Abstract
Chronic pulpal inflammation under caries appears to be elicited by bacterial antigens that diffuse into the pulp through dentinal tubules. This prompted the hypothesis that cytokines elicited by antigens from Streptococcus mutans, which frequently dominates shallow lesions, could play a major role in eliciting the initial T-cell response in the pulp. To test this, we examined the ability of S. mutans to stimulate T cells and elicit cytokines and used Lactobacillus casei, which often predominates in deep carious lesions where B cells and plasma cells predominate, as a control. In addition, the presence of cytokines in the pulp was analyzed at the mRNA level. S. mutans elicited potent gamma interferon (IFN-γ) responses in peripheral blood mononuclear cell cultures and reduced the CD4/CD8 ratio by promoting CD8+ T cells. Multiple inflammatory cytokine mRNAs (IFN-γ, interleukin 4 [IL-4], and IL-10) were detected in human dental pulp. A higher prevalence of IFN-γ (67%) than IL-4 (19%) or IL-10 (29%) was obtained in shallow caries, suggesting a type 1 cytokine mechanism in early pulpitis where S. mutans predominates. In contrast, in deep caries no differences in cytokine frequency were observed. Furthermore, the presence of IFN-γ in the pulp correlated with the presence of S. mutans. The extraordinary induction of type 1 cytokines and the preferential activation of CD8+ T cells by S. mutans offers an explanation for the etiology of the CD8+ T-cell-dominant lesion in early pulpitis and suggests that S. mutans may have a major impact on the initial lesion and pulpal pathology.
Although the dental pulp is equipped with cells of the immune system (16), the immune response in the pulp to caries pathogens is poorly understood. A chronic pulpal inflammation under caries is likely elicited by bacterial antigens that diffuse into the pulp through dentinal tubules (2, 3, 14). Immunohistological studies of dental pulps under shallow caries have revealed a lesion that is restricted almost exclusively to T cells, with CD8+ T cells predominating (9, 14). As the carious lesion enlarges and invades the inflamed dental pulp, CD8+ T cells continue to dominate but CD4+ T cells, B cells, and plasma cells appear in substantial numbers (9, 14). We are interested in understanding why the immune cell types shift during caries progression. We reasoned that antigens associated with the caries pathogens may preferentially elicit different immune cell types during caries invasion. The dominant organisms in the lesions, which likely elicit host responses, shift from Streptococcus mutans in shallow caries to Lactobacillus casei in deep carious lesions.
A type 1 cytokine response is defined as promoting cell-mediated response, with gamma interferon (IFN-γ) as the prototypic cytokine and a clear association with interleukin 2 (IL-2), tumor necrosis factor beta, and IL-12. A type 2 response is characterized as promoting one or more B-cell activities, with IL-4 as the prototypic cytokine and an association with IL-5, IL-6, IL-10, and IL-13 (18). This prompted us to reason that S. mutans might stimulate a highly polarized type 1 response while L. casei might be less polarized toward type 1. This could help explain the near-exclusive T-cell response, especially CD8+ T cells, in the shallow lesions while L. casei might maintain T cells but allow the B cells and plasma cells to appear in the deep lesions.
To begin testing the hypothesis that S. mutans promotes type 1 activity, we established the cytokine profile elicited by S. mutans and L. casei in peripheral blood mononuclear cells (PBMC). This was followed by analysis of cytokine mRNA expression in the inflamed pulps from shallow and deep caries. The effect of these two caries pathogens on CD4+ and CD8+ T cell populations was determined and a correlation between recovery of S. mutans and cytokine mRNA expression was sought to further test the hypothesis.
The data indicate that S. mutans is capable of eliciting potent type 1 responses in PBMC cultures and promoting CD8+ T-cell responses. Furthermore, IFN-γ mRNA was prominent in the inflamed pulps from shallow caries, supporting a type 1 pathology. L. casei also produced IFN-γ, but the level was much reduced, and IFN-γ mRNA was present in deep caries but was not dominant. In short, these data support the concept that antigens from S. mutans play a major role in promoting a type 1 response and may help explain pulpal pathology in shallow caries.
MATERIALS AND METHODS
Bacterial preparation.
S. mutans (ATCC 25175) and L. casei (ATCC 4646) were grown in brain heart infusion broth in an anaerobic chamber for 18 to 24 h before harvesting. Cells were then washed three times with phosphate-buffered saline, and the concentration of bacteria in suspension was determined using a Petroff-Hausser chamber. The bacterial suspension was exposed to 2.5 megarads of irradiation using a cesium-173 source, which killed the vast majority of the organisms, and then was stored at −70°C.
PBMC preparation.
Venous blood from medically healthy volunteers was collected, after obtaining an appropriately signed human consent form, in heparin (147 mg of sodium heparin per 50 ml of RPMI [0.2-μm-pore-size filter sterilized])-containing syringes. Blood was diluted 1:1 with RPMI (2 mM glutamine, 10 mM HEPES buffer, penicillin [100 U/ml] and streptomycin sulfate [100 μg/ml]), and this blood-RPMI mixture was layered on lymphocyte separation medium (20 ml per 30 ml of blood-RPMI mixture) and centrifuged at 400 × g for 30 min at room temperature. Mononuclear cells were collected at the medium interface and washed three times with RPMI at 200 × g at 4°C for 10 min. The cells were then suspended in enriched RPMI 1640 (supplemented with 10% heat-inactivated fetal bovine serum), and vital cell counts were determined using trypan blue. One million vital PBMC (106 cells/ml) were cultured with various concentrations of S. mutans or L. casei at 37°C in a humidified 5% CO2 chamber. Preliminary kinetic studies for each cytokine indicated that levels in supernatant fluids were near maximal after 3 days of culture. However, IFN-γ induced by S. mutans was higher on days 5 and 7 than on day 3, and IL-12 was significantly higher on day 7 for both S. mutans and L. casei. There were no significant differences in IL-10 and IL-4 levels in the period from day 3 to 7. Day 7 gave the maximum levels for all cytokines, and culture supernatants were therefore harvested on day 7 and stored in a −70°C freezer until assayed for cytokine concentration. Each experimental condition was performed in duplicate.
ELISA.
Cytokine concentrations in the culture supernatants were assayed with commercial cytokine enzyme-linked immunosorbent assay (ELISA) kits for human IFN-γ, IL-4, IL-10, and IL-12 according the manufacturer's instructions (Biosource Internationals, Camarillo, Calif.). The sensitivity of assays was 4 pg/ml for IFN-γ, 1 pg/ml for IL-12, 5 pg/ml for IL-10 and 0.27 pg/ml for IL-4. All cytokine assays were carried out in duplicate.
Flow cytometry.
PBMC stimulated with S. mutans and L. casei (104 to 106 bacteria, determined using a Petroff-Hausser chamber, per 106 PBMC per ml) for 7 days were harvested and washed once with 0.5% fetal calf serum in phosphate-buffered saline. Cells were then incubated with fluorescein isothiocyanate-conjugated, mouse anti-human CD4 and phycoerythrin-conjugated anti-human CD8 antibodies (BD Pharmingen, San Diego, Calif.) in ice for 20 min and washed once before flow cytometry. Cells with no staining and single-color controls were included. Cells were analyzed using the FACScan (Becton Dickinson, Mansfield, Calif.) equipped with Cyclops software (Cytomation Inc., Fort Collins, Colo.).
Selection of teeth.
Extracted molars, including impacted third molars, with vital pulps were obtained from the Oral Surgery clinic in the Dental School of Virginia Commonwealth University. Teeth were transported in sterile saline solution and processed within 2 h of extraction. Each tooth sample was split opened at the carious site with a pair of pliers, and individual pulp tissue was extirpated. The pulp tissues were then immediately placed in 1 ml of Ultraspec RNA Isolation solution (Biotecx, Houston, Tex.) and stored in a −70°C freezer until RNA extraction. The caries front was detected with an explorer, and the shortest distance between the caries front and the pulp chamber space was measured with a millimeter ruler. Tooth samples were then categorized into three groups: (i) control group: impacted third molars; (ii) shallow caries group: caries (enamel and dentinal caries) was more than 2 mm away from the pulp; (iii) deep caries group: caries was less than 1.5 mm from the pulp, including pulp exposure.
Total RNA extraction and RT-PCR.
Freshly removed pulpal tissues were cut into small pieces and homogenized in 1 ml of Ultraspec RNA Isolation solution with a sterile tissue grinder. The total RNA from each sample was extracted and precipitated with isopropanol according to the manufacturer's instruction. After dissolving in diethyl pyrocarbonate-treated water, the total RNA extract was quantified with a spectrophotometer at a wavelength of 260 nm. Due to the small amount of pulpal tissue and multiple cytokines that needed to be examined, we performed reverse transcriptase PCR (RT-PCR) to detect the presence of mRNA type 1 (IFN-γ) and type 2 (IL-4 and IL-10) cytokines in individual pulps. Reverse transcription and PCR were completed using the Superscript Preamplification system (Life Technology, Grand Island, N.Y.) as described by the manufacturer, with minor modifications. Briefly, 2 μg of total RNA with oligo(dT) primer was used for first-strand cDNA synthesis. The resulting DNA-RNA hybrid was treated with RNase H. The PCR mixture consisted of 2 μl of cDNA, 1 μl of 10 μM 5′ and 3′ primers for each cytokine (IFN-γ, IL-4 and IL-10; RT-PCR amplimer sets; Clontech, Palo Alto, Calif.), 2.5 U of Platinum Taq polymerase (Life Technology), and final concentrations of 10 mM Tris-Cl, 50 mM KCl, 2.5 mM MgCl2, and 0.2 mM deoxynucleoside triphosphate in 50 μl. Each reaction mixture was placed in a preheated (94°C) Perkin-Elmer (Norwalk, Conn.) DNA thermal cycler and denatured at 94°C for 3 min. PCR amplification was accomplished by 35 cycles of 94°C for 45 s, 60°C for 45 s, and 72°C for 2 min, followed by an extension at 72°C for 10 min. The amplified cDNA samples (17 μl) were then evaluated by gel electrophoresis (1.5% agarose gel) with ethidium bromide staining. Control PCR with primers for β-actin was run in parallel. φX174/HaeIII fragment was used as the base pair maker. Video images of the gels were obtained with an IS-1000 digital imaging system (Alpha Innotech Corporation).
Microbiological study of carious lesions.
Extracted teeth with shallow or deep carious lesions for microbiological analysis were transported in sterile saline and cracked opened with a pair of pliers in an anaerobic chamber. The dental pulps were stored in Ultraspec for RT-PCR study, and carious samples were cultured. The microbiological procedures were performed as described previously (8). In brief, the dentin shavings were removed with a sterile excavator from the deepest caries area and resuspended in reduced transported fluid to be sonicated, diluted, and plated on nonselective medium (MM10), mitis salivarius bacitracin-sucrose agar for S. mutans, and Rogosa medium for lactobacilli (8). Plates were incubated under anaerobic conditions for 7 days before colony count and identification. S. mutans and lactobacilli were identified by colony morphology, gram stain, and biochemical tests (API 20S and API 20A). The percentages of S. mutans and lactobacilli were calculated from the total CFU on the selective media divided by CFU on MM10 plates.
Statistical analysis.
A repeated-measures analysis of variance was used to test the cytokine type, bacterial concentration effects, and interaction between the two. The interaction was included to test for concentration effects separately for each cytokine effect. Because of the skewed nature of the data, a log transformation was chosen for analysis and the summary results were back-transformed into the original unit of measurement. The differences between S. mutans and L. casei in cytokine induction at each concentration were tested in the same repeated-measures analysis of variance model described above.
A total of 62 pulps from 49 individuals were included in the RT-PCR study (16 noncaries controls, 21 shallow caries, and 25 deep caries), and 22 caries samples were examined microbiologically (11 from shallow caries and 11 from deep caries). Differences of positive percentage between type 1 and type 2 cytokines in each caries type (shallow and deep caries) were analyzed with McNemar's chi-square test for paired comparisons of proportions. Differences between caries type for each cytokine were analyzed with the chi-square test. The correlation between the percent concentration of bacteria (S. mutans and lactobacilli) and positivity of IFN-γ cytokine expression was also analyzed using the Wilcoxon rank-sum test.
RESULTS
Induction of IFN-γ, IL-4, IL-10, and IL-12 by S. mutans.
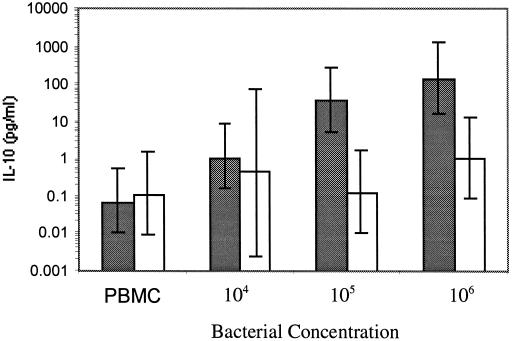
To begin testing the hypothesis that S. mutans promotes type 1 activity, we established the cytokine profile elicited by S. mutans and L. casei in PBMC from normal volunteers. As illustrated in Table 1, S. mutans stimulated production of nanogram levels of the prototypic type 1 cytokine IFN-γ, and only 10,000 organisms were required to get this high response. Detectable levels of IFN-γ were also apparent upon addition of L. casei, but the levels were 10- to 100-fold lower. The cultures were also examined for the type 2 cytokines IL-4 and IL-10. The levels of IL-4 were undetectable at the low bacterial concentrations and barely detectable at high concentrations (data not shown). Only background levels of IL-10 were detected at 104 S. mutans bacteria, but at 105 and 106 bacteria significant increases in IL-10 were observed (Fig. 1) (P < 0.0001). There were no significant increases in IL-10 induced with L. casei in the same range, but at 107 organisms IL-10 did reach statistical significance (mean = 47 pg/ml, 95% confidence interval [95% CI] = 546 to 43 pg/ml; P = 0.0027).
TABLE 1.
IFN-γ concentrations in supernatant fluids from PBMC culturesa stimulated by S. mutans and L. casei
| Bacterial concn (ml−1) | IFN-γ concn (ng/ml)b in culture stimulated with:
|
P | |
|---|---|---|---|
| S. mutans | L. casei | ||
| PBMC control | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.7878 |
| 104 | 1.88 (0.51–6.94) | 0.00 (0.00–0.02) | <0.0001 |
| 105 | 8.06 (2.28–28.55) | 0.02 (0.00–0.10) | <0.0001 |
| 106 | 6.76 (1.56–29.42) | 0.25 (0.06–1.01) | 0.0020 |
PBMC (106/ml) were stimulated with 104 to 106 S. mutans (n = 17) and L. casei (n = 14) bacteria per ml. The supernatant fluids were harvested after 7 days, and IFN-γ concentrations were assayed by ELISA.
Mean (95% CI).
FIG. 1.
Comparison of S. mutans and L. casei stimulation of IL-10 production in PBMC cultures. The IL-10 concentrations were determined in supernatant fluids from 7-day-old PBMC (106/ml) cultures stimulated with 104 to 106 S. mutans (shaded bars) (n = 6) and L. casei (open bars) (n = 4) bacteria per ml. The error bars represent 95% CIs.
Given the dramatic polarization toward IFN-γ production by S. mutans (especially at low concentrations of bacteria), we examined the cultures to see if IL-12 production might help explain this polarization. The IL-12 data were similar to the IFN-γ data (Table 2). Nanogram levels of IL-12 were produced by as few as 10,000 bacteria, whereas the levels induced by L. casei were barely detectable.
TABLE 2.
IL-12 concentrations in supernatant fluids from PBMC culturesa stimulated by S. mutans and L. casei
| Bacterial concn (ml−1) | IL-12 concn (ng/ml)b in culture stimulated with:
|
P | |
|---|---|---|---|
| S. mutans | L. casei | ||
| PBMC control | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.8820 |
| 104 | 1.36 (0.37–4.99) | 0.00 (0.00–0.01) | <0.0001 |
| 105 | 4.87 (1.32–17.93) | 0.01 (0.02–0.05) | <0.0001 |
| 106 | 3.46 (0.87–13.69) | 0.23 (0.06–0.92) | 0.0089 |
PBMC (106/ml) were stimulated with 104 to 106 S. mutans (n = 11) and L. casei (n = 10) bacteria per ml. The supernatant fluids were harvested after 7 days, and IL-12 concentrations were assayed by ELISA.
Mean (95% CI).
Cytokine mRNA profiles in shallow and deep carious lesions.
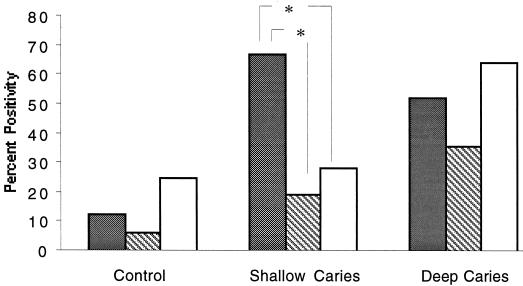
Given that S. mutans is a dominant organism in shallow caries that appears to stimulate a potent type 1 response, we reasoned that a message for IFN-γ should be prevalent in shallow caries. To test this, IFN-γ, IL-10, and IL-4 cytokine mRNA expression was examined in 62 pulp tissues by RT-PCR. In the control group without carious lesions, mRNA for IFN-γ, IL-10, and IL-4 was observed in some pulps but the frequency was low, with no statistical differences suggesting polarization (P > 0.05) (Fig. 2). In marked contrast, in the shallow-caries group the IFN-γ mRNA (67%) was about twofold more prevalent than IL-4 (19%) (P = 0.0098) and IL- 10 (29%) (P = 0.0433), supporting a type 1 polarization of the response (Fig. 2). Furthermore, in 43% of these samples the IFN-γ mRNA was the only cytokine message found. In the deep-caries group IFN-γ mRNA remained very common, but IL-4 and IL-10 were found in similar frequencies and a polarization toward type 1 was no longer apparent. However, the prevalence of IL-10 mRNA was significantly higher in the deep-caries than the shallow-caries (P = 0.0194) and control (P = 0.0187) groups.
FIG. 2.
Comparison of type 1 (IFN-γ) and type 2 (IL-4 and IL-10) cytokine mRNA in pulpal tissues from shallow and deep carious lesions. The presence of detectable cytokine mRNA in the pulps was determined using RT-PCR, and the percentage of positive pulps in the control, shallow-caries, and deep-caries groups is indicated for IFN-γ (shaded bars), IL-4 (striped bars), and IL-10 (open bars). The pulpal tissue was obtained from impacted third molars for controls (n = 16), molars with shallow caries (n = 21), and molars with deep caries (n = 25). ∗, P < 0.05.
Microbiological analysis.
Given the ability of S. mutans to stimulate production of IFN-γ it seemed possible that the IFN-γ mRNA in the lesions would correlate with the presence of S. mutans. Eleven samples each from the shallow- and deep- caries groups were examined, and IFN-γ mRNA was detected in 17 samples. A large variation of bacterial CFU (103 to 107 CFU) was observed on MM10 plates, but the difference in recovery of S. mutans in shallow and deep caries was not statistically significant in this relatively small sample. Nevertheless, the presence of S. mutans, which ranged from 0 to 13.5% of the CFU (data not shown), did correlate with the presence of IFN-γ mRNA (P = 0.0480). In contrast, the presence of lactobacilli was not statistically associated with IFN-γ expression (P = 0.1251).
Effect of S. mutans on CD4/CD8 ratios.
Immunohistological studies of dental pulp under shallow caries have described a lesion that is restricted almost exclusively to T cells, with CD8+ T cells predominating. To determine if S. mutans might influence CD4-to-CD8 ratios, the effect of increasing concentrations of S. mutans on CD4+ and CD8+ T cells was examined. Increasing concentrations of S. mutans were significantly associated with higher CD8+ levels (P = 0.0296), lower CD4+ T-cell percentages (P = 0.0014), and a reduction in the CD4/CD8 ratios (P = 0.0096) (Table 3). Neither CD4+, CD8+ T-cell percentages nor CD4/CD8 ratios were significantly related to L. casei concentrations (data not shown).
TABLE 3.
Differential stimulation of CD4+ and CD8+ T cells by S. mutansa
| Bacterial concn | Mean ± SE
|
||
|---|---|---|---|
| CD4 (%) | CD8 (%) | CD4/CD8 | |
| PBMC control | 48.40 ± 1.06 | 24.10 ± 0.67 | 2.11 ± 0.09 |
| S. mutans | |||
| 104 | 48.16 ± 1.06 | 25.71 ± 0.67 | 1.99 ± 0.09 |
| 105 | 42.71 ± 1.06b | 27.01 ± 0.67b | 1.71 ± 0.09b |
| 106 | 44.34 ± 1.06b | 26.46 ± 0.67b | 1.74 ± 0.09b |
PBMC (106/ml) from nine individuals were stimulated with 104 to 106 S. mutans bacteria/ml for 7 days, and CD4+ and CD8+ cells were identified by flow cytometry.
Significantly different (P < 0.03) from values for PBMC controls.
DISCUSSION
The lesions in dental pulp under shallow caries are T-cell dominated, with CD8+ T cells predominating (9, 14). As caries progresses and the deep lesion emerges, the CD8+ T cell continues to dominate, but CD4+ T cells, B cells, and plasma cells appear in substantial numbers (9, 14). We reasoned that antigens associated with carious pathogens might preferentially elicit different immune cell types during caries invasion. The dominant organism in shallow caries is S. mutans and shifts to L. casei in many deep carious lesions (8, 29). This prompted the hypothesis that S. mutans might stimulate type 1 responses while L. casei might be less polarized toward type 1. This could explain the near-exclusive T-cell response, especially CD8+ T cells, in the shallow lesions, while L. casei might maintain T cells but be less polarizing and allow the B cells and plasma cells to appear in the deep lesions. The results reported here are consistent with this view. The cytokine profile elicited by S. mutans in PBMC included large amounts of IFN-γ and polarization toward type 1. The high titers of IFN-γ induced by S. mutans in this study are in accordance with recent studies of others which included other S. mutans strains (15, 25). The polarization was most apparent when low numbers (104) of bacteria were used and nanogram levels of IFN-γ were produced in the absence of detectable IL-4 or IL-10 levels above background (Table 1 and Fig. 1). Analysis of cytokine mRNA expression in pulps from control, shallow, and deep caries lesions revealed that IFN-γ mRNA is generally present in shallow lesions, while mRNA for IL-4 and IL-10 are generally not present, supporting a type 1 polarization. In contrast, cytokine polarization was not apparent in pulps from impacted teeth, which served as the noncaries control, or from teeth with deep lesions (Fig. 2). Furthermore, the presence of IFN-γ in the pulp correlated with the presence of S. mutans, and S. mutans promoted CD8+ T cells in culture in preference to CD4+. The extraordinary induction of type 1 cytokines and the preferential activation of CD8+ by S. mutans offers an explanation for the etiology of the T-cell lesion and the CD8+ T-cell dominance in early pulpitis and suggests that S. mutans may have a major impact on the local inflammatory response.
The high titers of IL-12 induction by S. mutans agree with previous studies using different S. mutans strains (15, 25). Lipoteichoic acid and peptidoglycan are known to induce IL-12 by dendritic cells or monocytes and IL-12 promotes induction of Th1 responses and IFN-γ production (4, 26). A striking aggregation of pulpal dendritic cells is present in the dental pulps under shallow caries, suggesting that this highly efficient antigen-presenting cell may be a source of IL-12 in shallow lesions (27, 32). Bacterial antigens from S. mutans and Streptococcus sanguis have been ultrastructurally localized in the dentinal tubules and pulpal tissues (2). It is conceivable that fragments of cell wall from S. mutans could permeate through dentinal tubules during early caries to stimulate pulpal dendritic cells and macrophages and to set up a type 1 microenvironment. Our previous immunohistochemical study indicates lymphocytes are localized in the tissue near the caries front, and these cells likely produce the cytokines (9). A study correlating immunohistochemistry, including T-cell phenotype, and in situ hybridization with various cytokines should indicate the location and the cell type producing each cytokine.
Induction of IL-12 by S. mutans might further contribute to the preferential CD8+ maturation over CD4+ T cells (10, 11, 19). However, Plitnick et al. reported PBMC stimulated by S. mutans were CD4+, CD8+ T cells and NK cells with no differential effect (25). The reason for the discrepancy in data is not clear but might relate to fewer subjects (five) and a higher bacterium-to-cell ratio in their study. Interestingly, increased numbers of CD8+ T cells is thought to cause a depressed humoral immune response when animals are fed with high doses of S. mutans (17). Preliminary studies with two additional strains of S. mutans (v1310 and v1311) indicate a similar type 1 polarization (more IFN-γ than IL-4). Furthermore, other oral streptococci, including S. mitis (ATCC 903), S. oralis (ATCC 35037) and S. sorbrinus (v262), have been studied, and the data suggest that they are also potent IFN-γ inducers (data not shown). In preliminary studies, three clinical isolates of L. casei were also studied, and the levels of cytokines contrast with those stimulated by S. mutans but were comparable with those stimulated by the American Type Culture Collection strain of L. casei (ATCC 4646) (data not shown). It is speculated that antigens from streptococci and certain other gram-positive bacteria in shallow caries, permeating through dentinal tubules, are processed and presented by pulpal dendritic cells and induce type 1 cytokines, which orchestrate a cell-mediated immune response in the pulps.
When compared to S. mutans, L. casei is a weaker type 1 cytokine inducer. In lower concentrations, L. casei exhibits a type 1 cytokine profile, which is also reported by others (12, 20). Proprionibacteria, another major genus in deep dentinal caries (13), induce IFN-γ in vivo (30) and therefore contribute to the persistent IFN-γ expression in the deep-caries group. Higher concentrations of L. casei (107 and 108 per 106 PBMC) elicited a significant increase of IL-10 secretion, which reached a level comparable to IFN-γ and IL-12 (data not shown). A similar pattern is also reported with Lactobacillus rhamnosus (12). Some oral bacteria such as bifidobacterium and eubacterium also promote IL-10 (5, 21). The increased prevalence of IL-10 mRNA induced by higher concentrations of lactobacilli and/or other carious bacteria could help explain an elevated number of B and plasma cells in the pulps under deep caries. The polymicrobial nature of deep caries might help explain the high prevalence of both type 1 and type 2 cytokine mRNAs in this group.
This is the first paper to demonstrate the presence of multiple inflammatory cytokine mRNAs (IFN-γ, IL-4, and IL-10) in the human dental pulp in carious lesions. The background levels of IFN-γ, IL-4, and IL-10 in pulps from impacted third molars is not understood, but T cells are present in these pulps (9, 14), and these T cells may include some that were activated in remote sites. Minimal IL-4 induction by S. mutans agrees with a recent study by Plitnick et al. (25). IL-4 is important in B-cell development and is required for the production of immunoglobulin E and for promoting immunoglobulin G1 in mice (23, 24). Furthermore, IL-4 directly enhances the development of Th2 cells from naïve T cells (1, 28). A trend of higher prevalence of IL-4 was noted as caries invades pulpally. S. mutans and L. casei induced minimal amounts of IL-4 and did so only at high concentrations. However, Prevotella intermedia, a frequent isolate from deep caries and infected root canals, is capable of inducing IL-4 (31). Furthermore, the prostaglandin E2 concentration in inflamed pulps is known to be elevated (22), and this would also favor the induction of type 2 cytokines in deep caries (6, 7). Future quantification studies of each inflammatory cytokine in the inflamed pulp may contribute to our understanding of the delicate interactions among cytokines and their impact on pulpal immune responses, pathogenesis, and even healing. It is clear that interactions between oral bacteria and the immune system are complex and deserving of further attention if we hope to develop more efficacious modalities of therapy for carious lesions.
ACKNOWLEDGMENTS
This work was partially supported by the A. D. Williams Research Fund, Virginia Commonwealth University.
We thank Mingyen Yang and Tom Pasley for their valuable technical support in the RT-PCR study.
REFERENCES
- 1.Abbas A K, Murphy K M, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 2.Ackermans F, Klein J P, Frank R M. Ultrastructural location of Streptococcus mutans and Streptococcus sanguis antigens in carious human dentine. J Biol Bucc. 1981;9:203–217. [PubMed] [Google Scholar]
- 3.Brannstrom M, Lind P O. Pulpal response to early dental caries. J Dent Res. 1965;44:1045–1050. doi: 10.1177/00220345650440050701. [DOI] [PubMed] [Google Scholar]
- 4.Chehimi J, Trinchieri G. Interleukin-12: a bridge between innate resistance and adaptive immunity with a role in infection and acquired immunodeficiency. J Clin Immunol. 1994;14:149–161. doi: 10.1007/BF01533364. [DOI] [PubMed] [Google Scholar]
- 5.Chen T, Isomaki P, Rimpilainen M, Toivanen P. Human cytokine responses induced by gram-positive cell walls of normal intestinal microbiota. Clin Exp Immunol. 1999;118:261–267. doi: 10.1046/j.1365-2249.1999.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeure C E, Yang L P, Desjardins C, Raynauld P, Delespesse G. Prostaglandin E2 primes naive T cells for the production of anti-inflammatory cytokines. Eur J Immunol. 1997;27:3526–3531. doi: 10.1002/eji.1830271254. [DOI] [PubMed] [Google Scholar]
- 7.Gold K N, Weyand C M, Goronzy J J. Modulation of helper T cell function by prostaglandins. Arthritis Rheum. 1994;37:925–933. doi: 10.1002/art.1780370623. [DOI] [PubMed] [Google Scholar]
- 8.Hahn C L, Falkler W A, Jr, Minah G E. Microbiological studies of carious dentine from human teeth with irreversible pulpitis. Arch Oral Biol. 1991;36:147–153. doi: 10.1016/0003-9969(91)90077-8. [DOI] [PubMed] [Google Scholar]
- 9.Hahn C L, Falkler W A, Jr, Siegel M A. A study of T and B cells in pulpal pathosis. J Endod. 1989;15:20–26. doi: 10.1016/S0099-2399(89)80093-7. [DOI] [PubMed] [Google Scholar]
- 10.Hall S S. IL-12 at the crossroads. Science. 1995;268:1432–1434. doi: 10.1126/science.7770767. [DOI] [PubMed] [Google Scholar]
- 11.Halverson D C, Schwartz G N, Carter C, Gress R E, Fowler D H. In vitro generation of allospecific human CD8+ T cells of Tc1 and Tc2 phenotype. Blood. 1997;90:2089–2096. [PubMed] [Google Scholar]
- 12.Hessle C, Hanson L A, Wold A E. Lactobacilli from human gastrointestinal mucosa are strong stimulators of IL-12 production. Clin Exp Immunol. 1999;116:276–282. doi: 10.1046/j.1365-2249.1999.00885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoshino E. Predominant obligate anaerobes in human carious dentin. J Dent Res. 1985;64:1195–1198. doi: 10.1177/00220345850640100301. [DOI] [PubMed] [Google Scholar]
- 14.Izumi T, Kobayashi I, Okamura K, Sakai H. Immunohistochemical study on the immunocompetent cells of the pulp in human non-carious and carious teeth. Arch Oral Biol. 1995;40:609–614. doi: 10.1016/0003-9969(95)00024-j. [DOI] [PubMed] [Google Scholar]
- 15.Jiang Y, Magli L, Russo M. Bacterium-dependent induction of cytokines in mononuclear cells and their pathologic consequences in vivo. Infect Immun. 1999;67:2125–2130. doi: 10.1128/iai.67.5.2125-2130.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jontell M, Gunraj M, Bergenholtz G. Immunocompetent cells in the normal dental pulp. J Dent Res. 1986;66:1263–1266. doi: 10.1177/00220345870660061101. [DOI] [PubMed] [Google Scholar]
- 17.Lehner T, Caldwell J, Avery J. Sequential development of helper and suppressor functions, antibody titers and functional avidities to a streptococcal antigen in rhesus monkeys. Eur J Immunol. 1984;14:814–819. doi: 10.1002/eji.1830140909. [DOI] [PubMed] [Google Scholar]
- 18.Lucey D R, Clerici M, Shearer G M. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin Microbiol Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehrotra P T, Grant A J, Siegel J P. Synergistic effects of IL-7 and IL-12 on human T cell activation. J Immunol. 1995;154:5093–5102. [PubMed] [Google Scholar]
- 20.Miettinen M, Matikainen S, Vuopio-Varkila J, Pirhonen J, Varkila K, Kurimoto M, Julkunen I. Lactobacilli and streptococci induce interleukin-12 (IL-12), IL-18, and gamma interferon production in human peripheral blood mononuclear cells. Infect Immun. 1998;66:6058–6062. doi: 10.1128/iai.66.12.6058-6062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miettinen M, Vuopio-Varkila J, Varkila K. Production of human tumor necrosis factor alpha, interleukin-6, and interleukin-10 is induced by lactic acid bacteria. Infect Immun. 1996;64:5403–5405. doi: 10.1128/iai.64.12.5403-5405.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi T, Matsuo T, Ebisu S. Quantitative analysis of immunoglobulins and inflammatory factors in human pulpal blood from exposed pulps. J Endod. 1995;21:131–136. doi: 10.1016/s0099-2399(06)80438-3. [DOI] [PubMed] [Google Scholar]
- 23.Paul W E. Interleukin 4: signalling mechanisms and control of T cell differentiation. Ciba Found Symp. 1997;204:208–219. doi: 10.1002/9780470515280.ch14. [DOI] [PubMed] [Google Scholar]
- 24.Paul W E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991;77:1859–1870. [PubMed] [Google Scholar]
- 25.Plitnick L M, Banas J A, Jelley-Gibbs D M, O'Neil J, Christian T, Mudzinski S P, Gosselin E J. Inhibition of interleukin-2 by a Gram-positive bacterium, Streptococcus mutans. Immunology. 1998;95:522–528. doi: 10.1046/j.1365-2567.1998.00631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rissoan M C, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu Y J. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–1186. doi: 10.1126/science.283.5405.1183. [DOI] [PubMed] [Google Scholar]
- 27.Sakurai K, Okiji T, Suda H. Co-increase of nerve fibers and HLA-DR- and/or factor-XIIIa-expressing dendritic cells in dentinal caries-affected regions of the human dental pulp: an immunohistochemical study. J Dent Res. 1999;78:1596–1608. doi: 10.1177/00220345990780100401. [DOI] [PubMed] [Google Scholar]
- 28.Seder R A, Paul W E. Acquisition of lymphokine-producing phenotype by CD4+ T cells. Annu Rev Immunol. 1994;12:635–673. doi: 10.1146/annurev.iy.12.040194.003223. [DOI] [PubMed] [Google Scholar]
- 29.Shovlin F E, Gillis R E. Biochemical and antigenic studies of lactobacilli isolated from deep dentinal caries. II. Antigenic aspects. J Dent Res. 1972;51:583–587. doi: 10.1177/00220345720510025701. [DOI] [PubMed] [Google Scholar]
- 30.Smith S R, Terminelli C, Denhardt G, Narula S, Thorbecke G J. Administration of interleukin-10 at the time of priming protects Corynebacterium parvum-primed mice against LPS- and TNF-alpha-induced lethality. Cell Immunol. 1996;173:207–214. doi: 10.1006/cimm.1996.0269. [DOI] [PubMed] [Google Scholar]
- 31.Wassenaar A, Reinhardus C, Abraham-Inpijn L, Snijders A, Kievits F. Characteristics of Prevotella intermedia-specific CD4+ T cell clones from peripheral blood of a chronic adult periodontitis patient. Clin Exp Immunol. 1998;113:105–110. doi: 10.1046/j.1365-2249.1998.00630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoshiba N, Yoshiba K, Nakamura H, Iwaku M, Ozawa H. Immunohistochemical localization of HLA-DR-positive cells in unerupted and erupted normal and carious human teeth. J Dent Res. 1996;75:1585–1589. doi: 10.1177/00220345960750081001. [DOI] [PubMed] [Google Scholar]