Abstract
Idiopathic pulmonary fibrosis (IPF) is a fatal interstitial lung disease characterized by fibroblast activation, excessive deposition of extracellular matrix, and progressive scarring; the pathogenesis remains elusive. The present study explored the role of Tribbles pseudokinase 3 (TRIB3), a well-known stress and metabolic sensor, in IPF. TRIB3 is down-regulated in the lungs of IPF patients in comparison to control subjects. Deficiency of TRIB3 markedly inhibited A549 epithelial cells’ proliferation and migration, significantly reducing wound healing. Conversely, overexpression of TRIB3 promoted A549 cell proliferation and transmigration while it inhibited its apoptosis. Meanwhile, overexpressed TRIB3 inhibited fibroblast activation and decreased ECM synthesis and deposition in MRC5 cells. TRIB3 attenuated pulmonary fibrosis by negative regulation of ATF4, while TRIB3 expression markedly inhibited ATF4 promoter-driven transcription activity and down-regulated ATF4 expression. A co-culture system showed that TRIB3 is important to maintain the normal epithelial–mesenchymal crosstalk and regulate fibroblast activation. Taken together, our data suggested that an axis of TRIB3–ATF4 is a key mediator in IPF which might be a potential target for fibroproliferative lung disease treatment.
Keywords: idiopathic pulmonary fibrosis, TRIB3, ATF4, epithelial cell, fibroblast activation
1. Introduction
Idiopathic pulmonary fibrosis (IPF), one of the most common forms of interstitial lung disease (ILD), is a chronic, progressive, and usually lethal lung disease of unknown etiology and refractory to current therapeutic options [1]. The main pathological features of IPF include epithelial injury [2], the recruitment of inflammatory cells [3,4], the aberrant differentiation and proliferation of fibroblasts [5], and the persistence of apoptosis-resistant myofibroblasts [6] in fibrotic lesions. Resident lung fibroblasts-derived myofibroblasts are the major contributors to the processes of ECM deposition and tissue distortion in IPF. Stimulation with fibrotic factors, such as including transforming growth factor beta (TGF-β), platelet-derived growth factor (PDGF), and connective tissue growth factor (CTGF), promote fibroblast recruitment and myofibroblast activation, which are characterized by a spindle or stellate morphology with α-smooth muscle actin (α-SMA) stress fibers coupled with a hypersecretion phenotype, due to which they produce copious amounts of fibrillary extracellular matrix (ECM) proteins, such as collagen and fibronectin. Dysregulated crosstalk between the epithelium and the mesenchymal cells regulates lung development and homeostatic equilibrium [7] and further forms a profibrotic milieu and hampers the normal alveolar wound-repair process [8,9,10,11]. Until now, the molecular mechanisms by which alveolar epithelial cells (AECs) become activated and communicate with the fibroblast lead to fibrosis are not fully understood.
Tribbles pseudokinase 3 (TRIB3, also known as TRB3, NIPK, and SKIP3) is the mammalian homolog of Drosophila Tribbles [12]. TRIB3 is a pseudokinase, which contains a Ser/Thr protein kinase-like domain, but lacks the ATP-binding pocket and catalytic residues [13]. TRIB3 can serve as a molecular scaffold for the assembly of coactivator and corepressor complexes [14] and has been demonstrated to interact with several transcriptional mediators such as CCAAT-enhancer-binding protein homologous protein (CHOP), peroxisome proliferator-activated receptor alpha (PPARα), and activating transcription factor 4 (ATF4) [15,16]. Through those interactions, TRIB3 coordinates crucial cellular processes such as glucose and lipid metabolism, adipocyte differentiation, or apoptosis [15,16]. Alterations in TRIB3 gene expression have also recently been linked to numerous chronic diseases, including diabetes, hepatitis, and tissue fibrosis [17,18,19,20]. Previous study demonstrated that TRIB3 interacting with ATF4 inhibits its transcription activity [21,22,23], participating in regulating insulin exocytosis in human and mouse beta cells [21] and Parkinson’s disease process [24]. Elevated TRIB3 expression was observed in alveolar macrophages (AMs) from bleomycin challenged fibrotic mice, which contributes to pulmonary fibrosis (PF) [25].
Herein, we found that TRIB3 expression was down-regulated in the lungs of IPF and negatively associated with profibrotic gene expression. TRIB3 can enhance epithelial cell migration and inhibit lung fibroblast activation in vitro. The expression of TRIB3 was negatively correlated with disease severity in IPF patients. TRIB3 expression negatively regulated ATF4 expression, which promoted fibroblast proliferation, migration, and activation contributing to pulmonary fibrosis. These findings suggested that the TRIB3–ATF4 axis is a key mediator in IPF, which may provide novel insights into the pathogenesis of IPF.
2. Results
2.1. TRIB3 Expression Was Downregulated in the Lung Tissue of Patients with IPF
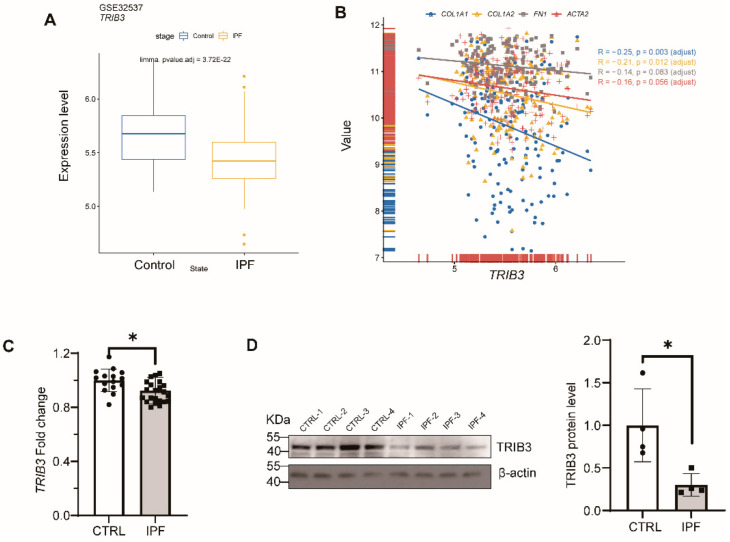
To verify expression of TRIB3, we initially evaluated the expression of TRIB3 in healthy and IPF lungs to properly describe human disease-relevant alterations from the Gene Expression Omnibus (GEO) RNA-seq dataset (GSE32537) and found the TRIB3 expression was significantly down-regulated in IPF patients in comparison to normal donors (Figure 1A). TRIB3 expression was negatively correlated with the expressions of COL1A1, COL1A2, FN1, and ACTA2 in the RNA-seq dataset (GSE32537) by Spearson correlation coefficient (Figure 1B). This significant decrease in the TRIB3 transcript level was validated by the qPCR (Figure 1C). Consistently, western blotting showed that TRIB3 protein levels in the lung tissue of patients with IPF dramatically decreased in comparison to normal lung tissues (Figure 1D). These findings exhibited that TRIB3 was stably repressed in IPF and negatively associated with pulmonary fibrosis markers.
Figure 1.
Down-regulation of TRIB3 in IPF lung. (A) TRIB3 was down-regulated in IPF patients, which was profoundly validated from the Gene Expression Omnibus (GEO) RNA-seq dataset (GSE32537). Statistical analysis of the microarray was performed by R package “limma”. (B) TRIB3 was negatively correlated with COL1A1, COL1A2, FN1, and ACTA2. Data were obtained from GSE32537 (Control = 50, IPF = 119) in the public database Gene Expression Omnibus (GEO). Spearson rank analysis was used to analyze the correlation between TRIB3 and FN1, ACTA2, COL1A1, and COL1A2 expression. (C) TRIB3 was down-regulated from patients with IPF in transcription level. The statistical test used was the Mann–Whitney U test for comparisons between two groups. * p < 0.05. (D) Western blot analysis showed reduced TRIB3 expression in IPF lungs (N = 4) compared with healthy lungs (N = 4). The statistical test used was the Mann–Whitney U test for comparisons between two groups. * p < 0.05.
2.2. TRIB3 Was Essential for Wound Healing in Epithelial Cells
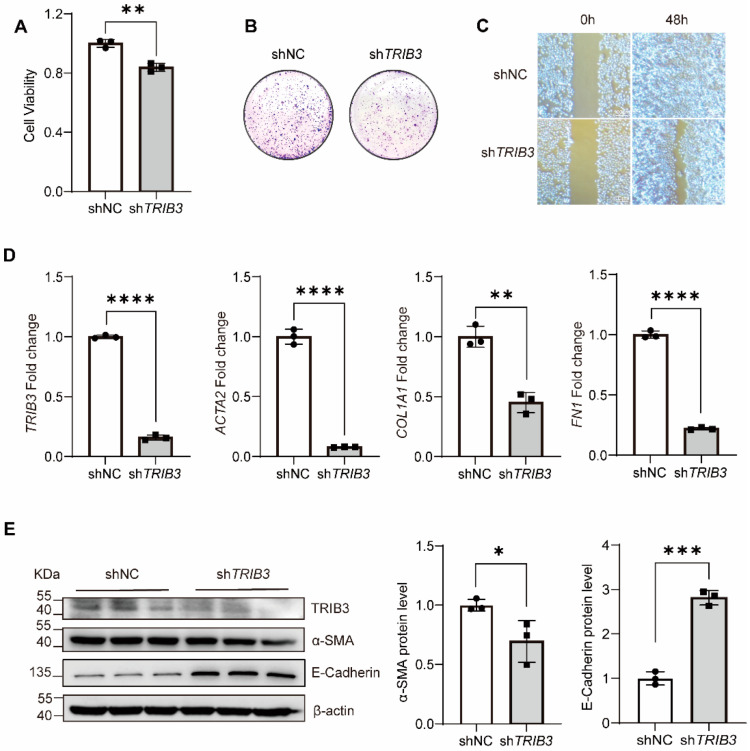
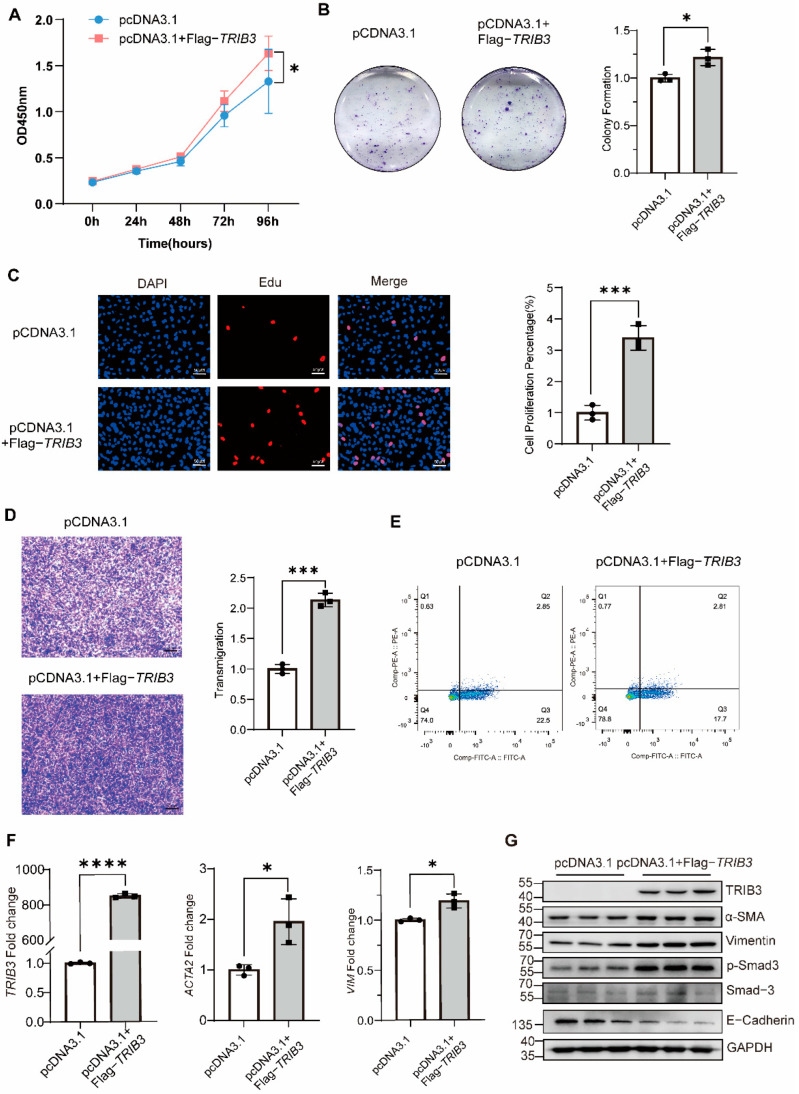
To determine the role of TRIB3 in epithelial cells, we examined the impact of TRIB3 deficiency on the proliferation and migration of A549 cells. Knockdown of TRIB3 by shRNA in the epithelial cells significantly inhibited A549 cells’ proliferation (Figure 2A,B). Moreover, TRIB3 knockdown significantly reduced wound healing after 48 h, indicating TRIB3 suppressed the epithelial cell migration (Figure 2C). In addition, TRIB3 knockdown inhibited profibrotic gene transcription (Figure 2D). Silencing of TRIB3 reduced the protein level of α-SMA, while it enhanced the E-Cadherin expression (Figure 2E). Conversely, overexpression of TRIB3 in A549 cells increased the proliferation and transmigration of A549 cells, while it inhibited apoptosis of A549 cells (Figure 3A–E), and TRIB3 overexpression promoted ACTA2 and VIM transcriptions and protein levels of α-SMA, VIM, and p-SMAD3, as well as inhibiting the expression of E-Cadherin (Figure 3F,G). These observations, taken together, suggested that TRIB3 is crucial in modulating epithelial cell proliferation, migration, and apoptosis.
Figure 2.
TRIB3 deficiency inhibited A549 cell proliferation, migration, and profibrotic gene expressions. (A) The effect of TRIB3 silencing on the viability of A549 cells was assessed by CCK8 assay. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. ** p < 0.01. (B) The effect of TRIB3 silencing on the colony formation of A549 cells (N = 3 independent experiments). (C) TRIB3-shRNA inhibited the migration of A549 cells. (D) Gene expression analysis by quantitative PCR (qPCR) demonstrates a significant decrease in ACTA2, COL1A1, and FN1 transcript levels in TRIB3-silenced normal epithelial cells. GAPDH was used as an internal control. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. ** p < 0.01, **** p < 0.0001. (E) TRIB3-shRNA decreased the protein level of TRIB3, α-SMA, E-Cadherin, and β-actin (N = 3 independent experiments). β-actin was used as an internal control. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. * p < 0.05 and *** p < 0.001.
Figure 3.
TRIB3 expression promoted A549 cell proliferation, migration, and profibrotic genes expression. (A) The effect of TRIB3 overexpression on the viability of A549 cells was assessed by CCK8 assay (N = 4 independent experiments). Significant differences between groups were evaluated by two-way ANOVA with Šídák’s multiple comparisons test for pairwise comparisons. * p < 0.05. (B) The effect of TRIB3 overexpression on the colony formation of A549 cells (N = 3 independent experiments). The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. * p < 0.05. (C) The EdU proliferation assay was used to measure A549 cell proliferation. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. *** p < 0.001. (D) Crystal violet staining of A549 cell is shown for the transwell assay (N = 3 independent experiments). The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. *** p < 0.001. (E) Cell apoptosis measured by cytometry, with quantification analysis (N = 3 independent experiments). (F) Gene expression analysis by qPCR demonstrated a significant increase in TRIB3, ACTA2, and VIM transcript levels in TRIB3-overexpressed normal epithelial cells. GAPDH was used as an internal control. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. * p < 0.05 and **** p < 0.0001 vs. control. (G) Western blot analysis showed that the expression of α-SMA, vimentin, E-Cadherin, and p-Smad3 in A549 cells increased after overexpressing TRIB3 (N = 3 independent experiments). For western blot, GAPDH was used as an internal control.
2.3. TRIB3 Overexpression Inhibited the Fibroblast Activation
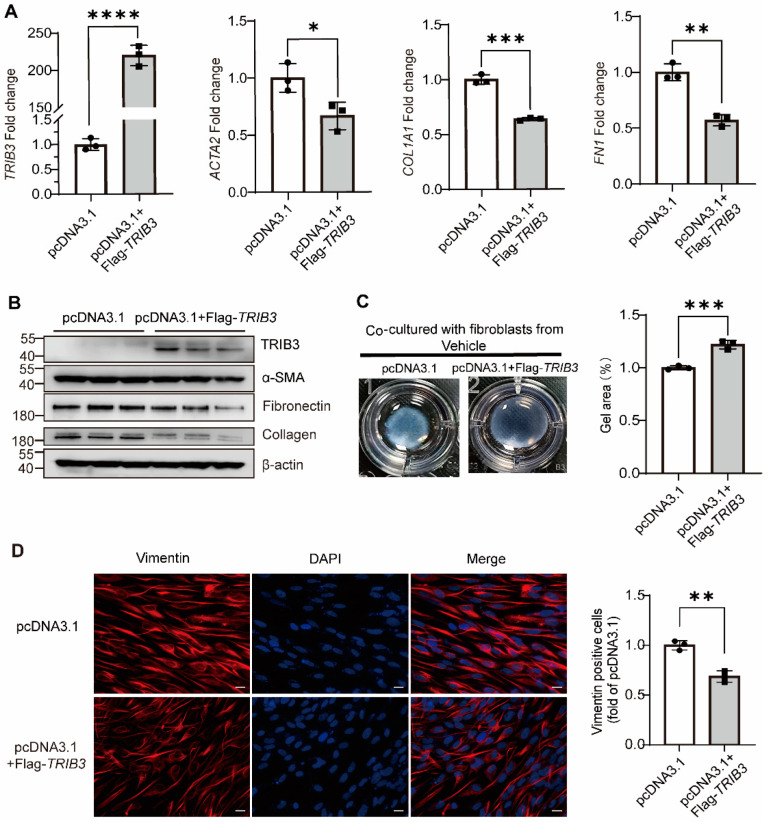
To further understand more about how TRIB3 could be involved in the development of pulmonary fibrosis in vitro, we overexpressed TRIB3 in MRC5 cells for 48 h and demonstrated that TRIB3 significantly decreased the profibrotic genes expression, including ACTA2, COL1A1, and FN1 at the mRNA level and the protein level of collagen I, fibronectin, and α-SMA in MRC5 cells (Figure 4A,B). The fibroblast-induced collagen gel contraction (CGC) assay demonstrated that TRIB3 overexpression inhibited the fibroblast contractility (Figure 4C) and the expression of vimentin, a marker for epithelial–mesenchymal transition (EMT) (Figure 4D). Taken together, our findings showed that increased TRIB3 expression may assist in reducing lung fibrosis in vitro.
Figure 4.
TRIB3 inhibits lung fibroblast activation. (A) Gene expression analysis by qPCR demonstrates a significant decrease in ACTA2, COL1A1, and FN1 transcript levels in TRIB3-overexpressing normal lung fibroblasts. GAPDH was used as an internal control. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001, and **** p < 0.0001. (B) Western blot analysis showed αSMA, collagen I, and fibronectin elevation in TRIB3-overexpressed lung fibroblasts (N = 3 independent experiments). β-actin was used as an internal control. (C) The activation of fibroblasts was examined based on fibroblast contraction in 3D collagen matrices (N = 3 independent experiments). We used ImageJ software to measure the area of gels. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. *** p < 0.001. (D) MRC5 cells were subjected to immunofluorescence with a vimentin antibody. Scale bars, 20 μm. ImageJ software was used to quantify the fluorescence intensity. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. ** p < 0.01.
2.4. TRIB3 Interaction with ATF4 Mediated Lung Fibroblast Activation
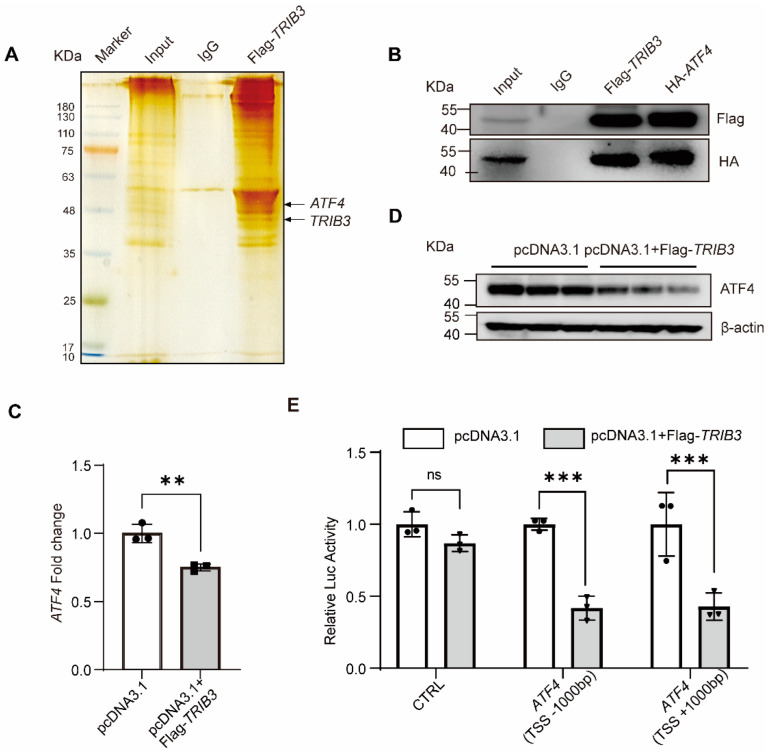
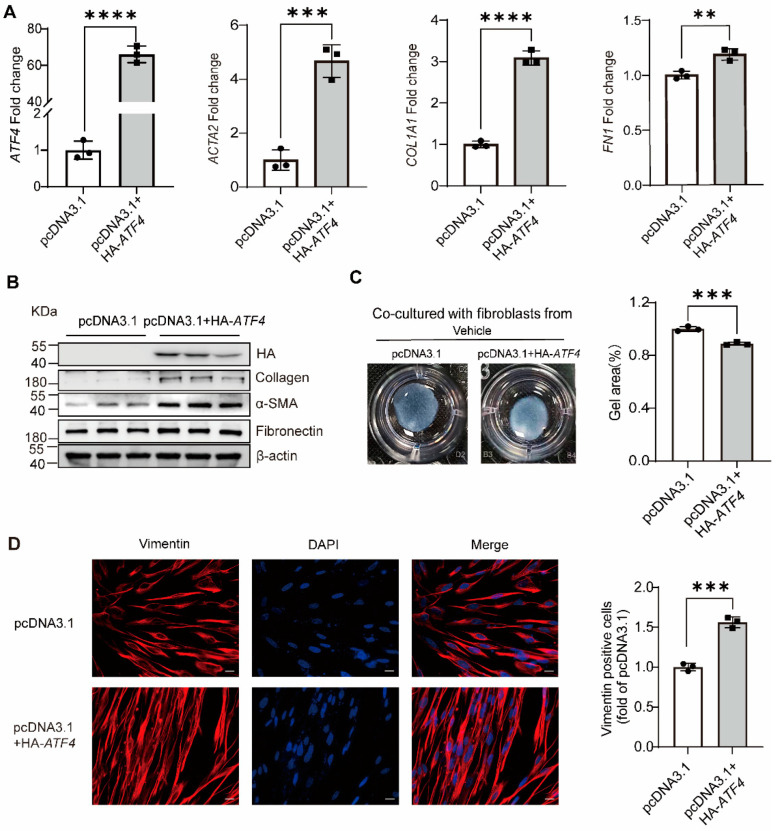
To investigate the molecular mechanisms of TRIB3 attenuating lung fibrosis, we postulated that TRIB3 plays a momentous role in IPF through regulation of ATF4. As shown in Figure 5A, immunoprecipitation and silver staining assay demonstrated that TRIB3 directly interacts with ATF4 in MRC5 cells. To further demonstrate this relationship, we examined the above associations using co-immunoprecipitation and western blotting and found that TRIB3 and ATF4 co-immunoprecipitated (Figure 5B). We also found that TRIB3 overexpression inhibited ATF4 expression at RNA and protein levels in MRC5 cells (Figure 5C,D). In addition, to further verify whether TRIB3 affects the transcription of ATF4, we established two luciferase-reporter vectors, including ATF4 transcription start sites (TSSs) (−1000 bp to +1000 bp), as described in Methods. Overexpression of TRIB3 markedly inhibited ATF4 promoter-driven luciferase activity (Figure 5E). We next tested whether ATF4 overexpression influences fibroblast activation and found that overexpression of ATF4 promoted ACTA2, COL1A1, and FN1 transcriptional activity (Figure 6A). Western blotting confirmed that ATF4 overexpression promotes ECM deposition and increases the proliferation and contraction of fibroblasts (Figure 6B,C), details as described in Methods. Furthermore, immunofluorescence staining showed that ATF4 overexpression increased the vimentin expression (Figure 6D). Altogether, these findings indicate that the enhanced expression of TRIB3 influences lung fibroblast activation by regulation of ATF4 expression.
Figure 5.
TRIB3 interacts with ATF4 and negatively regulates ATF4. (A) Immunoprecipitation for TRIB3 in MRC5 cells. TRIB3 interacts with ATF4 by silver staining. (B) Co-IP of flag-tagged TRIB3 and HA-tagged ATF4 protein from the transfected cell lysates. (C,D) Overexpression of TRIB3 decreased the expression of ATF4 both at RNA and protein levels (N = 3 independent experiments). GAPDH was used as an internal control for qPCR, and β-actin was used as an internal control for western blot. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. ** p < 0.01. (E) Relative expression of ATF4 promoter-driven luciferase reporters in TRIB3-overexpressing cells (N = 3 independent experiments). Results were normalized to Renilla luciferase activity and expressed as relative luciferase units. Significant differences between groups were evaluated by two-way ANOVA with Šídák’s multiple comparisons test for pairwise comparisons. *** p < 0.001. ns, no significance.
Figure 6.
ATF4 promotes lung fibroblast activation. (A) qPCR analysis of ATF4-overexpressing normal fibroblasts (pcDNA3.1+HA-ATF4) shows enhanced profibrotic transcript levels compared with normal lung fibroblasts transfected with an empty vector plasmid. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. ** p < 0.01, *** p < 0.001, and **** p < 0.0001. (B) HA-tagged ATF4, collagen I, α-SMA, fibronectin, and β-actin proteins were detected by western blot (N = 3 independent experiments). (C) The activation of fibroblasts was examined based on fibroblast contraction in 3D collagen matrices (N = 3 independent experiments). We used ImageJ software to measure the area of gels. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. *** p < 0.001. (D) MRC5 cells were subjected to immunofluorescence with a vimentin antibody. Scale bars, 20 μm. ImageJ software was used to quantify the fluorescence intensity. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. *** p < 0.001.
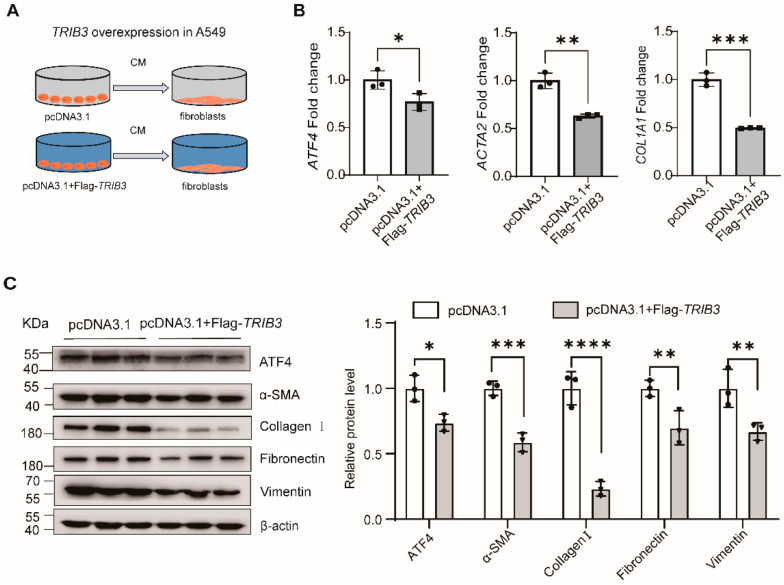
2.5. TRIB3 Maintained Epithelial-Mesenchymal Crosstalk and Attenuated Fibrosis by Negatively Regulation of ATF4
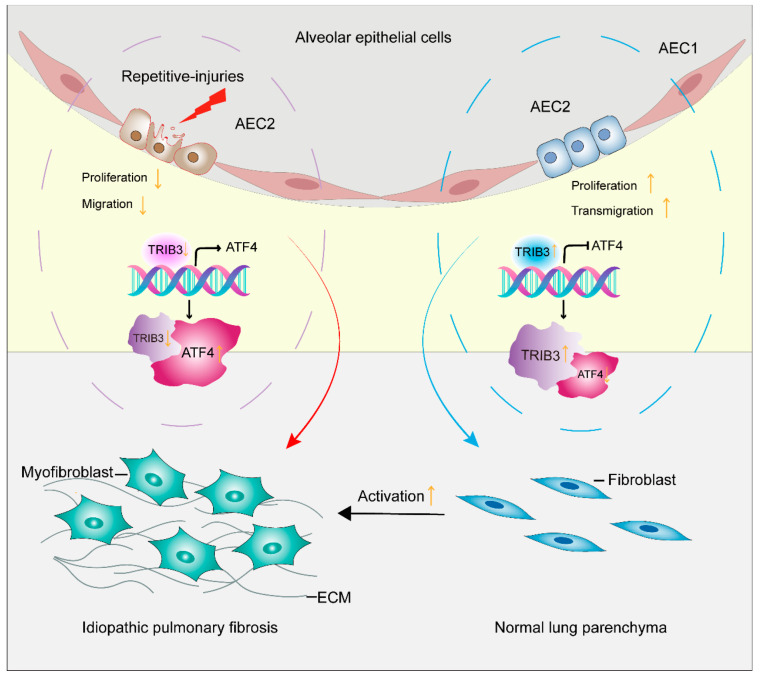
To evaluate whether alteration of TRIB3 expression in epithelial cells regulates the fibroblast activation through down-regulation of ATF4 expression, a co-culture system was established and the conditioned media (CM) were collected from A549 cells, for which TRIB3 was overexpressed, for fibroblast MRC5 cells culture (Figure 7A). Interestingly, this CM not only suppressed the ATF4 expression but also inhibited the profibrotic gene expressions in lung fibroblasts at both RNA and protein levels (Figure 7B,C). Putting it all together, these findings indicate that TRIB3 plays a pivotal role in maintaining normal epithelial–mesenchymal crosstalk and negatively attenuating fibrosis through interaction with ATF4 (Figure 8).
Figure 7.
TRIB3 attenuates lung fibrosis by negative regulation of ATF4. (A) Schematic of transfection of A549 cells before CM collection and transfer. CM from pcDNA3.1 or pcDNA3.1+Flag-TRIB3-transfected cells was collected 72 h after transfection and transferred to human lung fibroblast (MRC5) for an additional 72 h. (B) qPCR analysis showing that CM from pcDNA3.1+ Flag-TRIB3-transfected cells reduced the mRNA level of ATF4 and profibrotic genes in MRC5 cells. The results were analyzed by the unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD. * p < 0.05, ** p < 0.01 and *** p < 0.001. (C) Western blot analysis showing CM from pcDNA3.1+ Flag-TRIB3-transfected cells reduced the expression of ATF4, α-SMA, Collagen I, Fibronectin, and vimentin. Significant differences between groups were evaluated by two-way ANOVA with Šídák’s multiple comparisons test for pairwise comparisons. * p < 0.05, *** p < 0.001 and **** p < 0.0001.
Figure 8.
Antifibrotic mechanism of TRIB3 mediation of pulmonary fibrosis through inhibition of lung fibroblast activation. TRIB3 inhibits the transformation of pulmonary fibroblasts into myofibroblasts through interaction with ATF4 and inhibiting its expression.
3. Discussion
Increasing studies suggest that AECs injury predominately contributes to the development and progression of IPF. Dysfunction of AECs is considered to be involved in injury-impairment, an early event and cascade to fibroblast response in IPF. In the present study, we demonstrated that TRIB3 contributes to the A549 epithelial cells’ proliferation and migration and repair; TRIB3 attenuates pulmonary fibrosis by interacting with ATF4. TRIB3 is associated with the epithelial–mesenchymal crosstalk and regulates fibroblast activation. We provide a potential therapy strategy for fibroproliferative lung disease.
Little is known about the regulation of TRIB3 expression in IPF. A growing body of evidence suggests that TRIB3 is involved in the regulation of various biological processes that are relevant to cancers [26,27,28,29]. For instance, elevated TRIB3 links stress signals to induce breast cancer initiation and progression by supporting breast cancer stemness, and elevated TRIB3 is positively associated with epidermal growth factor receptor (EGFR) stability and non-small cell lung cancer (NSCLC) progression [26,29]. To wonder whether TRIB3 regulates IPF development through regulating EMT process, we altered TRIB3 expression in AEC2s and tested the EMT markers. In this work, we demonstrated that the down-regulated TRIB3 suppressed wound healing in epithelial cells, while up-regulation of TRIB3 promoted epithelial cell proliferation and migration. Meanwhile, blockage of TRIB3 inhibited the expression of α-SMA, conversely, TRIB3 overexpression increased the expression of α-SMA, vimentin, and p-Smad3 in epithelial cells. Hence, consistent with our initial hypothesis that TRIB3 may play an important role in epithelial cell migration by involvement of EMT and the down-regulation of TRIB3 in IPF, it may be detrimental to the repair of epithelial cells after injury. Furthermore, overexpressed TRIB3 in lung fibroblasts reduced α-SMA, vimentin, collagen I, and fibronectin expression, which are fibrotic markers and decreased ECM deposition. The co-culture system showed that overexpressed TRIB3 evidently inhibited fibroblast activation and ECM deposition, which is similar to overexpression of TRIB3 in fibroblasts. Previous study demonstrated that TRIB3 overexpression in AMs increased fibroblast activation [25]; our findings showed that TRIB3 may play different roles in various cells.
ATF4 is a basic leucine zipper transcription factor best known to be induced by stress responses. ATF4 has long been considered a profibrotic molecule because it enhances fibroblast proliferation, collagen synthesis, migration, and differentiation to myofibroblasts and induces fibroblasts’ apoptosis in tissue fibrosis [30,31,32,33]. Despite being less studied in lung disease, ATF4 was a profibrotic role in lung fibroblast activation and renal tubulointerstitial fibrosis [29,30]. For instance, ATF4 is an important regulator of metabolic reprogramming in myofibroblasts [29]. In fact, TRIB3 is a target of CHOP/ATF4; when up-regulating ATF4 expression, the level of TRIB3 was markedly increased [16,34]. Several studies have shown that TRIB3 interacts with ATF4 and inhibits ATF4 transcription activity [21,22,23]. To further explore the mechanism of TRIB3 attenuating fibroblast activation. We also demonstrated that TRIB3 interacts with ATF4 and inhibits ATF4 expression both at RNA and protein levels in a lung fibroblast and co-culture system through a negative feedback mechanism. Putting together, expression of TRIB3 by the epithelium is essential to recovery of the injured epithelium and suppression of mesenchymal cell activation by interaction with ATF4 and regulating its expression through a negatively regulated loop.
Even with our observations that TRIB3 regulation occurs in epithelium and fibroblast, we do acknowledge that our study has some limitations: whether TRIB3 mediated ATF4 signaling regulates EMT and fibrosis in IPF remains to be established in vivo. The possibility that TRIB3 functions through another signaling pathway in addition to the ATF4 signal in IPF has not been established yet. The underlying mechanism of TRIB3 in TGF-β-induced EMT is not fully unveiled and deserves further investigation. The role of TRIB3 in lung fibroblasts derived from IPF and its contribution to the progression of IPF requires an in-depth exploration in the future.
In conclusion, the key finding of our study is that TRIB3 promoted epithelial cell proliferation, migration, EMT, and attenuated lung fibroblasts’ abnormal activation. TRIB3 inhibited lung fibroblast activation by regulating ATF4 expression. Put together, these findings provide novel insights into the pathogenesis of the IPF and TRIB3–ATF4 axis, which might be an antifibrotic target for IPF management.
4. Material and Methods
4.1. Lung Tissue Sampling
Lung tissue samples for RT-qPCR and western blot analysis were obtained from the Henan Provincial Chest Hospital. Those included 23 lungs from patients with IPF and 15 normal lung histology samples from control (CTRL) subjects. The IPF samples were surgical remnants of biopsies or lungs explanted from patients with IPF undergoing pulmonary transplant. Controls were normal histology tissues obtained from normal disease-free margins of lung cancer resection specimens. IPF was diagnosed based on ATS/ERS/JRS/ALAT Clinical Practice Guidelines [35]. The clinical characteristics of all patients are summarized in Supporting Information Table S1. All studies were approved by the Henan Provincial Chest Hospital Medical Research Ethics Committee (No. 2020-03-06). The research conformed to the principles of the Declaration of Helsinki. Oral and written informed consent was obtained from all patients.
4.2. Cell Culture
A549 cells, MRC5 cells, and HEK293T cells were cultured in DME/F-12 and DMEM at 37 °C and 5% CO2. The medium was supplemented with 10% fetal bovine serum (FBS) 100 units/mL penicillin, 100μg/mL streptomycin, and 1 mM sodium pyruvate.
4.3. Plasmid Construction, Lentivirus Package and Stable-Infected Cell Lines Construction
Flag-tagged TRIB3 and HA-tagged ATF4 were cloned into pcDNA3.1 vector by a standard subcloning procedure. Silencing TRIB3 was achieved by targeting the sequences 5′ GATCTCAAGCTGTGTCGCTTT 3′ (shTRIB3) in the pLKO.1 vector. Concentrate lentivirus particles were used to infect sub-confluent cultures in the presence of 5 μg/mL polybrene overnight. Twenty-four hours post-transfection, cells were selected in media containing 2 μg/mL puromycin. Details for primers are provided in Table 1.
Table 1.
Primers used in the study.
| Gene | Primer | Sequence 5′-3′ |
|---|---|---|
| Flag-TRIB3 | Sense | CGCGGATCCGCCACCATGGATTACAAGGATGA |
| CGACGATAAGCGAGCCACCCCTCTGGCTGCT | ||
| Anti-sense | CCGCTCGAGCTAGCCATACAGAACCACTTCTC | |
| TGTCTCCCTCCTCTTCCCTG | ||
| HA-ATF4 | Sense | CGCGGATCCGCCACCATGACCCATACGACGTC |
| CCAGACTACGCTACCGAAATGAGCTTCCTGAG | ||
| Anti-sense | CCGCTCGAGCTAGGGGACCCTTTTCTTCCCCC | |
| TTGCCTTGCGGACCTCTTCT | ||
| shTRIB3 | Sense | CCGGGATCTCAAGCTGTGTCGCTTTCTCGAGA |
| AAGCGACACAGCTTGAGATCTTTTTG | ||
| Anti-sense | AATTCAAAAAGATCTCAAGCTGTGTCGCTTTCT | |
| CGAG AAAGCGACACAGCTTGAGATC | ||
| ATF4 (TSS-1000 bp to +100 bp) |
Anti-sense | CGGGGTACCTTCTGTGGCAGCCTTGCACTTGAG |
| CCGGATGAAAATTGTAAAAACCC | ||
| Sense | CCCAAGCTTCCCCTAATACGCCATGGTGGCCG | |
| TGGACCCTGAGGGCGGGGAGGAGG | ||
| ATF4 (TSS-100 bp to +1000 bp) |
Sense | CGGGGTACCCGGGAGGAGACGGTCACGTGGT |
| CGCGGCGGAAGGATGCGTCTGTGCT | ||
| Anti-sense | CCCAAGCTTCCTCCAGGTAATCATCTAAGAG | |
| ACCTAGGCTTTCTTCAGCCCCCAAA |
4.4. Analysis of Cell Survival and Apoptosis
Cell proliferation was measured by the CCK8 assay. Cells were plated into 96-well plates, and cell viability was detected by the CCK8 reagent purchased from the APExBLO company. Cells at a density of 2000 cells/well were plated in 6-well plates. After culturing for 14 days, the generated colonies were fixed with methanol for 30 min, stained with a hematoxylin solution, and photographed using a microscope. An Apollo567 in vitro Imaging Kit was purchased from RiboBio Corporation (Guangzhou, China) and was applied for the EdU incorporation assay. The apoptosis kit for TRIB3-overexpressed cells’ apoptosis detection is from Solarbio (CA1020). Annexin V and propidium iodide (PI) were used for labeling to assess cell apoptosis.
4.5. Migration and Wound Healing Assays
Cell migration and invasion were determined using a transwell assay. Briefly, cells were resuspended in serum-free DME/F12 and plated in the upper chamber of transwell inserts coated without BD Matrigel, and the lower chambers were filled with DME/F12 containing FBS (10%). The migrated cells were stained, imaged, and analyzed by calculating the cell numbers from five random fields. Cells were plated and grown into a confluent monolayer in six-well plates. Scratches were then generated using a pipette tip. After wounding, the cell migration process was visualized using a microscope at 0 and 48 h.
4.6. Real-Time PCR
Total RNA was extracted using Trizol reagent followed by Nanodrop concentration and purity analysis. cDNA was synthesized by M-MLV Reverse Transcriptase (Promega Corporation, Beijing, China (M1708)). RT-qPCR was conducted using a SYBR green kit (Yeasen Biotechnology, Shanghai, China (11201ES03)) according to the manufacturer’s instructions. Each sample was tested in triplicate. GAPDH was used for normalization. The data were evaluated by the 2−ΔΔCt method. RT-qPCR primers used are as described in Table 2.
Table 2.
Primers used in the study (qPCR).
| Gene | Primer | Sequence 5′-3′ |
|---|---|---|
| TRIB3 | Sense | GCGGTTGGAGTTGGATGA |
| Anti-sense | GCCACAGCAGTTGCACGA | |
| ATF4 | Sense | TCAAACCTCATGGGTTCTCC |
| Anti-sense | GTGTCATCCAACGTGGTCAG | |
| ACTA2 | Sense | CTCTGGACGCACAACTGGCATC |
| Anti-sense | CACGCTCAGCAGTAGTAACGAAGG | |
| COL1A1 | Sense | GAGGGCCAAGACGAAGACATC |
| Anti-sense | CAGATCACGTCATCGCACAAC | |
| FN1 | Sense | ACAACACCGAGGTGACTGAGAC |
| Anti-sense | GGACACAACGATGCTTCCTGAG | |
| VIM | Sense | TTGCCGTTGAAGCTGCTAACTACC |
| Anti-sense | AATCCTGCTCTCCTCGCCTTCC | |
| ACTB | Sense | CACCATTGGCAATGAGCGGTTC |
| Anti-sense | AGGTCTTTGCGGATGTCCACGT | |
| GAPDH | Sense | GTCTCCTCTGACTTCAACAGCG |
| Anti-sense | ACCACCCTGTTGCTGTAGCCAA |
4.7. Western Blotting
Proteins were obtained from cell lysates in lysis buffer and were used for protein quantification. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride membranes, and subjected to immunoprobing with specific antibodies. A chemiluminescence reagent kit purchased from Thermo Fisher Scientific company was used to detect the proteins. Images were obtained by the imager station (Gene Company Limited, Shanghai, China).
4.8. Isolation of Conditioned Medium (CM)
The medium was replaced 6 h after transfections and cells were incubated for 72 h. CM was collected, centrifuged, and immediately used for recipient cells incubation (72 h) or stored at −20 °C for later use.
4.9. Co-Immunoprecipitation (Co-IP) and Immunoprecipitation (IP) Analysis
Co-IP analysis was made as described [4] on 293T cells. The following antibodies were used: anti-ATF4 (Proteintech Group, Wuhan, China (10835-1-AP)), anti-Flag (Sigma, Shanghai, China (F1804)), and anti-HA (Sigma, Shanghai, China (H9658)). For Flag–TRIB3 and HA–ATF4 co-immunoprecipitation, we transfected the above two plasmids into 293T cells at the same time. After transfecting 48 h, cells were lysed with the Co-IP lysis buffer [150 mmol/L NaCl, 25 mmol/L Tris-HCI (pH 7.4), 2.5 mmol/L MgCl2, 0.5% NP-40, 0.5 mmol/L EDTA, 5% glycerol], the lysate was incubated with the specific antibodies at 4 °C overnight, and then with Protein A/G Plus-Agarose at 4 °C for 4 h. Interaction complexes were detached from the beads by boiling for 70 °C, 15 min, followed by SDS–PAGE and immunoblot analysis. We also did the IP analysis in MRC5 cells. We only transfected the Flag-TRIB3 plasmid in the MRC5 cells, and collect the cells also after transfecting 48 h, then repeat the above steps. TRIB3–ATF4 interaction in MRC5 cells was proved by silver staining. For silver staining, samples were run on SDS–PAGE gel and stained with a SilverQuest Silver Staining kit (thermo, Shanghai, China (WL 338261)) according to the manufacturer’s instructions.
4.10. Luciferase Reporter Analysis
Whether there was a direct target between the ATF4′ promoter and TRIB3 was confirmed by luciferase reporter gene assay. HEK293T cells were seeded in 24-well plates 24 h before transfection. Sequences of ATF4-TSSs (−1000 bp to +100 bp and −100 bp to +1000 bp) were inserted into the pGL3.0 luciferase reporter vector. Details for primers are provided in Table 2. To assess ATF4 transcription, TK plasmid, Flag-TRIB3 plasmid, and pGL3.0-ATF4 luciferase reporter plasmids were transfected into HEK293T cells. Luciferase activity was measured at 48 h post transfection with a Dual-Luciferase assay system as instruction described. The Dual-Luciferase Reporter kit (Yeasen Biotechnology, Shanghai, China (11402ES80)) was used to detect luciferase activity.
4.11. Collagen Gel Contraction (CGC) Assay
In order to detect the proliferation ability and contractility of fibroblasts, we designed the collagen gel contraction (CGC) assay. We transfected the Flag-TRIB3 (Figure 4C) or HA-ATF4 (Figure 6C) in the MRC5 cells. Then, 48 h after plasmid transfection, we performed the CGC assay to test the lung fibroblast activation. Transfected MRC5 cells were suspended in a serum-free medium and blended with 3 mg/mL neutralized rat tail type I collagen (ratio 2:1) after transfecting. Subsequently, the mixture was seeded in 24-well plates at 1 × 105/mL cell density. After collagen coagulating for 1 h at 37 °C, the gel edge was detached from well walls, and 1 mL 10% FBS containing culture medium was added to the gels. The incubation time depends on cell viability. Finally, the gel images were captured and then analyzed by ImageJ software. The gel area reflects the cell proliferation ability and contractility. The smaller the area of gel, the greater the cell contractility.
4.12. Gene Expression Data
Differentially expressed genes were determined by comparing IPF group to control groups using the “limma” package. The process of calculating Spearman correlation coefficient is: the variables X and Y are sorted from smallest to largest respectively, represented by the rank RX and RY; when the rank is the same due to value equality, the average rank is taken as the rank of each variable. To establish a test hypothesis, determine inspection level H0: ρ = 0; there is a correlation relationship between genes. If H1: ρ ≠ 0, there is no correlation between genes based on Benjamini and Hochberg control false positive rate. After correcting, the p value less than 0.1, H0 was rejected and H1 was accepted, and a correlation between the genes was constructed [36].
4.13. Statistical Analysis
The statistical methods used in the manuscript are summarized as follows. Statistical analysis of microarray (GSE32537) was performed by R package “limma”. Spearson rank analysis was used to analyze the correlation between TRIB3 and FN1, ACTA2, COL1A1, and COL1A2 expression. Multiple comparisons were addressed using the BH method. All experiments were carried out at least three times. The results in the control and experimental groups were analyzed by GraphPad software 9.0. The Shapiro–Wilk normality test was used to test normal distribution. The results were analyzed by the Mann–Whitney U test for comparisons between two groups when sample data were not normally distributed and by unpaired Student’s t-test for comparisons between two groups with normal distribution. Data are presented as mean ± SD and were considered statistically significant at p < 0.05. In particular, in Figure 3A, Figure 5E, and Figure 7C, significant differences between groups were evaluated by two-way ANOVA with Šídák’s multiple comparisons test for pairwise comparisons.
Abbreviations
| AECs | alveolar epithelial cells; |
| AEC1s | alveolar epithelial type 1 cells; |
| AEC2s | alveolar epithelial type 2 cells; |
| AMs | alveolar macrophages; |
| ILD | interstitial lung disease; |
| IPF | idiopathic pulmonary fibrosis; |
| PF | pulmonary fibrosis; |
| PDGF | platelet-derived growth factor; |
| CTGF | connective tissue growth factor; |
| CHOP | CCAAT-enhancer-binding protein homologous protein; |
| PPARα | peroxisome proliferator-activated receptor alpha; |
| TGF-β | transforming growth factor-β; |
| EGFR | epidermal growth factor receptor; |
| TRIB3 | tribbles homolog 3; |
| ATF4 | activating transcription factor 4; |
| TNF | tumor necrosis factor; CXCL12, |
| CXC | chemokine ligand 12; |
| NSCLC | non-small cell lung cancer; |
| CM | conditioned media; |
| CGC | Collagen gel contraction; |
| ECM | extracellular matrix; |
| EMT | epithelial–mesenchymal transition; |
| α-SMA | α-smooth muscle actin; |
| GEO | Gene Expression Omnibus; |
| PI | propidium iodide; |
| Co-IP | Co-immunoprecipitation. |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415705/s1, Table S1: Clinical information of all patients’ samples was used for RT-qPCR and western blot analyses in Figure 1A,D.
Author Contributions
L.W. and G.Y., conceptualization; W.Z. (Wenyu Zhao) and C.X., data curation; L.W., W.Z. (Wenyu Zhao) and C.X., formal analysis; W.Z. (Wenyu Zhao), C.X. and Z.L., investigation; K.X., W.Z. (Weiming Zhao), K.X., H.L. and N.W., methodology; L.W. and W.Z. (Wenyu Zhao), writing—original draft; L.W. and G.Y., resources; G.Y., supervision; G.Y., funding acquisition; G.Y., validation; G.Y., project administration; G.Y. and I.O.R., writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Henan Provincial Chest Hospital Medical Research Ethics Committee (No. 2020-03-06).
Informed Consent Statement
Oral and written informed consent was obtained from all patients.
Data Availability Statement
All data are contained within the manuscript.
Conflicts of Interest
The authors declare that they have no conflicts of interest with respect to the contents of this article.
Funding Statement
This work was supported by the Ministry of Science and Technology, PR China, 2019YFE0119500, Henan Province Science and Technology Project, 212102310894 and 222102310711, Xinxiang Major Project 21ZD002 and the 111 Project “State Innovation Base for Pulmonary Fibrosis”.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Richeldi L., Collard H.R., Jones M.G. Idiopathic pulmonary fibrosis. Lancet. 2017;389:1941–1952. doi: 10.1016/S0140-6736(17)30866-8. [DOI] [PubMed] [Google Scholar]
- 2.Chambers R.C., Mercer P.F. Mechanisms of alveolar epithelial injury, repair, and fibrosis. Ann. Am. Thorac. Soc. 2015;12((Suppl. 1)):S16–S20. doi: 10.1513/AnnalsATS.201410-448MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yao Y., Wang Y., Zhang Z., He L., Zhu J., Zhang M., He X., Cheng Z., Ao Q., Cao Y., et al. Chop Deficiency Protects Mice Against Bleomycin-induced Pulmonary Fibrosis by Attenuating M2 Macrophage Production. Mol. Ther. 2016;24:915–925. doi: 10.1038/mt.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J., Cai Z., Bai M., Yu X., Zhang C., Cao C., Hu X., Wang L., Su R., Wang D., et al. The RNA-binding protein ROD1/PTBP3 cotranscriptionally defines AID-loading sites to mediate antibody class switch in mammalian genomes. Cell Res. 2018;28:981–995. doi: 10.1038/s41422-018-0076-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnthaler T., Theiler A., Zabini D., Trautmann S., Stacher-Priehse E., Lanz I., Klepetko W., Sinn K., Flick H., Scheidl S., et al. Inhibiting eicosanoid degradation exerts antifibrotic effects in a pulmonary fibrosis mouse model and human tissue. J. Allergy Clin. Immunol. 2020;145:818–833.e11. doi: 10.1016/j.jaci.2019.11.032. [DOI] [PubMed] [Google Scholar]
- 6.Bellaye P.S., Shimbori C., Yanagihara T., Carlson D.A., Hughes P., Upagupta C., Sato S., Wheildon N., Haystead T., Ask K., et al. Synergistic role of HSP90alpha and HSP90beta to promote myofibroblast persistence in lung fibrosis. Eur. Respir. J. 2018;51:1700386. doi: 10.1183/13993003.00386-2017. [DOI] [PubMed] [Google Scholar]
- 7.Selman M., Pardo A., Kaminski N. Idiopathic pulmonary fibrosis: Aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mora A.L., Rojas M., Pardo A., Selman M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017;16:755–772. doi: 10.1038/nrd.2017.170. [DOI] [PubMed] [Google Scholar]
- 9.Noble P.W., Homer R.J. Back to the future: Historical perspective on the pathogenesis of idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2005;33:113–120. doi: 10.1165/rcmb.F301. [DOI] [PubMed] [Google Scholar]
- 10.Prasad S., Hogaboam C.M., Jarai G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrogenesis Tissue Repair. 2014;7:7. doi: 10.1186/1755-1536-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King T.E., Jr., Pardo A., Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 12.Hegedus Z., Czibula A., Kiss-Toth E. Tribbles: Novel regulators of cell function; evolutionary aspects. Cell Mol. Life Sci. 2006;63:1632–1641. doi: 10.1007/s00018-006-6007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosshans J., Wieschaus E. A genetic link between morphogenesis and cell division during formation of the ventral furrow in Drosophila. Cell. 2000;101:523–531. doi: 10.1016/S0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- 14.Boudeau J., Miranda-Saavedra D., Barton G.J., Alessi D.R. Emerging roles of pseudokinases. Trends Cell Biol. 2006;16:443–452. doi: 10.1016/j.tcb.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Du K., Herzig S., Kulkarni R.N., Montminy M. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver. Science. 2003;300:1574–1577. doi: 10.1126/science.1079817. [DOI] [PubMed] [Google Scholar]
- 16.Ohoka N., Yoshii S., Hattori T., Onozaki K., Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu J., Wu X., Franklin J.L., Messina J.L., Hill H.S., Moellering D.R., Walton R.G., Martin M., Garvey W.T. Mammalian Tribbles homolog 3 impairs insulin action in skeletal muscle: Role in glucose-induced insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2010;298:E565–E576. doi: 10.1152/ajpendo.00467.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tran S.C., Pham T.M., Nguyen L.N., Park E.M., Lim Y.S., Hwang S.B. Nonstructural 3 Protein of Hepatitis C Virus Modulates the Tribbles Homolog 3/Akt Signaling Pathway for Persistent Viral Infection. J. Virol. 2016;90:7231–7247. doi: 10.1128/JVI.00326-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tomcik M., Palumbo-Zerr K., Zerr P., Sumova B., Avouac J., Dees C., Distler A., Becvar R., Distler O., Schett G., et al. Tribbles homologue 3 stimulates canonical TGF-beta signalling to regulate fibroblast activation and tissue fibrosis. Ann. Rheum. Dis. 2016;75:609–616. doi: 10.1136/annrheumdis-2014-206234. [DOI] [PubMed] [Google Scholar]
- 20.Wang W., Sun A., Lv W., Cheng J., Lv S., Liu X., Guan G., Liu G. TRB3, up-regulated in kidneys of rats with type1 diabetes, mediates extracellular matrix accumulation in vivo and in vitro. Diabetes Res. Clin. Pract. 2014;106:101–109. doi: 10.1016/j.diabres.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 21.Liew C.W., Bochenski J., Kawamori D., Hu J., Leech C.A., Wanic K., Malecki M., Warram J.H., Qi L., Krolewski A.S., et al. The pseudokinase tribbles homolog 3 interacts with ATF4 to negatively regulate insulin exocytosis in human and mouse beta cells. J. Clin. Investig. 2010;120:2876–2888. doi: 10.1172/JCI36849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ord D., Ord T. Characterization of human NIPK (TRB3, SKIP3) gene activation in stressful conditions. Biochem. Biophys. Res. Commun. 2005;330:210–218. doi: 10.1016/j.bbrc.2005.02.149. [DOI] [PubMed] [Google Scholar]
- 23.Jousse C., Deval C., Maurin A.C., Parry L., Cherasse Y., Chaveroux C., Lefloch R., Lenormand P., Bruhat A., Fafournoux P. TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J. Biol. Chem. 2007;282:15851–15861. doi: 10.1074/jbc.M611723200. [DOI] [PubMed] [Google Scholar]
- 24.Aime P., Karuppagounder S.S., Rao A., Chen Y., Burke R.E., Ratan R.R., Greene L.A. The drug adaptaquin blocks ATF4/CHOP-dependent pro-death Trib3 induction and protects in cellular and mouse models of Parkinson’s disease. Neurobiol. Dis. 2020;136:104725. doi: 10.1016/j.nbd.2019.104725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S., Lv X., Wei X., Liu C., Li Q., Min J., Hua F., Zhang X., Li K., Li P., et al. TRIB3GSK-3beta interaction promotes lung fibrosis and serves as a potential therapeutic target. Acta Pharm. Sin. B. 2021;11:3105–3119. doi: 10.1016/j.apsb.2021.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu J.M., Sun W., Wang Z.H., Liang X., Hua F., Li K., Lv X.X., Zhang X.W., Liu Y.Y., Yu J.J., et al. TRIB3 supports breast cancer stemness by suppressing FOXO1 degradation and enhancing SOX2 transcription. Nat. Commun. 2019;10:5720. doi: 10.1038/s41467-019-13700-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shang S., Yang Y.W., Chen F., Yu L., Shen S.H., Li K., Cui B., Lv X.X., Zhang C., Yang C., et al. TRIB3 reduces CD8(+) T cell infiltration and induces immune evasion by repressing the STAT1-CXCL10 axis in colorectal cancer. Sci. Transl. Med. 2022;14:eabf0992. doi: 10.1126/scitranslmed.abf0992. [DOI] [PubMed] [Google Scholar]
- 28.Hua F., Li K., Yu J.J., Hu Z.W. The TRIB3-SQSTM1 interaction mediates metabolic stress-promoted tumorigenesis and progression via suppressing autophagic and proteasomal degradation. Autophagy. 2015;11:1929–1931. doi: 10.1080/15548627.2015.1084458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Leary E.M., Tian Y., Nigdelioglu R., Witt L.J., Cetin-Atalay R., Meliton A.Y., Woods P.S., Kimmig L.M., Sun K.A., Gokalp G.A., et al. TGF-beta Promotes Metabolic Reprogramming in Lung Fibroblasts via mTORC1-dependent ATF4 Activation. Am. J. Respir. Cell Mol. Biol. 2020;63:601–612. doi: 10.1165/rcmb.2020-0143OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liang Q., Liu T., Guo T., Tao W., Chen X., Chen W., Chen L., Xiao Y. ATF4 promotes renal tubulointerstitial fibrosis by suppressing autophagy in diabetic nephropathy. Life Sci. 2021;264:118686. doi: 10.1016/j.lfs.2020.118686. [DOI] [PubMed] [Google Scholar]
- 31.Luo X.H., Han B., Chen Q., Guo X.T., Xie R.J., Yang T., Yang Q. Expression of PERK-eIF2alpha-ATF4 pathway signaling protein in the progression of hepatic fibrosis in rats. Int. J. Clin. Exp. Pathol. 2018;11:3542–3550. [PMC free article] [PubMed] [Google Scholar]
- 32.Selvarajah B., Azuelos I., Plate M., Guillotin D., Forty E.J., Contento G., Woodcock H.V., Redding M., Taylor A., Brunori G., et al. mTORC1 amplifies the ATF4-dependent de novo serine-glycine pathway to supply glycine during TGF-beta1-induced collagen biosynthesis. Sci. Signal. 2019;12:eaav3048. doi: 10.1126/scisignal.aav3048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Chen X., Chen H., Li R., Xu P., Lv C., Liu B., Song X. Engeletin ameliorates pulmonary fibrosis through endoplasmic reticulum stress depending on lnc949-mediated TGF-beta1-Smad2/3 and JNK signalling pathways. Pharm. Biol. 2020;58:1105–1114. doi: 10.1080/13880209.2020.1834590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeon H.Y., Choi J., Kraaier L., Kim Y.H., Eisenbarth D., Yi K., Kang J.G., Kim J.W., Shim H.S., Lee J.H., et al. Airway secretory cell fate conversion via YAP-mTORC1-dependent essential amino acid metabolism. EMBO J. 2022;41:e109365. doi: 10.15252/embj.2021109365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raghu G., Remy-Jardin M., Myers J.L., Richeldi L., Ryerson C.J., Lederer D.J., Behr J., Cottin V., Danoff S.K., Morell F., et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018;198:e44–e68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 36.Ritchie M.E., Phipson B., Wu D., Hu Y., Law C.W., Shi W., Smyth G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43:e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are contained within the manuscript.