Abstract
PyK2 is a member of the proline-rich tyrosine kinase and focal adhesion kinase families and is ubiquitously expressed. PyK2 is mainly activated by stimuli, such as activated Src kinases and intracellular acidic pH. The mechanism of PyK2 activation in cancer cells has been addressed extensively. The up-regulation of PyK2 through overexpression and enhanced phosphorylation is a key feature of tumorigenesis and cancer migration. In this review, we summarized the cancer milieu, including acidification and cancer-associated molecules, such as chemical reagents, interactive proteins, chemokine-related molecules, calcium channels/transporters, and oxidative molecules that affect the fate of PyK2. The inhibition of PyK2 leads to a beneficial strategy to attenuate cancer cell development, including metastasis. Thus, we highlighted the effect of PyK2 on various cancer cell types and the distribution of molecules that affect PyK2 activation. In particular, we underlined the relationship between PyK2 and cancer metastasis and its potential to treat cancer cells.
Keywords: PyK2, migration, metastasis, acidic milieu, PyK2-interactive proteins
1. Introduction
1.1. General Pathway of PyK2 Phosphorylation
Proline-rich tyrosine kinase 2 (PyK2) is a member of the proline-rich cytoplasmic tyrosine kinase family, which is ubiquitously expressed and dominantly localized in neuronal cells, endothelial cells, and hematopoietic cells [1,2,3,4,5]. PyK2 is phosphorylated by the initiation of extracellular signals during recruitment to the perinuclear membrane or nucleus [6]. Activated PyK2 phosphorylates the tyrosine residues of the target proteins. The PyK2 has a FERM (F for 4.1 protein, E for ezrin, R for radixin, and M for moesin) domain, which is a regulating domain in the N-terminus, and a focal adhesion targeting (FAT) domain located in the C-terminus (Figure 1) [7]. As a focal adhesion kinase, PyK2 transduces extracellular signals and coordinates cellular adhesion and cytoskeletal dynamics to regulate cell migration, proliferation, and survival [8]. PyK2 has a critical role in various cellular mechanisms, such as the migration of immune cells, including lymphocytes, macrophages [9,10], and glioma cells [11]. PyK2 also regulates the adhesion of T cells [12] and lipopolysaccharide (LPS)-induced IL-8 production in human endothelial cells [13].
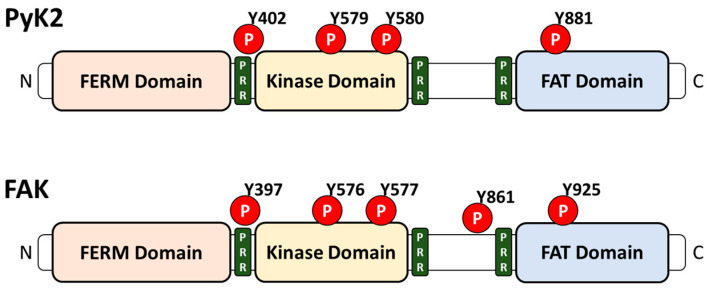
Figure 1.
Schematic structure of PyK2 and FAK. Similar structures of PyK2 and FAK, both of which contain a FERM domain, kinase domain, and FAT domain, from the N-terminal (N) to the C-terminal (C). PyK2 and FAK contain a proline-rich region (PRR) and a phosphorylation site (P).
In addition to PyK2, tyrosine kinases have two other members, focal adhesion kinase (FAK) and Src [14]. The structure of FAK shares a 65% similarity with the structure of PyK2 and contains the same three domains, including FERM, a central catalytic kinase domain, and a FAT domain (Figure 1) [3,5,15,16]. Although the structures of FAK and PyK2 are similar, they play distinct roles. The deletion of the FAK gene impaired mesodermal development, but the loss of PyK2 did not induce developmental impairments in mice [9,10,17]. While FAK is ubiquitous, PyK2 has limited expression [16]. PyK2 compensates for FAK expression. The deletion of FAK increased the expression of PyK2 in a mouse model [18,19]. Src is composed of family members that include Src, Lyn, Fyn, Yes, Hck, Fgr, Blk, and Lck [20]. The activity of Src has been studied in human lung, breast, colon, pancreatic, and gastric cancer [21,22,23,24,25]. Although the Y402 phosphorylation site of PyK2 is auto-phosphorylated [15], Src, another tyrosine kinase, is essential to the subsequent phosphorylation of PyK2 at other sites, including Y579, Y580, and Y881 [26,27,28]. In addition, Src has the SH2 domain which binds to PyK2 phosphorylation sites [7]. Especially in macrophages, LPS-stimulated IL-10 production is required to form a PyK2-Src complex with Src homology region 2 domain-containing phosphatase (SHP)-1 [29]. Although SHP-1 indirectly binds Src and directly binds PyK2, the inhibition of SHP-1 expression reduced the phosphorylation of Src and PyK2 [29]. Additionally, in SHP-1 knockout mice, macrophages showed the aberrant production of IL-10 after LPS stimulation [29]. Although the relationship between PyK2 and other kinases in the immune system has been extensively addressed, the roles of PyK2 in cancer progression, including the homeostasis of pH and intracellular Ca2+ signaling, have not been revealed. Thus, we elucidated the modulatory role of PyK2 and PyK2-associated molecular mechanisms in a cancer system.
1.2. PyK2 and Cancer-Favorable Acidified Milieu
The acidic pH of the extracellular milieu, which ranges from 6.4 to 7.0 [30], is a key feature of the cancer environment [31]. Extracellular acidosis induces numerous functions involved in cancer metabolism, especially cancer metastasis [31]. To develop metastasis, cancer cells proceed with the sequential steps of proliferation, epithelial-to-mesenchymal transition (EMT), invasion, transport, colonization, and angiogenesis [32]. Acidification of the cancer extracellular matrix induces EMT and invasion. For EMT, the loss of cell-to-cell adhesion and remodeled tight junctions must occur, and acidic pH triggers the dissociation of cancer cells [33,34]. With regard to the metastatic process in several cancer systems, acidic pH was shown to induce activation of acid-sensing ion channel (ASIC) with intracellular Ca2+ ([Ca2+]i) increases to activate EMT in pancreatic cancer cells [35]. Cancer cells need enzymes such as metalloproteinase (MMP) [36] and cathepsin [37] to penetrate tissue barriers for invasion. The enzymes secreted from cancer cells are activated by the sodium hydrogen exchanger (NHE)-induced acidification of the extracellular environment [38]. In addition, adaptation to the acidic pH triggers melanoma cell invasion [39,40,41]. Hwang et al. demonstrated that cancer cell migration was regulated by extracellular modulation through bicarbonate transporters, including anion exchanger 2 (AE2) and sodium bicarbonate cotransporter-n1 (NBCn1) [42,43]. Bicarbonate plays a role in intracellular pH maintenance to regulate physiological functions [44]. The activation of AE2 or NBCn1 transports bicarbonate ions, and the electrolyte flux was reported to induce A549 lung cancer cell migration [42,43]. Thus, adjustment of the extracellular pH is considered a critical strategy for treating cancer.
PyK2 has been associated with pH alterations. Li et al. demonstrated that PyK2 was a pH sensor and activator in the kidney [45]. The phosphorylation of PyK2, which is located on the basolateral side of renal epithelial cells, rapidly occurred in an acidic medium [45]. Activated PyK2 stimulated NHE3, followed by the release of H+ to acidify the luminal side of the renal epithelial cells [45]. No et al. demonstrated the precise mechanism of PyK2-induced NHE3 activation. In intestinal brush border cells, lysophosphatidic acid-induced epidermal growth factor receptor (EGFR) activation phosphorylated PyK2, and sequentially, p90 ribosomal S6 kinase (RSK) was phosphorylated through the involvement of PyK2 [46]. Phosphorylated RSK phosphorylated NHE3 to traffic NHE3 to the apical membrane of the intestine [46]. PyK2 increased ion movement through sodium-coupled dicarboxylate transporter-1 (NaDC-1) and H+-ATPase. NaDC-1 transported 3Na+ with citrate3−, which is used in the citric acid cycle [47,48]. Citrate plays important roles in the kidney, including the disruption of kidney stone formation [49,50], and is the principal base of urine [51]. Acidic media activated NaDC-1 in renal proximal tubule cells to uptake citrate [52]. The inhibition of PyK2 through a dominant-negative mutation decreased citrate uptake, and the deletion of PyK2 attenuated mouse blood citrate levels [52]. Reduced citrate levels are associated with potential kidney stone formation, which is affected by changes in the acid-base balance [52]. In addition, cellular adaptation to acidic pH was mediated by PyK2-associated H+-ATPase through the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 in mouse-derived outer medullary collecting ductal cells [53]. PyK2 is activated by ion channel ASIC1a. The acidosis of osteoclasts activated ASIC1a and subsequently increased the influx of Ca2+ [54]. The increased Ca2+ phosphorylated PyK2 and Src to up-regulate integrin, which induced cellular adhesion and migration [54]. Therefore, PyK2 triggers the acidification of extracellular pH and is modulated by extracellular pH. Thus, in this paper, we focused on the relationship between cancer (especially metastasis) and PyK2 with regard to pH alterations and the related mechanisms.
2. The Effect of PyK2 on Cancer Progression
The cancer environment is acidic, and this acidic microenvironment provides favorable conditions for cancer cell migration or invasion [31]. PyK2 is overexpressed in numerous cancers, including non-small lung cancer [55], breast cancer [56], colorectal cancer [57], and liver cancer [58]. PyK2 overexpression has a regulatory role in cancer tumorigenesis, including cell proliferation, migration, invasion, and metastasis. PyK2 activation is associated with the initiation of olfactory receptor signaling in prostate cancer cells [59], pre-malignant signaling in pancreatic ductal adenocarcinoma [60], and pituitary adenylate cyclase-induced phosphorylation-activating polypeptide receptors in non-small lung cancer cells [61]. In this section, we focused on the role of PyK2 as a signaling molecule in cellular metabolism, including proliferation, migration, and invasion, and highlighted the role of PyK2 as a therapeutic target in various cancers.
2.1. Role of PyK2 in Tumorigenesis and Proliferation
PyK2 is associated with the proliferation of cells such as fibroblasts, smooth muscle cells, and osteoblasts [62,63,64]. The knockdown of PyK2 decreased mouse embryo fibroblast growth, and deletion of the PyK2 gene attenuated the proliferation of megakaryocyte-induced osteoblasts in mice [62,64]. Platelet-derived growth factor (PDGF), which induces cellular proliferation, was shown to increase the phosphorylation of PyK2 in vascular smooth muscle cells [63]. In addition to fibroblasts and smooth muscle cells, the phosphorylated pY402 and pY881 forms of PyK2 are up-regulated in non-small cell lung cancer (NSCLC) tissues. However, the pY881 form was associated with different survival rates in patients with NSCLC [55]. Patients with a low expression of PyK2 (pY881) survived longer than patients with a high expression of PyK2 (pY881) [55]. Thus, the development of NSCLC is mainly regulated by the pY881 form of PyK2. PyK2 was also reported to phosphorylate the Y216 site of GSK3β to promote Wnt/β-catenin pathway signaling [57]. Wnt signaling is a major pathway in developing colorectal cancer [65]. Wnt signaling is activated by the inactivation of adenomatous polyposis coli (APC), which is a tumor suppressor, and the activation of β-catenin, which is a proto-oncogene [66]. The inactivation of APC resulted in the GSK3-induced phosphorylation of β-catenin [67]. The inhibition of PyK2 kinase activity attenuated adenoma formation in mice with APC inactivation [57]. Eph receptor 2 (EphA2), which is a tyrosine kinase, has been studied as a tumor suppressor [68]. Knocking down EphA2 induced skin cancer and ERK phosphorylation [69]. The tumorigenesis of cholangiocarcinoma was enhanced by EphA2 activation with the activation of PyK2 [70]. However, the relationship between PyK2 and cancer proliferation in prostate cancer is regulated by PyK2 expression, regardless of phosphorylation. PyK2 expression is up-regulated in prostate cancer cells and correlated with the enhanced expression of androgen receptors [71]. The inhibition of PyK2 expression attenuated the growth of prostate cancer cells and down-regulated androgen receptor expression and activity [71]. However, the overexpression of PyK2 and phosphorylation of the androgen receptor increased the growth of prostate cancer cells [71].
2.2. Migration, Invasion, and Metastasis
Several studies of PyK2 have verified that the over-expression and activation of PyK2 are related to cancer metastasis in numerous cancer cells, such as breast cancer, liver cancer, pancreatic cancer, prostate cancer, and glioma [19,58,72,73,74,75]. Not only does PyK2 mainly act as an up-regulated protein, but also the expression of PyK2 acts as an oncogenic protein for metastatic cancer. Cancer metastasis is initiated by EMT, which induces mobility to transform the shapes of cancer cells [76]. PyK2 promotes EMT or the migratory properties of various cancer cells. Briefly, PyK2 overexpressed in non-metastatic hepatocellular carcinoma (Hep3B) gave rise to EMT characteristics, which included enhanced membrane ruffle formation and the down-regulation of the cell adhesion molecule E-cadherin and the mechanical stress-associated protein cytokeratin [77]. In contrast, the knockdown of PyK2 modulated the morphology of BT-549 breast cancer cells to epithelial-like cells with the enhanced expression of E-cadherin [78]. The expression of PyK2 was increased by treating the epidermal growth factor (EGF) and transforming growth factor-β (TGF- β), which triggered EMT in MDA-MB-231 cells [78]. In high-grade breast cancer tissue, which manifests metastatic features, PyK2 expression was higher than in low-grade breast cancer tissue [78]. The migration of ovarian cancer and glioma cells was also stimulated by the up-regulation of PyK2. Chemokine ligand 18 (CCL18), which is a breast cancer cell migration stimulatory factor, enhanced the activation of PyK2 in ovarian cancer cells (CaOV3 and OVCAR3) [79]. The overexpression of PyK2 increased the migration of breast cancer cells, whereas the knockdown of PyK2 decreased breast cancer cell migration [79]. In addition, CCL18-induced increases in breast cancer cell (MDA-MB-231) migration, accompanied by the activation of PyK2 and Src [80], whereas CCL18-induced cell migration was attenuated by siRNA-PyK2 and siRNA-Src [80]. The overexpression of PyK2 enhanced glioma-cell (SF767 and G112) migration [81] and PyK2 siRNA-attenuated glioma-cell (A172, U87, HS683, and C6) migration [82]. Mutation of the FERM domain of PyK2 decreased the migration of glioma cells [83], suggesting that the FERM domain of PyK2 is involved in cellular migration.
PyK2-mediated invasion is evaluated using the Matrigel-coated Transwell assay. The application of siRNA-PyK2 decreased the invasion of A549 lung cancer cells [84], and the down-regulation of tropomyosin-related kinase B attenuated PyK2 phosphorylation (Y402) and subsequently decreased A549 migration [84]. The growth factors, EGF and heregulin (HRG), enhanced the invasion of breast cancer cells (MCF7, T47D, and SKBR3), accompanied by an increase in PyK2 phosphorylation [85]. The knockdown of PyK2 attenuated breast cancer cell migration via the down-regulation of MMP9, which degrades the ECM to penetrate the blood vessel barrier [85]. In non-cancer systems, the inhibition of PyK2 by the PyK2 inhibitor PF-4594755 decreased the migration of primary cultured mouse smooth muscle cells without a decrease in proliferation [86]. PyK2 regulates the migration of immune cells, including cytotoxic T lymphocytes (CTLs) and macrophages [87,88]. PyK2 Inhibition by PF-431396 decreased the migration of primary cultured mice CTLs [87] and attenuated the hydrogen sulfide-stimulated migration of RAW264.7 cells [88]. PyK2 overexpression stimulated the migration of mouse cortical neurons [89]. Thus, PyK2 plays a critical role in cell progression and migration (Figure 2), and strategies for regulating PyK2 might provide a new therapeutic approach against cancer.
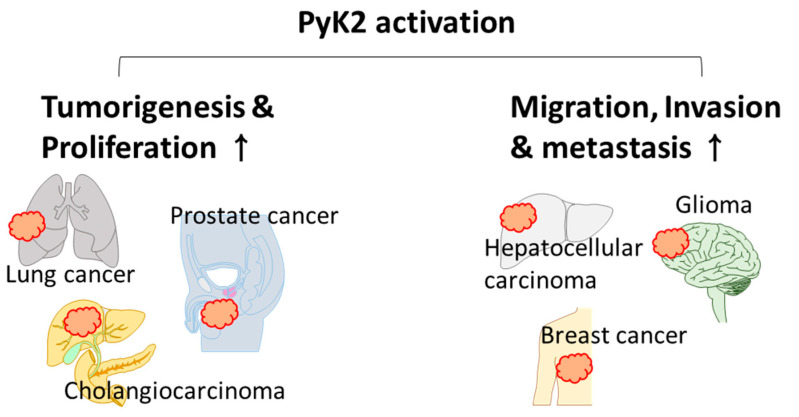
Figure 2.
Schematic illustration of the effect of PyK2 on various cancers. PyK2 activation enhances tumorigenesis, cancer proliferation, migration, invasion, and metastasis. The affected cancers are lung cancer, prostate cancer, cholangiocarcinoma, hepatocellular carcinoma, glioma, and breast cancer.
3. PyK2-Associated Molecules in Cancer
The acidic milieu is a favorable condition in cancer systems. Various evidence has shown that the activation of PyK2 regulated cancer progression and migration. Thus, in this chapter, we summarized the mechanism of molecular interaction in regulating PyK2 activity in cancer and PyK2-associated strategies against cancer.
3.1. Chemical Reagents
Kinase inhibitors, which decrease the phosphorylation of PyK2, suppress cancer viability and migration. Mitoxantrone, which targets the ATP-binding site of FAK and decreases the auto-phosphorylation of FAK, decreased PyK2 kinase activity in BT474 breast carcinoma cells [90]. Moreover, the tyrosine kinase inhibitor, SAR103168, decreased PyK2 phosphorylation by the downstream inhibition of Src in human myeloid leukemia cells (KG1) [91]. SKI-606, which is an Src inhibitor, decreased the phosphorylation of PyK2 and the migration and invasion of MDA-MB-468 breast cancer cells without affecting proliferation, suggesting that PyK2 induced the migration of breast cancer cells by activating Src [92]. The reactive oxygen species (ROS) inducer eicosapentaenoic acid (EPA), which dephosphorylates PyK2, exhibited anti-cancer effects by decreasing the proliferation and migration of PC3 prostate cancer cells [93]. PyK2 regulation ameliorated drug resistance to cisplatin and doxorubicin. The overexpression of PyK2 increased the effect of cisplatin in human hepatocellular carcinoma cells to decrease proliferation [94]. Alpha-naphthoflavone (ANF) decreased the phosphorylation of PyK2 in MCF-7 cells, and the combination of doxorubicin and ANF reduced breast cancer volume compared with a single treatment of doxorubicin or ANF in breast cancer-xenografted mice [95].
3.2. Interaction of Protein with PyK2 in Cancers
PyK2 interacts with various proteins, and its interactions with PyK2 have been developed in cancer systems. For example, the Csk homologous kinase (CHK), which inhibits the activation of Src family kinases, physically binds to PyK2 in T47D breast cancer cells [96]. A deficiency of heat shock cognate protein 70 (hsc70), which promotes the proliferation and migration of human glioma cells (U251 and U87), attenuated the phosphorylation of Src, FAK, and PyK2 [97]. Rb1-inducible coiled-coil 1 (RB1CC1) is a tumor suppressor that is considered to be a therapeutic target in renal carcinoma [98]. The overexpression of RB1CC1 decreased the phosphorylation of PyK2 and doxorubicin, which increased RB1CC1 expression and reduced the size of xenografted renal cell carcinoma tumors [99]. A decrease in PyK2 phosphorylation decreased cancer progression, and cancer migration and invasion were affected by PyK2 and its interactive proteins. Melatonin exerted an anti-cancer effect on brain tumor cells [100], and treatment with melatonin reduced the phosphorylation of PyK2 and the expression of alpha V beta 3 (αVβ3) integrin in U251 glioma cells [101]. The knockdown of αVβ3 decreased PyK2 phosphorylation and the migration of U251 cells [101].
3.3. Chemokine-Related Molecules
PyK2 is regulated by chemokine-related proteins, including the C-C motif chemokine ligand/receptor (CCL/CCR) and C-X-C motif chemokine ligand/receptor (CXCL/CXCR). CCL and CXCL recruit monocytes and neutrophils to the tumor site [102,103,104]. Thus CCL- and CXCL-related immune pathways have a close connection with cancer therapy. For example, CCL2 and CCL5, which are secreted by mesenchymal stem cells, induced PyK2-dependent chemoresistance in ovarian cancer cells (Skov3 and Ovcar3) [105]. CCL2- and CCL5-mediated chemoresistance was decreased through treatment with the PyK2 inhibitor PF-431396 [105]. PyK2 also plays a role in tumor viability and reactions with CCL2 and CCL5. ADP-ribosylation factor-GTPase activating protein (Arf-GAP), with an SH3 domain, ankyrin repeat, and PH domain-containing protein 1 (ASAP1, also called DDEF1 or AMAP1), is highly expressed on breast cancer cells and mediates breast cancer invasion and metastasis [106]. Treatment with CCL18 increased ASAP1 phosphorylation, and the knockdown of PyK2 prevented CCL18-induced increases in p-ASAP1 in MCF-7 cells [107]. p-ASAP1 trans-locates toward the plasma membrane to form a complex with PyK2 in the presence of CCL18 [107]. Treatment with CCL18 stimulated cellular adhesion, migration, and invasion, whereas the inhibition of ASAP1 through siRNA attenuated CCL18-induced cellular mobility features in MCF-7 cells [107]. CCR7 also plays a role in cancer migration and invasion. CCR7, which binds with CCL19, stimulated the phosphorylation of Janus kinase 2 (JAK2) and signal transducer and activator of transcription 3 (STAT3) in head and neck squamous cell carcinoma cell lines (PCI-4B and PCI-37B) [108]. The phosphorylation of JAK2 and STAT3 was attenuated by the PyK2 inhibitor A9 in PCI-4B and PCI-37B cells [108]. The inhibition of JAK2 and STAT3 decreased the migration and invasion of PCI-4B and PCI-37B cells [108], and treatment with CXCL12 induced the chemotaxis and chemoinvasion of MDA-MB-231 cells [109]. CXCL12, which binds with CXCR4, induced PyK2 phosphorylation in breast cancer cells (MDA-MB-231) [109]. The tyrosine phosphatase inhibitors vanadate and phenylarsine oxide attenuated the chemotaxis and chemo-invasion of MDA-MB-231 cells [109]. Although accumulating evidence has been reported, further verification of multiple chemokine/PyK2-associated mechanisms will provide potential strategies for treating cancer.
3.4. Ca2+ Channels and Transporters
PyK2 phosphorylation is also modulated by the signaling messenger, intracellular Ca2+. PyK2 senses Ca2+ signaling through calmodulin (CaM), and PyK2 has a CaM-binding motif [110]. In hypoxia, increases in the intracellular Ca2+ concentration ([Ca2+]i) induced PyK2 phosphorylation [111]. Treatment with the Ca2+ chelator BAPTA attenuated hydrogen peroxide (H2O2)-stimulated PyK2 phosphorylation [112]. Ca2+ signaling plays important roles in muscle contraction, neurotransmitter release, immune cell differentiation, fluid secretion, and cell proliferation [113,114,115,116]. Cancer progression and cancer cell death are especially affected by Ca2+ signaling [117,118,119,120,121,122,123]. In addition, the activation of Ca2+ channels and transporters regulates the interaction between PyK2 and cancer activity. [Ca2+]i is increased by the activation of various Ca2+ channels and transporters that are located on intracellular organelle and plasma membranes. Intracellular Ca2+ is stored in intracellular organelles, including the nucleus, mitochondria, and endoplasmic reticulum (ER), to maintain Ca2+ homeostasis. The mitochondrial protein Lon is involved in protein quality control and maintains mitochondrial homeostasis [124,125]. The overexpression of Lon induced the phosphorylation of PyK2, increased [Ca2+]i through the involvement of a mitochondrial Na2+/Ca2+ exchanger, and enhanced chemoresistance to cisplatin in human oral squamous carcinoma cells (OEC-M1) [126].
The ER, another intracellular Ca2+ store, contains a Ca2+ sensor protein called stromal interaction molecule 1 (STIM1) [127]. This Ca2+ sensor STIM1 recognizes depletions in ER Ca2+ by a STIM1-Orai1 complex on plasma membranes and mediates increases in [Ca2+]i in a process called store-operated Ca2+ (SOC) entry (SOCE) [127]. The down-regulation of STIM1 decreased the EGF-induced phosphorylation of PyK2 and enhanced the focal adhesion of cervical cancer cells (SiHa) [128]. The knockdown of STIM1 inhibited tumor progression in a cervical cancer mouse model [128]. Additionally, the inhibition of SOCE by the SOCE inhibitors shOrai1 and SKF96365 increased PyK2 dephosphorylation and focal adhesion in mouse glioma cells (C6), human glioma cells (U251 and SNB19), and human melanoma cells (WM793) [129,130,131]. Transient receptor potential melastatin 2 (TRPM2), which is located on plasma membranes, inhibited the effect of the anti-cancer drug doxorubicin in neuroblastoma [132]. The knockdown of TRPM2 enhanced the anti-cancer effects of doxorubicin to decrease PyK2 phosphorylation. Hirschler-Laszkiewicz et al. suggested the inhibition of TRPM2 as a target for cancer therapy in patients with doxorubicin chemoresistance [132]. Although the effect of modulating TRPM2 channels must be carefully verified because of conflicting views of TRPM2 (Ca2+ influx through TRPM2 induces apoptosis through goldnano-conjugated doxorubicin) [133], enhanced PyK2 phosphorylation through Ca2+ signaling presents further challenges in verifying the precise mechanism for cancer therapy.
3.5. Reactive Oxygen Species
In cancer cells, oxidative modification has pathological roles in protein alterations through the involvement of second messengers, including ROS, H2O2, reactive nitrogen species (RNS), and nitric oxide (NO) [134,135,136]. Oxidative stress has been considered a hallmark of cancer to increase cancer progression, including proliferation and invasion [137,138]. Oxidative stress also affects PyK2 activation in cancer cells. Treatment with estrogen produced ROS, and increased PyK2 phosphorylation in human breast cancer cells, including MCF-7, T47D, ZR75-1, and MDA-MB-468 cells [139]. Hypoxic conditions increased the phosphorylation of PyK2 in U251 glioma cells [101]. The migration and invasion of U251 cells were increased by hypoxic stimulation, and the knockdown of PyK2 inhibited hypoxia-induced U251 cell migration [101].
PyK2 was reported to bind with dihydronicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX) in KySE30 and KySE410 esophageal squamous cell carcinoma (ESCC) [140]. Hypoxia induced the phosphorylation of PyK2 and the production of H2O2 in ESCC [140]. NOX5 shRNA and PyK2 mutation decreased H2O2 levels in ESCC cells under hypoxic conditions and decreased ESCC proliferation [140]. Oxidation also plays a critical role in cardiovascular functions and CTLs [112,141,142,143,144]. Treatment with H2O2 enhanced the phosphorylation of PyK2 in mouse left ventricular myocytes [112] and H9c2 cardiomyocytes [142]. The deletion of PyK2 attenuated the production of NO in primary cultured-mouse endothelial cells from the aorta [141]. Additionally, treatment with H2O2 stimulated PyK2 phosphorylation, and the activation of PyK2 phosphorylation increased the production of ROS in CTLs [143]. Overall, oxidative stress induces PyK2 phosphorylation with tumor progression. Thus, the development of antioxidants and modulation of PyK2 phosphorylation provide potential strategies for cancer treatment. The mechanism of the various molecules involved in regulating PyK2 activity in cancer systems is shown in Figure 3.
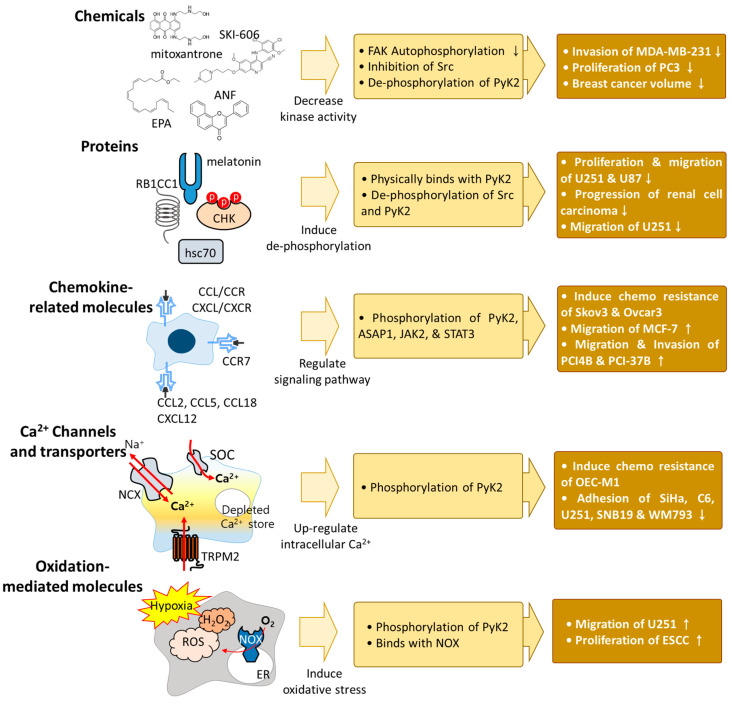
Figure 3.
Schematic illustration of PyK2-associated molecules. Various molecules affect PyK2 activation, including chemical reagents, interactive proteins, chemokine-related molecules, Ca2+ channels, transporters, and oxidation-mediated molecules. The phosphorylation of PyK2 induces cancer cell migration and proliferation. Various effector signals and chemicals exert different phosphorylation effects on PyK2. Thus, verification of the phosphorylation status of PyK2 could be a prognostic marker for evaluating cancer progression.
4. Future Perspectives
PyK2 has been studied as a key regulator of cancerous processes. pH-associated kinase PyK2 is regulated by various molecules such as chemical reagents and interactive proteins, including chemokine-related and Ca2+-related molecules, as well as oxidation-related molecules, in cancer cells. The features of the acidic microenvironment and Ca2+ signaling in PyK2-associated molecular mechanisms have been demonstrated. Thus, further investigation is required to include its multiple regulators. Cells possess various ion transporters and channels, including those discussed above, and electrolyte transporters such as potassium, sodium, and chloride are also involved in cellular systems. Thus, further experimental evidence, including the relationship between ion channels and transporters and cancer, should be determined. In addition, investigations of PyK2 as an extracellular milieu-sensing protein in cancerous processes might provide further information on the responsiveness to the cancer milieu.
Acknowledgments
All figures were developed by the authors.
Author Contributions
D.L. and J.-H.H. conceptualized and designed the study and acquired and interpreted the information. D.L. and J.-H.H. drafted the manuscript. J.-H.H. critically revised the manuscript for important intellectual content. J.-H.H. contributed to the funding acquisition and final approval of the published version and is responsible for all aspects of the work, including the accuracy and integrity of the study. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by a National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT; NRF-2022R1A2C1003890: J.-H.H. and NRF-2021R1A6A3A13044194: D.L.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Page T.H., Smolinska M., Gillespie J., Urbaniak A.M., Foxwell B.M. Tyrosine kinases and inflammatory signalling. Curr. Mol. Med. 2009;9:69–85. doi: 10.2174/156652409787314507. [DOI] [PubMed] [Google Scholar]
- 2.Giralt A., Brito V., Chevy Q., Simonnet C., Otsu Y., Cifuentes-Diaz C., de Pins B., Coura R., Alberch J., Gines S., et al. Pyk2 modulates hippocampal excitatory synapses and contributes to cognitive deficits in a Huntington’s disease model. Nat. Commun. 2017;8:15592. doi: 10.1038/ncomms15592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avraham S., London R., Fu Y., Ota S., Hiregowdara D., Li J., Jiang S., Pasztor L.M., White R.A., Groopman J.E., et al. Identification and characterization of a novel related adhesion focal tyrosine kinase (RAFTK) from megakaryocytes and brain. J. Biol. Chem. 1995;270:27742–27751. doi: 10.1074/jbc.270.46.27742. [DOI] [PubMed] [Google Scholar]
- 4.Lev S., Moreno H., Martinez R., Canoll P., Peles E., Musacchio J.M., Plowman G.D., Rudy B., Schlessinger J. Protein tyrosine kinase PYK2 involved in Ca2+-induced regulation of ion channel and MAP kinase functions. Nature. 1995;376:737–745. doi: 10.1038/376737a0. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki H., Nagura K., Ishino M., Tobioka H., Kotani K., Sasaki T. Cloning and characterization of cell adhesion kinase beta, a novel protein-tyrosine kinase of the focal adhesion kinase subfamily. J. Biol. Chem. 1995;270:21206–21219. doi: 10.1074/jbc.270.36.21206. [DOI] [PubMed] [Google Scholar]
- 6.Sieg D.J., Ilic D., Jones K.C., Damsky C.H., Hunter T., Schlaepfer D.D. Pyk2 and Src-family protein-tyrosine kinases compensate for the loss of FAK in fibronectin-stimulated signaling events but Pyk2 does not fully function to enhance FAK- cell migration. EMBO J. 1998;17:5933–5947. doi: 10.1093/emboj/17.20.5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao M., Finlay D., Zharkikh I., Vuori K. Novel Role of Src in Priming Pyk2 Phosphorylation. PLoS ONE. 2016;11:e0149231. doi: 10.1371/journal.pone.0149231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lipinski C.A., Loftus J.C. Targeting Pyk2 for therapeutic intervention. Expert Opin. Ther. Targets. 2010;14:95–108. doi: 10.1517/14728220903473194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinamard R., Okigaki M., Schlessinger J., Ravetch J.V. Absence of marginal zone B cells in Pyk-2-deficient mice defines their role in the humoral response. Nat. Immunol. 2000;1:31–36. doi: 10.1038/76882. [DOI] [PubMed] [Google Scholar]
- 10.Okigaki M., Davis C., Falasca M., Harroch S., Felsenfeld D.P., Sheetz M.P., Schlessinger J. Pyk2 regulates multiple signaling events crucial for macrophage morphology and migration. Proc. Natl. Acad. Sci. USA. 2003;100:10740–10745. doi: 10.1073/pnas.1834348100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoelzinger D.B., Mariani L., Weis J., Woyke T., Berens T.J., McDonough W.S., Sloan A., Coons S.W., Berens M.E. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia. 2005;7:7–16. doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapman N.M., Yoder A.N., Houtman J.C. Non-catalytic functions of Pyk2 and Fyn regulate late stage adhesion in human T cells. PLoS ONE. 2012;7:e53011. doi: 10.1371/journal.pone.0053011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anand A.R., Cucchiarini M., Terwilliger E.F., Ganju R.K. The tyrosine kinase Pyk2 mediates lipopolysaccharide-induced IL-8 expression in human endothelial cells. J. Immunol. 2008;180:5636–5644. doi: 10.4049/jimmunol.180.8.5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco M., Tamagnone L. Tyrosine phosphorylation in semaphorin signalling: Shifting into overdrive. Embo Rep. 2008;9:865–871. doi: 10.1038/embor.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Naser R., Aldehaiman A., Diaz-Galicia E., Arold S.T. Endogenous Control Mechanisms of FAK and PYK2 and Their Relevance to Cancer Development. Cancers. 2018;10:196. doi: 10.3390/cancers10060196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Avraham H., Park S.Y., Schinkmann K., Avraham S. RAFTK/Pyk2-mediated cellular signalling. Cell. Signal. 2000;12:123–133. doi: 10.1016/S0898-6568(99)00076-5. [DOI] [PubMed] [Google Scholar]
- 17.Ilic D., Furuta Y., Kanazawa S., Takeda N., Sobue K., Nakatsuji N., Nomura S., Fujimoto J., Okada M., Yamamoto T. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 18.Weis S.M., Lim S.T., Lutu-Fuga K.M., Barnes L.A., Chen X.L., Gothert J.R., Shen T.L., Guan J.L., Schlaepfer D.D., Cheresh D.A. Compensatory role for Pyk2 during angiogenesis in adult mice lacking endothelial cell FAK. J. Cell Biol. 2008;181:43–50. doi: 10.1083/jcb.200710038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fan H., Guan J.L. Compensatory function of Pyk2 protein in the promotion of focal adhesion kinase (FAK)-null mammary cancer stem cell tumorigenicity and metastatic activity. J. Biol. Chem. 2011;286:18573–18582. doi: 10.1074/jbc.M110.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei L., Yang Y., Zhang X., Yu Q. Altered regulation of Src upon cell detachment protects human lung adenocarcinoma cells from anoikis. Oncogene. 2004;23:9052–9061. doi: 10.1038/sj.onc.1208091. [DOI] [PubMed] [Google Scholar]
- 21.Mazurenko N.N., Kogan E.A., Zborovskaya I.B., Kisseljov F.L. Expression of pp60c-src in human small cell and non-small cell lung carcinomas. Eur. J. Cancer. 1992;28:372–377. doi: 10.1016/S0959-8049(05)80056-5. [DOI] [PubMed] [Google Scholar]
- 22.Egan C., Pang A., Durda D., Cheng H.C., Wang J.H., Fujita D.J. Activation of Src in human breast tumor cell lines: Elevated levels of phosphotyrosine phosphatase activity that preferentially recognizes the Src carboxy terminal negative regulatory tyrosine 530. Oncogene. 1999;18:1227–1237. doi: 10.1038/sj.onc.1202233. [DOI] [PubMed] [Google Scholar]
- 23.Windham T.C., Parikh N.U., Siwak D.R., Summy J.M., McConkey D.J., Kraker A.J., Gallick G.E. Src activation regulates anoikis in human colon tumor cell lines. Oncogene. 2002;21:7797–7807. doi: 10.1038/sj.onc.1205989. [DOI] [PubMed] [Google Scholar]
- 24.Lutz M.P., Esser I.B., Flossmann-Kast B.B., Vogelmann R., Luhrs H., Friess H., Buchler M.W., Adler G. Overexpression and activation of the tyrosine kinase Src in human pancreatic carcinoma. Biochem. Biophys. Res. Commun. 1998;243:503–508. doi: 10.1006/bbrc.1997.8043. [DOI] [PubMed] [Google Scholar]
- 25.Takeshima E., Hamaguchi M., Watanabe T., Akiyama S., Kataoka M., Ohnishi Y., Xiao H.Y., Nagai Y., Takagi H. Aberrant elevation of tyrosine-specific phosphorylation in human gastric cancer cells. Jpn. J. Cancer Res. 1991;82:1428–1435. doi: 10.1111/j.1349-7006.1991.tb01816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duong L.T., Lakkakorpi P.T., Nakamura I., Machwate M., Nagy R.M., Rodan G.A. PYK2 in osteoclasts is an adhesion kinase, localized in the sealing zone, activated by ligation of alpha(v)beta3 integrin, and phosphorylated by src kinase. J. Clin. Investig. 1998;102:881–892. doi: 10.1172/JCI3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lakkakorpi P.T., Bett A.J., Lipfert L., Rodan G.A., Duong L.T. PYK2 autophosphorylation, but not kinase activity, is necessary for adhesion-induced association with c-Src, osteoclast spreading, and bone resorption. J. Biol. Chem. 2003;278:11502–11512. doi: 10.1074/jbc.M206579200. [DOI] [PubMed] [Google Scholar]
- 28.Park S.Y., Avraham H.K., Avraham S. RAFTK/Pyk2 activation is mediated by trans-acting autophosphorylation in a Src-independent manner. J. Biol. Chem. 2004;279:33315–33322. doi: 10.1074/jbc.M313527200. [DOI] [PubMed] [Google Scholar]
- 29.Okenwa C., Kumar A., Rego D., Konarski Y., Nilchi L., Wright K., Kozlowski M. SHP-1-Pyk2-Src protein complex and p38 MAPK pathways independently regulate IL-10 production in lipopolysaccharide-stimulated macrophages. J. Immunol. 2013;191:2589–2603. doi: 10.4049/jimmunol.1300466. [DOI] [PubMed] [Google Scholar]
- 30.Griffiths J.R. Are cancer cells acidic? Br. J. Cancer. 1991;64:425–427. doi: 10.1038/bjc.1991.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boedtkjer E., Pedersen S.F. The Acidic Tumor Microenvironment as a Driver of Cancer. Annu. Rev. Physiol. 2020;82:103–126. doi: 10.1146/annurev-physiol-021119-034627. [DOI] [PubMed] [Google Scholar]
- 32.Gupta G.P., Massague J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Hofschroer V., Koch A., Ludwig F.T., Friedl P., Oberleithner H., Stock C., Schwab A. Extracellular protonation modulates cell-cell interaction mechanics and tissue invasion in human melanoma cells. Sci. Rep. 2017;7:42369. doi: 10.1038/srep42369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hwang S., Lee P.C.W., Shin D.M., Hong J.H. Modulated Start-Up Mode of Cancer Cell Migration Through Spinophilin-Tubular Networks. Front. Cell Dev. Biol. 2021;9:652791. doi: 10.3389/fcell.2021.652791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu S., Zhou H.Y., Deng S.C., Deng S.J., He C., Li X., Chen J.Y., Jin Y., Hu Z.L., Wang F., et al. ASIC1 and ASIC3 contribute to acidity-induced EMT of pancreatic cancer through activating Ca2+/RhoA pathway. Cell Death Dis. 2017;8:e2806. doi: 10.1038/cddis.2017.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartsch J.E., Staren E.D., Appert H.E. Matrix metalloproteinase expression in breast cancer. J. Surg. Res. 2003;110:383–392. doi: 10.1016/S0022-4804(03)00007-6. [DOI] [PubMed] [Google Scholar]
- 37.Mohamed M.M., Sloane B.F. Cysteine cathepsins: Multifunctional enzymes in cancer. Nat. Rev. Cancer. 2006;6:764–775. doi: 10.1038/nrc1949. [DOI] [PubMed] [Google Scholar]
- 38.Stock C., Pedersen S.F. Roles of pH and the Na+/H+ exchanger NHE1 in cancer: From cell biology and animal models to an emerging translational perspective? Semin. Cancer Biol. 2017;43:5–16. doi: 10.1016/j.semcancer.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 39.Moellering R.E., Black K.C., Krishnamurty C., Baggett B.K., Stafford P., Rain M., Gatenby R.A., Gillies R.J. Acid treatment of melanoma cells selects for invasive phenotypes. Clin. Exp. Metastasis. 2008;25:411–425. doi: 10.1007/s10585-008-9145-7. [DOI] [PubMed] [Google Scholar]
- 40.Estrella V., Chen T.A., Lloyd M., Wojtkowiak J., Cornnell H.H., Ibrahim-Hashim A., Bailey K., Balagurunathan Y., Rothberg J.M., Sloane B.F., et al. Acidity Generated by the Tumor Microenvironment Drives Local Invasion. Cancer Res. 2013;73:1524–1535. doi: 10.1158/0008-5472.CAN-12-2796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.MartinezZaguilan R., Seftor E.A., Seftor R.E.B., Chu Y.W., Gillies R.J., Hendrix M.J.C. Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis. 1996;14:176–186. doi: 10.1007/BF00121214. [DOI] [PubMed] [Google Scholar]
- 42.Hwang S., Shin D.M., Hong J.H. Drug Repurposing as an Antitumor Agent: Disulfiram-Mediated Carbonic Anhydrase 12 and Anion Exchanger 2 Modulation to Inhibit Cancer Cell Migration. Molecules. 2019;24:3409. doi: 10.3390/molecules24183409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang S., Shin D.M., Hong J.H. Protective Role of IRBIT on Sodium Bicarbonate Cotransporter-n1 for Migratory Cancer Cells. Pharmaceutics. 2020;12:816. doi: 10.3390/pharmaceutics12090816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee D., Hong J.H. The Fundamental Role of Bicarbonate Transporters and Associated Carbonic Anhydrase Enzymes in Maintaining Ion and pH Homeostasis in Non-Secretory Organs. Int. J. Mol. Sci. 2020;21:339. doi: 10.3390/ijms21010339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li S.Y., Sato S., Yang X.J., Preisig P.A., Alpern R.J. Pyk2 activation is integral to acid stimulation of sodium/hydrogen exchanger 3. J. Clin. Investig. 2004;114:1782–1789. doi: 10.1172/JCI200418046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.No Y.R., He P., Yoo B.K., Yun C.C. Regulation of NHE3 by lysophosphatidic acid is mediated by phosphorylation of NHE3 by RSK2. Am. J. Physiol. Cell Physiol. 2015;309:C14–C21. doi: 10.1152/ajpcell.00067.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simpson D.P. Citrate excretion: A window on renal metabolism. Am. J. Physiol. 1983;244:F223–F234. doi: 10.1152/ajprenal.1983.244.3.F223. [DOI] [PubMed] [Google Scholar]
- 48.Prot-Bertoye C., Vallet M., Houillier P. Urinary citrate: Helpful to predict acid retention in CKD patients? Kidney Int. 2019;95:1020–1022. doi: 10.1016/j.kint.2019.01.019. [DOI] [PubMed] [Google Scholar]
- 49.Moe O.W. Kidney stones: Pathophysiology and medical management. Lancet. 2006;367:333–344. doi: 10.1016/S0140-6736(06)68071-9. [DOI] [PubMed] [Google Scholar]
- 50.Hallson P.C., Kasidas G.P., Samuell C.T. The inhibitory activity of some citrate analogues upon calcium crystalluria: Observations using an improved urine evaporation technique. Urol. Int. 1996;57:43–47. doi: 10.1159/000282875. [DOI] [PubMed] [Google Scholar]
- 51.Moe O.W., Preisig P.A. Dual role of citrate in mammalian urine. Curr. Opin. Nephrol. Hypertens. 2006;15:419–424. doi: 10.1097/01.mnh.0000232882.35469.72. [DOI] [PubMed] [Google Scholar]
- 52.Zacchia M., Tian X., Zona E., Alpern R.J., Preisig P.A. Acid Stimulation of the Citrate Transporter NaDC-1 Requires Pyk2 and ERK1/2 Signaling Pathways. J. Am. Soc. Nephrol. 2018;29:1720–1730. doi: 10.1681/ASN.2017121268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fisher K.D., Codina J., Petrovic S., DuBose T.D., Jr. Pyk2 regulates H+-ATPase-mediated proton secretion in the outer medullary collecting duct via an ERK1/2 signaling pathway. Am. J. Physiol. Renal. Physiol. 2012;303:F1353–F1362. doi: 10.1152/ajprenal.00008.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poroca D.R., Pelis R.M., Chappe V.M. ClC Channels and Transporters: Structure, Physiological Functions, and Implications in Human Chloride Channelopathies. Front. Pharmacol. 2017;8:151. doi: 10.3389/fphar.2017.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kuang B.H., Zhang M.Q., Xu L.H., Hu L.J., Wang H.B., Zhao W.F., Du Y., Zhang X. Proline-rich tyrosine kinase 2 and its phosphorylated form pY881 are novel prognostic markers for non-small-cell lung cancer progression and patients’ overall survival. Br. J. Cancer. 2013;109:1252–1263. doi: 10.1038/bjc.2013.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behmoaram E., Bijian K., Jie S., Xu Y., Darnel A., Bismar T.A., Alaoui-Jamali M.A. Focal adhesion kinase-related proline-rich tyrosine kinase 2 and focal adhesion kinase are co-overexpressed in early-stage and invasive ErbB-2-positive breast cancer and cooperate for breast cancer cell tumorigenesis and invasiveness. Am. J. Pathol. 2008;173:1540–1550. doi: 10.2353/ajpath.2008.080292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gao C., Chen G., Kuan S.F., Zhang D.H., Schlaepfer D.D., Hu J. FAK/PYK2 promotes the Wnt/beta-catenin pathway and intestinal tumorigenesis by phosphorylating GSK3beta. eLife. 2015;4:e10072. doi: 10.7554/eLife.10072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun C.K., Ng K.T., Sun B.S., Ho J.W., Lee T.K., Ng I., Poon R.T., Lo C.M., Liu C.L., Man K., et al. The significance of proline-rich tyrosine kinase2 (Pyk2) on hepatocellular carcinoma progression and recurrence. Br. J. Cancer. 2007;97:50–57. doi: 10.1038/sj.bjc.6603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiese H., Gelis L., Wiese S., Reichenbach C., Jovancevic N., Osterloh M., Meyer H.E., Neuhaus E.M., Hatt H.H., Radziwill G., et al. Quantitative phosphoproteomics reveals the protein tyrosine kinase Pyk2 as a central effector of olfactory receptor signaling in prostate cancer cells. BBA-Proteins Proteom. 2015;1854:632–640. doi: 10.1016/j.bbapap.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Gao C., Chen G., Zhang D.H., Zhang J., Kuan S.F., Hu W., Esni F., Gao X., Guan J.L., Chu E., et al. PYK2 Is Involved in Premalignant Acinar Cell Reprogramming and Pancreatic Ductal Adenocarcinoma Maintenance by Phosphorylating beta-Catenin(Y654) Cell. Mol. Gastroenterol. Hepatol. 2019;8:561–578. doi: 10.1016/j.jcmgh.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moody T.W., Di Florio A., Jensen R.T. PYK-2 is Tyrosine Phosphorylated after Activation of Pituitary Adenylate Cyclase Activating Polypeptide Receptors in Lung Cancer Cells. J. Mol. Neurosci. 2012;48:660–666. doi: 10.1007/s12031-012-9785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lim S.T., Miller N.L.G., Nam J.O., Chen X.L., Lim Y., Schlaepfer D.D. Pyk2 Inhibition of p53 as an Adaptive and Intrinsic Mechanism Facilitating Cell Proliferation and Survival. J. Biol. Chem. 2010;285:1743–1753. doi: 10.1074/jbc.M109.064212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Perez J., Torres R.A., Rocic P., Cismowski M.J., Weber D.S., Darley-Usmar V.M., Lucchesi P.A. PYK2 signaling is required for PDGF-dependent vascular smooth muscle cell proliferation. Am. J. Physiol.-Cell Physiol. 2011;301:C242–C251. doi: 10.1152/ajpcell.00315.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng Y.H., Hooker R.A., Nguyen K., Gerard-O’Riley R., Waning D.L., Chitteti B.R., Meijome T.E., Chua H.L., Plett A.P., Orschell C.M., et al. Pyk2 regulates megakaryocyte-induced increases in osteoblast number and bone formation. J. Bone Miner. Res. 2013;28:1434–1445. doi: 10.1002/jbmr.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nie X., Liu H., Liu L., Wang Y.D., Chen W.D. Emerging Roles of Wnt Ligands in Human Colorectal Cancer. Front. Oncol. 2020;10:1341. doi: 10.3389/fonc.2020.01341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cancer Genome Atlas N. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang Y., Wang X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020;13:165. doi: 10.1186/s13045-020-00990-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brantley-Sieders D., Schmidt S., Parker M., Chen J. Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr. Pharm. Des. 2004;10:3431–3442. doi: 10.2174/1381612043383160. [DOI] [PubMed] [Google Scholar]
- 69.Guo H., Miao H., Gerber L., Singh J., Denning M.F., Gilliam A.C., Wang B. Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 2006;66:7050–7058. doi: 10.1158/0008-5472.CAN-06-0004. [DOI] [PubMed] [Google Scholar]
- 70.Cui X.D., Lee M.J., Kim J.H., Hao P.P., Liu L., Yu G.R., Kim D.G. Activation of mammalian target of rapamycin complex 1 (mTORC1) and Raf/Pyk2 by growth factor-mediated Eph receptor 2 (EphA2) is required for cholangiocarcinoma growth and metastasis. Hepatology. 2013;57:2248–2260. doi: 10.1002/hep.26253. [DOI] [PubMed] [Google Scholar]
- 71.Hsiao Y.H., Huang Y.T., Hung C.Y., Kuo T.C., Luo F.J., Yuan T.C. PYK2 via S6K1 regulates the function of androgen receptors and the growth of prostate cancer cells. Endocr.-Relat. Cancer. 2016;23:651–663. doi: 10.1530/ERC-16-0122. [DOI] [PubMed] [Google Scholar]
- 72.Lu H., Chen I., Shimoda L.A., Park Y., Zhang C., Tran L., Zhang H., Semenza G.L. Chemotherapy-Induced Ca2+ Release Stimulates Breast Cancer Stem Cell Enrichment. Cell Rep. 2017;18:1946–1957. doi: 10.1016/j.celrep.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 73.Huang H., Svoboda R.A., Lazenby A.J., Saowapa J., Chaika N., Ding K., Wheelock M.J., Johnson K.R. Up-regulation of N-cadherin by Collagen I-activated Discoidin Domain Receptor 1 in Pancreatic Cancer Requires the Adaptor Molecule Shc1. J. Biol. Chem. 2016;291:23208–23223. doi: 10.1074/jbc.M116.740605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yuan T.C., Lin F.F., Veeramani S., Chen S.J., Earp H.S., 3rd, Lin M.F. ErbB-2 via PYK2 upregulates the adhesive ability of androgen receptor-positive human prostate cancer cells. Oncogene. 2007;26:7552–7559. doi: 10.1038/sj.onc.1210570. [DOI] [PubMed] [Google Scholar]
- 75.Lipinski C.A., Tran N.L., Viso C., Kloss J., Yang Z., Berens M.E., Loftus J.C. Extended survival of Pyk2 or FAK deficient orthotopic glioma xenografts. J. Neurooncol. 2008;90:181–189. doi: 10.1007/s11060-008-9656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Skovierova H., Okajcekova T., Strnadel J., Vidomanova E., Halasova E. Molecular regulation of epithelial-to-mesenchymal transition in tumorigenesis (Review) Int. J. Mol. Med. 2018;41:1187–1200. doi: 10.3892/ijmm.2017.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun C.K., Ng K.T., Lim Z.X., Cheng Q., Lo C.M., Poon R.T., Man K., Wong N., Fan S.T. Proline-Rich Tyrosine Kinase 2 (Pyk2) Promotes Cell Motility of Hepatocellular Carcinoma through Induction of Epithelial to Mesenchymal Transition. PLoS ONE. 2011;6:e18878. doi: 10.1371/journal.pone.0018878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Verma N., Keinan O., Selitrennik M., Karn T., Filipits M., Lev S. PYK2 sustains endosomal-derived receptor signalling and enhances epithelial-to-mesenchymal transition. Nat. Commun. 2015;6:6064. doi: 10.1038/ncomms7064. [DOI] [PubMed] [Google Scholar]
- 79.Lane D., Matte I., Laplante C., Garde-Granger P., Carignan A., Bessette P., Rancourt C., Piche A. CCL18 from ascites promotes ovarian cancer cell migration through proline-rich tyrosine kinase 2 signaling. Mol. Cancer. 2016;15:58. doi: 10.1186/s12943-016-0542-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li H.Y., Cui X.Y., Wu W., Yu F.Y., Yao H.R., Liu Q., Song E.W., Chen J.Q. Pyk2 and Src mediate signaling to CCL18-induced breast cancer metastasis. J. Cell. Biochem. 2014;115:596–603. doi: 10.1002/jcb.24697. [DOI] [PubMed] [Google Scholar]
- 81.Lipinski C.A., Tran N.L., Menashi E., Rohl C., Kloss J., Bay R.C., Berens M.E., Loftus J.C. The tyrosine kinase pyk2 promotes migration and invasion of glioma cells. Neoplasia. 2005;7:435–445. doi: 10.1593/neo.04712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rolon-Reyes K., Kucheryavykh Y.V., Cubano L.A., Inyushin M., Skatchkov S.N., Eaton M.J., Harrison J.K., Kucheryavykh L.Y. Microglia Activate Migration of Glioma Cells through a Pyk2 Intracellular Pathway. PLoS ONE. 2015;10:e0131059. doi: 10.1371/journal.pone.0131059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lipinski C.A., Tran N.L., Dooley A., Pang Y.P., Rohl C., Kloss J., Yang Z., McDonough W., Craig D., Berens M.E., et al. Critical role of the FERM domain in Pyk2 stimulated glioma cell migration. Biochem. Biophys. Res. Commun. 2006;349:939–947. doi: 10.1016/j.bbrc.2006.08.134. [DOI] [PubMed] [Google Scholar]
- 84.Zhang S.Y., Guo D.W., Luo W.T., Zhang Q.F., Zhang Y., Li C.Y., Lu Y., Cui Z.S., Qiu X.S. TrkB is highly expressed in NSCLC and mediates BDNF-induced the activation of Pyk2 signaling and the invasion of A549 cells. BMC Cancer. 2010;10:43. doi: 10.1186/1471-2407-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Selitrennik M., Lev S. PYK2 integrates growth factor and cytokine receptors signaling and potentiates breast cancer invasion via a positive feedback loop. Oncotarget. 2015;6:22214–22226. doi: 10.18632/oncotarget.4257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Grossi M., Bhattachariya A., Nordstrom I., Turczynska K.M., Svensson D., Albinsson S., Nilsson B.O., Hellstrand P. Pyk2 inhibition promotes contractile differentiation in arterial smooth muscle. J. Cell. Physiol. 2017;232:3088–3102. doi: 10.1002/jcp.25760. [DOI] [PubMed] [Google Scholar]
- 87.Cheung S.M.S., Ostergaard H.L. Pyk2 Controls Integrin-Dependent CTL Migration through Regulation of De-Adhesion. J. Immunol. 2016;197:1945–1956. doi: 10.4049/jimmunol.1501505. [DOI] [PubMed] [Google Scholar]
- 88.Miao L., Xin X.M., Xin H., Shen X.Y., Zhu Y.Z. Hydrogen Sulfide Recruits Macrophage Migration by Integrin beta 1-Src-FAK/Pyk2-Rac Pathway in Myocardial Infarction. Sci. Rep. 2016;6:22363. doi: 10.1038/srep22363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fan L., Lu Y., Shen X., Shao H., Suo L., Wu Q. Alpha protocadherins and Pyk2 kinase regulate cortical neuron migration and cytoskeletal dynamics via Rac1 GTPase and WAVE complex in mice. eLife. 2018;7:e35242. doi: 10.7554/eLife.35242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Golubovskaya V.M., Ho B., Zheng M., Magis A., Ostrov D., Cance W.G. Mitoxantrone targets the ATP-binding site of FAK, binds the FAK kinase domain and decreases FAK, Pyk-2, c-Src, and IGF-1R in vitro kinase activities. Anticancer Agents Med. Chem. 2013;13:546–554. doi: 10.2174/1871520611313040003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bourrie B., Brassard D.L., Cosnier-Pucheu S., Zilberstein A., Yu K., Levit M., Morrison J.G., Perreaut P., Jegham S., Hilairet S., et al. SAR103168: A tyrosine kinase inhibitor with therapeutic potential in myeloid leukemias. Leuk. Lymphoma. 2013;54:1488–1499. doi: 10.3109/10428194.2012.745071. [DOI] [PubMed] [Google Scholar]
- 92.Vultur A., Buettner R., Kowolik C., Liang W., Smith D., Boschelli F., Jove R. SKI-606 (bosutinib), a novel Src kinase inhibitor, suppresses migration and invasion of human breast cancer cells. Mol. Cancer Ther. 2008;7:1185–1194. doi: 10.1158/1535-7163.MCT-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oono K., Ohtake K., Watanabe C., Shiba S., Sekiya T., Kasono K. Contribution of Pyk2 pathway and reactive oxygen species (ROS) to the anti-cancer effects of eicosapentaenoic acid (EPA) in PC3 prostate cancer cells. Lipids Health Dis. 2020;19:15. doi: 10.1186/s12944-019-1122-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Geng W., Ng K.T., Sun C.K., Yau W.L., Liu X.B., Cheng Q., Poon R.T., Lo C.M., Man K., Fan S.T. The role of proline rich tyrosine kinase 2 (Pyk2) on cisplatin resistance in hepatocellular carcinoma. PLoS ONE. 2011;6:e27362. doi: 10.1371/journal.pone.0027362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Datta A., Bhasin N., Kim H., Ranjan M., Rider B., Abd Elmageed Z.Y., Mondal D., Agrawal K.C., Abdel-Mageed A.B. Selective targeting of FAK-Pyk2 axis by alpha-naphthoflavone abrogates doxorubicin resistance in breast cancer cells. Cancer Lett. 2015;362:25–35. doi: 10.1016/j.canlet.2015.03.009. [DOI] [PubMed] [Google Scholar]
- 96.McShan G.D., Zagozdzon R., Park S.Y., Zrihan-Licht S., Fu Y., Avraham S., Avraham H. Csk homologous kinase associates with RAFTK/Pyk2 in breast cancer cells and negatively regulates its activation and breast cancer cell migration. Int. J. Oncol. 2002;21:197–205. doi: 10.3892/ijo.21.1.197. [DOI] [PubMed] [Google Scholar]
- 97.Sun G., Cao Y., Xu Y., Huai D., Chen P., Guo J., Li M., Dai Y. Overexpression of Hsc70 promotes proliferation, migration, and invasion of human glioma cells. J. Cell. Biochem. 2019;120:10707–10714. doi: 10.1002/jcb.28362. [DOI] [PubMed] [Google Scholar]
- 98.Lebovitz C.B., Robertson A.G., Goya R., Jones S.J., Morin R.D., Marra M.A., Gorski S.M. Cross-cancer profiling of molecular alterations within the human autophagy interaction network. Autophagy. 2015;11:1668–1687. doi: 10.1080/15548627.2015.1067362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen P.F., Duan Y.J., Lu X.S., Chen L.B., Zhang W., Wang H., Hu R., Liu S.M. RB1CC1 functions as a tumor-suppressing gene in renal cell carcinoma via suppression of PYK2 activity and disruption of TAZ-mediated PDL1 transcription activation. Cancer Immunol. Immun. 2021;70:3261–3275. doi: 10.1007/s00262-021-02913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Martin V., Sanchez-Sanchez A.M., Herrera F., Gomez-Manzano C., Fueyo J., Alvarez-Vega M.A., Antolin I., Rodriguez C. Melatonin-induced methylation of the ABCG2/BCRP promoter as a novel mechanism to overcome multidrug resistance in brain tumour stem cells. Br. J. Cancer. 2013;108:2005–2012. doi: 10.1038/bjc.2013.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xu C.S., Wang Z.F., Huang X.D., Dai L.M., Cao C.J., Li Z.Q. Involvement of ROS-alpha v beta 3 integrin-FAK/Pyk2 in the inhibitory effect of melatonin on U251 glioma cell migration and invasion under hypoxia. J. Transl. Med. 2015;13:95. doi: 10.1186/s12967-015-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Balkwill F.R. The chemokine system and cancer. J. Pathol. 2012;226:148–157. doi: 10.1002/path.3029. [DOI] [PubMed] [Google Scholar]
- 103.Caronni N., Savino B., Bonecchi R. Myeloid cells in cancer-related inflammation. Immunobiology. 2015;220:249–253. doi: 10.1016/j.imbio.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 104.Bonavita O., Massara M., Bonecchi R. Chemokine regulation of neutrophil function in tumors. Cytokine Growth Factor Rev. 2016;30:81–86. doi: 10.1016/j.cytogfr.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 105.Pasquier J., Gosset M., Geyl C., Hoarau-Vechot J., Chevrot A., Pocard M., Mirshahi M., Lis R., Rafii A., Touboul C. CCL2/CCL5 secreted by the stroma induce IL-6/PYK2 dependent chemoresistance in ovarian cancer. Mol. Cancer. 2018;17:47. doi: 10.1186/s12943-018-0787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hashimoto S., Hirose M., Hashimoto A., Morishige M., Yamada A., Hosaka H., Akagi K., Ogawa E., Oneyama C., Agatsuma T., et al. Targeting AMAP1 and cortactin binding bearing an atypical src homology 3/proline interface for prevention of breast cancer invasion and metastasis. Proc. Natl. Acad. Sci. USA. 2006;103:7036–7041. doi: 10.1073/pnas.0509166103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Li H.Y., Zhang D.W., Yu J.D., Liu H.L., Chen Z.P., Zhong H.F., Wan Y.L. CCL18-dependent translocation of AMAP1 is critical for epithelial to mesenchymal transition in breast cancer. J. Cell. Physiol. 2018;233:3207–3217. doi: 10.1002/jcp.26164. [DOI] [PubMed] [Google Scholar]
- 108.Liu F.Y., Safdar J., Li Z.N., Fang Q.G., Zhang X., Xu Z.F., Sun C.F. CCR7 Regulates Cell Migration and Invasion through JAK2/STAT3 in Metastatic Squamous Cell Carcinoma of the Head and Neck. Biomed. Res. Int. 2014;2014:415375. doi: 10.1155/2014/415375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Fernandis A.Z., Prasad A., Band H., Klosel R., Ganju R.K. Regulation of CXCR4-mediated chemotaxis and chemoinvasion of breast cancer cells. Oncogene. 2004;23:157–167. doi: 10.1038/sj.onc.1206910. [DOI] [PubMed] [Google Scholar]
- 110.Momin A.A., Mendes T., Barthe P., Faure C., Hong S., Yu P.A., Kadare G., Jaremko M., Girault J.A., Jaremko L., et al. PYK2 senses calcium through a disordered dimerization and calmodulin-binding element. Commun. Biol. 2022;5:800. doi: 10.1038/s42003-022-03760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Beitner-Johnson D., Ferguson T., Rust R.T., Kobayashi S., Millhorn D.E. Calcium-dependent activation of Pyk2 by hypoxia. Cell. Signal. 2002;14:133–137. doi: 10.1016/S0898-6568(01)00253-4. [DOI] [PubMed] [Google Scholar]
- 112.Miller B.A., Wang J., Song J., Zhang X.Q., Hirschler-Laszkiewicz I., Shanmughapriya S., Tomar D., Rajan S., Feldman A.M., Madesh M., et al. Trpm2 enhances physiological bioenergetics and protects against pathological oxidative cardiac injury: Role of Pyk2 phosphorylation. J. Cell. Physiol. 2019;234:15048–15060. doi: 10.1002/jcp.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Szent-Gyorgyi A.G. Calcium regulation of muscle contraction. Biophys. J. 1975;15:707–723. doi: 10.1016/S0006-3495(75)85849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Brini M., Cali T., Ottolini D., Carafoli E. Neuronal calcium signaling: Function and dysfunction. Cell. Mol. Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Vig M., Kinet J.P. Calcium signaling in immune cells. Nat. Immunol. 2009;10:21–27. doi: 10.1038/ni.f.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clapham D.E. Calcium signaling. Cell. 2007;131:1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 117.Roberts-Thomson S.J., Chalmers S.B., Monteith G.R. The Calcium-Signaling Toolkit in Cancer: Remodeling and Targeting. Cold Spring Harb. Perspect. Biol. 2019;11:a035204. doi: 10.1101/cshperspect.a035204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tennakoon S., Aggarwal A., Kallay E. The calcium-sensing receptor and the hallmarks of cancer. Biochim. Biophys. Acta. 2016;1863:1398–1407. doi: 10.1016/j.bbamcr.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 119.Bong A.H.L., Monteith G.R. Calcium signaling and the therapeutic targeting of cancer cells. Biochim. Biophys. Acta Mol. Cell Res. 2018;1865:1786–1794. doi: 10.1016/j.bbamcr.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 120.Varghese E., Samuel S.M., Sadiq Z., Kubatka P., Liskova A., Benacka J., Pazinka P., Kruzliak P., Busselberg D. Anti-Cancer Agents in Proliferation and Cell Death: The Calcium Connection. Int. J. Mol. Sci. 2019;20:3017. doi: 10.3390/ijms20123017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Santoni G., Morelli M.B., Marinelli O., Nabissi M., Santoni M., Amantini C. Calcium Signaling and the Regulation of Chemosensitivity in Cancer Cells: Role of the Transient Receptor Potential Channels. Adv. Exp. Med. Biol. 2020;1131:505–517. doi: 10.1007/978-3-030-12457-1_20. [DOI] [PubMed] [Google Scholar]
- 122.Romero-Garcia S., Prado-Garcia H. Mitochondrial calcium: Transport and modulation of cellular processes in homeostasis and cancer (Review) Int. J. Oncol. 2019;54:1155–1167. doi: 10.3892/ijo.2019.4696. [DOI] [PubMed] [Google Scholar]
- 123.Mundy G.R. Calcium and cancer. Life Sci. 1978;23:1735–1744. doi: 10.1016/0024-3205(78)90101-7. [DOI] [PubMed] [Google Scholar]
- 124.Pinti M., Gibellini L., Nasi M., De Biasi S., Bortolotti C.A., Iannone A., Cossarizza A. Emerging role of Lon protease as a master regulator of mitochondrial functions. BBA-Bioenergetics. 2016;1857:1300–1306. doi: 10.1016/j.bbabio.2016.03.025. [DOI] [PubMed] [Google Scholar]
- 125.Venkatesh S., Lee J., Singh K., Lee I., Suzuki C.K. Multitasking in the mitochondrion by the ATP-dependent Lon protease. Biochim. Biophys. Acta. 2012;1823:56–66. doi: 10.1016/j.bbamcr.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tangeda V., Lo Y.K., Babuharisankar A.P., Chou H.Y., Kuo C.L., Kao Y.H., Lee A.Y., Chang J.Y. Lon upregulation contributes to cisplatin resistance by triggering NCLX-mediated mitochondrial Ca2+ release in cancer cells. Cell Death Dis. 2022;13:241. doi: 10.1038/s41419-022-04668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang S.Y.L., Yu Y., Roos J., Kozak J.A., Deerinck T.J., Ellisman M.H., Stauderman K.A., Cahalan M.D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen Y.F., Chiu W.T., Chen Y.T., Lin P.Y., Huang H.J., Chou C.Y., Chang H.C., Tang M.J., Shen M.R. Calcium store sensor stromal-interaction molecule 1-dependent signaling plays an important role in cervical cancer growth, migration, and angiogenesis. Proc. Natl. Acad. Sci. USA. 2011;108:15225–15230. doi: 10.1073/pnas.1103315108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu M., Lv B., Ge W., Cui Z., Zhao K., Feng Y., Yang X. Suppression of store-operated Ca2+ entry regulated by silencing Orai1 inhibits C6 glioma cell motility via decreasing Pyk2 activity and promoting focal adhesion. Cell Cycle. 2020;19:3468–3479. doi: 10.1080/15384101.2020.1843814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zhu M., Chen L., Zhao P.F., Zhou H., Zhang C., Yu S.P., Lin Y., Yang X.J. Store-operated Ca2+ entry regulates glioma cell migration and invasion via modulation of Pyk2 phosphorylation. J. Exp. Clin. Cancer Res. 2014;33:98. doi: 10.1186/s13046-014-0098-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Lu F., Sun J., Zheng Q., Li J., Hu Y., Yu P., He H., Zhao Y., Wang X., Yang S., et al. Imaging elemental events of store-operated Ca2+ entry in invading cancer cells with plasmalemmal targeted sensors. J. Cell Sci. 2019;132:jcs224923. doi: 10.1242/jcs.224923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hirschler-Laszkiewicz I., Chen S.J., Bao L., Wang J.F., Zhang X.Q., Shanmughapriya S., Keefer K., Madesh M., Cheung J.Y., Miller B.A. The human ion channel TRPM2 modulates neuroblastoma cell survival and mitochondrial function through Pyk2, CREB, and MCU activation. Am. J. Physiol.-Cell Physiol. 2018;315:C571–C586. doi: 10.1152/ajpcell.00098.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee D.U., Park J.Y., Kwon S., Park J.Y., Kim Y.H., Khang D., Hong J.H. Apoptotic lysosomal proton sponge effect in tumor tissue by cationic gold nanorods. Nanoscale. 2019;11:19980–19993. doi: 10.1039/C9NR04323C. [DOI] [PubMed] [Google Scholar]
- 134.Kang S.W., Lee S., Lee E.K. ROS and energy metabolism in cancer cells: Alliance for fast growth. Arch. Pharm. Res. 2015;38:338–345. doi: 10.1007/s12272-015-0550-6. [DOI] [PubMed] [Google Scholar]
- 135.Miller T.W., Isenberg J.S., Roberts D.D. Molecular regulation of tumor angiogenesis and perfusion via redox signaling. Chem. Rev. 2009;109:3099–3124. doi: 10.1021/cr8005125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Jones D.P. Radical-free biology of oxidative stress. Am. J. Physiol. Cell Physiol. 2008;295:C849–C868. doi: 10.1152/ajpcell.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fiaschi T., Chiarugi P. Oxidative stress, tumor microenvironment, and metabolic reprogramming: A diabolic liaison. Int. J. Cell Biol. 2012;2012:762825. doi: 10.1155/2012/762825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Felty Q., Xiong W.C., Sun D.M., Sarkar S., Singh K.P., Parkash J., Roy D. Estrogen-induced mitochondrial reactive oxygen species as signal-transducing messengers. Biochemistry. 2005;44:6900–6909. doi: 10.1021/bi047629p. [DOI] [PubMed] [Google Scholar]
- 140.Chen J., Wang Y., Zhang W., Zhao D., Zhang L., Fan J., Li J., Zhan Q. Membranous NOX5-derived ROS oxidizes and activates local Src to promote malignancy of tumor cells. Signal Transduct. Target. Ther. 2020;5:139. doi: 10.1038/s41392-020-0193-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Matsui A., Okigaki M., Amano K., Adachi Y., Jin D., Takai S., Yamashita T., Kawashima S., Kurihara T., Miyazaki M., et al. Central role of calcium-dependent tyrosine kinase PYK2 in endothelial nitric oxide synthase-mediated angiogenic response and vascular function. Circulation. 2007;116:1041–1051. doi: 10.1161/CIRCULATIONAHA.106.645416. [DOI] [PubMed] [Google Scholar]
- 142.Bibli S.I., Szabo C., Chatzianastasiou A., Luck B., Zukunft S., Fleming I., Papapetropoulos A. Hydrogen Sulfide Preserves Endothelial Nitric Oxide Synthase Function by Inhibiting Proline-Rich Kinase 2: Implications for Cardiomyocyte Survival and Cardioprotection. Mol. Pharmacol. 2017;92:718–730. doi: 10.1124/mol.117.109645. [DOI] [PubMed] [Google Scholar]
- 143.Lysechko T.L., Cheung S.M.S., Ostergaard H.L. Regulation of the Tyrosine Kinase Pyk2 by Calcium Is through Production of Reactive Oxygen Species in Cytotoxic T Lymphocytes. J. Biol. Chem. 2010;285:31174–31184. doi: 10.1074/jbc.M110.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Martel-Gallegos G., Casas-Pruneda G., Ortega-Ortega F., Sanchez-Armass S., Olivares-Reyes J.A., Diebold B., Perez-Cornejo P., Arreola J. Oxidative stress induced by P2X7 receptor stimulation in murine macrophages is mediated by c-Src/Pyk2 and ERK1/2. BBA-Gen. Subj. 2013;1830:4650–4659. doi: 10.1016/j.bbagen.2013.05.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.