Abstract
Staphylococcus aureus invasion of mammalian cells, including epithelial, endothelial, and fibroblastic cells, critically depends on fibronectin bridging between S. aureus fibronectin-binding proteins (FnBPs) and the host fibronectin receptor integrin α5β1 (B. Sinha et al., Cell. Microbiol. 1:101–117, 1999). However, it is unknown whether this mechanism is sufficient for S. aureus invasion. To address this question, various S. aureus adhesins (FnBPA, FnBPB, and clumping factor [ClfA]) were expressed in Staphylococcus carnosus and Lactococcus lactis subsp. cremoris. Both noninvasive gram-positive microorganisms are genetically distinct from S. aureus, lack any known S. aureus surface protein, and do not bind fibronectin. Transformants of S. carnosus and L. lactis harboring plasmids coding for various S. aureus surface proteins (FnBPA, FnBPB, and ClfA) functionally expressed adhesins (as determined by bacterial clumping in plasma, specific latex agglutination, Western ligand blotting, and binding to immobilized and soluble fibronectin). FnBPA or FnBPB but not of ClfA conferred invasiveness to S. carnosus and L. lactis. Invasion of 293 cells by transformants was comparable to that of strongly invasive S. aureus strain Cowan 1. Binding of soluble and immobilized fibronectin paralleled invasiveness, demonstrating that the amount of accessible surface FnBPs is rate limiting. Thus, S. aureus FnBPs confer invasiveness to noninvasive, apathogenic gram-positive cocci. Furthermore, FnBP-coated polystyrene beads were internalized by 293 cells, demonstrating that FnBPs are sufficient for invasion of host cells without the need for (S. aureus-specific) coreceptors.
Staphylococcus aureus is generally considered an extracellular pathogen (12, 44). However, it was shown in vitro over several years that S. aureus can invade a variety of nonprofessional phagocytes of different species. Many studies have used cells of nonhuman origin, mainly bovine endothelial cells. S. aureus invasion of human cells has been described so far for endothelial cells (8, 38), epithelial cells (7, 47, 50), osteoblasts (24), and fibroblasts (50).
S. aureus is among the most important bacterial pathogens for humans. It commonly causes superficial skin infections. Upon bloodstream dissemination or by continuous spread, it can readily survive in various deep tissues and cause, among others, abscess formation, osteomyelitis, endocarditis, and sepsis. Since these S. aureus infections are frequently long lasting, are difficult to eradicate, and often relapse after antibiotic treatment, prolonged treatment, including the use of cell-permeating antimicrobial drugs, is often required (30, 31). Invasion of nonprofessional phagocytes may provide a protected niche for S. aureus, but the precise role of invasion of host cells in S. aureus pathology in vivo is still not known.
We have previously analyzed the process of cellular S. aureus invasion and elucidated the molecular mechanism of the internalization step (50). Bacterial entry proceeds by a “zipper-type” mechanism, which resembles professional phagocytosis, and requires bacterial surface proteins, namely, adhesins (also termed microbial surface components recognizing adhesive matrix molecules [MSCRAMMs] [13]). The internalization process if F actin dependent, as demonstrated by complete inhibition by cytochalasin D. By use of isogenic deletion mutants, we identified fibronectin-binding protein (FnBP) as the necessary S. aureus invasin. Using competition experiments, we mapped the region of interaction with the host cell to the major fibronectin-binding domain of the FnBP, the D1 to D4 repeats. Fibronectin acts as a bridging molecule between the FnBP and the fibronectin receptor integrin α5β1, an adhesion molecule on the host cell. Cellular invasion is highly conserved in clinical S. aureus isolates (15 out of 15 tested were invasive), and the mechanism elucidated for the epithelial cell model of 293 cells is also valid for ECV304 cells, skin fibroblasts (50), and human umbilical vein endothelial cells (B. Sinha, unpublished data). These data are compatible with the results obtained by four different groups, who found that FnBPs are essential either for adhesion to or invasion of human endothelial cells (42), bovine mammary epithelial cells (9, 27), and mouse fibroblastic cells (14).
Our previous data did not support a role for S. aureus invasins other than FnBPs but could not exclude necessary (S. aureus-specific) coreceptors. However, they largely excluded an efficient second invasin in S. aureus (50), unlike the situation found for streptococcal M proteins. This study was performed to address the questions of whether S. aureus FnBPs are sufficient to confer invasiveness for host cells when expressed in other gram-positive microorganisms and whether coreceptors are necessary for S. aureus invasion. We studied these questions using two different species of gram-positive cocci and using FnBP-coated polystyrene beads. We were able to express both types of S. aureus FnBPs and clumping factor (ClfA, the major fibrinogen adhesin) in Staphylococcus carnosus (strain TM300). This organism is a nonpathogenic coagulase-negative staphylococcal species which is found on domestic artiodactyls and dry meat products and which lacks most of the known S. aureus surface structures. In addition, we used Lactococcus lactis subsp. cremoris, formerly designated Streptococcus lactis, as a second nonpathogenic host [strain 1363(pIL253)]. This organism is another distantly related species of gram-positive cocci used in the processing of dairy products. These two microorganisms, when expressing S. aureus FnBPs on the surface, subsequently acquired the classical properties conferred by adhesins (i.e., binding of soluble proteins and adherence to immobilized proteins) and, at the same time, became invasive, a recently demonstrated novel function of FnBPs. FnBP-coated beads were internalized by 293 cells in a manner similar to that of bacterial transformants.
MATERIALS AND METHODS
Reagents and enzymes.
Phosphate-buffered saline without Ca2+ or Mg2+ (PBS) was obtained from Gibco-BRL (Karlsruhe, Germany). Recombinant lysostaphin (Ambicin; WAK Chemie/Applied Microbiology, Brooklyn, N.Y.), a murolytic enzyme, specifically degrades the staphylococcal cell wall by cleaving the pentaglycine bridge in peptidoglycan. Human serum albumin (HSA) was obtained from Behring (Marburg, Germany). Lyophilized bacterial culture media were obtained from Merck (M17 broth), Difco (tryptic soy agar [TSA]), and Mast (Mueller-Hinton broth [MHB]).
Bacterial strains, plasmid DNA isolation, and transformation.
All bacterial strains used are listed in Table 1. Strain Cowan 1 was used as a reference isolate of S. aureus. Strain DU5883 is an isogenic mutant of strain 8325-4 with deletions in fnbA and fnbB. The FnBP-encoding plasmids pFNBA4 and pFNBB4 (19) were isolated from the respective complemented S. aureus DU5883 strains (19), and the ClfA-encoding plasmid pCF4 was isolated from complemented strain 8325-4 (33) by modified alkaline lysis (including 10 μg of lysostaphin per ml in the resuspension buffer) and the Qiagen method (Midiprep columns). Plasmids were verified by agarose gel electrophoresis.
TABLE 1.
Bacterial strains used in this study
| Strain | Relevant genotype or plasmida | Propertiesb | Source or reference |
|---|---|---|---|
| S. aureus | |||
| Cowan 1 | WT | NCTC 8530 (isolated from septic arthritis) | ATCC 12598 |
| 8325-4 | WT | NCTC 8325 cured of prophages | 37 |
| 8325-4(pCF4) | ClfA +++ | 33 | |
| DU5883 | 8325-4 fnbA fnbB | FnBPA −, FnBPB − | 19 |
| DU5883(pFNBA4) | FnBPA8325-4 +++ | 19 | |
| DU5883(pFNBB4) | FnBPB8325-4 +++ | 19 | |
| Clones 1 and 2 | DU5883(pCF4) | ClfA +++ | This study |
| Newman D2C | WT | ClfA positive (high level); NCTC 10833 | ATCC 25904 |
| S. carnosus | |||
| TM300 | WT | No expression of adhesins | 46 |
| Clones 1 to 3 | TM300(pFNBA4) | FnBPA8325-4 +++ | This study |
| Clones 1 to 3 | TM300(pFNBB4) | FnBPB8325-4 +++ | This study |
| Clones 1 to 3 | TM300(pCF4) | ClfA +++ | This study |
| S. epidermidis N860187 | WT | Clinical isolate (endocarditis) | 11 |
| L. lactis subsp. cremoris 1363 | 1363(pIL253) | Empty vector, no expression of FnBPs | 17, 49 |
| 1363(pOri23-FnBPA) | FnBPA8325-4 +++ | Y.-A. Que et al., personal communication | |
| 1363(pOri23-ClfA) | ClfA +++ | 43 |
WT, wild type.
+++, overexpression; −, no expression. Subscript 8325-4 indicates the source of the protein (strain).
DU5883 and S. carnosus strain TM300 (46) were transformed by electroporation (29). Briefly, bacteria were grown to an optical density at 650 nm (OD650) of approximately 0.5 in basic 2 broth (peptone-yeast extract-glucose) and washed thoroughly on ice. Concentrated bacterial suspensions were incubated with plasmid DNA and electroporated in disposable cuvettes using a Bio-Rad Gene Pulser. Bacterial suspensions were diluted immediately in SMMP (saccharose-MgCl2-maleic acid-Bacto-Pennassay broth; adjusted for S. aureus and S. carnosus), supplemented with chloramphenicol (0.25 μg/ml; Boehringer Mannheim Biochemicals or Merck), incubated at 37°C for 1.5 h, and plated on TSA-chloramphenicol (10 μg/ml) plates. After 1 to 2 days, colonies were picked and tested by restriction analysis and gel electrophoresis of isolated plasmids obtained by modified alkaline lysis, with subsequent phenol-chloroform extraction. Three colonies each for S. carnosus with pFNBA4, pFNBB4, and pCF4 and two for S. aureus were kept for further analysis. L. lactis subsp. cremoris strain 1363 (17), containing plasmid pIL253 (49), was used as a wild-type strain.
S. aureus fnbA (48) was amplified by PCR from the chromosome of S. aureus strain 8325-4 prepared as described previously (32) and cloned into the BamHI site of the pOri23 expression vector (43). Reactions were started with 100 ng of template DNA, 0.3 mM deoxynucleoside triphosphate (dNTP), and 0.5 μM each forward primer (5′-CCG^GAT CCG CAT TTA AAG GGA GAT ATT ATA-3′) and reverse primer (5′-CCG^GAT CCC GGG CTT ACT TCA TAT AAT TAT GAA-3′) (^ indicates cleavage site; BamHI restriction sites in bold) (Microsynth, Balgach, Switzerland) in 10× PCR buffer–1.5 mM MgCl2 using 2 IU of Taq DNA polymerase (Life Technologies AG, Basel, Switzerland). fnbA was amplified for 30 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 3 min 15 s. The insertion and orientation of the fnb fragments were verified by restriction analysis. The constructs were transferred by electroporation into L. lactis as described previously (20). Construction of the ClfA-expressing strain is described elsewhere (43).
Bacterial cultures.
S. aureus strains and S. carnosus strain TM300 were cultured in MHB at 37°C. All transformants were grown in the presence of 10 μg of chloramphenicol per ml. Wild-type strains were kept on sheep blood agar plates, and transformants were kept on TSA plates supplemented with 10 μg of chloramphenicol per ml. L. lactis was grown in M17 broth supplemented with 0.5% glucose (Merck) and 5 μg of erythromycin (Serva or Merck) per ml at 30°C and kept on M17 agar plates supplemented with 0.5% glucose–5 μg of erythromycin per ml. All bacteria were grown in 5 ml of the respective broth overnight without shaking.
Slide agglutination tests.
All transformants of S. aureus, S. carnosus, and L. lactis were tested for the functional surface expression of adhesins semiquantitatively by two methods: a routine slide agglutination test with citrated rabbit plasma (Biomerieux, Marcy l'Etoile, France) and a commercial S. aureus identification latex agglutination kit (Pastorex; Sanofi-Pasteur, Marnes la Coquette, France), which recognizes ClfA, FnBPs, and capsular polysaccharide types 5 and 8.
Western ligand blots.
Fifty milliliters of exponential cultures of tested strains was washed in PBS and incubated for 10 min at 37°C in PBS–1.1 M sucrose supplemented with 50 μg of lysostaphin per ml. After centrifugation (10,000 × g, 30 min), protein extracts were ultrafiltered (5-kDa cutoff) in PBS to remove sucrose. Bacterial cell wall proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, blotted onto a polyvinylidene difluoride membrane (Millipore), and blocked in PBS containing 0.05% Tween 20 and 5% bovine serum albumin (BSA). Blots were incubated with 30 μg of human fibronectin solution per ml for 2 h at 4°C, followed by an anti–N-terminal fibronectin antibody (Chemicon, Temecula, Calif.). The secondary antibody was anti-mouse immunoglobulin G coupled to peroxidase (Amersham). The detection step was done with an ECL kit (Amersham). FnBPA and FnBPB were distinguished by their apparent molecular weights compared to those for reference S. aureus strains expressing either FnBPA or FnBPB.
Solid-phase adherence assay.
A radiometric adherence assay with log-phase cultures was performed as described previously (55). Briefly, polymethylmethacrylate (PMMA) coverslips (8 by 8 mm) were coated by incubation with various concentrations of purified human fibronectin (Chemicon; in PBS) for 60 min at 37°C and then rinsed in PBS. To optimize the absorption of fibronectin, PMMA coverslips were incubated in a 0.5-mg/ml solution of gelatin prior to fibronectin incubation (54). After the coverslips were rinsed, the number of attached bacteria (CFU per square centimeter) was calculated from radioactive counts.
Fluorescence labeling of fibronectin.
Human fibronectin and BSA were labeled with 5-fluorescein isothiocyanate (FITC; Molecular Probes, Leiden, The Netherlands) according to the manufacturer's instructions with minor modifications. Proteins (1 mg/ml) were coupled in 0.2 M sodium carbonate buffer (pH 9.2) supplemented with 10 μl of an FITC solution (10 mg/ml in dimethyl sulfoxide [DMSO]) by incubation at ambient temperature on a rotary shaker for 2 h. The reaction was stopped by the addition of 10 μl of ethanolamine for 30 min, and labeled proteins were purified by gel filtration (Sephadex G-25M column PD-10; Pharmacia-Biotech) with PBS for elution. Aliquots (0.5 ml) were monitored for protein content by spectrometry (280 nm) and for FITC content by fluorometry (excitation, 485 nm; emission, 515 nm). Fractions containing the fluorescent protein were pooled, and the protein content was determined by the Pierce method (2, 15).
Flow cytometric binding assay of soluble proteins.
Fibronectin binding was determined with formaldehyde-fixed late-logarithmic-phase bacteria and FITC-labeled soluble fibronectin (5 μg/ml) in a flow cytometric assay as previously described (15). Incubation was performed on an inverting shaker for 2 h at 4°C. Mean FL-1 height values for background fluorescence were determined by incubation with FITC-coupled HSA and were subtracted from the mean fluorescence signal obtained for each strain with FITC-labeled fibronectin (2, 15).
Preparation of FITC-labeled bacteria.
Bacteria were prepared as described previously (50). Bacteria were grown overnight without shaking, washed in 0.9% NaCl, fixed in 0.5% formaldehyde in PBS for at least 1 h, and washed in PBS. Subsequently, bacteria were labeled in 3 ml of 0.5 M NaHCO3 buffer (pH, ∼9.5) supplemented with 100 μg of FITC (isomer I; solubilized in 150 μl of DMSO) per ml for 1 h at 37°C. Finally, bacteria were resuspended in PBS–1% HSA and used within 24 h after preparation. Suspensions were normalized for OD540 after gentle sonication in a water bath.
Coating of fluorescent polystyrene beads.
Purified recombinant FnBPs were used to coat carboxylated highly green fluorescent polystyrene beads (1 μm; F-8823; Molecular Probes). FnBPs were then covalently coupled by the carbodiimide method as recommended by the bead manufacturer. Beads were gently sonicated and used in a manner similar to that used for fixed bacteria in the flow cytometric invasion assay (see below). Cell-associated fluorescence was read in the FL-3 channel (overflow), due to the extremely high difference between cellular autofluorescence and the signal of the beads.
Cell cultures.
All medium components were obtained from Gibco-BRL. 293 cells (adenovirus type 5 DNA-transformed primary human embryonic kidney cells) were obtained from the American Type Culture Collection (CRL-1573); maintained in Dulbecco's minimal essential medium (DMEM)–nutrient mixture F-12 (nut mix F-12) (containing Glutamax I, a stable glutamine dipeptide) supplemented with 10% fetal calf serum (FCS), 50 IU of penicillin per ml, and 50 μg of streptomycin per ml; and split 1:4 twice weekly by trypsinization. They were maintained in humidified air with 5% CO2 at 37°C and were used up to passage 35 after freezing.
Lysostaphin and penicillin protection assays.
The lysostaphin protection assay was performed as described previously (50) with modifications. The protocol is derived from a standard protocol for the gentamicin protection assay (10). 293 cells were plated at 0.2 × 106 cells 2 days before the assay. Bacterial suspensions were washed, and bacteria were counted with a Coulter counter and adjusted to 2 × 106 CFU/ml. Cell culture medium was replaced with 0.5 ml of bacterial suspensions in invasion medium (DMEM–nut mix F-12, 1% HSA, 10 mM HEPES), resulting in ∼106 CFU/well and yielding an estimated ratio of bacteria to cells (multiplicity of infection [MOI]) of ∼2.5:1. Cells were incubated for 1 h at 37°C, bacterial suspensions were replaced with lysostaphin medium (DMEM–nut mix F-12, 10% FCS, 20 μg of lysostaphin per ml), and cells were further incubated as described before for 30 min. Cells were subsequently lysed in 1 ml of sterile distilled H2O. Appropriate serial dilutions of the cell lysate in PBS were plated on Mueller-Hinton agar or TSA plates, and CFU were counted manually. Results for all dilutions were pooled and were corrected for the actual inoculum, as determined by a plating assay.
For the penicillin protection assay, lysostaphin was replaced with penicillin G at a concentration of 2 μg/ml (16 times the previously determined minimal bactericidal concentration for L. lactis).
Flow cytometric invasion assay.
The flow cytometric invasion assay was performed as described previously (50) with minor modifications. Briefly, 293 cells were plated at 0.4 × 106 cells in 24-well plates 1 day before the assay. Cells were washed with DMEM–nut mix F-12, and then 0.5 ml of 1% HSA–10 mM HEPES (pH 7.4) in DMEM–nut mix F-12 (invasion medium) was added. Cells were cooled on ice, and 50 μl of fixed, FITC-labeled bacterial suspensions was added, resulting in an estimated MOI of ∼25:1. Culture dishes were preincubated for 2 h at 4°C to allow sedimentation of bacteria and were shifted to 37°C for 2 h for invasion. Cells were then harvested and analyzed by flow cytometry as described previously (50).
Presentation of results.
For each experiment, a fresh bacterial culture was prepared. The results were expressed as the mean and standard error of the mean (SEM) for n independent experiments performed in duplicate (n as specified in the figure legends).
RESULTS
Phenotypic characterization of strains.
We tested the transformants of S. aureus, S. carnosus, and L. lactis for the correct surface expression and function of adhesins qualitatively by two methods: a slide agglutination test with citrated rabbit plasma and a commercial S. aureus identification latex agglutination kit (Pastorex), which recognizes ClfA, FnBPs, and capsular polysaccharide types 5 and 8. All clones of S. carnosus and L. lactis containing the respective plasmids tested positive in both agglutination tests, as opposed to the wild-type or parental strain. In general, the agglutination due to ClfA was more rapid and yielded larger aggregates than the agglutination due to FnBPs. Despite the fact that strain DU5883 expresses ClfA, the overexpression of ClfA enhanced and accelerated the agglutination reaction in the plasma slide agglutination test (Table 2).
TABLE 2.
Surface expression of adhesins in transformantsa
| Strain | Clone | Agglutination tested withb:
|
|||
|---|---|---|---|---|---|
| Rabbit plasma | NaCl | Latex | Control | ||
| Cowan I | +++ | − | +++ | − | |
| DU5883 | |||||
| None | ++c | − | +++c | − | |
| pFNBA4 | ++ | − | ++ | − | |
| pFNBB4 | ++ | − | ++ | − | |
| pCF4 | 1 or 2 | +++ | − | +++ | − |
| TM300 | |||||
| None | − | − | − | − | |
| pFNBA4 | 1 | ++ | − | +++ | − |
| 2 | ++ | − | +++ | − | |
| 3 | ++ | − | +++ | − | |
| pFNBB4 | 1 | + | − | +++ | − |
| 2 | + | − | +++ | − | |
| 3 | + | − | +++ | − | |
| pCF4 | 1 | +++ | − | +++ | − |
| 2 | +++ | − | +++ | − | |
| 3 | +++ | − | +++ | − | |
| 1363 | |||||
| pIL253d | − | − | − | − | |
| pOri23-FnBPA | +++ | − | ++ | − | |
| pOri23-ClfA | +++ | − | +++ | − | |
| N860187 | − | − | − | − | |
Surface expression of adhesins by transformants of S. aureus (DU5883), S. carnosus (TM300), and L. lactis [1363(pIL253)] was tested by induction of clumping in citrated rabbit plasma and a commercial latex agglutination kit (Pastorex) as described in Materials and Methods. Results were expressed semiquantitatively: −, no clumping; +, ++, and +++, increasing amounts of efficient and rapid clumping. S. aureus strain Cowan 1 was used as a positive control, and the clinical S. epidermidis isolate N860187 (11) was used as a negative control.
NaCl served as a negative control for citrated rabbit plasma. Control, control latex beads of the test kit.
Strain DU5883 expresses ClfA.
The parental strain 1363 contains pIL253.
Homologous expression of FnBPs in S. aureus confers invasiveness.
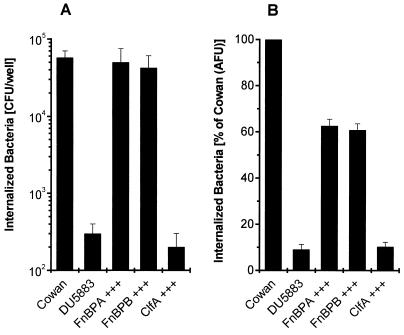
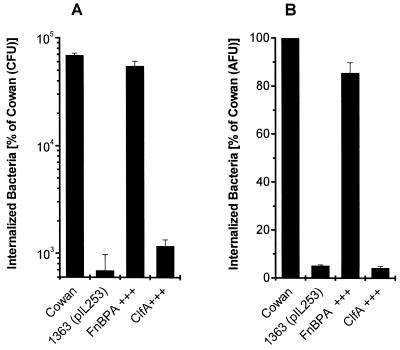
To test whether FnBPs are sufficient for the invasion of nonprofessional phagocytes by S. aureus when expressed in a homologous system, we used the barely invasive strain DU5883, a strain 8325-4-derived mutant, as a parental strain to provide an S. aureus background completely devoid of FnBPs. The expression of either FnBPA or FnBPB from a multicopy plasmid conferred invasiveness to a degree similar to that seen for the reference strain Cowan 1. This process was highly efficient, with up to 5 to 8% of the initial inoculum (106 CFU/well) being recovered after 1 h of coincubation for strain Cowan 1. The numbers of bacteria recovered were (5.74 ± 1.26) × 104 CFU and (0.03 ± 0.01) × 104 CFU for strains Cowan 1 and DU5883, respectively. In contrast, the expression of ClfA (clumping factor), the major fibrinogen adhesin, did not alter the phenotype with respect to invasion (identical results were obtained for a second ClfA-expressing clone; data not shown). The invasive phenotype of FnBP-expressing clones was found regardless of the method used, i.e., a lysostaphin protection assay with live bacteria (Fig. 1A) or a flow cytometric assay with formaldehyde-fixed bacteria (Fig. 1B) [slightly higher invasiveness, relative to that of Cowan 1, had been observed for DU5883(pFNBA4) and DU5883(pFNBB4) at a lower MOI in 293 cells (50); DU5883(pCF4) had not been tested at that time].
FIG. 1.
The adhesin FnBP confers invasiveness to an FnBP-deficient, noninvasive S. aureus mutant. Overexpression (+++) of adhesins in strain DU5883 by multicopy plasmids was tested for invasion of 293 cells. Strain DU5883 (an FnBPA- and FnBPB-deficient isogenic mutant of 8325-4) served as a parental strain to provide an S. aureus background completely devoid of FnBPs. (A) Total internalized bacteria were measured by lysostaphin (20 μg/ml) protection. Results are means and SEMs for four independent experiments run in duplicate and are expressed as recovered CFU, normalized for the actual inoculum. (B) Total internalized bacteria were measured by flow cytometry with fixed bacteria. Results are means and SEMs for three independent experiments run in duplicate and are expressed as invasiveness relative to that of strain Cowan 1. AFU, arbitrary fluorescence units.
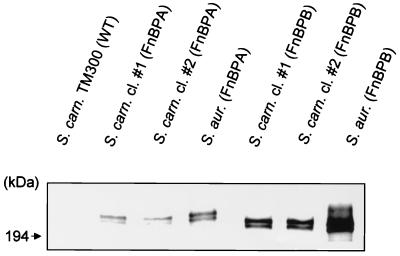
FnBPs expressed in S. carnosus bind fibronectin.
Western ligand blots confirmed that wild-type S. carnosus TM300 does not produce any factor with an affinity for fibronectin (Fig. 2). In contrast, cell wall protein extracts from all FnBP-expressing transformants contained a protein with an affinity for fibronectin. These bands corresponded to the bands obtained from cell wall extracts of S. aureus (strain DU5883) expressing the respective FnBP from a multicopy plasmid, which were used as positive controls (Fig. 2). Surprisingly, FnBPB yielded a stronger signal than FnBPA under these conditions. As seen previously (1), FnBPs migrated at an apparent molecular mass of >200 kDa, even though the predicted molecular masses for the mature forms of FnBPA and FnBPB are ∼110 and ∼100 kDa, respectively. As before, two bands, corresponding to the mature protein and the pro-protein, were detected (1).
FIG. 2.
FnBPs expressed in S. carnosus (S. carn.) specifically bind fibronectin. A Western ligand blot shows cell wall-associated proteins of S. carnosus transformants (strain TM300) expressing FnBPs. FnBPs were detected by a sandwich technique using fibronectin and then anti-N-terminal fibronectin antibodies as described in Materials and Methods. As a control, S. aureus (S. aur.) strains DU5883(pFNBA4) and DU5883(pFNBB4), expressing the respective FnBPs, were processed and run in parallel. Strain DU5883 did not yield any signal (data not shown). WT, wild type; cl, clone.
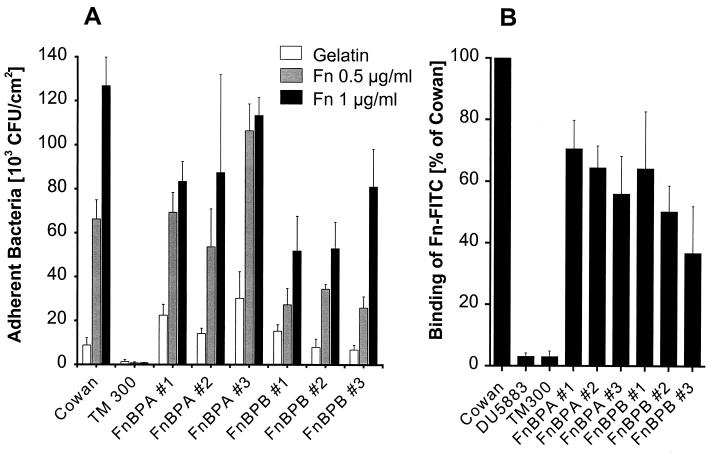
S. carnosus transformants expressing FnBPs bind to immobilized and soluble fibronectin.
Classically, two assays are used in order to assess the function of adhesins, i.e., binding to host proteins immobilized on surfaces (adherence) and to soluble proteins. Generally, for a given adhesin, these two properties correlate well in the same isolate, but there are exceptions, e.g., for strains 8325-4 and Newman (50). Therefore, we tested the transformants of S. carnosus for the correct surface expression and function of adhesins quantitatively by both methods. The interaction of proteins and strains was found to be as predicted upon the heterologous expression of FnBPs. The respective FnBPs effectively and specifically conferred adhesion to immobilized fibronectin in a dose-dependent manner (Fig. 3A) and binding to soluble fibronectin (Fig. 3B).
FIG. 3.
S. carnosus clones expressing FnBPs dose dependently bind to immobilized and soluble fibronectin. (A) Adherence of S. carnosus strain TM300 transformants to immobilized fibronectin (Fn). PMMA coverslips were precoated with 0.5 mg of gelatin per ml in order to optimize fibronectin adsorption (see Materials and Methods). Results are means and SEMs for four independent experiments run in duplicate. (B) Binding of soluble FITC-labeled fibronectin (Fn-FITC) (5 μg/ml) by S. carnosus strain TM300 transformants. Results are means and SEMs for four independent experiments run in duplicate.
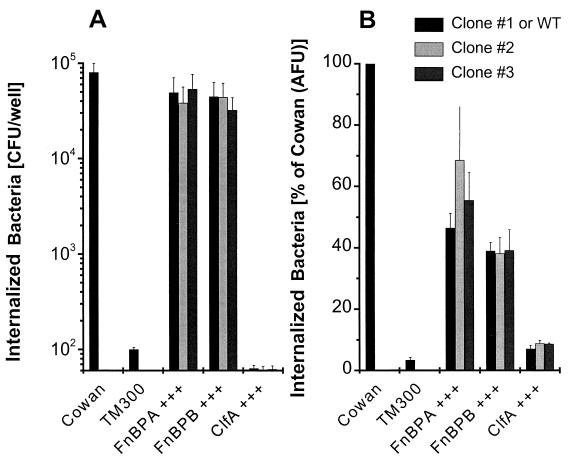
Heterologous expression of FnBPs in S. carnosus and L. lactis confers invasiveness.
In order to test whether a coreceptor(s) is required for FnBP-mediated invasion, we expressed both types of S. aureus FnBPs (from strain 8325-4) in S. carnosus strain TM300. Nearly identical results were obtained by both the lysostaphin protection (Fig. 4A) and the flow cytometric (Fig. 4B) assays. The expression of either FnBPA or FnBPB conferred invasiveness to strain TM300 (three clones each were tested for FnBPA and FnBPB) that was on the same order of magnitude as that seen for strain Cowan 1 (about 50%), whereas all three clones expressing ClfA did not acquire invasiveness. This process was highly efficient since, in the lysostaphin protection assay, about 5% of the initial inoculum (106 CFU/well) could be recovered after only 1 h of infection for FnBP-expressing S. carnosus. The numbers of bacteria recovered were (8.02 ± 1.91) × 104 CFU and (0.01 ± 0.00) × 104 CFU for strains Cowan 1 and TM300, respectively. Results similar to those for the lysostaphin protection assay were obtained when penicillin (2 μg/ml) was used instead of lysostaphin (data not shown). The zipper-type internalization was demonstrated by the nearly identical results obtained with live and fixed bacteria, indicating that no active secretion or de novo synthesis of invasogenic factors by the bacteria was necessary for invasion.
FIG. 4.
FnBPs confer invasiveness to noninvasive S. carnosus. Overexpression (+++) of adhesins in strain TM300 by multicopy plasmids was tested for invasion of 293 cells. (A) Total internalized bacteria were measured by lysostaphin (20 μg/ml) protection. Results are means and SEMs for five independent experiments (ClfA, n = 2) run in duplicate and are expressed as recovered CFU, normalized for the actual inoculum. Similar results were obtained when penicillin (2 μg/ml) was used instead of lysostaphin (data not shown). (B) Total internalized bacteria were measured by flow cytometry with fixed bacteria. Results are means and SEMs for three independent experiments (ClfA, n = 2) run in duplicate and are expressed as invasiveness relative to that of strain Cowan 1. AFU, arbitrary fluorescence units; WT, wild type.
In addition, we corroborated the role of FnBPs in cellular invasion by use of a species even less related to S. aureus. For this purpose, we used L. lactis and expressed two known surface proteins: FnBPA from strain 8325-4 and the major fibrinogen-binding protein, clumping factor (ClfA). As with S. aureus, the expression of surface proteins other than FnBPs did not confer invasiveness (cf. Fig. 1). In a penicillin (2 μg/ml) protection assay, (6.9 ± 0.3) × 104 and 5.5 ± 0.6 × 104 CFU/well were recovered for Cowan 1 and FnBPA-expressing L. lactis, respectively, as opposed to (0.07 ± 0.03) × 104 and (0.12 ± 0.02) × 104 CFU/well for wild-type L. lactis and ClfA-expressing L. lactis, respectively (Fig. 5A). Lactococci expressing FnBPA were ∼85% as invasive as the control strain Cowan 1 when measured by flow cytometry with fixed bacteria (Fig. 5B). In a second series of flow cytometric experiments, we directly compared L. lactis expressing FnBPB with L. lactis expressing FnBPA. L. lactis expressing FnBPB was ∼80% as invasive as L. lactis expressing FnBPA (n = 3; run in duplicate; data not shown).
FIG. 5.
FnBPs confer invasiveness to noninvasive L. lactis. Overexpression (+++) of adhesins in strain 1363(pIL253) led to invasion of 293 cells. (A) Total internalized bacteria were measured by penicillin (2 μg/ml) protection. Results are means and SEMs for four independent experiments (ClfA, n = 2) run in duplicate and are expressed as recovered CFU, normalized for the actual inoculum and relative to strain Cowan 1. (B) Total internalized bacteria were measured by flow cytometry with fixed bacteria. Results are means and SEMs for three independent experiments run in duplicate and are expressed as invasiveness relative to that of strain Cowan 1. AFU, arbitrary fluorescence units; WT, wild type.
FnBP-coated polystyrene beads are internalized by 293 cells.
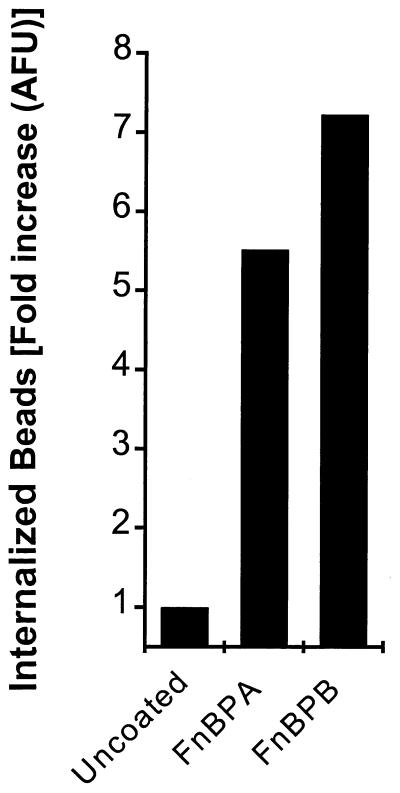
Finally, we tested the invasive property of S. aureus FnBPs by coating and covalently coupling fluorescent polystyrene beads with purified FnBPA or FnBPB. 293 cells were then incubated with the beads, harvested, and assayed by flow cytometry for trypsin-EDTA-resistant fluorescence similar to that described for fixed bacteria (Fig. 6). Both FnBPA and FnBPB were able to mediate substantial uptake of the beads in our model.
FIG. 6.
FnBP-coated beads are internalized by 293 cells. Carboxylated green fluorescent polystyrene beads coated with either FnBPA or FnBPB were incubated with 293 cells and assayed in the same way as fluorescent bacteria. Trypsin-EDTA-resistant cell-associated fluorescence was read by flow cytometry. Untreated beads served as a control but yielded a high background. Results were normalized to the mean fluorescence of preparation of the beads. AFU, arbitrary fluorescence units.
DISCUSSION
To our knowledge, this is the first study to address whether S. aureus FnBPs are sufficient to mediate fibronectin binding and invasion of host cells on their own or whether (possibly S. aureus-specific) coreceptors are required. Here we show that the expression of either FnBPA or FnBPB in two different gram-positive heterologous hosts, namely, S. carnosus and L. lactis, conferred the abilities to agglutinate in rabbit plasma and to invade mammalian cells. Based on DNA-DNA hybridization experiments under optimal conditions, S. aureus and S. carnosus display less than 25% relatedness (∼10% under stringent conditions) (26). L. lactis is even less related to S. aureus than is S. carnosus. The invasiveness of FnBP-expressing S. carnosus clones correlated well with the binding of soluble and immobilized fibronectin. This result might reflect the amount of accessible surface FnBPs and suggests that invasiveness is a direct function thereof. The expression of S. aureus ClfA, the major fibrinogen adhesin, in both species did not confer invasiveness, a result which excludes a role for ClfA and fibrinogen in S. aureus invasion, at least in this model. Since the results obtained for cellular invasion with live and fixed bacteria were nearly identical, we have provided further evidence for the internalization process being a zipper-type mechanism, as previously suggested for cells of epithelial, endothelial, and fibroblastic origins (50).
S. aureus appears to be critically dependent on either of the two known forms of FnBPs, FnBPA or FnBPB, to mediate efficient invasion. The lack of FnBPs largely abrogates efficient internalization of bacteria, with up to a 500-fold reduction (9, 14, 27, 42, 50), whereas it can be restored by the expression of either form of FnBPs in noninvasive strains of three different species (Fig. 1, 3, and 5). Previous data obtained with several isogenic S. aureus MSCRAMM deletion mutants of different strains (protein A, clumping factor, and coagulase [50]; protein A and clumping factor [9]) did not show a role for these MSCRAMMs in invasion of host cells. Thus, we have now proven our proposed model that S. aureus appears to critically depend on FnBPs as invasins (50). Nevertheless, it is conceivable that there may be other S. aureus surface structures besides FnBPA and FnBPB which are sufficient for cellular invasion under different conditions. Obviously, our data do not exclude accessory receptors in S. aureus invasion, which might, e.g., accelerate the rate of internalization or which might be required for efficient initial adhesion under certain circumstances. However, unlike streptococcal M proteins, such putative coreceptors apparently cannot induce invasion on their own.
Several other surface proteins of pathogenic bacteria (e.g., the gram-negative rod Yersinia, the gram-positive rod Listeria monocytogenes, and the gram-negative coccus Neisseria gonorrhoeae) have been shown to mediate invasion of host cells via a zipper-like mechanism. In most circumstances, there appears to be no need for an additional bridging molecule between the bacterium and the host cell (3, 21, 22, 28, 34, 52). Recently, L. monocytogenes was found to bind fibronectin specifically (18), a finding which probably makes the model for cellular invasion less clear-cut than previously thought.
Like several other bacteria, such as N. gonorrhoeae, S. aureus also binds proteoglycan receptors on mammalian cells (45). We can now formally exclude a requirement of glycosaminoglycan bridging for cellular invasion by S. aureus, since FnBP-coated polystyrene beads were internalized by 293 cells, apparently without the need for coreceptors. In addition, glycosaminoglycan bridging does not seem to be of importance for the following reasons. (i) S. aureus possesses a large number of adhesins, most of which are highly specific for a given host protein. These receptors would be costly if produced in parallel with an efficient, nonspecific mechanism. (ii) One of the three known heparin-binding sites on fibronectin is located on the 29-kDa N-terminal fragment of fibronectin and is identical or located extremely closely to the S. aureus-binding site, since there is competitive binding between heparin and S. aureus (53). Competition for binding between heparin and S. aureus is also known for vitronectin (16). This finding would suggest inhibition of invasion rather than enhancement by glycosaminoglycan binding. (iii) Unlike fibronectin, purified monomeric vitronectin (albeit without the addition of glycosaminoglycans) did not promote interactions between S. aureus and 293 cells which had been stripped of fibronectin and resuspended by trypsin-EDTA treatment (50).
To date, at least six Streptococcus pyogenes surface proteins have been implicated in cellular invasion (in different cellular models) (5); in addition, there appear to exist at least eight surface proteins in S. pyogenes which are able to bind fibronectin (25), but only three of them possess both abilities (i.e., to promote invasiveness and fibronectin binding). Protein F1 (PrtF1), an allelic variant of SfbI, is the streptococcal FnBP with homology to S. aureus FnBPs. In addition, other serotypes of M proteins, e.g., M1, also mediate streptococcal invasion and are able to directly bind to fibronectin (6). One of the best-characterized invasion mechanisms appears to be a two-component process (see reference 50 for an overview) which uses streptococcal protein M6 mainly for adherence and protein F1 mainly for internalization. However, strains expressing protein M6 in the absence of protein F1 still retain about 25% of their invasive capacity (23). The expression of F1 in weakly invasive Enterococcus faecalis strongly enhances invasiveness (39). SfbI is sufficient for invasion of HEp-2 cells, as demonstrated by coating of latex beads (3 μm, roughly three times the diameter of streptococci) with His-tagged recombinant protein (36). A limitation of these data is that the beads were resuspended in 1% FCS, so that they were coated with substantial amounts of adhesive host glycoproteins (besides fibrinogen and other components, plasma contains 200 to 400 μg of fibronectin and vitronectin per ml each [4]); this situation made it impossible to assess the requirement of serum proteins for the interaction. Furthermore, the overall efficiency of invasion for the native streptococcal strain was roughly 10 times lower with an MOI 20 times higher than that used in this study, in which conditions probably more comparable to the in vivo environment were used.
The major two fibronectin-binding domains of F1 are known (RD2 repeats and UR region) (41); they appear to act cooperatively in S. pyogenes invasion (39). However, in another study, the isolated UR region alone was able to completely inhibit streptococcal invasion (40). In S. aureus, the major high-affinity binding site, close to the C-terminal wall-spanning region, has been located to 3.5 tandem repeats of ∼38 amino acids and to an additional repeat ∼100 amino acids upstream of these (25). The role of these D repeats in cellular invasion was shown in competition experiments. Pretreatment of cells with a soluble recombinant fragment comprising the D1 to D4 repeats resulted in complete inhibition of invasion (50). Surprisingly, in a study using a similar approach with His-tagged proteins, the fragment comprising the (variable) A and B regions of FnBPA was as effective as the D1 to D3 repeats for reducing invasion; however, complete FnBPA was over 100 times more effective on a molar basis than either of the two fragments (14), suggesting a synergistic effect of the two adhesin domains.
The importance of FnBPs for S. aureus is evidenced by the high prevalence of FnBPs in clinical isolates: all 25 strains (35) and all but 1 of 30 strains (51) tested harbored at least one fnb gene, as detected by PCR analysis of the entire region encompassing the adjacent fnbA and fnbA genes and subsequent Southern blotting. In contrast, the prevalence of the allelic variants prtF1 or sfbI in S. pyogenes is estimated to be only approximately 70% (5), and the proportion of strains expressing them might be even lower. In accordance, all 15 clinical S. aureus isolates, as opposed to 5 out of 8 laboratory strains tested (62.5%), were invasive on a cellular level (50). If this role for FnBPs in invasion can be confirmed in vivo, it should allow a further understanding of the relationship between S. aureus pathogenicity and invasiveness for host cells.
ACKNOWLEDGMENTS
We thank Marion Reilly, Susanne Weber, Manuela Bento, and Elzbieta Huggler for excellent assistance with experiments.
B.S. holds a fellowship (Infektionsforschung) from Deutsches Krebsforschungszentrum (Heidelberg, Germany), funded by Bundesministerium für Forschung und Technologie, Bonn and Berlin, Germany. This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Collaborative Research Center 293, project A6, to M.H. and G.P.) and in part by the Swiss National Science Foundation—grants 31-52149.97 (to Y.-A.Q.), 31-55344.98 (to D.L.), and 3100.45891.95/1 (to K.-H.K.).
REFERENCES
- 1.Bisognano C, Vaudaux P E, Lew D P, Ng E Y, Hooper D C. Increased expression of fibronectin-binding proteins by fluoroquinolone-resistant Staphylococcus aureus exposed to subinhibitory levels of ciprofloxacin. Antimicrob Agents Chemother. 1997;41:906–913. doi: 10.1128/aac.41.5.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchara J P, Sanchez M, Chevailler A, Marot-Leblond A, Lissitzky J C, Tronchin G, Chabasse D. Sialic acid-dependent recognition of laminin and fibrinogen by Aspergillus fumigatus conidia. Infect Immun. 1997;65:2717–2724. doi: 10.1128/iai.65.7.2717-2724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun L, Ohayon H, Cossart P. The InlB protein of Listeria monocytogenes is sufficient to promote entry into mammalian cells. Mol Microbiol. 1998;27:1077–1087. doi: 10.1046/j.1365-2958.1998.00750.x. [DOI] [PubMed] [Google Scholar]
- 4.Chhatwal G S, Preissner K T. Extracellular matrix interactions with Gram-positive pathogens. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 2000. pp. 78–86. [Google Scholar]
- 5.Cue D, Dombek P E, Cleary P P. Intracellular invasion by Streptococcus pyogenes: invasins, host receptors, and relevance to human disease. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-positive pathogens. Washington, D.C.: ASM Press; 2000. pp. 27–33. [Google Scholar]
- 6.Cue D, Dombek P E, Lam H, Cleary P P. Streptococcus pyogenes serotype M1 encodes multiple pathways for entry into human epithelial cells. Infect Immun. 1998;66:4593–4601. doi: 10.1128/iai.66.10.4593-4601.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deitch E A, Haskel Y, Cruz N, Xu D, Kvietys P R. Caco-2 and IEC-18 intestinal epithelial cells exert bactericidal activity through an oxidant-dependent pathway. Shock. 1995;4:345–350. doi: 10.1097/00024382-199511000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Drake T A, Pang M. Staphylococcus aureus induces tissue factor expression in cultured human cardiac valve endothelium. J Infect Dis. 1988;157:749–756. doi: 10.1093/infdis/157.4.749. [DOI] [PubMed] [Google Scholar]
- 9.Dziewanowska K, Patti J M, Deobald C F, Bayles K W, Trumble W R, Bohach G A. Fibronectin binding protein and host cell tyrosine kinase are required for internalization of Staphylococcus aureus by epithelial cells. Infect Immun. 1999;67:4673–4678. doi: 10.1128/iai.67.9.4673-4678.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elsinghorst E A. Measurement of invasion by gentamicin resistance. Methods Enzymol. 1994;236:405–420. doi: 10.1016/0076-6879(94)36030-8. [DOI] [PubMed] [Google Scholar]
- 11.Etienne J, Eykyn S J. Increase in native valve endocarditis caused by coagulase negative staphylococci: an Anglo-French clinical and microbiological study. Br Heart J. 1990;64:381–384. doi: 10.1136/hrt.64.6.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. . (Erratum, 278:373.) [DOI] [PubMed] [Google Scholar]
- 13.Foster T J, Höök M. Surface protein adhesins of Staphylococcus aureus. Trends Microbiol. 1998;6:484–488. doi: 10.1016/s0966-842x(98)01400-0. [DOI] [PubMed] [Google Scholar]
- 14.Fowler T, Wann E R, Joh D, Johansson S, Foster T J, Höök M. Cellular invasion by Staphylococcus aureus involves a fibronectin bridge between the bacterial fibronectin-binding MSCRAMMs and host cell β1 integrins. Eur J Biochem, 2000;79:672–679. doi: 10.1078/0171-9335-00104. [DOI] [PubMed] [Google Scholar]
- 15.Francois P, Schrenzel J, Stoerman-Chopard C, Favre H, Herrmann M, Foster T J, Lew D P, Vaudaux P. Identification of plasma proteins adsorbed on hemodialysis tubing that promote Staphylococcus aureus adhesion. J Lab Clin Med. 2000;135:32–42. doi: 10.1016/s0022-2143(00)70018-7. [DOI] [PubMed] [Google Scholar]
- 16.Francois P P, Preissner K T, Herrmann M, Haugland R P, Vaudaux P, Lew D P, Krause K H. Vitronectin interaction with glycosaminoglycans. Kinetics, structural determinants, and role in binding to endothelial cells. J Biol Chem. 1999;274:37611–37619. doi: 10.1074/jbc.274.53.37611. [DOI] [PubMed] [Google Scholar]
- 17.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gilot P, Andre P, Content J. Listeria monocytogenes possesses adhesins for fibronectin. Infect Immun. 1999;67:6698–6701. doi: 10.1128/iai.67.12.6698-6701.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Greene C, McDevitt D, Francois P, Vaudaux P E, Lew D P, Foster T J. Adhesion properties of mutants of Staphylococcus aureus defective in fibronectin-binding proteins and studies on the expression of fnb genes. Mol Microbiol. 1995;17:1143–1152. doi: 10.1111/j.1365-2958.1995.mmi_17061143.x. [DOI] [PubMed] [Google Scholar]
- 20.Holo H, Nes I F. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isberg R R, Leong J M. Multiple β1 chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 22.Isberg R R, Voorhis D L, Falkow S. Identification of invasin: a protein that allows enteric bacteria to penetrate cultured mammalian cells. Cell. 1987;50:769–778. doi: 10.1016/0092-8674(87)90335-7. [DOI] [PubMed] [Google Scholar]
- 23.Jadoun J, Ozeri V, Burstein E, Skutelsky E, Hanski E, Sela S. Protein F1 is required for efficient entry of Streptococcus pyogenes into epithelial cells. J Infect Dis. 1998;178:147–158. doi: 10.1086/515589. [DOI] [PubMed] [Google Scholar]
- 24.Jevon M, Guo C, Ma B, Mordan N, Nair S P, Harris M, Henderson B, Bentley G, Meghji S. Mechanisms of internalization of Staphylococcus aureus by cultured human osteoblasts. Infect Immun. 1999;67:2677–2681. doi: 10.1128/iai.67.5.2677-2681.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joh D, Wann E R, Kreikemeyer B, Speziale P, Höök M. Role of fibronectin-binding MSCRAMMs in bacterial adherence and entry into mammalian cells. Matrix Biol. 1999;18:211–223. doi: 10.1016/s0945-053x(99)00025-6. [DOI] [PubMed] [Google Scholar]
- 26.Kloos W. Taxonomy and systematics of staphylococci indigenous to humans. In: Crossley K B, Archer G L, editors. The staphylococci in human disease. New York, N.Y: Churchill Livingstone; 1997. pp. 113–137. [Google Scholar]
- 27.Lammers A, Nuijten P J, Smith H E. The fibronectin binding proteins of Staphylococcus aureus are required for adhesion to and invasion of bovine mammary gland cells. FEMS Microbiol Lett. 1999;180:103–109. doi: 10.1111/j.1574-6968.1999.tb08783.x. [DOI] [PubMed] [Google Scholar]
- 28.Lecuit M, Ohayon H, Braun L, Mengaud J, Cossart P. Internalin of Listeria monocytogenes with an intact leucine-rich repeat region is sufficient to promote internalization. Infect Immun. 1997;65:5309–5319. doi: 10.1128/iai.65.12.5309-5319.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J C. Electrotransformation of staphylococci. Methods Mol Biol. 1993;47:209–216. doi: 10.1385/0-89603-310-4:209. [DOI] [PubMed] [Google Scholar]
- 30.Lew D P, Waldvogel F A. Osteomyelitis. N Engl J Med. 1997;336:999–1007. doi: 10.1056/NEJM199704033361406. [DOI] [PubMed] [Google Scholar]
- 31.Lowy F D. Staphylococcus aureus infections. N Engl J Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 32.Marmur J. A procedure for isolation of desoxyribonucleic acid from microorganisms. J Mol Biol. 1961;3:208–218. [Google Scholar]
- 33.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 34.Mengaud J, Ohayon H, Gounon P, Mege R-M, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenes into epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 35.Minhas T, Ludlam H A, Wilks M, Tabaqchali S. Detection by PCR and analysis of the distribution of a fibronectin-binding protein gene (fbn) among staphylococcal isolates. J Med Microbiol. 1995;42:96–101. doi: 10.1099/00222615-42-2-96. [DOI] [PubMed] [Google Scholar]
- 36.Molinari G, Talay S R, Valentin-Weigand P, Rohde M, Chhatwal G S. The fibronectin-binding protein of Streptococcus pyogenes, SfbI, is involved in the internalization of group A streptococci by epithelial cells. Infect Immun. 1997;65:1357–1363. doi: 10.1128/iai.65.4.1357-1363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967;33:155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- 38.Ogawa S K, Yurberg E R, Hatcher V B, Levitt M A, Lowy F D. Bacterial adherence to human endothelial cells in vitro. Infect Immun. 1985;50:218–224. doi: 10.1128/iai.50.1.218-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Okada N, Tatsuno I, Hanski E, Caparon M, Sasakawa C. Streptococcus pyogenes protein F promotes invasion of HeLa cells. Microbiology. 1998;144:3079–3086. doi: 10.1099/00221287-144-11-3079. [DOI] [PubMed] [Google Scholar]
- 40.Ozeri V, Rosenshine I, Mosher D F, Fässler R, Finlay B, Hanski E. Roles of integrins and fibronectin in the entry of Streptococcus pyogenes into cells via protein F1. Mol Microbiol. 1998;30:625–637. doi: 10.1046/j.1365-2958.1998.01097.x. [DOI] [PubMed] [Google Scholar]
- 41.Ozeri V, Tovi A, Burstein I, Natanson-Yaron S, Caparon M G, Yamada K M, Akiyama S K, Vlodavsky I, Hanski E. A two-domain mechanism for group A streptococcal adherence through protein F to the extracellular matrix. EMBO J. 1996;15:989–998. [PMC free article] [PubMed] [Google Scholar]
- 42.Peacock S J, Foster T J, Cameron B J, Berendt A R. Bacterial fibronectin-binding proteins and endothelial cell surface fibronectin mediate adherence of Staphylococcus aureus to resting human endothelial cells. Microbiology. 1999;145:3477–3486. doi: 10.1099/00221287-145-12-3477. [DOI] [PubMed] [Google Scholar]
- 43.Que Y-A, Haefliger J-A, Francioli P, Moreillon P. Expression of Staphylococcus aureus clumping factor A in Lactococcus lactis subsp. cremoris using a new shuttle vector. Infect Immun. 2000;68:3516–3523. doi: 10.1128/iai.68.6.3516-3522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankin S, Isberg R R, Leong J M. The integrin-binding domain of invasin is sufficient to allow bacterial entry into mammalian cells. Infect Immun. 1992;60:3909–3912. doi: 10.1128/iai.60.9.3909-3912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rostand K S, Esko J D. Microbial adherence to and invasion through proteoglycans. Infect Immun. 1997;65:1–8. doi: 10.1128/iai.65.1.1-8.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schleifer K H, Fischer U. Description of a new species of the genus Staphylococcus: Staphylococcus carnosus. Int J Syst Bacteriol. 1982;32:153–156. [Google Scholar]
- 47.Schmidt H, Bukholm G, Holberg-Petersen M. Adhesiveness and invasiveness of staphylococcal species in a cell culture model. APMIS. 1989;97:655–660. doi: 10.1111/j.1699-0463.1989.tb00457.x. [DOI] [PubMed] [Google Scholar]
- 48.Signäs C, Raucci G, Jönsson K, Lindgren P E, Anantharamaiah G M, Höök M, Lindberg M. Nucleotide sequence of the gene for a fibronectin-binding protein from Staphylococcus aureus: use of this peptide sequence in the synthesis of biologically active peptides. Proc Natl Acad Sci USA. 1989;86:699–703. doi: 10.1073/pnas.86.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Simon D, Chopin A. Construction of a vector plasmid family and its use for molecular cloning in Streptococcus lactis. Biochimie. 1988;70:559–566. doi: 10.1016/0300-9084(88)90093-4. [DOI] [PubMed] [Google Scholar]
- 50.Sinha B, Francois P P, Nüße O, Foti M, Hartford O M, Vaudaux P, Foster T J, Lew D P, Herrmann M, Krause K H. Fibronectin-binding protein acts as Staphylococcus aureus invasin via fibronectin bridging to integrin α5β1. Cell Microbiol. 1999;1:101–117. doi: 10.1046/j.1462-5822.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- 51.Smeltzer M S, Gillaspy A F, Pratt L, Jr, Thames M D, Iandolo J J. Prevalence and chromosomal map location of Staphylococcus aureus adhesin genes. Gene. 1997;196:249–259. doi: 10.1016/s0378-1119(97)00237-0. [DOI] [PubMed] [Google Scholar]
- 52.van Putten J P, Duensing T D, Cole R L. Entry of OpaA+ gonococci into HEp-2 cells requires concerted action of glycosaminoglycans, fibronectin and integrin receptors. Mol Microbiol. 1998;29:369–379. doi: 10.1046/j.1365-2958.1998.00951.x. [DOI] [PubMed] [Google Scholar]
- 53.Vaudaux P, Avramoglou T, Letourneur D, Lew D P, Jozefonvicz J. Inhibition by heparin and derivatized dextrans of Staphylococcus aureus adhesion to fibronectin-coated biomaterials. J Biomater Sci Polym Ed. 1992;4:89–97. [PubMed] [Google Scholar]
- 54.Vaudaux P, Pittet D, Haeberli A, Lerch P G, Morgenthaler J J, Proctor R A, Waldvogel F A, Lew D P. Fibronectin is more active than fibrin or fibrinogen in promoting Staphylococcus aureus adherence to inserted intravascular catheters. J Infect Dis. 1993;167:633–641. doi: 10.1093/infdis/167.3.633. [DOI] [PubMed] [Google Scholar]
- 55.Vaudaux P E, Waldvogel F A, Morgenthaler J J, Nydegger U E. Adsorption of fibronectin onto polymethylmethacrylate and promotion of Staphylococcus aureus adherence. Infect Immun. 1984;45:768–774. doi: 10.1128/iai.45.3.768-774.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]