Abstract
The endothelium is a monolayer of cells lining the inner blood vessels. Endothelial cells (ECs) play indispensable roles in angiogenesis, homeostasis, and immune response under normal physiological conditions, and their dysfunction is closely associated with pathologies such as cardiovascular diseases. Abnormal EC metabolism, especially dysfunctional fatty acid (FA) metabolism, contributes to the development of many diseases including pulmonary hypertension (PH). In this review, we focus on discussing the latest advances in FA metabolism in ECs under normal and pathological conditions with an emphasis on PH. We also highlight areas of research that warrant further investigation.
Keywords: endothelium, vascular biology, lipid, glycolysis
1. Introduction
Endothelial cell (EC) metabolism has gained remarkable attention in the last few years. Accumulating evidence on glycolysis and fatty acid oxidation (FAO) in ECs has revealed that metabolism abnormalities contribute to many pathological problems including cancers and cardiovascular diseases [1,2,3,4]. In this review, we focus on discussing the latest advances in fatty acid metabolism in ECs under normal and pathological conditions such as pulmonary hypertension (PH).
2. Role of Endothelial Cells in Angiogenesis, Homeostasis, Stimulus/Immune Response
ECs lining the inner blood vessels are essential for the vascular system [5,6]. In adults, ECs remain quiescent for years but still retain the capacity to switch rapidly from quiescent cells to activated sprout cells to initiate new vessel formation (angiogenesis) under certain conditions such as inflammation or injury [7]. This tightly regulated process starts with the migratory tip cells and is followed by proliferating stalk cells until new vessel sprouts are created. The quiescent phalanx cells stimulated by angiogenic signals such as vascular endothelial growth factor (VEGF) then proceed to line the established vessels to form the matured blood vessels [8].
ECs occupy an important location between circulating blood and tissues, which enables its quick response to changes in the environment via releasing a host of biologically active substances including relaxing factors prostaglandin I2 (PGI2), endothelium-derived relaxing factor (EDRF), nitric oxide (NO), and endothelium-derived hyperpolarizing factor (EDHF); epoxyeicosatrienoic acids (EET); and contracting factors arachidonic acid metabolites, endothelin-1 (ET-1) and angiotensin II (Ang II) [9]. These endothelium-derived factors are maintained in a balance to control blood flow and pressure and maintain the antithrombotic and anticoagulant balance in the bloodstream, which is extremely important for homeostasis [9].
Under hormonal and chemical signal stimulation, ECs produce mediators such as nitric oxide synthase (NOS) and phospholipase A2 to modify the responses of numerous cells, including vascular smooth muscle, platelets, and leukocytes [10,11]. For instance, NO synthesized from endothelial NOS (eNOS) directly regulates blood vessel dilation by stimulating soluble guanylyl cyclase, leading to an increasing level of cyclic guanosine monophosphate (cGMP) and subsequent relaxation of vascular smooth muscle, which plays essential roles in maintaining vascular homeostasis [3,10,12].
ECs act as a natural barrier inside blood vessels and are the first to recognize microbial components in the circulation, which indicates that ECs’ recognition and response may be essential to early innate immune system activation [13]. In addition, ECs express several innate immune receptors including the Toll-like receptor (TLR), NOD-like receptors (NLR), and chemokine receptors. For instance, in response to microbial stimulation, ECs could secrete pro-inflammatory cytokine interleukin-8 (IL-8) via NOD1-dependent signaling [14].
3. Endothelial Cell Metabolism
EC metabolism is confined to glucose, FAs, and amino acids (AAs), the three major substrates for adenosine triphosphate (ATP) production and biomass production in ECs, which have been widely studied and summarized [1,2,15]. The following sections will discuss the role of EC metabolism (predominately focusing on fatty acid metabolism) in maintaining endothelial functions and disease pathogenesis, with a focus on pulmonary hypertension (PH).
3.1. Endothelial Cell Glucose and Amino Acid Metabolism
Glycolysis is the main energy resource in cultured ECs, with higher rates of glycolysis and glucose consumption [16]. ECs prefer to utilize glycolysis instead of oxidative metabolism because the ECs need to maintain reactive oxygen species (ROS) levels in control [17] and enhance the diffusion of oxygen to perivascular cells [17,18]. Additional reasons are that glycolysis produces faster kinetic ATP under pro-angiogenic signals during angiogenesis [16], which is essential for ECs’ rapid proliferation and migration [16,19]. In addition to glucose-derived carbons, ECs also utilize glutamine to sustain proliferation and vascular expansion [20,21]. Glutamine, the most abundant circulating nonessential amino acid (NEAA), can supply 30% of the tricarboxylic acid (TCA) carbons, comparable to glycolysis and FAO-derived carbon [22]. Depletion of either glutamine or arginine makes ECs vulnerable to ROS-induced damage during proliferation and migration [21].
3.2. Endothelial Cell Fatty Acids (FAs) Metabolism
FA metabolism involves multiple processes including FA uptake and storage, FA transport, FA oxidation, and FA synthesis. FA metabolism is vitally important to sustain the function of organs such as the heart, skeletal muscle, and adipose tissue [15]. Heart and skeletal muscles utilize FAs as their top source for ATP production and therefore require an efficient supply system of FAs [23]. Uptake and transport of FAs by ECs are extremely important to numerous cellular processes, including membrane synthesis, intracellular signal transduction, ATP generation, protein posttranslational modifications, and metabolic gene transcriptional regulation in these high energy-demand organs [24]. This process requires the transport of FA across the EC barrier into the perivascular cells [25] (Figure 1). In addition, ECs can metabolize and synthesize FAs for maintaining vascular homeostasis.
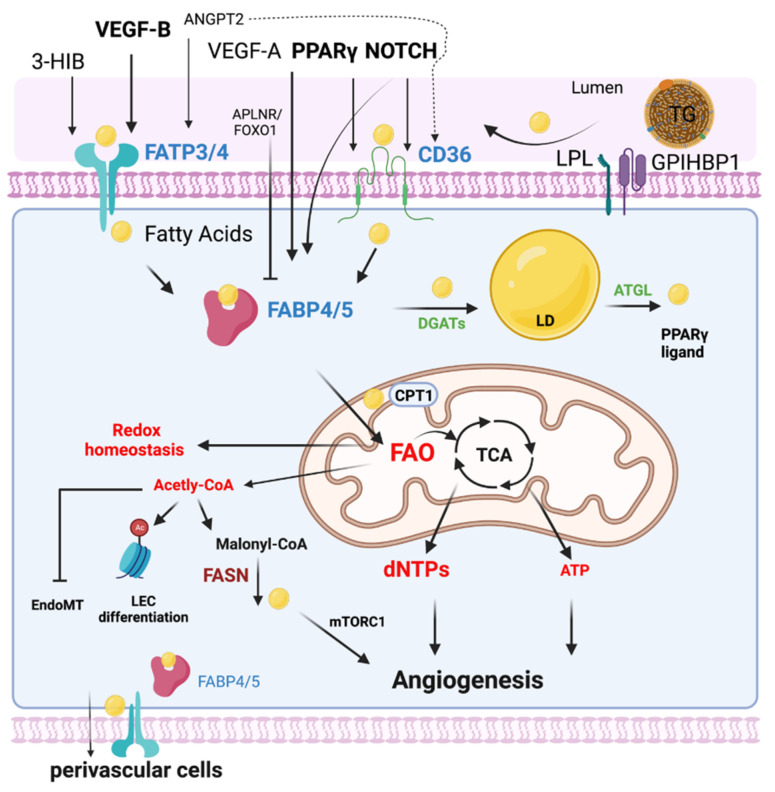
Figure 1.
EC FA metabolism. Fatty acids (FAs) are transported in the form of triglyceride (TG)-rich lipoproteins, which are released by lipoprotein lipase (LPL) and GPIHBP1 in the luminal side of ECs. Free FAs are transported across EC membranes by fatty acid transporter protein 3 and 4 (FATP3/4) and CD36. Intracellular FAs transportation is mediated by FABP3 and FABP4. Intracellular FAs could be synthesized by FA synthase (FASN) from Malonyl-CoA and affect mTORC1 activity for angiogenesis. DAGTs and ATGL are key enzymes regulating lipid droplet (LD) storage and lipolysis. FAs are transported into mitochondria via CPT1 for FA oxidation (FAO). Quiescent ECs utilize FAO to maintain redox homeostasis. FAO is required for the generation of dNTPs for EC spouting and angiogenesis. In certain conditions such as glucose depletion, FAO produces ATP in ECs. FAO also produces acetyl-CoA for epigenetic regulation, inhibiting EndoMT. The efflux of FAs to the perivascular cells could be mediated by FABP4/5 and FATP3/4. Many key endothelial signaling including VEGF, NOTCH, and PPAR γ control EC FA transport via regulating FATPs, FABPs, and CD36.
3.2.1. Endothelial Cell Fatty Acid Uptake and Transport
In mammals, lipids circulate as nonesterified free fatty acids (NEFAs) in the blood including medium-chain (with 6–12 carbons) and short-chain fatty acids (SCFAs) (with 6 carbons), but mostly (≈90%) as esterified FA, including long chain fatty acid (LCFAs) (with 12–20 carbons). The LCFAs are transported in the bloodstream in the form of triglyceride (TG)-rich lipoproteins, phospholipids, and cholesteryl esters in lipoproteins [26], while uptake LCFAs requires transfer from the circulation across the EC barrier, a process coordinated by several membrane proteins including lipoprotein lipase (LPL) and GPIHBP1 [27], CD36 [28], fatty acid transport proteins (FATPs) [29], and fatty acid-binding proteins (FABPs) [30,31,32]. The major fatty acid transporter including CD36, FATPs, and FABPs will be summarized in the following sections (Figure 1).
CD36
CD36 is the best-characterized FA transporter [33]. CD36 is expressed in platelets, monocytes, ECs, and parenchymal cells in white adipose tissue (WAT), brown adipose tissue (BAT), heart muscle, and skeletal muscle [34]. Besides its important biological roles in binding LCFAs and facilitating their uptake into cells [28], CD36 also recognizes lipoproteins, bacterial lipids, and nonlipid ligands (e.g.,thrombospondin-1) [34,35,36]. CD36 plays a very important role in FA import into cells and is involved in a series of cell signaling processes including apoptotic cell uptake, cellular adhesion, and angiogenesis [37,38,39,40]. EC-Cd36–KO mice had reduced uptake of FA in the heart, skeletal muscle, and BAT, suggesting that endothelial CD36 plays a critical role in FA delivery into parenchymal tissues [41]. ECs utilize FAs to accelerate their migration and invasion for new vessel formation following vascular injury in a CD36-dependent manner likely through AMPK signaling [42,43]. It has also been reported that EC-specific knockout of CD36 in mice had promoted glucose clearance compared with controls when fed with multiple diets and female EC CD36/LDLR-deficient mice have reduced atherosclerosis [44]. Other studies showed that intestinal lymphatic endothelial cells (LECs) CD36 regulates lymphatic integrity and optimizes lipid transport. Silencing CD36 in cultured LECs suppresses cell respiration via reducing VEGF-C-mediated VEGFR2/AKT phosphorylation and destabilizing VE–cadherin junctions [45]. Moreover, mice with specific deletion lymphatic Cd36 display slower transport of absorbed lipids, more permeable mesenteric lymphatics, accumulation of inflamed visceral fat, and impaired glucose disposal [45].
FATPs
FATPs are trans-membrane-spanning proteins, which allow and enhance the uptake of LCFA into cells [32]. Compared with other FATPs, FATP3 is specifically expressed in the vasculature in muscular tissues [23], whereas FATP4 is expressed in endothelium and muscle and WAT [23]. Previous studies demonstrated that VEGF-B specifically controlled endothelial uptake of FA via transcriptional regulation of vascular FATP3 and FATP4 in muscle, heart, and BAT through VEGF receptor 1 and neuropilin 1 [23]. Dr. Arany’s group later identified 3-hydroxyisobutyrate (3-HIB), a catabolic intermediate of the branched-chain amino acid (BCAA) valine, promotes trans-endothelial fatty acid transport through FATP3 and FATP4, stimulates muscle fatty acid uptake in vivo and promotes lipid accumulation in muscle, leading to insulin resistance in mice [46]. Another study found that Angpt2 activated integrin α5β1 signaling in the endothelium and triggered fatty acid transport via CD36 and FATP3 into subcutaneous adipose tissue (SAT) [47]. Surprisingly, mitochondrial ATP has been demonstrated to control endothelial FA uptake via endoplasmic reticulum (ER)-FATP4 through its ATP-dependent acyl-CoA synthetase activity [48].
The FABPs
The FABPs belong to a family that consists of at least 13 intracellular FA-handling proteins [32]. The FABPs can directly bind free LCFA and facilitate the delivery of LCFA to different intracellular sites for oxidation. Their expression pattern is tissue-specific [32]. Among the FABPs, FABP4, also known as A-FABP and aP2, is widely studied in ECs compared to other FABPs [49,50]. FABP5, also known as E-FABP and mal1, is found in the ECs of the microvasculature of the placenta, heart, skeletal muscle, small intestine, and lung [30,51]. FABP3 or h-FABP is found in the heart microvessels and aortic endothelial cells [38].
FABP4 and FABP5 share a 55% similarity of amino acid sequence and have a primarily microvascular expression pattern in both microvascular and large blood vessel ECs [51,52]. FABP4 is regulated by VEGF and contributes to EC proliferation, migration, and angiogenesis [50]. FABP4 exhibits a pro-angiogenic role in ECs by modulating the stem cell factor/c-kit pathway [53]. Other studies showed that exogenous FABP4 treatment induced EC dysfunction by impairing insulin-dependent eNOS activation and NO production through suppression of IRS1 and Akt signaling [54]. In addition, human coronary artery endothelial cells (HCAECs) derived FABP4 could increase inflammatory cytokines, and proliferation and adhesion-related molecules to promote human coronary smooth muscle cells proliferation and migration, which reveals the role of FABP4 in neointima formation after vascular injury [55]. Consistent with these studies, FABP4-specific inhibitor, BMS309403, exhibits therapeutic potential in the treatment of EC dysfunction-associated diseases including atherosclerosis and diabetes [56,57]. A recent study also showed that tumor endothelial cells (TECs)-derived FABP4 promoted lipid droplet formation in cancer cells and protected them from oxidative stress-induced ferroptosis [58].
There is a redundant function between FABP4 and FABP5, as Fabp4 and Fabp5 (Fabp4/5 double-knockout (DKO) mice) exhibited a dramatic phenotype related to protection against obesity, insulin resistance, atherosclerosis, and fatty liver disease compared to Fabp4-/- or Fabp5-/- mice [59,60]. Interestingly, even though FABP5 shares a similar expression pattern with FABP4 in microvascular beds of several tissues, and functions the same as FABP4—significantly enhancing EC proliferation, chemotactic migration, and angiogenic sprouting—FABP5 promotes apoptotic EC death under certain conditions, which is opposite from the anti-apoptotic effects of FABP4 [61]. FABP5 has been demonstrated to mediate the uptake of exogenous docosahexaenoic acid (DHA) in brain ECs and contributes to cognitive function [62].
Both FABP4 and FABP5 are co-expressed in capillary ECs across various organs, including the heart, and skeletal muscle. Key endothelial signaling has been shown to control FABP4 and/or 5 expressions for transendothelial FA transport. For example, endothelial PPARγ promotes FA uptake via CD36 and FABP4 [63]. EC Notch signaling induces FABP4/5 and CD36 to facilitate FA transport in the heart [64]. A recent study demonstrated that EC APLN/APLNR signaling suppressed the transcription factor Forkhead box protein O1 (FOXO1) in the endothelium and inhibited endothelial FABP4 expression, which limits the excess FA transport and accumulation in the tissue [65].
3.2.2. Endothelial Fatty Acid Storage and Lipolysis
Lipid droplets are present in most eukaryotic cells including ECs for cytosolic organelles fat storage [66]. Lipid droplets could buffer excess lipids to protect ECs from lipotoxicity and release them later according to cellular consumption [66]. Lipid droplets are strictly regulated under cellular needs and environmental signals, which is tightly balanced by LD biogenesis and lipolysis.
Large blood vessel ECs can form and degrade lipid droplets in response to changing levels of TG, in vivo. Combined with the pharmacological inhibition of DGAT1 and DGAT2, the Sessa group demonstrated that DGATs are essential for TG synthesis in ECs with a greater dependency on DGAT1 than on DGAT2 [67]. Inhibition of DGAT1 but not DGAT2 was sufficient to promote ER stress induced by exogenous oleic acid treatment, suggesting that the capability of EC to form lipid droplets is critical for the prevention of lipotoxicity [67].
Lipolysis is determined by the direct activation of LD-associated lipases, such as adipose triglyceride lipase (ATGL), monoglyceride lipase (MGL), and hormone-sensitive lipase (HSL) [67,68]. They further showed that ATGL inhibition but not HSL or MGL inhibition delayed lipid droplet degradation in ECs [67]. ATGL-deficient mice present severe micro- and macrovascular endothelial dysfunction [69], including impairment of vasodilatory, and reduction of eNOS/cGMP signaling. In human aortic endothelial cells (HAEC), silencing ATGL decreased the efficiency of stimulus-induced arachidonic acid (AA) release and prostacyclin secretion [70]. Adipose tissue microvascular endothelial cells (aMVECs) ATGL, but not HSL, plays an essential role in regulating adipose tissue lipid uptake through secreting PPARγ ligands [71].
3.2.3. Endothelial Cell Fatty Acid Oxidation
Glycolysis is the predominant bioenergetic pathway for ECs, as ECs generate 85% of their ATP via glycolysis [16]. Fatty acid oxidation (FAO) generates only 5% of the total amount of ATP in naive ECs [16]. Some studies showed that FAO compensates for ATP production up to 40% in ECs when glucose is removed or when lipid is provided in the presence of the AMPK activator AICAR [72]. Later studies showed that FAO-derived carbons sustain the TCA cycle to generate the precursor of nucleotides, aspartate, for deoxyribonucleotide (dNTP) synthesis during EC proliferation [22]. Silencing carnitine palmitoyltransferase 1 (CPT1A), the rate-limiting enzyme for FAO, disrupts EC sprouting in vitro and in vivo due to a decrease in the dNTP pool [22]. Surprisingly, quiescent ECs express a higher level of FAO genes to sustain the TCA cycle for redox homeostasis through NADPH regeneration. EC CPT1A deficiency in mice promotes EC dysfunction by increasing oxidative stress [73]. Indeed, inhibition of FAO via hyperoxia/air recovery leads to lung EC apoptosis and lung injury in mice [74]. However, other studies demonstrated that increased FAO in aortic ECs promotes the generation of superoxide, and inactivates prostacycline synthase and eNOS in the context of insulin resistance [75]. Moreover, lymphatic ECs proliferation during lymphatic development also requires FAO to produce dNTPs [76,77]. Interestingly, FAO-derived production of acetyl-CoA promotes histone acetylation of PROX1-target (lymphatic) genes and lymphatic EC differentiation [76]. Moreover, silencing EC FAO via carnitine palmitoyltransferase II deletion (Cpt2-KO) in mice causes augmented embryonic endothelial-to-mesenchymal transition (EndoMT), thickening cardiac valves and increased vascular permeability in these mice [78]. Taken together, ECs use FAO for redox homeostasis, DNA synthesis, acetyl-CoA generation/epigenetic regulation, and ATP production.
Although fatty acid synthesis is generally low in healthy cells, in vitro pharmacological inhibition of Fatty acid synthase (FASN), which converts malonyl-CoA to free FA, reduces EC proliferation and downregulation of vascular endothelial growth factor receptor 2 (VEGFR2) [79]. Endothelial-specific deletion of FASN impaired angiogenesis in mice, while pharmacological FASN blockade with a low dose of orlistat reduced pathological ocular neovascularization. The impairment of angiogenesis is not due to energy stress, redox imbalance, or palmitate loss, but malonylation modification of mTOR Complex 1 (mTORC1) [80]. In addition, the inactivation of endothelial FASN (Tie2Cre mediated FASN knockout) decreases eNOS palmitoylation (bioavailability) and increases vascular permeability in mice [81]. The role of lipogenesis in vascular physiopathology needs to be further investigated.
3.3. Fatty Acid Metabolism in Pulmonary Hypertension
Given multiple molecules and their complex regulation network involved in FA metabolism in ECs, any abnormality in these regulations will lead to a pathological state and even develop into a disease. Here, we will only discuss the role of FA metabolism and its related pathway in pulmonary hypertension (PH) (Figure 2).
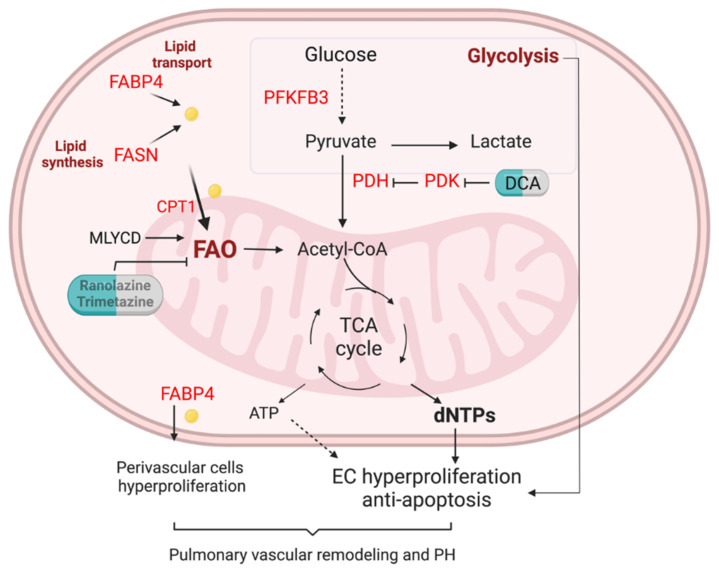
Figure 2.
FA metabolism in the lung EC of pulmonary hypertension. Enhanced glycolysis is evident in PAH ECs. Lipid transport via FABP4, lipid synthesis via fatty acid synthase (FASN), and fatty acid oxidation (FAO) are unregulated in pulmonary arterials of PAH patients. Elevation of FA could increase mitochondria FAO, contributing to the generation of dNTPs and ATP. Enhanced FAO and glycolysis lead to PAH EC hyperproliferation and anti-apoptotic phenotypes. Upregulation of FAPB4 could also increase FA efflux to perivascular cells such as pulmonary arterial smooth muscle cells and fibroblasts, and promote pulmonary vascular remodeling and PH.
Pulmonary arterial hypertension (PAH), or group 1 PH, is an incurable lung disease, characterized by increased resistance in the pulmonary vascular system, which will finally lead to elevated resting pulmonary artery pressure and end by right ventricular failure [82]. Accumulating evidence has demonstrated that EC dysfunction plays a crucial role in the initiation and progression of PAH, manifested by increased susceptibility to injury and enhanced proliferation, contributing to the formation of plexiform lesions [83,84,85,86]. Indeed, most PAH mutation-causing genes are mainly expressed in the lung ECs based on the human lung single-cell RNA sequencing analysis [87].
To date, increasing evidence indicates that EC metabolism dysfunction is closely associated with the pathogenesis of PAH [88,89,90]. The major observed metabolic alteration of EC metabolic change in PAH has increased glycolysis [88]. Glucose metabolic activities were higher in the lungs of PAH patients than in healthy individuals determined by in vivo fluorodeoxyglucose (FDG)-positron emission tomography (PET) scans [91,92]. Healthy ECs generate 85% of their energy from glycolysis. The glycolytic rate of PAH ECs is even greater compared to healthy ECs [91,92], suggesting that PAH ECs exhibit a further shift to aerobic glycolysis. Pulmonary vascular ECs sustain the proliferative and anti-apoptotic phenotypes depending on enhanced glycolysis. Mice with EC-specific deletion of PFKFB3, a key regulator of glycolysis, slowed PH development [93].
FA metabolism abnormality has been observed in PH. Metabolites profiling of the plasma from patients with PAH and healthy controls showed the dysregulated metabolic pathways. Metabolites representing lipid metabolism and fatty acid were closely associated with PAH [94]. Circulating levels of FABP4 were significantly elevated in PAH patients identified by plasma proteome profiling [94]. Lipid deposition is increased in the lungs of PH patients compared to control lungs [95]. FASN was upregulated in hypoxic pulmonary arterial smooth muscle cells (PASMCs) and pulmonary arterial ECs (PAECs), and monocrotaline (MCT)-treated rats (a widely used PH model) [79,96]. FASN inhibition using the C75 compound decreased right ventricular pressure, right heart hypertrophy, pulmonary vascular remodeling, and endothelial dysfunction in MCT-exposed rats [96] and hypoxic mice [97].
Previous studies showed that non-esterified free fatty acids and acylcarnitines in the circulation are upregulated in patients with PAH compared to healthy controls [98,99]. Using a combination of high-throughput liquid-and-gas-chromatography-based mass spectrometry analysis on human PAH lung, Zhao et al. observed that there were increased long- and medium-chain free fatty acid products accumulated in PAH tissues compared to control lung, a reflection of mitochondria -oxidation [100]. They also demonstrated an increase of omega-oxidation in fatty acids and upregulation of lipid oxidation in the lung of PAH [100]. Recent studies also demonstrated the upregulation of FA uptake, processing, and -oxidation-related genes in the laser-dissected pulmonary arteries from idiopathic PAH (IPAH) patients [101]. Our unpublished data showed that PAECs isolated from IPAH patients exhibited increased exogenous FAO compared to PAECs from failed donors using Seahorse XF Palmitate Oxidation Stress assay, suggesting upregulation of FAO in PAH EC. Other evidence showed that inhibition of FAO via genetic deletion of malonyl-CoA decarboxylase (MYLCD) in the whole body protected animal models from developing PH in mice [102]. Genetic knockdown of Cpt1a in mice or pharmacological inhibition (Oxfenicine) of Cpt1b in rats attenuated pulmonary vascular remodeling and PH [101].
In contrast, other studies showed a decrease in carnitine acyltransferase (CrAT), indicative of the reduction in FAO, in the endothelium of congenital heart disease and the PH lamb model [103]. Metabolomic analysis of bone morphogenetic protein receptor type 2 (BMPR2) mutations, which account for 80% of heritable PAH, in human pulmonary EC reveals the downregulation of the carnitine and FAO pathway [104].
PAH patients mostly die because of right heart failure. However, the role of FAO in RV failure is also controversial. Recent studies have demonstrated that intracellular lipid accumulation and the reduction of FAO are features of right heart failure secondary to PAH [98,99]. Human right ventricular (RV) long-chain FAs, myocardial triglyceride content, and ceramide were increased and long-chain acylcarnitines were markedly reduced in PAH versus controls [98]. Activation of FAO using PPAR- agonist pioglitazone or metformin or L-carnitine decreased lipid accumulation and improved right heart function in PH animal models [99,105,106]. However, another group demonstrated that partial inhibition of FAO using Trimetazidine restored pyruvate dehydrogenase (PDH) activity and glucose oxidation, and improved RV function in pulmonary artery banding rats [107] via manipulating Randle’s cycle, for example, inhibiting FAO increases glucose oxidation, or vice versa [107].
Taken together, the role of endothelial FA metabolism in the pathogenesis of PAH and RV failure is complicated and needed for further investigation.
4. Therapeutic Opportunities of FA Metabolism in PH
The current PAH therapies do not prevent the progression or cure the disease. The 5-year mortality of PAH is still as high as 40% [108]. The metabolic intervention via targeting glycolysis and FAO in PH patients has been explored in clinical trials. Pyruvate dehydrogenase kinase (PDK), a negative regulator of the mitochondrial enzyme pyruvate dehydrogenase (PDH), was upregulated in the wall of muscularized pulmonary arteries and remodeled RV from PAH patients compared to non-PAH donors, indicating the inhibition of glucose oxidation and upregulation of glycolysis in the pulmonary arteries and RV of PAH patients [109,110,111]. Perfusion of PDK inhibitor dichloroacetate (DCA) increased PDH activity and increased mitochondrial respiration in human PAH lung ex vivo [109]. DCA administration in 4 months to IPAH patients led to a reduction in mean PA pressure and pulmonary vascular resistance and improvement in functional capacity [109], suggesting PDK is a druggable target for PAH treatment.
Multiple FAO inhibitor trials are completed or on the way [112]. Ranolazine has been demonstrated to reduce calcium overload through inhibition of FAO and the in-ward late sodium current (INa) [113]. FAO inhibition using ranolazine treatment has been tested in a small number of PAH patients (n = 10) for 3 months [114]. Ranolazine treatment reduced RV size, and improved RV function, but did not affect hemodynamic parameters [114]. A recent completed double-blind, randomized, placebo-controlled Ranolazine trials (n = 9 Ranolazine, n = 6 placebo) showed that ranolazine treatment improved RV ejection fraction but not 6-min wall distance (6MWD), N-terminal pro-brain natriuretic peptide, or quality of life measures [115]. Another Ranolazine trials (NCT02133352) and Trimetazidine trial (NCT02102672) are still ongoing.
There is accumulated evidence that PAH is associated with systemic metabolic disorders including metabolic syndrome and insulin resistance [116]. Metformin is a widely used oral anti-diabetic drug that improves insulin sensitivity, increases fatty acid oxidation, and reduces oxidative stress [117]. The therapeutic role of metformin is investigated in both PAH and PH associated with congenital heart defects (PH-CHD) [118,119]. In a single-center, open-label 8-week phase II trial of metformin in PAH trial (n = 20), Metformin did not change the 6MWD but did significantly improve RV fractional area change and reduced RV triglyceride content [118]. In another study, Metformin was added to the Bosentan therapy in patients with PH-CHD. The improvements in 6MWD and pulmonary vascular resistance were significantly greater in PH-CHD patients treated with Metformin plus Bosentan therapy than in those who received Bosentan only [119].
The understanding of the metabolic mechanisms including FA metabolism in the pathogenesis of PAH is critical in the development of novel therapeutic strategies. The current understanding of EC FA metabolism remains elusive. The observation of FAO in the lung ECs and RV is inconsistent based on the studies from different groups (describe in Section 3.3, which might be due to the differences in the experimental models and approaches in individual laboratories. Further studies should employ comprehensive approaches such as single-cell omics, spatial transcriptomics, proteomes, and metabolomics to understand the metabolic alteration in PAH samples. To demonstrate the proof-of-concept of FA dysfunction theories in PH, genetic manipulation of a certain gene in the animal models are superior compared to pharmacological targeting of the protein of interest due to the potential off-target effect of chemical compounds [120]. In addition, severe PH mice models, for example, Egln1Tie2Cre [83,121] and IL6-Tg [122] mice, might be used as the PH phenotype of hypoxic mice model is mild which does not reproduce the human pathology [123].
In our perspective, EC FA transport signaling represents a novel therapeutic approach for the treatment of PAH. Limiting the EC FA transport will reduce FAO/dNTP axis and attenuate EC hyperproliferation in the lung ECs and pulmonary vascular remodeling and PH. On the other aspect, inhibition of EC FA uptake could potentially reduce the lipid deposit and lipotoxicity in the lung and RV cardiomyotes, and improve RV function. In conclusion, improved understanding of the EC FA metabolism responsible for the initiation and progression of PAH will facilitate the development of effective treatments for PAH.
Acknowledgments
We thank Karina Ramirez for the language editing.
Author Contributions
B.L. drafted the manuscript, and Z.D. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported in part by NIH grants R00HL138278, R01HL158596, AHA Career Development Award 20CDA35310084, The Cardiovascular Medical and Education Fund, and University of Arizona Startup funding to Z.D.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rohlenova K., Veys K., Miranda-Santos I., De Bock K., Carmeliet P. Endothelial Cell Metabolism in Health and Disease. Trends Cell Biol. 2018;28:224–236. doi: 10.1016/j.tcb.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Eelen G., de Zeeuw P., Simons M., Carmeliet P. Endothelial cell metabolism in normal and diseased vasculature. Circ. Res. 2015;116:1231–1244. doi: 10.1161/CIRCRESAHA.116.302855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pircher A., Treps L., Bodrug N., Carmeliet P. Endothelial cell metabolism: A novel player in atherosclerosis? Basic principles and therapeutic opportunities. Atherosclerosis. 2016;253:247–257. doi: 10.1016/j.atherosclerosis.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 4.Li X., Sun X., Carmeliet P. Hallmarks of Endothelial Cell Metabolism in Health and Disease. Cell Metab. 2019;30:414–433. doi: 10.1016/j.cmet.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Potente M., Gerhardt H., Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 6.Eelen G., Treps L., Li X., Carmeliet P. Basic and Therapeutic Aspects of Angiogenesis Updated. Circ. Res. 2020;127:310–329. doi: 10.1161/CIRCRESAHA.120.316851. [DOI] [PubMed] [Google Scholar]
- 7.Rafii S., Butler J.M., Ding B.S. Angiocrine functions of organ-specific endothelial cells. Nature. 2016;529:316–325. doi: 10.1038/nature17040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jakobsson L., Franco C.A., Bentley K., Collins R.T., Ponsioen B., Aspalter I.M., Rosewell I., Busse M., Thurston G., Medvinsky A., et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat. Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe E.A. Cell biology of endothelial cells. Hum. Pathol. 1987;18:234–239. doi: 10.1016/S0046-8177(87)80005-9. [DOI] [PubMed] [Google Scholar]
- 10.Steinberg H.O., Paradisi G., Hook G., Crowder K., Cronin J., Baron A.D. Free fatty acid elevation impairs insulin-mediated vasodilation and nitric oxide production. Diabetes. 2000;49:1231–1238. doi: 10.2337/diabetes.49.7.1231. [DOI] [PubMed] [Google Scholar]
- 11.Leo F., Suvorava T., Heuser S.K., Li J., LoBue A., Barbarino F., Piragine E., Schneckmann R., Hutzler B., Good M.E., et al. Red Blood Cell and Endothelial eNOS Independently Regulate Circulating Nitric Oxide Metabolites and Blood Pressure. Circulation. 2021;144:870–889. doi: 10.1161/CIRCULATIONAHA.120.049606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Förstermann U., Sessa W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012;33 doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pober J.S., Sessa W.C. Evolving functions of endothelial cells in inflammation. Nat. Rev. Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 14.Opitz B., Förster S., Hocke A.C., Maass M., Schmeck B., Hippenstiel S., Suttorp N., Krüll M. Nod1-mediated endothelial cell activation by Chlamydophila pneumoniae. Circ. Res. 2005;96:319–326. doi: 10.1161/01.RES.0000155721.83594.2c. [DOI] [PubMed] [Google Scholar]
- 15.Li X., Kumar A., Carmeliet P. Metabolic Pathways Fueling the Endothelial Cell Drive. Annu. Rev. Physiol. 2019;81:483–503. doi: 10.1146/annurev-physiol-020518-114731. [DOI] [PubMed] [Google Scholar]
- 16.De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R., Quaegebeur A., Ghesquière B., Cauwenberghs S., Eelen G., et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 17.De Bock K., Georgiadou M., Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Helmlinger G., Endo M., Ferrara N., Hlatky L., Jain R.K. Formation of endothelial cell networks. Nature. 2000;405:139–141. doi: 10.1038/35012132. [DOI] [PubMed] [Google Scholar]
- 19.Yu P., Wilhelm K., Dubrac A., Tung J.K., Alves T.C., Fang J.S., Xie Y., Zhu J., Chen Z., De Smet F., et al. FGF-dependent metabolic control of vascular development. Nature. 2017;545:224–228. doi: 10.1038/nature22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim B., Li J., Jang C., Arany Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J. 2017;36:2321–2333. doi: 10.15252/embj.201796436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang H., Vandekeere S., Kalucka J., Bierhansl L., Zecchin A., Brüning U., Visnagri A., Yuldasheva N., Goveia J., Cruys B., et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017;36:2334–2352. doi: 10.15252/embj.201695518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schoors S., Bruning U., Missiaen R., Queiroz K.C., Borgers G., Elia I., Zecchin A., Cantelmo A.R., Christen S., Goveia J., et al. Fatty acid carbon is essential for dNTP synthesis in endothelial cells. Nature. 2015;520:192–197. doi: 10.1038/nature14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hagberg C.E., Falkevall A., Wang X., Larsson E., Huusko J., Nilsson I., van Meeteren L.A., Samen E., Lu L., Vanwildemeersch M., et al. Vascular endothelial growth factor B controls endothelial fatty acid uptake. Nature. 2010;464:917–921. doi: 10.1038/nature08945. [DOI] [PubMed] [Google Scholar]
- 24.Kazantzis M., Stahl A. Fatty acid transport proteins, implications in physiology and disease. Biochim. Biophys. Acta. 2012;1821:852–857. doi: 10.1016/j.bbalip.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hülsmann W.C., Dubelaar M.L. Aspects of fatty acid metabolism in vascular endothelial cells. Biochimie. 1988;70:681–686. doi: 10.1016/0300-9084(88)90253-2. [DOI] [PubMed] [Google Scholar]
- 26.Dallinga-Thie G.M., Franssen R., Mooij H.L., Visser M.E., Hassing H.C., Peelman F., Kastelein J.J., Péterfy M., Nieuwdorp M. The metabolism of triglyceride-rich lipoproteins revisited: New players, new insight. Atherosclerosis. 2010;211:1–8. doi: 10.1016/j.atherosclerosis.2009.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davies B.S., Beigneux A.P., Barnes R.H., 2nd, Tu Y., Gin P., Weinstein M.M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin C., Chevrot M., Poirier H., Passilly-Degrace P., Niot I., Besnard P. CD36 as a lipid sensor. Physiol. Behav. 2011;105:36–42. doi: 10.1016/j.physbeh.2011.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Hagberg C., Mehlem A., Falkevall A., Muhl L., Eriksson U. Endothelial fatty acid transport: Role of vascular endothelial growth factor B. Physiology. 2013;28:125–134. doi: 10.1152/physiol.00042.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smathers R.L., Petersen D.R. The human fatty acid-binding protein family: Evolutionary divergences and functions. Hum. Genom. 2011;5:170–191. doi: 10.1186/1479-7364-5-3-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storch J., Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu. Rev. Nutr. 2008;28:73–95. doi: 10.1146/annurev.nutr.27.061406.093710. [DOI] [PubMed] [Google Scholar]
- 32.Furuhashi M., Hotamisligil G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008;7:489–503. doi: 10.1038/nrd2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Canton J., Neculai D., Grinstein S. Scavenger receptors in homeostasis and immunity. Nat. Rev. Immunol. 2013;13:621–634. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 34.Silverstein R.L., Febbraio M. CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2009;2:re3. doi: 10.1126/scisignal.272re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoebe K., Georgel P., Rutschmann S., Du X., Mudd S., Crozat K., Sovath S., Shamel L., Hartung T., Zähringer U., et al. CD36 is a sensor of diacylglycerides. Nature. 2005;433:523–527. doi: 10.1038/nature03253. [DOI] [PubMed] [Google Scholar]
- 36.Asch A.S., Barnwell J., Silverstein R.L., Nachman R.L. Isolation of the thrombospondin membrane receptor. J. Clin. Investig. 1987;79:1054–1061. doi: 10.1172/JCI112918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao J.W., Wang J., Guo H., Zhao Y.Y., Sun H.H., Li Y.F., Lai X.Y., Zhao N., Wang X., Xie C., et al. CD36 facilitates fatty acid uptake by dynamic palmitoylation-regulated endocytosis. Nat. Commun. 2020;11:4765. doi: 10.1038/s41467-020-18565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bastie C.C., Hajri T., Drover V.A., Grimaldi P.A., Abumrad N.A. CD36 in myocytes channels fatty acids to a lipase-accessible triglyceride pool that is related to cell lipid and insulin responsiveness. Diabetes. 2004;53:2209–2216. doi: 10.2337/diabetes.53.9.2209. [DOI] [PubMed] [Google Scholar]
- 39.Wang J., Hao J.W., Wang X., Guo H., Sun H.H., Lai X.Y., Liu L.Y., Zhu M., Wang H.Y., Li Y.F., et al. DHHC4 and DHHC5 Facilitate Fatty Acid Uptake by Palmitoylating and Targeting CD36 to the Plasma Membrane. Cell Rep. 2019;26:209–221.e5. doi: 10.1016/j.celrep.2018.12.022. [DOI] [PubMed] [Google Scholar]
- 40.Goldberg I.J., Eckel R.H., Abumrad N.A. Regulation of fatty acid uptake into tissues: Lipoprotein lipase- and CD36-mediated pathways. J. Lipid Res. 2009;50:S86–S90. doi: 10.1194/jlr.R800085-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Son N.H., Basu D., Samovski D., Pietka T.A., Peche V.S., Willecke F., Fang X., Yu S.Q., Scerbo D., Chang H.R., et al. Endothelial cell CD36 optimizes tissue fatty acid uptake. J. Clin. Investig. 2018;128:4329–4342. doi: 10.1172/JCI99315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bou Khzam L., Son N.H., Mullick A.E., Abumrad N.A., Goldberg I.J. Endothelial cell CD36 deficiency prevents normal angiogenesis and vascular repair. Am. J. Transl. Res. 2020;12:7737–7761. [PMC free article] [PubMed] [Google Scholar]
- 43.Ramakrishnan D.P., Hajj-Ali R.A., Chen Y., Silverstein R.L. Extracellular Vesicles Activate a CD36-Dependent Signaling Pathway to Inhibit Microvascular Endothelial Cell Migration and Tube Formation. Arter. Thromb. Vasc. Biol. 2016;36:534–544. doi: 10.1161/ATVBAHA.115.307085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rekhi U.R., Omar M., Alexiou M., Delyea C., Immaraj L., Elahi S., Febbraio M. Endothelial Cell CD36 Reduces Atherosclerosis and Controls Systemic Metabolism. Front. Cardiovasc. Med. 2021;8:768481. doi: 10.3389/fcvm.2021.768481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cifarelli V., Appak-Baskoy S., Peche V.S., Kluzak A., Shew T., Narendran R., Pietka K.M., Cella M., Walls C.W., Czepielewski R., et al. Visceral obesity and insulin resistance associate with CD36 deletion in lymphatic endothelial cells. Nat. Commun. 2021;12:3350. doi: 10.1038/s41467-021-23808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jang C., Oh S.F., Wada S., Rowe G.C., Liu L., Chan M.C., Rhee J., Hoshino A., Kim B., Ibrahim A., et al. A branched-chain amino acid metabolite drives vascular fatty acid transport and causes insulin resistance. Nat. Med. 2016;22:421–426. doi: 10.1038/nm.4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bae H., Hong K.Y., Lee C.K., Jang C., Lee S.J., Choe K., Offermanns S., He Y., Lee H.J., Koh G.Y. Angiopoietin-2-integrin α5β1 signaling enhances vascular fatty acid transport and prevents ectopic lipid-induced insulin resistance. Nat. Commun. 2020;11:2980. doi: 10.1038/s41467-020-16795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ibrahim A., Yucel N., Kim B., Arany Z. Local Mitochondrial ATP Production Regulates Endothelial Fatty Acid Uptake and Transport. Cell Metab. 2020;32:309–319.e7. doi: 10.1016/j.cmet.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee C.H., Lui D.T.W., Lam K.S.L. Adipocyte Fatty Acid-Binding Protein, Cardiovascular Diseases and Mortality. Front. Immunol. 2021;12:589206. doi: 10.3389/fimmu.2021.589206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmasri H., Karaaslan C., Teper Y., Ghelfi E., Weng M., Ince T.A., Kozakewich H., Bischoff J., Cataltepe S. Fatty acid binding protein 4 is a target of VEGF and a regulator of cell proliferation in endothelial cells. FASEB J. 2009;23:3865–3873. doi: 10.1096/fj.09-134882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Masouyé I., Hagens G., Van Kuppevelt T.H., Madsen P., Saurat J.H., Veerkamp J.H., Pepper M.S., Siegenthaler G. Endothelial cells of the human microvasculature express epidermal fatty acid-binding protein. Circ. Res. 1997;81:297–303. doi: 10.1161/01.RES.81.3.297. [DOI] [PubMed] [Google Scholar]
- 52.Iso T., Maeda K., Hanaoka H., Suga T., Goto K., Syamsunarno M.R., Hishiki T., Nagahata Y., Matsui H., Arai M., et al. Capillary endothelial fatty acid binding proteins 4 and 5 play a critical role in fatty acid uptake in heart and skeletal muscle. Arter. Thromb. Vasc. Biol. 2013;33:2549–2557. doi: 10.1161/ATVBAHA.113.301588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elmasri H., Ghelfi E., Yu C.W., Traphagen S., Cernadas M., Cao H., Shi G.P., Plutzky J., Sahin M., Hotamisligil G., et al. Endothelial cell-fatty acid binding protein 4 promotes angiogenesis: Role of stem cell factor/c-kit pathway. Angiogenesis. 2012;15:457–468. doi: 10.1007/s10456-012-9274-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aragonès G., Saavedra P., Heras M., Cabré A., Girona J., Masana L. Fatty acid-binding protein 4 impairs the insulin-dependent nitric oxide pathway in vascular endothelial cells. Cardiovasc. Diabetol. 2012;11:72. doi: 10.1186/1475-2840-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fuseya T., Furuhashi M., Matsumoto M., Watanabe Y., Hoshina K., Mita T., Ishimura S., Tanaka M., Miura T. Ectopic Fatty Acid-Binding Protein 4 Expression in the Vascular Endothelium is Involved in Neointima Formation After Vascular Injury. J. Am. Heart Assoc. 2017;6:e006377. doi: 10.1161/JAHA.117.006377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee M.Y., Li H., Xiao Y., Zhou Z., Xu A., Vanhoutte P.M. Chronic administration of BMS309403 improves endothelial function in apolipoprotein E-deficient mice and in cultured human endothelial cells. Br. J. Pharmacol. 2011;162:1564–1576. doi: 10.1111/j.1476-5381.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Furuhashi M., Tuncman G., Görgün C.Z., Makowski L., Atsumi G., Vaillancourt E., Kono K., Babaev V.R., Fazio S., Linton M.F., et al. Treatment of diabetes and atherosclerosis by inhibiting fatty-acid-binding protein aP2. Nature. 2007;447:959–965. doi: 10.1038/nature05844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luis G., Godfroid A., Nishiumi S., Cimino J., Blacher S., Maquoi E., Wery C., Collignon A., Longuespée R., Montero-Ruiz L., et al. Tumor resistance to ferroptosis driven by Stearoyl-CoA Desaturase-1 (SCD1) in cancer cells and Fatty Acid Biding Protein-4 (FABP4) in tumor microenvironment promote tumor recurrence. Redox Biol. 2021;43:102006. doi: 10.1016/j.redox.2021.102006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Boord J.B., Maeda K., Makowski L., Babaev V.R., Fazio S., Linton M.F., Hotamisligil G.S. Combined adipocyte-macrophage fatty acid-binding protein deficiency improves metabolism, atherosclerosis, and survival in apolipoprotein E-deficient mice. Circulation. 2004;110:1492–1498. doi: 10.1161/01.CIR.0000141735.13202.B6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Maeda K., Cao H., Kono K., Gorgun C.Z., Furuhashi M., Uysal K.T., Cao Q., Atsumi G., Malone H., Krishnan B., et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 61.Yu C.W., Liang X., Lipsky S., Karaaslan C., Kozakewich H., Hotamisligil G.S., Bischoff J., Cataltepe S. Dual role of fatty acid-binding protein 5 on endothelial cell fate: A potential link between lipid metabolism and angiogenic responses. Angiogenesis. 2016;19:95–106. doi: 10.1007/s10456-015-9491-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pan Y., Short J.L., Choy K.H., Zeng A.X., Marriott P.J., Owada Y., Scanlon M.J., Porter C.J., Nicolazzo J.A. Fatty Acid-Binding Protein 5 at the Blood-Brain Barrier Regulates Endogenous Brain Docosahexaenoic Acid Levels and Cognitive Function. J. Neurosci. 2016;36:11755–11767. doi: 10.1523/JNEUROSCI.1583-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goto K., Iso T., Hanaoka H., Yamaguchi A., Suga T., Hattori A., Irie Y., Shinagawa Y., Matsui H., Syamsunarno M.R., et al. Peroxisome proliferator-activated receptor-γ in capillary endothelia promotes fatty acid uptake by heart during long-term fasting. J. Am. Heart Assoc. 2013;2:e004861. doi: 10.1161/JAHA.112.004861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jabs M., Rose A.J., Lehmann L.H., Taylor J., Moll I., Sijmonsma T.P., Herberich S.E., Sauer S.W., Poschet G., Federico G., et al. Inhibition of Endothelial Notch Signaling Impairs Fatty Acid Transport and Leads to Metabolic and Vascular Remodeling of the Adult Heart. Circulation. 2018;137:2592–2608. doi: 10.1161/CIRCULATIONAHA.117.029733. [DOI] [PubMed] [Google Scholar]
- 65.Hwangbo C., Wu J., Papangeli I., Adachi T., Sharma B., Park S., Zhao L., Ju H., Go G.W., Cui G., et al. Endothelial APLNR regulates tissue fatty acid uptake and is essential for apelin’s glucose-lowering effects. Sci. Transl. Med. 2017;9:eaad4000. doi: 10.1126/scitranslmed.aad4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jarc E., Petan T. Lipid Droplets and the Management of Cellular Stress. Yale J. Biol. Med. 2019;92:435–452. [PMC free article] [PubMed] [Google Scholar]
- 67.Kuo A., Lee M.Y., Sessa W.C. Lipid Droplet Biogenesis and Function in the Endothelium. Circ. Res. 2017;120:1289–1297. doi: 10.1161/CIRCRESAHA.116.310498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kaushik S., Cuervo A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015;17:759–770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schrammel A., Mussbacher M., Wölkart G., Stessel H., Pail K., Winkler S., Schweiger M., Haemmerle G., Al Zoughbi W., Höfler G., et al. Endothelial dysfunction in adipose triglyceride lipase deficiency. Biochim. Biophys. Acta. 2014;1841:906–917. doi: 10.1016/j.bbalip.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Riederer M., Lechleitner M., Köfeler H., Frank S. Reduced expression of adipose triglyceride lipase decreases arachidonic acid release and prostacyclin secretion in human aortic endothelial cells. Arch. Physiol. Biochem. 2017;123:249–253. doi: 10.1080/13813455.2017.1309052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gogg S., Nerstedt A., Boren J., Smith U. Human adipose tissue microvascular endothelial cells secrete PPARγ ligands and regulate adipose tissue lipid uptake. JCI Insight. 2019;4:e125914. doi: 10.1172/jci.insight.125914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dagher Z., Ruderman N., Tornheim K., Ido Y. Acute regulation of fatty acid oxidation and amp-activated protein kinase in human umbilical vein endothelial cells. Circ. Res. 2001;88:1276–1282. doi: 10.1161/hh1201.092998. [DOI] [PubMed] [Google Scholar]
- 73.Kalucka J., Bierhansl L., Conchinha N.V., Missiaen R., Elia I., Brüning U., Scheinok S., Treps L., Cantelmo A.R., Dubois C., et al. Quiescent Endothelial Cells Upregulate Fatty Acid β-Oxidation for Vasculoprotection via Redox Homeostasis. Cell Metab. 2018;28:881–894.e13. doi: 10.1016/j.cmet.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 74.Yao H., Gong J., Peterson A.L., Lu X., Zhang P., Dennery P.A. Fatty Acid Oxidation Protects against Hyperoxia-induced Endothelial Cell Apoptosis and Lung Injury in Neonatal Mice. Am. J. Respir. Cell Mol. Biol. 2019;60:667–677. doi: 10.1165/rcmb.2018-0335OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Du X., Edelstein D., Obici S., Higham N., Zou M.H., Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. J. Clin. Investig. 2006;116:1071–1080. doi: 10.1172/JCI23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong B.W., Wang X., Zecchin A., Thienpont B., Cornelissen I., Kalucka J., García-Caballero M., Missiaen R., Huang H., Brüning U., et al. The role of fatty acid β-oxidation in lymphangiogenesis. Nature. 2017;542:49–54. doi: 10.1038/nature21028. [DOI] [PubMed] [Google Scholar]
- 77.Zecchin A., Wong B.W., Tembuyser B., Souffreau J., Van Nuffelen A., Wyns S., Vinckier S., Carmeliet P., Dewerchin M. Live imaging reveals a conserved role of fatty acid β-oxidation in early lymphatic development in zebrafish. Biochem. Biophys. Res. Commun. 2018;503:26–31. doi: 10.1016/j.bbrc.2018.04.233. [DOI] [PubMed] [Google Scholar]
- 78.Xiong J., Kawagishi H., Yan Y., Liu J., Wells Q.S., Edmunds L.R., Fergusson M.M., Yu Z.X., Rovira I.I., Brittain E.L., et al. A Metabolic Basis for Endothelial-to-Mesenchymal Transition. Mol. Cell. 2018;69:689–698.e7. doi: 10.1016/j.molcel.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Singh N., Singh H., Jagavelu K., Wahajuddin M., Hanif K. Fatty acid synthase modulates proliferation, metabolic functions and angiogenesis in hypoxic pulmonary artery endothelial cells. Eur. J. Pharmacol. 2017;815:462–469. doi: 10.1016/j.ejphar.2017.09.042. [DOI] [PubMed] [Google Scholar]
- 80.Bruning U., Morales-Rodriguez F., Kalucka J., Goveia J., Taverna F., Queiroz K.C.S., Dubois C., Cantelmo A.R., Chen R., Loroch S., et al. Impairment of Angiogenesis by Fatty Acid Synthase Inhibition Involves mTOR Malonylation. Cell Metab. 2018;28:866–880.e15. doi: 10.1016/j.cmet.2018.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei X., Schneider J.G., Shenouda S.M., Lee A., Towler D.A., Chakravarthy M.V., Vita J.A., Semenkovich C.F. De novo lipogenesis maintains vascular homeostasis through endothelial nitric-oxide synthase (eNOS) palmitoylation. J. Biol. Chem. 2011;286:2933–2945. doi: 10.1074/jbc.M110.193037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thenappan T., Ormiston M.L., Ryan J.J., Archer S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ. 2018;360:j5492. doi: 10.1136/bmj.j5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dai Z., Li M., Wharton J., Zhu M.M., Zhao Y.Y. Prolyl-4 Hydroxylase 2 (PHD2) Deficiency in Endothelial Cells and Hematopoietic Cells Induces Obliterative Vascular Remodeling and Severe Pulmonary Arterial Hypertension in Mice and Humans Through Hypoxia-Inducible Factor-2α. Circulation. 2016;133:2447–2458. doi: 10.1161/CIRCULATIONAHA.116.021494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dai Z., Zhu M.M., Peng Y., Jin H., Machireddy N., Qian Z., Zhang X., Zhao Y.Y. Endothelial and Smooth Muscle Cell Interaction via FoxM1 Signaling Mediates Vascular Remodeling and Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2018;198:788–802. doi: 10.1164/rccm.201709-1835OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu B., Peng Y., Yi D., Machireddy N., Dong D., Ramirez K., Dai J., Vanderpool R., Zhu M.M., Dai Z., et al. Endothelial PHD2 deficiency induces nitrative stress via suppression of caveolin-1 in pulmonary hypertension. Eur. Respir. J. 2022;60:2102643. doi: 10.1183/13993003.02643-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Evans C.E., Cober N.D., Dai Z., Stewart D.J., Zhao Y.Y. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur. Respir. J. 2021;58:2003957. doi: 10.1183/13993003.03957-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Travaglini K.J., Nabhan A.N., Penland L., Sinha R., Gillich A., Sit R.V., Chang S., Conley S.D., Mori Y., Seita J., et al. A molecular cell atlas of the human lung from single-cell RNA sequencing. Nature. 2020;587:619–625. doi: 10.1038/s41586-020-2922-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chan S.Y., Rubin L.J. Metabolic dysfunction in pulmonary hypertension: From basic science to clinical practice. Eur. Respir. Rev. 2017;26:170094. doi: 10.1183/16000617.0094-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xu W., Janocha A.J., Erzurum S.C. Metabolism in Pulmonary Hypertension. Annu. Rev. Physiol. 2021;83:551–576. doi: 10.1146/annurev-physiol-031620-123956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi X.F., Su Y.C. Vascular Metabolic Mechanisms of Pulmonary Hypertension. Curr. Med. Sci. 2020;40:444–454. doi: 10.1007/s11596-020-2198-9. [DOI] [PubMed] [Google Scholar]
- 91.Xu W., Koeck T., Lara A.R., Neumann D., DiFilippo F.P., Koo M., Janocha A.J., Masri F.A., Arroliga A.C., Jennings C., et al. Alterations of cellular bioenergetics in pulmonary artery endothelial cells. Proc. Natl. Acad. Sci. USA. 2007;104:1342–1347. doi: 10.1073/pnas.0605080104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu W., Erzurum S.C. Endothelial cell energy metabolism, proliferation, and apoptosis in pulmonary hypertension. Compr. Physiol. 2011;1:357–372. doi: 10.1002/cphy.c090005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cao Y., Zhang X., Wang L., Yang Q., Ma Q., Xu J., Wang J., Kovacs L., Ayon R.J., Liu Z., et al. PFKFB3-mediated endothelial glycolysis promotes pulmonary hypertension. Proc. Natl. Acad. Sci. USA. 2019;116:13394–13403. doi: 10.1073/pnas.1821401116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen C., Luo F., Wu P., Huang Y., Das A., Chen S., Chen J., Hu X., Li F., Fang Z., et al. Metabolomics reveals metabolite changes of patients with pulmonary arterial hypertension in China. J. Cell Mol. Med. 2020;24:2484–2496. doi: 10.1111/jcmm.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Umar S., Ruffenach G., Moazeni S., Vaillancourt M., Hong J., Cunningham C., Cao N., Navab S., Sarji S., Li M., et al. Involvement of Low-Density Lipoprotein Receptor in the Pathogenesis of Pulmonary Hypertension. J. Am. Heart Assoc. 2020;9:e012063. doi: 10.1161/JAHA.119.012063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Singh N., Manhas A., Kaur G., Jagavelu K., Hanif K. Inhibition of fatty acid synthase is protective in pulmonary hypertension. Br. J. Pharmacol. 2016;173:2030–2045. doi: 10.1111/bph.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hou C., Chen J., Zhao Y., Niu Y., Lin S., Chen S., Zong Y., Sun X., Xie L., Xiao T. The Emerging Role of Fatty Acid Synthase in Hypoxia-Induced Pulmonary Hypertensive Mouse Energy Metabolism. Oxidative Med. Cell. Longev. 2021;2021:9990794. doi: 10.1155/2021/9990794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brittain E.L., Talati M., Fessel J.P., Zhu H., Penner N., Calcutt M.W., West J.D., Funke M., Lewis G.D., Gerszten R.E., et al. Fatty Acid Metabolic Defects and Right Ventricular Lipotoxicity in Human Pulmonary Arterial Hypertension. Circulation. 2016;133:1936–1944. doi: 10.1161/CIRCULATIONAHA.115.019351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hemnes A.R., Brittain E.L., Trammell A.W., Fessel J.P., Austin E.D., Penner N., Maynard K.B., Gleaves L., Talati M., Absi T., et al. Evidence for right ventricular lipotoxicity in heritable pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2014;189:325–334. doi: 10.1164/rccm.201306-1086OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao Y., Peng J., Lu C., Hsin M., Mura M., Wu L., Chu L., Zamel R., Machuca T., Waddell T., et al. Metabolomic heterogeneity of pulmonary arterial hypertension. PLoS ONE. 2014;9:e88727. doi: 10.1371/journal.pone.0088727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee M.H., Sanders L., Kumar R., Hernandez-Saavedra D., Yun X., Ford J.A., Perez M.J., Mickael C., Gandjeva A., Koyanagi D.E., et al. Contribution of fatty acid oxidation to the pathogenesis of pulmonary hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2022;323:L355–l371. doi: 10.1152/ajplung.00039.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sutendra G., Bonnet S., Rochefort G., Haromy A., Folmes K.D., Lopaschuk G.D., Dyck J.R., Michelakis E.D. Fatty acid oxidation and malonyl-CoA decarboxylase in the vascular remodeling of pulmonary hypertension. Sci. Transl. Med. 2010;2:44ra58. doi: 10.1126/scitranslmed.3001327. [DOI] [PubMed] [Google Scholar]
- 103.Sun X., Sharma S., Fratz S., Kumar S., Rafikov R., Aggarwal S., Rafikova O., Lu Q., Burns T., Dasarathy S., et al. Disruption of endothelial cell mitochondrial bioenergetics in lambs with increased pulmonary blood flow. Antioxid. Redox Signal. 2013;18:1739–1752. doi: 10.1089/ars.2012.4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fessel J.P., Hamid R., Wittmann B.M., Robinson L.J., Blackwell T., Tada Y., Tanabe N., Tatsumi K., Hemnes A.R., West J.D. Metabolomic analysis of bone morphogenetic protein receptor type 2 mutations in human pulmonary endothelium reveals widespread metabolic reprogramming. Pulm. Circ. 2012;2:201–213. doi: 10.4103/2045-8932.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Agrawal V., Hemnes A.R., Shelburne N.J., Fortune N., Fuentes J.L., Colvin D., Calcutt M.W., Talati M., Poovey E., West J.D., et al. l-Carnitine therapy improves right heart dysfunction through Cpt1-dependent fatty acid oxidation. Pulm. Circ. 2022;12:e12107. doi: 10.1002/pul2.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Legchenko E., Chouvarine P., Borchert P., Fernandez-Gonzalez A., Snay E., Meier M., Maegel L., Mitsialis S.A., Rog-Zielinska E.A., Kourembanas S., et al. PPARγ agonist pioglitazone reverses pulmonary hypertension and prevents right heart failure via fatty acid oxidation. Sci. Transl. Med. 2018;10:eaao0303. doi: 10.1126/scitranslmed.aao0303. [DOI] [PubMed] [Google Scholar]
- 107.Fang Y.H., Piao L., Hong Z., Toth P.T., Marsboom G., Bache-Wiig P., Rehman J., Archer S.L. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: Exploiting Randle’s cycle. J. Mol. Med. 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hassoun P.M. Pulmonary Arterial Hypertension. N. Engl. J. Med. 2021;385:2361–2376. doi: 10.1056/NEJMra2000348. [DOI] [PubMed] [Google Scholar]
- 109.Michelakis E.D., Gurtu V., Webster L., Barnes G., Watson G., Howard L., Cupitt J., Paterson I., Thompson R.B., Chow K., et al. Inhibition of pyruvate dehydrogenase kinase improves pulmonary arterial hypertension in genetically susceptible patients. Sci. Transl. Med. 2017;9:eaao4583. doi: 10.1126/scitranslmed.aao4583. [DOI] [PubMed] [Google Scholar]
- 110.Michelakis E.D., McMurtry M.S., Wu X.C., Dyck J.R., Moudgil R., Hopkins T.A., Lopaschuk G.D., Puttagunta L., Waite R., Archer S.L. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 111.McMurtry M.S., Bonnet S., Wu X., Dyck J.R., Haromy A., Hashimoto K., Michelakis E.D. Dichloroacetate prevents and reverses pulmonary hypertension by inducing pulmonary artery smooth muscle cell apoptosis. Circ. Res. 2004;95:830–840. doi: 10.1161/01.RES.0000145360.16770.9f. [DOI] [PubMed] [Google Scholar]
- 112.Harvey L.D., Chan S.Y. Emerging Metabolic Therapies in Pulmonary Arterial Hypertension. J. Clin. Med. 2017;6:43. doi: 10.3390/jcm6040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stone P.H. Ranolazine: New paradigm for management of myocardial ischemia, myocardial dysfunction, and arrhythmias. Cardiol. Clin. 2008;26:603–614. doi: 10.1016/j.ccl.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 114.Khan S.S., Cuttica M.J., Beussink-Nelson L., Kozyleva A., Sanchez C., Mkrdichian H., Selvaraj S., Dematte J.E., Lee D.C., Shah S.J. Effects of ranolazine on exercise capacity, right ventricular indices, and hemodynamic characteristics in pulmonary arterial hypertension: A pilot study. Pulm. Circ. 2015;5:547–556. doi: 10.1086/682427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han Y., Forfia P., Vaidya A., Mazurek J.A., Park M.H., Ramani G., Chan S.Y., Waxman A.B. Ranolazine Improves Right Ventricular Function in Patients with Precapillary Pulmonary Hypertension: Results From a Double-Blind, Randomized, Placebo-Controlled Trial. J. Card. Fail. 2021;27:253–257. doi: 10.1016/j.cardfail.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 116.Zamanian R.T., Hansmann G., Snook S., Lilienfeld D., Rappaport K.M., Reaven G.M., Rabinovitch M., Doyle R.L. Insulin resistance in pulmonary arterial hypertension. Eur. Respir. J. 2009;33:318–324. doi: 10.1183/09031936.00000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Eurich D.T., McAlister F.A., Blackburn D.F., Majumdar S.R., Tsuyuki R.T., Varney J., Johnson J.A. Benefits and harms of antidiabetic agents in patients with diabetes and heart failure: Systematic review. BMJ. 2007;335:497. doi: 10.1136/bmj.39314.620174.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Brittain E.L., Niswender K., Agrawal V., Chen X., Fan R., Pugh M.E., Rice T.W., Robbins I.M., Song H., Thompson C., et al. Mechanistic Phase II Clinical Trial of Metformin in Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2020;9:e018349. doi: 10.1161/JAHA.120.018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liao S., Li D., Hui Z., McLachlan C.S., Zhang Y. Metformin added to bosentan therapy in patients with pulmonary arterial hypertension associated with congenital heart defects: A pilot study. ERJ Open Res. 2018;4:00060–2018. doi: 10.1183/23120541.00060-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rao M.S., Gupta R., Liguori M.J., Hu M., Huang X., Mantena S.R., Mittelstadt S.W., Blomme E.A.G., Van Vleet T.R. Novel Computational Approach to Predict Off-Target Interactions for Small Molecules. Front. Big Data. 2019;2:25. doi: 10.3389/fdata.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dai Z., Zhao Y.Y. Discovery of a murine model of clinical PAH: Mission impossible? Trends Cardiovasc. Med. 2017;27:229–236. doi: 10.1016/j.tcm.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Steiner M.K., Syrkina O.L., Kolliputi N., Mark E.J., Hales C.A., Waxman A.B. Interleukin-6 overexpression induces pulmonary hypertension. Circ. Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. 228p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dignam J.P., Scott T.E., Kemp-Harper B.K., Hobbs A.J. Animal models of pulmonary hypertension: Getting to the heart of the problem. Br. J. Pharmacol. 2022;179:811–837. doi: 10.1111/bph.15444. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.