Abstract
Ischemia-Reperfusion Injury (IRI) is responsible for adverse outcomes in patients with ST-Elevation Myocardial Infarction (STEMI). Oxidative stress, resulting from the production of Reactive Oxygen Species (ROS) and low availability of Glutathione (GSH), are the two main mediators of IRI. The effectiveness of exogenous antioxidant therapy in this scenario is still debated, since the encouraging results obtained in animal models have not been fully reproduced in clinical studies. In this review we focus on the role of GSH, specifically on the biomolecular mechanisms that preserve myocardial cells from damage due to reperfusion. In this regard, we provide an extensive discussion about GSH intrinsic antioxidant properties, its current applications in clinical practice, and the future perspectives.
Keywords: glutathione, STEMI, primary PCI, angioplasty, reperfusion injury
1. Introduction
In the last 40 years, medical research in the field of ischemic heart disease has mostly followed a dichotomous trend, from ischemia to reperfusion. Specifically, in the early 1980s research focused on “Ischemia” and its treatment, first with fibrinolytic therapy [1] and then with primary percutaneous coronary intervention [2,3,4] (pPCI). The effectiveness of pPCI in the ST-Elevation Myocardial Infarction (STEMI) setting has been well established [5], currently representing the gold standard reperfusion technique [6]. However, the early advantage of the restored coronary artery patency is amended by the so called “Ischemia-Reperfusion Injury” (IRI), which is responsible for adverse outcomes [5,7,8]. To date, IRI is still a concern, since we do not have a well-established therapy yet [9].
2. STEMI and IRI: A Misbalance between ROS Activity and GSH Availability
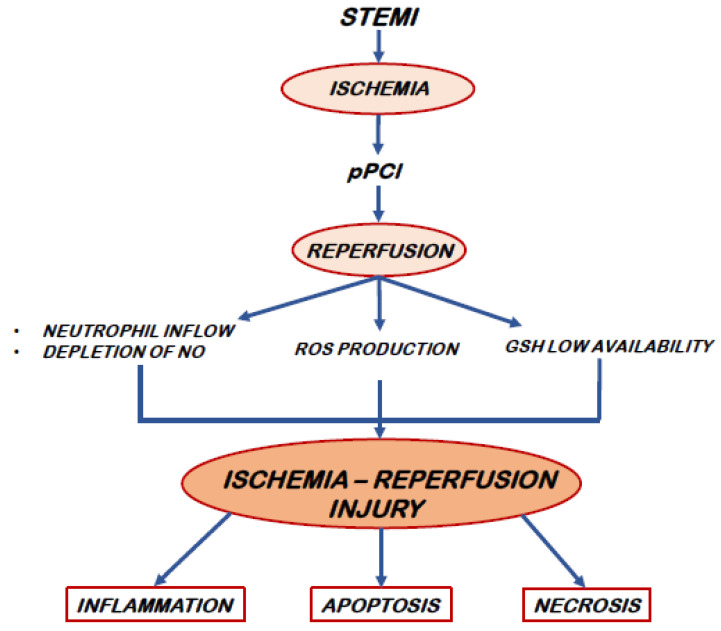
Despite timely adequate reperfusion strategies, STEMI-related mortality is currently high (6–12%), as it is the prevalence of heart failure hospitalizations within one year (14–36%) [10]. Myocardial damage resulting from reperfusion and the subsequent death of cardiomyocytes largely explain these adverse outcomes. The experimental evidence from both animal models and clinical investigations suggests that IRI accounts for up to 50% of the final myocardial scar extension [11,12]. Cellular calcium overload and oxidative stress with production of ROS are the main mediators of IRI [9]. The sources of ROS are essentially three: mitochondrial electron transport chain, xanthine oxidase (derived from endothelial cells) and reduced Nicotinamide Adenine Dinucleotide Phosphate (NADPH) oxidase (from activated neutrophils) [9,11,12]. ROS induce the opening of Mitochondrial Permeability Transition Pore (MPTP) that, in turn, results in necrosis and apoptosis of cardiomyocytes. Moreover, ROS also plays a chemotactic action on white blood cells [13,14,15]. In particular, the oxidative burst promotes a long lasting free-radical activity that induces a sustained inflammatory response with a significant increase in leukocytes adherence in the reperfused areas and further massive leukocyte infiltration into the injured myocardium [16,17]. The optimal healing requires a sequential and coordinated recruitment of cells that remove necrotic tissue and improve tissue repair, thereby hindering the expansion of the infarcted area [18,19]. The early phase is characterized by massive neutrophils infiltration [20,21] and additional damage on myocardial cells provoked by neutrophils themselves as a primary source of ROS [22]. Thereafter, monocytes and their descendants’ macrophages dominate the cellular infiltration, promoting an effective tissue regeneration and resolution of the inflammation [23]. Experimental investigations revealed that a misbalance of these sequential phases, as a consequence of an expanded systemic supply of inflammatory cells, could affect infarct healing, leading to adverse outcomes [16]. In this scenario, lymphocytes play a pivotal role by modulating innate immune cell recruitment to the infarcted myocardium. In a mice experimental model of myocardial infarction, CD4+ T lymphocytes cell deficiency delays the monocyte transition and impairs the healing of the heart [24]. Furthermore, an enhanced metabolic activity accompanying T cells activation drives increased production of ROS, that requires anti-oxidative GSH up-regulation to prevent cellular damage [25]. Upon early T cell activation, GSH tissue content allows adequate scavenging activity. The subsequent burst of oxidative status linked to massive lymphocytes proliferation overwhelms GSH anti-oxidant potential affecting T cell growth and differentiation [26,27]. An adequate level of GSH is therefore of paramount importance for the maintenance of an appropriate cell redox environment, thus avoiding or repairing oxidative modifications that involve cell function and survival [28]. Consequently, a reduced availability of this endogenous antioxidant during the early phase of myocardial reperfusion, negatively impacts the cellular repair process, facilitating ROS-mediated myocytic damage [29] [Figure 1]. However, ROS can also be protective as signal preconditioning defense and induce stress responses that guide it to survival [30]. It is possible that cells are largely protected by antioxidants while preconditioning and adaptation signals are prevalent in tissues next to the most ischemic portion. The latter may be injured by addition of antioxidants which hindering in adaptive natural cardio-protection [30]. Thus, the correct balance between ROS production and antagonization is essential for the maintenance of cellular homeostasis.
Figure 1.
The pathophysiology of IRI. Abbreviations. STEMI: ST-elevation myocardial infarction, pPCI: primary PCI, NO: nitric oxide, ROS: Reactive Oxygen Species, and GSH: glutathione.
3. Antioxidant Agents in IRI Protection: The Unfinished Controversy
To date, the use of exogenous antioxidant therapy in IRI is still debated. Despite encouraging results obtained from animal models, these benefits have not been fully reproduced in the clinical scenario, leaving uncertainties about its implementation in clinical practice [31]. The reason of inconclusive data probably arises from differences in the use of the various antioxidants (often alone), dissimilarities of drug dosage, time and method of administration between studies [32]. Indeed, for antioxidants to be successful, the agent must be delivered in an appropriate time window (as soon as possible), be able to enter the target tissue and cells, and directed to the sensitive intracellular compartment (e.g., mitochondria and/or lysosomes) [30]. Obviously having all three of these faculties at the same time is very difficult. This would explain the reduced success rate of antioxidant therapy in the everyday medical practice. Antioxidants are classified as hydrophilic, such as ascorbic acid (vitamin C) with ROS scavenger activity, and hydrophobic, such as vitamin E. The latter, as a membrane-bound product, protects against lipid peroxidation [33]. Ascorbate scavenging is a dose-dependent phenomenon and requires intravenous administration to react with superoxide anion radicals, as its plasma concentration is strictly regulated by dose-related excretion [32]. Its administration before elective coronary angioplasty was associated with a reduction of oxidative stress and an improvement in reperfusion parameters [34]. However, the evaluation of clinical outcomes in the setting of IRI remain inconclusive [32]. Similarly, observational and epidemiological data regarding vitamin E in reducing myocardial reperfusion injury are contradictory. Furthermore, its adequate administration on top of the oxidative burden can be beneficial [35], although high doses (>400 IU/d) have been related to an excess of all-cause mortality [36]. The results of N-Acetylcysteine (NAC) administration for cardio-protection are similarly inconclusive [37]. Its acts mainly as GSH-donor. Some studies in the setting of pPCI highlighted a decrease in oxidative stress, with no differences in infarct size [37,38]. A better preservation of the left ventricular function and a decrease in biomarkers of oxidative stress were found when NAC infusion was associated with nitroglycerine and streptokinase [39]. Side effects related to intravenous administration are not to be underestimated, especially after initial loading. Indeed, serious adverse effects, such as anaphylactoid reactions, despite uncommon, were found in up to 8.2% of patients [40]. In addition, iron-chelators, such as deferoxamine, have been tested before and after angioplasty in patients with AMI but were ineffective in determining significant differences in scar extension vs controls [41]. Even preconditioning, postconditioning and remote ischemic conditioning (RIC) studies gave inconclusive results [11,42]. Indeed, the unpredictability of STEMI makes the use of preconditioning difficult in this context, although it may play a role in planned cardiac surgery [43,44]. Whether RIC can actually improve clinical outcomes following pPCI is currently unknown, despite initial promising results before hospital admission in patients with AMI [45]. Therefore, in spite of at least four decades of research, results concerning the use of antioxidant as well as pre/post conditioning therapeutic strategies in limiting IRI are still elusive.
4. Antioxidant Properties of GSH
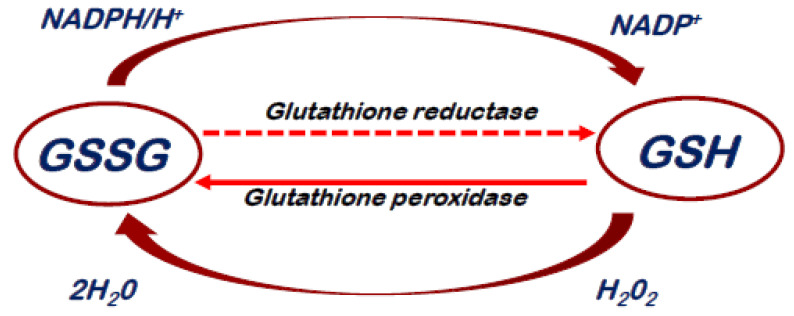
GSH is a water-soluble tripeptide. Its chemical structure is composed by a combination of three single amino acids: cysteine, glycine and glutamine [28]. GSH is present in all mammalian cells and plays a role in transport of amino acids, maintenance of the sulfhydryl groups of proteins and protection against oxidizing agents. Its antioxidant power is linked to the sulfur chemical group, a potent reducing agent [28]. GSH is the principal intracellular antioxidant that acts directly by scavenging reactive oxygen and nitrogen species or indirectly by supporting enzymatic activity as a cofactor [46,47]. Glutathione S-transferases and peroxidases catalyze the conjugation of the reduced form of GSH to xenobiotic substrates for the purpose of detoxification [28]. The isoenzymes are cytosolic, mitochondrial and microsomal. The oxidized state (GSSG) could be chemically reverted through the isoenzyme glutathione reductase and NADPH [Figure 2]. An increased GSSG-to-reduced GSH ratio is indicative of oxidative stress [28]. The total cellular GSH content and the GSH/GSSG ratio are controlled by a GSH-negative feedback loop, in response to fluctuating oxidative stress levels [48]. The intracellular and extracellular GSH concentrations are regulated by the balance between its synthesis and catabolism, as well as by its transport between the cytosol and the different organelles or the extracellular area [47]. Of note, GSH could pass through the innermost layer of the mitochondrial membrane and accumulate into the endoplasmic reticulum [49,50]. Much of GSH synthetized in cells is exported across the plasma membrane into the extracellular spaces, especially during oxidative conditions [51]. The synthesis of GSH from glutamate, cysteine, and glycine is catalyzed by two enzymes, those being γ-glutamylcysteine synthetase (GCS) and GSH synthetase [52]. This process is regulated primarily by GCS activity, cysteine availability and GSH feedback inhibition. This pathway occurs in virtually all cell types, with the liver being the major producer and exporter of GSH [52]. Both animal and human studies demonstrated that adequate protein nutrition was determined for the maintenance of GSH homeostasis. Most of acute events that lead to oxidative and/or inflammatory burst increase GCS transcription or activity in a variety of cells [52].
Figure 2.
Glutathione Redox cycle. Abbreviations. GSSG: Oxidized Glutathione, GSH: Reduced Glutathione.
5. Biomolecular Mechanisms of GSH Protection against IRI: From Experimental Studies to Clinical Practice
In acute coronary syndrome ST-elevation myocardial infarction (ACS-STEMI), ROS-mediated myocytic cell damage starts in the first minutes of acute reoxygenation (secondary to reperfusion) and lasts for weeks or months through the activation of apoptosis and autophagy processes [53,54]. Among ROS, Hydrogen Peroxide (H2O2) is produced by many enzymes including xanthine oxidase, lipoxygenase and, specifically, NADPH oxidase [55]. H2O2 plays an important role in the pathogenesis of myocardial reperfusion injury. Indeed, exposure of cultured human cardiomyocytes to H2O2 has been shown to determine the rapid onset and progressive oxidative cell death [56]. The antioxidant capacity of GSH is related to its ability to donate hydrogen atoms from its thiol group to most carbon-, oxygen-, and nitrogen-centered radicals [57]. H2O2 is removed by GSH, a reaction catalyzed by GSH peroxidase, a selenium-dependent enzyme [58]. GSH is then regenerated from glutathione disulfide (GSSG) by GSH reductase, a reaction using NADPH as a cofactor. This redox cycle is important for the cell to combat oxidant stress. Furthermore, GSH is able to limit damage to the lipid membrane by inhibiting lipid peroxidation [59] and can be effective against some highly reactive species such as hydroxyl radical [60,61] and singlet oxygen [54]. As already mentioned, a reduction of the myocardial content of GSH has been observed during ischemia and reperfusion of the ischemic myocardium [62] and it is associated with future cardiac events [63]. This results in a reduction in cellular defenses against the heightened oxidative burden. Animal studies [62] have shown that an increase in myocardial GSH through GSH supplementation is beneficial in protecting against reperfusion injury. They observed a significantly smaller infarct size compared to animals with lower myocardial GSH [62]. Preclinical study of ischemia/reperfusion showed that timely application of GSH provides better cardio-protection at higher doses [64]. Tanzilli et al. demonstrated, within the context of a randomized clinical trial [29,65], that an early (immediately before pPCI) and prolonged (up to 72 h after angioplasty) administration of glutathione sodium salt in patients with STEMI was able to significantly reduce H2O2 production and decrease isoprostanes serum levels. GSH infusion also promoted early and sustained increase of serum H2O2 breakdown activity (HBA) and NO bioavailability [65], which was highly related to progressive significant reduction of serological markers of myocardial injury. In addition, the authors showed a progressive significant decrease of serum cardiac Troponin T release during the 5 days of reperfusion in the GSH-treated patients compared with the control group, resulting in a 21% reduction of myocardial damage [65]. Specifically, prophylactic and prolonged GSH infusion was able to reduce NADPH oxidase 2 activation and modulate inflammatory effector cell response, as assessed by changes of differential leukocytes count and TNF-α production over the first 5 days after STEMI [29]. In treated patients, the balance of innate and adaptive immune response determined an improvement of left ventricular function at follow-up [29] and a significant decrease in the hospital length of stay [66]. Based on these results, it could be hypothesized that direct GSH infusion, through both activation of endogenous antioxidant protection mechanisms and attenuation of inflammatory cell recruitment, was able to preserve vital myocardial components and endothelial cell function against a harmful pro-oxidant and inflammatory environment [29].
6. Future Perspectives
A targeted antioxidant therapy that does not interfere with other signaling pathways (such as preconditioning ones), and is readily available for use is a medical goal that must be pursued. The easy availability, low cost and the absence of interactions or relevant side effects [67] make GSH a good candidate for its widespread and safe use for the protection of IRI. Moreover, GSH supplementation during STEMI does not exclude the use other antioxidants such as ascorbic acid that demonstrated beneficial effects in contrast-induced nephropathy [68]. Indeed, acute renal failure following STEMI results from oxidative stress, vasoconstriction and hypoperfusion due to left ventricular dysfunction [69]. Therefore, the scavenging action of the ascorbic acid, clearly demonstrated in contrast-induced nephropathy [68], could be enhanced by an increase in NO bioavailability resulting from the GSH supplementation [65,70]. Based on previous results [29,34,65], it is plausible that a combination of the two molecules applying the same modalities of administration (such as early and prolonged administration over time) may be effective. Future studies, adequately addressing this purpose, will be able to give us an answer to this hypothesis.
7. Conclusions
The ischemic/reperfusion myocardial injury negatively impacts on STEMI outcomes after successful pPCI. The oxidative burst [71] determined by an increased ROS production and reduced GSH availability act as the main players of this phenomenon [9,29,65]. The scavenging activity of GSH maintains thiol groups of enzymes and other proteins in their reduced state, thus preventing cell membrane lipid peroxidation and limiting cardiomyocytes loss [72]. The effects of GSH supplementation are promising in IRI setting, not only based on laboratory findings, such as the observed reduction of the oxidative stress burden [65], but also on clinical ones, such as the decrease in length of hospital stay [66] and the reduced incidence of the adverse left ventricle remodeling at follow-up [29]. Administration in combination with other antioxidants with proven scavenging activity could be hopeful and call for further research on the topic.
Acknowledgments
The authors thank Nicola Bier for the graphic support to the figures’ elaboration.
Author Contributions
Conceptualization, F.B., A.A. and E.M.; writing—original draft preparation, A.A.; writing—review and editing A.A., F.B., R.C., M.S., G.P., G.T., F.R.P. and E.M.; figures: A.A., F.B. and F.R.P.; supervision, F.B., F.R.P. and E.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rentrop K.P., Blanke H., Karsch K.R., Wiegand V., Köstering H., Oster H., Leitz K. Acute myocardial infarction: Intracoronary application of nitroglycerin and streptokinase. Clin. Cardiol. 1979;2:354–363. doi: 10.1002/clc.4960020507. [DOI] [PubMed] [Google Scholar]
- 2.Andersen H.R., Nielsen T.T., Rasmussen K., Thuesen L., Kelbaek H., Thayssen P., Abildgaard U., Pedersen F., Madsen J.K., Grande P., et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N. Engl. J. Med. 2003;349:733–742. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 3.Widimský P., Budesínský T., Vorác D., Groch L., Želízko M., Aschermann M., Branny M., Št’ásek J., Formánek P., On Behalf of the ‘PRAGUE’ Study Group Investigators Long distance transport for primary angioplasty vs immediate thrombolysis in acute myocardial infarction. Final results of the randomized national multicentre trial–PRAGUE-2. Eur. Heart J. 2003;24:94–104. doi: 10.1016/S0195-668X(02)00468-2. [DOI] [PubMed] [Google Scholar]
- 4.Keeley E.C., Boura J.A., Grines C.L. Primary angioplasty versus intravenous thrombolytic therapy for acute myocardial infarction: A quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 5.Hausenloy D.J., Yellon D.M. Time to take myocardial reperfusion injury seriously. N. Engl. J. Med. 2008;359:518–520. doi: 10.1056/NEJMe0803746. [DOI] [PubMed] [Google Scholar]
- 6.Ibanez B., James S., Agewall S., Antunes M.J., Bucciarelli-Ducci C., Bueno H., Caforio A.L.P., Crea F., Goudevenos J.A., ESC Scientific Document Group et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC) Eur. Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 7.Ekeløf S., Jensen S.E., Rosenberg J., Gögenur I. Reduced oxidative stress in STEMI patients treated by primary percutaneous coronary intervention and with antioxidant therapy: A systematic review. Cardiovasc. Drugs Ther. 2014;28:173–181. doi: 10.1007/s10557-014-6511-3. [DOI] [PubMed] [Google Scholar]
- 8.Yellon D.M., Hausenloy D.J. Myocardial reperfusion injury. N. Engl. J. Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667. [DOI] [PubMed] [Google Scholar]
- 9.Rout A., Tantry U.S., Novakovic M., Sukhi A., Gurbel P.A. Targeted pharmacotherapy for ischemia reperfusion injury in acute myocardial infarction. Expert Opin. Pharmacother. 2020;21:1851–1865. doi: 10.1080/14656566.2020.1787987. [DOI] [PubMed] [Google Scholar]
- 10.Benjamin E.J., Muntner P., Alonso A., Bittencourt M.S., Callaway C.W., Carson A.P., Chamberlain A.M., Chang A.R., Cheng S., Das S.R., et al. American heart association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. heart disease and stroke statistics-2019 update: A report from the American heart association. Circulation. 2019;139:e56–e528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- 11.Hausenloy D., Yellon D. Myocardial ischemia-reperfusion injury: A neglected therapeutic target. J. Clin. Investig. 2013;123:92–100. doi: 10.1172/JCI62874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausenloy D.J., Barrabes J.A., Botker H.E., Davidson S.M., Di Lisa F., Downey J., Engstrom T., Ferdinandy P., Carbrera-Fuentes H.A., Heusch G., et al. Ischaemic conditioning and targeting reperfusion injury: A 30 year voyage of discovery. Basic Res. Cardiol. 2016;111:170. doi: 10.1007/s00395-016-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu M., Yiang G., Liao W., Tsai A.P., Cheng Y., Cheng P., Li C.Y., Li C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018;46:1650–1667. doi: 10.1159/000489241. [DOI] [PubMed] [Google Scholar]
- 14.Ma S., Wang Y., Chen Y., Cao F. The role of the autophagy in myocardial ischemia/reperfusion injury. Biochim. Biophys. Acta. 2015;1852:271–276. doi: 10.1016/j.bbadis.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Kalogeris T., Bao Y., Korthuis R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs. preconditioning. Redox. Biol. 2014;2:702–714. doi: 10.1016/j.redox.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swirski F.K., Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yan X., Anzai A., Katsumata Y., Matsuhashi T., Ito K., Fukuda K., Sano M. Temporal dynamics of cardiac immune cell accumulation following acute myocardial infarction. J. Mol. Cell. Cardiol. 2013;62:24–35. doi: 10.1016/j.yjmcc.2013.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Dentali F., Nigro O., Squizzato A., Gianni M., Zuretti F., Grandi A.M., Guasti L. Impact of neutrophils to lymphocytes ratio on major clinical outcomes in patients with acute coronary syndromes: A systematic review and meta-analysis of the literature. Int. J. Cardiol. 2018;266:31–37. doi: 10.1016/j.ijcard.2018.02.116. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S., Diao J., Qi C., Jin J., Li L., Gao X., Gong L., Wu W. Predictive value of neutrophil to lymphocyte ratio in patients with acute ST segment elevation myocardial infarction after percutaneous coronary intervention: A meta-analysis. BMC Cardiovasc. Disord. 2018;18:75. doi: 10.1186/s12872-018-0812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tourki B., Halade G. Leukocyte diversity in resolving and nonresolving mechanisms of cardiac remodeling. FASEB J. 2017;31:4226–4239. doi: 10.1096/fj.201700109R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vinten-Johansen J. Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc. Res. 2004;61:481–497. doi: 10.1016/j.cardiores.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 22.Duilio C., Ambrosio G., Kuppusamy P., DiPaula A., Becker L.C., Zweier J.L. Neutrophils are primary source of 02 radicals during reperfusion after prolonged myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2649–H2657. doi: 10.1152/ajpheart.2001.280.6.H2649. [DOI] [PubMed] [Google Scholar]
- 23.Leoni G., Soehnlein O. (Re) Solving Repair After Myocardial Infarction. Front. Pharmacol. 2018;9:1342. doi: 10.3389/fphar.2018.01342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hofmann U., Beyersdorf N., Weirather J., Podolskaya A., Bauersachs J., Ertl G., Kerkau T., Frantz S. Activation of CD4+ T lymphocytes improves wound healing and survival after experimental myocardial infarction in mice. Circulation. 2012;125:1652–1663. doi: 10.1161/CIRCULATIONAHA.111.044164. [DOI] [PubMed] [Google Scholar]
- 25.Mak T.W., Grusdat M., Duncan G.S., Dostert C., Nonnenmacher Y., Cox M., Binsfeld C., Hao Z., Brüstle A., Itsumi M., et al. Glutathione Primes T Cell Metabolism for Inflammation. Immunity. 2017;46:675–689. doi: 10.1016/j.immuni.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 26.Frauwirth K.A., Riley J.L., Harris M.H., Parry R.V., Rathmell J.C., Plas D.R., Elstrom R.L., June C.H., Thompson C.B. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/S1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 27.Pollizzi K.N., Powell J.D. Integrating canonical and metabolicsignalling programmes in the regulation of T cell responses. Nat. Rev. Immunol. 2014;14:435–446. doi: 10.1038/nri3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Truscelli G., Tanzilli G., Viceconte N., Dominici M., Arrivi A., Sommariva L., Granatelli A., Gaudio C., Mangieri E. Glutathione sodium salt as a novel adjunctive treatment for acute myocardial infarction. Med. Hypotheses. 2017;102:48–50. doi: 10.1016/j.mehy.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 29.Tanzilli G., Arrivi A., Placanica A., Viceconte N., Cammisotto V., Nocella C., Barillà F., Torromeo C., Pucci G., Acconcia M.C., et al. Glutathione Infusion Before and 3 Days After Primary Angioplasty Blunts Ongoing NOX2-Mediated Inflammatory Response. J. Am. Heart Assoc. 2021;10:e020560. doi: 10.1161/JAHA.120.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Becker L.B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 31.González-Montero J., Brito R., Gajardo A.I., Rodrigo R. Myocardial reperfusion injury and oxidative stress: Therapeutic opportunities. World J. Cardiol. 2018;10:74–86. doi: 10.4330/wjc.v10.i9.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodrigo R., Prieto J.C., Aguayo R., Ramos C., Puentes Á., Gajardo A., Panieri E., Rojas-Solé C., Lillo-Moya J., Saso L. Joint Cardioprotective Effect of Vitamin C and Other Antioxidants against Reperfusion Injury in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Molecules. 2021;26:5702. doi: 10.3390/molecules26185702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Naito H., Nojima T., Fujisaki N., Tsukahara K., Yamamoto H., Yamada T., Aokage T., Yumoto T., Osako T., Nakao A. Therapeutic strategies for ischemia reperfusion injury in emergency medicine. Acute Med. Surg. 2020;7:e501. doi: 10.1002/ams2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Basili S., Tanzilli G., Mangieri E., Raparelli V., Di Santo S., Pignatelli P., Violi F. Intravenous ascorbic acid infusion improves myocardial perfusion grade during elective percutaneous coronary intervention: Relationship with oxidative stress markers. JACC Cardiovasc. Interv. 2010;3:221–229. doi: 10.1016/j.jcin.2009.10.025. [DOI] [PubMed] [Google Scholar]
- 35.Niki E. Evidence for beneficial effects of vitamin E. Korean J. Intern. Med. 2015;30:571–579. doi: 10.3904/kjim.2015.30.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller E.R., 3rd, Pastor-Barriuso R., Dalal D., Riemersma R.A., Appel L.J., Guallar E. Meta-analysis: High-dosage vitamin E supplementation may increase all-cause mortality. Ann. Intern. Med. 2005;142:37–46. doi: 10.7326/0003-4819-142-1-200501040-00110. [DOI] [PubMed] [Google Scholar]
- 37.Winterbourn C.C., Metodiewa D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 1999;27:322–328. doi: 10.1016/S0891-5849(99)00051-9. [DOI] [PubMed] [Google Scholar]
- 38.Thiele H., Hildebrand L., Schirdewahn C., Eitel I., Adams V., Fuernau G., Erbs S., Linke A., Diederich K.W., Nowak M., et al. Impact of high-dose N-acetylcysteine versus placebo on contrast-induced nephropathy and myocardial reperfusion injury in unselected patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. The LIPSIA-N-ACC (Prospective, Single-Blind, Placebo-Controlled, Randomized Leipzig Immediate PercutaneouS Coronary Intervention Acute Myocardial Infarction N-ACC) Trial. J. Am. Coll. Cardiol. 2010;55:2201–2209. doi: 10.1016/j.jacc.2009.08.091. [DOI] [PubMed] [Google Scholar]
- 39.Arstall M.A., Yang J., Stafford I., Betts W.H., Horowitz J.D. N-Acetylcysteine in Combination with Nitroglycerin and Streptokinase for the Treatment of Evolving Acute Myocardial Infarction. Circulation. 1995;92:2855–2862. doi: 10.1161/01.CIR.92.10.2855. [DOI] [PubMed] [Google Scholar]
- 40.Tenório M.C.D.S., Graciliano N.G., Moura F.A., Oliveira A.C.M., Goulart M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants. 2021;10:967. doi: 10.3390/antiox10060967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chan W., Taylor A.J., Ellims A.H., Lefkovits L., Wong C., Kingwell B.A., Natoli A., Croft K.D., Mori T., Kaye D.M., et al. Effect of iron chelation on myocardial infarct size and oxidative stress in ST-elevation-myocardial infarction. Circ. Cardiovasc. Interv. 2012;5:270–278. doi: 10.1161/CIRCINTERVENTIONS.111.966226. [DOI] [PubMed] [Google Scholar]
- 42.Ferrari R., Balla C., Malagù M., Guardigli G., Morciano G., Bertini M., Biscaglia S., Campo G. Reperfusion Damage—A Story of Success, Failure, and Hope. Circ. J. 2017;81:131–141. doi: 10.1253/circj.CJ-16-1124. [DOI] [PubMed] [Google Scholar]
- 43.Kloner R.A. Current state of clinical translation of cardioprotective agents for acute myocardial infarction. Circ. Res. 2013;113:451–463. doi: 10.1161/CIRCRESAHA.112.300627. [DOI] [PubMed] [Google Scholar]
- 44.Yellon D.M., Opie L.H. Postconditioning for protection of the infarcting heart. Lancet. 2006;367:456–458. doi: 10.1016/S0140-6736(06)68157-9. [DOI] [PubMed] [Google Scholar]
- 45.Botker H.E., Kharbanda R., Schmidt M.R., Bøttcher M., Kaltoft A.K., Terkelsen C.J., Munk K., Andersen N.H., Hansen T.M., Trautner S., et al. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: A randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 46.Sies H. Glutathione and its role in cellular functions. Free Radic. Biol. Med. 1999;27:916–921. doi: 10.1016/S0891-5849(99)00177-X. [DOI] [PubMed] [Google Scholar]
- 47.Gaucher C., Boudier A., Bonetti J., Clarot I., Leroy P., Parent M. Glutathione: Antioxidant properties dedicated to nanotechnologies. Antioxidants. 2018;7:62. doi: 10.3390/antiox7050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bajic V.P., Van Neste C., Obradovic M., Zafirovic S., Radak D., Bajic V.B., Essack M., Isenovic E.R. Glutathione “Redox Homeostasis” and Its Relation to Cardiovascular Disease. Oxid. Med. Cell. Longev. 2019;2019:5028181. doi: 10.1155/2019/5028181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lash L.H. Mitochondrial glutathione transport: Physiological, pathological and toxicological implications. Chem. Biol. Interact. 2006;163:54–67. doi: 10.1016/j.cbi.2006.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bánhegyi G., Csala M., Nagy G., Sorrentino V., Fulceri R., Benedetti A. Evidence for the transport of glutathione through ryanodine receptor channel type 1. Biochem. J. 2003;376:807–812. doi: 10.1042/bj20031419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belcastro E., Wu W., Fries-Raeth I., Corti A., Pompella A., Leroy P., Lartaud I., Gaucher C. Oxidative stress enhances and modulates protein S-nitrosation in smooth muscle cells exposed to S-nitrosoglutathione. Nitric Oxide Biol. Chem. 2017;69:10–21. doi: 10.1016/j.niox.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 52.Wu G., Fang Y.Z., Yang S., Lupton J.R., Turner N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004;134:489–492. doi: 10.1093/jn/134.3.489. [DOI] [PubMed] [Google Scholar]
- 53.McCully J.D., Wakiyama H., Hsieh Y.J., Jones M., Levitsky S. Differential contribution of necrosis and apoptosis in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H1923–H1935. doi: 10.1152/ajpheart.00935.2003. [DOI] [PubMed] [Google Scholar]
- 54.Dong Y., Undyala V.V., Gottlieb R.A., Mentzer R.M., Jr., Przyklenk K. Autophagy: Definition, molecular machinery, and potential role in myocardial ischemia reperfusion injury. J. Cardiovasc. Pharmacol. Ther. 2010;15:220–230. doi: 10.1177/1074248410370327. [DOI] [PubMed] [Google Scholar]
- 55.Byon C.H., Heath J.M., Chen Y. Redox signaling in cardiovascular pathophysiology: A focus on hydrogen peroxide and vascular smooth muscle cells. Redox. Biol. 2016;9:244–253. doi: 10.1016/j.redox.2016.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tanimoto T., Parseghian M.H., Nakahara T., Kawai H., Narula N., Kim D., Nishimura R., Weisbart R.H., Chan G., Richieri R.A., et al. Cardioprotective Effects of HSP72 Administration on Ischemia-Reperfusion Injury. J. Am. Coll. Cardiol. 2017;70:1479–1492. doi: 10.1016/j.jacc.2017.07.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kosower E.M. Chemical properties of glutathione. In: Arias I.M., Jakoby W.B., editors. Glutathione: Metabolism and Function. Raven Press; New York, NY, USA: 1976. pp. 1–15. [Google Scholar]
- 58.Flohe L., Gunzler W.A. Glutathione-dependent enzymatic oxidoreduction reactions. In: Arias I.M., Jakoby W.B., editors. Glutathione: Metabolism and Function. Raven Press; New York, NY, USA: 1976. pp. 17–34. [Google Scholar]
- 59.Weiss S.J. Oxygen, ischemia and inflammation. Acta Physiol. Scand. Suppl. 1986;548:9–37. [PubMed] [Google Scholar]
- 60.Meister A. New aspects of glutathione biochemistry and transport: Selective alteration of glutathione metabolism. Fed. Proc. 1984;43:3031–3042. [PubMed] [Google Scholar]
- 61.Bernard G.R., Lucht W.D., Niedermeyer M.E., Snapper J.R., Ogletree M.L., Brigham K.L. Effect of N-acetylcysteine on pulmonary response to endotoxin in the awake sheep and upon in vitro granulocyte function. J. Clin. Investig. 1984;73:1772–1784. doi: 10.1172/JCI111386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Singh A., Lee K.J., Lee C.Y., Goldfarb R.D., Tsan M.F. Relation between myocardialmglutathione content and extent of ischemia-reperfusion injury. Circulation. 1989;80:1795–1804. doi: 10.1161/01.CIR.80.6.1795. [DOI] [PubMed] [Google Scholar]
- 63.De Chiara B., Mafrici A., Campolo J., Famoso G., Sedda V., Parolini M., Cighetti G., Lualdi A., Fiorentini C., Parodi O. Low plasma glutathione levels after reperfused acute myocardial infarction are associated with late cardiac events. Coron. Artery Dis. 2007;18:77–82. doi: 10.1097/01.mca.0000236294.32672.26. [DOI] [PubMed] [Google Scholar]
- 64.Hinkel R., Boekstegers P., Kupatt C. Adjuvant early and late cardioprotective therapy: Access to the heart. Cardiovasc. Res. 2012;94:226–236. doi: 10.1093/cvr/cvs075. [DOI] [PubMed] [Google Scholar]
- 65.Tanzilli G., Truscelli G., Arrivi A., Carnevale R., Placanica A., Viceconte N., Raparelli V., Mele R., Cammisotto V., Nocella C., et al. Glutathione infusion before primary percutaneous coronary intervention: A randomised controlled pilot study. BMJ Open. 2019;9:e025884. doi: 10.1136/bmjopen-2018-025884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arrivi A., Pucci G., Placanica A., Bier N., Sordi M., Dominici M., Carnevale R., Tanzilli G., Mangieri E. Early and prolonged glutathione infusion favourably impacts length of hospital stay in ST-elevation myocardial infarction patients: A sub-analysis of the GSH2014 trial. Minerva Cardiol. Angiol. 2022. ahead of print . [DOI] [PubMed]
- 67.TAD 600 mg/4 mL Polvere e Solvente per Soluzione Iniettabile. [(accessed on 10 November 2022)]. Available online: https://mediately.co/it/drugs/AL8f4kqgmJkMiohLCPJpZ0cangy/tad-600-mg-4-ml-polvere-e-solvente-per-soluzione-iniettabile.
- 68.Sadat U., Usman A., Gillard J.H., Boyle J.R. Does ascorbic acid protect against contrast-induced acute kidney injury in patients undergoing coronary angiography: A systematic review with meta-analysis of randomized, controlled trials. J. Am. Coll. Cardiol. 2013;62:2167–2175. doi: 10.1016/j.jacc.2013.07.065. [DOI] [PubMed] [Google Scholar]
- 69.Schmucker J., Fach A., Becker M., Seide S., Bünger S., Zabrocki R., Fiehn E., Würmann-Busch B., Pohlabeln H., Günther K., et al. Predictors of acute kidney injury in patients admitted with ST-elevation myocardial infarction—results from the Bremen STEMI-Registry. Eur. Heart J. Acute Cardiovasc. Care. 2018;7:710–722. doi: 10.1177/2048872617708975. [DOI] [PubMed] [Google Scholar]
- 70.Arrivi A., Pucci G., Dominici M., Mangieri E., Tanzilli G. Contrast-induced acute kidney injury and nitric oxide depletion in patients undergoing primary percutaneous coronary intervention: Insights from GSH 2014 trial. J. Cardiovasc. Med. 2021;22:788–789. doi: 10.2459/JCM.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 71.Calvieri C., Tanzilli G., Bartimoccia S., Cangemi R., Arrivi A., Dominici M., Cammisotto V., Viceconte N., Mangieri E., Frati G., et al. Interplay between Oxidative Stress and Platelet Activation in Coronary Thrombus of STEMI Patients. Antioxidants. 2018;7:83. doi: 10.3390/antiox7070083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J. Biol. Chem. 1994;269:9397–9400. doi: 10.1016/S0021-9258(17)36891-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.