Abstract
Clostridioides difficile is an obligate anaerobic pathogen among the most common causes of healthcare-associated infections. It poses a global threat due to the clinical outcomes of infection and resistance to antibiotics recommended by international guidelines for its eradication. In particular, C. difficile infection can lead to fulminant colitis associated with shock, hypotension, megacolon, and, in severe cases, death. It is therefore of the utmost urgency to fully characterize this pathogen and better understand its spread, in order to reduce infection rates and improve therapy success. This review aims to provide a state-of-the-art overview of the genetic variation of C. difficile, with particular regard to pathogenic genes and the correlation with clinical issues of its infection. We also summarize the current typing techniques and, based on them, the global distribution of the most common ribotypes. Finally, we discuss genomic surveillance actions and new genetic engineering strategies as future perspectives to make it less difficile.
Keywords: Clostridioides difficile, PaLoc, antibiotic resistance, worldwide spread, ribotypes, genomic surveillance, genetic engineering
1. Introduction
Clostridioides difficile, formerly known as Clostridium difficile [1], is an obligate anaerobic pathogen among the most common causes of healthcare-associated infections [2]. It was first described in 1935 by Hall and O’Toole, following isolation from a newborn’s stool, and initially called Bacillus difficilis due to the difficulty of cultivating it [3].
C. difficile is a Gram-positive, spore-forming, rod-shaped bacterium. Its spores are able to resist oxygen, heat, and common disinfectants (e.g., ethanol) thanks to their low water content and other properties, such as high levels of dipicolinic acid and saturation of DNA with soluble proteins [4,5,6,7].
This pathogen is transmitted via the fecal–oral route: if ingested, C. difficile spores withstand the acidic conditions of the stomach and then germinate in the intestine. This happens in the lower gastrointestinal tract where the oxygen concentration is lower [8]. Spore germination is triggered by certain compounds such as the primary bile acid, cholic acid, and cholesterol derivatives, including taurocholate. On the other hand, chenodeoxycholate is able to inhibit the germination of C. difficile spores [9,10,11]. Once germination has begun, the vegetative cells are able to colonize the colon and start producing toxins [12].
C. difficile generally produces two exotoxins, namely toxin A (TcdA) and toxin B (TcdB), which are both enterotoxic and cytotoxic. In addition, some C. difficile strains are capable of producing C. difficile transferase (CDT or binary toxin). These toxins damage the cytoskeleton of epithelial cells, causing breakdown of tight junctions, fluid secretion, and adhesion of neutrophils, thus leading to disruption of the intestinal barrier integrity, loss of function and local inflammation. In addition to toxin production, C. difficile virulence is also attributable to enzymes, such as collagenase, chondroitin-sulfatase, and hyaluronidase, which are capable of disrupting tight junctions as well, leading to fluid secretion and promoting inflammation [2,13,14].
With these prerequisites, the clinical picture of C. difficile infection (CDI) ranges from mild asymptomatic carrier status and diarrhea to fulminant colitis associated with shock, hypotension, or megacolon [15]. In the most severe cases of CDI, symptoms are critical (e.g., colon perforation, intestinal paralysis, septicemia) and can lead to death [15]. The mortality rate due to CDI is estimated at 5%, but the mortality associated with CDI complications ranges from 15% to 25% (up to 34% in intensive care units) [16,17,18].
Among all the potential risk factors for CDI, we can list increasing age (>65 years) and disorders such as inflammatory bowel disease, malignant tumors, diabetes mellitus and suppression of gastric acidity. However, the strongest risk factor for CDI is antibiotic therapy because it allows for the disruption of the gut barrier integrity [19]. In the study by Webb et al. [20], it is suggested that cumulative exposure to antibiotics, prior to admission for hospitalized patients, is the main contributor to CDI risk. Another risk concerns antibiotic classes: late-generation cephalosporins and carbapenems present a higher risk [21], but even relatively narrow-spectrum antibiotics, such as ampicillin, are associated with CDI. A recent work by Anjewierden et al. [22] has identified risks for asymptomatic CDI, such as hospitalization within six months prior to infection, nasogastric tube feeding, use of gastric acid suppression therapies, as well as use of corticosteroids in the previous eight weeks.
The gut microbiota is also known to play an important role in the pathogenesis of CDI. For example, the presence of bacterial consortia consisting of Porphyromonadaceae, Lachnospiraceae, Lactobacillus, and Alistipes might limit the growth of C. difficile [23]. On the other hand, in the case of dysbiosis exacerbated especially by the loss of primary fermenters, such as Lachnospiridae and Lactobacillus, a niche is established in which alternative primary fermenters such as Bacteroides thetaiotaomicron can proliferate. These microorganisms can produce various metabolic end-products that have been shown to be used by C. difficile as a carbon source [23,24,25]. Furthermore, some members of the gut microbiota are responsible for the generation of secondary bile acids, which, as anticipated above, can have an impact on the germination of C. difficile spore [23]. In particular, bacterial 7alpha-dehydroxylation of primary bile acids appears to protect against CDI [26], potentially representing a biomarker for a C. difficile-resistant environment. However, cefoperazone and other broad-spectrum antimicrobials have been shown to disrupt the bile acid-modifying activity of the gut microbiota, thus facilitating the colonization and outgrowth of C. difficile [27]. As described by Spigaglia et al. [28], the administration of certain antimicrobials (e.g., cephalosporins and carbapenems) is in fact more commonly associated with the induction of CDI than other pharmacological treatments.
One of the most common problems with CDI is recurrent CDI, which is generally associated with poor clinical outcomes. Some of the risk factors for recurrent CDI, recently described by Alrahmany et al. [29], are over 76 years of age, the total length of hospital stay (>7 days), previous exposure to clindamycin, and concomitant use of aztreonam.
Constituting one of the most important nosocomial infections, several guidelines have been developed to counter the spread of C. difficile [15,30]. However, ironically, the use of antibiotics is still considered first-line therapy. Of course, prolonged administration of antibiotics leads to the onset of antimicrobial resistance. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) has established epidemiological cut-off values (ECOFFs), i.e., values that distinguish wildtype microorganisms from those with phenotypically detectable acquired resistance mechanisms (non-wildtype) to a given pharmacological agent [31]. According to EUCAST data, in some cases, C. difficile shows minimum inhibitory concentration (MIC) values above the ECOFF limit, especially against antimicrobials recommended by European and American guidelines, such as vancomycin, fidaxomicin and metronidazole [31]. However, fortunately, the resistance to these antimicrobials remains quite rare (see paragraph on “Antimicrobial resistance genes”) [32,33]. It should also be noted that a correlation between vancomycin treatment to counteract intestinal colonization of C. difficile and the acquisition of vancomycin-resistant Enterococcus (VRE) species has been demonstrated [34,35,36]. In particular, Fujitani et al. [35] found that the rate of VRE exceeded 50% in CDI patients, and patients with VRE had a higher rate of co-infection with multi-drug resistant pathogens. In an epidemiological study of hospital-acquired CDI, involving intensive care units, nearly one-third of patients with CDI were found to be colonized with VRE [37]. The use of intravenous vancomycin or metronidazole could be the main contributing factor to the risk of VRE colonization or infection [35].
Several studies focusing on both human and animal isolates have suggested a possible role for animals as sources of C. difficile [38,39,40,41,42,43]. A small Italian study, conducted on 39 samples, identified 14 different C. difficile ribotypes (RTs, see paragraph “Molecular typing techniques for C. difficile strains”), of which four (i.e., RT020, RT078, RT106, RT126) were detected in both animal and human samples. It should be noted that RT018 and RT078 were the two most frequently identified RTs, making up nearly 50% of all animal and human strains [44]. However, transmission of C. difficile can occur not only in a hospital setting or via animals. There is indeed evidence that C. difficile spores can also persist in the food chain, from farm to fork [45].
In light of the serious health implications, it is of the utmost urgency to better understand how to treat and contain the spread of this pathogen. The present review aims to investigate the genetic variation of C. difficile pathogenic genes and their relationship with the clinical issues of CDI. Then, we discuss the current typing techniques and the global distribution of the most common RTs, putting forward some hypotheses on the absence of hypervirulent RTs in some countries. Finally, this review summarizes actual genomic surveillance with a focus on novel genetic engineering strategies to make it less difficile.
2. Genetic Evolution of C. difficile Virulence
The C. difficile genome is characterized by a high level of plasticity. In particular, the evolution of its virulence concerns a specific genomic region, namely the Pathogenicity Locus (PaLoc). PaLoc consists of 19.6 kb of a chromosomally located element [46], which includes tcdA and tcdB, the genes encoding the two main toxins (TcdA and TcdB, respectively). PaLoc also comprises three accessory genes, namely tcdR, tcdC and tcdE. Specifically, tcdR encodes a positive regulator of toxin expression, while tcdC a negative regulator. tcdE encodes a putative holin-like protein, responsible for the secretion of microbial toxins [46,47]. Another toxin produced by C. difficile is the binary toxin (CDT), encoded by the Ctd Locus (CdtLoc), a distinct chromosomal region that carries cdtA and cdtB, the genes for catalytic and binding/translocation proteins, as well as cdtR, coding for a regulatory protein. The CdtLoc can be found in two versions, whole or truncated. In strains lacking CdtLoc, a unique 68-bp sequence is found inserted in the same genomic location [48,49]. In non-toxigenic strains, such as the recently discovered NTCD-035 by Maslanka et al. [42], PaLoc is replaced by a conserved non-coding sequence of 115 bp [46,50,51,52]. Ongoing works on the comparative genomics of C. difficile are highlighting some important features on the evolution of these two pathogenic regions. The entire PaLoc region appears to have a modular structure and its variability may be due to the substitution of single nucleotides and to recombination events that played a pivotal role in the evolution of PaLoc variants. This structure also affects the tcdA and tcdB genes. Interestingly, the CdtLoc region appears to be more conserved than the PaLoc region, but it is mainly observed that full-length CdtLoc is associated with C. difficile strains exhibiting significantly altered PaLoc [53]. Mansfield et al. [54] highlighted a difference between tcdA and tcdB: while the evolutionary history of tcdB may depend on extensive homologous recombination, tcdA shows a greater degree of sequence variation and a greater number of subtypes [54]. Therefore, the authors suggest that the extreme recombination events observed in tcdB, but not in tcdA, could lead to increased selective pressure for tcdB diversification, highlighting the potential role of tcdB in the pathogenesis of C. difficile. This observation seems to be confirmed also by studies focused on the use of monoclonal antibodies (e.g., bezlototumab and actoxumab) both in animal models (i.e., gnotobiotic piglets) [55] and in humans [54,56,57].
One of the few works so far focused on the evolutionary history of C. difficile is a study by Dingle et al. [52], which paves the way for understanding the genomic background of this bacillus. According to the authors, PaLoc acquisition occurred at separate times between the C. difficile clades. Based on multi-locus sequence typing (MLST), C. difficile strains can be stratified into at least eight phylogenetic clades: clade 1–5 and clades C-I, C-II and C-III [58]. Clade 1 includes over 200 toxigenic and non-toxigenic sequence type (STs). Clade 2 also contains several highly virulent strains (e.g., ST1). Little is known about clade 3, although it includes ST5, a toxigenic CDT-producing strain [58]. Clade 4 contains ST37, which is responsible for much of the endemic CDI burden in Asia [59] despite the absence of the tcdA gene. Clade 5 contains several CDT-producing strains (e.g., ST11), which are highly prevalent in production animals worldwide [60]. Clades C-I, C-II, and C-III, known as “cryptic clades”, were first described in 2012 [52,61] and contain more than 50 STs [52,61,62,63,64]; however, their evolution remains poorly understood [58,63]. In the study by Dingle et al. [52], the authors suggest that each lineage acquired its current PaLoc variant after the divergence and that the common ancestor of all modern C. difficile strains may have been non-toxigenic. In particular, clade 1, which includes the greatest diversity of toxigenic genotypes, may exemplify the most ancient acquisition; this fact would also explain the emergence of non-toxigenic strains within this clade, as sufficient time has elapsed for occasional PaLoc losses to occur. Moreover, the most recent PaLoc loss event occurred about 30 years ago within a genotype belonging to clade 1. In contrast, clades 4 and 5 exemplify the most recent PaLoc acquisition (about 500 years ago), because of their narrow genotypic diversity [52].
3. Antimicrobial Resistance Genes
One of the major issues related to CDI (and recurrent CDI) is antimicrobial resistance, which plays a crucial role in the pathogenesis and spread of C. difficile [33]. Resistance to specific antimicrobials may actually support the spread of C. difficile, as in the case of tetracycline resistance in RT078 [65] and clindamycin resistance in RT017 [66]. Although resistance to antimicrobials recommended by guidelines for the treatment of CDI remains quite rare [32,33], these guidelines have been modified and reshaped over the years, also to address this issue. In particular, the first guidelines (1995–1997) were focused on the administration of vancomycin and metronidazole, while from 2014 to date they also include treatment with fidaxomicin [67]. Fortunately, most countries in a pan-European study [32] reported metronidazole, vancomycin and fidaxomicin resistance below 10% (most of them reported 0%), and a species-wide genomic study [33] could identify pCD-METRO plasmid (conferring metronidazole resistance) in only 15 of > 10,000 strains tested. Below, we discuss the antimicrobial resistance mechanisms for medications commonly recommended for the treatment of CDI. Regarding the mechanisms of resistance, C. difficile strains can encode a vanG-type gene cluster (vanGCd), conferring resistance to vancomycin, an antimicrobial glycopeptide that inhibits bacterial cell wall biosynthesis [67]. Specifically, resistance is conferred by the production of an alternative lipid II carrying a D-alanine-D-serine terminus that is seven times less bound by vancomycin than the D-alanine-D-alanine terminus [68]. Constitutive expression of vanGCd has been shown in vancomycin-resistant strains isolated in the clinical setting as well as in laboratory-generated mutants, which carry mutations in the two-component VanSR system that regulates vanGCd [68].
Metronidazole-resistant C. difficile strains are occasionally isolated in clinical practice, but some strains appear to need the heme cofactor to be detectable [69,70]. The authors suggest that C. difficile may use heme in oxidative stress responses to metronidazole, as a source of iron and cofactor for redox-associated proteins, as shown in other bacteria [70,71]. Metronidazole belongs to the nitroimidazole class of antimicrobials that can block the helical structure of DNA, leading to strand breakage, inhibition of protein synthesis and cell death [67]. As anticipated above, resistance to metronidazole appears to be mediated by a high-copy-number plasmid, pCD-METRO [72]. However, so far, there are no data available as to which plasmid gene may actually be responsible for the resistance. Another genetic mechanism involved in metronidazole resistance in clinical isolates is the modification of the catalytic domains of pyruvate-ferredoxin/flavodoxin oxidoreductase (PFOR), a protein encoded by the nifJ gene [73]. This modification is mediated by a synonymous codon change in putative xanthine dehydrogenase, which is likely to affect mRNA stability, and frameshift and point mutations that inactivate the iron-sulfur cluster regulator [73].
Another drug commonly used to treat CDI is fidaxomicin, a narrow-spectrum macrocyclic antimicrobial that inhibits RNA synthesis. This drug acts through lipiarmycin A3 (Lpm), its active component, which inhibits bacterial RNA polymerase [74]. Hence, resistance to fidaxomicin is conferred by mutations affecting RNA polymerase, especially the β-subunit of the RNA polymerase [75,76]. Alternative therapies for the treatment of CDI recommended by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), the Infectious Diseases Society of America (IDSA), and the Society for Healthcare Epidemiology of America (SHEA) [30,77] include rifaximin and tigecycline. Like fidaxomicin, rifaximin, which belongs to the rifamycin family, inhibits bacterial RNA polymerase, especially the β subunit [28,78]. The β subunit appears to be the primary site of mutations responsible for modifying the rifamycin binding pocket, thus limiting the interaction between the target and the antimicrobial [79]. Tigecycline, a glycylcycline chemically and structurally similar to tetracyclines, has been proposed for the treatment of CDI due to its ability to inhibit spore formation and reduce the toxin production by the pathogen [80]. It should be noted that some tet genes (i.e., tet(X3) and tet(X4), encoding a flavin-dependent monooxygenase), which [81,82,83,84,85,86,87] typically confer tetracycline resistance, also appear to mediate tigecycline resistance in various microorganisms of clinical relevance [81,82], thus challenging the clinical efficacy of the entire family of tetracycline antibiotics, including any derivatives.
4. Molecular Typing Techniques for C. difficile Strains
Several molecular methods are available for typing C. difficile, and routine typing is not performed with the same techniques across countries. The most widely used C. difficile typing technique is PCR ribotyping. Specifically, this technique consists in the amplification of the intergenic spacer region (ISR) of the 16S-23S rRNA gene using primers targeting the 3′ end of the 16S rRNA gene and the 5′ end of the 23S rRNA gene [83]. Due to its high discrimination capacity, PCR ribotyping is currently recommended by the European Centre for Disease Prevention and Control (ECDC) for surveillance of the C. difficile spread [84]. However, this molecular technique has a delivery time of up to one week and often requires in-house protocol optimization, thus not being fully transferable between laboratories [85]. Another technique in use is pulsed-field gel electrophoresis (PFGE). This technique is based on DNA digestion, through enzymatic restriction, and separation of DNA fragments on gel. This method therefore provides for the clonal assignment of the bacterial strain based on PFGE banding patterns [86]. The criticality of this method can be identified in the tendency of C. difficile DNA to rapid degradation, resulting in non-typable isolates. To overcome this problem, a modified PGFE method has been designed, with different plate cultures compared to the standard protocol, but its application is rare in light of the other methods adopted for molecular typing [87]. Among these, restriction endonuclease analysis (REA) is a technique based on the use of restriction enzymes (e.g., HindIII) but, unlike PFGE, the digestion fragments obtained are separated by classical electrophoresis on agarose or polyacrylamide gels [83]. Other noteworthy typing methods are Multilocus VNTR Analysis (MLVA) and MLST. Specifically, the target of MLVA are variable number tandem repeats (VNTR), disseminated throughout the genome, while MLST uses PCR amplification of housekeeping genes to generate a complete allelic profile [88].
Since there are a multitude of different typing techniques, a web-accessible database [89] (always updated) was set up in 2011 by Griffiths et al. [90]: they typed a total of 49 isolates by MLST and classified them into 40 STs. Since MLST and PCR ribotyping are very similar in discriminatory abilities, they found a correspondence between RTs and STs: multiple RTs for the same ST and multiple STs for the same RT usually had very similar profiles. Some STs correspond to a single RT (e.g., ST54/RT012), while others to multiple (e.g., ST02/RT014, RT020, RT076 and RT220). However, it should be noted that RTs were not always predictive of STs [90].
In recent years, with the advancement of whole genome sequencing (WGS) techniques, the scientific community is moving towards these methods instead of standard PCR ribotyping and is trying to develop new methods for the characterization of C. difficile strains [85,91]. The two main approaches to discover genomic variations are single nucleotide variant (SNV) analysis and core genome or whole genome MLST (cgMLST, or wgMLST). The first technique is based on comparing the differences in single nucleotide polymorphisms (SNPs) while the second is based on the analysis of multiple genes across the whole genome. Cg- or wgMLST typing works according to the same principles as the classic MLST [91]. Three publicly available schemes for C. difficile are available for cg- and/or wgMLST typing, and analysis can be performed using commercial software (e.g., BioNumerics, Ridom) or freely accessible online resources (e.g., EnteroBase). Eyre et al. [92] were the first to use WGS of C. difficile genomes on benchtop sequencing platforms to investigate its transmission, demonstrating that the use of these technologies could improve infection control and patient outcomes in routine clinical practice. Since then, WGS typing has been widely adopted for CDI surveillance and has revealed some novel insights concerning the spread of C. difficile [91].
5. Worldwide Distribution of C. difficile Ribotypes
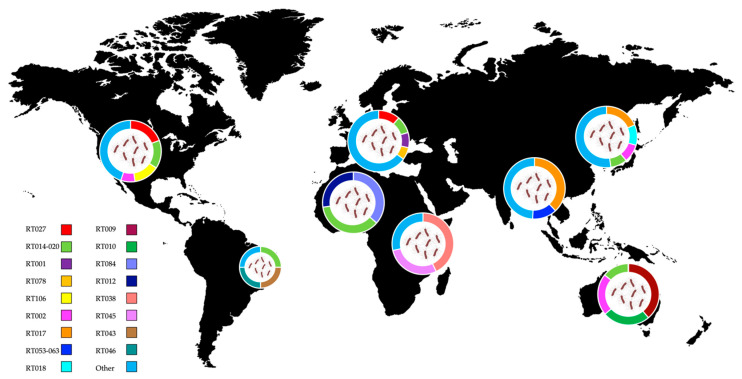
In the following chapters, we will offer a glimpse into the global distribution of the C. difficile RTs, divided by country. These data are summarized in Figure 1, while the toxin gene profile of the mentioned RTs is shown in Table S1.
Figure 1.
Distribution of C. difficile ribotypes in the United States, South America, Europe, North and Central Africa, Asia and Australia. Data are shown in pie charts as percentage, from studies covering different countries [93,94,95] or single country [96,97,98,99,100]. The smaller pie chart is representative of a single hospital study. The world map was obtained from Freepik.com. See also Table S1 for the toxin gene profile of listed ribotypes.
5.1. Europe
In Europe, several studies have highlighted a common genetic profile of C. difficile [93,101,102,103]. In particular, in 2021, a global study by Zhao et al. [101], based on data obtained from the MODIFY I (NCT01241552) and MODIFY II (NCT01513239) clinical studies using a WGS approach [104], found that in Europe there is a predominance of clade 1, with the exception of Poland where clade 2 predominates. Clade 2 was found to be one of the most virulent, along with clade 5. Interestingly, these two hypervirulent clades show the lowest recombination rates, while clade 3 and clade 4 show similar recombination rates [101]. Regarding the classification into RTs, clade 1 includes the non-toxigenic RT009, RT010, and RT039 [105], while the hypervirulent RT027 belongs to clade 2 [101]. The study conducted by Zhao et al. offers a broad view of the C. difficile genotype distribution at the global level but lacks comprehensive data. In fact, the study took into consideration only 1501 clinical isolates distributed globally. Another less recent but more accurate study analyzed 3499 isolates from 40 sites across Europe [93]. This study describes the 2011–2016 epidemiological framework. In particular, there was a well-defined predominance of RT027 during the five years, followed by RT001 in 2011 and RT014 in 2012, 2013 and 2014 (both RTs are toxigenic). In 2015, a predominance of RT014 was observed, followed by RT106 and RT002 [93]. These results appear to be in contrast with the observation by Zhao et al. [101], but they are probably more accurate due to the greater number of isolates analyzed. It is also worth mentioning the two works by Abdrabou et al., who considered Germany in the periods 2014–2019 and 2019–2021 [102,103]. The authors noted that in the first period there was a prevalence of RT027, with a decrease in the following years [102,103]. As discussed by the European Medicines Agency (EMA), the Centers for Disease Control and Prevention (CDC) and the U.S. Food and Drug Administration (FDA), such a decrease could be due to a potential reduction in fluoroquinolone administration [106,107,108]. Fluoroquinolones have in fact been associated with CDI outbreaks with RT027, which is highly resistant to them [109,110].
Similarly, comparing these results with other studies, a decrease in the prevalence of RT001 in Germany over time was observed [102,111,112]. It is also interesting to note that certain foods, such as potatoes, could be a vector for the introduction of C. difficile spores into the food chain or household environment. Indeed, potatoes have the highest rates of C. difficile contamination tested to date [113,114]. A recent study analyzed the positivity rate and distribution of RTs on potatoes in 12 European countries in the first half of 2018. Thirty-three of the 147 samples tested were positive for C. difficile, and the most common RTs were RT126, RT023, RT010, and RT014, in part overlapping what was discovered for human samples. The multiplicity of RTs was found to be substantial and the overlap between countries moderate.
5.2. America
In the USA, clinical specimens acquired from 2011 to 2015 showed a prevalence of RT027, belonging to the hypervirulent clade 2, as well as a prevalence of clade 1 [101]. Interestingly, the prevalence of clade 2 was higher on the East Coast and West Coast than inland. This observation was reversed for clade 1, which was more prevalent inland [101]. Another recent study [94] focusing on stool specimens recovered from 2011 to 2016 in the states of Illinois, Minnesota, New York, Massachusetts, California, and Virginia showed a marked decrease in the prevalence of RT027 over these six years in agreement with what was seen in Europe and Canada [94,112,115,116,117,118]. This decrease was also found in a 2020 study [119], covering the 2011–2018 period in Texas, where the most common RT was RT027, followed by RT014-020, RT106, and RT002. Curiously, the authors found a novel emerging RT, RT255 [119]. The complete RT255 genome has recently been made publicly available [120] and has occasionally been isolated [94]. However, its attributes and associated clinical outcomes are not yet well described [119].
Considering the transmission of C. difficile between humans and animals, an interesting study of samples collected from 13 Ohio swine farms (from farrowing rooms, nursery rooms and workers’ breakrooms) showed high contamination with toxigenic C. difficile [121]. The same C. difficile RTs recovered from most of the farm breakrooms were also recovered from at least one swine environment in those same farms. Furthermore, three RTs (i.e., RT078, RT005 and RT412) identified in the environment had previously been found in association with CDI in humans [122,123] and with animal-to-human transmission in Europe (i.e., Italy) [44]. Water and soil are also an important reservoir for this pathogen. At the Flagstaff site (Arizona), researchers found potential novel strains belongings to clades C-I, C-II and C-III and a hypothetical additional clade (C-V) [124].
In contrast to the literature describing the situation in North America, few recent articles are available on what concerns South America, mainly from Brazil. Regarding the latter, a study using MLST found that patient stool samples from different hospitals were positive for C. difficile distributed in 14 STs [125]. In particular, it was the first description of the STs 15 and 54 in the Brazilian country. ST15 has already been described in the UK [90], but as it is a non-toxigenic ST, there are not many data in the literature, while ST54 is already widespread in South America [125]. Unlike Europe and North America, the RT027 epidemic has never been reported in Brazil and the epidemiology of CDI is still underexplored. This is partly due to the lack of specialized technologies and facilities for the detection of obligate anaerobic bacteria, which is not a routine procedure in clinical laboratories [126]. However, numerous C. difficile RTs involved in CDI cases have been detected in Brazilian hospitals (e.g., RT014, RT043, RT046, RT106, RT132-135, RT142, and RT143) [96,126,127,128].
5.3. Asia and Middle East
While for Europe and North America there are numerous studies on the genetics and epidemiology of C. difficile strains, Asia appears to be split in half. For example, there is a lack of scientific reports on the prevalence of C. difficile in South Asian countries such as India, while these works appear to be abundant in East and Southeast Asian countries such as China and Japan. In India, a recent study using MLST on samples from a tertiary care center found that the most common STs were ST17, ST54, and ST63, with ST17 being the most prominent [129]. In Bangladesh, the first report on the prevalence of CDI in the hospital setting found that the most common RTs in stools (i.e., present in ≥10% of isolates) were RT017, RT053–163 (the same RTs found in environmental isolates), and a new RT (i.e., FP435) [130].
Furthermore, another study conducted in 2020 on the antimicrobial susceptibility of C. difficile in the 2014–2015 period in Asia-Pacific countries highlighted the prevalence of RT017 [95], confirming what was observed in a previous study [130]. Interestingly, the hypervirulent RT027 and RT078 have only rarely been isolated in the Asia-Pacific region [131,132,133,134]. In Japan, evidence suggests that the most common RT is RT018 [133,134,135,136]. In China, one of the dominant circulating RTs is RT017 [59,137,138]. However, another study published in 2021 on samples from different sources (e.g., soil, animals) found that the predominant RTs in China are RT001, RT046, and RT596 [139]. In 2021, the study by Zhao et al. found that the most common clade in the Asian country is clade 4 [101]. Furthermore, another report focusing on antibiotic resistance and molecular features of economic animals in China showed that RT126 is the most prevalent in Shandong province [140]. As for the Middle East region, RT001 is the most prevalent in Iran, followed by RT126, while RT258 is widespread in Qatar, RT139 in Kuwait and RT014 in Lebanon [141,142,143,144].
5.4. Oceania
Most of the data on the incidence of CDI come from Australia, the largest country in Oceania, particularly Western Australia. However, probably due to the variety of patient characteristics such as age, there is a discrepancy between the studies available to date.
For example, a study of C. difficile isolated from pediatric patients hospitalized in Perth, capital of Western Australia, in the period 2019–2020, reported a prevalence of RT002 (toxigenic) and RT009 (non-toxigenic) (on a total of 427 stool samples) [98]. On the other hand, a study conducted in the earlier period 2013–2018 in a geographically larger area covering 10 diagnostic laboratories from five states in Australia (Western Australia, New South Wales, Victoria, South Australia, and Queensland), reported 203 different RTs in predominantly elderly subjects, with RT014/020 being the most common, while RT027 and RT078 only rarely found [145]. Additionally, toxigenic RT014/020 was found to be more common among clinical cases in a tertiary hospital in Perth, while non-toxigenic RT010 was prevalent among floor samples and shoe soles of hospital staff, visitors and patients [146]. Outside of Australia, few research works are available. In particular, for New Zealand, a 2021 study reported that the two most common RTs were the same found in Australia (i.e., RT014, RT020) [147]. Interestingly, the dominance of RT014 has also been reported in Europe, particularly in 2015 [93]. The dominance of RT014 was also found in Auckland in 2014 [148]. In the study by Zhao et al., clade 1 was found to be the predominant one in Oceania [101].
5.5. Africa
In Africa, C. difficile is generally considered a minor pathogen, while the most common causative agents of diarrhea are Escherichia coli, Cryptosporidium, Shigella, and rotavirus [149]. No less important, there is a lack of recent literature on ribotyping and molecular characterization of C. difficile. For example, for Northern Africa, the latest PCR ribotyping report was released in 2018 in two Algerian hospitals, revealing the predominance of RT014, RT020 and non-toxigenic RT084, but only in 11 out of 159 stool samples collected between 2013 and 2015 [99]. In sub-Saharan Africa (i.e., Tanzania), a 2015 study identified RT038 among non-toxigenic strains, RT045 among toxigenic strains, and an unknown RT for two strains resulted positive for both tcdA and tcdB. Further analyses conducted on one of the two unknown strains highlighted a similarity with RT228 and RT043 [100]. Another study conducted in rural Ghana showed a high rate of non-toxigenic strains (e.g., RT084) isolated from patients with diarrhea and no hypervirulent strains [150]. A 2018 multi-centric cross-sectional study conducted between Germany, Ghana, Tanzania, and Indonesia showed that non-toxigenic strains were more abundant in Africa, with a prevalence of RT084 in the Ghanaian site and RT038 and RT045 in the Tanzanian site [151]. In light of this evidence, there appears to be a shortage of hypervirulent RTs in Africa (as opposed to Europe and the USA), while most of the CDIs found are attributable to non-toxigenic strains. Finally, it should be noted that in the Zimbabwe region, RT084 was the most common in human samples, while RT103, RT025, and RT070 were prevalent in chicken isolates, and RT025 and RT070 in soil samples [152]. Based on the study by Zhao et al., in South Africa, there is a prevalence of clade 1 [101].
6. Future Perspectives to Make It Less difficile
6.1. Genomic Surveillance
Recently, the COVID-19 pandemic has exposed the challenges for genomics in public health surveillance systems, given the rapid spread of new viral variants [153]. Genomic surveillance is transforming public health action by providing a deeper understanding of pathogens, their evolution and circulation. As mentioned earlier, new technologies in sequencing and bioinformatics have recently emerged and we have now reached a point where genomic surveillance has a clear role to play for public health. Specialized laboratory techniques such as WGS are increasingly used according to the WHO Global Surveillance 2022 report, in the investigation and acute management of diseases that could constitute public health emergencies, including cholera, influenza, Ebola virus disease, bacterial meningitis and poliomyelitis [154]. Public health responsiveness to pathogens could also benefit from larger-scale genomic surveillance, for example to monitor the occurrence and spread of C. difficile variants. However, for this to happen, several actions would be required. First, there is a strong need to improve access to tools for a better geographical representation of the pathogen spreading, sampling not only hospitalized patients with symptoms, but also environmental hotspots that can be enriched in human-derived pathogenic microorganisms, such as wastewater. Recently, sewage systems have gained increasing attention for health surveillance purposes due to their relevance in improving responsiveness to SARS-CoV-2 infection peaks, enabling early detection of viral loads and the emergence of new viral variants [155,156]. C. difficile is commonly found in raw sewage and survives the wastewater treatment process, thus being disseminated into the environment via effluent and land application of biosolids [157]. A genomic investigation conducted by Moradigaravand et al. [158] in 65 patients showed that CDI was attributable not to hospital-derived spores, but rather to strains isolated from effluent water from nearby treatment facilities, bolstering the need for bacterial genomic control in the environment [158]. This first action implies the need to strengthen the workforce to support this monitoring burden between countries and improve data sharing in a local-to-global fashion, enabling shared decision-making. Furthermore, the production of big data on a global scale encompassing genomic data, geographic origin, and spread of the isolates in the population will serve as a starting set to train a genomic-informed, real-time, global pathogen predictive model that might allow for greater control of infection outbreaks and conscious management of wastewater and other pathogen reservoir hotspots. Surveillance, outbreak detection, and response fully adhere to the One Health concept, which encompasses the domains of human, animal, and environmental health [159]. A deep global genomic characterization of C. difficile isolates could definitely help improve not only the profiling, but also the treatment of infected patients in a more rational, knowledge-based way.
6.2. Genetic Engineering
In recent years, a second-line approach leveraging synthetic biology is receiving particular attention, as an innovative strategy to limit C. difficile outbreaks by reducing its pathogenicity. Indeed, a wide range of tools are now available to precisely delete genes, change single nucleotides, complement deletions, integrate novel DNA, or overexpress genes [160]. Among these, the CRISPR/Cas system is of great interest for its ability to produce targeted modifications with high precision and efficiency, and to act on multiple strains [161]. CRISPR-mediated genome editing has been successfully implemented to tackle C. difficile infectious process, by hijacking the native system to introduce selective knock-outs [154] of several virulence factors involved in its pathogenicity in humans, deepening our knowledge over certain pathogenic processes [162]. For example, the study of the flagellin FliW-CsrA-fliC/FliC regulation mechanism implementing a CRISPR-based deletion of fliW, csrA and fliW-csrA genes demonstrated that the RNA-binding protein CsrA negatively modulates fliC expression, whilst FliW indirectly affects fliC expression through inhibition of CsrA post-transcriptional regulation [163].
Understanding C. difficile virulence regulation and pathogenicity factors through gene editing techniques also paves the way for efficient attenuation and possible therapeutic strategies. For example, toxicity and early-stage adhesion can be counteracted by knocking-out the tcdA and cpw84 genes, respectively [164]. While tcdA, as previously mentioned, encodes toxin A, cpw84 encodes a cell wall protein, the main protease of C. difficile, important in the early stages of colonization [164]. Another example of effective attenuation of C. difficile virulence was proposed by McAllister et al. [165], who produced a CRISPR-Cas9 mutant of C. difficile lacking the selenophosphate synthetase gene selD, resulting in a growth-deficient mutant [165]. Similarly, Wang et al. [166] achieved the complete deletion of the sporulation protein spo0A in a wildtype strain of C. difficile, undermining its ability to survive environmental stresses. Finally, Selle et al. [167] proposed an interesting delivery system for in vivo targeting of C. difficile, by developing a phage-delivered CRISPR/Cas3-mediated antimicrobial [167]. Phage infection was selective and apparently safe, activating a repurposing response of the endogenous CRISPR/Cas system that induced self-targeting endonuclease activity.
Such synthetic biology studies pave the way for new treatments in the field of personalized medicine, with in vivo strain-specific targeted genomic modifications to make C. difficile more vulnerable to therapies, thus providing the patient with a rapid and relapse-free recovery. Furthermore, such strategies can be virtually translated to other bacterial infections and could provide powerful and reliable strategies for overcoming the challenges of infections with multidrug-resistant microorganisms directly in the context of complex microbial communities in vivo.
7. Conclusions
This review gives a glimpse on the worldwide spread of C. difficile from a genetic standpoint. C. difficile is nowadays a global threat due to the clinical outcomes of its infection and growing concern about resistance to antimicrobials. From a methodological point of view, it should be remembered that C. difficile is not easy to grow and, although the ECDC guidelines suggest using PCR ribotyping as the main typing technique, many studies from different countries have been conducted with other methods, obviously challenging the overlap of results. Additionally, typing data are absent in Northern and Central Africa or some Asian countries, making the view of what C. difficile burden really represents even more fragmented. However, to date, as far as we know, there is a majority of toxigenic RTs in Western countries (e.g., RT027 in Europe), as well as in Asian countries (e.g., RT017), while in Africa, the most abundant strains are non-toxigenic (e.g., RT084). Failure to detect hypervirulent RTs could be explained by multiple factors, related to populations and their lifestyle, as well as poor typing effort. For example, as regards the African continent, CDI patients are often younger than in European and North American contexts, but typing data are rarely reported [168]. Furthermore, co-infection with the causative agents of tuberculosis or malaria has been reported to affect the virulence of C. difficile RTs [169], although the underlying mechanisms are unknown. Finally, the low availability of antimicrobials in some Asian and African countries and the suggested reduction in their use by international agencies [170,171] could certainly be linked to lower selective pressure on circulating C. difficile strains, potentially helping to foster the emergence of non-hypervirulent RTs.
Another relevant issue in CDI management is the lack of data on non-clinical transmission, i.e., through the food chain or the environment. In addition to a homogenization of methodological approaches, future studies should therefore aim at a better sampling and geographic representation of the C. difficile spread, in order to better understand (and interfere with) its transmission dynamics. With specific regard to therapies, in recent years, genome editing technologies such as the CRISPR/Cas system are demonstrating their potential in improving the susceptibility of C. difficile to treatments, thus opening up unprecedented opportunities for personalized medicine. Coupled with the mapping effort, advances in genomics and bioinformatics could lead to a better understanding of what this pathogen is and, more importantly, how to make it less difficile.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13122200/s1, Table S1: Toxin gene profile of the ribotypes mentioned in the main text.
Author Contributions
Writing—original draft preparation, M.M. and M.F.; writing—review and editing, M.B., S.T. and F.D.; visualization, M.M.; supervision, P.B., S.T. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lawson P.A., Citron D.M., Tyrrell K.L., Finegold S.M. Reclassification of Clostridium Difficile as Clostridioides Difficile (Hall and O’Toole 1935) Prévot 1938. Anaerobe. 2016;40:95–99. doi: 10.1016/j.anaerobe.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Czepiel J., Dróżdż M., Pituch H., Kuijper E.J., Perucki W., Mielimonka A., Goldman S., Wultańska D., Garlicki A., Biesiada G. Clostridium Difficile Infection: Review. Eur. J. Clin. Microbiol. Infect. Dis. 2019;38:1211–1221. doi: 10.1007/s10096-019-03539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hall I.C., O’Toole E. Intestinal Flora in New-Born Infants: With a Description of a New Pathogenic Anaerobe, Bacillus Difficilis. Am. J. Dis. Child. 1935;49:390–402. doi: 10.1001/archpedi.1935.01970020105010. [DOI] [Google Scholar]
- 4.Rodriguez-Palacios A., LeJeune J.T. Moist-Heat Resistance, Spore Aging, and Superdormancy in Clostridium Difficile. Appl. Environ. Microbiol. 2011;77:3085–3091. doi: 10.1128/AEM.01589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dawson L.F., Valiente E., Donahue E.H., Birchenough G., Wren B.W. Hypervirulent Clostridium Difficile PCR-Ribotypes Exhibit Resistance to Widely Used Disinfectants. PLoS ONE. 2011;6:e25754. doi: 10.1371/journal.pone.0025754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Setlow P. I Will Survive: DNA Protection in Bacterial Spores. Trends Microbiol. 2007;15:172–180. doi: 10.1016/j.tim.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Paredes-Sabja D., Shen A., Sorg J.A. Clostridium Difficile Spore Biology: Sporulation, Germination, and Spore Structural Proteins. Trends Microbiol. 2014;22:406–416. doi: 10.1016/j.tim.2014.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kochan T.J., Foley M.H., Shoshiev M.S., Somers M.J., Carlson P.E., Hanna P.C. Updates to Clostridium Difficile Spore Germination. J. Bacteriol. 2018;200:e00218-18. doi: 10.1128/JB.00218-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson K.H., Kennedy M.J., Fekety F.R. Use of Sodium Taurocholate to Enhance Spore Recovery on a Medium Selective for Clostridium Difficile. J. Clin. Microbiol. 1982;15:443–446. doi: 10.1128/jcm.15.3.443-446.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sorg J.A., Sonenshein A.L. Inhibiting the Initiation of Clostridium Difficile Spore Germination Using Analogs of Chenodeoxycholic Acid, a Bile Acid. J. Bacteriol. 2010;192:4983–4990. doi: 10.1128/JB.00610-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu C.W., Tsai P.J., Lee C.C., Ko W.C., Hung Y.P. Inhibition of Spores to Prevent the Recurrence of Clostridioides Difficile Infection—A Possibility or an Improbability? J. Microbiol. Immunol. Infect. 2021;54:1011–1017. doi: 10.1016/j.jmii.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Sandhu B.K., McBride S.M. Clostridioides Difficile. Trends Microbiol. 2018;26:1049–1050. doi: 10.1016/j.tim.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baktash A., Terveer E.M., Zwittink R.D., Hornung B.V.H., Corver J., Kuijper E.J., Smits W.K. Mechanistic Insights in the Success of Fecal Microbiota Transplants for the Treatment of Clostridium Difficile Infections. Front. Microbiol. 2018;9:1242. doi: 10.3389/fmicb.2018.01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smits W.K., Lyras D., Lacy D.B., Wilcox M.H., Kuijper E.J. Clostridium Difficile Infection. Nat. Rev. Dis. Prim. 2016;2:16020. doi: 10.1038/nrdp.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald L.C., Gerding D.N., Johnson S., Bakken J.S., Carroll K.C., Coffin S.E., Dubberke E.R., Garey K.W., Gould C.V., Kelly C., et al. Clinical Practice Guidelines for Clostridium Difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) Clin. Infect. Dis. 2018;66:e1–e48. doi: 10.1093/cid/cix1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sidler J.A., Battegay M., Tschudin-Sutter S., Widmer A.F., Weisser M. Enterococci, Clostridium Difficile and ESBL-Producing Bacteria: Epidemiology, Clinical Impact and Prevention in ICU Patients. Swiss Med. Wkly. 2014;144:w14009. doi: 10.4414/smw.2014.14009. [DOI] [PubMed] [Google Scholar]
- 17.Vincent J.L., Rello J., Marshall J., Silva E., Anzueto A., Martin C.D., Moreno R., Lipman J., Gomersall C., Sakr Y., et al. International Study of the Prevalence and Outcomes of Infection in Intensive Care Units. JAMA. 2009;302:2323–2329. doi: 10.1001/jama.2009.1754. [DOI] [PubMed] [Google Scholar]
- 18.Czepiel J., Kędzierska J., Biesiada G., Birczyńska M., Perucki W., Nowak P., Garlicki A. Epidemiology of Clostridium Difficile Infection: Results of a Hospital-Based Study in Krakow, Poland. Epidemiol. Infect. 2015;143:3235–3243. doi: 10.1017/S0950268815000552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adelman M.W., Woodworth M.H., Shaffer V.O., Martin G.S., Kraft C.S. Critical Care Management of the Patient with Clostridioides Difficile HHS Public Access. Crit. Care Med. 2021;49:127–139. doi: 10.1097/CCM.0000000000004739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webb B.J., Subramanian A., Lopansri B., Goodman B., Jones P.B., Ferraro J., Stenehjem E., Brown S.M. Antibiotic Exposure and Risk for Hospital-Associated Clostridioides Difficile Infection. Antimicrob. Agents Chemother. 2020;64:e02169-19. doi: 10.1128/AAC.02169-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watson T., Hickok J., Fraker S., Korwek K., Poland R.E., Septimus E. Evaluating the Risk Factors for Hospital-Onset Clostridium Difficile Infections in a Large Healthcare System. Clin. Infect. Dis. 2018;66:1957–1959. doi: 10.1093/cid/cix1112. [DOI] [PubMed] [Google Scholar]
- 22.Anjewierden S., Han Z., Brown A.M., Donskey C.J., Deshpande A. Risk Factors for Clostridioides Difficile Colonization among Hospitalized Adults: A Meta-Analysis and Systematic Review. Infect. Control Hosp. Epidemiol. 2021;42:565–572. doi: 10.1017/ice.2020.1236. [DOI] [PubMed] [Google Scholar]
- 23.Jukes C.A., Ijaz U.Z., Buckley A., Spencer J., Irvine J., Candlish D., Li J.V., Marchesi J.R., Douce G. Bile Salt Metabolism Is Not the Only Factor Contributing to Clostridioides (Clostridium) Difficile Disease Severity in the Murine Model of Disease. Gut Microbes. 2020;11:481–496. doi: 10.1080/19490976.2019.1678996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreyra J.A., Wu K.J., Hryckowian A.J., Bouley D.M., Weimer B.C., Sonnenburg J.L. Gut Microbiota-Produced Succinate Promotes C. Difficile Infection after Antibiotic Treatment or Motility Disturbance. Cell Host Microbe. 2014;16:770–777. doi: 10.1016/j.chom.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curtis M.M., Hu Z., Klimko C., Narayanan S., Deberardinis R., Sperandio V. The Gut Commensal Bacteroides Thetaiotaomicron Exacerbates Enteric Infection through Modification of the Metabolic Landscape. Cell Host Microbe. 2014;16:759–769. doi: 10.1016/j.chom.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aguirre A.M., Yalcinkaya N., Wu Q., Swennes A., Tessier M.E., Roberts P., Miyajima F., Savidge T., Sorg J.A. Bile Acid-Independent Protection against Clostridioides Difficile Infection. PLoS Pathog. 2021;17:e1010015. doi: 10.1371/journal.ppat.1010015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Theriot C.M., Bowman A.A., Young V.B. Antibiotic-Induced Alterations of the Gut Microbiota Alter Secondary Bile Acid Production and Allow for Clostridium Difficile Spore Germination and Outgrowth in the Large Intestine. mSphere. 2016;1:e00045-15. doi: 10.1128/mSphere.00045-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spigaglia P., Mastrantonio P., Barbanti F. Antibiotic Resistances of Clostridium Difficile. Adv. Exp. Med. Biol. 2018;1050:137–159. doi: 10.1007/978-3-319-72799-8_9. [DOI] [PubMed] [Google Scholar]
- 29.Alrahmany D., Ereshefsky B.J., el Nekidy W.S., Harb G., Pontiggia L., Ghazi I.M. Risk Factors for Recurrence of Clostridioides Difficile in Hospitalized Patients. J. Infect. Public Health. 2021;14:1642–1649. doi: 10.1016/j.jiph.2021.09.016. [DOI] [PubMed] [Google Scholar]
- 30.Van Prehn J., Reigadas E., Vogelzang E.H., Bouza E., Hristea A., Guery B., Krutova M., Norén T., Allerberger F., Coia J.E., et al. European Society of Clinical Microbiology and Infectious Diseases: 2021 Update on the Treatment Guidance Document for Clostridioides Difficile Infection in Adults. Clin. Microbiol. Infect. 2021;27:S1–S21. doi: 10.1016/j.cmi.2021.09.038. [DOI] [PubMed] [Google Scholar]
- 31.MIC EUCAST. [(accessed on 21 July 2022)]. Available online: https://mic.eucast.org/
- 32.Freeman J., Vernon J., Morris K., Nicholson S., Todhunter S., Longshaw C., Wilcox M.H., Pfeiffer S., Delmee M., Muytjens L., et al. Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium Difficile Ribotypes. Clin. Microbiol. Infect. 2015;21:248-e9. doi: 10.1016/j.cmi.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 33.Imwattana K., Rodríguez C., Riley T.V., Knight D.R. A Species-Wide Genetic Atlas of Antimicrobial Resistance in Clostridioides Difficile. Microb. Genom. 2021;7:696. doi: 10.1099/mgen.0.000696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jang M.-O., An J.H., Jung S.-I., Park K.-H. Refractory Clostridium Difficile Infection Cured With Fecal Microbiota Transplantation in Vancomycin-Resistant Enterococcus Colonized Patient. Intest. Res. 2015;13:80–84. doi: 10.5217/ir.2015.13.1.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujitani S., George W.L., Morgan M.A., Nichols S., Murthy A.R. Implications for Vancomycin-Resistant Enterococcus Colonization Associated with Clostridium Difficile Infections. Am. J. Infect. Control. 2011;39:188–193. doi: 10.1016/j.ajic.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 36.Özsoy S., İlki A. Detection of Vancomycin-Resistant Enterococci (VRE) in Stool Specimens Submitted for Clostridium Difficile Toxin Testing. Braz. J. Microbiol. 2017;48:489–492. doi: 10.1016/j.bjm.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marra A.R., Edmond M.B., Wenzel R.P., Bearman G.M.L. Hospital-Acquired Clostridium Difficile-Associated Disease in the Intensive Care Unit Setting: Epidemiology, Clinical Course and Outcome. BMC Infect. Dis. 2007;7:42. doi: 10.1186/1471-2334-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bauer M.P., Notermans D.W., van Benthem B.H., Brazier J.S., Wilcox M.H., Rupnik M., Monnet D.L., van Dissel J.T., Kuijper E.J. Clostridium Difficile Infection in Europe: A Hospital-Based Survey. Lancet. 2011;377:63–73. doi: 10.1016/S0140-6736(10)61266-4. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez C., Taminiau B., van Broeck J., Delmée M., Daube G. Clostridium Difficile in Food and Animals: A Comprehensive Review. Adv. Exp. Med. Biol. 2016;932:65–92. doi: 10.1007/5584_2016_27. [DOI] [PubMed] [Google Scholar]
- 40.Jhung M.A., Thompson A.D., Killgore G.E., Zukowski W.E., Songer G., Warny M., Johnson S., Gerding D.N., McDonald L.C., Limbago B.M. Toxinotype V Clostridium Difficile in Humans and Food Animals. Emerg. Infect. Dis. 2008;14:1039–1045. doi: 10.3201/eid1407.071641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Goorhuis A., Bakker D., Corver J., Debast S.B., Harmanus C., Notermans D.W., Bergwerff A.A., Dekker F.W., Kuijper E.J. Emergence of Clostridium Difficile Infection Due to a New Hypervirulent Strain, Polymerase Chain Reaction Ribotype 078. Clin. Infect. Dis. 2008;47:1162–1170. doi: 10.1086/592257. [DOI] [PubMed] [Google Scholar]
- 42.Freeman D.M. Invertible Carnot Groups. Anal. Geom. Metr. Spaces. 2014;2:248–257. doi: 10.2478/agms-2014-0009. [DOI] [Google Scholar]
- 43.Knetsch C.W., Connor T.R., Mutreja A., van Dorp S.M., Sanders I.M., Browne H.P., Harris D., Lipman L., Keessen E.C., Corver J., et al. Whole Genome Sequencing Reveals Potential Spread of Clostridium Difficile between Humans and Farm Animals in the Netherlands, 2002 to 2011. Eurosurveillance. 2014;19:20954. doi: 10.2807/1560-7917.ES2014.19.45.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tramuta C., Spigaglia P., Barbanti F., Bianchi D.M., Boteva C., di Blasio A., Zoppi S., Zaccaria T., Proroga Y.T.R., Chiavacci L., et al. Comparison of Clostridioides Difficile Strains from Animals and Humans: First Results after Introduction of C. Difficile Molecular Typing and Characterization at the Istituto Zooprofilattico Sperimentale of Piemonte, Liguria e Valle d’Aosta, Italy. Comp. Immunol. Microbiol. Infect. Dis. 2021;75:101623. doi: 10.1016/j.cimid.2021.101623. [DOI] [PubMed] [Google Scholar]
- 45.Marcos P., Whyte P., Rogers T., McElroy M., Fanning S., Frias J., Bolton D. The Prevalence of Clostridioides Difficile on Farms, in Abattoirs and in Retail Foods in Ireland. Food Microbiol. 2021;98:103781. doi: 10.1016/j.fm.2021.103781. [DOI] [PubMed] [Google Scholar]
- 46.Braun V., Hundsberger T., Leukel P., Sauerborn M., von Eichel-Streiber C. Definition of the Single Integration Site of the Pathogenicity Locus in Clostridium Difficile. Gene. 1996;181:29–38. doi: 10.1016/S0378-1119(96)00398-8. [DOI] [PubMed] [Google Scholar]
- 47.Martin-Verstraete I., Peltier J., Dupuy B. The Regulatory Networks That Control Clostridium Difficile Toxin Synthesis. Toxins. 2016;8:153. doi: 10.3390/toxins8050153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stare B.G., Delmée M., Rupnik M. Variant Forms of the Binary Toxin CDT Locus and TcdC Gene in Clostridium Difficile Strains. J. Med. Microbiol. 2007;56:329–335. doi: 10.1099/jmm.0.46931-0. [DOI] [PubMed] [Google Scholar]
- 49.Carter G.P., Lyras D., Allen D.L., Mackin K.E., Howarth P.M., O’Connor J.R., Rood J.I. Binary Toxin Production in Clostridium Difficile Is Regulated by CdtR, a LytTR Family Response Regulator. J. Bacteriol. 2007;189:7290. doi: 10.1128/JB.00731-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maslanka J.R., Gu C.H., Zarin I., Denny J.E., Broadaway S., Fett B., Mattei L.M., Walk S.T., Abt M.C. Detection and Elimination of a Novel Non-Toxigenic Clostridioides Difficile Strain from the Microbiota of a Mouse Colony. Gut Microbes. 2020;12:1851999. doi: 10.1080/19490976.2020.1851999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Monot M., Eckert C., Lemire A., Hamiot A., Dubois T., Tessier C., Dumoulard B., Hamel B., Petit A., Lalande V., et al. Clostridium Difficile: New Insights into the Evolution of the Pathogenicity Locus. Sci. Rep. 2015;5:15023. doi: 10.1038/srep15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dingle K.E., Elliott B., Robinson E., Griffiths D., Eyre D.W., Stoesser N., Vaughan A., Golubchik T., Fawley W.N., Wilcox M.H., et al. Evolutionary History of the Clostridium Difficile Pathogenicity Locus. Genome Biol. Evol. 2014;6:36–52. doi: 10.1093/gbe/evt204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janezic S., Dingle K., Alvin J., Accetto T., Didelot X., Crook D.W., Borden Lacy D., Rupnik M. Comparative Genomics of Clostridioides Difficile Toxinotypes Identifies Module-Based Toxin Gene Evolution. Microb. Genom. 2020;6:1–13. doi: 10.1099/mgen.0.000449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mansfield M.J., Tremblay B.J.M., Zeng J., Wei X., Hodgins H., Worley J., Bry L., Dong M., Doxey A.C. Phylogenomics of 8,839 Clostridioides Difficile Genomes Reveals Recombination-Driven Evolution and Diversification of Toxin A and B. PLoS Pathog. 2020;16:e1009181. doi: 10.1371/journal.ppat.1009181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Steele J., Mukherjee J., Parry N., Tzipori S. Antibody Against TcdB, but Not TcdA, Prevents Development of Gastrointestinal and Systemic Clostridium Difficile Disease. J. Infect. Dis. 2013;207:323–330. doi: 10.1093/infdis/jis669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gupta S.B., Mehta V., Dubberke E.R., Zhao X., Dorr M.B., Guris D., Molrine D., Leney M., Miller M., Dupin M., et al. Antibodies to Toxin B Are Protective Against Clostridium Difficile Infection Recurrence. Clin. Infect. Dis. 2016;63:730–734. doi: 10.1093/cid/ciw364. [DOI] [PubMed] [Google Scholar]
- 57.Rounds J., Strain J. Bezlotoxumab for Preventing Recurrent Clostridium Difficile Infections. J. South Dak. State Med. Assoc. 2017;70:422–423. [PubMed] [Google Scholar]
- 58.Knight D.R., Imwattana K., Kullin B., Guerrero-Araya E., Paredes-Sabja D., Didelot X., Dingle K.E., Eyre D.W., Rodríguez C., Riley T.V. Major Genetic Discontinuity and Novel Toxigenic Species in Clostridioides Difficile Taxonomy. Elife. 2021;10:e64325. doi: 10.7554/eLife.64325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Imwattana K., Knight D.R., Kullin B., Collins D.A., Putsathit P., Kiratisin P., Riley T.V. Clostridium Difficile Ribotype 017—Characterization, Evolution and Epidemiology of the Dominant Strain in Asia. Emerg. Microbes Infect. 2019;8:796–807. doi: 10.1080/22221751.2019.1621670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knight D.R., Kullin B., Androga G.O., Barbut F., Eckert C., Johnson S., Spigaglia P., Tateda K., Tsai P.J., Riley T.V. Evolutionary and Genomic Insights into Clostridioides Difficile Sequence Type 11: A Diverse Zoonotic and Antimicrobial-Resistant Lineage of Global One Health Importance. mBio. 2019;10:e00446-19. doi: 10.1128/mBio.00446-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Didelot X., Eyre D.W., Cule M., Ip C.L.C., Ansari M.A., Griffiths D., Vaughan A., O’Connor L., Golubchik T., Batty E.M., et al. Microevolutionary Analysis of Clostridium Difficile Genomes to Investigate Transmission. Genome Biol. 2012;13:R118. doi: 10.1186/gb-2012-13-12-r118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Janezic S., Potocnik M., Zidaric V., Rupnik M. Highly Divergent Clostridium Difficile Strains Isolated from the Environment. PLoS ONE. 2016;11:e0167101. doi: 10.1371/journal.pone.0167101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramírez-Vargas G., López-Ureña D., Badilla A., Orozco-Aguilar J., Murillo T., Rojas P., Riedel T., Overmann J., González G., Chaves-Olarte E., et al. Novel Clade C-I Clostridium Difficile Strains Escape Diagnostic Tests, Differ in Pathogenicity Potential and Carry Toxins on Extrachromosomal Elements. Sci. Rep. 2018;8:13951. doi: 10.1038/s41598-018-32390-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramirez-Vargas G., Rodriguez C. Putative Conjugative Plasmids with TcdB and CdtAB Genes in Clostridioides Difficile. Emerg. Infect. Dis. 2020;26:2287. doi: 10.3201/eid2609.191447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dingle K.E., Didelot X., Quan T.P., Eyre D.W., Stoesser N., Marwick C.A., Coia J., Brown D., Buchanan S., Ijaz U.Z., et al. A Role for Tetracycline Selection in Recent Evolution of Agriculture-Associated Clostridium Difficile PCR Ribotype 078. MBio. 2019;10:e02790-18. doi: 10.1128/mBio.02790-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kuijper E., Weerdt J., Kato H., Kato N., Dam A., Vorm E., Weel J., Rheenen C., Dankert J. Nosocomial Outbreak of Clostridium Difficile-Associated Diarrhoea Due to a Clindamycin-Resistant Enterotoxin A-Negative Strain. Eur. J. Clin. Microbiol. Infect. Dis. 2001;20:528–534. doi: 10.1007/s100960100550. [DOI] [PubMed] [Google Scholar]
- 67.Chaar A., Feuerstadt P. Evolution of Clinical Guidelines for Antimicrobial Management of Clostridioides Difficile Infection. Ther. Adv. Gastroenterol. 2021;14:17562848211011953. doi: 10.1177/17562848211011953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shen W.J., Deshpande A., Hevener K.E., Endres B.T., Garey K.W., Palmer K.L., Hurdle J.G. Constitutive Expression of the Cryptic VanGCd Operon Promotes Vancomycin Resistance in Clostridioides Difficile Clinical Isolates. J. Antimicrob. Chemother. 2020;75:859–867. doi: 10.1093/jac/dkz513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Boekhoud I.M., Sidorov I., Nooij S., Harmanus C., Bos-Sanders I.M.J.G., Viprey V., Spittal W., Clark E., Davies K., Freeman J., et al. Haem Is Crucial for Medium-Dependent Metronidazole Resistance in Clinical Isolates of Clostridioides Difficile. J. Antimicrob. Chemother. 2021;76:1731–1740. doi: 10.1093/jac/dkab097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu X., Shen W.J., Deshpande A., Olaitan A.O., Palmer K.L., Garey K.W., Hurdle J.G. The Integrity of Heme Is Essential for Reproducible Detection of Metronidazole-Resistant Clostridioides Difficile by Agar Dilution Susceptibility Tests. J. Clin. Microbiol. 2021;59:e0058521. doi: 10.1128/JCM.00585-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Giardina G., Rinaldo S., Johnson K.A., di Matteo A., Brunori M., Cutruzzolà F. NO Sensing in Pseudomonas Aeruginosa: Structure of the Transcriptional Regulator DNR. J. Mol. Biol. 2008;378:1002–1015. doi: 10.1016/j.jmb.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 72.Boekhoud I.M., Hornung B.V.H., Sevilla E., Harmanus C., Bos-Sanders I.M.J.G., Terveer E.M., Bolea R., Corver J., Kuijper E.J., Smits W.K. Plasmid-Mediated Metronidazole Resistance in Clostridioides Difficile. Nat. Commun. 2020;11:598. doi: 10.1038/s41467-020-14382-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Deshpande A., Wu X., Huo W., Palme K.L., Hurdl J.G. Chromosomal Resistance to Metronidazole in Clostridioides Difficile Can Be Mediated by Epistasis between Iron Homeostasis and Oxidoreductases. Antimicrob. Agents Chemother. 2020;64:e00415-20. doi: 10.1128/AAC.00415-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lin W., Das K., Degen D., Mazumder A., Duchi D., Wang D., Ebright Y.W., Ebright R.Y., Sineva E., Gigliotti M., et al. Structural Basis of Transcription Inhibition by Fidaxomicin (Lipiarmycin A3) Mol. Cell. 2018;70:60–71. doi: 10.1016/j.molcel.2018.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Babakhani F., Seddon J., Sears P. Comparative Microbiological Studies of Transcription Inhibitors Fidaxomicin and the Rifamycins in Clostridium Difficile. Antimicrob. Agents Chemother. 2014;58:2934–2937. doi: 10.1128/AAC.02572-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Babakhani F., Gomez A., Robert N., Sears P. Killing Kinetics of Fidaxomicin and Its Major Metabolite, OP-1118, against Clostridium Difficile. J. Med. Microbiol. 2011;60:1213–1217. doi: 10.1099/jmm.0.029470-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Johnson S., Lavergne V., Skinner A.M., Gonzales-Luna A.J., Garey K.W., Kelly C.P., Wilcox M.H. Clinical Practice Guideline by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA): 2021 Focused Update Guidelines on Management of Clostridioides Difficile Infection in Adults. Clin. Infect. Dis. 2021;73:755–757. doi: 10.1093/cid/ciab718. [DOI] [PubMed] [Google Scholar]
- 78.Peng Z., Jin D., Kim H.B., Stratton C.W., Wu B., Tang Y.W., Suna X. Update on Antimicrobial Resistance in Clostridium Difficile: Resistance Mechanisms and Antimicrobial Susceptibility Testing. J. Clin. Microbiol. 2017;55:1998. doi: 10.1128/JCM.02250-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baines S.D., Wilcox M.H. Antimicrobial Resistance and Reduced Susceptibility in Clostridium Difficile: Potential Consequences for Induction, Treatment, and Recurrence of C. Difficile Infection. Antibiotics. 2015;4:267–298. doi: 10.3390/antibiotics4030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlson T.J., Gonzales-Luna A.J. Antibiotic Treatment Pipeline for Clostridioides Difficile Infection (CDI): A Wide Array of Narrow-Spectrum Agents. Curr. Infect. Dis. Rep. 2020;22:20. doi: 10.1007/s11908-020-00730-1. [DOI] [Google Scholar]
- 81.Sun J., Chen C., Cui C.Y., Zhang Y., Liu X., Cui Z.H., Ma X.Y., Feng Y., Fang L.X., Lian X.L., et al. Plasmid-Encoded Tet(X) Genes That Confer High-Level Tigecycline Resistance in Escherichia Coli. Nat. Microbiol. 2019;4:1457–1464. doi: 10.1038/s41564-019-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He T., Wang R., Liu D., Walsh T.R., Zhang R., Lv Y., Ke Y., Ji Q., Wei R., Liu Z., et al. Emergence of Plasmid-Mediated High-Level Tigecycline Resistance Genes in Animals and Humans. Nat. Microbiol. 2019;4:1450–1456. doi: 10.1038/s41564-019-0445-2. [DOI] [PubMed] [Google Scholar]
- 83.Collins D.A., Elliott B., Riley T.V. Molecular Methods for Detecting and Typing of Clostridium Difficile. Pathology. 2015;47:211–218. doi: 10.1097/PAT.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 84.Krutova M., Kinross P., Barbut F., Hajdu A., Wilcox M.H., Kuijper E.J., Allerberger F., Delmée M., van Broeck J., Vatcheva-Dobrevska R., et al. How to: Surveillance of Clostridium Difficile Infections. Clin. Microbiol. Infect. 2018;24:469–475. doi: 10.1016/j.cmi.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 85.Seth-Smith H.M.B., Biggel M., Roloff T., Hinic V., Bodmer T., Risch M., Casanova C., Widmer A., Sommerstein R., Marschall J., et al. Transition From PCR-Ribotyping to Whole Genome Sequencing Based Typing of Clostridioides Difficile. Front. Cell Infect. Microbiol. 2021;11:681518. doi: 10.3389/fcimb.2021.681518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neoh H.M., Tan X.E., Sapri H.F., Tan T.L. Pulsed-Field Gel Electrophoresis (PFGE): A Review of the “Gold Standard” for Bacteria Typing and Current Alternatives. Infect. Genet. Evol. 2019;74:103935. doi: 10.1016/j.meegid.2019.103935. [DOI] [PubMed] [Google Scholar]
- 87.Alonso R., Martín A., Peláez T., Marín M., Rodríguez-Creixéms M., Bouza E. An Improved Protocol for Pulsed-Field Gel Electrophoresis Typing of Clostridium Difficile. J. Med. Microbiol. 2005;54:155–157. doi: 10.1099/jmm.0.45808-0. [DOI] [PubMed] [Google Scholar]
- 88.Pérez-Losada M., Cabezas P., Castro-Nallar E., Crandall K.A. Pathogen Typing in the Genomics Era: MLST and the Future of Molecular Epidemiology. Infect. Genet. Evol. 2013;16:38–53. doi: 10.1016/j.meegid.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 89.PubMLST Clostridioides Difficile. [(accessed on 23 September 2022)]. Available online: https://pubmlst.org/organisms/clostridioides-difficile.
- 90.Griffiths D., Fawley W., Kachrimanidou M., Bowden R., Crook D.W., Fung R., Golubchik T., Harding R.M., Jeffery K.J.M., Jolley K.A., et al. Multilocus Sequence Typing of Clostridium Difficile. J. Clin. Microbiol. 2010;48:770–778. doi: 10.1128/JCM.01796-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Janezic S., Rupnik M. Development and Implementation of Whole Genome Sequencing-Based Typing Schemes for Clostridioides Difficile. Front. Public Health. 2019;7:309. doi: 10.3389/fpubh.2019.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Eyre D.W., Golubchik T., Gordon N.C., Bowden R., Piazza P., Batty E.M., Ip C.L.C., Wilson D.J., Didelot X., O’Connor L., et al. A Pilot Study of Rapid Benchtop Sequencing of Staphylococcus Aureus and Clostridium Difficile for Outbreak Detection and Surveillance. BMJ Open. 2012;2:e001124. doi: 10.1136/bmjopen-2012-001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Freeman J., Vernon J., Pilling S., Morris K., Nicolson S., Shearman S., Clark E., Palacios-Fabrega J.A., Wilcox M. Five-Year Pan-European, Longitudinal Surveillance of Clostridium Difficile Ribotype Prevalence and Antimicrobial Resistance: The Extended ClosER Study. Eur. J. Clin. Microbiol. Infect. Dis. 2020;39:169–177. doi: 10.1007/s10096-019-03708-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Snydman D.R., McDermott L.A., Jenkins S.G., Goldstein E.J.C., Patel R., Forbes B.A., Johnson S., Gerding D.N., Thorpe C.M., Walk S.T. Epidemiologic Trends in Clostridioides Difficile Isolate Ribotypes in United States from 2011 to 2016. Anaerobe. 2020;63:102185. doi: 10.1016/j.anaerobe.2020.102185. [DOI] [PubMed] [Google Scholar]
- 95.Lew T., Putsathit P., Sohn K.M., Wu Y., Ouchi K., Ishii Y., Tated K., Riley T.V., Collins D.A. Antimicrobial Susceptibilities of Clostridium Difficile Isolates from 12 Asia-Pacific Countries in 2014 and 2015. Antimicrob. Agents Chemother. 2020;64:e00296-20. doi: 10.1128/AAC.00296-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Secco D.A., Balassiano I.T., Boente R.F., Miranda K.R., Brazier J., Hall V., dos Santos-Filho J., Lobo L.A., Nouér S.A., Domingues R.M.C.P. Clostridium Difficile Infection among Immunocompromised Patients in Rio de Janeiro, Brazil and Detection of Moxifloxacin Resistance in a Ribotype 014 Strain. Anaerobe. 2014;28:85–89. doi: 10.1016/j.anaerobe.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 97.Sofjan A.K., Islam M.A., Halder K., Kabir N.D., Saleh A.A., Miranda J., Lancaster C., Begum K., Alam M.J., Garey K.W. Molecular Epidemiology of Toxigenic Clostridioides Difficile Isolates from Hospitalized Patients and the Hospital Environment in Dhaka, Bangladesh. Anaerobe. 2020;61:102081. doi: 10.1016/j.anaerobe.2019.102081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Perumalsamy S., Lim S.C., Riley T.V. Clostridioides (Clostridium) Difficile Isolated from Paediatric Patients in Western Australia 2019–2020. Pathology. 2022;54:460–465. doi: 10.1016/j.pathol.2021.10.009. [DOI] [PubMed] [Google Scholar]
- 99.Djebbar A., Sebaihia M., Kuijper E., Harmanus C., Sanders I., Benbraham N., Hacène H. First Molecular Characterisation and PCR Ribotyping of Clostridium Difficile Strains Isolated in Two Algerian Hospitals. J. Infect. Dev. Ctries. 2018;12:15–21. doi: 10.3855/jidc.9580. [DOI] [PubMed] [Google Scholar]
- 100.Seugendo M., Mshana S.E., Hokororo A., Okamo B., Mirambo M.M., von Müller L., Gunka K., Zimmermann O., Groß U. Clostridium Difficile Infections among Adults and Children in Mwanza/Tanzania: Is It an Underappreciated Pathogen among Immunocompromised Patients in Sub-Saharan Africa? New Microbes New Infect. 2015;8:99–102. doi: 10.1016/j.nmni.2015.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhao H., Nickle D.C., Zeng Z., Law P.Y.T., Wilcox M.H., Chen L., Peng Y., Meng J., Deng Z., Albright A., et al. Global Landscape of Clostridioides Difficile Phylogeography, Antibiotic Susceptibility, and Toxin Polymorphisms by Post-Hoc Whole-Genome Sequencing from the MODIFY I/II Studies. Infect. Dis. Ther. 2021;10:853–870. doi: 10.1007/s40121-021-00426-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abdrabou A.M.M., Ul Habib Bajwa Z., Halfmann A., Mellmann A., Nimmesgern A., Margardt L., Bischoff M., von Müller L., Gärtner B., Berger F.K. Molecular Epidemiology and Antimicrobial Resistance of Clostridioides Difficile in Germany, 2014–2019. Int. J. Med. Microbiol. 2021;311:151507. doi: 10.1016/j.ijmm.2021.151507. [DOI] [PubMed] [Google Scholar]
- 103.Abdrabou A.M.M., Bischoff M., Mellmann A., von Müller L., Margardt L., Gärtner B.C., Berger F.K., Haase G., Häfner H., Hoffmann R., et al. Implementation of a Clostridioides Difficile Sentinel Surveillance System in Germany: First Insights for 2019–2021. Anaerobe. 2022;77:102548. doi: 10.1016/j.anaerobe.2022.102548. [DOI] [PubMed] [Google Scholar]
- 104.Johnson S., Citron D.M., Gerding D.N., Wilcox M.H., Goldstein E.J.C., Sambol S.P., Best E.L., Eves K., Jensen E., Dorr M.B. Efficacy of Bezlotoxumab in Trial Participants Infected With Clostridioides Difficile Strain BI Associated With Poor Outcomes. Clin. Infect. Dis. 2021;73:e2616–e2624. doi: 10.1093/cid/ciaa1035. [DOI] [PubMed] [Google Scholar]
- 105.Romano V., Pasquale V., Krovacek K., Mauri F., Demarta A., Dumontet S. Toxigenic Clostridium Difficile PCR Ribotypes from Wastewater Treatment Plants in Southern Switzerland. Appl. Environ. Microbiol. 2012;78:6643. doi: 10.1128/AEM.01379-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Skinner A.M., Petrella L., Siddiqui F., Sambol S.P., Gulvik C.A., Gerding D.N., Donskey C.J., Johnson S. Unique Clindamycin-Resistant Clostridioides Difficile Strain Related to Fluoroquinolone-Resistant Epidemic BI/RT027 Strain. Emerg. Infect. Dis. 2020;26:247–254. doi: 10.3201/eid2602.181965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.FDA FDA Updates Warnings for Fluoroquinolone Antibiotics. [(accessed on 9 November 2022)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-updates-warnings-fluoroquinolone-antibiotics.
- 108.European Medicines Agency Fluoroquinolone and Quinolone Antibiotics: PRAC Recommends New Restrictions on Use Following Review of Disabling and Potentially Long-Lasting Side Effects. [(accessed on 9 November 2022)]; Available online: https://www.ema.europa.eu/en/news/fluoroquinolone-quinolone-antibiotics-prac-recommends-new-restrictions-use-following-review.
- 109.Wieczorkiewicz J.T., Lopansri B.K., Cheknis A., Osmolski J.R., Hecht D.W., Gerding D.N., Johnson S. Fluoroquinolone and Macrolide Exposure Predict Clostridium Difficile Infection with the Highly Fluoroquinolone- and Macrolide-Resistant Epidemic C. Difficile Strain BI/NAP1/027. Antimicrob. Agents Chemother. 2016;60:418–423. doi: 10.1128/AAC.01820-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tabak Y.P., Srinivasan A., Yu K.C., Kurtz S.G., Gupta V., Gelone S., Scoble P.J., Mcdonald L.C. Hospital-Level High-Risk Antibiotic Use in Relation to Hospital-Associated Clostridioides Difficile Infections: Retrospective Analysis of 2016-2017 Data from US Hospitals HHS Public Access. Infect. Control Hosp. Epidemiol. 2019;40:1229–1235. doi: 10.1017/ice.2019.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Berger F.K., Rasheed S.S., Araj G.F., Mahfouz R., Rimmani H.H., Karaoui W.R., Sharara A.I., Dbaibo G., Becker S.L., von Müller L., et al. Molecular Characterization, Toxin Detection and Resistance Testing of Human Clinical Clostridium Difficile Isolates from Lebanon. Int. J. Med. Microbiol. 2018;308:358–363. doi: 10.1016/j.ijmm.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 112.Davies K.A., Ashwin H., Longshaw C.M., Burns D.A., Davis G.L., Wilcox M.H. Diversity of Clostridium Difficile PCR Ribotypes in Europe: Results from the European, Multicentre, Prospective, Biannual, Point-Prevalence Study of Clostridium Difficile Infection in Hospitalised Patients with Diarrhoea (EUCLID), 2012 and 2013. Eurosurveillance. 2016;21:30294. doi: 10.2807/1560-7917.ES.2016.21.29.30294. [DOI] [PubMed] [Google Scholar]
- 113.Tkalec V., Janezic S., Skok B., Simonic T., Mesaric S., Vrabic T., Rupnik M. High Clostridium Difficile Contamination Rates of Domestic and Imported Potatoes Compared to Some Other Vegetables in Slovenia. Food Microbiol. 2019;78:194–200. doi: 10.1016/j.fm.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 114.Lim S.C., Foster N.F., Elliott B., Riley T.V. High Prevalence of Clostridium Difficile on Retail Root Vegetables, Western Australia. J. Appl. Microbiol. 2018;124:585–590. doi: 10.1111/jam.13653. [DOI] [PubMed] [Google Scholar]
- 115.Freeman J., Vernon J., Pilling S., Morris K., Nicholson S., Shearman S., Longshaw C., Wilcox M.H. The ClosER Study: Results from a Three-Year Pan-European Longitudinal Surveillance of Antibiotic Resistance among Prevalent Clostridium Difficile Ribotypes, 2011–2014. Clin. Microbiol. Infect. 2018;24:724–731. doi: 10.1016/j.cmi.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 116.Cheknis A., Johnson S., Chesnel L., Petrella L., Sambol S., Dale S.E., Nary J., Sears P., Citron D.M., Goldstein E.J.C., et al. Molecular Epidemiology of Clostridioides (Clostridium) Difficile Strains Recovered from Clinical Trials in the US, Canada and Europe from 2006–2009 to 2012–2015. Anaerobe. 2018;53:38–42. doi: 10.1016/j.anaerobe.2018.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Eyre D.W., Davies K.A., Davis G., Fawley W.N., Dingle K.E., de Maio N., Karas A., Crook D.W., Peto T.E.A., Walker A.S., et al. Two Distinct Patterns of Clostridium Difficile Diversity Across Europe Indicating Contrasting Routes of Spread. Clin. Infect. Dis. 2018;67:1035–1044. doi: 10.1093/cid/ciy252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Piepenbrock E., Stelzer Y., Berger F., Jazmati N. Changes in Clostridium (Clostridioides) Difficile PCR-Ribotype Distribution and Antimicrobial Resistance in a German Tertiary Care Hospital Over the Last 10 Years. Curr. Microbiol. 2019;76:520–526. doi: 10.1007/s00284-019-01654-3. [DOI] [PubMed] [Google Scholar]
- 119.Gonzales-Luna A.J., Carlson T.J., Dotson K.M., Poblete K., Costa G., Miranda J., Lancaster C., Walk S.T., Tupy S., Begum K., et al. PCR Ribotypes of Clostridioides Difficile across Texas from 2011 to 2018 Including Emergence of Ribotype 255. Emerg. Microbes Infect. 2020;9:341–347. doi: 10.1080/22221751.2020.1721335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Spinler J.K., Gonzales-Luna A.J., Raza S., Runge J.K., Luna R.A., Savidge T.C., Garey K.W. Complete Genome Sequence of Clostridioides Difficile Ribotype 255 Strain Mta-79, Assembled Using Oxford Nanopore and Illumina Sequencing. Microbiol. Resour. Announc. 2019;8:e00935-19. doi: 10.1128/MRA.00935-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.O’shaughnessy R.A., Habing G.G., Gebreyes W.A., Bowman A.S., Weese J.S., Rousseau J., Stull J.W. Clostridioides Difficile on Ohio Swine Farms (2015): A Comparison of Swine and Human Environments and Assessment of on-Farm Risk Factors. Zoonoses Public Health. 2019;66:861–870. doi: 10.1111/zph.12637. [DOI] [PubMed] [Google Scholar]
- 122.Fawley W.N., Davies K.A., Morris T., Parnell P., Howe R., Wilcox M.H. Enhanced Surveillance of Clostridium Difficile Infection Occurring Outside Hospital, England, 2011 to 2013. Eurosurveillance. 2016;21:30295. doi: 10.2807/1560-7917.ES.2016.21.29.30295. [DOI] [PubMed] [Google Scholar]
- 123.Cheng A.C., Collins D.A., Elliott B., Ferguson J.K., Paterson D.L., Thean S., Riley T.V. Laboratory-Based Surveillance of Clostridium Difficile Circulating in Australia, September–November 2010. Pathology. 2016;48:257–260. doi: 10.1016/j.pathol.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 124.Williamson C.H.D., Stone N.E., Nunnally A.E., Roe C.C., Vazquez A.J., Lucero S.A., Hornstra H., Wagner D.M., Keim P., Rupnik M., et al. Identification of Novel, Cryptic Clostridioides Species Isolates from Environmental Samples Collected from Diverse Geographical Locations. Microb. Genom. 2022;8:000742. doi: 10.1099/mgen.0.000742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saldanha G.Z., Pires R.N., Rauber A.P., de Lima-Morales D., Falci D.R., Caierão J., Pasqualotto A.C., Martins A.F. Genetic Relatedness, Virulence Factors and Antimicrobial Resistance of C. Difficile Strains from Hospitalized Patients in a Multicentric Study in Brazil. J. Glob. Antimicrob Resist. 2020;22:117–121. doi: 10.1016/j.jgar.2020.01.007. [DOI] [PubMed] [Google Scholar]
- 126.Balassiano I.T., Miranda K.R., Boente R.F., Pauer H., Oliveira I.C.M., Santos-Filho J., Amorim E.L.T., Caniné G.A., Souza C.F., Gomes M.Z.R., et al. Characterization of Clostridium Difficile Strains Isolated from Immunosuppressed Inpatients in a Hospital in Rio de Janeiro, Brazil. Anaerobe. 2009;15:61–64. doi: 10.1016/j.anaerobe.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 127.Alcides A.P.P., Brazier J.S., Pinto L.J.F., Balassiano I.T., Boente R.F., Paula G.R., Ferreira E.O., Avelar K.E.S., Miranda K.R., Ferreira M.C.S., et al. New PCR Ribotypes of Clostridium Difficile Detected in Children in Brazil. Antonie Van Leeuwenhoek. 2007;92:53–59. doi: 10.1007/s10482-006-9134-2. [DOI] [PubMed] [Google Scholar]
- 128.Carneiro L.G., Pinto T.C.A., Moura H., Barr J., Domingues R.M.C.P., Ferreira E.d.O. MALDI-TOF MS: An Alternative Approach for Ribotyping Clostridioides Difficile Isolates in Brazil. Anaerobe. 2021;69:102351. doi: 10.1016/j.anaerobe.2021.102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chaudhry R., Sharma N., Bahadur T., Khullar S., Agarwal S.K., Gahlowt A., Gupta N., Kumar L., Kabra S.K., Dey A.B. Molecular Characterization of Clostridioides Difficile by Multi-Locus Sequence Typing (MLST): A Study from Tertiary Care Center in India. Anaerobe. 2022;75:102545. doi: 10.1016/j.anaerobe.2022.102545. [DOI] [PubMed] [Google Scholar]
- 130.Collins D.A., Sohn K.M., Wu Y., Ouchi K., Ishii Y., Elliott B., Riley T.V., Tateda K. Clostridioides Difficile Infection in the Asia-Pacific Region. Emerg. Microbes Infect. 2020;9:42–52. doi: 10.1080/22221751.2019.1702480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Collins D.A., Hawkey P.M., Riley T.V. Epidemiology of Clostridium Difficile Infection in Asia. Antimicrob. Resist. Infect. Control. 2013;2:21. doi: 10.1186/2047-2994-2-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Collins D.A., Riley T.V. Clostridium Difficile Guidelines. Clin. Infect. Dis. 2018;67:1639. doi: 10.1093/cid/ciy249. [DOI] [PubMed] [Google Scholar]
- 133.Kato H., Kato H., Ito Y., Akahane T., Izumida S., Yokoyama T., Kaji C., Arakawa Y. Typing of Clostridium Difficile Isolates Endemic in Japan by Sequencing of SlpA and Its Application to Direct Typing. J. Med. Microbiol. 2010;59:556–562. doi: 10.1099/jmm.0.016147-0. [DOI] [PubMed] [Google Scholar]
- 134.Senoh M., Kato H., Fukuda T., Niikawa A., Hori Y., Hagiya H., Ito Y., Miki H., Abe Y., Furuta K., et al. Predominance of PCR-Ribotypes, 018 (Smz) and 369 (Trf) of Clostridium Difficile in Japan: A Potential Relationship with Other Global Circulating Strains? J. Med. Microbiol. 2015;64:1226–1236. doi: 10.1099/jmm.0.000149. [DOI] [PubMed] [Google Scholar]
- 135.Kuwata Y., Tanimoto S., Sawabe E., Shima M., Takahashi Y., Ushizawa H., Fujie T., Koike R., Tojo N., Kubota T., et al. Molecular Epidemiology and Antimicrobial Susceptibility of Clostridium Difficile Isolated from a University Teaching Hospital in Japan. Eur. J. Clin. Microbiol. Infect. Dis. 2015;34:763–772. doi: 10.1007/s10096-014-2290-9. [DOI] [PubMed] [Google Scholar]
- 136.Aoki K., Takeda S., Miki T., Ishii Y., Tateda K. Antimicrobial Susceptibility and Molecular Characterization Using Whole-Genome Sequencing of Clostridioides Difficile Collected in 82 Hospitals in Japan between 2014 and 2016. Antimicrob. Agents Chemother. 2019;63:e01259-19. doi: 10.1128/AAC.01259-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jin D., Luo Y., Huang C., Cai J., Ye J., Zheng Y., Wang L., Zhao P., Liu A., Fang W., et al. Molecular Epidemiology of Clostridium Difficile Infection in Hospitalized Patients in Eastern China. J. Clin. Microbiol. 2017;55:801–810. doi: 10.1128/JCM.01898-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang C., Cui L., Xu Y., Xie L., Sun P., Liu C., Xia W., Liu G. The Incidence and Drug Resistance of Clostridium Difficile Infection in Mainland China: A Systematic Review and Meta-Analysis. Sci. Rep. 2016;6:37865. doi: 10.1038/srep37865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhou Y., Zhou W., Xiao T., Chen Y., Lv T., Wang Y., Zhang S., Cai H., Chi X., Kong X., et al. Comparative Genomic and Transmission Analysis of Clostridioides Difficile between Environmental, Animal, and Clinical Sources in China. Emerg. Microbes Infect. 2021;10:2244–2255. doi: 10.1080/22221751.2021.2005453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang W.Z., Li W.G., Liu Y.Q., Gu W.P., Zhang Q., Li H., Liu Z.J., Zhang X., Wu Y., Lu J.X. The Molecular Characters and Antibiotic Resistance of Clostridioides Difficile from Economic Animals in China. BMC Microbiol. 2020;20:70. doi: 10.1186/s12866-020-01757-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baghani A., Mesdaghinia A., Kuijper E.J., Aliramezani A., Talebi M., Douraghi M. High Prevalence of Clostridiodes Diffiicle PCR Ribotypes 001 and 126 in Iran. Sci. Rep. 2020;10:4658. doi: 10.1038/s41598-020-61604-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Sharara A., Karaoui W.R., Shayto R.H., Rimmani H., Chalhoub J.M., Zahreddine N., el Sabbagh R., Kanj S.S., Mahfouz R., Araj G., et al. Hypervirulent Clostridium Difficile Strains Are Rarely Associated With Nosocomial CDI in Adults in Lebanon: Results From a Prospective Study on the Incidence, Risk Factors for Relapse, and Outcome of Nosocomial CDI. Am. J. Gastroenterol. 2017;112:S51. doi: 10.14309/00000434-201710001-00104. [DOI] [Google Scholar]
- 143.Jamal W., Pauline E., Rotimi V. A Prospective Study of Community-Associated Clostridium Difficile Infection in Kuwait: Epidemiology and Ribotypes. Anaerobe. 2015;35:28–32. doi: 10.1016/j.anaerobe.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 144.Al-Thani A.A., Hamdi W.S., Al-Ansari N.A., Doiphode S.H. Polymerase Chain Reaction Ribotyping of Clostridium Difficile Isolates in Qatar: A Hospital-Based Study. BMC Infect. Dis. 2014;14:502. doi: 10.1186/1471-2334-14-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Hong S., Putsathit P., George N., Hemphill C., Huntington P.G., Korman T.M., Kotsanas D., Lahra M., McDougall R., Moore C.V., et al. Laboratory-Based Surveillance of Clostridium Difficile Infection in Australian Health Care and Community Settings, 2013 to 2018. J. Clin. Microbiol. 2020;58:e01552-20. doi: 10.1128/JCM.01552-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lim S.C., Collins D.A., Imwattana K., Knight D.R., Perumalsamy S., Hain-Saunders N.M.R., Putsathit P., Speers D., Riley T.V. Whole-Genome Sequencing Links Clostridium (Clostridioides) Difficile in a Single Hospital to Diverse Environmental Sources in the Community. J. Appl. Microbiol. 2021;133:1156–1168. doi: 10.1111/jam.15408. [DOI] [PubMed] [Google Scholar]
- 147.Johnston M., Irwin J., Roberts S., Leung A., Andersson H.S., Orme G., Deroles-Main J., Bakker S. Clostridioides Difficile Infection in a Rural New Zealand Secondary Care Centre: An Incidence Case–Control Study. Intern. Med. J. 2021;52:1009–1015. doi: 10.1111/imj.15220. [DOI] [PubMed] [Google Scholar]
- 148.Sathyendran V., Mcauliffe G.N., Swager T., Freeman J.T., Taylor S.L., Roberts S.A. Clostridium Difficile as a Cause of Healthcare-Associated Diarrhoea among Children in Auckland, New Zealand: Clinical and Molecular Epidemiology. Eur. J. Clin. Microbiol. Infect. Dis. 2014;33:1741–1747. doi: 10.1007/s10096-014-2139-2. [DOI] [PubMed] [Google Scholar]
- 149.Kotloff K.L. The Burden and Etiology of Diarrheal Illness in Developing Countries. Pediatr. Clin. N. Am. 2017;64:799–814. doi: 10.1016/j.pcl.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 150.Janssen I., Cooper P., Gunka K., Rupnik M., Wetzel D., Zimmermann O., Groß U. High Prevalence of Nontoxigenic Clostridium Difficile Isolated from Hospitalized and Non-Hospitalized Individuals in Rural Ghana. Int. J. Med. Microbiol. 2016;306:652–656. doi: 10.1016/j.ijmm.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 151.Seugendo M., Janssen I., Lang V., Hasibuan I., Bohne W., Cooper P., Daniel R., Gunka K., Kusumawati R.L., Mshana S.E., et al. Prevalence and Strain Characterization of Clostridioides (Clostridium) Difficile in Representative Regions of Germany, Ghana, Tanzania and Indonesia—A Comparative Multi-Center Cross-Sectional Study. Front. Microbiol. 2018;9:1843. doi: 10.3389/fmicb.2018.01843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Berger F.K., Mellmann A., Bischoff M., von Müller L., Becker S.L., Simango C., Gärtner B. Molecular Epidemiology and Antimicrobial Resistance of Clostridioides Difficile Detected in Chicken, Soil and Human Samples from Zimbabwe. Int. J. Infect. Dis. 2020;96:82–87. doi: 10.1016/j.ijid.2020.04.026. [DOI] [PubMed] [Google Scholar]
- 153.Robishaw J.D., Alter S.M., Solano J.J., Shih R.D., DeMets D.L., Maki D.G., Hennekens C.H. Genomic Surveillance to Combat COVID-19: Challenges and Opportunities. Lancet Microbe. 2021;2:e481–e484. doi: 10.1016/S2666-5247(21)00121-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.World Health Organization Global Genomic Surveillance Strategy for Pathogens with Pandemic and Epidemic Potential, 2022–2032. [(accessed on 27 July 2022)]. Available online: https://www.who.int/publications/i/item/9789240046979. [DOI] [PMC free article] [PubMed]
- 155.Jahn K., Dreifuss D., Topolsky I., Kull A., Ganesanandamoorthy P., Fernandez-Cassi X., Bamp C., Devaux A.J., Stachler E., Caduff L., et al. Early Detection and Surveillance of SARS-CoV-2 Genomic Variants in Wastewater Using COJAC. Nat. Microbiol. 2022;7:1151–1160. doi: 10.1038/s41564-022-01185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schumann V.-F., Cuadrat R.R.d.C., Wyler E., Wurmus R., Deter A., Quedenau C., Dohmen J., Faxel M., Borodina T., Blume A., et al. SARS-CoV-2 Infection Dynamics Revealed by Wastewater Sequencing Analysis and Deconvolution (Preprint) Sci. Total Environ. 2022;853:158931. doi: 10.1016/j.scitotenv.2022.158931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu C., Weese J.S., Flemming C., Odumeru J., Warriner K. Fate of Clostridium Difficile during Wastewater Treatment and Incidence in Southern Ontario Watersheds. J. Appl. Microbiol. 2014;117:891–904. doi: 10.1111/jam.12575. [DOI] [PubMed] [Google Scholar]
- 158.Moradigaravand D., Gouliouris T., Ludden C., Reuter S., Jamrozy D., Blane B., Naydenova P., Judge K., Aliyu S.H., Hadjirin N.F., et al. Genomic Survey of Clostridium Difficile Reservoirs in the East of England Implicates Environmental Contamination of Wastewater Treatment Plants by Clinical Lineages. Microb. Genom. 2018;4:e000162. doi: 10.1099/mgen.0.000162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gardy J.L., Loman N.J. Towards a Genomics-Informed, Real-Time, Global Pathogen Surveillance System. Nat. Rev. Genet. 2017;19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kuehne S.A., Rood J.I., Lyras D. Clostridial Genetics: Genetic Manipulation of the Pathogenic Clostridia. Microbiol. Spectr. 2019;7:20. doi: 10.1128/microbiolspec.GPP3-0040-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Joseph R.C., Kim N.M., Sandoval N.R. Recent Developments of the Synthetic Biology Toolkit for Clostridium. Front. Microbiol. 2018;9:154. doi: 10.3389/fmicb.2018.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Maikova A., Kreis V., Boutserin A., Severinov K., Soutourina O. Using an Endogenous CRISPR-Cas System for Genome Editing in the Human Pathogen Clostridium Difficile. Appl. Environ. Microbiol. 2019;85:e01416-19. doi: 10.1128/AEM.01416-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhu D., Wang S., Sun X. FliW and CsrA Govern Flagellin (FliC) Synthesis and Play Pleiotropic Roles in Virulence and Physiology of Clostridioides Difficile R20291. Front. Microbiol. 2021;12:735616. doi: 10.3389/fmicb.2021.735616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Hong W., Zhang J., Cui G., Wang L., Wang Y. Multiplexed CRISPR-Cpf1-Mediated Genome Editing in Clostridium Difficile toward the Understanding of Pathogenesis of C. Difficile Infection. ACS Synth. Biol. 2018;7:1588–1600. doi: 10.1021/acssynbio.8b00087. [DOI] [PubMed] [Google Scholar]
- 165.McAllister K.N., Bouillaut L., Kahn J.N., Self W.T., Sorg J.A. Using CRISPR-Cas9-Mediated Genome Editing to Generate C. Difficile Mutants Defective in Selenoproteins Synthesis. Sci. Rep. 2017;7:14672. doi: 10.1038/s41598-017-15236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Wang S., Hong W., Dong S., Zhang Z.T., Zhang J., Wang L., Wang Y. Genome Engineering of Clostridium Difficile Using the CRISPR-Cas9 System. Clin. Microbiol. Infect. 2018;24:1095–1099. doi: 10.1016/j.cmi.2018.03.026. [DOI] [PubMed] [Google Scholar]
- 167.Selle K., Fletcher J.R., Tuson H., Schmitt D.S., McMillan L., Vridhambal G.S., Rivera A.J., Montgomery S.A., Fortier L.C., Barrangou R., et al. In Vivo Targeting of Clostridioides Difficile Using Phage- Delivered CRISPR-Cas3 Antimicrobials. mBio. 2020;11:e00019-20. doi: 10.1128/mBio.00019-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Roldan G.A., Cui A.X., Pollock N.R. Assessing the Burden of Clostridium Difficile Infection in Low- and Middle-Income Countries. J. Clin. Microbiol. 2018;56:e01747-17. doi: 10.1128/JCM.01747-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kullin B., Abratt V.R., Reid S.J., Riley T.V. Clostridioides Difficile Infection in Africa: A Narrative Review. Anaerobe. 2022;74:102549. doi: 10.1016/j.anaerobe.2022.102549. [DOI] [PubMed] [Google Scholar]
- 170.Antimicrobial Stewardship. [(accessed on 10 November 2022)]. Available online: https://www.ecdc.europa.eu/en/publications-data/directory-guidance-prevention-and-control/prudent-use-antibiotics/antimicrobial.
- 171.CDC What CDC Is Doing to Reduce C. Diff Infections. [(accessed on 10 November 2022)]; Available online: https://www.cdc.gov/cdiff/reducing.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.