Abstract
Colonization of the plaque biofilm by the oral pathogen Porphyromonas gingivalis is favored by the presence of antecedent organisms such as Streptococcus gordonii. Coadhesion between P. gingivalis and S. gordonii can be mediated by the SspB protein of S. gordonii; however, the P. gingivalis cognate receptor for this protein has not been identified. In this study, we identified a surface protein of P. gingivalis that interacts with the SspB protein. Coprecipitation between P. gingivalis outer membrane proteins and purified SspB protein demonstrated that a 100-kDa P. gingivalis protein bound to SspB. The 100-kDa protein also bound to an engineered strain of Enterococcus faecalis that expresses the SspB protein on the cell surface. Monospecific polyclonal antibodies to the 100-kDa protein inhibited the binding between P. gingivalis and S. gordonii in a dose-dependent manner up to 86%. Amino acid sequencing of the 100-kDa protein showed homology to a protein previously identified as the P. gingivalis minor fimbria. The minor fimbrial protein may exist as a complex with a hemagglutinin-like protein since the genes encoding these proteins are adjacent on the chromosome and are cotranscribed. Thus, the P. gingivalis receptor for S. gordonii SspB is a 100-kDa protein that structurally may be a minor fimbria-protein complex and functionally effectuates coadhesion.
Porphyromonas gingivalis, a gram-negative anaerobic coccobacillus, is an etiologic agent of severe adult periodontitis, a chronic inflammatory disease that can cause destruction of periodontal tissues and resorption of the alveolar bone, with eventual exfoliation of teeth (17, 21). Colonization of the oral cavity by P. gingivalis is facilitated by adherence to various oral surfaces, including epithelial cells, the salivary pellicle that coats tooth surfaces, and other oral bacteria that comprise the plaque biofilm. P. gingivalis is a secondary colonizer of plaque, adhering to the primary colonizers including Actinomyces species and oral streptococci such as Streptococcus gordonii (11, 20, 22). In vivo studies have demonstrated that P. gingivalis preferentially colonizes preformed early plaque over other oral sites, suggesting that the interaction between the early colonizers and P. gingivalis is important in the development of pathogenic plaque (20). In vitro, P. gingivalis adheres avidly to sessile S. gordonii and, once attached, rapidly forms a biofilm comprising towering microcolonies separated by fluid-filled channels (3).
Adhesion between P. gingivalis and S. gordonii is multimodal, involving a number of distinct adhesin-receptor pairs on the surfaces of both organisms. These molecules include the major fimbriae and a 35-kDa protein of P. gingivalis and the Ssp proteins of S. gordonii (10, 12). The Ssp proteins are members of the antigen I/II family of major streptococcal surface proteins and are multifunctional adhesins (2). In S. gordonii, tandem genes encode the SspA and SspB polypeptides, which are highly similar with respect to structure and function (6). The P. gingivalis-binding domain of the SspB protein has been mapped to the C terminus within amino acid residues 1167 to 1250 (2).
The P. gingivalis cognate receptor for the SspB protein has not been identified. In this study we present evidence that a 100-kDa surface protein of P. gingivalis binds SspB and that this interaction is important for cellular coadhesion. The 100-kDa protein may represent a component of the minor fimbrial structure of P. gingivalis.
MATERIALS AND METHODS
Bacteria and culture conditions.
P. gingivalis 33277, Escherichia coli DH5α, and S. gordonii M5 were maintained as frozen stock cultures. Enterococcus faecalis EB5 was generated by transformation of E. faecalis strain S161 with shuttle vector pAM401 containing a 5.3-kb insert encoding the S. gordonii SspB peptide (4). E. faecalis 401 was generated by transformation of S161 with pAM401 that did not contain a streptococcal insert. P. gingivalis cells were cultured in Trypticase soy broth (BBL), supplemented with 1 g of yeast extract per liter, 5 mg of hemin per liter, and 1 mg of menadione per liter, under anaerobic conditions (85% N2, 10% H2, 5% CO2) at 37°C overnight. When necessary, P. gingivalis cells were metabolically labeled by including [3H]thymidine (10 μCi/ml) in the culture medium. S. gordonii M5 and enterococci were grown in Trypticase peptone broth (BBL), supplemented with yeast extract (5 g/liter) and 0.5% glucose as a carbon source, at 37°C under static conditions. E. coli DH5α cells were cultured in Luria-Bertani broth (10 g of tryptone per liter, 5 g of yeast extract per liter, 5 g of sodium chloride per liter) at 37°C with shaking. When necessary, the broth was supplemented with 100 μg of ampicillin (Sigma) per ml. Bacterial numbers were determined in a Klett-Summerson photometer.
Biotinylation and extraction of P. gingivalis surface molecules.
P. gingivalis surface molecules were labeled with biotin as previously described (12). P. gingivalis cells were washed twice in buffered KCl (5 mM KCl, 2 mM K2HPO4, 1 mM CaCl2 [pH 6.0]), resuspended in 0.1 M NaHCO3 (pH 8.1), and surface labeled with N-hydroxysuccinimidobiotin (Sigma) (3 mg/1010 cells) for 3 h at room temperature. The bacteria were washed in buffered KCl and resuspended in buffered KCl plus 10 mM EDTA. After the mixture was shaken at 37°C for 1 h and subjected to mild sonication as previously described (12), EDTA-extracted biotinylated surface molecules in the supernatant were recovered by centrifugation at 12,000 × g for 20 min. The integrity of the P. gingivalis cells following the extraction procedure was confirmed by Gram staining and by measuring viable counts.
Purification of SspB protein.
SspB was expressed in the periplasm of E. coli DH5α transformed with pUC19 containing a 5.3-kb insert encoding the S. gordonii M5 SspB peptide (4). Crude periplasmic preparations were generated by osmotically shocking washed E. coli cells as described by Heppel (8). The SspB polypeptides were further purified by chromatography of the crude periplasmic protein samples on Sepharose 6B (Pharmacia) and DEAE-Sephadex (Sigma Chemical Co.) as described by Demuth et al. (5). Purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and a single band of SspB was detected.
Coprecipitation.
Purified SspB protein (50 μg) was incubated with biotin-labeled P. gingivalis extract (109 cells) for 2 h at room temperature and then with SspB antibodies (4)(1:500 dilution) for 30 min at room temperature. Protein A-Sepharose beads (10 μg) (Amersham Pharmacia) were added and reacted at 4°C for 1 h. The beads with bound antibody molecules were recovered by centrifugation at 2,500 × g for 5 min, washed four times in phosphate-buffered saline (PBS), and resuspended in 100 μl of SDS-PAGE sample buffer. The suspension was subjected to 10% SDS-PAGE and Western blotting as previously described (14). The blots were visualized with avidin-peroxidase conjugate and 3,3′-diaminobenzidine tetrahydrochloride (Sigma) to detect biotinylated P. gingivalis membrane components that bound to SspB and were subsequently sedimented with SspB antibodies.
Binding of P. gingivalis surface molecules to engineered E. faecalis strains.
Biotinylated P. gingivalis surface molecules were reacted with E. faecalis EB5 and 401 (100 μg of protein/1010 cells) for 2 h at room temperature. The cells were washed four times with PBS containing 0.1% Tween 20 and recovered by centrifugation. The cells with adsorbed P. gingivalis molecules were resuspended in 50 μl of SDS-PAGE sample buffer, subjected to SDS-PAGE and blotted onto nitrocellulose paper as above. The membrane was blocked at room temperature for 30 min with PBS containing 0.1% Tween 20, and biotinylated P. gingivalis molecules bound to SspB were visualized by developing with avidin-peroxidase conjugate and 3,3′-diaminobenzidine tetrahydrochloride.
Purification of monospecific antibodies.
Monospecific polyclonal antibodies to the 100-kDa protein and to a control 70-kDa protein in the P. gingivalis extract were prepared by affinity purification from blots as previously described (12, 19). Briefly, the region of the blot (unstained) containing the protein of interest was excised and reacted with anti-P. gingivalis (whole-cell) antibodies diluted 1:100. After the blot was washed, bound antibodies were eluted with 0.05 M glycine hydrochloride (pH 2.3) and dialyzed against 0.05 M Tris–0.15 M NaCl (pH 8.6). The specificity of the antibodies was confirmed in an immunoblot assay with P. gingivalis extract following SDS-PAGE on a 7.5% polyacrylamide gel.
Interbacterial binding assay.
Adherence of P. gingivalis to S. gordonii M5 was determined by the nitrocellulose blot assay (13). S. gordonii cells were suspended in buffered KCl, and 108 bacteria were deposited on nitrocellulose paper in a dot-blot apparatus. The blot was washed three times in KCl containing 0.1% Tween 20 (KCl-Tween). Adsorbed bacteria were incubated for 2 h at room temperature with [3H]thymidine-labeled P. gingivalis (108 cells) suspended in KCl-Tween. After the blot was washed to remove unbound organisms, the experimental areas of the nitrocellulose were excised and the amount of interbacterial binding was measured by scintillation spectroscopy. Antibody inhibition of binding was investigated by incubating (for 1 h at 37°C) radiolabeled P. gingivalis with the antibody prior to the assay. P. gingivalis cells were collected by centrifugation (10,000 × g for 10 min), resuspended in KCl-Tween, and tested for coadhesion as above.
Amino acid sequencing.
After P. gingivalis outer membrane proteins were separated by SDS-PAGE (10% polyacrylamide), protein bands were blotted onto a polyvinylidene difluoride membrane and visualized with Coomassie brilliant blue, and the 100-kDa band was excised from the membrane. On-membrane tryptic digestion followed by high-performance liquid chromatography (HPLC) separation and peptide sequence analysis were performed at the Protein Structure Core Facility at the University of Nebraska Medical Center. Briefly, the protein band was digested with trypsin (12.5 ng/μl) for 3 h at 37°C, and the resulting peptides were separated on a Michrom MAGIC HPLC apparatus with a photo-diode array detector. The HPLC fractions were analyzed with a PerSeptive Voyager Maldi-Tof mass spectrometer to determine the approximate number of amino acids for setup of the protein sequencer. Amino acid sequence was determined by Edman degradation using an ABI 477A Sequencer.
RT-PCR.
The oligonucleotides used in the PCR are listed in Table 1. Reverse transcription (RT) was performed in the presence of 2 μg of RNA, 50 ng of antisense primer, 50 U of reverse transcriptase (Ambion), 13 U of RNase inhibitor, 10 mM deoxynucleoside triphosphate, and 1× RT buffer. Annealing of the primer and RNA was carried out at 72°C for 2 min and then at 48°C for 1 h. The resulting cDNA was amplified, with each 100 μl of PCR mixture containing 1× PCR buffer, 3 μl of cDNA, 1.5 mM MgCl2, 10 mM deoxynucleoside triphosphate, 100 ng of each primer, and 2.5 U of Herculase DNA polymerase (Stratagene). The amplification conditions were 30 cycles of denaturation at 96°C for 1 min, annealing at 45°C for 30 s, and elongation at 72°C for 3 min.
TABLE 1.
Oligonucleotide sequences used in PCR
| Oligonucleotide | ORF locationb | Sequence |
|---|---|---|
| ORF1-1 | ORF upstream of the area encoding the SspB-binding protein (bp 139127–139147) | 5′-GTA GAA GAT GGT GGA ATA ATG-3′ |
| ORF2-1 | ORF encoding the SspB-binding protein (bp 140031–140050) | 5′-GGT GCT CAA TGA CAT CGC AG-3′ |
| ORF2-2a | ORF encoding the the SspB-binding protein (bp 141143–141163) | 5′-GAT TGT TAG GAT CCG GAT CCG-3′ |
| ORF3-2a | ORF downstream of the area encoding the SspB-binding protein (bp 141963–141982) | 5′-GGA TGA GGC AGA CTA TCT AC-3′ |
Antisense oligonucleotide.
Base pair location from the TIGR database (http://www.tigr.org).
RESULTS
Coprecipitation of P. gingivalis molecules with purified SspB protein.
To identify molecules of P. gingivalis that interact with SspB protein, surface proteins were first labeled with biotin and extracted with EDTA and mild sonication. Biotinylation has been demonstrated to predominantly label cell surface molecules (1, 15). In addition, EDTA and mild agitation has been shown to extract specifically outer membrane components from gram-negative bacteria including P. gingivalis (9, 12). Coupled with the finding that the extraction procedure did not cause noticeable cell disruption, as determined by Gram staining and by measuring viable counts, it is reasonable to conclude that the biotinylated molecules in this preparation are surface exposed. Biotinylated P. gingivalis molecules were reacted with purified SspB protein followed by SspB antibodies and protein A-Sepharose beads. Figure 1A shows that a 100-kDa band from P. gingivalis interacted with purified SspB protein.
FIG. 1.
(A) Western blot analysis of the biotinylated P. gingivalis 33277 outer membrane extract coprecipitated with purified SspB protein. Lanes: 1, outer membrane extract only; 2, outer membrane extract coprecipitated with SspB protein; 3, SspB protein without outer membrane extract. (B) Western blot analysis of the biotinylated P. gingivalis 33277 outer membrane extract binding to E. faecalis strains. Lanes: 1, outer membrane extract only; 2, outer membrane extract reacted with E. faecalis EB5; 3: outer membrane extract reacted with E. faecalis 401.
Binding of biotinylated P. gingivalis extract to E. faecalis strains.
To determine whether the 100-kDa molecule reacts with SspB when the latter is presented on a gram-positive cell surface, outer membrane extract was reacted with E. faecalis EB5 and 401. Interactive molecules were identified by SDS-PAGE followed by Western blotting. The 100-kDa protein from P. gingivalis interacted with E. faecalis EB5, which expresses SspB on the cell surface, but not with control E. faecalis 401 (Fig. 1B).
Inhibition of binding between P. gingivalis and S. gordonii by 100-kDa antibodies.
To examine the role of the interaction between SspB and the 100-kDa protein in coadhesion between P. gingivalis and S. gordonii whole cells, monospecific polyclonal antibodies to the 100-kDa protein were tested for inhibition of cell-cell binding. As a control, antibodies to a P. gingivalis 70-kDa protein that did not interact with SspB were also tested. Western blotting confirmed that these antibody preparations were specific for their target antigens (Fig. 2). The 100-kDa protein antibodies were able to inhibit the binding between P. gingivalis and S. gordonii by up to 86% in a dose-dependent manner (Fig. 3). Antibodies to the 70-kDa protein did not affect the binding between P. gingivalis and S. gordonii. In an enzyme-linked immunosorbent assay, the 100-kDa antibodies also reacted with formalinized whole P. gingivalis cells, providing additional evidence that the 100-kDa molecule is located on the surface (data not shown).
FIG. 2.
Western blot of P. gingivalis outer membrane extract reacted with antibodies to whole P. gingivalis cells (lane 1), the 100-kDa protein (lane 2), and the 70-kDa protein (lane 3).
FIG. 3.
Dose-dependent inhibition of coadhesion between P. gingivalis and S. gordonii by 100-kDa antibodies or by 70-kDa control antibodies (Ab). The number of P. gingivalis cells bound to streptococci in the absence of antibodies was 4.1 × 107 from an input cell number of 1 × 108. Error bars represent standard errors of the means of three experiments.
Sequence analysis.
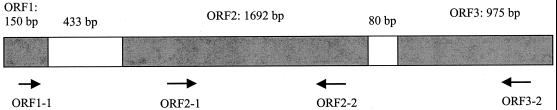
The amino acid sequence of an internal region of the 100-kDa outer membrane protein of P. gingivalis was VLYXAWLNPSTXSPDSGXN, where X signifies an amino acid that could not be determined. Table 2 shows that this amino acid sequence has 78% identity to the 67-kDa minor fimbrillin from P. gingivalis strain 33277 (GenBank accession no. BAA86887). Similar homology was also found to P. gingivalis proteins reported as outer membrane components of 67 and 72 kDa (GenBank accession no. ABO16284 and 1089992). Thus, these deposited sequences would all appear to represent the same protein independently isolated from different strains. The degree of homology may be greater than we were able to determine, given that 3 of the 19 amino acids could not be identified. A search of the P. gingivalis strain W83 database in The Institute for Genomic Research (http://www.tigr.org) with the National Center for Biotechnology Information ORF-Finder revealed additional open reading frames (ORFs) upstream and downstream of the gene of interest (Fig. 4). ORF3 (80 bp downstream) has a 30-amino-acid region with 46% identity to the genes encoding hemagglutinin proteins HagB and HagC (GenBank accession no. CAA84627 and CAA81786, respectively). No homologies were observed for the putative product of ORF1 (433 bp upstream). The theoretical molecular masses of these ORF products was estimated using the ExPASy (Expert Protein Analysis System; Swiss Institute of Bioinformatics, Geneva, Switzerland) Compute pI/Mw Tool. The estimated molecular mass of the ORF2 product is 61 kDa, and that of the ORF3 product is 35 kDa. Thus, the protein of approximately 100 kDa that we detected may be a complex of the products of ORF2 and ORF3 (theoretically 96 kDa).
TABLE 2.
Homologies of amino acid sequence from the 100-kDa protein to previously published P. gingivalis proteins
| Protein (GenBank accession no.) | Sequencea | Identity |
|---|---|---|
| 67-kDa minor fimbrillin (BAA86887) | VLYXAWLNPSTXSPDSGXN | 15/19 (78%) |
| VLYYAWLNPSTTSPDSWWN | ||
| 67-kDa major outer membrane protein (ABO16284) | VLYXAWLNPSTXSPDSGXN | 15/19 (78%) |
| VLYYAWLNPSTTSPDSWWN | ||
| 72-kDa major cell surface protein (1089992) | VLYXAWLNPSTXSPDSGXN | 15/19 (78%) |
| VLYYAWLNPSTTSPDSWWN |
The 100-kDa protein sequence is given in bold letters. X denotes an amino acid that could not be determined. Identical amino acids are underlined.
FIG. 4.
Arrangement and the sizes of the ORF containing SspB binding protein (ORF2) and the ORFs immediately upstream and downstream in strain W83. White areas indicate a noncoding region, and shaded areas indicate ORFs. Arrows indicate the positions and directions of the primers used in RT-PCR.
RT-PCR.
Since the data indicated that the products of ORF2 and ORF3 may be complexed together, we tested for cotranscription by using RT-PCR with the primers depicted in Fig. 4. Figure 5 shows that mRNA can be detected that spans ORF2 and ORF3 (the product of primers ORF2-1 and ORF3-2). In contrast, no PCR product was obtained with primers ORF1-1 and ORF2-2, indicating that ORF1 is not transcribed with ORF2.
FIG. 5.
RT-PCR of strain 33277 mRNA expressed from the ORF encoding the SspB-binding protein and the ORFs upstream and downstream. Lanes: 1, primers ORF1-1 and ORF2-2; 2, primers ORF2-1 and ORF2-2; 3, primers ORF2-1 and ORF3-2.
DISCUSSION
Bacterial accretion through coadhesion drives the temporal development of the plaque biofilm, a process characterized by bacterial successions occurring over a time frame of weeks. Interbacterial coadhesion is a common occurrence that has been demonstrated for over 700 strains representing 14 genera (22) and is readily visible in undisturbed plaque (16). Primary commensal colonizers, such as S. gordonii, can provide attachment sites for colonization by P. gingivalis, and this attachment is mediated, at least in part, by the Ssp proteins of S. gordonii (2). This study identified a 100-kDa protein of P. gingivalis as a receptor for the S. gordonii SspB protein. Several lines of evidence suggested that the 100-kDa protein is the cognate receptor for SspB. First, the 100-kDa protein coprecipitated with purified SspB protein. Second, the 100-kDa protein interacted with an E. faecalis strain expressing SspB on the cell surface but not with a control strain devoid of SspB. Furthermore, antibodies to the 100-kDa protein inhibited the binding between whole cells of P. gingivalis and S. gordonii in a dose-dependent manner. Collectively, these data provide strong support for the notion that the 100-kDa protein is a functional receptor for SspB.
SspB-mediated binding is only one component of the multimodal interaction between P. gingivalis and S. gordonii. Other adhesins include the major fimbriae and a 35-kDa outer membrane protein of P. gingivalis. Nonetheless, 100-kDa antibodies were capable of inhibiting coadhesion by up to 86%, a level of inhibition similar to that observed with Ssp antibodies (12). This indicates that the SspB–100-kDa protein interaction may be the most important component of the overall adhesive interaction. We can postulate, therefore, that the major fimbriae of P. gingivalis may be required for the initial association between the two organisms and that binding is then stabilized by Ssp–100-kDa protein interactions. This concept is supported by our preliminary observations that a mutant of P. gingivalis that lacks the major fimbriae (18) is unable to attach to S. gordonii when mild shear forces are present but will attach to almost wild-type levels when initially centrifuged onto S. gordonii (Y. Park, J. W. Costerton, G. S. Cook, D. R. Demuth, and R. J. Lamont, Abstr. 77th Meet. Int. Assoc. Dent. Res., abstr. 2514, 1999).
The identity of the 100-kDa protein was suggested by a BLAST search of the GenBank database (http://www.ncbi.nlm.nih.gov/BLAST). Of 19 amino acids sequenced from the 100-kDa protein, 15 (78%) are identical to a 67-kDa protein identified as the minor fimbrial subunit protein of P. gingivalis (7). The P. gingivalis minor fimbriae were first discovered as proteinaceous appendages on the surface of a mutant of strain 381 deficient in production of fimbrillin (FimA, the structural component of the major fimbriae). This secondary fimbrial structure is thus distinct from the 43-kDa FimA in size and in antigenicity (7). Analysis of the nucleotide sequence in the TIGR P. gingivalis W83 database in the region containing the gene for the 67-kDa minor fimbrial protein revealed additional ORFs upstream and downstream. RT-PCR demonstrated that the minor fimbrial gene and the downstream gene are cotranscribed in strain 33277 and could potentially produce a multimeric protein of 96 kDa, a size consistent with the molecular mass of the protein we identified. This downstream ORF is 975 bp long and has homology to the genes encoding the hemagglutinin proteins HagB and HagC. Thus, it would appear that the minor fimbriae and a protein with potential hemagglutinating activity are cotranscribed and exist as a complex on the cell surface. Functionally, this complex (which may contain additional molecules) is capable of binding to SspB. There is a 433-bp gap between the ORF encoding the 67-kDa minor fimbrillin and the next upstream ORF. This upstream ORF is only 150 bp in size and may be too small to code for a functional protein.
In conclusion, we identified a P. gingivalis outer membrane receptor for the SspB protein of S. gordonii. This protein may be the structural component of the minor fimbriae in association with a hemagglutinin. The binding reaction mediated by this protein is a major component of the coadhesion between cells of P. gingivalis and S. gordonii.
ACKNOWLEDGMENT
This research was funded by NIDCR grant DE12505.
REFERENCES
- 1.Bradburne J A, Godfrey P, Choi J H, Mathis J N. In vivo labeling of Escherichia coli cell envelope proteins with N-hydroxysuccinimide esters of biotin. Appl Environ Microbiol. 1993;59:663–668. doi: 10.1128/aem.59.3.663-668.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks W, Demuth D R, Gil S, Lamont R J. Identification of a Streptococcus gordonii SspB domain that mediates adhesion to Porphyromonas gingivalis. Infect Immun. 1997;65:3753–3758. doi: 10.1128/iai.65.9.3753-3758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cook G S, Costerton J W, Lamont R J. Biofilm formation by Porphyromonas gingivalis and Streptococcus gordonii. J Periodontal Res. 1998;33:323–327. doi: 10.1111/j.1600-0765.1998.tb02206.x. [DOI] [PubMed] [Google Scholar]
- 4.Demuth D R, Berthold P, Leboy P S, Golub E E, Davis C A, Malamud D. Saliva-mediated aggregation of Enterococcus faecalis transformed with a Streptococcus sanguisgene encoding the SSP-5 surface antigen. Infect Immun. 1989;57:1470–1475. doi: 10.1128/iai.57.5.1470-1475.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demuth D R, Davis C A, Corner A M, Lamont R J, Leboy P S, Malamud D. Cloning and expression of a Streptococcus sanguissurface antigen that interacts with a human salivary agglutinin. Infect Immun. 1988;56:2484–2490. doi: 10.1128/iai.56.9.2484-2490.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demuth D R, Duan Y, Brooks W, Holmes A R, McNab R, Jenkinson H F. Tandem genes encode cell-surface polypeptides SspA and SspB which mediate adhesion of the oral bacterium Streptococcus gordoniito human and bacterial receptors. Mol Microbiol. 1996;20:403–413. doi: 10.1111/j.1365-2958.1996.tb02627.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamada N, Sojar H T, Cho M, Genco R J. Isolation and characterization of a minor fimbria from Porphyromonas gingivalis. Infect Immun. 1996;64:4788–4794. doi: 10.1128/iai.64.11.4788-4794.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heppel L A. The concept of periplasmic enzymes. In: Rothfield L I, editor. Structure and function of biological membranes. New York, N.Y: Academic Press, Inc.; 1971. pp. 223–247. [Google Scholar]
- 9.Hofstra H, Dankert J. Major outer membrane proteins: common antigens in Enterobacteriaceae species. J Gen Microbiol. 1980;119:123–131. doi: 10.1099/00221287-119-1-123. [DOI] [PubMed] [Google Scholar]
- 10.Lamont R J, Bevan C A, Gil S, Persson R E, Rosan B. Involvement of Porphyromonas gingivalis fimbriae in adherence to Streptococcus gordonii. Oral Microbiol Immunol. 1993;8:272–276. doi: 10.1111/j.1399-302x.1993.tb00573.x. [DOI] [PubMed] [Google Scholar]
- 11.Lamont R J, Hersey S G, Rosan B. Characterization of the adherence of Porphyromonas (Bacteroides) gingivalisto oral streptococci. Oral Microbiol Immunol. 1992;7:193–197. doi: 10.1111/j.1399-302x.1992.tb00024.x. [DOI] [PubMed] [Google Scholar]
- 12.Lamont R J, Hsiao G W, Gil S. Identification of a molecule of Porphyromonas gingivalis that binds to Streptococcus gordonii. Microb Pathog. 1994;17:355–360. doi: 10.1006/mpat.1994.1081. [DOI] [PubMed] [Google Scholar]
- 13.Lamont R J, Rosan B. Adherence of mutans streptococci to other oral bacteria. Infect Immun. 1990;58:1738–1743. doi: 10.1128/iai.58.6.1738-1743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lamont R J, Rosan B, Murphy G M, Baker C T. Streptococcus sanguissurface antigens and their interactions with saliva. Infect Immun. 1988;56:64–70. doi: 10.1128/iai.56.1.64-70.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levy-Toledano R, Caro H, Hindman N, Taylor S I. Streptavidin blotting: a sensitive technique to study cell surface proteins; application to investigate autophosphorylation and endocytosis of biotin-labeled insulin receptors. Endocrinology. 1993;133:1803–1808. doi: 10.1210/endo.133.4.8404622. [DOI] [PubMed] [Google Scholar]
- 16.Listgarten M A, Mayo H E, Tremblay R. Development of dental plaque on epoxy resin crowns in man. J Periodontol. 1975;46:10–26. doi: 10.1902/jop.1975.46.1.10. [DOI] [PubMed] [Google Scholar]
- 17.Loesche W J. Bacterial mediators in periodontal disease. Clin Infect Dis. 1993;16:S203–S210. doi: 10.1093/clinids/16.supplement_4.s203. [DOI] [PubMed] [Google Scholar]
- 18.Love R M, McMillan M D, Park Y, Jenkinson H F. Coinvasion of dentinal tubules by Porphyromonas gingivalis and Streptococcus gordoniidepends upon binding specificity of streptococcal antigen I/II adhesin. Infect Immun. 2000;68:1359–1365. doi: 10.1128/iai.68.3.1359-1365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olmsted J B. Affinity purification of antibodies from diazotized paper blots of heterogeneous protein samples. J Biol Chem. 1981;256:11955–11957. [PubMed] [Google Scholar]
- 20.Slots J, Gibbons R J. Attachment of Bacteroides melaninogenicus subsp. asaccharolyticusto oral surfaces and its possible role in colonization of the mouth and periodontal pockets. Infect Immun. 1978;19:254–264. doi: 10.1128/iai.19.1.254-264.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Socransky S S, Haffajee A D. The bacterial etiology of destructive periodontal disease: current concepts. J Periodontol. 1992;63:322–331. doi: 10.1902/jop.1992.63.4s.322. [DOI] [PubMed] [Google Scholar]
- 22.Whittaker C J, Klier C M, Kolenbrander P E. Mechanisms of adhesion by oral bacteria. Annu Rev Microbiol. 1996;50:513–552. doi: 10.1146/annurev.micro.50.1.513. [DOI] [PubMed] [Google Scholar]