Abstract
Pneumothorax was previously considered as a complication of severe coronavirus disease 2019 (COVID-19) pneumonia. However, it is now known that pneumothorax can develop in other cases. Here, we describe the case of a patient who developed tension pneumothorax after release from isolation from COVID-19 pneumonia. The patient was admitted to our hospital with severe COVID-19 pneumonia on the 10th day after onset. Ventilatory management was carried out on the first day of admission; however, the patient was weaned off the next day. The treatment course was uneventful. On the morning of discharge from the hospital, the patient experienced sudden dyspnea. Chest radiography revealed a large left-tension pneumothorax with a mediastinal shift to the right. As this finding required immediate attention, a chest tube was inserted. Chest computed tomography (CT) showed an airspace in the left thoracic cavity and subpleural thin-walled cystic lesions, such as bullae in the left lobe. One month later, chest CT showed resolution of the cystic lesions. The development of pneumothorax in COVID-19 pneumonia should be considered not only in cases of severe illness, but also after release from isolation. Recently, revisions to measures against COVID-19 have been considered worldwide, including shortening of the isolation period and reviewing the identification of all cases. This is an educational report demonstrating that life-threatening pneumothorax may develop after release from isolation due to COVID-19 pneumonia.
Keywords: Bulla, COVID-19, Isolation, Pneumothorax
Background
Since the World Health Organization announced the coronavirus disease 2019 (COVID-19) pandemic due to SARS-CoV-2 infection in March 2020 [1], the patient population has increased exponentially. In patients who develop acute respiratory distress syndrome due to COVID-19, the complication rate of pneumothorax is much higher (> 10%) [2]. However, recent studies have described that pneumothorax can develop in patients with mild COVID-19 without underlying comorbidities [3,4]. Herein, we report our experience with a case of COVID-19 pneumonia that resulted in tension pneumothorax after release from isolation.
Case presentation
A 48-year-old Asian man was admitted to our hospital with severe COVID-19 pneumonia on the 10th day after symptom onset. The antigen test result for COVID-19 (Fujirebio, Inc., Tokyo, Japan) was positive. The patient had a smoking index of 34 pack-years; however, no pulmonary comorbidities, such as chronic obstructive pulmonary disease and interstitial pneumonia were noted, and the patient was unvaccinated. Percutaneous oxygen saturation (SpO2) was 85% on supplemental oxygen (3 L/min). Chest computed tomography (CT) showed bilateral subpleural ground-glass opacity (GGO) in the entire lung (Fig. 1). The patient was extubated after mechanical ventilation for 24 hours. He received therapeutic-dose heparin for 2 weeks from the first day of admission and 6 mg dexamethasone once daily from the first to the 10th day, 3 mg once daily from the 11th to the 13th day, and 1.5 mg once daily from the 14th to the 16th day. Although dry cough persisted, other symptoms gradually resolved. Acute chest pain with dyspnea, hemoptysis, and palpitations developed at discharge on the 25th day after hospitalization. His vital signs included a body temperature of 36.6°C, blood pressure of 100/80 mmHg, heart rate of 142 beats per minute, respiratory rate of 32 breaths per minute, and SpO2 of 75% on room air. No left-sided breath sounds were detected. A chest radiograph showed a large left-tension pneumothorax with a mediastinal shift to the right (Fig. 2). As this finding required immediate attention, a chest tube was inserted. Chest CT showed non-segmentally distributed intralobular septal thickening, pleural indentation, and consolidation, leading to volume loss in the left lung, with several subpleural thin-walled cystic lesions and intrathoracic air (Fig. 3). These lesions were consistent with the distribution of GGO on the chest CT at admission. His laboratory blood workup was mostly uneventful, apart from a slight elevation in the C-reactive protein level (0.72 mg/dL, reference range was < 0.26 mg/L). The polymerase chain reaction test for COVID-19 (BD SARS-CoV-2/Flu for BD MAX TM System; Becton, Dickinson and Company, New Jersey, USA) showed negative conversion after 2 confirmations. Continuous air leakage through the chest tube was observed during the following 8 days. Eventually, the patient was discharged after a 35-day inpatient care period. One month later, a subsequent chest CT scan showed resolution of all cystic lesions, leaving mild GGO in the lung fields (Fig. 4).
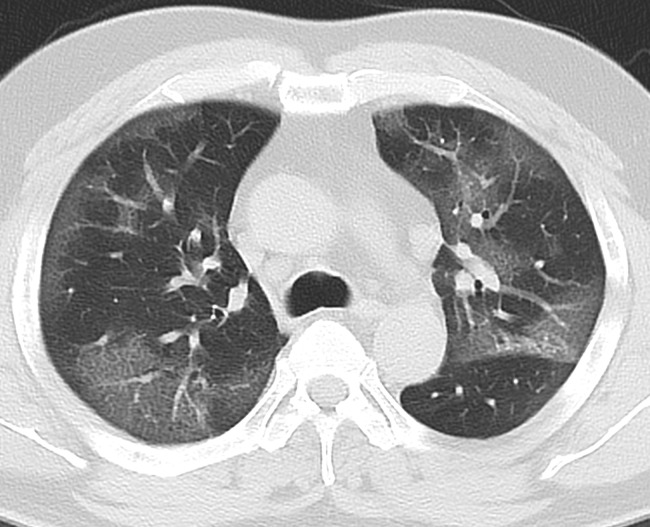
Fig. 1.
Chest computed tomography (CT) on admission. Chest CT showed bilateral subpleural ground-glass opacity (GGO) in the entire lung.
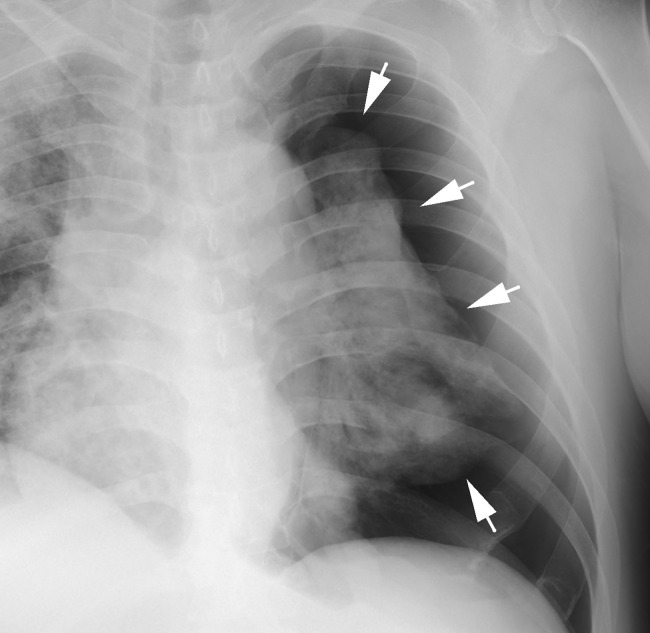
Fig. 2.
Chest radiographic images of the pneumothorax. Chest radiograph, anteroposterior view showing a large left-tension pneumothorax with a mediastinal shift to the right. White arrows indicate thin white visceral pleural lines.
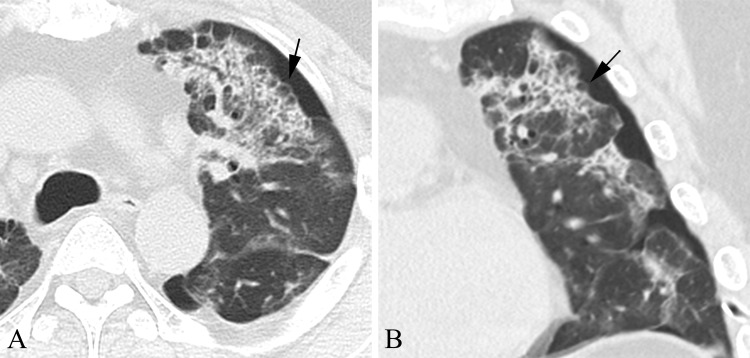
Fig. 3.
Chest CT of the thin-walled cystic air lesions such as bullae. (A) Computerized axial tomography scan. (B) Computerized coronal tomography scan. Chest CT showing several thin-walled cystic air lesions (black arrows) and left-sided pneumothorax revealed intralobular septal thickening and consolidation in the left lung field.
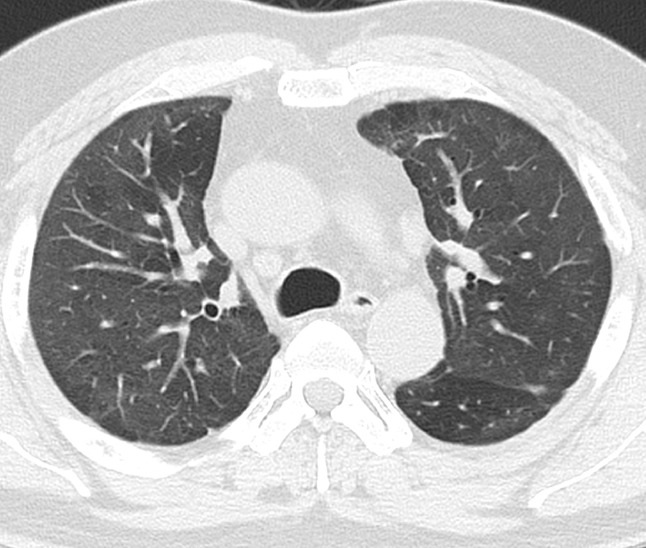
Fig. 4.
Chest CT 1 month after discharge showing absence of thin-walled cystic lesions with remaining mild ground-glass opacity in the lung fields.
Discussion
Pneumothorax is prominently known to be a potentially fatal complication of patients with severe COVID-19. Wang et al. [2] reported that 56% of patients with critical COVID-19 on invasive mechanical ventilation developed pneumothorax. However, with the continuation of the COVID-19 pandemic, non-severe pneumonia cases have also been reported to develop into pneumothorax during the treatment and observation periods [3]. At our hospital, the convalescence for severe COVID-19 pneumonia is set to at least 15 days after onset and 48 h after symptom resolution because the virus shedding period is longer [5] and the viral load shed is higher than that observed in milder cases [6]. Recently, revisions to the measures against COVID-19 infection are being considered worldwide, including shortening of the isolation period and reviewing the identification of all cases. Considering these global trends, this case highlights the importance of careful follow-up because of the risk of developing pneumothorax, even after the release of isolation.
COVID-19 pneumonia with cavitary lesions on computed tomography (CT) in the initial stages is rarely observed [7]. Similarly, in our case, there were no cavitary lesions in the lung field on CT at the initial visit. The chest CT at the development of pneumothorax showed several subpleural thin-walled cavitary lesions, such as bullae. At follow-up, the chest CT scan after discharge revealed that these peripheral cavitary lesions had resolved in the left lung. The reports of Nunna et al. suggest that rupture of the pneumatocele, complicated during the healing process, is associated with pneumothorax in COVID-19 pneumonia [8]. Rapid growth of pneumatocele can trigger the development of pneumothorax. It almost always resolves spontaneously and can be managed conservatively [9]. In this case as well, all subpleural thin-walled cavities spontaneously disappeared without any specific treatment on CT one month after discharge, while these lesions were approximately 1 cm in diameter, and chest radiographs during hospitalization could not confirm their presence, let alone rapid enlargement.
Common lung histopathological findings in patients with COVID-19 include capillary congestion caused by alveolar capillary microthrombi and reactive alveolar epithelial cells associated with disrupted cell membranes [10]. In this case, although no histological examination was performed, the formation of subpleural thin-walled cavitary lesions may have been associated with damage to the pulmonary acinus structure. Nonsegmental intralobular septal thickening and massive consolidation indicate organizing pneumonia and pulmonary fibrosis, which may induce alveolar collapse and low pulmonary compliance. In addition, the alveolar space destroyed, and peripheral airways narrowed by inflammation and edema may function as a check valve mechanism, and aggressive prolonged coughing, a powerful Valsalva maneuver, may have played a role in increasing the intra-alveolar pressure, causing the rupture of gas-filled lesions.
Eastridge et al. [11] demonstrated that steroid treatment reduced the vascular permeability, inhibited inflammatory cell migration and fibroblast proliferation, and decreased collagen fibrils, resulting in the delayed repair of pleural damage. Thus, it is considered that inflammatory cell infiltration within the subpleural thin-walled cyst wall is suppressed by steroids, which attenuates the inflammatory response and inhibits fibrosis, leading to the weakening and rupture of the cyst wall. In contrast, Agrafiotis et al. [3] reported a steroid administration rate of 25.0% in patients with pneumothorax. Furthermore, dexamethasone is effective in patients with severe COVID-19 [12]. Ultimately, it is unclear whether steroid therapy is related to the risk of pneumothorax in patients with COVID-19 pneumonia. It is necessary to discuss the risks and benefits of steroid therapy for this infection, including the type, duration of administration, duration, and dosage.
Conclusion
In this case, a thin-walled cavity lesion and pneumothorax developed on day 35 after onset and resolved one month later. The long-term course of COVID-19 pneumonia remains unclear, and the pathogenesis of pneumothorax seems to differ depending on the time of onset. Recently, the declaration of the end of the COVID-19 pandemic has been suggested and a symbiotic relationship is being explored. However, COVID-19 infection can be widespread at any time, and countermeasures must always be sought. In general practice, post-COVID-19 infection is listed as one of the causes of pneumothorax. If the coexistent relationship with coronavirus progresses, this will provide important information when administering treatment for COVID-19 in general medical practice.
Patient consent
Written informed consent was obtained from the patients for the anonymized information to be published in this article.
Ethical approval
Not required.
Acknowledgments
We thank the patient and his family for their patience and cooperation. We would like to convey our gratitude to the clinicians of the COVID-19 infectious team at Osaka City General Hospital for supporting us with this project. We thank Dr. Okuma for their radiological expertise.
Footnotes
Funding: None declared.
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): coronavirus disease 2019 (COVID-19): situation report, 51. 2020. Available at: https://apps.who.int/iris/handle/10665/331475, August, 15, 2022. [PubMed]
- 2.Wang XH, Duan J, Han X, Liu X, Zhou J, Wang X, et al. High incidence and mortality of pneumothorax in critically ill patients with COVID-19. Heart Lung. 2021;50:37–43. doi: 10.1016/j.hrtlng.2020.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agrafiotis AC, Rummens P, Lardinoid I. Pneumothorax in otherwise healthy non-intubated patients suffering from COVID-19 pneumonia: a systematic review. J Thorax Dis. 2021;13:4519–4529. doi: 10.21037/jtd-21-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi H, Han X, Jiang N, Cao Y, Alwalid O, Gu J, et al. Radiological findings from 81 patients with COVID-19 pneumonia in Wuhan, China: a descriptive study. Lancet Infect Dis. 2020;20:425–434. doi: 10.1016/S1473-3099(20)30086-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Qi T, Liu L, Ling Y, Qian Z, Li T, et al. Clinical progression of patients with COVID-19 in Shanghai, China. J Infect. 2020;80:e1–e6. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To KK, Tsang OT, Leung WS, Tam AR, Wu TC, Lung DC, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan CS, Lv ZB, Yan S, Du YN, Chen H, Wei LG, et al. Imaging features of coronavirus disease 2019 (COVID-19): evaluation on thin-section CT. Acad Radiol. 2020;27:609–613. doi: 10.1016/j.acra.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nunna K, Braun AB. Development of a large spontaneous pneumothorax after recovery from mild COVID-19 infection. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2020-238863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quigley MJ, Fraser RS. Pulmonary pneumatocele: pathology and pathogenesis. AJR. 1988;150:1275–1277. doi: 10.2214/ajr.150.6.1275. [DOI] [PubMed] [Google Scholar]
- 10.Beigee FS, Toutkaboni MP, Khalili N, Nadji SA, Dorudinia A, Rezaei M, et al. Diffuse alveolar damage and thrombotic microangiopathy are the main histopathological findings in lung tissue biopsy samples of COVID-19 patients. Pathol Res Pract. 2020;216 doi: 10.1016/j.prp.2020.153228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eastridge CE, Hamman JL. Pneumothorax complicated by chronic steroid treatment. Am J Surg. 1973;126:784–787. doi: 10.1016/s0002-9610(73)80071-6. [DOI] [PubMed] [Google Scholar]
- 12.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Recovery Collaborative Group. Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. 2020;25:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]