Abstract
Baicalein (BA) is a flavonoid with wide-ranging pharmacological activity. However, its biological evaluation is hampered by its low solubility in aqueous medium, making forms of incorporation that improve its solubility necessary. In the present study, BA was combined with a solution of silk fibroin protein (SF), a biomaterial used too as a drug carrier, to evaluate the anti-inflammatory potential of this combination, in vivo, in an experimental model, zebrafish (Danio rerio). Baicalein-silk fibroin (BASF) improved the DPPH (2,2‐diphenyl‐1‐picryl‐hydrazyl‐hydrate) free radical scavenging rate (95%) in comparison with BA in solution. The acute toxicity study and histopathological analysis in zebrafish showed that BASF has low cytotoxic potential, except for the maxim dose of 2000 mg/kg. The use of BA in combination with SF enhanced the anti-inflammatory effect of flavonoids by inducing inflammatory peritoneal edema through carrageenan and achieved 77.6% inhibition of abdominal edema at a dose of 75 mg/kg. The results showed that the BASF, significantly increases the bioavailability and therapeutic effect of flavonoids and several results observed in this study may help in the development of new drugs.
Keywords: Silk cocoon, Advanced biopolymer, Flavonoid, Drug delivery, Flavonoid
Introduction
The flavonoid baicalein (5,6,7-trihydroxyflavone) extracted from the root of Scutellaria Baicalenses and Scutellaria Lateriflora (Zhao et al. 2019) (Yoshino et al. 1997) represent the major bioactive flavone constituents of Oraxylum Indicum leaf (Mat Ali et al. 1998). These herbs are traditionally used in chinese medicine and in other Asian countries to treat various diseases, such as diarrhea, hepatitis, inflammation, hypertension, insomnia and respiratory infections (Zhao et al. 2016). The biological activities involving this flavonoid include, neuroprotective (Gu et al. 2016; Sowndhararajan et al. 2017; Guo et al. 2019; Yan et al. 2020), anticancer, antitumor (Bie et al. 2017; Dinda et al. 2017; Yu et al. 2018), antiviral (Sithisarn et al. 2013; Luo et al. 2020), action against SARS-CoV-2 (Song et al. 2021), antioxidant (Yoshino et al. 1997) and anti-inflammatory (Iio et al. 1986; Yin et al. 2018; Pu et al. 2019; Wu et al. 2020) activity. Formulations containing baicalein are effectively absorbed by the stomach and small intestine (Psimadas et al. 2012). Like baicalein, other flavonoids showed anti-inflammatory action in vivo, such as quercetin, kaempferol, luteolin, anthocyanidin and genistein, through different mechanisms, acting for example in the inhibition of COX, LOX, PLA2 and in the production of free radicals, in the modulation of transcription factors, such as NF-k, GATA-3, SATAT-6, in addition to participating in cell activation, maturation and signaling processes (Maleki et al. 2019).
Although baicalein is a biologically promising substance for medicine, has limitations such as low bioavailability, due to insolubility in an aqueous system, which hinders the development of pharmaceutical formulations (Liu et al. 2012). To enhance the biological effects of baicalein, formulations using encapsulation or nanoformulation technologies, along with a drug delivery system to increase its bioavailability, are already being used (Jong 2008; Gao 2017; Erdoğar et al. 2018). Encapsulation of baicalein in chitosan nanoparticles for the treatment of asthma (Wang et al. 2021b), hydroxyapatite nanoparticles with baicalein with antimicrobial properties used in metallic implants (Palierse et al. 2021), solid baicalein lipid nanoparticles against ionizing radiation-induced apoptosis (Joshi et al. 2021), baicalein encapsulated in supercritical fluid with hydroxypropyl-β-cyclodextrin (Li et al. 2018), baicalein in a self-micro emulsifying delivery system with a blend of surfactants, co-surfactants, and oils (Liu et al. 2012), among other recent studies, are examples of methods used to enhance the properties of baicalein and other flavonoids.
Therefore, silk fibroin (SF) is increasingly considered an alternative to conventional drug carriers. SF is a natural polymeric biomaterial produced by a variety of silkworm larvae, including Bomboxy mori (Ferreira et al. 2017a), that meets the requirements of drug delivery systems by exhibiting advantageous properties such as low toxicity, biodegradability, biocompatibility, mechanical strength, self-assembly ability, dissolution resistance, low manufacturing cost, resistance to thermal and enzymatic degradation (Mottaghitalab et al. 2015). This biomaterial has been shown to favor the biological efficacy of various substances and also to control release rates (Sarquis et al. 2020; Marinho et al. 2022). Due to the large number of amino acids and functional groups, fibroin can be tested in combination with a wide variety of isolated substances (Ferreira et al. 2017b; Araújo et al. 2020).
Among other features, controlled biodegradation and low cell toxicity make SF highly suitable for use in drug delivery systems (Hosseinkhani and Hosseinkhani 2009). The combination of SF in drug delivery systems with flavonoids has already been tested, as in a study of SF nanoparticles loaded with naringenin used in cancer therapy (Fuster et al. 2020), in the use of quercetin in an efficient controlled-release system (Lozano-Pérez et al. 2017) and in the encapsulation of a herbal extract containing BA with a high release rate (Chan et al. 2017).
The zebrafish animal model (Danio rerio) is commonly used for drug screening (Lawrence 2007; Victor et al. 2016). Due to its many advantages, including large-scale handling, small size, optical transparency, organ sensitivity, high genetic similarity to humans, rapid development and low relative cost, this model has well-established protocols for several biomedical studies (Jeevanandam et al. 2019). The zebrafish model has excelled in research studies into various types of inflammation in recent years (Huang et al. 2014; Brugman 2016; Novoa et al. 2019; Wu et al. 2020; Quitian-Useche et al. 2021).
Therefore, in the present study, baicalein—silk fibroin solution (BASF) to enhance its anti-inflammatory effect in vivo in zebrafish, as an alternative vehicle for flavonoids with low water solubility.
Material and methods
Reagents and solvents
All reagents and solvents used in the in vivo tests, as well as in the preparation of the baicalein flavonoid formulation (98%) and in the silk fibroin solution, were purchased from Sigma-Aldrich (St. Louis, MO, USA). The list includes 99.5% sodium carbonate (Vetec®), 99% ethanol, 74–78% calcium chloride (Alphatec®) and 99.5% isopropyl alcohol (Quimex®).
Preparation of the silk fibroin solution
The silk fibroin solution was prepared based on the method developed by Maciel Ferreira et al. 2014, Silkworm cocoon (3.0 g, from Bratac, Brazil) were degummed in a boiling (2%, w/v) Na2CO3 solution for 30 min. The resultant fibers were filtrated and washed with distilled water (3 × 500 mL). Subsequently, the silk fiber was dissolved in a ternary solution (50 mL) of H2O:EtOH:CaCl2 (8:2:1 molar proportions) at 30 ºC for 4 h. This mixture was then dialyzed (cellulose tube with an exclusion limit of 16 kDa, from Viskase, Brazil) for 3 days at room temperature and the water changed every 24 h. The fibroin solution was centrifuged (6000 rpm for 10 min) to remove impurities and larger particles. The concentration of the silk fibroin solution was adjusted to 2% (w/w).
Preparation of BASF solution
The BASF was prepared by adding the silk fibroin solution to isopropanol BA. The BA (2%) was solubilized in isopropanol (6.5% w/v) and water (85% w/v). All the components were mixed at room temperature using a magnetic stirrer for 30 min. Next, silk fibroin (6.5%) was added with continuous agitation for 30 min to obtain the BASF solution, which was stored at 4 ºC for later use.
Characterization of BASF
Droplet size, polydispersity and zeta potential were determined by photographic correlation spectroscopy using a Zetasizer 5000 (Malvern Instruments, Malvern, UK). Each BASF and BA in methanol sample was diluted using distilled water (1:25). Measurements were performed in triplicate. Average droplet size was expressed as the average diameter. All analyzes were performed at 25 ºC.
Determination of adsorption efficiency by UV–vis.
The adsorption efficiency (%AE) of the BASF formulation was determined according to the techniques previously described by Li et al., 2016. After BASF preparation, the solution was shaken and then centrifuged (KASVI) at 3000 rpm for 15 min to remove the excess baicalein that was not adsorbed on the SF. The supernatant was collected and quantified in a UV–Vis spectrometer (model Lambda 35, PerkinElmer) with a scan range of 200–600 nm.
The amount of free BASF was determined in triplicate using a standard curve generated with different concentrations. The concentration was calculated using the linear regression equation: y = 1595.5x + 91.742, and the correlation coefficient was R2 = 0.9759.
The adsorption efficiency percentage of BASF was calculated using the following equation:
where, Total BA corresponds to the total baicalein used in the solution and Free BA corresponds to the amount of baicalein not adsorbed in the biopolymer.
Scavenging of free radicals (% SRL) in DPPH
A methanolic solution of DPPH at a 40 µg/mL, was prepared to evaluate the radical scavenging ability of BASF, monitored at 517 nm by a spectrophotometer. The sample (BASF) was diluted at different concentrations (10, 25, 50, 75, 100 and 125 µg/mL), pipetted 0.3 mL of each sample and added to 2.7 mL of the DPPH solution. The solution was kept in the dark for 30 min and was finally read in spectrophotometer. A blank control was prepared using 3 mL of methyl alcohol. The test was performed in triplicate and the absorbance values were applied to determine the percentage of DPPH consumption according to the following formula:
The effective concentration (EC50) was obtained through the application of the percentages of consumption to the straight-line equation:
Baicalein stock solution was used as positive control, at the same concentrations as the sample. This procedure was performed according to Lopes-Lutz et al., 2008.
Inflammatory edema reduction study in Zebrafish (Danio rerio)
Animals
Ninety adult animals (Danio rerio) weighing 300–1000 mg, purchased from Acqua New Aquarium and Fish LTDA, ME (Itagassu-PE, Brazil) were used. After quarantine, the animals were acclimatized under the following controlled conditions: pH (6.8–8.5), temperature (24–28 °C), alkalinity (50–100 mg/L), salinity (0.5–2.0 g/L) and dissolved oxygen (above 4 mg/L), with a light/dark photoperiod (12/12 h), as described by Carvalho et al., 2018.
The animals were treated according to the standards of the protocol approved by the Ethics Committee for the Use of Animals-CEUA-of the Federal University of Amapá-UNIFAP-under protocol number 003/2020.
Acute Toxicity
The acute toxicity study was conducted on adult zebrafish, according to the 425 OECD (Organization for Economic Co-operation and Development) and behavioral and histopathological analysis were performed according to Souza et al. 2016. The animals received doses of the BASF compound orally and were divided according to the experimental groups described below: group 1—animals that received the initial dose of 175 mg/Kg; group 2—animals that received an intermediate dose of 550 mg/Kg of the compound; group 3—animals that received the limit dose of 2000 mg/Kg of the compound and group 4—animals that did not receive the compound (control group). Each experimental group consisted of 5 animals and were observed for 48 h after oral administration.
Behavioral assessment of acute toxicity
The behavioral assay was evaluated in parallel with the acute toxicity assay and was divided into 3 stages: Stage I: 1) increased swimming activity; 2) presence of tail tremors; Stage II: 1) circular swimming; 2) loss of balance; Stage III: 1) loss of motility; 2) deposition of the animal at the bottom of the aquarium; 3) death. The animals were observed in the period of 0, 3, 6, 12, 24 and 48 h after the oral administration of the BASF solution (Souza et al. 2016).
Treatment groups and routes of administration
Assays were performed with administration of the formulation BASF, fibroin (control, 18 mg/mL) and baicalein (control) solutions by gavage (oral), one hour before the intraperitoneal administration of carrageenan to induce inflammatory edema.
The animals were divided into six main groups (n = 12/group): group A—nimals that received only saline solution via gavage (2 μL oral rout) and PBS (diluent carrageenan) intraperitoneally (20 μL), control group; group B—negative control, animals that received fibroin solution via gavage (2 μL) and Cg intraperitoneally (20 μL); group C—animals that received non-steroidal anti-inflammatory NSAID used for comparative purposes (indomethacin 10 mg/kg) via gavage and Cg intraperitoneally (20 μL); group D—animals that received BASF formulation, with subgroups according to doses (5, 10, 50, 75 and 100 mg/kg) via gavage and Cg intraperitoneally (20 μL); group E—animals that received baicalein (BA) at 100 mg/kg via gavage and Cg intraperitoneally (20 μL).
Induction of inflammation with carrageenan and measurement of edema
The process of inflammation induction was performed as described by Borges et al., 2018. First, zebrafish were collected and anesthetized in cold water (8–10 °C) for approximately 2 min and 300 µg/µL of carrageenan (in PBS) was injected intraperitoneally. Animals were individually weighed on an analytical balance at the beginning and end of the experiment (6 h after carrageenan injection) to measure edema.
The animals were euthanized, photographed, immediately placed in Bouin's solution for fixation and histopathological processing.
For the analysis of abdominal edema induced by intraperitoneal injection of carrageenan after treatment with BASF at different doses, all animals were individually weighed on an analytical balance (FA2104N, Bioprecisa Co., São Paulo, Brazil), before starting the experiment (PI = initial body weight) and at the end of the experiment (FP = final body weight), as described by Borges et al., (2018) and Carvalho et al., (2018). For this study, the final weight was determined at the maximum peak of edema for each inflammatory stimulus used: carrageenan (after 6 h). The evaluation of the inhibitory effect, for all tests, was performed from the difference in body weight (≠ BW = PF—PI) of each animal, being used to calculate the percentage of inhibition of inflammation (% II) that was calculated, according to: % II = 100%—[Mean (BASF treatment) 100%/Mean [negative control (Cg)].
Histopathological analysis
For histopathological analysis of the liver, intestine and kidneys, animals were fixed in Bouin's solution for 24 h and decalcified in EDTA solution (ethylenediaminetetraacetic acid, Sigma Co., São Paulo, Brazil) for 24 h. Samples were dehydrated in increasing ethanol series (70, 80, 90, and 100%) and then diaphanized by impregnation with xylene and embedded in paraffin. Samples were sectioned using a microtome (Brand Rotary Microtome Cut 6062, Slee Medical, Germany) at 5 µm and histopathological analysis was performed after staining the tissue sections with hematoxylin and eosin (Souza et al. 2016). Images were analyzed using an Olympus BX41 Micron Microscope and photographed using an MDCE-5C USB 2.0 digital camera.
Assessment of histopathological alterations
The histopathological alterations index (HAI) was determined by the extent of tissue alterations observed in the liver, intestine and kidneys. The alterations can be classified into stages I, II and III. The HAI value indicates whether the organ is healthy (0–10), has mild to moderate alterations (11–20), moderate to severe alterations (21 and 50) or contains irreversible alterations (> 100) (Poleksic and Mitrovic-Tutundzic 1994; Carvalho et al. 2018). Thus, the indices were calculated according to the following equation:
Where: a: first stage alterations; b: second stage alterations; c: third stage alterations; na: number of alterations considered as the first stage; nb: number of alterations considered as the second stage; nc: number of alterations considered as the third stage; N: number of fishes analyzed per treatment.
Statistical analysis
Data were expressed as mean ± standard error of the mean (SD). For statistical analysis, one-way ANOVA (analysis of variance) was used, followed by the Tukey–Kramer post hoc test. P ≤ 0.05 was considered a statistically significant difference. The Graph Pad Prism® (version 5.03) software, was used.
Results and discussion
Silk fibroin (SF) has been broadly explored as vehicle or biomaterial due to its biocompatibility, permeability, morphological flexibility, low immunogenicity, non-cytotoxicity, and advantageous mechanical and structural properties (Mirzadegan et al. 2020). Considering these advantages of SF was selected to improve the solubility of the flavonoid in aqueous media and enhance its anti-inflammatory properties as a replacement for conventional surfactants in combination with BA, which can eliminate reactive oxygen species (ROS). Such species possess great potential for causing cell damage (Shao et al. 2002) and are associated with various diseases such as cancer, neurodegenerative diseases, atherosclerosis and liver damage (Gao et al. 1999).
BASF formulation characteristics
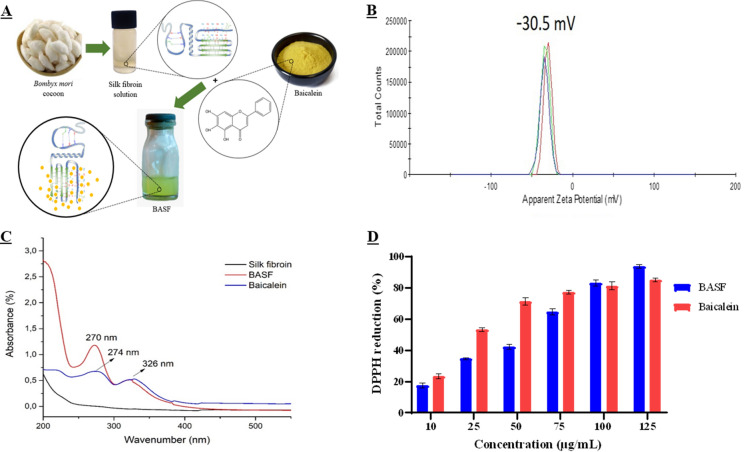
After preparation, BASF exhibited a homogeneous solution with no precipitates and a yellowish color (Fig. 1A). BASF exhibited a zeta potential of − 30.5 ± 0.59 mV (Fig. 1B and Table 1), a negative zeta potential resulting from the interaction of BA with SF with a strong electrostatic bond, which may allow good dispersibility (Zhang et al. 2008). The non-interaction between BA and SF showed an evident reduction in the mean value of the negative charge of the zeta potential for − 24.5 ± 1.63 mV (Table 1). Therefore, a higher negative potential (up − 30 mV) on the surface of BA particles indicates stronger interparticle repulsion, and, consequently, the non-occurrence of an agglomeration process (Park et al. 2015; Ren et al. 2016) and good solution stability (Mishra et al. 2019) (Zhang et al. 2011; Tsai et al. 2012; Li et al. 2016). Moreover, the presence of anionic ions in BA molecules and the protonation process in the surface groups (Kavithaa et al. 2016) indicate good interaction with the cell membrane and a higher efficiency for the delivery of BASF. Another viable hypothesis for cell-BASF interaction is the drug entry through endocytosis. The internalization of BA, incorporated into a formulation with negative zeta potential, makes the membrane more intuitively negative or may also promote active cellular signaling pathways through specific cell–BASF interactions. Such modifications of charge, therefore, expected to alter the interaction between drug molecules and cell medium.
Fig. 1.
In A, Simplified illustration of the preparation of BASF solution containing baicalein combined with silk fibroin; In B, BASF Zeta Potential Value; In C, the absorption spectra of SF, BASF and BA; In D, DPPH free radical inhibiting activity (%) at various concentration of the BASF and BA
Table 1.
Zeta potential (mV), index and polydispersity (PDI) values of free baicalein solution (BA), silk fibroin solution (SF) and BASF
| Zeta potential (mV) | PDI | |
|---|---|---|
| BA | − 24.5 (± 1.63) | 0.40 (± 0.06) |
| SF | − 24.7 (± 1.33) | 0.77 (± 0.21) |
| BASF | − 30.5 (± 0.59) | 0.33 (± 0.06) |
The zeta potential and polydispersity index (PDI) of the free BA solution, SF solution and BASF are presented in the table below.
The result of the analysis revealed that the average PDI of BASF was 0.33 ± 0.06, of the solution containing only BA was PDI 0.4 ± 0.06, and the SF solution presented a PDI 0.77 ± 0.21. Notably, BASF's average particle size and distribution (PDI) were smaller than in the solution containing only free BA.
The evaluation of the PDI has great relevance in the evaluation of the distribution of particles in the formulation, the average value presented by BASF (0.33 ± 0.06) was slightly lower than that of the free BA (0.4 ± 0.06). This variation of values attributed to the adsorption of BA in SF represents a better homogeneous distribution of BASF. The PDI values of the order of 0.3 indicate a homogeneous size distribution of the dispersed particles, in addition to the presentation of uniformity of the formulation (Lemarchand et al. 2003), the average value of BASF PDI indicates the non-agglomeration of particles (Li et al. 2016), the same did not occur with the BA solution without the presence of SF.
The adsorption efficiency (% AE) of BA in SF was 76%. Based on this result, it can be said that the amount of BA adsorbed on SF was satisfactory and more efficient than other previously mentioned forms of capturing and encapsulating BA (liposomes, nanoliposomes, nanostructured lipid carriers, chitosan nanoparticles), (Tsai et al. 2012; Li et al. 2016; Zhang et al. 2020b, c; Wang et al. 2021a). It has been shown that BA interacts on the surface of SF, which is associated with the formation of a hydrogen bond between the protein and the flavonoid. Amines, alcohols, phenols, carboxyl groups, and thiols, present in SF, are potentially active side groups for the chemical interactions with BA (Sofia et al. 2001).
Since BA is a photosensitive substance, the interaction of the drug with SF most probably occurs in its hydrophobic blocks (β-sheet). These fibroin regions are responsible for its high strength and thermal stability caused by the protective effect of BA (Liu et al. 2017; Chen et al. 2019). These properties are due to the main structure of SF formed by a Gly-Ser-Gly-Ala-Gly-Ala type amino acid sequence, resulting in a secondary structure in a crystalline antiparallel β-sheet conformation (two-thirds of the protein) and another amorphous region of the fibers (Meinel et al. 2006; Pham and Tiyaboonchai 2020).
The UV–vis absorption spectra of BASF, BA and SF (Fig. 1C) show that the absorption maximum of BA occurs at 274 nm and 326 nm (blue curve) in the absence of SF. On the other hand, the BASF solution shows an absorption spectrum at 270 nm and 326 nm (red curve), while the SF solution shows no absorption in the range of 200 to 600 nm, indicating that BA changes to a medium with lower polarity upon chemical interaction with SF (Zhang et al. 2014). Based on these results, it can be assumed that there is an interaction between BA and the surface of SF, which is associated with the formation of a hydrogen bond between the protein and the flavonoid (Chan et al. 2017).
DPPH antioxidant potential (% SRL)
The evaluation of BASF in the DPPH solution indicates the antioxidant activity of the formulation, through % SRL and shows the successful reducing effect of the DPPH radical (Fig. 2). The EC50 values determined for BASF and BA were 54.1 µg/mL and 29.8 µg/mL consecutively. At lower concentrations (10 to 75 µg/mL), the free flavonoid in the medium SF was more efficient than the effect of BASF. However, at higher concentrations (100 to 125 µg/mL), BASF is more efficient and reduces the initial free radical concentration by 50% (Fig. 1D).
Fig. 2.
A Index of histopathological changes in the liver at each dose of BASF. Data are presented as mean ± SD (n = 3/groups); B Index of histopathological changes in the intestine at each dose of BASF. Data are presented as mean ± SD (n = 3/groups); C Index of histopathological changes in the kidney at each dose of BASF. Data are presented as mean ± SD (n = 3/groups); * p < 0.05 to the fibroin solution. Statistical analysis was performed through one-way ANOVA followed by the post hoc Tukey test
Tests at a 125 µg/mL BASF concentration showed SRL of 94%, indicating the high profile of this activity compared to standard substances (Mrcp et al. 2013). Samples containing only BA at the same concentration achieved 85% of the reducing activeness of DPPH radical. It is likely that the use of SF in the formulation increased the availability of the BA molecule in aqueous media and consequently resulted in an improved transfer of hydrogen atoms and electrons in the antioxidant mechanism. Based on these results, BASF was found to maintain one of the main properties of baicalein, namely a high % SRL activity, at all concentrations used (10 to 125 µg/mL) (Fig. 1D). Several studies on the antioxidant potential of BA have shown its ability to transfer electrons followed by proton transfer (Martínez-Flórez et al. 2002; Kim et al. 2016).
Behavioral assessment of acute toxicity
After oral administration of BASF at doses of 175, 550 and 2000 mg/kg, the groups were observed for 48 h, with no death at any of the doses tested, thus concluding that BASF did not present acute toxicity in the animals. As for behavioral changes during the experiment, BASF induced few changes compared to the normal pattern when observed within 48 h. These changes may indicate the toxic action of the substance tested, since when in contact with it, they tend to trigger a series of physiological, tissue and behavioral changes. Several authors corroborate that the use of adult zebrafish is a reliable and reproductive model to test the acute toxicity of a substance (Borges et al. 2018; Carvalho et al. 2018; Jeevanandam et al. 2019; Quitian-Useche et al. 2021). The adult zebrafish, when in contact with a toxic substance or an unfavorable environmental condition, can present physiological responses by the stress mechanism, in an attempt to survive, such responses can cause tissue and behavioral changes and even the death of the animal (Santos, 2019).
The main changes presented during the observations were loss of motility and rest at the bottom of the aquarium (stage III) in 90% of the animals soon after oral administration (0 h) and until the third hour of the experiment at all doses (Souza et al. 2016), this behavior occurs due to a way of saving metabolic and energy expenditure so that the animal can maintain physiological homeostasis. Only 10% of the animals showed circular movement or loss of balance (stage II) in the first hours of observations (0-1 h). There is no clear relationship between these changes and increasing doses of BASF. During the subsequent evaluations (12, 24 and 48 h), no behavioral changes were observed.
Five adult animals were used as a control group for comparison between the groups that received BASF doses, and during the observations, they served as a parameter to assess behavioral changes. The control group showed no behavioral changes during the 48 h of observation. The normal standard swimming behavior of adult zebrafish is typically characterized by slow or still movements (Spence et al. 2008).
Another important factor to be highlighted is the non-occurrence of stage I alterations, since the increase in swimming activity is among the most important behavioral changes, as it indicates some internal alteration in the animal (Little et al. 1993), as a mechanism defense to reduce the probability of death (Victor et al. 2016). There was no mortality in the groups, confirming the low toxicity of other natural products tested on zebrafish (Abbate et al. 2021).
From this behavioral analysis associated with the indices of histopathological alterations, it is possible to conclude that BASF does not have an acute toxic effect on animals, since the flavonoid BA is capable of producing protective effects against induced toxicity, already proven in zebrafish larvae (Zhang et al. 2020a).
Histopathological Evaluation of the Acute Toxicity Test
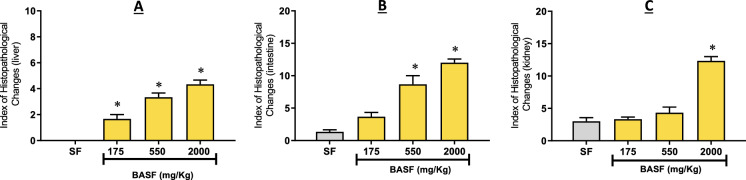
After histopathological analysis, it was possible to observe that BASF treatment (175, 550 and 2000 mg/kg) promoted alterations in the liver, intestines and kidneys (Fig. 3). The index of histopathological alterations was calculated, which indicates the degree of severity and the impairment of the functions of each organ, after the use of BASF (Fig. 2).
Fig. 3.
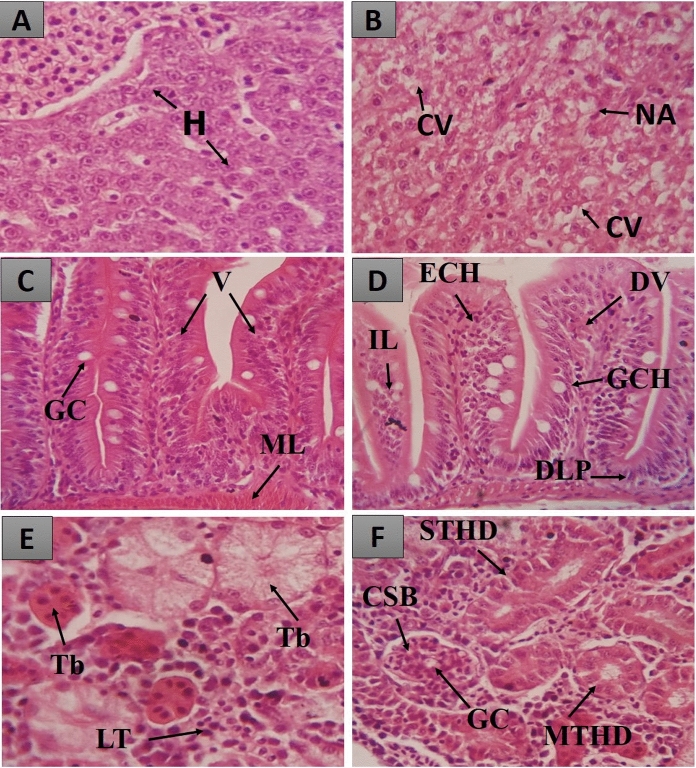
Histopathological changes in the organs of the zebrafish from the acute toxicity study. In A, liver of a normal animal showing hepatocytes (H); In B, liver of a treated animal showing cytoplasmic vacuolization (CV) and nuclear atrophy (NA); In C, intestine of a normal animal showing goblet cells (GC), villi (V) and muscle layer (ML); In D, intestine of treated animal showing dilation of vessels in villi (DV), leukocyte infiltration (LI), goblet cell hyperplasia (GCH), epithelial cell hypertrophy (ECH) and displacement of lamina propria (DLP). In E, kidneys of normal animals where lymphoid tissue (LT) and tubules (Tb) are observed; In F, kidneys of treated animals where glomerulus capillaries (GC), Bowman's capsular space (BCS), mild tubular hyaline degeneration (MTHD) and severe tubular hyaline degeneration (STHD) are observed
According to Holden et al. (Holden et al. 2013), the zebrafish liver resembles the mammalian liver in its main physiological processes, although its structure is different. According to Carvalho et al. (Carvalho et al. 2018), this similarity includes the pathways of drug metabolism, cytochrome P450 activity, which enables metabolic, hydroxylation, conjugation, oxidation, and demethylation reactions, a factor already demonstrated in a pharmacological prediction study conducted by Souza et al. 2019 and Hiacyenth et al. (Souza et al. 2019a; de Sá Hyacienth et al. 2020).
The liver is essential in biliary synthesis, in the storage of lipids and glycogen, as well as in the production of Vitellogenin, a protein present in the chorion (Goksøyr 1995; Souza et al. 2019a; de Sá Hyacienth et al. 2020). It is also the most fundamental site in synthesis and biotransformation (Borges et al. 2018; Santos et al. 2018; Souza et al. 2019b, a).
Recent studies have shown that the oral treatment of zebrafish from flavonoid extracts, even with little toxic action, can alter the typical characteristics of liver cells, and histopathology can be compared to liver lesions in mammals (Vliegenthart et al. 2014; Souza et al. 2019a, b).
The livers of animals treated with the fibroin solution did not show histopathological alterations (Fig. 2A), with an HAI equal to 0.0 ± 0.0, showing that this substance did not cause changes capable of compromising the normal functioning of this organ. As it is a non-toxic biopolymer, this result corroborates another study with SF.
In the other BASF groups (175, 550, and 2000 mg/kg), the histopathological alterations were mild and did not compromise the normal functioning. The metabolic potential and the glycogen storage capacity remained efficient, with HAI equal to 1.6 ± 0.57, 3.33 ± 0.51, 4.33 ± 0.57, respectively, characterizing this organ as normal (Fig. 2A). The alterations observed were only level I, such as nuclear and cell contour atypia, cytoplasmic vacuolization and increase in cell volume (Fig. 3B).
Several studies have reported the occurrence of cytoplasmic vacuolization, an alteration associated with reduced glycogen stores in hepatocytes, and the accumulation of lipids combined with toxic agents, which is known to alter the normal functioning of the organ (Souza et al. 2016, 2019b; Borges et al. 2018; Carvalho et al. 2018; de Sá Hyacienth et al. 2020). Despite the frequent occurrence of this alteration, the present study showed that it was not enough to interfere with the normal functioning of the organ.
The zebrafish does not have a stomach, so the intestinal bulb, which precedes the esophagus, has an absorption function and acts as a food reservoir. The mucous layer is made by goblet cells, dispersed inflammatory cells, and enterocytes, which probably absorb lipids. Through these cells, the intestinal epithelium performs nutrient absorption and acts on the immune response (Holden et al. 2013). It is also a recycling site for enzymes and macronutrients (Alvarez-Pellitero and Sitja-Bobadilla 1993).
In this study, the zebrafish intestine was little affected in the groups treated with fibroin (1.33 ± 0.57), and at doses of 175 mg/kg (3.66 ± 1.15) and 550 mg/kg (8.66 ± 2.30), with the HAI < 10 characterizing this organ as normal (Fig. 2B), as the observed alterations did not change the normal functioning of the organ. The observed alterations were levels I and II: vessel dilatation (I), epithelial cell hypertrophy (I), goblet cell hyperplasia (I), and displacement of the lamina propria (II) (Fig. 3D).
The group of animals treated with the 2000 mg/kg dose of BASF had a HAI of 12.0 ± 1.0, which characterizes this organ as suffering mild to moderate alterations (Fig. 2B). The observed alterations were levels I and II: vessel dilatation (I), epithelial cell hypertrophy (I), goblet cell hyperplasia (I), lymphocyte and leukocyte infiltration (I), and displacement of the lamina propria (II) (Fig. 3D). According to Carvalho et al. and Souza et al. (Carvalho et al. 2018; Souza et al. 2019b), oral treatment with substances at high doses can cause damage to the intestine as it is the first organ in contact with the substance.
The presence of alterations such as infiltration of lymphocytes and leukocytes observed in this study indicates that the dose of 2000 mg/kg of BASF was toxic, leading to an increase in the number of defense cells in the intestinal epithelium, which may cause an inflammatory process in the lamina propria (Roberts and Ellis 2012).
Another important organ analyzed was the kidney of the zebrafish. This organ has a renal corpuscle, a proximal and distal convoluted tubule, and nephrons responsible for filtering blood waste (Takshima and Hibiya 1995; Souza et al. 2019a). It is the organ responsible for physiological tasks that maintain homeostasis in the body, such as excretion of metabolites through the urine, nutrient conservation, regulation of osmosis, and the acid/base balance in the blood (Takshima and Hibiya 1995). Furthermore, it plays the vital role of excreting the volume of water that enters the animal through its mouth. It also filters waste and absorbs salt and water (Takshima and Hibiya 1985; Holden et al. 2013). It is one of the organs most affected by toxic substances, according to Carvalho et al. (Carvalho et al. 2018).
In animals treated with fibroin solution (3.00 ± 1.00) and at doses of 175 mg/kg (3.33 ± 0.57) and 550 mg/kg (4.33 ± 1.52), the HAI < 10 defines this organ as normal (Fig. 2C), as the observed alterations are not capable of changing how it functions. The alterations observed were only level I: mild tubular hyaline degeneration, tubular cell hypertrophy and the enlargement of the tubular lumen (Fig. 3F).
According to Carvalho et al., 2018, tubular alterations observed in zebrafish kidneys may be caused indirectly by metabolic dysfunction induced by exposure to toxic substances. In this study, the tubular alterations observed did not change the normal functioning of the organ.
The most significant damage occurred in the kidney of animals treated with the 2000 mg/kg dose of BASF, which presented a HAI of 12.00 ± 1.15, indicating mild to moderate organ alteration. The main change observed was severe tubular hyaline degeneration (level II). According to Takashima & Hibiya, 1995, tubular hyaline degeneration consists of an increase in the number of eosinophilic granules in the cytoplasm of the cell.
Anti-inflammatory activity of BASF in zebrafish model through inhibition of edema
To evaluate the anti-inflammatory effect of the BASF formulation, treatment was conducted with the formulation, BA, SF, PBS, and Indomethacin, administered 30 min before the induction of the inflammatory process. This process was induced by the administration of carrageenan, a bio product derived from marine algae, which, in addition to the formation of edema, leads to increased immune stimulation characterized by cell migration and a continuous inflammatory process (Prata et al. 2020). This method to assess substances with anti-inflammatory potential is currently an excellent option for screening this class of drugs (Huang et al. 2014).
From an extensive view of the results, the inhibition of inflammatory edema obtained values > 65% in group BASF, which received the baicalein-fibroin formulation treatment at different doses (5, 10, 50, 75, and 100 mg/ kg), clearly demonstrating the anti-inflammatory potential of BASF. The best results for reducing inflammatory edema in zebrafish were ascertained at BASF doses of 75 and 100 mg/kg, achieving 77.6 and 75.3% inhibition, respectively (Fig. 4).
Fig. 4.
A Inflammatory abdominal edema inhibition index at each dose of BASF. A -PBS for control, substance to dilute carrageenan (Cg); B—Cg + SF (carrageenan via intraperitoneal and silk fibroin via gavage) BASF negative control; C—NSAID nonsteroidal anti-inflammatory used for comparison purposes (indomethacin 10 mg/kg); BASF baicalein-fibroin formulation (group D), with subgroups by dose (5, 10, 50, 75, 100 mg/kg); E – baicalein BA (100 mg/kg), comparative control group. B Mean percentage of inhibition of abdominal edema on the effect of the oral treatment on animals. Data were expressed as mean ± standard error of the mean (n = 12 / group). *P ≤ 0.05 vs negative control; and One-way ANOVA statistical analysis was followed by Tukey–Kramer post hoc test. C The effect of the oral treatment on animals: A – PBS; B – fibroin solution on edema induced by carrageenan (300 µg intraperitoneal); D – BASF 75 mg/kg; E – baicalein 100 mg/kg
The reduction of abdominal edema by BASF probably occurs through the inhibition of the levels of TNF-α and iNOS (induced nitric oxide synthase), as these are pro-inflammatory cytokines responsible for the formation of this type of edema induced by carrageenan in zebrafish (Huang et al. 2014). The results obtained corroborate the anti-inflammatory activity of baicalein, proven by different authors (Mat Ali et al. 1998; Patwardhan et al. 2016; Dinda et al. 2017). With a known mechanism of action, baicalein works by attenuating the expression levels of pro-inflammatory cytokines, such as tumor necrosis factor (TNF-α) and interleukins (IL-1β, IL-6, IL-8) and COX-2 (Pu et al. 2019; Song et al. 2021), via expression of HMGB1, TRL4, Myd88, NF-kβ and Ikβ proteins (Yin et al. 2018).
Baicalein mainly acts via down-regulation of the transcription factor NF-Kβ, one of the primary gene regulators of inflammatory mediators, including the aforementioned cytokines (Patwardhan et al. 2016), and the chemokines MCP-1, NO, and adhesion molecules (Patwardhan et al. 2016). Baicalein acts by inactivating thioredoxin reductase (Trx R), which is responsible for reducing thioredoxin (Trx), which facilitates the binding of NF-Kβ in DNA for transcription, thereby blocking cytokine production, cell proliferation, and membrane signaling (Pu et al. 2019).
It is important to highlight the anti-inflammatory action of BA via arachidonic acid metabolism, through the attenuation of the levels of cyclooxygenase enzymes (COX-2) and lipoxygenase (5-LOX e 12-LOX), that are responsible for the synthesis of several chemical mediators such as prostaglandins, lipoxins and leukotrienes that act in the inflammatory response (Martel-Pelletier et al. 2003; Selvam and Jachak 2004; Favacho et al. 2011; Pineda-peña et al. 2020). BA studies in vivo have shown promising therapeutic action in rats with osteoporosis (Saul et al. 2017) and with diabetic cognitive dysfunction, acting as a neuroprotector (Li et al. 2019) both works associated with attenuation of LOX levels. Chandrashekar et al., 2012, investigated the therapeutic efficacy of BA on inflammatory cytokines (TNF-α, IL, iNOS) through the induction of pulmonary carcinogenesis in mice and revealed the interruption of factor activation NF-kβ and in parallel with the inhibition of enzyme expression COX-2.
Group A (PBS control) did not receive the dose of carrageenan, and therefore, there was no induction of inflammatory edema. This comparative group received only a carrageenan extender (PBS) intraperitoneally and was treated orally with saline (Borges et al. 2018).
The animals in group C (NSAID), which represented the positive control, were treated with indomethacin (10 mg/kg), and all animals were weighed 6 h after edema induction. The decrease in inflammatory edema in this group was 78.5% compared with the control group, which received only the fibroin solution as treatment (group B). It should be noted that BASF achieved levels of inhibition of abdominal edema (77.6%) similar to a commonly used anti-inflammatory drug (Mohamed et al. 2020). Indomethacin is a non-steroidal anti-inflammatory drug, widely used as a comparison for screening new anti-inflammatory molecules. In this case, it had a good response in inhibiting edema in zebrafish (Fasolo et al. 2021).
In group E with a solution containing only baicalein (single dose of 100 mg/kg), the inhibition of inflammatory edema was 51.6%, a 24% lower than the anti-inflammatory activity obtained with BASF at the same dose. Furthermore, at doses lower than 100 mg/kg (5, 10, 50 and 75 mg/kg) tested, the BA solution did not present a statistically significant difference in reducing inflammatory edema in zebrafish compared to the control group.
It can be inferred from these results that the solution prepared with SF potentiated the anti-inflammatory effect of baicalein (BA) due to the remarkable advantages of this biomaterial as a drug carrier and in the controlled release of drugs (Mottaghitalab et al. 2015; Pham and Tiyaboonchai 2020), since the preparation of pharmaceutical formulations with BA is difficult because of its low bioavailability and insolubility in water (Liu et al. 2012). The increased therapeutic effect of BASF might be related to the greater bioavailability of BA when using SF.
Histopathological evaluation of the inflammation test
Carvalho et al. (Carvalho et al. 2018) reported that due to the small size of zebrafish, the injection of an inflammatory agent in the abdominal region, such as carrageenan, can promove reactions in other vital organs, such as the liver (Fig. 5A), intestine (Fig. 5C) and kidneys (Fig. 5E).
Fig. 5.
A index of histopathological changes in the liver at each dose of BASF. Data are presented as mean ± SD (n = 5/groups); B index of histopathological changes in the intestine at each dose of BASF. Data are presented as mean ± SD (n = 3/groups); C index of histopathological changes in the kidney at each dose of BASF. Data are presented as mean ± SD (n = 3/groups); * p < 0.05 to the fibroin solution. Statistical analysis was performed through one-way ANOVA followed by the post hoc Tukey test
In group A animals (intraperitoneal PBS + oral saline solution), the HAI value was 0.66 ± 0.577 (Fig. 5A), indicating that the organ remained normal after the application of PBS. Histopathological analysis of the livers of group A animals showed only grade I alterations, such as cytoplasmic vacuolization (Fig. 6B). A study conducted by Borges et al. (Borges et al. 2018) revealed a similar result where intraperitoneal injection with PBS and oral saline solution caused only level I alterations.
Fig. 6.
Histopathological changes in the organs of the zebrafish in the study of inflammatory activity. A liver of a normal animal showing hepatocytes (H); B liver of a treated animal showing cytoplasmic vacuolization (CV), hyperemia (Hp) and nuclear atrophy (NA); C intestine of a normal animal showing goblet cells (GC), villi (V) and muscle layer (ML); D intestine of a treated animal showing dilation of vessels in villi (DV), stromal lymphocyte infiltration (SLI), leukocyte infiltration (LI) and displacement of lamina propria (DLP). E kidneys of normal animals in which lymphoid tissue (LT), tubules (Tb), glomeruli (G) and capsular space of the Bowman capsule (CSB) are observed; F kidneys of treated animals in which dilated glomerular capillary (DGC), capsular space of the Bowman capsule reduction (CSBR), tubular lumen increase (TLI) and mild tubular hyaline degeneration (MTHD) are observed. (H&E)
Group B (treated with Fibroin solution and an intraperitoneal injection of carrageenan) had a HAI of 23.5 ± 0.70 (Fig. 5A), which classified this organ as undergoing moderate to severe alterations. In terms of histopathology, group B presented significant histopathological alterations (levels I and II), such as loss of cell and nuclear contour (I), cytoplasmic vacuolization (I), glycogen decrease (I), nuclear atrophy (II), and hyperemia (II) (Fig. 6B). Our results agree with what was previously observed in the inflammation test, where group B presented bigger inflammatory edema, showing that the fibroin solution was unable to prevent the appearance of histopathological alterations caused by carrageenan. As the liver is A vital organ for substance detoxification, any dysfunction in its tissue can be harmful to the animal and even cause death (Carvalho et al. 2018).
Hyperemia, an alteration observed in animals treated with fibroin, occurs as an attempt to increase the general blood flow in the liver and to increase the release of nutrients and oxygen to the affected areas, avoiding hypoxia (Takshima and Hibiya 1995; Borges et al. 2018; Souza et al. 2019b).
The liver of animals in treatment group D – BASF (5, 10, 50, 75 and 100 mg/kg) showed normal liver tissue, with a HAI < 10 (9 ± 1.73; 8.33 ± 1.57; 4.66 ± 1.15; 3.66 ± 0.57; 3.33 ± 0.57) respectively, which classifies this organ as normal (Fig. 5A). The observed histopathological changes were loss of cell and nuclear contour (I), increase in cell volume (I), cytoplasmic vacuolization (I) and nuclear atrophy (II) (Fig. 6B).
Group E (treated with a dose of 100 mg/kg baicalein and intraperitoneal injection of carrageenan) and group C (treated with 10 mg/kg indomethacin) exhibited normal liver tissue, with a HAI of (3.33 ± 0.57 and 5.0 ± 1.57, respectively). This organ was therefore classified as normal. The histopathological changes observed were loss of cell and nuclear contours (I) and cytoplasmic vacuolization (I) (Fig. 6B).
According to Carvalho et al., 2018, the vacuolization observed in all the treatment groups is caused by a reduction of glycogen in the liver or an accumulation of toxic substances in the hepatocytes (or a combination of these two phenomena). In certain cases, vacuolization can alter liver function. The metabolism of the zebrafish is highly agile and greater than that of other larger fish species. This fact may help explain the presence of intense vacuolization of cytoplasm in hepatocytes, which in some cases indicates the onset of a degenerative process that may lead to disruption of metabolic processes after exposure to toxic substances (Takshima and Hibiya 1985). No cell degeneration was observed in this study, suggesting that the components tested did not cause sufficient changes to cause dysfunction of this organ.
According to Carvalho et al., 2018, in addition to its roles in nutrient absorption, the zebrafish intestinal epithelium is also an important site for immune responses and osmotic balance. It is also an important recycling site for enzymes and macronutrients (Alvarez-Pellitero and Sitja-Bobadilla 1993). Studies carried out by Borges et al., 2018, report histopathological changes in the zebrafish intestine after intraperitoneal injection of carrageenan. Previously, bowel studies were based only on informal observations (Roberts and Ellis 2012).
The intestinal tissue of group A (intraperitoneal PBS and treated with saline solution) did not show histopathological changes, and the HAI was 0.0 ± 0.0, classifying this organ as normal.
The groups whose HAI classified the intestine as having mild to moderate alterations were group B (carrageenan injection and treated with fibroin solution), BASF 5 mg/kg and 10 mg/kg. The calculated indexes were 17.33 ± 2.51 (group B), 11.33 ± 2.30 (BASF 5 mg/kg) and 10.33 ± 2.51 (BASF 10 mg/kg) (Fig. 5 B). These groups showed several histopathological changes: increased leukocyte infiltration (I), vessel dilation (I), lymphocyte infiltration (I), goblet cell hypertrophy (I), enterocyte vacuolization (I), displacement of lamina propria (II) (Fig. 6D). Vacuolization is a type of change that can compromise the body's ability to absorb nutrients. According to Borges et al. (Borges et al. 2018), the incidence of these changes may be related to the invasive technique of intraperitoneal injection, which can damage the intestine. Invasive procedures in fish can generate inflammation in the intestinal lamina propria and cause infiltration of leukocytes into the epithelial tissue of the intestine (Carvalho et al. 2018), in addition to causing an increase in defense cells (Roberts and Ellis 2012).
Groups BASF 50–100 mg/kg, D (100 mg/kg baicalein) and C (10 mg/kg indomethacin) have a HAI < 10, which classifies the intestine as normal, as the recorded changes could not affect the normal function of the organ. The most frequently observed histopathological changes: increased leukocyte infiltration (I), vessel dilation (I), lymphocyte infiltration (I), goblet cell hypertrophy (I), and epithelial cell hypertrophy (I) (Fig. 6D). The hypertrophy of epithelial cells observed in these groups may be considered a defense mechanism that serves as a barrier to reduce the penetration of these substances into the intestinal epithelium (Takshima and Hibiya 1985).
The kidney was also analyzed (Fig. 6E). This contains the nephrons, structures responsible for filtering blood debris and absorbing salt and water, with lymphoid, hematopoietic and steroidogenic areas, and endocrine cells (Borges et al. 2018). According to Holden et al., 2013, the main function of the kidneys in freshwater teleost fish is to excrete the large volume of water entering through the mouth without having to store it.
In group A kidneys (intraperitoneal PBS treated with saline solution) no histopathological damage was observed, with a HAI equal to 0.0 ± 0 (Fig. 5C). This result agrees with the observations of Borges et al., 2018, where intraperitoneal injection with PBS and oral saline did not cause renal damage.
Group B (carrageenan injection and treated with fibroin solution) showed level I and II histopathological changes: mild tubular hyaline degeneration (I); tubular cell hypertrophy (I), glomerular capillary dilatation (I), Bowman's capsule space reduction (I), increased tubular lumen (I), tubular degeneration (II), and severe tubular hyaline degeneration (II) (Fig. 6F). The HAI was 24.33 ± 0.57 (Fig. 5C), which classified the organ as having moderate to extreme alterations. According to Carvalho et al., 2018, the kidney is one of the organs most affected by toxic substances. This result shows that the fibroin solution was unable to prevent the onset of histopathological changes in this organ.
Groups BASF 5 and 10 mg/kg showed similar histopathological changes, such as mild tubular hyaline degeneration (I); tubular cell hypertrophy (I) tubular lumen increase (I), and severe tubular hyaline degeneration (II) (Fig. 6F). The HAI was 12.00 ± 1.73 and 11.33 ± 2.30 respectively (Fig. 5C), classifying this organ as suffering mild to moderate alterations.
According to Borges et al., 2018, hyaline degeneration consists of an increase in the number of eosinophilic granules in the cytoplasm of these cells and may be related to the reabsorption of excess proteins synthesized by the glomerulus (Takshima and Hibiya 1985).
The groups BASF 50—100 mg/kg, D—E (100 mg/kg baicalein), and C (10 mg/kg indomethacin) had a HAI < 10, which classifies the kidneys as normal as the changes observed are not capable of affecting the normal function of the organ. The most common histopathological changes observed: mild tubular hyaline degeneration (I), tubular cell hypertrophy (I), glomerular capillary dilatation (I), Bowman's capsule space reduction (I) and tubular lumen increase (I) (Fig. 6F).
With the advantages of using SF as a vehicle for drug delivery and sustained release due to its physicochemical properties (Wenk et al., 2011; Werner and Meinel 2015), the results obtained in this study significantly increased the bioavailability of BA at the site of action, thereby enhancing its therapeutic properties. We have also expanded the possibilities for the perfecting of innovative formulations by combining SF with BA.
Conclusions
This study addressed the use of silk fibroin (SF) as a baicalein carrier, as an alternative to conventional carrier polymers, favoring its antioxidant and anti-inflammatory activity, in addition to opening new perspectives for future formulations. The formulation developed, BASF, showed good stability through the assessment of zeta potential, and acute toxicity tests revealed that this combination is safe for oral administration in zebrafish, with few histopathological changes in the kidney, intestine, and liver of the animal.
Evidently, the inhibition of inflammatory edema induced by carrageenan with the groups treated with BASF demonstrated satisfactory results at all doses used. Histopathological and behavioral analyzes with zebrafish for anti-inflammatory assays confirm the proposal of the study, namely the creation of a simple, stable, and safe formulation. SF proved to be a promising, low-cost alternative for the development of innovative pharmaceutical formulations.
Funding
The authors would like to acknowledge the Research Support Foundation of the State of Amapá (Fundação de Amparo à Pesquisa do Estado do Amapá, FAPEAP); grant no. 34568.515.22257.28052017, the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES), for the financing of part of the present work and the Pharmaceutical Innovation Graduate Program (PPGIF) for their financial support.
Declarations
Conflict of interest
The authors have not disclosed any competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abbate F, Maugeri A, Laur R, et al. Zebrafish as a useful model to study oxidative stress-linked disorders: focus on flavonoids. Antioxidants (Basel) 2021 doi: 10.3390/antiox10050668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Pellitero P, Sitja-Bobadilla A. Pathology of myxosporea in marine fish culture. Dis Aquat Organ. 1993;17:229–238. doi: 10.3354/dao017229. [DOI] [Google Scholar]
- Araújo IF, Loureiro HA, Marinho VHS, et al. Larvicidal activity of the methanolic, hydroethanolic and hexanic extracts from Acmella oleracea, solubilized with silk fibroin, against Aedes aegypti. Biocatal Agric Biotechnol. 2020;24:101550. doi: 10.1016/j.bcab.2020.101550. [DOI] [Google Scholar]
- Bie B, Sun J, Guo Y, et al. Baicalein: a review of its anti-cancer effects and mechanisms in Hepatocellular Carcinoma. Biomed Pharmacother. 2017;93:1285–1291. doi: 10.1016/j.biopha.2017.07.068. [DOI] [PubMed] [Google Scholar]
- Borges RS, Keita H, Ortiz BLS, et al. Anti-inflammatory activity of nanoemulsions of essential oil from Rosmarinus officinalis L.: in vitro and in zebrafish studies. Inflammopharmacology. 2018;26:1057–1080. doi: 10.1007/s10787-017-0438-9. [DOI] [PubMed] [Google Scholar]
- Brugman S. The zebrafish as a model to study intestinal inflammation. Dev Comp Immunol. 2016;64:82–92. doi: 10.1016/j.dci.2016.02.020. [DOI] [PubMed] [Google Scholar]
- Carvalho JCT, Keita H, Santana GR, et al. Effects of Bothrops alternatus venom in zebrafish: a histopathological study. Inflammopharmacology. 2018;26:273–284. doi: 10.1007/s10787-017-0362-z. [DOI] [PubMed] [Google Scholar]
- Chan WP, Huang KC, Bai MY. Silk fibroin protein-based nonwoven mats incorporating baicalein Chinese herbal extract: preparation, characterizations, and in vivo evaluation. J Biomed Mater Res - Part B Appl Biomater. 2017;105:420–430. doi: 10.1002/jbm.b.33560. [DOI] [PubMed] [Google Scholar]
- Chandrashekar N, Selvamani A, Subramanian R, et al. Baicalein inhibits pulmonary carcinogenesis-associated inflammation and interferes with COX-2, MMP-2 and MMP-9 expressions in-vivo. Toxicol Appl Pharmacol. 2012;261:10–21. doi: 10.1016/j.taap.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Chen L, Batjikh I, Hurh J, et al. Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik (Stuttg) 2019;184:324–329. doi: 10.1016/j.ijleo.2019.03.051. [DOI] [Google Scholar]
- de Jong WH. Drug delivery and nanoparticles : applications and hazards. Int J Nanomed. 2008;3:133–149. doi: 10.2147/IJN.S596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de SáHyacienthTavares Picanço BMKR, Sánchez-Ortiz BL, et al. Hydroethanolic extract from Endopleura uchi (Huber) cuatrecasas and its marker bergenin: toxicological and pharmacokinetic studies in silico and in vivo on zebrafish. Toxicol Reports. 2020;7:217–232. doi: 10.1016/j.toxrep.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza GC, Duarte JL, Fernandes CP, et al. Obtainment and study of the toxicity of perillyl alcohol nanoemulsion on zebrafish danio rerio. J Nanomedicine Res. 2016;4:00093. [Google Scholar]
- de Souza GC, Matias Pereira AC, Viana MD, et al. Acmella oleracea (L) R K. jansen reproductive toxicity in zebrafish: an in vivo and in silico assessment. Evidence-based Complement Altern Med. 2019 doi: 10.1155/2019a/1237301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza GC, da Silva IDR, Viana MD, et al. Acute toxicity of the hydroethanolic extract of the flowers of Acmella oleracea l In zebrafish (Danio rerio): behavioral and histopathological studies. Pharmaceuticals. 2019 doi: 10.3390/ph12040173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinda B, Dinda S, DasSharma S, et al. Therapeutic potentials of baicalin and its aglycone, baicalein against inflammatory disorders. Eur J Med Chem. 2017;131:68–80. doi: 10.1016/j.ejmech.2017.03.004. [DOI] [PubMed] [Google Scholar]
- dos Santos SB. Toxicidade aguda do pesticida clorpirifós de formulação comercial em tambaqui (Colossoma macropomum. CUVIER. 2019;1818:89. [Google Scholar]
- Erdoğar N, Akkın S, Bilensoy E. Nanocapsules for drug delivery: an updated review of the last decade. Recent Pat Drug Deliv Formu. 2018 doi: 10.2174/1872211313666190123153711. [DOI] [PubMed] [Google Scholar]
- Fasolo JMMA, Vizuete AFK, Rico EP, et al. Anti-inflammatory effect of rosmarinic acid isolated from Blechnum brasiliense in adult zebrafish brain. Comp Biochem Physiol Part - C Toxicol Pharmacol. 2021;239:108874. doi: 10.1016/j.cbpc.2020.108874. [DOI] [PubMed] [Google Scholar]
- Favacho HAS, Oliveira BR, Santos KC, et al. Anti-inflammatory and antinociceptive activities of Euterpe oleracea oil. Rev Bras Farmacogn. 2011;21:105–114. doi: 10.1590/S0102-695X2011005000007. [DOI] [Google Scholar]
- Ferreira IM, de Ganzeli L S, LRosset IG, et al. Ethylic biodiesel production using lipase immobilized in silk fibroin-alginate spheres by encapsulation. Catalysis Letters. 2017;147(1):269–80. doi: 10.1007/s10562-016-1917-0. [DOI] [Google Scholar]
- Ferreira IM, Yoshioka SA, Comasseto JV, Porto ALM. Immobilization of amano lipase from Pseudomonas fluorescens on silk fibroin spheres: an alternative protocol for the enantioselective synthesis of halohydrins. RSC Adv. 2017;7:12650–12658. doi: 10.1039/c7ra00083a. [DOI] [Google Scholar]
- Fuster MG, Carissimi G, Montalbán MG, Víllora G. Improving anticancer therapy with naringenin-loaded silk fibroin nanoparticles. Nanomaterials. 2020 doi: 10.3390/nano10040718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H. The progress of novel drug delivery systems. Eye. 2017;25(5):578–586. [PubMed] [Google Scholar]
- Gao Z, Huang K, Yang X, Xu H. Free radical scavenging and antioxidant activities of flavonoids extracted from the radix of Scutellaria baicalensis Georgi. Biochim Biophys Acta - Gen Subj. 1999;1472:643–650. doi: 10.1016/S0304-4165(99)00152-X. [DOI] [PubMed] [Google Scholar]
- Goksøyr A. Use of cytochrome P450 1A (CYP1A) in fish as a biomarker of aquatic pollution. Arch Toxicol Suppl. 1995;17:80–95. doi: 10.1007/978-3-642-79451-3_7. [DOI] [PubMed] [Google Scholar]
- Gu XH, Xu LJ, Liu ZQ, et al. The flavonoid baicalein rescues synaptic plasticity and memory deficits in a mouse model of Alzheimer’s disease. Behav Brain Res. 2016;311:309–321. doi: 10.1016/j.bbr.2016.05.052. [DOI] [PubMed] [Google Scholar]
- Guo LT, Wang SQ, Su J, et al. Baicalin ameliorates neuroinflammation-induced depressive-like behavior through inhibition of toll-like receptor 4 expression via the PI3K/AKT/FoxO1 pathway. J Neuroinflammation. 2019;16:1–21. doi: 10.1186/s12974-019-1474-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden JA, Layfield LJ, Matthews JL. The Zebrafish: atlas of macroscopic and microscopic anatomy. Cambridge Univ Press Cambridge. 2013 doi: 10.1017/CBO9781139198431. [DOI] [Google Scholar]
- Hosseinkhani H, Hosseinkhani M. Biodegradable polymer-metal complexes for gene and drug delivery. Curr Drug Saf. 2009;4:79–83. doi: 10.2174/157488609787354477. [DOI] [PubMed] [Google Scholar]
- Huang SY, Feng CW, Hung HC, et al. A novel zebrafish model to provide mechanistic insights into the inflammatory events in carrageenan-induced abdominal edema. PLoS One. 2014 doi: 10.1371/journal.pone.0104414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iio M, Ishimoto S, Nishida Y, et al. Effects of baicalein, a flavonoid, and other anti-inflammatory agents on glyoxalase-i activity. Agric Biol Chem. 1986;50:1073–1074. doi: 10.1080/00021369.1986.10867521. [DOI] [Google Scholar]
- Jeevanandam J, Chan YS, Danquah MK. Zebrafish as a model organism to study nanomaterial toxicity. Emerg Sci J. 2019;3:195–208. doi: 10.28991/esj-2019-01182. [DOI] [Google Scholar]
- Joshi HA, Patwardhan RS, Sharma D, et al. Pre-clinical evaluation of an innovative oral nano-formulation of baicalein for modulation of radiation responses. Int J Pharm. 2021;595:120181. doi: 10.1016/j.ijpharm.2020.120181. [DOI] [PubMed] [Google Scholar]
- Kavithaa K, Paulpandi M, Padma PR, Sumathi S. Induction of intrinsic apoptotic pathway and cell cycle arrest: Via baicalein loaded iron oxide nanoparticles as a competent nano-mediated system for triple negative breast cancer therapy. RSC Adv. 2016;6:64531–64543. doi: 10.1039/c6ra11658b. [DOI] [Google Scholar]
- Kim H, Yiluo H, Park S, et al. Characterization and enhanced antioxidant activity of the cysteinyl β-cyclodextrin-baicalein inclusion complex. Molecules. 2016 doi: 10.3390/molecules21060703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. The husbandry of zebrafish (Danio rerio): a review. Aquaculture. 2007;269:1–20. doi: 10.1016/j.aquaculture.2007.04.077. [DOI] [Google Scholar]
- Lemarchand C, Couvreur P, Vauthier C, et al. Study of emulsion stabilization by graft copolymers using the optical analyzer turbiscan. Int J Pharm. 2003;254:77–82. doi: 10.1016/S0378-5173(02)00687-7. [DOI] [PubMed] [Google Scholar]
- Li K, Zhang H, Gao L, et al. Preparation and characterization of baicalein-loaded nanoliposomes for antitumor therapy. J Nanomater 2016. 2016 doi: 10.1155/2016/2861915. [DOI] [Google Scholar]
- Li Y, He ZD, Zheng QE, et al. Hydroxypropyl-β-cyclodextrin for delivery of baicalin via inclusion complexation by supercritical fluid encapsulation. Molecules. 2018 doi: 10.3390/molecules23051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chen Q, Ran D, et al. Changes in the levels of 12/15-lipoxygenase, apoptosis-related proteins and inflammatory factors in the cortex of diabetic rats and the neuroprotection of baicalein. Free Radic Biol Med. 2019;134:239–247. doi: 10.1016/j.freeradbiomed.2019.01.019. [DOI] [PubMed] [Google Scholar]
- Little EE, Fairchild JF, DeLonay AJ. Behavioral methods for assessing impacts of contaminants on early life stage fishes. Am Fish Soc Symp. 1993;14:67–76. [Google Scholar]
- Liu W, Tian R, Hu W, et al. Preparation and evaluation of self-microemulsifying drug delivery system of baicalein. Fitoterapia. 2012;83:1532–1539. doi: 10.1016/j.fitote.2012.08.021. [DOI] [PubMed] [Google Scholar]
- Liu Q, Liu H, Fan Y. Preparation of silk fibroin carriers for controlled release. Microsc Res Tech. 2017;80:312–320. doi: 10.1002/jemt.22606. [DOI] [PubMed] [Google Scholar]
- Lopes-Lutz D, Alviano DS, Alviano CS, Kolodziejczyk PP (2008) Screening of chemical composition, antimicrobial and antioxidant activities of Artemisia essential oils. Phytochemistry 69:1732–1738. 10.1016/j.phytochem.2008.02.014 [DOI] [PubMed]
- Lozano-Pérez AA, Rivero HC, del Pérez Hernández M, C, et al. Silk fibroin nanoparticles: Efficient vehicles for the natural antioxidant quercetin. Int J Pharm. 2017;518:11–19. doi: 10.1016/j.ijpharm.2016.12.046. [DOI] [PubMed] [Google Scholar]
- Luo Z, Kuang XP, Zhou QQ, et al. Inhibitory effects of baicalein against herpes simplex virus type 1. Acta Pharm Sin B. 2020;10:2323–2338. doi: 10.1016/j.apsb.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel Ferreira I, Coutinho Rocha L, Akinobo Yoshioka S, et al. Chemoselective reduction of chalcones by whole hyphae of marine fungus Penicillium citrinum CBMAI 1186, free and immobilized on biopolymers. Biocatal Agric Biotechnol. 2014;3:358–364. doi: 10.1016/j.bcab.2014.04.001. [DOI] [Google Scholar]
- Maleki SJ, Crespo Jesus F, Cabanillas Beatriz. Anti-inflammatory effects of flavonoids. Food chemistry. 2019;299:125124. doi: 10.1016/j.foodchem.2019.125124. [DOI] [PubMed] [Google Scholar]
- Marinho VHS, Neves FB, Jimenez DEQ, et al. Development of an environmentally friendly formulation of silk fibroin combined with fatty acid from astrocaryum murumuru Mart. effective against aedes aegypti larvae. J Drug Deliv Sci Technol. 2022 doi: 10.1016/j.jddst.2022.103626. [DOI] [Google Scholar]
- Martel-Pelletier J, Lajeunesse D, Reboul P, Pelletier JP. Therapeutic role of dual inhibitors of 5-LOX and COX, selective and non-selective non-steroidal anti-inflammatory drugs. Ann Rheum Dis. 2003;62:501–509. doi: 10.1136/ard.62.6.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Flórez S, González-Gallego J, Culebras JM, Tuñón MJ. Los flavonoides: propiedades y acciones antioxidantes. Nutr Hosp. 2002;17:271–278. [PubMed] [Google Scholar]
- Mat Ali R, Houghton PJ, Raman A, Hoult JRS. Antimicrobial and antiinflammatory activities of extracts and constituents of Oroxylum indicum (L.) Vent. Phytomedicine. 1998;5:375–381. doi: 10.1016/s0944-7113(98)80020-2. [DOI] [PubMed] [Google Scholar]
- Meinel L, Betz O, Fajardo R, et al. Silk based biomaterials to heal critical sized femur defects. Bone. 2006;39:922–931. doi: 10.1016/j.bone.2006.04.019. [DOI] [PubMed] [Google Scholar]
- Mirzadegan E, Golshahi H, Kazemnejad S. Current evidence on immunological and regenerative effects of menstrual blood stem cells seeded on scaffold consisting of amniotic membrane and silk fibroin in chronic wound. Int Immunopharmacol. 2020;85:106595. doi: 10.1016/j.intimp.2020.106595. [DOI] [PubMed] [Google Scholar]
- Mishra P, Dutta S, Haldar M, et al. Enhanced mosquitocidal efficacy of colloidal dispersion of pyrethroid nanometric emulsion with benignity towards non-target species. Ecotoxicol Environ Saf. 2019;176:258–269. doi: 10.1016/j.ecoenv.2019.03.096. [DOI] [PubMed] [Google Scholar]
- Mohamed MFA, Marzouk AA, Nafady A, et al. Design, synthesis and molecular modeling of novel aryl carboximidamides and 3-aryl-1,2,4-oxadiazoles derived from indomethacin as potent anti-inflammatory iNOS/PGE2 inhibitors. Bioorg Chem. 2020;105:104439. doi: 10.1016/j.bioorg.2020.104439. [DOI] [PubMed] [Google Scholar]
- Mottaghitalab F, Farokhi M, Shokrgozar MA, et al. Silk fibroin nanoparticle as a novel drug delivery system. J Control Release. 2015;206:161–176. doi: 10.1016/j.jconrel.2015.03.020. [DOI] [PubMed] [Google Scholar]
- Mrcp SJP, Katz A, Wang Y, et al. Vitamin C as an Antioxidant : Evaluation of Its Role in Disease Prevention. J Am Collage Nutr. 2013 doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- Novoa B, Pereiro P, López-Muñoz A, et al. Rag1 immunodeficiency-induced early aging and senescence in zebrafish are dependent on chronic inflammation and oxidative stress. Aging Cell. 2019;18:1–17. doi: 10.1111/acel.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palierse E, Hélary C, Krafft JM, et al. Baicalein-modified hydroxyapatite nanoparticles and coatings with antibacterial and antioxidant properties. Mater Sci Eng C. 2021;118:111537. doi: 10.1016/j.msec.2020.111537. [DOI] [PubMed] [Google Scholar]
- Park CE, Park DJ, Kim BK. Effects of a chitosan coating on properties of retinol-encapsulated zein nanoparticles. Food Sci Biotechnol. 2015;24:1725–1733. doi: 10.1007/s10068-015-0224-7. [DOI] [Google Scholar]
- Patwardhan RS, Sharma D, Thoh M, et al. Baicalein exhibits anti-inflammatory effects via inhibition of NF-κB transactivation. Biochem Pharmacol. 2016;108:75–89. doi: 10.1016/j.bcp.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Pham DT, Tiyaboonchai W. Fibroin nanoparticles: a promising drug delivery system. Drug Deliv. 2020;27:431–448. doi: 10.1080/10717544.2020.1736208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pineda-peña EA, Orona-ortiz A, Velázquez-moyado JA, et al. Anti-inflammatory, and gaso-protective mechanism of 3 α -hydroxymasticadienoic acid and diligustilide combination on indomethacin gastric damage. Naunyn-Schmiedeberg’s Arch Pharmacol. 2020;393:1501–1513. doi: 10.1007/s00210-020-01857-7. [DOI] [PubMed] [Google Scholar]
- Poleksic V, Mitrovic-Tutundzic V (1994) Fish gills as a monitor of sublethal and chronic effects of pollution. In Müller R, Lloyd R (eds) Sublethal and Chronic Effects of Pollutants on Freshwater Fish. Fishing New Books ltd., Farnham. pp. 339–352
- Prata MNL, Charlie-Silva I, Gomes JMM, et al. Anti-inflammatory and immune properties of the peltatoside, isolated from the leaves of Annona crassiflora Mart., in a new experimental model zebrafish. Fish Shellfish Immunol. 2020;101:234–243. doi: 10.1016/j.fsi.2020.03.044. [DOI] [PubMed] [Google Scholar]
- Psimadas D, Georgoulias P, Valotassiou V, Loudos G. Molecular nanomedicine towards cancer. J Pharm Sci. 2012;101:2271–2280. doi: 10.1002/jps. [DOI] [PubMed] [Google Scholar]
- Pu WL, Bai RY, Zhou K, Peng YF, Zhang MY, Hottiger MO, Li WH, Gao XM, Sun LK. Baicalein attenuates pancreatic inflammatory injury through regulating MAPK, STAT 3 and NF-κB activation. Int immunopharmacol. 2019;72:204–10. doi: 10.1016/j.intimp.2019.04.018. [DOI] [PubMed] [Google Scholar]
- Quitian-Useche YF, Sánchez-Ortiz BL, Borges SF, et al. Fatty ethanolamide of Bertholletia excelsa triglycerides (Brazil nuts): anti-inflammatory action and acute toxicity evaluation in zebrafish (Danio rerio) Inflammopharmacology. 2021 doi: 10.1007/s10787-021-00867-y. [DOI] [PubMed] [Google Scholar]
- Ren C, Park EY, Kim JY, Lim ST. Enhancing dispersion stability of alpha-tocopherol in aqueous media using maize starch and ultrasonication. LWT - Food Sci Technol. 2016;68:589–594. doi: 10.1016/j.lwt.2016.01.001. [DOI] [Google Scholar]
- Roberts RJ, Ellis AE. The anatomy and physiology of teleosts. Fish Pathol Fourth Ed. 2012 doi: 10.1002/9781118222942.ch2. [DOI] [Google Scholar]
- Santos IVF, de Souza GC, Santana GR, et al. Histopathology in Zebrafish (Danio rerio) to Evaluate the Toxicity of Medicine: An Anti-Inflammatory Phytomedicine with Janaguba Milk (Himatanthus drasticus Plumel) Intech i:13. 2018 doi: 10.5772/intechopen.76670. [DOI] [Google Scholar]
- Sarquis IR, Sarquis RSFR, Marinho VHS, et al. Carapa guianensis Aubl. (Meliaceae) oil associated with silk fibroin, as alternative to traditional surfactants, and active against larvae of the vector Aedes aegypti. Ind Crops Prod. 2020 doi: 10.1016/j.indcrop.2020.112931. [DOI] [Google Scholar]
- Saul D, Gleitz S, Nguyen HH, et al. Effect of the lipoxygenase-inhibitors baicalein and zileuton on the vertebra in ovariectomized rats. Bone. 2017;101:134–144. doi: 10.1016/j.bone.2017.04.011. [DOI] [PubMed] [Google Scholar]
- Selvam C, Jachak SM. A cyclooxygenase (COX) inhibitory biflavonoid from the seeds of Semecarpus anacardium. J Ethnopharmacol. 2004;95:209–212. doi: 10.1016/j.jep.2004.07.026. [DOI] [PubMed] [Google Scholar]
- Shao ZH, Vanden Hoek TL, Qin Y, et al. Baicalein attenuates oxidant stress in cardiomyocytes. Am J Physiol - Hear Circ Physiol. 2002;282:999–1006. doi: 10.1152/ajpheart.00163.2001. [DOI] [PubMed] [Google Scholar]
- Sithisarn P, Michaelis M, Schubert-Zsilavecz M, Cinatl J. Differential antiviral and anti-inflammatory mechanisms of the flavonoids biochanin A and baicalein in H5N1 influenza A virus-infected cells. Antiviral Res. 2013;97:41–48. doi: 10.1016/j.antiviral.2012.10.004. [DOI] [PubMed] [Google Scholar]
- Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::AID-JBM17>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Song J, Zhang L, Xu Y, et al. The comprehensive study on the therapeutic effects of baicalein for the treatment of COVID-19 in vivo and in vitro. Biochem Pharmacol. 2021 doi: 10.1016/j.bcp.2020.114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowndhararajan K, Deepa P, Kim M, et al. Baicalein as a potent neuroprotective agent: a review. Biomed Pharmacother. 2017;95:1021–1032. doi: 10.1016/j.biopha.2017.08.135. [DOI] [PubMed] [Google Scholar]
- Spence R, Gerlach G, Lawrence C, Smith C. The behaviour and ecology of the zebrafish, Danio rerio. Biol Rev. 2008;83:13–34. doi: 10.1111/j.1469-185X.2007.00030.x. [DOI] [PubMed] [Google Scholar]
- Takshima F, Hibiya T. An atlas of fish histology normal and phatology feature. 2. Tokyo: Kodansha Ltd; 1985. [Google Scholar]
- Takshima F, Hibiya T. An atlas of fish histology normal and phatology feature. Tokyo: Kodansha Ltd; 1995. [Google Scholar]
- Tsai MJ, Wu PC, Bin Huang Y, et al. Baicalein loaded in tocol nanostructured lipid carriers (tocol NLCs) for enhanced stability and brain targeting. Int J Pharm. 2012;423:461–470. doi: 10.1016/j.ijpharm.2011.12.009. [DOI] [PubMed] [Google Scholar]
- Victor I, Duarte JL, Fernandes CP, et al. Use of zebrafish (Danio rerio) in experimental models for biological assay with natural products. Afr J Pharm Pharmacol. 2016;10:883–891. doi: 10.5897/AJPP2016.4662. [DOI] [Google Scholar]
- Vliegenthart ADB, Tucker CS, DelPozo J, Dear JW. Zebrafish as model organisms for studying drug-induced liver injury. Br J Clin Pharmacol. 2014;78:1217–1227. doi: 10.1111/bcp.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, MehrabiNasab E, Athari SS. Study effect of baicalein encapsulated/loaded chitosan-nanoparticle on allergic asthma pathology in mouse model. Saudi J Biol Sci. 2021 doi: 10.1016/j.sjbs.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Nasab EM, Athari SS. Study effect of chitosan-nanoparticle encapsulated/loaded baicalein on allergic Asthma pathology in mouse model. Saudi J Biol Sci. 2021 doi: 10.1016/j.sjbs.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenk E, Merkle H, P, Meinel L (2011) Silk fibroin as a vehicle for drug delivery applications. J Control Release 150:128–141. 10.1016/j.jconrel.2010.11.007 [DOI] [PubMed]
- Werner V, Meinel L. From silk spinning in insects and spiders to advanced silk fibroin drug delivery systems. Eur J Pharm Biopharm. 2015;97:392–399. doi: 10.1016/j.ejpb.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Wu CC, Chen YR, Lu DH, et al. Evaluation of the post-treatment anti-inflammatory capacity of osteoarthritic chondrocytes: An in vitro study using baicalein. Regen Ther. 2020;14:177–183. doi: 10.1016/j.reth.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan JJ, Du GH, Qin XM, Gao L. Baicalein attenuates the neuroinflammation in LPS-activated BV-2 microglial cells through suppression of pro-inflammatory cytokines, COX2/NF-κB expressions and regulation of metabolic abnormality. Int Immunopharmacol. 2020;79:106092. doi: 10.1016/j.intimp.2019.106092. [DOI] [PubMed] [Google Scholar]
- Yin H, Huang L, Ouyang T, Chen L. Baicalein improves liver inflammation in diabetic db/db mice by regulating HMGB1/TLR4/NF-κB signaling pathway. Int Immunopharmacol. 2018;55:55–62. doi: 10.1016/j.intimp.2017.12.002. [DOI] [PubMed] [Google Scholar]
- Yoshino M, Ito M, Okajima H, et al. Role of baicalein compounds as antioxidant in the traditional herbal medicine. Biomed Res. 1997;18:349–352. doi: 10.2220/biomedres.18.349. [DOI] [Google Scholar]
- Yu X, Tang W, Yang Y, et al. Long noncoding RNA NKILA enhances the anti-cancer effects of baicalein in hepatocellular carcinoma via the regulation of NF-κB signaling. Chem Biol Interact. 2018;285:48–58. doi: 10.1016/j.cbi.2018.02.027. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Yang M, Portney NG, et al. Zeta potential: A surface electrical characteristic to probe the interaction of nanoparticles with normal and cancer human breast epithelial cells. Biomed Microdevices. 2008;10:321–328. doi: 10.1007/s10544-007-9139-2. [DOI] [PubMed] [Google Scholar]
- Zhang J, Lv H, Jiang K, Gao Y. Enhanced bioavailability after oral and pulmonary administration of baicalein nanocrystal. Int J Pharm. 2011;420:180–188. doi: 10.1016/j.ijpharm.2011.08.023. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Wang L, et al. Interactions of the baicalin and baicalein with bilayer lipid membranes investigated by cyclic voltammetry and UV-Vis spectroscopy. Bioelectrochemistry. 2014;95:29–33. doi: 10.1016/j.bioelechem.2013.10.008. [DOI] [PubMed] [Google Scholar]
- Zhang J, Deng Y, Cheng B, et al. Protective effects and molecular mechanisms of baicalein on thioacetamide-induced toxicity in zebrafish larvae. Chemosphere. 2020;256:127038. doi: 10.1016/j.chemosphere.2020.127038. [DOI] [PubMed] [Google Scholar]
- Zhang X, Xie J, Xu Z, et al. The interaction between cucurbit[8]uril and baicalein and the effect on baicalein properties. Beilstein J Org Chem. 2020;16:71–77. doi: 10.3762/bjoc.16.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Liu S, Wan J, et al. Preparation, characterization and in vivo study of borneol-baicalin-liposomes for treatment of cerebral ischemia-reperfusion injury. Int J Nanomedicine. 2020;15:5977–5989. doi: 10.2147/IJN.S259938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Fu L, Yang W, et al. Cardioprotective effects of baicalein on heart failure via modulation of Ca2 + handling proteins in vivo and in vitro. Life Sci. 2016;145:213–223. doi: 10.1016/j.lfs.2015.12.036. [DOI] [PubMed] [Google Scholar]
- Zhao T, Tang H, Xie L, et al. Scutellaria baicalensis Georgi (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J Pharm Pharmaco. 2019 doi: 10.1111/jphp.13129. [DOI] [PubMed] [Google Scholar]