Abstract
The constituents of mycobacteria are an effective immune adjuvant, as observed with complete Freund's adjuvant. In this study, we demonstrated that the cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guérin (BCG-CWS), a purified noninfectious material consisting of peptidoglycan, arabinogalactan, and mycolic acids, induces maturation of human dendritic cells (DC). Surface expression of CD40, CD80, CD83, and CD86 was increased by BCG-CWS on human immature DC, and the effect was similar to those of interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), heat-killed BCG, and viable BCG. BCG-CWS induced the secretion of TNF-α, IL-6, and IL-12 p40. CD83 expression was increased by a soluble factor secreted from BCG-CWS-treated DC and was completely inhibited by monoclonal antibodies against TNF-α. BCG-CWS-treated DC stimulated extensive allogeneic mixed lymphocyte reactions. The level of TNF-α secreted through BCG-CWS was partially suppressed in murine macrophages with no Toll-like receptor 2 (TLR 2) or TLR4 and was completely lost in TLR2 and TLR4 double-deficient macrophages. These results suggest that the BCG-CWS induces TNF-α secretion from DC via TLR2 and TLR4 and that the secreted TNF-α induces the maturation of DC per se.
The Mycobacterium bovis bacillus Calmette-Guérin (BCG) strain is a tuberculosis vaccine strain which is almost nonpathogenic yet retains the immunogenic properties of tuberculosis (22). Several reports have suggested that phagocytosis of viable BCG mycobacteria is a potent inducer of maturation of dendritic cells (DC). Infection of DC with BCG or Mycobacterium tuberculosis facilitated secretion of inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), and IL-12, and up-regulation of CD40, CD80, CD83, CD86, and major histocompatibility complex (MHC) class I molecules. Immature dendritic cells (iDC) exhibit potent antigen-presenting ability through uptake of BCG (11, 19, 20, 23, 39). The potent antigen-presenting activity of these DC can be used on soluble antigens other than BCG itself. Thus, the consensus is that BCG mycobacteria serve as an immune potentiator of lymphocytes, namely an adjuvant, via the maturation of iDC.
These recent studies may also explain a previous observation. Live BCG has been used as an effective adjuvant for the active immunotherapy of various cancers (12, 26). In humans, BCG immunotherapy has been employed for the treatment and prophylaxis of transitional cell carcinoma of the bladder and urinary tract for more than 20 years and has been associated with good prognoses (1). Live BCG was also effective as a vaccination adjuvant for the immunization of irradiated colon cancer cells (33, 40). For tumors transplanted into mice and guinea pigs, injection of BCG into tumors was reported to be effective in stimulating tumor regression (7, 16, 25, 29). However, no clear concept has been proposed regarding the constituents of BCG that are responsible for antitumor immunity or the mechanisms by which BCG can potentiate the host immune system.
On the other hand, it is also commonly recognized that host immune responses are enhanced by complete Freund's adjuvant (CFA) containing dead mycobacteria. The cell wall skeleton of mycobacteria consists of a peptidoglycan that is covalently linked to arabinogalactan and mycolic acids (4, 8, 9) and conserves adjuvant effects in vivo, antibody production in animal studies, and induction of typical delayed-type hypersensitivity via intracutaneous injection of the cell wall skeleton of BCG (BCG-CWS), as seen with viable BCG (4, 41, 44). Furthermore, immune therapy with BCG-CWS has led to a good prognosis without any signs of infection for many cancer patients (17, 18).
The recent establishment of a human DC culturing protocol enabled us to analyze the effect of immune modulators on DC functions in vitro (31, 32, 34, 35, 45). We used the human DC culture system for the elucidation of BCG-CWS function and have proposed that noninfectious BCG-CWS is capable of converting iDC to mature DC. These functions of BCG-CWS may represent the adjuvant feature of mycobacteria in CFA and in immune therapy for cancer.
MATERIALS AND METHODS
Reagents, ELISA kits, and antibodies.
The following materials were obtained as indicated: fetal bovine serum (FBS) from Bio Whittaker (Walkersville, Md.), human AB serum from ICN Biomedicals, Inc. (Aurora, Ohio), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1β, and IL-4 from Pepro Tech EC, Ltd. (London, United Kingdom), TNF-α from Gibco BRL (Rockville, Md.), lipopolysaccharide (LPS) (Escherichia coli O127:B8) from Difco Laboratories (Detroit, Mich.), [3H]thymidine from NEN Life Science Products, Inc. (Boston, Mass.), N-acetyl-d-glucosaminyl-(β1-4)-acetyl-l-alanyl-d-isoglutamine (GMDP) from Calzyme Laboratories, Inc. (San Luis Obispo, Calif.).
The following enzyme-linked immunosorbent assay (ELISA) kits were obtained: gamma interferon (IFN-γ), IL-1β, IL-6, and TNF-α from Amersham Pharmacia Biotech (Buckinghamshire, United Kingdom), total IL-12 (p40 plus p70) and mouse TNF-α from Genzyme Co. (Cambridge, Mass.), IL-12 p70 from Endogen, Inc. (Woburn, Mass.), IL-18 from Medical & Biological Laboratories Co., Ltd. (Nagoya, Japan), and an endotoxin-specific assay kit (Endospecy ES-6 set) from Seikagaku Co. (Tokyo, Japan). The following antibodies were obtained: anti-CD1a (B17.20.9), anti-CD80 (MAB104), and anti-HLA-DR (Immu-357) from Immunotech (Marseille, France), anti-CD11c (S-HCL-3) from Becton-Dickinson Monoclonal Center, Inc. (Mountain View, Calif.), anti-CD14 (UCHM-1), immunoglobulin G1 (IgG1) (MOPC-21), IgG2a (UPC-10), and IgG2b (MOPC-141) from Sigma Chemical Co. (Saint Louis, Mo.), anti-CD40 (5C3) and anti-CD64 (10.1) from PharMingen (San Diego, Calif.), anti-CD71 (Ber-T9) from DakoPatts (Glostrup, Denmark), anti-CD83 (HB15A) from Cosmo Bio Co. (Tokyo, Japan), anti-CD86 (BU63) from Ancell Co. (Bayport, Minn.), anti-TNF-α, anti-IL-1β, and anti-IL-12 (clone C8.6) from Genzyme Co. (Cambridge, Mass.), and fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG F(ab′)2 from American Qualex (San Clemente, Calif.).
BCG-CWS.
BCG-CWS was prepared as described previously (4). The purity of the lot used in this study (lot 10-2) was also described (4). Briefly, the constituents related to peptidoglycan, arabinogalactan, and mycolic acid made up more than 97%. Minimal amounts of phospholipid (∼0.2%) and amino acids (<2%) contaminated this preparation. No mannan and glucose were detected.
Since BCG-CWS is insoluble in water and organic solvent, oil-in-water emulsion forms of BCG-CWS particles were used throughout this study. Dried BCG-CWS was resuspended at a concentration of 1 mg/ml in emulsion buffer (phosphate-buffered saline [PBS] containing 1% drakeol and 1% Tween 80) with a Potter homogenizer and sterilized by heating for 30 min at 60°C. For FITC labeling of BCG-CWS, dried BCG-CWS was resuspended in 50 mM HEPES-buffered saline, pH 8.5, at a concentration of 1 mg/ml. Thereafter, 10 μl of 10-mg/ml FITC in dimethyl sulfoxide was added to the suspension and incubated for 15 min at 37°C. FITC-labeled BCG-CWS was collected by centrifugation (20,000 × g, 10 min) and washed once with HEPES-buffered saline (pH 7.0). FITC–BCG-CWS was resuspended in emulsion buffer at 1 mg/ml with a Potter homogenizer and sterilized by heating for 30 min at 60°C.
BCG.
BCG cells were picked up from a colony of BCG Tokyo and suspended in saline containing 1% Tween 80. The concentration of BCG was calculated from the absorbance at 600 nm. Heat-killed BCG cells were obtained by heating for 30 min at 70°C.
Cells.
Peripheral blood mononuclear cells (PBMC) were isolated by standard density gradient centrifugation with Ficoll-Paque (Amersham Pharmacia Biotech AB) from heparinized whole blood or buffy coat of normal healthy donors. CD14+ monocytes were separated from PBMC by anti-CD14-coated microbeads and magnet cell separation columns (Miltenyi Biotec GmBH). iDC were generated from monocytes (5 × 105 cells/ml) cultured for 6 days in RPMI 1640 medium containing 10% heat-inactivated FBS, 500 IU of granulocyte-macrophage colony-stimulating factor (GM-CSF)/ml, and 100 IU of IL-4/ml (31, 32, 35, 45), with a change of medium every 3 days. Lymphocytes for mixed lymphocyte reactions were prepared from fresh PBMC that were depleted of monocytes by anti-CD14-coated microbeads and magnet cell separation columns.
DC maturation.
iDC were prepared as described above. These cells were further cultured (5 × 105 cells/ml) for 2 days in RPMI 1640 medium containing 10% heat-inactivated FBS and 500 IU of GM-CSF/ml with either of 100 IU of IL-4/ml, 100 ng of IL-1β/ml, 100 IU of TNF-α/ml, 10 ng of LPS/ml, 15 μl of emulsion buffer (vehicle of BCG-CWS)/ml, 15 μg of BCG-CWS/ml, 5 × 105 heat-killed BCG cells/ml, or 5 × 105 viable BCG cells/ml. After 2 days, the adherent cells were collected at 4°C by gentle pipetting in PBS containing 10 mM EDTA.
Phagocytosis assay by flow cytometry.
iDC were cultured for 6 days (5 × 105 cells/ml) as described above and were incubated with 15 μg of FITC–BCG-CWS/ml in RPMI 1640 medium containing 10% heat-inactivated FBS and 500 IU of GM-CSF/ml at 37°C for 0.5 or 7 h. Cells were harvested at 4°C by gentle pipetting in PBS containing 0.9 mM CaCl2, 0.5 mM MgCl2, 0.1% sodium azide, and 0.1% bovine serum albumin and washed with the same buffer. These cells were analyzed by flow cytometry (FACSCalibur; Becton-Dickinson). Total fluorescence reflected bound and phagocytosed FITC–BCG-CWS. For quenching the fluorescence of uningested FITC–BCG-CWS, the cell suspension was mixed in an equivalent 50 mM acetate buffered saline (pH 4.5) containing 2 mg of trypan blue/ml and analyzed by flow cytometry (38). The levels of fluorescence reflected phagocytosed FITC–BCG-CWS. Fluorescence analysis was also performed with a fluorescence microscope (Olympus; IX-70, BX-60). The fluorescence of extracellular FITC–BCG-CWS was completely quenched by this analysis.
Flow-cytometric analysis of cell surface antigens.
Cells were resuspended in PBS containing 0.1% sodium azide and 0.1% bovine serum albumin and then incubated for 30 min at 4°C with saturating concentrations of monoclonal antibodies (MAbs). Cells were washed and counterstained with FITC-conjugated goat anti-mouse IgG F(ab′)2 for 30 min at 4°C. Fluorescence intensity was then determined by flow-cytometric analysis.
ELISA.
DC culture supernatants were collected, cleared by centrifugation, and stored at −30°C. IL-1β, IL-6, IL-12 p40, IL-12 p70, IL-18, IFN-γ, and TNF-α concentrations were measured by commercial ELISA kits.
Transwell assay.
Conventional iDC (3.5 × 105 cells/well) were cultured for 2 days on upper (100 μl) or lower (600 μl) wells of a transwell apparatus (6.5-mm-diameter, 0.4-μm-pore size, polycarbonate membrane; Corning Costar Co.) in RPMI 1640 containing 10% heat-inactivated FBS and 500 IU of GM-CSF/ml with either IL-4 (100 IU/ml), emulsion buffer (15 μl/ml), BCG-CWS (15 μg/ml), IgG1 (MOPC-21) (10 μg/ml), anti-IL-1β (10 μg/ml), or anti-TNF-α (10 μg/ml). Cells on the lower well were harvested at 4°C by gentle pipetting in PBS containing 10 mM EDTA and were analyzed by flow cytometry as described above.
MLR assay.
iDC for the mixed lymphocyte reaction (MLR) assay were generated from monocytes (5 × 105 cells/ml) that were cultured for 6 days in RPMI 1640 medium containing 10% heat-inactivated human AB serum, 500 IU of GM-CSF/ml, and 100 IU of IL-4/ml. Then the cells were cultured for 2 days in the same medium (iDC) or in the medium containing 15 μg of BCG-CWS/ml instead of IL-4 (BCG-CWS-treated DC). iDC and BCG-CWS-treated DC were irradiated (3,000 rads; 137Cs source) and cultured for 4 days with 2 × 105 allogeneic lymphocytes in 96-well cell culture plates in 200 μl of RPMI 1640 medium containing 10% human heat-inactivated AB serum. During the last 24 h of culturing, half of the medium was replaced with fresh medium containing [3H]thymidine (1 μCi/well). Then the cells and medium were harvested separately with a cell harvester, and the radioactivity was measured by a liquid scintillation counter (Aloca).
Measurement of TNF-α secreted by peritoneal macrophages in TLR-deficient mice.
Peritoneal macrophages were prepared from Toll-like receptor (TLR)-deficient mice as previously described (37). Mice were intraperitoneally injected with 2 ml of 4% thioglycolate medium (Difco). Three days later, peritoneal exudate cells were isolated from the peritoneal cavity by washing with ice-cold Hanks' buffered salt solution. Cells were cultured for 2 h and washed with Hanks' buffered salt solution to remove nonadherent cells. Adherent monolayer cells were used as peritoneal macrophages. The cells were cultured for 24 h with RPMI 1640 medium containing 10% FBS, IFN-γ (30 U/ml), and BCG-CWS (10 μg/ml). The concentration of TNF-α in culture supernatants was measured by ELISA.
RESULTS
Surface marker profiles of DC treated by BCG-CWS.
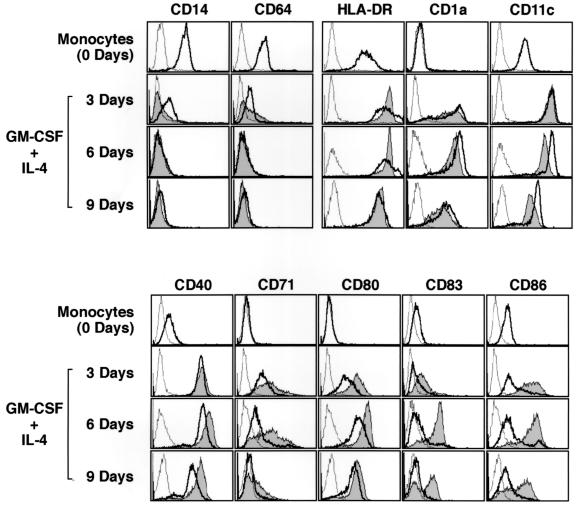
Previous studies of surface markers have suggested that during differentiation of cells from monocytes to iDC, CD1a, CD40, and CD80 levels are elevated, while levels of CD14 and CD64 are decreased on cells (32). Actually, in our experiments (Fig. 1), iDC, resulting from treatment with IL-4 and GM-CSF for either 3, 6, or 9 days, showed high levels of HLA-DR, CD1a, CD11c, CD40, CD71, and CD80 and low levels of CD14 and CD64 compared to monocytes, while the levels of CD83 and CD86 were marginally changed (Fig. 1). Most typical features were obtained with cells cultured for 6 days. Interestingly, the typical surface marker profiles of the iDC were altered by treatment with BCG-CWS. Two days after treatment with BCG-CWS, levels of CD40, CD71, CD80, and CD86 were further increased. Strikingly, CD83, a marker of mature DC, appeared on the cell surface after BCG-CWS treatment, though iDC express this marker only minimally (Fig. 1).
FIG. 1.
Cell surface phenotypes of DC exposed to BCG-CWS. iDC were prepared by culture for either 3, 6, or 9 days and were also incubated for an additional 2 days in medium with GM-CSF (500 IU/ml) and BCG-CWS (15 μg/ml). Broken lines in the histograms show nonspecific fluorescence by subclass control MAb. Fluorescence for the indicated antigens on DC before and after BCG-CWS treatment is shown by bold lines and shaded areas, respectively. This experiment was repeated three times with similar results, and representative results are shown.
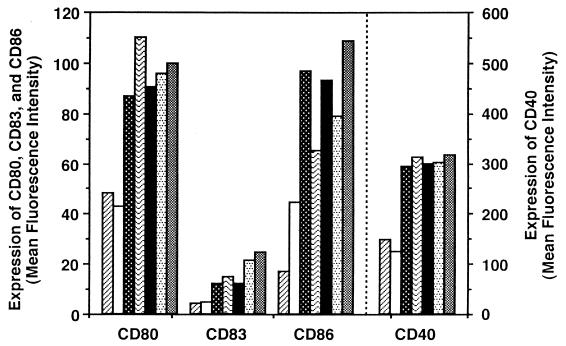
The levels of surface markers on DC after BCG-CWS treatment were compared to those of DC treated with other stimuli (Fig. 2). BCG-CWS induced the increases of CD40, CD80, CD83, and CD86 on DC, similarly to TNF-α and IL-1β. BCG-CWS also showed an effect similar to that of heat-killed BCG or viable BCG. Under our experimental conditions, we could not find a difference in stimulation among heat-killed BCG, viable BCG, and BCG-CWS. Treatment with LPS, another DC maturation inducer, gave rise to a three- to fivefold greater increase in the level of these markers from that of treatment with BCG-CWS or TNF-α (data not shown).
FIG. 2.
Levels of surface markers on iDC with and without
treatment with reagents. iDC were incubated for 2 days in medium
containing GM-CSF (500 IU/ml) and the following materials: GM-CSF alone
(none) (▨), emulsion buffer (15 μl/ml) (□), TNF-α (100 IU/ml)
(▩), IL-1β (100 ng/ml) ( ),
BCG-CWS (15 μg/ml) (■), heat-killed BCG (iDC/bacterium ratio =
1:1) (
), or viable BCG (iDC/bacterium ratio = 1:1) (▩).
Values are expressed as the mean fluorescence intensities measured by a
flow cytometer, and the mean values of subclass control MAbs were
negligible (∼3 to 4). This experiment was repeated three times with
similar results, and representative results are shown.
),
BCG-CWS (15 μg/ml) (■), heat-killed BCG (iDC/bacterium ratio =
1:1) (
), or viable BCG (iDC/bacterium ratio = 1:1) (▩).
Values are expressed as the mean fluorescence intensities measured by a
flow cytometer, and the mean values of subclass control MAbs were
negligible (∼3 to 4). This experiment was repeated three times with
similar results, and representative results are shown.
We determined the concentrations of contaminating LPS in the medium containing BCG-CWS, the emulsion buffer, or saline by the endotoxin-specific assay kit. The LPS contents were 10.2 ± 0.2, 8.7 ± 0.2, and 6.3 ± 0.2 pg/ml, respectively. DC was not activated by LPS concentrations of less than 20 pg/ml. Moreover, polymyxin B, an LPS inhibitor, did not inhibit the effect of BCG-CWS (data not shown). Thus, the effect of LPS contamination on DC maturation was negligible. In addition, it has been reported that GMDP is a consensus unit of peptidoglycan in the mycobacterial cell wall skeleton that is in part responsible for the adjuvant activity in BCG-CWS in vivo (3, 5, 13). However, synthetic GMDP (15 μg/ml) showed no effect on iDC maturation (data not shown).
Induction of cytokine in iDC by BCG-CWS.
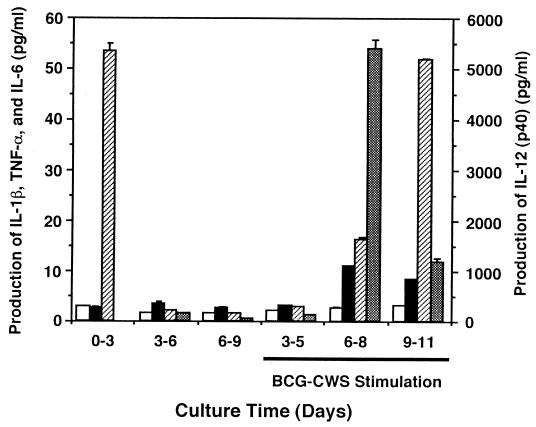
We next determined the levels of cytokines secreted by DC into the culture media (Fig. 3). iDC secreted very low levels of IL-1β, TNF-α, and IL-12 p40 at each time point during culturing. Monocytes released a large amount of IL-6 with treatment with IL-4 plus GM-CSF, although this ability was abrogated after the cells' differentiation to iDC. This cytokine liberation profile was markedly altered by BCG-CWS treatment, which induced the secretion of TNF-α, IL-6, and IL-12 p40 in the cells treated for more than 6 days with IL-4 plus GM-CSF, namely iDC. Although the levels of the liberated cytokines differed depending on the IL-4 plus GM-CSF culturing interval, the levels of IL-6 and IL-12 p40 were both high. It is notable that the time course curves of secretion of IL-12 p40 and TNF-α paralleled that of the CD83 expression level (Fig. 1). On iDC treated for 6 days, the concentration of IL-12 p70 was also minimal in spite of BCG-CWS treatment (Table 1). Similar results were obtained with DC that had been treated with TNF-α and IL-1β. In contrast, DC that matured with LPS produced high levels of IL-12 p70. IL-18 and IFN-γ were not detected in the culture supernatant at any time (data not shown).
FIG. 3.
Cytokine production by iDC and DC treated with BCG-CWS. Monocytes were cultured in medium containing GM-CSF and IL-4, and the medium was collected every 3 days (0-3, 3-6, 6-9). iDC cultured for 3, 6, or 9 days were incubated in medium with GM-CSF (500 IU/ml) and BCG-CWS (15 μg/ml), and each medium was collected after 2 days (3-5, 6-8, 9-11). The concentrations of each cytokine in the medium (IL-1β [□], TNF-α [■], IL-6 [▨], and IL-12 p40 [▩]) were determined by ELISA. Values are the means ± standard deviations (SD) of triplicate determinations. The concentrations of IL-12 p40 are on the right vertical axis.
TABLE 1.
Secretion of IL-12 by DC with various stimulia
| Treatment of iDC | Secretion (pg/ml) of:
|
|
|---|---|---|
| IL-12 (p40 + p70) | IL-12 (p70) | |
| IL-4 + GM-CSF | 17 ± 2 | 3.8 ± 0.1 |
| GM-CSF alone | 51 ± 5 | 4.2 ± 0.1 |
| TNF-α + GM-CSF | 4,900 ± 130 | 8.3 ± 0.1 |
| IL-1β + GM-CSF | 32,250 ± 1,200 | 7.0 ± 0.1 |
| LPS + GM-CSF | 130,000 ± 6,000 | 653 ± 52 |
| Emulsion buffer + GM-CSF | 14 ± 4 | 2.9 ± 0.1 |
| BCG-CWS + GM-CSF | 5,400 ± 210 | 6.4 ± 0.1 |
iDC differentiated with GM-CSF and IL-4 for 6 days were incubated for 2 days in medium containing GM-CSF (500 IU/ml) and the following material: IL-4 (100 IU/ml), GM-CSF alone, TNF-α (100 IU/ml), IL-1β (100 ng/ml), LPS (10 ng/ml), emulsion buffer (15 μl/ml), or BCG-CWS (15 μg/ml). The culture supernatants were collected, cleared by centrifugation, and measured by ELISA kits. Values are the means ± SD of triplicate determinations. The experiments were repeated twice, and results of one experiment are shown.
Autocrine activation of DC by TNF-α induced by BCG-CWS.
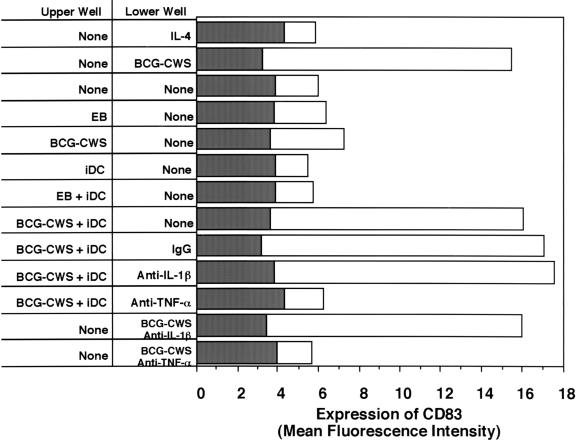
We next wanted to determine the factors that were either directly or indirectly responsible for DC maturation after BCG-CWS treatment. A transwell apparatus was employed for this analysis. Stimulators or mediator sources (including iDC) were placed in the upper wells, and iDC were added to the lower wells with or without antibodies against mediators. The maturation of iDC in the lower wells was evaluated by expression of CD83, which was used as a DC maturation marker (Fig. 4). Expression of CD83 was not induced in the lower-well cells when either emulsion buffer or BCG-CWS was added to the upper wells. BCG-CWS could not pass through the intercepting membrane, since BCG-CWS was insoluble material, and CD83 expression was not increased on the lower-well cells. iDC in the lower wells also did not express CD83 when the upper wells were filled with iDC or iDC plus emulsion buffer. The lower-well iDC expressed CD83 only when iDC and BCG-CWS were simultaneously added to the upper well. The expression of CD83 in the lower-well iDC was not suppressed by the addition of control IgG or anti-IL-1β to the lower well but was abrogated by the addition of anti-TNF-α to the lower well. Moreover, CD83 was not induced by the addition of anti-TNF-α plus BCG-CWS in lower-well iDC. These results suggest that BCG-CWS-mediated maturation of iDC is induced by TNF-α secreted from the stimulated iDC per se and that the direct contact of BCG-CWS is not a factor in CD83 expression. IL-12 did not affect DC maturation, since neither anti-IL-12 p70 nor recombinant IL-12 affected the levels of CD83 expression in BCG-CWS-treated DC or iDC, respectively (data not shown).
FIG. 4.
The levels of CD83 in the lower-well DC in the transwell system. iDC were incubated for 2 days in a transwell apparatus with GM-CSF (500 IU/ml) and the reagents indicated in the figure. The cells in the lower well were harvested, and levels of CD83 were measured by a flow cytometer. Values are expressed as the mean fluorescence intensity (open bars, levels of CD83; closed bars, background levels by subclass control MAb). Emulsion buffer is abbreviated EB. These experiments were repeated three times with similar results, and representative results are shown.
Antigen-presenting ability of DC matured by BCG-CWS.
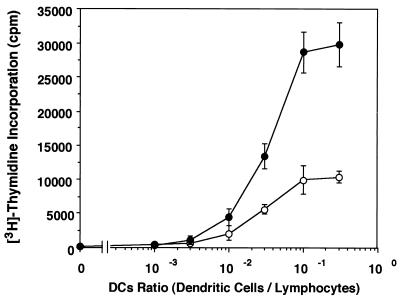
The antigen-presenting activity of iDC and DC treated with BCG-CWS was assessed by MLR (Fig. 5). iDC facilitated an increase of allogeneic lymphocytes, and addition of BCG-CWS induced approximately a threefold more effective increase in lymphocytes. DC treated with BCG-CWS were allowed to amplify lymphocyte proliferation but did not increase the sensitivity to the ratio of lymphocytes.
FIG. 5.
Allogeneic MLR using iDC and DC treated with BCG-CWS. iDC were incubated for 2 days in medium with GM-CSF plus IL-4 (○) or GM-CSF plus BCG-CWS (●). These DC were irradiated and cultured for 4 days with allogeneic lymphocytes, and [3H]thymidine incorporation was measured. Values are expressed as the means ± SD of triplicate determinations. Similar experiments were repeated twice, and representative results are shown.
Direct binding of BCG-CWS to DC.
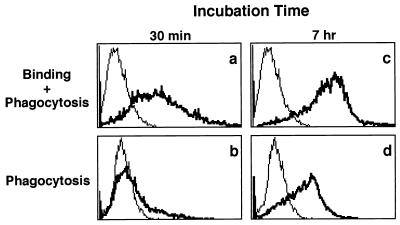
The binding properties of BCG-CWS for iDC were analyzed with FITC-labeled BCG-CWS, and phagocytosis properties were evaluated by the trypan blue quenching method (38). FITC-BCG-CWS bound efficiently to iDC within 30 min (Fig. 6a), and then the labeled particles were not phagocytosed because the fluorescence was quenched by the addition of trypan blue (Fig. 6b). Seven hours later, the cell-associated BCG-CWS particles appeared to have increased (Fig. 6c) concomitantly with augmentation of intracellular uptake (Fig. 6d).
FIG. 6.
Flow-cytometric analysis of phagocytosis of FITC–BCG-CWS by iDC. iDC were incubated for 0.5 or 7 h in medium with GM-CSF (500 IU/ml) and FITC–BCG-CWS (15 μg/ml). Thin lines show self-fluorescence of cells, and bold lines reflect fluorescence of FITC-BCG-CWS particles which are bound and/or phagocytosed. Total fluorescence intensities (panels a and c) and those quenched with trypan blue (panels b and d) are shown. This experiment was repeated three times with similar results, and representative results are shown.
Analysis of receptors for BCG-CWS.
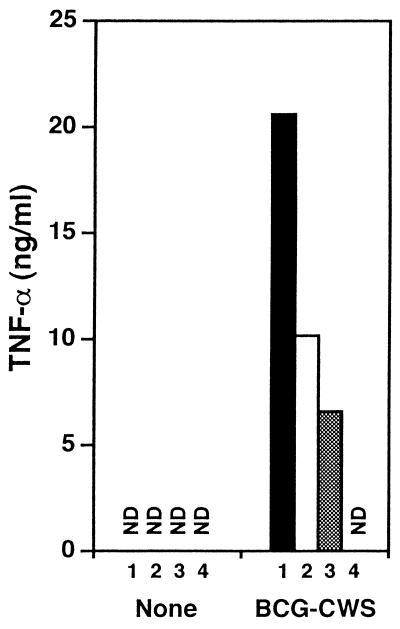
The proteins of the TLR family are currently considered to be receptors for materials of bacterial origin. To analyze the signaling receptor for BCG-CWS, we investigated the response to BCG-CWS of peritoneal macrophages from TLR-deficient mice (Fig. 7). BCG-CWS induced TNF-α secretion to the macrophages in wild-type mice but not in TLR2 and TLR4 double-deficient mice. TNF-α secretion by BCG-CWS was partially suppressed in macrophages from TLR2 or TLR4 single-deficient mice. Thus, TNF-α responsiveness was exclusively attributable to TLR2 and TLR4.
FIG. 7.
Signaling of BCG-CWS via TLR. Thioglycolate-elicited peritoneal macrophages from wild-type (1; ■), TLR2-deficient (2; □), TLR4-deficient (3; ▩), or TLR2- and TLR4-deficient (4) mice were cultured with IFN-γ (30 U/ml) in the presence or absence of BCG-CWS (10 μg/ml) for 24 h. Concentrations of TNF-α in the culture supernatants were measured by ELISA. ND, not detected. This experiment was repeated twice with similar results, and representative results are shown.
The expressions of TLR2 and TLR4 were examined with human iDC by reverse transcription-PCR. Both TLR2 and TLR4 mRNAs were detected in iDC (data not shown). This, together with the results for TLR-deficient mice, indicates that the BCG-CWS-inducible secretion of TNF-α is likely to depend upon TLR2 and TLR4 in iDC.
DISCUSSION
The mycobacterial cell wall is known to be a strong adjuvant and contains CWS, lipoarabinomannan (LAM), trehalose 6,6′-dimycolate (TDM), and other various lipooligosaccharides and lipoproteins. CWS is peptidoglycan that is covalently linked to arabinogalactan via a phosphodiester bond, and mycolic acids are in turn attached to the arabinogalactan (4, 8, 9). Here we demonstrated that BCG-CWS is able to activate human iDC. BCG-CWS induced increases of the costimulatory molecules CD80 and CD86 on DC, similarly to TNF-α, IL-1β, and BCG bacteria, and also induced an increase of allogeneic MLR. These results indicate that antigen presentation and T-cell stimulation would be enhanced by BCG-CWS. BCG-CWS also induced the up-regulation of the DC maturation marker CD83 and the secretion of inflammatory cytokines, such as IL-6, IL-12, and TNF-α. These responses and the increases of antigen-presenting ability indicate that the activation and maturation of DC is induced by CWS containing mycobacterial peptidoglycan.
The complete CFA is a typical immuno-adjuvant containing dead M. tuberculosis cells. CFA induces T cell-mediated immune responses and antibody production more potently than incomplete Freund's adjuvant, which is mineral oil without bacterial components, so that CFA is traditionally used as a primary adjuvant (14). Immune activation or adjuvant potency by CFA has mainly been assessed by in vivo animal responses. However, the factors required for potent adjuvant activity are not yet well defined. Since a part of adjuvant activity is attributable to the increase of antigen-presenting ability through maturation of DC, our results suggest that mycobacterial CWS is an essential adjuvant factor in CFA.
It has been reported that DC are activated by mycobacteria (11, 19, 20, 23, 39). LAM and TDM are known as immuno-modulatory factors in the mycobacterial cell wall (6, 8, 15, 21, 24, 30). However, we could not find evidence of the activation and maturation of DC by LAM or TDM (data not shown). Moreover, BCG-CWS without LAM and TDM showed an effect similar to that for BCG bacteria (Fig. 2). These results suggest that CWS is a DC maturation factor in mycobacteria. We also investigated whether GMDP reserves the function of DC maturation, since GMDP is a consensus unit of peptidoglycan in CWS of mycobacteria (3, 13) and has an adjuvant activity in vivo (5). However, GMDP showed no effect on DC activation (data not shown). From these results, we surmise that DC activation by BCG-CWS depends on the structure of the GMDP polymer or the addition of arabinogalactans and/or mycolic acids.
Recently it has been reported that TLR2 and TLR4 are implicated in the recognition of various bacterial cell wall components, such as LPS, LAM, lipoteichoic acid, lipoproteins, and soluble peptidoglycan (2, 10, 27, 28, 36, 37, 43). The recent studies of TLR overexpression and TLR-deficient mice showed that gram-positive bacterial peptidoglycan induced TLR2-dependent cell activation (36, 37, 43). Means et al. reported that viable M. tuberculosis induced the signaling via both TLR2 and TLR4, whereas heat-killed bacteria induced the signaling via only TLR2 (28). Our analysis on TLR-deficient mice showed that BCG-CWS induced the signaling via both TLR2 and TLR4, because the induction of TNF-α secretion by BCG-CWS was partially suppressed on macrophages from TLR2 or TLR4 single-deficient mice, and it was completely lost on TLR2 and TLR4 double-deficient mice. On BCG-CWS stimulation, the signaling via TLR on DC would not directly induce DC maturation, since induction of CD83 expression by BCG-CWS was inhibited by the neutralization of TNF-α (Fig. 4).
Two results were reported in relation to the association of TNF-α with DC maturation in response to infection by BCG mycobacteria; Kim et al. reported that BCG-induced CD83 expression was not inhibited by the neutralization of TNF-α, which used TNF-binding protein (23), and Thurnher et al. reported that it was inhibited more than 50% by the neutralizing antibody against TNF-α (39). This difference may be caused by the various uncertain factors in BCG bacteria. We demonstrated that BCG-CWS-induced CD83 expression is completely inhibited by the neutralizing monoclonal antibody against TNF-α (Fig. 4). Thus, we favor the interpretation that DC maturation by purified CWS of BCG is dependent on TNF-α secreted from BCG-CWS-stimulated DC per se.
Live BCG bacteria have been used for immune therapy for bladder cancer in humans (1). Although not widely accepted, immune therapy with BCG-CWS has also yielded a good prognosis without any sign of infection for many cancer patients (17, 18, 42). Since the stimulation and the maturation of DC induce the increases of antigen-presenting ability, our present study may explain a part of the molecular mechanism in the tumor immunotherapy with BCG-CWS.
REFERENCES
- 1.Alexandroff A B, Jackson A M, O'Donnell M A, James K. BCG immunotherapy of bladder cancer: 20 years on. Lancet. 1999;353:1689–1694. doi: 10.1016/S0140-6736(98)07422-4. [DOI] [PubMed] [Google Scholar]
- 2.Aliprantis A O, Yang R, Mark M R, Suggett S, Devaux B, Radolf J D, Klimpel G R, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through Toll-like receptor-2. Science. 1999;285:736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 3.Azuma I, Thomas D W, Adam A, Ghuysen J M, Bonaly R, Petit J F, Lederer G. Occurrence of N-glycolylmuramic acid in bacterial cell walls. Biochim Biophys Acta. 1970;208:444–451. doi: 10.1016/0304-4165(70)90217-5. [DOI] [PubMed] [Google Scholar]
- 4.Azuma I, Ribi E E, Meyer T J, Zbar B. Biologically active components from mycobacterial cell walls. I. Isolation and composition of cell wall skeleton and component P3. J Natl Cancer Inst. 1974;52:95–101. doi: 10.1093/jnci/52.1.95. [DOI] [PubMed] [Google Scholar]
- 5.Azuma I, Okumura H, Saiki I, Tanio Y, Kiso M, Hasegawa A, Yamamura Y. Adjuvant activity of 6-amino-6-deoxy-muramyldipeptides and their acylamino derivatives on the induction of delayed hypersensitivity to azobenzenearsonate-N-acetyl-l-tyrosine in guinea pigs. Infect Immun. 1981;32:1305–1308. doi: 10.1128/iai.32.3.1305-1308.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnes P F, Chatterjee D, Abrams J S, Lu S, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan. Relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 7.Bartlett G L, Kreider J W, Purnell D M. Relationship between intradermal tumor suppression and tumor immunity. J Natl Cancer Inst. 1976;57:1297–1303. doi: 10.1093/jnci/57.6.1297. [DOI] [PubMed] [Google Scholar]
- 8.Besra G S, Chatterjee D. Lipids and carbohydrates of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 285–306. [Google Scholar]
- 9.Brennan P J, Draper P. Ultrastructure of Mycobacterium tuberculosis. In: Bloom B R, editor. Tuberculosis: pathogenesis, protection, and control. Washington, D.C.: American Society for Microbiology; 1994. pp. 271–284. [Google Scholar]
- 10.Brightbill H D, Libraty D H, Krutzik S R, Yang R, Belisle J T, Bleharski J R, Maitland M, Norgard M V, Plevy S E, Smale S T, Brennan P J, Bloom B R, Godowski P J, Modlin R L. Host defense mechanisms triggered by microbial lipoproteins through Toll-like receptors. Science. 1999;285:732–736. doi: 10.1126/science.285.5428.732. [DOI] [PubMed] [Google Scholar]
- 11.Demangel C, Bean A G D, Martin E, Feng C G, Kamath A T, Britton W J. Protection against aerosol Mycobacterium tuberculosis infection using Mycobacterium bovis bacillus Calmette Guérin-infected dendritic cells. Eur J Immunol. 1999;29:1972–1979. doi: 10.1002/(SICI)1521-4141(199906)29:06<1972::AID-IMMU1972>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 12.DeVita V T, Jr, Hellman S, Rosenberg S A. Biological therapy of cancer. J. B. Philadelphia, Pa: Lippincott; 1991. [Google Scholar]
- 13.Ellouz F, Adam A, Ciorubaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan subunits. Biochem Biophys Res Commun. 1974;59:1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 14.Freund J. The mode of action of immunomologic adjuvants. Adv Tuberc Res. 1956;7:130–148. [PubMed] [Google Scholar]
- 15.Grand-Perret T, Lepoivre M, Petit J F, Lemaire G. Macrophage activation by trehalose dimycolate requirement for an expression signal in vitro for antitumoral activity; biochemical markers distinguishing primed and fully activated macrophages. Eur J Immunol. 1986;16:332–338. doi: 10.1002/eji.1830160403. [DOI] [PubMed] [Google Scholar]
- 16.Hawrylko E. BCG immunopotentiation of an antitumor response: evidence for a cell-mediated mechanism. J Natl Cancer Inst. 1975;55:413–423. doi: 10.1093/jnci/55.2.413. [DOI] [PubMed] [Google Scholar]
- 17.Hayashi A. Interferon-g as a marker for the effective cancer immunotherapy with BCG-cell wall skeleton. Proc Jpn Acad. 1994;70(B):205–209. [Google Scholar]
- 18.Hayashi A, Doi O, Azuma I, Toyoshima K. Immuno-friendly use of BCG-cell wall skeleton remarkably improves the survival rate of various cancer patients. Proc Jpn Acad. 1998;74(B):50–55. [Google Scholar]
- 19.Henderson R A, Watkins S C, Flynn J L. Activation of human dendritic cells following infection with Mycobacterium tuberculosis. J Immunol. 1997;159:635–643. [PubMed] [Google Scholar]
- 20.Inaba K, Inaba M, Naito M, Steinman R M. Dendritic cell progenitors phagocytose particulates, including bacillus Calmette-Guerin organisms, and sensitize mice to mycobacterial antigens in vivo. J Exp Med. 1993;178:479–488. doi: 10.1084/jem.178.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kaplan G, Gandhi R R, Weinstein D E, Levis W R, Patarroyo M E, Brennan P J, Cohn Z A. Mycobacterium leprae antigen-induced suppression of T cell proliferation in vitro. J Immunol. 1987;138:3028–3034. [PubMed] [Google Scholar]
- 22.Kaufmann S H E, van Embden J D A. Tuberculosis, a neglected disease strikes back. Trends Microbiol. 1993;1:2–5. doi: 10.1016/0966-842x(93)90015-j. [DOI] [PubMed] [Google Scholar]
- 23.Kim K D, Lee H G, Kim J K, Park S N, Choe I S, Choe Y-K, Kim S J, Lee E, Lim J-S. Enhanced antigen-presenting activity and tumor necrosis factor-α-independent activation of dendritic cells following treatment with Mycobacterium bovis bacillus Calmette-Guérin. Immunology. 1999;97:626–633. doi: 10.1046/j.1365-2567.1999.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Knutson K L, Hmama Z, Herrera-Velit P, Rochford R, Reiner N E. Lipoarabinomannan of Mycobacterium tuberculosis promotes protein tyrosine dephosphorylation and inhibition of mitogen-activated protein kinase in human mononuclear phagocytes. Role of the Src homology 2 containing tyrosine phosphatase 1. J Biol Chem. 1998;273:645–652. doi: 10.1074/jbc.273.1.645. [DOI] [PubMed] [Google Scholar]
- 25.Lamensans A, Chedid L, Lederer E, Rosselet J P, Gustafson R H, Spencer H J, Ludwig B, Berger F M. Enhancement of immunity against murine syngeneic tumors by a fraction extracted from non-pathogenic mycobacteria. Proc Natl Acad Sci USA. 1975;72:3656–3660. doi: 10.1073/pnas.72.9.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathe G, Halle-Pannenko O, Bourut C. BCG in cancer immunotherapy: results obtained with various BCG preparations in a screening study for systemic adjuvants applicable to cancer immuno-prophylaxis or immunotherapy. Natl Cancer Inst Monogr. 1973;39:107–113. [PubMed] [Google Scholar]
- 27.Means T K, Lien E, Yoshimura A, Wang S, Golenbock D T, Fenton M J. The CD14 ligands lipoarabinomannan and lipopolysaccharide differ in their requirement for Toll-like receptors. J Immunol. 1999;163:6748–6755. [PubMed] [Google Scholar]
- 28.Means T K, Wang S, Lien E, Yoshimura A, Golenbock D T, Fenton M J. Human Toll-like receptors mediate cellular activation by Mycobacterium tuberculosis. J Immunol. 1999;163:3920–3927. [PubMed] [Google Scholar]
- 29.Minden P, Mathews H L. Suppression and immunotherapy of the guinea pig line 10 hepatocarcinoma mediated by heat-killed disrupted Mycobacterium bovis strain Bacillus Calmette-Guerin. Cancer Res. 1980;40:3214–3217. [PubMed] [Google Scholar]
- 30.Oswald I P, Dozois C M, Petit J F, Lemaire G. Interleukin-12 synthesis is a required step in trehalose dimycolate-induced activation of mouse peritoneal macrophages. Infect Immun. 1997;65:1364–1369. doi: 10.1128/iai.65.4.1364-1369.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palucka K A, Taquet N, Sanchez-Chapuis F, Gluckman J C. Dendritic cells as the terminal stage of monocytes differentiation. J Immunol. 1998;160:4587–4595. [PubMed] [Google Scholar]
- 32.Pickl W F, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, Bello-Fernandez C, Knapp W. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+peripheral blood monocytes. J Immunol. 1996;157:3850–3859. [PubMed] [Google Scholar]
- 33.Ransom J H, Pelle B A, Hubers H, Keynton L M, Hanna M G, Jr, Pomato N. Identification of colon-tumor-associated antigens by T-cell lines derived from tumor-infiltrating lymphocytes and peripheral-blood lymphocytes from patients immunized with an autologous tumor-cell/bacillus Calmette-Guerin vaccine. Int J Cancer. 1993;54:734–740. doi: 10.1002/ijc.2910540505. [DOI] [PubMed] [Google Scholar]
- 34.Romani N, Gruner S, Brang D, Kakumpgen E, Lenz A, Trockenbacher B, Konwalinka G, Fritsch P O, Steinman R M, Schuler G. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93. doi: 10.1084/jem.180.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwandner R, Dziarski R, Wesche H, Rothe M, Kirschning C J. Peptidoglycan- and lipoteichoic acid-induced cell activation is mediated by Toll-like receptor 2. J Biol Chem. 1999;274:17406–17409. doi: 10.1074/jbc.274.25.17406. [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takeda H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of Gram-negative and Gram-positive bacterial cell wall components. Immunity. 1999;11:443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 38.Takizawa F, Tsuji S, Nagasawa S. Enhancement of macrophage phagocytosis upon iC3b deposition on apoptotic cells. FEBS Lett. 1996;397:269–272. doi: 10.1016/s0014-5793(96)01197-0. [DOI] [PubMed] [Google Scholar]
- 39.Thurnher M, Ramoner R, Gastl G, Radmayr C, Beck G, Herold M, Klocker H, Bartsch G. Bacillus Calmette-Guerin mycobacteria stimulate human blood dendritic cells. Int J Cancer. 1997;70:128–134. doi: 10.1002/(sici)1097-0215(19970106)70:1<128::aid-ijc19>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 40.Vermorken J B, Claessen A M, van Tinteren H, Gall H E, Ezinga R, Meijer S, Scheper R J, Meijer C J, Bloemena E, Ransom J H, Hanna M G, Jr, Pinedo H M. Active specific immunotherapy for stage II and stage III human colon cancer: a randomised trial. Lancet. 1999;353:345–350. doi: 10.1016/S0140-6736(98)07186-4. [DOI] [PubMed] [Google Scholar]
- 41.Yamamura Y, Azuma I, Taniyama T, Sugimura K, Hirao F, Tokuzen R, Okabe M, Nakahara W, Yasumoto K, Ohta M. Immunotherapy of cancer with cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin: experimental and clinical results. Ann N Y Acad Sci. 1976;277:209–227. doi: 10.1111/j.1749-6632.1976.tb41699.x. [DOI] [PubMed] [Google Scholar]
- 42.Yasumoto K, Manabe H, Yanagawa E, Nagano N, Ueda H, Hirota N, Ohta M, Nomoto K, Azuma I, Yamamura Y. Nonspecific adjuvant immunotherapy of lung cancer with cell wall skeleton of Mycobacterium bovis bacillus Calmette-Guerin. Cancer Res. 1979;39:3262–3267. [PubMed] [Google Scholar]
- 43.Yoshimura A, Lien E, Ingalls R R, Tuomanen E, Dziarski R, Golendock D. Recognition of Gram-positive bacterial cell wall components by the innate immune system occurs via Toll-like receptor 2. J Immunol. 1999;163:1–5. [PubMed] [Google Scholar]
- 44.Zbar B, Ribi E E, Meyer T J, Azuma I, Rapp H J. Immunotherapy of cancer: regression of established intradermal tumors after intralesional injection of mycobacterial cell walls attached to oil droplets. J Natl Cancer Inst. 1974;52:1571–1577. doi: 10.1093/jnci/52.5.1571. [DOI] [PubMed] [Google Scholar]
- 45.Zhou L, Tedder T F. CD14+ blood monocytes can differentiate into functionally mature CD83+dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–2592. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]