Abstract
A randomized controlled trial (RCT) was conducted to evaluate the effect of fermented sarco oysters (FSO) on muscle strength in postmenopausal females with low muscle mass. Fifty-two female participants were randomly divided into the experiment group (EG) or control group (CG). For 12 weeks, the EG was subjected to 1000 mg of FSO extract daily while the CG consumed the placebo extract. The muscle extension and flexion at an angular velocity of 60°/s and with respect to grip strength, body composition, and muscle growth-related blood factors were measured at the baseline and after the trial. The difference in the quadriceps muscle extension at an angular velocity of 60°/s, grip strength on both the left and right side, and insulin-like growth factor-1(IGF-1) between groups were significantly higher in the EG compared with the CG. However, no differences were found in body composition, blood pyruvate, lactate, or high-sensitivity C-reactive protein (hsCRP) concentration between the two groups. In conclusion, FSO supplements may improve muscle strength in postmenopausal females with relatively reduced muscle strength without a change in muscle mass.
Keywords: fermented oyster, γ-aminobutyric acid, insulin growth factor-1, muscle strength, postmenopausal female, dietary supplements, sarcopenia
1. Introduction
Sarcopenia is a degenerative disease with the involuntary loss of skeletal muscle mass, strength, and dysfunction with age. As a type of senile disease, sarcopenia can have profound consequences on both health and quality of life, including reduced muscle function, restricted mobility, disability and frailty, and rheumatoid arthritis (RA) [1,2,3]. The pathophysiology of sarcopenia involves many factors, including low levels of physical activity, low intake of protein, and age-related changes in the hormonal factors [3]. Studies have shown that a decline in the levels of growth hormone (GH) and insulin-like growth factor 1 (IGF-1), the major mediator of GH action, has a strong association with the reduction in muscle function [4]. Both GH and IGF-1 are important factors responsible for the anabolic effects on skeletal muscle tissue. IGF-1 plays a key role and has been shown to stimulate muscle cell proliferation and act as a regulator for both muscle protein synthesis and degradation [5]. Studies have proven that γ-aminobutyric acid (GABA) can directly or indirectly stimulate GH release and IGF-1 expression [6,7].
Menopause occurs due to a decline in estrogen level, which leads to a decrease in bone density, muscles mass, and muscle strength; this is known as sarcopenia, which frequently occurs in postmenopausal women [3]. A previous statistic reported that 10–40% of postmenopausal females had the prevalence of sarcopenia [8]. Hence, preventing sarcopenia can prevent physical impairment and related disabilities and thus enhance the quality of life of people. At present, resistance training has been an effective method for attenuating muscle loss and strength in postmenopausal women [3]; an effective method for the prevention and treatment of sarcopenia is needed.
The Crassostrea gigas oyster is one of the world’s most industrially important seafood due to its high sustainability and due it being a great source of protein, vitamins, minerals (such as zinc, iron, calcium, and selenium), and, especially, a large amount of glutamic acid, a precursor of GABA [9,10]. GABA is the main inhibitory neurotransmitter in the central nervous system, and by blocking the chemical messages and delaying the stimulation of the nerve cells, it can affect several functions in the body, including muscle health. Previous studies have shown that enriched GABA contents after being fermented by Lactobacillus brevis BJ-20 (L. brevis BJ-20) had a positive effect on height growth [11], bone formation [12], and muscle endurance [13].
Thus, fermented sarco oyster (FSO) extract was expected to improve the management of sarcopenia. In our pilot study, an oral intake of 1000 mg of FSO supplementation for 8 weeks increased the muscle endurance and concentration of IGF-1 in the experimental group (EG). Based on the previous studies, FSO may have a beneficial effect on the improvement of skeletal muscle mass and strength in postmenopausal women.
Therefore, this clinical trial was undertaken to evaluate the effect of FSO on muscle strength in postmenopausal females over 12 weeks.
2. Materials and Methods
2.1. Study Design and Ethics
This study was conducted at Kyungsung University and was designed as a randomized, placebo-controlled, double-blind clinical trial. All participants were provided a signed consent form prior to the study, and their responses were treated confidentially and anonymously.
2.2. Participant Eligibility
Participants aged 65 years or older, female, with a body mass index (BMI) between 18.5 and 30.0 kg/m2, and with a relatively low skeletal muscles mass (<110% of the standard lean mass) were eligible for the study. Participants with abnormal liver or renal function (aspartate aminotransferase (AST) or alanine aminotransferase (ALT) ≥ 60 IU/L, creatinine level ≥ 1.2 mg/dL, urinalysis dipstick reading of ≥2+), uncontrolled hypertension (blood pressure (BP) ≥ 160/100 mmHg), uncontrolled hyperthyroidism or hypothyroidism, uncontrolled diabetes (fasting glucose level ≥ 160 mg/dL), a history of gastrectomy, mental disorder, known allergies, addiction to alcohol or drugs, and those with a notable cardiovascular disease or central bone fracture within the past 6 months were excluded. Fifty-two females participated in our study.
2.3. Randomization
A double-blind, randomized placebo-controlled trial was conducted. The subjects were assigned a 1:1 allocation ratio to the experimental group (EG) and control group (CG) using the block randomization method. The block randomization method in SPSS Statistics for Windows, Version 22.0 (IBM Corp., Armonk, NY, USA) was used. Because the size of the block was arbitrarily set by a third-party statistician, the size and number of blocks were unknown to all investigators. The random list was offered in a sealed, non-permeable envelope, and all participants, investigators, and assessors were blinded to the treatment allocation until the end of the study.
The two study populations were analyzed as the intention-to-treat (ITT) and per-protocol (PP) populations. All subjects with at least one primary or secondary efficacy endpoint datum obtained after the first visit were included in the ITT analysis. Subjects who consumed more than 80% of the total without skipping more than 5 consecutive days of the test product were included in the PP analysis. Accordingly, the total number of subjects in the ITT analysis group was 52 (EG n = 26, CG n = 26), and the total number of subjects in the PP analysis group was 46 (EG n = 23, CG n = 23), excluding 6 dropouts.
Every participant was asked to visit the center four times during the study (visit 1: for screening; visit 2: for randomized supplement distribution; visit 3: 6 weeks after the intervention; visit 4: 12 weeks after for the final observation).
2.4. Intervention
The FSO extract and placebo were manufactured by Marine Bioprocess Co. Ltd. (Busan, Republic of Korea). The EG participants were given 1000 mg of FSO/day orally in the form of a 250 mg capsule. Four capsules were taken 30 min after a meal with water for 12 weeks. The placebo capsule was identical to the FSO capsule in appearance and was filled with dextrin. In a previous animal study, no toxicity was observed with 100 mg/kg and 200 mg/kg admission of FSO for 28 days [14]. Therefore, a safe and effective dose of 200 mg/kg was converted to the dose appropriate for a 60 kg adult human based on the body surface area and was calculated as 960 mg/day. In the previous preliminary clinical study, the established safe dose of FSO extract was 1000 mg/day [15]. Therefore, a dose of 1000 mg/day was selected as the final dose for the convenience of intake. The content of FSO and placebo capsules are presented in Table 1. The composition of FSO used in this study is equivalent to that of the previous study.
Table 1.
Ingredients and formulation of the test supplement and placebo supplement.
| FSO-Experiment | Placebo-Control | |
|---|---|---|
| Common Name | Fermented Sarco Oyster extract | Dextrin |
| Ingredients and Contents | Fermented Sarco Oyster extract 1000 mg/day | Dextrin 1000 mg/day |
| Type | 250 mg Capsule | Same as left |
| Administration Method | Take 4 capsules once a day after a meal | Same as left |
| Packing Unit | 196 capsules in 1 bottle | Same as left |
| Storage Method | Room temperature storage | Same as left |
| Expiration Period | 2 years | Same as left |
2.5. Procedures
2.5.1. Efficacy Measurement
The primary efficacy endpoint was the change in quadriceps muscle strength measured by the extension and flexion motions at angular velocities at 60°/s, which represented the muscle function at 12 weeks of FSO or placebo use. The secondary efficacy endpoints were the changes in the handgrip strength, body composition, and creatinine, pyruvate, lactate, IGF-1, and high-sensitivity C-reactive protein (hsCRP) concentrations [16], along with the quality of life questionnaire (Euro-Qol-5D (EQ-5D)) score [17].
2.5.2. Biomarker Measurement
Blood samples from all participants were collected after a 12-h fasting at baseline and at 12 weeks to explore the potential effect of the FSO extract. Pyruvate concentrations were measured by an enzymatic assay kit (Determiner PA, Kyowa Medex Co. Ltd., Milano, Italy) on a URIT-800 chemistry analyzer (URIT, Guanxi, China). Lactate concentrations were measured by a colorimetry method kit (Lactate Gen.2., Roche, Manheim, Germany), and hsCRP concentrations were measured by a turbidmetric immunoassay (TIA) kit (CRP4, Roche, Manheim, Germany) on a Cobas C702 chemistry analyzer (Roche, Manheim, Germany). IGF-1 concentrations were measured by an electrochemiluminescence immunoassay (ECLIA) kit (Elecsys IFG-1, Roche, Manheim, Germany) on a Cobas-e801 chemistry analyzer (Roche, Manheim, Germany).
2.5.3. Body Composition Analysis
The body composition analysis was determined by using a multi-frequency bioelectrical impedance analysis (BIA) (InbodyS10, Inbody Co., Ltd., Gangnam, Seoul, Republic of Korea) in the standing posture as per the guidelines of the manufacturer. The appendicular skeletal mass (ASM) was defined as the sum of the muscle masses of the 4 limbs. In this study, the appendicular skeletal mass index (ASMI) was determined by using height squared (ASM/height²) and weight (ASM/weight × 100). All participants were asked to remove their shoes and stand on the platform for 10 minutes prior to the measurements. The measurements were obtained at baseline and after 12 weeks.
2.5.4. Dietary Intake and Physical Activity Assessment
The participants were asked to maintain their usual diet and were required to walk from 30 min to 1 h per day and > 3 times/week during the 12-week trial period. A walking log was used to assess the walking activity. At baseline and after 12 weeks, the participants were asked to answer a questionnaire on physical activities and dietary intake. The International Physical Activity Questionnaire (IPAQ) was used to assess physical activity [18] and the 24-h dietary recall method was used to assess food intake.
2.6. Sample Size Calculation
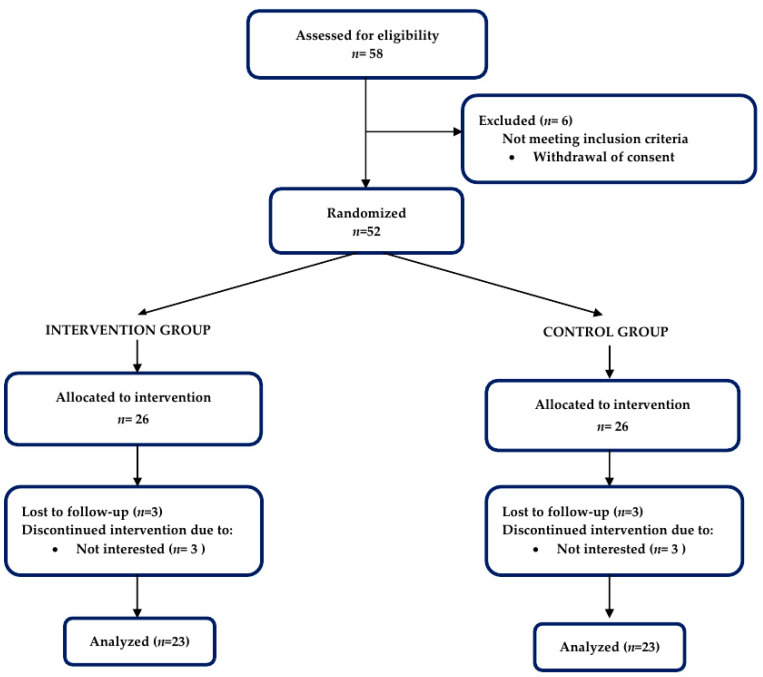
The sample size was calculated using G*Power 3.1.9.2. [19]. Previous nutritional intervention studies with a similar outcome variable (angular velocity of 60°/s measured by the Biodex) were used to estimate the sample size. We derived a small-to-medium effect size of intervention from these studies (Cohen’s f = 0.29). Thus, with a statistical power of 0.80, probability level of 0.05, and effect size of 0.20, a sample size of 45 participants was deemed necessary to achieve sufficient power. Considering a 30% dropout, we recruited a total of 52 participants and randomly allocated them into two groups (n = 26 in each group): (1) intervention and (ii) control. Figure 1 describes the study design, with participant recruitment and exclusion criteria. The study procedures were approved by the Research Ethics Committee of Kyungsung University (IRB NO. KSU-21-03-004, 17 June 2021).
Figure 1.
CONSORT flow diagram.
2.7. Safety Assement
For safety assessment, participants were queried about adverse events and tested for abnormalities at the baseline and 12th week. A paired t-test was performed for continuous data, and a chi–square test was performed for categorical data. For the ITT population (test group 26, control group 26), with an intake of the experiment supplement more than once, the safety was evaluated by evaluating the biological signs and by conducting clinical laboratory tests, using adverse reactions and laboratory tests as variables during the test period. The clinical laboratory test results were compared between the groups for the amount of change compared with the baseline.
2.8. Statistical Analysis
We analyzed the results based on the PP analysis. For all statistical analysis, SPSS Version 22 (SPSS Statistics for Windows Version 22.0, IBM Corp., Armonk, NY, USA) was used, and a two-sided test was performed under a significance level of 0.05. Intergroup comparisons of the baseline characteristics were performed using a chi-square test for the categorical variables or using Fisher’s exact test for non-parametric categorical variables; an independent t-test was performed for continuous variables, and the Mann–Whitney U test was performed for non-parametric categorical variables. As for intragroup comparison, we performed a paired t-test or Wilcoxon’s rank-sum test for the non-parametric variables. The differences between each endpoint before and after the treatments were analyzed.
3. Results
3.1. Baseline Demogrpahic Characteristics
We screened 58 participants aged between 65 and 80 years old, and 52 participants were determined to be eligible for the study. The participants were separated into two groups: EG (n = 26) or CG (n = 26). Six participants (EG: n = 3, CG: n = 3) withdrew consent for personal reasons and were excluded from the study. Hence, the ITT and PP populations were 52 and 46, respectively. Overall, 46 female subjects completed the trial. The flow of participants is depicted in a CONSORT (Consolidated Standards of Reporting Trials) conform diagram [5] (Figure 1). The demographic and clinical data had no significant difference at the baseline between the two groups (Table 2).
Table 2.
Baseline demographic characteristics.
| Variables | Intention-to-Treat Population | Per-Protocol Population | ||||
|---|---|---|---|---|---|---|
| CG (n = 26) | EG (n = 26) | p | CG (n = 23) | EG (n = 23) | p | |
| Age (years) | 73.8 | 72.3 | 0.295 | 73.6 | 72.1 | 0.247 |
| Weight (kg) | 58.3 | 57.9 | 0.854 | 58.4 | 58.0 | 0.838 |
| Height (cm) | 153.5 | 153.4 | 0.949 | 153.5 | 153.6 | 0.930 |
p values were derived from the chi-square test; p values were derived from the independent t-test to compare between the groups.
3.2. Primary Outcome
The primary efficacy analysis demonstrated that after 12 weeks, there was a significant increase in the right (dominant) quadriceps muscle strength (60°/s, extension) in the EG (p < 0.02) compared with the CG, based on the PP analysis (Table 3). Furthermore, the ITT analysis also showed an increase in right quadriceps muscle strength (60°/s, extension) in the EG (p < 0.01) (Table 4), (See Supplementary Materials).
Table 3.
Primary outcome comparisons between and within each group (PP population).
| Variable | Observed Value | Change from the Baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| CG (n = 23) |
EG (n = 23) |
p ** | CG (n = 23) |
p * | EG (n = 23) |
p * | p ** | |
| 60°/s knee extension peak TQ (R), Nm | 0.736 | 6.33 ± 11.10 | 0.015 | 0.037 | ||||
| Visit 2 | 53.45 ± 12.13 | 54.83 ± 12.01 | 0.628 | −1.41 ± 10.38 | ||||
| Visit 4 | 52.05 ± 14.29 | 61.17 ± 15.23 | 0.056 | |||||
| 60°/s knee extension peak TQ (L), Nm | 0.516 | −3.74 ± 12.66 | 0.171 | 0.734 | ||||
| Visit 2 | 57.17 ± 17.03 | 62.00 ± 14.80 | 0.310 | −2.26 ± 16.42 | ||||
| Visit 4 | 54.91 ± 12.99 | 58.26 ± 16.88 | 0.455 | |||||
| 60°/s knee flexion peak TQ (R), Nm | 0.096 | 1.50 ± 9.87 | 0.506 | 0.477 | ||||
| Visit 2 | 28.23 ± 9.44 | 32.50 ± 9.29 | 0.512 | 3.63 ± 9.46 | ||||
| Visit 4 | 31.86 ± 11.89 | 34.00 ± 13.66 | 0.956 | |||||
| 60°/s knee flexion peak TQ (L), Nm | 0.013 | −3.74 ± 8.23 | 0.007 | 0.824 | ||||
| Visit 2 | 31.8 ± 12.4 | 32.3 ± 13.5 | 0.892 | −4.26 ± 7.59 | ||||
| Visit 4 | 27.6 ± 10.7 | 27.9 ± 12.3 | 0.919 | |||||
p *: Values were compared within each group; p **: values were compared between groups.
Table 4.
Primary outcome comparisons between and within each group (ITT population).
| Variable | Observed Value | Change from the Baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| CG (n = 26) |
EG (n = 26) |
p ** | CG (n = 26) |
p * | EG (n = 26) |
p * | p ** | |
| 60°/s knee extension peak TQ (R), Nm | 0.985 | 5.77 ± 10.58 | 0.010 | 0.048 | ||||
| Visit 2 | 52.88 ± 12.15 | 56.27 ± 12.13 | 0.320 | −0.04 ± 10.13 | ||||
| Visit 4 | 52.85 ± 13.66 | 62.04 ± 14.35 | 0.023 | |||||
| 60°/s knee extension peak TQ (L), Nm | 0.368 | −4.19 ± 11.98 | 0.087 | 0.625 | ||||
| Visit 2 | 57.58 ± 16.48 | 62.27 ± 14.89 | 0.287 | −2.31 ± 15.42 | ||||
| Visit 4 | 55.27 ± 12.70 | 58.08 ± 16.84 | 0.500 | |||||
| 60°/s knee flexion peak TQ (R), Nm | 0.118 | 1.50 ± 9.33 | 0.607 | 0.567 | ||||
| Visit 2 | 28.46 ± 9.24 | 31.00 ± 9.90 | 0.344 | 2.99 ± 9.43 | ||||
| Visit 4 | 31.46 ± 11.86 | 32.50 ± 13.29 | 0.768 | |||||
| 60°/s knee flexion peak TQ (L), Nm | 0.090 | −0.38 ± 5.32 | 0.230 | 0.203 | ||||
| Visit 2 | 29.73 ± 13.08 | 31.31 ± 13.29 | 0.668 | −2.88 ± 8.33 | ||||
| Visit 4 | 26.85 ± 10.34 | 10.31 ± 12.87 | 0.290 | |||||
p *: Values were compared within each group; p **: values were compared between groups.
3.3. Secondary Outcome
In the secondary efficacy analysis, the grip force strength in both the left (p < 0.04) and right (p < 0.01) hands and the IGF-1 concentration (p < 0.02) were significantly higher in the EG compared with the CG. However, a comparison between the groups with respect to the change in body composition (total body fat, visceral fat area, and relative muscle mass) using the BIA and amount of change in blood pyruvate, lactate, and hsCRP concentrations using a blood analysis did not yield any significant differences between the two groups throughout the study period, based on both the PP and ITT analyses (Table 5 and Table 6), (See Supplementary Materials).
Table 5.
Secondary outcome comparisons between and within each group (PP population).
| Variable | Observed Value | Change from the Baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| CG (n = 23) | EG (n = 23) | p ** | CG (n = 23) | p * | EG (n = 23) | p * | p ** | |
| Grip Force (R), Nm | ||||||||
| visit 2 | 20.26 ± 2.46 | 19.91 ± 2.93 | 0.667 | 1.30 ± 2.07 | 0.006 | 2.70 ± 1.47 | 0.000 | 0.011 |
| visit 4 | 21.56 ± 3.56 | 22.61 ± 3.46 | 0.315 | |||||
| Grip Force (L), Nm | ||||||||
| visit 2 | 19.46 ± 2.49 | 19.34 ± 3.65 | 0.901 | 1.48 ± 2.31 | 0.006 | 3.00 ± 2.49 | 0.000 | 0.036 |
| visit 4 | 20.94 ± 3.09 | 22.35 ± 3.36 | 0.146 | |||||
| Total Body Fat, % | ||||||||
| visit 2 | 37.67 ± 4.56 | 35.40 ± 6.54 | 0.177 | 0.70 ± 1.61 | 0.051 | 0.87 ± 1.13 | 0.001 | 0.674 |
| visit 4 | 38.37 ± 4.62 | 36.27 ± 6.29 | 0.202 | |||||
| Visceral Fat Area, cm2 | ||||||||
| visit 2 | 118.4 ± 28.4 | 106.7 ± 39.8 | 0.260 | 9.77 ± 16.39 | 0.009 | 8.93 ± 7.96 | 0.000 | 0.827 |
| visit 4 | 128.2 ± 25.9 | 115.7 ± 41.3 | 0.226 | |||||
| ASM/height2 | ||||||||
| visit 2 | 118.4 ± 28.4 | 106.7 ± 39.8 | 0.260 | −0.01 ± 0.11 | 0.695 | −0.01 ± 0.13 | 0.592 | 0.882 |
| visit 4 | 128.2 ± 25.9 | 115.7 ± 41.3 | 0.226 | |||||
| ASM/weight x100 | ||||||||
| visit 2 | 10.45 ± 0.75 | 10.84 ± 1.05 | 0.152 | −0.17 ± 0.46 | 0.094 | −0.11 ± 6.62 | 0.388 | 0.729 |
| visit 4 | 10.28 ± 0.64 | 10.73 ± 0.92 | 0.061 | |||||
| IGF-1 (ng/mL) | ||||||||
| visit 2 | 104.04 ± 29.59 | 109.31 ± 45.91 | 0.646 | 7.13 ± 25.18 | 0.188 | 12.91 ± 23.36 | 0.015 | 0.424 |
| visit 4 | 111.17 ± 26.67 | 122.22 ± 46.43 | 0.329 | |||||
| hsCRP | ||||||||
| visit 2 | 0.15 ± 0.25 | 0.16 ± 0.36 | 0.924 | −0.032 ± 0.167 | 0.371 | 0.05 ± 0.343 | 0.369 | 0.228 |
| visit 4 | 0.12 ± 0.11 | 0.22 ± 0.69 | 0.473 | |||||
| EQ-5D-3L | ||||||||
| visit 2 | 0.822 ± 0.186 | 0.798 ± 0.178 | 0.663 | 0.049 ± 0.156 | 0.144 | 0.061 ± 0.124 | 0.027 * | 0.775 |
| visit 4 | 0.871 ± 0.173 | 0.859 ± 0.157 | 0.812 | |||||
p *: Values were compared within each group; p **: values were compared between groups.
Table 6.
Secondary outcome comparisons between and within each group (ITT population).
| Variable | Observed Value | Change from the Baseline | ||||||
|---|---|---|---|---|---|---|---|---|
| CG (n = 26) | EG (n = 26) | p ** | CG (n = 26) | p * | EG (n = 26) | p * | p ** | |
| Grip Force (R), Nm | ||||||||
| visit 2 | 20.59 ± 2.83 | 19.97 ± 2.80 | 0.439 | 0.79 ± 2.94 | 0.192 | 2.58 ± 1.44 | 0.000 | 0.010 |
| visit 4 | 21.38 ± 3.47 | 22.55 ± 3.28 | 0.221 | |||||
| Grip Force (L), Nm | ||||||||
| visit 2 | 19.51 ± 2.79 | 19.34 ± 3.71 | 0853 | 1.15 ± 2.87 | 0.057 | 2.85 ± 2.39 | 0.000 | 0.025 |
| visit 4 | 20.66 ± 3.12 | 22.19 ± 3.62 | 0.112 | |||||
| Total Body Fat, % | ||||||||
| visit 2 | 37.63 ± 4.63 | 35.05 ± 6.51 | 0.066 | 0.66 ± 1.53 | 0.037 | 0.83 ± 1.09 | 0.001 | 0.674 |
| visit 4 | 38.29 ± 4.65 | 35.88 ± 6.37 | 0.074 | |||||
| Visceral Fat Area, cm2 | ||||||||
| visit 2 | 121.58 ± 30.87 | 107.59 ± 39.33 | 0.160 | 9.54 ± 15.84 | 0.008 | 9.68 ± 7.78 | 0.000 | 0.968 |
| visit 4 | 131.30 ± 28.53 | 117.27 ± 40.96 | 0163 | |||||
| ASM/height2 | ||||||||
| visit 2 | 2.59 ± 0.26 | 2.65 ± 0.24 | 0.428 | −0.02 ± 0.11 | 0.465 | −0.01 ± 0.12 | 0.696 | 0.839 |
| visit 4 | 2.58 ± 0.26 | 2.64 ± 0.22 | 0.357 | |||||
| ASM/weight x100 | ||||||||
| visit 2 | 10.40 ± 0.74 | 10.83 ± 1.03 | 0.085 | 0.18 ± 0.46 | 0.053 | −0.10 ± 0.53 | 0.379 | 0.580 |
| visit 4 | 10.22 ± 0.66 | 10.73 ± 0.91 | 0.023 | |||||
| IGF-1 (ng/mL) | ||||||||
| visit 2 | 105.75 ± 30.12 | 109.54 ± 44.92 | 0.733 | 7.03 ± 24.64 | 0.176 | 11.80 ± 22.77 | 0.013 | 0.422 |
| visit 4 | 112.77 ± 27.23 | 122.13 ± 45.41 | 0.391 | |||||
| hsCRP | ||||||||
| visit 2 | 0.15 ± 0.25 | 0.16 ± 0.35 | 0.830 | −0.03 ± 0.16 | 0.371 | 0.05 ± 0.33 | 0.440 | 0.276 |
| visit 4 | 0.12 ± 0.11 | 0.22 ± 0.68 | 0.463 | |||||
| EQ-5D-3L | ||||||||
| visit 2 | 0.827 ± 0.187 | 0.783 ± 0.205 | 0.426 | 0.041 ± 0.148 | 0.169 | 0.066 ± 0.128 | 0.014 * | 0.524 |
| visit 4 | 0.868 ± 0.179 | 0.849 ± 0.164 | 0.688 | |||||
p *: Values were compared within each group; p **: values were compared between groups.
3.4. Safety and Adverse Event
In the safety assessment, all subjects completed the experiment without any adverse events (AEs). There were no dropouts due to AEs. In addition, as a result of the safety evaluation through clinical laboratory test values along with physical examinations, FSO supplementation is not likely to be associated in any AEs.
4. Discussion
The Crassostrea gigas oyster is one of the most industrially important seafood in the world as a versatile food source and excellent nutrient source. They are rich in protein, minerals, and various essential amino acids [9]. GABA is a non-protein amino acid that functions as a primary inhibitory neurotransmitter for the central nervous system and contributes to motor control, GH secretion, and emotion regulation [7,20]. Oysters contain a small amount of GABA, but not enough to promote their use as a functional food. Hence, fermentation with lactic acid bacteria can enhance the GABA content in oysters [5,21].
The present study investigated whether FSO supplementation improves skeletal muscle strength in postmenopausal women. Women who had ingested FSO for 12 weeks showed a significant increase in right quadriceps muscle strength and grip force strength on both the right and left sides compared with women in the CG. The mean difference (MD) between pre- and post-trial within each group was greater in the EG than in the CG by 7.74 Nm in muscle strength and by 1.52 kg and 1.4 kg in grip force in both the right and left sides, respectively. However, the body composition showed no significant change. A previous pilot RCT with postmenopausal women also reported an increase in 60°/s knee extension and 60°/s flexion of the quadriceps muscle without a change in body composition in the EG group [15]. With the comparable results of an increase in muscle strength and muscular endurance in both studies in addition to an increase in grip strength as obtained from the present study, it may be considered that FSO is effective in improving the muscle strength of postmenopausal women via the activation of the muscular nervous system without the improvement of muscle mass.
Previous studies have reported that fermented oyster (FO) extract contains high GABA and lactate contents, which has the potential for improvements in mitochondrial metabolism and biogenesis and exercise endurance indicators in mice [13]. Furthermore, the administration of FO extract increased the insulin–like growth factor binding protein-3 (IGFBP-3) level, which mediates the action of IGF-1. Hence, the amount of change in the blood IGF-1 concentrations may induce quantitative growth in muscle improvement. The results of the current study show an increase in IGF-1 levels in both the EG and CG after 12 weeks of ingesting FSO. However, the level of IGF-1 in the EG was significantly higher, further supporting that the positive effect of FSO on muscle strength improvement is due to a functional change via the activation of the nervous system rather than a structural change.
Our study had some limitations, including a lack of biological confirmation for FSO, including environmental factors such as sleeping hours and eating habits. Furthermore, the focus of this study was only postmenopausal women; therefore the effect of FSO on people of different sexes, ages, and living conditions are still unknown. Secondly, considering the differences between the current study and the pilot study, further follow-up study time is necessary for more accurate results. Despite the above limitations, FSO can be recommended for muscle improvement.
5. Conclusions
In conclusion, the efficacy and safety of FSO extract for 12 weeks to increase muscle function in postmenopausal women was approved. Furthermore, there was a significant difference in IGF-1 concentration within the experimental group by FSO ingestion for 12 weeks. Hence, our findings provide evidence for the enhancing effect of FSO on muscle strength enhancement.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1660-4601/19/24/16450/s1. Figure S1: Measurement comparison of the 60°/s right knee extension peak TQ (Nm) in the PP population. Each bar represents the mean ± SD; Figure S2: Measurement comparison of the left hand grip force (Nm) in the PP population. Each bar represents the mean ± SD; Figure S3: Measurement comparison of the right hand grip force (Nm) in the PP population. Each bar represents the mean ± SD; Figure S4: Measurement comparison of the IGF-1 level in the PP population. Each bar represents the mean ± SD; Figure S5: Measurement comparison of the 60°/s right knee extension peak TQ (Nm) in the ITT population. Each bar represents the mean ± SD; Figure S6: Measurement comparison of the left hand grip force (Nm) in the ITT population. Each bar represents the mean ± SD; Figure S7: Measurement comparison of the right hand grip force (Nm) in the ITT population. Each bar represents the mean ± v; Figure S8: Measurement comparison of the IGF-1 level in the ITT population. Each bar represents the mean ± SD; Table S1: Comparison between and within each group (PP population); Table S2: Comparison between and within each group (ITT population).
Author Contributions
Conceptualization: B.-J.L., B.-H.J., H.-T.P. and M.-S.H.; investigation and data curation: W.-H.S. and D.-S.K.; writing and original draft: K.-M.R. and B.-H.J.; review and editing: B.-H.J., H.-T.P. and M.-S.H.; supervision: B.-H.J.; resources: W.-H.S., D.-S.K. and B.-H.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Research Ethics Committee of Kyungsung University (IRB No. KSU-21-03-004, 17 June 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Conflicts of Interest
The authors and funders declare no conflict of interest. The funders had no role in the designing of the study or in the collection or analyses. However, the funder had a role in the interpretation and the writing of the manuscript.
Funding Statement
This research was part of the Marine Bio Strategic Material Development and Commercialization Support Project funded by the Korea Institute of Marine Science & Technology Promotion grant funded by the Korean government (Grant No. ACP6047-1525010967).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dufour A.B., Hannan M.T., Murabito J., Kiel D., McLean R.R. Sarcopenia Definitions Considering Body Size and Fat Mass Are Associated with Mobility Limitations: The Framingham Study. J. Gerontol. Ser. A. 2012;68:168–174. doi: 10.1093/gerona/gls109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xue Q.L., Walston J.D., Fried L.P., Beamer B.A. Prediction of risk of falling, physical disability, and frailty by rate of decline in grip strength: The women’s health and aging study. Arch. Intern. Med. 2011;171:1119–1121. doi: 10.1001/archinternmed.2011.252. [DOI] [PubMed] [Google Scholar]
- 3.Giles J.T., Ling S.M., Ferrucci L., Bartlett S., Andersen R.E., Towns M., Muller D., Fontaine K.R., Bathon J.M. Abnormal body composition phenotypes in older rheumatoid arthritis patients: Association with disease characteristics and pharmacotherapies. Arthritis Care Res. 2008;59:807–815. doi: 10.1002/art.23719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherlock M., Toogood A.A. Aging and the growth hormone/insulin like growth factor-I axis. Pituitary. 2007;10:189–203. doi: 10.1007/s11102-007-0039-5. [DOI] [PubMed] [Google Scholar]
- 5.Schulz K.F., Altman D.G., Moher D., CONSORT Group CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010;8:18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers M. GABA Supplementation and Growth Hormone Response. Med. Sport. Sci. 2012;59:36–46. doi: 10.1159/000341944. [DOI] [PubMed] [Google Scholar]
- 7.Athapaththu A.M.G.K., Molagoda I.M.N., Jayasooriya R.G.P.T., Choi Y.H., Jeon Y.-J., Park J.-H., Lee B.-J., Kim G.-Y. Gamma-Aminobutyric Acid (GABA) Promotes Growth in Zebrafish Larvae by Inducing IGF-1 Expression via GABAA and GABAB Receptors. Int. J. Mol. Sci. 2021;22:11254. doi: 10.3390/ijms222011254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marini M., Sarchielli E., Brogi L., Lazzeri R., Salerno R., Sgambati E., Monaci M. Role of adapted physical activity to prevent the adverse effects of the sarcopenia. A pilot study. Ital. J. Anat. Embryol. = Arch. Ital. Di Anat. Ed Embriol. 2008;113:217–225. [PubMed] [Google Scholar]
- 9.Lee Y.L., Lee S.Y. Effect of fermented oyster (Crassostrea gigas) extracts and regular walking on muscle strength and mass in older adults with relatively low muscle mass: A randomized controlled trial. Front. Nutr. 2022;9:935395. doi: 10.3389/fnut.2022.935395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang J.S., Choung S.-Y. Inhibitory effect of oyster hydrolysate on wrinkle formation against UVB irradiation in human dermal fibroblast via MAPK/AP-1 and TGFβ/Smad pathway. J. Photochem. Photobiol. B: Biol. 2020;209:111946. doi: 10.1016/j.jphotobiol.2020.111946. [DOI] [PubMed] [Google Scholar]
- 11.Molagoda I.M.N., Athapaththu A.M.G.K., Park E.K., Choi Y.H., Jeon Y.-J., Kim G.-Y. Fermented Oyster (Crassostrea gigas) Extract Cures and Prevents Prednisolone-Induced Bone Resorption by Activating Osteoblast Differentiation. Foods. 2022;11:678. doi: 10.3390/foods11050678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jeong A., Park B.-C., Kim H.-Y., Choi J.-Y., Cheon J., Park J.-H., Lee B.-J., Kim K., Jeong A., Park B.-C., et al. Efficacy and safety of fermented oyster extract for height of children with short stature: A randomized placebo-controlled trial. Integr. Med. Res. 2021;10:100691. doi: 10.1016/j.imr.2020.100691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reid S.N.S., Park J.-H., Kim Y., Kwak Y.S., Jeon B.H. In Vitro and In Vivo Effects of Fermented Oyster-Derived Lactate on Exercise Endurance Indicators in Mice. Int. J. Environ. Res. Public Health. 2020;17:8811. doi: 10.3390/ijerph17238811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oh S., Choi C.H., Lee B.-J., Park J.-H., Son K.-H., Byun K. Fermented Oyster Extract Attenuated Dexamethasone-Induced Muscle Atrophy by Decreasing Oxidative Stress. Molecules. 2021;26:7128. doi: 10.3390/molecules26237128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park J.H., Jeon B.H., Kim D.S., Lee B.J., Her J.S. Effects of Fermented Oyster Extract Supplementation on Body Composition, Muscular Strengths and Blood Muscle Growth Factors in Elderly Women. J. Mar. Biosci. Biotechnol. 2021;13:76–85. [Google Scholar]
- 16.Rabinowitz J.D., Enerbäck S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020;2:566–571. doi: 10.1038/s42255-020-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim S.-H., Ahn J., Ock M., Shin S., Park J., Luo N., Jo M.-W. The EQ-5D-5L valuation study in Korea. Qual. Life Res. 2016;25:1845–1852. doi: 10.1007/s11136-015-1205-2. [DOI] [PubMed] [Google Scholar]
- 18.Chun M.Y. Validity and Reliability of Korean Version of International Physical Activity Questionnaire Short Form in the Elderly. Korean J. Fam. Med. 2012;33:144–151. doi: 10.4082/kjfm.2012.33.3.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faul F., Erdfelder E., Buchner A., Lang A.-G. Statistical power analyzes using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods. 2009;41:1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- 20.Allen M.J., Sabir S., Sharma S. StatPearls. StatPearls Publishing; Tampa, FL, USA: 2022. GABA Receptor. [PubMed] [Google Scholar]
- 21.Harnedy P.A., FitzGerald R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Funct. Foods. 2012;4:6–24. doi: 10.1016/j.jff.2011.09.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.