SUMMARY
RNA granules are cytoplasmic condensates that organize biochemical and signaling complexes in response to cellular stress. Functional proteomic investigations under RNA granule-inducing conditions are needed to identify protein sites involved in coupling stress response with ribonucleoprotein regulation. Here, we apply chemical proteomics using sulfonyl-triazole (SuTEx) probes to capture cellular responses to oxidative and nutrient stress. The stress responsive tyrosine and lysine sites detected mapped to known proteins involved in processing body (PB) and stress granule (SG) pathways, including LSM14A, FUS, and EDC3. Notably, disruption of EDC3 tyrosine 475 (Y475) resulted in hypo-phosphorylation at S161 and S131, and altered protein-protein interactions (PPI) with decapping complex components (DDX6, DCP1A/B) and 14-3-3 proteins. This resulting mutant form of EDC3 was capable of rescuing the PB-deficient phenotype of EDC3 knockout cells. Taken together, our findings identify Y475 as an arsenic-responsive site that regulates RNA granule formation by coupling EDC3 post-translational modification and PPI states.
Keywords: EDC3, P-bodies, stress granules, LLPS, stress, chemoproteomics, condensate, RNA granule, tyrosine, SuTEx
Graphical Abstract

eTOC Blurb
Ciancone et al. discover stress-responsive residues on proteins involved in RNA granule modulation using tyrosine/lysine-reactive SuTEx probes. The Y475 site that emerged from these chemoproteomic studies was found to regulate P-body response to arsenic by controlling protein-protein interactions and phosphorylation state of EDC3 in cells.
INTRODUCTION
Processing-bodies (P-bodies or PBs)1 and stress granules (SGs)2,3 are cytoplasmic foci that concentrate proteins and RNA through a process known as liquid-liquid phase separation (LLPS)4–6. PBs localize the 5’ mRNA decapping complexes and can function as sites of mRNA degradation7. Emerging studies have shown that PBs can also sequester RNA and prevent degradation8,9. Thus, PBs are speculated to aggregate RNA in the regulation of translational output under times of cellular stress9,10. PBs are critical for IL-6 production in macrophages, and compounds that dissociate PBs can sensitize cells to radiation and chemotherapeutics11–14. Furthermore, PBs have been shown to affect certain viral RNA through distinct mechanisms15. In contrast with SGs, the role of PBs in mammalian physiology and disease remains poorly understood16.
Enhancer of mRNA-decapping protein 3 (EDC3) is a protein that undergoes LLPS into PBs. EDC3 assists in the removal of the 7-methylguanosine (7MG) cap on mRNA that is used for translational recognition and protection from 5’-to-3’ degradation4,17. EDC3 comprises four functional domains: 1) an N-terminal LSm, 2) an intrinsically disordered central region, 3) an FDF, and 4) a C-terminal YjeF_N. The LSm domain mediates EDC3 PB localization and interactions with DCP1A. The intrinsically disordered region (IDR) is highly phosphorylated and contains serine 161 (S161), a target for PIM kinases17. This disordered region has previously been shown to assist in RNA binding. The FDF domain is critical for mediating EDC3 binding to the ATP-dependent RNA helicase DDX6 (DDX6). Finally, the YjeF_N mediates homodimerization, a function which assists in protein-protein interactions and provides an RNA binding surface for the 7MG decapping complex4,18–21. Recent work has implicated the YjeF_N domain as critical for seeding the formation of PBs in S. cerevisiae through dimerization of the protein, which contributes to increased valency for the P-body network and competes with the P-body disassembly protein Sbp122.
The exact function of EDC3 in regulation of PBs is still under active investigation. Although EDC3 is important for PB formation in S. cerevisiae, genetic disruption of the mammalian counterpart does not impact PB formation4,23. Recent work has shown that EDC3 phosphorylation at S161 by PIM kinases is important for regulating PB formation, proliferation, and migration in cancer cells17. Additionally, EDC3 localization to PBs is known to degrade certain RNA24. Finally, EDC3 may function as a pre-stress ‘seed’ protein for SG formation25. Collectively, the range of functions carried out by EDC3 in regulation of PB biology appears diverse but remains ill-defined.
We discovered that tyrosine 475 (Y475) on EDC3 displayed enhanced nucleophilicity using sulfonyl-triazole (i.e., SuTEx) probes26. EDC3 Y475 is located in the YjeF_N domain, which is important for homodimerization, RNA binding, and 7MG decapping. However, the exact role of Y475 and the importance of enhanced reactivity at this site remain unclear4,27. Here, we combine SuTEx chemical proteomics28 and immunofluorescence (IF) to discover that Y475 functions as a key site for regulating cellular PB formation in response to oxidative stress. Phospho- and affinity-proteomics revealed alterations in EDC3 phosphorylation state and protein-protein interaction (PPI) networks upon perturbation of Y475 including enhanced binding to DCP1A that was further validated by increased colocalization of Y475F mutant protein with DCP1A in IF studies. Collectively, our findings position EDC3 Y475 as a key stress-responsive site for regulation of PB composition and formation in cells.
RESULTS
Functional profiling of the RNA granule proteome under stressed cell states
SuTEx probe-modified peptides and corresponding proteins are enriched for binding in protein domains involved in nucleotide recognition and PPIs26. Many of these same domains (e.g., RNA recognition motif and zinc finger C3H1-type) are known to localize to RNA granules9,26,29. Furthermore, SuTEx preferentially modifies tyrosine (Y) and lysine (K) residues, which are found at a higher abundance in nucleotide-binding domains26,30–32. We surmised that SuTEx probes can capture functional alterations in the RNA granule proteome as determined by the probe modification state on Y and K sites.
Stable isotope labeling by amino acids in cell culture (SILAC) light and heavy HEK293T and HeLa cells were subjected to vehicle or oxidative stress treatments (arsenic: 1 mM for 45 min; H2O2: 200 μM for 15 min). We also included glucose deprivation to compare nutrient and oxidative stress effects on SuTEx probe binding profiles (Figure 1A and S1A; see STAR Methods for additional details). These treatment conditions have been previously shown to form SGs or PBs in cells, albeit utilizing different mechanisms6,33,34. Cells were subsequently treated with the SuTEx probe HHS-465 because of its ability to profile tyrosine and lysine sites (100 μM, 2 h). Cells were lysed, proteomes extracted, and subjected to copper-catalyzed azide-alkyne cycloaddition (CuAAC)35 conjugation of desthiobiotin for quantitative liquid chromatography-tandem mass spectrometry (LC-MS) chemical proteomics as previously described36. We confirmed these stress conditions induced RNA granule formation by immunofluorescence using confocal microscopy antibody markers of PBs (α-EDC4) and SGs (α-G3BP1; Figure 1B, S1B and S1C)6,23,37. As a control, we included a treatment condition that utilized emetine (50 nM, 2 h) as a method to dissolve RNA granules as previously described in literature(Figure S1D and E)6.
Figure 1. Global discovery of RNA granule induction-sensitive lysines and tyrosines (RISKYs) in cells.

A) Workflow for RISKY chemoproteomic discovery. HEK293T and HeLa cells were induced to form RNA granules (arsenic, glucose-deprivation, H2O2) followed by SuTEx probe (HHS-465) labeling to capture resulting tyrosine and lysine covalent binding profiles. LC-MS coupled with previously published bioinformatics analysis identified RISKYs as determined by reduced probe binding in stressed (heavy, blue) compared with matching control (light, red) cells (light/heavy SILAC ratio or SR > 2, n = 2). B) Representative immunofluorescence images for cells (nuclei stained with DAPI) exposed to oxidative (arsenic, H2O2) or nutrient stress (glucose-deprived). Arsenic-treated cells form PBs (α-EDC4) and SGs (α-G3BP1), glucose-deprived cells are enriched for PBs, and H2O2-treated cells are enriched for SGs (white scale bar represents 38μm; n = 2). C) Plot of HHS-465-modified Y and K sites (represented by individual circles) as a function of SILAC ratios, SR (light (− stress)/heavy (+ stress)). RISKYs are highlighted as red dots (SR >2). D) Domain enrichment analysis of RISKYs. Analyses were performed as previously described36. E) Representative MS1 extracted ion chromatograms (EICs) for arsenic-, glucose-, and peroxide-RISKY peptides. Data shown are representative of n = 2 biologically independent experiments.
Overall, we detected over 6,000 distinct probe-modified tyrosine and lysine sites from ~1,800 proteins (Figure 1C, see Table S1 for full list). We identified a collection of sites that showed altered SuTEx probe labeling upon RNA granule induction (SILAC ratio or SR > 2). The accessibility of these sites for SuTEx probe labeling is likely affected because of post-translational modifications (PTMs), protein-protein or protein-RNA binding, or partitioning by phase separation during RNA granule formation. We compared these RNA-granule induction-sensitive lysines and tyrosines (RISKYs) to the reported SG and PB proteomes (376 sites from 264 proteins, Table S2)9,29. Overall, we identified substantial overlap between proteins containing RISKYs with reported RNA granule proteins (~37%, Table S2). These proteins include LSM14A, FUS, G3BP2, and EDC3, which are well-annotated components of RNA granules9,29.
Domain enrichment analysis of the RISKYs revealed that the HMG boxes A and B domain (PRU00267) was highly enriched, along with several RNA-binding domains, such as the KH (PRU00117), RNA recognition motif (PRU00176), nudix hydrolase (PRU00794), and zinc-finger CHY-type domains (PRU00601, Figure 1D and Table S2)38,39. Notably, we identified several protein sites that appeared to respond exclusively to arsenic (K30, high mobility group protein B1 or HMGB1), glucose-deprivation (Y528, PALS1), or peroxide treatments (K110, malate dehydrogenase or MDHC; Figure 1E).
Identification of EDC3 Y475 as a hyper-reactive, arsenic-responsive RISKY
Next, we compared RISKY sites with reported hyper-reactive-tyrosine-containing proteins to prioritize sites for functional validation26. We identified 14 proteins that overlapped across RISKYs, annotated RNA granule proteomes9,29, and were previously annotated to contain a hyper-reactive tyrosine26 (designated as Hyper-RISKYs; Figure 2A and Table S2). From this list, EDC3 emerged as a key candidate because this protein is known to localize to PBs and was recently implicated as a seed for SG formation (Figure 2B)4,25.
Figure 2. Identification of EDC3 Y475 as an arsenic-responsive RISKY.

A) Venn diagram showing overlap of proteins reported in RNA granule proteomes9,29, meet the criteria of a RISKY site, or proteins containing a hyper-reactive tyrosine26. The combined overlap contains a subset of hyper-reactive RISKY sites (14 Hyper-RISKY sites) found on proteins in the RNA granule proteome. B) Schematic of EDC3 domain topology. Y475 is located at the C-terminus, near the end of the YjeF_N domain, which mediates self-dimerization, RNA binding, and PPIs4,27. C) MS1 EIC abundances of the EDC3 Y475 probe-modified light (red, control) and heavy (blue, stress) peptides under different stress induction conditions. EDC3 Y475 probe labeling is substantially decreased (SR = 5.6) in cells pre-treated with arsenic, but not other induction methods (n = 5). D) Plot of RISKY site SR (y-axis) from SuTEx probe binding as a function of respective protein abundance SR (x-axis) from unenriched proteomic analysis (n = 2). The median ratio of detected tryptic peptides derived from each protein was used for analysis. See STAR Methods for additional details. Linear regression indicates no correlation (R2 = 0.0005) between changes in probe labeling and protein expression in cells evaluated under the experimental conditions tested. EDC3 Y475 is highlighted as a red star. The dotted line represents the SR cut-off for RISKY designation.
Probe labeling at EDC3 Y475 was inhibited by arsenic treatment but not by other induction methods (Figure 2C). Alterations in probe binding of EDC3 Y475 could be due to exclusion of HHS-465 from PBs and/or SGs. The insensitivity of EDC3 Y475 site to emetine treatment, which dissolves RNA granules6, supports probe binding is not likely due to differences in target protein accessibility from compartmentalization (Figure S1D, E, and S2A). The lack of changes from hydrogen peroxide treatment further supports arsenic-specific effects, which has been shown to induce oxidative stress through a distinct stress response pathway34. Critically, non-enriched proteomics confirmed that alterations in EDC3 probe binding profiles were not due principally to differences from protein expression (Figure 2D and Table S3). In conclusion, we discovered that EDC3 Y475 functions as a RISKY site that responds to cellular stress in an arsenic-specific, protein expression-independent manner as measured by SuTEx probe labeling.
Disrupting EDC3 Y475 alters P-body response to stress
Next, we investigated the effects of mutating EDC3 Y475 on PB formation. Recombinant EDC3 wild-type (WT)- or tyrosine-475-to-phenylalanine (Y475F) mutant-expressing HEK293T cells were subjected to cellular stress followed by evaluation of the number of PBs per cell by immunofluorescence. We compared PB response to both oxidative (arsenic treatments; 1 mM, 45 min) and nutrient stress (glucose deprivation; 15 min) using antibodies for PBs (α-EDC4) and recombinant protein expression (α-FLAG, Figure 3A). A negative control sample lacking antibody staining was used to set the threshold to remove background signals. As a control, emetine treatment (50 nM, 2 h) was used to confirm PB depletion matched previously reported observations6 (Figure S1D). Comparable expression of recombinant EDC3 WT and Y475F was confirmed by western blot (Figure 3B). Control data from mock and basal samples confirmed both glucose-deprivation and arsenic increased the total number of PBs per cell (Figure 3C and D). See STAR Methods for additional details of the confocal microscopy experiments. All quantified immunofluorescence data can be found in Table S4.
Figure 3. EDC3 Y475 is critical for stress-induced P-body formation.

A) Representative immunofluorescence images showing HEK293T cells expressing recombinant EDC3 WT or Y475F mutant stained for cell nuclei (DAPI, blue), PBs (α-EDC4, green) and recombinant expression (α-FLAG, cyan). Image of a zoomed-in region is highlighted by a gold box. B) Western blots verifying comparable expression of EDC3 WT and Y475F mutant protein (anti-FLAG). GAPDH loading controls are also included. C) Enhanced PB formation was observed in EDC3 Y475F cells treated with arsenic (1 mM, 45 min) compared with PBS vehicle treated controls (***P < 0.001, Dunnett’s multiple comparisons, n = 3; data shown as mean +/− S.E.M.). White scale bars represent 38 μm. D) Glucose deprivation results in reduced PB formation in EDC3 WT-expressing cells compared with (+)glucose controls (**P < 0.01, Dunnett’s multiple comparisons, n = 3; data shown as mean +/− S.E.M.). Overexpression of EDC3 Y475F had no effect on glucose-deprived PB response.
Overexpression of recombinant EDC3 WT in HEK293T cells resulted in higher basal levels of PBs and this effect was not further augmented in response to arsenic treatments (Figure 3A and C). In contrast, the PB response was increased >2-fold in EDC3 Y475F-expressing cells under the same oxidative stress conditions (~6.0 vs 2.8 PBs/cell, P < 0.001; Figure 3C). We observed negligible differences in the number of PBs per cell in EDC3 WT- compared with Y475F-expressing cells under basal conditions (Figure 3A and C). In contrast, glucose deprivation of EDC3 WT-expressing HEK293T cells resulted in a statistically significant decrease in the number of PBs/cell (~6 versus 2, P < 0.01; Figure 3D). This inhibitory effect on nutrient stress-induced PB formation was blunted with mutation of Y475 (EDC3 Y475F-expressing cells, Figure 3D).
Taken together, these findings support a role for the EDC3 Y475 site in modulating cellular PB responses to stress. Mutation of Y475 (Y-to-F) appears to reverse the PB formation effects seen when WT EDC3 is recombinantly overexpressed in cells under different stress conditions.
Mutation of EDC3 Y475 enhances DCP1A and DDX6 binding and alters the EDC3 interactome
The Y475 site is located in the YjeF_N domain, which has been shown to be important for homodimerization, 7MG decapping, and for RNA binding of EDC34,27. In yeast, Edc3p dimerization is necessary for binding to the C-terminal end of Dcp2p, a process required to enhance 7MG decapping efficiency4. We reasoned that a potential mechanism explaining the cell biological outcomes from mutating EDC3 Y475 is the alteration in EDC3 PPI networks. Recent work from the Parker and Brangwynne groups has demonstrated that PPIs are important for RNP composition and formation37,40.
SILAC light and heavy HEK293T cells expressing recombinant EDC3 (WT or Y475F mutant; mock-transfected cells were used as a negative control) were subjected to arsenic treatments (1 mM, 45 min) or glucose-deprivation (15 min) followed by cell lysis and preparation of proteomes for immunopurification followed by LC-MS analysis. In brief, light and heavy soluble proteomes were separately incubated with magnetic anti-FLAG tag beads, beads were washed to remove non-EDC3 interacting proteins, and bead-bound proteins (EDC3 and interactome) were eluted. Light and heavy elution fractions were mixed and subjected to a filter-aided sample preparation (FASP) method41. Tandem LC-MS/MS analysis and protein identifications were performed as previously described26. Tryptic peptides that met quality control criteria were used for identification of binding partners of EDC3 WT and Y475F. See STAR Methods for additional details of LC-MS/MS data acquisition and analyses.
Tryptic peptides matching to EDC3 WT and Y475F mutant (IYLCDIGIPQQVFQEVGINYHSPFGCK and IFLCDIGIPQQVFQEVGINYHSPFGCK, respectively) were detected prominently in recombinant HEK293T cells, but not in mock-transfected cells as expected (SR > 20 in EDC3 WT or Y475F (light) / mock-transfected (heavy); Figure S3A). In total, we detected 487 proteins in our affinity proteomic studies (see Table S5 for complete list). Analysis of proteins that displayed substantial enrichment (SR > 4, Table S6) identified several known EDC3 binding partners, including mRNA-decapping enzyme 1A (DCP1A), and 1B (DCP1B), and DDX6. EDC3, DDX6, DCP1A, and DCP1B comprise components of the 7MG decapping complex, and this PPI network has been previously reported18,42,43. In addition to 7MG decapping complex binding partners, we also observed an enrichment of 14-3-3 protein members. 14-3-3 proteins have been reported to bind to phosphorylated EDC3 (S161) in response to insulin signaling44.
Next, we manually reintegrated the peak areas of peptides corresponding to prominently detected EDC3 binding partners (Table S7). In total, we detected 30 unique peptides for EDC3 and 18 unique peptides for DDX6 (Table S7). This analysis found that almost all EDC3 binding partners were detected in higher abundances in Y475F arsenic-treated but not glucose-deprived samples compared to WT EDC3, with the greatest changes arising from DCP1A/B, 1433F, and DDX6 (SR > 1.5, Figure 4, Table S6). Taken together, these data suggests that the EDC3 interactome is dependent on the cell stress conditions and perturbations of the Y475 site can further alter the PPI network.
Figure 4. Perturbation of Y475 alters the EDC3 interactome in response to stress.

A) Plot of protein ratios from all proteins detected in EDC3 WT and Y475F IP studies: EDC3 Y475F/WT SR (x-axis, log2-scale) vs. relative enrichment (EDC3/Mock SR, y-axis). EDC3 is highlighted as a red star, while annotated binding partners are highlighted as blue dots. Other proteins are shown as black dots. B) EDC3 binding partners are identified by SR >4 in EDC3 WT-expressing compared with mock transfected proteomes. Protein ratios were calculated as an average of the protein SR from independent biological replicates (median of ≥ 2 distinct peptides per protein, n = 3). Bar plot depicts the relative enrichment of EDC3 binding partners in Y475F mutant (light) compared with WT proteome (heavy). The EDC3 interactome was distinct based on the stress condition and whether the Y475 site was perturbed. C) Representative MS1 EICs for peptides used to calculate the overall protein values in B. The SRs below each MS1 peak set are calculated as the light (red) to heavy (blue) for HEK293T cells expressing the listed recombinant protein under arsenic activating conditions (n = 3).
In summary, we discover that mutation of Y475 alters EDC3 PPIs in cells exposed to arsenic. The changes in EDC3 PPIs under arsenic-induced stress include enhanced DDX6, DCP1A/B, and 14-3-3 interactions that provide clues to how the Y475 site on EDC3 influences PB composition in response to cellular stress.
Perturbation of Y475 results in a hypo-phosphorylated state of EDC3
We performed phosphoproteomic analyses to test whether the Y475 site is involved in regulating the phosphorylation state of EDC3, which could include phosphorylation of Y475 and/or crosstalk with the IDR of EDC3 that contains a PIM kinase phosphorylation site (serine 161 or S161)17,21. Phosphopeptides from EDC3 WT- or Y475F-expressing SILAC HEK293T cells exposed to PBS vehicle or arsenic (1 mM, 45 min) were enriched using commercial titanium dioxide (TiO2) affinity chromatography followed by LC-MS/MS analysis as described previously45 and in the STAR Methods. In total, we quantified 1,337 reproducible phosphopeptides (identified in at least 2 biologically independent replicates) corresponding to 645 proteins that included phospho-serine (82%), -threonine (15%), and -tyrosine (3%) with distributions that matched reported phosphoproteomic studies (Figure 5A and Table S8)46,47.
Figure 5. The EDC3 Y475F mutant is hypo-phosphorylated at S161 and S131.
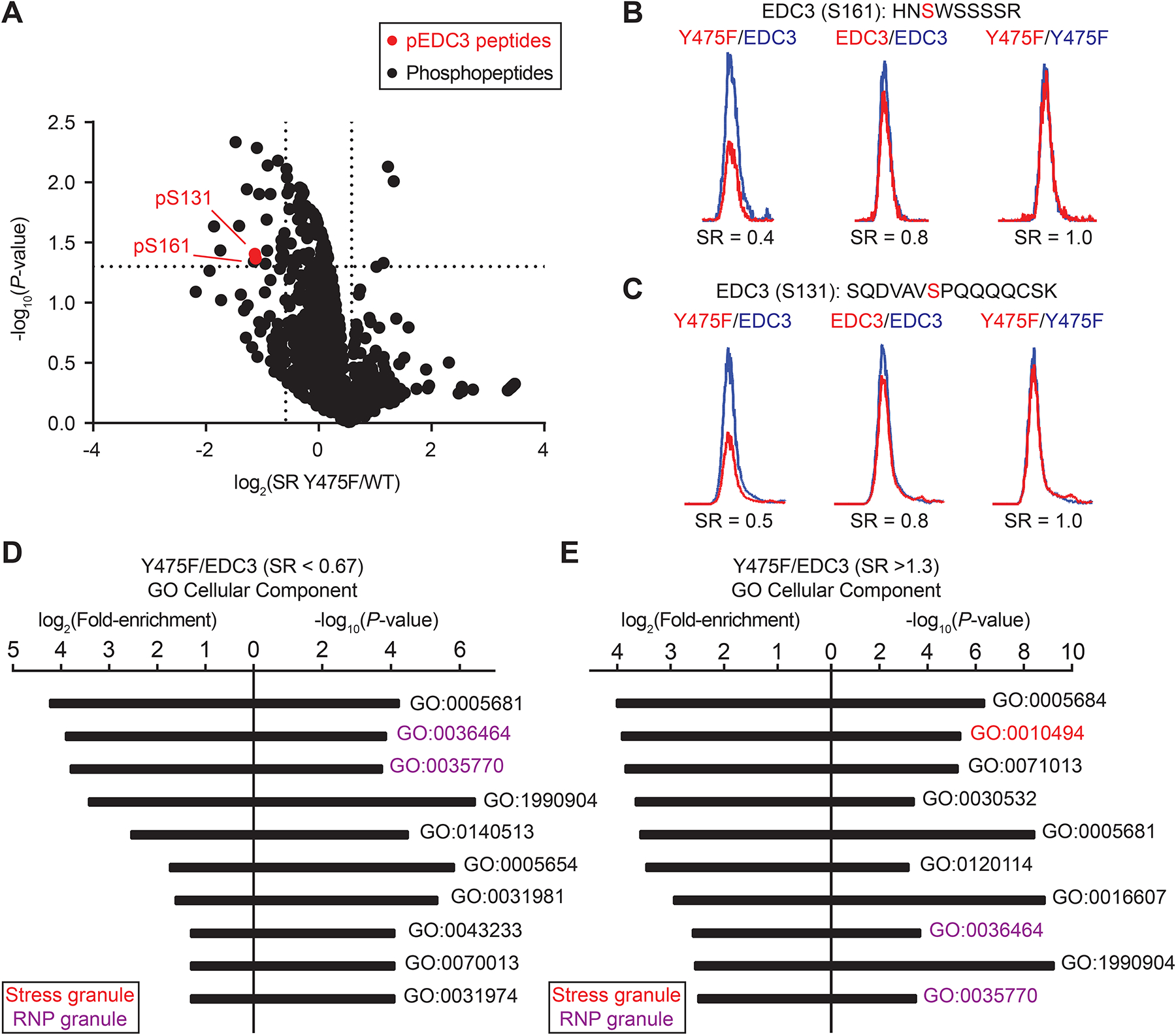
A) Volcano plot comparing detected phosphopeptide levels in EDC3 Y475F (light)- compared with WT (heavy)-overexpressing HEK293T cells treated with arsenic (1 mM, 45 min). The log2 of fold change (SR) was plotted as a function of statistical signficance (−log10 P value) for enriched phosphopeptides that passed our quality control criteria (see STAR Methods for additional details). General phosphopeptides are represented by black dots; statistically significant, EDC3-specific phosphopeptides are highlighted in red. Vertical dotted lines represent a cutoff for phosphopeptides with a change of ~33% (increase or decrease in SR). Horizontal dotted line represents a cutoff of statistical significance (P < 0.05). Representative MS1 EICs depicting changes in relative levels of EDC3 phospho-serine 161 (pS161, B) and pS131 (C) in Y475F (light, red) compared with WT EDC3 protein (heavy, blue) in phosphoproteomes from arsenic-treated cells. Gene Ontology enrichment analysis (PANTHER) for cellular component of proteins with a SR <0.67 (D) or SR >1.3 (E) in EDC3 Y475F compared with WT phosphoproteomes. Data are representative of n = 2 biologically independent replicates.
We mixed light and heavy samples to evaluate alterations in phosphorylation state of EDC3 Y475F mutant (L) compared with that of the WT (H) protein under arsenic treatment conditions. We also included appropriate 1:1 mixing controls to identify artefactual changes in phosphopeptide abundances. We discovered that EDC3 Y475F was hypo-phosphorylated at both S161 (SR = 0.4) and S131 (SR = 0.5) compared with WT counterparts (Figure 5B and C). Importantly, the S131 and S161 site on both EDC3 WT and Y475F mutant respond to arsenic with ~2-fold increase in phosphorylation under stressed conditions (Figure S4). Notably, we did not detect phosphorylation on Y475 of EDC3 in our phosphoproteomic analyses, which agrees with its lack of annotation in the PhosphoSitePlus database48. Gene ontology (GO) analysis of phosphorylation changes that were altered for EDC3 Y475 compared with WT-expressing cells in response to arsenic revealed enrichment for cellular components related to RNP granules (Figure 5D and E).
Collectively, these phosphoproteomic studies reveal a role for the Y475 site in altering the phosphorylation state of EDC3 that can be principally localized to S161 and S131 residues. The inability to detect phosphorylation on Y475 is consistent with hyper-reactive tyrosines like Y475 showing a lower frequency of phosphorylation26.
Rescue of P-body deficient EDC3 knockout cells by the hypo-phosphorylated Y475F mutant
Next, we evaluated the impact of EDC3 Y475 perturbation on stress induced PB formation in a loss of function system. We utilized CRISPR-Cas949,50 to generate an EDC3 knockout (KO) HEK293T cell line to test whether reinstatement of EDC3 WT or Y475F mutant can reverse the resulting phenotypes from absence of endogenous protein. These studies are important for direct assessment of EDC3 Y475 function using the mutant protein, which could dimerize with endogenous EDC3. To generate an EDC3 KO cell line, two CRISPR guide vectors containing Cas9 were designed based on Benchling server to target exon 2 of human EDC3 gene (hEDC3, Figure S5A). The alteration in the genomic region of hEDC3 was confirmed using a surveyor assay (Figure S5B). We confirmed the absence of EDC3 protein from positive clones by western blot and immunofluorescence analyses (Figure S5C–E).
Next, we performed IF assays to monitor PB and SG formation in EDC3 WT compared with KO cells. EDC3 KO cells contained approximately half the number of PBs per cell after arsenic treatment (1 mM, 45 min) when compared to a PBS treated control (Figure S5F and G). In contrast, arsenic treatment did not elicit an altered SG response in EDC3 KO compared with WT cells (6.4 vs 7.2 SGs/cell, respectively; Figure S5F and H). Restoration of EDC3 WT in KO cells (+EDC3) through recombinant expression did not affect PB response to arsenic compared with mock-transfected KO cells (Figure 6). Intriguingly, the reinstatement of EDC3 Y475F mutant (+Y475F) resulted in a statistically significant increase in the number of PBs per cell compared with mock KO cells under arsenic conditions (~4 vs 1 PBs/cell for +Y475F vs mock; Figure 6B).
Figure 6. Restoration of EDC3 Y475F rescues the PB-deficient EDC3-KO cell phenotype.

A) Immunofluorescence images (respresentative) for EDC3 KO cells transiently transfected with recombinant EDC3 WT or Y475F mutant and treated with PBS vehicle or arsenic (1 mM, 45 min). Cells (DAPI, blue) were stained for PBs (α-EDC4, green) and for recombinant protein expression (α-FLAG, cyan). White scale bar represents 38 μm. B) Bar plot comparing the number of PBs per cell for mock-, EDC3-, or Y475F-transfected EDC3-KO treated with either PBS or arsenic. Mock EDC3-KO cells contain fewer PBs per cell when treated with arsenic compared to PBS (~1 vs 2 PBs per cell, respectively). Expression of EDC3 Y475F in KO cells results in a statistically significant increase in PBs per cell compared with mock KO cells in response to arsenic treatment (**P < 0.01). An ANOVA analysis with Dunnett’s multiple comparisons test was performed; data shown are mean +/− S.E.M. A separate comparison of mock KO (−arsenic) vs mock KO (+arsenic) resulted in a statistically significant decrease in PBs per cell (Welch’s t-test, ****P < 0.0001, data not shown). Data are representative of n = 3 biologically independent replicates.
Collectively, these data support a role for EDC3 Y475 in arsenic-dependent PB response of cells. We demonstrate that reinstatement of a hypo-phosphorylated EDC3 Y475F mutant in EDC3 KO cells enhances PB formation compared with KO cells alone in response to arsenic. These findings agree with previous reports that reduced EDC3 S161 phosphorylation using kinase inhibitors increased PB formation and reduced tumor cell growth17. Future work will determine whether EDC3 Y475 represents a pharmacological site for modulating PB formation in stress response of tumor cells.
EDC3 Y475F mutant shows enhanced colocalization with DCP1A
To provide further mechanistic insights into Y475 function, we performed IF colocalization experiments to visualize PPIs between EDC3 WT and Y475F mutant with binding partners in live cells exposed to arsenic. Recombinant EDC3 (WT or Y475F mutant) was co-expressed with annotated binding partners (Figure 4B) in EDC3 KO cells and exposed to arsenic treatments. Western blots were used to verify co-expression of recombinant proteins (Figure 7A). Immunofluorescence and colocalization assessments were performed as described in the STAR Methods.
Figure 7. Enhanced colocalization of EDC3 Y475F with DCP1A in arsenic-treated cells.

A) Western blots showing recombinant expression of EDC3 WT or Y475F with identified binding partner in cells treated with arsenic. Data are representative of n = 2 biologically independent replicates. B) Representative images for EDC3-KO cells (DAPI, dark blue) co-expressing EDC3 WT (left panels) or Y475F protein (right panels; FLAG, light blue) and binding partner (HA-tag, red; BFP, blue; GFP, green) treated with arsenic (1 mM, 45 min). For cells expressing recombinant BFP-DCP1A, a GAPDH stain (red) was used in place of DAPI. Overlap of recombinant protein can be seen as white for HA-tagged proteins (DDX6) or a different shade of blue for DCP1A/B. Data are representative of n = 2 biologically independent replicates. A complete panel of immunofluorescence images can be found in Figure S6. C) Bar plot comparing colocalization (Pearson’s coefficient, y-axis) of recombinant EDC3 WT (grey) or Y475F protein (white) with annotated binding partner (x-axis). EDC3 Y475F shows statistically significantly increased colocalization with DCP1A compared with WT counterpart (****P < 0.0001; data shown as mean +/− S.E.M.). ANOVA analysis with Dunnett’s multiple comparisons test was performed. Data are representative of n = 2 biologically independent replicates.
Our immunofluorescence studies revealed colocalization between EDC3 and the annotated binding partners, albeit to varying degrees (Pearson’s coefficient values of 0.3–0.8, Figure 7B–C and S6). The differences in colocalization between EDC3 WT and Y475F protein with binding partners were not readily apparent except for DCP1A. Importantly, DCP1A colocalized with EDC3 Y475F to a statistically significantly higher degree compared with WT protein, which matched our affinity proteomics that identified DCP1A as the binding partner most affected by mutation of Y475 on EDC3 (Figure 4B and C; 7C). Interestingly, the FLAG staining of EDC3 Y475F mutant was less diffuse and appeared to be more concentrated with DCP1A-containing foci51 compared to cells co-expressing other binding partners (Figure 7B).
In summary, we discovered DCP1A and EDC3 Y475F showed enhanced colocalization under arsenic stress conditions. We also found colocalization between EDC3 and its annotated binding partners identified from our PPI studies. These findings further strengthen our hypothesis that SuTEx chemoproteomics can identify functional sites on proteins that respond to stress and RNA granule formation.
DISCUSSION
Functional investigations of RNA granule proteomes are important for identifying protein activities regulating RNP composition and formation. Here, we applied chemical proteomics to identify EDC3 Y475 as an arsenic-responsive site for modulating phosphorylation status and PPI networks involved in PB formation. The ability to restore deficient PB formation in EDC3 KO cells using the hypo-phosphorylated EDC3 Y475F mutant but not WT protein further supports the functional relevance of this reactive tyrosine site for PB formation.
We defined functional changes in the RNA granule proteome through alterations in covalent binding to reactive tyrosine and lysine residues (376 RISKYs). These RISKYs were enriched for protein functions involved in protein folding and RNA processing (>250 proteins, Figure 1 and 2). Among the candidate RISKYs, we identified proteins critical for RNA granules, including LSM14A, EDC3 and FUS23,37. The decreased SuTEx probe binding at stress responsive sites could reflect competition from PTMs, PPIs, and/or protein-RNA binding. Further comparison of proteins containing RISKY sites with the reported PB and SG proteome revealed substantial overlap (~37%, Table S2).
We compared the RISKY sites with previously annotated hyper-reactive tyrosine residues to prioritize EDC3 for follow-up studies. Disruption of EDC3 Y475 through mutagenesis resulted in disinhibition or activation of PB formation in cells exposed to nutrient or oxidative stress, respectively (Figure 3). We show through affinity proteomics that EDC3 Y475 is critical for EDC3 PPIs in response to arsenic and perturbation of this site alters EDC3 binding partner profiles including enhanced DCP1A binding, which we could directly visualize by immunofluorescence (Figure 4 and 7). We complement our EDC3 interactome studies with phosphoproteomics to discover that EDC3 Y475F is hypo-phosphorylated compared with WT protein at both well-annotated (S161) and relatively unexplored (S131) phosphoserine sites (Figure 5). The functional impact of altered PPI and phosphorylation states of EDC3 Y475F was revealed in the ability of this mutant, but not WT protein to ‘rescue’ deficient PB formation of EDC3 KO cells (Figure 6 and S5F and G).
A key question that emerged from our current studies is why the S161 and S131 phosphorylation sites are impacted by Y475 disruption on EDC3. We find it interesting that the IDR of EDC3, which contains S131 and S161, was reported to interact with the YjeF_N domain (containing Y475) in an irreversible LLPS maturation step21. The phosphorylation of S161 in the EDC3 IDR was recently found to be critical for PB formation17. Notably, the phosphorylation-deficient EDC3 S161A mutant increased P-body formation, which resembles the phenotype of our Y475F mutant in both WT and EDC3 KO cells exposed to arsenic stress (Figure 3 and 6). Thus, the EDC3 Y475 site appears to couple IDR phosphorylation state with PPI networks (DCP1A, DDX6, 14-3-3s) to facilitate stress-induced PB formation of cells.
In summary, we identify a stress-responsive tyrosine site involved in coupling phosphorylation state with PPI networks of EDC3 in stress-induced PB formation. Our findings serve as a template for additional discovery efforts aimed at liganding tyrosines and lysines – residues found at high frequency in RNA recognition domains32 – for chemical control of RNA granule response in stressed cells.
LIMITATIONS OF THE STUDY
We are not able to formally rule out the possibility that the Y475 site is phosphorylated to further contribute to EDC3 biochemical and cell biological functions. The inability to detect phosphorylated Y475 could be due to limitations of our current LC-MS/MS methodology. We envision future studies will couple our current phosphoproteomic method with more sophisticated workflows tailored for detection and quantitation of phosphotyrosines52. Additional PTMs could occur on Y475 (e.g., sulfation and nitration) to further diversify the biochemistry and cell biology regulated by this EDC3 site. The inclusion of appropriate proteomic workflows customized for these PTMs, (e.g., sulfotyrosines53) will be important for enabling these future investigations on EDC3. We are cognizant that genetic knockout of EDC3 can produce compensatory effects in cells, which can be addressed in future studies through development of EDC3 Y475-directed SuTEx ligands to enable complementary pharmacology.
SIGNIFICANCE
This work investigates cellular stress responses through chemical proteomic evaluation of covalent probe binding to tyrosine and lysines under known RNA granule induction conditions. We detected a subset of sites in the proteome that showed altered SuTEx probe labeling in response to oxidative (arsenic, H2O2) and nutrient stress (glucose deprivation). Our rationale for this approach was to capture functional changes on tyrosines and lysines – residues at higher natural abundance in RNA binding domains – due to post-translational modifications, protein-protein interactions (PPIs), or protein-RNA binding. The enhancer of mRNA decapping protein 3 (EDC3) tyrosine 475 (Y475) site emerged from our global profiling studies because of its well-annotated role in processing body (PB) formation and our previous characterization of Y475 as a highly nucleophilic tyrosine site. We used a combination of immunofluorescence, affinity proteomics, and global phosphoproteomics to discover that perturbation of Y475, located in the poorly defined YjeF_N domain, alters EDC3 phosphorylation state (phospho-S161 and -S131 specifically) and PPI networks in cells. The phospho-deficient mutant form of EDC3 (Y475F) was able to effectively rescue a PB-deficient phenotype observed in EDC3 knockout cells and establish the functional relevance of this key arsenic-responsive site. Future work will evaluate additional stress-responsive tyrosine and lysine sites identified from our chemical proteomics to further investigate regulation of ribonucleoprotein composition and formation in the cellular stress response.
STAR METHODS
RESOURCE AVAILABILITY
LEAD CONTACT
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Prof. Ku-Lung Hsu (kenhsu@virginia.edu).
MATERIALS AVAILABILITY
Experimental materials generated for this manuscript are available upon reasonable request.
DATA AND CODE AVAILABILITY
Proteomics data have been deposited at ProteomeXchange via the PRIDE database (http://www.proteomexchange.org) and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| EDC4 | Cell Signaling Technologies | cat#2548S |
| G3BP1 | Santa Cruz Biotechnology | cat#sc-365338 |
| EDC3 | Cell Signaling Technologies | cat#14495 |
| FLAG-tag | Cell Signaling Technologies, Sigma Aldrich | cat#8146, cat#SAB4301135 |
| HA-tag | Invitrogen | cat#26183 |
| GFP | GenScript | cat#A01388 |
| GAPDH | Cell Signaling Technologies | cat#2118 |
| Alexa Fluor® 488 Conjugate Anti-Rabbit IgG (H+L) F(ab’)2 Fragment | Cell Signaling Technologies | cat#4412S |
| IgG (H+L) Goat anti-Mouse, DyLight™ 550 | Invitrogen | cat#84540 |
| Goat anti-Mouse IgG (H+L) Secondary Antibody, DyLight™ 650 | Invitrogen | cat#84545 |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, DyLight™ 550 | Invitrogen | cat#84541 |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, DyLight™ 650 | Invitrogen | cat#84546 |
| Bacterial and virus strains | ||
| XL1-Blue | Agilent | cat#200228 |
| Chemicals, peptides, and recombinant proteins | ||
| Polyethylenimine | Polysciences | cat#24765-1 |
| PBS | Cytiva | cat#SH30378.03 |
| Sodium (meta)arsenite | Sigma-Aldrich | cat#S7400 |
| Hydrogen peroxide | Macron | cat#MK5240120 |
| HHS-465 | Hahm, et al.26 | N/A |
| DAPI | Tocris | cat#5748 |
| Lipofectamine 2000 | Thermo Fisher Scientific | cat#11668019 |
| QuickExtract DNA Extraction Solution | Lucigen Corporation | cat#QE09050 |
| Protease and Phosphatase Inhibitor Mini Tablets | Pierce | cat#A32959 |
| μ Columns | Miltenyi Biotec | cat#130-042-701 |
| Trypsin/Lys-C (LC-MS grade) | Promega | cat#V5073 |
| Dithiothreitol | Fisher bioreagents | cat#BP172-5 |
| Iodoacetamide | Thermo Fisher Scientific | cat#AC122270250 |
| Formaldehyde solution | Sigma-Aldrich | cat#252549 |
| Triton™ X-100 | Sigma-Aldrich | cat#X100 |
| Tween™ 20 | Thermo Fisher Scientific | cat#bp337500 |
| VECTASHIELD® HardSet™ Antifade Mounting Medium | Vector Laboratories | cat#H-1400-10 |
| VECTASHIELD Vibrance® Antifade Mounting Medium | Vector Laboratories | cat#H-1700 |
| Emetine dihydrochloride | Abcam | cat#ab141478 |
| Desthiobiotin-PEG-3-azide | Hahm, et al.26 | N/A |
| Critical commercial assays | ||
| μMACS™ DYKDDDDK Isolation Kit | Miltenyi Biotec | cat#130-101-591 |
| High-Select™ TiO2 Phosphopeptide Enrichment Kit | Thermo Scientific | cat#A32993 |
| QIAprep Spin Miniprep Kit | Qiagen | cat#27106X4 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | cat#CRL-3216 |
| HeLa | Gift from Dr. Ralph Kleiner (Princeton University) | N/A |
| HEK293T EDC3-KO | This paper | N/A |
| Oligonucleotides | ||
| CACCGTCAGGGAAGAGTGTCAGCTG (Oligo 1 fWd) | Integrated DNA Technologies | N/A |
| AAACCAGCTGACACTCTTCCCTGAC (Oligo 1 rev) | Integrated DNA Technologies | N/A |
| CACCGAACATGGCTACAGATTGGCT (Oligo 2 fWd) | Integrated DNA Technologies | N/A |
| AAACAGCCAATCTGTAGCCATGTTC (Oligo 2 rev) | Integrated DNA Technologies | N/A |
| ACACTTGGTACAGAGCCTGAT (Surveyor oligo 1 fWd) | Integrated DNA Technologies | N/A |
| AAGGGGAGGCCAAGGAATGAA (Surveyor oligo 1 rev) | Integrated DNA Technologies | N/A |
| Recombinant DNA | ||
| EDC3 in pcDNA3.1+/C-(K)DYK | Hahm, et al.26 | N/A |
| EDC3 Y475F in pcDNA3.1+/C-(K)DYK | Hahm, et al.26 | N/A |
| pcDNA3-HA-14-3-3 beta | pcDNA3-HA-14-3-3 beta was a gift from Michael Yaffe (Addgene plasmid # 13270; http://n2t.net/addgene:13270; RRID:Addgene 13270) | cat#13270 |
| HA-14-3-3 zeta | HA-14-3-3 zeta was a gift from FENG-QIAN Li & Ken-Ichi Takemaru (Addgene plasmid # 116888; http://n2t.net/addgene:116888; RRID:Addgene 116888) | cat#116888 |
| HA-14-3-3 epsilon | HA-14-3-3 epsilon was a gift from FENG-QIAN Li & Ken-Ichi Takemaru (Addgene plasmid # 116886; http://n2t.net/addgene:116886; RRID:Addgene 116886) | cat#116886 |
| HA-14-3-3 eta | HA-14-3-3 eta was a gift from FENG-QIAN Li & Ken-Ichi Takemaru (Addgene plasmid # 116887; http://n2t.net/addgene:116887; RRID:Addgene 116887) | cat#116887 |
| BFP-Dcp1a | BFP-Dcp1a was a gift from Gia Voeltz (Addgene plasmid # 153973; http://n2t.net/addgene:153973; RRID:Addgene 153973) | cat#153973 |
| GFP-Dcp1b | GFP-Dcp1b was a gift from Gia Voeltz (Addgene plasmid # 153976; http://n2t.net/addgene:153976; RRID:Addgene 153976) | cat#153976 |
| Software and algorithms | ||
| Custom LC-MS Bioinformatics Pipeline | Hahm, et al.26 | N/A |
| Skyline | MacCoss Lab Software | https://skyline.ms/project/home/software/Skyline/begin.view |
| Byonic | Protein Metrics | https://proteinmetrics.com/byos/ |
| Fiji | ImageJ | https://github.com/fiji/fiji |
| GraphPad PRISM | GraphPad | https://www.graphpad.com/ |
| RawConverter | RawConverter | http://fields.scripps.edu/rawconv/ |
| Adobe Illustrator | Adobe | https://www.adobe.com/products/illustrator.html |
| Microsoft Office | Microsoft | https://www.office.com/ |
| Image Lab | Bio-rad | https://www.bio-rad.com/en-us/product/image-lab-software?ID=KRE6P5E8Z |
| MestreNova | Mestrelab Research | https://mestrelab.com/download/mnova/ |
| Leica Applications Suite | Leica Microsystems | https://www.leica-microsystems.com/products/microscope-software/p/leica-application-suite/ |
| Deposited data | ||
| 10.6019/PXD038010 | This paper | PXD038010 |
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Cell culture
HEK293T cells (female origin) were grown in Dulbecco’s Modified Eagle Media (DMEM, high glucose, no l-glutamine, with sodium pyruvate; GE Healthcare, cat#SH30285.01) supplemented with sterilized 10% fetal bovine serum (FBS, US Source, Omega Scientific, cat#FB01) and 1% l-glutamine (Caisson Labs, cat#GLL01). HeLa cells were a generous gift from Dr. Ralph Kleiner at Princeton University. HeLa cells (female origin) were grown in DMEM with FBS, L-glutamine, and 1% penicillin-streptomycin (Sigma-Aldrich, cat#P0781). Cultures were seeded at 400,000 cells and grown in 10 mL of media in 10 cm BioLite dishes (Fisher Scientific, cat#12556002) or 1e6 cells in 20 mL of media in 15 cm BioLite dishes (CELLTREAT®, cat#229651) and were experimented on or collected at around 90% confluency. Prior to treatments, cells were washed twice with warm PBS and exchanged with relevant serum-free media (supplemented with 1% l-glutamine) for at least 30 minutes at 37 °C with 5% CO2. For microscopy experiments, cultures were seeded at 150,000 cells in 3 mL of media in 6-well BioLite dishes (Fisher Scientific, cat#12556004). Cells never exceeded twenty passages and were grown at 37°C with 5% CO2. For collection, media was aspirated and cells were washed once with cold PBS before scraping from plates. Cells were centrifuged at 500 × g at 4 °C for 5 min and supernatant was removed. Cell pellets were resuspended in cold PBS (1 mL) and centrifuged at 1,400 × g at 4 °C for 3 min. Supernatant was again removed and pellets were then snap-frozen in liquid nitrogen and stored at −80 °C until used.
SILAC cell culture
SILAC HEK293T cells were grown in 10mL of ‘heavy’ or ‘light’ DMEM in 10cm BioLite dishes at 37°C with 5% CO2. DMEM was supplemented with 10% dialyzed FBS (Omega Scientific, cat#FB-03), 1% l-glutamine, and isotopically labelled amino acids. ‘Light’ media was supplemented with 100 μg/mL of both l-arginine and l-lysine. ‘Heavy’ media was supplemented with 100 μg/mL of both [13C615N4] l-arginine and [13C615N2] l-lysine. SILAC HeLa cells were grown in the same media with added 1% penicillin-streptomycin. Labelled amino acids were incorporated into SILAC cells for at least 5 passages prior to cell experimentation.
Generation of EDC3 KO cells using CRISPR-Cas9
The guide sequences were created using the human EDC3 exon 2 sequence as a starting point. Two guide locations were chosen based on various targets and on- and off-target scores generated by the Benchling server (https://www.benchling.com/). The sequences of guide 1 and guide 2 oligoes are Guide 1 oligo forward CACCGTCAGGGAAGAGTGTCAGCTG, Guide 1 oligo reverse AAACCAGCTGACACTCTTCCCTGAC, Guide 2 oligo forward CACCGAACATGGCTACAGATTGGCT and Guide 2 oligo reverse AAACAGCCAATCTGTAGCCATGTTC. For generation of guide, 413 guide vector was digested using BSMB1 enzyme. In the next step, the oligos for guide 1 and 2 were annealed and ligated into the digested P413 vector using T4 ligase. The sequences of each guide vectors were then confirmed by sanger sequencing. To generate the EDC3 knockout cell line, the HEK293T cells were transfected with the respective guide vectors using Lipofectamine 2000 (ThermoFisher). The transfected cells were selected using 4 μg/ml puromycin for 5 days. To have single cell clones, transfected cells were diluted and a single cell was seeded into a 96-well plate. The single cell genomic DNA was obtained using a quick extract buffer based on the manufacture protocol (Lucigen, cat#QE09050). The presence of any genomic alteration in EDC3 exon 2 was investigated using the Surveyor assay with the primers listed below (forward primer: ACACTTGGTACAGAGCCTGAT and reverse primer: AAGGGGAGGCCAAGGAATGAA. In brief, the EDC3 region was amplified and a DNA heteroduplex was formed. The heteroduplex DNA was cleaved using 1 unit of T7 endonuclease for 1 hour. The possible cleavage at hEDC3 was studied using a 1% agarose gel. To confirm knockout condition at protein level, a western blot experiment was carried out with selected positive clones.
METHOD DETAILS
Plasmid constructs
Information about the EDC3 and EDC3 Y475F plasmid constructs can be found as previously described26. Information about BFP-DCP1A and GFP-DCP1B plasmids can be found as previously described51. The EDC3 plasmid was obtained from GenScript in pcDNA3.1+/C-(K)DYK. The EDC3-Y475F plasmid was a custom gene synthesis obtained from Gene Universal in pcDNA3.1(+) with a C-terminal FLAG tag. The following agar stabs were obtained from Addgene (backbone in parentheses): HA-14-3-3 beta (pcDNA3), HA-14-3-3 zeta (pCS2+HA), HA-14-3-3 epsilon (pCS2+HA), HA-14-3-3 eta (pCS2+HA), GFP-Dcp1b (pAcGFP-c1), and BFP-Dcp1a (pAcGFP-c1). Gene sequences were confirmed using a commercial Sanger sequencing service (Azenta Life Sciences).
Transient transfection
Transient transfection of recombinant protein was performed as previously described26. In brief, for a 10 cm dish, cells were transfected with 2.6 μg of plasmid, 20 μL of polyethylenimine (PEI), and 600 μL of serum-free media for 48 hours at 37 °C with 5% CO2. For a 3 cm dish, corresponding amounts of plasmid, PEI, and serum-free media were scaled for 1 μg of plasmid. For the dual recombinant expression experiments, 0.5 μg of each plasmid was used to reach a total of 1 μg of plasmid. Mock samples received only serum-free media and PEI.
Western blotting
Western blot analysis of protein expression was performed as previously described26. In brief, soluble lysates were separated by SDS-PAGE (Bio-rad, cat#5678095) at 150V for 60 minutes. Gels were subsequently removed from cassettes and transferred to nitrocellulose blots (Bio-rad, cat#1704271), which were shaken in blocking solution (3% BSA in TBST, 30 mL) for one hour at RT. Blocking solution was removed and blots were incubated with primary antibody solution shaken overnight at 4°C. Blots were washed with TBST with shaking at RT five times (20 mL, 5 min) before incubating with secondary antibody in TBST (30 mL) shaking for one hour at RT. TBST washes were repeated and blots were imaged using the ChemiDoc MP Imaging System.
Immunoprecipitation
Cell pellets were resuspended in “lysis buffer” (50mM Tris-HCl, 150mM NaCl, 1% NP-40, Pierce Protease and Phosphatase Inhibitor Mini Tablets (cat#A32959) in purified water at pH 7.4) and homogenized before incubating on ice for 20 min. Lysates were centrifuged at 16,000 × g for 30 min at 4 °C and the supernatant was normalized to 2 mg/mL in lysis buffer. 250 μL of soluble lysate fraction was incubated with 50 μL of μMACS™ DYKDDDDK Isolation Kit anti-FLAG beads for 30 minutes at 4 °C. The lysate-bead mix was added to a μColumn (Miltenyi Biotec) that had been prewashed with lysis buffer and was attached to a magnetic MACS MultiStand. Beads were washed three times with lysis buffer and five times with 50 mM Tris-HCl/150 mM NaCl in water (all at 4 °C) and incubated with 20 μL of boiling elution buffer (from kit) for five minutes followed by addition of 50 μL of boiling elution buffer and elution (this elution was repeated twice); all elution fractions were combined.
Filter-aided sample preparation (FASP)
75 μL of both heavy and light lysate fractions (elution fractions for IP) were combined in a 10kDa cutoff spin column (Microcon) and centrifuged for 15 min at 14,000×G (“spin”). Samples were spun with 300 μL of 6 M urea/25 mM ammonium bicarbonate (“UA”), followed by two additional spins with 200 μL of UA. Proteins were incubated with dithiothreitol (5 mM, 60 μL, 30 min, 56 °C), diluted with 200 μL of UA and spun (repeated twice), and incubated with iodoacetamide (50 mM, 100 μL, 20 mins RT, in dark) before diluting with 100 μL of UA and spinning through (UA was repeated once more). Proteins on columns were spun with 100 μL of 25 mM ammonium bicarbonate (“ambic”) three times total and columns were incubated with trypsin/Lys-C (Promega, Mass Spec Grade, 5 μg in 40 μL ambic) for 15 hours at 37°C. Fractions were then spun (10 min) and collected in a lo-bind tube with 40 μL of ambic followed by an additional spin and collection with 50 μL of a 500 mM NaCl aqueous solution. Peptides were acidified with acetic acid (Optima™ grade, Fisher Chemical) and desalted using C18 stage-tip clean up protocol as previously described26,41,54. Internal angiotensin and vasoactive peptide standards were added, peptides were snap-frozen, dried, and stored at −80 °C until usage.
Preparation of SILAC proteomes for LC-MS/MS
SILAC cell proteomes were fractionated and normalized to 2.3 mg/mL in PBS. ‘Heavy’ and ‘light’ proteomes were aliquoted (427 μL) and probe-modified proteins were conjugated to desthiobiotin-PEG-3-azide using CuAAC, proteomes were mixed and proteins were extracted, reduced, alkylated, enriched using avidin-agarose beads, trypsinized into peptides, and prepared for nano-electrospray ionization-LC-MS/MS analysis as previously described26.
TiO2 affinity chromatography phosphoproteomics
SILAC HEK293T cells were transiently transfected, treated with PBS or arsenic, and pelleted as previously described. Cell pellets were resuspended in 6 M/25 mM urea/ammonium bicarbonate with protease and phosphatase inhibitor tablet (Pierce, cat#A32959), lysed via sonication, and centrifuged at 100,000 × g at 4 °C for 45 min. The supernatant was collected and 1 mg of soluble proteome was reduced, alkylated, and trypsin digested as previously described26. Tryptic peptides were transferred to a bio-spin column (BIO-RAD, cat#7326207), acidified with 16 μL of formic acid, and dried. Peptides were resuspended in 50 μL of water with 0.1% formic acid and 20 μL were desalted using a C18 stage-tip clean up protocol41. Desalted peptides were dried and phosphopeptides were enriched using the High-Select™ TiO2 Phosphopeptide Enrichment Kit (Thermo Scientific, cat#A32993) following manufacturer protocols. Phosphopeptides were dried before resuspension and analysis using LC-MS as written above.
Immunofluorescence
HEK293T or HeLa cells were grown in 6-well BioLite dishes with 3 mL to roughly 95% confluency on 22 mm circular glass cover slips (Carolina Biological, cat#633035). The glass cover slips were sterilized before use by incubating with methanol (Optima™ grade, Fisher Scientific, cat#A454SK) at room temperature for at least 15 min and allowing the slips to air-dry, all in a sterile environment. All cells were washed twice with warm PBS (2 mL) and incubated for 30 min with serum-free or complete media prior to any treatments. After treatments, cells were washed once with warm PBS (2 mL), the PBS was aspirated, a 4% (wt/v%) formaldehyde (Sigma-Aldrich, cat#252549) in PBS solution was added (1 mL), and cells were incubated in this solution at RT for 20 min under a chemical hood. Following formaldehyde incubation, the solution was aspirated from the wells and the cells were washed with PBS (2 mL) at RT while shaking for 3 minutes. PBS was aspirated and this process was repeated. Triton™ X-100 (Sigma-Aldrich, cat#X100) was diluted in PBS to 0.125% (v/v%) and this solution (1 mL) was incubated with cells for 5 min at room temperature while shaking. The same washing procedure was repeated. See following sections for details on blocking and staining cells. Between the primary and secondary antibody incubations and after the secondary antibody incubation, the general washing procedure was repeated for a total of 5 times, except cells were washed with a 0.1% (v/v%) solution of Tween™ 20 (Thermo Fisher Scientific, cat#bp337500) diluted in PBS (2 mL, PBST). After the final wash, glass slips were mounted on frosted glass slides (Sigma-Aldrich, cat#S8400) using antifade mounting media (VECTASHIELD® Vibrance™, cat#H-1700, 40 μL) and allowed to set overnight in the dark. The following morning, the outer edges of the mounted cover slips were coated with a layer of nail polish (Seche, Clear Base) to prevent the samples from drying out, and to preserve brightness. Samples were blinded and imaged on a Leica SP5X Confocal/Spectral Imaging Microscopy System (equipped with UV, argon-ion, and white-light lasers) using the 63X (oil) objective lens. Each laser was used in a separate analysis channel to excite and detect signals from a singular image. The UV laser (405 nm) was used to excite both DAPI and BFP-DCP1A. The argon-ion laser was used to excite the Alexa Fluor® 488 Conjugate Anti-Rabbit IgG (H+L) F(ab’)2 Fragment (Cell Signaling Technology, cat#4412S) and GFP-DCP1B at 488 nm. The white-light laser was used to excite both the IgG (H+L) Goat anti-Mouse, DyLight™ 550 (Invitrogen, cat#84540) at 550 nm and the DyLight™ 650 Goat polyclonal anti-mouse IgG (H+L) Secondary Antibody (Invitrogen, cat#84545) at 650nm. A z-stack of five images was used to capture the cells throughout the total perceived thickness.
P-body staining
A solution containing 5% (wt/v%) bovine serum albumin dissolved in PBS was added to cells and incubated for 1 h at RT while shaking. The solution was aspirated and a new solution containing 5% (wt/v%) bovine serum albumin and EDC4/GE-1 rabbit antibody (Cell Signaling Technology, cat#2548S; 1:400) dissolved in PBS was incubated with the cells at 4 °C overnight with shaking. The cells were washed and a solution containing Alexa Fluor® 488 Conjugate Anti-Rabbit IgG (H+L) F(ab’)2 Fragment (Cell Signaling Technology, cat#4412S; 1:1,000) and DAPI (Tocris, 1:2,000) dissolved in PBST was incubated with cells (1 mL) at 37 °C with 5% CO2 for 1 h. The cells were washed and mounted as described in the above protocols.
Stress granule staining
A solution containing 5% (wt/v%) bovine serum albumin dissolved in PBS was added to cells and incubated for 1 h at RT while shaking. The solution was aspirated and a new solution containing 5% (wt/v%) bovine serum albumin and G3BP1 mouse antibody (Santa Cruz, cat#sc-365338, 1:500) dissolved in PBS was incubated with the cells at 4 °C overnight with shaking. The cells were washed and a solution containing IgG (H+L) Goat anti-Mouse, DyLight™ 550 (Invitrogen, cat#84540, 1:250) and DAPI (Tocris, 1:2,000) dissolved in PBST was incubated with cells (1 mL) at 37 °C with 5% CO2 for 1 h. The cells were washed and mounted as described in the above protocols.
Anti-FLAG staining
The same protocol was followed as stated above for EDC4 except the antibody solutions and times used were as follows: primary antibody solution: 5% (wt/v%) bovine serum albumin, DYKDDDDK Tag mouse mAb, (Cell Signaling Technology, cat#8146, 1:1,000), and EDC4/GE-1 rabbit antibody (Cell Signaling Technology, cat#2548S; 1:400) dissolved in PBS were added to wells (1 mL) and cells were shaken overnight at 4°C; secondary antibody solution: Alexa Fluor® 488 Conjugate Anti-Rabbit IgG (H+L) F(ab’)2 Fragment (Cell Signaling Technology, cat#4412S; 1:1,000), Goat anti-Mouse IgG (H+L) Secondary Antibody, DyLight 650 (Invitrogen, cat#84545, 1:500) and DAPI (Tocris, 1:2,000) dissolved in PBST was incubated with cells (1 mL) at 37°C with 5% CO2 for 1 hour.
Anti-EDC3 staining
The same immunostaining protocol was followed above for EDC3 except the primary stain contained 5% (wt/v%) bovine serum albumin and EDC3 rabbit mAb (Cell Signaling Technology, cat#14495; 1:1,000) and cells were shaken overnight at 4°C.
Anti-HA tag staining
The same immunostaining protocol was followed above for HA-tagged proteins except the primary stain contained HA Tag Monoclonal Antibody (Invitrogen, cat#26183, 1:250) and cells were shaken overnight at 4°C.
Anti-GAPDH staining
The same immunostaining protocol was followed above for staining of endogenous GAPDH, except the primary stain contained GAPDH Monoclonal Antibody (Cell Signaling Technologies, cat#2118, 1:100) and cells were shaken overnight at 4 °C.
RNA granule induction methods
Media amounts are 10 mL for 10 cm dishes, 20 mL for 15 cm dishes, and 3 mL for 6-well dishes unless otherwise specified. PBS washes are 5 mL for 10 cm dishes, 10 mL for 15 cm dishes, and 2 mL for 6-well dishes unless otherwise specified. For non-probe-treated cells, DMEM supplemented with FBS was used in place of serum-free DMEM.
Arsenic:
After serum-free media exchange, cells were washed twice with warm PBS and incubated with either PBS or sodium (meta)arsenite (Sigma-Aldrich, cat#S7400; as a 1000x PBS stock; 1 mM final) in DMEM for 45 min at 37 °C with 5% CO2.
Glucose deprivation:
After serum-free media exchange, cells were washed twice with warm PBS and incubated with either DMEM or glucose free DMEM (Thermo Fisher Scientific, cat#11966025) supplemented 10% FBS and 1% l-glutamine, at 37 °C with 5% CO2 for 15 min.
Hydrogen Peroxide:
After serum-free media exchange, cells were washed twice with warm PBS and incubated with either water or hydrogen peroxide (200 μM in water as a 1000x stock, Macron, cat#MK5240120) in DMEM at 37 °C with 5% CO2 for 15 min.
Emetine:
After serum-free media exchange, cells were washed twice with warm PBS and incubated with either water or emetine dihydrochloride (50 nM in water as a 1000x stock, Abcam, cat#ab141478) in DMEM at 37 °C with 5% CO2 for 2 h.
LC-MS data collection
Raw LC-MS data was collected as previously described for probe-modified peptides.26 For IP and unenriched proteomics experiments, a top-10 DDMS method was performed over 180 min in the positive mode. A full MS was performed at 70,000 resolution, with an AGC target of 1e6, a maximum IT of 100 ms, a scan range of 350 to 2,000 m/z, centroid, with a dynamic exclusion time of 30 seconds. A subsequent DDMS was performed at 17,500 resolution, with an AGC target of 1e5, a maximum IT of 100ms, a loop count of 10, a TopN of 10, an isolation window of 1.5m/z, a fixed first mass of 80.0m/z, and a normalized collision energy of 26, centroid. Either an EASY-nLC™ 1200 System or a Dionex Ultimate 3000 RSLCnano System was used to apply the following gradient using previously described mobile phases (%B) at a flowrate of 300nL/min: 0–8 min. (1%), 8–107 min. (21%), 107–142 min. (38%), 142–143 min. (80%), 143–149 min. (80%), 149–150 min. (1%), 150–180 min. (1%)26. A data-independent acquisition (parallel reaction monitoring) was utilized to generate quality MS2 spectra for the following peptides: DCP1A: SASPYHGFTIVNR - m/z: 483.5809, 486.9170 (heavy) +3 charge state, ASSPSPLTIGTPESQR - m/z: 814.4179, 819.4221 (heavy) +2 charge state; DCP1B: DISLAALQR - m/z: 493.7851, 498.7892 (heavy) +2 charge state, APTSVTPVAPGK - m/z: 562.8191, 566.8262 (heavy) +2 charge state; EDC4: ALQDVQIR - m/z: 471.7720, 476.7761 (heavy) +2 charge state. The method utilized a 400 ms injection time, an isolation window of 2.0 m/z, and a normalized collision energy of 35.
QUANTIFICATION AND STATISTICAL ANALYSIS
Chemical proteomics.
Identification and analysis of peptides, proteins, and MS1s using LC-MS raw data was accomplished using bioinformatics software and quality control parameter protocols as previously described26. For IP, unenriched, and phosphoproteomics experiments, the following EDC3 Y475F peptide was added to the human proteome database file used in the Byonic™ searches: IFCLDIGIPQQVFQEVGINYHSPFGCK, named ‘EDC3_Y475F_HUMAN’. For IP, unenriched, and phosphoproteomics experiments, data were searched were the following parameters: cleavage after lysine and arginine (C-terminal, fully specific), max 2 missed cleavages, precursor mass tolerance of 10 ppm, a fragment mass tolerance of 50 ppm, carbamidomethyl (+57.021464, C, fixed), oxidation (+15.994915, M, common1), phospho (+79.966331, H, S, T, Y, rare1), with no more than 2 total common and rare mods. All SR values were capped at a maximum value of 20. Median protein values were calculated from quality peptide matches and an average of medians across multiple bioreplicates was used as the final values.
PB/SG Counting.
All slides were blinded for imaging. Leica SP5X images were analyzed using Fiji (ImageJ) software. Custom macros were written for analysis and can be obtained upon request. To elaborate, images were split into channels based on the different laser wavelengths measured. Image stacks were then combined using ‘Z-Project’ with an average intensity parameter and a threshold was applied to exclude noise based on the negative control (no primary antibody). The ‘Analyze Particles’ function was used with relevant size and circularity constraints (0.85–1.00) to count the number of foci, with total PBs having a diameter => 150 nm. For stress granules, the same general counting protocol was used (with smaller diameter), except the circularity constraint was lowered to 0.50–1.00. The number of foci was normalized to the number of cells (defined by a DAPI stain count) in each image, and the number of foci per cell was averaged across technical replicates. Every sample has at least three technical replicates. Data was entered manually into a Microsoft Excel file. For plots, the number of foci per cell was normalized to 2 in the basal or unstressed condition.
Colocalization.
For determining colocalization of different markers by immunofluorescence microscopy, the ‘Coloc 2’ function in ImageJ was utilized. Each image was Z-projected using the ‘Sum Slices’ algorithm and the relevant channels were isolated using the ‘Split Channels’ function. A Costes threshold regression was utilized with a PSF of 10.0. The Pearson’s R value (no threshold) was then extracted and used for analysis. At least 100 cells (as determined by DAPI count) per sample condition were analyzed to determine Pearson’s R values. For the BFP-DCP1A-containing samples, a GAPDH stain was used in place of DAPI to count cells, as tagBFP and DAPI have overlapping excitation spectra.
Phosphopeptide analysis.
Phosphopeptide data was exported from Skyline and peptides were filtered by an idotp > 0.8, a |ppm error| < 5, and a SR 0.5 < x < 2.0 for the WT/WT arsenic mixing condition. The YF/WT SRs were then normalized to the relevant WT/WT SRs for the corresponding peptide. Peptides were then filtered for a 33% change in SR in either direction (x < 0.667 or x > 1.33) and a P-value was calculated using the T.TEST(SR1:SR2, AVG1:AVG2, 2, 3) function in excel comparing the independent bioreplicates to the overall dataset averages as a two-tailed t-test assuming unequal variances. Max SR values were capped at 20 and minimum SRs had to be greater than zero.
Statistics.
GraphPad PRISM (ver. 7) software was used to perform statistical tests on the generated plots. Outliers were removed via Grubbs test (α = 0.05) unless otherwise stated.
Venn diagrams.
Venn diagrams were first generated with InteractiVenn online tool (http://www.interactivenn.net/) to find overlap numbers55. Subsequent Venn diagrams were generated using Meta-Chart’s Venn Diagram Maker Online (https://www.meta-chart.com/venn).
Bar plots.
Unless otherwise stated, all bar plots are shown as the mean +/− SEM for at least two independent bioreplicates.
Supplementary Material
Table S1. All probe modified protein identifications as determined by mass spectrometry, related to Figures 1 and 2
Table S2. List of RISKYs and Hyper RISKYs along with domain enrichment analysis, related to Figure 1 and 2
Table S3. Unenriched proteomics data, related to Figure 2
Table S5. Immunoprecipitation proteomics data, related to Figure 4
Table S6. EDC3 IP binding partner proteomics data analysis, related to Figure 4
Table S7. EDC3 IP binding partner proteomics data analysis all quantified peptides for calculation, related to Figure 4
Table S8. Phosphoproteomics data analysis, related to Figure 5
Highlights.
SuTEx chemoproteomics identifies stress-responsive Y/K sites in RNA granule biology
EDC3 Y475 regulates P-body (PB) formation in cellular response to arsenic
Mutagenesis of Y475 affects EDC3 phosphorylation state and interactome under stress
EDC3 Y475F rescues PB-deficient KO cells and shows enhanced DCP1A colocalization
ACKNOWLEDGMENTS
We thank Mark Ross and all members of the Hsu Lab for helpful discussions and review of the manuscript. This work was supported by the National Institutes of Health grant nos. DA043571 (K.-L.H.), GM144472 (K.-L.H.), CA009109 (A.L.B.), University of Virginia Cancer Center (NCI Cancer Center Support Grant No. 5P30CA044579-27 to K.-L.H.), the Robbins Family MRA Young Investigator Award from the Melanoma Research Alliance (http://doi.org/10.48050/pc.gr.80540 to K.-L.H.) and the Mark Foundation for Cancer Research (Emerging Leader Award to K.-L.H). We acknowledge the Keck Center for Cellular Imaging for the usage of the Leica SP5X microscopy system (PI:AP, NIH-RR025616). We thank Adam Libby for assistance in synthesis of SuTEx probes. We thank Heung-Sik Hahm and Emmanuel Toroitich for the original SuTEx inspiration. A special thanks to Timothy B. Ware and various members of Hsu lab for helping to blind the microscopy studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.Sheth U, and Parker R (2003). Decapping and decay of messenger RNA occur in cytoplasmic processing bodies. Science 300, 805–808. 10.1126/science.1082320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collier NC, and Schlesinger MJ (1986). The dynamic state of heat shock proteins in chicken embryo fibroblasts. J Cell Biol 103, 1495–1507. 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nover L, Scharf KD, and Neumann D (1989). Cytoplasmic heat shock granules are formed from precursor particles and are associated with a specific set of mRNAs. Mol Cell Biol 9, 1298–1308. 10.1128/mcb.9.3.1298-1308.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ling SH, Decker CJ, Walsh MA, She M, Parker R, and Song H (2008). Crystal structure of human Edc3 and its functional implications. Mol Cell Biol 28, 5965–5976. 10.1128/MCB.00761-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luo Y, Na Z, and Slavoff SA (2018). P-Bodies: Composition, Properties, and Functions. Biochemistry 57, 2424–2431. 10.1021/acs.biochem.7b01162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kedersha N, and Anderson P (2007). Mammalian Stress Granules and Processing Bodies. In Translation Initiation: Cell Biology, High - Throughput Methods, and Chemical - Based Approaches, pp. 61–81. 10.1016/s0076-6879(07)31005-7. [DOI] [PubMed] [Google Scholar]
- 7.Lykke-Andersen J (2002). Identification of a human decapping complex associated with hUpf proteins in nonsense-mediated decay. Mol Cell Biol 22, 8114–8121. 10.1128/MCB.22.23.8114-8121.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aizer A, Kalo A, Kafri P, Shraga A, Ben-Yishay R, Jacob A, Kinor N, and Shav-Tal Y (2014). Quantifying mRNA targeting to P-bodies in living human cells reveals their dual role in mRNA decay and storage. J Cell Sci 127, 4443–4456. 10.1242/jcs.152975. [DOI] [PubMed] [Google Scholar]
- 9.Hubstenberger A, Courel M, Benard M, Souquere S, Ernoult-Lange M, Chouaib R, Yi Z, Morlot JB, Munier A, Fradet M, et al. (2017). P-Body Purification Reveals the Condensation of Repressed mRNA Regulons. Mol Cell 68, 144–157 e145. 10.1016/j.molcel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Standart N, and Weil D (2018). P-Bodies: Cytosolic Droplets for Coordinated mRNA Storage. Trends Genet 34, 612–626. 10.1016/j.tig.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Seto E, Yoshida-Sugitani R, Kobayashi T, and Toyama-Sorimachi N (2015). The Assembly of EDC4 and Dcp1a into Processing Bodies Is Critical for the Translational Regulation of IL-6. PLoS One 10, e0123223. 10.1371/journal.pone.0123223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davidson VJ, Patel D, Flanigan R, Gupta GN, and Foreman KE (2017). Emetine reduces the effective dose of cisplatin or carboplatin required to inhibit bladder cancer cell proliferation. Bladder 4, e31. 10.14440/bladder.2017.570. [DOI] [Google Scholar]
- 13.Foreman KE, Jesse JN 3rd, Kuo PC, and Gupta GN (2014). Emetine dihydrochloride: a novel therapy for bladder cancer. J Urol 191, 502–509. 10.1016/j.juro.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 14.Moller M, and Wink M (2007). Characteristics of apoptosis induction by the alkaloid emetine in human tumour cell lines. Planta Med 73, 1389–1396. 10.1055/s-2007-990229. [DOI] [PubMed] [Google Scholar]
- 15.Kanakamani S, Suresh PS, and Venkatesh T (2021). Regulation of processing bodies: From viruses to cancer epigenetic machinery. Cell Biol Int 45, 708–719. 10.1002/cbin.11527. [DOI] [PubMed] [Google Scholar]
- 16.Anderson P, Kedersha N, and Ivanov P (2015). Stress granules, P-bodies and cancer. Biochim Biophys Acta 1849, 861–870. 10.1016/j.bbagrm.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bearss JJ, Padi SK, Singh N, Cardo-Vila M, Song JH, Mouneimne G, Fernandes N, Li Y, Harter MR, Gard JM, et al. (2021). EDC3 phosphorylation regulates growth and invasion through controlling P-body formation and dynamics. EMBO Rep 22, e50835. 10.15252/embr.202050835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tritschler F, Eulalio A, Truffault V, Hartmann MD, Helms S, Schmidt S, Coles M, Izaurralde E, and Weichenrieder O (2007). A divergent Sm fold in EDC3 proteins mediates DCP1 binding and P-body targeting. Mol Cell Biol 27, 8600–8611. 10.1128/MCB.01506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fromm SA, Truffault V, Kamenz J, Braun JE, Hoffmann NA, Izaurralde E, and Sprangers R (2012). The structural basis of Edc3- and Scd6-mediated activation of the Dcp1:Dcp2 mRNA decapping complex. EMBO J 31, 279–290. 10.1038/emboj.2011.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Decker CJ, Teixeira D, and Parker R (2007). Edc3p and a glutamine/asparagine-rich domain of Lsm4p function in processing body assembly in Saccharomyces cerevisiae. J Cell Biol 179, 437–449. 10.1083/jcb.200704147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schutz S, Noldeke ER, and Sprangers R (2017). A synergistic network of interactions promotes the formation of in vitro processing bodies and protects mRNA against decapping. Nucleic Acids Res 45, 6911–6922. 10.1093/nar/gkx353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roy R, Das G, Kuttanda IA, Bhatter N, and Rajyaguru PI (2022). Low complexity RGG-motif sequence is required for Processing body (P-body) disassembly. Nat Commun 13, 2077. 10.1038/s41467-022-29715-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayache J, Benard M, Ernoult-Lange M, Minshall N, Standart N, Kress M, and Weil D (2015). P-body assembly requires DDX6 repression complexes rather than decay or Ataxin2/2L complexes. Mol Biol Cell 26, 2579–2595. 10.1091/mbc.E15-03-0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tibble RW, Depaix A, Kowalska J, Jemielity J, and Gross JD (2021). Biomolecular condensates amplify mRNA decapping by biasing enzyme conformation. Nat Chem Biol 17, 615–623. 10.1038/s41589-021-00774-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmor-Kollet H, Siany A, Kedersha N, Knafo N, Rivkin N, Danino YM, Moens TG, Olender T, Sheban D, Cohen N, et al. (2020). Spatiotemporal Proteomic Analysis of Stress Granule Disassembly Using APEX Reveals Regulation by SUMOylation and Links to ALS Pathogenesis. Mol Cell 80, 876–891 e876. 10.1016/j.molcel.2020.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahm HS, Toroitich EK, Borne AL, Brulet JW, Libby AH, Yuan K, Ware TB, McCloud RL, Ciancone AM, and Hsu KL (2020). Global targeting of functional tyrosines using sulfur-triazole exchange chemistry. Nat Chem Biol 16, 150–159. 10.1038/s41589-019-0404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paquette DR, Tibble RW, Daifuku TS, and Gross JD (2018). Control of mRNA decapping by autoinhibition. Nucleic Acids Res 46, 6318–6329. 10.1093/nar/gky233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borne AL, Brulet JW, Yuan K, and Hsu KL (2021). Development and biological applications of sulfur-triazole exchange (SuTEx) chemistry. RSC Chem Biol 2, 322–337. 10.1039/d0cb00180e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youn JY, Dyakov BJA, Zhang J, Knight JDR, Vernon RM, Forman-Kay JD, and Gingras AC (2019). Properties of Stress Granule and P-Body Proteomes. Mol Cell 76, 286–294. 10.1016/j.molcel.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 30.King OD, Gitler AD, and Shorter J (2012). The tip of the iceberg: RNA-binding proteins with prion-like domains in neurodegenerative disease. Brain Res 1462, 61–80. 10.1016/j.brainres.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maris C, Dominguez C, and Allain FH (2005). The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J 272, 2118–2131. 10.1111/j.1742-4658.2005.04653.x. [DOI] [PubMed] [Google Scholar]
- 32.Kruger DM, Neubacher S, and Grossmann TN (2018). Protein-RNA interactions: structural characteristics and hotspot amino acids. RNA 24, 1457–1465. 10.1261/rna.066464.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwaki A, and Izawa S (2012). Acidic stress induces the formation of P-bodies, but not stress granules, with mild attenuation of bulk translation in Saccharomyces cerevisiae. Biochem J 446, 225–233. 10.1042/BJ20120583. [DOI] [PubMed] [Google Scholar]
- 34.Emara MM, Fujimura K, Sciaranghella D, Ivanova V, Ivanov P, and Anderson P (2012). Hydrogen peroxide induces stress granule formation independent of eIF2alpha phosphorylation. Biochem Biophys Res Commun 423, 763–769. 10.1016/j.bbrc.2012.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rostovtsev VV, Green LG, Fokin VV, and Sharpless KB (2002). A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl 41, 2596–2599. . [DOI] [PubMed] [Google Scholar]
- 36.Huang T, Hosseinibarkooie S, Borne AL, Granade ME, Brulet JW, Harris TE, Ferris HA, and Hsu KL (2021). Chemoproteomic profiling of kinases in live cells using electrophilic sulfonyl triazole probes. Chem Sci 12, 3295–3307. 10.1039/d0sc06623k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sanders DW, Kedersha N, Lee DSW, Strom AR, Drake V, Riback JA, Bracha D, Eeftens JM, Iwanicki A, Wang A, et al. (2020). Competing Protein-RNA Interaction Networks Control Multiphase Intracellular Organization. Cell 181, 306–324 e328. 10.1016/j.cell.2020.03.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown RS (2005). Zinc finger proteins: getting a grip on RNA. Curr Opin Struct Biol 15, 94–98. 10.1016/j.sbi.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 39.Hall TM (2005). Multiple modes of RNA recognition by zinc finger proteins. Curr Opin Struct Biol 15, 367–373. 10.1016/j.sbi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 40.Decker CJ, Burke JM, Mulvaney PK, and Parker R (2022). RNA is required for the integrity of multiple nuclear and cytoplasmic membrane-less RNP granules. EMBO J, e110137. 10.15252/embj.2021110137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wisniewski JR, Zougman A, and Mann M (2009). Combination of FASP and StageTip-based fractionation allows in-depth analysis of the hippocampal membrane proteome. J Proteome Res 8, 5674–5678. 10.1021/pr900748n. [DOI] [PubMed] [Google Scholar]
- 42.Tritschler F, Braun JE, Eulalio A, Truffault V, Izaurralde E, and Weichenrieder O (2009). Structural basis for the mutually exclusive anchoring of P body components EDC3 and Tral to the DEAD box protein DDX6/Me31B. Mol Cell 33, 661–668. 10.1016/j.molcel.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Arribas-Layton M, Wu D, Lykke-Andersen J, and Song H (2013). Structural and functional control of the eukaryotic mRNA decapping machinery. Biochim Biophys Acta 1829, 580–589. 10.1016/j.bbagrm.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larance M, Rowland AF, Hoehn KL, Humphreys DT, Preiss T, Guilhaus M, and James DE (2010). Global phosphoproteomics identifies a major role for AKT and 14-3-3 in regulating EDC3. Mol Cell Proteomics 9, 682–694. 10.1074/mcp.M900435-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thingholm TE, Jorgensen TJ, Jensen ON, and Larsen MR (2006). Highly selective enrichment of phosphorylated peptides using titanium dioxide. Nat Protoc 1, 1929–1935. 10.1038/nprot.2006.185. [DOI] [PubMed] [Google Scholar]
- 46.Humphrey SJ, Yang G, Yang P, Fazakerley DJ, Stockli J, Yang JY, and James DE (2013). Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab 17, 1009–1020. 10.1016/j.cmet.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lundby A, Secher A, Lage K, Nordsborg NB, Dmytriyev A, Lundby C, and Olsen JV (2012). Quantitative maps of protein phosphorylation sites across 14 different rat organs and tissues. Nat Commun 3, 876. 10.1038/ncomms1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hornbeck PV, Zhang B, Murray B, Kornhauser JM, Latham V, and Skrzypek E (2015). PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res 43, D512–520. 10.1093/nar/gku1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. 10.1126/science.1225829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ran FA, Hsu PD, Wright J, Agarwala V, Scott DA, and Zhang F (2013). Genome engineering using the CRISPR-Cas9 system. Nat Protoc 8, 2281–2308. 10.1038/nprot.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee JE, Cathey PI, Wu H, Parker R, and Voeltz GK (2020). Endoplasmic reticulum contact sites regulate the dynamics of membraneless organelles. Science 367. 10.1126/science.aay7108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gerritsen JS, and White FM (2021). Phosphoproteomics: a valuable tool for uncovering molecular signaling in cancer cells. Expert Rev Proteomics 18, 661–674. 10.1080/14789450.2021.1976152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yu Y, Hoffhines AJ, Moore KL, and Leary JA (2007). Determination of the sites of tyrosine O-sulfation in peptides and proteins. Nat Methods 4, 583–588. 10.1038/nmeth1056. [DOI] [PubMed] [Google Scholar]
- 54.Ishihama Y, Rappsilber J, and Mann M (2006). Modular stop and go extraction tips with stacked disks for parallel and multidimensional Peptide fractionation in proteomics. J Proteome Res 5, 988–994. 10.1021/pr050385q. [DOI] [PubMed] [Google Scholar]
- 55.Heberle H, Meirelles GV, da Silva FR, Telles GP, and Minghim R (2015). InteractiVenn: a web-based tool for the analysis of sets through Venn diagrams. BMC Bioinformatics 16, 169. 10.1186/s12859-015-0611-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. All probe modified protein identifications as determined by mass spectrometry, related to Figures 1 and 2
Table S2. List of RISKYs and Hyper RISKYs along with domain enrichment analysis, related to Figure 1 and 2
Table S3. Unenriched proteomics data, related to Figure 2
Table S5. Immunoprecipitation proteomics data, related to Figure 4
Table S6. EDC3 IP binding partner proteomics data analysis, related to Figure 4
Table S7. EDC3 IP binding partner proteomics data analysis all quantified peptides for calculation, related to Figure 4
Table S8. Phosphoproteomics data analysis, related to Figure 5
Data Availability Statement
Proteomics data have been deposited at ProteomeXchange via the PRIDE database (http://www.proteomexchange.org) and are publicly available as of the date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| EDC4 | Cell Signaling Technologies | cat#2548S |
| G3BP1 | Santa Cruz Biotechnology | cat#sc-365338 |
| EDC3 | Cell Signaling Technologies | cat#14495 |
| FLAG-tag | Cell Signaling Technologies, Sigma Aldrich | cat#8146, cat#SAB4301135 |
| HA-tag | Invitrogen | cat#26183 |
| GFP | GenScript | cat#A01388 |
| GAPDH | Cell Signaling Technologies | cat#2118 |
| Alexa Fluor® 488 Conjugate Anti-Rabbit IgG (H+L) F(ab’)2 Fragment | Cell Signaling Technologies | cat#4412S |
| IgG (H+L) Goat anti-Mouse, DyLight™ 550 | Invitrogen | cat#84540 |
| Goat anti-Mouse IgG (H+L) Secondary Antibody, DyLight™ 650 | Invitrogen | cat#84545 |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, DyLight™ 550 | Invitrogen | cat#84541 |
| Goat anti-Rabbit IgG (H+L) Secondary Antibody, DyLight™ 650 | Invitrogen | cat#84546 |
| Bacterial and virus strains | ||
| XL1-Blue | Agilent | cat#200228 |
| Chemicals, peptides, and recombinant proteins | ||
| Polyethylenimine | Polysciences | cat#24765-1 |
| PBS | Cytiva | cat#SH30378.03 |
| Sodium (meta)arsenite | Sigma-Aldrich | cat#S7400 |
| Hydrogen peroxide | Macron | cat#MK5240120 |
| HHS-465 | Hahm, et al.26 | N/A |
| DAPI | Tocris | cat#5748 |
| Lipofectamine 2000 | Thermo Fisher Scientific | cat#11668019 |
| QuickExtract DNA Extraction Solution | Lucigen Corporation | cat#QE09050 |
| Protease and Phosphatase Inhibitor Mini Tablets | Pierce | cat#A32959 |
| μ Columns | Miltenyi Biotec | cat#130-042-701 |
| Trypsin/Lys-C (LC-MS grade) | Promega | cat#V5073 |
| Dithiothreitol | Fisher bioreagents | cat#BP172-5 |
| Iodoacetamide | Thermo Fisher Scientific | cat#AC122270250 |
| Formaldehyde solution | Sigma-Aldrich | cat#252549 |
| Triton™ X-100 | Sigma-Aldrich | cat#X100 |
| Tween™ 20 | Thermo Fisher Scientific | cat#bp337500 |
| VECTASHIELD® HardSet™ Antifade Mounting Medium | Vector Laboratories | cat#H-1400-10 |
| VECTASHIELD Vibrance® Antifade Mounting Medium | Vector Laboratories | cat#H-1700 |
| Emetine dihydrochloride | Abcam | cat#ab141478 |
| Desthiobiotin-PEG-3-azide | Hahm, et al.26 | N/A |
| Critical commercial assays | ||
| μMACS™ DYKDDDDK Isolation Kit | Miltenyi Biotec | cat#130-101-591 |
| High-Select™ TiO2 Phosphopeptide Enrichment Kit | Thermo Scientific | cat#A32993 |
| QIAprep Spin Miniprep Kit | Qiagen | cat#27106X4 |
| Experimental models: Cell lines | ||
| HEK293T | ATCC | cat#CRL-3216 |
| HeLa | Gift from Dr. Ralph Kleiner (Princeton University) | N/A |
| HEK293T EDC3-KO | This paper | N/A |
| Oligonucleotides | ||
| CACCGTCAGGGAAGAGTGTCAGCTG (Oligo 1 fWd) | Integrated DNA Technologies | N/A |
| AAACCAGCTGACACTCTTCCCTGAC (Oligo 1 rev) | Integrated DNA Technologies | N/A |
| CACCGAACATGGCTACAGATTGGCT (Oligo 2 fWd) | Integrated DNA Technologies | N/A |
| AAACAGCCAATCTGTAGCCATGTTC (Oligo 2 rev) | Integrated DNA Technologies | N/A |
| ACACTTGGTACAGAGCCTGAT (Surveyor oligo 1 fWd) | Integrated DNA Technologies | N/A |
| AAGGGGAGGCCAAGGAATGAA (Surveyor oligo 1 rev) | Integrated DNA Technologies | N/A |
| Recombinant DNA | ||
| EDC3 in pcDNA3.1+/C-(K)DYK | Hahm, et al.26 | N/A |
| EDC3 Y475F in pcDNA3.1+/C-(K)DYK | Hahm, et al.26 | N/A |
| pcDNA3-HA-14-3-3 beta | pcDNA3-HA-14-3-3 beta was a gift from Michael Yaffe (Addgene plasmid # 13270; http://n2t.net/addgene:13270; RRID:Addgene 13270) | cat#13270 |
| HA-14-3-3 zeta | HA-14-3-3 zeta was a gift from FENG-QIAN Li & Ken-Ichi Takemaru (Addgene plasmid # 116888; http://n2t.net/addgene:116888; RRID:Addgene 116888) | cat#116888 |
| HA-14-3-3 epsilon | HA-14-3-3 epsilon was a gift from FENG-QIAN Li & Ken-Ichi Takemaru (Addgene plasmid # 116886; http://n2t.net/addgene:116886; RRID:Addgene 116886) | cat#116886 |
| HA-14-3-3 eta | HA-14-3-3 eta was a gift from FENG-QIAN Li & Ken-Ichi Takemaru (Addgene plasmid # 116887; http://n2t.net/addgene:116887; RRID:Addgene 116887) | cat#116887 |
| BFP-Dcp1a | BFP-Dcp1a was a gift from Gia Voeltz (Addgene plasmid # 153973; http://n2t.net/addgene:153973; RRID:Addgene 153973) | cat#153973 |
| GFP-Dcp1b | GFP-Dcp1b was a gift from Gia Voeltz (Addgene plasmid # 153976; http://n2t.net/addgene:153976; RRID:Addgene 153976) | cat#153976 |
| Software and algorithms | ||
| Custom LC-MS Bioinformatics Pipeline | Hahm, et al.26 | N/A |
| Skyline | MacCoss Lab Software | https://skyline.ms/project/home/software/Skyline/begin.view |
| Byonic | Protein Metrics | https://proteinmetrics.com/byos/ |
| Fiji | ImageJ | https://github.com/fiji/fiji |
| GraphPad PRISM | GraphPad | https://www.graphpad.com/ |
| RawConverter | RawConverter | http://fields.scripps.edu/rawconv/ |
| Adobe Illustrator | Adobe | https://www.adobe.com/products/illustrator.html |
| Microsoft Office | Microsoft | https://www.office.com/ |
| Image Lab | Bio-rad | https://www.bio-rad.com/en-us/product/image-lab-software?ID=KRE6P5E8Z |
| MestreNova | Mestrelab Research | https://mestrelab.com/download/mnova/ |
| Leica Applications Suite | Leica Microsystems | https://www.leica-microsystems.com/products/microscope-software/p/leica-application-suite/ |
| Deposited data | ||
| 10.6019/PXD038010 | This paper | PXD038010 |


