Abstract
Since chronic inflammation can be seen in severe, long-lasting diseases such as cancer, there is a high demand for effective methods to modulate inflammatory responses. Among many therapeutic candidates, lignans, absorbed from various plant sources, represent a type of phytoestrogen classified into secoisolariciresionol (Seco), pinoresinol (Pino), matairesinol (Mat), medioresinol (Med), sesamin (Ses), syringaresinol (Syr), and lariciresinol (Lari). Lignans consumed by humans can be further modified into END or ENL by the activities of gut microbiota. Lignans are known to exert antioxidant and anti-inflammatory activities, together with activity in estrogen receptor-dependent pathways. Lignans may have therapeutic potential for postmenopausal symptoms, including cardiovascular disease, osteoporosis, and psychological disorders. Moreover, the antitumor efficacy of lignans has been demonstrated in various cancer cell lines, including hormone-dependent breast cancer and prostate cancer, as well as colorectal cancer. Interestingly, the molecular mechanisms of lignans in these diseases involve the inhibition of inflammatory signals, including the nuclear factor (NF)-κB pathway. Therefore, we summarize the recent in vitro and in vivo studies evaluating the biological effects of various lignans, focusing on their values as effective anti-inflammatory agents.
Keywords: lignans, antioxidant, chronic inflammation, menopausal symptoms, cancers
1. Introduction
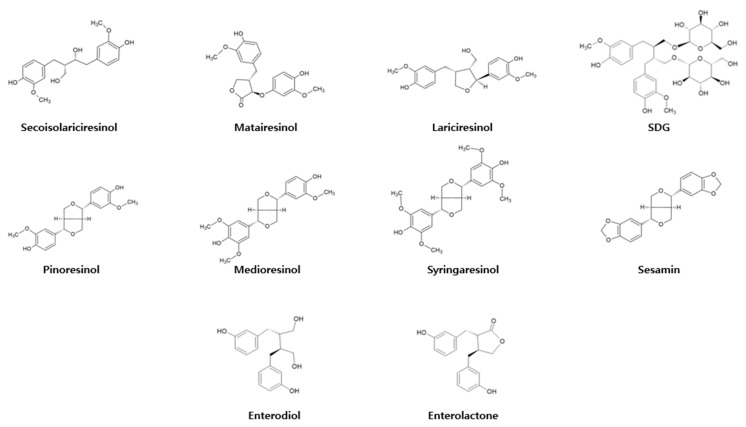
As the name suggests, phytoestrogens are estrogen analogs. These can be extracted from flax seeds, sesame seeds, fruits, and vegetables [1]. They have polyphenolic structures and can bind to estrogen receptors in the human body, mimicking the effects of estrogen. Therefore, many reports have discussed the therapeutic potential of phytoestrogens for estrogen replacement. Phytoestrogens include isoflavonoids, flavonoids, stilbenoids, and lignans [2]. Lignans are classified into seven main types: secoisolariciresionol (Seco), pinoresinol (Pino), matairesinol (Mat), medioresinol (Med), sesamin (Ses), syringaresinol (Syr), and lariciresinol (Lari) (Figure 1) [3]. Many lignans exhibit therapeutic effects, including antioxidant, anti-cancer, anti-inflammation, anti-bacterial, and anti-fungal properties [4,5,6,7]. Most bioactivities of lignans arise following their chemical transformation by gut microbiota. For instance, undigested flax or sesame seeds contain a secoisolariciresionol diglucoside (SDG) conjugate [8]. The human gut microbiota is responsible for the conversion of SDG to Seco by deglycosylation. The unconjugated Seco can be demethylated or dehydroxylated by two fecal bacterial strains, Peptostreptococcus productus and Eggerthella lenta, to produce enterodiol (END) or enterolactone (ENL) metabolites that are easily absorbed from the gastrointestinal tract [9]. Similarly, microbial digestion of other lignans within the gastrointestinal tract may also lead to production of the END or ENL metabolites in the colon.
Figure 1.
Chemical structures of lignans and their metabolites (END and ENL).
Inflammatory processes are initiated by binding of pathogen-associated molecular patterns (PAMPs) to pattern recognition receptors (PRRs) [10,11,12]. Major PRRs include toll-like receptors (TLRs), which activate TGF-β-activated kinase 1 (TAK1) to induce the phosphorylation of downstream molecules of the nuclear factor (NF)-κB and activator protein (AP)-1 pathways [13]. NF-κB subunits, including Rel A (p65) and p50, bind to IκB inhibitory protein [14,15,16]. However, TAK1-mediated phosphorylation of IκB kinase (IKK) activates the kinase activity of IKK, inducing the proteasomal degradation of IκB. This triggers the release NF-κB subunits and phosphorylation of p50 and p65 [17,18]. TAK1 also increases phosphorylation of mitogen-activated protein kinase (MAPK), including p38, extracellular signal-regulated kinase (ERK), and c-Jun N-terminal kinase (JNK), resulting in the continuous phosphorylation of AP-1 subunits, e.g., c-Jun and c-Fos [19,20,21,22,23]. Both NF-κB and AP-1 translocate into the nucleus after their phosphorylation, acting as a pivotal transcription factor for inflammatory responses [24,25]. Immune cells utilize these processes to secrete pro-inflammatory cytokines that trigger neutrophil infiltration and activation of lymphocytes for further immunological activation [26,27]. However, chronic inflammation may result in damage to normal tissues, causing necrosis of non-infected cells [28,29,30]. Excessive inflammation also recruits reactive oxygen species (ROS) that may alter tissue metabolism [31,32]. In postmenopausal women suffering from estrogen deficiency, chronic inflammation can be significant in the reproductive organs, aorta, bone, brain, and other organs. Progression of most symptoms during the menopausal period is modulated by the NF-κB pathway [33,34]. Increased cytokine production induced by NF-κB pathway activation in various organs may eventually lead to the progression of atherosclerosis, osteoporosis, and psychological disorders such as depression [35,36,37,38,39,40]. In addition, it is also reported that NF-κB activation is known to trigger cancer cell proliferation and angiogenesis by regulating the gene expression of B-cell lymphoma 2 (Bcl-2), vascular endothelial growth factor (VEGF), and colony stimulating factor 1 (CSF1) [41,42]. Long-term secretion of pro-inflammatory cytokines, which can be induced by NF-κB and AP-1, may also activate various pathways of cell migration, apoptosis, and proliferation, associated with cancer survival [43]. For instance, elevated tumor necrosis factor alpha (TNF-α) and interleukin-6 (IL-6) secretion is known to promote the metastasis and malignancy of tumor cells [18,44,45,46]. Therefore, the development of novel methods to alleviate chronic inflammation remains a therapeutic target in many diseases [12].
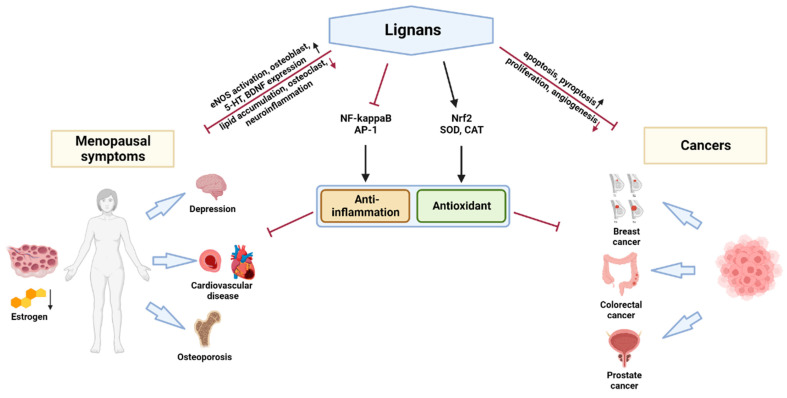
In this review, we summarize recent studies of the therapeutic properties of various lignans, especially those targeting chronic inflammatory diseases (Figure 2). For this, we referred to the experimental papers published within the last five years that show the efficacy of lignans. We set various lignan compounds, including the seven classes and their metabolites, as key words and searched studies in Pubmed and Embase. To be specific, the efficacy of lignans in both in vitro and in vivo models of menopausal disorders and cancers areis highlighted in a discussion on the outlook of lignan research.
Figure 2.
Schematic analysis of the therapeutic properties of lignans.
2. Antioxidant Properties of Lignans
ROS, including free radicals such as the superoxide (O2*) or hydroxyl (HO*) radicals and non-radical molecules such as hydrogen peroxide (H2O2), are crucial factors for pathogen resistance and tumor removal, in that elevated ROS concentrations precede programmed cell death of pathogen-infected or cancer cells [47,48]. However, excessive or unfavorable production of ROS often leads to the progression of severe inflammatory diseases [31]. For instance, inflammatory bowel disease (IBD; characterized by ulcerative colitis) is associated with the uncontrolled release of cytotoxic ROS, which contributes to the increased expression of pro-inflammatory molecules, such as leukotriene B4 [49]. Therefore, many antioxidant reagents, e.g., ascorbic acid or riboflavin, have been examined as therapeutic agents in inflammatory diseases [50,51]. Interestingly, many experiments have demonstrated the antioxidant effects of lignans (Figure 3). Below, we summarize how each type of lignan ameliorates oxidative stress in vitro and in vivo.
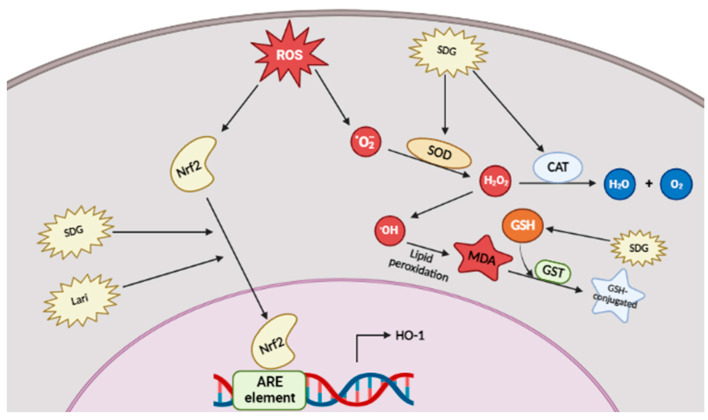
Figure 3.
Antioxidant properties of lignans. Intracellular radical scavenging process can be stimulated by SDG by enhancing the expression of SOD and CAT as well as MDA reduction. Lari and SDG target Nrf2-mediated expression of HO-1.
It has been found that SDG, the glycosylated form of Seco, was able to upregulate the expression of superoxide dismutase (SOD) and catalase (CAT) in damaged mouse liver and kidney [52,53]. Since both enzymes scavenge ROS, we can assume that SDG induces free radical scavenging [54,55]. Moreover, glutathione concentrations in both the liver and kidney increased to normal due to SDG treatment, while malondialdehyde (MDA) concentrations were reduced. Ralph A. Pietrofesa et al. confirmed that synthetic SDG ameliorates ROS production through activation of nuclear factor erythroid 2-related factor 2 (Nrf2), which binds to the AU-rich element (ARE element) to induce the expression of genes for antioxidant proteins, such as heme oxygenase (HO-1) [56,57]. The Lari lignin has two enantiomer structures, (+)-lariciresinol and (−)-lariciresinol: the antioxidant activity of the (+)-lariciresinol enantiomer extracted from Rubia philippinensis increased Nrf2–induced HO-1 expression in RAW264.7 murine macrophage cells [58].
3. Anti-Inflammatory Properties of Lignans
Inflammatory responses play an important role in eliminating invasive pathogens during the early stages of infection. However, chronic inflammation accompanies many diseases including cancer [59,60,61,62,63]. Moreover, conventional anti-inflammatory agents, e.g., the corticosteroid dexamethasone, exert severe side effects, e.g., muscle atrophy [64], while nonsteroid anti-inflammatory drugs (NSAIDs) specifically target cyclooxygenases (COXs) [65]. Therefore, many studies addressing modulation of inflammatory signaling pathways by natural compounds have been conducted. As a result, lignans derived from many plants have been found to possess anti-inflammatory activities.
Various molecular mechanisms contribute to pro-inflammatory processes. For instance, the Janus kinase (JAK)-signal transduction and activator of transcription (STAT) signaling pathway is related to T-cell differentiation and B-cell class switching by receiving signals from both type I and type II cytokines [66]. Meanwhile, the NF-κB and MAPK pathways can be activated by phosphorylation and translocation of NF-κB and AP-1 subunits, respectively [67,68]. Both transcription factors upregulate pro-inflammatory cytokines, including TNF-α, interleukin-1 beta (IL-1β), IL-6, COX-2, and inducible NO synthase (iNOS) [69,70,71]. Interestingly, it has been reported that dietary lignans and their metabolites derived from gut microbiota control the inflammatory response through suppression of these pathways [72,73].
Zhen Wang et al. explored whether SDG could attenuate intestinal inflammation in C57BL/6 mice [74]. The research group discovered that, in a dextran-sulfate-sodium-salt (DSS)-induced colitis model, mice supplemented with 200 mg/kg of SDG in their diets had reduced pathological severity. Administration of SDG blocked the NLR family pyrin domain containing 1 (NLRP1) inflammasome due to NF-κB pathway inhibition. Moreover, oral administration of SDG increased the serum enterolactone concentration and attenuated atopic dermatitis in a mouse model [75]. Increased enterolactone attenuated the Th2 cell response by targeting the JAK-STAT6 signaling pathway. The Pino-mediated anti-inflammatory response was dependent on NF-κB pathway inhibition accompanied by phosphorylation of upstream molecules like IκBα [76]. Meanwhile, Syr treatment attenuated the expression of inflammatory cytokines, such as TNF-α, IL-1β, COX-2, and iNOS in BV2 microglial cells [77]. These effects were induced by inhibition of translocation of the p65 subunit of NF-κB to the nucleus. Along with the effects of Syr, dose-dependent inhibitory effects of Ses on p65 phosphorylation accompanied the downregulation of TNF-α, IL-1β, and interleukine-8 (IL-8) secretion in an vivo carrageenan-induced model of inflammation [78]. Schisandrin B, the lignan found in Schisandra chinensis, also exerted anti-inflammatory effects by modulating both the NF-κB and MAPK pathways [79].
Taken together, the reported findings indicate lignans as potential therapeutic agents in inflammatory conditions (Figure 4).
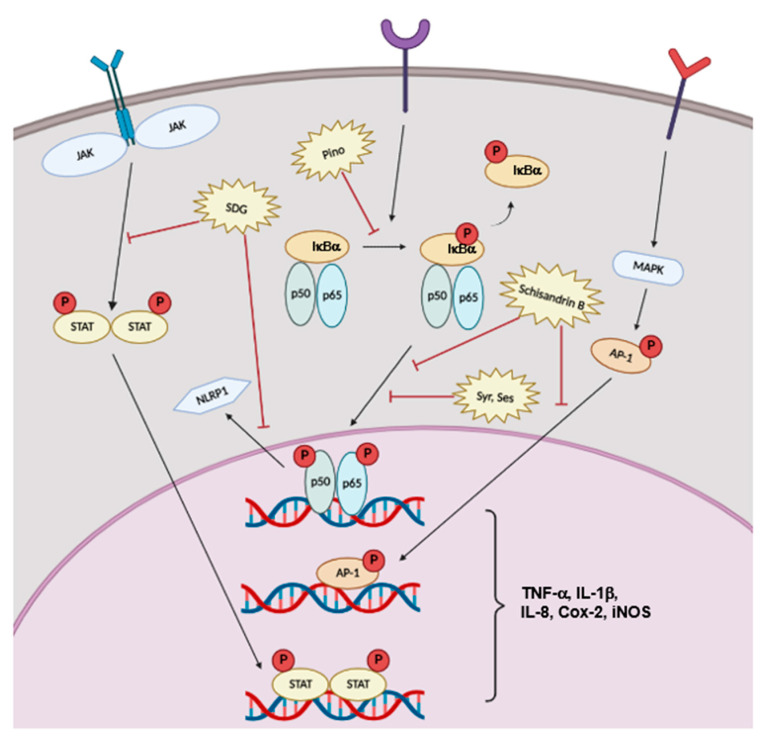
Figure 4.
Anti-inflammatory properties of lignans. Various lignans block the expression of pro-inflammatory cytokines via downregulating JAK/STAT, NF-κB, and AP-1 pathways.
4. Anti-Menopausal Effects of Lignans
The ovary plays a crucial role in the female reproductive, cardiovascular, skeletal, and central nervous systems in that it, along with the brain, is the major organ that secretes estrogen [80]. In general, estrogen secretion is mediated by luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which are produced based on a signal from gonadotropin synthesized in the hypothalamus [81]. The process is regulated by negative feedback in that secreted estrogen downregulates the secretion of LH, FSH, and gonadotropin. However, estrogen deficiency due to ovarian aging blocks the negative feedback system, leading to various menopausal symptoms. Such symptoms include face flushing, skin dryness, and anxiety and may be accompanied by more severe conditions such as cardiovascular disorders, osteoporosis, and depression. Most symptoms are correlated with chronic inflammation as estrogen deficiency often leads to a dramatic increase in pro-inflammatory cytokine concentrations in serum, liver, bone, and brain. To alleviate postmenopausal symptoms, hormone therapies with direct injection of estrogen have been developed based on certain guidelines from the US FDA. However, treatment-related breast and uterine cancer has limited the use of direct estrogen administration as a mainstream treatment [82]. Numerous studies have been conducted to evaluate the potential of lignans as therapeutic agents for menopausal symptoms.
4.1. Cardiovascular Disease
Most women in menopause suffer from a vasomotor system disorder [83]. Symptoms last several years in some cases and are accompanied by redness of the skin and night sweating. The symptoms are not life-threatening but significantly reduce the quality of life.
Cardiovascular disease is less prevalent in premenopausal women than in men, but estrogen deficiency increases the prevalence of cardiovascular disease [84]. This change is a function of estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ), which are activated by estrogen and phosphorylate endothelial nitric oxide synthase (eNOS) [85]. Activation of eNOS induces nitric oxide (NO) production, which mediates vascular relaxation. According to several studies, estrogen also increases serum high-density lipoprotein (HDL) and reduces serum low-density lipoprotein (LDL) [86]. Increased LDL level induces chronic injury to the aorta and liver by recruiting and activating macrophages to increase production of cytokines including TNF-α, IL-1β, and plasma platelet activating factor-acetyl hydrolase (PAF-AH) [87]. Since excessive LDL causes chronic inflammation in the aorta and liver, estrogen deficiency can lead to hypercholesterolemia and atherosclerosis.
Hui-Hui Xiao et al. showed that the lignan-rich fraction from Sambucus Williamsii Hance attenuated the imbalance between HDL and LDL levels in an ovariectomized (OVX) mouse model, as well as reducing total cholesterol (TC) and triglyceride (TG) concentrations. The extract significantly reduced serum and liver LDL concentrations. The extract also reduced serum total cholesterol and triglycerides in OVX mice by reducing serum concentrations of various cytokines, including TNF-α, interleukin-22 (IL-22), and monocyte chemoattractant protein-1 (MCP-1), and modulating the gut microbiota population [88]. In light of the effects of lignans on menopause-related cardiovascular diseases, a clinical investigation of 214,108 people was undertaken, and long-term intake of lignans was found to significantly downregulate the risk of total coronary heart disease in both men and women [89]. Surprisingly, steady intake of 40 μg/day of Mat reduced the hazard ratio of coronary heart disease by almost half compared to the group that did not consume the lignan [89]. In addition, oral administration of 50 mg/kg of sesame lignans attenuated oxidized LDL in rabbits fed a fat- and cholesterol-enriched diet and reduced inflammatory lesions in liver induced by a high-fat diet [90]. Among the sesame lignans, Ses also alleviated cardiovascular injury in rats by reducing secretion of lactate dehydrogenase (LDH), creatine kinase (CK), and CK-MB in the heart and serum [91]. In addition, Ses reduced iron accumulation in the heart by recovering the proteins related to ferroptosis inhibition including solute carrier family 7 member 11 (SLC7A11) and glutathione peroxidase 4 (GPX4). The Ses lignin also activated the transient receptor potential vanilloid type 1 (TRPV1) that phosphorylates downstream molecules including protein kinase A (PKA), protein kinase B (Akt), and AMP-activated protein kinase (AMPK), which stimulate eNOS for NO secretion [92]. Interestingly, Ses-mediated eNOS activation inhibited the TNF-α-induced inflammatory response by downregulating NF-κB signaling and intracellular adhesion molecule-1 (ICAM-1) expression. This finding indicated that Ses may prove useful to prevent hypertension and coronary inflammation. Summary of the result of published pharmacological experiments conducted to evaluate the effects of lignans on cardiovascular diseases is included in Table 1.
4.2. Osteoporosis
Osteoporosis results from an imbalance between the activities of osteoblasts and osteoclasts [93] and is characterized by reduced bone mineral density and degradation of trabecular tissues, resulting in fragility fractures.
Bone marrow stromal cells differentiate into osteoblasts in the presence of transcription factors, such as osterix (OSX), SRY-box9 (SOX9), and runt-related transcription factor 2 (RUNX2). Osteoblasts then secrete alkaline phosphatase (ALP), osteopontin (OPN), and osteocalcin (OCN) for bone formation. Bone marrow stem cells or bone marrow-derived macrophages differentiate into osteoclasts in the presence of macrophage-colony stimulating factor (M-CSF) and receptor activator of nuclear factor-κB ligand (RANKL) [94]. The interaction between RANK from osteoclasts and RANKL precedes a downstream signal along the MAPK or NF-κB pathway to stimulate nuclear factor of activated T cells 1 (NFATc1) [95,96]. Then, NFATc1-mediated expression of protease-like cathepsin K (CTSK) or matrix metalloproteinase-9 (MMP-9) triggers the degradation of the collagen structure in bone, which recruits C-telopeptide of collagen Type 1 (CTX-1) in blood [34,97]. The activity of osteoclasts can be blocked by the actions of estrogen, while estrogen upregulates the production of osteoprotegerin (OPG) in osteoblasts [98]. Since OPG is a decoy receptor that competitively binds to RANKL against RANK, estrogen-mediated OPG expression reduces bone resorption by inhibiting RANKL-induced differentiation of osteoclasts and further downstream signals [99]. Unfortunately, estrogen deficiency in menopausal women results in significant permanent bone loss and absence of osteoclast regulating factor.
Although other lignans such as Mat repress osteoclastogenic activity by targeting the MAPK pathway of osteoclasts, recent studies of the activity of lignans in osteoporosis have mainly focused on the effects of Ses [100]. When Ses was added to bone marrow stem cell cultures, it promoted gene expression of osteoblast differentiation markers like RUNX2 or osteocalcin. Moreover, Ses blocked protein expression of glycogen synthase kinase 3 beta (GSK3β), enhancing the wingless-related integration site (Wnt)/β-catenin signaling pathway to increase osteoblast proliferation [101]. Zhengmeng Yang et al. also discovered the anti-osteoclastogenesis effect of Ses to be mediated by NF-κB pathway inhibition, which reduces the expression of CTSK and tartaric-acid phosphatase (TRAP) in osteoclasts [102]. Moreover, healing of osteoporotic fractures in C57BL/6 mice following OVX surgery was improved by oral administration of Ses [103]. Ses upregulated the expression of VEGF, an angiogenesis marker, along with bone morphogenetic protein 2 (BMP2) and SOX9, both chondrogenesis markers. These effects promoted cartilage formation, supporting bone stability and the synthesis of trabecular bone tissues in OVX mice. After Ses, sesamolin is the second major lignan derived from sesame [104]. Intraperitoneal injection of sesamolin in OVX mice improved the volume and number of trabecular tissues in the femur [105] due to downregulation of phosphorylation of p65 and MAP kinases including ERK, JNK, and p38. These studies indicate a potentially significant role for lignans, and especially those derived from sesame seeds, as therapeutic agents for postmenopausal osteoporosis. Summary of the result of published pharmacological experiments conducted to evaluate the effects of lignans on osteoporosis is included in Table 1.
4.3. Psychological Disorders
Although the mechanism is not clearly understood, postmenopausal women often suffer from depression accompanied by irritability, fatigue, and loss of confidence. Recently, several effects of estrogen and its receptors have been identified in serotonergic and dopaminergic systems. Specifically, estrogen activates the tryptophan hydroxylase responsible for synthesis of serotonin (5-HT) [106]. Moreover, estrogen mediates the mRNA expression of monoamine oxidases (MAO), which act as serotonin degradation enzymes [107]. In the postmenopausal period, reduced 5-HT in the brain increases the feeling of anxiety, eventually leading to depression. Ovariectomy-induced estrogen deficiency increases neuroinflammation together with increased TNF-α and IL-1β levels in the neurons of the dorsal root ganglion, hippocampus, and spinal dorsal horn [108]. Neuroinflammation reduces magnesium ion concentrations in neurons, eventually leading to memory loss and chronic pain.
When designing an animal model of depression, researchers often create chronic unpredictable mild stress (CUMS) among the test animals for several weeks [109]. Yihang Zhao et al. developed a CUMS protocol consisting of food/water deprivation, overnight illumination, forced swimming, cage tilting, and tail clipping. In mice subjected to these stressors for six weeks, 50 mg/kg/day of Ses upregulated 5-HT level and decreased norepinephrine level in the striatum [110]. Moreover, CUMS-induced loss of brain-derived neurotrophic factor (BDNF) expression was strongly attenuated by Ses administration. Qianxu Wang et al. also found that Ses-mediated anti-depressant effects are strongly correlated with its inhibitory effects on neuroinflammation [111]. Ses downregulated cytokine expression in the mouse cortex and protected the brain from inflammatory injuries. Summary of the result of published pharmacological experiments conducted to evaluate the effects of lignans on psychological disorders is included in Table 1.
Table 1.
Summary of the result of published pharmacological experiments conducted to evaluate the effects of lignans on postmenopausal symptoms.
| Disease | Lignan | Source | Test Type and Dose | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Cardiovascular | Lignan-rich Fraction | S. Williamsii | In vivo (140, 280 mg/kg) |
Gut microbiota modulation TNF-α ↓ LDL, TG, TC ↓ |
[88] |
| Total Lignan, Mat, Seco, Pino, Lari | Dietary Lignans | Clinical test (Diet was repeatedly assessed using questionnaire) |
Circulating ENL↑ Total fiber intake ↑ Coronary head disease risk ↓ |
[89] | |
| Lignans (Sesamin:Episesamin = 1:1) |
Purchased (Takemoto Oil & Fat) |
In vivo (50 mg/kg) |
LDL ↓ PAF-AH ↓ IL-1β, macrophage infiltration ↓ |
[90] | |
| Ses | Purchased (Aladdin) |
In vivo (40, 80, 160 mg/kg) |
LDH, CK, CK-MB ↓ TNF-α, IL-1β ↓ SOD, GSH ↑ MDA ↓ SLC7A11, GPX4 ↑ |
[91] | |
| Ses | Purchased (Sigma-Aldrich) |
In vitro (20 μM) |
TRPV1, PKA, Akt, AMPK ↑ eNOS ↑ p65, ICAM-1 ↓ |
[92] | |
| Osteoporosis | Mat | Purchased (Sigma-Aldrich) |
In vitro (10 μM) |
TRAP ↓ NFATc1 ↓ p38, ERK ↓ |
[100] |
| Ses | Purchased (Sigma-Aldrich) |
In vitro (1, 10 μM) In vivo (80 mg/kg) |
RUNX2, OCN ↑ β-catenin, LRP5 ↑ GSK-3β ↓ ALP, OSX ↑ |
[101] | |
| Ses | Purchased (Selleck Chemicals) |
In vitro (2.5, 5, 10 μM) In vivo (80, 160 mg/kg) |
ALP, OCN, OPN, β-catenin ↑ TRAP, c-FOS, CTSK, NFATc1 ↓ p65, IκBα ↓ |
[102] | |
| Ses | Purchased (Sigma-Aldrich) |
In vitro (0.5 μM) In vivo (80 mg/kg) |
VEGF ↑ SOX9, BMP2 ↑ |
[103] | |
| Sesamolin | Purchased (Chegdu DeSiTe Biological Technology Co.) |
In vitro (5, 10 μM) In vivo (5 mg/kg) |
TRAP, CTSK, MMP-9, c-Fos, NFATc1 ↓ p65, IκBα, ERK, JNK, p38 ↓ |
[105] | |
| Psychological disorder | (+)-Ses | Purchased (Yuanye Biotechnology) |
In vivo (50 mg/kg) |
5-HT, BDNF ↑ COX-2, iNOS, TNF-α, IL-1β ↓ |
[110] |
| Ses | Purchased (Yuanye Biotechnology) |
In vivo (50 mg/kg) |
TNF-α, IL-6 ↓ | [111] |
5. Anticancer Effects of Lignans
Cancer is the leading cause of death in Korea. Globally, approximately 10 million patients die due to cancer annually [112]. However, conventional methods, e.g., chemotherapy or radiotherapy, carry a large patient burden and can show low efficacy. Recently, therapies directly targeting cancer-associated antigens and use of immunotherapy to modulate the immune system to avoid antigen resistance have provided new options for cancer treatment [113]. Nevertheless, the development of novel anti-tumor drugs from natural compounds with fewer adverse effects may improve cancer treatment outcomes: lignans, with excellent anti-inflammatory effects, may be one such category of cancer drugs derived from natural compounds.
5.1. Breast Cancer
Approximately 15% of breast cancer patients died within five years regardless of therapeutic treatment, according to the statistics from American Cancer Society [114]. Some of the patients suffer from hormone receptor-positive breast cancer receiving drugs that block estrogen receptors. Since dietary lignans mimic estrogen and are active in estrogen-receptor-mediated signaling pathways, many studies have examined the effects of dietary lignan consumption on breast cancer [115]. Interestingly however, Seco isolated from flax seeds downregulates the proliferation of MCF-7 cancer cells by exerting inhibitory activity on ERα [116]. In addition, SDG, the glycosylated form of Seco, synergistically supported the anti-cancer effect of doxorubicin, a conventional chemotherapeutic agent [117]. High-dose SDG treatment increased serum END and ENL concentrations, which led to downregulation of the transcriptive activity of NF-κB, inhibiting the survival and proliferation of MDA-MB-231 and MCF-7 cells in mice [118]. Trans-(-)-kusunokinin is a lignan compound derived from Piper nigrum known to target various breast cancer cell lines [119]. Interestingly, it exerted anti-tumor effects on breast cancer cell lines by cell-cycle arrest and induction of apoptosis [120].
5.2. Colorectal Cancer
Colorectal cancer is the third major cause of death globally in both men and women [114]. The lethality of colorectal cancer arises from its propensity to metastasize to the liver [121]. However, dietary lignans isolated from flax and sesame seeds may act as inhibitors of such metastasis. The postulated mechanism is based on the regulatory role of Lar, Med, and Pino on the cellular autophagy system that is directly linked with cancer survival and metastasis [122]. Dietary lignans are able to block UNC-51-like autophagy activating kinase 1/2 (ULK1/2), key regulators of metastatic colorectal cancer [123]. Surprisingly, Yefei Huang et al. discovered that Ses can inhibit metastasis of colorectal cancer by blocking angiogenesis [124]. After conducting both an in vivo angiogenesis assay and an in vitro tube-formation assay, the researchers confirmed that hypoxia-induced angiogenesis was suppressed by Ses treatment, which inhibited the NF-κB/hypoxia-inducible factor 1-alpha (HIF-1α)/VEGFA axis. In addition, SDG induced apoptosis of SW480 human colorectal cancer cells by upregulating apoptosis-inducing factor (AIF) and caspase 3 gene expression [125]. Consistent with these results, Tuo Chen et al. found that SDG mediated caspase 1 activation to induce pyroptosis of HCT116 colorectal cancer cells [126].
5.3. Prostate Cancer
The second leading cause of death among men worldwide is prostate cancer, with a mortality rate of 11% annually [114]. Androgens and their receptors play crucial roles in the progression of prostate cancer. The expression of androgen receptors is mainly regulated by the NF-κB pathway, and elevated NF-κB expression is detected in most prostate cancer patients [127]. Certain phytoestrogens are excellent modulators of inflammation, inhibitors of NF-κB activity, and androgen receptor (AR) antagonists, blocking the effects of androgens, such as testosterone or dihydrotestosterone (DHT) [72]. The Syr lignan, which suppresses the NF-κB pathway, has a greater binding affinity for Ars according to molecular dynamics studies [77,128]. In addition, Syr may target mutant Ars found in castration-resistant prostate cancer (CRPC), which often shows resistance to androgen-deprivation therapy [129].
5.4. Other Cancers
Lignans also have therapeutic effects in other cancers, notably with regard to the programmed-cell death pathway or phosphoinositide 3-kinase (PI3K)/Akt-mediated cancer cell proliferation (Table 2). For instance, Ses exerted anti-tumor effects in the EL4 mouse lymphoma cell line [130]. The Ses lignan induced the intrinsic apoptosis pathway by deactivating Bcl-2 and initiated pyroptosis by increasing expression of IL-1β. The flax seed lignan metabolite ENL inhibited proliferation of the KG-1 and Monomac-1 acute myeloid leukemia cell lines [131]; 100 μM of ENL triggered DNA fragmentation and apoptosis in both cell lines.
Table 2.
Summary of the results of published pharmacological experiments conducted to evaluate the effects of lignans in cancers.
| Disease | Lignan | Source | Test Type and Dose | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Breast | Seco | L. usitatissimum | In vitro | PARP cleavage ↑ Erα ↓ |
[116] |
| SDG | L. usitatissimum | In vitro (1, 10 μM) In vivo (25, 74 mg/kg) |
END, ENL ↑ p65 ↓ |
[118] | |
| (-)-Kusunokinin | P. nigrum | In vitro (1.6, 3.2, 6.4 μM) |
p53, p21 ↑ Bcl-2 ↓ Bax, Cytochrome c, caspase 7/8 ↑ |
[120] | |
| Colorectal cancer | Mat, Pino, Lari, Seco, Medi |
Structures from pubchem database | In silico | ULK1/2 ↓ | [123] |
| Ses | Purchased (Sellek Chemicals) |
In vitro (10 μM) |
IκBα, p65 ↓ HIF-1α ↓ VEGFA ↓ |
[124] | |
| SDG | Purchased (Sigma-Aldrich) |
In vitro (100, 150 μM) |
AIF, caspase 3 ↑ | [125] | |
| SDG | Purchased (MedChemExpress) |
In vitro (50 μM) |
GSDMD, caspase-1, cytochrome c, Bax ↑ PI3K, Akt ↓ |
[126] | |
| Prostate cancer | Syr | Structure from pubchem database | In silico | AR ↓ | [128] |
| T cell Lymphoma | Ses | Purchased (TargetMol) |
In vivo (10 mg/kg) In vitro (10, 20, 40 μM) |
Cyclin D1 ↓ caspase 3, Bax ↑ Bcl-2 ↓ caspase 1, NLPR3 ↑ Atg5, LC3 II/I ↑ p62 ↓ |
[130] |
| Acute Myeloid Leukemia | ENL | Purchased (Sigma-Aldrich) |
In vitro (40, 100 μM) |
Cytochrome c, PARP, Bax, caspase 3/9 ↑ Bcl-2 ↓ |
[131] |
6. Conclusions and Perspectives
In this review, we aimed to describe the recent research addressing the antioxidant, anti-inflammatory, anti-menopause, and anti-cancer effects of lignans. Previously published data indicated the antioxidant properties of lignans are mainly related to the regulation of radical scavenging enzymes, e.g., SOD and CAT. Administration of SDG alleviated liver and kidney damage by enhancing the expression of SOD and CAT. Moreover, upregulation of the Nrf2-ARE axis, which leads to expression of HO-1, has been reported following SDG or Lari administration. Especially, Lari showed remarkable inhibition capability of cellular ROS generation comparable to gallic acid [58]. The anti-inflammatory effects of lignans are the result of NF-κB and MAPK pathway inhibition. Induction of pro-inflammatory cytokines, such as TNF-α, IL-6, IL-8, and IL-1β, by activation of the p50/p65 and c-Jun/c-Fos transcription factors was downregulated by lignans in various in vitro and in vivo models. Since the molecular mechanism of the lignans on inflammatory response has been elucidated in detail, further applicability to chronic inflammatory disease was addressed in this review.
Lignans have been suggested as possible therapeutic agents to reduce symptoms related to menopause. Many researchers have emphasized the value of lignans as a substitute for estrogen. However, recent studies showed that anti-inflammatory properties of lignans mediated by deactivation of the NF-κB and AP-1 signaling pathways can also alleviate severe bone diseases caused by estrogen deficiency in female. Moreover, their role as eNOS activators suggests the potential of lignans against cardiovascular diseases often seen in menopausal women. Lignans also alleviate neuroinflammation, reducing brain damage by preserving BDNF expression and modulating neurotransmitter levels. Surprisingly, some of the lignans showed impressive efficacy on postmenopausal symptoms compared to extrinsic hormones such as parathyroid hormone or estrogen [88,103]. It was confirmed that TNF-α expression was more strongly suppressed when treated with lignans derived from Sambucus Willaimsii compared to parathyroid hormone treatment. Osteoporosis alleviating factors were also upregulated by Ses treatment to the same extent as estrogen treatment. These findings indicate that there is the specific curative mechanism of lignans in the treatment of postmenopausal disorders and these compounds can substitute hormonal therapies. Lignans also target cancer cells by suppressing the NF-κB pathway and modulating apoptotic pathways. Interestingly, although breast cancer patients are counselled to avoid consuming dietary lignans, as they upregulate the estrogen receptor-mediated pathway, some lignans, e.g., SDG or trans-(-)-kusunokinin, have antitumor effects on breast cancer cells by inhibiting Akt, Cyclin D1, and CDK to reduce proliferation. Their role as inflammation modulators also emphasizes the function of lignans in other cancers like colorectal, prostate, and blood cancers.
Although this review does not cover entire studies of lignans on inflammatory diseases, we hope that the review presents the direction of therapeutic approaches on lignans against several major diseases, such as cancerous, inflammatory, and cardiovascular diseases. Lignans are typically formulated as functional food items and administered orally. Few studies have been conducted to develop drugs based upon lignans and to design specific carriers to increase the delivery efficiency of lignans, possibly because of the uncertainty regarding the efficacies of lignan polymers in various chronic diseases. However, recent studies have shown that parent lignans also exert numerous curative effects on human chronic diseases, not just the active END or ENL metabolites. Therefore, more efforts should be made to develop lignan-based drugs against chronic inflammatory diseases. Based on previous studies with differing target concentrations and lignans, a specific formulation should be determined for a pharmaceutical preparation containing various lignan compounds. We expect that lignans will soon not only be used as functional foods, but also in the pharmaceutical industry.
Abbreviations
| Seco | secoisolariciresinol |
| Pino | pinoresinol |
| Mat | matairesinol |
| Ses | sesamin |
| Syr | syringaresinol |
| Lari | lariciresinol |
| SDG | secoisolariciresinol diglucoside |
| END | enterodiol |
| ENL | enterolactone |
| PAMPs | pathogen-associated molecular patterns |
| PRRs | pattern recognition receptors |
| TLRs | toll-like receptors |
| TAK1 | TGF-b-activated kinase 1 |
| NF | nuclear factor |
| AP | activator protein |
| p65 | Rel A |
| IKK | IκB kinase |
| MAPK | mitogen-activated protein kinase |
| ERK | extracellular signal-regulated kinase |
| JNK | c-Jun N-terminal kinase |
| ROS | reactive oxygen species |
| Bcl-2 | B-cell lymphoma 2 |
| VEGF | vascular endothelial growth factor |
| CSF1 | colony stimulating factor 1 |
| TNF-α | tumor necrosis factor alpha |
| IL-6 | interleukin-6 |
| O2* | superoxide |
| HO* | hydroxyl |
| H2O2 | hydrogen peroxide |
| IBD | inflammatory bowel disease |
| SOD | superoxide dismutase |
| CAT | catalase |
| MDA | malondialdehyde |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| ARE element | AU-rich element |
| HO-1 | heme oxygenase-1 |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| COXs | cyclo-oxygenases |
| JAK | janus family tyrosine k |
| STAT | signal transducers and activator |
| IL-1β | interleukin-1 beta |
| iNOS | inducible nitric oxide synthase |
| DSS | dextran-sulfate sodium-salt |
| NLRP1 | NLR family pyrin domain containing 1 |
| IL-8 | interleukin 8 |
| LH | luteinizing hormone |
| FSH | follicle stimulating hormone |
| ERα | estrogen receptor alpha |
| ERβ | estrogen receptor beta |
| eNOS | endothelial nitric oxide synthase |
| NO | nitric oxide |
| HDL | high density lipoprotein |
| LDL | low density lipoprotein |
| PAF-AH | platelet activating factor-acetyl hydrolase |
| OVX | ovariectomy |
| TC | total cholesterol |
| TG | triglyceride |
| IL-22 | interleukin-22 |
| MCP-1 | monocyte chemoatrractant protein-1 |
| LDH | lactate dehyrogenase |
| CK | creatine phosphokinase |
| SLC7A11 | solute carrier family 7 member 11 |
| GPX4 | glutathione peroxidase 4 |
| TRPV1 | transient receptor potential vanilloid 1 |
| PKA | protein kinase A |
| Akt | protein kinase B |
| AMPK | AMP-activated protein kinase |
| ICAM-1 | intracellular adhesion molecule-1 |
| OSX | osterix |
| SOX9 | SRY-box9 |
| RUNX2 | runt-related transcription factor 2 |
| ALP | alkaline phosphatase |
| OPN | osteopontin |
| OCN | osteocalcin |
| M-CSF | macrophage-colony stimulating factor |
| RANKL | receptor activator of nuclear factor kappa B ligand |
| NFATc1 | nuclear factor of activated T cells 1 |
| CTSK | cathepsin K |
| MMP-9 | matrix metalloprotease-9 |
| CTX-1 | C-telopeptide of collagen Type 1 |
| OPG | osteoprotegerin |
| RUNX2 | runt-related transcription factor 2 |
| OCN | osteocalcin |
| Wnt | wingless-related integration site |
| GSK3β | glycogen synthase kinase 3 beta |
| TRAP | tartrate resistant acid phosphatase |
| BMP2 | bone morphogenetic protein 2 |
| 5-HT | serotonin |
| MAO | monoamine oxidase |
| CUMS | chronic unpredictable mild stress |
| BDNF | brain-derived neurotrophic factor |
| ULK1/2 | UNC51-like autophagy activating kinase 1/2 |
| HIF-1α | hypoxia-inducible factor 1-alpha |
| AIF | apoptosis-inducing factor |
| AR | androgen receptor |
| DHT | dihydrotestosterone |
| CRPC | castration-resistant prostate cancer |
| PI3K | phosphoinositide 3-kinase |
Author Contributions
W.Y.J., Conceptualization, formal analysis, methodology, visualization, and writing-original draft. M.-Y.K. and J.Y.C., Conceptualization, formal analysis, funding acquisition, project administration, visualization, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Ministry of Science and ICT; the International Cooperative R&D Program under Grant no. P0019158 through the Ministry of Trade, Industry and Energy (MOTIE) and Korea Institute for Advancement of Technology (KIAT), Korea.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lephart E.D. Phytoestrogens (Resveratrol and Equol) for Estrogen-Deficient Skin-Controversies/Misinformation versus Anti-Aging In Vitro and Clinical Evidence via Nutraceutical-Cosmetics. Int. J. Mol. Sci. 2021;22:11218. doi: 10.3390/ijms222011218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sirotkin A.V., Harrath A.H. Phytoestrogens and their effects. Eur. J. Pharm. 2014;741:230–236. doi: 10.1016/j.ejphar.2014.07.057. [DOI] [PubMed] [Google Scholar]
- 3.Adlercreutz H. Lignans and human health. Crit. Rev. Clin. Lab. Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- 4.Fang X., Hu X. Advances in the Synthesis of Lignan Natural Products. Molecules. 2018;23:3385. doi: 10.3390/molecules23123385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teodor E.D., Moroeanu V., Radu G.L. Lignans from Medicinal Plants and their Anticancer Effect. Mini Rev. Med. Chem. 2020;20:1083–1090. doi: 10.2174/1389557520666200212110513. [DOI] [PubMed] [Google Scholar]
- 6.Li K.M., Dong X., Ma Y.N., Wu Z.H., Yan Y.M., Cheng Y.X. Antifungal coumarins and lignans from Artemisia annua. Fitoterapia. 2019;134:323–328. doi: 10.1016/j.fitote.2019.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Parikh M., Maddaford T.G., Austria J.A., Aliani M., Netticadan T., Pierce G.N. Dietary Flaxseed as a Strategy for Improving Human Health. Nutrients. 2019;11:1171. doi: 10.3390/nu11051171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kezimana P., Dmitriev A.A., Kudryavtseva A.V., Romanova E.V., Melnikova N.V. Secoisolariciresinol Diglucoside of Flaxseed and Its Metabolites: Biosynthesis and Potential for Nutraceuticals. Front. Genet. 2018;9:641. doi: 10.3389/fgene.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clavel T., Henderson G., Engst W., Doré J., Blaut M. Phylogeny of human intestinal bacteria that activate the dietary lignan secoisolariciresinol diglucoside. FEMS Microbiol. Ecol. 2006;55:471–478. doi: 10.1111/j.1574-6941.2005.00057.x. [DOI] [PubMed] [Google Scholar]
- 10.Song C., Kim M.Y., Cho J.Y. Olea europaea Suppresses Inflammation by Targeting TAK1-Mediated MAP Kinase Activation. Molecules. 2021;26:1540. doi: 10.3390/molecules26061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y., Ho L., Tergaonkar V. sORF-Encoded MicroPeptides: New players in inflammation, metabolism, and precision medicine. Cancer Lett. 2021;500:263–270. doi: 10.1016/j.canlet.2020.10.038. [DOI] [PubMed] [Google Scholar]
- 12.Goncalves R.M., Delgobo M., Agnes J.P., das Neves R.N., Falchetti M., Casagrande T., Garcia A.P.V., Vieira T.C., Somensi N., Bruxel M.A., et al. COX-2 promotes mammary adipose tissue inflammation, local estrogen biosynthesis, and carcinogenesis in high-sugar/fat diet treated mice. Cancer Lett. 2021;502:44–57. doi: 10.1016/j.canlet.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Li H., Chen R., Jiang X., He J., Li C. TAK1 confers antibacterial protection through mediating the activation of MAPK and NF-κB pathways in shrimp. Fish. Shellfish Immunol. 2022;123:248–256. doi: 10.1016/j.fsi.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Yi H., Liang L., Wang H., Luo S., Hu L., Wang Y., Shen X., Xiao L., Zhang Y., Peng H., et al. Albendazole inhibits NF-kappaB signaling pathway to overcome tumor stemness and bortezomib resistance in multiple myeloma. Cancer Lett. 2021;520:307–320. doi: 10.1016/j.canlet.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Yang J., Li Y., Sun Z., Zhan H. Macrophages in pancreatic cancer: An immunometabolic perspective. Cancer Lett. 2021;498:188–200. doi: 10.1016/j.canlet.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Mirzaei S., Zarrabi A., Hashemi F., Zabolian A., Saleki H., Ranjbar A., Seyed Saleh S.H., Bagherian M., Sharifzadeh S.O., Hushmandi K., et al. Regulation of Nuclear Factor-KappaB (NF-kappaB) signaling pathway by non-coding RNAs in cancer: Inhibiting or promoting carcinogenesis? Cancer Lett. 2021;509:63–80. doi: 10.1016/j.canlet.2021.03.025. [DOI] [PubMed] [Google Scholar]
- 17.Suhail M., Tarique M., Muhammad N., Naz H., Hafeez A., Zughaibi T.A., Kamal M.A., Rehan M. A Critical Transcription Factor NF-κB as a Cancer Therapeutic Target and its Inhibitors as Cancer Treatment Options. Curr. Med. Chem. 2021;28:4117–4132. doi: 10.2174/0929867327666201111142307. [DOI] [PubMed] [Google Scholar]
- 18.Jiang L., Chen Y., Min G., Wang J., Chen W., Wang H., Wang X., Yao N. Bcl2-associated athanogene 4 promotes the invasion and metastasis of gastric cancer cells by activating the PI3K/AKT/NF-kappaB/ZEB1 axis. Cancer Lett. 2021;520:409–421. doi: 10.1016/j.canlet.2021.08.020. [DOI] [PubMed] [Google Scholar]
- 19.Mihaly S.R., Ninomiya-Tsuji J., Morioka S. TAK1 control of cell death. Cell Death Differ. 2014;21:1667–1676. doi: 10.1038/cdd.2014.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L.T., Liu K.Y., Chiou S.S., Huang S.K., Hsu S.H., Wang S.N. Phosphorylation of intestine-specific homeobox by ERK1 modulates oncogenic activity and sorafenib resistance. Cancer Lett. 2021;520:160–171. doi: 10.1016/j.canlet.2021.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Ma Q., Xu Q., Zhao J., Zhang W., Wang Q., Fang J., Lu Z., Liu J., Ma L. Coupling HDAC4 with transcriptional factor MEF2D abrogates SPRY4-mediated suppression of ERK activation and elicits hepatocellular carcinoma drug resistance. Cancer Lett. 2021;520:243–254. doi: 10.1016/j.canlet.2021.07.049. [DOI] [PubMed] [Google Scholar]
- 22.Liu M., Qin Y., Hu Q., Liu W., Ji S., Xu W., Fan G., Ye Z., Zhang Z., Xu X., et al. SETD8 potentiates constitutive ERK1/2 activation via epigenetically silencing DUSP10 expression in pancreatic cancer. Cancer Lett. 2021;499:265–278. doi: 10.1016/j.canlet.2020.11.023. [DOI] [PubMed] [Google Scholar]
- 23.Hong Y.H., Aziz N., Park J.G., Lee D., Kim J.K., Kim S.A., Choi W., Lee C.Y., Lee H.P., Huyen Trang H.T., et al. The EEF1AKMT3/MAP2K7/TP53 axis suppresses tumor invasiveness and metastasis in gastric cancer. Cancer Lett. 2022;544:215803. doi: 10.1016/j.canlet.2022.215803. [DOI] [PubMed] [Google Scholar]
- 24.Ha A.T., Kim M.Y., Cho J.Y. TAK1/AP-1-Targeted Anti-Inflammatory Effects of Barringtonia augusta Methanol Extract. Molecules. 2021;26:3053. doi: 10.3390/molecules26103053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jang J., Hunto S.T., Kim J.W., Lee H.P., Kim H.G., Cho J.Y. Anti-Inflammatory Activities of an Anti-Histamine Drug, Loratadine, by Suppressing TAK1 in AP-1 Pathway. Int. J. Mol. Sci. 2022;23:3986. doi: 10.3390/ijms23073986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong C. Cytokine Regulation and Function in T Cells. Annu. Rev. Immunol. 2021;39:51–76. doi: 10.1146/annurev-immunol-061020-053702. [DOI] [PubMed] [Google Scholar]
- 27.Marzano A.V., Ortega-Loayza A.G., Heath M., Morse D., Genovese G., Cugno M. Mechanisms of Inflammation in Neutrophil-Mediated Skin Diseases. Front. Immunol. 2019;10:1059. doi: 10.3389/fimmu.2019.01059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta S.C., Kunnumakkara A.B., Aggarwal S., Aggarwal B.B. Inflammation, a Double-Edge Sword for Cancer and Other Age-Related Diseases. Front. Immunol. 2018;9:2160. doi: 10.3389/fimmu.2018.02160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X., Shao C., Duan L., Hou X., Huang Y., Gao L., Zong C., Liu W., Jiang J., Ye F., et al. Oncostatin M promotes hepatic progenitor cell activation and hepatocarcinogenesis via macrophage-derived tumor necrosis factor-alpha. Cancer Lett. 2021;517:46–54. doi: 10.1016/j.canlet.2021.05.039. [DOI] [PubMed] [Google Scholar]
- 30.Lu F., Zhao Y., Pang Y., Ji M., Sun Y., Wang H., Zou J., Wang Y., Li G., Sun T., et al. NLRP3 inflammasome upregulates PD-L1 expression and contributes to immune suppression in lymphoma. Cancer Lett. 2021;497:178–189. doi: 10.1016/j.canlet.2020.10.024. [DOI] [PubMed] [Google Scholar]
- 31.Mittal M., Siddiqui M.R., Tran K., Reddy S.P., Malik A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014;20:1126–1167. doi: 10.1089/ars.2012.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang S.F., Chang Y.L., Tzeng Y.D., Wu C.L., Wang Y.Z., Tseng L.M., Chen S., Lee H.C. Mitochondrial stress adaptation promotes resistance to aromatase inhibitor in human breast cancer cells via ROS/calcium up-regulated amphiregulin-estrogen receptor loop signaling. Cancer Lett. 2021;523:82–99. doi: 10.1016/j.canlet.2021.09.043. [DOI] [PubMed] [Google Scholar]
- 33.Liu T., Ma Y., Zhang R., Zhong H., Wang L., Zhao J., Yang L., Fan X. Resveratrol ameliorates estrogen deficiency-induced depression- and anxiety-like behaviors and hippocampal inflammation in mice. Psychopharmacology. 2019;236:1385–1399. doi: 10.1007/s00213-018-5148-5. [DOI] [PubMed] [Google Scholar]
- 34.Hong G., Zhou L., Han X., Sun P., Chen Z., He W., Tickner J., Chen L., Shi X., Xu J. Asiatic Acid Inhibits OVX-Induced Osteoporosis and Osteoclastogenesis Via Regulating RANKL-Mediated NF-κb and Nfatc1 Signaling Pathways. Front. Pharm. 2020;11:331. doi: 10.3389/fphar.2020.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Monaco C., Paleolog E. Nuclear factor kappaB: A potential therapeutic target in atherosclerosis and thrombosis. Cardiovasc. Res. 2004;61:671–682. doi: 10.1016/j.cardiores.2003.11.038. [DOI] [PubMed] [Google Scholar]
- 36.Miklowitz D.J., Portnoff L.C., Armstrong C.C., Keenan-Miller D., Breen E.C., Muscatell K.A., Eisenberger N.I., Irwin M.R. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 2016;241:315–322. doi: 10.1016/j.psychres.2016.04.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abdelzaher W.Y., Mohammed H.H., Welson N.N., Batiha G.E., Baty R.S., Abdel-Aziz A.M. Rivaroxaban Modulates TLR4/Myd88/NF-Kβ Signaling Pathway in a Dose-Dependent Manner With Suppression of Oxidative Stress and Inflammation in an Experimental Model of Depression. Front. Pharm. 2021;12:715354. doi: 10.3389/fphar.2021.715354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alles N., Soysa N.S., Hayashi J., Khan M., Shimoda A., Shimokawa H., Ritzeler O., Akiyoshi K., Aoki K., Ohya K. Suppression of NF-kappaB increases bone formation and ameliorates osteopenia in ovariectomized mice. Endocrinology. 2010;151:4626–4634. doi: 10.1210/en.2010-0399. [DOI] [PubMed] [Google Scholar]
- 39.Abu-Amer Y. NF-κB signaling and bone resorption. Osteoporos Int. 2013;24:2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y.Y., Hung A.C., Lo S., Yuan S.F. Adipocytokines visfatin and resistin in breast cancer: Clinical relevance, biological mechanisms, and therapeutic potential. Cancer Lett. 2021;498:229–239. doi: 10.1016/j.canlet.2020.10.045. [DOI] [PubMed] [Google Scholar]
- 41.Karin M., Greten F.R. NF-kappaB: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005;5:749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 42.Li X., Wei Z., Yu H., Xu Y., He W., Zhou X., Gou X. Secretory autophagy-induced bladder tumour-derived extracellular vesicle secretion promotes angiogenesis by activating the TPX2-mediated phosphorylation of the AURKA-PI3K-AKT axis. Cancer Lett. 2021;523:10–28. doi: 10.1016/j.canlet.2021.09.036. [DOI] [PubMed] [Google Scholar]
- 43.Sheta M., Hassan G., Afify S.M., Monzur S., Kumon K., Abu Quora H.A., Farahat M., Zahra M.H., Fu X., Seno A., et al. Chronic exposure to FGF2 converts iPSCs into cancer stem cells with an enhanced integrin/focal adhesion/PI3K/AKT axis. Cancer Lett. 2021;521:142–154. doi: 10.1016/j.canlet.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 44.Huang P., Han J., Hui L. MAPK signaling in inflammation-associated cancer development. Protein Cell. 2010;1:218–226. doi: 10.1007/s13238-010-0019-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagumo Y., Kandori S., Tanuma K., Nitta S., Chihara I., Shiga M., Hoshi A., Negoro H., Kojima T., Mathis B.J., et al. PLD1 promotes tumor invasion by regulation of MMP-13 expression via NF-kappaB signaling in bladder cancer. Cancer Lett. 2021;511:15–25. doi: 10.1016/j.canlet.2021.04.014. [DOI] [PubMed] [Google Scholar]
- 46.Yang F., Duan M., Zheng F., Yu L., Wang Y., Wang G., Lin J., Han S., Gan D., Meng Z., et al. Fas signaling in adipocytes promotes low-grade inflammation and lung metastasis of colorectal cancer through interaction with Bmx. Cancer Lett. 2021;522:93–104. doi: 10.1016/j.canlet.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 47.Perillo B., Di Donato M., Pezone A., Di Zazzo E., Giovannelli P., Galasso G., Castoria G., Migliaccio A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020;52:192–203. doi: 10.1038/s12276-020-0384-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roy R.V., Pratheeshkumar P., Son Y.O., Wang L., Hitron J.A., Divya S.P., Zhang Z., Shi X. Different roles of ROS and Nrf2 in Cr(VI)-induced inflammatory responses in normal and Cr(VI)-transformed cells. Toxicol. Appl. Pharm. 2016;307:81–90. doi: 10.1016/j.taap.2016.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Z., Ren Z., Zhang J., Chuang C.C., Kandaswamy E., Zhou T., Zuo L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018;9:477. doi: 10.3389/fphys.2018.00477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yan H., Wang H., Zhang X., Li X., Yu J. Ascorbic acid ameliorates oxidative stress and inflammation in dextran sulfate sodium-induced ulcerative colitis in mice. Int. J. Clin. Exp. Med. 2015;8:20245–20253. [PMC free article] [PubMed] [Google Scholar]
- 51.von Martels J.Z.H., Bourgonje A.R., Klaassen M.A.Y., Alkhalifah H.A.A., Sadaghian Sadabad M., Vich Vila A., Gacesa R., Gabriëls R.Y., Steinert R.E., Jansen B.H., et al. Riboflavin Supplementation in Patients with Crohn’s Disease [the RISE-UP study] J. Crohns Colitis. 2020;14:595–607. doi: 10.1093/ecco-jcc/jjz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.You L., Cho J.Y. The regulatory role of Korean ginseng in skin cells. J. Ginseng Res. 2021;45:363–370. doi: 10.1016/j.jgr.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moree S.S., Kavishankar G.B., Rajesha J. Antidiabetic effect of secoisolariciresinol diglucoside in streptozotocin-induced diabetic rats. Phytomedicine. 2013;20:237–245. doi: 10.1016/j.phymed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 54.Wang Y., Branicky R., Noë A., Hekimi S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018;217:1915–1928. doi: 10.1083/jcb.201708007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge J., Hao R., Rong X., Dou Q.P., Tan X., Li G., Li F., Li D. Secoisolariciresinol diglucoside mitigates benzo[a]pyrene-induced liver and kidney toxicity in mice via miR-101a/MKP-1-mediated p38 and ERK pathway. Food Chem. Toxicol. 2022;159:112733. doi: 10.1016/j.fct.2021.112733. [DOI] [PubMed] [Google Scholar]
- 56.Pietrofesa R.A., Chatterjee S., Kadariya Y., Testa J.R., Albelda S.M., Christofidou-Solomidou M. Synthetic Secoisolariciresinol Diglucoside (LGM2605) Prevents Asbestos-Induced Inflammation and Genotoxic Cell Damage in Human Mesothelial Cells. Int. J. Mol. Sci. 2022;23:10085. doi: 10.3390/ijms231710085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Villavicencio Tejo F., Quintanilla R.A. Contribution of the Nrf2 Pathway on Oxidative Damage and Mitochondrial Failure in Parkinson and Alzheimer’s Disease. Antioxidants. 2021;10:1069. doi: 10.3390/antiox10071069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bajpai V.K., Alam M.B., Quan K.T., Kwon K.R., Ju M.K., Choi H.J., Lee J.S., Yoon J.I., Majumder R., Rather I.A., et al. Antioxidant efficacy and the upregulation of Nrf2-mediated HO-1 expression by (+)-lariciresinol, a lignan isolated from Rubia philippinensis, through the activation of p38. Sci. Rep. 2017;7:46035. doi: 10.1038/srep46035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lucafò M., Curci D., Franzin M., Decorti G., Stocco G. Inflammatory Bowel Disease and Risk of Colorectal Cancer: An Overview From Pathophysiology to Pharmacological Prevention. Front. Pharm. 2021;12:772101. doi: 10.3389/fphar.2021.772101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Oshima H., Ishikawa T., Yoshida G.J., Naoi K., Maeda Y., Naka K., Ju X., Yamada Y., Minamoto T., Mukaida N., et al. TNF-α/TNFR1 signaling promotes gastric tumorigenesis through induction of Noxo1 and Gna14 in tumor cells. Oncogene. 2014;33:3820–3829. doi: 10.1038/onc.2013.356. [DOI] [PubMed] [Google Scholar]
- 61.Hoesel B., Schmid J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu C.H., Chen Z., Chen K., Liao F.T., Chung C.E., Liu X., Lin Y.C., Keohavong P., Leikauf G.D., Di Y.P. Lipopolysaccharide-Mediated Chronic Inflammation Promotes Tobacco Carcinogen-Induced Lung Cancer and Determines the Efficacy of Immunotherapy. Cancer Res. 2021;81:144–157. doi: 10.1158/0008-5472.CAN-20-1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tang S., Zhang Z., Oakley R.H., Li W., He W., Xu X., Ji M., Xu Q., Chen L., Wellman A.S., et al. Intestinal epithelial glucocorticoid receptor promotes chronic inflammation-associated colorectal cancer. JCI Insight. 2021;6:e151815. doi: 10.1172/jci.insight.151815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cea L.A., Balboa E., Puebla C., Vargas A.A., Cisterna B.A., Escamilla R., Regueira T., Sáez J.C. Dexamethasone-induced muscular atrophy is mediated by functional expression of connexin-based hemichannels. Biochim. Biophys. Acta. 2016;1862:1891–1899. doi: 10.1016/j.bbadis.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 65.Bhandari K., Venables B. Ibuprofen bioconcentration and prostaglandin E2 levels in the bluntnose minnow Pimephales notatus. Comp. Biochem. Physiol. C Toxicol. Pharm. 2011;153:251–257. doi: 10.1016/j.cbpc.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Banerjee S., Biehl A., Gadina M., Hasni S., Schwartz D.M. JAK-STAT Signaling as a Target for Inflammatory and Autoimmune Diseases: Current and Future Prospects. Drugs. 2017;77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jang W.Y., Lee H.P., Kim S.A., Huang L., Yoon J.H., Shin C.Y., Mitra A., Kim H.G., Cho J.Y. Angiopteris cochinchinensis de Vriese Ameliorates LPS-Induced Acute Lung Injury via Src Inhibition. Plants. 2022;11:1306. doi: 10.3390/plants11101306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li L., Chen J., Lin L., Pan G., Zhang S., Chen H., Zhang M., Xuan Y., Wang Y., You Z. Quzhou Fructus Aurantii Extract suppresses inflammation via regulation of MAPK, NF-κB, and AMPK signaling pathway. Sci. Rep. 2020;10:1593. doi: 10.1038/s41598-020-58566-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Higham A., Singh D. Dexamethasone and p38 MAPK inhibition of cytokine production from human lung fibroblasts. Fundam Clin. Pharm. 2021;35:714–724. doi: 10.1111/fcp.12627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Son M., Wang A.G., Tu H.L., Metzig M.O., Patel P., Husain K., Lin J., Murugan A., Hoffmann A., Tay S. NF-κB responds to absolute differences in cytokine concentrations. Sci. Signal. 2021;14:eaaz4382. doi: 10.1126/scisignal.aaz4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.You L., Huang L., Jang J., Hong Y.H., Kim H.G., Chen H., Shin C.Y., Yoon J.H., Manilack P., Sounyvong B., et al. Callerya atropurpurea suppresses inflammation in vitro and ameliorates gastric injury as well as septic shock in vivo via TLR4/MyD88-dependent cascade. Phytomedicine. 2022;105:154338. doi: 10.1016/j.phymed.2022.154338. [DOI] [PubMed] [Google Scholar]
- 72.Li D., Luo F., Guo T., Han S., Wang H., Lin Q. Targeting NF-κB pathway by dietary lignans in inflammation: Expanding roles of gut microbiota and metabolites. Crit. Rev. Food Sci. Nutr. 2022 doi: 10.1080/10408398.2022.2026871. in press. [DOI] [PubMed] [Google Scholar]
- 73.Bajpai V.K., Alam M.B., Quan K.T., Ju M.K., Majumder R., Shukla S., Huh Y.S., Na M., Lee S.H., Han Y.K. Attenuation of inflammatory responses by (+)-syringaresinol via MAP-Kinase-mediated suppression of NF-κB signaling in vitro and in vivo. Sci. Rep. 2018;8:9216. doi: 10.1038/s41598-018-27585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Z., Chen T., Yang C., Bao T., Yang X., He F., Zhang Y., Zhu L., Chen H., Rong S., et al. Secoisolariciresinol diglucoside suppresses Dextran sulfate sodium salt-induced colitis through inhibiting NLRP1 inflammasome. Int. Immunopharmacol. 2020;78:105931. doi: 10.1016/j.intimp.2019.105931. [DOI] [PubMed] [Google Scholar]
- 75.Yu L., Xu Q., Wang P., Luo J., Zheng Z., Zhou J., Zhang L., Sun L., Zuo D. Secoisolariciresinol diglucoside-derived metabolite, enterolactone, attenuates atopic dermatitis by suppressing Th2 immune response. Int. Immunopharmacol. 2022;111:109039. doi: 10.1016/j.intimp.2022.109039. [DOI] [PubMed] [Google Scholar]
- 76.Zhang Y., Lei Y., Yao X., Yi J., Feng G. Pinoresinol diglucoside alleviates ischemia/reperfusion-induced brain injury by modulating neuroinflammation and oxidative stress. Chem. Biol. Drug Des. 2021;98:986–996. doi: 10.1111/cbdd.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang L., Jiang X., Zhang J., Gao H., Yang L., Li D., Zhang Q., Wang B., Cui L., Wang X. (-)-Syringaresinol suppressed LPS-induced microglia activation via downregulation of NF-κB p65 signaling and interaction with ERβ. Int. Immunopharmacol. 2021;99:107986. doi: 10.1016/j.intimp.2021.107986. [DOI] [PubMed] [Google Scholar]
- 78.Ye H., Sun L., Li J., Wang Y., Bai J., Wu L., Han Q., Yang Z., Li L. Sesamin attenuates carrageenan-induced lung inflammation through upregulation of A20 and TAX1BP1 in rats. Int. Immunopharmacol. 2020;88:107009. doi: 10.1016/j.intimp.2020.107009. [DOI] [PubMed] [Google Scholar]
- 79.Wang H.Q., Wan Z., Zhang Q., Su T., Yu D., Wang F., Zhang C., Li W., Xu D., Zhang H. Schisandrin B targets cannabinoid 2 receptor in Kupffer cell to ameliorate CCl(4)-induced liver fibrosis by suppressing NF-κB and p38 MAPK pathway. Phytomedicine. 2022;98:153960. doi: 10.1016/j.phymed.2022.153960. [DOI] [PubMed] [Google Scholar]
- 80.Tang Z.R., Zhang R., Lian Z.X., Deng S.L., Yu K. Estrogen-Receptor Expression and Function in Female Reproductive Disease. Cells. 2019;8:1123. doi: 10.3390/cells8101123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shaw N.D., Histed S.N., Srouji S.S., Yang J., Lee H., Hall J.E. Estrogen negative feedback on gonadotropin secretion: Evidence for a direct pituitary effect in women. J. Clin. Endocrinol. Metab. 2010;95:1955–1961. doi: 10.1210/jc.2009-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rozenberg S., Di Pietrantonio V., Vandromme J., Gilles C. Menopausal hormone therapy and breast cancer risk. Best Pract. Res. Clin. Endocrinol. Metab. 2021;35:101577. doi: 10.1016/j.beem.2021.101577. [DOI] [PubMed] [Google Scholar]
- 83.Thurston R.C., Joffe H. Vasomotor symptoms and menopause: Findings from the Study of Women’s Health across the Nation. Obs. Gynecol. Clin. N. Am. 2011;38:489–501. doi: 10.1016/j.ogc.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mosca L., Barrett-Connor E., Wenger N.K. Sex/gender differences in cardiovascular disease prevention: What a difference a decade makes. Circulation. 2011;124:2145–2154. doi: 10.1161/CIRCULATIONAHA.110.968792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chambliss K.L., Yuhanna I.S., Mineo C., Liu P., German Z., Sherman T.S., Mendelsohn M.E., Anderson R.G., Shaul P.W. Estrogen receptor alpha and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ. Res. 2000;87:E44–E52. doi: 10.1161/01.RES.87.11.e44. [DOI] [PubMed] [Google Scholar]
- 86.Ghaffari S., Naderi Nabi F., Sugiyama M.G., Lee W.L. Estrogen Inhibits LDL (Low-Density Lipoprotein) Transcytosis by Human Coronary Artery Endothelial Cells via GPER (G-Protein-Coupled Estrogen Receptor) and SR-BI (Scavenger Receptor Class B Type 1) Arter. Thromb. Vasc. Biol. 2018;38:2283–2294. doi: 10.1161/ATVBAHA.118.310792. [DOI] [PubMed] [Google Scholar]
- 87.Frostegård J., Huang Y.H., Rönnelid J., Schäfer-Elinder L. Platelet-activating factor and oxidized LDL induce immune activation by a common mechanism. Arter. Thromb. Vasc. Biol. 1997;17:963–968. doi: 10.1161/01.ATV.17.5.963. [DOI] [PubMed] [Google Scholar]
- 88.Xiao H.H., Lu L., Poon C.C., Chan C.O., Wang L.J., Zhu Y.X., Zhou L.P., Cao S., Yu W.X., Wong K.Y., et al. The lignan-rich fraction from Sambucus Williamsii Hance ameliorates dyslipidemia and insulin resistance and modulates gut microbiota composition in ovariectomized rats. Biomed. Pharm. 2021;137:111372. doi: 10.1016/j.biopha.2021.111372. [DOI] [PubMed] [Google Scholar]
- 89.Hu Y., Li Y., Sampson L., Wang M., Manson J.E., Rimm E., Sun Q. Lignan Intake and Risk of Coronary Heart Disease. J. Am. Coll Cardiol. 2021;78:666–678. doi: 10.1016/j.jacc.2021.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakamura Y., Okumura H., Ono Y., Kitagawa Y., Rogi T., Shibata H. Sesame lignans reduce LDL oxidative susceptibility by downregulating the platelet-activating factor acetylhydrolase. Eur. Rev. Med. Pharm. Sci. 2020;24:2151–2161. doi: 10.26355/eurrev_202002_20395. [DOI] [PubMed] [Google Scholar]
- 91.Ren J.Y., Yin B.W., Li X., Zhu S.Q., Deng J.L., Sun Y.T., Zhang Z.A., Guo Z.H., Pei H.T., Zhang F., et al. Sesamin attenuates PM(2.5)-induced cardiovascular injury by inhibiting ferroptosis in rats. Food Funct. 2021;12:12671–12682. doi: 10.1039/D1FO02913D. [DOI] [PubMed] [Google Scholar]
- 92.Pham T.H., Jin S.W., Lee G.H., Park J.S., Kim J.Y., Thai T.N., Han E.H., Jeong H.G. Sesamin Induces Endothelial Nitric Oxide Synthase Activation via Transient Receptor Potential Vanilloid Type 1. J. Agric. Food Chem. 2020;68:3474–3484. doi: 10.1021/acs.jafc.9b07909. [DOI] [PubMed] [Google Scholar]
- 93.Kim J.M., Lin C., Stavre Z., Greenblatt M.B., Shim J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells. 2020;9:2073. doi: 10.3390/cells9092073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Udagawa N., Koide M., Nakamura M., Nakamichi Y., Yamashita T., Uehara S., Kobayashi Y., Furuya Y., Yasuda H., Fukuda C., et al. Osteoclast differentiation by RANKL and OPG signaling pathways. J. Bone Min. Metab. 2021;39:19–26. doi: 10.1007/s00774-020-01162-6. [DOI] [PubMed] [Google Scholar]
- 95.Kim M.H., Choi Y., Chung J.Y., Kim E.J., Yang W.M. Auraptene ameliorates osteoporosis by inhibiting RANKL/NFATc1 pathway-mediated bone resorption based on network pharmacology and experimental evaluation. Bone Jt. Res. 2022;11:304–316. doi: 10.1302/2046-3758.115.BJR-2021-0380.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Long F., Chen R., Su Y., Liang J., Xian Y., Yang F., Lian H., Xu J., Zhao J., Liu Q. Epoxymicheliolide inhibits osteoclastogenesis and resists OVX-induced osteoporosis by suppressing ERK1/2 and NFATc1 signaling. Int. Immunopharmacol. 2022;107:108632. doi: 10.1016/j.intimp.2022.108632. [DOI] [PubMed] [Google Scholar]
- 97.Tao H., Li W., Zhang W., Yang C., Zhang C., Liang X., Yin J., Bai J., Ge G., Zhang H., et al. Urolithin A suppresses RANKL-induced osteoclastogenesis and postmenopausal osteoporosis by, suppresses inflammation and downstream NF-κB activated pyroptosis pathways. Pharm. Res. 2021;174:105967. doi: 10.1016/j.phrs.2021.105967. [DOI] [PubMed] [Google Scholar]
- 98.Cheng C.H., Chen L.R., Chen K.H. Osteoporosis Due to Hormone Imbalance: An Overview of the Effects of Estrogen Deficiency and Glucocorticoid Overuse on Bone Turnover. Int. J. Mol. Sci. 2022;23:1376. doi: 10.3390/ijms23031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ono T., Hayashi M., Sasaki F., Nakashima T. RANKL biology: Bone metabolism, the immune system, and beyond. Inflamm. Regen. 2020;40:2. doi: 10.1186/s41232-019-0111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Choi S.W., Park K.I., Yeon J.T., Ryu B.J., Kim K.J., Kim S.H. Anti-osteoclastogenic activity of matairesinol via suppression of p38/ERK-NFATc1 signaling axis. BMC Complement. Altern. Med. 2014;14:35. doi: 10.1186/1472-6882-14-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ma Z.P., Zhang Z.F., Yang Y.F., Yang Y. Sesamin Promotes Osteoblastic Differentiation and Protects Rats from Osteoporosis. Med. Sci. Monit. 2019;25:5312–5320. doi: 10.12659/MSM.915529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Z., Feng L., Wang H., Li Y., Lo J.H.T., Zhang X., Lu X., Wang Y., Lin S., Tortorella M.D., et al. DANCR Mediates the Rescuing Effects of Sesamin on Postmenopausal Osteoporosis Treatment via Orchestrating Osteogenesis and Osteoclastogenesis. Nutrients. 2021;13:4455. doi: 10.3390/nu13124455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang Z., Feng L., Wang M., Li Y., Bai S., Lu X., Wang H., Zhang X., Wang Y., Lin S., et al. Sesamin Promotes Osteoporotic Fracture Healing by Activating Chondrogenesis and Angiogenesis Pathways. Nutrients. 2022;14:2106. doi: 10.3390/nu14102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michailidis D., Angelis A., Aligiannis N., Mitakou S., Skaltsounis L. Recovery of Sesamin, Sesamolin, and Minor Lignans From Sesame Oil Using Solid Support-Free Liquid-Liquid Extraction and Chromatography Techniques and Evaluation of Their Enzymatic Inhibition Properties. Front. Pharm. 2019;10:723. doi: 10.3389/fphar.2019.00723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang X., Liang J., Wang Z., Su Y., Zhan Y., Wu Z., Li J., Li X., Chen R., Zhao J., et al. Sesamolin Protects Mice From Ovariectomized Bone Loss by Inhibiting Osteoclastogenesis and RANKL-Mediated NF-κB and MAPK Signaling Pathways. Front. Pharm. 2021;12:664697. doi: 10.3389/fphar.2021.664697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rybaczyk L.A., Bashaw M.J., Pathak D.R., Moody S.M., Gilders R.M., Holzschu D.L. An overlooked connection: Serotonergic mediation of estrogen-related physiology and pathology. BMC Womens Health. 2005;5:12. doi: 10.1186/1472-6874-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Paredes S., Cantillo S., Candido K.D., Knezevic N.N. An Association of Serotonin with Pain Disorders and Its Modulation by Estrogens. Int. J. Mol. Sci. 2019;20:5729. doi: 10.3390/ijms20225729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang J., Mai C.L., Xiong Y., Lin Z.J., Jie Y.T., Mai J.Z., Liu C., Xie M.X., Zhou X., Liu X.G. The Causal Role of Magnesium Deficiency in the Neuroinflammation, Pain Hypersensitivity and Memory/Emotional Deficits in Ovariectomized and Aged Female Mice. J. Inflamm. Res. 2021;14:6633–6656. doi: 10.2147/JIR.S330894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Antoniuk S., Bijata M., Ponimaskin E., Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci. Biobehav. Rev. 2019;99:101–116. doi: 10.1016/j.neubiorev.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 110.Zhao Y., Wang Q., Jia M., Fu S., Pan J., Chu C., Liu X., Liu X., Liu Z. (+)-Sesamin attenuates chronic unpredictable mild stress-induced depressive-like behaviors and memory deficits via suppression of neuroinflammation. J. Nutr. Biochem. 2019;64:61–71. doi: 10.1016/j.jnutbio.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 111.Wang Q., Jia M., Zhao Y., Hui Y., Pan J., Yu H., Yan S., Dai X., Liu X., Liu Z. Supplementation of Sesamin Alleviates Stress-Induced Behavioral and Psychological Disorders via Reshaping the Gut Microbiota Structure. J. Agric. Food Chem. 2019;67:12441–12451. doi: 10.1021/acs.jafc.9b03652. [DOI] [PubMed] [Google Scholar]
- 112.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 113.Vanneman M., Dranoff G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer. 2012;12:237–251. doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 115.Velentzis L.S., Cantwell M.M., Cardwell C., Keshtgar M.R., Leathem A.J., Woodside J.V. Lignans and breast cancer risk in pre- and post-menopausal women: Meta-analyses of observational studies. Br. J. Cancer. 2009;100:1492–1498. doi: 10.1038/sj.bjc.6605003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scherbakov A.M., Stasevich O.V., Salnikova D.I., Andreeva O.E., Mikhaevich E.I. Antiestrogenic and antiproliferative potency of secoisolariciresinol diglucoside derivatives on MCF-7 breast cancer cells. Nat. Prod. Res. 2021;35:6099–6105. doi: 10.1080/14786419.2020.1826479. [DOI] [PubMed] [Google Scholar]
- 117.Argenziano M., Gigliotti C.L., Clemente N., Boggio E., Ferrara B., Trotta F., Pizzimenti S., Barrera G., Boldorini R., Bessone F., et al. Improvement in the Anti-Tumor Efficacy of Doxorubicin Nanosponges in In Vitro and in Mice Bearing Breast Tumor Models. Cancers. 2020;12:162. doi: 10.3390/cancers12010162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bowers L.W., Lineberger C.G., Ford N.A., Rossi E.L., Punjala A., Camp K.K., Kimler B.K., Fabian C.J., Hursting S.D. The flaxseed lignan secoisolariciresinol diglucoside decreases local inflammation, suppresses NFκB signaling, and inhibits mammary tumor growth. Breast Cancer Res. Treat. 2019;173:545–557. doi: 10.1007/s10549-018-5021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rattanaburee T., Tanawattanasuntorn T., Thongpanchang T., Tipmanee V., Graidist P. Trans-(-)-Kusunokinin: A Potential Anticancer Lignan Compound against HER2 in Breast Cancer Cell Lines? Molecules. 2021;26:4537. doi: 10.3390/molecules26154537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sriwiriyajan S., Sukpondma Y., Srisawat T., Madla S., Graidist P. (-)-Kusunokinin and piperloguminine from Piper nigrum: An alternative option to treat breast cancer. Biomed. Pharm. 2017;92:732–743. doi: 10.1016/j.biopha.2017.05.130. [DOI] [PubMed] [Google Scholar]
- 121.Valderrama-Treviño A.I., Barrera-Mera B., Ceballos-Villalva J.C., Montalvo-Javé E.E. Hepatic Metastasis from Colorectal Cancer. Euroasian J. Hepatogastroenterol. 2017;7:166–175. doi: 10.5005/jp-journals-10018-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burada F., Nicoli E.R., Ciurea M.E., Uscatu D.C., Ioana M., Gheonea D.I. Autophagy in colorectal cancer: An important switch from physiology to pathology. World J. Gastrointest. Oncol. 2015;7:271–284. doi: 10.4251/wjgo.v7.i11.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sain A., Kandasamy T., Naskar D. Targeting UNC-51-like kinase 1 and 2 by lignans to modulate autophagy: Possible implications in metastatic colorectal cancer. Mol. Divers. 2022. in press . [DOI] [PubMed]
- 124.Huang Y., Liu Z., Li L., Jiang M., Tang Y., Zhou L., Li J., Chen Y. Sesamin inhibits hypoxia-stimulated angiogenesis via the NF-κB p65/HIF-1α/VEGFA signaling pathway in human colorectal cancer. Food Funct. 2022;13:8989–8997. doi: 10.1039/D2FO00345G. [DOI] [PubMed] [Google Scholar]
- 125.Özgöçmen M., Bayram D., Yavuz Türel G., Toğay V.A., Şahin Calapoğlu N. Secoisolariciresinol diglucoside induces caspase-3-mediated apoptosis in monolayer and spheroid cultures of human colon carcinoma cells. J. Food Biochem. 2021;45:e13719. doi: 10.1111/jfbc.13719. [DOI] [PubMed] [Google Scholar]
- 126.Chen T., Wang Z., Zhong J., Zhang L., Zhang H., Zhang D., Xu X., Zhong X., Wang J., Li H. Secoisolariciresinol diglucoside induces pyroptosis by activating caspase-1 to cleave GSDMD in colorectal cancer cells. Drug Dev. Res. 2022;83:1152–1166. doi: 10.1002/ddr.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang L., Altuwaijri S., Deng F., Chen L., Lal P., Bhanot U.K., Korets R., Wenske S., Lilja H.G., Chang C., et al. NF-kappaB regulates androgen receptor expression and prostate cancer growth. Am. J. Pathol. 2009;175:489–499. doi: 10.2353/ajpath.2009.080727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Selvaraj D., Muthu S., Kotha S., Siddamsetty R.S., Andavar S., Jayaraman S. Syringaresinol as a novel androgen receptor antagonist against wild and mutant androgen receptors for the treatment of castration-resistant prostate cancer: Molecular docking, in-vitro and molecular dynamics study. J. Biomol. Struct. Dyn. 2021;39:621–634. doi: 10.1080/07391102.2020.1715261. [DOI] [PubMed] [Google Scholar]
- 129.Min K., Chung J.W., Ha Y.S., Lee J.N., Kim B.S., Kim H.T., Kim T.H., Yoo E.S., Kwon T.G., Chung S.K., et al. Efficacy of Androgen Deprivation Therapy in Patients with Metastatic Castration-Resistant Prostate Cancer Receiving Docetaxel-Based Chemotherapy. World J. Mens Health. 2020;38:226–235. doi: 10.5534/wjmh.190029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Meng Z., Liu H., Zhang J., Zheng Z., Wang Z., Zhang L., Jia Z., Sui Y. Sesamin promotes apoptosis and pyroptosis via autophagy to enhance antitumour effects on murine T-cell lymphoma. J. Pharm. Sci. 2021;147:260–270. doi: 10.1016/j.jphs.2021.08.001. [DOI] [PubMed] [Google Scholar]
- 131.Tannous S., Haykal T., Dhaini J., Hodroj M.H., Rizk S. The anti-cancer effect of flaxseed lignan derivatives on different acute myeloid leukemia cancer cells. Biomed. Pharm. 2020;132:110884. doi: 10.1016/j.biopha.2020.110884. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are contained within the article.