Abstract
This work provides consensus guidance regarding clinical diagnosis and early medical management of endometriosis within Asia. Clinicians with expertise in endometriosis critically evaluated available evidence on clinical diagnosis and early medical management and their applicability to current clinical practices. Clinical diagnosis should focus on symptom recognition, which can be presumed to be endometriosis without laparoscopic confirmation. Transvaginal sonography can be appropriate for diagnosing pelvic endometriosis in select patients. For early empiric treatment, management of women with clinical presentation suggestive of endometriosis should be individualized and consider presentation and therapeutic need. Medical treatment is recommended to reduce endometriosis-associated pelvic pain for patients with no immediate pregnancy desires. Hormonal treatment can be considered for pelvic pain with a clinical endometriosis diagnosis; progestins are a first-line management option for early medical treatment, with oral progestin-based therapies generally a better option compared with combined oral contraceptives because of their safety profile. Dienogest can be used long-term if needed and a larger evidence base supports dienogest use compared with gonadotropin-releasing hormone agonists (GnRHa) as first-line medical therapy. GnRHa may be considered for first-line therapy in some specific situations or as short-term therapy before dienogest and non-steroidal anti-inflammatory drugs as add-on therapy for endometriosis-associated pelvic pain.
Keywords: endometriosis, diagnosis, medical management
1. Introduction
Endometriosis is an estrogen-dependent, progesterone-resistant gynecologic condition characterized by the presence of ectopic endometrial-like tissue outside the uterine cavity; endometriosis is strongly affected by cyclic changes in response to steroid hormones and is associated with an inflammatory response in the peritoneal cavity [1,2,3]. Endometriosis is characterized by chronic pelvic pain, with common clinical presentations of dysmenorrhea, dyspareunia, dyschezia, dysuria, and infertility [1,4]. Accordingly, it is an important cause of morbidity that can detrimentally affect the quality of life (QoL) in women of reproductive age [5,6].
The exact etiology and pathogenesis of endometriosis continue to be elucidated, with environmental and genetic factors implicated [4,7,8,9]. Similarly, the true burden of endometriosis is unknown although the prevalence rate in Western populations is estimated to range from 2% to 10% of women of reproductive age, with an estimated 50% of infertile women affected [10,11]. The burden of endometriosis in the Asian population has been poorly characterized but is considered in some studies to be greater in Asian women than women from other continents [12,13].
Delay in diagnosis of endometriosis is commonly reported, some as long as 11 years [10,14,15,16,17,18,19,20]. Studies of diagnostic delays in Asia are less common, but it is possible that diagnosis in Asia may occur earlier including because of cultural and socioeconomic barriers limiting access to care. These delays can result in ongoing symptoms that detrimentally affect QoL and fertility [14]. Limitations in current approaches for diagnosis of endometriosis may be contributing to these delays.
The diagnosis and treatment of endometriosis has undergone considerable changes in recent years with an increasing focus on patient-centered care that includes more frequent clinical diagnosis and early medical management [14,21]. Additionally, improved understanding of the underlying associated hormonal and inflammatory abnormalities and therapeutic targets for endometriosis has led to the availability of new treatments [22,23]. While this changing paradigm for clinical diagnosis and medical management of endometriosis necessitates consideration of how best to deliver patient-centered care, available guidelines and recommendations do not necessarily reflect current practice and the emerging evidence base, including within Asia.
In this context, a group of clinicians from Asia and Europe with expertise in the diagnosis and treatment of endometriosis met to critically evaluate recent international guidelines and consensus reports [as summarized in Table 1 [7,8,10,22,24,25,26,27]. Literature on clinical diagnosis and early medical management of endometriosis and their applicability to current clinical practice, with a predominant focus within Asia, was also considered. This work is a summation of these deliberations and provides consensus guidance regarding clinical diagnosis and early medical management of endometriosis within Asia.
Table 1.
Guidelines and consensus reports referred to in the development of the current consensus recommendations for Asia.
| Group | Title | Reference |
|---|---|---|
| Global | ||
| World Endometriosis Society Montpellier Consortium | Consensus on current management of endometriosis | Johnson et al., 2013 [8] |
| European | ||
| The European Society of Human Reproduction and Embryology (ESHRE) | ESHRE guideline: management of women with endometriosis |
Dunselman et al., 2014 [10] |
| National Institute for Health and Care Excellence (NICE) | Endometriosis: diagnosis and management | NICE 2017 [24] |
| National German Guideline | National German guideline (S2k): Guideline for the diagnosis and treatment of endometriosis | Ulrich et al., 2014 [25] |
| North American | ||
| American Society for Reproductive Medicine (ASRM) | Treatment of pelvic pain associated with endometriosis: a committee opinion |
The Practice Committee of the American Society for Reproductive Medicine 2014 [22] |
| Asian | ||
| Korean Society of Endometriosis (KSE) | Clinical evaluation and management of endometriosis: guideline for Korean patients from Korean Society of Endometriosis | Hwang et al., 2018 [26] |
| Obstetrical and Gynaecological Society of Malaysia | Clinical guidelines for the management of endometriosis 2016 | Subramaniam et al., 2016 [7] |
| The Federation of Obstetric and Gynaecological Societies of India (FOGSI) | Good Clinical Practice Recommendations on Endometriosis | FOGSI 2017 [27] |
2. Clinical Diagnosis
2.1. Consensus: Focus Should Be Directed towards the Recognition of Symptoms That May Lead to the Diagnosis of Endometriosis, Such as Abdominal-Pelvic Pain and Infertility. These Symptoms Can Be Presumed to Be Endometriosis without the Need for Laparoscopy
Definitive diagnosis of endometriosis relied previously on laparoscopic findings with histological verification [7,10,22,25,26]. However, limitations of laparoscopy include surgical risks and reliance on identifiable pelvic lesions rather than consideration of endometriosis as a systemic disease with variable presentations [14]. While endometriosis is commonly defined histologically, the presence of lesions does not preclude other causes for patients’ symptoms. Conversely, the lack of clinically identified lesions does not necessarily exclude a diagnosis of endometriosis [28]. Further, endometriosis cannot be identified currently by pathogenic features or biomarkers, and the key symptoms of endometriosis like pain and infertility can mistakenly be attributed to other causes.
We propose instead focusing on the patient and her clinical symptoms, which can decrease diagnostic delay in women from low resource settings. A clinical approach to diagnosis also considers that endometriosis can occur without pelvic pain symptoms and that pelvic pain might be attributed to causes other than endometriosis [21,29].
The presence of symptoms suggestive of endometriosis warrants further investigation to support diagnosis. Importantly, diagnosis of endometriosis should not be predominantly focused on pain, as the perception of pain is subjective and varies globally [30], but rather clinicians should exert efforts to recognize both gynecologic and non-gynecologic symptoms of endometriosis. Endometriosis should be suspected in reproductive-aged women with chronic and/or cyclic pelvic pain (e.g., dysmenorrhea, deep dyspareunia, dyschezia), pelvic mass (e.g., ovarian endometrioma and adenomyosis), and/or subfertility. It should also be suspected for unexplained fatigue, weariness, depression, anxiety, hematuria, rectal bleeding, and other catamenial symptoms outside the genitourinary system. Patient history is another important consideration in the diagnosis of endometriosis, with infertility history in conjunction with clinical signs and symptoms being strongly associated with endometriosis [11,31,32,33,34,35]. Other patient characteristics suggestive of endometriosis include previous laparoscopic diagnosis or a positive family history [12,16,33,36].
Women with endometriosis are diagnosed typically in their 20s and 30s [19,37], but endometriosis should also be considered in adolescents suffering from intractable pain unresponsive to non-steroidal anti-inflammatory drugs (NSAIDs) [38]. This is especially so in adolescents with a strong family history [38]. Thus, adolescents should be encouraged to report their experience with menstruation, as normalizing painful menstruation among this age group may result in delayed diagnosis.
Physical examination findings are able to identify endometriosis with high accuracy dependent on location of the lesions [39,40,41] and should be included as a component of clinical diagnosis. Inspection and palpation of the abdomen, and depending on patient age and sexual history, physical examination of the pelvis is recommended to identify abdominal masses and pelvic symptoms (e.g., decreased organ mobility and enlargement, visible vaginal lesions, nodules in the posterior vaginal fornix, retroverted uterus) [42]. Pelvic examination should include speculum examination and vaginal palpation, investigation of the position, mobility, size, fixation, or tenderness of the uterus, and evaluation of pelvic tenderness [14,43]. Rectovaginal examination or palpation of the contents of the pouch of Douglas (POD), which can sensitively detect deep endometriosis (DE), should be considered [28]. Notably, the sensitivity and specificity of the pelvic examination depend on only palpable lesions (e.g., ovarian endometriomas enlarged beyond normal ovarian volume and cul de sac or POD masses noted on rectovaginal examination) and may be insufficiently sensitive for other phenotypes [39,40,41].
2.2. Consensus: Transvaginal Sonography Is an Appropriate Imaging Technique in the Diagnosis of Pelvic Endometriosis
Imaging must now be considered as a major component of clinical diagnosis to further investigate underlying symptoms, localize the disease, and determine disease severity of endometriosis [21]. Imaging can be used to detect endometrioma, ovarian cysts, and other nodules, masses, and pelvic disorders [7,14,44]. However, its accuracy for the assessment of some pathologies, such as superficial lesions and ovarian foci, is limited [14,44,45].
For some endometriosis subtypes, transvaginal sonography (TVS) improves accuracy when used in conjunction with symptoms, patient history, and/or physical findings [14,39,46]. The International Deep Endometriosis Analysis (IDEA) group has provided 4 basic steps to be used for the sonographic examination of suspected endometriosis including evaluation or assessment of (i) the uterus and adnexa to identify signs of adenomyosis or the presence of endometrioma; (ii) TVS markers such as site-specific tenderness and ovarian mobility; (iii) the status of the POD; and (iv) the presence of DE nodules in the anterior and posterior compartments [47]. Another important consideration with TVS is that it has to be performed by highly experienced sonologists, as ultrasound findings are highly operator dependent [10]. However, proficiency for this technique can be achieved after examining less than 50 patients [48].
Consideration of the appropriateness of TVS for individual patients is required. For those in whom TVS is not appropriate, use of alternative imaging approaches, such as transabdominal or transrectal sonography (TRS), should be considered. TRS may be a sensitive and useful approach, particularly in Asia where cultural norms may make this imaging technique more appropriate. In a diagnostic accuracy study conducted in Asian patients with symptoms of endometriosis, the sensitivity of TRS in diagnosing DE was comparable to that of TVS and magnetic resonance imaging, although its cost limits its utilization in low resource settings [49].
3. Early Empiric Medical Management
3.1. Consensus: Management of Women with a Presumptive Clinical Presentation Suggestive of Endometriosis Depends on the Individual Patient and Should Consider Her Presentation at That Time and the Need for Therapy
A definitive diagnosis is not required before commencing treatment in patients with pelvic pain who are not desirous of immediate pregnancy. A large percentage of adolescents with chronic pelvic pain or dysmenorrhea are reported to have endometriosis on laparoscopy [50]. The detrimental effect of endometriosis on patient well-being, ovarian reserve, and QoL [5,6,21] further emphasizes the importance of initiating treatment even in the absence of histologic diagnosis of endometriosis.
We recommend that the primary focus of endometriosis treatment should be the management of a patient’s presenting symptoms. Furthermore, treatment should also be individually tailored, accounting for patient- and disease-related factors (e.g., age, disease severity and extent, fertility requirements, contraception, patient wishes) and treatment-related characteristics (e.g., side effects, compliance, cost) [51].
3.2. Consensus: Medical Treatment Is Recommended to Reduce Endometriosis-Associated Pelvic Pain for Patients Who Have No Immediate Desire for Pregnancy
Medical therapies for endometriosis induce atrophy within hormonally dependent ectopic endometrium, leading to a decrease in the number and size of lesions [52], ultimately controlling pain and suppressing the hormonally active endometriotic tissue [23]. As endometriosis is an estrogen-dependent systemic disease [1,2,3], we recommend that women with symptoms presumed to be due to endometriosis receive medical therapy, which includes hormonal (e.g., combined oral contraceptives [COCs], gonadotropin-releasing hormone [GnRH] agonists/antagonists, and progestins or anti-progestins) medications. Non-hormonal (e.g., NSAIDs) medications can also be considered, with the choice of therapy dependent on tolerability profile, cost, availability, and patient characteristics.
3.3. Consensus: Hormonal Treatment Is a More Effective Option in the Treatment of Pelvic Pain from Clinically Diagnosed Endometriosis. Progestins Are among the First-Line Management Options for Early Medical Treatment
Hormonal treatment for women with suspected or confirmed endometriosis can have a beneficial effect on pain and is not associated with a detrimental effect on subsequent fertility [24]. Of the available hormonal treatments, we consider progestins one of the first-line treatment options for early medical management of endometriosis.
The effect of progesterone on the pathophysiology of endometriosis is multifactorial [23], leading to the development of several progestin-based therapies for the medical treatment of endometriosis. Progestins are believed to exert their effects by decidualization followed by atrophy of endometrial tissue, with suppression of matrix metalloproteinases and angiogenesis proposed as contributory mechanisms [22,23]. The effect of progesterone on inflammatory pathways has also been reported [53]. For a subset of women, impaired action of progesterone on the endometrium may render some hormonal treatments ineffective, although synthetic progestins may overcome this resistance through effects on progesterone receptors and proinflammatory cytokines [54].
Based on a 2012 systematic review of 13 randomized controlled trials (RCTs), progestins or anti-progestins (i.e., medroxyprogesterone acetate, dienogest, cyproterone acetate, norethisterone acetate, danazol) were found to reduce endometriosis-associated pain compared with other interventions, placebo, or no treatment [52]. Notably, most RCTs assessing the treatment of endometriosis-associated pain were conducted in patients with laparoscopically diagnosed endometriosis and side effect profiles of treatments have confounded results [22,26]. Large placebo effects have also been observed and evaluation of treatment durations of 6 months or longer are limited, although such long-term data are emerging as described below. Accordingly and based on current evidence, several guidelines recommend that effective treatment of endometriosis-associated pain can be achieved with progestins in women with suspected or confirmed endometriosis and without a detrimental effect on subsequent fertility [7,10,24,26].
The tolerability profile of progestins is another important attribute of the medication, as better tolerated progestins may be more appropriate for long-term use because of the chronicity of the condition. Therefore, we recommend that the differing tolerability profiles of progestins and anti-progestins be considered when selecting a particular medication, including transient (e.g., vaginal bleeding, weight gain, headache, mood change, decreased libido) and irreversible (e.g., thrombosis) adverse effects [10,26]. Additionally, we recommend that women of reproductive age requiring treatment be encouraged to start treatment with progestins to preserve fertility potential as available non-hormonal medical therapies do not suppress the progression of endometriosis [8,10,22,55].
3.4. Consensus: Oral Progestin-Based Therapies Are Generally a Better Option Compared with COCs Because of Their Safety Profile
Hormonal contraceptives exert their effects in endometriosis through ovarian and pituitary suppression, or through a general suppression of the hypothalamic–pituitary–ovarian (HPO) axis; estrogen and progesterone combinations or progestins alone lead to decidualization of the endometriotic tissue and decreased disease activity [22,23]. COCs decrease endometriosis-associated dyspareunia, dysmenorrhea, and non-menstrual pain [56]. Despite limited evidence of their efficacy and a lack of license for this specific indication [55], COCs have been widely used cyclically or continuously to treat endometriosis-associated symptoms; this is thought to be related, at least in part, to their non-endometriosis-specific benefits, including contraceptive protection and control of the menstrual cycle [57,58,59].
However, COCs may not be appropriate for all patients and are contraindicated in women older than 35 years who smoke or are at increased risk of myocardial infarction, stroke, or venous thromboembolism [55]. Furthermore, as endometriosis is highly estrogen dependent [60,61], supplementation of endogenous estrogen through the use of estrogen-containing COCs may cause exacerbation of the disease [62]. Additionally, while COCs are effective in thinning the eutopic endometrium, insufficient evidence is available of its effectiveness in diminishing the activity of endometrial implants [55,63,64,65]. In the context of these limitations, oral progestin-based therapies are generally a better option compared with COCs for the medical management of endometriosis, as oral progestins are not contraindicated according to patient age and smoking status; neither increase the risk of thrombosis nor induce amenorrhea; and have a generally favorable tolerability profile [55].
3.5. Consensus: Dienogest Can Be Used Long-Term If Needed
Endometriosis is typically considered a chronic disease [66,67], which therefore may require a lifelong management plan [21]. The use of medical treatment to avoid repeated surgical procedures is recommended, as surgeries are associated with inherent risks and repeated procedures might lead to pain-causing adhesions and adversely affect ovarian reserve [22]. Therefore, patients may require long-term medical therapy.
Dienogest is a selective progestin that combines the pharmacological properties of 19-nortestosterone and derivatives of progesterone, with high specificity for progesterone receptors and minimal androgenic, estrogenic, glucocorticoid, and mineralocorticoid activity [23,68]. Additionally, dienogest has in vitro anti-inflammatory and progesterone receptor upregulation activity, supporting its efficacy in improving patient response to medical management [69,70,71].
Currently, long-term follow-up for dienogest is at least 60 months in clinical studies that include those from Japan and patients from adolescence to women in their fifth decade (Table 2) [72,73,74,75,76,77,78,79,80]. A 5-year study found that dienogest (2 mg/day) effectively reduced endometriosis-associated pelvic pain and avoided pain recurrence post-surgery [78]. The treatment was well tolerated with clinically manageable adverse effects. Dienogest was also reported to decrease recurrence after endometrioma excision, although metrorrhagia and decreased bone mineral density (BMD) were observed [79].
Table 2.
Overview of studies supporting long-term treatment with dienogest.
| Study | Population (Age) | Intervention a (Setting) | Treatment Length | Outcomes |
|---|---|---|---|---|
| Cosson et al. [77] |
n = 130 (mean: 28.5–30.3 y) |
DNG vs. GnRH b
(post-surgical consolidation therapy) |
16 wks |
|
| Harada et al. [76] |
n = 271 (mean: 33.5–33.8 y) |
DNG vs. GnRH c | 24 wks |
|
| Strowitzki et al. [75] |
n = 229 (mean: 30.6–31.0 y) |
DNG vs. GnRH d | 24 wks |
|
| Köhler et al. [74] |
n = 68 (mean: 27.6–33.5 y) |
DNG e
(dose-finding study) |
24 wks |
|
| Momoeda et al. [73] |
n = 135 (mean: 34.1 y) |
DNG | 52 wks |
|
| Petraglia et al. [72] |
n = 152 (18–45 y f) |
DNG | 36–52 wks |
|
| Ebert et al. [80] |
n = 111 (adolescents; median [range] 16.0 [12,13,14,15,16,17] y) |
DNG | 52 wks |
|
| Römer [78] |
n = 37 (39 y) |
DNG | 60 mo |
|
| Ota et al. [79] |
n = 151 (32.6 y) |
DNG vs. no therapy (post-surgical therapy) |
60 mo |
|
AE, adverse event; AFS, American Fertility Society; BMD, bone mineral density; DNG, dienogest; EAPP, endometriosis-associated pelvic pain; GnRH, gonadotropin-releasing hormone; POD, pouch of Douglas; VAS, visual analog score. a DNG was administered at a dose of 2 mg/day unless otherwise indicated. b Triptorelin 3.75 mg/month. c Intranasal buserelin acetate 900 µg/day. d Leuprolide acetate 3.75 mg/month. e 1, 2, or 4 mg/day. f Inclusion criteria.
3.6. Consensus: A Large Evidence Base Exists Supporting the Use of Dienogest Compared with GnRH agonists as First-Line Medical Therapy for Endometriosis
A systematic review of 9 RCTs comparing dienogest to other medical therapies for endometriosis treatment found that dienogest was significantly better than placebo and as effective as GnRH agonists in reducing pelvic pain symptoms. Dienogest was also effective in reducing endometriotic lesions and frequency of hot flushes [68]. However, there was a higher frequency of irregular vaginal bleeding with dienogest compared with GnRH agonists. These results are generally consistent with another systematic review of 5 RCTs of dienogest versus placebo and GnRH agonists [81]. Notably, this second systematic review found that dienogest and buserelin intranasal spray appeared equally effective in improving QoL, but the comparative QoL effects of dienogest with other GnRH agonists could not be determined as no RCTs meeting the authors’ inclusion criteria considered this comparison. However, a RCT of 24 weeks of dienogest versus leuprolide acetate found a pronounced improvement in QoL measures with dienogest [82].
3.7. Consensus: GnRH Agonists May Be Considered for First-Line Therapy Only in Some Specific Situations or as Short-Term Therapy before Dienogest
GnRH agonists bind to receptors in the pituitary gland, thereby downregulating the pituitary–ovarian axis and causing hypoestrogenism [22], with the subsequent induction of amenorrhea and progressive endometrial atrophy thought to inactivate pelvic lesions and relieve endometriosis-associated pain [7,8,10,22,83]. However, GnRH agonists cause symptoms of estrogen deficiency, including BMD depletion as well as breakthrough bleeding, vaginal dryness, irritability, fatigue, headaches, depression, and skin problems [7,22]. Given the chronic nature of endometriosis, the adverse effects of GnRH agonists preclude its long-term use for this indication, and there is insufficient evidence of the benefits of using lower GnRH agonist doses (i.e., ‘draw-back’ therapy) [51,83]. Accordingly, we only recommend short courses of GnRH agonist therapy because of the risk of BMD loss. Additionally, we recommend hormonal add-back therapy to prevent bone loss and hypoestrogenic symptoms during GnRH agonist treatment. This recommendation is supported by data from a prospective, non-randomized trial of women with chronic pelvic pain associated with recurrent endometriosis, who achieved pelvic pain relief after 4–6 months of treatment with a GnRH agonist followed by 12 months of therapy with dienogest (1–2 mg/day) [84]. The use of GnRH agonists in young women and adolescents who have not reached maximum bone density requires careful consideration [7,10,26]. Therefore, we recommend that GnRH agonists be considered as a first-line, short-term therapy only for carefully selected patients.
3.8. Consensus: NSAIDs May Be Considered as Add-on Therapy for Endometriosis-Associated Pelvic Pain
Limited evidence exists regarding the use of NSAIDs for endometriosis treatment, apart from a single trial of NSAIDs versus placebo that found no evidence of a beneficial pain-relieving effect of NSAIDs in 24 women with endometriosis [85]. However, the favorable effect of NSAIDs on primary dysmenorrhea [86] supports its use for analgesia of endometriosis-associated pain, and may be considered particularly for young patients solely with dysmenorrhea and the absence of other endometriosis symptoms.
Limitations of NSAIDs include potential inhibition of ovulation, the risk of gastric ulceration and cardiovascular disease, and their inability to alter the disease course [85,86,87]. Additionally, NSAIDs are generally insufficient for treatment of patients with a confirmed diagnosis of endometriosis or with symptoms other than dysmenorrhea. Therefore, we recommend that NSAIDs may be considered only as add-on, short-term therapy for endometriosis-associated pelvic pain.
4. Conclusions
This review and consensus deliberations considered clinical diagnosis and early medical management of endometriosis within Asia. The diagnosis and treatment of endometriosis are evolving, with a greater emphasis on patient-centered care that includes clinical diagnosis and early medical management [21]. Furthermore, new therapies for endometriosis are available and several others are in development. This changing paradigm for clinical diagnosis and medical management of endometriosis necessitates consideration of how best to deliver patient-centered care to women with endometriosis.
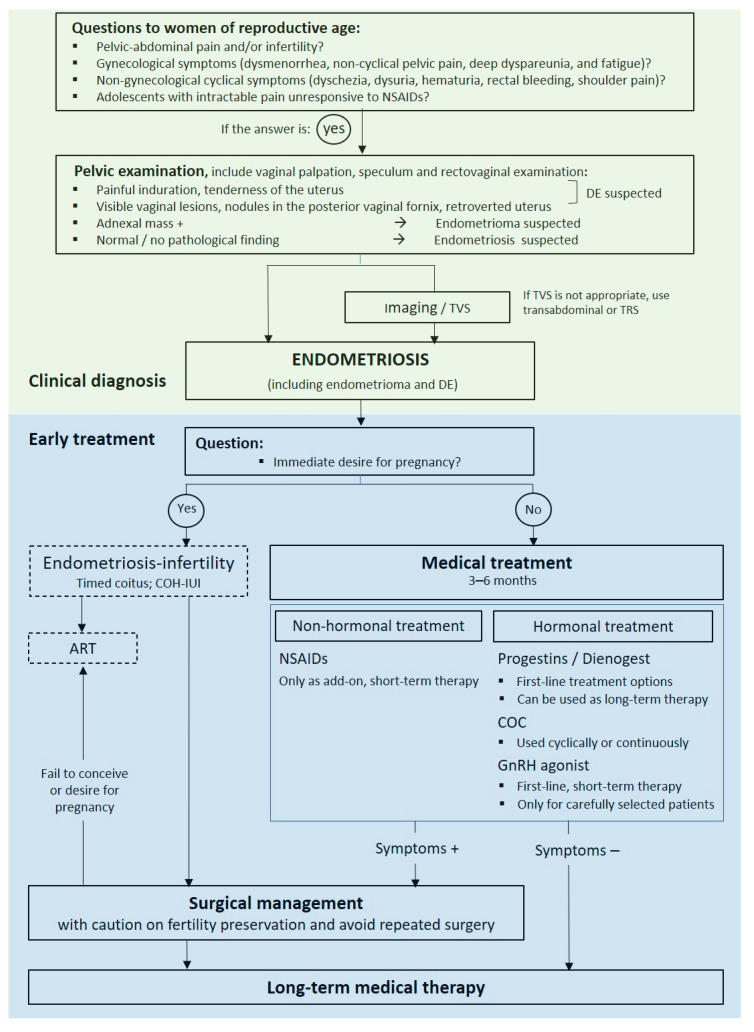
The evolving paradigm emphasizes the importance of early clinical diagnosis. However, although clinical diagnosis is used in practice [14], the approach has not been standardized [11]. A consistent approach to clinical diagnosis and treatment is necessary to optimize patient care and outcomes. A validated algorithm that utilizes both clinical diagnosis and early medical therapy using contemporary treatment approaches is not available currently. Based on our consideration of the available evidence from recent international guidelines and consensus reports and the literature on clinical diagnosis and early medical management of endometriosis, we propose an algorithm that incorporates clinical diagnosis and early medical management for endometriosis in Asia (Figure 1). Notably, further evaluation of such an algorithm and incorporation into routine practice will require consideration of its effect on diagnosis rates and patient outcomes. Additionally, because the role of surgery and medical management before surgery and the role of medical management before assisted reproductive technology (ART) are important aspects of the patient journey (i.e., the ‘endometriosis life’ [21]), these were included within the algorithm, but their in-depth consideration were beyond the scope of our review, which focused on clinical diagnosis and early medical management of endometriosis.
Figure 1.
Algorithm for the clinical diagnosis and early treatment of endometriosis in Asia. ART, assisted reproductive technology; COC, combined oral contraceptive; COH, controlled ovarian hyperstimulation; DE, deep endometriosis; GnRH, gonadotropin-releasing hormone; IUI, intra-uterine insemination; NSAID, nonsteroidal anti-inflammatory drug; TRS, transrectal sonograph; TVS, transvaginal sonography.
The strength of our work is that it provides a contemporary assessment of current practice and treatments. Additionally, our recommendations are based on the results of a consensus meeting of many specialists across several Asian countries, which was the first consensus meeting that we are aware of spanning several Asian countries and addressing clinical diagnosis and early medical therapy; we note from our clinical experience that this concept has been recently accepted in Asia.
Limitations of the available dataset for clinical diagnosis and early medical management are noted. For instance, the duration of follow-up of many studies is limited and few comparative studies of medical management, or combined medical treatments, and of studies within Asian populations are available. Additionally, as described above, important aspects of the journey for a patient with endometriosis were not considered and warrant future consideration within the Asian population, including the role of surgery, the importance of medical management before ART, and medical treatment of specific phenotypes (e.g., extra-genital endometriosis).
In conclusion, in the context of the changing paradigm of diagnosis and management, this consensus guidance recommends that early clinical diagnosis and medical treatment of endometriosis be considered, including within Asia, as a means of delivering patient-centered care to women with endometriosis.
Acknowledgments
Editorial support was provided by Huntsworth Health and funded by Bayer Inc. Medical writing services were provided by Tricia Newell, and were funded by Bayer Inc in accordance with Good Publication Practice (GPP3) guidelines (http://ismpp.org/gpp3 accessed on 10 December 2022).
Author Contributions
All authors participated in the meeting to critically evaluate recent international guidelines and consensus reports, participated in the writing of the manuscript or critically revised the manuscript for important intellectual content, and approved of the final version of the manuscript for submission. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Mee-Ran Kim has nothing to disclose. Charles Chapron has nothing to disclose. Thomas Römer reports personal fees from Bayer, during the conduct of the study; personal fees from Gedeon Richter, personal fees from Theramex, personal fees from Myovant Sciens, and personal fees from Exceltis outside the submitted work. Angela Aguilar has nothing to disclose. Amphan Chalermchockcharoenkit has nothing to disclose. Siddharta Chatterjee has nothing to disclose. Le Thi Anh Dao has nothing to disclose. Yoke Fai Fong reports personal fees from Bayer, during the conduct of the study. Hendy Hendarto reports personal fees from Bayer and grants from Universitas Airlangga, during the conduct of the study; and personal fees from Bayer and grants from Universitas Airlangga outside the submitted work. Syarief Taufik Hidayat has nothing to disclose. Su Yen Khong reports personal fees and non-financial support from Bayer to attend advisory boards and to speak at Bayer-sponsored meetings outside the submitted work. Li Ma has nothing to disclose. Pratap Kumar has no conflict of Interest with any company nor received financial incentives. Relly Yanuari Primariawan has nothing to disclose. Anthony Siow has nothing to disclose. Areepan Sophonsritsuk reports personal fees from Bayer during the conduct of the study and personal fees from Bayer, outside the submitted work. Ramani Devi Thirunavukarasu has nothing to disclose. Bui Chi Thuong has nothing to disclose. Chih-Feng Yen has nothing to disclose.
Funding Statement
This work was supported by Bayer Inc.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Giudice L.C. Clinical practice. Endometriosis. N. Engl. J. Med. 2010;362:2389. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parente Barbosa C., Bentes De Souza A.M., Bianco B., Christofolini D.M. The effect of hormones on endometriosis development. Minerva Ginecol. 2011;63:375–386. [PubMed] [Google Scholar]
- 3.Donnez J., Binda M.M., Donnez O., Dolmans M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016;106:1011–1017. doi: 10.1016/j.fertnstert.2016.07.1075. [DOI] [PubMed] [Google Scholar]
- 4.Greene A.D., Lang S.A., Kendziorski J.A., Sroga-Rios J.M., Herzog T.J., Burns K.A. Endometriosis: Where are we and where are we going? Reproduction. 2016;152:R63–R78. doi: 10.1530/REP-16-0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marinho M.C.P., Magalhaes T.F., Fernandes L.F.C., Augusto K.L., Brilhante A.V.M., Bezerra L. Quality of Life in Women with Endometriosis: An Integrative Review. J. Womens Health (Larchmt) 2018;27:399–408. doi: 10.1089/jwh.2017.6397. [DOI] [PubMed] [Google Scholar]
- 6.Mehedintu C., Plotogea M.N., Ionescu S., Antonovici M. Endometriosis still a challenge. J. Med. Life. 2014;7:349–357. [PMC free article] [PubMed] [Google Scholar]
- 7.Subramaniam R., Sinthamoney E., Damodaran P., Kumarasamy S., Wai T.S. Obstetrical and Gynecological Society of Malaysia Clinical Guidelines for the Management of Endometriosis. 2016. [(accessed on 4 August 2019)]. Available online: http://www.ogsm.org.my.
- 8.Johnson N.P., Hummelshoj L., World Endometriosis Society Montpellier Consortium. Abrao M.S., Adamson G.D., Allaire C., Amelung V., Andersson E., Becker C., Birna Árdal K.B., et al. Consensus on current management of endometriosis. Hum. Reprod. 2013;28:1552–1568. doi: 10.1093/humrep/det050. [DOI] [PubMed] [Google Scholar]
- 9.Rogers P.A., D’Hooghe T.M., Fazleabas A., Giudice L.C., Montgomery G.W., Petraglia F., Taylor R.N. Defining future directions for endometriosis research: Workshop report from the 2011 World Congress of Endometriosis in Montpellier, France. Reprod Sci. 2013;20:483–499. doi: 10.1177/1933719113477495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunselman G.A.J., Vermeulen N., Becker C., Calhaz-Jorge C., D’Hooghe T., De Bie B., Heikinheimo O., Horne A.W., Kiesel L., Nap A., et al. ESHRE guideline: Management of women with endometriosis. Hum. Reprod. 2014;29:400–412. doi: 10.1093/humrep/det457. [DOI] [PubMed] [Google Scholar]
- 11.Fuldeore M.J., Soliman A.M. Prevalence and Symptomatic Burden of Diagnosed Endometriosis in the United States: National Estimates from a Cross-Sectional Survey of 59,411 Women. Gynecol. Obstet. Investig. 2017;82:453–461. doi: 10.1159/000452660. [DOI] [PubMed] [Google Scholar]
- 12.Yen C.F., Kim M.R., Lee C.L. Epidemiologic Factors Associated with Endometriosis in East Asia. Gynecol. Minim. Invasive Ther. 2019;8:4–11. doi: 10.4103/GMIT.GMIT_83_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yasui T., Hayashi K., Nagai K., Mizunuma H., Kubota T., Lee J.-S., Suzuki S. Risk profiles for endometriosis in Japanese women: Results from a repeated survey of self-reports. J. Epidemiol. 2015;25:194–203. doi: 10.2188/jea.JE20140124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarwal S.K., Chapron C., Giudice L.C., Laufer M.R., Leyland N., Missmer S.A., Singh S.S., Taylor H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019;220:354.e1–354.e12. doi: 10.1016/j.ajog.2018.12.039. [DOI] [PubMed] [Google Scholar]
- 15.Moradi M., Parker M., Sneddon A., Lopez V., Ellwood D. Impact of endometriosis on women’s lives: A qualitative study. BMC Womens Health. 2014;14:123. doi: 10.1186/1472-6874-14-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nnoaham K.E., Hummelshoj L., Webster P., d’Hooghe T., de Cicco Nardone F., de Cicco Nardone C., Jenkinson C., Kennedy S.H., Zondervan K.T. World Endometriosis Research Foundation Global Study of Women’s Health consortium Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011;96:366–373.e8. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudelist G., Fritzer N., Thomas A., Niehues C., Oppelt P., Haas D., Tammaa A., Salzer H. Diagnostic delay for endometriosis in Austria and Germany: Causes and possible consequences. Hum. Reprod. 2012;27:3412–3416. doi: 10.1093/humrep/des316. [DOI] [PubMed] [Google Scholar]
- 18.Fourquet J., Sinaii N., Stratton P., Khayel F., Alvarez-Garriga C., Bayona M., Ballweg M.L., Flores I. Characteristics of women with endometriosis from the USA and Puerto Rico. J. Endometr. Pelvic. Pain Disord. 2015;7:129–135. doi: 10.5301/je.5000224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staal A.H., van der Zanden M., Nap A.W. Diagnostic Delay of Endometriosis in the Netherlands. Gynecol. Obstet. Invest. 2016;81:321–324. doi: 10.1159/000441911. [DOI] [PubMed] [Google Scholar]
- 20.Soliman A.M., Fuldeore M., Snabes M.C. Factors Associated with Time to Endometriosis Diagnosis in the United States. J. Women’s Health. 2017;26:788–797. doi: 10.1089/jwh.2016.6003. [DOI] [PubMed] [Google Scholar]
- 21.Chapron C., Marcellin L., Borghese B., Santulli P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019;15:666–682. doi: 10.1038/s41574-019-0245-z. [DOI] [PubMed] [Google Scholar]
- 22.Practice Committee of the American Society for Reproductive Medicine Treatment of pelvic pain associated with endometriosis: A committee opinion. Fertil. Steril. 2014;101:927–935. doi: 10.1016/j.fertnstert.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Rafique S., Decherney A.H. Medical Management of Endometriosis. Clin. Obstet. Gynecol. 2017;60:485–496. doi: 10.1097/GRF.0000000000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institute for Health and Care Excellence . Endometriosis: Diagnosis and management (ng73) National Institute for Health and Care Excellence; London, UK: 2017. [Google Scholar]
- 25.Ulrich U., Buchweitz O., Greb R., Keckstein J., von Leffern I., Oppelt P., Renner S.P., Sillem M., Stummvoll W., De Wilde R.L. National German Guideline (S2k): Guideline for the Diagnosis and Treatment of Endometriosis: Long Version—AWMF Registry No. 015-045. Geburtshilfe Frauenheilkd. 2014;74:1104–1118. doi: 10.1055/s-0034-1383187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang H., Chung Y.J., Lee S.R., Park H.T., Song J.Y., Kim H., Lee D.Y., Lee E.J., Kim M.R., Oh S.T. Clinical evaluation and management of endometriosis: Guideline for Korean patients from Korean Society of Endometriosis. Obstet. Gynecol. Sci. 2018;61:553–564. doi: 10.5468/ogs.2018.61.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.FOGSI Good Clinical Practice Recommendations on Endometriosis. 2017. [(accessed on 10 December 2022)]. Available online: https://www.fogsi.org/wp-content/uploads/2017/01/GCRP-2017-final.pdf.
- 28.Chapron C., Dubuisson J.-B., Pansini V., Vieira M., Fauconnier A., Barakat H., Dousset B. Routine clinical examination is not sufficient for diagnosing and locating deeply infiltrating endometriosis. J. Am. Assoc. Gynecol. Laparosc. 2002;9:115–119. doi: 10.1016/S1074-3804(05)60117-X. [DOI] [PubMed] [Google Scholar]
- 29.Hurd W.W. Criteria that indicate endometriosis is the cause of chronic pelvic pain. Obstet. Gynecol. 1998;92:1029–1032. doi: 10.1016/s0029-7844(98)00283-x. [DOI] [PubMed] [Google Scholar]
- 30.Chapron C., Lang J.-H., Leng J.-H., Zhou Y., Zhang X., Xue M., Popov A., Romanov V., Maisonobe P., Cabri P. Factors and Regional Differences Associated with Endometriosis: A Multi-Country, Case-Control Study. Adv. Ther. 2016;33:1385–1407. doi: 10.1007/s12325-016-0366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saha R., Marions L., Tornvall P. Validity of self-reported endometriosis and endometriosis-related questions in a Swedish female twin cohort. Fertil. Steril. 2017;107:174–178.e2. doi: 10.1016/j.fertnstert.2016.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Flores I., Abreu S., Abac S., Fourquet J., Laboy J., Rios-Bedoya C. Self-reported prevalence of endometriosis and its symptoms among Puerto Rican women. Int. J. Gynaecol. Obstet. 2008;100:257–261. doi: 10.1016/j.ijgo.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ballard K.D., Seaman H.E., de Vries C.S., Wright J.T. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case-control study—Part 1. BJOG. 2008;115:1382–1391. doi: 10.1111/j.1471-0528.2008.01878.x. [DOI] [PubMed] [Google Scholar]
- 34.Lafay Pillet M.C., Huchon C., Santulli P., Borghese B., Chapron C., Fauconnier A. A clinical score can predict associated deep infiltrating endometriosis before surgery for an endometrioma. Hum. Reprod. 2014;29:1666–1676. doi: 10.1093/humrep/deu128. [DOI] [PubMed] [Google Scholar]
- 35.Matalliotakis I.M., Cakmak H., Fragouli Y.G., Goumenou A.G., Mahutte N.G., Arici A. Epidemiological characteristics in women with and without endometriosis in the Yale series. Arch. Gynecol. Obstet. 2008;277:389–393. doi: 10.1007/s00404-007-0479-1. [DOI] [PubMed] [Google Scholar]
- 36.Ashrafi M., Sadatmahalleh S.J., Akhoond M.R., Talebi M. Evaluation of Risk Factors Associated with Endometriosis in Infertile Women. Int. J. Fertil. Steril. 2016;10:11–21. doi: 10.22074/ijfs.2016.4763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arruda M.S., Petta C.A., Abrao M.S., Benetti-Pinto C.L. Time elapsed from onset of symptoms to diagnosis of endometriosis in a cohort study of Brazilian women. Hum. Reprod. 2003;18:756–759. doi: 10.1093/humrep/deg136. [DOI] [PubMed] [Google Scholar]
- 38.ACOG Committee Opinion No.760: Dysmenorrhea and Endometriosis in the Adolescent. Obstet. Gynecol. 2018;132:e249–e258. doi: 10.1097/AOG.0000000000002978. [DOI] [PubMed] [Google Scholar]
- 39.Hudelist G., Oberwinkler K., Singer C., Tuttlies F., Rauter G., Ritter O., Keckstein J. Combination of transvaginal sonography and clinical examination for preoperative diagnosis of pelvic endometriosis. Hum. Reprod. 2009;24:1018–1024. doi: 10.1093/humrep/dep013. [DOI] [PubMed] [Google Scholar]
- 40.Bazot M., Lafont C., Rouzier R., Roseau G., Thomassin-Naggara I., Darai E. Diagnostic accuracy of physical examination, transvaginal sonography, rectal endoscopic sonography, and magnetic resonance imaging to diagnose deep infiltrating endometriosis. Fertil. Steril. 2009;92:1825–1833. doi: 10.1016/j.fertnstert.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 41.Hudelist G., Ballard K., English J., Wright J., Banerjee S., Mastoroudes H., Thomas A., Singer C.F., Keckstein J. Transvaginal sonography vs. clinical examination in the preoperative diagnosis of deep infiltrating endometriosis. Ultrasound Obs. Gynecol. 2011;37:480–487. doi: 10.1002/uog.8935. [DOI] [PubMed] [Google Scholar]
- 42.Riazi H., Tehranian N., Ziaei S., Mohammadi E., Hajizadeh E., Montazeri A. Clinical diagnosis of pelvic endometriosis: A scoping review. BMC Womens Health. 2015;15:39. doi: 10.1186/s12905-015-0196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Long W.N. Pelvic Examination. In: A division of Reed Publishing; Walker H.K., Hall W.D., Hurst J.W., editors. Clinical Methods: The History, Physical, and Laboratory Examinations. Butterworth Publishers; Boston, MA, USA: 1990. [PubMed] [Google Scholar]
- 44.Foti P.V., Farina R., Palmucci S., Vizzini I.A.A., Libertini N., Coronella M., Spadola S., Caltabiano R., Iraci M., Basile A., et al. Endometriosis: Clinical features, MR imaging findings and pathologic correlation. Insights Imaging. 2018;9:149–172. doi: 10.1007/s13244-017-0591-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bianek-Bodzak A., Szurowska E., Sawicki S., Liro M. The importance and perspective of magnetic resonance imaging in the evaluation of endometriosis. Biomed. Res. Int. 2013;2013:436589. doi: 10.1155/2013/436589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marasinghe J.P., Senanayake H., Saravanabhava N., Arambepola C., Condous G., Greenwood P. History, pelvic examination findings and mobility of ovaries as a sonographic marker to detect pelvic adhesions with fixed ovaries. J. Obstet. Gynaecol. Res. 2014;40:785–790. doi: 10.1111/jog.12234. [DOI] [PubMed] [Google Scholar]
- 47.Guerriero S., Condous G., van den Bosch T., Valentin L., Leone F.P.G., Van Schoubroeck D., Exacoustos C., Installé A.J.F., Martins W.P., Abrao M.S., et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. 2016;48:318–332. doi: 10.1002/uog.15955. [DOI] [PubMed] [Google Scholar]
- 48.Eisenberg V.H., Alcazar J.L., Arbib N., Schiff E., Achiron R., Goldenberg M., et al. Applying a statistical method in transvaginal ultrasound training: Lessons from the learning curve cumulative summation test (LC-CUSUM) for endometriosis mapping. Gynecol. Surg. 2017;14:19. doi: 10.1186/s10397-017-1022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alborzi S., Rasekhi A., Shomali Z., Madadi G., Alborzi M., Kazemi M., Nohandani A.H. Diagnostic accuracy of magnetic resonance imaging, transvaginal, and transrectal ultrasonography in deep infiltrating endometriosis. Medicine. 2018;97:e9536. doi: 10.1097/MD.0000000000009536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssen E.B., Rijkers A.C., Hoppenbrouwers K., Meuleman C., D’Hooghe T.M. Prevalence of endometriosis diagnosed by laparoscopy in adolescents with dysmenorrhea or chronic pelvic pain: A systematic review. Hum. Reprod. Update. 2013;19:570–582. doi: 10.1093/humupd/dmt016. [DOI] [PubMed] [Google Scholar]
- 51.Becker C.M., Bokor A., Heikinheimo O., Horne A., Jansen F., Kiesel L., King K., Kvaskoff M., Nap A., Petersen K. ESHRE guideline: Endometriosis. Hum. Reprod. Open. 2022;2022:hoac009. doi: 10.1093/hropen/hoac009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown J., Kives S., Akhtar M. Progestagens and anti-progestagens for pain associated with endometriosis. Cochrane Database Syst. Rev. 2012;2012:CD002122. doi: 10.1002/14651858.CD002122.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Adur M.K., Kannan A., Davila J., Zhao Y., Nowak R.A., Bagchi M.K., Bagchi I.C., Li Q. Progesterone Alleviates Endometriosis via Inhibition of Uterine Cell Proliferation, Inflammation and Angiogenesis in an Immunocompetent Mouse Model. PLoS ONE. 2016;11:e0165347. doi: 10.1371/journal.pone.0165347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel B.G., Rudnicki M., Yu J., Shu Y., Taylor R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017;96:623–632. doi: 10.1111/aogs.13156. [DOI] [PubMed] [Google Scholar]
- 55.Casper R.F. Progestin-only pills may be a better first-line treatment for endometriosis than combined estrogen-progestin contraceptive pills. Fertil. Steril. 2017;107:533–536. doi: 10.1016/j.fertnstert.2017.01.003. [DOI] [PubMed] [Google Scholar]
- 56.Vercellini P., Trespidi L., Colombo A., Vendola N., Marchini M., Crosignani P.G. A gonadotropin-releasing hormone agonist versus a low-dose oral contraceptive for pelvic pain associated with endometriosis. Fertil. Steril. 1993;60:75–79. doi: 10.1016/S0015-0282(16)56039-7. [DOI] [PubMed] [Google Scholar]
- 57.Hillard P.A. Menstrual suppression: Current perspectives. Int. J. Womens Health. 2014;6:631–637. doi: 10.2147/IJWH.S46680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grimes D.A., Lopez L.M., O’Brien P.A., Raymond E.G. Progestin-only pills for contraception. Cochrane Database Syst. Rev. 2013;2013:CD007541. doi: 10.1002/14651858.CD007541.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edelman A., Micks E., Gallo M.F., Jensen J.T., Grimes D.A. Continuous or extended cycle vs. cyclic use of combined hormonal contraceptives for contraception. Cochrane Database Syst. Rev. 2014;2014:CD004695. doi: 10.1002/14651858.CD004695.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitawaki J., Kado N., Ishihara H., Koshiba H., Kitaoka Y., Honjo H. Endometriosis: The pathophysiology as an estrogen-dependent disease. J. Steroid. Biochem. Mol. Biol. 2002;83:149–155. doi: 10.1016/S0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 61.Noël J.-C., Chapron C., Bucella D., Buxant F., Peny M.-O., Fayt I., Borghese B., Anaf V. Estrogen and progesterone receptors in smooth muscle component of deep infiltrating endometriosis. Fertil. Steril. 2010;93:1774–1777. doi: 10.1016/j.fertnstert.2008.12.114. [DOI] [PubMed] [Google Scholar]
- 62.Zeun S., Lu M., Uddin A., Zeiler B., Morrison D., Blode H. Pharmacokinetics of an oral contraceptive containing oestradiol valerate and dienogest. Eur. J. Contracept Reprod Health Care. 2009;14:221–232. doi: 10.1080/13625180902850039. [DOI] [PubMed] [Google Scholar]
- 63.Vercellini P., Eskenazi B., Consonni D., Somigliana E., Parazzini F., Abbiati A., Fedele L. Oral contraceptives and risk of endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update. 2011;17:159–170. doi: 10.1093/humupd/dmq042. [DOI] [PubMed] [Google Scholar]
- 64.Chapron C., Souza C., Borghese B., Lafay-Pillet M.-C., Santulli P., Bijaoui G., Goffinet F., de Ziegler D. Oral contraceptives and endometriosis: The past use of oral contraceptives for treating severe primary dysmenorrhea is associated with endometriosis, especially deep infiltrating endometriosis. Hum. Reprod. 2011;26:2028–2035. doi: 10.1093/humrep/der156. [DOI] [PubMed] [Google Scholar]
- 65.Meresman G.F., Auge L., Baranao R.I., Lombardi E., Tesone M., Sueldo C. Oral contraceptives suppress cell proliferation and enhance apoptosis of eutopic endometrial tissue from patients with endometriosis. Fertil. Steril. 2002;77:1141–1147. doi: 10.1016/S0015-0282(02)03099-6. [DOI] [PubMed] [Google Scholar]
- 66.OHara R., Rowe H., Roufeil L., Fisher J. Should endometriosis be managed within a chronic disease framework? An analysis of national policy documents. Aust. Health Rev. 2018;42:627–634. doi: 10.1071/AH17185. [DOI] [PubMed] [Google Scholar]
- 67.Canis M., Bourdel N., Houlle C., Gremeau A.S., Botchorishvili R., Matsuzaki S. Endometriosis may not be a chronic disease: An alternative theory offering more optimistic prospects for our patients. Fertil. Steril. 2016;105:32–34. doi: 10.1016/j.fertnstert.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 68.Andres Mde P., Lopes L.A., Baracat E.C., Podgaec S. Dienogest in the treatment of endometriosis: Systematic review. Arch. Gynecol. Obstet. 2015;292:523–529. doi: 10.1007/s00404-015-3681-6. [DOI] [PubMed] [Google Scholar]
- 69.Grandi G., Mueller M., Bersinger N.A., Cagnacci A., Volpe A., McKinnon B. Does dienogest influence the inflammatory response of endometriotic cells? A systematic review. Inflamm. Res. 2016;65:183–192. doi: 10.1007/s00011-015-0909-7. [DOI] [PubMed] [Google Scholar]
- 70.Hayashi A., Tanabe A., Kawabe S., Hayashi M., Yuguchi H., Yamashita Y., Okuda K., Ohmichi M. Dienogest increases the progesterone receptor isoform B/A ratio in patients with ovarian endometriosis. J. Ovarian. Res. 2012;5:31. doi: 10.1186/1757-2215-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Flores V.A., Vanhie A., Dang T., Taylor H.S. Progesterone Receptor Status Predicts Response to Progestin Therapy in Endometriosis. J. Clin. Endocrinol. Metab. 2018;103:4561–4568. doi: 10.1210/jc.2018-01227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Petraglia F., Hornung D., Seitz C., Faustmann T., Gerlinger C., Luisi S., Lazzeri L., Strowitzki T. Reduced pelvic pain in women with endometriosis: Efficacy of long-term dienogest treatment. Arch. Gynecol. Obstet. 2012;285:167–173. doi: 10.1007/s00404-011-1941-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Momoeda M., Harada T., Terakawa N., Aso T., Fukunaga M., Hagino H., Taketani Y. Long-term use of dienogest for the treatment of endometriosis. J. Obstet. Gynaecol. Res. 2009;35:1069–1076. doi: 10.1111/j.1447-0756.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 74.Kohler G., Faustmann T.A., Gerlinger C., Seitz C., Mueck A.O. A dose-ranging study to determine the efficacy and safety of 1, 2, and 4 mg of dienogest daily for endometriosis. Int. J. Gynaecol. Obstet. 2010;108:21–25. doi: 10.1016/j.ijgo.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 75.Strowitzki T., Marr J., Gerlinger C., Faustmann T., Seitz C. Dienogest is as effective as leuprolide acetate in treating the painful symptoms of endometriosis: A 24-week, randomized, multicentre, open-label trial. Hum. Reprod. 2010;25:633–641. doi: 10.1093/humrep/dep469. [DOI] [PubMed] [Google Scholar]
- 76.Harada T., Momoeda M., Taketani Y., Aso T., Fukunaga M., Hagino H., Terakawa N. Dienogest is as effective as intranasal buserelin acetate for the relief of pain symptoms associated with endometriosis--a randomized, double-blind, multicenter, controlled trial. Fertil. Steril. 2009;91:675–681. doi: 10.1016/j.fertnstert.2007.12.080. [DOI] [PubMed] [Google Scholar]
- 77.Cosson M., Querleu D., Donnez J., Madelenat P., Konincks P., Audebert A., Manhes H. Dienogest is as effective as triptorelin in the treatment of endometriosis after laparoscopic surgery: Results of a prospective, multicenter, randomized study. Fertil. Steril. 2002;77:684–692. doi: 10.1016/S0015-0282(01)03270-8. [DOI] [PubMed] [Google Scholar]
- 78.Romer T. Long-term treatment of endometriosis with dienogest: Retrospective analysis of efficacy and safety in clinical practice. Arch. Gynecol. Obstet. 2018;298:747–753. doi: 10.1007/s00404-018-4864-8. [DOI] [PubMed] [Google Scholar]
- 79.Ota Y., Andou M., Yanai S., Nakajima S., Fukuda M., Takano M., Kurotsuchi S., Ebisawa K., Hada T., Ota I. Long-Term Administration of Dienogest Reduces Recurrence after Excision of Endometrioma. J. Endometr. Pelvic Pain Disord. 2015;7:63–67. doi: 10.5301/je.5000219. [DOI] [Google Scholar]
- 80.Ebert A.D., Dong L., Merz M., Kirsch B., Francuski M., Böttcher B., Roman H., Suvitie P., Hlavackova O., Gude K., et al. Dienogest 2 mg Daily in the Treatment of Adolescents with Clinically Suspected Endometriosis: The VISanne Study to Assess Safety in ADOlescents. J. Pediatr. Adolesc. Gynecol. 2017;30:560–567. doi: 10.1016/j.jpag.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Ferrero S., Remorgida V., Venturini P.L., Bizzarri N. Endometriosis: The effects of dienogest. BMJ Clin. Evid. 2015;2015:0802. [PMC free article] [PubMed] [Google Scholar]
- 82.Strowitzki T., Marr J., Gerlinger C., Faustmann T., Seitz C. Detailed analysis of a randomized, multicenter, comparative trial of dienogest versus leuprolide acetate in endometriosis. Int. J. Gynaecol. Obstet. 2012;117:228–233. doi: 10.1016/j.ijgo.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 83.Brown J., Pan A., Hart R.J. Gonadotrophin-releasing hormone analogues for pain associated with endometriosis. Cochrane Database Syst. Rev. 2010;2010:CD008475. doi: 10.1002/14651858.CD008475.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kitawaki J., Kusuki I., Yamanaka K., Suganuma I. Maintenance therapy with dienogest following gonadotropin-releasing hormone agonist treatment for endometriosis-associated pelvic pain. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011;157:212–216. doi: 10.1016/j.ejogrb.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 85.Brown J., Crawford T.J., Allen C., Hopewell S., Prentice A. Nonsteroidal anti-inflammatory drugs for pain in women with endometriosis. Cochrane Database Syst. Rev. 2017;1:CD004753. doi: 10.1002/14651858.CD004753.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Marjoribanks J., Ayeleke R.O., Farquhar C., Proctor M. Nonsteroidal anti-inflammatory drugs for dysmenorrhoea. Cochrane Database Syst. Rev. 2015;2015:CD001751. doi: 10.1002/14651858.CD001751.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salman S., Sherif B., Al-Zohyri A. OP0131 Effects of Some Non Steroidal Anti-Inflammatory Drugs on Ovulation in Women with Mild Musculoskeletal Pain. Ann. Rheum. Dis. 2015;74:117–118. doi: 10.1136/annrheumdis-2015-eular.1062. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.