Summary
Background
A nationwide Severe Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) vaccination campaign was initiated in Brazil in January 2021 with CoronaVac (Sinovac Biotech) and ChAdOx1 nCoV-19 (AstraZeneca) followed by BNT162b2 mRNA (Pfizer–BioNTech) and Ad26.COV2.S (Johnson & Johnson–Janssen) vaccines. Here we provide estimates of the number of severe cases and deaths due to coronavirus disease (COVID-19) averted during the first year of the mass vaccination campaign in Brazil.
Methods
Data on COVID-19 vaccination and COVID-19-related illness and death were obtained from the Brazilian Ministry of Health and used to estimate the direct effects of the vaccination campaign on the number of severe cases and deaths due to COVID-19 occurring between January 17, 2021 and January 31, 2022. To this end, we compared the daily age-specific rates between the unvaccinated population and the “at least partly vaccinated” population (received at least one dose of a two-dose vaccine), as well as other two vaccination subgroups, “fully vaccinated” (completed the one- or two-dose vaccine schedule), and “boosted-vaccinated” (fully vaccinated and recipients of booster dose) populations.
Findings
We estimated that 74% (n = 875,846; 95% confidence interval, CI 843,383–915,709) of total expected cases of severe COVID-19 and 82% (n = 303,129; 95% CI 284,019–321,681) of total expected deaths due to COVID-19 were averted in the first year of the national vaccination campaign. The averted burden was heterogeneous between age groups and higher in the more populous states. However, outcome rate differences between vaccinated and unvaccinated groups were higher in the less populated states.
Interpretation
The first year of the COVID-19 vaccination program in Brazil saved the lives of at least 303,129 adults. The results highlight the need for future vaccination campaigns, including those required in the current pandemic, to rapidly achieve high uptake, particularly among the elderly and residents of the least populous regions.
Funding
Ministry of Health (Brazil).
Keywords: Pandemic, SARS-CoV-2, COVID-19, Immunization, Vaccine, Serious disease, Death
Research in context.
Evidence before this study
After a comprehensive literature search for publications on the estimated averted burden of COVID-19 due to the mass vaccination campaign in Brazil (performed using PubMed on December 12, 2021), we identified only one study that estimated the impact of COVID-19 vaccination in averting mortality due to COVID-19 in the elderly population. Thus, there was a clear need for more comprehensive studies to estimate the impact of the mass vaccination campaign on not only deaths but also severe cases of COVID-19 in Brazil, especially among other age groups.
Added value of this study
We retrospectively analyzed official nationwide records on vaccination, severe COVID-19 cases, and COVID-19-related deaths for the 27 states and 5,569 municipalities of Brazil, starting from the date of initiation of the vaccination campaign (January 17, 2021) to January 31, 2022. Our estimates show that COVID-19 vaccination averted nearly 900,000 cases of severe COVID-19 and about 300,000 deaths during the first year of vaccination.
Implications of all the available evidence
Our findings provide real-world estimates of the benefits of COVID-19 vaccination, as suggested by formal vaccine trials and cohort studies. We estimated that COVID-19 vaccines averted a 74% and an 82% increase in the number of severe cases and deaths, respectively. Our results provide a lower bound of the remarkable impact of the first year of the mass vaccination campaign on the COVID-19 burden in Brazil and highlight the overall differences between the outcomes in specific age groups and regions of residence.
Introduction
Since the start of the current Severe Acute Respiratory Syndrome-associated Coronavirus 2 (SARS-CoV-2) pandemic, more than 631 million confirmed cases and 6.5 million deaths due to coronavirus disease (COVID-19) have been reported worldwide (as of November 02, 2022).1,2 In Brazil, more than 34 million COVID-19 cases and 688 thousand deaths were reported, placing Brazil as the fifth in the number of cases and second in the death toll, reaching more than 4,148 daily deaths in April 2021.1,2
Vaccination was a key measure in controlling the COVID-19 health burden. Brazil started a nationwide COVID-19 vaccination campaign on January 17, 2021, prioritizing people at relatively high risk of severe disease (the elderly and people with chronic health conditions), vulnerable populations (e.g., homeless and indigenous people), healthcare workers, and essential workers, and then extending to the entire population by decreasing age.
The campaign in Brazil began with CoronaVac (Sinovac) and ChAdOx1 nCov-19 (AstraZeneca/Oxford University), which were administered in two doses scheduled 28 days apart and 12 weeks apart, respectively.3,4 On April 29, 2021, the BNT162b2 mRNA vaccine (Pfizer–BioNTech) was added to the campaign (two doses, 12 weeks apart) and, finally, on June 15, 2021, the single-dose Ad26.COV2.S (Janssen) vaccine was made available.3,4 Thus, the introduction of the four vaccines was staggered over the first 6 months of 2021.
During 2021, Brazil experienced successive waves of SARS-CoV-2 infections caused by the Gamma (P.1) variant, which predominated from January through August and peaked in May; the Delta (B.1.617.2) variant, which predominated through December 2021; and the Omicron (B.1.1.529) variant which emerged in Brazil in late November, 2021.1,2,5
Brazil has a population of more than 220 million people.6 By February 18, 2022, more than 300 million doses of the vaccines had been administered, corresponding to approximately 81% of the population being partly or fully immunized.4,6
Previous analyses have found that the vaccination campaign in Brazil was highly effective, with protection against severe cases reaching 86.4% and 61.2%, for the most used vaccines in the primary course of vaccination, ChAdOx1 nCov-19 and CoronaVac, respectively.7, 8, 9 These findings corroborated data from real-world effectiveness studies in other countries, such as the United Kingdom, United States, and Israel.9, 10, 11, 12
Despite the speed and breadth of coverage achieved by this nationwide mass vaccination campaign, the precise number of COVID-19 severe cases and deaths averted in Brazil remains unknown. The only estimates come from two studies that evaluated the early impact of vaccination among the elderly13,14 and residents of specific Brazilian states.15,16 Quantifying the nationwide disease burden averted by the vaccination campaign would be valuable not only to help elucidate and assess the public health benefits of vaccination but also to inform the design of mass vaccination campaigns for future pandemics and even possibly during the ongoing COVID-19 pandemic. In the present study, we estimated the COVID-19 severe cases and deaths due to COVID-19 averted during the first year of the nationwide vaccination campaign in Brazil.
Methods
Study design, population, and data source
This was an ecological study using retrospective observational data to estimate the number of severe cases and deaths from COVID-19 averted from January 17, 2021, the initiation date of the mass vaccination campaign, until January 31, 2022. We included all individuals aged ≥20 years who had received at least one dose of CoronaVac, ChAdOx1 nCov-19, BNT162b2, or Ad26.COV2.S vaccines, or who had a laboratory-confirmed SARS-CoV-2 infection.
We analyzed data curated from three national databases: (i) COVID-19 Vaccination Campaign (SI-PNI), which includes anonymized individual-level data on COVID-19 vaccination17; (ii) Severe Acute Respiratory Infection/Illness (SARI) from the Influenza Epidemiological Surveillance System (SIVEP-Gripe), which holds data on all COVID-19 severe cases that led to hospitalization and deaths due to COVID-1918; and (iii) Brazilian Institute for Geography and Statistics (IBGE) data on age-specific population estimates at the national, state, and municipality level.17 Data from SI-PNI and SIVEP-Gripe were probabilistically linked by the Brazilian Ministry of Health and further information is provided in Supplementary Material 1.
We excluded individuals who: (i) were vaccinated but lacked a record of the vaccination dates; (ii) had a laboratory-confirmed infection but lacked a record of the symptom onset date; and (iii) were vaccinated but had inconsistent vaccination records, such as a discordance of first and second vaccines or lack of a record for the second dose for those on a two-dose vaccine schedule, or lack of a record for the any of the two first doses in boosted individuals (flow diagram illustrating the steps to reach the final population are presented in Supplementary Material 1).
Outcomes
We estimated the averted burden for two outcomes: severe COVID-19 cases and deaths due to COVID-19. We defined COVID-19 severe cases and deaths in vaccinated as those individuals with laboratory-confirmed RT-PCR or rapid antigen testing positivity at least 14 days after vaccination, following the definitions given by the Brazilian Ministry of Health (detailed in Supplementary Material 1). The unvaccinated group were defined as individuals with severe disease or death records but no record of vaccination, or individuals with vaccination records whose symptom onset before the first dose of the vaccine. Outcomes with symptom onset occurring ≤14 days after the vaccination date were excluded from the analysis.
Vaccination groups
We calculated the number of severe COVID-19 cases and deaths averted (i) among individuals who were “at least partly vaccinated,” defined as those who had received at least one dose of a two-dose vaccine (i.e., CoronaVac, ChAdOx1 nCov-19, BNT162b2 vaccines); and two other vaccination subsets: (ii) “fully vaccinated,” defined as those who had received one dose of Ad26.COV2.S or two doses of the other three vaccines; and (iii) “boosted-vaccinated,” defined as those who were fully vaccinated and received a booster dose. Only one booster dose was approved in Brazil during the period covered by this study.
Statistical analysis
To estimate the averted burden of COVID-19, we used the steps described by Haas.19 Initially, we calculated the daily susceptible population in age-specific categories (20–29, 30–39, 40–49, 50–59, 60–69, 70–79, 80–89, and ≥90 years) by subtracting the number of new cases from the population estimates within each age group (IBGE). We then assigned the susceptible population into the unvaccinated group or the vaccinated groups, for each of the above-mentioned vaccination categories. Then, we calculated daily, age-specific outcome incidence rates for the unvaccinated and vaccination groups for each outcome (severe cases and deaths). The number of person-days for each vaccination group was calculated by multiplying the daily proportion of the susceptible population in each vaccination group by the IBGE age-group population estimates.6,20 The number of person-days for the unvaccinated group was calculated by subtracting the number of person-days of the vaccinated groups from the IBGE population estimates. Next, for each outcome and vaccination group, we calculated the daily age-specific rate differences between the unvaccinated and vaccinated and their respective 95% confidence interval (CI).
Finally, for each age group, we multiplied the daily rate differences by the susceptible population (i.e., the population without previous evidence of COVID-19) and by the proportion of individuals who received at least one dose of the vaccine. This process was repeated and summed for all days of follow-up for each outcome to estimate the burden of COVID-19 averted by the mass vaccination. The abovementioned steps were initially determined nationally and then repeated for each of the 27 Brazilian states and their 5569 cities.
All analyses were performed using R statistical software (version 4.1.1).21
Role of the funding source
The funders had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. All the authors had final responsibility for the decision to submit it for publication.
Results
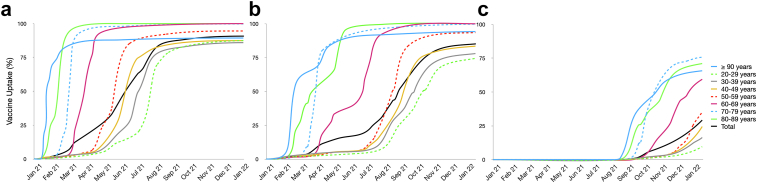
By the end of the first year of the mass vaccination, more than 91% (∼137 million) of the Brazilian population aged ≥20 years had received at least one dose of a COVID-19 vaccine, of whom about 129 million (85%) and about 44 million (29%) were fully vaccinated and boosted-vaccinated, respectively. The vaccination campaign in Brazil initially prioritized those at higher risk of severe disease (the elderly and those with chronic health conditions); consequently, vaccination occurred earlier and vaccine coverage was higher in the ≥60-year age groups (Fig. 1). For instance, by the end of 2021, >99% of the population aged ≥60 years received at least one dose of a COVID-19 vaccine, 98% were fully vaccinated, and 65% were boosted-vaccinated.
Fig. 1.
Cumulative vaccine uptake for receiving any vaccine in Brazil, stratified by age group, January 2021 to January 2022. (a) Cumulative proportion of the population of individuals at least partially vaccinated, i.e., those who received at least one dose of the COVID-19 vaccine. (b) Fully vaccinated, individuals that complete the vaccination schedule. (c) Boosted-vaccinated, i.e., individuals who received the booster dose.
During 2021, a total of 1,169,972 cases of severe COVID-19 were recorded among the ≥20-year-old population, resulting in 369,034 deaths. We estimated that 875,846 severe cases and 303,129 deaths were averted in the at least partly vaccinated population, which would correspond to approximately 74% more severe COVID-19 cases and 82% more COVID-19-related deaths compared with the observed numbers (Table 1 and Fig. 2). Although individuals aged ≥60 years represented only 20% (30,763,655) of the estimated susceptible population and 21% (30,130,737) of the at least partly vaccinated population, they made up nearly 45% (391,207) of averted severe COVID-19 cases and 61% (187,890) of averted deaths. A different pattern was observed in the fully vaccinated and boosted-vaccinated population groups, where the younger age groups (20–29, 30–39, 40–49, and 50–59 years) accounted for the higher proportions of averted severe cases and deaths.
Table 1.
Estimated outcomes averted through COVID-19 mass vaccination program by the vaccination status and age group, Brazil January 31, 2021, to January 31, 2022.
| Age group | Population estimate | Severe COVID-19 cases averted | COVID-19 deaths averted |
|---|---|---|---|
| Individuals at least partially vaccinateda | |||
| 20–29 years | 34,069,012 | 28,663·9 (22,183·7–37,654·14) | 5,008·9 (646·3–9,064·2) |
| 30–39 years | 34,259,369 | 103,031·5 (94,904·1–112,048·45) | 16,824·9 (12,507·5–20,950·4) |
| 40–49 years | 29,854,837 | 161,651·9 (154,595·4–169,433·8) | 35,093·7 (31,420·9–38,707·5) |
| 50–59 years | 24,234,960 | 190,931·9 (185,403·6–197,265·6) | 58,311·7 (55,346·5–61,277·0) |
| 60–69 years | 17,295,908 | 249,724·3 (246,192·3–254,204·2) | 108,441·5 (106,344·1–110,538·9) |
| 70–79 years | 9,416,919 | 119,119·4 (117,944·3–121,547·0) | 65,898·9 (64,762·37–67,035·4) |
| 80–89 years | 3,761,197 | 20,507·2 (20,323·2–21,479·8) | 12,314·2 (11,858·9–12,759·5) |
| ≥ 90 years | 856,211 | 1,856·1 (1,837·1–2,076·1) | 1,235·3 (1,132·3–1,338·33) |
| ≥ 20 years | 153,748,413 | 875,486·5 (843,383·0–915,709·4) | 303,129·4 (284,019·1–321,681·5) |
| Individuals fully vaccinatedb | |||
| 20–29 years | 34,069,012 | 44,486·3 (39,933·0–49,346·8) | 5,906·9 (3,059·6–8,583·5) |
| 30–39 years | 34,259,369 | 132,291·0 (128,878·3–138,320·0) | 20,852·1 (18,088·89–23,629·1) |
| 40–49 years | 29,854,837 | 196,781·1 (195,074·2–203,222·8) | 41,847·1 (39,778·5–44,560·0) |
| 50–59 years | 24,234,960 | 235,022·3 (234,898·4–241,530·5) | 68,241·6 (67,241–71,132·8) |
| 60–69 years | 17,295,908 | 137,469·8 (136,689·35–144,380·3) | 62,659·0 (60,814·0–65,566·6) |
| 70–79 years | 9,416,919 | 72,460·7 (72,369·4–77,411·4) | 44,431·1 (42,293·2–46,784) |
| 80–89 years | 3,761,197 | 19,175·0 (19,069·3–22,225·7) | 16,469·1 (14,573·5–18,171·0) |
| ≥ 90 years | 856,211 | 3,451·1 (3,461·2–3,691·7) | 3,731·1 (3,100·3–4,235·5) |
| ≥ 20 years | 153,748,413 | 841,137·68 (838,011·6–880,129·4) | 264,138·4 (257,949·5–282,663·8) |
| Individuals boosted-vaccinatedc | |||
| 20–29 years | 34,069,012 | 6,641·0 (6,107·3–7,404·9) | 802·3 (−121·2 to 2,249·9) |
| 30–39 years | 34,259,369 | 12,951·7 (8,625·9–19,948·3) | 2,116·3 (−187·7 to 2,591·7) |
| 40–49 years | 29,854,837 | 15,155·8 (9,141·4–18,285·1) | 3,744·6 (745·32–4,144·14) |
| 50–59 years | 24,234,960 | 19,138·1 (12,657·7–23,588·6) | 6,456·3 (2,463·6–10,416·0) |
| 60–69 years | 17,295,908 | 7,486·3 (7,334·6–7,818·1) | 2,490·9 (1,800·4–3,181·3) |
| 70–79 years | 9,416,919 | 1,834·7 (1,809·6–2,014·5) | 755·1 (380·9–1,129·28) |
| 80–89 years | 3,761,197 | 12,516·3 (6,591·8–16,728·5) | 8,777·8 (5,335·7–9,635·5) |
| ≥ 90 years | 856,211 | 2,062·8 (1,344·3–7,372·8) | 2,781·2 (1,860·5–2,928·3) |
| ≥ 20 years | 153,748,413 | 77,787·0 (51,612·8–97,161·27) | 27,924·89 (13,717·00–39,558·95) |
Data are the number of outcomes averted with 95% CI presented in parentheses.
Individuals at least partially vaccinated were those who received one or more doses of any two-dose COVID-19 vaccine.
Individuals who were fully vaccinated were those who completed the vaccine schedule.
Individuals who received the booster dose (i.e., 3rd dose).
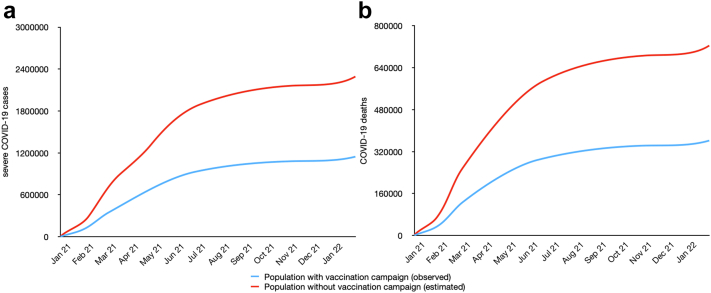
Fig. 2.
Comparison of cumulative COVID-19 outcomes observed in the presence of vaccination campaign (in blue) and predicted COVID-19 outcomes in the absence of vaccination (in red) in Brazil· January 2021 to January 2022. (a) COVID-19 severe cases; (b) COVID-19 deaths.
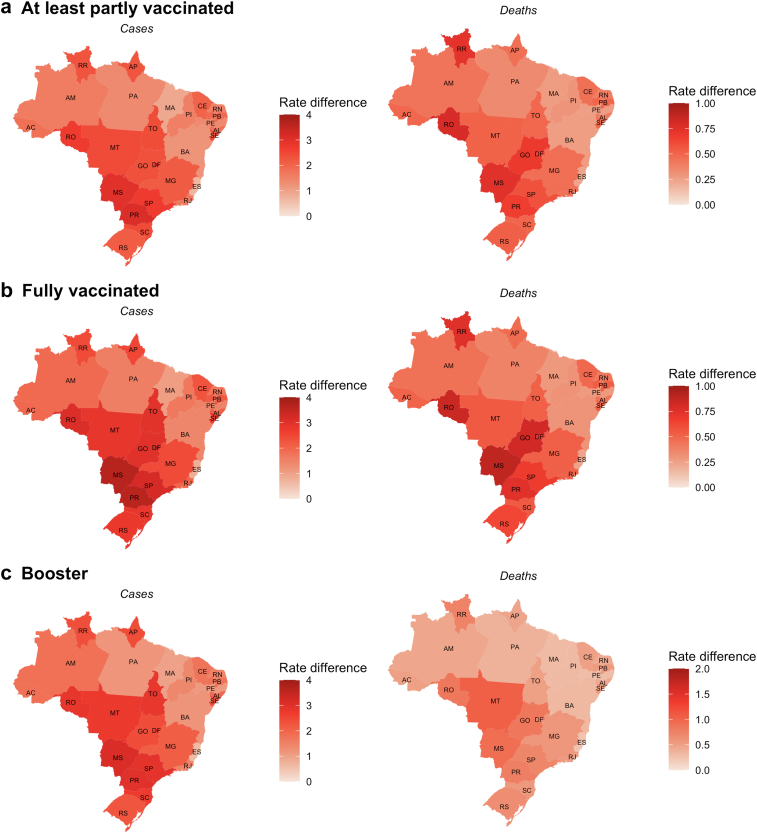
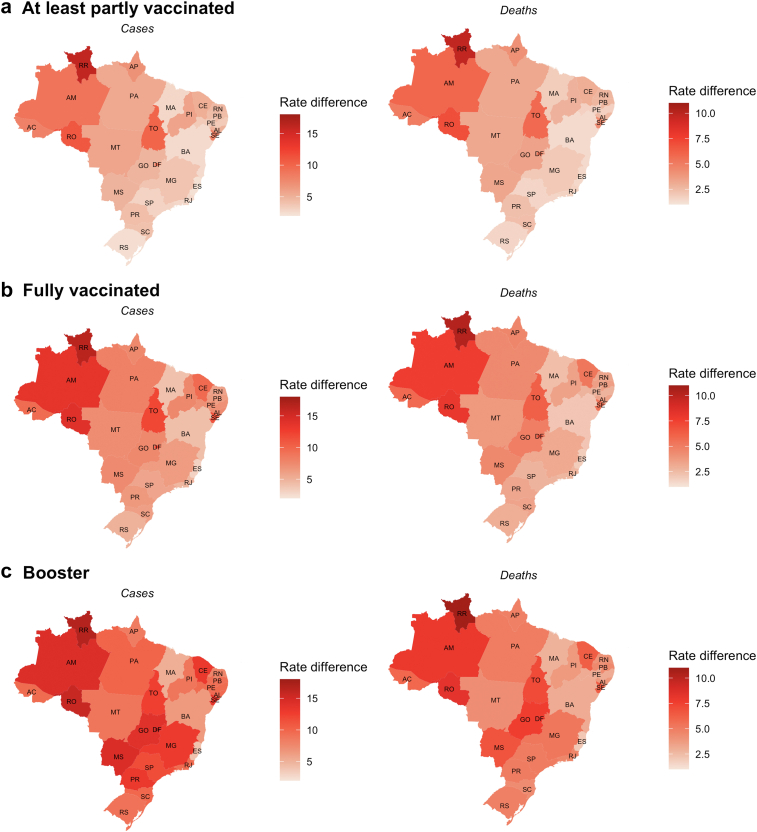
As expected, the highest number of averted cases were estimated to occur in the most densely populated states of São Paulo, Minas Gerais, Rio de Janeiro, and Bahia (Table 2). Comparing the differences in outcome rates (as a proportion of the population) between the vaccinated and unvaccinated groups in the 27 Brazilian states, we observed the highest rate differences in severe COVID-19 cases and deaths were in the 20- to 59-year-old age group (Fig. 3) in the Midwest region states and in the ≥60-year-old age groups in the North region states (Fig. 4). The estimates of the averted severe cases and deaths for the 5569 Brazilian municipalities are shown in Supplementary Material 2 and are publicly available at https://github.com/epicleber.
Table 2.
Estimated COVID-19 outcomes averted through mass vaccination program by the vaccination status and state, Brazil January 31, 2021, to January 31, 2022.
| State | Estimated population | Individuals at least partially vaccinateda |
Individuals fully vaccinatedb |
Individuals boosted-vaccinatedc |
|||
|---|---|---|---|---|---|---|---|
| Averted severe cases | Averted deaths | Averted severe cases | Averted deaths | Averted severe cases | Averted deaths | ||
| Acre | 559,201 | 3,185·7 | 1,103·0 | 3,060·7 | 961·1 | 283·0 | 101·6 |
| Alagoas | 2,284,354 | 13,013·9 | 4,505·9 | 12,503·3 | 3,926·3 | 1,156·2 | 415·0 |
| Amazonas | 2,672,986 | 15,227·9 | 5,272·5 | 14,630·5 | 4,594·3 | 1,353·0 | 485·7 |
| Amapá | 552,340 | 3,146·6 | 1,089·5 | 3,023·2 | 949·3 | 279·5 | 100·3 |
| Bahia | 10,669,688 | 60,785·1 | 21,046·3 | 58,400·3 | 18,339·1 | 5,400·7 | 1,938·7 |
| Ceará | 6,568,565 | 37,421·0 | 12,956·7 | 35,952·9 | 11,290·1 | 3,324·8 | 1,193·5 |
| Distrito Federal | 2,256,510 | 12,855·3 | 4,451·0 | 12,350·9 | 3,878·5 | 1,142·1 | 410·0 |
| Espírito Santo | 2,973,566 | 16,940·3 | 5,865·4 | 16,275·7 | 5,110·9 | 1,505·1 | 540·3 |
| Goiás | 5,151,411 | 29,347·5 | 10,161·3 | 28,196·1 | 8,854·2 | 2,607·5 | 936·0 |
| Maranhão | 4,680,216 | 26,663·1 | 9,231·8 | 25,617·0 | 8,044·3 | 2,369·0 | 850·4 |
| Minas Gerais | 15,977,820 | 91,025·5 | 31,516·7 | 87,454·2 | 27,462·8 | 8,087·6 | 2,903·3 |
| Mato Grosso do Sul | 2,017,276 | 11,492·4 | 3,979·1 | 11,041·5 | 3,467·3 | 1,021·1 | 366·5 |
| Mato Grosso | 2,475,387 | 14,102·2 | 4,882·7 | 13,548·9 | 4,254·7 | 1,252·9 | 449·7 |
| Pará | 5,758,306 | 32,805·0 | 11,358·4 | 31,517·9 | 9,897·4 | 2,914·7 | 1,046·3 |
| Paraíba | 2,883,545 | 16,427·5 | 5,687·8 | 15,783·0 | 4,956·2 | 1,459·5 | 523·9 |
| Pernambuco | 6,805,645 | 38,771·7 | 13,424·3 | 37,250·5 | 11,697·6 | 3,444·8 | 1,236·6 |
| Piauí | 2,288,668 | 13,038·5 | 4,514·4 | 12,526·9 | 3,933·7 | 1,158·4 | 415·8 |
| Paraná | 8,397,724 | 47,841·8 | 16,564·7 | 45,964·7 | 14,434·0 | 4,250·7 | 1,525·9 |
| Rio de Janeiro | 13,066,646 | 74,440·6 | 25,774·3 | 71,520·0 | 22,459·0 | 6,614·0 | 2,374·3 |
| Rio Grande do Norte | 2,557,213 | 14,568·4 | 5,044·1 | 13,996·8 | 4,395·3 | 1,294·4 | 464·6 |
| Rondônia | 1,256,434 | 7,157·9 | 2,478·3 | 6,877·0 | 2,159·5 | 635·9 | 228·3 |
| Roraima | 427,856 | 2,437·0 | 843·0 | 2,341·0 | 735·0 | 216·0 | 77·0 |
| Rio Grande do Sul | 8,655,734 | 49,311·6 | 17,073·7 | 47,376·9 | 14,877·5 | 4,381·3 | 1,572·8 |
| Santa Catarina | 5,466,747 | 31,144·0 | 10,783·3 | 29,922·1 | 9,396·2 | 2,767·1 | 993·3 |
| Sergipe | 1,637,385 | 9,328·1 | 3,229·7 | 8,962·1 | 2,814·3 | 828·8 | 297·5 |
| São Paulo | 34,544,205 | 196,798·2 | 68,139·5 | 189,077·0 | 59,374·9 | 17,485·5 | 6,276·9 |
| Tocantins | 1,089,560 | 6,207·2 | 2,149·1 | 5,963·6 | 1,872·7 | 551·5 | 197·9 |
Individuals at least partially vaccinated were those who received one or more doses of the COVID-19 vaccine.
Individuals who were fully vaccinated were those who completed the vaccine schedule.
Individuals who received the booster dose (i.e., 3rd dose).
Fig. 3.
Rate difference of averted COVID-19 severe cases and deaths between vaccinated and unvaccinated individuals in Brazilian states in the population between 20 and 59 years, from January 31, 2021, to January 31, 2022. Data are the mean daily rate difference per 100,000 population. (a) Individuals at least partially vaccinated were those who received one or more doses of the COVID-19 vaccine. (b) Individuals who were fully vaccinated were those who completed the vaccine schedule. (c) Individuals who received the booster shot.
Fig. 4.
Rate difference of averted COVID-19 severe cases and deaths between vaccinated and unvaccinated individuals in Brazilian states in the population ≥60 years, from January 31, 2021, to January 31, 2022. Data are the mean daily rate difference per 100,000 population. (a) Individuals at least partially vaccinated were those who received one or more doses of the COVID-19 vaccine. (b) Individuals who were fully vaccinated were those who completed the vaccine schedule. (c) Individuals who received the booster shot.
Discussion
Between January 17, 2021, and the end of January 2022, nearly 23 million doses of COVID-19 vaccines were administered per month in Brazil. Brazil is recognized worldwide for the efficiency of its vaccination programs and has a successful history of inclusive governmental vaccination policies, sustained through effective communication strategies and excellent geographical coverage of the Unified Health System (Sistema Único de Saúde - SUS), and good population adherence.22,23 For instance, during the 2009 H1N1 influenza pandemic, Brazil vaccinated nearly 90 million people within 3 months. Nevertheless, despite the high level of vaccine uptake in the first year of the COVID-19 campaign, Brazil has not been able to surpass the pace of vaccination observed during the 2009 H1N1 influenza pandemic. The relatively slow pace of COVID-19 vaccination could be due to a variety of factors, such as underfunding of SUS over the past several years, the delay in vaccine acquisition and, consequently, the vaccine supply shortages, and the widespread dissemination of misinformation regarding the disease severity, the effectiveness of non-pharmaceutical interventions and the vaccine safety, especially by the federal government.14,24, 25, 26
From the first recorded case of COVID-19 (February 26, 2020) through January 31, 2022, a total of 2,021,646 cases of severe COVID-19 and 636,873 deaths due to COVID-19 were recorded in Brazil, which was the third and second-highest number of cases and deaths due to COVID-19, respectively, by country.1,2 Nearly 57% of the outcomes occurred during the study period examined here: from January 17, 2021 to January 31, 2022, and we estimated that an additional 875,846 severe cases and 303,129 deaths were averted by the vaccination campaign over the same period. Our estimates indicated that the decision to prioritize vaccination of the older population had important effects on averting the outcomes. Thus, although the population aged ≥60 years represented only 20% of the estimated susceptible population, they comprised almost 45% of severe cases and 61% of deaths averted by the vaccination campaign.
We estimated that the vaccination campaign averted both severe COVID-19 cases and deaths at higher numbers in the more populated states of Brazil. Interestingly, however, the rate differences between vaccinated and unvaccinated (per 100,000 inhabitants) were higher in the states with higher COVID-19 incidence rates, specifically in the 20- to 59-year-old group in the states from the Midwest and South regions and in the ≥60-year-old group in the states from the North region.3
To the best of our knowledge, this is the first study to provide comprehensive estimates of the number of severe cases and deaths averted by the COVID-19 vaccination campaign in Brazil. Nevertheless, our study has some limitations. First, there is an inherent limitation in using secondary data, and the rate differences between the unvaccinated and vaccinated populations rely on observational data. To control for confounding, we stratified the data by date and age. However, we do not have information on other variables, such as socioeconomic variables, comorbidities, COVID-19 testing rate and positivity, health-seeking behaviour, and adoption of preventive strategies (e.g., social distancing, mask use) that may differ between the comparison groups. However, the databases we used are the best available evidence on both COVID-19 outcomes and vaccination and were largely used in many studies during the pandemic in Brazil.7,8,25,27, 28, 29, 30, 31, 32, 33 The analysis here does not consider stratification by sex for two main reasons, first, given the absence of difference observed in the interim analysis, and second, previous ecological studies with similar methodology in other countries did not choose to sex-stratify.19 A second methodological limitation is that our analysis does not incorporate the indirect effects of the vaccination campaign that might have benefited the unvaccinated individuals due to the reduction of transmission rates. Vaccines can affect not only an individual's susceptibility to infection but also reduce symptomatic infection, the likelihood of progression to severe disease and death, and may also decrease the transmissibility potential.34, 35, 36 Given the preliminary evidence for the transmission-blocking effects of the current COVID-19 vaccines, our results may have underestimated the effects of vaccination on averting severe cases and deaths from COVID-19.37 Still, they do provide a lower bound of the disease burden averted by the mass vaccination campaign.37, 38, 39, 40 Furthermore, we did not assess the effects of infection with specific SARS-CoV-2 variants, mainly because genomic surveillance is not widely adopted or regularly performed in Brazil, or of the impact of lockdowns, which were not uniformly recommended and did not occur with any geographic or temporal consistency throughout Brazil. Future work should disentangle the direct and indirect effects of vaccination on other crucial outcomes, such as the economic benefits and the number of disability-adjusted life years averted.
Contributors
C.V.B.d.S., C.J.S., and D.A.M.V. conceived the idea for the study. C.V.B.d.S., D.A.M.V. had access to the anonymized microdata. C.V.B.d.S. conducted the analysis. C.V.B.d.S., G.L.W., D.A.M.V., and C.J.S. drafted the manuscript. T.G.d.N. coordinate the project and secured funding. All authors contributed to the interpretation of the study findings and contributed to the final version of the manuscript.
Data sharing statement
Data supporting this manuscript comes from databases maintained by the Brazilian Ministry of Health, which linked the vaccination and surveillance data.
The database resulting from the linkage cannot be publicly shared. Requests for these data should be made to the Brazilian Ministry of Health. Not merged individual data are open and freely available at https://opendatasus.saude.gov.br.
Ethics statement
The study was conducted in accordance with fundamental ethical principles of the Declaration of Helsinki and the Brazilian National Health Council on research involving human beings. The study protocol was approved by the Research Ethics Committee of the Evandro Chagas National Institute of Infectious Diseases- Fiocruz (CAAE: 51567721.9.0000.5262).
Editor's note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
D.A.M.V. and T.G.d.N. are affiliated with Fundação Oswaldo Cruz, which manufactures the ChAdOx nCoV-19 vaccine in Brazil through a full technology transfer agreement with AstraZeneca. T.G.d.N. received payment from the Ministry of Health to coordinate a national network to monitor the safety and effectiveness of the Covid-19 vaccination campaign in Brazil. All other authors declare no competing interests.
Acknowledgements
C.J.S., G.L.W. and C.V.B.d.S. acknowledge support from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ). D.A.M.V. is grateful for support from CNPQ/Brazil (Ref. 441057/2020-9, 309569/2019-2). G.L.W. acknowledges support from FAPERJ (project E-26/210.180/2020). We also thank to the Coordenação Geral do Programa Nacional de Imunizações, part of the Brazilian Ministry of Health for providing the anonymized database, and Luis Antônio Bastos Camacho for his valuable comments in the early versions of the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lana.2022.100418.
Appendix A. Supplementary data
References
- 1.Roser M., Ritchie H., Ortiz-Ospina E., Hasell J. 2020. Coronavirus pandemic (COVID-19) [Google Scholar]
- 2.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ministério da Saúde Painel coronavírus. 2020. https://covid.saude.gov.br/
- 4.Ministério da Saúde Vacinômetro. 2022. https://www.gov.br/saude/pt-br/vacinacao
- 5.Shu Y., McCauley J. GISAID: global initiative on sharing all influenza data – from vision to reality. Euro Surveill. 2017;22:2–4. doi: 10.2807/1560-7917.ES.2017.22.13.30494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Instituto Brasileiro de Geografia e Estatistica (IBGE) 2020. Cidades e Estados. [Google Scholar]
- 7.Katikireddi S.V., Cerqueira-Silva T., Vasileiou E., et al. Two-dose ChAdOx1 nCoV-19 vaccine protection against COVID-19 hospital admissions and deaths over time: a retrospective, population-based cohort study in Scotland and Brazil. Lancet. 2022;399:25–35. doi: 10.1016/S0140-6736(21)02754-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ranzani O.T., Hitchings M.D.T., Dorion M., et al. Effectiveness of the CoronaVac vaccine in older adults during a gamma variant associated epidemic of covid-19 in Brazil: test negative case-control study. BMJ. 2021;374 doi: 10.1136/bmj.n2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haas E.J., Angulo F.J., McLaughlin J.M., et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalisations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021;397:1819–1829. doi: 10.1016/S0140-6736(21)00947-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng C., Shao W., Chen X., Zhang B., Wang G., Zhang W. Real-world effectiveness of COVID-19 vaccines: a literature review and meta-analysis. Int J Infect Dis. 2022;114:252–260. doi: 10.1016/j.ijid.2021.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall V.J., Foulkes S., Saei A., et al. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet. 2021;397:1725–1735. doi: 10.1016/S0140-6736(21)00790-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin D.-Y., Gu Y., Wheeler B., et al. Effectiveness of covid-19 vaccines over a 9-month period in North Carolina. N Engl J Med. 2022;386:933–941. doi: 10.1056/NEJMoa2117128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Victora C.G., Castro M.C., Gurzenda S., Medeiros A.C., França G.V.A., Barros A.J.D. Estimating the early impact of vaccination against COVID-19 on deaths among elderly people in Brazil: analyses of routinely-collected data on vaccine coverage and mortality. EClinicalMedicine. 2021;38:101036. doi: 10.1016/j.eclinm.2021.101036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferreira LS, Darcie Marquitti FM, Lopes R, et al. Estimating the impact of implementation and timing of COVID-19 vaccination programme in Brazil: a counterfactual analysis. medRxiv. 10.1101/2021.12.24.21268384. [DOI] [PMC free article] [PubMed]
- 15.Izbicki R., Bastos L.S., Izbicki M., Lopes H.F., dos Santos T.M. How many hospitalizations has the Covid-19 vaccination already prevented in São Paulo? Clinics (Sao Paulo) 2021;76 doi: 10.6061/clinics/2021/e3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valiati N.C.M., Villela D.A.M. Modelling policy combinations of vaccination and transmission suppression of SARS-CoV-2 in Rio de Janeiro, Brazil. Infect Dis Model. 2022;7:231–242. doi: 10.1016/j.idm.2021.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ministério da Saúde Dados da campanha Nacional de Vacinação contra Covid-19. 2022. https://opendatasus.saude.gov.br/dataset/covid-19-vacinacao
- 18.Ministério da Saúde SRAG 2021 - Banco de Dados de Síndrome Respiratória Aguda Grave - incluindo dados da COVID-19. 2021. 2022. https://opendatasus.saude.gov.br/dataset/srag-2021-e-2022
- 19.Haas E.J., McLaughlin J.M., Khan F., et al. Infections, hospitalisations, and deaths averted via a nationwide vaccination campaign using the Pfizer–BioNTech BNT162b2 mRNA COVID-19 vaccine in Israel: a retrospective surveillance study. Lancet Infect Dis. 2022;22:357–366. doi: 10.1016/S1473-3099(21)00566-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Instituto Brasileiro de Geografia e Estatística Projeção da população brasileira. 2021. https://www.ibge.gov.br/apps/populacao/projecao/index.html
- 21.R Core Team . 2022. R: a language and environment for statistical computing. [Google Scholar]
- 22.Barreto M.L., Teixeira M., Bastos F.I., Ximenes R.A.A., Barata R.B., Rodrigues L.C. Successes and failures in the control of infectious diseases in Brazil: social and environmental context, policies, interventions, and research needs. Lancet. 2011;377:1877–1889. doi: 10.1016/S0140-6736(11)60202-X. [DOI] [PubMed] [Google Scholar]
- 23.Domingues C.M.A.S., de Oliveira W.K. Uptake of pandemic influenza (H1N1)-2009 vaccines in Brazil, 2010. Vaccine. 2012;30:4744–4751. doi: 10.1016/j.vaccine.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Pacheco F.C., França G.V.A., Elidio G.A., Oliveira C.M., Guilhem D.B. Decrease in the coverage of measles-containing vaccines and the risk of reestablishing endemic transmission of measles in Brazil. Int J Infect Dis. 2019;82:51–53. doi: 10.1016/j.ijid.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa Libotte G., dos Anjos L., Célia Cerqueira De Almeida R., Mara Cardoso Malta S., de Andrade Medronho R. Impacts of a delayed and slow-paced vaccination on cases and deaths during the COVID-19 pandemic: a modelling study. J R Soc Interface. 2022;19:821–826. doi: 10.1098/rsif.2022.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xavier D.R., Lima e Silva E., Lara F.A., et al. Involvement of political and socio-economic factors in the spatial and temporal dynamics of COVID-19 outcomes in Brazil: a population-based study. Lancet Reg Health Am. 2022;10:1–16. doi: 10.1016/j.lana.2022.100221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cerqueira-Silva T., Katikireddi S.V., de Araujo Oliveira V., et al. Vaccine effectiveness of heterologous CoronaVac plus BNT162b2 in Brazil. Nat Med. 2022;28:838–843. doi: 10.1038/s41591-022-01701-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Castro M.C., Gurzenda S., Macário E.M., França G.V.A. Characteristics, outcomes and risk factors for mortality of 522 167 patients hospitalised with COVID-19 in Brazil: a retrospective cohort study. BMJ Open. 2021;11:1–9. doi: 10.1136/bmjopen-2021-049089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Souza M.L., Ichihara M.Y.T., Sena S.O.L. 2020. Sistemas de informação para a COVID-19. [DOI] [Google Scholar]
- 30.Santos C.V.B., Cavalcante J.R., Pungartnik P.C., Guimarães R.M. Transição da idade de casos, internações e óbitos em internações por Covid-19 no município do Rio de Janeiro. Rev Bras Estud Popul. 2022;39:1–10. [Google Scholar]
- 31.Santos C.V.B., Cavalcante J.R., Pungartnik P.C., Guimarães R.M. Space-time analysis of the first year of Covid-19 pandemic in the city of Rio de Janeiro, Brazil. Rev Bras Epidemiol. 2021;24 doi: 10.1590/1980-549720210046. [DOI] [PubMed] [Google Scholar]
- 32.Cavalcante J.R., Xavier D.R., dos Santos C.V.B., Pungartnik P.C., Guimarães R.M. Spatial analysis of the origin-destination flow of admissions for severe acute respiratory syndrome caused by COVID-19 in the metropolitan region of Rio de Janeiro. Rev Bras Epidemiol. 2021;24:1–6. doi: 10.1590/1980-549720210054. [DOI] [PubMed] [Google Scholar]
- 33.Silva G.A.E., Jardim B.C., dos Santos C.V.B. Excess mortality in Brazil in times of covid-19. Ciên Saúde Colet. 2020;25:3345–3354. doi: 10.1590/1413-81232020259.23642020. [DOI] [PubMed] [Google Scholar]
- 34.Halloran M.E., Longini I.M., Struchiner C.J. 2010. Design and analysis of vaccine studies. [Google Scholar]
- 35.Halloran M.E., Struchiner C.J. Study designs for dependent happenings. Epidemiology. 1991;2:331–338. doi: 10.1097/00001648-199109000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Halloran M.E., Haber M., Longini I.M., Struchiner C.J. 1991. Direct and indirect effects in vaccine efficacy and effectiveness. [DOI] [PubMed] [Google Scholar]
- 37.Salo J., Hägg M., Kortelainen M., et al. The indirect effect of mRNA-based Covid-19 vaccination on unvaccinated household members. medRxiv. 2021 doi: 10.1101/2021.05.27.21257896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Monge S., Olmedo C., Alejos B., Lapeña M.F., Sierra M.J., Limia A. Direct and indirect effectiveness of mRNA vaccination against severe acute respiratory syndrome coronavirus 2 in long-term care facilities, Spain. Emerg Infect Dis. 2021;27:2595–2603. doi: 10.3201/eid2710.211184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Milman O., Yelin I., Aharony N., et al. SARS-CoV-2 infection risk among unvaccinated is negatively associated with community-level vaccination rates. medRxiv. 2021 doi: 10.1101/2021.03.26.21254394. [DOI] [Google Scholar]
- 40.Harris R.J., Hall J.A., Zaidi A., Andrews N.J., Dunbar J.K., Dabrera G. Effect of vaccination on household transmission of SARS-CoV-2 in England. N Engl J Med. 2021;385:759–760. doi: 10.1056/NEJMc2107717. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.