fig. 1). This finding is consistent with the glucuronic acid determinations for these two strains.
FIG. 1.
Immunoelectron micrographs of K2 (A), its capsule-switched derivative K2(K36) (C), and the parent strain K36 (B) grown on agar supplemented with 1% lactose and labeled with rabbit anti-K36 capsule antibody. Note that the anti-K36 serum labeled only the K36 parent strain and the derivative K2(K36).
The MSHA and MR/K-HA activities per mass unit of bacteria harvested after 48 h of growth (four passages) were the same as those of bacteria harvested after 2 h of growth in Luria broth inoculated with the 48-h-grown bacteria (Table 1). A previous study showed that encapsulation may interfere with the assembly of type 1 fimbrial subunits into structural fimbriae (22). Consistent with this notion are the results showing that MSHA activity was very low or undetectable for all capsulated strains. All of the noncapsulated variants derived from K21a and K50 capsulated parent strains exhibited potent MSHA activities, suggesting that they were fully fimbriated (Table 1). In contrast, the noncapsulated variants derived from K26 and K36 parent strains exhibited weak activity, suggesting that the majority of the bacterial cells are nonfimbriated probably because they are in the off phase (22). The minimal bacterial count required for MR/K-HA activity was very high (≥3 × 109 CFU/ml) for the parent strains K21a, K36, and K2. The MR/K-HA activity of the corresponding noncapsulated variants did not differ significantly from that of their corresponding capsulated parent strains. The capsulated K26 and K50 strains were more active, showing minimal MR/K-HA activity at 7.5 × 108 CFU; the activity of the corresponding noncapsulated variants was similar except for one K26-derived noncapsulated variant (KPTA 51), which exhibited activity at 7 × 107 CFU/ml (P < 0.01).
Relationship between capsule formation and adhesion and internalization into epithelial cells.
For each pair of strains, the percent internalization of capsulated strains by both types of epithelial cells was significantly lower than that of the noncapsulated variants (P < 0.01) (Table 2). Likewise, the percent adhesion to the two types of epithelial cells was significantly lower for capsulated than noncapsulated strains for each pair (P < 0.02) except K26 and K26/1. Nevertheless, the overall adhesion values for all capsulated parent strains were significantly lower than those for the noncapsulated variants (Table 2). It should be noted, however, that although the magnitude of adhesion of either the noncapsulated or capsulated strains was in the same range for both types of cell lines, invasion into T24 bladder epithelial cells was considerably lower than that into intestinal cells. The host cell tropism was further examined by using two other human cell lines, the embryonic intestinal cell line INT 407 and the bladder cell line RT112. The invasion of all capsulated strains and their noncapsulated phase variants in these cell lines was below the level of detection.
TABLE 2.
Adhesion to and internalization by epithelial cells of capsulated parent strains and their corresponding noncapsulated variantsa
| Parental and noncapsulated variant pairs | % Adhesion
|
% Invasion
|
||
|---|---|---|---|---|
| Ileocecal cells | Bladder cells | Ileocecal cells | Bladder cells | |
| K21a | 16.5 | 11.2 | 2.4 | 0.4 |
| K21/3 | 31.7 | 33.0 | 7.8 | 0.8 |
| K26 | 60.0 | 15.0 | 0.7 | 0.2 |
| K26/1 | 57.4 | 15.5 | 1.8 | 0.6 |
| K36 | 14.0 | 21.5 | 2.9 | 0.4 |
| K36/3 | 30.6 | 49.0 | 4.3 | 0.8 |
| K50 | 42.0 | 40.0 | 2.7 | 0.9 |
| K50/3 | 99.0 | 81.0 | 9.5 | 1.5 |
Confluent monolayers of ileocecal (HCT-8) and bladder (T24) epithelial cell lines were exposed to the indicated Klebsiella strains, and percents adhesion to and internalization by the cell lines were estimated as described in Materials and Methods. Data shown represent mean values of at least triplicate experiments; standard deviations of the mean values did not exceed 20%.
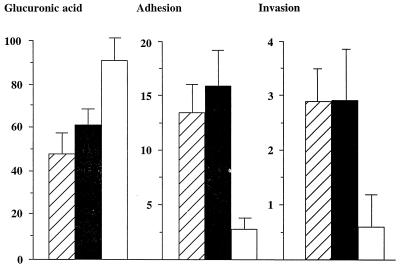
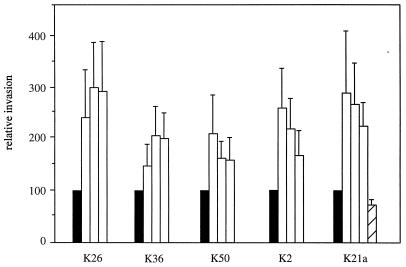
To further examine the relationship between adhesion, internalization, and capsulation, three additional noncapsulated phase variants of each serotype were obtained. Furthermore, a revertant capsulated strain derived from the noncapsulated variant and a capsule-switched derivative were used. The amount of capsule formed by the K2(K36) recombinant strain was significantly higher than that formed by the parent strain (92 ± 10 versus 62 ± 7 μg of glucuronic acid/109 bacteria, P < 0.01). The values of percent adhesion and invasion for the recombinant K2(K36) were markedly lower than those for the parent K2 strain (Fig. 2). All of the three noncapsulated variants exhibited significantly more extensive invasion into ileocecal epithelial cells compared to their corresponding capsulated parent strains (Fig. 3). Although the internalization in bladder cells was significantly lower than that in ileocecal cells, there was a significant difference between the ability of the noncapsulated variants to invade bladder epithelial cells compared to their corresponding parent strains (data not shown).
FIG. 2.
Capsule formation, cellular adhesion, and internalization of the K2 and K36 parent strains and their capsule-switched derivative. Confluent monolayers of HCT-8 cells were exposed to K2 (closed box) and K36 (striped box) capsulated parent strains and their derivative K2(K36) (open box). The amount of glucuronic acid (micrograms per 109 bacteria), percent adhesion to, and percent internalization into the cell lines were estimated as described in Materials and Methods. Data represent mean values and standard deviations of at least quadruplicate experiments for each strain. Mean values for the K2 and K36 parent strains were significantly higher than those for K2(K36) (P > 0.01) for both the adhesion and internalization assays.
FIG. 3.
Internalization of noncapsulated variants of Klebsiella into HCT-8 cells. Relative invasion represents the percent increase in invasion of three noncapsulated variants (open boxes) from each of the indicated Klebsiella serotypes compared to the corresponding capsulated parent strains (=100%; closed boxes). The hatched box is the relative invasion of the K21a capsulated revertant strain derived from a noncapsulated variant strain. Percents invasion of the capsulated parent strains were 0.75, 2.94, 4.55, 3.58, and 2.74 for K26, K36, K50, K2, and K21a, respectively. All values for the noncapsulated variants are significantly higher (P < 0.01) than those for the corresponding capsulated parent strains. Data shown represent mean values and standard deviations.
Bacterial intracellular persistence and replication.
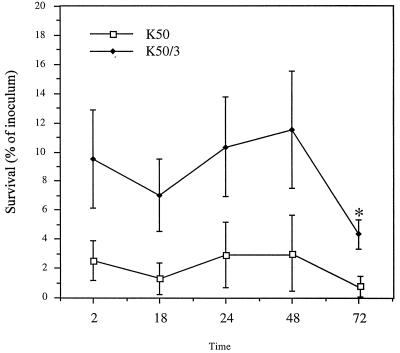
Following the initial 2-h gentamicin killing period, the number of intracellular capsulated parent strain and noncapsulated variants of K50 recovered in the presence of 10 μg of gentamicin per ml did not changed significantly during 48 h of incubation. Thereafter the number of intracellular bacteria of the noncapsulated variant declined significantly, while that of the capsulated bacteria did not change significantly (Fig. 4).
FIG. 4.
Intracellular survival of capsulated parent strain K50 and its noncapsulated variant K50/3. Monolayers of HCT-8 cells were infected with the indicated K. pneumoniae strains. After invasion, the medium was replaced with fresh medium containing 10 μg of gentamicin ml, and the monolayers were lysed at the indicated times to determine the number of the intracellular bacteria expressed as a percentage of inoculum. The asterisk denotes a value significantly different from that obtained after 2 h (P < 0.01). Data shown represent mean values and standard deviations.
DISCUSSION
K. pneumoniae, a classic extracellular pathogen, has been shown to be internalized by nonphagocytic cells (20, 24). In the present study, this ability was found to be markedly hampered by the presence of capsular material on the bacterial surface. This notion is supported by our findings showing that (i) all of the noncapsulated variants were internalized in significantly higher numbers than the corresponding parent strains by both types of epithelial cells, (ii) a revertant K21a capsulated variant derived from a noncapsulated strain invaded epithelial cells poorly, and (iii) a genetically manipulated strain exhibiting a larger capsule and forming more capsular material invaded the intestinal epithelial cell line poorly compared to the corresponding parent strain. Although the isogenic identity of the noncapsulated variants with their parent strains has not been fully elucidated, it is unlikely that the observed reduced internalization of the capsulated parent strain is due to factors other than capsule formation because (i) the DNA patterns as well as phenotypic behaviors of parents and their corresponding noncapsulated variants were identical, (ii) even if there is a hypothetical difference in the genetic background of the noncapsulated variants derived from a capsulated clone, the probability that such other hypothetical factors contributed to the difference in their invasion ability is anticipated to be low because in all cases the noncapsulated strains behaved similarly with respect to invasion ability, and (iii) a K21a revertant capsulated strain derived from a noncapsulated variant exhibited reduced invasion similar to that of the original parent capsulated strain. A major prerequisite for the pathogen to invade epithelial cells is its ability to adhere to the target cells. The results showing that capsulated bacteria which invaded epithelial cells poorly also adhered significantly less to these cells is consistent with this notion. The results appear to suggest that capsule interferes in some manner with the ability of Klebsiella to adhere to the epithelial cells and consequently to be internalized by these cells. There may be a number of mechanisms through which capsule could interfere with adhesion of Klebsiella to epithelial cells. One mechanism may be a masking effect in which the adhesins on the bacterial surface are not accessible for binding to their cognate receptors on epithelial cells.
Another mechanism may involve interference of capsule precursors or regulatory elements that precede biosynthesis or secretion with the ability of the capsulated strain to assemble type 1 fimbrial subunits into mature fimbriae on the bacterial surface (22). This may be the case for the capsulated parent strains K21a and K50 because their corresponding noncapsulated variants K21a/3 and K50/3 exhibited potent type 1 fimbrial activity (Table 1). It has been shown that 48-h broth cultures after several passages provide the best conditions for the expression of both type 1 and type 3 fimbriae (31). In the invasion assay, we used bacteria grown in Luria broth for 2 h. When Luria broth was inoculated from passaged 48-h cultures, the MSHA and MR/K-HA activities per mass unit after a 2-h incubation were the same as that of the inoculum, suggesting that the culture conditions for bacteria used in the invasion assay were permissible for the expression of both MSHA and MR/K-HA. Adhesion via type 1 fimbriae has been shown recently to promote invasion of Citrobacter spp. into epithelial cells (N. Daryab, C. Wass, J. Badger, K. S. Kim, and T. A. Oelschlaeger, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. B/D 176, p. 63, 1999). Capsule may also modulate the transcription of the recently described CF29K adhesin of K. pneumoniae (19). It is also possible that additional adhesins not affected by capsule formation play a role in the invasion process. In this regard, the relatively high activities of both the capsulated and noncapsulated phases of serotype K26 may be mediated by such putative adhesins.
Taken together, the results suggest that capsule formation by K. pneumoniae interferes with the expression of a number of adhesins and thus impairs the ability of the bacteria to be internalized by nonphagocytic cells. Furthermore, it is tempting to speculate on the dual role of the capsule in virulence in the context of the site of infection. In the phagocyte-poor environment of mucosal surfaces, capsule is deleterious to the infectious process irrespective of its chemical structure. This notion is supported by the fact that epithelial cells are not known to express a lectin such as the mannose receptor that recognizes any of the capsular serotypes of K. pneumoniae. In the phagocyte-rich environment of deep tissues such as the lung, the cleansing mechanisms are ineffective and clearance of invading bacteria is dependent on phagocytosis. In this regard, some CPS (e.g., containing d-mannose or dirhamnose repeating units) are deleterious to the infectious process because they are recognized by the mannose receptor on the phagocytic cells (25).
Adhesion by itself is probably not enough to enable the pathogen to invade nonphagocytic cells. Entry of bacteria into animal cells generally has been found to require the coexpression of multiple components and to involve signal transduction via receptors distinct from those required for adhesion (8, 13, 14). Klebsiella species also seem to require distinct components to be internalized by epithelial cells because internalization of the bacteria by mature intestinal cells was much greater than internalization by embryonic intestinal cells or bladder epithelial cells. Previous studies have also shown marked differences in the invasion of a urinary isolate of a K. pneumoniae strain into various cell lines (24). Obviously, various epithelial cells may lack one or more of the components required for the internalization of the different Klebsiella strains. At this point, the possibility that the observed enhanced internalization of Klebsiella by the HCT-8 ileocecal cells is cell line specific rather than tropism for mature intestinal cells cannot be excluded.
Invasion of nonphagocytic cells by bacteria is now considered to be a major virulence factor in the infectious process (18). While this notion is well documented for intracellular pathogens such as Yersinia spp., Listeria spp., Salmonella spp., and Shigella spp., it is less clear for those pathogens whose lifestyle is primarily extracellular. Nevertheless, the list of classic extracellular pathogens that are capable of being internalized by nonphagocytic cells is growing. We have shown that following invasion the Klebsiella organisms persist for at least 72 h, albeit in small numbers. Internalization of bacterial pathogens by nonphagocytic cells such as epithelial cells probably enables the bacteria to escape the effects of deleterious agents such as antibodies and antibiotics. Recently it was suggested that in the case of Streptococcus pyogenes, internalization may also lead to asymptomatic carriage (23). It has been suggested that although Klebsiella colonizes asymptomatically a number of body sites, the main reservoir for severe symptomatic infections is the large bowel (7, 34). The observation, therefore, that the tested Klebsiella serotypes invade intestinal cells better than bladder cells supports the notion that K. pneumoniae surviving within epithelial cells may serve as a critical reservoir from which reinfection of the host can take place and capsule formation may modulate this process. Thus, although Klebsiella strains are more pathogenic in the urinary tract, the latter is not the habitat of this or in fact of any other bacteria.
ACKNOWLEDGMENTS
This work was supported by Deutsche Forschungsgemeinschaft grants SA 730/1-1 and SA730/1-2.
REFERENCES
- 1.Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- 2.Campbell W, Hendrix E, Cryz S J, Cross A. Immunogenicity of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered to victims of acute trauma. Clin Infect Dis. 1996;23:179–181. doi: 10.1093/clinids/23.1.179. [DOI] [PubMed] [Google Scholar]
- 3.Casewell M, Talsania H G. Predominance of certain Klebsiella capsular types in hospitals in the United Kingdom. J Infect. 1979;1:77–79. [Google Scholar]
- 4.Cryz S J, Fürer E, Germanier R. Experimental Klebsiella pneumoniae burn wound sepsis: role of capsular polysaccharide. Infect Immun. 1984;43:440–441. doi: 10.1128/iai.43.1.440-441.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cryz S J, Jr, Fürer E, Germanier R. Safety and immunogenicity of Klebsiella pneumoniae K1 capsular polysaccharide vaccine in humans. J Infect Dis. 1985;151:665–671. doi: 10.1093/infdis/151.4.665. [DOI] [PubMed] [Google Scholar]
- 6.Cryz S J, Mortimer P M, Mansfield V, Germanier R. Seroepidemiology of Klebsiella bacteremic isolates and implications for vaccine development. J Clin Microbiol. 1986;23:687–690. doi: 10.1128/jcm.23.4.687-690.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Champ C, Sauvant M P, Chanal C, Sirot D, Gazuy N, Malhuret R, Baguet J C, Sirot J. Prospective survey of colonization and infection caused by extended spectrum-beta-lactamase-producing members of the family Enterobacteriaceae in intensive care units. J Clin Microbiol. 1989;27:2887–2890. doi: 10.1128/jcm.27.12.2887-2890.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vries F P, van der Ende A, van Putten J P M, Dankert J. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect Immun. 1996;64:2998–3006. doi: 10.1128/iai.64.8.2998-3006.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domenico P, Johanson W G, Straus D C. Lobar pneumonia in rats produced by clinical isolates of Klebsiella pneumoniae. Infect Immun. 1982;37:327–335. doi: 10.1128/iai.37.1.327-335.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domenico P, Landolphi D R, Cunha B A. Reduction of capsular polysaccharide and potentiation of aminoglycoside inhibition in Gram-negative bacteria by bismuth salicylate. J Antimicrob Chemother. 1991;28:801–810. doi: 10.1093/jac/28.6.801. [DOI] [PubMed] [Google Scholar]
- 11.Domenico P, Schwartz S, Cunha B A. Reduction of capsular polysaccharide production in Klebsiella pneumoniae by sodium salicylate. Infect Immun. 1989;57:3778–3782. doi: 10.1128/iai.57.12.3778-3782.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domenico P, Tomas J M, Merino S, Rubires X, Cunha B A. Surface antigen exposure by bismuth dimercaprol suppression of Klebsiella pneumoniae capsular polysaccharide. Infect Immun. 1999;67:664–669. doi: 10.1128/iai.67.2.664-669.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg M S. Interactions between enteropathogenic Escherichia coli and epithelial cells. Clin Infect Dis. 1999;28:451–455. doi: 10.1086/515159. [DOI] [PubMed] [Google Scholar]
- 14.Donnenberg M S, Kaper J K, Finlay B. Interaction between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 15.Dutton G G S, Ng S K, Parolis L A S, Parolis H. A re-investigation of the structure of the capsular polysaccharides of Klebsiella K10. Carbohydr Res. 1989;193:147–155. doi: 10.1016/0008-6215(89)85114-6. [DOI] [PubMed] [Google Scholar]
- 16.Edelman R, Taylor D N, Wasserman S S, McClain J B, Cross A S, Sadoff J C, Que J U, Cryz S J. Phase 1 trial of a 24-valent Klebsiella capsular polysaccharide vaccine and an eight-valent Pseudomonas O-polysaccharide conjugate vaccine administered simultaneously. Vaccine. 1994;12:1288–1294. doi: 10.1016/s0264-410x(94)80054-4. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenwort L, Baer H. The pathogenicity of Klebsiella pneumoniae for mice: the relationship to the quantity and rate of production of type-specific capsular polysaccharide. J Bacteriol. 1956;72:713–717. doi: 10.1128/jb.72.5.713-717.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falkow S. Perspectives series: host/pathogen interactions. Invasion and intracellular sorting of bacteria: searching for bacterial genes expressed during host/pathogen interactions. J Clin Investig. 1997;100:239–243. doi: 10.1172/JCI119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Favre-Bonte S, Joly B, Forestier C. Consequences of reduction of Klebsiella pneumoniae capsule expression on interaction of this bacterium with epithelial cells. Infect Immun. 1999;67:554–561. doi: 10.1128/iai.67.2.554-561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fumagalli O, Tall B, Schipper C, Oelschlaeger T A. N-glycosylated proteins are involved in efficient internalization of Klebsiella pneumoniae by cultured human epithelial cells. Infect Immun. 1997;65:4445–4451. doi: 10.1128/iai.65.11.4445-4451.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Highsmith A K, Jarvis W R. Klebsiella pneumoniae: selected virulence factors that contribute to pathogenicity. Infect Control. 1985;6:75–77. doi: 10.1017/s0195941700062640. [DOI] [PubMed] [Google Scholar]
- 22.Matatov R, Ofek I, Skutelsky E, Schechter I, Perry R, Podschun R, Sahly H, Thankavel K, Abraham S N, Goldhar J. Inability of encapsulated Klebsiella pneumoniae to assemble functional type 1 fimbriae on their surface. FEMS Microbiol Lett. 1999;179:123–130. doi: 10.1111/j.1574-6968.1999.tb08717.x. [DOI] [PubMed] [Google Scholar]
- 23.Neeman R, Keller N, Brazilai A, Korenman Z, Sela S. Prevention of internalisation-associated gene, prtF1, among persisting group-A Streptococcus strains isolated from asymptomatic carriers. Lancet. 1998;352:1974–1977. doi: 10.1016/S0140-6736(97)12452-7. [DOI] [PubMed] [Google Scholar]
- 24.Oelschlaeger T, Tall B. Invasion of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect Immun. 1997;65:2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ofek I, Goldhar J, Keisari Y, Sharon N. Nonopsonic phagocytosis of microorganisms. Annu Rev Microbiol. 1995;49:239–276. doi: 10.1146/annurev.mi.49.100195.001323. [DOI] [PubMed] [Google Scholar]
- 26.Ofek I, Kabha K, Athamna A, Frankel G, Wozniak D J, Hasty L D, Ohman E D. Genetic exchange of determinants for capsular polysaccharide biosynthesis between Klebsiella pneumoniae strains expressing serotypes K2 and K21a. Infect Immun. 1993;61:4208–4216. doi: 10.1128/iai.61.10.4208-4216.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ørskov I, Fife-Asbury M A. New Klebsiella capsular antigen, K82, and the deletion of five of those previously assigned. Int J Syst Bacteriol. 1979;27:386–387. [Google Scholar]
- 28.Podschun R. Phenotypic properties of Klebsiella pneumoniae and K. oxytoca isolated from different sources. Zentbl Hyg. 1990;189:527–535. [PubMed] [Google Scholar]
- 29.Podschun R, Heineken P, Ullmann U, Sonntag H G. Comparative investigations of Klebsiella species of clinical origin: plasmid patterns, biochemical reactions, antibiotic resistances and serotypes. Zentbl Bakteriol Hyg A. 1986;262:335–345. doi: 10.1016/s0176-6724(86)80006-2. [DOI] [PubMed] [Google Scholar]
- 30.Podschun R, Penner I, Ullmann U. Interaction of Klebsiella capsular type 7 with human polymorphonuclear leucocytes. Microb Pathog. 1992;13:371–379. doi: 10.1016/0882-4010(92)90080-8. [DOI] [PubMed] [Google Scholar]
- 31.Podschun R, Sahly H. Hemagglutinins of Klebsiella pneumoniae and Klebsiella oxytoca isolated from different sources. Zentbl Hyg. 1991;191:46–52. [PubMed] [Google Scholar]
- 32.Podschun R, Ullmann U. Bacteriocin typing of environmental Klebsiella isolates. Zentbl Hyg. 1993;195:22–26. [PubMed] [Google Scholar]
- 33.Podschun R, Ullmann U. Klebsiella capsular type K7 in relation to toxicity, susceptibility to phagocytosis and resistance to serum. J Med Microbiol. 1992;36:250–254. doi: 10.1099/00222615-36-4-250. [DOI] [PubMed] [Google Scholar]
- 34.Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11:589–603. doi: 10.1128/cmr.11.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riser E, Noone P. Klebsiella capsular type versus site of isolation. J Clin Pathol. 1981;34:552–555. doi: 10.1136/jcp.34.5.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sahly H, Podschun R. Clinical, bacteriological, and serological aspects of Klebsiella infections and their spondylarthopathic sequelae. Clin Diagn Lab Immunol. 1997;4:393–399. doi: 10.1128/cdli.4.4.393-399.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sahly H, Podschun R, Sass R, Bröcker B, Gross W L, Kekow J, Ullmann U. Serum antibodies to the 77 Klebsiella capsular types in ankylosing spondylitis. Arthritis Rheum. 1994;37:754–759. doi: 10.1002/art.1780370521. [DOI] [PubMed] [Google Scholar]
- 38.Simoons-Smit A M, Verweij-van Vught A M J J, Kanis I Y R, MacLaren D M. Chemiluminescence of human leucocytes stimulated by clinical isolates of Klebsiella. J Med Microbiol. 1985;19:333–338. doi: 10.1099/00222615-19-3-333. [DOI] [PubMed] [Google Scholar]
- 39.Simoons-Smit A M, Verweij-van Vught A M J J, MacLaren D M. The role of K antigens as virulence factors in Klebsiella. J Med Microbiol. 1986;21:133–137. doi: 10.1099/00222615-21-2-133. [DOI] [PubMed] [Google Scholar]
- 40.Weller T M A, Mackenzie F M, Forbes K J. Molecular epidemiology of a large outbreak of multiresistant Klebsiella pneumoniae. J Med Microbiol. 1997;46:921–926. doi: 10.1099/00222615-46-11-921. [DOI] [PubMed] [Google Scholar]
- 41.Williams P, Lambert P A, Brown M R W, Jones R J. The role of the O and K antigens in determining the resistance of Klebsiella aerogenes to serum killing and phagocytosis. J Gen Microbiol. 1983;129:2181–2191. doi: 10.1099/00221287-129-7-2181. [DOI] [PubMed] [Google Scholar]