Abstract
As enteric pathogens, the salmonellae have developed systems by which they can sense and adapt appropriately to deleterious intestinal components that include bile. Previously, growth in the presence of bile was shown to repress the transcription of prgH, a locus encoding components of the Salmonella pathogenicity island I (SPI-1) type III secretion system (TTSS) necessary for eukaryotic cell invasion. This result suggested an existing interaction between salmonellae, bile, and eukaryotic cell invasion. Transcription assays demonstrated that invasion gene regulators (e.g., sirC and invF) are repressed by bile. However, bile does not interact with any of the invasion regulators directly but exerts its effect at or upstream of the two-component system at the apex of the invasion cascade, SirA-BarA. As suggested by the repression of invasion gene transcription in the presence of bile, Western blot analysis demonstrated that proteins secreted by the SPI-1 TTSS were markedly reduced in the presence of bile. Furthermore, Salmonella enterica serovar Typhimurium grown in the presence of bile was able to invade epithelial cells at only 4% of the level of serovar Typhimurium grown without bile. From these data, we propose a model whereby serovar Typhimurium uses bile as an environmental signal to repress its invasive capacity in the lumen of the intestine, but upon mucous layer penetration and association with intestinal epithelial cells, where the apparent bile concentration would be reduced, the system would become derepressed and invasion would be initiated.
Enteric pathogens, such as Salmonella spp., interact with bile in the human intestine. Bile is a detergent that aids in dispersion and degradation of fats and can also degrade the lipid bilayers of bacteria. Salmonella spp. encounter bile not only in the intestine but also in the gallbladder, where the organism resides in those 3% of infected individuals who become chronic carriers. Salmonellae alter protein production when grown in the presence of bile, and they are able to adapt to high bile concentrations by pregrowth in sublethal concentrations (30); however, mechanisms by which salmonellae sense and respond to bile are not known.
Enterics possess various means of avoiding the harsh effects of bile, which include membrane components such as lipopolysaccharide, porins, transport proteins, and efflux pumps (reviewed in reference 9). Bacteria with mutations in the lipopolysaccharide that cause an incomplete O antigen (rough strains) have been shown to be more sensitive to antibiotics and detergents, including bile salts. Also, porins and the Tol proteins have been shown to be important in maintaining a selectively permeable membrane that excludes molecules such as bile salts. Finally, bile salts that do enter into the cytoplasm can be pumped out of the cell by efflux pumps, which include the mar and acr loci in Escherichia coli.
In the salmonellae, it is known that the two-component system PhoP-PhoQ (PhoPQ) regulates virulence phenotypes such as survival within macrophages and is necessary for disease in humans and mice (7, 16, 23). PhoPQ is also necessary for high level resistance to bile in Salmonella enterica serovar Typhi and S. enterica serovar Typhimurium (30). PhoP is a response regulator protein that controls the expression of a number of loci, and PhoQ is a membrane-bound histidine kinase (8, 11, 23). Previously, our laboratory screened a number of PhoP-activated gene (pag) and PhoP-repressed gene (prg) MudJ or TnphoA mutants for an effect on bile resistance and for regulation by bile (30). While none of the loci examined were involved in bile resistance, the prgH locus, a component of a type III secretion system (TTSS) apparatus used for invasion (25), showed bile-mediated transcriptional repression independent of PhoP (3). These data suggested that bile might repress invasion in serovar Typhimurium.
Salmonellae use a contact-dependent TTSS for invasion, which is encoded within Salmonella pathogenicity island 1 (SPI-1) at centisome 63 (17, 21). This TTSS appears to be regulated by genes both inside and outside SPI-1 and encodes both the apparatus as well as the proteins that are secreted directly from the cytoplasm to the cytosol of epithelial cells. These secreted Salmonella proteins are effector molecules that interact with host proteins causing, for example, actin rearrangement, “ruffling,” and bacterial uptake (17).
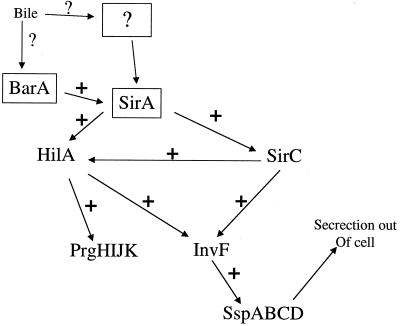
Regulation of invasion gene transcription appears to be very complex. There are numerous invasion regulatory proteins, which include SirA, SirC (HilC), SirB, HilD, HilA, and InvF (19, 20, 27, 28). This complex regulatory network may be in place to integrate numerous environmental signals. It appears that SirA (and its proposed kinase, BarA [1]) are located at the top of this cascade, while HilA and InvF are the terminal effectors that activate transcription of genes encoding the TTSS apparatus and secreted proteins.
We hypothesize that bile negatively affects invasion regulatory protein transcription, such that the SPI-1 TTSS is shut down in the lumen of the intestine where there is a high apparent bile concentration. However, once the bacteria cross the mucous layer of the intestinal epithelium, the apparent bile concentration should decrease, which would derepress the SPI-1 TTSS and initiate invasion. Therefore, this study was designed to examine the effect of bile on serovar Typhimurium epithelial cell invasion and to determine the point at which bile may act as a signal in the invasion regulatory cascade.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and reagents.
Strains used for this study are described in Table 1. Cultures were grown under various conditions as described below. When required, medium was supplemented with chloramphenicol (25 μg/ml), ampicillin (50 μg/ml), kanamycin (45 μg/ml), or tetracycline (15 μg/ml). The bile used in this study comes from Sigma Chemical (St. Louis, Mo.) under the label of sodium choleate or crude ox bile extract and contains taurocholic, glycocholic, deoxycholic, and cholic acids.
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype or relevant phenotype | Source or reference |
|---|---|---|
| JSG210 | 14028S (wild type, parent of all strains) | ATCCa |
| JSG205 | msgA::MudJ | 10 |
| JSG648 | prgH::TnphoA | 3 |
| JSG940 | sirA::Tn10d | 19 |
| JSG941 | invF::Tn5lacZY | 2 |
| JSG943 | sirC::luc | 27 |
| JSG971 | sirA::Tn10d sirC::luc | This study |
| JSG1100 | ΔbarA | R. Maurer (1) |
| JSG1102 | pCJ13d (psirA) | 19 |
| JSG1103 | pCJ20 (psirC) | 19 |
| JSG1104 | pCJ22 (psirB) | 19 |
| JSG1113 | ΔbarA invF::Tn5lacZY | This study |
| JSG1127 | ΔbarAinvF::Tn5lacZY pCJ13d (psirA) | This study |
| JSG1128 | ΔbarAinvF::Tn5lacZY pCJ22 (psirB) | This study |
| JSG1129 | ΔbarAinvF::Tn5lacZY pCJ20 (psirC) | This study |
ATCC, American Type Culture Collection.
Transcription assays.
Strains carrying luc (firefly luciferase) were grown first overnight in Luria-Bertani (LB) broth or LB broth plus bile ranging in concentration from 0.1 to 7.5% at 37°C with aeration and then back-diluted into the same medium and grown to logarithmic phase (optical density at 600 nm [OD600] = 0.6) at 37°C. Cells grown in bile were collected by centrifugation and washed twice in LB broth. Cultures were then assayed for luciferase activity as previously described (12). For β-galactosidase assays, cells were grown and washed as described above. β-Galactosidase activity was determined and expressed in units as described by Miller (22).
Western blot.
Strains were grown overnight in 12 ml of LB broth or LB broth with 10% bile at 37°C with aeration. Cultures were centrifuged for 1 h 10 min at 221,000 × g and 4°C to separate whole cells from the supernatant so that both could be assayed. Supernatant (secreted) proteins of cultures grown in LB broth were concentrated using trichloroacetic acid (TCA) precipitation as previously described (25). The whole-cell extracts were prepared by boiling pelleted cells for 10 min in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (0.125 M Tris [pH 6.8], 20% glycerol, 4% SDS, 10% β-mercaptoethanol, 0.1% bromophenol blue). Direct culture supernatants and the concentrated supernatant (secreted) proteins (see Fig. 5) were boiled for 10 min in 2× SDS-PAGE sample buffer, and samples were loaded onto a 10% polyacrylamide gel for separation of proteins. Antibodies used for the Western blot include anti-SspA (1:30,000) and anti-β-galactosidase (1:5,000) (Chemicon International Inc., Temecula, Calif.).
FIG. 5.
Growth in bile dramatically reduces the secretion of SspA. SspA is an 87-kDa protein secreted by the SPI-1 TTSS. Supernatants from centrifuged overnight cultures (45 μl) (lanes 3 to 5) were directly loaded on SDS-PAGE gels. SspA was detected by Western blotting. Lane 1, LB broth alone; lane 2, LB broth plus 10% bile; lane 3, supernatant from wild-type serovar Typhimurium; lane 4, supernatant from wild-type serovar Typhimurium grown with 10% bile; lane 5, supernatant from a prgH::TnphoA strain; lane 6, secreted proteins isolated by TCA precipitation from a wild-type serovar Typhimurium supernatant.
Invasion assays.
Cultures were grown with or without 3% bile in LB broth overnight at 37°C without aeration and washed twice with LB broth prior to use in the invasion assay. HeLa cells were grown in 1× Dulbecco's Modification of Eagle's Medium (DMEM) (Cellgro) with 10% fetal bovine serum (FBS) and penicillin-streptomycin (100 IU of penicillin and 100 μg of streptomycin per ml). When confluent, the HeLa cells were detached and washed by centrifugation in DMEM-FBS. HeLa cells were then diluted to 2 × 105 cells/ml and incubated overnight in 24-well titer plates at 37°C in 5% CO2. Bacteria were added to the cells at a multiplicity of infection of 100:1 and allowed to invade the HeLa cells for 1 h at 37°C in 5% CO2. The cells were washed three times in 1× phosphate-buffered saline (PBS), and gentamicin (100 μg/ml) was added for 15 min to kill extracellular bacteria. Cells were washed again three times in 1× PBS and lysed with 1% Triton X-100. Lysates and the original bacterial cultures were appropriately diluted and plated to determine the number of bacteria present.
Ex vivo invasion assays were conducted using jejunal sections of BALB/c mouse small intestine. Mice were deprived of food for 24 h, after which they were euthanized and the small intestine was removed and placed in DMEM-FBS. One-inch sections were cut from the jejunal region, and the lumen was thoroughly washed with 1× PBS. One end was tied off, and 50 μl of bacterial culture grown overnight without aeration at 37°C was injected into the intestinal section. The other end was tied off, and the tissue was placed in DMEM-FBS and allowed to incubate for 1 h at 37°C in 5% CO2. The ends were then cut and the tissue was washed with gentamicin in DMEM-FBS (400 μg/ml). After washing, the intestine was opened longitudinally and allowed to incubate in gentamicin-DMEM-FBS for 1 h at 37°C in 5% CO2. The intestinal tissue was then washed three times using centrifugation to remove all traces of gentamicin, resuspended in 1 ml of LB broth, homogenized in 2 ml of 0.5% Triton-X 100, diluted, and plated. Original bacterial cultures were also appropriately diluted and plated.
Genetic constructs.
P22HT-int transduction was used to move the invF::Tn5lacZY mutation into the ΔbarA strain constructing strain JSG1113. The plasmids pSirA, pSirB, and pSirC were transformed into JSG1113 by electroporation to create strains JSG1127 to JSG1129. P22HT-int transduction was also used to create strain JSG971 by the movement of the chromosomal sirC::luc fusion into strain JSG940 (sirA::Tn10d).
RESULTS
Transcription of invasion gene regulators is repressed in the presence of bile.
Our previous data showed that prgH was repressed by environmental bile independent of PhoP (30). It is thought that hilA encodes the direct transcriptional activator of prgH in a complex cascade of regulatory factors (27) (Fig. 1). To determine whether bile-mediated repression of prgH is occurring at the hilA-prgH interface or higher in the cascade, transcriptional assays were performed on sirC, a regulatory gene above hilA in the invasion pathway (19). A strain containing a sirC firefly luciferase fusion (constructed so that the wild-type gene was not disrupted) was grown to logarithmic phase in LB broth without bile or with bile ranging from 0.1 to 7.5%. It was found that sirC transcription was significantly repressed in the presence of all concentrations of bile tested, with maximum repression ranging from 8- to 10-fold (Fig. 2). This suggests that bile-mediated repression of prgH is not a direct effect on hilA but is due to transcriptional repression of genes higher in the invasion pathway.
FIG. 1.
Simplified cascade of regulatory factors necessary for the induction of eukaryotic cell invasion. The SirA-BarA two-component system is thought to be at the apex of this pathway, which, when activated, induces a cascade of transcriptional activation of regulatory factors such as SirC, HilA, and InvF. Although many other regulatory factors are involved in serovar Typhimurium invasion, for simplicity, those regulatory factors not investigated in this work are not included in this figure. A + indicates transcriptional activation. Bile and bile salts enter the regulatory pathway at SirA-BarA or at an unknown factor that affects the activity of SirA-BarA or the transcription of genes encoding SirA-BarA (box with question mark). Bile results in the lack of transcription of the regulatory proteins in the above cascade, which results in a marked reduction in SPI-1-mediated type III secretion and invasion of epithelial cells.
FIG. 2.
sirC transcription is repressed by growth in the presence of bile. Cultures were grown to log phase (OD600 = 0.6) in LB broth or in LB broth with the addition of bile (ranging from 0.1 to 7.5% bile), washed, and examined for firefly luciferase activity. The data correspond to a single experiment of three independent assays that gave similar results.
Bile-mediated repression of sirC is dependent upon sirA and barA.
Recent data suggests that SirA, along with its proposed sensor kinase, BarA, whose genes are located outside of SPI-1, are at the apex of the invasion cascade (1, 19). To determine whether bile-mediated repression of sirC is dependent on sirA, transcriptional assays were performed on sirC::luc strains in a SirA− background. Although the loss of SirA decreases the overall transcription of sirC, transcriptional activity is still easily monitored. As shown in Fig. 3, the bile-mediated repression of sirC::luc does not occur in the strain background without a functional SirA (∼2-fold repression versus 8- to 10-fold repression). Therefore, the bile-mediated repression of the transcription of invasion gene regulators can be traced to proteins known to be at the top of the invasion regulatory cascade.
FIG. 3.
Bile-mediated repression of sirC transcription is dependent upon SirA. Cultures were grown to log phase (OD600 = 0.6) in LB broth or in LB broth with the addition of 3% bile, washed, and examined for firefly luciferase activity. The data correspond to a single experiment of three independent assays that gave similar results.
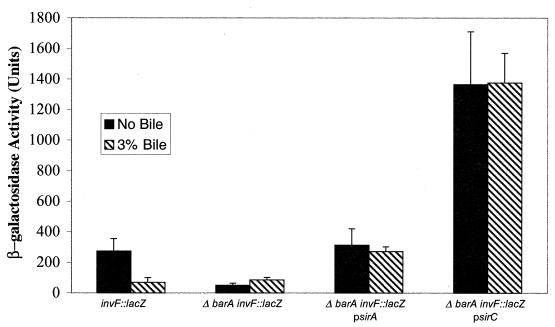
To both confirm and extend the above results, a barA deletion strain containing an invF::lacZ reporter was complemented with plasmids carrying the wild-type sirA, sirB (data not shown), or sirC genes, all of which produce invasion gene regulatory proteins that have been shown to complement the loss of sirA (and therefore also likely barA) when in multicopy (19, 27). InvF is thought to be the most-distal regulator in the invasion pathway that activates transcription of genes such as those encoding the secreted effectors (4, 6, 20). Although the plasmids restored different amounts of invF transcription, none of these strains demonstrated the ability to be repressed by bile in the medium (Fig. 4). These results demonstrate that bile does not interact with SirA, SirB, or SirC directly to affect the protein's regulatory activity and that the effect of bile on invasion gene transcription is occurring at or above SirA-BarA.
FIG. 4.
Bile-mediated repression of invF transcription does not act through direct interaction with invasion gene regulators but is dependent upon BarA. Cultures were grown to stationary phase in LB broth or in LB broth with the addition of 3% bile, washed, and examined for β-galactosidase activity. Plasmids psirA and psirC complement the reduction in invF expression in the ΔbarA strain, but these strains are unresponsive to bile.
SPI-1-mediated type III secretion is markedly reduced in the presence of bile.
The transcriptional repression of invasion gene regulators in the presence of bile suggests that SPI-1-mediated type III secretion will be dramatically reduced or eliminated when serovar Typhimurium is grown in the presence of bile. To test this hypothesis, we analyzed culture supernatants for the presence of a known SPI-1 secreted protein, SspA (18, 25). Although we attempted to look at the entire repertoire of secreted proteins, we were unable to effectively separate bile (or even individual bile salts such as deoxycholate) from the secreted proteins so that bile salts and bile protein components were not coprecipitated with the secreted proteins from the supernatant using TCA. Therefore, to examine SPI-1 mediated secretion, bile-containing and non-bile-containing supernatants were electrophoresed directly on polyacrylamide gels, and Western blot analysis was performed with anti-SspA antibody. As seen in Fig. 5, the level of SspA was significantly reduced in the supernatants of the wild type grown in 10% bile versus those of a wild-type control. In addition, only small amounts of SspA were observed in Western blots of whole-cell lysates, corroborating the data concerning the transcriptional repression of invasion gene regulators (data not shown). The supernatant of a PrgH− strain (Fig. 5, lane 5) was also examined as a negative control because it is defective in the formation of the secretion apparatus.
To determine if the small amount of SspA (and other, likely cross-reactive proteins) visible in Fig. 5 (lane 4) was due to detergent (bile salt)-mediated membrane damage and leakage of cytoplasmic contents, Western blotting was performed on a strain producing β-galactosidase grown with or without bile in the medium. Using an antibody to β-galactosidase, it was shown that the wild type grown in bile had a slight amount (∼1%) of leakage of cytoplasmic proteins into the supernatant (data not shown). The non-SspA bands in lane 4 of Fig. 5 are therefore most likely a result of leakage of cross-reactive proteins. These data further support the hypothesis that the level of SspA is reduced in the presence of bile, as the SspA band seen in lane 4 may be due more to leakage than actual secretion.
Invasion of epithelial cells in vitro is reduced in the presence of bile.
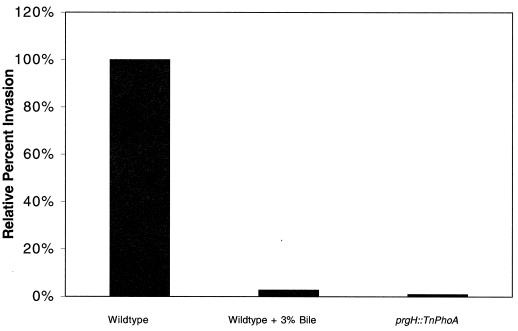
It is likely that if the genes of the invasion pathway are transcriptionally repressed in the presence of bile and the SPI-1-mediated TTSS is repressed, then serovar Typhimurium invasion of epithelial cells will be markedly reduced. A wild-type strain was grown overnight without aeration in LB broth with or without 3% bile. A PrgH− strain was also grown under the same conditions to serve as a negative control. Invasion assays were performed using HeLa cells and washed bacterial cultures. The results show that serovar Typhimurium grown in bile is able to invade at only 4% of the level of the wild-type strain grown without bile. This invasion defect can be compared to the prgH negative control, which invades at 0.8% of the rate of a wild-type strain grown without bile (Fig. 6). Therefore, bile represses serovar Typhimurium invasion of epithelial cells in vitro.
FIG. 6.
Epithelial cell invasion is markedly reduced by prior growth in bile. Cells from overnight, nonaerated cultures were washed, diluted, and added to HeLa cells at a multiplicity of infection of 100:1. Following the standard gentamicin invasion assay, the invasion percentage of wild-type serovar Typhimurium grown in LB broth was set at 100%. Growth in 3% bile reduced the percent invasion to 4% of that of the wild type, while a prgH mutant (negative control) invaded at 0.8% of the rate of the wild type.
To determine if this result also applied to live, ex vivo intestinal tissue, jejunal sections were removed from mice, washed extensively, and used in invasion assays. Bacteria grown overnight with or without bile were washed and added to the lumens of these tissues. The results of this experiment corroborate those results observed in the tissue culture invasion assay, as serovar Typhimurium grown in bile invaded these ex vivo tissues at only 3% of the rate of strains grown in LB broth alone (data not shown).
DISCUSSION
Exit of salmonellae from the intestinal lumen involves invasion of intestinal epithelial cells, and this invasion relies upon the production of a type III secretory apparatus and protein effector molecules (including prgH and its cotranscribed genes) from SPI-1. These secreted effector molecules mediate changes in host epithelial cells, causing them to ruffle and engulf the bacterium. Our previous work showed that the transcription of prgH, which is part of an operon encoding members of the SPI-1-encoded secretion apparatus, is repressed in the presence of bile. Based on this preliminary observation, in this work, we show that the interaction of serovar Typhimurium with bile dramatically affects its invasive ability by downregulating the transcription of invasion gene regulators, which results in a marked decrease in the transcription of SPI-1 genes involved in epithelial cell entry.
A complex set of transcriptional activators interact to regulate serovar Typhimurium invasion of epithelial cells. SirA (19), SirC (19), HilA (2), and likely HilD (28) and SsrA (14) have all been shown to affect bacterial invasion and transcription of prgH. It is likely that HilA is the protein that binds to the prgHIJK promoter to activate transcription. SirC was identified as a multicopy suppressor of a SirA− strain and positively affects both hilA and invF transcription (invF produces an activator downstream of SirC and HilA in the cascade) (19, 27). The SirA-BarA two-component system is at the top of the regulatory cascade involving these proteins. Therefore, in very simplistic terms, one functioning cascade is thought to be: SirA→SirC→HilA→InvF→type III secretion genes. To determine if the bile effect on prgHIJK transcription was due to an effect on HilA only or if the effect was upstream of HilA, we measured sirC transcription in the presence or absence of bile in a wild-type strain (sirC was not disrupted). These data showed that sirC transcription was markedly (eightfold or more) repressed by bile (and deoxycholate [data not shown]). Therefore, these data suggested that the bile effect was at the level of SirC or upstream of SirC. Because SirA positively regulates the expression of sirC (27), we tested whether repression of sirC transcription by bile was dependent upon a functional SirA. This could be accomplished because there is still transcription of sirC even in the absence of the positive activator SirA. This experiment demonstrated that the bile-mediated repression of sirC was dependent upon SirA (more than eightfold repression of sirC transcription in a wild-type background versus only a twofold repression in a background lacking sirA.
To confirm and extend the finding that the bile effect occurs upstream of SirC, we examined the transcription of genes far downstream in the invasion pathway in a BarA− background complemented with plasmids carrying either sirA or sirC. Both plasmids, as has been shown previously (19, 27), increased transcription of the downstream gene reporter, but these strains were unresponsive to bile. Therefore, bile does not directly interact with regulatory factors in the cascade but results in repression of invasion gene transcription in a manner that requires a functional SirA-BarA system.
Experiments were also conducted to demonstrate that proteins normally secreted by the SPI-1 system were markedly reduced in supernatants of cultures grown in the presence of bile. Using polyclonal antibody to the SspA product (an 87-kDa protein that effects host cell actin rearrangement [18, 31]), the secretion of this protein was shown to be dramatically reduced in strains grown in bile. In fact, the secretion defect is likely even greater than that observed because we could demonstrate slight leakage of cytoplasmic proteins into the supernatant in the presence of bile or bile salts using β-galactosidase as a marker (data not shown). Therefore, type III secretion is dramatically reduced by exposure of serovar Typhimurium to bile.
Because of the lack of invasion gene transcription and the reduction in SPI-1-mediated type III secretion, we predicted that invasion of epithelial cells would also be dramatically reduced. Indeed, in the standard gentamicin invasion assay, cells grown in the presence of bile invaded at 3% of the rate of those grown in LB broth. This phenomenon was also observed in washed, ex vivo intestinal tissues removed from mice. Because of the relevance of ex vivo tissues, future studies will utilize live intestine to examine the influence of coinjected factors such as bile and bile salts on the temporal nature of the repression and derepression of invasion. It will also be important to attempt studies of the effect of bile on salmonella invasion in the mouse model. It is possible to decrease bile (by ligation of the bile duct) or increase bile (by preinoculation with a fatty substance such as cream or by hormone treatment) in the mouse to examine its effect on salmonella invasion and virulence. However, care must be taken when performing and evaluating such studies as bile concentration is unlikely to be the only effect of these experimental manipulations.
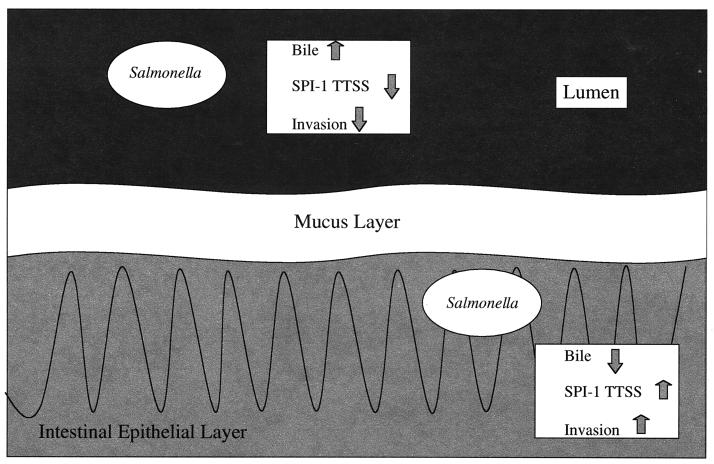
Based on the above results, we have formulated the following hypothesis of the role of bile in salmonella pathogenesis (Fig. 7). After emerging from the stomach and entering the small intestine, the organism encounters bile released into the duodenum. While in the lumen of the anterior small bowel, the relatively high bile concentration represses the invasion pathway. Upon transit to the distal ileum, the lumenal bile concentration will be reduced (15), and the organisms will begin to transit the mucous layer covering the epithelium. Once within or beyond the mucous layer, the apperant bile concentration will decrease, allowing for derepression of the invasion pathway. Therefore, bile may represent a signal allowing the bacterium to know if it is lumenal or close to the epithelium, where invasion factors must be synthesized.
FIG. 7.
Model of the effect of bile on serovar Typhimurium invasion. When the bacterium is within the intestinal lumen, bile inhibits invasion gene transcription, which markedly reduces SPI-1-mediated type III secretion and therefore the invasive abilities of the organism. However, upon migration through the mucous layer overlaying the intestinal epithelial cells, the apparent bile concentration should decrease, allowing a derepression of the invasion gene transcription, type III secretion, and epithelial cell invasion.
It is becoming increasingly clear that bile affects virulence properties of intestinal pathogens (9). In Campylobacter spp., a peritrichous pilus-like appendage was shown to be activated by growth in bile, which led to a highly aggregative phenotype (5). In addition, a mutant of this locus responsible for the phenotype resulted in less symptomology in a ferret model of disease. Bile affects numerous virulence properties in Vibrio spp., including hemolysin production (24), motility (13), cholera toxin and toxin-coregulated pilus production (13), and the activity of the regulator ToxT (29). Bile affects invasion and type III secretion in Shigella spp.; however, surprisingly, the effect is opposite to that observed in serovar Typhimurium. Pope et al. (26) demonstrated that certain bile salts resulted in increased Ipa secretion and increased epithelial cell invasion. Interestingly, these authors also showed that enteroinvasive E. coli did not exhibit bile salt-enhanced adherence and invasion even though bile induced the secretion of proteins into the supernatant. Thus, it appears that enteric pathogens have evolved to sense and utilize bile to their advantage in different ways. Regardless of their various effects on these bacteria, it is clear that bile salts not only are an antimicrobial substance which enteric bacteria must avoid but also represent a signal to help localize and temporally regulate invasion factors.
ACKNOWLEDGMENTS
This work was supported by grants AI43521 (J.S.G.) and T32AI07271-15 (A.M.P.) from the National Institutes of Health.
We thank Michael Hantman for the SspA antibody and Sam Miller, Jen Rakeman, and Russ Maurer for strains and experimental advice.
REFERENCES
- 1.Altier C, Suyemoto M, Ruiz A I, Burnham K D, Maurer R. Characterization of two novel regulatory genes affecting Salmonella invasion gene expression. Mol Microbiol. 2000;35:635–646. doi: 10.1046/j.1365-2958.2000.01734.x. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj V, Hwang C, Lee C A. hilA is a novel ompR/toxR family member that activates the expression of Salmonella typhimurium invasion genes. Mol Microbiol. 1995;18:715–727. doi: 10.1111/j.1365-2958.1995.mmi_18040715.x. [DOI] [PubMed] [Google Scholar]
- 3.Behlau I, Miller S I. A PhoP-repressed gene promotes Salmonella typhimurium invasion of epithelial cells. J Bacteriol. 1993;175:4475–4484. doi: 10.1128/jb.175.14.4475-4484.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Darwin K H, Miller V L. InvF is required for expression of genes encoding proteins secreted by the SPI1 type III secretion apparatus in Salmonella typhimurium. J Bacteriol. 1999;181:4949–4954. doi: 10.1128/jb.181.16.4949-4954.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolg P, Yao R, Burr D H, Guerry P, Trust T J. An environmentally regulated pilus-like appendage involved in Campylobacter pathogenesis. Mol Microbiol. 1996;20:885–894. doi: 10.1111/j.1365-2958.1996.tb02526.x. [DOI] [PubMed] [Google Scholar]
- 6.Eichelberg K, Galan J E. Differential regulation of Salmonella typhimurium type III secreted proteins by pathogenicity island 1 (SPI-1)-encoded transcriptional activators InvF and HilA. Infect Immun. 1999;67:4099–4105. doi: 10.1128/iai.67.8.4099-4105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fields P I, Swanson R V, Haidaris C G, Heffron F. Mutants of Salmonella typhimurium that cannot survive within the macrophage are avirulent. Proc Natl Acad Sci USA. 1986;83:5189–5193. doi: 10.1073/pnas.83.14.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Groisman E A, Chiao E, Lipps C J, Heffron F. Salmonella typhimurium phoP virulence gene is a transcriptional regulator. Proc Natl Acad Sci USA. 1989;86:7077–7081. doi: 10.1073/pnas.86.18.7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gunn, J. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2:907–913. [DOI] [PubMed]
- 10.Gunn J S, Alpuche-Aranda C A, Loomis W P, Miller S I. Characterization of the S. typhimurium pagC/pagD chromosomal region. J Bacteriol. 1995;177:5040–5047. doi: 10.1128/jb.177.17.5040-5047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunn J S, Hohmann E L, Miller S I. Transcriptional regulation of Salmonella virulence: a PhoQ periplasmic domain mutation results in increased net phosphotransfer to PhoP. J Bacteriol. 1996;178:6369–6373. doi: 10.1128/jb.178.21.6369-6373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gunn J S, Miller S I. PhoP/PhoQ activates transcription of pmrA/B, encoding a two-component system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996;178:6857–6864. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta S, Chowdhury R. Bile affects production of virulence factors and motility of Vibrio cholerae. Infect Immun. 1997;65:1131–1134. doi: 10.1128/iai.65.3.1131-1134.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hensel M, Shea J E, Gleeson C, Jones M D, Dalton E, Holden D W. Simultaneous identification of bacterial virulence genes by negative selection. Science. 1995;269:400–403. doi: 10.1126/science.7618105. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann A F. Bile secretion and the enterohepatic circulation of bile acids. In: Feldman M, Scharschmidt B F, Sleisenger M H, editors. Sleisenger and Fordtran's gastrointestinal and liver disease. 6th ed. Philadelphia, Pa: W. B. Saunders, Co.; 1998. pp. 937–948. [Google Scholar]
- 16.Hohmann E L, Oletta C A, Killeen K P, Miller S I. phoP/phoQ-deleted Salmonella typhi (TY800) is a safe and immunogenic single dose typhoid fever vaccine in volunteers. J Infect Dis. 1996;173:1408–1414. doi: 10.1093/infdis/173.6.1408. [DOI] [PubMed] [Google Scholar]
- 17.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hueck C J, Hantman M J, Bajaj V, Johnston C, Lee C A, Miller S I. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 19.Johnston C, Pegues D A, Hueck C J, Lee A, Miller S I. Transcriptional activation of Salmonella typhimurium invasion genes by a member of the phosphorylated response-regulator superfamily. Mol Microbiol. 1996;22:715–727. doi: 10.1046/j.1365-2958.1996.d01-1719.x. [DOI] [PubMed] [Google Scholar]
- 20.Kaniga K, Bossio J C, Galán J E. The Salmonella typhimurium invasion genes invF and invG encode homologues of the AraC and PulD family of proteins. Mol Microbiol. 1994;13:555–568. doi: 10.1111/j.1365-2958.1994.tb00450.x. [DOI] [PubMed] [Google Scholar]
- 21.Lee C A, Jones B D, Falkow S. Identification of a Salmonella typhimurium invasion locus by selection for hyperinvasive mutants. Proc Natl Acad Sci USA. 1992;89:1847–1851. doi: 10.1073/pnas.89.5.1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 23.Miller S I, Kukral A M, Mekalanos J J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci USA. 1989;86:5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osawa R, Yamai S. Production of thermostable direct hemolysin by Vibrio parahaemolyticus enhanced by conjugated bile acids. Appl Environ Microbiol. 1996;62:3023–3025. doi: 10.1128/aem.62.8.3023-3025.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pegues D A, Hantman M J, Behlau I, Miller S I. PhoP/PhoQ transcriptional repression of Salmonella typhimurium invasion genes: evidence for a role in protein secretion. Mol Microbiol. 1995;17:169–181. doi: 10.1111/j.1365-2958.1995.mmi_17010169.x. [DOI] [PubMed] [Google Scholar]
- 26.Pope L M, Reed K E, Payne S M. Increased protein secretion and adherence to HeLa cells by Shigella spp. following growth in the presence of bile salts. Infect Immun. 1995;63:3642–3648. doi: 10.1128/iai.63.9.3642-3648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rakeman J L, Bonifield H R, Miller S I. A HilA-independent pathway to Salmonella typhimurium invasion gene transcription. J Bacteriol. 1999;181:3096–3104. doi: 10.1128/jb.181.10.3096-3104.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schechter L M, Damrauer S M, Lee C A. Two AraC/XylS family members can independently counteract the effect of repressing sequences upstream of the hilA promoter. Mol Microbiol. 1999;32:629–642. doi: 10.1046/j.1365-2958.1999.01381.x. [DOI] [PubMed] [Google Scholar]
- 29.Schuhmacher D A, Klose K E. Environmental signals modulate ToxT-dependent virulence factor expression in Vibrio cholerae. J Bacteriol. 1999;181:1508–1514. doi: 10.1128/jb.181.5.1508-1514.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Velkinburgh J C, Gunn J S. PhoP-PhoQ-regulated loci are required for enhanced bile resistance in Salmonella spp. Infect Immun. 1999;67:1614–1622. doi: 10.1128/iai.67.4.1614-1622.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou D, Mooseker M S, Galan J E. Role of the S. typhimurium actin-binding protein SipA in bacterial internalization. Science. 1999;283:2092–2095. doi: 10.1126/science.283.5410.2092. [DOI] [PubMed] [Google Scholar]