Abstract
Lipopolysaccharide (LPS) and related bacterial products can be recognized by host inflammatory cells in a particulate, bacterium-bound form, as well as in various soluble, released forms. In the present study we have compared the mechanisms used by LPS, detoxified LPS (DLPS), and mannuronic acid polymers (M-polymers), in solution or covalently linked to particles, in stimulating monocytes to tumor necrosis factor (TNF) production. The addition of recombinant LPS binding protein (LBP) and/or soluble CD14 (sCD14) enhanced the production of TNF from monocytes stimulated with soluble LPS, DLPS, or M-polymer, but did not affect the response to M-polymer or DLPS attached to particles. Treatment of monocytes with antibody to CD14, CD18, or CD11b showed that CD14, but not CR3 (CD11b/CD18), mediated monocyte TNF production in response to the soluble antigens. In contrast, anti-CD14, anti-CD11b and anti-CD18 monoclonal antibodies all inhibited the response to the particulate stimuli. On the other hand, B975, a synthetic analog of Rhodobacter capsulatus lipid A, completely abrogated the monocyte TNF response induced by LPS but did not affect the TNF induction by DLPS or M-polymer, either in soluble or particulate forms. These data demonstrate that the engagement of immune receptors by bacterial products such as LPS, DLPS, and M-polymer is dependent upon the presentation form of their constituent carbohydrates, and that factors such as aggregation state, acylation, carbohydrate chain length, and solid versus liquid phase of bacterial ligands influence the mechanisms used by cells in mediating proinflammatory responses.
Lipopolysaccharide (LPS), a glycolipid present in the outer membrane of gram-negative bacteria, is a potent inducer of proinflammatory responses from cells of the monocytic lineage. LPS stimulation of monocytes results in cytokine production, one of the key events in the pathogenesis of gram-negative sepsis (4). The cell surface glycoprotein CD14 (membrane CD14 [mCD14]) has been identified as the principal LPS receptor on phagocytic leukocytes, enabeling them to be stimulated with picogram amounts of LPS (42, 47). This process is facilitated by the catalytic activity of the blood protein LPS binding protein (LBP), which accelerates the binding of LPS to mCD14 (20). CD14 exists in two forms; in myeloid cells it is expressed as a glycosylphosphatidylinositol (GPI)-anchored glycoprotein (21), whereas a soluble form of CD14 (sCD14) lacking a GPI tail is present in blood (2). We have previously reported that uronic acid polymers with a β (1→4) glycosidic linkage are able to stimulate monocytes to produce tumor necrosis factor (TNF) in an mCD14-dependent manner; polymers of high mannuronic acid content (M-polymers) were found to be the most potent (14). Several reports subsequently implicated CD14 in responses to a variety of bacterial compounds (36, 37, 44), suggesting that the role of CD14 is not limited to LPS recognition.
In addition to CD14, other proteins described as LPS receptors include the β2-integrins CR3 (CD11b/CD18, Mac-1) and CR4 (CD11c/CD18, p150,95) (46). Wright and coworkers reported that CR3 and CR4 function in the recognition of Escherichia coli by binding to the lipid A portion of LPS (46). However, cells from patients genetically deficient in CD18 expression responded normally to LPS (45), suggesting that CD18 is not essential for cellular responses to LPS. On the other hand, Ingalls et al. found that Chinese hamster ovary (CHO)-K1 cells transfected with CR3 or CR4 acquire LPS responsiveness, as evidenced by inducible NF-κB translocation (24, 25). Furthermore, components from group B streptococcus (GBS) type III can activate human monocytes to TNF production through a CD18-dependent mechanism (8, 31), suggesting that under certain defined conditions, engagement of the β2-integrins by bacterial ligands is proinflammatory.
Previously we have reported that covalently linking detoxified LPS (DLPS) and M-polymers to particles increased their TNF-inducing potency 2,000 to 60,000 times compared to that of the polymers in soluble form (3). In the present work we have investigated the mechanisms by which soluble LPS, DLPS, and M-polymers (350 kDa) stimulate monocytes to produce TNF compared to DLPS covalently attached to particles (DLPS-particles) or M-polymers (∼3 kDa) covalently attached to particles (M-particles). The data suggest that phagocytes utilize membrane CD14 for LPS-, DLPS-, and M-polymer-induced TNF production, both in solution and attached to particles. In contrast, the β2-integrin CR3 only participates in the response to the particulate form of the polymers. These data suggest that different membrane receptors are used by soluble and particulate forms of DLPS and M-polymers in mediating TNF production from human monocytes.
MATERIALS AND METHODS
Reagents.
Alginate highly enriched in mannuronic acid (M-polymer) was isolated from agar colonies of Pseudomonas aeruginosa strain 8830 grown at 18°C as described previously (27). Alginate was radiolabeled by adding 30 μCi of 14C-labeled fructose/petri dish (Amersham, Little Chalfont, Buckinghamshire, England). The radiolabeled material was deacetylated by treatment with 0.1 M NaOH for 1 h at room temperature (RT) and then comprehensively dialyzed against distilled water. This product was then purified by precipitation with 50% ethanol and repeated extraction of the precipitate in 70% ethanol and in chloroform. M-polymer was subjected to 0.1 M NaOH for 30 min at 45°C in order to inactivate trace amounts of endotoxin by base hydrolysis (33). The polymer was then utterly purified by 2 rounds of precipitation with ethanol followed by treatment with 0.1 M HCl at RT (cleaves the acid-labile 2-keto-3-deoxyoctulosonic acid [KDO] linkage), dissolved in pyrogen-free water, filtered through a 0.22-μm-pore-size membrane filter (Millipore), and lyophilized. The content of mannuronic acid in the M-polymer was estimated to be 92% by 1H nuclear magnetic resonance (NMR) spectroscopy (18, 19), and the average molecular size was determined to be about 350 kDa by viscometry (Scott-Geräte). M-polymers of low molecular weight were prepared by acid hydrolysis of 350 kDa M-polymer for 1 h at 100°C and pH 5.6 and then 1.5 h at 100°C and pH 3.8. This procedure yielded M-polymers with an average molecular size of 3 kDa and 94% d-ManA. Endotoxin contamination was 0.25 ng/mg in the 350-kDa M-polymer preparation and 0.2 ng/mg in the 3-kDa M-polymer preparation, as measured by the Limulus amebocyte lysate assay (Chromogenix AB, Mölndal, Sweden).
LPS L-2137 and detoxified LPS L-1523 (prepared by mild alkaline deacylation of LPS to remove ester-linked fatty acids) from smooth Salmonella minnesota were purchased from Sigma (St. Louis, Mo.). Protein contamination of DLPS was less than 0.5% as measured by the Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, Calif.). Recombinant sCD14 and LBP were provided by H. Lichenstein (Amgen, Thousand Oaks, Calif.). Hybridoma supernatants containing immunoglobulin M (IgM) monoclonal antibodies (MAbs) IIE10 (27) and 2G8 (14), specific for M-polymer, were prepared as previously described. Anti-human CD14 MAb 3C10 (IgG2b) and anti-human CD18 MAb IB4 (IgG2a) were purified on Sepharose goat anti-mouse IgG as described by the manufacturer from supernatants of the respective hybridoma cell lines (American Type Culture Collection [ATCC], Manassas, Va.). The anti-CD11b MAbs Mn41 (IgG1) (12) and OKM-1 (IgG2b) (6) were kindly provided by G. D. Ross (University of Louisville, Louisville, Ky.). MAb 6H8 (IgG1), which recognizes a widely distributed 180-kDa glycoprotein (T. Espevik and B. Naume, unpublished observation), was used as a control. A synthetic disaccharide analog of Rhodobacter capsulatus lipid A (B975) was provided by D. P. Rossignol and W. J. Christ (Eisai Research Institute, Andover, Mass.) (34). B975 is a potent LPS inhibitor (34). B975 was dissolved in dimethyl sulfoxide (DMSO) to a stock solution of 10−3 M. Human recombinant TNF (specific activity, 7.6 × 107 U/mg) was supplied by Genentech Inc. (South San Francisco, Calif.).
Preparation of covalent DLPS- and M-particles.
Magnetic monodisperse polystyrene particles L-1658 (4 μm) were prepared as described elsewhere (41). Low-molecular-size M-polymer (3 kDa) and DLPS were covalently coupled to the particles through formation of amide bonds between carboxylate groups on the M-polymer and DLPS (KDO sugars), and primary amino groups on the particles. The coupling was carried out in 0.1 M phosphate buffer, pH 7.3, by adding 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC; Fluka Chemie AG, Buchs, Switzerland) and sulfo-NHS (N-hydroxysulfosuccinimide sodium salt) (Fluka) as described elsewhere (23). After the oligosaccharides were linked to the particles, they were extensively washed in 0.1 M and 1.0 M sodium phosphate buffer, pH 7.3, and 0.1 M sodium carbonate buffer, pH 10, in order to remove noncovalently bound polymers. The amount of M-polymer covalently linked to the beads was estimated to be 40 ng of M-polymer per 106 particles, by measuring incorporated 14C. Although the amount of DLPS bound to the particle surface was not measured, it was estimated to be equal to or less than the amount of M-polymer attached to the beads. DLPS can form amide bonds to the particles only through the KDO sugars, and therefore has fewer residues to attack than the M-polymer, which contains available carboxylate groups at each monomer.
Preparation of noncovalent M-particles.
Monodisperse magnetic Dynabeads containing M-450 rat anti-mouse (RAM) IgM were purchased from Dynal (Oslo, Norway). Low-molecular-mass M-polymer (3 kDa) was attached to the particles through a secondary IgM MAb, IIE10, specific to the polymer (27). The particles were washed in 0.1% bovine serum albumin (BSA)–phosphate-buffered saline (PBS) then incubated for 30 min at +4°C with hybridoma supernatant containing 2 μg of MAb IIE10 per mg of particles, or 0.1% BSA-PBS. After thorough washing of the particles in 0.1% BSA-PBS, they were incubated at 37°C for 1 h with either 4 mg of M-polymer/ml (M-particles) or 0.1% BSA-PBS (control particles) and washed again. The amount of M-polymer noncovalently attached to the beads was not measured.
Cell lines and culture conditions.
The following stably transfected CHO cell lines are described elsewhere: CHO/neo (CHO-K1 transfected with pCDNA1/neo) (17); CHO/CD14, CHO/CR3, and CHO/CR4 (CHO-K1 transfected with human CD14 [17]), human CD11b and CD18 [24], or human CD11c and CD18 [25], respectively). Transfectants were maintained in RPMI 1640 medium (Gibco, Paisley, United Kingdom) with 0.01% l-glutamine and 40 μg of gentamicin/ml (referred to below as RPMI), 10% heat-inactivated (HI) fetal calf serum (FCS) (HyClone, Logan, Utah), and 1 mg of G418 (Sigma)/ml at 37°C under 5% CO2.
Isolation of human monocytes.
Monocytes were isolated from human A+ buffy coats (The Bloodbank, University Hospital, Trondheim, Norway) as described previously (5). Adherent cell monolayers (1 × 105 to 2 × 105 monocytes/well) were cultured in 24-well plates in AIM serum-free medium (Gibco) supplemented with 0.01% l-glutamine and 40 μg of gentamicin/ml. The monocytes were stimulated for 8 h at 37°C under 5% CO2 with the indicated preparations. In some experiments, the cells were preincubated with MAbs, B975, or an equivalent amount of DMSO for 30 min at RT prior to addition of the agonists. Supernatants were collected and stored at −20°C until assayed for TNF activity in the WEHI clone 13 bioassay, as described previously (13).
Flow cytometric quantification of M-polymer binding to CHO transfectants.
All steps were performed at 0 to 4°C as described in detail elsewhere (14). Briefly, adherent CHO transfectants were detached by 0.02% EDTA-PBS, washed twice in 0.1% BSA-PBS, and incubated with 100 μg of M-polymer/ml in 0.1% BSA–10% HI normal human A+ serum (HS)–PBS for 45 min. After two washes, the cells were incubated for 30 min with 50 μl of 2G8 hybridoma supernatant (specific for M-polymer [14]), washed twice, and stained with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse MAbs (GAM-FITC; Becton Dickinson, Lincoln Park, N.J.) for 30 min. Controls without M-polymer were incubated either with 2G8 hybridoma supernatant and GAM-FITC or with GAM-FITC alone. Analysis was performed with a FACscan flow cytometer (Becton Dickinson).
Binding of particles to fluorescently labeled CHO transfectants.
To assess binding of the DLPS- and M-particles, CHO transfectants were first stained with a PKH26 Red Fluorescence Cell Linker Kit (Sigma). One million suspended cells were washed in PBS and incubated with 1 μM PKH26 in dilution buffer (supplied by the manufacturer) for 5 min at RT. Two hundred microliters of HI FCS was then added, and then incubation was allowed to proceed for 1 additional minute before the cells were washed three times in RPMI–10% HI FCS. The cells were seeded onto coverslips in 24-well plates at a density of 2 × 104 cells/well in RPMI–10% HI FCS and incubated overnight at 37°C in a 5% humidified atmosphere. The following day, the adherent cells were washed three times with Hank's balanced salt solution (BSS) (Gibco) and incubated with particles at a ratio of 10:1 (particles to cells) for 2 h at 37°C in RPMI–1% HS. Finally, the coverslips were washed in PBS, immersed in 3.7% formalin for cell fixation, and mounted on glass slides in Mowiol (Hoechst, Frankfurt, Germany). The glass slides were examined by microscopy, and the number of particles associated per cell were determined for at least 100 cells. Data are expressed as the mean values of bound particles per cell for triplicate determinations.
RESULTS
Attaching mannuronic acid polymers to particles increases their ability to induce TNF.
Monocytes were exposed to M-polymer (3 kDa) linked covalently or via MAbs to particles, and the amount of released TNF was determined.
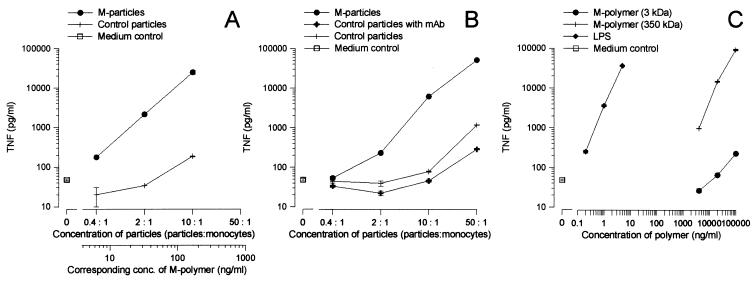
In agreement with previously reported results (3), Fig. 1A and C demonstrate that M-polymers presented to cells as surface particulates are more efficacious than the soluble form of the polymer in stimulating monocytes to produce TNF. To verify that the observed enhancement is not caused by chemical changes of the polymers during the coupling reaction, M-polymers were noncovalently attached to particles via a specific MAb, IIE10 (27). As evident from the data in Fig. 1, the noncovalent M-particles (Fig. 1B) stimulated monocytes to release TNF comparably to the covalently coupled M-particles (Fig. 1A).
FIG. 1.
Attaching mannuronic acid polymers to particles, either covalently or via MAbs, increases their TNF-inducing potency. Human monocytes were stimulated with serial dilutions of M-polymers (3 kDa) covalently linked to particles (M-particles) or particles without polymer (control particles) (A); M-polymers (3 kDa) noncovalently attached via a MAb, IIE10, to particles (M-particles), particles with IIE10 (control particles with MAb), or particles without antibody or polymer (control particles) (B); and M-polymers (3 and 350 kDa) or LPS in soluble forms (C). Supernatants were collected after 8 h of stimulation and assayed for TNF activity. The level of spontaneous TNF release (medium control) is indicated. The mean TNF levels ± standard deviations of three replicates from a representative experiment are shown, and similar data were obtained in two other independent experiments.
The potentiating effect of serum in stimulating monocytes with DLPS- or M-particles is not due to LBP or sCD14.
In the next series of experiments, human monocytes were exposed to the soluble and particulate antigens in the presence or absence of either HS, recombinant LBP (rLBP), sCD14, or a combination of rLBP and sCD14.
HS not only potentiated LPS- and M-polymer-induced TNF production, but also had a comparable effect on DLPS, DLPS-particles, and M-particles (Table 1). Heat inactivation equally reduced the potentiating effect of serum on the various samples, although the subsequent TNF release was higher than that without the addition of serum (data not shown). As reported previously (27, 28), the addition of either rLBP or sCD14 increased TNF production from monocytes exposed to LPS or M-polymer. This effect was further enhanced when rLBP and sCD14 were added together (Table 1). Similar results were also obtained for DLPS (Table 1). In contrast, neither rLBP nor sCD14, alone or in combination, affected the level of TNF induced by the particulate antigens. Thus, while the potentiating effect of serum on the soluble polymers can be explained in part by the presence of LBP and sCD14, other serum components may be responsible for the enhanced TNF production induced by M-particles and DLPS-particles.
TABLE 1.
Effects of sCD14 and rLBP on TNF production by human monocytesa
| Samples | TNF release (pg/ml) (mean ± SD)
|
||||
|---|---|---|---|---|---|
| Medium | + rLBP (0.1 μg/ml) | + sCD14 (0.1 μg/ml) | + rLBP-sCD14 (0.1 μg/ml) | + HS (10%) | |
| M-particles | 6,028 ± 181 | 4,816 ± 222 | 5,112 ± 943 | 7,018 ± 443 | 41,645 ± 3,514 |
| DLPS-particles | 519 ± 15 | 527 ± 63 | 517 ± 17 | 610 ± 32 | 7,090 ± 358 |
| LPS | 241 ± 19 | 8,797 ± 848 | 3,370 ± 84 | 28,440 ± 1,276 | 39,439 ± 3,535 |
| DLPS | 394 ± 17 | 763 ± 4 | 1,313 ± 171 | 2,451 ± 102 | 3,704 ± 791 |
| M-polymer | 5,396 ± 440 | 19,109 ± 1,648 | 37,751 ± 1,587 | 46,811 ± 4,076 | 57,338 ± 5,282 |
| Control particles | 118 ± 23 | 120 ± 8 | 160 ± 4 | 213 ± 18 | 198 ± 11 |
| Medium | 166 ± 4 | 151 ± 14 | 203 ± 15 | 290 ± 10 | 89 ± 10 |
Human monocytes were incubated with M-particles (2:1, particles to monocytes), DLPS-particles (2:1, particles:monocytes), LPS (0.2 ng/ml), DLPS (20 μg/ml), M-polymer (20 μg/ml), or control particles (2:1, particles:monocytes) in the presence or absence of HS, rLBP, sCD14, or a combination of rLBP and sCD14 for 8 h at 37°C. Cell supernatants were harvested and assayed for TNF as described in Materials and Methods, and results are shown as mean TNF release ± standard deviations of three parallel samples from one representative among four independent experiments.
Expression of CR3 or CR4 on CHO cell transfectants promotes binding of DLPS- and M-particles.
CHO cell transfectants expressing either CD14, CR3, or CR4 were used to assess the binding of M-polymer, M-particles, and DLPS-particles in order to distinguish the individual roles of each of these receptors.
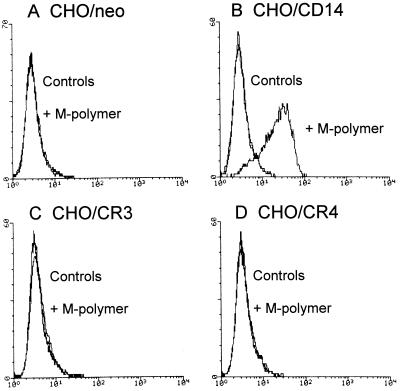
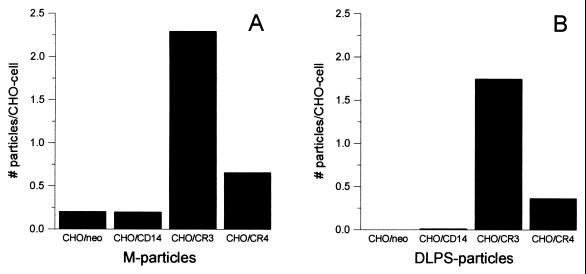
Binding of M-polymer to CHO transfectants was assessed by flow cytometry with an M-polymer specific MAb, 2G8 (14). The results demonstrate that M-polymer bound only to CD14-transfected CHO cells, and not to CR3-, CR4-, or control (neo) transfected cells (Fig. 2). Binding of DLPS- and M-particles was quantified by microscopy by counting the number of particles attached to or ingested by fluorescently labeled CHO cells. As shown in Fig. 3, both M- and DLPS-particles bound specifically to CR3- and CR4-transfected cells, although the binding to CHO/CR3 cells was about threefold more efficient than the binding to CHO/CR4 cells. Some unspecific binding of the M-particles to control CHO/neo cells explains the apparently higher number of M-particles attached to all the CHO transfectants compared to the DLPS-particles. Despite specific binding of the particles to β2-integrin-transfected CHO-cells, DLPS- and M-particles failed to activate NF-κB translocation in CHO/CD14/CR3 or CHO/CD14/CR4 cells, whereas high concentrations of the soluble polymers weakly induced NF-κB activation in CHO/CD14/β2-integrin transfectants (data not shown). Thus, expression of β2-integrins together with CD14 was not sufficient to enable responses to DLPS- and M-particles in CHO cells.
FIG. 2.
M-polymer binds to CHO/CD14, but not to CHO/neo, CHO/CR3, or CHO/CR4 cells. CHO/neo (A), CHO/CD14 (B), CHO/CR3 (C), and CHO/CR4 (D) cells were incubated on ice with 100 μg of M-polymer/ml in 0.1% BSA–PBS–10% HI HS for 45 min, and binding was assessed by flow cytometry with 2G8 hybridoma supernatant specific for M-polymer. Results from one of three experiments are shown, and controls represent either binding of 2G8 hybridoma supernatant and GAM-FITC or binding of GAM-FITC only.
FIG. 3.
Binding of M-particles and DLPS-particles to CHO cells transfected with CD14, CR3, or CR4. Fluorescently stained CHO/neo, CHO/CD14, CHO/CR3, and CHO/CR4 cells were incubated with M-particles (A) or DLPS-particles (B) at concentrations of 10:1 (particles to cells) in RPMI–1% HS for 2 h at 37°C and were then fixated and mounted on glass slides. An average value of the number of particles per cell for triplicate slides was determined for each cell type by use of light and fluorescence microscopy. Shown are the results from one experiment representative of three independent experiments.
DLPS- and M-particles activate human monocytes through an mCD14- and CR3-dependent pathway.
We next wanted to elucidate the importance of CD14 and the β2-integrins CR3 and CR4 in signaling TNF production induced by M-particles and DLPS-particles. Human monocytes were preincubated with MAbs to CD14 (3C10), CD18 (IB4), or CD11b (Mn41 and OKM-1) under serum-free conditions, prior to the addition of soluble or particulate stimulants. MAb 6H8 served as a control.
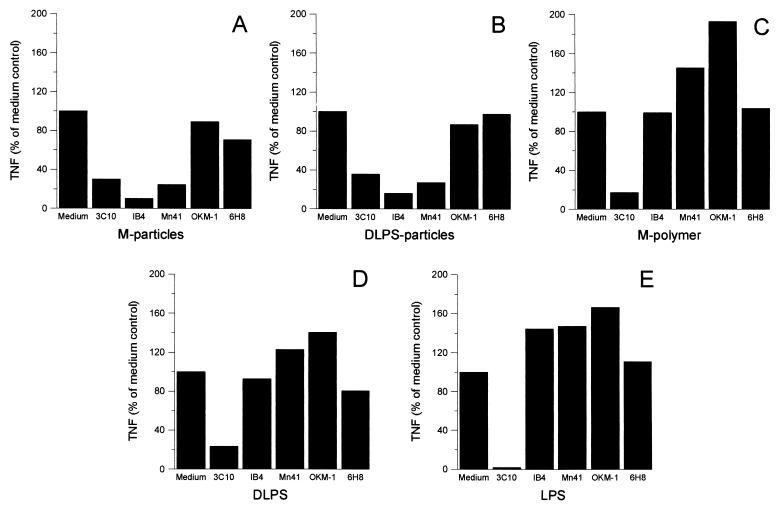
In accordance with previous findings (14, 47), the anti-CD14 MAb 3C10 almost completely blocked TNF production from monocytes stimulated with M-polymer or LPS (Fig. 4C and E). In addition, 3C10 also abrogated the TNF response induced by DLPS (Fig. 4D). The MAbs to CD18 and CD11b had no inhibiting effect on either of the soluble antigens (Fig. 4C through E).
FIG. 4.
Effects of anti-CD14, anti-CD18, and anti-CD11b MAbs on TNF production from human monocytes. Human monocytes were pretreated with either a CD14 MAb (3C10), a CD18 MAb (IB4), CD11b MAbs (Mn41 or OKM-1), or a control MAb, 6H8, at 10 μg/ml for 30 min at RT, prior to addition of M-particles (10:1, particles to monocytes) (A), DLPS-particles (10:1, particles to monocytes) (B), M-polymer at 100 μg/ml (C), DLPS at 100 μg/ml (D), or LPS at 1 ng/ml under serum-free conditions (E). The cells were incubated for 8 h at 37°C before bioactive TNF was assayed in the supernatants. After correcting for the spontaneous TNF production, the results were calculated as percentages of the TNF level produced in the absence of MAbs (Medium). Results are presented as means of four independent experiments.
Addition of 3C10 reduced the TNF production from monocytes exposed to M-particles and DLPS-particles to about 30% of the initial value (Fig. 4A and B), and did not completely block the response even after the concentration of MAbs was raised to 40 μg/ml (data not shown). Both the anti-CD18 MAb IB4 and the anti-CD11b MAb Mn41, which recognizes the I domain (11, 39), profoundly inhibited the TNF response to M- and DLPS-particles. A second MAb, OKM-1, which recognizes the C-terminal lectin domain of CD11b (11, 39), did not inhibit TNF release in response to the particulate stimuli (Fig. 4A and B). The control MAb, 6H8, did not influence any of the samples tested. The results suggest that β2-integrins are signaling receptors on monocytes for DLPS- and M-particles that function in a coordinated manner with CD14. This conclusion is reinforced by our observations that combinations of MAbs 3C10 and IB4 inhibited particle-induced TNF production to a greater extent than either MAb alone (data not shown).
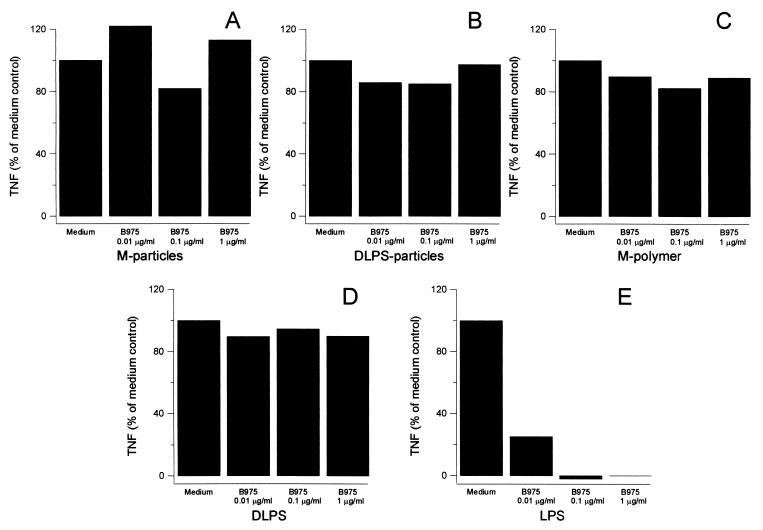
B975 antagonizes LPS but has no effect on TNF production induced by DLPS, M-polymer, DLPS-particles, or M-particles.
Several lipid A structural analogs antagonize LPS responses in human cells. This effect is likely due to the inhibitory activity of these compounds on an LPS signal-transducing component and is independent of CD14 (10, 26). B975 is a synthetic analog of R. capsulatus lipid A and a potent LPS antagonist (34). We examined the inhibiting action of B975 under serum-free conditions in order to study the mechanisms involved in signaling TNF production from monocytes.
As little as 10 ng of B975/ml significantly inhibited TNF production by LPS-stimulated monocytes (Fig. 5E). The antagonistic action of B975 seemed to require an intact lipid ligand, as evidenced by the lack of inhibition of soluble and particulate DLPS (Fig. 5D and B). Furthermore, B975 failed to inhibit M-polymer and M-particles, which lack a lipid component (Fig. 5C and A). The lack of inhibition of DLPS- and M-polymer-induced TNF production by B975 could be due to the higher concentration of these polymers (100 μg/ml) compared to LPS (1 ng/ml). However, in additional experiments we found that 10 ng of B975/ml gave 50% reduction of the TNF level induced by a 100-fold-higher concentration (1 μg/ml) of E. coli LPS, whereas 1 μg of B975/ml did not affect the TNF production induced by a 10-fold-higher concentration of DLPS or M-polymer (10 μg/ml) (data not shown). Moreover, subjecting the polymers to 100°C for 2 min did not alter their TNF-inducing potency, excluding a possible interference from protein contamination (not shown). Thus, although sharing the involvement of CD14, the subsequent signaling events seem to differ for LPS compared to DLPS and mannuronic acid polymers.
FIG. 5.
Effects of the LPS antagonist B975 on TNF production from human monocytes. Human monocytes were pretreated with the indicated concentrations of B975 for 30 min at RT prior to addition of M-particles (10:1, particles to monocytes) (A), DLPS-particles (10:1, particles to monocytes) (B), M-polymer at 100 μg/ml (C), DLPS at 100 μg/ml (D), or LPS at 1 ng/ml under serum free conditions (E). The cells were incubated for 8 h at 37°C before bioactive TNF was assayed in the supernatants. After correcting for the spontaneous TNF production, the results were calculated as percentages of the TNF level produced in the absence of MAbs (Medium). Results are presented as means of three independent experiments.
DISCUSSION
During sepsis and inflammation the host cells may encounter intact bacteria as well as various soluble, released bacterial compounds. By covalently coupling bacterial carbohydrates to a microbead, the resulting particle mimics the surface of an extremely simplified model bacterium with the advantage of no shedding of bacterial components. In the present study we show that M-polymers noncovalently attached to particles via a MAb, IIE10, stimulate TNF production from monocytes to an extent comparable with the covalently linked M-particles. Thus, the increased biological activity observed when DLPS and M-polymers are linked to particles (3) seems to be caused by changes in the physical presentation form, and not by the chemical treatment of the polymers.
Our results show that while soluble LPS, DLPS, and M-polymer used mCD14 for signaling TNF release, the DLPS- and M-particles in addition exploited CR3 and/or CR4. The preference for β2-integrins in stimulation of monocytes with immobilized polysaccharides resembles observations that encapsulated GBS type III bacteria induce TNF production from monocytes through a CD18-dependent mechanism, whereas GBS cell wall fragments use both CD14 and CD18, and soluble GBS type III polysaccharides preferentially stimulate monocytes through a CD14 pathway (8, 9, 31). Also of interest is a recent study by Troelstra et al. showing that free LPS binds to neutrophils via CD14, whereas whole S. minnesota interacts mainly independently of CD14, and LPS-coated erythrocytes activate neutrophils via CD14 and subsequently bind to CR3 (40).
LBP and sCD14, proteins known to enhance LPS effects both in mCD14-negative and mCD14-positive cells (16, 20), had similar effects on LPS, M-polymer, and DLPS in potentiating the TNF response from monocytes. In contrast to this, neither rLBP nor sCD14, alone or in combination, had any effect on the TNF level induced by DLPS- or M-particles. This may be explained by sCD14 and rLBP acting as carriers in transporting the soluble antigens to mCD14 or other membrane structures on the monocytes. When these polymers are present on a particle surface, such transport could be superfluous. Heat inactivation reduced the potentiating effect of serum on both soluble and particulate stimuli (data not shown). Both LPB (32) and sCD14 (our unpublished observations) are heat-sensitive proteins, and this might explain the effect on the soluble stimuli. Preincubating the particles in serum prior to serum-free stimulation of monocytes did not result in increased TNF production (data not shown). Thus, the reduced effect of HI serum on the particles cannot be explained by complement inactivation, and further experiments are necessary to clarify what heat-sensitive serum components impart the increased TNF production induced by the particles.
Some CD14 MAbs and several lipid A structural analogs have been shown to block cellular activation by LPS but not LPS binding, and high concentrations of LPS activate cells in a CD14-independent manner (10). CD14 lacks transmembrane and cytoplasmic parts; thus the main role of CD14 may be to concentrate LPS and other bacterial components at the cell surface to interact with other, signal-transducing molecules. Although the β2-integrins are transmembrane receptors, Ingalls et al. have shown that the cytoplasmic part is not necessary for signaling LPS-induced NF-κB activation in CHO/CR3 cells (24). Thus, the β2-integrins may have functions similar to CD14 in bringing LPS, M-particles, and DLPS-particles into closer contact with putative signal transducers. Both Delude et al. (10) and Ingalls et al. (26) have suggested the existence of a lipid A signal transducer, and the recent discovery that Toll-like receptor 4 (TLR4) can mediate lipid A and LPS cell activation has verified this theory (29, 35). In humans, TLR4 is a signal transducer for LPS, and recently it has been shown that TLR2 functions as a signal-transducing receptor for diverse microbial products such as gram-positive bacterial components (15, 30, 38), lipoproteins (1, 7, 30) and zymosan (43). The results obtained with DLPS and DLPS-particles suggest the existence of signal-transducing mechanisms recognizing the O-chain part of LPS which was not blocked by the lipid A analog B975. This suggests that, with a defective lipid A part, LPS behaves similarly to the polysaccharide M-polymer in that B975 did not affect the TNF induction in monocytes. It is possible that intact LPS signals through TLR4, whereas some of the other TLRs, like TLR2, recognize DLPS and/or M-polymer. Moreover, although DLPS- and M-particles used both CD14 and the β2-integrins for signaling TNF production in monocytes, they were incapable of signaling NF-κB translocation in CHO/CD14/CR3 and CHO/CD14/CR4 cells. If a signal transducer is missing in CHO cells but present in monocytes, or if other crucial factors are not working efficiently in CHO cells, such as the ability to ingest and degrade particulate antigens, this could explain the apparently conflicting results. A similar phenomenon has been observed with GBS cell wall fragments, as GBS-induced TNF production was inhibited with antibodies to CD18, but GBS failed to induce NF-κB in CHO/CR3 or CHO/CR4 cells (31). Of interest is the finding that CHO cells express a nonfunctional TLR2, which could be associated with the lack of responses observed with the particles (22). A potential role of TLRs in recognition of DLPS and mannuronic acid polymers is currently under investigation.
In summary, our results show that both chemical differences and physical presentation forms of various stimuli influence what monocyte receptors are used in signaling TNF production. Further studies in comparing the mechanisms of stimulation of various cells with intact bacteria and isolated bacterial compounds will help bring a better understanding of the events underlying inflammation and the often fatal sepsis syndrome.
ACKNOWLEDGMENTS
This work was supported by a research grant (to T.H.F.) from the Norwegian University of Science and Technology and by Pronova Biopolymer, the Norwegian Cancer Society, and the Norwegian Research Council.
REFERENCES
- 1.Aliprantis A O, Yang R B, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 2.Bazil V, Horejsi V, Baudys M, Kristofova H, Strominger J L, Kostka W, Hilgert I. Biochemical characterization of a soluble form of the 53-kDa monocyte surface antigen. Eur J Immunol. 1986;16:1583–1589. doi: 10.1002/eji.1830161218. [DOI] [PubMed] [Google Scholar]
- 3.Berntzen G, Flo T H, Medvedev A, Kilaas L, Skjak-Braek G, Sundan A, Espevik T. The tumor necrosis factor-inducing potency of lipopolysaccharide and uronic acid polymers is increased when they are covalently linked to particles. Clin Diagn Lab Immunol. 1998;5:355–361. doi: 10.1128/cdli.5.3.355-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bone R C. The pathogenesis of sepsis. Ann Intern Med. 1991;115:457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- 5.Boyum A. Isolation of lymphocytes, granulocytes and macrophages. Scand J Immunol. 1976;5(Suppl 5):9–15. [PubMed] [Google Scholar]
- 6.Breard J, Reinherz E L, Kung P C, Goldstein G, Schlossman S F. A monoclonal antibody reactive with human peripheral blood monocytes. J Immunol. 1980;124:1943–1948. [PubMed] [Google Scholar]
- 7.Brightbill H D, Libraty D H, Krutzik S R, Yang R B, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 8.Cuzzola M, Mancuso G, Beninati C, Biondo C, Genovese F, Tomasello F, Flo T H, Espevik T, Teti G. β2 Integrins are involved in cytokine responses to whole gram-positive bacteria. J Immunol. 2000;164:5871–5876. doi: 10.4049/jimmunol.164.11.5871. [DOI] [PubMed] [Google Scholar]
- 9.Cuzzola M, Mancuso G, Beninati C, Biondo C, von Hunolstein C, Orefici G, Espevik T, Flo T H, Teti G. Human monocyte receptors involved in tumor necrosis factor responses to group B streptococcal products. Infect Immun. 2000;68:994–998. doi: 10.1128/iai.68.2.994-998.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delude R L, Savedra R, Jr, Zhao H, Thieringer R, Yamamoto S, Fenton M J, Golenbock D T. CD14 enhances cellular responses to endotoxin without imparting ligand-specific recognition. Proc Natl Acad Sci USA. 1995;92:9288–9292. doi: 10.1073/pnas.92.20.9288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diamond M S, Garcia-Aguilar J, Bickford J K, Corbi A L, Springer T A. The I domain is a major recognition site on the leukocyte integrin Mac-1 (CD11b/CD18) for four distinct adhesion ligands. J Cell Biol. 1993;120:1031–1043. doi: 10.1083/jcb.120.4.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddy A, Newman S L, Cosio F, LeBien T, Michael A. The distribution of the CR3 receptor on human cells and tissue as revealed by a monoclonal antibody. Clin Immunol Immunopathol. 1984;31:371–389. doi: 10.1016/0090-1229(84)90090-4. [DOI] [PubMed] [Google Scholar]
- 13.Espevik T, Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986;95:99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- 14.Espevik T, Otterlei M, Skjak-Braek G, Ryan L, Wright S D, Sundan A. The involvement of CD14 in stimulation of cytokine production by uronic acid polymers. Eur J Immunol. 1993;23:255–261. doi: 10.1002/eji.1830230140. [DOI] [PubMed] [Google Scholar]
- 15.Flo T H, Halaas O, Lien E, Ryan L, Teti G, Golenbock D T, Sundan A, Espevik T. Human toll-like receptor 2 mediates monocyte activation by Listeria monocytogenes, but not by group B streptococci or lipopolysaccharide. J Immunol. 2000;164:2064–2069. doi: 10.4049/jimmunol.164.4.2064. [DOI] [PubMed] [Google Scholar]
- 16.Frey E A, Miller D S, Jahr T G, Sundan A, Bazil V, Espevik T, Finlay B B, Wright S D. Soluble CD14 participates in the response of cells to lipopolysaccharide. J Exp Med. 1992;176:1665–1671. doi: 10.1084/jem.176.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golenbock D T, Liu Y, Millham F H, Freeman M W, Zoeller R A. Surface expression of human CD14 in Chinese hamster ovary fibroblasts imparts macrophage-like responsiveness to bacterial endotoxin. J Biol Chem. 1993;268:22055–22059. [PubMed] [Google Scholar]
- 18.Grasdalen H. High-field 1H NMR spectroscopy of alginate: sequential structure and linkage conformation. Carbohydr Res. 1983;118:255–260. [Google Scholar]
- 19.Grasdalen H, Larsen B, Smidsrod O. A PMR study of composition and sequence of uronate residues in alginate. Carbohydr Res. 1979;68:23–31. [Google Scholar]
- 20.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haziot A, Chen S, Ferrero E, Low M G, Silber R, Goyert S M. The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol. 1988;141:547–552. [PubMed] [Google Scholar]
- 22.Heine H, Kirschning C J, Lien E, Monks B G, Rothe M, Golenbock D T. Cutting edge: cells that carry a null allele for toll-like receptor 2 are capable of responding to endotoxin. J Immunol. 1999;162:6971–6975. [PubMed] [Google Scholar]
- 23.Hermanson G T, Mallia A K, Smith P K. Immobilized affinity ligand techniques. San Diego, Calif: Academic Press; 1992. [Google Scholar]
- 24.Ingalls R R, Arnaout M A, Golenbock D T. Outside-in signaling by lipopolysaccharide through a tailless integrin. J Immunol. 1997;159:433–438. [PubMed] [Google Scholar]
- 25.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ingalls R R, Monks B G, Savedra R, Jr, Christ W J, Delude R L, Medvedev A E, Espevik T, Golenbock D T. CD11/CD18 and CD14 share a common lipid A signaling pathway. J Immunol. 1998;161:5413–5420. [PubMed] [Google Scholar]
- 27.Jahr T G, Ryan L, Sundan A, Lichenstein H S, Skjak-Braek G, Espevik T. Induction of tumor necrosis factor production from monocytes stimulated with mannuronic acid polymers and involvement of lipopolysaccharide-binding protein, CD14, and bactericidal/permeability-increasing factor. Infect Immun. 1997;65:89–94. doi: 10.1128/iai.65.1.89-94.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jahr T G, Sundan A, Lichenstein H S, Espevik T. Influence of CD14, LBP and BPI in the monocyte response to LPS of different polysaccharide chain length. Scand J Immunol. 1995;42:119–127. doi: 10.1111/j.1365-3083.1995.tb03634.x. [DOI] [PubMed] [Google Scholar]
- 29.Lien E, Means T K, Heine H, Yoshimura A, Kusumoto S, Fukase K, Fenton M J, Oikawa M, Qureshi N, Monks B, Finberg R W, Ingalls R R, Golenbock D T. Toll-like receptor 4 imparts ligand-specific recognition of bacterial lipopolysaccharide. J Clin Investig. 2000;105:497–504. doi: 10.1172/JCI8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lien E, Sellati T J, Yoshimura A, Flo T H, Rawadi G, Finberg R W, Carroll J D, Espevik T, Ingalls R R, Radolf J D, Golenbock D T. Toll-like receptor 2 functions as a pattern recognition receptor for diverse bacterial products. J Biol Chem. 1999;274:33419–33425. doi: 10.1074/jbc.274.47.33419. [DOI] [PubMed] [Google Scholar]
- 31.Medvedev A E, Flo T H, Ingalls R R, Golenbock D T, Teti G, Vogel S N, Espevik T. Involvement of CD14 and complement receptors CR3 and CR4 in nuclear factor-κB activation and TNF production induced by lipopolysaccharide and group B streptococcal cell walls. J Immunol. 1998;160:4535–4542. [PubMed] [Google Scholar]
- 32.Meszaros K, Aberle S, White M, Parent J B. Immunoreactivity and bioactivity of lipopolysaccharide-binding protein in normal and heat-inactivated sera. Infect Immun. 1995;63:363–365. doi: 10.1128/iai.63.1.363-365.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niwa M, Milner K C, Ribi E, Rudbach J A. Alteration of physical, chemical, and biological properties of endotoxin by treatment with mild alkali. J Bacteriol. 1969;97:1069–1077. doi: 10.1128/jb.97.3.1069-1077.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perera P Y, Qureshi N, Christ W J, Stutz P, Vogel S N. Lipopolysaccharide and its analog antagonists display differential serum factor dependencies for induction of cytokine genes in murine macrophages. Infect Immun. 1998;66:2562–2569. doi: 10.1128/iai.66.6.2562-2569.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poltorak A, He X, Smirnova I, Liu M Y, Huffel C V, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in the Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 36.Pugin J, Heumann I D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 37.Savedra R, Jr, Delude R L, Ingalls R R, Fenton M J, Golenbock D T. Mycobacterial lipoarabinomannan recognition requires a receptor that shares components of the endotoxin signaling system. J Immunol. 1996;157:2549–2554. [PubMed] [Google Scholar]
- 38.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 39.Thornton B P, Vetvicka V, Pitman M, Goldman R C, Ross G D. Analysis of the sugar specificity and molecular location of the β-glucan-binding lectin site of complement receptor type 3 (CD11b/CD18) J Immunol. 1996;156:1235–1246. [PubMed] [Google Scholar]
- 40.Troelstra A, Graaf-Miltenburg L A, van Bommel T, Verhoef J, Van Kessel K P, Van Strijp J A. Lipopolysaccharide-coated erythrocytes activate human neutrophils via CD14 while subsequent binding is through CD11b/CD18. J Immunol. 1999;162:4220–4225. [PubMed] [Google Scholar]
- 41.Ugelstad J, Berge A, Ellingsen T, Schmid R, Nilsen T N, Mork P C, Stenstad P, Hornes E, Olsvik O. Preparation and application of new monosized polymer particles. Prog Polym Sci. 1992;17:87–161. [Google Scholar]
- 42.Ulevitch R J. Recognition of bacterial endotoxins by receptor-dependent mechanisms. Adv Immunol. 1993;53:267–289. doi: 10.1016/s0065-2776(08)60502-7. [DOI] [PubMed] [Google Scholar]
- 43.Underhill D M, Ozinsky A, Hajjar A M, Stevens A, Wilson C B, Bassetti M, Aderem A. The Toll-like receptor 2 is recruited to macrophage phagosomes and discriminates between pathogens. Nature. 1999;401:811–815. doi: 10.1038/44605. [DOI] [PubMed] [Google Scholar]
- 44.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H D, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wright S D, Detmers P A, Aida Y, Adamowski R, Anderson D C, Chad Z, Kabbash L G, Pabst M J. CD18-deficient cells respond to lipopolysaccharide in vitro. J Immunol. 1990;144:2566–2571. [PubMed] [Google Scholar]
- 46.Wright S D, Jong M T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]