Abstract
Most studies of human T-cell responses in tuberculosis have focused on persons with either active disease or latent infection. Although this work has been critical in defining T-cell correlates of successful versus failed host containment, little is known about the development of Mycobacterium-specific T-cell responses in uninfected persons. To explore this issue, naive T cells from uninfected donors were sensitized in vitro with avirulent Mycobacterium tuberculosis-infected autologous macrophages. T-cell lines primed in this manner proliferated and produced cytokines after challenge with mycobacterial antigens. Of 11 such lines, 8 were high Th1 responders, 2 were low Th1 responders, and 1 was a Th2 responder. Furthermore, similar patterns and magnitudes of proliferative and cytokine responses were seen when Mycobacterium infection-primed lines were challenged with recombinant antigen 85 (Ag85) proteins. The addition of interleukin 12 (IL-12) during the initial sensitization increased the magnitude of Th1 responses; however, antibody to IL-12 did not eliminate Th1 responses, suggesting that additional factors contributed to the differentiation of these cells. Finally, in the presence of IL-12, recombinant Ag85B was able to prime naive T cells for Th1 responses upon challenge with Mycobacterium-infected macrophages or Ag85B. Therefore, under the appropriate conditions, priming with whole bacteria or a subunit antigen can stimulate Mycobacterium-specific Th1 effector cell development. Further definition of the antigens and conditions required to drive naive human T cells to differentiate into Th1 effectors should facilitate the development of an improved tuberculosis vaccine.
Tuberculosis remains one of the leading infectious diseases of humans, causing an estimated 6.7 million new cases and 2.4 million deaths in 1998 (27). Despite bacillus Calmette-Guérin (BCG) vaccination programs and increasing use of directly observed therapy in many developing countries, it is predicted that there will be 225 million new cases and 79 million deaths from tuberculosis between 1998 and 2030. A safe, inexpensive, and effective vaccine is of the highest priority. The development of an improved vaccine will depend on a more thorough understanding of protective host immune responses to Mycobacterium tuberculosis as well as the identification and characterization of any antigen(s) that induces those protective responses.
Both mouse models of infection and human patient material have provided evidence that protective immune responses to Mycobacterium are T-cell dependent (2, 28). It has been shown that CD4+ Th1 cells play an important role in the development of resistance to disease (13, 32), primarily through the production of macrophage-activating cytokines, such as gamma interferon (IFN-γ) or granulocyte-macrophage colony-stimulating factor (1, 4). In addition, CD8+ T cells may contribute to disease resistance either through the elaboration of cytokines or through direct cytotoxicity for Mycobacterium-infected cells (10, 21, 22). Most studies of human T cells in tuberculosis have focused on persons with either active disease or latent infection. These studies have been invaluable in defining the T-cell correlates of unsuccessful versus successful human immune responses to M. tuberculosis. However, the roles of the different T-cell subsets and their interactions with one another to mediate protection against disease remain unclear.
More relevant to the issue of vaccine development are the types of responses that occur when T cells from tuberculosis-naive individuals are exposed to mycobacterial antigens. The antigen(s), adjuvants, and other conditions required to effectively sensitize and induce naive T cells to differentiate into protective effector cells are largely unknown but clearly warrant further investigation if a successful vaccine effort is to be undertaken.
To this end, we have focused our studies on naive human T-cell sensitization with mycobacterial antigens by developing two in vitro vaccine models. The first uses live attenuated Mycobacterium (HR37a)-infected macrophages (MIM) to sensitize naive human T cells. We believe that the T-cell responses observed in this system reflect what occurs in vivo on initial infection of a naive host with M. tuberculosis, with responses ranging from beneficial (i.e., correlating with host containment of infection) to permissive (i.e., correlating with failure to contain infection). However, since sensitization occurs in vitro, the parameters affecting T-cell priming leading to the differentiation of beneficial T cells can be manipulated and investigated. In addition, the functions of and interactions between T-cell subsets can be studied. Here, we report the use of this system to investigate certain factors affecting the development of responses to antigen 85 (Ag 85) complex proteins during infection.
The second in vitro model we developed represents a subunit vaccine system. Here, naive T cells were sensitized with defined mycobacterial antigens under various conditions. The effector T cells that developed were tested for antimycobacterial functions. Using this system, we found that sensitization with the vaccine candidate, recombinant Ag85B (rAg85B), in the presence of interleukin 12 (IL-12) effectively primes naive human T cells to become Th1 effectors against M. tuberculosis-infected macrophages. These two in vitro sensitization models represent novel approaches to investigating questions concerning naive human T-cell responses to mycobacterial antigens that cannot be readily addressed using murine or human in vivo systems but that may ultimately contribute to vaccine development.
MATERIALS AND METHODS
Mycobacteria.
M. tuberculosis (H37Ra; American Type Culture Collection) was grown on Lowenstein-Jensen medium at 37°C and then transferred for further cultivation to Middlebrook 7H9 broth with ADC enrichment (Difco Laboratories, Detroit, Mich.). The culture was kept in a shaking 37°C incubator until an optical density of 0.6 (mid-log phase) was obtained (∼2 weeks). The bacteria were frozen in aliquots and stored at −70°C until used. The titers in the culture were determined by colony counts of serial dilutions on Middlebrook 7H10 agar plates supplemented with OADC (Difco). Heat-killed M. tuberculosis (H37Ra) was obtained from Difco.
Expression, purification, and resolubilization of recombinant proteins.
The genes encoding Ag85A, Ag85B, and Ag85C were cloned into the Escherichia coli expression vector pRSETB (Invitrogen, Carlsbad, Calif.), which was transformed into E. coli JM109(DE3) (Promega, Madison, Wis.). The genes for Ag85A and Ag85B were altered to replace low-usage codons for the purpose of increasing the expression of rAg85 (22a). Flasks containing 50 ml of SOB broth and 200 μg of ampicillin per ml were inoculated with 300 μl of an overnight culture of transformed JM109(DE3) cells and incubated at 37°C with shaking to an absorbance at 600 nm of 0.6. Recombinant protein expression was induced by the addition of isopropyl-β-d-thiogalactopyranoside (Promega) at a final concentration of 1 mM and incubation for an additional 5 h.
To purify rAg85 proteins, the 5-h culture was pelleted by centrifugation, and the cells were lysed in 10 ml of 25 mM Tris-HCl (pH 7.8)–500 mM NaCl–1% Triton X-100–0. 1 mg of lysozyme per ml on ice for 30 min. This lysate was sonicated (VirTis Inc., Gardiner, N.Y.), frozen in liquid nitrogen, and thawed at 37°C. Insoluble inclusion bodies were harvested by centrifugation at 7,000 rpm for 15 min and solubilized in 6 M guanidine-HCl–30 mM Tris-HCl (ph 7.8)–500 mM NaCl–10 mM dithiothreitol–2 mM EDTA. The solubilized antigen fraction was filtered through a 0.4-μm-pore-size filter (Millipore) and slowly diluted to 100 ml with 4 M urea–30 mM Tris-HCl–500 mM NaCl–6 mM reduced glutathione (Sigma Chemical Co., St. Louis, Mo.)–0.6 mM oxidized glutathione (Sigma) (pH 7.8). After 1 h of incubation at room temperature, insoluble protein was removed by filtration. The filtrate was slowly added to 1 liter of 100 mM Tris-HCl–1 M NaCl–6 mM reduced glutathione–0.6 mM oxidized glutathione (pH 7.8). This solution was concentrated to 30 ml by ultrafiltration using a stirred cell and a YM10 membrane (Millipore). After filtration through a 0.22- μm-pore-size filter, the concentrated protein was dialyzed against 100 mM Tris-HCl (pH 7.8)–1 M NaCl. Following resolubilization, polyacrylamide gel electrophoresis of rAg85B showed a single band at the expected molecular weight. The refolded Ag85 proteins were freed of endotoxin by two passages over Detoxigel affinity columns (Pierce Chemical, Rockford, Ill.), after which endotoxin contamination was less than 0.8 endotoxin units/mg of protein (Limulus amebocyte assay; BioWhittaker, Walkersville, Md.).
PBMC.
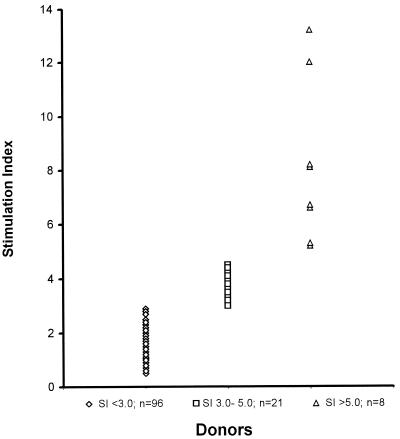
Human buffy coats were obtained from healthy individuals by the American Red Cross (Pacific Northwest Regional Services, Portland, Oreg.). Donated blood was serologically screened for human immunodeficiency virus, hepatitis B and C viruses, cytomegalovirus, human T-cell leukemia virus type 1, and other pathogens. Only nonreactive samples were used. Peripheral blood mononuclear cells (PBMC) were isolated by density centrifugation through Ficoll-Paque (Life Technologies, Grand Island, N.Y.) and stored frozen in liquid nitrogen. Differential white blood cell counts and fluorescence-activated cell sorting analysis of common cell surface markers indicated that the samples fell within the normal ranges for lymphocytes, monocytes, and granulocytes as well as for the frequency of CD4+ and CD8+ T cells. All of the samples used were nonreactive (stimulation index [SI], <3.0) to M. tuberculosis antigens, as determined by testing in a primary proliferation assay with 25 μg of heat-killed M. tuberculosis (H37Ra) per ml. Of samples from 125 donors tested, ∼77% were nonreactive by this criterion, 17% had an SI of >3.0 but <5.0, and 6% had an SI of >5.0. A subset of this last population may correspond to persons who are latently infected with M. tuberculosis and whose samples are possibly reactive with purified protein derivative (PPD). This suggestion is supported by U.S. data indicating that ∼4% of the population has latent tuberculosis infection (7).
In vitro sensitization and propagation of Mycobacterium-specific T cells from normal PBMC.
Monocytes were enriched from PBMC by adherence to plastic for 45 min in 48-well tissue culture plates and then extensively washed. The cells matured into macrophages during culturing for 5 to 7 days in endotoxin-free RPMI 1640 (BioWhittaker) supplemented with gentamicin, 5 × 10−5 M 2-mercaptoethanol, l-glutamine, and 10% heat-inactivated fetal bovine serum (Hyclone Laboratories, Inc., Logan, Utah). Mature macrophages were infected with M. tuberculosis at a ratio of 20:1 for 4 h at 37°C. The macrophages were washed, fed with fresh medium, and recultured at 37°C for an additional 2 days. They were washed again just prior to T-cell sensitization. Histological analysis of cells adherent to LabTek slides which were stained with DiffQuik revealed that 100% of adherent cells present after 5 days of maturation and 2 days of infection were macrophages, as evidenced by morphology. No contaminating lymphocytes were detected. Slides stained with reagents to detect acid-fast bacilli demonstrated that 50 to 70% of the macrophages were infected with M. tuberculosis. These infected cells remained viable for up to 7 days in culture.
Autologous PBMC (107) in complete medium containing 10% heat-inactivated human serum (Pel-Freez Clinical Systems, Brown Deer, Wis.) were added to M. tuberculosis-infected macrophages for a 48-h sensitization period. Five days after sensitization, complete medium supplemented with 10 U of recombinant IL-2 (Life Technologies) per ml was added to the cultures. Thereafter, the cells were maintained in IL-2-containing medium that was changed every 3 to 4 days. Every 12 to 16 days, the T-cell lines were boosted with autologous MIM at an approximate ratio of 10 T cells to 1 MIM. T-cell lines underwent two to four cycles of stimulation with MIM before being tested for functional activity or specificity. Infection-sensitized T cells propagated in this manner were designated Mtb T-cell lines. For some experiments, Mtb T-cell lines were sensitized in the presence of IL-12 (1 to 5 ng/ml; Pharmingen, San Diego, Calif.) or neutralizing antibody to IL-12 (100 μg/ml; Biosource International, Camarillo, Calif.) during the first exposure to MIM.
Ag85B-specific T-cell lines were generated from normal PBMC and propagated as follows. Naive PBMC were sensitized with 20 μg of rAg85B per ml in the absence (N-85B lines) or presence (T-85B lines) of 1 ng of IL-12 per ml. After 7 days, the T cells were boosted with autologous macrophages pulsed with rAg85B. Thereafter, the lines were propagated in IL-2-containing medium that was changed every 3 to 4 days and then were boosted with autologous macrophages pulsed with rAg85B every 12 to 14 days. IL-12 was given only during the initial sensitization phase.
Depletion of CD45R0-positive cells from PBMC.
PBMC were depleted of CD45R0-positive cells using the MiniMacs system (Miltenyi Biotech, Inc., Sunnyvale, Calif.). Briefly, PBMC were incubated with an affinity-purified mouse anti-human CD45R0 antibody (Pharmingen) and washed. Cells were then incubated with goat anti-mouse immunoglobulin G-conjugated beads and passed over the magnetic separation column. Flow cytometry indicated that the negatively enriched populations were 94 to 98% CD45RA (naive cells), with contaminating CD45R0-positive cells ranging from 0% (n = 3) to 8% (n = 1). PBMC and PBMC depleted of CD45R0-positive cells, obtained from the same donors, were used to generate Mtb T-cell lines as described above.
In vitro proliferation assays.
Resting cells from Mtb or rAg85B-specific T-cell lines were cultured in quadruplicate in 96-well flat-bottom plates at 3 × 104 cells/well with MIM, uninfected macrophages pulsed with heat-killed Mycobacterium (HKM) or rAg85, or uninfected macrophages alone. Chicken ovalbumin (OVA; Sigma) or Leishmania lysate was used as a control antigen. After 3 days of culturing, the cells were pulsed with 1 μCi of 3H-thymidine (6.7 Ci/mmol; NEN Research Products, Boston, Mass.) for 18 h and harvested on glass fiber filter mats. Radioactive incorporation was assessed by liquid scintillation counting. The SI was calculated as the mean counts per minute for cells cultured with antigen divided by the mean counts per minute for cells cultured without antigen. MIM cultured without T cells did not incorporate significant radioactivity (< 300 cpm).
Cytokine assays.
Supernatants from proliferation assays were collected for the determination of cytokine levels. Preliminary kinetic experiments revealed that peak IL-4 production was detected in 24-h supernatants, while peak IFN-γ and IL-10 production was detected in 72-h supernatants. Cytokine levels were quantified by an enzyme-linked immunosorbent assay (ELISA) with commercial anti-cytokine antibody pairs (Pharmingen) according to the manufacturer's protocol. Human recombinant IL-4, IFN-γ, and IL-10 (Pharmingen) were used to generate standard curves. Supernatants were harvested from quadruplicate cultures and pooled and run as duplicate samples in the ELISA. Supernatants from infected or uninfected macrophages (triplicate cultures) were harvested at 24 and 48 h and tested for IL-12 using a p70-specific IL-12 ELISA (Quantikine; Pharmingen) in duplicate.
Immunofluorescence and flow cytometry.
Cells from Mtb T-cell lines were tested for CD3, CD8 (Leu-2a), and CD4 (Leu-3a) (Becton Dickinson Immunocytometry Systems, Mountain View, Calif.) surface marker expression by three-color analysis using standard procedures. Data were collected using a FACScan (Becton Dickinson) and analyzed with LYSUS II software. At least 5,000 cells were analyzed per sample.
RESULTS
Mycobacterium-specific T-cell lines sensitized in vitro develop predominantly Th1 profiles.
Tuberculosis-naive donors were selected by testing the proliferative responses of PBMC to heat-killed M. tuberculosis H37Ra. T-cell lines used in these studies were generated from nonreactive donors (SI, <3.0) and were considered naive. Figure 1 shows the proliferative responses of samples from 125 donors to H37Ra. Ninety-six donors (77%) were considered tuberculosis naive by this criterion. The 17 donors whose samples were used for the following studies were chosen from this group. The average mean primary proliferation SI of these samples was 2.0. Using the methods described above, the generation of in vitro-sensitized T-cell lines was 100% efficient. Thus, the reactivity to M. tuberculosis antigens was an intrinsic capability of the cells and was not attributable to prior infection.
FIG. 1.
Screening of donors for M. tuberculosis reactivity by proliferation. PBMC were tested in a standard proliferation assay using 25 μg of H37Ra per ml. The mean (standard deviation) SIs were 1.53 (0.62), 3.8 (0.45), and 8.2 (3.0), respectively, for the groups shown on the figure, from left to right.
Long-term-infection-induced T-cell lines (Mtb T-cell lines) were prepared from 11 donors by cultivating PBMC from naive donors on autologous MIM. These lines were extensively analyzed. Table 1 demonstrates that all such Mtb lines proliferated specifically in response to in vitro challenge with MIM or HKM-pulsed autologous normal macrophages. SIs ranged from 3.0 to 34. As might be predicted, the cells proliferated more in response to live rather than dead Mycobacterium antigens. Analysis of cytokine production demonstrated that the majority of the T-cell lines developed Th1 profiles, producing significant levels of IFN-γ but little or no IL-4. However, the range of IFN-γ production among these Th1 cell lines varied widely, with some producing high quantities and others producing very low quantities. In most instances, IL-10 production (data not shown) paralleled that of IL-4. Only one T-cell line, Mtb.94, produced more IL-4 than IFN-γ; it was thus designated a Th2 responder. Duplicate lines generated from the same donors at a later time showed similar cytokine profiles. Not surprisingly, the levels of cytokines produced did not correlate with proliferation (Table 1) or with the percentage of CD4+ cells found in the lines (Table 2). For example, the percentages of CD4+ and CD8+ cells were very similar in lines Mtb.90, Mtb.94, and Mtb.101, but their cytokine profiles were quite different (Tables 1 and 2). All of the Mtb lines contained both CD4 and CD8 cells. In addition, some lines contained significant numbers of CD3+, CD4−, and CD8− (double-negative) T cells (Table 2).
TABLE 1.
Proliferation and cyokine profiles of Mycobacterium-specific T-cell lines
| T-cell line | Results obtained in the presence of:
|
Profile | |||||
|---|---|---|---|---|---|---|---|
| MIMa
|
HKMb
|
||||||
| SIc | Leveld (pg/ml) of:
|
SIc | Leveld (pg/ml) of:
|
||||
| IFN-γ | IL-4 | IFN-γ | IL-4 | ||||
| Mtb.90 | 23 | 236 | <5 | 10.6 | 236 | <5 | Th1 |
| Mtb.94 | 15 | 19 | 67 | 7.6 | <10 | 8 | Th2 |
| Mtb.101 | 14 | 157 | 20 | 9.2 | 813 | <5 | Th1 |
| Mtb.102 | 28 | 331 | 37 | 8.5 | 54 | <5 | Th1 |
| Mtb.104 | 10.4 | 193 | <5 | 3.2 | 85 | 18 | Th1 |
| Mtb.106 | 33.5 | 215 | <5 | 10.2 | 528 | <5 | Th1 |
| Mtb.107 | 12.5 | 597 | 37 | 8.9 | 512 | <1 | Th1 |
| Mtb.108 | 8.0 | 50 | <5 | ND | <5 | 12 | Th1 |
| Mtb.184 | 5.1 | 600 | <5 | 4.0 | 644 | <5 | Th1 |
| Mtb.186 | 6.1 | 116 | <5 | 5.2 | 264 | <5 | Th1 |
| Mtb.187 | 19.4 | 30 | 7 | 23.2 | 91 | 5 | Th1 |
MIM ratio, 20:1.
HKM (15 μg/ml)-pulsed macrophages.
Background counts per minute for SI (mean and standard deviation), 674 ± 434. ND, not determined.
Supernatants were harvested from antigen-stimulated and nonstimulated cultures (T cells plus normal macrophages) and tested for cytokines. Data are reported as levels of cytokines from stimulated cultures minus unstimulated cultures. The amount of IFN-γ produced from unstimulated cultures was 1.6 ± 5.1 pg/ml (mean and standard deviation; 95% confidence interval upper limit, 5.1), and that of IL-4 was 7.7 ± 19.4 pg/ml (95% confidence interval upper limit, 20).
TABLE 2.
Cell surface phenotype of Mycobacterium-specific T-cell lines
| T-cell line | % of cells that werea:
|
||
|---|---|---|---|
| CD4+ | CD8+ | DN | |
| Mtb.90 | 87 | 4 | 2 |
| Mtb.94 | 86 | 4 | 6 |
| Mtb.101 | 87 | 3 | 5 |
| Mtb.102 | 71 | 11 | 9 |
| Mtb.104 | 66 | 19 | 4 |
| Mtb.106 | 66 | 19 | 9 |
| Mtb.107 | 67 | 17 | 7 |
| Mtb.108 | 75 | 11 | 7 |
| Mtb.184 | 63 | 19 | 16 |
| Mtb.186 | 69 | 14 | 14 |
Mtb T-cell lines were stained with antibodies to CD3-PerCP, CD4 fluorescein isothiocyanate, and CD8-phycoerythrin. DN, double negative (CD4− and CD8−).
In order to evaluate the contribution of naive versus memory cells to the response to M. tuberculosis, infection-induced T-cell lines (Mtb T-cell lines) were prepared by cultivating PBMC (from naive donors; SI, <3.0) or PBMC depleted of CD45R0-positive cells (from the same donors) on autologous MIM. CD45R0-positive cells ranged from 21 to 26% of normal PBMC in the donors tested. In CD45R0-depleted PBMC, this population was reduced to 0% (n = 3) or 8% (n = 1; donor 229). Four weeks later, the cells from both types of lines were challenged in vitro with mycobacterial antigens. Table 3 demonstrates that in three of four donors, IFN-γ production from lines generated with PBMC did not differ significantly from that in lines generated with CD45R0-depleted PBMC. In one donor, IFN-γ levels were somewhat higher in lines generated from CD45R0-depleted PBMC. Of interest, the sample from donor 234 exhibited no IFN-γ or IL-4 (data not shown) production but was Mycobacterium specific, as evidenced by proliferation (SIs ranged from 3 to 13). Thus, the contribution of CD45R0-positive cells to the responses seen were minimal.
TABLE 3.
IFN-γ production by Mtb T-cell lines generated in the presence or absence of CD45R0-positive cellsa
| T-cell lines | IFN-γ produced (pg/ml) with the following challenge antigen:
|
||
|---|---|---|---|
| MIM | H37Ra | rAg85B | |
| Mtb.231 | 76 | 122 | 111 |
| Mtb.231 R0 depleted | 42 | 260 | 66 |
| Mtb.232 | 135 | 747 | 55 |
| Mtb.232 R0 depleted | 140 | 477 | 126 |
| Mtb.229 | 37 | 98 | 67 |
| Mtb.229 R0 depleted | 260 | 226 | 99 |
| Mtb.234b | <10 | <10 | <10 |
| Mtb.234 R0 depletedb | <10 | <10 | <10 |
Mtb T-cell lines were generated with normal PBMC or with PBMC depleted of CD45R0-positive cells (R0 depleted) and propagated as described in the text. Four weeks later, the cells were challenged in vitro with mycobacterial antigens, and supernatants were tested for IFN-γ production.
These T-cell lines were specific for mycobacterial antigens, as determined by proliferation (SI, 3 to 13).
Mtb T-cell lines respond to challenge with Ag85 complex proteins.
We analyzed cytokine production from Mtb T-cell lines that were stimulated in vitro with normal autologous macrophages pulsed with rAg85A, rAg85B, and rAg85C. All Mtb T-cell lines responded to challenge with Ag85 complex proteins by production of IFN-γ or IL-4 (Table 4). Cells from Mtb lines challenged with OVA or Leishmania lysate did not proliferate (SI, <2) or produce IFN-γ (<20 pg/ml). Mtb lines which produced high concentrations of IFN-γ in response to MIM also produced high quantities of IFN-γ in response to rAg85 proteins. Mtb.94, the one Th2 cell line generated, produced only IL-4 in response to rAg85A. Responses to Ag85 proteins were of a very high magnitude, ranging from 50 to 150% the cytokine production seen in response to MIM. Mtb lines in which the three recombinant proteins were tested individually showed no greater response to one Ag85 protein than to the others.
TABLE 4.
Cytokine profiles of Ag85-elicited Mycobacterium-specific T-cell linesa
| T-cell line | Level (pg/ml) of the indicated cytokine produced with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| rAg85A
|
rAg85B
|
rAg85C
|
MIM
|
|||||
| IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | IFN-γ | IL-4 | |
| Mtb.186 | 11 | <5 | 27 | 6 | 831 | 13 | 101 | <5 |
| Mtb.144 | 169 | <5 | 73 | <5 | ND | ND | 129 | <5 |
| Mtb.149 | 1,178 | <1 | 746 | <5 | ND | ND | 420 | <5 |
| Mtb.173 | 300 | <5 | 270 | <1 | 400 | <1 | 500 | <5 |
| Mtb.183 | 250 | <5 | 300 | <5 | 300 | <5 | 501 | <5 |
| Mtb.184 | 41 | <5 | 20 | <5 | 129 | <5 | 319 | <5 |
| Mtb.191 | 34 | <5 | 82 | <5 | 30 | <5 | 65 | 11 |
| Mtb.193 | <10 | <5 | 22 | <5 | 141 | <5 | 82 | <5 |
| Mtb.94 | <10 | 55 | ND | ND | ND | ND | 19 | 50 |
Resting T cells from Mycobacterium-specific T-cell lines were cultured with MIM or with autologous macrophages pulsed with 15 μg of rAg85A, rAg85B, rAg85C, or no antigen. Supernatants were harvested from triplicate cultures at 24 h (IL-4) and 72 h (IFN-γ) and tested by an ELISA. Results are expressed as levels of cytokines from stimulated cultures minus unstimulated cultures. ND, not determined.
Regulation of Mtb T-cell line Th1 responses by IL-12.
In order to determine if IL-12 affected the cytokine profile of Mycobacterium-specific T cells in this model, new Mtb T-cell lines were generated in the presence or absence of IL-12 or neutralizing antibody to IL-12. Mtb lines sensitized in the presence of IL-12 produced 3- to 30-fold more IFN-γ upon subsequent challenge with MIM or rAg85B-pulsed macrophages (Table 5) than Mtb lines sensitized in the absence of IL-12. However, the addition of antibody to IL-12 to cultures at the initiation of the Mtb lines did not reduce or eliminate IFN-γ production below the levels seen in Mtb lines initiated in the absence of IL-12. Therefore, the elimination of endogenous IL-12 during sensitization did not inhibit the development of Mycobacterium-specific Th1 cells. This conclusion is further supported by the fact that endogenous IL-12 production from MIM in both the presence and the absence of PBMC did not correlate with IFN-γ production by Mtb lines generated in the absence of exogenous IL-12 (Table 5).
TABLE 5.
IL-12 potentiates Mycobacterium-specific IFN-γ production
| T-cell line | IFN-γ production a (pg/ml) with:
|
||||||
|---|---|---|---|---|---|---|---|
| MIM
|
rAg85B
|
IL-12 productionb (pg/ml) with:
|
|||||
| − IL-12 | + IL-12 | + anti– IL-12 | − IL-12 | + IL-12 | MIM | MIM + PBMCb | |
| Mtb.187 | <5 | 174 | <5 | ND | ND | 7 | <5 |
| Mtb.191 | 65 | 184 | 63 | ND | ND | <5 | 10 |
| Mtb.184 | 34 | 599 | 50 | 97 | 206 | 30 | 17 |
| Mtb.193 | 82 | 397 | 141 | 22 | 77 | 7 | 14 |
| Mtb.186 | 129 | 1,510 | ND | 12 | 394 | <5 | <5 |
Mtb T-cell lines were generated by stimulating normal PBMC with MIM in the absence (−) or presence (+) of IL-12 (1 ng/ml) or neutralizing antibody to IL-12 (100 μg). They were challenged in vitro with MIM or rAg85B-pulsed normal macrophages for 3 days. Supernatants were harvested at 72 h, and IFN-γ production was determined by an ELISA. ND, not determined.
Mature macrophages (from donors 187, 191, 184, 193, and 186) were infected with MIM (ratio, 20:1) or mock infected (data not shown). After 2 days, autologous naive PBMC were cocultured with MIM. Supernatants were harvested at 48 h and tested for IL-12 p70 by an ELISA.
Immunization with rAg85B can prime naive T cells for Mycobacterium-specific responses.
In order to test the immunogenicity of rAg85B in a human vaccine system, naive T cells from six naive donors were sensitized with rAg85B in the presence or absence of IL-12 (1 ng/ml). These cells were propagated as continuous Ag85B-specific T-cell lines by periodic boosting with rAg85B-pulsed normal macrophages. IL-12 was given only at the time of initial sensitization. Ag85B-specific T-cell lines were tested for specificity by proliferation and cytokine production after 4 to 6 weeks in cultures. All T-cell lines proliferated in response to MIM and HKM- or rAg85B-pulsed normal macrophages (Table 6). Ag85B-specific T-cell lines generated in the absence of IL-12 proliferated to mycobacterial antigens, demonstrating specificity, but produced little or no IFN-γ. In contrast, five of the six Ag85B-specific T-cell lines sensitized in the presence of IL-12 produced high quantities of IFN-γ; little or no IL-4 production was detected (data not shown). Thus, in the presence of IL-12, rAg85B can prime human Th1 cells which recognize the natural ligand present on MIM. We found similar results when using rAg85A as the sensitizing antigen in this system (data not shown). Surface marker analysis of the Ag85B-expressing T-cell lines demonstrated that they contained 80 to 85% CD4+ cells and 10 to 15% CD8+ cells. Of interest, no significant surface phenotype differences between lines generated in the absence (N-85B) or the presence (T-85B) of IL-12 were detected.
TABLE 6.
Immunization with rAg85B induces Mycobacterium-specific human T-cell responsesa
| T-cell line | Results obtained in the presence of:
|
|||||
|---|---|---|---|---|---|---|
| MIM
|
HKM
|
rAg85B
|
||||
| SI | IFN-γ level | SI | IFN-γ level | SI | IFN-γ level | |
| N-85B.149 | 5.0 | <5 | 2.0 | 71 | 2.0 | <5 |
| T-85B.149 | 3.2 | 40 | 2.0 | 223 | 2.1 | 92 |
| N-85B.159 | 1.0 | 62 | ND | ND | 3.8 | 240 |
| T-85B.159 | 2.0 | 190 | ND | ND | 4.6 | 641 |
| N-85B.173 | ND | 43 | ND | 61 | ND | <5 |
| T-85B.173 | ND | 864 | ND | 3,106 | ND | 336 |
| N-85B.186 | 10.0 | <5 | 10.7 | <5 | 4.5 | 12 |
| T-85B.186 | 6.8 | <5 | 10.4 | 31 | 15 | 107 |
| N-85B.187 | 10.7 | 5 | 3.6 | <5 | 6.6 | <5 |
| T-85B.187 | 3.8 | 594 | 1.5 | 260 | 2.7 | 734 |
| N-85B.191 | 4.2 | <5 | 2.3 | <5 | 2.4 | <5 |
| T-85B.191 | 3.3 | 50 | 2.3 | 36 | 2.3 | 48 |
Resting cells from rAg85B-specific T-cell lines were stimulated in vitro with MIM (20:1) or HKM (15 μg/ml)- or rAg85B (10 μg/ml)-pulsed normal macrophages for 3 days. Supernatants were harvested and tested for the IFN-γ concentration (picograms per milliliter) by an ELISA. Proliferation was assessed by measuring 3H-thymidine incorporation. ND, not determined. T-cell lines designated with “N” were generated by immunization of normal PBMC with rAg85B. T-cell lines designated with “T” were generated by immunization of normal PBMC with rAg85B plus IL-12 (1 ng/ml).
DISCUSSION
The development of resistance to disease caused by M. tuberculosis is still poorly understood and probably involves multiple innate as well as acquired immune components. T-cell responses, in particular, have been shown to be critical. Specifically, these include the CD4+ Th1 subset, which secretes IFN-γ, as well as CD8+ cells, which produce cytokines and directly lyse infected cells. Roles for other T-cell subsets, including CD1-restricted CD3+ cells (12, 33) and γδ T cells (5, 16), are also being investigated.
We were interested in studying vaccine-induced human T-cell responses to M. tuberculosis. To this end, we developed an in vitro system whereby naive human T cells were sensitized and propagated with an attenuated live vaccine (Mtb T-cell lines) or a subunit vaccine (Ag85B lines). These models may be useful for investigating the antigens and other conditions required to induce the differentiation of naive T cells into beneficial effector cells against M. tuberculosis. The advantages of this in vitro system are (i) the sensitization parameters can be controlled and easily manipulated and (ii) early events and cell interactions can be delineated. Neither of these objectives can be easily accomplished using human cells sensitized in vivo.
Mtb T-cell lines mounted largely beneficial responses, as expected. Mtb lines proliferated and produced cytokines in response to challenge with live or dead bacteria. Of 11 Mtb lines generated from different donors, 10 developed a Th1 profile of cytokine production, although 2 of these lines produced very low levels of IFN-γ (<50 pg/ml). Only one line (Mtb.94) showed an overt Th2 cytokine profile. In general, cytokine levels produced by Mtb lines were low, but this result is not surprising, as these cells were primed in vitro for a limited period of time.
The type of immune responses seen with this system appeared to be mounted largely by the CD45RA or naive cell population. Depletion of CD45R0-positive (memory) cells from the initial PBMC population did not affect the type or magnitude of responses which developed. Therefore, the contribution of cross-reacting memory cells, which may have originally been primed by related bacterial antigens, appeared minimal.
Although the number of naive donors tested was small, the profiles seen in our Mtb T-cell lines in vitro appeared to closely fit the T-cell profiles that accompany natural infection in humans. Normally, about 90% of individuals newly infected with M. tuberculosis develop protective immune responses, whereas less than 10% develop primary progressive disease. While we have no direct knowledge of how these donors would respond to in vivo infection, we speculate that the moderate to high Th1 responders might be at low risk for the development of active disease, while the Th2 responder (Mtb.94) might be at high risk. The two low Th1 responders (Mtb.108 and Mtb.187) are more difficult to classify, but their clinical status might be more dependent on the presence of other protective responses (cytotoxic T cells). Thus, the majority of responses seen in this model are beneficial ones and may be analogous to those seen in PPD-positive (PPD+) asymptomatic individuals.
Since sensitization in vitro with avirulent M. tuberculosis induces T-cell responses which parallel at least some of those seen during vivo sensitization, it may represent an alternative to the use of patient material for certain studies, particularly those investigating early T-cell responses to new infection. We have used a similar in vitro model to study early human T-cell responses to Leishmania infection (31). Like infection with Mycobacterium, infection with Leishmania usually results in resistance to disease. Using an in vitro model of human Leishmania infection, we found that only 50% of individuals developed Th1 responses to Leishmania. However, >75% of individuals mounted cytotoxic responses which lysed Leishmania-infected macrophages. This result suggested that cytotoxic effector activity, in addition to Th1 responses, contributed to resistance to human disease caused by Leishmania. Further investigation is needed to determine if the CD8+ T cells present within Mtb T-cell lines are cytotoxic. Using a similar in vitro system, involving short-term coculturing of M. tuberculosis-infected macrophages with autologous PBMC from tuberculosis patients, PPD+ asymptomatic individuals, or naive donors, other investigators demonstrated cytokine differences between the groups. In the naive PBMC cocultures, high-level IFN-γ production in association with low-level IL-2 production suggested a role for natural killer cell-derived cytokines in the development of a successful M. tuberculosis-specific immune response (20).
When Mtb T-cell lines were challenged with rAg85 proteins, the quantities of IFN-γ produced in response to the Ag85 proteins correlated with the quantities produced in response to MIM or HKM. However, in some instances, more IFN-γ was elicited by rAg85 than by MIM, demonstrating the potency of Ag85 proteins. Not surprisingly, IL-4 was produced by the one Th2 Mtb T-cell line in response to rAg85A. These results support the immunodominance of Ag85 proteins which has been shown by other investigators, primarily by using cells from PPD+ asymptomatic individuals (6, 15, 26). Of interest, a comparison of the immunogenicities of rAg85 proteins done with four Mtb lines revealed no significant differences in the ability of the three proteins to elicit strong IFN-γ production from Th1 responders. However, in three other Mtb lines, rAg85C elicited much higher levels of IFN-γ production, suggesting a differential response to these proteins in some individuals.
Work from several laboratories has shown that IL-12 contributes to the magnitude of anti-Mycobacterium Th1 responses (39, 40). We demonstrated that Mtb T-cell lines sensitized in the presence of IL-12 showed dramatically increased IFN-γ production in response to challenge with mycobacterial antigens, including rAg85B. These data support work by other investigators showing that IL-12 increased IFN-γ production in cultures from PPD+ individuals (25). Furthermore, in patients with multidrug-resistant tuberculosis, the addition of IL-12 to in vitro cultures of PBMC restored IFN-γ production (25). However, when we generated Mtb lines in the presence of antibody to IL-12, IFN-γ production was not reduced to levels below those seen in Mtb lines generated in the absence of IL-12. Also, endogenous IL-12 production by MIM in the presence or absence of PBMC did not correlate with the amount of IFN-γ production seen in Mtb lines. These data support observations demonstrating that inherent resistance to Mycobacterium in BCG-resistant mice does not depend on optimal IL-12 levels (35). Together, the results suggest that other cytokines (e.g., IL-18 and MIP-1β) also contribute to the development of M. tuberculosis-specific Th1 responses (11, 34; N. Kozlova, D. L. Lakey, T. Winn, D. Kernodle, and D. M. Russo, Abstr. 34th Tuberculosis-Leprosy Conf. Tuber. Lung Dis., p. 262, 1999).
Human T-cell responses to secreted Ag85 proteins appear to be immunodominant in PPD+ latently infected individuals (6, 15, 26) and are downregulated in those with active tuberculosis (3, 15, 26). These findings suggest an association with protective responses. Furthermore, strong Th1 responses have been elicited in vitro from PPD+ asymptomatic individuals using native, recombinant, or synthetic peptide forms of Ag85 proteins (15, 23, 26). In addition, Ag85 proteins have been shown to induce partial protection in murine models of infection (17, 18). These findings have provided hope that an efficacious subunit vaccine can be developed. However, recombinant proteins expressed in bacterial systems can be problematic in terms of immunogenicity due to improper processing and folding. We used an in vitro sensitization system to derive important information regarding a vaccine candidate (gp63) for Leishmania (30). We showed that the immunogenic potential of E. coli-expressed recombinant gp63 was limited due to its inability to prime T cells which could subsequently recognize native antigen. This result provided one explanation as to why recombinant gp63 failed to protect mice (14, 24), even though native gp63, other recombinant forms of gp63, and peptides of gp63 had all induced some degree of protection (8, 19, 29, 37, 38).
Since naive T-cell sensitization studies with rAg85 proteins had not been reported previously, we were concerned that a similar problem might be observed with tubercular rAg85. Therefore, we tested the immunogenicity of rAg85B in a human vaccine model where naive T cells were immunized with rAg85B in the presence or absence of IL-12. We found that rAg85B-specific T-cell lines generated in the presence of IL-12 responded to challenge with MIM by proliferation and, more importantly, by enhanced production of IFN-γ. Thus, rAg85B expressed in E. coli can prime Mycobacterium-specific human Th1 cells which recognize the naturally expressed ligand on MIM. This result suggests that rAg85B in combination with an appropriate adjuvant (IL-12 or an IL-12-inducing substance) can induce the human immune responses required of a potential candidate vaccine for M. tuberculosis.
In summary, we conclude that naive human T cells react with whole M. tuberculosis cells and defined subunit antigens in vitro, reproducibly generating predominantly Th1 responses, although some variability based on the individual T-cell donor was observed. T-cell lines generated using rAg85B subsequently responded to live tubercle bacilli, validating the concept that humans vaccinated with a single mycobacterial antigen might develop beneficial T-cell responses. Further definition of the antigens and conditions required to drive naive human T cells to differentiate into Th1 effectors should facilitate the development of an improved tuberculosis vaccine.
ACKNOWLEDGMENTS
We thank Frank Hatcher for help analyzing the flow cytometry data and James M. Burns, Jr., and Radiah Corn for critically reviewing the manuscript.
This work was supported by the Department of Veteran Affairs.
REFERENCES
- 1.Barnes P F, Lu S, Abrams J S, Wang E, Yamumura M, Modlin R L. Cytokine production at the site of disease in human tuberculosis. Infect Immun. 1993;61:3482–3489. doi: 10.1128/iai.61.8.3482-3489.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnes P F, Modlin R L, Ellner J J. T-cell responses and cytokines. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 417–435. [Google Scholar]
- 3.Boesen H, Jensen B N, Wilcke T, Anderson P. Human T-cell responses to secreted antigen fractions of Mycobacterium tuberculosis. Infect Immun. 1995;63:1491–1497. doi: 10.1128/iai.63.4.1491-1497.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boom W H, Wallis R S, Chervenak K A. Human Mycobacterium tuberculosis-reactive CD4+ T-cell clones: heterogeneity in antigen recognition, cytokine production, and cytotoxicity for mononuclear phagocytes. Infect Immun. 1991;59:2737–2743. doi: 10.1128/iai.59.8.2737-2743.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boom W H, Chervenal K A, Mincek M A, Ellner J J. Role of the mononuclear phagocyte as an antigen-presenting cell for human γδ T cells activated by live Mycobacterium tuberculosis. Infect Immun. 1992;60:3480–3488. doi: 10.1128/iai.60.9.3480-3488.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carlucci S, Beshin A, Tuosto L, Ameglio F, Fandolfo F, Cocito C, Fiorucci F, Saltini C, Piccolella E. Mycobacterial antigen complex A60-specific T-cell repertoire during the course of pulmonary tuberculosis. Infect Immun. 1993;61:439–447. doi: 10.1128/iai.61.2.439-447.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Tuberculosis elimination revisited: obstacles, opportunities and renewed commitment—Advisory Council for the Elimination of Tuberculosis (ACET) Morb Mortal Wkly Rep. 1999;48:1–13. [PubMed] [Google Scholar]
- 8.Connell N, Medina-Acosta E, McMaster W R, Bloom B, Russell D. Effective immunization against cutaneous leishmaniasis with recombinant bacille Calmette-Guerin expressing the Leishmania surface proteinase gp63. Proc Natl Acad Sci USA. 1993;90:11473–11477. doi: 10.1073/pnas.90.24.11473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Content A, De La Cuvellerie A, De Wit L, Vincent-Levy-Frebault V, Ooms J, De Bruyn J. The genes coding for the antigen 85 complexes of Mycobacterium tuberculosis and Mycobacterium bovis BCG are members of a gene family: cloning, sequence determination, and genomic organization of the gene coding for antigen 85C of M. tuberculosis. Infect Immun. 1991;59:3205–3212. doi: 10.1128/iai.59.9.3205-3212.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flynn J L, Goldstein M M, Triebold K J, Koller B, Bloom B R. Major histocompatibility complex class I restricted T cells are required for resistance to Mycobacterium tuberculosis infection. Proc Natl Acad Sci USA. 1992;89:12013–12017. doi: 10.1073/pnas.89.24.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia V E, Uyemura K, Sieling P A, Ochoa M T, Morita C T, Okamura H, Kurimoto M, Rea T H, Modlin R L. IL-18 promotes type 1 cytokine production from NK cells and T cells in human intracellular infection. J Immunol. 1999;162:6114–6121. [PubMed] [Google Scholar]
- 12.Gong J, Stenger S, Zack J A, Jones B E, Bristol G C, Modlin R L, Morrissey P J, Barnes P F. Isolation of Mycobacterium reactive CD1-restricted T cells from patients with human immunodeficiency virus infection. J Clin Investig. 1998;101:383–389. doi: 10.1172/JCI318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haanen J B, de Waal Malefit R, Res P C, Kraakman E M, Ottenhoff T H, de Vries R R, Spits H. Selection of a human T helper type 1 subset by mycobacteria. J Exp Med. 1991;174:583–592. doi: 10.1084/jem.174.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Handman E, Button L L, McMaster R W. Leishmania major: production of recombinant gp63, its antigenicity and immunogenicity in mice. Exp Parasitol. 1990;70:427–432. doi: 10.1016/0014-4894(90)90127-x. [DOI] [PubMed] [Google Scholar]
- 15.Havlir D V, Wallis R S, Boom W H, Daniel T M, Chervenak K, Ellner J J. Human immune response to Mycobacterium tuberculosis antigens. Infect Immun. 1991;59:665–670. doi: 10.1128/iai.59.2.665-670.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Havlir D V, Ellner J J, Chervenak K A, Boom W H. Selective expansion of human γδ T cells by monocytes infected by live Mycobacterium tuberculosis. J Clin Investig. 1991;87:729–733. doi: 10.1172/JCI115053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horwitz M A, Lee B W E, Dillionn B J, Harth G. Protection immunity against tuberculosis induced by vaccination with major extracellular proteins of Mycobacterium tuberculosis. Proc Natl Acad Sci USA. 1995;92:1530–1534. doi: 10.1073/pnas.92.5.1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huygen K, Content J, Denis O, Montgomery D L, Yawman A M, Deck R R, Dewitt C M, Orme I M, Baldwin S, D'Souza C, Drowart A, Lozes E, Vandenbussche P, Van Vooren J P, Liu M, Ulmer J B. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat Med. 1996;2:893–898. doi: 10.1038/nm0896-893. [DOI] [PubMed] [Google Scholar]
- 19.Jardim A, Alexander J, Teh H S, Ou D, Olafson R W. Immunoprotective Leishmania major synthetic T cell epitopes. J Exp Med. 1990;172:645. doi: 10.1084/jem.172.2.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson B J, McMurray D N. Cytokine gene expression by cultures of human lymphocytes with autologous Mycobacterium tuberculosis-infected monocytes. Infect Immun. 1994;62:1444–1450. doi: 10.1128/iai.62.4.1444-1450.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaufmann S H E. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988;9:168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- 22.Kumararatne D S, Pithie A S, Drysdale P, Gaston J S, Kiessling R, Iyes P B, Ellis C J, Innes J, Wise R. Specific lysis of mycobacterial antigen bearing macrophages by class II MHC restricted polyclonal T cell lines in healthy donors or patients with tuberculosis. Clin Exp Immunol. 1990;80:314–323. doi: 10.1111/j.1365-2249.1990.tb03287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.Lakey, D. L., R. K. R. Voladri, K. M. Edwards, C. Hager, B. Samten, R. S. Wallis, P. F. Barnes, and Douglas S. Kernddle. 2000. Enhanced production of recombinant Mycobacterium tuberculosis antigens in Escherichia coli by replacement of low-usage codons. 68:233–238. [DOI] [PMC free article] [PubMed]
- 23.Launois P, DeLeys R, Niang M, Srowart A, Andrien M, Dierckx P, Cartel J-L, Sarthou J-L, Van Vooren J-P, Huygen K. T cell mapping of the major secreted mycobacterial antigen Ag85A in tuberculosis and leprosy. Infect Immun. 1994;62:3679–3687. doi: 10.1128/iai.62.9.3679-3687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez J, Reins H, Etges R, Button L, McMaster W R, Overathe P, Klein J. Genetic control of the immune response in mice to Leishmania mexicana surface protease. J Immunol. 1991;146:1328. [PubMed] [Google Scholar]
- 25.McDyer J F, Hackley M N, Walsh T E, Cook J L, Seder R A. Patients with multidrug-resistent tuberculosis with low CD4+ T cell counts have impaired Th1 responses. J Immunol. 1997;158:492–500. [PubMed] [Google Scholar]
- 26.Mehra V, Gong J, Iyer D, Lin Y G, Boylen C T, Bloom B R, Barnes P F. J. Infect. Dis:431–434. 1996. Immune response to recombinant mycobacterial proteins in patients with tuberculosis infection and disease. [DOI] [PubMed] [Google Scholar]
- 27.Murray C J, Salomon J A. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95:13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orme I M, Anderson P, Boom W H. T cell response to Mycobacterium tuberculosis. J Infect Dis. 1993;167:1481–1497. doi: 10.1093/infdis/167.6.1481. [DOI] [PubMed] [Google Scholar]
- 29.Russell D G, Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988;140:1274–1280. [PubMed] [Google Scholar]
- 30.Russo D M, Burns J M, Jr, Carvalho E, Barral A, McMaster R, Button L, Reed S G. Human T cell responses to native and recombinant gp63. J Immunol. 1990;47:3575. [PubMed] [Google Scholar]
- 31.Russo D M, Chakrabarti P, Burns J M., Jr Naive human T cells develop into Th1 or Th2 effectors and exhibit cytotoxicity early after stimulation with Leishmania infected macrophages. J Infect Dis. 1998;177:1345. doi: 10.1086/515284. [DOI] [PubMed] [Google Scholar]
- 32.Sanchez F O, Rodriguez J I, Agudelo G, Garcia L F. Immune responsiveness and lymphokine production in patients with tuberculosis and healthy controls. Infect Immun. 1994;62:5673–5678. doi: 10.1128/iai.62.12.5673-5678.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stenger S R, Mazzaccaro J, Uyemura K, Cho S, Barnes P F, Rosat J-P, Sette A, Brenner M B, Porcelli S A, Bloom B R, Modlin R L. Differential effects of cytolytic T cell subsets on intracellular infection. Science. 1997;276:1684–1687. doi: 10.1126/science.276.5319.1684. [DOI] [PubMed] [Google Scholar]
- 34.Sugawara I, Yamada H, Kaneko H, Mizuno S, Takeda K, Akira S. Role of interleukin 18 (IL-18) in mycobacterial infection in IL-18 gene-disrupted mice. Infect Immun. 1999;67:2585–2589. doi: 10.1128/iai.67.5.2585-2589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thompson-Snipes L, Skamene E, Radzioch D. Acquired resistance but not innate resistance to Mycobacterium bovis bacillus Calmette-Guérin is compromised by interleukin-12 ablation. Infect Immun. 1998;66:5268–5274. doi: 10.1128/iai.66.11.5268-5274.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wiker H G, Harboe M. The antigen 85 complex: a major secreted product of Mycobacterium tuberculosis. Microbiol Rev. 1992;56:648–661. doi: 10.1128/mr.56.4.648-661.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang D M, Fairweather N, Button L L, McMaster W R, Kahl LP, Liew FY. Oral Salmonella typhimurlum (AroA−) vaccine expressing a major leishmanial surface protein (gp63) preferentially induces T helper 1 cells and protective immunity against leishmaniasis. J Immunol. 1990;145:2281. [PubMed] [Google Scholar]
- 38.Yang D M, Rogers M, Liew F Y. Identification and characterization of host-protective T cell epitopes of a major surface glycoprotein (gp63) from Leishmania major. Immunology. 1991;72:3–10. [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M, Gong J, Iyer D V, Jones B E, Modlin R L, Barnes P F. T cell cytokine responses in persons with tuberculosis and human immunodeficiency disease. J Clin Investig. 1994;93:1733–1739. doi: 10.1172/JCI117611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang M, Gately M K, Wang E, Wolf S F, Lu S, Modlin R L, Barnes P F. IL-12 at the site of disease in tuberculosis. J Clin Investig. 1994;94:2435–2442. doi: 10.1172/JCI117157. [DOI] [PMC free article] [PubMed] [Google Scholar]