Abstract
Background
Children of parents who were overweight/obese prior to pregnancy face a variety of neurodevelopmental challenges. The goal of this meta-analysis is to compile evidence about the impact of parental overweight/obesity on their children’s mental health.
Methods
The databases Cochrane Library, EMBASE, Pubmed, PsycINFO, and Web of Science were searched until May 2022. The pooled effect size was calculated using the fixed and random effect models. We also performed I2 index, subgroup analyses, sensitivity analyses, quality assessment, and publication bias analysis. The protocol was registered on the PROSPERO database (CRD42022334408).
Results
For maternal exposure (35 studies), both maternal overweight [OR 1.14 (95% CI 1.10,1.18)] and maternal obesity [OR 1.39 (95% CI (1.33, 1.45)] were significantly associated with offspring’s mental disorders. Maternal pre-pregnancy overweight/obesity increased the risk of attention-deficit/hyperactivity disorder (ADHD) [OR 1.55 (95% CI 1.42,1.70)], autism spectrum disorder (ASD) [OR 1.37 (95% CI 1.22,1.55)], cognitive/intellectual delay [OR 1.40 (95% CI 1.21,1.63)], behavioral problems [OR 1.50 (95% CI 1.35,1.66)] and other mental diseases [OR 1.30 (95% CI 1.23,1.37)]. For paternal exposure (6 studies), paternal obesity [OR 1.17 (95% CI 1.06, 1.30)] but not overweight [OR 1.03 (95% CI 0.95,1.11)] was significantly associated with offspring’s mental disorders.
Conclusions
Parental overweight/obesity might have negative consequences on offspring’s mental health and pre-pregnancy weight control is advised.
Introduction
The prevalence of mental illness is clearly on the rise. According to the Global Burden of Diseases 2019 report, there is no global indication of a decline in mental illnesses since 1990, placing them among the top 10 primary sources of burden globally [1]. A diverse set of genetic and/or environmental risk factors play an important role in the etiology of mental illness by altering brain structure and/or function. Notably, the incidence of parental obesity has been rising together with the prevalence of mental illness, suggesting a possible link between the two phenomena [2]. Studies have shown children born to obese mothers may have a higher risk of ASD and ADHD, as well as perform worse in terms of intellectual disability (ID) than children born to normal weight mothers. Paternal obesity may also negatively impact cognitive and behavioral outcomes in offspring [3–5]. The underlying mechanisms causing these later-life detrimental consequences in offspring are not clear. Potential pathways whereby metabolized nutritional components may affect child development. Both maternal and paternal obesity can impact several processes involved in the conception, as well as intrauterine and postnatal development of the offspring [3, 6].
Previous meta-analysis established a link between maternal obesity and offspring’s neurodevelopment. All of them reported offspring exposed to maternal overweight/obesity, might raise the risk of psychiatric disorders such as ADHD, ASD, negative emotion, schizophrenia, and behavior problems [7–12]. But they seldom comprehensively assessed their offspring’s mental health and failed to carefully check maternal weight exposure prior to pregnancy. Despite the equal prevalence of obesity in both sexes [13–15], the vast majority of research has focused on maternal nutritional influences on children during gestation and lactation. However, limited attention has been paid to the effect of paternal weight on offspring [16]. None of the previous meta analyses considered the effect of paternal overweight/obesity on offspring’s mental health. Only one analyzed the relationship between paternal body mass index (BMI) and ASD in the offspring [9] and stated that paternal BMI has no association with offspring’s ASD because of insufficient evidence. More importantly, a large number of studies on this topic have been published in recent years and have explored the effect of paternal BMI, with inconsistent outcomes [17–19].To better summarize the expanding research, we performed a meta-analysis to fully evaluate the association between parental obesity/overweight and offspring’s mental health.
Methods
We adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [20] and registered our meta-analysis on PROSPERO(CRD42022334408).
Search strategy
Cochrane Library, EMBASE, Pubmed, PsycINFO and Web of Science databases were searched up to May 2022. Medical Subject Headings (MeSH), Emtree Headings and other relevant key words were used to find studies related to parental BMI and the risk of mental diseases in offspring. We searched using the following general terms: (parent) AND (BMI) AND (offspring) AND (mental illness) AND (case–control OR cohort study). We used truncations and wildcards to accommodate synonyms and variations. Additionally, we looked for further studies by manually scanning review papers that came up in our searches. Two authors (Zhang and Lin) checked reference lists to find research that would be possibly eligible for our inclusion criteria. The full search strategy can be found in S1 Table.
Study selection
Two authors screened records separately. First, titles and abstracts of articles were examined and full texts would be retrieved if necessary. Inclusion criteria:(1) Population: parents and their offspring;(2) Exposure: evaluated either the paternal or maternal pre-pregnancy weight status;(3) Outcome: addressed the mental health of offspring;(4) Study design: original observational study. Exclusion criteria:(1) Target population involved parents with mental disorder or children not born at full term;(2) The parental BMI used was not measured at pre-pregnancy; (3) The study reported continuous outcome data. Disagreements were resolved in a group discussion.
Data extraction
Data extraction was completed by two investigators (Zhang and Lin). The following information was extracted: (1) Basic research information about authorship and date of publication; (2) Cohort characteristics included study design, country, sample size, cohort name and covariates; (3) Exposure information included parental weight status and BMI criteria; (4) Outcome information included the offspring’s age at evaluation, neurodevelopmental outcomes and their diagnostic methods (5) Odds Ratios(OR), Risk Ratio (RR), or Hazard Ratio(HR) with corresponding 95% confidence interval (CI) was also extracted from each included study.
Quality assessment
The Newcastle–Ottawa Quality Assessment Scale (NOS) was used to assess the quality of the included studies, which is recommended by Cochrane Collaboration [21]. We regarded studied as high quality when they had 6 or more stars.
Statistical analysis
In this meta-analysis, the following issues with repeated measurements in the same sample should be taken into account: (1) Data from studies with two cohorts were analyzed as independent samples; (2) The meta-analysis only included studies reporting separate data for pre-pregnancy overweight and obesity;(3)When neurodevelopment was examined using several tests within the same cohort, we took into account the values for all of the tests and if a test provided a total score value, this was the only value taken into account for the pooled estimate; (4) When several estimates were reported within the same study, the most adjusted model was used for the pooled estimate.
For all outcomes, estimates were converted into OR (where possible) and pooled OR were estimated using the fixed and random effect models. Heterogeneity was assessed using the I2 statistic. I2 values of <25%, 25–50% and >50% usually correspond to small, medium and large heterogeneity, respectively [22].
The following subgroup analyses were conducted: (1) Analyze the relationship between parental BMI pre-pregnancy categories and the risk of mental illness in offspring; (2) Analyze the relationship between maternal BMI pre-pregnancy categories and the risk of specific outcomes (ADHD, ASD, cognitive/intellectual delay, behavioral problems, and other mental diseases including schizophrenia, negative emotions, psychomotor development, etc.). The presence of publication bias was quantified using the Begg’s test and Egger’s test.
We performed three kinds of sensitivity analyses to examine the robustness of our results:(1) We defined higher-quality studies as those that had high scores on the quality measure;(2) We grouped studies by their study design;(3) We removed studies one by one to assess the robustness of the summary estimates. All statistical analyses were conducted using Stata, version 16.0 (Stata Corp, College Station, TX, USA).
Results
Literature search
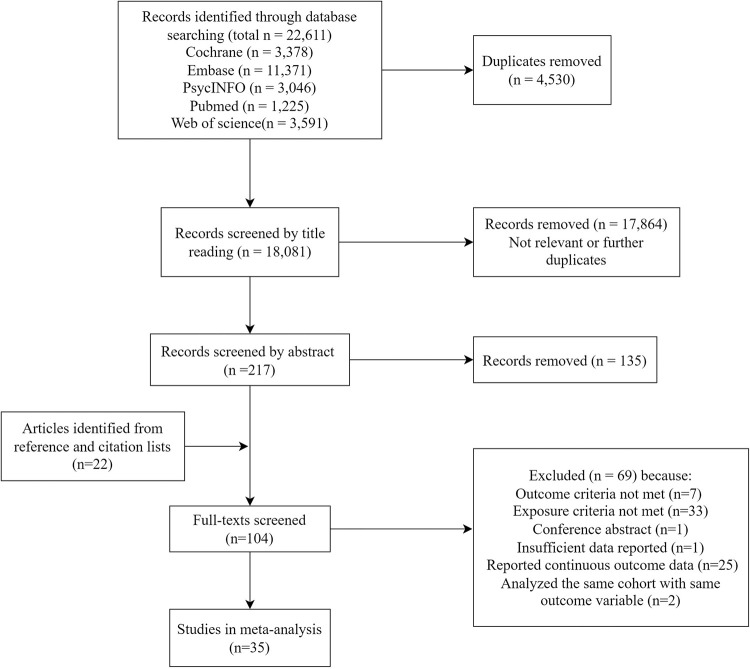
A total of 22 611 records were identified in the above databases (Fig 1). After removing duplicates (4 530), 18 081 records were yielded. Of these, most records were removed from the title (n = 17 864) and abstract screening (135) because they were non-relevant study designs or further duplicates. Besides, 22 additional articles were found during manual searching from the reference lists of relevant records. Thus, 104 articles were considered for full-text screening, and 69 were excluded, giving final 35 studies included in the meta-analysis.
Fig 1. Study selection flowchart.
Study characteristics
Of the 35 included studies, 24 were prospective studies [23–46], 6 were retrospective studies [47–52]and 5 were case–control studies [53–57] (Table 1). Note that 4 articles included in their analysis two cohorts. The included samples ranged from 197 to 620 795, belonging to 39 cohorts started between 1959 and 2008. For the geographical area of included studies, 18 studies were from the America, 14 from Europe, 2 from Australasia and 1 from Asia. There were 30 high quality studies and 5 low quality studies.
Table 1. Descriptive summary of the included 35 studies.
| General character | No. of studies (%) |
|---|---|
| Study design | |
| Prospective | 24 (68.6%) |
| Retrospective | 6 (17.1%) |
| Case–Control | 5 (14.3%) |
| Sample size | |
| <1000 | 3 (8%) |
| 1000–5000 | 16 (46%) |
| >5000 | 16 (46%) |
| Origin of sample | |
| Australasia | 2 (6%) |
| Asia | 1 (3%) |
| Europe | 14 (40%) |
| America | 18 (51%) |
| Quality grade of study | |
| High (NOS≥6) | 30 (86%) |
| Low (NOS<6) | 5 (14%) |
| Exposure character | |
| Maternal exposure | |
| Overweight | 31 (89%) |
| Obesity | 32 (91%) |
| Overweight/Obesity | 3 (9%) |
| Paternal exposure | |
| Overweight | 4 (11%) |
| Obesity | 5 (14%) |
| Overweight/Obesity | 1 (3%) |
| Outcome character | |
| ADHD | 10 (29%) |
| ASD | 15 (43%) |
| Cognitive/intellectual delay | 7 (20%) |
| Behavioral problems | 9 (26%) |
| Other mental diseases | 14 (40%) |
Note: NOS, Newcastle–Ottawa Quality Assessment Scale; ADHD, Attention-Deficit/Hyperactivity Disorder; ASD, Autism Spectrum Disorder.
All studies assessed the exposure to maternal prepregnant overweight and obesity, while six studies also investigated the exposure to fathers. All but 8 studies [29, 30, 34, 37, 38, 41, 53, 54] defined weight groups according to World Health Organization categories(WHO).4 articles [24, 32, 35, 38] collapsed underweight and normal weight into the same category (BMI < 24.99), which was analyzed collectively as normal weight,9 studies [24, 32, 39, 40, 46, 49–51, 54] further divided obesity into Obese Class I, II and III (or II/III), 3 articles [44, 45, 53] grouped overweight and obese mothers together. Thus, these were analyzed only as a combined obese and overweight category. Of the 35 studies reporting maternal exposure, 31 involved maternal overweight and 32 involved maternal obesity. Among the 6 studies [24, 28, 32, 35, 43, 44] reported paternal exposure, 4 examined paternal overweight [28, 32, 35, 43] and 5 examined paternal obesity [24, 28, 32, 35, 43]. Different diagnostic methods were used to determine each mental health outcome (S2 Table). Studies reporting maternal exposure were further grouped into the categories according to offspring outcomes: ADHD (n = 10), ASD (n = 15), cognitive and intellectual development(n = 7), behavioral problems (n = 9) and other mental diseases (n = 14). Table 2 contains summary characteristics for all studies included in meta-analyses.
Table 2. Characteristics of the included 35 studies.
| Study | Design; Country(start year);n | Cohort name | Parent exposure | BMI standard(year); categories | BMI source | Offspring’s outcome(age) | Adjusted covariates | NOS score |
|---|---|---|---|---|---|---|---|---|
| Schaefer et al. (2000) [29] | Prospective study; USA(1959); 6,633 |
Child Health and Development Study | Mother | Institute of Medicine (1990); low ≤19.9 average 20.0–26.9 above average 27.0–29.9 high ≥30.0 |
Medical record | Other mental diseases: schizophrenia spectrum disorder (adolescent and adult) | Maternal age, parity, education, race, cigarette smoking, and gender of the offspring | 8 |
| Heikura et al. (2008) [41] | Prospective study; Finland(1966), Finland(1986) 12,058; 9,432 |
Northern Finland Birth Cohorts | Mother | NR; thin <20.0 normal 20.0–24.9 overweight 25.0–29.9 obese ≥30.0 |
Self-report and medical staff | Cognitive/intellectual delay (<11.5y) | Age, smoking, maternal education, parity and family structure, marital status, place of residence, number of visits to maternity health center | 5 |
| Rodriguez et al. (2008) [30] | Prospective study; Sweden(2001) Denmark(2001) Finland(1993); 12,556 |
Nordic Network on ADHD | Mother | NR; underweight<18 normal 19–26 overweight>26 |
Medical record and questionnaire | ADHD(7-12y) | Maternal smoking, weight gain, gestational age, birth weight, infant sex, maternal age, maternal education and family structure | 6 |
| Rodriguez et al. (2010) [31] | Prospective study; Sweden(1999); 1,009 |
Pregnancy Cohort from Sweden | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical record | ADHD; other mental diseases: negative emotion; behavioral problems (5y) | Maternal smoking during pregnancy, maternal education, maternal age, gestational age, birth weight, infant sex, family structure at follow-up, family structure, depressive symptoms, life events and current: child overweight, parental ADHD symptoms, and maternal depressive symptoms | 8 |
| Brion et al. (2011) [44] | Prospective study; British(1991),Dutch(2002); 4,712, 2,046 |
British Avon Longitudinal Study of Parents and Children; Dutch Generation R | Mother; father |
WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | Behavioral problems; cognitive/intellectual delay (3.2–3.9y;4.9–8.0y) | Maternal education, paternal education, family income, social class, and maternal smoking. | 7 |
| Lyall et al. (2011) [37] | Prospective study; USA(1989); 61,596 |
Nurses’ Health Study II | Mother | NR; Underweight <22 normal 22–25 overweight 25–30 obesity ≥30 |
Self-report | ASD(NR) | Age at baseline, race, income; BMI, body shape at age 20, and cycle length and regularity models also included age at menarche | 5 |
| Hinkle et al. (2012) [51] | Retrospective cohort study; USA(2001) 6,850 |
Early Childhood Longitudinal Study Birth Cohort | Mother | WHO(1998); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II 35.0–39.9 class III ≥40.0 |
Self-report | Cognitive/intellectual delay; other mental diseases: psychomotor development(0.8–2y) | Maternal age (continuous), race-ethnicity, marital status, parity, schooling (continuous), smoking during pregnancy, household poverty status and child sex | 7 |
| Krakowiak et al. (2012) [55] | Case-control study; USA (2003); 1,004 |
Childhood Autism Risks from Genetics and the Environment | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical record and medical staff | ASD; other mental diseases: DD without ASD(2-5y) | Mother’s age at delivery, race/ethnicity, education level, delivery payer, calendar time, child’s age at enrollment and gender, and catchment area | 8 |
| Hinkle et al. (2013) [50] | Retrospective cohort study; USA (2001); 5,200 |
Early Childhood Longitudinal Study Birth Cohort | Mother | WHO(2000); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II and III ≥35.0 |
Self-report | Behavioral problems, other mental diseases: psychomotor development (4.8–7.1y) | Demographics, smoking, enrichment and current child weight status | 7 |
| Mann et al. (2013) [49] | Retrospective cohort study; USA(2004); 78,675 |
South Carolina Medicaid Programme | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II 35.0–39.9 class III ≥40.0 |
Self-report | Cognitive/intellectual delay (3–6y) | Maternal age, race, ethnicity, and level of education, maternal tobacco use, gonorrhea, chlamydia, syphilis, intrapartum fever, epilepsy, hypertension, diabetes mellitus, child’s sex, gestational age at delivery, and birthweight | 7 |
| Robinson et al. (2013) [33] | Prospective study; Australian(1989); 2,765 |
Western Australian Pregnancy Cohort (Raine) | Mother | WHO(2004); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report and research staff | Other mental diseases: affective problems (5–17y) | Maternal age at conception, maternal education, total family income, maternal smoking in pregnancy, maternal alcohol consumption in pregnancy, the presence of the biological father in the family home, the maternal experience of stressful events and perinatal data | 6 |
| Antoniou et al. (2014) [45] | Prospective study; UK (2008); 788 |
Twins and Multiple Birth Association Heritability Study | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | Behavioral problems (1.5–5y) | Gestational age, maternal educational level and smoking, twins‘ age, sex, birth weight | 5 |
| Chen et al. (2014) [42] | Prospective study; Sweden(1973); 620,795 |
Sweden population-based cohort | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical record | ADHD(>3y) | Offspring sex, birth order, year of birth, mother’s country of birth, maternal education, maternal age at delivery, smoking during pregnancy, cohabitation with child’s father at childbirth | 8 |
| Moss et al. (2014) [48] | Retrospective cohort study; USA (2001); 4,800 |
Early Childhood Longitudinal Study Birth Cohort | Mother | WHO(1995); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | ASD(0.8–2y) | Maternal age, child sex, birthweight, rates of height growth and weight gain | 5 |
| Surén et al. (2014) [28] | Prospective study; Norway (1999); 50,116 |
Norwegian Mother and Child Cohort | Mother, father |
WHO(2013); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | ASD(4–13.1y) | Parental education, parental age, parental smoking, parental psychiatric disorders, maternal parity, maternal use of folic acid supplements, use of hormone treatment or in vitro fertilization to become pregnant, maternal diabetes, preeclampsia, child’s year of birth, child’s gestational age at birth, and child’s birth weight | 9 |
| Tanda et al. (2014) [27] | Prospective study; USA (1976); 2,127(White); 1,268(African-American) |
National Longitudinal Survey of Youth | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | Behavioral problems (8–9.2y) | Child’s birth weight, gestational age, and mother’s smoking during pregnancy, maternal education, child’s home environment, household income, and marital status of their mothers, child’s race, gender, birth order, child’s age in month, and child’s weight status | 5 |
| Jo et al. (2015) [40] | Prospective study; USA (2005); 1,311 |
Infant Feeding Practices Study II | Mother | WHO(2000); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II 35.0–39.9 class III ≥40.0 |
Self-report | ADHD; ASD; behavioral problems; cognitive/intellectual delay; other mental diseases: depression or anxiety, motor skills (6y) | Maternal age, maternal race or ethnicity, marital status, mother’s education, poverty-to-income ratio, smoking during third trimester, parity, child’s gender, child’s current BMI, and child’s enrichment, birth weight, pregnancy weight gain, gestational diabetes, breastfeeding, and postpartum depression. | 8 |
| Torres-Espinola et al. (2015) [26] | Prospective study; Spain(2007) 215; 197 |
PREOBE Cohort | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical record and questionnaire | Cognitive/intellectual delay; other mental diseases: psychomotor development, composite socio emotion (0.5y,1.5y) | Maternal age, maternal educational level, placental weight, and weight gain during pregnancy | 8 |
| Xiang et al. (2015) [47] | Retrospective cohort study; USA (1995); 68,837 |
Kaiser Permanente Southern California longitudinal cohort | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical records | ASD(1.5 and 2y) | Maternal age at delivery, parity, education, self-reported maternal race/ethnicity, median family household income based on census tract of residence, history of comorbidity, and sex of the child | 9 |
| Connolly et al. (2016) [57] | Case–Control Study; USA (2006); 39,313 |
Cincinnati Children’s Hospital Medical Center’s Cohort | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical records | ASD(5.5y) | Maternal age at birth, maternal race, year of birth | 7 |
| Getz et al. (2016) [56] | Case–Control Study; UK (1993); 4,419 |
General Practice Research Database | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical records | ASD(6.2y) | Maternal age (continuous), maternal pre-pregnancy depression, diabetes, smoking status, drug abuse, alcoholism, in addition to matching factors (birth year, sex, and general practice) | 6 |
| Li et al. (2016) [38] | Prospective study; USA (1998); 1,767 |
Boston Birth Cohort | Mother | CDC(2014); underweight /normal<25.0 overweight 25.00–29.99 obese ≥30.00 |
Self-report | ADHD; ASD; cognitive/intellectual delay; other mental diseases: DD(3.6–9.0y) | Child year of birth, child gender, maternal age, parity, smoking during pregnancy and preterm birth | 8 |
| Casas et al. (2017) [43] | Prospective study; Spanish(2003); 1,827 |
INfancia y Medio Ambiente—Environment and Childhood | Mother, father |
WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | ADHD; ASD (mean:4.8 y) | Age and sex of the child, maternal and paternal education and social class, maternal age, parity, maternal employment status during pregnancy and at 5 years, maternal IQ, breast feeding duration, daycare attendance, and child physical activity paternal or maternal BMI | 7 |
| Mikkelsen et al. (2017) [35] | Prospective study; Denmark(1996); 38,314 |
Danish National Birth Cohort | Mother, father |
WHO(2000); underweight/normal (<25.0) overweight 25.00–29.99 obese ≥30.00 |
Self-report | Behavioral problems(7y) | Parental age, marital status, socioeconomic status, smoking, parity, birth year, sex, parental BMI, and parental hyperactivity | 7 |
| Yeung et al. (2017) [24] | Prospective study; USA (2008); 4,821 |
Upstate KIDS Study | Mother, father |
WHO(NR); underweight/normal (<25.1) overweight 25.00–29.99 obese class I 30.0–34.9 obese class II 35.0–39.9 class III ≥40.0 |
Medical records and self-report | Other mental diseases: DD(0.3-3y) | Maternal age, race, education, insurance, married, previous live birth, pregnancy smoking and parent BMI | 7 |
| Andersen et al. (2018) [46] | Prospective study; Denmark(1996); 81,892 |
Danish National Birth Cohort | Mother | WHO(1993); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II 35.0–39.9 class III ≥40.0 |
Self-report | ADHD;ASD(mean 13.3 y) | Socioeconomic status, maternal smoking, maternal psychiatric diagnoses, parental age, gestational age and birth weight | 9 |
| Menting et al. (2018) [36] | Prospective cohort study; Netherlands(2003); 4,094 |
Amsterdam Born Children and their Development | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | Behavioral problems (5-7y) | Sex and age of the child, maternal age, maternal ethnicity, maternal educational level and parity, maternal mental health disorder, paternal mental health disorder, smoking during pregnancy, alcohol use during pregnancy and child BMI z-score | 7 |
| Shen et al. (2018) [53] | Case–Control Study; China(NR); 2,897 |
Elim Training Center for Children with Autism | Mother | BMI classification standards for the Chinese population(2002); underweight <18.5 normal 18.5–24.0 overweight 24.0–28.0 obese ≥28.0 |
Self-report | ASD(2–9y) | Child’s gender, child age, parental age, maternal history of alcoholism/drug use during pregnancy, family annual income | 7 |
| Grudzinski et al. (2019) [52] | Retrospective cohort study; Canada(1989); 38,211 |
Nova Scotia population-based cohort | Mother | WHO(2000); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Medical records | ADHD; other mental diseases: any mental health disorder(0-18y) | Maternal age, area of residence, area-level income quintile, marital status, parity, maternal psychiatric disorders during pregnancy, and smoking in pregnancy | 7 |
| Varcin et al. (2019) [25] | Prospective study; Australian(1989); 1,238 |
Western Australian Pregnancy Cohort (Raine) Study | Mother | WHO(2000); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report and research staff | ASD(19-20y) | Paternal obesity at time of pregnancy, maternal age at conception, maternal smoking during pregnancy, alcohol consumption during pregnancy, maternal hypertensive diseases of pregnancy, maternal education, family income at time of pregnancy, threatened abortion, offspring gender, diabetes, and parity | 7 |
| Robinson et al. (2020) [32] | Prospective cohort study; USA (2008); 2,870 |
Upstate KIDS | Mother, father |
WHO(NR); underweight/normal <25.1 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II ≥35.0 |
Medical records | ADHD; other mental diseases: any mental health disorder(7-8y) | Mother’s age, race/ethnicity, education, insurance status, smoking, alcohol intake, marital status, and PCOS diagnosis; parental age difference and BMI; parental history of affective disorders; and child’s sex | 7 |
| Kong et al. (2020) [39] | Prospective study; Finland(2004); 34,892 |
Finland population-based registry cohort | Mother | WHO(NR); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II / III ≥35.0 |
Medical records | Other mental diseases: any mental health disorder(0-11y) | Offspring birth year, sex, perinatal problems and offspring birth weight according to gestational age, number of fetuses, mode of delivery, maternal age at delivery, parity, family situation, mother’s country of birth, and maternal smoking were obtained from the drugs and pregnancy database, maternal psychiatric disorders | 8 |
| Matias et al. (2021) [54] | Case–control study; USA(2003); 4,409 |
Study to Explore Early Development | Mother | NIH(2000) underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese class I 30.0–34.9 obese class II/ III≥35.0 |
Medical records | ASD; other mental diseases: DD without ASD(2-5y) | Maternal age, education, race/ethnicity, parity, smoking, income and site | 7 |
| Yim et al. (2021) [23] | Prospective study; USA(2001); 44,720 |
Nurses’ Mothers’ Cohort Study | Mother | WHO(2000); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | ADHD;ASD(NR) | Grandmother (G0) race/ethnicity, grandmother and grandfather educational levels, grandfather occupation, grandmother lifetime history of depression, maternal (G1) year of birth, and G1 smoking status at baseline | 7 |
| Parker et al. (2022) [34] | Prospective study; USA(NA); 484 |
NR | Mother | CDC(2019); underweight <18.5 normal 18.50–24.99 overweight 25.00–29.99 obese ≥30.00 |
Self-report | Behavioral problems (5-12y) | Maternal age, maternal education, marital status, periconceptional alcohol use, periconceptional smoking, and weighted by stabilized inverse probabilities of participation | 6 |
Note: ADHD, Attention-Deficit/Hyperactivity Disorder; ASD, Autism Spectrum Disorder; BMI, Body Mass Index; CDC, Centers for Disease Control and Prevention; DD, Development Delay; NIH, National Institutes of Health; IQ, Intelligence Quotient; NR, Not Report; PCOS, Polycystic Ovary Syndrome; PREOBE, a prospective observational cohort study (NCT01634464); USA, United States of America; WHO, World Health Organization.
Risk of any mental illness
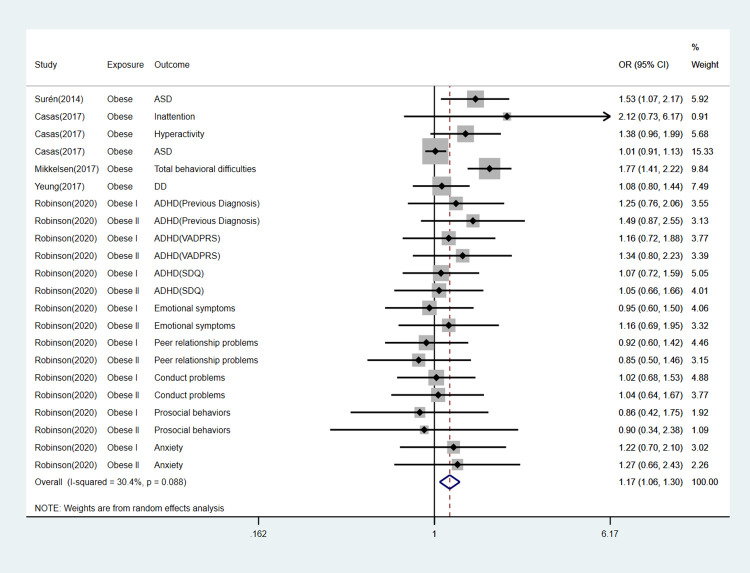
We pooled the risk of any mental illness in offspring exposed to maternal and paternal overweight and obesity separately. For overweight mothers, there were 14% higher odds of having a child with any adverse mental outcome (OR, 1.14; 95% CI, 1.10–1.18; P < 0.001), For obese mothers, across 32 studies, there were 39% higher odds to have a child with any adverse mental outcome (OR, 1.39; 95% CI, 1.33–1.45; P < 0.001). As for paternal exposure, the pooled OR for paternal overweight was 1.03(95%CI, 0.95–1.11; P = 0.44), indicating that there was no significant association between paternal overweight and offspring mental illness. However, results across 5 studies indicated that fathers who were obese prior to pregnancy had 17% higher odds of having a child with any adverse neurodevelopmental outcome (OR, 1.17; 95% CI, 1.06–1.30; P < 0.001; Fig 2; Table 3).
Fig 2. Forest plot for obese fathers and their offspring’s risk of any mental diseases.
Table 3. Summary of meta-analyses.
| Subgroup | No. of studies | Overall effect | Heterogeneity | Publication bias | ||
|---|---|---|---|---|---|---|
| OR (95%) | P value | I2 | Begg’s test | Egger’s test | ||
| Maternal BMI weight group | ||||||
| Overweight | 31 | 1.14(1.10,1.18) | <0.001 | 34.9% | 0.20 | 0.17 |
| Obesity | 32 | 1.39(1.33, 1.45) | <0.001 | 57.3% | 0.75 | 0.70 |
| Overweight + Obesity | 35 | 1.24(1.20, 1.28) | <0.001 | 71.8% | 0.17 | 0.18 |
| Paternal BMI weight group | ||||||
| Overweight | 4 | 1.03(0.95,1.11) | 0.44 | 11.7% | 0.71 | 0.02 |
| Obesity | 5 | 1.17(1.06,1.30) | 0.003 | 30.4% | 0.74 | 0.39 |
| Overweight + Obesity | 6 | 1.04(1.00, 1.09) | 0.056 | 29.8% | 0.23 | 0.39 |
| Maternal-Offspring mental diseases(Overweight) | ||||||
| ADHD | 10 | 1.21(1.16,1.26) | <0.001 | 9.0% | 0.50 | 0.92 |
| ASD | 13 | 1.09(1.04,1.15) | 0.001 | 0% | 0.30 | 0.50 |
| Cognitive/intellectual delay | 7 | 1.13(1.04, 1.24) | 0.006 | 1.6% | 0.09 | 0.26 |
| Behavioral problems | 9 | 1.28 (1.16, 1.41) | <0.001 | 10.8% | 0.74 | 1.00 |
| Other mental diseases | 14 | 1.06(1.04,1.09) | <0.001 | 0% | 0.77 | 0.62 |
| Maternal-Offspring mental diseases(Obesity) | ||||||
| ADHD | 10 | 1.55(1.42,1.70) | <0.001 | 45.9% | 0.70 | 0.45 |
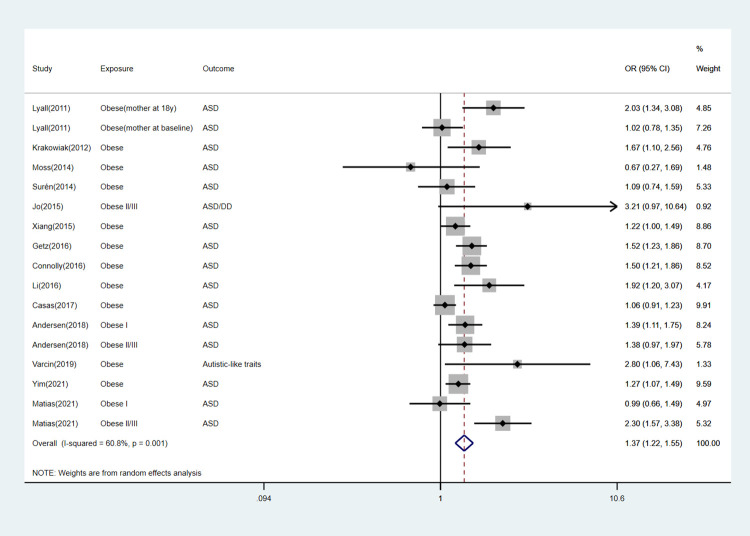
| ASD | 15 | 1.37(1.22,1.55) | <0.001 | 60.8% | 0.23 | 0.10 |
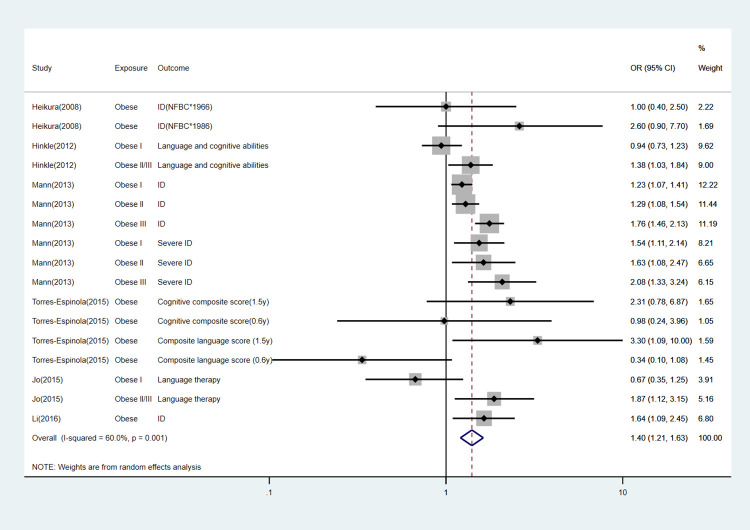
| Cognitive/intellectual delay | 7 | 1.40(1.21,1.63) | <0.001 | 60.0% | 0.59 | 0.69 |
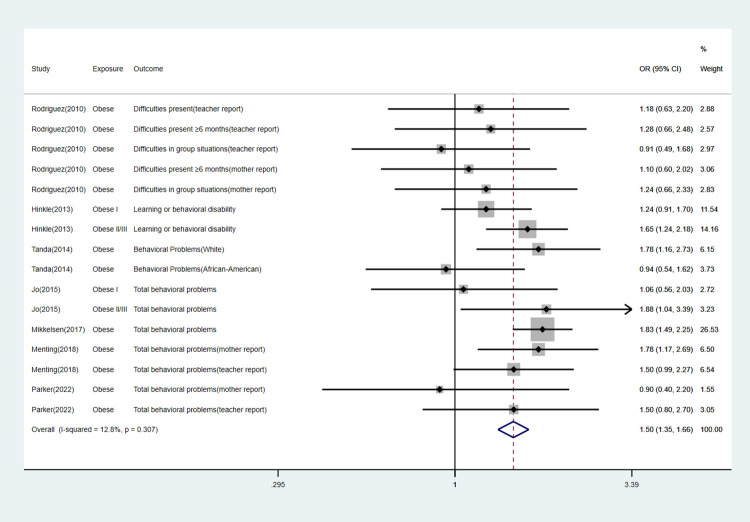
| Behavioral problems | 9 | 1.50(1.35, 1.66) | <0.001 | 12.8% | 0.07 | 0.004 |
| Other mental diseases | 15 | 1.30(1.23,1.37) | <0.001 | 28.6% | 0.88 | 0.68 |
Note: ADHD, Attention-Deficit/Hyperactivity Disorder; ASD, Autism Spectrum Disorder; BMI, Body Mass Index.
Risk of specific mental illness
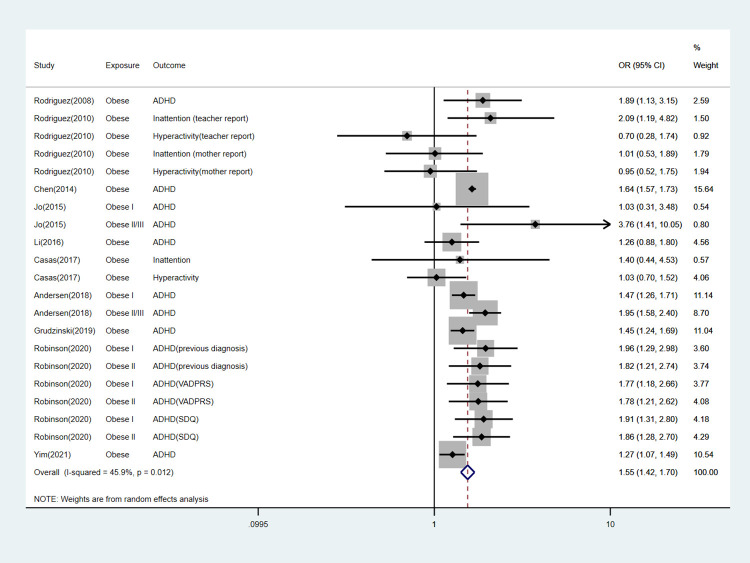
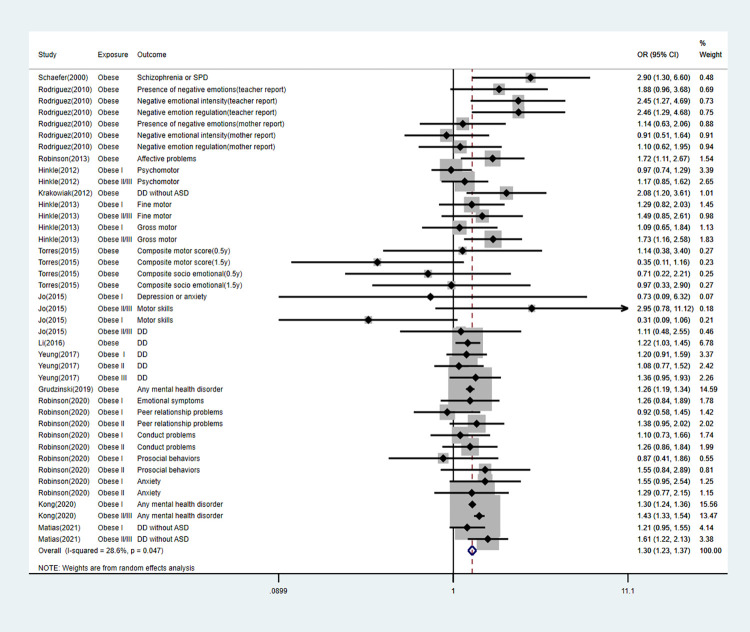
We performed subgroup analyses according to specific offspring outcomes. The detailed information can be found in Table 3 and Figs 3–7.
Fig 3. Forest plot for obese mothers and their offspring’s risk of ADHD.
Fig 7. Forest plot for obese mothers and their offspring’s risk of other mental diseases.
Fig 4. Forest plot for obese mothers and their offspring’s risk of ASD.
Fig 5. Forest plot for obese mothers and their offspring’s risk of cognitive/intellectual delay.
Fig 6. Forest plot for obese mothers and their offspring’s risk of behavioral problems.
Publication bias
There was no evidence of publication bias according to Begg’s test and Egger’s test (all P>0.05) except the pooled analysis which show inconclusive evidence for an association between paternal overweight and offspring mental illnesses.
Sensitivity analysis
Sensitivity analysis showed the results of the main analyses are robust. Studies with cohort study design produced an overall OR that was lower than those with case-control design. Slightly attenuated OR was observed for those studies ranked as having a lower quality score than those ranked as having a higher quality score (Table 4). We also investigated the influence of a single study on the overall risk estimate by performing leave-one-out analysis. The combined ORs of overall risk estimates were consistent and without apparent fluctuation (S3 Table).
Table 4. Sensitivity analysis of the effects of maternal pre-pregnancy overweight and obesity on offspring mental health.
| No. of studies | Overall effect(95%CI) | P | I2 | |
|---|---|---|---|---|
| Study design | ||||
| Prospective | 24 | 1.24(1.19,1.29) | <0.001 | 72.5% |
| Retrospective | 6 | 1.22(1.14,1.30) | <0.001 | 67.4% |
| Case–Control | 5 | 1.30(1.13,1.48) | <0.001 | 68.8% |
| Quality grade of study | ||||
| High (NOS≥6) | 30 | 1.24(1.20,1.28) | <0.001 | 73.1% |
| Low (NOS<6) | 5 | 1.19(1.05,1.35) | 0.008 | 19.9% |
Note: NOS, Newcastle–Ottawa Quality Assessment Scale.
Discussion
The findings of this meta-analysis are consistent with previous reviews that revealed a negative link between maternal pre-pregnancy overweight/obesity and the neurodevelopmental outcomes in offspring. Several inconsistencies should also be mentioned. First, to date, this is the first comprehensive meta-analysis not only investigated the effect of maternal overweight/obesity on offspring mental health but also fathers. Although far fewer studies have investigated paternal exposure than maternal, to some extent, we still conclude that paternal obesity has adverse effects on the neurodevelopment of their offspring. Second, for maternal weight exposure, we excluded studies that examined weight during pregnancy because of the neurological effects of gestational weight gain (GWG) on offspring. Evidence showed that whether GWG is insufficient or excessive may be important to fetal neurodevelopment [58–60]. However, the degree to which GWG and pre-pregnancy obesity independently or synergistically relate to an increased risk of offspring’s mental illnesses is not clear [61, 62]. Third, because we adhere to strict definitions of study design, the estimates of effect sizes are more accurate than they would be with weaker study designs, including cross-sectional studies. Forth, the included studies come from heterogeneous samples, countries, measures and designs so the effect size in the main analysis showed appropriate representativeness.
Multiple mechanisms have been proposed to explain the impact of maternal obesity on the offspring neurodevelopment. To our knowledge, obesity during pregnancy changes neuroendocrine, metabolic and inflammatory status [4].The increased cytokines, oxidative stress, and circulating hormones disorder “mother–placenta–fetus” system [63, 64]and the composition of lactational milk composition [65], resulting in potential changes to child’s neurodevelopment which in turn influences behaviour, emotion and cognition [66]. In addition to neuroinflammation, altered neuronal plasticity(brain-derived neurotrophic factor, notch signaling genes, proliferation of neuronal progenitor cells, abnormal synaptic stability) [67], impaired reward circuitry(dopaminergic and serotonergic signaling) [68] and dysregulated brain metabolism (leptin, insulin and oxytocin) and likely contributors. The alterations in the gut microbiome’s composition are another mechanism that has recently received attention [69]. Microbial metabolites may affect neuroendocrine regulation and brain development via passing through the placenta, vertically transmitting and lactation [65]. However, the detailed dynamics remain poorly understood.
The mechanisms explaining how the father may affect the neurodevelopment of offspring are still under debate [70].Obesity causes a variety of symptoms in men, including changes in their gut microbiome, hormone levels, and sperm health, which can lead to abnormalities in fertilization and embryonic development [71–73]. Additionally, seminal plasma has a distinct microbiome that can be altered by a high-fat diet(HFD), which means it can indirectly affect the offspring’s neurodevelopment and later brain function by passing along information about the father’s eating habits and metabolic condition [74].
Moreover, maternal and paternal obesity predispose offspring to poor neurodevelopment by epigenetic molecular mechanism, which is studied intensively in the field of neurobiology in animals [63]. The underlying molecular mechanisms involve DNA methylation, post-translational modifications of chromatin and/or histone proteins, and small noncoding RNAs (sncRNAs) alterations, which change the expression of genes involved in neuroplasticity [3]. Among the classical epigenetic mechanisms, DNA methylation is an attractive target for research. It has been reported that offspring of obese females exhibited DNA hypomethylation in gene promoters including those encoding proteins involved in dopamine uptake, and serotonergic and opioid signaling [3, 75, 76] and may be associated with the decreased folic acid in obese pregnant women [67]. Zhou et al. report that paternal HFD results in cognitive impairments in the F1 potentially due to the increased methylation of the BDNF gene promoter transmitted by F0 spermatozoa [77]. The recent clinical research found a link between paternal BMI and altered DNA methylation in cord blood nucleated cells, indicating that there are environment-sensitive parts of the sperm epigenome that respond to food and may transmit an ’epigenomic map’ that impacts offspring development [78]. Furthermore, sncRNAs have been hypothesized as another pathway of epigenetic transfer, particularly through the paternal line. These sperm-derived RNAs appear to be sensitive to a diverse range of psychological and physiological conditions, including stress exposure and nutrition, and are able to transmit intergenerational information through the paternal lineage [70, 72, 73]. Obesity-caused hyperglycemia, insulin resistance, and a proinflammatory lipotoxic environment could be the immediate source of such epigenetic changes, resulting in developmental brain problems in offspring [3].
Here, we detail the limitations that might influence the combined results. First, lack of resources meant that we excluded papers that were not in English and studies with continuous variables. Besides, it was very possible that studies with bad outcomes were unlikely to be published. Second, the exposure and outcome factors might have resulted in disparate results. The parental weight groupings and diagnostic criteria of neurodevelopment were variable, which may affect the results and contribute to the heterogeneity across several risk factors. Third, all the identified studies came from observational settings and were therefore subjected to confounding bias. Forth, the limited number of studies investigated fathers (only 6 studies) might affect the combined results. Also, 91% included studies were conducted in the America (51%) and Europe (40%), which limit generalizability to other populations across the world. Future studies should be performed in more countries outside of America and Europe.
Conclusion
We found that the most recent evidence indicates the detrimental connections between parental pre-pregnancy overweight/obesity and offspring mental health. To reduce the incidence of mental problems in children, it may be prudent to conduct measures targeted at avoiding overweight and obesity in both mother and father. However, given the constraints discussed above, more research employing a range of study designs such as sibling comparison, children-of-twins designs, and within-family Mendelian randomization would be helpful in identifying the magnitude of the overall impact size. Also, the recent study showed grandmother underweight prior to pregnancy is associated with an increased risk of ADHD among grandchildren independent of maternal pre-pregnancy weight status, future work should focus on discovering mechanisms linking weight status and offspring mental health across generations.
Supporting information
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Acknowledgments
We would like to acknowledge the authors of the included articles.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the National Natural Science Foundation of China (82088102), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-064), Collaborative Innovation Program of Shanghai Municipal Health Commission (2020CXJQ01), Clinical Research Plan of SHDC (SHDC2020CR1008A) and Shanghai Frontiers Science Research Base of Reproduction and Development.
References
- 1.Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet. Psychiatry, 2022. 9(2): p. 137–150. doi: 10.1016/S2215-0366(21)00395-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bluher M., Obesity: global epidemiology and pathogenesis. Nat Rev Endocrinol, 2019. 15(5): p. 288–298. doi: 10.1038/s41574-019-0176-8 [DOI] [PubMed] [Google Scholar]
- 3.Mattson M.P., An Evolutionary Perspective on Why Food Overconsumption Impairs Cognition. Trends Cogn Sci, 2019. 23(3): p. 200–212. doi: 10.1016/j.tics.2019.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cirulli F., Musillo C., and Berry A., Maternal Obesity as a Risk Factor for Brain Development and Mental Health in the Offspring. Neuroscience, 2020. 447: p. 122–135. [DOI] [PubMed] [Google Scholar]
- 5.Bliddal M., et al., Maternal pre-pregnancy BMI and intelligence quotient (IQ) in 5-year-old children: a cohort based study. PloS One, 2014. 9(4): p. e94498. doi: 10.1371/journal.pone.0094498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bodden C., Hannan A.J., and Reichelt A.C., Of ’junk food’ and ’brain food’: how parental diet influences offspring neurobiology and behaviour. Trends Endocrinol Metab, 2021. 32(8): p. 566–578. doi: 10.1016/j.tem.2021.04.001 [DOI] [PubMed] [Google Scholar]
- 7.Sanchez C.E., et al., Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. Obes Rev, 2018. 19(4): p. 464–484. doi: 10.1111/obr.12643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L., et al., Maternal pre-pregnancy overweight/obesity and the risk of attention-deficit/hyperactivity disorder in offspring: a systematic review, meta-analysis and quasi-experimental family-based study. Int J Epidemiol, 2020. 49(3): p. 857–875. doi: 10.1093/ije/dyaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei X.Y., et al., Association between parental body mass index and autism spectrum disorder: a systematic review and meta-analysis. Eur Child Adolesc Psychiatry, 2019. 28(7): p. 933–947. doi: 10.1007/s00787-018-1259-0 [DOI] [PubMed] [Google Scholar]
- 10.Alvarez-Bueno C., et al., Association between pre-pregnancy overweight and obesity and children’s neurocognitive development: a systematic review and meta-analysis of observational studies. Int J Epidemiol, 2017. 46(5): p. 1653–1666. [DOI] [PubMed] [Google Scholar]
- 11.Adane A.A., Mishra G.D., and Tooth L.R., Maternal pre-pregnancy obesity and childhood physical and cognitive development of children: a systematic review. Int J Obes (Lond), 2016. 40(11): p. 1608–1618. [DOI] [PubMed] [Google Scholar]
- 12.Li Y.-M., et al., Association Between Maternal Obesity and Autism Spectrum Disorder in Offspring: A Meta-analysis. Journal of Autism and Developmental Disorders, 2016. 46(1). doi: 10.1007/s10803-015-2549-8 [DOI] [PubMed] [Google Scholar]
- 13.Hales C.M., et al., Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018. NCHS Data Brief, 2020(360): p. 1–8. [PubMed] [Google Scholar]
- 14.Loos R.J.F. and Yeo G.S.H., The genetics of obesity: from discovery to biology. Nat Rev Genet, 2022. 23(2): p. 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abarca-Gómez L., et al., Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. The Lancet, 2017. 390(10113): p. 2627–2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soubry A., Epigenetics as a Driver of Developmental Origins of Health and Disease: Did We Forget the Fathers? Bioessays, 2018. 40(1). doi: 10.1002/bies.201700113 [DOI] [PubMed] [Google Scholar]
- 17.Marmorstein N.R. and Iacono W.G., Associations Between Depression and Obesity in Parents and Their Late-Adolescent Offspring: A Community-Based Study. Psychosom Med, 2016. 78(7): p. 861–6. doi: 10.1097/PSY.0000000000000334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lainez N.M. and Coss D., Obesity, Neuroinflammation, and Reproductive Function. Endocrinology, 2019. 160(11): p. 2719–2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton S., et al., The menace of obesity to depression and anxiety prevalence. Trends Endocrinol Metab, 2022. 33(1): p. 18–35. [DOI] [PubMed] [Google Scholar]
- 20.Page M.J., et al., The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 2021. 372: p. n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells G.A., et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. [Google Scholar]
- 22.Higgins J.P.T. and Thompson S.G., Quantifying heterogeneity in a meta-analysis. Statistics In Medicine, 2002. 21(11): p. 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 23.Yim G., et al., Association Between Periconceptional Weight of Maternal Grandmothers and Attention-Deficit/Hyperactivity Disorder in Grandchildren. Jama Network Open, 2021. 4(7). doi: 10.1001/jamanetworkopen.2021.18824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeung E.H., et al., Parental obesity and early childhood development. Pediatrics, 2017. 139(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varcin K.J., Newnham J.P., and Whitehouse A.J.O., Maternal pre-pregnancy weight and autistic-like traits among offspring in the general population. Autism Research, 2019. 12(1): p. 80–88. doi: 10.1002/aur.1973 [DOI] [PubMed] [Google Scholar]
- 26.Torres-Espinola F.J., et al., Maternal Obesity, Overweight and Gestational Diabetes Affect the Offspring Neurodevelopment at 6 and 18 Months of Age—A Follow Up from the PREOBE Cohort. PLoS One, 2015. 10(7): p. e0133010. doi: 10.1371/journal.pone.0133010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanda R. and Salsberry P.J., Racial differences in the association between maternal prepregnancy obesity and children’s behavior problems. Journal of Developmental and Behavioral Pediatrics, 2014. 35(2): p. 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Surén P., et al., Parental obesity and risk of autism spectrum disorder. Pediatrics, 2014. 133(5): p. e1128–e1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer C.A., et al., Maternal prepregnant body mass and risk of schizophrenia in adult offspring. Schizophrenia Bulletin, 2000. 26(2): p. 275–286. doi: 10.1093/oxfordjournals.schbul.a033452 [DOI] [PubMed] [Google Scholar]
- 30.Rodriguez A., et al., Maternal adiposity prior to pregnancy is associated with ADHD symptoms in offspring: Evidence from three prospective pregnancy cohorts. International Journal of Obesity, 2008. 32(3): p. 550–557. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez A., Maternal pre-pregnancy obesity and risk for inattention and negative emotionality in children. Journal of Child Psychology and Psychiatry, 2010. 51(2): p. 134–143. [DOI] [PubMed] [Google Scholar]
- 32.Robinson S.L., et al., Parental Weight Status and Offspring Behavioral Problems and Psychiatric Symptoms. Journal of Pediatrics, 2020. 220: p. 227–236.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Robinson M., Pre-pregnancy maternal overweight and obesity increase the risk for affective disorders in offspring. Journal of developmental origins of health and disease, 2013. 4(1): p. 42–48. [DOI] [PubMed] [Google Scholar]
- 34.Parker S.E., et al., Pre-pregnancy body mass index and parent and teacher-reported behavioral outcomes among offspring in childhood. Neurotoxicology and Teratology, 2022. 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mikkelsen S.H., et al., Parental Body Mass Index and Behavioral Problems in Their Offspring: A Danish National Birth Cohort Study. American Journal of Epidemiology, 2017. 186(5): p. 593–602. [DOI] [PubMed] [Google Scholar]
- 36.Menting M.D., et al., The association between pre-pregnancy overweight/obesity and offspring’s behavioral problems and executive functioning. Early Human Development, 2018. 122: p. 32–41. doi: 10.1016/j.earlhumdev.2018.05.009 [DOI] [PubMed] [Google Scholar]
- 37.Lyall K., et al., Maternal early life factors associated with hormone levels and the risk of having a child with an autism spectrum disorder in the nurses health study II. Journal of Autism and Developmental Disorders, 2011. 41(5): p. 618–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li M., et al., The association of maternal obesity and diabetes with autism and other developmental disabilities. Pediatrics, 2016. 137(2): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kong L., et al., Associations of Different Types of Maternal Diabetes and Body Mass Index With Offspring Psychiatric Disorders. JAMA Netw Open, 2020. 3(2): p. e1920787. doi: 10.1001/jamanetworkopen.2019.20787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jo H., et al., Maternal prepregnancy body mass index and child psychosocial development at 6 years of age. Pediatrics, 2015. 135(5): p. e1198–e1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heikura U., et al., Variations in prenatal sociodemographic factors associated with intellectual disability: a study of the 20-year interval between two birth cohorts in northern Finland. American Journal of Epidemiology, 2008. 167(2): p. 169–177. [DOI] [PubMed] [Google Scholar]
- 42.Chen Q., et al., Maternal pre-pregnancy body mass index and offspring attention deficit hyperactivity disorder: a population-based cohort study using a sibling-comparison design. International Journal of Epidemiology, 2014. 43(1): p. 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casas M., et al., Maternal pre-pregnancy obesity and neuropsychological development in pre-school children: A prospective cohort study. Pediatric Research, 2017. 82(4): p. 596–606. [DOI] [PubMed] [Google Scholar]
- 44.Brion M.J., et al., Intrauterine effects of maternal prepregnancy overweight on child cognition and behavior in 2 cohorts. Pediatrics, 2011. 127(1): p. e202–e211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antoniou E.E., et al., Maternal pre-pregnancy weight and externalising behaviour problems in preschool children: A UK-based twin study. BMJ Open, 2014. 4(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Andersen C.H., et al., Maternal body mass index before pregnancy as a risk factor for ADHD and autism in children. European Child & Adolescent Psychiatry, 2018. 27(2): p. 139–148. doi: 10.1007/s00787-017-1027-6 [DOI] [PubMed] [Google Scholar]
- 47.Xiang A.H., et al., Association of maternal diabetes with autism in offspring. JAMA, 2015. 313(14): p. 1425–1434. [DOI] [PubMed] [Google Scholar]
- 48.Moss B.G. and Chugani D.C., Increased risk of very low birth weight, rapid postnatal growth, and autism in underweight and obese mothers. American Journal of Health Promotion, 2014. 28(3): p. 181–188. [DOI] [PubMed] [Google Scholar]
- 49.Mann J.R., et al., Pre-pregnancy body mass index, weight change during pregnancy, and risk of intellectual disability in children. Bjog-an International Journal of Obstetrics and Gynaecology, 2013. 120(3): p. 309–319. [DOI] [PubMed] [Google Scholar]
- 50.Hinkle S.N., et al., Maternal prepregnancy weight status and associations with children’s development and disabilities at kindergarten. International Journal of Obesity, 2013. 37(10): p. 1344–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hinkle S.N., et al., Associations between maternal prepregnancy body mass index and child neurodevelopment at 2 years of age. International Journal of Obesity, 2012. 36(10): p. 1312–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grudzinski A., et al., Maternal pre-pregnancy weight status and health care use for mental health conditions in the offspring. Eur Child Adolesc Psychiatry, 2019. 28(11): p. 1499–1506. doi: 10.1007/s00787-019-01312-w [DOI] [PubMed] [Google Scholar]
- 53.Shen Y., et al., Associations among maternal pre-pregnancy body mass index, gestational weight gain and risk of autism in the Han Chinese population. BMC Psychiatry, 2018. 18. doi: 10.1186/s12888-018-1593-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matias S.L., et al., Maternal prepregnancy weight and gestational weight gain in association with autism and developmental disorders in offspring. Obesity, 2021. 29(9): p. 1554–1564. doi: 10.1002/oby.23228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krakowiak P., et al., Maternal metabolic conditions and risk for autism and other neurodevelopmental disorders. Pediatrics, 2012. 129(5): p. e1121–e1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Getz K.D., et al., Maternal Pre-pregnancy Body Mass Index and Autism Spectrum Disorder among Offspring: A Population-Based Case-Control Study. Paediatric and Perinatal Epidemiology, 2016. 30(5): p. 479–487. doi: 10.1111/ppe.12306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connolly N., et al., Maternal metabolic risk factors for autism spectrum disorder-An analysis of electronic medical records and linked birth data. Autism Research: Official Journal of the International Society For Autism Research, 2016. 9(8): p. 829–837. [DOI] [PubMed] [Google Scholar]
- 58.Tore E.C., et al., Gestational Weight Gain by Maternal Pre-pregnancy BMI and Childhood Problem Behaviours in School-Age Years: A Pooled Analysis of Two European Birth Cohorts. Matern Child Health J, 2020. 24(10): p. 1288–1298. doi: 10.1007/s10995-020-02962-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mackay E., et al., Association of Gestational Weight Gain and Maternal Body Mass Index in Early Pregnancy With Risk for Nonaffective Psychosis in Offspring. JAMA Psychiatry, 2017. 74(4): p. 339–349. [DOI] [PubMed] [Google Scholar]
- 60.Gardner R.M., et al., Maternal body mass index during early pregnancy, gestational weight gain, and risk of autism spectrum disorders: Results from a Swedish total population and discordant sibling study. Int J Epidemiol, 2015. 44(3): p. 870–83. doi: 10.1093/ije/dyv081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bilder D.A., et al., Maternal prenatal weight gain and autism spectrum disorders. Pediatrics, 2013. 132(5): p. e1276–e1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee P., et al., Association of maternal gestational weight gain with intellectual developmental disorder in the offspring: a nationwide follow-up study in Sweden. BJOG, 2022. 129(4): p. 540–549. doi: 10.1111/1471-0528.16887 [DOI] [PubMed] [Google Scholar]
- 63.Shook L.L., Kislal S., and Edlow A.G., Fetal brain and placental programming in maternal obesity: A review of human and animal model studies. Prenat Diagn, 2020. 40(9): p. 1126–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Evsyukova I.I., The impact of maternal obesity and diabetes on fetal brain development (mechanisms and prevention). Journal of obstetrics and women’s diseases, 2020. 69(3): p. 33–38. [Google Scholar]
- 65.Urbonaite G., et al., The impact of maternal high-fat diet on offspring neurodevelopment. Front Neurosci, 2022. 16: p. 909762. doi: 10.3389/fnins.2022.909762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell A.J., Dunn G.A., and Sullivan E.L., The Influence of Maternal Metabolic State and Nutrition on Offspring Neurobehavioral Development: A Focus on Preclinical Models. Biol Psychiatry Cogn Neurosci Neuroimaging, 2022. 7(5): p. 450–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Contu L. and Hawkes C.A., A Review of the Impact of Maternal Obesity on the Cognitive Function and Mental Health of the Offspring. Int J Mol Sci, 2017. 18(5). doi: 10.3390/ijms18051093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Edlow A.G., Maternal obesity and neurodevelopmental and psychiatric disorders in offspring. Prenat Diagn, 2017. 37(1): p. 95–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hasebe K., Kendig M.D., and Morris M.J., Mechanisms Underlying the Cognitive and Behavioural Effects of Maternal Obesity. Nutrients, 2021. 13(1). doi: 10.3390/nu13010240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ornellas F., et al., Obese fathers lead to an altered metabolism and obesity in their children in adulthood: review of experimental and human studies. J Pediatr (Rio J), 2017. 93(6): p. 551–559. [DOI] [PubMed] [Google Scholar]
- 71.Chavarro J.E., et al., Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil Steril, 2010. 93(7): p. 2222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bodden C., Hannan A.J., and Reichelt A.C., Diet-Induced Modification of the Sperm Epigenome Programs Metabolism and Behavior. Trends Endocrinol Metab, 2020. 31(2): p. 131–149. [DOI] [PubMed] [Google Scholar]
- 73.Billah M.M., et al., Effects of paternal overnutrition and interventions on future generations. Int J Obes (Lond), 2022. 46(5): p. 901–917. doi: 10.1038/s41366-021-01042-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Javurek A.B., et al., Consumption of a high-fat diet alters the seminal fluid and gut microbiomes in male mice. Reprod Fertil Dev, 2017. 29(8): p. 1602–1612. [DOI] [PubMed] [Google Scholar]
- 75.Vucetic Z., et al., Maternal high-fat diet alters methylation and gene expression of dopamine and opioid-related genes. Endocrinology, 2010. 151(10): p. 4756–4764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sullivan E.L., et al., Chronic consumption of a high-fat diet during pregnancy causes perturbations in the serotonergic system and increased anxiety-like behavior in nonhuman primate offspring. The Journal of Neuroscience: the Official Journal of the Society For Neuroscience, 2010. 30(10): p. 3826–3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou Y., et al., Diet-Induced Paternal Obesity Impairs Cognitive Function in Offspring by Mediating Epigenetic Modifications in Spermatozoa. Obesity (Silver Spring, Md.), 2018. 26(11): p. 1749–1757. doi: 10.1002/oby.22322 [DOI] [PubMed] [Google Scholar]
- 78.Noor N., et al., Association of Periconception Paternal Body Mass Index With Persistent Changes in DNA Methylation of Offspring in Childhood. JAMA Netw Open, 2019. 2(12): p. e1916777. doi: 10.1001/jamanetworkopen.2019.16777 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(XLSX)
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.