Abstract
The objective of this study was to investigate the effects of tributyrin supplementation on liver fat metabolism in broiler chickens. Two hundred and forty broilers were randomly allocated into two experimental groups (6 replicates per treatment; 20 chickens in each replicate): the control group (CN), which received a basal diet, and the tributyrin group (TB), which received a basal diet supplemented with 1 g/kg of tributyrin. The experimental period lasted 37 days. The results showed that in the liver, broilers supplemented with tributyrin had higher content of high-density lipoprotein cholesterol (HDL-C) (p < 0.05). Liver hepatic lipase (HL), lipoprotein lipase (LPL) and total lipid (TL) activity were significantly lower than in the TB group than that in the NC group. Meanwhile, the diet supplemented with tributyrin had more lipid droplets than the NC group, whereas the TB and NC groups showed no histological abnormalities in the liver. Furthermore, the mRNA expression levels of peroxisome proliferators-activated receptor α (PPARα), proliferators-activated receptor γ (PPARγ), fatty acid synthase (FAS), LPL and adipose triglyceride lipase (ATGL) in the liver were significantly upregulated in the TB group (p < 0.05), while those of the long-chain acyl-CoA-synthetase 1 (ACSL1) mRNA between the TB group and the NC group were not different (p > 0.05). These findings indicated that the diet supplemented with tributyrin could increase fat deposition appropriately by promoting fat synthesis without causing liver tissue damage, which demonstrated that tributyrin can be considered a valid feed additive for broiler chickens.
Keywords: tributyrin, fat deposition, lipid metabolism, gene expression, broiler chicken
1. Introduction
Chickens are important components of human food, and appropriate fat deposition in chicken meat contributes significantly to its quality attributes, such as juiciness, flavor, taste and other organoleptic properties. However, excessive fat deposition in broiler chickens will not only induce broiler ascites syndrome, sudden death and other metabolic diseases, but could also lead to adverse effects in the consumer’s health [1]. Therefore, it is imperative to find suitable additives for fat deposition.
Tributyrin (TB) is a triglyceride containing three butyrate moieties, and one molecule of tributyrin releases three molecules of butyrate directly in the small intestine, which could be rapidly adsorbed [2,3]. Supplementation of tributyrin showed positive effects on growth performance and gut health in pig and rat [4,5,6]. In pig, a basal diet supplemented with tributyrin significantly increased production traits and nutrient metabolism to regulate lipid metabolism [4]. In rat, treatment with tributyrin attenuated diet-induced obesity and associated insulin resistance [6]. Moreover, regarding beneficial effects, tributyrin administration can also attenuate lipopolysaccharide-induced liver injury through gut regulation [7,8]. Therefore, these findings indicated that tributyrin could modulate liver lipid metabolism. However, there are no other studies investigating the effects of dietary tributyrin on lipid metabolites in healthy chickens.
In birds, the liver is the main site of lipogenesis [9], contributing 80 to 85% of the fatty acids stored in adipose tissue [10]. As a result, most of the endogenous body lipids are of hepatic origin, and the development of adipose tissue depends on the availability of plasma triglycerides that are hydrolyzed prior to their utilization by adipocytes [11,12,13]. Liver fatty acid metabolism, as the most important lipid metabolic pathway, is widely involved in fat deposition [14,15]. These results prompted studies on gene expression in the liver, especially those genes involved in lipogenesis and lipolysis [16,17,18], including peroxisome proliferators-activated receptor α (PPARα), peroxisome proliferators-activated receptor γ (PPARγ), fatty acid synthase (FAS), adipose triglyceride lipase (ATGL) and lipoprotein lipase (LPL). However, little is known about the effect of tributyrin on hepatic gene expression in poultry, especially in broiler chickens.
Broiler chickens have a high propensity for lipid biosynthesis. It is reported that total body lipids of growing chickens double every 5.5 days, and they reach the maximum rate of hepatic fatty acid synthesis at 7 weeks of age [19]. The aim of the present study was to explore the effect of tributyrin on hepatic lipid metabolism and lipid metabolism-related gene expression in broiler chickens, which might help to identify the underlying mechanism of tributyrin in modulating fat deposition in liver tissue.
2. Materials and Methods
2.1. Ethics Statement
Animals used in this study were raised and slaughtered in accordance with the national standard of Laboratory animal Guideline for ethical review of animal welfare (GB/T 35892-2018), issued by General Administration of Quality Supervision, Inspection and Quarantine of the people’s Republic of China and Standardization Administration of the People’s Republic of China. All experiment procedures were approved by the Institute of Animal Husbandry and Veterinary Science, Zhejiang Academy of Agricultural Sciences (Hangzhou, China).
2.2. Animals and Experimental Model
Two hundred and forty 26-day-old Hexi dwarf female broilers were randomly allotted into two experimental groups with similar conditions to those under which commercial farm animals were kept before the first day of the trial. After one week of adaptation, the control group (NC) received a basal diet, while the TB group received the same basal diet supplemented with 1 g/kg of dietary tributyrin [20] (Jinfulai Technology Development Co., Ltd., Harbin, China). All groups consisted of 6 replicates per treatment and 20 chickens in each replicate. The dietary nutrient levels were based on National Research Council recommended nutrient requirements for broiler chickens (Table 1). The experimental period lasted 37 days. After the experimental period, six broilers in each group were randomly selected for blood and liver sample collection.
Table 1.
The composition and nutrient levels of the experimental diets.
| Items | 26~40 d | 41~62 d |
|---|---|---|
| Ingredient (%) | ||
| Wheat | 77.96 | 81.51 |
| Soybean meal | 5.65 | 0 |
| Sunflower meal | 3.5 | 4.5 |
| Peanut meal | 4 | 4.25 |
| Corn gluten meal | 1.56 | 1.28 |
| Feather meal | 0 | 1 |
| Lard | 3.14 | 3.46 |
| Calcium bicarbonate | 0.63 | 0.47 |
| Stone powder | 1.36 | 1.33 |
| Premix 1 | 2.2 | 2.2 |
| Nutrient composition, calculated | ||
| Nitrogen-corrected apparent metabolizable energy (MJ/kg) | 12.76 | 12.97 |
| Crude protein (%) | 17.50 | 16.50 |
| Crude fat (%) | 4.76 | 5.17 |
| Calcium (%) | 0.8 | 0.75 |
| Phosphorus (%) | 0.3 | 0.28 |
| Lysine (%) | 0.90 | 0.85 |
| Methionine (%) | 0.45 | 0.37 |
1 Premix supplied the following per kilogram of diet: NaHCO3 90 g; NaCl 90 g; vitaminA 10,000 IU; vitaminD 33,000 IU; vitaminE 30 mg; vitaminK 31.3 mg; vitaminB 120.013 mg; thiamine 2.2 mg; riboflavin 8 mg; nicotinamide 40 mg; choline chloride 600 mg; calcium pantothenate 10 mg; pyridoxine·HCl 4 mg; biotin 0.04 mg; folic acid 1 mg; Fe 80 mg; Cu 7.5 mg; Mn 110 mg; Zn 65 mg; I 1.1 mg; Se 0.3 mg. Moreover, 26 to 40 days includes methionine 90 g; threonine 70 g; lysinesulphate 361 g, while 41 to 62 days includes methionine 68 g; threonine 89 g; lysinesulphate 406 g.
2.3. Sample Collection
After 37 days, blood samples from the jugular vein of six broiler chickens per treatment (1 broiler per replicate) were collected into pro-coagulation tubes and maintained for 2 h at room temperature. All samples were centrifuged at 3000 rpm for 10 min at 4 °C. Serum was removed and the aliquots were stored at −20 °C for further analysis. Broilers were euthanized with an ear intravenous injection of sodium pentobarbital (200 mg/kg BW). The liver tissue samples were collected and immediately snap-frozen in liquid nitrogen and fixed in 4% paraformaldehyde for RNA and histological analysis, respectively.
2.4. Biochemical Analysis
Liver total cholesterol (TC), triglycerides (TG), high-density lipoproteincholesterol (HDL-C) and low-density lipoproteincholesterol (LDL-C) were measured using commercially available kits (Jiancheng Biotechnology Inc., Nanjing, China), following the manufacturers’ instructions. Hepatic lipase (HL), LPL and total lipid (TL) activities were determined using liver tissue HL, LPL and TL kits (Jiancheng Biotechnology Inc., Nanjing, China), following the manufacturers’ instructions.
2.5. Histological Observation
Liver tissues fixed in 4% paraformaldehyde were subjected to a standard hematoxylin and eosin (H&E) staining and then cut into 10 μm thin layers. The obtained slices were subjected to a dry-wash cycle (60 min); after that, the dried slices were rinsed in 60% isopropanol and then stained with Oil Red O solutions (10 min). After differentiation in 60% isopropanol, distilled water was used to wash twice, then restained with Mayer’s hematoxylin (2 min), washed twice with distilled water (10 min), dried and embedded with the aqueous medium. The slices were observed using a Nikon E100 microscope, and pictures were taken at a magnification of 400×.
2.6. Gene Expression Analysis
Total RNA was isolated from the liver samples using TRIzol reagent (TAKARA, Dalian, China), and then the single-stranded cDNA was synthesized. All cDNA samples were stored at −20 °C until used. A 20 μL reaction mixture contained 10 μL of 2× Power SYBR® Green Master Mix (Applied Biosystems, Waltham, MA, USA), 0.5 μL of each primer, 1 μL of template cDNA, and 8 μL of double-distilled water (ddH2O). The amplification reaction consisted of 95 °C for 30 s, then 40 cycles at 95 °C for 15 s and 63 °C for 25 s. Glyceraldehyde 3-phosphatedehydrogenase (GAPDH) was used as the housekeeping gene, and the normalized target gene expression level in the sample was calculated using the formula 2−ΔΔCt. The primers were designed using Primer Premier 5.0 software and synthesized by Sangon Biotech (Shanghai, China), and they are listed in Table 2.
Table 2.
Primers used in the study.
| Gene | Genbank Accession | Primer Sequences (5′→3′) | Size (bp) | Annealing (°C) |
|---|---|---|---|---|
| β-actin | NM_205518.1 | CTGAACCCCAAAGCCAACAGA | 120 | 60 |
| AGTGGTACGACCAGAGGCATACA | ||||
| LPL | NM_205282.2 | CAGTGTCTGCTGCTTACACGAA | 101 | 60 |
| CAAGTGGACATTGTTGAGAGGGTAA | ||||
| ACSL1 | XM_040698931.1 | CGGACAGAGCAGAGTATGTG | 74 | 60 |
| GCCTACGTACTGGCTGTGA | ||||
| PPARγ | NM_001001460.1 | CATGCATCACCACTGCAGGAA | 83 | 60 |
| ACTGCCTCCACAGAGCGAAA | ||||
| PPARα | NM_001001464.1 | GGAGTACATGCTTGTGAAGGTTG | 148 | 60 |
| CTGAAAGGCACTTCTGAAAACGACA | ||||
| FAS | NM_205155.3 | CAAGCCTGGAGATGTGGAGTAT | 154 | 60 |
| CTCTGGATGACCCATGTTTGAC | ||||
| ATGL | EU240627.2 | CTGACAACTTGCCACGATATGAG | 149 | 60 |
| GAGGTTGCGAAGGTTGAATTGGA |
2.7. Statistical Analysis
Data are presented as the mean ± standard error (SE) and processed by the statistical package for the SPSS 25.0 software (Chicago, IL, USA). t-test analysis was used to assess the differences between groups. Differences were considered statistically significant at p < 0.05.
3. Results
3.1. Effect of Tributyrin on Lipid Levels in Liver Tissues
To determine whether dietary addition of TB could affect fat deposition in the liver, the contents of TC, TG, HDL-C and LDL-C were observed. As indicated in Table 3, TB treatment significantly increased the content of HDL-C (p < 0.05), while no difference between the control and TB groups in the liver content of TC, TG and LDL-C (p > 0.05) was observed. On the other hand, broilers fed the diet supplemented with TB had significantly decreased values of the liver TL, HL and LPL activity.
Table 3.
Effect of TB on liver lipid parameters in broiler chickens.
| NC | TB | p-Value | |
|---|---|---|---|
| TC (mmol/L) | 0.0645 ± 0.0079 | 0.0578 ± 0.0059 | 0.129 |
| TG (mmol/L) | 0.1248 ± 0.0187 | 0.1073 ± 0.0135 | 0.095 |
| HDL-C (mmol/L) | 0.0049 ± 0.0008 b | 0.0071 ± 0.0005 a | <0.001 |
| LDL-C (mmol/L) | 0.0532 ± 0.0058 | 0.0480 ± 0.0057 | 0.149 |
TC, total cholesterol; TG, triglycerides; HDL-C, high-density lipoproteincholesterol; LDL-C, low-density lipoproteincholesterol. Data are presented as means ± SE (n = 6). Different superscripts indicate significant differences between groups (a, b: p ≤ 0.05).
3.2. Effect of Tributyrin on Enzymatic Activity in Liver Tissues
As shown in Table 4, the hepatic activity of TL in the TB supplementation group was significantly lower than in the control group (p < 0.05). Meanwhile, the activity of HL and LPL in the liver were also significantly lower in the TB supplementation group (p < 0.05).
Table 4.
Effect of TB on liver lipid parameters in broiler chickens.
| NC | TB | p-Value | |
|---|---|---|---|
| TL (U/mg.prot) | 9.14 ± 0.73 a | 6.30 ± 0.96 b | <0.001 |
| HL (U/mg.prot) | 1.78 ± 0.32 a | 1.23 ± 0.14 b | 0.003 |
| LPL (U/mg.prot) | 7.36 ± 0.47 a | 5.08 ± 0.84 b | <0.001 |
TL, totallipase; HL, hepatic lipase; LPL, lipoproteinlipase. Data are presented as means ± SE (n = 6). Different superscripts indicate significant differences between groups (a, b: p ≤ 0.05).
3.3. Histological Observations of Liver Tissues
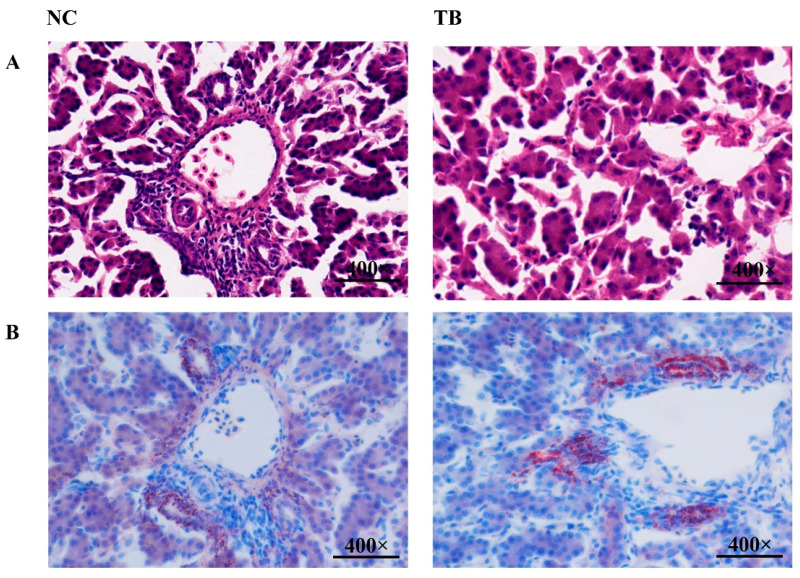
To confirm the changes in liver tissue after TB supplementation, histological analysis was performed, and the NC and TB groups showed no histological abnormalities in the liver (Figure 1A). To further confirm the distribution and abundance of lipid droplets, the liver tissue was subjected to Oil Red O staining. It was found that the TB group had more lipid droplets than the NC group (Figure 1B).
Figure 1.
TB increased fat deposition in the liver tissue. (A) H&E staining of liver tissue. (B) Oil Red O staining.
3.4. Effect of Tributyrin on Liver mRNA Expression of Lipogenesis and Lipolysis-Related Genes
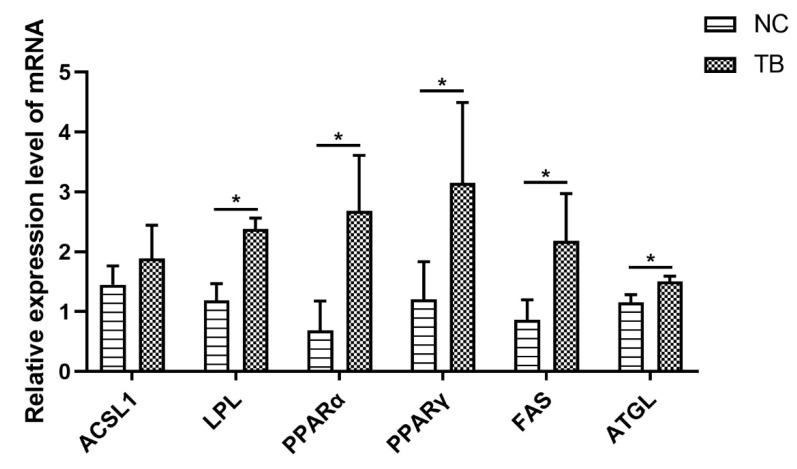
To further determine the underlying mechanisms of tributyrin involved in liver lipid deposition, the expression levels of genes that are related to lipogenesis and lipolysis were measured (Figure 2). Among lipid uptake genes, the mRNA expression levels of LPL in the TB group were significantly higher than that in the control group (p < 0.05), while there was no difference between the control and TB groups in ACSL1 mRNA expression (p > 0.05). The key lipogenesis genes, including PPARα, PPARγ and FAS, were also compared in the two groups. It was found that in comparison to the control group, TB treatment significantly increased PPARα, PPARγ and FAS mRNA expression levels (p < 0.05). Finally, TB was found to increase the expression of the ATGL gene related to lipolysis in the liver (p < 0.05).
Figure 2.
TB improved the lipid metabolism in the liver. Values are expressed as the mean ± SE (n = 4). * indicates significant difference (p < 0.05).
4. Discussion
With its long history as a delicious food source, chicken has proven highly popular with consumers. Meanwhile, appropriate fat deposition in broiler chickens will improve the flavor of meat quality, which makes the meat more delicious [21]. Glyceryl butyrate has beneficially improved the growth performance and carcass yield in broiler chickens [22,23,24]. Moreover, TB contains the butyrate moieties and could be rapidly adsorbed by releasing the butyrate directly in the small intestine [2,3]. In birds, lipogenesis takes place primarily in the liver, whereas adipocyte serves as the storage site for triglycerides [9]. Despite the wealth of information indicating that tributyrin alters lipid metabolism in animals [4,6], little is known about the effect of tributyrin on lipid metabolism and hepatic gene expression in poultry, especially in broiler chickens.
The HDL-C can transport the free cholesterol accumulated in peripheral tissues and the lipoproteins in circulation to the liver cells, accelerating the removal of cholesterol and thus playing an important role in minimizing atherosclerosis [25,26]. In the present study, broiler chickens fed with TB-containing diets showed increased content of HDL-C in liver tissue, while no statistically significant differences were observed for TC, TG and LDL-C content. These results indicated that TB could accelerate the accumulation of HDL cholesterol in circulation.
The lipid mobilization includes lipogenesis and lipolysis, which are in a dynamic balance under normal conditions [27]. The HL, as an important lipid metabolic enzyme produced primarily by the liver, mainly modulates the reaction responsible for transferring cholesterol from the peripheral tissue to the liver [28]. The LPL is synthesized in parenchymal cells in fat, myocardium, skeletal muscle and breast tissues, which has functional similarities with HL. HL has been found to catalyze and break the TG into fatty acids and monoglycerides for use in aerobic metabolism or for fat storage. The liver takes up the unesterified cholesterol accumulated in HDL with the help of HL. This could prevent excess cholesterol accumulation in the liver’s peripheral tissues [29,30]. This study demonstrated a pronounced increase in HL, LPL and TL activity after the administration of TB in broiler chickens, which resulted in increased lipid synthesis and accelerated lipolysis [28,31]. Histological analysis and Oil Red O staining further demonstrated that TB dietary supplementation accelerated liver fat deposition with no adverse effects on hepatic functionality. We speculated that TB might positively affect liver lipid regulation based on the findings mentioned above.
Fat accumulation is a complex process characterized by many gene expression changes controlling lipogenesis and lipolysis [1]. LPL, a classical lipid metabolic enzyme, is involved in liver fatty acid metabolism [32], and ACSL1 is also involved in the regulation of lipid metabolism uptake [33]. Our study showed that the mRNA expression level of LPL in the TB group was higher than in the NC group, while the ACSL1 mRNA level showed no difference between the TB and NC groups. The result suggested that TB may improve lipid uptake of chicken broilers by regulating the LPL level. The PPARs are a superfamily of nuclear receptors that play a significant role in adipocyte cell differentiation and intra- and extracellular transportation of fatty acid [34,35]. Both PPARα and PPARγ mainly influence fatty acid metabolism and modulate the lipid accumulation via increased expression of LPL [36]. In the present study, we found that TB treatment significantly upregulated the LPL, PPARα and PPARγ mRNA levels, showing similarities with a previous study where oral tributyrin increased the hepatic PPARα and PPARγ gene expression to attenuate LPS-induced lipid metabolism abnormalities [37]. Based on these results, supplementation with TB could play a positive role in liver lipogenesis in chicken broilers. Moreover, ATGL is the key lipase involved in the lipolysis process, and these enzymes hydrolyze the triacylglycerols to monoacylglycerols and other lipids in various tissues [38,39]. The TB group had a higher ATGL mRNA expression than the NC group, which indicated that TB might improve the liver lipolysis of chicken broilers. These data suggest that TB plays a more relevant role in balancing lipogenesis and lipolysis.
5. Conclusions
The present study showed that supplementation with tributyrin significantly elevated HDL-C content and decreased the HL, LPL and TL activity. Broiler chickens treated with tributyrin promoted fat deposition without negative effects on liver morphology. Moreover, the PPARα, PPARγ, FAS, LPL and ATGL mRNA levels were significantly upregulated. All these data suggested that tributyrin could promote liver fat deposition without resulting in excessive accumulation. For these reasons, it would be interesting to evaluate and investigate the meat quality in detail.
Author Contributions
Conceptualization, T.G.; methodology, M.D. and J.L.; formal analysis, T.G. and L.C.; investigation, T.G.; resources, Y.T. and W.X.; writing—original draft preparation, T.G.; writing—review and editing, T.G., T.Z. and L.L.; supervision, L.L.; project administration, L.L.; funding acquisition, T.Z. and L.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Animals used in this study were raised and slaughtered in accordance with the national standard of Laboratory animal Guideline for ethical review of animal welfare (GB/T 35892-2018) issued by General Administration of Quality Supervision, Inspection and Quarantine of the people’s Republic of China and Standardization Administration of the People’s Republic of China. All experiment procedures were approved by Institute of Animal Husbandry and Veterinary Science, Zhejiang Academy of Agricultural Sciences (Hangzhou, China).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the Key R&D Program of Zhejiang Province (2021C02034) and Zhejiang Major Scientific and Technological Project of Agricultural (Livestock) Breeding (2021C02068).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Han J., Li L., Wang D., Ma H. (−)-Hydroxycitric acid reduced fat deposition via regulating lipid metabolism-related gene expression in broiler chickens. Lipids Health Dis. 2016;15:37–50. doi: 10.1186/s12944-016-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakdee J., Poeikhamph T., Rakangthon C., Poungpong K., Bunchasak C. Effect of Tributyrin Supplementation in Diet on Production Performance and Gastrointestinal Tract of Healthy Nursery Pigs. Pakistan J. Nutr. 2012;15:954–962. doi: 10.3923/pjn.2016.954.962. [DOI] [Google Scholar]
- 3.Dong L., Zhong X., He J., Zhang L., Bai K., Xu W., Wang T., Huang X. Supplementation of tributyrin improves the growth and intestinal digestive and barrier functions in intrauterine growth-restricted piglets. Clin. Nutr. 2016;35:399–407. doi: 10.1016/j.clnu.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Sotira S., Dell’Anno M., Caprarulo V., Hejna M., Pirrone F., Callegari M.L., Tucci T.V., Rossi L. Effects of Tributyrin Supplementation on Growth Performance, Insulin, Blood Metabolites and Gut Microbiota in Weaned Piglets. Animals. 2020;10:726. doi: 10.3390/ani10040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nguyen T.D., Prykhodko O., Fåk Hållenius F., Nyman M. Effects of monobutyrin and tributyrin on liver lipid profile, caecal microbiota composition and SCFA in high-fat diet-fed rats. J. Nutr. Sci. 2017;6:e51. doi: 10.1017/jns.2017.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vinolo M.A., Rodrigues H.G., Festuccia W.T., Crisma A.R., Alves V.S., Martins A.R., Amaral C.L., Fiamoncini J., Hirabara S.M., Sato F.T., et al. Tributyrin attenuates obesity-associated inflammation and insulin resistance in high-fat-fed mice. Am. J. Physiol. Endocrinol. Metab. 2012;303:272–282. doi: 10.1152/ajpendo.00053.2012. [DOI] [PubMed] [Google Scholar]
- 7.Miyoshi M., Sakaki H., Usami M., Iizuka N., Shuno K., Aoyama M., Usami Y. Oral administration of tributyrin increases concentration of butyrate in the portal vein and prevents lipopolysaccharide-induced liver injury in rats. Clin. Nutr. 2011;30:252–258. doi: 10.1016/j.clnu.2010.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Cresci G., Glueck B., McMullen M., Xin W., Allende D., Nagy L. Prophylactic tributyrin treatment mitigates chronic-binge alcohol-induced intestinal barrier and liver injury. J. Gastroenterol. Hepatol. 2017;32:1587–1597. doi: 10.1111/jgh.13731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cogburn L.A., Wang X., Carre W., Rejto L., Aggrey S.E., Duclos M.J., Simon J., Porter T.E. Functional genomics in chickens: Development of integrated-systems microarrays for transcriptional profiling and discovery of regulatory pathways. Comp. Funct. Genom. 2004;5:253–261. doi: 10.1002/cfg.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Richards M.P., Poch S.M., Coon C.N., Rosebrough R.W., Ashwell C.M., McMurtry J.P. Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J. Nutr. 2003;133:707–715. doi: 10.1093/jn/133.3.707. [DOI] [PubMed] [Google Scholar]
- 11.Goodridge A.G., Ball E.G. Lipogenesis in the pigeon: In vivo studies. Am. J. Physiol. 1967;213:245–249. doi: 10.1152/ajplegacy.1967.213.1.245. [DOI] [PubMed] [Google Scholar]
- 12.O’Hea E.K., Leveille G.A. Lipogenesis in isolated adipose tissue of the domestic chick (Gallus domesticus) Comp. Biochem. Physiol. 1968;26:111–120. doi: 10.1016/0010-406X(68)90317-4. [DOI] [PubMed] [Google Scholar]
- 13.Cai Y., Song Z., Zhang X., Wang X., Jiao H., Lin H. Increased de novo lipogenesis in liver contributes to the augmented fat deposition in dexamethasone exposed broiler chickens (Gallus gallus domesticus) Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009;150:164–169. doi: 10.1016/j.cbpc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 14.Leclercq B., Hermier D., Guy G. Metabolism of very low density lipoproteins in genetically lean or fat lines of chicken. Reprod. Nutr. Dev. 1990;30:701–715. doi: 10.1051/rnd:19900607. [DOI] [PubMed] [Google Scholar]
- 15.Legrand P., Hermier D. Hepatic delta 9 desaturation and plasma VLDL level in genetically lean and fat chickens. Int. J. Obes. Relat. Metab. Disord. 1992;16:289–294. [PubMed] [Google Scholar]
- 16.Douaire M., Le Fur N., el Khadir-Mounier C., Langlois P., Flamant F., Mallard J. Identifying genes involved in the variability of genetic fatness in the growing chicken. Poult. Sci. 1992;71:1911–1920. doi: 10.3382/ps.0711911. [DOI] [PubMed] [Google Scholar]
- 17.Daval S., Lagarrigue S., Douaire M. Messenger RNA levels and transcription rates of hepatic lipogenesis genes in genetically lean and fat chickens. Genet. Sel. Evol. 2000;32:521–531. doi: 10.1186/1297-9686-32-5-521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H.B., Li H., Wang Q.G., Zhang X.Y., Wang S.Z., Wang Y.X., Wang X.P. Profiling of chicken adipose tissue gene expression by genome array. BMC Genom. 2007;8:193–206. doi: 10.1186/1471-2164-8-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Royan M., Meng G.Y., Othman F., Sazili A.Q., Navidshad B. Effects of conjugated linoleic acid, fish oil and soybean oil on PPARs (α & γ) mRNA expression in broiler chickens and their relation to body fat deposits. Int. J. Mol. Sci. 2011;12:8581–8595. doi: 10.3390/ijms12128581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang J., Zhang H., Bai S., Zeng Q., Su Z., Zhuo Y., Mao X., Yin H., Feng B., Liu J., et al. Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult. Sci. 2021;100:101429–101437. doi: 10.1016/j.psj.2021.101429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zerehdaran S., Vereijken A.L., van Arendonk J.A., van der Waaijt E.H. Estimation of genetic parameters for fat deposition and carcass traits in broilers. Poult. Sci. 2004;83:521–525. doi: 10.1093/ps/83.4.521. [DOI] [PubMed] [Google Scholar]
- 22.Yin F., Yu H., Lepp D., Shi X., Yang X., Hu J., Leeson S., Yang C., Nie S., Hou Y., et al. Transcriptome Analysis Reveals Regulation of Gene Expression for Lipid Catabolism in Young Broilers by Butyrate Glycerides. PLoS ONE. 2016;11:e0160751. doi: 10.1371/journal.pone.0160751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mansoub N.H., Rahimpour K., Asl L.M., Nezhady M.A.M., Kalhori M.M. Effect of Different Level of Butyric Acid Glycerides on Performance and Serum Composition of Broiler Chickens. World J. Zool. 2010;6:179–182. [Google Scholar]
- 24.Leeson S., Namkung H., Antongiovanni M., Lee E.H. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005;84:1418–1422. doi: 10.1093/ps/84.9.1418. [DOI] [PubMed] [Google Scholar]
- 25.Khera A.V., Cuchel M., de la Llera-Moya M., Rodrigues A., Burke M.F., Jafri K., French B.C., Phillips J.A., Mucksavage M.L., Wilensky R.L., et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mora S., Buring J.E., Ridker P.M., Cui Y. Association of high-density lipoprotein cholesterol with incident cardiovascular events in women, by low-density lipoprotein cholesterol and apolipoprotein B100 levels: A cohort study. Ann. Intern Med. 2011;155:742–750. doi: 10.7326/0003-4819-155-11-201112060-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lafontan M., Langin D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009;48:275–297. doi: 10.1016/j.plipres.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Kuusi T., Saarinen P., Nikkilä E.A. Evidence for the role of hepatic endothelial lipase in the metabolism of plasma high density lipoprotein2 in man. Atherosclerosis. 1980;36:589–593. doi: 10.1016/0021-9150(80)90251-8. [DOI] [PubMed] [Google Scholar]
- 29.Connolly J.M., Gilhooly E.M., Rose D.P. Effects of reduced dietary linoleic acid intake, alone or combined with an algal source of docosahexaenoic acid, on MDA-MB-231 breast cancer cell growth and apoptosis in nude mice. Nutr. Cancer. 1999;35:44–49. doi: 10.1207/S1532791444-49. [DOI] [PubMed] [Google Scholar]
- 30.Perret B., Mabile L., Martinez L., Tercé F., Barbaras R., Collet X. Hepatic lipase: Structure/function relationship, synthesis, and regulation. J. Lipid. Res. 2002;43:1163–1169. doi: 10.1194/jlr.R100020-JLR200. [DOI] [PubMed] [Google Scholar]
- 31.Herbst K.L., Amory J.K., Brunzell J.D., Chansky H.A., Bremner W.J. Testosterone administration to men increases hepatic lipase activity and decreases HDL and LDL size in 3 wk. Am. J. Physiol. Endocrinol. Metab. 2003;284:1112–1118. doi: 10.1152/ajpendo.00524.2002. [DOI] [PubMed] [Google Scholar]
- 32.Xia J., Yu P., Zeng Z., Ma M., Zhang G., Wan D., Gong D., Deng S., Wang J. Lauric Triglyceride Ameliorates High-Fat-Diet-Induced Obesity in Rats by Reducing Lipogenesis and Increasing Lipolysis and β-Oxidation. J. Agric. Food Chem. 2021;69:9157–9166. doi: 10.1021/acs.jafc.0c07342. [DOI] [PubMed] [Google Scholar]
- 33.Digel M., Ehehalt R., Stremmel W., Füllekrug J. Acyl-CoA synthetases: Fatty acid uptake and metabolic channeling. Mol. Cell Biochem. 2009;326:23–28. doi: 10.1007/s11010-008-0003-3. [DOI] [PubMed] [Google Scholar]
- 34.Royan M., Navidshad B. Peroxisome proliferator-activated receptor γ (PPARγ), a key regulatory gene of lipid metabolism in chicken. World Poul. Sci. J. 2016;72:773–784. doi: 10.1017/S0043933916000684. [DOI] [Google Scholar]
- 35.Spiegelman B.M. Peroxisome proliferator-activated receptor γ: A key regulator of adipogenesis and systemic insulin sensitivity. Eur. J. Med. Res. 1997;2:457–464. [PubMed] [Google Scholar]
- 36.Bocher V., Pineda-Torra I., Fruchart J.C., Staels B. PPARs: Transcription factors controlling lipid and lipoprotein metabolism. Ann. N. Y. Acad. Sci. 2002;967:7–18. doi: 10.1111/j.1749-6632.2002.tb04258.x. [DOI] [PubMed] [Google Scholar]
- 37.Miyoshi M., Iizuka N., Sakai S., Fujiwara M., Aoyama-Ishikawa M., Maeshige N., Hamada Y., Takahashi M., Usami M. Oral tributyrin prevents endotoxin-induced lipid metabolism disorder. Clin. Nutr. ESPEN. 2015;10:83–88. doi: 10.1016/j.clnesp.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Lampidonis A.D., Rogdakis E., Voutsinas G.E., Stravopodis D.J. The resurgence of Hormone-Sensitive Lipase (HSL) in mammalian lipolysis. Gene. 2011;477:1–11. doi: 10.1016/j.gene.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 39.Morak M., Schmidinger H., Riesenhuber G., Rechberger G.N., Kollroser M., Haemmerle G., Zechner R., Kronenberg F., Hermetter A. Adipose triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) deficiencies affect expression of lipolytic activities in mouse adipose tissues. Mol. Cell Proteom. 2012;11:1777–1789. doi: 10.1074/mcp.M111.015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.