Abstract
Caladiums are promising colorful foliage plants due to their dazzling colors of the leaves, veins, stripes, and patches, which are often cultivated in pots or gardens as decorations. Four wild species, including C. bicolor, C. humboldtii, C. praetermissum, and C. lindenii, were employed in this study, where their chloroplast (cp) genomes were sequenced, assembled, and annotated via high-throughput sequencing. The whole cp genome size ranged from 162,776 bp to 168,888 bp, and the GC contents ranged from 35.09% to 35.91%. Compared with the single large copy (LSC) and single small copy (SSC) regions, more conserved sequences were identified in the inverted repeat regions (IR). We further analyzed the different region borders of nine species of Araceae and found the expansion or contraction of IR/SSC regions might account for the cp genome size variation. Totally, 131 genes were annotated in the cp genomes, including 86 protein-coding genes (PCGs), 37 tRNAs, and eight rRNAs. The effective number of codons (ENC) values and neutrality plot analyses provided the foundation that the natural selection pressure could greatly affect the codon preference. The GC3 content was significantly lower than that of GC1 and GC2, and codons ending with A/U had higher usage preferences. Finally, we conducted phylogenetic relationship analysis based on the chloroplast genomes of twelve species of Araceae, in which C. bicolor and C. humboldtii were grouped together, and C. lindenii was furthest from the other three Caladium species occupying a separate branch. These results will provide a basis for the identification, development, and utilization of Caladium germplasm.
Keywords: Caladium, ornamental foliage plant, chloroplast genome, natural selection pressure, phylogenetic analysis
1. Introduction
The genus Caladium Vent. (family Araceae) includes perennial herbs native to the tropical regions of South and Central America [1,2,3]. Most Caladium species are distributed in the Amazon rainforest either in open areas or beside streams [4,5]. Caladium spp. is regarded as the most promising colorful foliage plant with great variation in leaf color [6,7]. Because of the dazzling colors of the leaves, veins, stripes, and patches, as well as the long ornamental period, they are often cultivated in pots or gardens as decorations [8]. The ornamental effect for urban areas is excellent, and hence this genus is collectively known as the “Queen of Foliage Plants” [9,10]. In many countries of Europe and America, Caladium is grown as a replacement for traditional grasses and flowers for the purposes of arranging flower beds and flower borders or creating a unique landscape effect of “no flowers is better than flowers” [11,12,13]. In recent years, Caladium has become a new favorite foliage plant due to its colorful leaves, short production cycle, and high selling profit which conferred its high popularity in domestic and foreign markets [14,15].
Over nearly 150 years of selection and breeding, more than 2000 varieties have been cultivated [16,17,18]. At present, there are more than 90 varieties on the market, among which more than 50 varieties are grown for large-scale flower production [19]. Since the beginning of this century, China has successively introduced some varieties of colorful Caladium as ornamental plants, which are greatly appreciated by retailers and young people [20].
The number of native species of the genus Caladium has always been controversial, ranging from 7 to 17. It has been reported earlier that C. marmoratum, C. picturatum, and C. steudnirifolium should be more appropriately merged into C. bicolor [21]. However, Croat recommended keeping the three species separate [22]. In 2013, C. clavatum and C. praetermissum were added to the list of species of wild Caladium, jointly released by Kew Gardens in the UK and the Missouri Botanical Garden in the US [23]. This increased the number of native species of Caladium to 14. Cao and Deng investigated the genome size and chromosome numbers of ten native species and 53 cultivars of Caladium [24]. They found that there are two genome types, thus providing some evidence for the evolutionary origin of Caladium germplasm.
Genetic and kinship analyses of Caladium germplasm have also received some attention. Loh et al. used 17 pairs of amplified fragment length polymorphism (AFLP) markers to accurately distinguish two wild species (C. bicolor and C. schomburgkii) and six cultivars of Caladium [25]. Deng et al. used simple sequence repeat (SSR) markers to analyze the genetic relationships among 45 major cultivars and seven wild species of Caladium [23]. The authors reported that the genetic variation rate among cultivars was low (44.4%). However, the two wild species C. bicolor and C. schomburgkii, exhibited high genetic consistencies with the cultivars, whereas the genetic consistencies between other wild species and cultivars were low. Moreover, C. steudnirifolium and C. lindenii were less genetically related to other wild species.
The chloroplast is the site of photosynthetic activity and is also one of the most important organelles of green plants, playing an important role in the inheritance and expression of plant genetic material [26,27,28,29]. The chloroplast has its own genome with an independent genetic system consisting of multiple copies of circular DNA molecules (110–210 kb), although few plant species have multiple linear copies of chromosomes [30,31,32]. The chloroplast genome has a typical tetrad structure featuring a large single-copy region, a small single-copy region, and a pair of inverted repeats (IR) regions [33,34]. The differences among plant species are attributed to the expansion and contraction of the IR regions [35]. Compared with the nuclear genome, the chloroplast genome shows maternal uni-parental inheritance, highly conserved structure, and less gene recombination [36,37,38]. Therefore, the chloroplast genome sequence is widely used in plant systematics, phylogeny, and population genomics [39,40].
Next-generation sequencing (NGS) technology has been widely used to identify functional genes and DNA markers massively due to its high throughput, high accuracy, and low cost. With the recent improvement of sequencing technology, the ongoing upgrading of sequencing platforms, and the development of a series of dynamic assembly and annotation software such as plasmaSPAdes, NOVOPlasty, and GetOrganelle, the chloroplast genomes of more than 3000 plant species have been sequenced and analyzed [41,42,43,44]. Remarkably, the chloroplast genome of Caladium has not yet been sequenced, and few phylogenetic studies based on chloroplast genomics have been reported.
In this study, four wild species of Caladium were used, and their chloroplast genomes were sequenced, assembled, and annotated via high-throughput sequencing. The characteristics of IR boundary and spacer loci in the chloroplast genomes were analyzed, and their genetic diversity and phylogenetic relationship were investigated. These results will provide a basis for the identification, development, and utilization of Caladium germplasm.
2. Results
2.1. The Structure of the Chloroplast Genomes of the Four Caladium Species
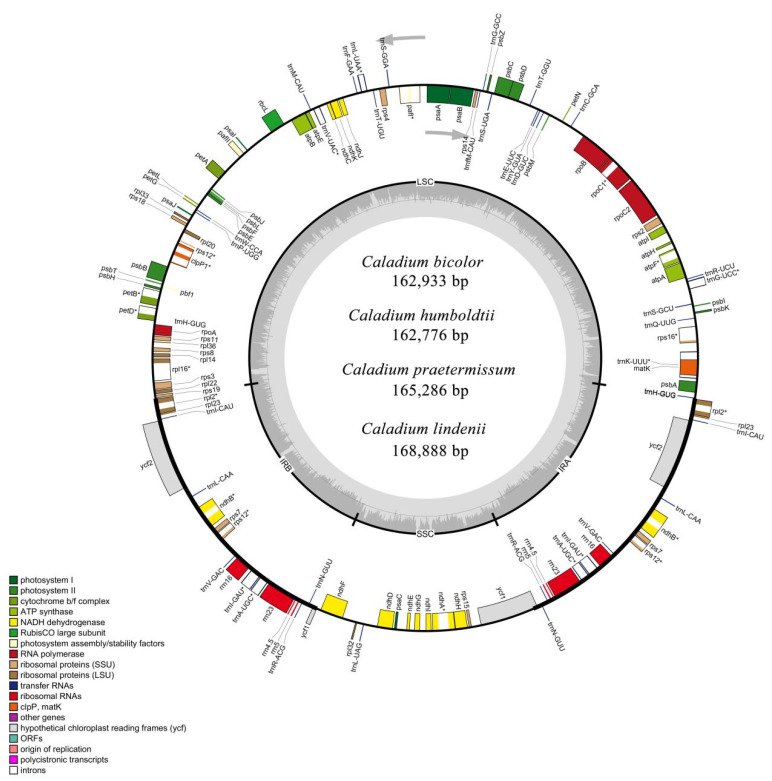
The chloroplast genome sizes of C. bicolor and C. humboldtii showed high similarity (162,933 bp and 162,776 bp, respectively) with a 157 bp difference, whereas those of C. praetermissum and C. lindenii were slightly larger (165,286 bp and 168,888 bp, respectively) (Figure 1). The GC contents of the four cp genomes were 35.87%, 35.91%, 35.64%, and 35.09%, respectively (Table 1). The cpDNA of the four samples showed a typical tetrad ring structure, consisting of a pair of inverted repeat regions (IRa and IRb; 26,277, 26,277, 26,484 and 26,472 bp in length, respectively), a large single copy region (LSC; 89,209, 88,986, 91,168 and 93,162 bp in length, respectively) and a small single copy region (SSC; 21,170, 21,236, 21,150 and 22,782 bp in length, respectively). Table 1 also shows that the LSC region of C. humboldtii was also significantly shorter than that of the other species, whereas that of C. lindenii was the longest. In terms of SSC length, Zamioculcas zamiifolia showed the smallest, but C. lindenii had the greatest SSC. For IR size, Z. amazonica exhibited the shortest while Z. zamiifolia had the longest IR. Moreover, we compared the differences in chloroplast genome sequences between the ON7070731 and NC_060474 (Table S1). Results showed there were only some variations in the noncoding region and one SNP in the coding region, which did not affect the genetic structure.
Figure 1.
Circular gene map of the complete chloroplast genomes of four Caladium species.
Table 1.
Comparison of the general information of nine species of Araceae.
| Species Names | Size (bp)/GC Content (%) | Number of Gene | ||||||
|---|---|---|---|---|---|---|---|---|
| Genome | LSC | SSC | IR | PCGs | RNA/rRNA | RNA/tRNA | Total Genes | |
| C. bicolor | 162,933/35.87 | 89,209/34.17 | 21,170/29.07 | 26,277/41.5 | 86 | 37 | 8 | 131 |
| C. humboldtii | 162,776/35.91 | 88,986/34.26 | 21,236/28.99 | 26,277/41.5 | 86 | 37 | 8 | 131 |
| C. praetermissum | 165,286/35.64 | 91,168/33.76 | 21,150/29.27 | 26,484/41.42 | 86 | 37 | 8 | 131 |
| C. lindenii | 168,888/35.09 | 93,162/33.26 | 22,782/27.87 | 26,472/41.43 | 86 | 37 | 8 | 131 |
| Syngonium angustatum | 164,929/35.72 | 90,714/33.94 | 21,559/28.98 | 26,328/41.53 | 86 | 37 | 8 | 131 |
| Zomicarpella amazonica | 162,729/35.82 | 90,811/34.33 | 21,656/28.76 | 25,131/41.55 | 85 | 37 | 8 | 130 |
| Zamioculcas zamiifolia | 167,405/35.7 | 91,357/34.02 | 19,326/29.47 | 28,361/40.52 | 86 | 37 | 8 | 131 |
| Xanthosoma helleborifolium | 164,418/35.84 | 90,833/33.88 | 20,705/41.53 | 26,440/41.53 | 86 | 37 | 8 | 131 |
| Xanthosoma sagittifolium | 165,169/35.67 | 91,122/33.79 | 21,079/29.36 | 26,484/41.42 | 86 | 37 | 8 | 131 |
A total of 131 genes were identified in the chloroplast genome of Caladium, including 86 protein-coding genes (PCGs), 37 tRNAs, and eight rRNAs (Table S2). Most genes appeared in the LSC or SSC region in single copy form. Among them, 12 genes were assigned to the SSC region, including 11 PCGs (ndhF, rpl32, ccsA, ndhD, psaC, ndhE, ndhG, ndhI, ndhA, ndhH, and rps15) and one tRNA (trnL-UAG). There were 83 genes in the LSC region, including 61 PCGs and 22 tRNAs. Only 16 genes were detected in the IR region, including five PCGs (rpl2, rpl23, ycf2, ndhB, and rps7), seven tRNAs (trnI-CAU, trnL-CAA, trnV-GAC, trnI-GAU, trnA-UGC, trnI-ACG, and trnA-GUU) and four rRNAs (rrn16, rrn23, rrn4.5 and rrn5). The ycf1 gene spanned the SSC region and the IR region. The rps12 had two copies, each having three exons, and the two copies shared the first exon, which was in the LSC region, while the other two exons were in the IR region.
2.2. Analysis of Contraction and Expansion of the IR Region
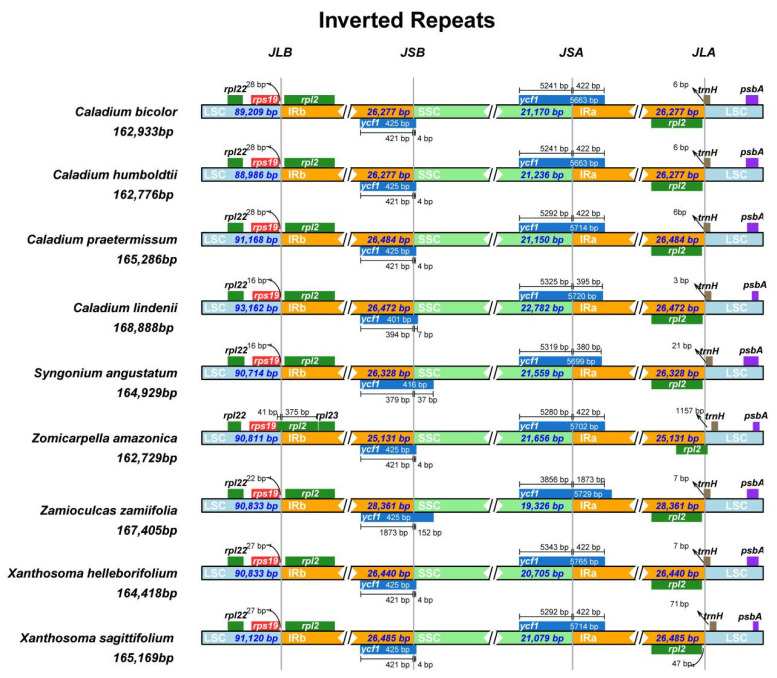
By comparing the gene distribution of IR/LSC and IR/SSC border regions in the chloroplast genomes of four Caladium species and those of related species, the expansion or contraction of IR/SSC boundary regions was assessed. As shown in Figure 2, the nine sequences had similar gene structures and sequences and the genes distributed near the boundaries of IR/LSC and IR/SSC were rps19, rpl22, rpl2, ycf1, trnH, and psbA. Among them, the IR boundaries of C. bicolor and C. humboldtii were identical, whereas C. praetermissum differed only in the SSC/IRa boundary. In C. bicolor and C. humboldtii, the sizes of the ycf1 gene were 5241 and 422 bp in the SSC and IRa regions, respectively, while these sizes in C. praetermissum were 5292 bp and 422 bp, respectively. As for C. lindenii, it was quite different from the other three Caladium species, and its boundaries were different, indicating that this species may have undergone a unique evolutionary process.
Figure 2.
Comparison of the borders of the LSC, SSC, and IR regions of nine species of Araceae. The numbers above the gene features denote the distance between the gene borders, either the start or end of genes, and the junction sites.
2.3. GView Analysis
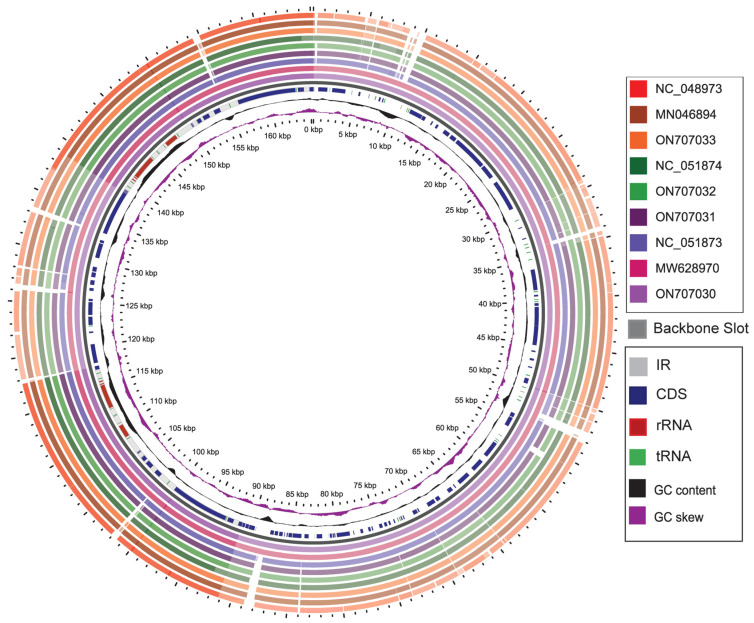
To gain a deeper understanding of the phylogenetic relationships among the different species of Caladium and the differences from other closely related species, we used the GView tool to create a circle map of the chloroplast genomes with the assembled C. bicolor genome as a reference. The characteristics and structural variation of all chloroplast genomes were evaluated. Figure 3 shows that the nine genomes had similar structures. Compared with the IR region, the LSC and SSC regions varied greatly among different species. Even within the same genus, the chloroplast genomes of the studied four species of Caladium showed inconsistencies. Particularly, there existed tiny variations among the intergenic regions. In terms of the genome structures, C. bicolor and C. humboldtii were identical. However, the cp genome of C. praetermissum was similar to that of Xanthosoma sagittifolium. Moreover, the cp genome of C. lindenii was similar to that of Syngonium angustatum.
Figure 3.
The graphical map shows nine circular plastome assemblies using C. bicolor plastome alignment as a reference. The innermost ring shows the genome size in kbp, followed by GC skew (purple) and GC content (black). From the inner to outer: C. praetermissum (ON707030), X. sagittifolium (MW628970), X. helleborifolium (NC_051873), C. bicolor (ON707031), C. humboldtii (ON707032), Z. amazonica (NC_051874), C. lindenii (ON707033), S. angustatum (MN046894), Z. zamiifolia (NC_048973). The similar and divergent locations are represented by continuous and interrupted track lines, respectively.
2.4. Analysis of Chloroplast Microsatellites and Repeat Sequences
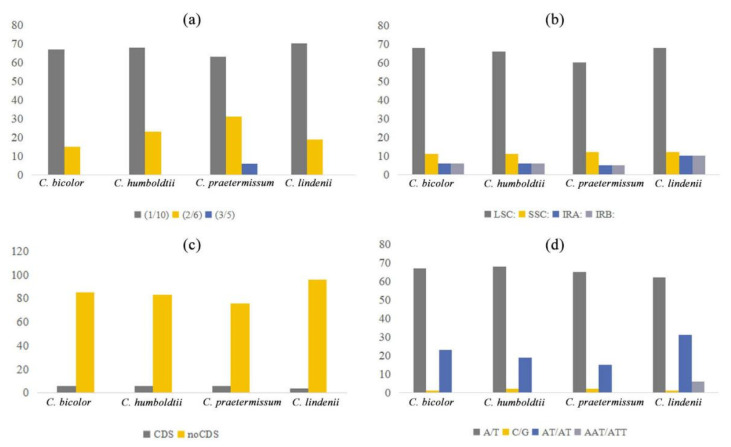
In this study, we analyzed the distribution of SSRs in the cp genomes of four Caladium species. As shown in Figure 4, there were only three types of SSRs, including single-base, two-base, and three-base repeats. Three-base repeats were only present in C. lindenii but not in the other three species. Most SSRs were single-base repeats, accounting for 63.0–81.7% of the total SSRs, which was more than all other repeat types combined. The total numbers of SSRs in C. bicolor and C. humboldtii were similar (91 and 89, respectively). C. praetermissum had the least number of SSRs, whereas C. lindenii had the highest. The SSRs of the four Caladium genomes were mostly distributed in the LSC region and the non-coding region, compared to other regions. In terms of repeating units, A/T repeats were significantly more than C/G repeats. All two-base and three-base repeats were AT/AT repeats and AAT/ATT, respectively, but there were no other types of repeats.
Figure 4.
Comparison of repeat sequences in chloroplast genome distribution of four Caladium species. (a) The frequencies of different SSR repeat classes; (b) The SSR distribution in different chloroplast genome regions; (c) The SSR distribution in CDS and noCDS; (d) The SSR distributions of mono-, di-, and trinucleotide motifs.
2.5. Analysis of Selection Pressure and Codon Bias
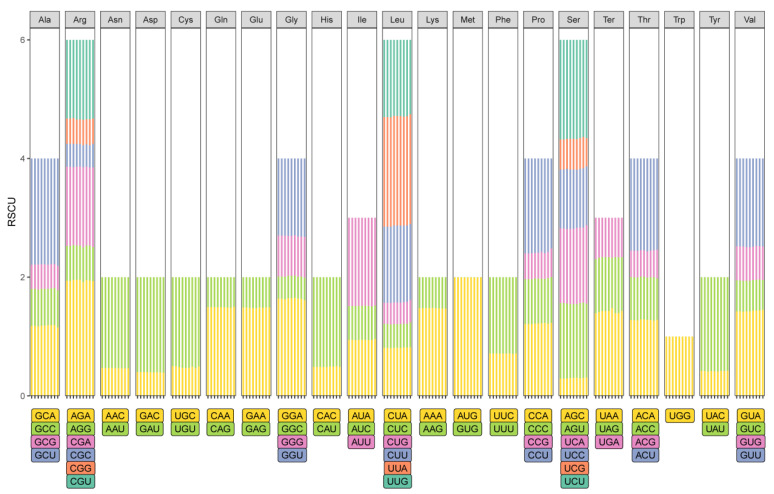
The relative synonymous codon usage (RSCU) tool was used to evaluate the use of synonymous codons in coding regions, where a larger RSCU indicated a stronger bias. Our data showed that the content of leucine was highest in the chloroplast genomes, followed by serine and arginine, whereas the number of codons of tryptophan was the least. As shown in Figure 5, all amino acids except tryptophan used two or more synonymous codons. For example, isoleucine was encoded by three synonymous codons (alanine, glycine, and proline). Threonine and valine were encoded by four synonymous codons, and leucine, serine, and arginine were encoded by six synonymous codons. There were 32 RSCU values greater than one, of which 13 of them ended with A and 16 end with U. These findings were consistent with previous studies, which showed that codons ending with A/U in plants had higher usage preference. As shown in Table S3, the codon preferences of different Caladium species showed high conservation, where two codons exhibited the consistent RSCU value (AUG, 1.997; GUG, 0.003) and represented the extreme value in the four Caladium species. However, there were still some discrepancies among different materials, which mainly focused on the number of codons. For most codons, the codon preferences of C. bicolor and C. humboldtii were almost identical, significantly differing from those of the other two species, especially in C. lindenii.
Figure 5.
Relative synonymous codon usage (RSCU) analysis in chloroplast genes of Caladium species.
In order to analyze the trend of codon usage bias in the cp genomes of nine species, the values of the effective number of codons (ENC) were investigated. As in Table S4, the ENC values varied from 17.158 to 61.000, showing different extents of codon preferences among the species and indicating that the codon preference was weak. To further explore the details, the distribution of the ENC values of the coding genes in the genomes was exhibited in Figure S1.
GC1-3 means the GC content of three different positions of each codon. The overall GC content of the cp genomes varied among the nine species and ranged from 29.06% to 46.21% (Table S5). As expected, the GC1, GC2, and GC3 contents varied significantly across species and also among genes in the genomes. The average value of GC3 in the cp genomes was 28.63%, and the GC1 and GC2 were 46.07%, and 40%, respectively. The GC3 content was significantly lower than that of GC1 and GC2. We also found that the greatest difference in GC content existed in GC3, which was widely applied to better illustrate the codon usage variation. The neutrality plot was shown in Figure S2, which revealed little correlation between GC3 and GC12. These results provided the foundation that the natural selection pressure could greatly affect codon preference.
2.6. Phylogenetic Relationship Analysis
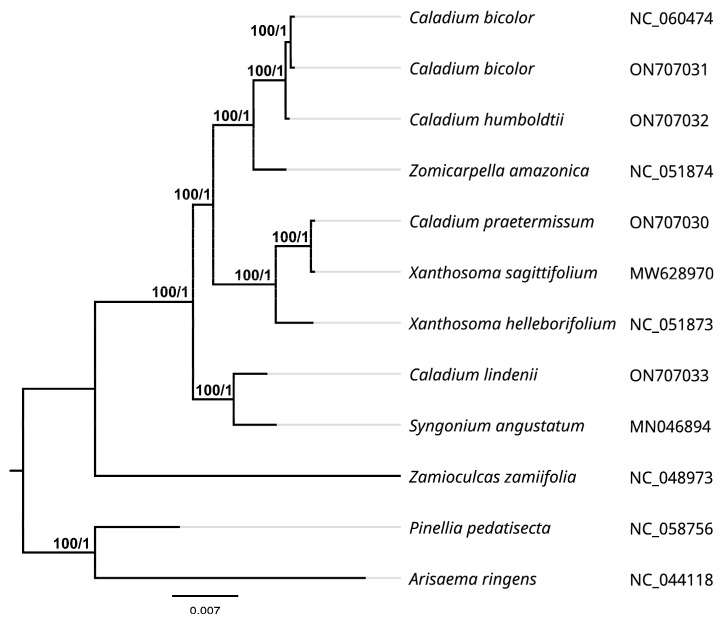
We selected the chloroplast genomes of twelve species of Araceae to explore the genetic relationship between Caladium and its relatives. The phylogenetic tree was constructed based on the complete chloroplast genome sequences using maximum likelihood (ML) and Bayesian inference (BI) methods. The results showed that the topological structures of the ML and BI analyses were identical and that most clades had high posterior probabilities and bootstrap values. As shown in Figure 6, four Caladium species were divided into different branches, in which two C. bicolor (ON7070731 and NC_060474) and C. humboldtii were grouped together, with Z. amazonica being relatively close. Furthermore, C. praetermissum and X. sagittifolium were categorized into a branch, while C. lindenii was furthest from the other three Caladium species occupying a separate branch and being relatively closely related to Syngonium angustatum. These findings are consistent with previous reports, in which C. lindenii was later classified as Caladium genus, and its appearance and resistance were more similar to those of S. angustatum.
Figure 6.
Maximum likelihood (ML) phylogenetic tree construction, including twelve species based on concatenated sequences from all chloroplast genomes. The obtained bootstrap values (BS) and Bayesian inference posterior probabilities (PP) are marked at the tree node (BS/PP).
3. Discussion
The cp genomes of the four Caladium species all had the typical circular tetrad structure of angiosperm cp genomes and were quite different in length. In terms of genome sequence length and number of annotated genes, except for Z. amazonica, which has 130 genes, the other Caladium species, as well as the related species, had 131 genes, indicating that the cp genome of Caladium had a certain degree of conservation. The GC content was reported as an important indicator for judging the genetic relationship among species [45,46]. In our study, the gene types, numbers, and order of genes encoded by the genomes of the four Caladium species were identical, with highly similar G + C content. Based on the G + C content in the region sequence, the rank from high to low was IR, LSC, and SSC, which is a ubiquitous phenomenon in many plant species [47,48,49]. The IR boundary was different among different species, and the fluctuation of the IR boundary was the main reason for the difference [50]. The cp genomes of C. bicolor and C. humboldtii showed the smallest difference in the IR regions among those of the four Caladium species. There was no significant difference in the contraction and expansion of the IR regions between the two. Therefore, the GC content and the IR region boundary conditions, to a large extent, indicate that C. bicolor and C. humboldtii are very closely related but are relatively distinct from C. humboldtii and C. lindenii.
Codon bias affects translation initiation, elongation, and accuracy, as well as mRNA splicing and protein folding [51,52,53]. Therefore, codon preference can also reflect kinship to a certain extent. Among the chloroplast genes of the four Caladium species, the number of codons of leucine was the largest, the number of tryptophan occurrences was the least, and codons ending in A/T were preferred. This feature is consistent with that of most plant species [54,55]. The codon preferences of C. bicolor and C. humboldtii showed almost no difference, suggesting that the two species are closely related, while the codon preferences of C. humboldtii and C. lindenii were markedly different, indicating that they may have unique evolutionary positions.
Microsatellite sequences (microsatellite DNA), also known as SSR, are 1–6 bp repeats that are widely distributed in the cp genome. SSR is highly polymorphic and specific and is a valuable marker for studying gene flow, population genetics, and genetic mapping [56]. Except for C. lindenii, the repeat types and distribution numbers of the other three Caladium species were roughly the same. Identification of these SSR loci can provide candidate molecular markers for research on the genetic diversity and conservation genetics of Caladium. The nodes in the LSC, SSC, and IR regions of the four Caladium species were highly conserved, indicating that the cp genome structure of Caladium is highly conserved. Phylogenetic analysis classified C. lindenii as a single clade that was far from the other three Caladium species, which is consistent with previous reports [23,24,25]. As for the limited research on the phylogenetic analysis, AFLP and SSR markers were often used to identify their kinship among Caladium cultivars. In this paper, we provided more accurate findings on the phylogenetic relationship analysis among different species of Caladieae. Based on the above results, we conclude that C. lindenii has greater specificities in cp genome structure, IR region contraction and expansion, SSR distribution, and codon preference, being relatively close to S. angustatum. Therefore, this species is suggested to be classified into the genus Synaptocarpus.
4. Materials and Methods
4.1. Plant Materials
Plants of four Caladium species were collected from the Environmental Horticulture Research Institute of Guangdong Academy of Agricultural Sciences (23° 23′ N, 113° 26′ E), namely, C. bicolor, C. humboldtii ‘Mini White’, C. praetermissum ‘Hilo Beauty,’ and C. lindenii. The phenotypic characteristics are shown in Figure 7. Young leaves were collected and rinsed thoroughly with tap water. Subsequently, they were washed several times with sterile water and dried quickly in a sampling bag containing silica gel. The samples were then stored at −80 °C until used.
Figure 7.
The four Caladium species used in the present study. (a) C. bicolor; (b) C. humboldtii; (c) C. praetermissum; (d) C. lindenii.
4.2. DNA Extraction and High-Throughput Sequencing
Total DNA was extracted from frozen leaf samples by the modified cetyltrimethylammonium bromide (CTAB) method, and the quality of DNA was assessed by 1.5% agarose gel electrophoresis. The DNA was fragmented by mechanical interruption (ultrasound) and then purified, and end repaired. PolyA tails were added to the 3′ ends, and the fragments were ligated with sequencing adapters. The required fragment size was selected by agarose gel electrophoresis. PCR amplification was performed to form a sequencing library, and the qualified library was sequenced using the BGISEQ-500 platform with PE150 read lengths according to the manufacturer’s instructions. DNA extraction and sequencing were all performed by Guangzhou Bio&Data Biotechnologies Co., Ltd. (Guangzhou, China).
4.3. Chloroplast Genome Assembly and Annotation
At least 5 G of raw data were obtained for each species. After data filtering, adapter sequences and low-quality reads were removed to obtain high-quality clean data. First, NOVOPlasty software (k-mer = 39) was used for assembly and splicing, where the size of the insert was set to 250 bp [57]. Subsequently, the online program GeSeq was employed to annotate the chloroplast genome sequence, and Geneious v9.0.2 was used for visualizing the annotated sequence with manual corrections [58,59]. The annotated sequencing data for the four species were uploaded to the NCBI database with serial numbers ON707030, ON707031, ON707032, and ON707033, respectively. Finally, with the help of the online program Organellar Genome DRAW, the genome maps of the Caladium chloroplast genomes were constructed [60].
4.4. Comparative Analysis of Chloroplast Genomes
Relative synonymous codon usage (RSCU) analysis for every codon in each genome was conducted to determine codon bias [61]. The expansion and contraction of the IR borders of the four chloroplast genomes of Caladium were mapped with the aid of an IRscope [62]. Chloroplast genome similarity was assessed using BLAST Atlas on the GView server (http://server.gview.ca/, accessed on 26 October 2022) with 50 kbp connection windows with C. bicolor genome as a reference [63]. The Perl program provided by MIcroSAtellite Identification Tool (MISA) was used to analyze simple repeat sequence (SSR) sites. For mononucleotides, dinucleotides, trinucleotides, tetranucleotides, pentanucleotides, and hexanucleotides, the repetition thresholds were set to 10, 5, 4, 3, 3, and 3, respectively.
4.5. Phylogenetic Analysis
Published and fully annotated chloroplast genome-wide data of another eight species of Araceae were downloaded from the NCBI database, including C. Bicolor (NC_060474), Syngonium angustatum (MN046894), Zomicarpella amazonica (NC_051874), Zamioculcas zamiifolia (NC_048973), Xanthosoma helleborifolium (NC_051873), Xanthosoma sagittifolium (MW628970), Pinellia pedatisecta (NC_058756) and Arisaema ringens (NC_044118). A phylogenetic tree was constructed based on the complete chloroplast genome sequences of 12 species with maximum likelihood (ML) and Bayesian inference (BI) methods [64]. The ML tree was conducted using IQ-TREE v.2.1.4 [65]. The best-fitting nucleotide substitution model TVM+F+R3 was determined using the Bayesian Information Criterion (BIC) by ModelFinder in the IQ-TREE package, and 1000 bootstrap replicates [66]. The Bayesian inference was performed with MrBayes v.3.2.7, employing the GTR+G model of nucleotide substitution [67]. After the phylogenetic tree was exported, it was viewed using FigTree version 1.4.2.z.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13122180/s1, Figure S1: Analysis of ENC-plot in the cp genomes of nine species of Araceae; Figure S2: Neutrality analysis performed by plotting GC12 values against GC3 values for the cp genomes of nine species of Araceae. The diagonal line on the neutrality plot shows that the value of GC12 is equal to GC3; Table S1: The comparison between the ON707031 and NC_060474; Table S2: Functional classification of Caladium chlroplast genome; Table S3: Determination of optimal codons in the chloroplast genomes of Caladium; Table S4: The ENC values of the coding genes in the cp genomes of nie species of Araceae; Table S5: The GC contents of three positions of codons (GC1, GC2 and GC3) in the cp genomes of nine species of Araceae.
Author Contributions
Conceptualization, Y.Y. and Y.X.; methodology, Y.Z. and J.L.; software, Y.Z. and Y.Y.; validation, G.Z., J.T., Y.Z. and Y.Y.; formal analysis, Y.Y.; investigation, J.T. and J.L.; resources, Y.X.; data curation, Y.Z.; writing-original draft preparation, Y.Y.; writing-review and editing, Y.Y.; visualization, Y.Y.; supervision, G.Z. and Y.X.; project administration, Y.X.; funding acquisition, G.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The four-chloroplast genome sequence data generated in this study are available in GenBank of the National Center for Biotechnology Information (NCBI) under the access numbers: ON707030-ON707033.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by The Project of Collaborative Innovation Center of GDAAS, grant number XT202212, The National Overseas Study Fund Program, grant number 202008440096, and Guangdong Science and Technology Planning Project, grant number 2022B0202160002.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhang Y.S., Chen J.J., Cao Y.M., Duan J.X., Cai X.D. Induction of tetraploids in ‘Red Flash’ Caladium using colchicine and oryzalin: Morphological, cytological, photosynthetic and chilling tolerance analysis. Sci. Hortic. 2020;275:109524. doi: 10.1016/j.scienta.2020.109524. [DOI] [Google Scholar]
- 2.Cao Z., Deng Z.A. Morphological, cytological and molecular marker analyses of ‘Tapestry’ Caladium variants reveal diverse genetic changes and enable association of leaf coloration pattern loci with molecular markers. Plant Cell Tiss. Org. 2020;143:363–375. doi: 10.1007/s11240-020-01922-2. [DOI] [Google Scholar]
- 3.Chen J.J., Zhang Y.S., Duan J.X., Cao Y.M., Cai X.D. Morphological, cytological, and pigment analysis of leaf color variants regenerated from long-term subcultured Caladium callus. In Vitro Cell Dev. Biol.-Plant. 2021;57:60–71. doi: 10.1007/s11627-020-10106-8. [DOI] [Google Scholar]
- 4.Croat T.B., Delannay X., Ortiz O.O., Jiménez P.D. A review of the Aroid Tribe Caladieae with the description of three new species of Caladium and seven new species of Syngonium (Araceae) Novon J. Bot. Nomencl. 2019;27:38–64. doi: 10.3417/2018283. [DOI] [Google Scholar]
- 5.Akhigbemen A.M., Ozolua R.I., Bafor E.E., Okwuofu E.O. Evaluation of some neuropharmacological effects of Caladium bicolor aiton (Araceae) leaf extracts in mice. Metab. Brain Dis. 2019;34:537–544. doi: 10.1007/s11011-019-0390-z. [DOI] [PubMed] [Google Scholar]
- 6.Isah T. Changes in the biochemical parameters of albino, hyperhydric and normal green leaves of Caladium bicolor cv. “Bleeding hearts” in vitro long-term cultures. J. Photoch. Photobiol. B. 2019;191:88–98. doi: 10.1016/j.jphotobiol.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 7.Deng Z.A., Harbaugh B.K. Independent inheritance of leaf shape and main vein color in Caladium. J. Am. Soc. Hortic. Sci. 2006;131:53–58. doi: 10.21273/JASHS.131.1.53. [DOI] [Google Scholar]
- 8.Ahmed E.U., Hayashi T., Yazawa S. Leaf color stability during plant development as an index of leaf color variation among micropropagated Caladium. HortScience. 2004;39:328–332. doi: 10.21273/HORTSCI.39.2.328. [DOI] [Google Scholar]
- 9.Kokalis-Burelle N., Brito J.A., Hartman R.D. Susceptibility of seven Caladium (Caladium × hortulanum) cultivars to Meloidogyne arenaria, M. enterolobii, M. floridensis, M. incognita, and M. javanica. J. Nematol. 2017;49:457–461. [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Z., Deng Z.A. De novo assembly, annotation, and characterization of root transcriptomes of three Caladium cultivars with a focus on necrotrophic pathogen resistance/defense-related genes. Int. J. Mol. Sci. 2017;18:712. doi: 10.3390/ijms18040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain R., Younis A., Riaz A., Tariq U., Ali S., Ali A., Raza S. Evaluating sustainable and environment friendly substrates for quality production of potted Caladium. Int. J. Recycl. Org. 2017;6:13–21. doi: 10.1007/s40093-016-0148-0. [DOI] [Google Scholar]
- 12.Cao Z., Sui S.Z., Cai X.D., Yang Q., Deng Z.A. Somaclonal variation in ‘Red Flash’ Caladium: Morphological, cytological and molecular characterization. Plant Cell Tiss. Org. 2016;126:269–279. doi: 10.1007/s11240-016-0996-3. [DOI] [Google Scholar]
- 13.Cao Z., Sui S.Z., Yang Q., Deng Z.A. Inheritance of rugose leaf in Caladium and genetic relationships with leaf shape, main vein color, and leaf spotting. J. Am. Soc. Hortic. Sci. 2016;141:527–534. doi: 10.21273/JASHS03854-16. [DOI] [Google Scholar]
- 14.Ekeke C., Agbagwa I.O. Anatomical characteristics of Nigerian variants of Caladium bicolor (Aiton) Vent. (Araceae) Afr. J. Plant Sci. 2016;10:121–129. [Google Scholar]
- 15.Ahmed E.U., Hayashi T., Yazawa S. Auxins increase the occurrence of leaf-colour variants in Caladium regenerated from leaf explants. Sci. Hortic. 2004;100:153–159. doi: 10.1016/j.scienta.2003.08.012. [DOI] [Google Scholar]
- 16.Deng Z.A., Peres N.A. ‘Sea Foam Pink’ Caladium. HortScience. 2019;54:1637–1640. doi: 10.21273/HORTSCI14186-19. [DOI] [Google Scholar]
- 17.Yu J.L., Boyd N.S. Tolerance of Caladium cultivars florida cardinal and florida fantasy to sulfonylurea herbicides. HortScience. 2018;53:850–858. doi: 10.21273/HORTSCI12863-18. [DOI] [Google Scholar]
- 18.Cai X.D., Cao Z., Xu S.X., Deng Z.A. Induction, regeneration and characterization of tetraploids and variants in ‘Tapestry’ Caladium. Plant Cell Tiss. Org. 2015;120:689–700. doi: 10.1007/s11240-014-0636-8. [DOI] [Google Scholar]
- 19.Maia A.C.D., Schlindwein C. Caladium bicolor (Araceae) and Cyclocephala celata (Coleoptera, Dynastinae): A well-established pollination system in the northern atlantic rainforest of pernambuco, Brazil. Plant Biol. 2006;8:529–534. doi: 10.1055/s-2006-924045. [DOI] [PubMed] [Google Scholar]
- 20.Deng Z.A., Goktepe F., Harbaugh B.K. Inheritance of leaf Spots and their genetic relationships with leaf shape and vein color in Caladium. J. Am. Soc. Hortic. Sci. 2008;133:78–83. doi: 10.21273/JASHS.133.1.78. [DOI] [Google Scholar]
- 21.Madison M. Notes on Caladium (Araceae) and its allies. Selbyana. 1981;5:342–377. [Google Scholar]
- 22.Croat T. Taxonomic status of neotropical Araceae. Aroideana. 1994;17:33–60. [Google Scholar]
- 23.Deng Z.A., Goktepe F., Harbaugh B.K., Hu J.G. Assessment of genetic diversity and relationships among Caladium cultivars and species using molecular markers. J. Am. Soc. Hortic. Sci. 2007;132:219–229. doi: 10.21273/JASHS.132.2.219. [DOI] [Google Scholar]
- 24.Cao Z., Deng Z.A. Interspecific genome size and chromosome number variation shed new light on species classification and evolution in Caladium. J. Am. Soc. Hortic. Sci. 2014;139:449–459. doi: 10.21273/JASHS.139.4.449. [DOI] [Google Scholar]
- 25.Loh J.P., Kiew R., Kee A., Gan L.H., Gan Y.Y. Amplified fragment length polymorphism (AFLP) provides molecular markers for the identification of Caladium bicolor cultivars. Ann. Bot. 1999;84:155–161. doi: 10.1006/anbo.1999.0903. [DOI] [Google Scholar]
- 26.Chumley T.W., Palmer J.D., Mower J.P., Fourcade H.M., Calie P.J., Boore J.L., Jansen R.K. The complete chloroplast genome sequence of Pelargonium × hortorum: Organization and evolution of the largest and most highly rearranged chloroplast genome of land plants. Mol. Biol. Evol. 2006;23:2175–2190. doi: 10.1093/molbev/msl089. [DOI] [PubMed] [Google Scholar]
- 27.Firetti F., Zuntini A.R., Gaiarsa J.W., Oliveira R.S., Lohmann L.G., Van Sluys M.A. Complete chloroplast genome sequences contribute toplant species delimitation: A case study of the Anemopaegma species complex. Am. J. Bot. 2017;104:1493–1509. doi: 10.3732/ajb.1700302. [DOI] [PubMed] [Google Scholar]
- 28.Lu R.S., Li P., Qiu Y.X. The complete chloroplast genomes of three Cardiocrinum (Liliaceae) species: Comparative genomic and phylogenetic analyses. Front. Plant Sci. 2017;7:2054. doi: 10.3389/fpls.2016.02054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee S.R., Kim K., Lee B.Y., Lim C.E. Complete chloroplast genomes of all six Hosta species occurring in Korea: Molecular structures, comparative, and phylogenetic analyses. BMC Genom. 2019;20:833. doi: 10.1186/s12864-019-6215-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daniell H., Lin C.S., Yu M., Chang W.J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu M.L., Li Q., Hu Z.G., Li X.W., Chen S.L. The complete Amomum kravanh chloroplast genome sequence and phylogenetic analysis of the commelinids. Molecules. 2017;22:1875. doi: 10.3390/molecules22111875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou Y.X., Nie J., Xiao L., Hu Z.G., Wang B. Comparative chloroplast genome analysis of rhubarb botanical origins and development of specific identification markers. Molecules. 2018;23:2811. doi: 10.3390/molecules23112811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J.H., Shi S., Li J.L., Jing Y., Wang L., Yang X.Y., Guo L., Zhou S.L., Sun F.J. Phylogeny of Maleae (Rosaceae) based on multiple chloroplast regions: Implications to genera Circumscription. Biomed. Res. Int. 2018;2018:7627191. doi: 10.1155/2018/7627191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin M.M., Qi X.J., Chen J.Y., Sun L.M., Zhong Y.P., Fang J.B., Hu C.G. The complete chloroplast genome sequence of Actinidia arguta using the PacBio RS II platform. PLoS ONE. 2018;13:e0197393. doi: 10.1371/journal.pone.0197393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li D.M., Zhao C.Y., Liu X.F. Complete chloroplast genome sequences of Kaempferia galanga and Kaempferia elegans: Molecular structures and comparative analysis. Molecules. 2019;24:474. doi: 10.3390/molecules24030474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fonseca L.H.M., Lohmann L.G. Plastome rearrangements in the “Adenocalymma-Neojobertia” clade (Bignonieae, Bignoniaceae) and its phylogenetic implications. Front. Plant Sci. 2017;8:1875. doi: 10.3389/fpls.2017.01875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thode V.A., Lohmann L.G. Comparative chloroplast genomics at low taxonomic levels: A case study using Amphilophium (Bignonieae, Bignoniaceae) Front. Plant Sci. 2019;10:796. doi: 10.3389/fpls.2019.00796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chiapella J.O., Barfuss M.H.J., Xue Z.Q., Greimler J. The plastid genome of Deschampsia cespitosa (Poaceae) Molecules. 2019;24:216. doi: 10.3390/molecules24020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cui Y.X., Chen X.L., Nie L.P., Sun W., Hu H.Y., Lin Y.L., Li H.T., Zheng X.L., Song J.Y., Yao H. Comparison and phylogenetic analysis of chloroplast genomes of three medicinal and edible Amomum species. Int. J. Mol. Sci. 2019;20:4040. doi: 10.3390/ijms20164040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cui Y.X., Nie L.P., Sun W., Xu Z.C., Wang Y., Yu J., Song J.Y., Yao H. Comparative and phylogenetic analyses of ginger (Zingiber offcinale) in the family Zingiberaceae based on the complete chloroplast genome. Plants. 2019;8:283. doi: 10.3390/plants8080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barrett C.F., Baker W.J., Comer J.R., Conran J.G., Lahmeyer S.C., Leebens-Mack J.H., Li J., Lim G.S., MayfieldJones D.R., Perez L., et al. Plastid genomes reveal support for deep phylogenetic relationships and extensive rate variation among palms and other commelinid monocots. N. Phytol. 2016;209:855–870. doi: 10.1111/nph.13617. [DOI] [PubMed] [Google Scholar]
- 42.Ferrarini M., Moretto M., Ward J.A., Surbanovski N., Stevanovic V., Giongo L., Viola R., Cavalieri D., Velasco R., Cestaro A., et al. An evaluation of the PacBio RS platform for sequencing and de novo assembly of a chloroplast genome. BMC Genom. 2013;14:670. doi: 10.1186/1471-2164-14-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Z.H., Gui S.T., Guan Z.W., Pan L., Wang S.Z., Ke W.D., Liang D.Q., Ding Y. A precise chloroplast genome of Nelumbo nucifera (Nelumbonaceae) evaluated with Sanger, Illumina MiSeq, and PacBio RS II sequencing platforms: Insight into the plastid evolution of basal eudicots. BMC Plant Biol. 2014;14:289. doi: 10.1186/s12870-014-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li X.W., Hu Z.G., Lin X.H., Li Q., Gao H.H., Luo G.A., Chen S.L. High-throughput pyrosequencing of the complete chloroplast genome of Magnolia officinalis and its application in species identification. Acta Pharm. Sin. 2012;47:124–130. [PubMed] [Google Scholar]
- 45.Li Y.F., Sylvester S.P., Li M., Zhang C., Li X., Duan Y.F., Wang X.R. The complete plastid genome of Magnolia zenii and genetic comparison to Magnoliaceae species. Molecules. 2019;24:261. doi: 10.3390/molecules24020261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Brunkard J.O., Runkel A.M., Zambryski P.C. Chloroplast extend stromules independently and in response to internal redox signals. Proc. Natl. Acad. Sci. USA. 2015;112:10044–10049. doi: 10.1073/pnas.1511570112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gao C.M., Deng Y.F., Wang J. The complete chloroplast genomes of Echinacanthus species (Acanthaceae): Phylogenetic relationships, adaptive evolution, and screening of molecular markers. Front. Plant Sci. 2019;9:1989. doi: 10.3389/fpls.2018.01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pauwels M., Vekemans X., Godé C., Frérot H., Castric V., Saumitou-Laprade P. Nuclear and chloroplast DNA phylogeography reveals vicariance among european populations of the model species for the study of metal tolerance, Arabidopsis halleri (Brassicaceae) N. Phytol. 2012;193:916–928. doi: 10.1111/j.1469-8137.2011.04003.x. [DOI] [PubMed] [Google Scholar]
- 49.Wu L.W., Nie L.P., Wang Q., Xu Z.C., Wang Y., He C.N., Song J.Y., Yao H. Comparative and phylogenetic analyses of the chloroplast genomes of species of Paeoniaceae. Sci. Rep. 2021;11:14643. doi: 10.1038/s41598-021-94137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li L., Wu Q.P., Fang L., Wu K.L., Li M.Z., Zeng S.J. Comparative chloroplast genomics and phylogenetic analysis of Thuniopsis and closely related genera within Coelogyninae (Orchidaceae) Front. Genet. 2022;13:850201. doi: 10.3389/fgene.2022.850201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hanson G., Coller J. Codon optimality, bias and usage in translation and mRNA decay. Nat. Rev. Mol. Cell Biol. 2017;19:2030. doi: 10.1038/nrm.2017.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tang D., Wei F., Cai Z., Wei Y., Khan A., Miao J., Wei K. Analysis of codon usage bias and evolution in the chloroplast genome of Mesona chinensis Benth. Dev. Genes Evol. 2021;231:1–9. doi: 10.1007/s00427-020-00670-9. [DOI] [PubMed] [Google Scholar]
- 53.Wei L., He J., Jia X., Qi Q., Liang Z., Zheng H., Ping Y., Liu S., Sun J. Analysis of codon usage bias of mitochondrial genome in Bombyx moriand its relation to evolution. BMC Evol. Biol. 2014;14:262. doi: 10.1186/s12862-014-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zheng G., Wei L.L., Ma L., Wu Z.Q., Gu C.H., Chen K. Comparative analyses of chloroplast genomes from 13 Lagerstroemia (Lythraceae) species: Identification of highly divergent regions and inference of phylogenetic relationships. Plant Mol. Biol. 2020;102:659–676. doi: 10.1007/s11103-020-00972-6. [DOI] [PubMed] [Google Scholar]
- 55.Li N., Sun M.H., Jiang Z.S., Shu H.R., Zhang S.Z. Genome-wide analysis of the synonymous codon usage patterns in apple. J. Integr. Agr. 2016;15:983–991. doi: 10.1016/S2095-3119(16)61333-3. [DOI] [Google Scholar]
- 56.Ye Y.J., Xu Y.C., Li D.M., Tan J.J., Liu J.M. Characterization of EST-SSR markers in Curcuma kwangsiensis S. K. Lee & C. F. Liang based on RNA sequencing and its application for phylogenetic relationship analysis and core collection construction. Genet. Resour. Evol. 2021;68:1503–1516. [Google Scholar]
- 57.Dierckysens N., Mardulyn P., Smits G. NOVOPlasty: De novo assembly of organelle genomes from whole genome data. Nucleic Acids Res. 2017;45:e18. doi: 10.1093/nar/gkw955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tillich M., Lehwark P., Pellizzer T., Ulbricht-Jones E.S., Fischer A., Bock R., Greiner S. GeSeq-versatile and accurate annotation of organelle genomes. Nucleic Acids Res. 2017;45:W6–W11. doi: 10.1093/nar/gkx391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., et al. Geneious basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Greiner S., Lehwark P., Bock R. Organellar Genome DRAW (OGDRAW) version 1.3.1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019;47:W59–W64. doi: 10.1093/nar/gkz238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meade J.C., Shah P.H., Lushbaugh W.B. Trichomonas vaginalis: Analysis of codon usage. Exp. Parasitol. 1997;87:73–74. doi: 10.1006/expr.1997.4185. [DOI] [PubMed] [Google Scholar]
- 62.Amiryousefi A., Hyvonen J., Poczai P. Irscope: An online program to visualize the junction sites of chloroplast genomes. Bioinformatics. 2008;34:3030–3031. doi: 10.1093/bioinformatics/bty220. [DOI] [PubMed] [Google Scholar]
- 63.Petkau A., Stuart-Edwards M., Stothard P., Domselaar G.V. Interactive microbial genome visualization with GView. Bioinformatics. 2010;26:3125–3126. doi: 10.1093/bioinformatics/btq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stamatakis A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nguyen L.T., Schmidt H.A., Haeseler A.V., Minh B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., von Haeseler A., Jermiin L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The four-chloroplast genome sequence data generated in this study are available in GenBank of the National Center for Biotechnology Information (NCBI) under the access numbers: ON707030-ON707033.