Abstract
In a previous study we showed that the involvement of EP4 subtype of the prostaglandin E (PGE) receptor is crucial for lipopolysaccharide (LPS)-induced osteoclast formation in vitro. The present study was undertaken to test whether EP4 is actually associated with LPS-induced bone resorption in vivo. In wild-type (WT) mice, osteoclast formation in vertebrae and tibiae increased 5 days after systemic LPS injection, and urinary excretion of deoxypyridinoline, a sensitive marker for bone resorption, statistically increased 10 days after injection. In EP4 knockout (KO) mice, however, LPS injection caused no significant changes in these parameters throughout the experiment. LPS exposure for 4 h strongly induced osteoclast differentiation factor (ODF) mRNA expression in primary osteoblastic cells (POB) both from WT and EP4 KO mice, and this expression was not inhibited by indomethacin, suggesting prostaglandin (PG) independence. LPS exposure for 24 h further induced ODF expression in WT POB, but not in EP4 KO POB. Indomethacin partially inhibited ODF expression in WT POB, but not in EP4 KO POB. These data suggest that ODF is induced both PG dependently and PG independently. LPS exposure for 24 h induced slightly greater osteoclastgenesis inhibitory factor (OCIF) mRNA expression in EP4 KO than in WT POB. These findings suggest that the reduced ODF expression and apparently increased OCIF expression also are responsible for the markedly reduced LPS-induced osteoclast formation in EP4 KO mice. Our results show that the EP4 subtype of the PGE receptor is involved in LPS-induced bone resorption in vivo also. Since LPS is considered to be largely involved in bacterially induced bone loss, such as in periodontitis and osteomyelitis, our study is expected to help broaden our understanding of the pathophysiology of these conditions.
Osteomyelitis, bacterial arthritis, and periodontal diseases are all caused by bacterial infection (13, 24). Since bacteria do not invade the periodontal tissues, the release of soluble bacterial factors is thought to be involved in the pathogenesis of these forms of bacterially induced bone destruction. Lipopolysaccharide (LPS) is the most likely candidate for mediating these processes. LPS has been reported to potently stimulate bone resorption in both in vitro and in vivo studies (12, 16, 26). LPS was reported to induce osteoclast formation in bone marrow culture, and local injection of LPS into the femur was found to lead to a rapid increase in the number of osteoclasts and the area of eroded surface. These findings indicate that increased osteoclast formation is involved in LPS-stimulated bone resorption. Osteoclast formation is an important step in bone resorption, since only osteoclasts can actively resorb bone and have a short life span, less than 2 weeks (8, 23). Osteoclasts originate from hematopoietic stem cells and belong to the monocyte/macrophage lineage (36, 39). Differentiation into osteoclasts is influenced by many systemic and local factors, such as hormones, cytokines, growth factors, and eicosanoids. LPS has been known to stimulate the production of many local factors, including tumor necrosis factor α (TNF-α), interleukin-1 (IL-1), and prostaglandin E2 (PGE2) from macrophages, fibroblasts, and osteoblastic cells in inflamed periodontal tissues (2, 3, 24). Despite rigorous studies by many researchers (4, 5, 11, 22, 46), however, the molecular mechanism of LPS-induced osteoclast formation remains unknown. We therefore focused our attention on the involvement of prostaglandin (PG) in LPS-induced osteoclast formation, based on the recently cloned PG receptor and the results of our recent studies on the skeletal action of PGs (see below).
PGs, especially of the E series, are potent bone-active substances, PGE2 was reported to potently resorb bone and enhance bone formation both in vitro and in vivo (27, 29). Until recently, however, the mechanism of action of PGs remained largely unknown. Recently, though, PG receptors have been cloned and thoroughly characterized. Four PGE receptor subtypes have been cloned in mice. They are coupled with different intracellular signaling mechanisms: EP1 with calcium mobilization, EP2 and EP4 with the stimulation of adenylate cyclase, and EP3 mainly with the inhibition of adenylate cyclase (10, 14, 18, 37, 42). On the basis of these recent findings, we aimed to determine through which subtype of PGE receptor each of the various skeletal actions of PGE2 is exerted.
We recently studied the mechanism of PGE2-induced osteoclast formation in the coculture (30), and EP4 agonist was found to be far more potent in osteoclast formation than any other agonists. Therefore, we studied EP4 subtype-deficient (EP4 knockout [KO]) mice, which have been recently generated by us, to clarify the importance of EP4 in bone. Most EP4 KO mice died shortly after birth because of failure to close of the ductus arteriosus, a blood vessel which bypasses pulmonary circulation during fetal life (25, 32). EP4 KO mice which survived to adulthood had no skeletal abnormalities as seen by their gross appearance, soft X-ray analysis, or histological examination. Since these results suggest that EP4 is not essential for the bone function under the basal condition, we then studied the role of EP4 under the disease state, where PGE2 production is increased. We first studied the involvement of the EP4 subtype in exogenously added PGE2-induced osteoclast formation. Our data clearly showed that PGE2 enhances osteoclast formation through the EP4 subtype on primary osteoblastic cells (POB), not on osteoclast precursors. We then studied the possible involvement of the EP4 subtype in osteoclast formation induced by IL-1α, TNF-α, basic fibroblast growth factor (bFGF), and LPS. All these molecules markedly enhanced osteoclast formation in the coculture from wild-type (WT) mice but not in the coculture from EP4 KO mice. Furthermore, they significantly induced prostaglandin G/H synthase-2 (PGHS-2) (which is the inducible form of the rate-limiting enzyme in PG synthesis) mRNA expression in POB, and osteoclast formation by these molecules was almost completely eliminated by the PGHS inhibitor indomethacin. Therefore, the likely mechanism of osteoclast formation by IL-1α, TNF-α, bFGF, and LPS is that they increase PG production by inducing PGHS-2 in POB and that PGE2, in turn, stimulates osteoclast formation mainly via the EP4 subtype on osteoblasts.
In order to follow up on this evidence that the EP4 subtype of the PGE receptor is crucially involved in LPS-induced osteoclast formation in vitro, the aim of the study reported here was to test the in vivo relevance of this finding.
MATERIALS AND METHODS
Reagents.
LPS (Escherichia coli 0127:B8) was purchased from Difco Laboratories (Detroit, Mich.). The osteoclast differentiation factor (ODF) and osteoclast inhibitory factor (OCIF) cDNAs were prepared by reverse transcription-PCR as previously described (20, 45). β-Actin cDNA probe and indomethacin were purchased from Wako Chemical Industry (Tokyo, Japan).
Generation of EP4 KO mice.
Details of the generation of EP4 KO mice have been described elsewhere (25, 32). In brief, a 1.6-kb fragment containing the coding region from Asn-32 in the N-terminal region to Val-317 in the sixth transmembrane region was replaced with a neomycin resistance gene. The targeting vector was then electroporated into E14-1 ES cells. Chimeric males were mated to C57BL/6 females, and homozygous mutant mice were obtained by interbreeding of heterozygous mice.
Treatment.
Each experiment included the WT and EP4 KO groups, consisting of seven mice each. The mice were male, 11 weeks old, weighed approximately 25 g, and were age matched. There was no significant difference in body weight between WT and EP4 KO mice. LPS (20 mg/kg of body weight) diluted on sterile 0.01 M phosphate-buffered saline (PBS) or PBS was injected subcutaneously into the back of WT mice or EP4 KO mice (1). Urine was collected before and on days 5, 7, 10, and 14 after injection. Urinary excretion of deoxypyridinoline (D-Pyr) was measured with an enzyme immunoassay kit (Pyrilinks-D; Metra Biosystems Inc., Mountain View, Calif.) and was corrected by simultaneously measured urinary creatinine concentration (40). Serum was collected before and on days 3, 5, 7, 10, and 14 after injection. Serum osteocalcin levels were measured with a radioimmunoassay kit (mouse osteocalcin RIA reagents; Biomedical Technologies Inc., Stoughton, Mass.).
Histomorphometry.
Vertebrae (lumbar 2 to 4) and tibiae from WT and EP4 KO mice were prepared before and on days 5 and 10 after LPS injection. The vertebrae and tibiae were fixed in 4% paraformaldehyde–PBS (pH 7.2) for 24 h, decalcified by means of 10% EDTA–PBS (pH 7.2) for 7 days, dehydrated in a graded concentration of ethanol, and embedded in paraffin according to the standard procedure. Five-micrometer-thick sections were placed on APS-coated glass slides (Matsunami, Osaka, Japan) and stained for tartrate-resistant acid phosphate (TRAP) using a naphthol ASTR (Sigma Chemical Co., St. Louis, Mo.) (9). For each lumbar vertebra and tibia, two rectangular areas of interest with a width of 0.45 mm were randomly assigned for analysis, extending 0.2 to 0.4 mm from the growth plate. For each group of seven mice, the results were averaged. Osteoclasts were identified as TRAP-positive cells when they had three or more nuclei, directly faced a bone surface, or were located in a resorption cavity.
Cell culture, RNA extraction, and Northern blot analysis.
POB were isolated from the calvariae of 1-day-old WT and EP4 KO mice by sequential digestion with bacterial collagenase and dispase (34). POB (3.5 × 103 cells/cm2) from WT mice and EP4 KO mice were seeded in 6-cm-diameter plates, and cultured in α-minimum Eagle's medium containing 10% fetal bovine serum (JRH Biosciences, Lenexa, Kans.). At confluency, cells were cultured in a starvation medium (α-minimum Eagle's medium containing 0.1% fetal bovine serum) for 24 h and exposed to LPS (100 ng/ml) with or without indomethacin (10−7 M). After a 4- or 24-h incubation, total RNA was extracted with an RNA Easy Kit (QIAGEN). The RNA was then electrophoresed on a 1.2% agarose gel and transferred onto a Biodyne B membrane (Pall Biosupport, East Hills, N.Y.) and hybridized with ODF, OCIF, and β-actin probes labeled with [α-32P]dCTP. Hybridization was carried out at 42°C overnight in a hybridization buffer (50% deionized formamide, 5× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 5× Denhardt's solution, 0.2% sodium dodecyl sulfate [SDS], 200 μg of sonicated salmon sperm DNA per ml). The membrane was washed with a washing buffer (2× SSC–0.1% SDS and 0.2× SSC–0.1% SDS), and the radioactivity was analyzed by a bioimage analyzer (BAS 2000; Fuji Film, Tokyo, Japan). The levels of ODF and OCIF mRNAs were analyzed relative to that of β-actin.
Statistical analysis.
To test statistical significance between two groups, Student's t test was used. For more than two groups, an analysis of variance was used to determine which groups were significantly different. A P value of <0.05 was considered to be statistically significant.
RESULTS
Urinary excretion of D-Pyr.
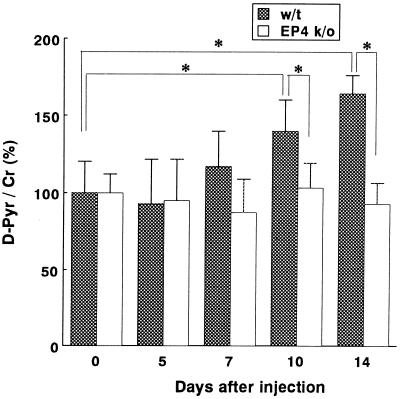
Systemic LPS injection into WT mice caused a statistically significant increase in the urinary excretion of D-Pyr, which is a sensitive marker for bone resorption. It was approximately 140% of the initial value on day 10 and approximately 160% on day 14 in WT mice (Fig. 1). In contrast, there was no significant increase of D-Pyr in EP4 KO mice throughout the experiment (day 0 through 14) (Fig. 1).
FIG. 1.
Effect of systemic LPS injection on urinary excretion of D-Pyr. LPS (20 mg/kg of body weight) was injected subcutaneously into 11-week-old WT mice or EP4 KO mice. The ratios of D-Pyr (nanomolar) creatinine (Cr) (millimolar) before injection (day 0) and on days 5, 7, 10, and 14 after injection are shown. Values for the basal level (before injection) were assigned 100% in each case. Data are expressed as the mean + standard deviation (error bar). (n = 7). ∗, P < 0.05.
Histomorphometric analysis of lumbar vertebrae and tibiae.
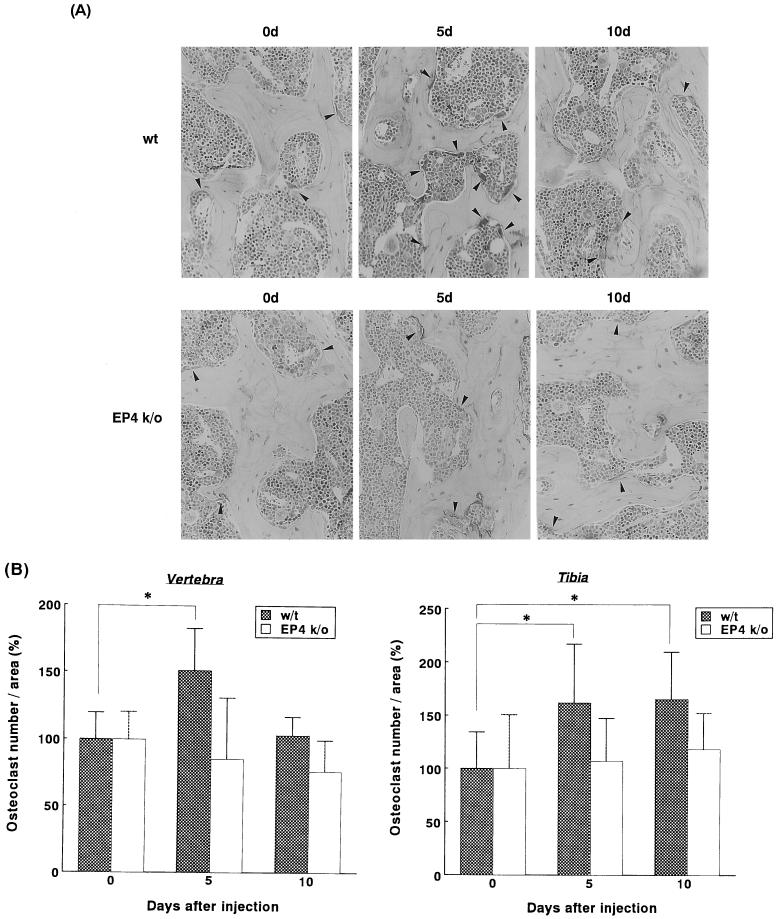
Osteoclast formation was evaluated by counting the number of cells which were positively stained with TRAP. After systemic injection of LPS into the WT mice, the number of osteoclasts of the lumbar vertebrae statistically significantly increased to approximately 150% of the basal level on day 5 and returned to almost the basal level on day 10. In sharp contrast, LPS injection into the EP4 KO mice caused no significant increase in the number of osteoclasts throughout the experiment (Fig. 2).
FIG. 2.
(A) Histological analysis of vertebrae from WT mice (top) and EP4 KO mice (bottom). Figures show representative photographs of TRAP staining of lumbar vertebrae before (0d) and on days 5 and 10 (5d and 10d, respectively) after systemic LPS injection into WT or EP4 KO mice. Cells stained with red (showed by arrows) represent osteoclasts. (B) Osteoclast formation in sections of the vertebrae and the tibiae from WT mice and EP4 KO mice after systemic LPS injection. Mice were sacrificed before injection (day 0) and on days 5 and 10, and their vertebrae and tibiae were processed for histomorphometry. Values for the basal level (before injection) were assigned 100% in each case. Data are expressed as the mean + standard deviation (error bar). ∗, P < 0.05.
The number of osteoclasts of the tibiae, in WT mice, statistically significantly increased to approximately 160 and 165% of the basal level on days 5 and 10, respectively. In contrast, LPS injection into the EP4 KO mice caused less increase in the number of osteoclasts of the tibiae (107 and 118% on days 5 and 10, respectively) (Fig. 2B).
Serum osteocalcin levels.
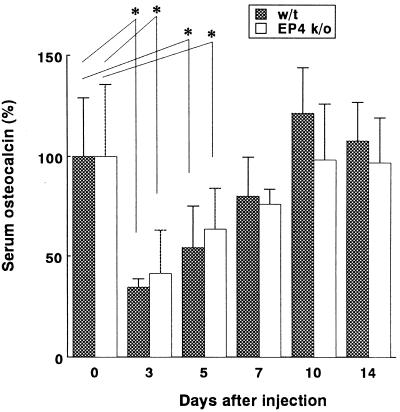
After systemic injection of LPS, serum osteocalcin levels had decreased to approximately 40% of the basal level by day 3 after injection and returned to the initial values 10 days after injection. Throughout the experiment, there was no significant difference between WT and EP4 KO mice (Fig. 3).
FIG. 3.
Serum osteocalcin levels in WT or EP4 KO mice before (day 0) and on days 3, 5, 7, 10, and 14 after injection. Levels are expressed as the percentage of the value before injection for either group. Data are expressed as the mean + standard deviation (error bar). (n = 7). ∗, P < 0.05.
ODF and OCIF mRNA expressions in POB.
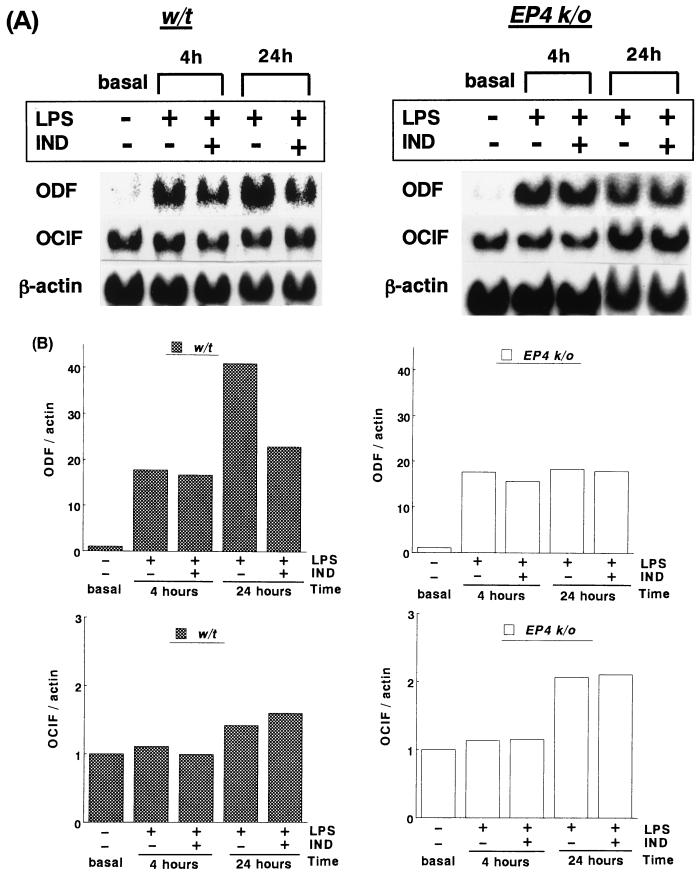
ODF mRNA expression was hardly detectable under the basal condition (after 24 h of starvation) in POB from either WT or EP4 KO POB. LPS (100 ng/ml) exposure for 4 and 24 h markedly and time dependently induced ODF mRNA expression in POB from WT mice. In POB from EP4 KO mice, LPS exposure for 4 h induced ODF mRNA at almost the same level as WT, and LPS exposure for 24 h increased ODF expression, though this increase was minimal (Fig. 4A).
FIG. 4.
Effect of LPS (100 ng/ml) on ODF and OCIF mRNA expression in POB from WT and EP4 KO mice. At confluency, POB were cultured in a starvation medium for 24 h and exposed to LPS with or without indomethacin (IND) (10−7 M) for 4 or 24 h. Fifteen micrograms of total RNA from each of the POB cultures was hybridized to ODF, OCIF, and β-actin probes. (A) Representative results of ODF and OCIF expression among similar results from four independent experiments. (B) The levels of ODF and OCIF mRNAs were normalized to that of β-actin at each time point by means of northern blot hybridization. Values for the basal level (after 24 h of starvation) were assigned 1.0 in each case.
The ODF level normalized to β-actin induced by LPS exposure for 4 h was approximately 15 times the basal level (average of four independent experiments) in POB from WT and EP4 KO mice. The ODF level induced by LPS exposure for 24 h was 240% ± 43% (from four independent experiments) of that induced by LPS exposure for 4 h in POB from WT mice. In contrast, the ODF level induced by LPS exposure for 24 h was 118% ± 24% of that induced by LPS exposure for 4 h in EP4 KO mice, which was significantly lower than that in WT mice (Fig. 4B). The ODF level induced by LPS exposure for 4 h was not inhibited by indomethacin either in WT or EP4 KO POB. In contrast, the ODF level by LPS exposure for 24 h was inhibited 34% ± 15% by indomethacin in WT POB, but it was not inhibited by indomethacin in EP4 KO POB.
OCIF mRNA expression was not significantly different under the basal condition between POB from WT and that from EP4 KO mice. LPS (100 ng/ml) exposure for 4 h caused no appreciable changes in OCIF mRNA expression in POB from either WT or EP4 KO mice. LPS exposure for 24 h, however, increased OCIF mRNA expression in POB from both WT and EP4 KO mice (Fig. 4A). OCIF levels normalized to β-actin induced by LPS exposure for 24 h were approximately 150% ± 27% of the basal level in WT mice and 215% ± 49% of the basal level in EP4 KO mice (average of four independent experiments) (Fig. 4B). OCIF levels induced by LPS exposure were not affected by indomethacin, irrespective of the cell origin (WT or EP4 KO), or duration (4 or 24 h).
DISCUSSION
Some studies have suggested the involvement of PGE2 in LPS-induced bone resorption (15, 31). Inhibition of LPS-induced bone resorption by inhibitors of PG synthesis has been reported by several investigations. LPS was found to increase 45Ca release, hence bone resorption, from neonatal mouse calvariae, and this increase was partly inhibited by indomethacin. More specifically, the involvement of PG in LPS-induced osteoclast formation has also been suggested. For example, Ueda et al. showed that indomethacin inhibited LPS-induced osteoclast formation in bone marrow cultures (41). Elevation of the number of osteoclasts after LPS injection was reduced by about 50% by indomethacin (10−7 M) in mouse calvariae (46). Furthermore, flurbiprofen, a nonsteroidal anti-inflammatory drug and PGHS inhibitor, reduced the alveolar bone loss associated with periodontitis in beagles in vivo (43). In addition, Plotquin et al. reported that PGE2 production was 5 to 30 times higher in osteomyelitic human bones compared with control bones, whereas prostacyclin production remained the same in these two types of bones (28). These reports indicate that it is likely that PGE2 at least partially mediates LPS-induced bone resorption. However, these findings constitute indirect evidence and do not provide information on the action mechanism of PGE2. To follow up on our recent observation that the involvement of the EP4 subtype of the PGE receptor is essential for LPS-induced osteoclast formation in vitro (30), the aim of the study reported here was to determine whether this mechanism also functions in vivo. For this purpose, LPS was injected systemically into WT and EP4 KO mice.
The overall bone resorption was evaluated by urinary excretion of D-Pyr. D-Pyr is a degradation product of the cross-link structure of triple helical type I collagen and is a very sensitive marker for bone resorption (40). LPS injection resulted in a marked increase in urinary D-Pyr excretion in WT but not in EP4 KO mice. Since these data suggest that the EP4 subtype of the PGE receptor is essential for LPS-induced bone resorption in vivo, we then studied whether the EP4 subtype is involved in LPS-induced osteoclast formation in vivo. Histological sections of the lumbar vertebrae and tibiae were stained for TRAP, which is a representative marker for osteoclasts. Histomorphometric analysis revealed that systemic injection of LPS significantly increased the number of osteoclasts of WT vertebrae and tibiae. Interestingly, on day 10 after LPS injection, osteoclast numbers in vertebrae returned to the basal level, in contrast, osteoclast numbers in tibiae remained increased. These results suggest that LPS produces generalized increases in osteoclasts, although each bone may respond to LPS with different time courses. In contrast, LPS caused little changes in osteoclast numbers in vertebrae or tibiae of EP4 KO mice. These results clearly show that the EP4 subtype is involved in LPS-induced osteoclast formation and bone resorption in vivo.
Our results indicated that the number of osteoclasts statistically increased 5 days after LPS injection but that the increase in urinary D-Pyr excretion did not become obvious until day 10 and had only slightly increased by day 7. A recent report also showed that the peak of osteoclast formation and of the area of the eroded surface covered by osteoclasts occurred on day 5 after LPS injection (9). At present, there is no clear explanation for this apparent discrepancy in the time course of the number of osteoclasts and urinary D-Pyr excretion. Although D-Pyr has not been studied in LPS-injected mice, similar findings have been reported in an ovariectomy model, with recent reports (7, 21) showing that deterioration in the trabecular bone connectivity and volume already occurred on day 5 after ovariectomy, while urinary D-Pyr excretion increased more slowly (on day 13). Probably some period is required before D-Pyr excretion increases, which reflects an increase in bone resorption by newly formed osteoclasts. Of further interest is the reduction in serum osteocalcin levels in both WT and EP4 KO mice after LPS injection. Since osteocalcin is a differentiation marker of osteoblasts, and hence of bone formation, these results suggest that LPS negatively affects bone formation both in WT and EP4 KO mice. Recently, LPS was reported to inhibit osteoblastic cell differentiation in rat calvarial cells (17). Although further studies are required, these data suggest that the EP4 subtype is involved in LPS-induced bone resorption but not in the negative influence of LPS on bone formation.
We subsequently studied the downstream mechanism of EP4. Osteoclast formation is known to be enhanced by three major classes of stimuli: the 1,25(OH)2 vitamin D3 pathway, the cyclic AMP-mediated pathway (parathyroid hormone and PGE2), and the gp-130 mediated pathway (IL-6, IL-11, and oncostatin M). Suda et al. postulated that these molecules commonly induce a putative factor to enhance osteoclast formation on osteoblasts (35). Recently this putative common factor, the ODF (also known as osteoprotegerin ligand, OPGL, RANKLE, or TRANCE) was cloned by several groups of researchers. ODF is a TNF-α-like molecule, is induced on the osteoblast plasma membrane by the stimuli mentioned earlier, and enhances osteoclast differentiation through the interaction with its receptor on osteoclast precursor cells (20, 38, 45). Another molecule involved in this cell-cell interaction was cloned: the OCIF (also known as osteoprotegerin or OPG). OCIF functions as a decoy receptor and interferes with the interaction of ODF and its receptor (33, 44). Their functional relevance is clearly shown by the finding that ODF KO mice develop osteopetrosis and OCIF KO mice develop severe osteoporosis (6, 19). In the present study, LPS exposure for 4 h strongly induced ODF mRNA expression in POB from WT mice and EP4 KO mice. ODF levels in WT and EP4 KO were almost the same, and this may be due to a PG-independent pathway, because the levels were not inhibited by indomethacin. LPS exposure for 24 h further increased ODF mRNA expression level (240% of that by LPS exposure for 4 h) in WT POB, and this induction was inhibited about 34% by indomethacin. These results suggest that ODF induction was induced by not only a PG-dependent pathway but also a PG-independent pathway such as a direct effect of LPS. Although LPS exposure for 24 h increased ODF mRNA expression in EP4 KO mice (118% of that by LPS exposure for 4 h), this increase was minimal, and this induction was not inhibited by indomethacin. From these results, a small increase in ODF expression in EP4 KO POB is likely to be due to a PG-independent pathway rather than a PG-dependent pathway via another receptor except EP4 subtype. LPS induced slightly more OCIF mRNA expression in POB from EP4 KO mice than WT mice. These findings suggest that the reduced ODF expression and apparently increased OCIF expression also are responsible for the aforementioned finding that bone resorption induced by LPS is markedly reduced in EP4 KO mice.
Our previous in vitro data showed, in the coculture using cells from EP4 KO mice, little osteoclast was formed by PGE2, IL-1α, TNF-α, bFGF, and LPS, which suggests that osteoclast formation by these molecules is mostly PG dependent. In contrast, osteoclast formation by 1,25(OH)2 vitamin D3 and parthyroid hormone in the coculture from EP4 KO mice was only partially decreased, which suggests that osteoclast formation by 1,25(OH)2 vitamin D3 and parathyroid hormone is largely PG independent. The above findings, together with the current data, suggest that impaired LPS-induced osteoclast formation is a specific phenomenon in EP4 KO mice and not the nonspecific or indirect consequence of the lack of EP4 receptor.
In conclusion, the involvement of the EP4 subtype of the PGE receptor is important for LPS-induced bone resorption in vivo. Antagonizing EP4 action may thus prove to be clinically useful for the treatment of bacterially induced bone loss, such as in periodontitis and osteomyelitis.
ACKNOWLEDGMENTS
This work was supported by a grant-in-aid from the Ministry of Sciences, Education, and Culture and grants from the Research Association for the Metabolic Bone Diseases and the Smoking Research Foundation.
REFERENCES
- 1.Abu-Amer Y, Ross F P, Edwards J, Teitelbaum S L. Lipopolysaccharide-stimulated osteoclastogenesis is mediated by tumor necrosis factor via its p55 receptor. J Clin Investig. 1997;100:1557–1565. doi: 10.1172/JCI119679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agarwall S, Piesco N P, Johns L P, Riccelli A E. Differential expression of IL-1 beta, TNF-alpha, IL-6, IL-8 in human monocytes in response to lipopolysaccharides from different microbes. J Dent Res. 1995;74:1057–1065. doi: 10.1177/00220345950740040501. [DOI] [PubMed] [Google Scholar]
- 3.Aznar C, Fitting C, Cavaillon J M. LPS-induced production of cytokines by bone marrow-derived macrophages: destruction between intracellular IL-1 production and IL-1 release. Cytokine. 1990;2:259–265. doi: 10.1016/1043-4666(90)90026-p. [DOI] [PubMed] [Google Scholar]
- 4.Birkeadal-Hansen H. Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res. 1993;28:500–510. doi: 10.1111/j.1600-0765.1993.tb02113.x. [DOI] [PubMed] [Google Scholar]
- 5.Bom-van Noorloos A A, van der Merr J W, van de Gevel J S, Schepens E, van Steenbergen T J, Burger E H. Bacteroides gingivalis stimulates bone resorption via interleukin production by mononuclear cells. The relative role for B. gingivalis endotoxin. J Clin Periodontol. 1990;17:409–413. doi: 10.1111/j.1600-051x.1990.tb02338.x. [DOI] [PubMed] [Google Scholar]
- 6.Bucay N, Sasori I, Dundtan C R, Morony S, Tarpley J, Capparelli C, Scully S, Tan H L, Xu W, Lacey D L, Boyle W J, Simonet W S. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998;12:1260–1268. doi: 10.1101/gad.12.9.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casari E, Alfano M, Clarke G D, Ferni G, Grazioli B. Ovarectomy in the rat induces a rapid increase in the urinary excretion of hydroxylysine glycosides and non-reducible crosslink residues. Osteoporos Int. 1997;7:539–543. doi: 10.1007/BF02652559. [DOI] [PubMed] [Google Scholar]
- 8.Chambers T J. Regulation of osteoclast development and function. In: Rifkin B R, Gay C V, editors. Biology and physiology of the osteoclast. Boca Raton, Fla: CRC Press; 1991. pp. 105–128. [Google Scholar]
- 9.Chiang C H, Kyritsis G, Graves D T, Amar S. Interleukin-1 and tumor necrosis factor avtivities partially account for calvarial bone resorption induced by local injection of lipopolysaccharide. Infect Immun. 1999;67:4231–4236. doi: 10.1128/iai.67.8.4231-4236.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman R A, Smith W L, Narumiya S. Classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol Rev. 1994;46:205–209. [PubMed] [Google Scholar]
- 11.Hausmann E, Weinfeld N, Miller W A. Effect of lipopolysaccharide on bone resorption in tissue culture. Calcif Tissue Res. 1972;9:272–282. doi: 10.1007/BF02061967. [DOI] [PubMed] [Google Scholar]
- 12.Hausmann E, Raisz L G, Miller W A. Endotoxin: stimulation of bone resorption in tissue culture. Science. 1970;168:862–864. doi: 10.1126/science.168.3933.862. [DOI] [PubMed] [Google Scholar]
- 13.Holt S C, Ebersole J L. The surface of selected periodontopathic bacteria: possible role in virulence. In: Hamada S, Holt S C, McGhee J R, editors. Periodontal disease. 79. Pathogens and host immune responses. Tokyo, Japan: Quintessence Publishing Co., Ltd.; 1991. pp. 981–991. [Google Scholar]
- 14.Honda A, Sugimoto Y, Namba T, Watabe A, Irie A, Negishi M, Narumiya S, Ishikawa A. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP2 subtype. J Biol Chem. 1993;268:7759–7762. [PubMed] [Google Scholar]
- 15.Howell T H, Williams R C. Nonsteroidal antiflammatory drugs as inhibitors of periodontal disease progression. Crit Rev Oral Biol Med. 1993;4:177–196. doi: 10.1177/10454411930040020301. [DOI] [PubMed] [Google Scholar]
- 16.Ishihara Y, Nishihara T, Maki E, Noguchi T, Koga T. Role of interlrukin-1 and prostaglandin in in-vitro bone resorption induced by actinobacillus-actinomycetemcomitans lipopolysaccharide. J Periodontal Res. 1991;26:155–160. doi: 10.1111/j.1600-0765.1991.tb01639.x. [DOI] [PubMed] [Google Scholar]
- 17.Kadono H, Kido J, Kataoka M, Yamauchi N, Nagata T. Inhibition of osteoblastic cell differentiation by lipopolysaccharide extract from Porphyromonas gingivalis. Infect Immun. 1999;67:2841–2846. doi: 10.1128/iai.67.6.2841-2846.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katsuyama M, Nishigaki N, Sugimoto Y, Morimoto K, Negishi M, Narumiya S, Ichikawa A. The mouse prostaglandin E receptor EP2 subtypes: cloning, expression and Northern blot analysis. FEBS Lett. 1995;372:151–156. doi: 10.1016/0014-5793(95)00966-d. [DOI] [PubMed] [Google Scholar]
- 19.Kong Y Y, Yoshida H, Sarosi I, Tan H L, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos A J, Van G, Itie A, Khoo W, Wakeham A, Dunstan C R, Lacey D L, Mak T W, Boyle W J, Penninger J M. OPGL is a key factor of osteoclastgenesis, lymphocyte development and lymph-node organogenesis. Nature. 2000;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 20.Lacey D L, Timms E, Tan H L, Kelly M J, Dunstan C R, Burgess T, Elicott R, Colombero A, Scully G E S, Hsu H, Sullivan J, Hawkins N, Davy E, Capparelli C, Eli A, Qian Y X, Kaufman S, Sarosi I, Shalhoub V, Senaldi G, Delaney J, Boyle W J. Osteoprogerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 21.Lane N E, Thompson J M, Haupt D, Kimmel D B, Modin G, Kinney J H. Acute changes in travecular bone connectivity and osteoclast activity in the ovarectomized rat in vivo. J Bone Miner Res. 1998;13:229–236. doi: 10.1359/jbmr.1998.13.2.229. [DOI] [PubMed] [Google Scholar]
- 22.Meryon S D, Perris A D. Lipopolysaccharide-induced bone resorption is mediated by prostaglandins. Life Sci. 1981;28:1061–1065. doi: 10.1016/0024-3205(81)90754-2. [DOI] [PubMed] [Google Scholar]
- 23.Mundy G R, Roodman G D. Osteoclast ontogeny and function, annual 5. In: Peck W A, editor. Bone and mineral research. Amsterdam, The Netherlands: Elsevier; 1987. pp. 209–279. [Google Scholar]
- 24.Nair S P, Meghji S M, Wilson M, Reddi K, White P, Henderson B. Bacterial induced bone destruction: mechanisms and misconceptions. Infect Immun. 1996;64:2371–2380. doi: 10.1128/iai.64.7.2371-2380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen M, Camenisch T, Snouwaert J N, Hicks E, Coffman T M, Anderson P A W, Malouf N N, Koller B H. The prostaglandin receptor EP4 triggers remodelling of the cardiovascular system at birth. Nature. 1997;390:78–81. doi: 10.1038/36342. [DOI] [PubMed] [Google Scholar]
- 26.Orcel P, Feuga M, Bielakoff J, De Vernejoul M C. Local bone injections of LPS and M-CSF increase bone resorption by different pathways in vivo in rats. Am J Physiol. 1993;264:E391–397. doi: 10.1152/ajpendo.1993.264.3.E391. [DOI] [PubMed] [Google Scholar]
- 27.Pilbeam C C, Harrison J R, Raisz L G. Prostaglandins and bone metabolism. In: Bilezikian J P, Raisz L G, Rodan G A, editors. Principles of bone biology. San Diego, Calif: Academic Press; 1996. pp. 715–728. [Google Scholar]
- 28.Plotquin D, Dekel S, Katz S, Danon A. Prostaglandin release by normal and osteomyelitic human bones. Prostaglandins Leukot Essent Fatty Acids. 1991;43:13–15. doi: 10.1016/0952-3278(91)90126-p. [DOI] [PubMed] [Google Scholar]
- 29.Raisz L G. Physiologic and pathologic roles of prostaglandins and other eicosanoids in bone metabolism. J Nutr. 1995;125:2024S–2027S. doi: 10.1093/jn/125.suppl_7.2024S. [DOI] [PubMed] [Google Scholar]
- 30.Sakuma Y, Tanaka K, Suda M, Yasoda A, Natsui K, Tanaka I, Ushikubi F, Narumiya S, Segi E, Sugimoto Y, Ichikawa A, Nakao K. Crucial involvement of the EP4 subtype of prostaglandin E (PGE) receptor in osteoclast formation by proinflammatory cytokines and lipopolysaccharide. J Bone Miner Res, 2000;15:218–227. doi: 10.1359/jbmr.2000.15.2.218. [DOI] [PubMed] [Google Scholar]
- 31.Salvi G E, Williams R C, Offenbacher S. Nonsteroidal anti-inflammatory drugs as adjuncts in the manegement of periodontal diseases and peri-implantitis. Curr Opin Periodontol. 1997;4:51–58. [PubMed] [Google Scholar]
- 32.Segi E, Sugimoto Y, Yamasaki A, Aze Y, Oida H, Nishimura T, Murata T, Matsuoka T, Ushikubi F, Hirose M, Tanaka T, Yoshida N, Narumiya S, Ichikawa A. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 33.Simonet W S, Lacey D L, Dunstan C R, Kelley M, Chang M S, Luethy R, Nguen H Q, Wooden S, Bennett L, Boone T. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;64:693–702. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 34.Suda T, Jimi E, Nakamura I, Takahashi N. Role of 1α,25-dihydroxyvitamin D3 in osteoclast differentiation and function. Methods Enzymol. 1997;282:223–235. doi: 10.1016/s0076-6879(97)82110-6. [DOI] [PubMed] [Google Scholar]
- 35.Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie M T, Martin T J. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocrinol Rev. 1999;20:345–357. doi: 10.1210/edrv.20.3.0367. [DOI] [PubMed] [Google Scholar]
- 36.Suda T, Udagawa N, Takahashi N. Cells of bone: osteoclast generation. In: Bilezikian J P, Raisz L G, Rodan G A, editors. Principles of bone biology. San Diego, Calif: Academic Press; 1996. pp. 87–102. [Google Scholar]
- 37.Sugimoto Y, Namba T, Honda T, Hayashi Y, Negishi M, Ichikawa A, Narumiya S. Cloning and expression of a cDNA for mouse prostaglandin E receptor EP3 subtype. J Biol Chem. 1992;267:6463–6466. [PubMed] [Google Scholar]
- 38.Tsukii K, Shima N, Mochizuki S, Yamaguchi K, Kinosaki M, Yano K, Shibata O, Udagawa N, Yasuda H, Suda T, Higashio K. Osteoclast differentiation factor mediates an essential signal for bone resorption induced by 1α,25-dihydroxyvitamin D3, prostaglandin E2, or parathyroid hormone in the microenvironment of bone. Biochem Biophys Res Commun. 1998;246:337–341. doi: 10.1006/bbrc.1998.8610. [DOI] [PubMed] [Google Scholar]
- 39.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin T J, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Uebelhart D, Gineyts E, Chapuy M C, Delmas P D. Urinary excretion of pyridinium crosslinks: a new marker of bone resorption in metabolic bone desease. Bone Miner. 1990;8:87–96. doi: 10.1016/0169-6009(91)90143-n. [DOI] [PubMed] [Google Scholar]
- 41.Ueda N, Koide M, Ohguchi M, Ishihara Y, Noguchi T, Okahashi N, Nishihara T. Involvement of prostaglandin E2 and interleukin-1 alpha in the differentiation and survival of osteoclasts induced by lipopolysaccharide from Actinobacillus actinomycetemcomitans Y4. J Periodontal Res. 1998;33:509–516. doi: 10.1111/j.1600-0765.1998.tb02351.x. [DOI] [PubMed] [Google Scholar]
- 42.Watabe A, Sugimoto Y, Honda A, Irie A, Namba T, Negishi M, Ito M, Narumiya S, Ichikawa A. Cloning and expresion of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J Biol Chem. 1993;268:20175–20178. [PubMed] [Google Scholar]
- 43.Williams R C, Jeffcoat M K, Kaplan M L, Goldhaber P, Johnson H G, Wechter W J. Flurbiprofen: a potent inhibitor of alveolar bone resorption in beagles. Science. 1985;227:640–642. doi: 10.1126/science.3969553. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda H, Shima N, Nakagawa N, Mochizuki S, Yano K, Fujise N, Sato Y, Goto M, Yamaguchi K, Kuriyama M, Kanno T, Murakami A, Tsuda E, Morinaga T, Higashio K. Identify of osteoclast inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–1337. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 45.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci USA. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zubery Y, Dunstan C R, Story M, Kesavalu L, Ebersole J L, Holt S C, Boyce B F. Bone resorption caused by three periodontal pathogens in vivo in mice is mediated in part by prostaglandin. Infect Immun. 1999;66:4158–4162. doi: 10.1128/iai.66.9.4158-4162.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]