Abstract
Scutellaria baicalensis Georgi is an annual herb from the Scutellaria genus that has been extensively used as a traditional medicine for over 2000 years in China. Baicalin and other flavonoids have been identified as the principal bioactive ingredients. The biosynthetic pathway of baicalin in S. baicalensis has been elucidated; however, the specific functions of R2R3-MYB TF, which regulates baicalin synthesis, has not been well characterized in S. baicalensis to date. Here, a S20 R2R3-MYB TF (SbMYB12), which encodes 263 amino acids with a length of 792 bp, was expressed in all tested tissues (mainly in leaves) and responded to exogenous hormone methyl jasmonate (MeJA) treatment. The overexpression of SbMYB12 significantly promoted the accumulation of flavonoids such as baicalin and wogonoside in S. baicalensis hairy roots. Furthermore, biochemical experiments revealed that SbMYB12 is a nuclear-localized transcription activator that binds to the SbCCL7-4, SbCHI-2, and SbF6H-1 promoters to activate their expression. These results illustrate that SbMYB12 positively regulates the generation of baicalin and wogonoside. In summary, this work revealed a novel S20 R2R3-MYB regulator and enhances our understanding of the transcriptional and regulatory mechanisms of baicalin biosynthesis, as well as sheds new light on metabolic engineering in S. baicalensis.
Keywords: Scutellaria baicalensis, R2R3-MYB, SbMYB12, flavonoids biosynthesis, baicalin, positive regulation
1. Introduction
In eukaryotes, transcription factors (TFs) play a critical role in plant development, growth, and stress responses through the self-regulation and control of the expression of target genes [1]. A class of transcription factors referred to as MYB contains the MYB domain, which was first identified in maize and is involved in anthocyanin biosynthesis [2]. Myeloblastosis (MYB) proteins can be divided into the R1-, R2R3-, R1R2R3- (3R-), and 4R-MYB proteins [2,3], with the R2R3-MYB family of higher plants being unique and the largest in size. Recently, whole-genome sequencing has enabled the identification of the R2R3-MYB gene family in a variety of plants (e.g., Arabidopsis thaliana [4], Populus trichocarpa [5], Brassica napu [6], Zea mays [7], Ananas comosus [8], Oryza sativa [9], Hypericum perforatum [10], etc.). These genes influence a variety of processes including the regulation of biotic and abiotic stress responses, secondary metabolites, plant growth and development, as well as cell fate and identity [11,12].
Flavonoids are ubiquitous in nature and extensively distributed in various plant parts. Studies have revealed that flavonoids are highly conserved in nature, with their synthetic pathway being one of the best-known secondary metabolic pathways in plants today [13]. A particular subfamily of MYB (R2R3-MYB) plays a critical role in the positive regulation of flavonoid synthesis, which for Arabidopsis includes S6 (AtMYB11/12/111) and S7 (AtMYB75/90/114) [14,15]. Studies have shown that AtMYB112, which is a member of the stress-resistant S20 family, can positively regulate the accumulation of anthocyanin in Arabidopsis [16].
Scutellariae Radix is a traditional Chinese remedy derived from the dried roots of S. baicalensis, which is widely used in China. Modern pharmacological studies have shown that baicalin and other flavonoids are the primary pharmacodynamic constituents of S. baicalensis, which have antitumor, antiviral, analgesic, and other beneficial pharmacological effects [17]. Thus, it is referred to as the “antibiotic of traditional Chinese medicine” due to its excellent inhibitory impacts on bacteria, fungi, and viruses. The baicalin content of S. baicalensis is typically > 10%, which has garnered the attention of many researchers. Recent studies have revealed that baicalin and baicalein can effectively inhibit the replication of the new coronavirus (SARS-CoV-2) [18,19]. The baicalein content of S. baicalensis was observed to increase following water stress, and moderate drought can generally promote the accretion of flavonoids. Suspension cultures of S. baicalensis cells were shown to stimulate the accumulation of flavonoids following light and PEG treatments. The exogenous administration of hormones was observed to stimulate the accumulation of baicalin, which is an active constituent of Baicalaria [20]. With the publication of the complete S. baicalensis genome, the synthetic pathways of baicalin and other flavonoids were also elucidated. In contrast, very few investigations into the molecular kinetics responsible for the synthesis of flavonoids in S. baicalensis have been undertaken.
R2R3-MYB transcription factors such as AtMYB75, AtMYB90, AtMYB113, and AtMYB114 [14,15]; MdMYBA, MdMYB1, MdMYB10, and MdMYB110a [21]; and SlMYB12 [22], StAN1 [23,24], and SmMYB98 [25] etc., have been shown to play roles in flavonoid biosynthesis. Qi et al. [26] identified 19 MYB genes at the transcriptome level and found that SbMYB2 and SbMYB7 negatively affected the phenylpropane content in tobacco through the activation of genes involved in flavonoid biosynthesis. However, to date, no studies have investigated the members of R2R3-MYB in S. baicalensis that participate in the regulation of specific flavonoids such as baicalin and wogonoside.
In one of our earlier studies based on the whole-genome data of S. baicalensis, we identified 95 members of the R2R3-MYB family, of which the gene expression levels of SbMYB12 and SbMYB74 were significantly increased following ABA and MeJA treatments [27]. AtMYB112, which belongs to the S20 family in A. thaliana, positively regulated the accumulation of anthocyanins and other flavonoids [16]. Furthermore, another S20 R2R3-MYB member (SmMYB98) was reported to positively regulate the synthesis and accumulation of salvianolic acid in the transgenic roots of S. miltiorrhiza. The sequence structure of SbMYB12 is akin to that of AtMYB112 and SmMYB98, which suggests that these proteins may perform similar functions in regulating secondary metabolites.
To address this knowledge gap, in this study, a novel member of S20 R2R3-MYB (SbMYB12) was isolated and characterized from S. baicalensis, which strongly responded to the exogenous MeJA treatment. The regulatory properties of SbMYB12 in overexpressed S. baicalensis hairy root cultures revealed that it enhanced the accumulation of baicalin and wogonoside via the upregulation of a series of key structural genes involved in flavonol biosynthesis. To the best of our knowledge, this is the first report to demonstrate that R2R3-MYB from S. baicalensis has the capability to enhance the accumulation of baicalin and wogonoside.
2. Results
2.1. Expression Patterns of SbMYB12 in S. baicalensis
Based on our previous research [27], SbMYB12 was classified in subgroup S20 with AtMYB112, which is responsible for the accumulation of flavonoids and anthocyanins in Arabidopsis [16]. Combined with the RNA-seq results of different tissues and qRT-PCR data under different hormone and abiotic stresses, SbMYB12 attracted our attention, as it appeared to play an important role in the regulation of flavonoid biosynthesis in S. baicalensis [27]. In particular, our earlier study revealed that SbMYB12 was significantly upregulated in response to MeJA stress, with its expression reaching a peak after 3 h and being upregulated ~10-fold [27]. The induction expression profile suggested that SbMYB12 was responsive to MeJA.
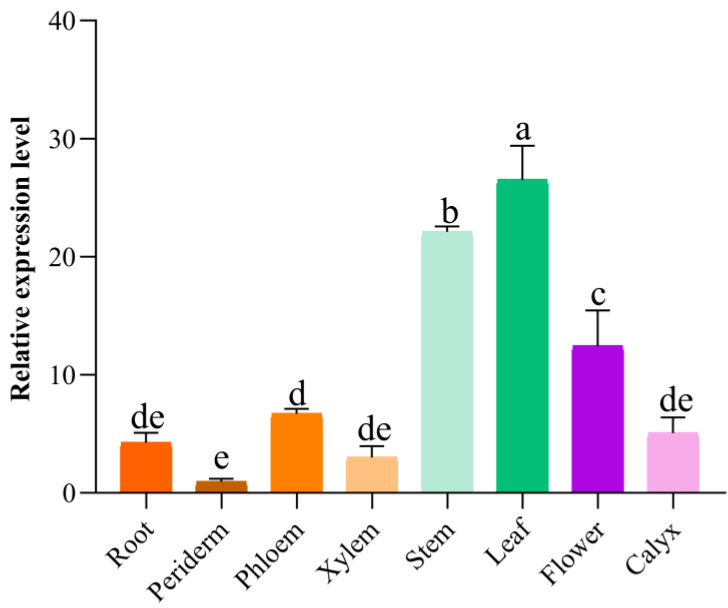
To evaluate the tissue-specific expression patterns of SbMYB12, the roots, root pericytes, root phloem, root xylem, stems, leaves, flowers, and calyx of two-year-old S. baicalensis plants were assessed by qRT-PCR. Although the expression of SbMYB12 was verified for all tested tissues, the highest expression occurred in the leaves (Figure 1).
Figure 1.
Expression profiles of SbMYB12 in different tissues of S. baicalensis. The data represent the means of three biological replicates, and the error bars indicate the standard deviation (SD). Significant differences between means were identified (depicted by different letters; p < 0.05) using one-way ANOVA (followed by Tukey’s comparisons).
2.2. Extraction and Sequence Analysis of SbMYB12
As shown in Figure S1, the full-length SbMYB12 gene derived from S. baicalensis encodes 263 amino acids with a length of 792 bp. Based on the results of the ExPASy ProtParam tool, the encoded protein has a theoretical molecular weight of 30.249 kDa and a theoretical isoelectric point of 6.19. In the amino acid composition of the SbMYB12 protein, Ser (11.8%), Leu (10.3%), Asn (7.6%), Glu (7.2%), Arg (6.5%), and Asp (6.1%) were prominent. There are 35 negatively charged residues (Asp + Glu) and 32 positively charged residues (Arg + Lys). SbMYB12 exhibited an overall average hydropathicity of −0.786, which implies that the protein is hydrophilic. The protein’s instability index (II) was 62.72, meaning that it was unstable. According to the TMHMM and SignalP web software, SbMYB12 lacked signal peptides and transmembrane sites, which suggests that the protein is a non-secretory and/or non-transmembrane protein (Figure S2).
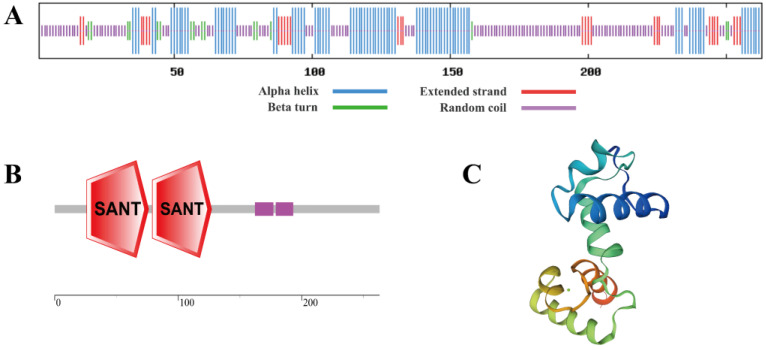
SbMYB12 had a secondary structure that contained 33.08% α helix, 11.03% extended strand, 6.08% turn, and 49.81% random coils, as shown in Figure 2A. Two conserved SANT domains were found in SbMYB12’s amino acid sequence (Figure 2B), which suggests that it is an R2R3-MYB member. Based on the SWISS-MODEL online analysis tool, the tertiary structure of SbMYB12 was predicted as having an HTH structure, which aligned with its secondary structure prediction (Figure 2C).
Figure 2.
Prediction of SbMYB12 protein structure and domains. (A) Predicted protein secondary structure. (B) Predicted protein domains. (C) Predicted tertiary structure.
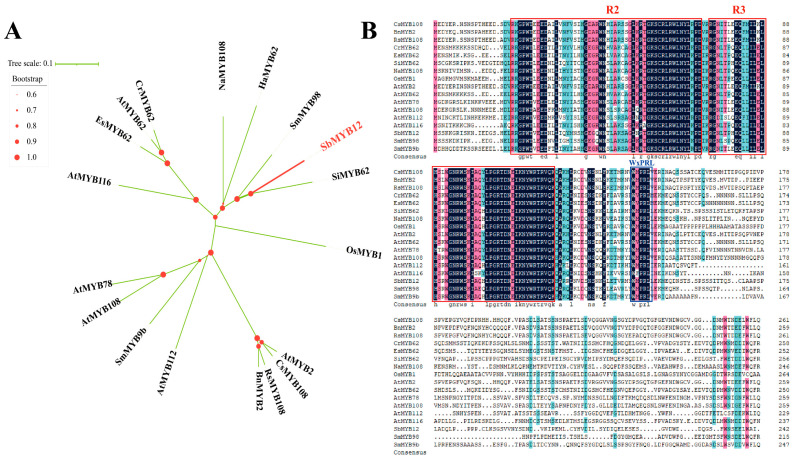
The determination of the MYB protein family’s evolution in plants (based on phylogenetic analysis of 18 SbMYB12-related genes from different species) revealed that SbMYB12 has higher homology with SiMYB62, SmMYB98, HaMYB62, and NaMYB62 (Figure 3A). Furthermore, the alignment of SbMYB12 with other S20 R2R3-MYB subgroup proteins from other species revealed that SbMYB12 shares the conserved R2 domain, R3 domain, and WxPRL motif, which characterizes the S20 R2R3-MYB subgroup protein domain (Figure 3B). According to these results, SbMYB12 possesses a highly conserved domain [25,28,29].
Figure 3.
Analysis of SbMYB12 compared with related sequences. (A) SbMYB12 gene phylogenetic analysis along with 17 representatives of the R2R3-MYB S20 subgroup. (B) Analysis of sequence alignments between SbMYB12 and other R2R3-MYB S20 subgroup proteins from different plants. Red frames indicate conserved R2 and R3 domains, and blue frames indicate the WxPRL core sequence.
2.3. Subcellular Localization and Transactivating Assays of SbMYB12
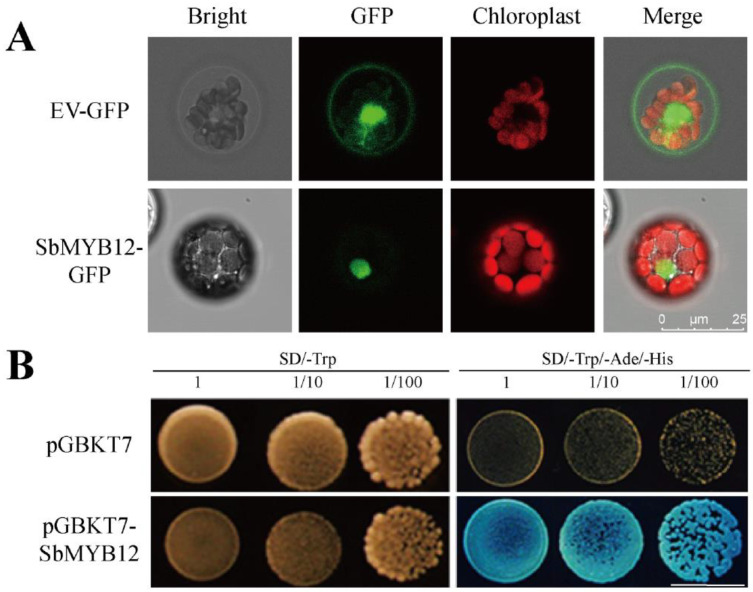
To further elucidate the classification of SbMYB12, we amplified the coding region of SbMYB12 and fused it to the HBT-GFP-NOS and pGBKT7 vectors, respectively. Subcellular localization analysis indicated that SbMYB12 was specifically localized within the nucleus (Figure 4A), which was consistent with our prediction [27]. Transactivating assays indicated that SbMYB12 had strong transcriptional activities in a yeast system (Figure 4B), likely functioning as a TF. These results indicate that SbMYB12 is a functional nuclear-localized transcription activator.
Figure 4.
Subcellular localization and transactivation activities of the SbMYB12 protein. (A) Subcellular location of SbMYB12 in Arabidopsis mesophyll protoplasts. (B) Transcriptional activation activity analysis of BD-SbMYB12 in yeast (Scale bar: 8mm).
2.4. Transgenic Hairy Roots of S. baicalensis Overexpressing SbMYB12
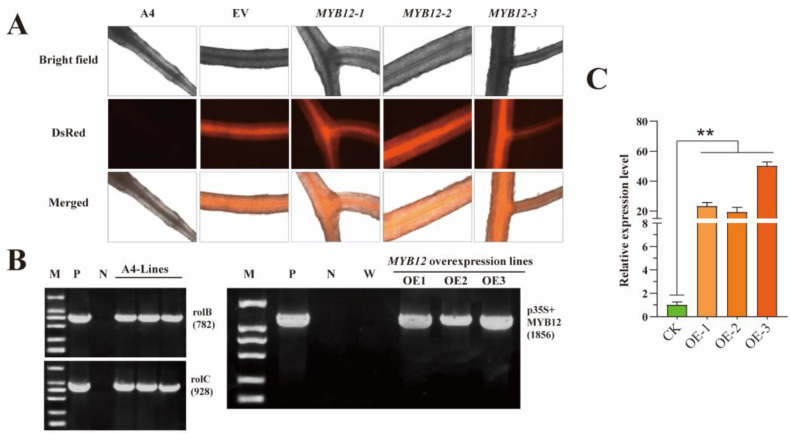
To validate the functions of SbMYB12 in flavonoid biosynthesis, we obtained three independent SbMYB12-overexpressing (OE) transgenic hairy roots via the A. rhizogenes A4-strain-mediated transformation method (Figure S3), which were verified at the genomic DNA level via the fluorescence of the DsRed protein and PCR using the rolB, rolC, and SbMYB12 specific primers (Figure 5A,B). The results of qRT-PCR revealed that the transcript level of SbMYB12 in the three OE lines were significantly higher than that of the control (CK) (Figure 5C). OE-1, OE-2, and OE-3 increased by 23.2, 19.2, and 50.1-fold, respectively, compared with the CK; thus, they were selected for further investigation.
Figure 5.
Identification of transgenic hairy roots of S. baicalensis. (A) Transgenic hairy root lines and observations with red fluorescent protein. A4: ArA4 lines (negative control); EV: ArA4 strain that harbors the pK7WG2R plasmids (positive control); MYB12-1~3: SbMYB12 overexpression hairy root lines. (B) PCR identification of transgenic hairy roots of S. baicalensis. PCR screening of SbMYB12 overexpressing lines. M: DNA marker (DL2000); P: ArA4 strain containing recombinant plasmid (positive control); N: S. baicalensis sterile plantlet (negative control); W: wild seedling of S. baicalensis. (C) Real-time quantitative PCR analysis of SbMYB12 in transgenic lines. CK1-3: ArA4 lines; OE1–3: SbMYB12 overexpression transgenic line; ** represents significant difference (p < 0.01) via Student’s t-test.
2.5. SbMYB12 Positively Regulates the Biosynthesis of Flavonoids in S. baicalensis
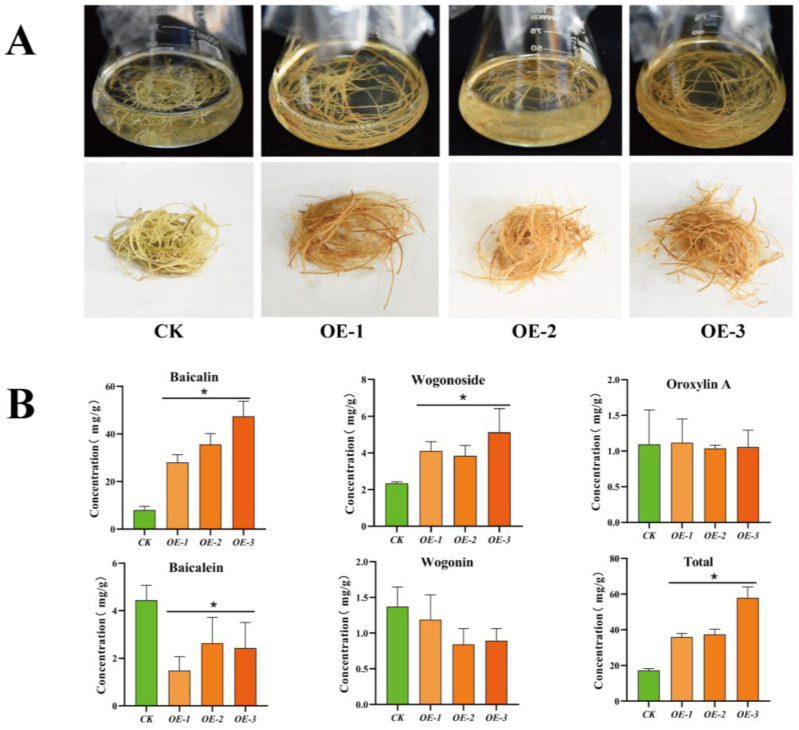
Subsequently, we investigated the phenotype of SbMYB12-OE hairy roots and the CK. As shown in Figure 6A, the color of the SbMYB12-OE lines was quite different from that of the CK, which may have been caused by different concentrations of secondary metabolites. We quantified the concentrations of five flavonoids (baicalin, wogonoside, baicalein, wogonin, and oroxylin A) in the SbMYB12-OE lines and the CK (Figure 6). The contents of baicalin, wogonoside, and total flavonoids (the sum of five flavonoids) in the SbMYB12-OE transgenic hairy roots were significantly increased. Compared with the CK, the baicalin content in OE-1, OE-2, and OE-3 increased by 3.48-, 4.44-, and 6.03-times, respectively; the content of wogonoside increased by 1.76-, 1.65-, and 2.20-times, respectively; the content of the total flavonoids increased by 2.08-, 2.55-, and 3.36-times, respectively. Conversely, the content of baicalein in OE-1, OE-2, and OE-3 decreased by 2.99-, 1.69-, and 1.83-times, respectively, and wogonin and oroxylin A did not exhibit significant changes. Overall, these results demonstrate that SbMYB12 is a positive regulator for flavonoid biosynthesis, especially for baicalin and wogonoside.
Figure 6.
SbMYB12 promoted the accumulation of flavonoids (baicalin and wogonoside) in S. baicalensis transgenic hairy roots. (A) Phenotypes of the two-month-old SbMYB12-overexpression (OE) lines and the control (CK). (B) Concentrations of flavonoids in the transgenic hairy roots and the control (CK). Significant differences between the OE lines and the CK were identified (depicted by * p < 0.05) via Student’s t-test.
2.6. SbMYB12 Directly Activated Enzyme Genes of Flavonoid Biosynthesis Pathways
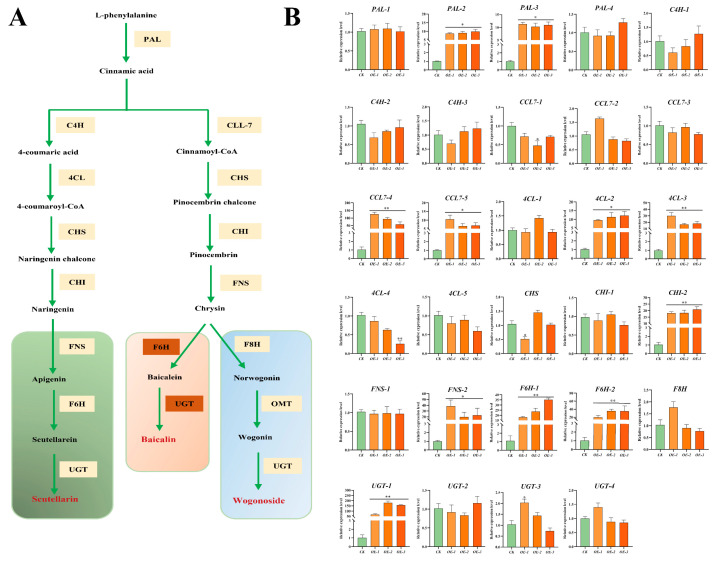
To reveal the molecular mechanisms behind the SbMYB12 regulation of flavonoid biosynthesis, we analyzed the transcriptional levels of 24 enzyme genes involved in the flavonoid synthesis pathway via qRT-PCR (Figure 7). Most of these genes, including SbPAL2, SbPAL3, SbCCL7-4, SbCCL7-5, Sb4CL-2, Sb4CL-3, SbCHI-2, SbFNS-2, SbF6H-1, SbF6H-2, and SbUGT, were significantly upregulated in the SbMYB12-OE lines at the p < 0.05 level. Of these genes, the expression levels of SbCCL7-4, Sb4CL-3, SbCHI-2, SbF6H-1, SbF6H-2, and SbUGT-1 were significantly upregulated at the p < 0.01 level. The other enzyme genes had no significant changes in the transgenic lines. These results confirm that SbMYB12 positively regulates the accumulation of flavonoids such as baicalin and wogonoside by activating its biosynthetic pathway.
Figure 7.
Expression analyses of enzyme genes for the flavonoid biosynthesis pathway in SbMYB12 overexpressed transgenic hairy roots. (A) Proposed biosynthetic pathway for flavonoids in S. baicalensis. (B) Flavonoid biosynthesis pathway gene expression analysis in SbMYB12 overexpressed strain. Significant differences between the OE lines and the CK were identified (depicted by * p < 0.05, ** p < 0.01) via Student’s t-test.
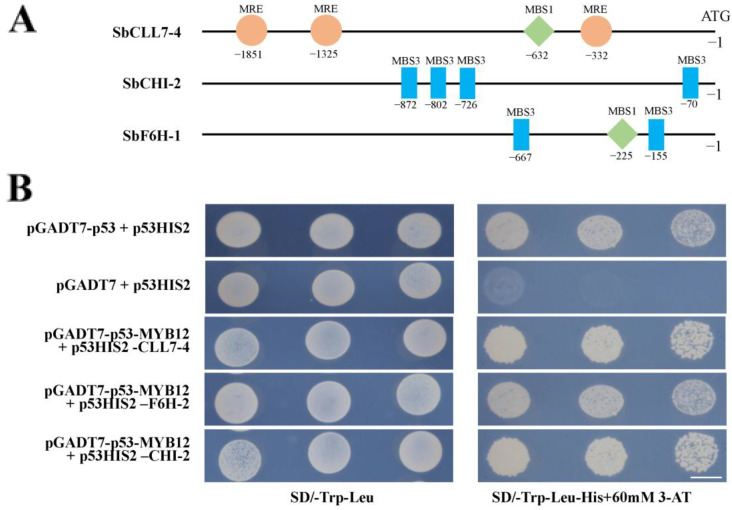
MYB proteins typically bind to the cis-elements of enzyme gene promoters to activate or repress their expression to regulate the biosynthesis of secondary metabolites. To further investigate the potential mechanism of how SbMYB12 regulates flavonoid biosynthesis, we analyzed cis-elements in the promoter regions of enzyme genes in the flavonoid biosynthesis pathway. We found MYB-binding elements (e.g., MRE, MBS1, and MBS3) in the promoter regions of SbCLL7-4, SbCHI-2, and SbF6H-1. Y1H assays were performed, which revealed that SbMYB12 interacts with the promoter regions of SbCLL7-4, SbCHI-2, and SbF6H-1 (Figure 8), indicating that SbMYB12 binds directly to the promoters of these genes and activates their transcription.
Figure 8.
SbMYB12 binds to the SbCCL7-4, SbCHI-2, and SbF6H-1 promoters. (A) Distribution of the MYB-binding sites in the SbCCL7-4, SbCHI-2, and SbF6H-1 promoters. (B) Y1H assays indicated interactions between SbMYB12 and the promoter regions of SbCCL7-4, SbCHI-2, and SbF6H-1. MRE: AACCTAA; MBS1: CAACTG; MBS3: TAACTG. (Scale bar: 6 mm).
3. Discussion
As one of the largest TF families in plants, the MYB family was divided into four subfamilies, namely 1R, R2R3, 3R, and 4R-MYBs [30]. Among them, the R2R3- MYB subfamily consisting of the conserved R2 and R3 MYB domains was the largest MYB subfamily. According to current research, the R2R3-MYBs have been well characterized for their functions in plant developmental processes, stress responses, as well as primary and secondary metabolism [2]. For S. baicalensis, 95 R2R3-MYB proteins have been identified and categorized into 34 subgroups [27]. Among them, SbMYB12 was classified into the S20 subgroups. In Arabidopsis, six S20 members (e.g., AtMYB2/62/78/108/112/116) were reported to be primarily involved in stress responses and the regulation of secondary metabolism [2]. For example, AtMYB2 was responsible for ABA-induced salt and drought response genes [31]; AtMYB62 regulated phosphate starvation and gibberellic acid biosynthesis [29]; AtMYB108 was involved in both biotic and abiotic stress responses [32]. Furthermore, AtMYB112 (S20) was engaged with regulating the accumulation of anthocyanins and flavonoids in the phenylpropanoid biosynthetic pathway [16]. In S. miltiorrhiza, SmMYB9b-OE (a S20 R2R3-MYB) efficiently increased the tanshinone concentration in S. miltiorrhiza transgenic roots [28]. Subsequently, Hao et al. [25] reported that another S20 R2R3-MYB member (SmMYB98) positively regulated the synthesis and accumulation of salvianolic acid and tanshinone by activating the transcription of PAL1, GGPPS1, and RAS1 in the transgenic roots of S. miltiorrhiza [25]. As a member of the S20 family, it was speculated that SbMYB12 may have functions akin to the homologous genes SmMYB98 and AtMYB112, which are involved in the regulation of secondary metabolites [16,25]. Moreover, our earlier studies revealed that SbMYB12 responded to different exogenous hormone and abiotic stress treatments, particularly exogenous MeJA, and may play important roles in the regulation of flavonoid biosynthesis in S. baicalensis
Since it was speculated that SbMYB12 may be a key regulator of flavonoid biosynthesis, we conducted further investigations. As described earlier, SbMYB12 transcripts were expressed in all tested tissues and preferentially expressed in S. baicalensis leaves (Figure 1). The length of the SbMYB12 nucleotide sequence was found to be 792 bp, encoding 263 amino acids that were predicted to produce a relatively hydrophilic protein (Figure S1). According to an analysis of the conserved domains, the SbMYB12 protein contained two SANT-MYB DNA-binding domains (Figure 2), which implies that the protein is a typical R2R3-MYB member [3]. Multiple sequence alignments between the SbMYB12 protein sequence and other reported S20 R2R3-MYB subgroup proteins from other species revealed that they had a high similarity in the conserved sequence, including the R2 and R3 domains and the WxPRL motif, but were completely different in the non-conserved region (Figure 3). This result was consistent with the transcription factor characteristics [33]. Additionally, phylogenetic analysis revealed that SbMYB12 was phylogenetically closest to SiMYB62 and SmMYB98 (Figure 3A).
The flavonoids (e.g., baicalin, wogonoside, baicalein, wogonin, and oroxylin A) were confirmed to be the main active components of Scutellariae Radix [34], which possesses significant antibacterial, anti-inflammatory, anti-COVID-19 virus, and anti-HIV activities [18,19,35]. Baicalin and wogonoside are considered as important evaluation indices for the quality of Scutellariae Radix [20]. The functions of three R2R3-MYB genes from S. baicalensis (SbMYB2, SbMYB7, and SbMYB8) were characterized based on a non-reference transcriptome, and transgenic tobacco plants overexpressing SbMYB2, SbMYB7, or SbMYB8 exhibited a higher phenylpropane content by activating the expression of flavonoid-biosynthesis-related genes [26,36]. However, the heterologous expression verification in tobacco did not directly confirm that SbMYBs participated in the regulation of S. baicalensis-specific flavonoids such as baicalin and wogonoside. Of particular note was that the SbMYB2, SbMYB7, and SbMYB8 mentioned above were renamed as SbMYB45, SbMYB95, and SbMYB18, respectively, in the latest study based on their sequence similarity [27]. Thus, based on the above comprehensive analysis, we speculated that SbMYB12 most likely functions as an activator of some of the S. baicalensis-specific flavonoid biosynthesis in S. baicalensis. However, additional experimental evidence is required to verify this.
Subcellular location results demonstrated that SbMYB12 was localized within the nucleus (Figure 5A). Transcription factors were functionally related to their localization [12,25,28]. According to these results, SbMYB12 may act as a transcription factor during transcriptional regulation. Subsequently, transactivation activity assays demonstrated that SbMYB12 is a nuclear-localized transcriptional activator, which suggests that it has the capacity to regulate the transcription of target genes in the nucleus on its own, as reported previously [16].
To further uncover the role of SbMYB12 in S. baicalensis, we generated transgenic hairy roots that overexpressed SbMYB12 and found that the concentrations of baicalin, wogonoside, and total flavonoids were significantly increased in the SbMYB12-OE hairy roots (Figure 6). Furthermore, the expression levels of these key flavonoid biosynthesis enzyme genes were significantly upregulated in the SbMYB12-OE lines, particularly SbCCL7-4, SbCHI-2, SbF6H-1, SbUGT-1, etc. (Figure 7). As reported, these genes are responsible for the biosynthesis of S. baicalensis-specific flavonoids [34]. In addition, the Y1H assay indicated that SbMYB12 could bind to the promoters of the key enzyme genes (SbCCL7-4, SbCHI-2, and SbF6H-1) and activate their expression (Figure 8). Therefore, we concluded that SbMYB12 is responsible for the biosynthesis of flavonoids through the direct activation of SbCCL7-4, SbCHI-2, and SbF6H-1 transcription in S. baicalensis. SbMYB12 is a potential manipulation target for improving the flavonoid content via genetic engineering.
4. Materials and Methods
4.1. Plant Materials
Sterile S. baicalensis seedlings were obtained by using stem segments as explants. We selected vigorously growing S. baicalensis seedlings, removed the leaves, sterilized them, followed by inoculation in an MS solid medium with 0.5mg/L NAA and 1.0mg/L 6-BA, and cultivation in an incubator at 22 ± 2 °C (14 h light/10 h dark cycle, 60 ± 5% humidity). When the lateral buds of S. baicalensis germinated and grew to 2 cm, they were removed and transferred to an MS solid medium (Solarbio, Beijing, China) without hormones and cultivated in an incubator to obtain sterile seedlings of S. baicalensis for the transformation experiments.
Various tissues including roots, pericytes, phloem, xylem, stems, leaves, flowers, and calyx were harvested from two-year-old S. baicalensis plants at the germplasm resource garden of Shaanxi Normal University, respectively, for RNA extraction.
4.2. Isolation and Cloning of SbMYB12
The total RNA was extracted from three-month-old S. baicalensis plantlets using the TIANGEN RNA Prep Pure Plant kit (Tiangen, Beijing, China). TransScript® First-Strand cDNA Synthesis SuperMix (TransGen Biotech, Beijing, China) was used for cDNA synthesis. SbMYB12 (evm.model.contig76.33) full-length cDNA was employed as the reference sequence, and the primers (Table S1) were designed with Primer Premier 5. As the resulting PCR products were linked to a pMD19-T vector (TaKaRa, Dalian, China) and confirmed by sequencing, the results were positively determined.
4.3. Bioinformatics Analysis of SbMYB12
The ExPASy-ProtParam tool (https://web.expasy.org/protparam/ (accessed on 20 September 2022)) was employed to predict the primary structure of the SbMYB12 protein, as well as its amino acid composition, molecular weight, theoretical isoelectric point (pI), and stability [37]. ProtScale (https://web.expasy.org/protscale/ (accessed on 20 September 2022)) was used to analyze its hydrophobic properties and charge distributions [38]. For analyzing transmembrane domains, the TMHMM server software (https://services.healthtech.dtu.dk/service.php?TMHMM-2.0 (accessed on 20 September 2022)) was used [39], while the SignalP 5.0 Server (https://services.healthtech.dtu.dk/service.php?SignalP-5.0 (accessed on 20 September 2022)) was employed to estimate signaling peptides [40]. Using the SMART website (http://smart.embl-heidelberg.de (accessed on 20 September 2022)) [41], the protein domains of SbMYB12 were predicted, while its tertiary structure was predicted using SWISS-MODEL (https://swissmodel.expasy.org/ (accessed on 20 September 2022)) [42].
The SbMYB12 and 17 members from the S20 subgroup R2R3-MYB were used to construct a phylogenetic tree with MEGA11 (https://www.megasoftware.net/dload_mac_beta (accessed on 20 September 2022)) using the neighbor-joining method (NJ) [43]. DNAMAN v. 9.0 (https://www.lynnon.com/dnaman.html (accessed on 20 September 2022)) was used to perform multiple sequence alignments between SbMYB12 and other MYB TF species. Table S2 lists the amino acid sequences of SbMYB12 and other MYB proteins.
4.4. Expression Analysis Using qRT-PCR
Based on previously described procedures [44], the total RNA was isolated and qRT-PCR analysis was performed, using the SbACT7 gene as a reference [44]. SYBR green qPCR Mix (Vazyme, Nanjing, China) was employed for qRT-PCR with a real-time fluorescence quantitative PCR detection system (Roche LightCycler® 96 Instrument, Basel, Switzerland). Based on the qRT-PCR data, SbMYB12 and other corresponding genes were calculated using the 2−ΔΔCt method [27]. All primers used for qRT-PCR and plasmid construction can be found in Table S1.
4.5. Subcellular Location Analyses of SbMYB12
To determine the locations of SbMYB12 proteins in cells, SbMYB12-GFP fusion protein expression vectors were developed by amplifying the SbMYB12 coding sequence and fusing it with HBT-GFP-NOS vectors. Arabidopsis leaves were cultured under 12 h light/12 h darkness for four weeks, and mesophyll protoplasts were prepared as previously described [45]. The transformed protoplasts were grown at 21 °C for 12 h and observed using a confocal laser microscope (Leica TCS SP5, Wetzlar, Germany).
4.6. Transcriptional Activation Assays of SbMYB12
Using a Gateway recombinatorial cloning system [46], the ORF of SbMYB12 was cloned and integrated into the pGBKT7 (BD) vector. Saccharomyces cerevisiae strain AH109 (Weidi Biotechnology, Shanghai, China) was transformed with recombinant vector BD-SbMYB12 and cultured for 2–3 days at 28 °C on SD/-Trp (Coolaber, Beijing, China), then screened on SD/-Trp/-Ade/-His/X-α-gal (Coolaber, Beijing, China) media to assay the transactivation activity.
4.7. Overexpression of SbMYB12 in Hairy Roots of S. baicalensis
The full-length CDS of SbMYB12 was cloned into the pK7WG2R (with a dsRed marker gene) to generate the overexpression vector pK7WG2R-SbMYB12 using the Gateway recombinatorial cloning system (Invitrogen, Carlsbad, CA, USA) [46]. Transgenic hairy roots were obtained from the stem segment explants of S. baicalensis infected by the Agrobacterium rhizogenes A4 strain (Weidi Biotechnology, Shanghai, China) containing the pK7WG2R-SbMYB12 as described previously [47]. In parallel, hairy roots infested with the A4 strain were used as a control. The SbMYB12-overexpressed hairy roots were confirmed by DsRed protein fluorescence and the presence of rolB, rolC, and SbMYB12 at the genomic DNA level, as described earlier [48]. For liquid culturing, 3–5 cm-long hairy roots were inoculated in a 250 mL conical flask that was filled with 50 mL of a B5 liquid medium (Coolaber, Beijing, China) and propagated in a steady temperature shaking incubator (22 °C at 100 rpm). The hairy roots were gathered after two months and used for qRT-PCR and HPLC analysis.
4.8. Measurement of Flavonoid Content in Hairy Roots by HPLC
The flavonoid content was measured using previously described techniques [49]. Briefly, the transgenic hairy roots and control, which were cultured in liquid medium for three months, were harvested and naturally dried at room temperature. The dried hairy root samples were powdered and extracted with 70% ethanol for 1 h under ultrasonication (200 W, 40 kHz). Subsequently, the extracts were filtered through a 0.22 µm microporous membrane for HPLC (Thermo Fisher Scientific UltiMate 3000 HPLC, Waltham, MA, USA).
4.9. Yeast One-Hybrid Assay
The complete SbMYB12 CDS was cloned into the BamH I and Xho I (TaKaRa, Beijing, China) sites of the pGADT7(AD) vector to generate AD-SbMYB12. The promoter fragments of SbCLL7-4, SbF6H-1, and SbCHI-2 were inserted into the EcoR I and Sac II (TaKaRa, Beijing, China) sites of the pHIS2 vector to generate pHIS2-pSbCLL7-4, pHIS2-pSbF6H-1, and pHIS2-pSbCHI-2, respectively. The Yeast One-Hybrid (Y1H) assay was performed as described in our previous protocols [50].
4.10. Statistical Analysis
The SPSS software (version 26.0) was utilized to analyze the differences via one-way ANOVA (followed by Tukey’s comparisons) and Student’s t-tests. Each bar represents the mean and standard deviation (SD) of three replicates. The significance of a statistical difference was defined as * p ≤ 0.05 and ** p ≤ 0.01, respectively.
5. Conclusions
For this study, a novel R2R3-MYB subgroup 20 TF from S. baicalensis (SbMYB12) was isolated and functionally characterized. Further, SbMYB12 was found to be a nuclear-localized transcription activator that positively regulates the accumulation of flavonoids, such as baicalin and wogonoside, by directly binding to the promoter regions of the key enzyme genes SbCCL7-4, SbCHI-2, and SbF6H-1. Together, these results enrich the understanding of the function of the R2R3-MYB gene in medicinal plants and illustrate the exploitation of R2R3-MYB in flavonoid biosynthesis, which provides a feasible strategy for enhancing the baicalin and wogonoside concentrations via MYB proteins in S. baicalensis. In addition, SbMYB12 was also the first transcription factor cloned and verified gene function in the hairy root transgenic system of S. baicalensis, which lays the foundation for further research on regulating the functional genes of S. baicalensis.
Acknowledgments
We thank all those who participated in this research.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415452/s1.
Author Contributions
Conceptualization, W.W., S.H., X.C. and Z.W.; Methodology, W.W. and S.H.; Validation, S.H., J.Y. and D.W.; Formal analysis, W.W., T.Z. and X.C.; Investigation, J.Y., C.Z. and T.Z.; Resources, T.Z. and D.W.; Data curation, C.Z.; Writing—original draft, W.W. and J.Y.; Writing—review and editing, X.C. and Z.W.; Visualization, W.W. and C.Z.; Project administration, Z.W.; Funding acquisition, X.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that there are no conflicts of interest in this paper.
Funding Statement
This research was funded by the National Key Technologies R&D Program for Modernization of Traditional Chinese Medicine (2017YFC1701300); the Special Project of the Administration of Traditional Chinese Medicine of Shaanxi (2021-QYZL-02); the Key Industry Chain Project of Shaanxi province (2022ZDLSF05-01); and the Fundamental Research Funds for the Central Universities (GK202205006).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wang Z., Tang J., Hu R., Wu P., Hou X.L., Song X.M., Xiong A.S. Genome-wide analysis of the R2R3-MYB transcription factor genes in Chinese cabbage (Brassica rapassp. pekinensis) reveals their stress and hormone responsive patterns. BMC Genom. 2015;16:17. doi: 10.1186/s12864-015-1216-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dubos C., Stracke R., Grotewold E., Weisshaar B., Martin C., Lepiniec L. MYB transcription factors in Arabidopsis. Trends Plant Sci. 2010;15:573–581. doi: 10.1016/j.tplants.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Millard P.S., Kragelund B.B., Burow M. R2R3 MYB Transcription Factors—Functions outside the DNA-Binding Domain. Trends Plant Sci. 2019;24:934–946. doi: 10.1016/j.tplants.2019.07.003. [DOI] [PubMed] [Google Scholar]
- 4.Stracke R., Werber M., Weisshaar B. The R2R3-MYB gene family in Arabidopsis thaliana. Curr. Opin. Plant Biol. 2001;4:447–456. doi: 10.1016/S1369-5266(00)00199-0. [DOI] [PubMed] [Google Scholar]
- 5.Wilkins O., Nahal H., Foong J., Provart N.J., Campbell M.M. Expansion and diversification of the Populus R2R3-MYB family of transcription factors. Plant Physiol. 2009;149:981–993. doi: 10.1104/pp.108.132795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajiebrahimi A., Owji H., Hemmati S. Genome-wide identification, functional prediction, and evolutionary analysis of the R2R3-MYB superfamily in Brassica napus. Genome. 2017;60:797–814. doi: 10.1139/gen-2017-0059. [DOI] [PubMed] [Google Scholar]
- 7.Du H., Feng B.R., Yang S.S., Huang Y.B., Tang Y.X. The R2R3-MYB transcription factor gene family in maize. PLoS ONE. 2012;7:e37463. doi: 10.1371/journal.pone.0037463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu C., Xie T., Chen C., Luan A., Long J., Li C., Ding Y., He Y. Genome-wide organization and expression profiling of the R2R3-MYB transcription factor family in pineapple (Ananas comosus) BMC Genom. 2017;18:503. doi: 10.1186/s12864-017-3896-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katiyar A., Smita S., Lenka S.K., Rajwanshi R., Chinnusamy V., Bansal K.C. Genome-wide classification and expression analysis of MYB transcription factor families in rice and Arabidopsis. BMC Genom. 2012;13:544. doi: 10.1186/1471-2164-13-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou W., Zhang Q., Sun Y., Yang L., Wang Z. Genome-wide identification and characterization of R2R3-MYB family in Hypericum perforatum under diverse abiotic stresses. Int. J. Biol. Macromol. 2020;145:341–354. doi: 10.1016/j.ijbiomac.2019.12.100. [DOI] [PubMed] [Google Scholar]
- 11.Chezem W.R., Memon A., Li F.S., Weng J.K., Clay N.K. SG2-Type R2R3-MYB Transcription Factor MYB15 Controls Defense-Induced Lignification and Basal Immunity in Arabidopsis. Plant Cell. 2017;29:1907–1926. doi: 10.1105/tpc.16.00954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czemmel S., Stracke R., Weisshaar B., Cordon N., Harris N.N., Walker A.R., Robinson S.P., Bogs J. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries. Plant Physiol. 2009;151:1513–1530. doi: 10.1104/pp.109.142059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yonekura-Sakakibara K., Higashi Y., Nakabayashi R. The Origin and Evolution of Plant Flavonoid Metabolism. Front Plant Sci. 2019;10:943. doi: 10.3389/fpls.2019.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gonzalez A., Zhao M., Leavitt J.M., Lloyd A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/Myb transcriptional complex in Arabidopsis seedlings. Plant J. 2008;53:814–827. doi: 10.1111/j.1365-313X.2007.03373.x. [DOI] [PubMed] [Google Scholar]
- 15.Stracke R., Ishihara H., Huep G., Barsch A., Mehrtens F., Niehaus K., Weisshaar B. Differential regulation of closely related R2R3-MYB transcription factors controls flavonol accumulation in different parts of the Arabidopsis thaliana seedling. Plant J. 2007;50:660–677. doi: 10.1111/j.1365-313X.2007.03078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lotkowska M.E., Tohge T., Fernie A.R., Xue G.P., Balazadeh S., Mueller-Roeber B. The Arabidopsis Transcription Factor MYB112 Promotes Anthocyanin Formation during Salinity and under High Light Stress. Plant Physiol. 2015;169:1862–1880. doi: 10.1104/pp.15.00605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z.L., Wang S., Kuang Y., Hu Z.M., Qiao X., Ye M. A comprehensive review on phytochemistry, pharmacology, and flavonoid biosynthesis of Scutellaria baicalensis. Pharm Biol. 2018;56:465–484. doi: 10.1080/13880209.2018.1492620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu H., Ye F., Sun Q., Liang H., Li C., Li S., Lu R., Huang B., Tan W., Lai L. Scutellaria baicalensis extract and baicalein inhibit replication of SARS-CoV-2 and its 3C-like protease in vitro. J Enzym. Inhib. Med. Chem. 2021;36:497–503. doi: 10.1080/14756366.2021.1873977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su H., Yao S., Zhao W., Li M., Xu Y. Discovery of baicalin and baicalein as novel, natural product inhibitors of SARS-CoV-2 3CL protease in vitro. bioRxiv. 2020 doi: 10.1101/2020.04.13.038687. [DOI] [Google Scholar]
- 20.Bai C., Yang J., Cao B., Xue Y., Gao P., Liang H., Li G. Growth years and post-harvest processing methods have critical roles on the contents of medicinal active ingredients of Scutellaria baicalensis. Ind. Crop. Prod. 2020;158:112985. doi: 10.1016/j.indcrop.2020.112985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chagné D., Lin-Wang K., Espley R.V., Volz R.K., How N.M., Rouse S., Brendolise C., Carlisle C.M., Kumar S., De Silva N., et al. An ancient duplication of apple MYB transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol. 2013;161:225–239. doi: 10.1104/pp.112.206771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballester A.R., Molthoff J., de Vos R., Hekkert B., Orzaez D., Fernández-Moreno J.P., Tripodi P., Grandillo S., Martin C., Heldens J., et al. Biochemical and molecular analysis of pink tomatoes: Deregulated expression of the gene encoding transcription factor SlMYB12 leads to pink tomato fruit color. Plant Physiol. 2010;152:71–84. doi: 10.1104/pp.109.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung C.S., Griffiths H.M., De Jong D.M., Cheng S., Bodis M., Kim T.S., De Jong W.S. The potato developer (D) locus encodes an R2R3 MYB transcription factor that regulates expression of multiple anthocyanin structural genes in tuber skin. Appl. Genet. 2009;120:45–57. doi: 10.1007/s00122-009-1158-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y., Lin-Wang K., Espley R.V., Wang L., Yang H., Yu B., Dare A., Varkonyi-Gasic E., Wang J., Zhang J., et al. Functional diversification of the potato R2R3 MYB anthocyanin activators AN1, MYBA1, and MYB113 and their interaction with basic helix-loop-helix cofactors. J. Exp. Bot. 2016;67:2159–2176. doi: 10.1093/jxb/erw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao X., Pu Z., Cao G., You D., Zhou Y., Deng C., Shi M., Nile S.H., Wang Y., Zhou W., et al. Tanshinone and salvianolic acid biosynthesis are regulated by SmMYB98 in Salvia miltiorrhiza hairy roots. J. Adv. Res. 2020;23:1–12. doi: 10.1016/j.jare.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qi L., Yang J., Yuan Y., Huang L., Chen P. Overexpression of two R2R3-MYB genes from Scutellaria baicalensis induces phenylpropanoid accumulation and enhances oxidative stress resistance in transgenic tobacco. Plant Physiol. Biochem. 2015;94:235–243. doi: 10.1016/j.plaphy.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Wang W., Hu S., Zhang C., Yang J., Zhang T., Wang D., Cao X., Wang Z. Systematic Analysis and Functional Characterization of R2R3-MYB Genes in Scutellaria baicalensis Georgi. Int. J. Mol. Sci. 2022;23:9342. doi: 10.3390/ijms23169342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang J., Zhou L., Zheng X., Zhang J., Yang L., Tan R., Zhao S. Overexpression of SmMYB9b enhances tanshinone concentration in Salvia miltiorrhiza hairy roots. Plant Cell Rep. 2017;36:1297–1309. doi: 10.1007/s00299-017-2154-8. [DOI] [PubMed] [Google Scholar]
- 29.Devaiah B.N., Madhuvanthi R., Karthikeyan A.S., Raghothama K.G. Phosphate starvation responses and gibberellic acid biosynthesis are regulated by the MYB62 transcription factor in Arabidopsis. Mol. Plant. 2009;2:43–58. doi: 10.1093/mp/ssn081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan H., Pei X., Zhang H., Li X., Zhang X., Zhao M., Chiang V.L., Sederoff R.R., Zhao X. MYB-Mediated Regulation of Anthocyanin Biosynthesis. Int. J. Mol. Sci. 2021;22:3103. doi: 10.3390/ijms22063103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia T., Zhang K., Li F., Huang Y., Fan M., Huang T. The AtMYB2 inhibits the formation of axillary meristem in Arabidopsis by repressing RAX1 gene under environmental stresses. Plant Cell Rep. 2020;39:1755–1765. doi: 10.1007/s00299-020-02602-3. [DOI] [PubMed] [Google Scholar]
- 32.Mandaokar A., Browse J. MYB108 acts together with MYB24 to regulate jasmonate-mediated stamen maturation in Arabidopsis. Plant Physiol. 2009;149:851–862. doi: 10.1104/pp.108.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weidemüller P., Kholmatov M., Petsalaki E., Zaugg J.B. Transcription factors: Bridge between cell signaling and gene regulation. Proteomics. 2021;21:e2000034. doi: 10.1002/pmic.202000034. [DOI] [PubMed] [Google Scholar]
- 34.Pei T., Yan M., Huang Y., Wei Y., Martin C., Zhao Q. Specific Flavonoids and Their Biosynthetic Pathway in Scutellaria baicalensis. Front Plant Sci. 2022;13:866282. doi: 10.3389/fpls.2022.866282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao Q., Chen X.Y., Martin C. Scutellaria baicalensis, the golden herb from the garden of Chinese medicinal plants. Sci. Bull. 2016;61:1391–1398. doi: 10.1007/s11434-016-1136-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yuan Y., Qi L., Yang J., Wu C., Liu Y., Huang L. A Scutellaria baicalensis R2R3-MYB gene, SbMYB8, regulates flavonoid biosynthesis and improves drought stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. Int. J. Vitr. Cult. High. Plants. 2015;120:961–972. doi: 10.1007/s11240-014-0650-x. [DOI] [Google Scholar]
- 37.Duvaud S., Gabella C., Lisacek F., Stockinger H., Ioannidis V., Durinx C. Expasy, the Swiss Bioinformatics Resource Portal, as designed by its users. Nucleic Acids Res. 2021;49:W216–W227. doi: 10.1093/nar/gkab225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilkins M.R., Gasteiger E., Bairoch A., Sanchez J.C., Williams K.L., Appel R.D., Hochstrasser D.F. Protein identification and analysis tools in the ExPASy server. Methods Mol. Biol. 1999;112:531–552. doi: 10.1385/1-59259-584-7:531. [DOI] [PubMed] [Google Scholar]
- 39.Wu Y., Li T., Cheng Z., Zhao D., Tao J. R2R3-MYB Transcription Factor PlMYB108 Confers Drought Tolerance in Herbaceous Peony (Paeonia lactiflora Pall.) Int. J. Mol. Sci. 2021;22:11884. doi: 10.3390/ijms222111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcion C., Béven L., Foissac X. Comparison of Current Methods for Signal Peptide Prediction in Phytoplasmas. Front. Microbiol. 2021;12:661524. doi: 10.3389/fmicb.2021.661524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Letunic I., Khedkar S., Bork P. SMART: Recent updates, new developments and status in 2020. Nucleic Acids Res. 2021;49:D458–D460. doi: 10.1093/nar/gkaa937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Waterhouse A., Bertoni M., Bienert S., Studer G., Tauriello G., Gumienny R., Heer F.T., de Beer T.A.P., Rempfer C., Bordoli L., et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura K., Stecher G., Kumar S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021;38:3022–3027. doi: 10.1093/molbev/msab120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang W., Hu S., Cao Y., Chen R., Wang Z., Cao X. Selection and evaluation of reference genes for qRT-PCR of Scutellaria baicalensis Georgi under different experimental conditions. Mol. Biol. Rep. 2021;48:1115–1126. doi: 10.1007/s11033-021-06153-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoo S.D., Cho Y.H., Sheen J. Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2007;2:1565–1572. doi: 10.1038/nprot.2007.199. [DOI] [PubMed] [Google Scholar]
- 46.Reece-Hoyes J.S., Walhout A.J.M. Gateway Recombinational Cloning. Cold Spring Harb. Protoc. 2018;2018:pdb.top094912. doi: 10.1101/pdb.top094912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao Q., Zhang Y., Wang G., Hill L., Weng J.K., Chen X.Y., Xue H., Martin C. A specialized flavone biosynthetic pathway has evolved in the medicinal plant, Scutellaria baicalensis. Sci. Adv. 2016;2:e1501780. doi: 10.1126/sciadv.1501780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ding K., Pei T., Bai Z., Jia Y., Ma P., Liang Z. SmMYB36, a Novel R2R3-MYB Transcription Factor, Enhances Tanshinone Accumulation and Decreases Phenolic Acid Content in Salvia miltiorrhiza Hairy Roots. Sci. Rep. 2017;7:5104. doi: 10.1038/s41598-017-04909-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu R.X., Song G.H., Wu P.G., Zhang X.W., Hu H.J., Liu J., Miao X.S., Hou Z.Y., Wang W.Q., Wei S.L. Distribution patterns of the contents of five biologically activate ingredients in the root of Scutellaria baicalensis. Chin. J. Nat. Med. 2017;15:152–160. doi: 10.1016/S1875-5364(17)30030-4. [DOI] [PubMed] [Google Scholar]
- 50.Cao Y., Chen R., Wang W.T., Wang D.H., Cao X.Y. SmSPL6 Induces Phenolic Acid Biosynthesis and Affects Root Development in Salvia miltiorrhiza. Int. J. Mol. Sci. 2021;22:7895. doi: 10.3390/ijms22157895. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.