Abstract
The significance of delivery systems in modern vaccine design strategies is underscored by the fact that a promising vaccine formulation may fail in vivo due to an inappropriate delivery method. We evaluated the immunogenicity and efficacy of a candidate vaccine comprising the major outer membrane protein (MOMP) of Chlamydia trachomatis delivered with the lipophilic immune response-stimulating complexes (ISCOMs) as a vehicle with adjuvant properties, in a murine model of chlamydial genital infection. Immunocompetent BALB/c mice were immunized intranasally (IN) or intramuscularly (IM) with MOMP, MOMP-ISCOMs, and live or heat-inactivated C. trachomatis serovar D. The level of local genital mucosal Th1 response was measured by assaying for antigen-specific Th1 cell induction and recruitment into the genital mucosa at different times after immunization. Immunization with MOMP-ISCOMs by the IM route induced the greatest and fastest local genital mucosal Th1 response, first detectable 2 weeks after exposure. Among the other routes and regimens tested, only IN immunization with MOMP-ISCOMs induced detectable and statistically significant levels of local genital mucosal Th1 response during the 8-week test period (P < 0.001). In addition, when T cells from immunized mice were adoptively transferred into syngeneic naive animals and challenged intravaginally with Chlamydia, recipients of IM immunization of MOMP-ISCOMs cleared their infection within 1 week and were resistant to reinfection. Animals that received IN immunization of MOMP-ISCOMs were partially protected, shedding fewer chlamydiae than did control mice. Altogether, the results suggested that IM delivery of MOMP-ISCOMs may be a suitable vaccine regimen potentially capable of inducing protective mucosal immunity against C. trachomatis infection.
An important goal in controlling the spread of sexually transmitted diseases is the development and administration of protective vaccines that induce long-term local genital mucosal immunity. This combined requirement is crucial because even the most promising vaccine formulations may fail to establish the desired protective immunity due to an inadequate delivery vehicle that does not optimize or adversely affects mucosal immune elicitation and maintenance. The pathological consequence of genital infection by Chlamydia trachomatis include major sequelae such as Pelvic inflammatory disease and infertility. The urgent need to develop an efficacious vaccine underscores efforts to define the requirements for induction and maintenance of protective genital mucosal immunity. The immunological control of several intracellular pathogens is due to adequate Th1 responses, including major histocompatibility complex (MHC) class I-mediated CD8 T-cell function (1, 15, 28). It has been established that the induction and recruitment of Th1 cells into the local genital mucosae are crucial for immunity against Chlamydia (24, 26, 40, 47, 60). Thus, important objectives in designing protective anti-chlamydia vaccines include the identification of an appropriate antigen(s) and the development of effective delivery vehicles such as adjuvants to optimize the induction and recruitment of chlamydia-specific Th1 cells into the genital mucosa.
Lipophilic immune response-stimulating complexes (ISCOMs) are negatively charged cage-like assemblies of complex micelles, composed of the saponin, Quil A, cholesterol, and phospholipids, into which particulate antigens can be incorporated during synthesis (30, 33, 38). ISCOM particles can be used as vehicles for amphipathic macromolecules, especially membrane proteins, to substantially enhance immune response against antigens (33, 42). The adjuvant properties of ISCOMs have been demonstrated in animal models of several infectious diseases, where both protective humoral and T-cell immunity has been observed (33). The adjuvanticity of ISCOMs derives partly from the hemolytic and local inflammatory effects of Quil A, possibly due to complex formation with membrane cholesterol, causing an influx of leukocytes to the site of antigen deposition, as well as direct B- and T-cell stimulation (33). The accumulation of ISCOMs in secondary lymphoid tissues also favors contact of antigen-presenting cells (APCs) with lymphocytes (33). ISCOMs have been used in immunogenicity and protection studies involving viral, bacterial, and parasitic proteins, including pore protein 1 from Neisseria gonorrhoeae (31, 32), the major outer membrane protein (MOMP) of Neisseria meningitis (42, 49), pili of enterotoxigenic Escherichia coli, and detergent-solubilized Mycoplasma gallisepticum antigens (54). Both systemic and mucosal immune responses have been detected.
The identification of an immunogenic and protective antigen(s) that can serve as a subunit or peptide vaccine has been a major focus of chlamydia research for almost three decades (9). There are eight major serologically defined chlamydial antigens recognized during human infection by immunoblotting analysis of sera from women with cervical C. trachomatis infections (7, 8, 58). The antigens range in size from 10 through 75 kDa, and most of the encoding genes have been cloned (8). The dnaK and groEL genes encode the 75- and 60-kDa heat shock proteins, respectively, while omp-1 and omp-2 encode the 40- and 57-kDa membrane proteins, respectively. The 40-kDa Omp-1 antigen, also called the MOMP, is regarded as the most promising vaccine candidate. First, MOMP is both highly immunogenic and immunoaccessible and elicits T-cell responses and neutralizing antibodies. Second, MOMP is the dominant surface protein (60% of the total protein mass in the outer membrane) and is expressed in all phases of the developmental cycle of Chlamydia (8, 10, 58). Functionally, MOMP contributes to the structural integrity of the chlamydial elementary body (EB) through disulfide bonding with other membrane components, and it is also a porin (6) as well as an adhesin (53). Typically, the MOMP (omp-1) gene has a 1,182-bp open reading frame which encodes a 394-amino-acid polypeptide with eight cysteine residues and a 22-amino-acid signal peptide, and it harbors two or more tandem promoters (50, 51). Comparative sequence analysis revealed that MOMP is 84 to 97% identical in nucleotides and amino acids among several C. trachomatis serovars, but variation in amino acid sequence is clustered into four variable sequence domains (VD1, VD2, VD3, and VD4) that are interspersed among invariable sequences (4, 14, 27). Immunologic analysis has shown that MOMP harbors extensive species- and genus-specific immunogenic epitopes, suggesting that a MOMP-based vaccine with either a narrow- or broad-spectrum effect is feasible (8). In previous MOMP-based vaccine studies, whole subunits, oligopeptides, or cloned recombinant fragments have been delivered with or without bacterial, viral, and phage vectors, and at best relatively low immunogenicity or partial protective immunity was observed (5, 8, 12, 19, 34, 39, 52, 55–57, 61, 63, 64). Previous reports indicated that ISCOMs have a predilection for inducing MHC class I-mediated, as well as T-cell-mediated, and humoral immune responses against several viruses in experimental immunogenicity and protection studies of mice, rabbits, dogs, pigs, calves, ewes, cats, dogs, and cotton-top tamarin and rhesus monkeys (33). Besides, intramuscular (IM) vaccination with MOMP-ISCOMs could boost the immunogenicity of a DNA vaccine comprising the MOMP and it enhanced protective immunity against pulmonary chlamydial infection (13). This suggests that the adjuvanticity of ISCOMs could foster T-cell-mediated immunity against intracellular pathogens such as C. trachomatis. In the present study, the immunogenicity of a MOMP-ISCOMs candidate vaccine was evaluated to determine its ability to induce a local genital Th1 response and to confer protection against chlamydial genital infection in mice. The results revealed that MOMP-ISCOMs delivered via certain routes are potent inducers of genital mucosal, specific, anti-chlamydial Th1 response and that T cells from immunized mice protected recipients from genital chlamydial infection.
MATERIALS AND METHODS
Chlamydia stocks and antigens.
Stocks of the human isolate of C. trachomatis serovar D used to infect mice in vivo were prepared by propagating EBs in McCoy cells, as previously described (45). The titers of the stocks of chlamydiae were determined by infecting McCoy cells with varying dilutions of EBs, and the infectious titer was expressed as the number of inclusion-forming units (IFU) per milliliter. Chlamydial antigen was prepared by purifying HeLa-grown EBs over renografin gradients, followed by inactivation under UV light for 3 h.
Animals, infection, and assessment of protective immunity.
Female BALB/c mice, 5 to 8 weeks old, were obtained from the Jackson Laboratory, Bar Harbor, Maine. All animals were fed with food and water ad libitum and maintained in laminar flow racks under pathogen-free conditions of 12-h light and 12-h darkness. Mice were challenged with C. trachomatis serovar D 24 h after the adoptive transfer of T cells by intravaginal infection with 104 IFU of chlamydiae per mouse in a volume of 30 μl of phosphate-buffered saline while under phenobarbitol anesthesia (45). The intensity of infection was monitored by periodic (every 3 days) cervicovaginal swabbing of individual animals and isolation of Chlamydia from swabs in tissue culture according to standard methods (45). Animals were monitored for 3 weeks, a time period that covers the acute phase of chlamydial infection in mice, when relatively high titers of chlamydiae are shed into the vaginocervical vault (40).
Preparation of MOMP-ISCOMs.
MOMP-ISCOMs of C. trachomatis serovar D were prepared as a proportionate admixture of purified MOMP and Quil A-based ISCOMs, as recently described (13). Briefly, Sarkosyl and dithiothreitol fractions of EBs were further separated by centrifugation at 15 × g for 1 h at 20°C, and the pellet composed of the outer membrane complexes was extracted with 10 mM phosphate buffer (Mega 10) and/or n-octylglucopyranoside at a total combined concentration of 1%. Following incubation, the soluble and insoluble fractions were separated by centrifugation at 150,000 × g for 1 h at 20°C. MOMP is the predominant protein component of the soluble fraction (>90%). To prepare MOMP-ISCOMs, the MOMP solution was diluted to 0.2 mg/ml with 10 mM phosphate buffer (pH 6.8). Phosphatidylcholine and cholesterol were dissolved at concentrations of 5 mg/ml each, and Quil A was added to a concentration of 1 mg/ml. A 20% Mega 10 solution was added to bring the final concentration in the mixture to 1%. The mixture was rocked at 20 to 25°C overnight and then dialyzed against three changes of 10 mM phosphate buffer for ∼8 to 16 h per change. When prepared by this method, ISCOMs are uniform particles ∼40 to 50 nm in diameter. Circular dichroism studies showed that the MOMP in the MOMP-ISCOMs preparation exists in a predominantly β-sheet conformation (A. Murdin and K. Sokoll, unpublished data).
Immunization protocol.
Eight groups of mice (six mice/group) were vaccinated three times every 3 weeks as follows: group 1 received 105 IFU of live serovar D by the intranasal (IN) route. Group 2 received 105 IFU of UV-inactivated serovar D by the IN route. Group 3 received 2 μg of MOMP by the IN route. Group 4 received 2 μg of MOMP-ISCOMs by the IN route. Group 5 received 0.2 μg of MOMP-ISCOMs by the IN route. Group 6 received ISCOMs alone by the IN route. Group 7 received 2 μg of MOMP-ISCOMs by the IM route via the hind limbs; group 8 mice received ISCOMs alone by the IM route. All preparations were delivered in 0.03 ml of phosphate-buffered saline.
Cytokine assays, monoclonal antibodies, and other reagents.
Enzyme-linked immunosorbent assay (ELISA) kits for quantitating the amounts of murine cytokines in biological and culture fluids were purchased from BioSource International, Camarillo, Calif. Isolation of chlamydia from cervicovaginal swabs in tissue culture was assayed by staining infected monolayers of McCoy cells with fluorescein isothiocyanate-labeled, genus-specific, anti-chlamydia antibodies (Kallestad Diagnostics, Chaska Minn.) to detect chlamydial inclusions by direct immunofluorescence (45).
Assessment of genital mucosal and systemic anti-chlamydia Th1 response.
The level of genital mucosal and systemic Th1 response was determined by measuring the response of chlamydia-specific, gamma interferon (IFN-γ)-secreting T cells from genital tissues and spleens of immunized mice, as previously described (24). Briefly, immune T-cell-enriched cells were prepared from the genital tract tissues of immunized and infected mice by the collagenase digestion and nylon wool enrichment method (23, 24) as follows: at the indicated time after immunization or infection, animals in each group were sacrificed, and the genital tract between the vagina and ovaries (i.e., the cervix, uterus, and fallopian tubes) was excised and placed in sterile HEPES (N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid)-buffered RPMI 1640 culture medium (Atlanta Biologicals, Norcross, Ga.). Explants were transferred to 7 ml of 0.6 mg of filter-sterilized type I collagenase (Atlanta Biologicals) per ml. The tissues were minced, incubated at 37°C for 45 to 60 min, then teased with forceps, and passed through a cell strainer. After being washed, the cells were enriched for T cells by the nylon wool adherence method (22, 23). Purified splenic or genital cells contained at least 90% CD3+ cells, as determined by fluorescence-activated cell sorter analysis. The level of response of chlamydia-specific cells induced was assessed by seeding purified cells into 96-well tissue culture plates (Costar, Cambridge, Mass.) at 105 cells per well with syngeneic antigen-presenting cells (2 × 105 cells per well), in the presence or absence of UV-inactivated EBs as antigen (at a multiplicity of infection of 5, EBs:APC). APCs were X-irradiated (2,000 rads) spleen cells from syngeneic wild-type mice. After 5 days of incubation in humidified incubators at 37°C and 5% CO2, the supernatants were collected and stored at −70°C until assayed for IFN-γ content. The amounts of IFN-γ contained in supernatants derived from culture-stimulated cells and controls were measured by specific ELISA assays. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± standard deviation [SD]) of triplicate cultures for each experiment. It was previously shown that culture-derived IFN-γ obtained by this procedure possesses biological activity as determined by the ability of IFN-γ-containing supernatants to protect L929 cells from infection by encephalomyocarditis virus (22).
Systemic Th1 or Th2 responses were determined by assaying the supernatants from stimulated splenic T cells isolated from immunized mice for either IFN-γ or interleukin-4 (IL-4), respectively, with the ELISA kits from the same supplier as previously described above.
Protection studies.
Five groups of mice (12 mice/group) were vaccinated three times every 3 weeks as follows: group 1 received 2 μg of MOMP by the IN route; group 2 had 2 μg of MOMP-ISCOMs by the IN route; group 3 had 2 μg of MOMP-ISCOMs by the IM route; and groups 4 and 5 received ISCOMs alone by the IN or IM route, respectively. Eleven days after the last immunization, T cells were isolated from the spleens by the nylon wool adherence method, and 25 × 106 cells were adoptively transferred into naive mice corresponding to each group. All mice were challenged intravaginally with 104 IFU of live serovar D after 24 h. Infections were monitored by cervicovaginal swabbing and isolation of live chlamydiae in tissue culture (45).
Statistical analysis. The levels of IFN-γ in samples from different experiments were analyzed and compared by performing a one- or two-tailed t test, and the relationship between different experimental groupings was assessed by analysis of variance. Minimal statistical significance was judged at a P value of <0.05.
RESULTS
Ability of a MOMP-ISCOMs vaccine candidate to induce local genital mucosal specific anti-chlamydia Th1 response.
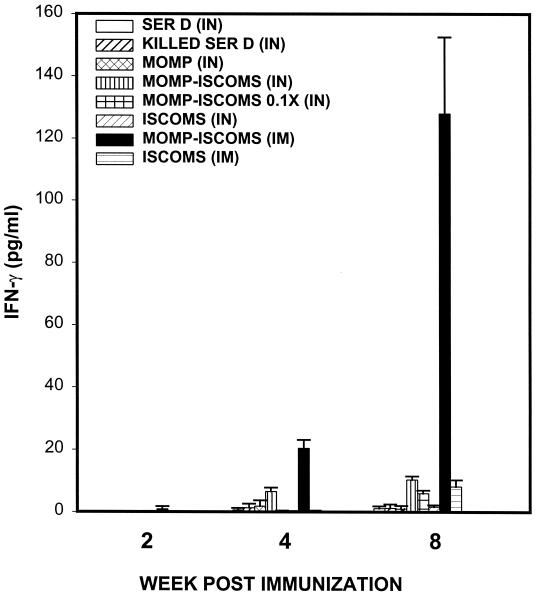
Purified T cells isolated from the genital tracts of mice immunized with C. trachomatis serovar D MOMP-ISCOMs and T cells from control groups were evaluated for levels of Th1 cells by in vitro restimulation and assessment of antigen-specific Th1 response, as previously described (21, 24). Immunization with MOMP-ISCOMs by the IM route induced the greatest and fastest local genital mucosal Th1 response, barely detectable (∼1.0 ± 0.6 pg/ml) by 2 weeks after exposure (Fig. 1), but increased to 20.45 and 127.95 pg/ml by 4 and 8 weeks, respectively. Of the other routes and regimens tested, only IN immunization with MOMP-ISCOMs induced detectable and statistically significant levels of local genital mucosal Th1 response (P < 0.001) that measured 6.56 ± 1.21 and 10.39 ± 1.1 pg of IFN-γ per ml at 4 and 8 weeks, respectively, postimmunization with 2.0 μg of MOMP-ISCOMs. Immunization with 0.2 μg of MOMP-ISCOMs produced a diluted specific anti-chlamydia Th1 effect of 0.2 and 5.98 pg of IFN-γ per ml during the same time periods, respectively. These data revealed that MOMP-ISCOMs vaccine formulation is capable of inducing genital mucosal anti-chlamydia Th1 response, suggesting that ISCOMs constitute an appropriate vehicle for the administration of a potential MOMP-based candidate vaccine against C. trachomatis. Also, these data support the hypothesis that the route of administration of a vaccine formulation plays a major role in the efficacy of a potential vaccine, as previously observed (24, 29).
FIG. 1.
Induction of specific genital mucosal Th1 response against C. trachomatis by immunization with MOMP-ISCOMs. Groups of mice (six mice/group) were vaccinated three times every 3 weeks with the indicated regimens, as described in Materials and Methods. The level of Th1 response induced and amount of Th1 recruitment into the genital mucosa were determined by measuring the response of chlamydia-specific, IFN-γ-secreting T cells from genital tract tissues of infected mice, as previously described (24). The antigen used in culture was UV-inactivated EBs (at a multiplicity of infection of 5 EBs:APC) in each stimulated well. The amounts of IFN-γ contained in supernatants derived from culture-stimulated cells and controls were measured by ELISA. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± SD) of triplicate cultures for each experiment. The control cultures without antigens did not contain detectable levels of the cytokine, and so the data were excluded from the results.
Ability of MOMP-ISCOMs to induce systemic anti-chlamydia Th1 response.
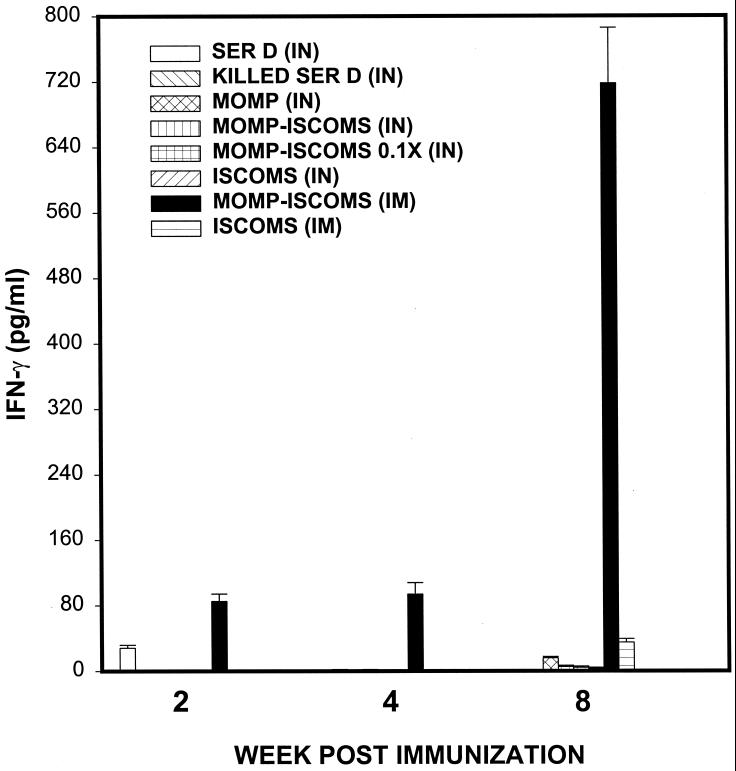
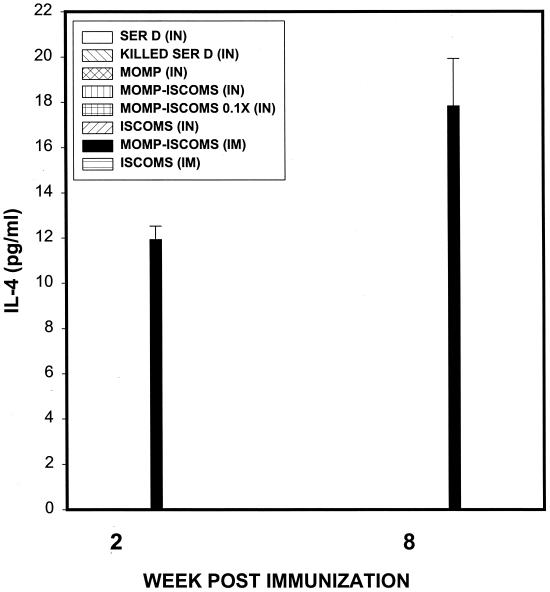
We determined whether IN or IM administration of MOMP-ISCOMs could also lead to the induction of systemic anti-chlamydia Th1 responses. The rationale is that clinical evaluation of the efficacy of anti-chlamydia immunity induced by a potential vaccine in humans is more likely and conveniently performed by analysis of systemic Th1 immune status than genital mucosal Th1 response. For the assessment of systemic anti-chlamydia Th1 response following immunization with MOMP-ISCOMs, splenic T cells from immunized mice were analyzed as previously described above. Figure 2 shows that IM immunization with MOMP-ISCOMs induced a specific systemic Th1 response measured by early (2 weeks) and late (8 weeks) IFN-γ levels of 85.16 ± 9.0 and 717.99 ± 67.88 pg/ml, respectively. Moreover, whereas the induction of mucosal Th2 response (measured by antigen-specific IL-4 production by stimulated T cells) was undetectable in genital tract tissues during this period (data not shown), systemic Th2 responses were detected early (11.93 ± 7.4 pg/ml) and late (17.83 ± 6.2 pg/ml) after IM immunization with MOMP-ISCOMs (Fig. 3). The data suggested that IM immunization with MOMP-ISCOMs is capable of inducing both genital mucosal and systemic anti-chlamydia Th1 responses. Thus, systemic evaluation of specific anti-chlamydia Th1 status following IM administration of a MOMP-ISCOMs vaccine could give an indication of the presence of mucosal anti-chlamydia Th1 response. While specific genital mucosal Th1 response has been correlated with resolution of genital chlamydial infection (24, 40, 47), it is uncertain whether the systemically detectable Th1 cells are functional clonotypes capable of conferring anti-chlamydia immunity and protecting the host against an infection.
FIG. 2.
Induction of specific systemic Th1 response against C. trachomatis by immunization with MOMP-ISCOMs. Groups of mice (six mice/group) were vaccinated three times every 3 weeks with the indicated regimens, as described in Materials and Methods. The level of Th1 response induced was determined by measuring the response of chlamydia-specific, IFN-γ-secreting T cells from spleen cells of infected mice (24). The amounts of IFN-γ contained in supernatants derived from culture-stimulated cells and controls were measured by ELISA. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± SD) of triplicate cultures for each experiment. The control cultures without antigens did not contain detectable levels of the cytokine, and so the data were excluded from the results.
FIG. 3.
Induction of specific systemic Th2 response against C. trachomatis by immunization with MOMP-ISCOMs. Eight groups of mice (six mice/group) were vaccinated three times every 3 weeks with the indicated regimens, as described in Materials and Methods. The level of Th2 response induced was determined by measuring the response of chlamydia-specific, IL-4-secreting T cells from spleen cells of infected mice, as previously described (24). The amounts of IL-4 contained in supernatants derived from culture-stimulated cells and controls were measured by ELISA. The concentration of the cytokine in each sample was obtained by extrapolation from a standard calibration curve generated simultaneously. Data were calculated as the mean values (± SD) of triplicate cultures for each experiment. The control cultures without antigens did not contain detectable levels of the cytokine, and so the data were excluded from the results.
Ability of systemically derived chlamydia-reactive T cells to confer adoptive immunity against genital chlamydial infection.
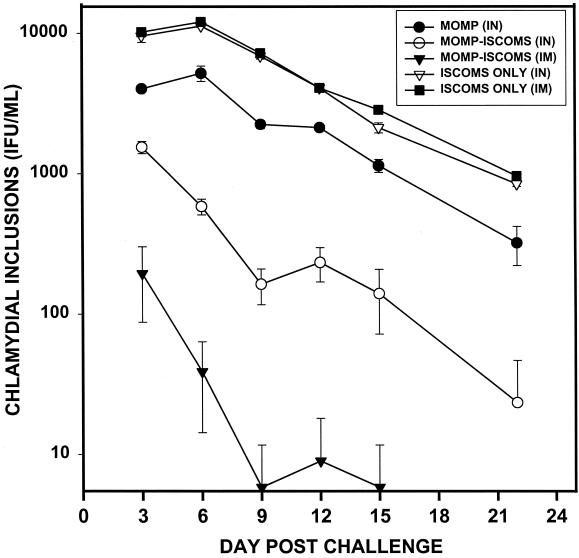
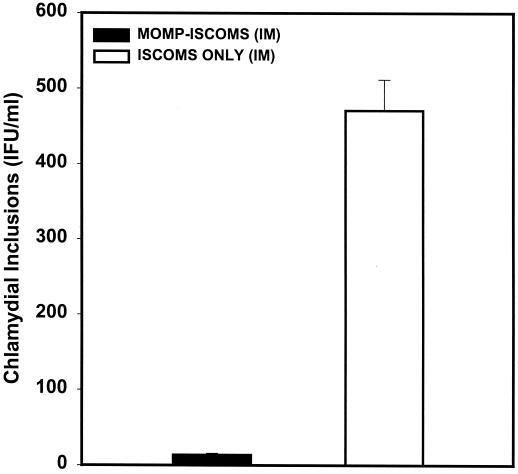
The ability of MOMP-ISCOMs to induce protective immunity that is measurable by systemic assessment of a specific Th1 response was determined by investigating the hypothesis that systemically derived chlamydia-reactive T cells from immunized animals showing vigorous mucosal Th1 response would transfer protection against C. trachomatis. Splenic T cells from immune mice were selected as systemically derived chlamydia-reactive T cells because the spleen is the major draining lymphoid tissue of the systemic circulation. Purified splenic T cells from mice immunized with MOMP (IN), MOMP-ISCOMs (IN), MOMP-ISCOMs (IM), ISCOMs (IN), or ISCOMs (IM) were adoptively transferred into naive animals corresponding to each immunization regimen, and after 24 h the animals were challenged intravaginally with homologous live C. trachomatis serovar D. The level of protective immunity was assessed by cervicovaginal swabbing of the animals and isolation of chlamydiae in tissue culture as previously described (45). As shown in Fig. 4, all recipients of T cells from IM MOMP-ISCOMs-immunized mice were highly resistant to infection, with 32.5, 6.5, 1.0, 4.2, 1.0, and 0.0 IFU/mouse on days 3, 6, 9, 12, 15, and 22 after challenge, as compared to the control group that received IM ISCOMs alone that had 2,041.0, 2,412.2, 1,446.5, 819.2, 571.1, and 192.1 IFU/mouse at the same time points. Recipients of T cells from IN MOMP-ISCOMs-immunized mice were moderately immune, although to a lesser degree. Finally, recipients of T cells from IN MOMP-immunized mice were protected to a limited extent that was statistically higher than controls that received T cells from mice immunized with IN ISCOMs alone (P < 0.01) but much lower than the recipients of T cells from mice immunized with either IN or IM MOMP-ISCOMs. The results revealed that IN immunization with MOMP could elicit T cells capable of conferring limited protection against C. trachomatis genital infection, but immunization with MOMP-ISCOMs offered better protection.
FIG. 4.
Ability of immune T cells from MOMP-ISCOMs-immunized mice to transfer protective immunity against genital chlamydial infection following adoptive transfer into naive animals. T cells were isolated from the spleens of mice immunized as indicated by the nylon wool adherence method, and 25 × 106 cells were adoptively transferred into naive mice corresponding to each group. All mice were challenged intravaginally with 104 IFU of live serovar D after 24 h. The infection was monitored by cervicovaginal swabbing and isolation of live chlamydiae in tissue culture by standard procedures (45).
To determine the duration of the protective immunity conferred by T cells from mice immunized with IM MOMP-ISCOMs, recipients and controls were challenged with a large dose of innoculum (105 IFU/mouse) 8 weeks after the primary infection. Results presented in Fig. 5 show that recipients of T cells from IM MOMP-ISCOMs-vaccinated mice enjoyed higher-level resistance to reinfection than control mice that received T cells from ISCOMs-vaccinated animals. These findings would suggest that IM immunization with MOMP-ISCOMs may elicit a Th1 response capable of conferring enduring protective immunity against C. trachomatis that would surpass the protection conferred by exposure to the primary infection alone.
FIG. 5.
Assessment of long-term protective immunity against genital chlamydial infection after the adoptive transfer of immune T cells from MOMP-ISCOMs-immunized mice. T cells were isolated from the spleens of mice immunized as indicated by the nylon wool adherence method, and 25 × 106 cells were adoptively transferred into naive mice corresponding to each group. All mice were challenged intravaginally with 104 IFU of live serovar D after 24 h. The infection was monitored by cervicovaginal swabbing and isolation of live chlamydiae in tissue culture by standard procedures (45). After 8 weeks, the mice were reinfected with a relative large amount of innoculum of the homologous strain of C. trachomatis (105 IFU/mouse), and the status of reinfection was assessed after 5 days, as described in Materials and Methods. A total of five to six animals were used per experimental group.
DISCUSSION
The development of protective vaccines that induce long-term local genital mucosal immunity is an important approach to preventing sexually transmitted diseases. In general, the efficacy of a vaccine is influenced by the immunogenicity of the antigen and the immune status of the host. In particular, the mucosal immune response to a vaccine directed at mucosally laden pathogens can be affected by additional factors that include the vector, adjuvant, delivery vehicle or route, and hormones associated with the estrous cycle (for genital mucosal response) (44, 59). Despite considerable efforts, and clinical and experimental evidence suggesting that at least partial protective immunity is feasible in humans (9, 17), the development of reliable chlamydia vaccines using conventional immunization strategies have proven to be elusive. Among other setbacks, vaccine effectiveness was relatively limited because of poor immunogenicity; more importantly, the use of inactivated whole-chlamydia agents appears to be unattractive due to likely immunopathogenic components (8).
Progress made in molecular and cellular immunology and genetic bioengineering in the last two decades has led to a gradual shift in the philosophy of vaccine development from classical whole vaccines consisting of inactivated organisms (i.e., rabies, pertussis, cholera, and Salk poliovirus vaccines) or live attenuated intact pathogens (i.e., measles, mumps, rubella, tuberculosis, and Sabin poliovirus vaccines) or their inactivated toxins (i.e., toxoids of tetanus and diphtheria). The new era of vaccinology focuses on epitope, peptide, oligosaccharide, oligoglycopeptide, or subunit vaccines, due partly to the availability of technology enabling the identification, isolation, and production of relevant antigenic determinants of a complex antigen as well as the mass production of such reagents for administration to humans. In a growing list of accomplishments in these new approaches to vaccinology, human subunit vaccines are currently available against pneumococci, meningococci, Haemophilus influenzae, and hepatitis B and influenza viruses. While the era of the epitope or subunit vaccines have obviated the concerns inherent in inactivated or live attenuated whole pathogen vaccines (35–37), including infectious, noxious, or integrating nucleic acid contents, induction of nonprotective blocking antibodies (41), epitope destruction during inactivation (16), and the presence of pathogenic antigenic determinants (18, 37), it has also encountered a major setback associated with the relatively poor immunogenicity of such preparations. Thus, the preference for epitopic or subunit vaccines has necessitated the search for more-efficient delivery vehicles, such as adjuvants, to boost immune responses against less-complex antigens.
The design of an immunization regimen capable of inducing sustained genital mucosal Th1 response is the current goal for a vaccine for humans to control the severe complications of genital infection by C. trachomatis (9, 26, 43, 60). In the present study, we evaluated the immunogenicity and efficacy of a candidate vaccine comprising the MOMP of the human isolate of C. trachomatis serovar D delivered with the lipophilic ISCOMs as a vehicle in a urine model of genital chlamydial infection. The results demonstrated that the MOMP-ISCOMs formulation is a highly immunogenic anti-chlamydia vaccine regimen, capable of inducing high levels of specific genital mucosal Th1 response. Th1 responses, including MHC class I-mediated CD8 T-cell function, are important for controlling several intracellular pathogens by mechanisms that include metabolic inhibition and cellular cytotoxicity (1, 15, 28). The ability of MOMP to induce a T-cell response has been previously established (2, 3, 25, 46, 52), and MOMP-ISCOMs could enhance the partial protection conferred by a MOMP-based DNA vaccine (13). However, our finding appears to be the first report of MOMP or a regimen containing a MOMP capable of inducing genital mucosal Th1 response. IN and more particularly IM immunizations were highly effective routes for MOMP-ISCOMs administration leading to the induction of high levels of genital mucosal Th1 response. Besides, although previous reports have indicated that MOMP delivered as a purified outer membrane protein, recombinant protein, or DNA vaccine could confer partial protection against certain complications of chlamydial infection (5, 55, 57, 61), the present study shows that MOMP-ISCOMs would constitute an efficacious vaccine formulation for inducing protective immunity against a primary genital chlamydial infection. Of special prognostic interest was the finding that of different vaccine regimens and routes of immunization investigated, only IM immunization with MOMP-ISCOMs induced system Th1 response that correlated in time and intensity with the presence of a genital mucosal Th1 response. This observation would suggest that systemic assessment of an anti-chlamydia Th1 response would constitute a reliable approach to following or determining the status of a genital mucosal Th1 response after vaccination with MOMP-ISCOMs. More so, the specific genital mucosal Th1 immune status of immunized patients is less likely to be conveniently determined by testing genital tract-derived T cells. However, assessment of a delayed-type hypersensitivity reaction by skin test and PCR analysis of vaginal and/or genital scrapings for expression of certain Th1-associated cytokines could be acceptable approaches.
To be of widespread attraction and application, the MOMP-ISCOMs vaccine regimen should not be toxic to humans, should elicit long-term protective immunity (preferably with cross-protection from other C. trachomatis serovars or species), and should possess no long-term adverse effects such as induction of autoreactive immune effectors. Detailed systematic studies will be required to establish the safety of ISCOM-based vaccines before their extension to humans. Also, it is unclear at this time whether the present technology for the experimental preparation of MOMP-ISCOMs is suitable for mass production for human use. Besides, MOMP is known to possess both genus- and species-specific epitopes (11, 20, 58, 62), which may include those capable of inducing cross-species Th1 responses, since partially protective cross-reactive cytotoxic T lymphocytes against C. trachomatis in mice have been generated (48). Furthermore, the inductive sites containing the appropriate APCs responsible for inducting Th1 cells that are recruited into the genital mucosa following IM or IN immunization with MOMP-ISCOMs are unknown. It is likely that the iliac lymph node and spleen, as well as dendritic cells present in these sites, may play a role in Th1 induction. Further studies will investigate these sites and analyze the molecular elements expressed on Th1 cells that foster their recruitment into the genital mucosa.
ACKNOWLEDGMENTS
This study was supported by a research support from Pasteur Merieux Connaught Canada, Toronto, Ontario M2R 3T4, Canada, and institutional research support from PHS grants AI41231, RR03034, GM08248, and RR011598 from the National Institutes of Health.
REFERENCES
- 1.Abbas A K, Williams M E, Burstein H J, Chang T L, Bossu P, Lichtman T. Activation of CD4+ T cell subsets. Immunol Rev. 1991;123:5–22. doi: 10.1111/j.1600-065x.1991.tb00603.x. [DOI] [PubMed] [Google Scholar]
- 2.Allen J E, Locksley R M, Stephens R S. A single peptide from the major outer membrane protein of Chlamydia trachomatis elicits T cell help for the production of antibodies to protective determinants. J Immunol. 1991;147:674–679. [PubMed] [Google Scholar]
- 3.Allen J E, Stephens R S. An intermolecular mechanism of T cell help for the production of antibodies to the bacterial pathogen, Chlamydial trachomatis. Eur J Immunol. 1993;23:1169–1172. doi: 10.1002/eji.1830230529. [DOI] [PubMed] [Google Scholar]
- 4.Baehr W, Zhang Y-X, Joseph T, Su H, Nano F E, Everett K D E, Caldwell H D. Mapping antigenic domains expressed by Chlamydia trachomatis major outer membrane protein genes. Proc Natl Acad Sci USA. 1988;85:4000–4004. doi: 10.1073/pnas.85.11.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Batteiger B E, Rank R G, Bavoil P M, Soderberg L S F. Partial protection against genital reinfection by immunization of guinea-pigs with isolated outer-membrane proteins of the chlamydial agent of guinea-pig inclusion conjunctivitis. J Gen Microbiol. 1993;139:2965–2972. doi: 10.1099/00221287-139-12-2965. [DOI] [PubMed] [Google Scholar]
- 6.Bavoil P, Ohlin A, Schachter J. Role of disulfide bonding in outer membrane structure and permeability in Chlamydia trachomatis. Infect Immun. 1984;44:479–485. doi: 10.1128/iai.44.2.479-485.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunham R C, Peeling R, Maclean I, McDowell J, Persson K, Osser S. Postabortal Chlamydia trachomatis salpingitis: correlating risk with antigen-specific serological responses and with neutralization. J Infect Dis. 1987;155:749–755. doi: 10.1093/infdis/155.4.749. [DOI] [PubMed] [Google Scholar]
- 8.Brunham R C, Peeling R W. Chlamydia trachomatis antigens: role in immunity and pathogenesis. Infect Agents Dis. 1994;3:218–233. [PubMed] [Google Scholar]
- 9.Byrne G I. Immunity to chlamydia. In: Stephens R S, Byrne G I, Christiansen G, Clarke I, N, Grayston J T, Rank R G, Ridgway G L, Saikku P, Schachter J, Stamm W E, editors. Chlamydial infections. Berkeley: University of California; 1998. pp. 365–374. [Google Scholar]
- 10.Caldwell H D, Kromhout J, Schachter J. Purification and partial characterization of the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1982;31:1161–1176. doi: 10.1128/iai.31.3.1161-1176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Conlan J W, Clarke I N, Ward M E. Epitope mapping with solid-phase peptides: identification of type-, subspecies-, species-, and genus-reactive antibody binding domains on the major outer membrane protein of Chlamydia trachomatis. Mol Microbiol. 1988;2:673–679. doi: 10.1111/j.1365-2958.1988.tb00076.x. [DOI] [PubMed] [Google Scholar]
- 12.Conlan J W, Ferris S, Clarke I N, Ward M E. Isolation of recombinant fragments of the major outer-membrane protein of Chlamydia trachomatis: their potential as subunit vaccines. J Gen Microbiol. 1990;136:2013–2020. doi: 10.1099/00221287-136-10-2013. [DOI] [PubMed] [Google Scholar]
- 13.Dong-Ji Z, Yang X, Shen C, Lu H, Murdin A, Brunham R C. Priming with Chlamydia trachomatis major outer membrane protein (MOMP) DNA followed by MOMP-ISCOM boosting enhances protection and is associated with increased immunoglobulin A and Th1 cellular immune responses. Infect Immun. 2000;68:3074–3078. doi: 10.1128/iai.68.6.3074-3078.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fitch W M, Peterson E M, de la Maza L M. Phylogenetic analysis of the outer membrane protein of Chlamydiae, and its implication for vaccine development. Mol Biol Evol. 1993;10:892–913. doi: 10.1093/oxfordjournals.molbev.a040048. [DOI] [PubMed] [Google Scholar]
- 15.Fresno M, Kopf M, Rivas L. Cytokines and infectious diseases. Immunol Today. 1997;18:56. doi: 10.1016/s0167-5699(96)30069-8. [DOI] [PubMed] [Google Scholar]
- 16.Fulginiti V A, Eller J J, Downie A W, Kempe C H. Altered reactivity to measles virus: atypical measles in children previously immunized with inactivated measles virus vaccines. JAMA. 1967;202:1075–1080. doi: 10.1001/jama.202.12.1075. [DOI] [PubMed] [Google Scholar]
- 17.Grayston J T, Wang S P. The potential for vaccine against infection of the genital tract with Chlamydia trachomatis. Sex Transm Dis. 1978;5:73–77. doi: 10.1097/00007435-197804000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Grayston J T, Wang S-P, Yeh L J, Kuo C. Importance of reinfection in the pathogenesis of trachoma. Rev Infect Dis. 1985;7:717–725. doi: 10.1093/clinids/7.6.717. [DOI] [PubMed] [Google Scholar]
- 19.Hayes L J, Conlan J W, Everson J S, Ward M E, Clarke I N. Chlamydia trachomatis major outer membrane protein epitopes expressed as fusions with LamB in an attenuated aroA strain of Salmonella typhimurium: their application as potential immunogens. J Gen Microbiol. 1991;137:1557–1564. doi: 10.1099/00221287-137-7-1557. [DOI] [PubMed] [Google Scholar]
- 20.Hayes L J, Pickett M A, Conlan J W, Ferris S, Everson J S, Ward M E, Clarke I N. The major outer-membrane proteins of Chlamydia trachomatis serovars A and B: intra-serovar amino acid changes do not alter specificities of serovar- and C subspecies-reactive antibody-binding domains. J Gen Microbiol. 1990;136:1559–1566. doi: 10.1099/00221287-136-8-1559. [DOI] [PubMed] [Google Scholar]
- 21.Igietseme J U, Ananaba G A, Bolier J, Bowers S, Moore T, Belay T, Lyn D, Black C M. The intercellular adhesion molecule type-1 is required for rapid activation of T helper type (Th1) lymphocytes that control early acute phase of genital chlamydial infection in mice. Immunology. 1999;98:510–519. doi: 10.1046/j.1365-2567.1999.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Igietseme J U, Ramsey K H, Magee D M, Williams D M, Kincy T J, Rank R G. Resolution of murine chlamydial genital infection by the adoptive transfer of a biovar-specific, Th1 lymphocyte clone. Reg Immunol. 1993;5:317–324. [PubMed] [Google Scholar]
- 23.Igietseme J U, Rank R G. Susceptibility to reinfection after a primary chlamydial genital infection is associated with a decrease of antigen-specific T cells in the genital tract. Infect Immun. 1991;59:1346–1351. doi: 10.1128/iai.59.4.1346-1351.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Igietseme J U, Uriri I M, Kumar S N, Ananaba G A, Ojior O O, Momodu I A, Candal D H, Black C M. Route of infection that induces a high intensity of gamma interferon-secreting T cells in the genital tract produces optimal protection against Chlamydia trachomatis infection in mice. Infect Immun. 1998;66:4030–4035. doi: 10.1128/iai.66.9.4030-4035.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishizaki M, Allen J E, Beatty P R, Stephens R S. Immune specificity of murine T cell lines to the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1992;60:3714–3718. doi: 10.1128/iai.60.9.3714-3718.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johansson M, Schon K, Ward M, Lycke N. Studies in knockout mice reveal that anti-chlamydial protection requires TH1 cells producing IFN-gamma: is this true for human? Scand J Immunol. 1997;46:546–552. doi: 10.1046/j.1365-3083.1997.d01-167.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaltenboeck B, Kousoulas K G, Storz J. Structures of and allelic diversity and relationships among the major outer membrane protein (ompA) genes of the four chlamydial species. J Bacteriol. 1993;175:487–502. doi: 10.1128/jb.175.2.487-502.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaufmann S H E. CD8+ lymphocytes in intracellular microbial infections. Immunol Today. 1988;9:168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- 29.Kelly K A, Robinson E A, Rank R G. Initial route of antigen administration alters the T-cell cytokine profile produced in response to the mouse pneumonitis biovar of Chlamydia trachomatis following genital infection. Infect Immun. 1996;64:4976–4983. doi: 10.1128/iai.64.12.4976-4983.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersten G F, Spiekstra A, Beuvery E C, Crommelin D J. On the structure of immune-stimulating saponin-lipid complexes (iscoms) Biochim Biophys Acta. 1991;1062:165–171. doi: 10.1016/0005-2736(91)90388-o. [DOI] [PubMed] [Google Scholar]
- 31.Kersten G F, Teerlink T, Derks H J, Verskleij A J, van Wezel T L, Crommelin D J, Beuvery E C. Incorporation of the major outer membrane protein of Neisseria gonorrhoeae in saponin-lipid complexes (iscoms): chemical analysis, some structural features, and comparison of their immunogenicity with three other antigen delivery systems. Infect Immun. 1988;56:432–438. doi: 10.1128/iai.56.2.432-438.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kersten G F, van de Put A M, Teerlink T, Beuvery E C, Crommelin D J. Immunogenicity of liposomes and iscoms containing the major outer membrane protein of Neisseria gonorrhoeae: influence of protein content and liposomal bilayer composition. Infect Immun. 1988;56:1661–1664. doi: 10.1128/iai.56.6.1661-1664.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kersten G F A, Crommelin D J A. Liposomes and ISCOMS as vaccine formulations. Biochim Biophys Acta. 1995;1241:117–138. doi: 10.1016/0304-4157(95)00002-9. [DOI] [PubMed] [Google Scholar]
- 34.Knight S C, Iqball S, Woods C, Stagg A, Ward M E, Tuffrey M. A peptide of Chlamydia trachomatis shown to be a primary T-cell epitope in vitro induces cell-mediated immunity in vivo. Immunology. 1995;85:8–15. [PMC free article] [PubMed] [Google Scholar]
- 35.Macadam A J, Pollard S R, Ferguson G, Skuce R, Wood D, Almond J W, Minor P D. Genetic basis of attenuation of the Sabin type 2 strain of poliovirus in primates. Virology. 1993;192:18–26. doi: 10.1006/viro.1993.1003. [DOI] [PubMed] [Google Scholar]
- 36.Minor P D. Attenuation and reversion of the Sabin vaccine strains of poliovirus. Dev Biol Stand. 1993;78:17–26. [PubMed] [Google Scholar]
- 37.Minor P D. The molecular biology of poliovaccines. J Gen Virol. 1992;73:3065–3077. doi: 10.1099/0022-1317-73-12-3065. [DOI] [PubMed] [Google Scholar]
- 38.Mowat A M, Donachie A M. ISCOMS—a novel strategy for mucosal immunization. Immunol Today. 1991;12:383–385. doi: 10.1016/0167-5699(91)90133-E. [DOI] [PubMed] [Google Scholar]
- 39.Murdin A D, Su H, Manning D S, Klein M H, Parnell M J, Caldwell H D. A poliovirus hybrid expressing a neutralization epitope from the major outer membrane protein of Chlamydia trachomatis is highly immunogenic. Infect Immun. 1993;61:4404–4414. doi: 10.1128/iai.61.10.4406-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patton D L, Rank R G. Animal models for the study of pelvic inflammatory disease. In: Quinn T C, editor. Sexually transmitted diseases. New York, N.Y: Raven Press, Ltd.; 1992. pp. 85–111. [Google Scholar]
- 41.Pedersen N C, Johnson L, Birch D, Theilen G H. Possible immunoenhancement of persistent viremia by feline leukemia virus envelope glycoprotein vaccines in challenge-exposure situations where whole inactivated virus vaccines were protective. Vet Immunol Immunopathol. 1986;11:123–148. doi: 10.1016/0165-2427(86)90093-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Peeters C C, Claassen I J, Schuller M, Kersten G F, van der Voort E M, Poolman J T. Immunogenicity of various presentation forms of PorA outer membrane protein of Neisseria meningitidis in mice. Vaccine. 1999;17:2702–2712. doi: 10.1016/s0264-410x(99)00011-0. [DOI] [PubMed] [Google Scholar]
- 43.Perry L L, Su H, Feilzer K, Messer R, Hughes S, Whitmire W, Caldwell H D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J Immunol. 1999;162:3541–3548. [PubMed] [Google Scholar]
- 44.Prabhala R H, Wira C R. Sex hormone and IL-6 regulation of antigen presentation in the female reproductive mucosal tissues. J Immunol. 1995;155:5566–5573. [PubMed] [Google Scholar]
- 45.Ramsey K H, Soderberg L S F, Rank R G. Resolution of chlamydial genital infection in B-cell-deficient mice and immunity to reinfection. Infect Immun. 1988;56:1320–1325. doi: 10.1128/iai.56.5.1320-1325.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stagg A J, Elsley W A J, Pickett M A, Ward M E, Knight S C. Primary human T-cell responses to the major outer membrane protein of Chlamydia trachomatis. Immunology. 1993;79:1–9. [PMC free article] [PubMed] [Google Scholar]
- 47.Stagg A J, Tuffrey M, Woods C, Wunderink E, Knight S C. Protection against ascending infection of the genital tract by Chlamydia trachomatis is associated with recruitment of major histocompatibility complex class II antigen-presenting cells into uterine tissue. Infect Immun. 1998;66:3535–3544. doi: 10.1128/iai.66.8.3535-3544.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Starnbach M N, Bevan M J, Lampe M F. Murine cytotoxic T lymphocytes induced following Chlamydia trachomatis intraperitoneal or genital tract infection respond to cells infected with multiple serovar. Infect Immun. 1995;63:3527–3530. doi: 10.1128/iai.63.9.3527-3530.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Steeghs L, Kuipers B, Hamstra H J, Kersten G, van Alphen L, van der Ley P. Immunogenicity of outer membrane proteins in a lipopolysaccharide-deficient mutant of Neisseria meningitidis: influence of adjuvants on the immune response. Infect Immun. 1999;67:4988–4993. doi: 10.1128/iai.67.10.4988-4993.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens R S, Mullenbach G, Sanchez-Pescador R, Agabian N. Sequence analysis of the major outer membrane protein gene from Chlamydia trachomatis serovar L2. J Bacteriol. 1986;168:1277–1282. doi: 10.1128/jb.168.3.1277-1282.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stephens R S, Wagar E A, Edman U. Development regulation of tandem promoters for the major outer membrane protein gene of Chlamydia trachomatis. J Bacteriol. 1988;170:744–750. doi: 10.1128/jb.170.2.744-750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Su H, Caldwell H D. Immunogenicity of a chimeric peptide corresponding to T helper and B cell epitopes of the Chlamydia trachomatis major outer membrane protein. J Exp Med. 1992;175:227–235. doi: 10.1084/jem.175.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Su H, Watkins N G, Zhang Y-X, Caldwell H D. Chlamydia trachomatis-host cell interactions: role of the chlamydial major outer membrane protein as an adhesin. Infect Immun. 1990;58:1017–1025. doi: 10.1128/iai.58.4.1017-1025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sundquist B G, Czifra G, Stipkovits L. Protective immunity induced in chicken by a single immunization with Mycoplasma gallisepticum immunostimulating complexes (ISCOMS) Vaccine. 1996;14:892–897. doi: 10.1016/0264-410x(95)00262-y. [DOI] [PubMed] [Google Scholar]
- 55.Taylor H R, Whittum-Hudson J, Schachter J, Caldwell H D, Prendergast R A. Oral immunization with chlamydial major outer membrane protein (MOMP) Investig Ophthalmol Vis Sci. 1988;29:1847–1853. [PubMed] [Google Scholar]
- 56.Toye B, Zhong G, Peeling R, Brunham R C. Immunologic characterization of a cloned fragment containing the species-specific epitope from the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1990;58:3909–3913. doi: 10.1128/iai.58.12.3909-3913.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tuffrey M, Alexander I, Conlan W, Woods C, Ward M. Hetrotypic protection of mice against chlamydial salpingitis and colonization of the lower genital tract with a human serovar F isolate of Chlamydia trachomatis by prior immunization with recombinant serovar L1 major outer membrane protein. Eur J Immunol. 1992;23:1169–1172. doi: 10.1099/00221287-138-8-1707. [DOI] [PubMed] [Google Scholar]
- 58.Ward M E. Chlamydial vaccine—future trends. J Infect. 1992;25(Suppl. 1):11–26. doi: 10.1016/0163-4453(92)91882-c. [DOI] [PubMed] [Google Scholar]
- 59.Wira C R, O'Mara B, Richardson J, Prabhala R. The mucosal immune system in the female reproductive tract: influence of sex hormones and cytokines on immune recognition and responses to antigen. Vaccine Res. 1992;1:151–167. [Google Scholar]
- 60.Yang X, Brunham R C. Role of T cell-mediated immunity in host defense against Chlamydia trachomatis and its implication for vaccine development. Can J Infect Dis. 1998;9:99. doi: 10.1155/1998/395297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang D J, Yang X, Berry J, Shen C, McClarty G, Brunham R C. DNA vaccination with the outer membrane protein gene induces acquired immunity to Chlamydia trachomatis (mouse pneumonitis) infection. J Infect Dis. 1997;176:1035–1040. doi: 10.1086/516545. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y-X, Stewart S J, Caldwell H D. Protective monoclonal antibodies to Chlamydia trachomatis serovar- and serogroup-specific major outer membrane protein determinants. Infect Immun. 1989;57:636–638. doi: 10.1128/iai.57.2.636-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhong G, Smith G P, Berry J, Brunham R C. Conformational mimicry of a chlamydial neutralization epitope on filamentous phage. J Biol Chem. 1994;269:24183–24188. [PubMed] [Google Scholar]
- 64.Zhong G, Toth I, Reid R, Brunham R C. Immunogenicity evaluation of a lipidic amino acid-based synthetic peptide vaccine for Chlamydia trachomatis. J Immunol. 1993;151:3728–3736. [PubMed] [Google Scholar]