Abstract
Aims
Simplified detection of atrial arrhythmias via consumer-electronics would enable earlier therapy in at-risk populations. Whether this is feasible and effective in older populations is not known.
Methods and results
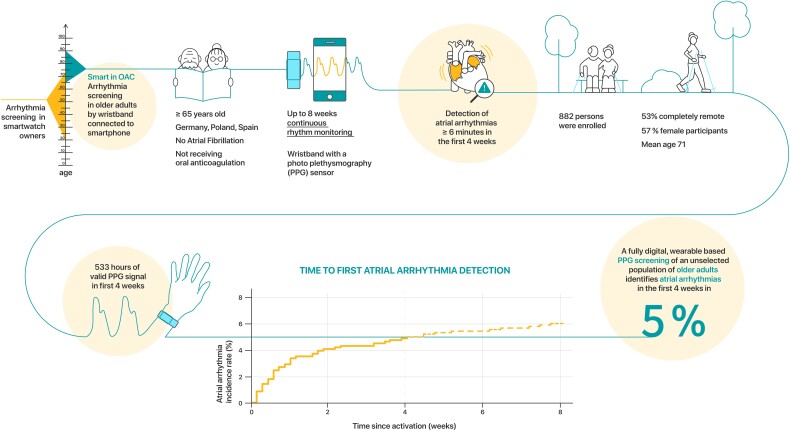
The fully remote, investigator-initiated Smartphone and wearable detected atrial arrhythmia in Older Adults Case finding study (Smart in OAC—AFNET 9) digitally enrolled participants ≥65 years without known atrial fibrillation, not receiving oral anticoagulation in Germany, Poland, and Spain for 8 weeks. Participants were invited by media communications and direct contacts. Study procedures adhered to European data protection. Consenting participants received a wristband with a photoplethysmography sensor to be coupled to their smartphone. The primary outcome was the detection of atrial arrhythmias lasting 6 min or longer in the first 4 weeks of monitoring. Eight hundred and eighty-two older persons (age 71 ± 5 years, range 65–90, 500 (57%) women, 414 (47%) hypertension, and 97 (11%) diabetes) recorded signals. Most participants (72%) responded to adverts or word of mouth, leaflets (11%) or general practitioners (9%). Participation was completely remote in 469/882 persons (53%). During the first 4 weeks, participants transmitted PPG signals for 533/696 h (77% of the maximum possible time). Atrial arrhythmias were detected in 44 participants (5%) within 28 days, and in 53 (6%) within 8 weeks. Detection was highest in the first monitoring week [incidence rates: 1st week: 3.4% (95% confidence interval 2.4–4.9); 2nd–4th week: 0.55% (0.33–0.93)].
Conclusion
Remote, digitally supported consumer-electronics-based screening is feasible in older European adults and identifies atrial arrhythmias in 5% of participants within 4 weeks of monitoring (NCT04579159).
Keywords: Atrial arrhythmia, Consumer electronics, Elderly population, Early detection, Digital trial
Graphical Abstract
Graphical Abstract.
Introduction
Earlier initiation of anticoagulation could prevent strokes and cardiovascular deaths in patients with atrial fibrillation (AF).1–4 Recently controlled clinical trials demonstrate that population-based screening for AF and subsequent initiation of oral anticoagulation can prevent some strokes.5,6 These trials led to recent recommendations in a practical guide of the European Heart Rhythm Association (EHRA) to intermittently screen individuals aged ≥ 75 years and consider systematic screening in individuals aged ≥ 65 years with additional comorbidities contributing to stroke risk.7 However, these studies also illustrate relatively high numbers needed to screen. Recent trials have shown that patient-operated ECG monitors can be rolled out to preselected screening populations.5 Implanted cardiac monitors are associated with a high analysable monitoring time,6 but involve invasive procedures. Simple, scalable methods to identify atrial arrhythmias in at-risk populations are needed to enable the timely detection of AF and initiation of therapy.
Continuous rhythm screening using implanted pacemakers or ECG monitors detects short atrial arrhythmias in up to 30% of elderly participants,6,8 but is limited by its invasive nature. Atrial arrhythmias that are only detected during many months of monitoring must statistically occur less often or be of shorter durations than arrhythmias that occur more often and are longer and therefore are more likely to be detected in shorter monitoring periods.6,9,10 Indeed, subclinical AF detected in implantable cardiac devices is associated with a lower stroke risk than clinical AF,9–11 although a cut-off point for increased stroke risk remains to be found and validated.12–14 Modern consumer electronics, including smartphones and smartwatches or wearable-based devices,15–17 enable recording of pulse plethysmography (PPG). Combined with validated analysis algorithms,18,19 this can be applied to monitor for arrhythmias.19 Wearable-based screening for atrial arrhythmias is feasible when company-owned data are analysed in relatively young, early adopters.15–17,19,20 An analysis of previously reported atrial arrhythmia detection rates with wearables is summarized in Table 2 and Supplementary material online, Figure S1. The US Screening Task Force and an EHRA practical guide recognized the potential of PPG-based arrhythmia screening,7 but noted that more evidence was needed before it could be recommended,1 especially regarding arrhythmia screening in older populations.1,5,6 Inclusive methods offering PPG-based arrhythmia screening to older participants are therefore required.7
Table 2.
Studies using different heart rate monitoring techniques to detect atrial arrhythmias in older adults with focus on wearables
| Study | Monitoring | Technique | Author, study acronym subgroup/own analysis | Population | Design, intervention | Mean age (+/− Std.) | Female (%) | n | Atrial arrhythmia IR (%) |
|---|---|---|---|---|---|---|---|---|---|
| General population/community based/at risk cohorts, remote | Continuous monitoring | single lead ECG | Bowman/Casadei et al. AMALFI From ISRCTN | General population based Region: K Age: ≥ 64 Inclusion: CHA2DS2-VASc ≥ 4 in woman or ≥ 3 in men Exclusion: Known AF: excluded | Sequence: continuous, 14 days Biodata: single lead ECG Device: ZioPatch | Analysis ongoing/unpublished | |||
| Langer et al. 202129 AWARE-AF program | Community based, recruited from outpatient cardiology Region: Canada Age: ≥ 65 Inclusion: ≥ 1 of poAF, ST/TIA or ≥ 75 years old + ≥ 2 of HF, HT, DM, CKD, OSAS, CVD, EF < 35%, significant DDys Exclusion: implanted PM/ICD Known AF: excluded | Sequence: continuous, 7 days Biodata: single lead ECG Device: Cardiostat, Canada | 78.7 ± 6.1 | 42% | 942 | 2.7 (1.8–4.0) | |||
| Gladstone et al. 202130 SCREEN-AF | General population based, recruited from GP practices Region: Canada, Germany Age: ≥ 75 years Inclusion: CHADS2 ≥ 2 Exclusion: implantable PM/ICD/ILR, contraindication for OAC Known AF: excluded | Sequence: continuous, 14 days Biodata: single lead ECG Device: ZioPatch | 80 ± 4 | 57% | 434 | 5.3 (3.4–8) | |||
| Kemp Gudmundsdottir et al. 202031 STROKESTOP II High-risk subgroup | General population based Region: Stockholm Age: 75 or 76 years old Inclusion: NT-proBNP ≥ 125 ng/L Exclusion: -Known AF: excluded | Sequence: continuous, 14 days Biodata: single lead ECG Device: Novacor R-test 4 evolution | N/A | 60% | 3766 | 4.4 (3.8–5.1) | |||
| Rooney et al. 201932 ARIC | Community based Region: USA Age: ≥ 75 years Inclusion: Exclusion: implantable PM/ICD/ILR, skin allergic Known AF: separated | Sequence: continuous, 14 days Biodata: single lead ECG Device: ZioPatch | 79.2 ± 4.6 | 58% | 2244 | 2.5 (1.9–3.2) | |||
| Steinhubl et al. 201833 mSToPS | General population based (all subscribers of a certain health care plan9 Region: USA Age: ≥ 75 years or ≥ 65 years + Female + 1 comorbidity or ≥ 55 years + Male + 1 comorbidity Inclusion: -Exclusion: implantable PM/ICD, on OAC, known AT, known Aflutter Known AF: excluded | Sequence: continuous, 2 × 14 days Biodata: single lead ECG Device: ZioPatch | 73.7 ± 7.0 | 41% | 1738 | 2.9 (2.2–3.8) | |||
| Heckbert et al. 201834 MESA (substudy) | General population based (6 communities) Region: USA Age: ≥ 18 years Inclusion: -Exclusion: skin allergy to tape/adhesives Known AF: excluded | Sequence: continuous, 14 days Biodata: single lead ECG Device: ZioPatch | 75 ± 8 | 48% | 804 | 4.0 (2.7–5.6) | |||
| PPG | Perez et al. 201915 Apple Heart Study own analysis of participants aged ≥ 65 years | General population based Region: USA Age: ≥ 18 years Inclusion: Apple Watch owner Exclusion: -Known AF: included | Sequence: continuous Biodata: PPG + ECG patch Device: Apple Watch | N/A | 42%c | 24 626 | 3.1 (2.9–3.3)a | ||
| Guo et al. 201916 Pre-MAFA II (Huawei Heart Study) own analysis of participants aged ≥ 65 years | General population based Region: China Age: ≥ 18 years Inclusion: Huawei smartphone owner Exclusion: -Known AF: included | Sequence: continuous Biodata: PPG Device: Huawei Watch or Honor Watch or Honor Band | N/A | 13%c | 3419 | 2.8 (2.3–3.4) | |||
| Lubitz et al. 202120 FITBIT Heart Study Design paper | General population based Region: USA Age: ≥ 22 years Inclusion: Fitbit device owner Exclusion: on OAC, implanted PM/ICD Known AF: excluded (AF/AFlutter) | Sequence: continuous Biodata: PPG + ECG patch Device: Fitbit Ionic, Charge 3, Charge 4, Versa, Versa Lite, Versa 2, Versa 3, Sense, Inspire HR, Inspire 2 | Analysis ongoing/unpublished | ||||||
| intermittent monitoring | single lead ECG | Berge et al. 201835 ACE1950 Follow-Up | Community based Region: Sweden Age: 65 years Inclusion: ≥ 1 of HT, DM, HF, ST/TIA, VD Exclusion: -Known AF: excluded | Sequence: intermittent, 2 × daily, 14 days Biodata: single lead ECG Device: Zenicor | 65 | 44% | 1510 | 0.9 (0.5–1.5) | |
| Ghazal et al. 202036 | Community based Region: Stockholm regional, Sweden Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: excluded | Sequence: intermittent, 3 × daily, 14 days Biodata: single lead ECG Device: Zenicor | 72.1 (68.7, 75.9)b | 61% | 1010 | 2.7 (1.8–3.9) | |||
| Halcox et al. 201737 REHEARSE-AF | Community based, recruited from GP practices Region: Aberdeen region, UK Age: ≥ 65 Inclusion: CHA2DS2-VASc ≥ 2 (non-sex) Exclusion: on OAC, known CI for OAC, implanted PM/ICD Known AF: excluded | Sequence: intermittent, 2 × weekly, 1 year Biodata: single lead ECG Device: AliveCor | 72.6 ± 5.4 | 52% | 500 | 3.4 (2.0–5.4) | |||
| Svennberg et al. 201538 STROKESTOP (screening results) | General population based Region: Stockholm + Halland county, Sweden Age: 75 or 76 years old Inclusion: -Exclusion: -Known AF: separate | Sequence: intermittent, 2 × daily, 14 days Biodata: single lead ECG Device: Zenicor | N/A | 55% | 6507 | 3.8 (3.3–4.3) | |||
| once | single lead ECG | Poulsen et al. 202239 | Community based, at preventive home visits Region: 3 Danish municipals Age: ≥ 65 years Inclusion: understanding Danish Exclusion: on OAC, home care or living in nursing home Known AF: excluded | Sequence: once per patient (2 repeated measures) Biodata: single lead ECG Device: Zenicor | 80.9 (4.7) | 56% | 477 | 1.5 (0.6–3) | |
| Senoo et al. 202240 | Community based, at AF awareness campaign symposiums Region: Kyoto, Japan Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: separate | Sequence: once per patient, 30 s Biodata: single lead ECG Device: Complete, Omron, Kyoto | 72.4 ± 5.8 | 52% | 1607 | 0.9 (0.5–1.5) | |||
| Systematic, GP-/Pharmacy-based, stationary | Once, cross-sectional | single lead ECG | Chen et al. 202041 AF-CATCH, pre-phase | Community based, community health centres Region: Shanghai, China Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: separate | Sequence: once per patient, 30 s Biodata: single lead ECG Device: AliveCor | 71.6 ± 6.3 | 56% | 4370 | 0.5 (0.3–0.8) |
| Zaprutko et al. 202042 | General population based, recruited from 10 pharmacies Region: 6 cities in 3 regions of Poland Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: excluded | Sequence: once per patient, 30 s Biodata: single lead ECG Device: AliveCor | 73.7 ± 6.5 | 68% | 525 | 1.4 (0.6–2.8) | |||
| Orchard et al. 202043 AF SMART II | Community based, recruited at GP practices Region: rural Australia Age: ≥ 65 years Inclusion: -Exclusion: implantable PM/ICD, on OAC Known AF: excluded | Sequence: once per patient, 30 s Biodata: single lead ECG Device: AliveCor | 75.1 ± 6.8 | 53% | 3103 | 1.2 (0.8–1.7) | |||
| Gwynn et al. 202044 own analysis of participants aged ≥ 65 years | Community based at 16 Aboriginal Community Health Organizations Region: rural Australia Age: ≥ 45 years Inclusion: Aboriginal heritage Exclusion: -Known AF: separated | Sequence: once per patient, 30 s Biodata: single lead ECG Device: AliveCor | N/A | 56% | 146 | 0.6 (0.0–3.7) | |||
| Sun et al. 202245 Hong Kong Outpatient AF screening study | Outpatient clinics (2 cardiology, 2 IM, 1 geriatric) Region: Hong Kong region Age: ≥ 65 years Inclusion: -Exclusion: dementia, terminal illness, unable to understand informed consent Known AF: separated | Sequence: once per patient, 30 s Biodata: single lead ECG Device: AliveCor | 76.4 ± 7.8 | 49% | 9734 | 3.0 (2.7–3.4) | |||
| combination | Verbiest-van Gurp et al. 202246 | Outpatient clinics (2 cardiology, 2 IM, 1 geriatric) Region: Hong Kong region Age: ≥ 65 years Inclusion: Exclusion: known AF Known AF: separated | Sequence: once per patient Biodata: single lead ECG/BP/palpation Device: MyDiagnostick, Microlife BP Monitor, Radial pulse palpation | 73.5 ± 5.5 | 54% | 4339 | 0.8 (0.6–1.1) | ||
| BP monitor | Jatau et al. 202247 What’s Your Beat? | General population based, health centers, recruitment via media campaigns Region: Tasmania, Australia Age: ≥ 65 years Inclusion:—Exclusion: severe dementia, known cardiac arrhythmia, implantable PM/ICD Known AF: excluded | Sequence: once per patient Biodata: BP monitor Device: Microlife | 71.0 (68.0–76.0) | 59% | 1704 | 0.9 (0.5–1.5) | ||
| Intermittent | BP monitor | Denas et al. 202048 | General population based, GP practices Region: Veneto, Italy Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: excluded | Sequence: 3 consecutive measurements per visit, unsystematically intermittent at visits over in average 410 days per patient Biodata: BP monitor Device: Microlife | 75.5 ± 7.0 | 58% | 14 987 | 2.5 (2.3–2.8) | |
| single lead ECG | Zhang et al. 202149 AF-CATCH Quarterly-screened subgroup | Community based, community health centers Region: Shanghai Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: excluded | Sequence: intermittent, quarterly Biodata: single lead ECG Device: AliveCor | 71.3 ± 6.1 | 56% | 2841 | 1.4 (1.0–1.9) | ||
| Lubitz et al. 202250 VITAL-AF | Community based at GP practices Region: Massachusetts, USA Age: ≥ 65 years Inclusion: -Exclusion: -Known AF: excluded | Sequence: intermittent over 12 months at GP visits Biodata: single lead ECG Device: AliveCor | 73.9 ± 6.8 | 60% | 15 393 | 1.7 (1.5–1.9) | |||
| combination | Watanabe et al. 202251 SCAN-AF | Hospital-affiliated outpatient clinics Region: Japan Age: ≥ 65 years Inclusion: CHA2DS2-VASc ≥ 2 or CHADS2 ≥ 1 Exclusion: known AF, use of AAD, inability to use the monitoring devices Known AF: excluded | Sequence: intermittent over 24 weeks Biodata: BP oscillogram, single lead ECG Device: Omron; myBeat | 74.0 (69.0–79.0) | 50% | 1148 | 0.8 (0.4–1.5) | ||
| Cardiology patients without history of AF | continuous | Single lead ECG (ILR) | Svendsen et al. 20216 LOOP | Cardiology patients, 4 centers Region: Denmark Age: 70–90 years Inclusion: ≥ 1 of HT, DM, ST/TIA, HF Exclusion: on OAC, known CI for OAC, implanted PM/ICD Known AF: excluded | Sequence: continuous, 64.5 months (59.3, 69.8)bBiodata: single lead ECG (ILR) Device: Medtronic Reveal LINQ ILR | 74.7 ± 4.1 | 47% | 1501 | 31.8 (29.0–34.8) |
| Philippsen et al. 201752 | Diabetes and cardiology outpatient clinics at the Hospital of Southern Jutland Region: USA Age: ≥ 65 years Inclusion: HT (treated with ≥ 2 antihypertensives) and DM (treated with any antidiabetics or insulin) Exclusion: on OAC, EF < 45%, valvular disease needing intervention, implanted PM/ICD, known ischemic heart disease, ST/TIA, PAD, thyrotoxicosis, end-stage renal failure, severe obesity Known AF: excluded | Sequence: continuous, until ERI Biodata: PPG (ILR) Device: Medtronic ILR (Reveal XT or Reveal LINQ) | 71.3 (67.4, 75.1)b | 37% | 82 | 20.7 (12.0–33.2) | |||
The table lists the name of the study, the population studied, the design, age, and sex of the population, the number of participants, and the incidence rate of atrial arrhythmias with a 95% confidence interval. When age subgroup data was available or calculable, the study was included and parameters are reported for the population aged ≥ 65 years. Two studies are included of which only the design has been published (Lubitz et al.) or reported on a clinical trials website (AMALFI). Two implantable loop recorder studies are added at the bottom of the table.
Abbreviations: AAD, antiarrhythmic drug(s); BP, blood pressure; 95% CI, 95% confidence interval; CI, contraindication; CVD, any cardiovascular disease; DDys, diastolic dysfunction in echocardiography; DM, diabetes mellitus; EF, ejection fraction; GP, general practitioner; HF, heart failure; HT, hypertension; ICD, implantable cardioverter-defibrillator; ILR, implantable loop recorder; IM, internal medicine; IR, Incidence rate; IR/100 000, Incidence rate per 100 000 screened; n, Population size used for incidence rate calculation; OAC, oral anticoagulation; OSAS, obstructive sleep apnea syndrome; PAD, peripheral artery disease; PM, pacemaker; poAF, postoperative AF; ST/TIA, stroke or TIA; VD, vascular disease (in CHA2DS2-VASc).
This study included participants without knowledge of the history of atrial fibrillation but was included in the review due to its sample size and population-wide approach. Reported is the incidence rate of screen-positive participants (irregular pulse notification). Of these, 34% were diagnosed on a subsequent Holter ECG.
Median age and interquartile range (IQR) are reported.
Proportion of females not available for reported age group.
To address this societal need, the Smartphone and wearable detected atrial arrhythmia in Older Adults Case finding study (Smart in OAC—AFNET 9) evaluated the usability of a fully digital, PPG-based detection system for atrial arrhythmias in older European adults.21
Methods
Study design
Smart in OAC—AFNET 9 is an investigator-initiated, single-arm, international, multicentre case-finding study in an at-risk population without previously known AF using a low-threshold, digitally enhanced screening platform (https://clinicaltrials.gov/ct2/show/NCT04579159). Details of the study design have been published.21 The study has been approved by the local Ethics Committees in all participating sites [Hamburg 2020–10260-BO-ff, Dresden (Markkleeberg) EK-BR-95/21–1, Barcelona HCB/2021/0255, Krakow/Nowy Sasz 298/KBL/OIL/2020, Birmingham, UK IRAS 292218]. To capture societal and health care realities in different parts of Europe, the study was planned in Germany (Central Europe), Poland (Eastern Europe), Spain (Western Europe), and the UK (central NHS system). In the UK, administrative delays due to COVID-19 prevented the study from commencement in time. Sponsor of the trial is AFNET (https://www.kompetenznetz-vorhofflimmern.de). Financial support came from Daiichi-Sankyo Europe in the form of an unrestricted grant and by Preventicus, Jena, Germany, as an in-kind contribution.
Participants
Potential participants aged 65 years or older without known AF and not on oral anticoagulation were made aware of the study using newspaper and television advertisements targeting audiences of older adults, senior citizen interest groups, personal contacts in the sites, general physicians in the community, leaflets, and a website.
Study intervention. Within the limitations of a case finding study requiring consent, the system was designed for simplicity. After expressing interest and agreeing to be contacted using digital, oral, or written communication, potential participants were offered participation. Informed consent was obtained digitally. Paper versions were available on demand and were required in Spain. A wristband with a PPG sensor (Corsano 287, MMT SA, Switzerland) was shipped to consenting participants or collected at the site. Participants installed the Corsano Preventicus Smart app onto their smartphone (operating system requirements Apple iOS version 12.2 or higher or Android 8.0 or higher) and coupled the wristband via Bluetooth for app-transferal of PPG data. The wearable technology records and transfers passively around the clock, operating for up to 5 days between recharging. Participants were asked to wear the wristband and use the system for 4 weeks with the possibility to extend monitoring for up to 8 weeks if atrial arrhythmias had not been found. Analysis of the pulse waves for atrial arrhythmias used a validated algorithm (Class IIa CE certified medical product, Preventicus Heartbeats®, Jena, Germany, www.preventicus.com,18,19). All signals were centrally analysed by a cloud-based and device-agnostic analytic service (Preventicus Heartbeats, CE marked certified medical device.21) Although the Corsano wristband was used and the app was adapted to Corsano technology, any other high-quality PPG wristband could be used in the future.
The PPG was continuously recorded with the wristband and split in 1 min-long segments, each of them analysed via the atrial arrhythmia detection algorithm. One-minute recordings were excluded automatically if more than 10% of the signal had poor quality, e.g. from movement artefacts. Length of atrial arrhythmia episodes was estimated via consecutive positive one-minute segments and atrial arrhythmias in this study were defined as periods of an irregular PPG signal lasting six minutes or longer or a burden of 1.5% per 24 h or more.21 Atrial arrhythmias of this duration detected by implanted devices are associated with an increased risk of stroke.10,22 PPG analysis was stopped after the detection of atrial arrhythmias.
To ensure that participants would be reassured or receive a diagnosis of AF and subsequent treatment as required despite restrictions of health services during the pandemic, all participants with positive PPG atrial arrhythmia screening were offered a 14-day external loop recorder Holter ECG (CardioMem® CM 100 XT), delivered by post or handed out on site. The same loop recorder was also planned to be offered to a random sample of participants without PPG detection of atrial arrhythmias, while the delivery of Holter ECG recorders to positively PPG screened participants was prioritized.
Data collection
Information on name, mobile number, date of birth, known AF, and current oral anticoagulation was entered by the participants via their smartphone at enrolment and during the screening process (Table 1). The results of the PPG analyses and the Holter ECG were captured on the systems described above and integrated into the final data set for analysis. The results of this investigation were made available to the site teams for medical action.
Table 1.
Clinical characteristics of the study participants and of participants with and without atrial arrhythmia (AA)
| Total (n = 882) | without atrial arrhythmias (n = 838) | with atrial arrhythmias (n = 44) | P-value | |
|---|---|---|---|---|
| Age | 0.0081 | |||
| Mean ± SD, | 70.9 ± 4.9 | 70.8 ± 4.8 | 72.8 ± 5.7 | |
| Median (Q1, Q3) | 70.0 (67.0, 74.0) | 69.0 (67.0, 74.0) | 71.5 (68.8, 75.2) | |
| Range | 65.0–90.0 | 65.0–90.0 | 65.0–86.0 | |
| Sex | 0.7972 | |||
| female | 500 (56.7%) | 473 (56.4%) | 27 (61.4%) | |
| male | 381 (43.2%) | 364 (43.4%) | 17 (38.6%) | |
| other | 1 (0.1%) | 1 (0.1%) | 0 (0.0%) | |
| Country | 0.9142 | |||
| Germany | 575 (65.2%) | 546 (65.2%) | 29 (65.9%) | |
| Poland | 277 (31.4%) | 263 (31.4%) | 14 (31.8%) | |
| Spain | 30 (3.4%) | 29 (3.5%) | 1 (2.3%) | |
| data source | 0.9632 | |||
| GP | 80 (9.1%) | 76 (9.1%) | 4 (9.1%) | |
| Hospital | 20 (2.3%) | 19 (2.3%) | 1 (2.3%) | |
| Leaflet | 96 (10.9%) | 92 (11.0%) | 4 (9.1%) | |
| Other | 633 (71.8%) | 600 (71.6%) | 33 (75.0%) | |
| Pharmacy | 15 (1.7%) | 15 (1.8%) | 0 (0.0%) | |
| Website | 38 (4.3%) | 36 (4.3%) | 2 (4.5%) | |
| Measurement bracelet received | 0.6192 | |||
| Post | 469 (53.2%) | 444 (53.0%) | 25 (56.8%) | |
| Site | 413 (46.8%) | 394 (47.0%) | 19 (43.2%) | |
| Ethnic origin | 0.9652 | |||
| Arab | 2/851 (0.2%) | 2/808 (0.2%) | 0/43 (0.0%) | |
| Asian | 2/851 (0.2%) | 2/808 (0.2%) | 0/43 (0.0%) | |
| Mixed | 2/851 (0.2%) | 2/808 (0.2%) | 0/43 (0.0%) | |
| Other | 62/851 (7.3%) | 58/808 (7.2%) | 4/43 (9.3%) | |
| White | 783/851 (92.0%) | 744/808 (92.1%) | 39/43 (90.7%) | |
| Hypertension | 0.5432 | |||
| No | 439/853 (51.5%) | 416/812 (51.2%) | 23/41 (56.1%) | |
| Yes | 414/853 (48.5%) | 396/812 (48.8%) | 18/41 (43.9%) | |
| Diabetes mellitus | 0.0572 | |||
| No | 762/859 (88.7%) | 720/816 (88.2%) | 42/43 (97.7%) | |
| Yes | 97/859 (11.3%) | 96/816 (11.8%) | 1/43 (2.3%) | |
| EQ-5D: mobility | 0.5223 | |||
| Nmiss | 331 | 313 | 18 | |
| I have no problems in walking about | 471 (85.5%) | 449 (85.5%) | 22 (84.6%) | |
| I have slight problems in walking about | 51 (9.3%) | 50 (9.5%) | 1 (3.8%) | |
| I have moderate problems in walking about | 26 (4.7%) | 23 (4.4%) | 3 (11.5%) | |
| I have severe problems in walking about | 3 (0.5%) | 3 (0.6%) | 0 (0.0%) | |
| I am unable to walk about | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| EQ-5D: self-care | 0.1583 | |||
| Nmiss | 331 | 313 | 18 | |
| I have no problems washing or dressing myself | 538 (97.6%) | 513 (97.7%) | 25 (96.2%) | |
| I have slight problems washing or dressing myself | 11 (2.0%) | 11 (2.1%) | 0 (0.0%) | |
| I have moderate problems washing or dressing myself | 2 (0.4%) | 1 (0.2%) | 1 (3.8%) | |
| I have severe problems washing or dressing myself | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| I am unable to wash or dress myself | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| EQ-5D: usual activities | 0.6663 | |||
| Nmiss | 330 | 312 | 18 | |
| I have no problems doing my usual activities | 498 (90.2%) | 475 (90.3%) | 23 (88.5%) | |
| I have slight problems doing my usual activities | 41 (7.4%) | 39 (7.4%) | 2 (7.7%) | |
| I have moderate problems doing my usual activities | 13 (2.4%) | 12 (2.3%) | 1 (3.8%) | |
| I have severe problems doing my usual activities | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| I am unable to do my usual activities | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | |
| EQ-5D: Pain and discomfort | 0.5093 | |||
| Nmiss | 334 | 316 | 18 | |
| I have no pain or discomfort | 316 (57.7%) | 300 (57.5%) | 16 (61.5%) | |
| I have slight pain or discomfort | 172 (31.4%) | 164 (31.4%) | 8 (30.8%) | |
| I have moderate pain or discomfort | 48 (8.8%) | 46 (8.8%) | 2 (7.7%) | |
| I have severe pain or discomfort | 11 (2.0%) | 11 (2.1%) | 0 (0.0%) | |
| I have extreme pain or discomfort | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |
| EQ-5D: anxiety and depression | 0.2323 | |||
| Nmiss | 329 | 311 | 18 | |
| I am not anxious or depressed | 447 (80.8%) | 423 (80.3%) | 24 (92.3%) | |
| I am slightly anxious or depressed | 83 (15.0%) | 82 (15.6%) | 1 (3.8%) | |
| I am moderately anxious or depressed | 21 (3.8%) | 20 (3.8%) | 1 (3.8%) | |
| I am severely anxious or depressed | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |
| I am extremely anxious or depressed | 1 (0.2%) | 1 (0.2%) | 0 (0.0%) | |
| EQ-5D VAS | 0.4541 | |||
| Nmiss | 331 | 313 | 18 | |
| Mean ± SD | 82.9 ± 12.8 | 83.0 ± 12.6 | 81.1 ± 17.6 | |
| Median (Q1, Q3) | 85.0 (80.0, 91.0) | 85.0 (80.0, 91.0) | 86.0 (80.0, 90.0) | |
| Range | 20.0–100.0 | 29.0–100.0 | 20.0–100.0 | |
| EQ-5D 5L VT score | 0.5661 | |||
| Nmiss | 339 | 321 | 18 | |
| Mean ± SD | 0.95 ± 0.08 | 0.95 ± 0.09 | 0.96 ± 0.06 | |
| Median (Q1, Q3) | 0.97 (0.92, 1.00) | 0.97 (0.92, 1.00) | 1.00 (0.94, 1.00) | |
| Range | 0.28–1.00 | 0.28–1.00 | 0.80–1.00 |
Baseline characteristics of the study population grouped by AA detection within the first 28 days. Categorical data are n (%) or n/valid n (%) in case of missing values. Age is presented as mean ± SD, EQ-5D VAS, and EQ-5D 5L VT Score as median (IQR). EQ-5D VAS was missing for total n = 331, without AA n = 313, with AA n = 18, EQ-5D 5L VT Score was missing for n = 339/321/18. (1) Linear Model ANOVA, (2) Pearson’s χ2 test, (3) Trend test for ordinal variables.
(1) Student’s t-test, (2) Pearson’s χ2, (3) Armitage trend test for ordinal variables, Nmiss, number of missing values; GP, general practitioner.
Preventicus data management and data protection comply with General Data Protection Regulations. Personal data (declarations of consent, contact information, etc.) were stored exclusively in a defined cloud workspace (Preventicus Caresafe). The data in the Caresafe were end-to-end encrypted, limiting access to personal data to study site staff. Preventicus did not have any access to the personal data of participants.
Statistical considerations
Sample size
AA are detected in circa 30–40% of elderly populations when continuous monitoring is applied for 2–3 years using implantable loop recorders.6,8,23 Integrating the estimated effects of shorter monitoring times (1 month), considering that the wearable will not record continuously due to noise and the need for charging, and based on the known effects of intermittent and shorter ECG monitoring on detection rates of short AA,9,17,24 we assumed a detection rate of AA of 3–6% in the screening population.21 A sample size of 1000 participants would allow us to estimate a rate of detection of 5% with a precision of 2.8% (width of the two-sided 95% Clopper-Pearson confidence interval (CI), PASS 16.0.3), a sample size of 750 gives a precision of 3.3%.
Primary outcome
The primary outcome parameter of this study is the prevalence of PPG-detected atrial arrhythmias (lasting six minutes or longer), calculated as the number of participants with AA detected by the wearable in relation to all included participants. The primary analysis assessed atrial arrhythmias detected in 4 weeks of monitoring.
Secondary outcomes
Secondary outcomes include the total number of participants with atrial arrhythmias over the entire 8-week recording; time from enrolment to AA detection with death as a competing risk; regional differences in AA detection and differences by route of invitation; quality of life estimated by EQ-5D-5L in participants with and without AA; detection of AF by ECG, compliance, and reasons for non-participation.
Adverse events
SMART in OAC—AFNET 9 is a low-risk study using approved procedures to screen for atrial arrhythmias. Adverse events of interest related to the study procedures (e.g. unwanted effects of the wearable, in this case, a wristband) were noted by study centres if voiced by participants and collected in a questionnaire for participants following an invitation to a Holter ECG.
Statistical analyses
All analyses were prespecified in a dedicated statistical analysis plan signed on 31 January 2022 before accessing the data. The primary analysis was based on the full analysis data set (FAS), consisting of all participants that consented to screening and provided at least one data point. A sensitivity analysis was performed in a per protocol population including all participants that used the wearable as intended, i.e. used the device until screening rendered a positive result or in whom an analysable PPG signal was available for at least 300 h in the first 4 weeks of monitoring. Demographics and baseline characteristics are summarized using descriptive statistics. The detection rate of AA was calculated together with the corresponding two-sided 95% Clopper-Pearson CI. If participants discontinued participation, the information gathered until discontinuation was analysed. Time to first AA detection was analysed by taking death as a competing risk into account using Aalen-Johansen curves. A multivariable logistic model utilizing Firth’s bias-reduced penalized-likelihood was used to simultaneously identify predictors of AA. All analyses were carried out using R v4.0.5 (R Core Team, Vienna).
Role of the funding source
Smart in OAC—AFNET 9 is an investigator-initiated trial designed and executed by the authors. AFNET oversaw the trial as the legal sponsor, with U.S. serving as sponsor representative on the steering committee. Daiichi-Sankyo Europe provided funding for the study to AFNET and held a non-voting seat on the steering committee. Preventicus provided access to their PPG-based AF screening technology and Telecare Health system.
Data sharing
The protocol, informed consent in its written form, and statistical analysis plan are available in this paper. Study data will be made available for research purposes for at least 5 years after the completion of the study. Please direct inquiries including an outline of the planned analyses to info@kompetenznetz-vorhofflimmern.de; info@af-net.eu. Data will be made available by AFNET on reasonable request.
Results
Participants
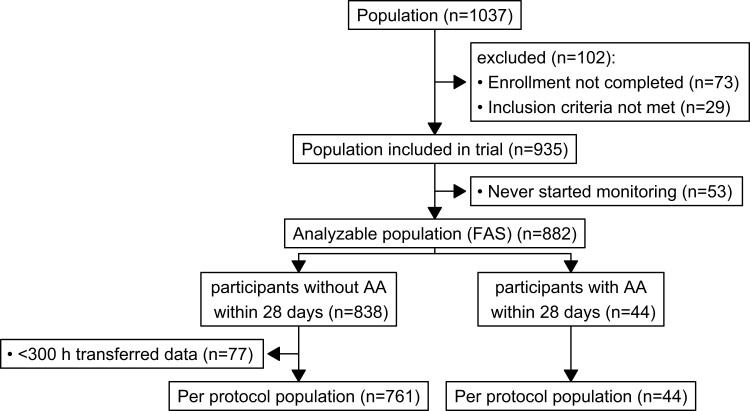
A total of 882 participants were PPG-screened in Germany, Poland, and Spain between 01 February 2021 and 31 January 2022 (Figure 1). In Spain, the Barcelona ethics committee required an in-person consent process with a hand-signed consent form. Five hundred (57%) participants were female, 414 (47%) participants reported known hypertension, and 97 (11%) reported known diabetes (Table 1). The age ranged from 65 to 90 years (mean age 71 ± 5 years). The majority of participants (72%) were reached by media campaigns in newspapers and television or by word of mouth and town hall meetings for senior citizens (category ‘other’ in Supplementary material online, Figure S2). The remaining participants were attracted by leaflets (11%), identified by general practitioners made aware of the study (9%), a website (4%), the site team hospital ambulatory settings (2%), or pharmacies (2%). Communication about the study in targeting audiences of older adults, including newspaper and television adverts, video messages, and town hall meetings, were associated with high recruitment rates (see Supplementary material online, Figure S2).
Figure 1.
STROBE flow chart of the study. FAS, full analysis sample.
Primary outcome
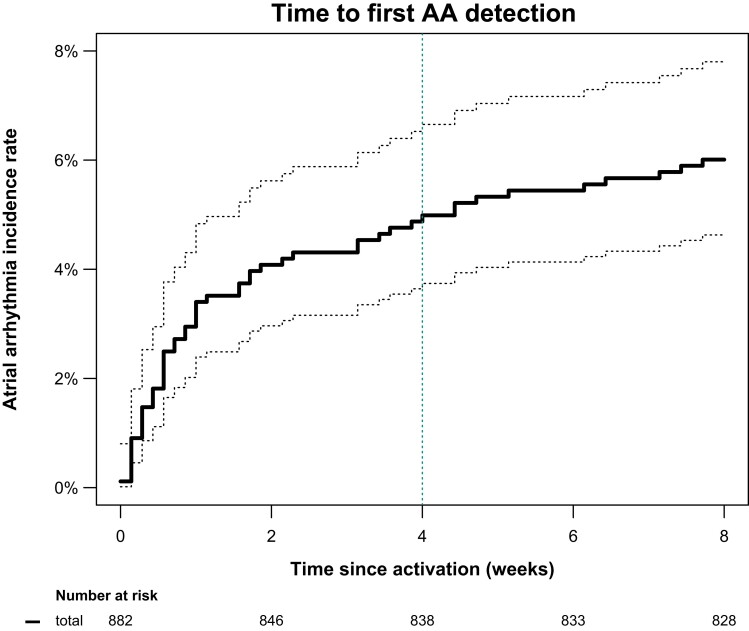
Atrial arrhythmias were detected in 44/882 participants [5.0%, 95% CI (3.6–6.6)] within 28 days of monitoring (Figure 2). Arrhythmia detection rate was higher in the 1st week of monitoring compared with subsequent weeks: The atrial arrhythmia incidence rate was 3.4 participants with atrial arrhythmias/100 monitored weeks (95% CI 2.4–4.9) in the 1st week of monitoring and between 0.12 and 0.71 in subsequent weeks [average incidence rate for week 2–4 was 0.55 (0.33–0.93), P < 0.001 for incidence rate in the 1st week vs. weeks 2–4, Figure 2].
Figure 2.
Detection of atrial arrhythmias over time in the study population. The bold continuous black line shows the Kaplan-Meier estimated cumulative event rate with corresponding 95% confidence interval (dotted black lines). The dotted green vertical line identifies the time point of the primary outcome, detection of atrial arrhythmias within 28 days of screening. Screening beyond this time point identified only a few additional cases.
Secondary outcomes
Atrial arrhythmias were detected in 53/882 participants (6%) within 8 weeks of monitoring (Figure 2). The time from initiation of monitoring to detection of atrial arrhythmias was relatively short, confirming the higher detection rate early during monitoring (Figure 2).
A prespecified sensitivity analysis confined to participants who used the device per protocol within the first 4 weeks of monitoring, found a similar detection rate of 44/805 5.5% (95% CI 4.0–7.3).
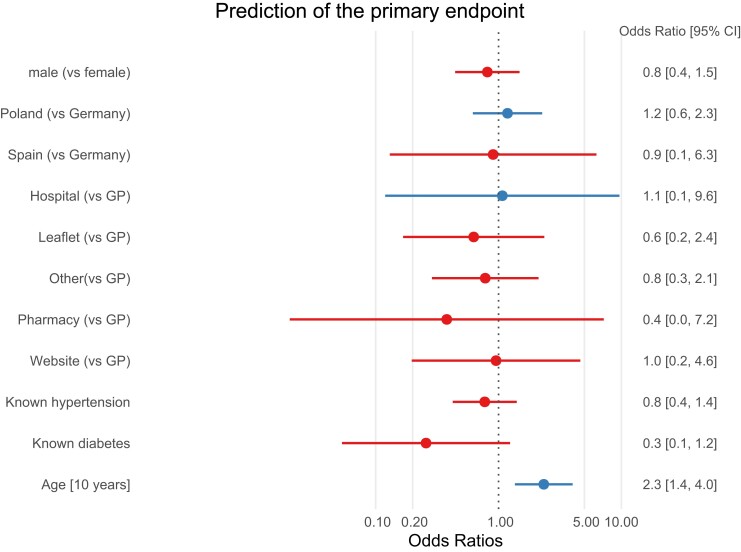
Participants with atrial arrhythmias were older than those without atrial arrhythmias (Table 1 and Figure 4). There were no differences in the detection of atrial arrhythmias by region, by route of invitation to the study, or by sex (Table 1 and Figure 4). Quality of life was similar in participants with atrial arrhythmias compared with those without atrial arrhythmias (Table 1). Older age was the only parameter associated with atrial arrhythmia detection in this study (Figure 4).
Figure 4.
Forest plot of factors associated with AA. Older age was the only factor associated with atrial arrhythmias in this study.
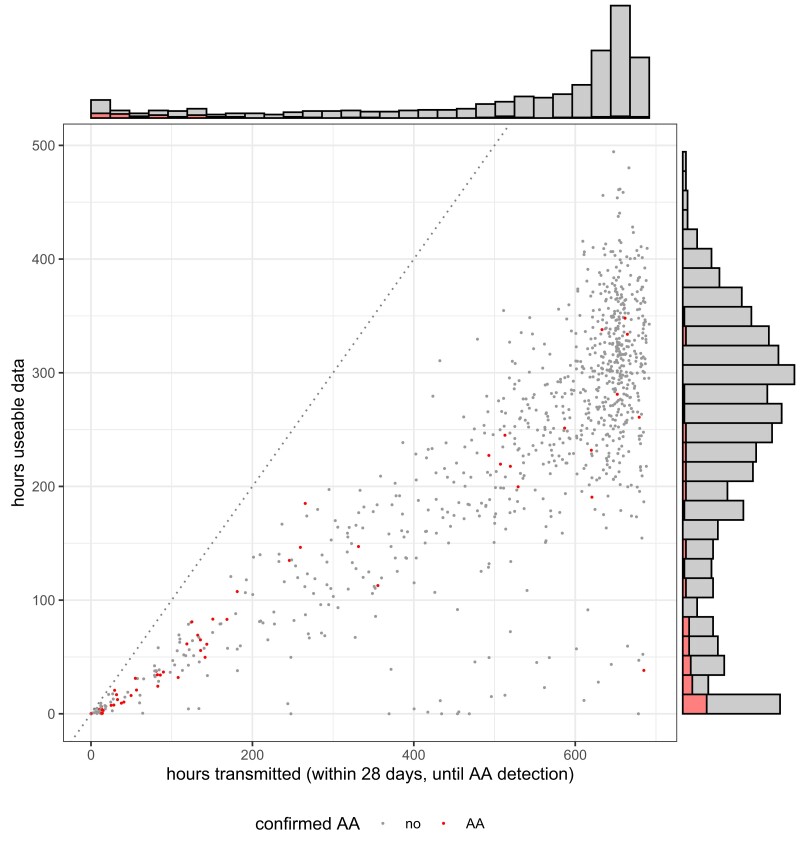
Participants transmitted a mean of 530 h of PPG recordings over the first 696 h of monitoring (76% of the maximal monitoring duration within 4 weeks plus the inclusion day. Of these, 240 h (45%) were of sufficient quality for rhythm analyses (Figure 3). The transmission rate dropped slightly to 400 h/28 days in weeks 5–8 of monitoring.
Figure 3.
Distribution of wearable screening duration (hours transmitted on x-axis, hours usable on y-axis) PPG screening analysis stopped after AA was detected and confirmed by the analysis service.
Time of day of AA detection was evaluated in participants with any AA detection (53/882, 6%): There were no differences between the number of transmitted PPG-minutes observed between daytime (6:00 am to 10:00 pm) and nighttime. While 73% of recorded PPG-minutes during nighttime could be used for AA detection, only 26% of recorded PPG-minutes during daytime were analysable (P < 0.0001). In participants with any AA detection, AA burden at night was 1.57-fold higher than during the daytime, with daytime AA burden of 9 min/h and nighttime AA burden of 14 min/h of analysable recording [(95% CI 1.15–2.14), P = 0.004]. Just over half of the participants (53%) participated without any in-person contact, while 47% of participants received personal assistance with the device. At the Barcelona site, the 30 participants were required to sign a written informed consent. Technical problems with Bluetooth coupling and recoupling, omission of recharging the smartphone, or local skin irritation during the summer heat, were reasons for queries to technical support and study sites and discontinuation of monitoring. In addition to communication routes via the app, SMS, email, and staff at the study sites, a central technical telephone support hotline was provided. About half of the participants (51%) contacted the central telephone hotline for queries. Most of the queries regarded pairing and coupling for data transfer.
All 53 participants with PPG-detected atrial arrhythmias were invited to undergo a 14-day event recorder ECG. Of these, 45 later underwent a 14-day event recorder ECG as part of the study. Eight participants did not have that test as part of the study, as they either had symptoms and were diagnosed with AF in the hospital or aimed to receive further diagnostics elsewhere with results not known. An additional random control sample without PPG-detected atrial arrhythmias underwent the same event monitoring. Event monitoring started with a median delay of 31 days (IQR 21, 48) after AA detection in PPG. Event-recorder Holter ECGs identified AF in 27/45 participants with previously PPG-detected arrhythmias, and none (0/7) participants without PPG-detected arrhythmias.
Participants undergoing the study Holter ECG filled in a second questionnaire depicted in Supplementary material online, Table S2. The estimated CHA2DS2-VASc-Score was 2.6 ± 1.4, sufficient to decide on oral anticoagulation.
Discussion
Main findings. Smartphone and wearable detected atrial arrhythmia in Older Adults Case finding study (Smart in OAC—AFNET 9) successfully deployed a fully digital, consumer-electronics based system to detect atrial arrhythmias in older adults in several European countries. The main results are:
A fully digital wearable system worn for 4 weeks identifies atrial arrhythmias in 5% of older adults (> 65 years of age).
The majority of participants were identified using targeted public media communications or direct contacts. Offers of remote technical assistance were accepted and compliance was high, showing feasibility and scalability for this age group if targeted.
Detection rates for atrial arrhythmias are high in the 1st week of PPG monitoring, and taper off thereafter, suggesting that relatively short monitoring periods may be sufficient to detect older adults with atrial arrhythmias.
These findings encourage the use of fully digital, consumer-electronics based PPG systems to screen for atrial arrhythmias in older adults.
Atrial arrhythmia detection in Smart in OAC—AFNET 9. We systematically reviewed the performance of electronic devices to screen for atrial arrhythmias in older adults 65 years of age and above. Five systematic reviews of arrhythmia detection via mobile health applications published between 2020 and 202225–28 yielded 28 potentially eligible studies. A MEDLINE search conducted on 25 February 2022 (search terms see Supplementary material online) revealed 235 unique records published between 2020 and 2022, containing 23 already found eligible via the initial five systematic reviews. During revision, the MEDLINE search was repeated and yielded additional 70 potentially eligible articles. Overall, 71 full texts were assessed which yielded 26 included studies (Table 2, Supplementary material online, Figure S1). In cases where age subgroup data were reported in a trial, it was still included and the incidence rate was calculated from the reported patient numbers.15,16 The same was performed when the original study only reported comparative outcomes such as hazard ratio but counts of diagnosed patients and totally screened patients were also reported.37
Published studies in populations and cohorts including a subgroup with a comparable age range and mostly comparable screening technologies reported atrial arrhythmia detection rates between 2.8 and 3.1%,15,16 less than the smartphone and wearable PPG-based incidence rate in Smart in OAC—AFNET 9 of 5% in 4 weeks. When screened populations were enriched using clinical risk factors or elevated NT-proBNP concentrations,31 incidence rates increased (2.7–4.4%,29,31,33) Published reports suggest that continuous PPG monitoring is associated with higher (2.5 -5.3%,30,32,34) arrhythmia detection rates than intermittent monitoring (0.9–3.8%,35–38) confirmed in this study. The rate of ECG-confirmed AF in the Smart in OAC substudy via Holter or the clinical setting was of 3.1–3.4% of the overall study population, within the range we had estimated in this age group, but lower than previous PPG-detected arrhythmias in the same study, pointing to the paroxysmal character of AF. However, the confirmation rate of 60% (27 AF-positive out of 45 AA-positively screened participants) is higher than in the younger population of the Apple Heart Study.15 This has several potential explanations. One reason could be that Smart in OAC only screened for arrhythmia episodes lasting 6 min or longer, while Apple Heart accepted shorter arrhythmia durations. Three remotely conducted, large and population- and consumer-technology based landmark trials in AF screening via continuous PPG monitoring are the Apple Heart Study,15 the Pre-MAFA II trial (Huawei Heart Study)16 and the Fitbit Heart Study.20 The AA screen positive rates were 0.52%, 0.23%, and 1.0% in the overall screened population and 3.1, 2.8, and 3.6% in those aged 65 years and older.
Much higher detection rates were observed when opportunistic screening was performed or when data from implantable loop recorders were used to screen pre-selected, multimorbid patient populations.6,52 Subclinical AF episodes lasting longer than 6 min were detected in 26% of patients in a study of continuous single lead ECG monitoring.34 Studies employing implantable loop recorders also employed the cut-off of 6 min6 and a recent meta-analysis suggests that stroke risk is very low in patients with episodes shorter than 6 min.9
The AA detection yield in Smart in OAC—AFNET 9 was slightly higher than in similar published screening trials in a comparable population only preselected by age above 65 years. Reasons for high AA yield could include the near continuous monitoring with a wearable PPG-sensor, and high compliance with wearing the device during nighttime. In participants in which AA was detected, the yield was nearly 1.6-fold elevated during nighttime (10 p.m to 6 a.m) compared with daytime even after correcting for better signal quality at night. In line with this observation, Deguchi et al.53 reported an elevated probability of AF onset around midnight from Holter-monitoring data of 217 patients with paroxysmal AF.
In 83% of participants, AA was detected within the first 28 days of monitoring and in most participants AA was detected within the first 14 days.
The minimal duration for arrhythmia detection used in Smart in OAC—AFNET 9 was 6 min.21 This is longer than the ESC guidelines definition of AF when detected on a clinical ECG, and longer than the minimal arrhythmia duration suggested for AF screening using consumer electronics in a recent EHRA guide.7 Rare arrhythmias of 6 min duration or more, detected within three months of screening using an implanted device, are associated with an increased risk of stroke.10,22 Six minutes of atrial arrhythmias are also sufficiently long to allow good differentiation of atrial arrhythmias from artefacts or other rhythm irregularities in wearables.19 These considerations informed our decision to screen for atrial arrhythmias of 6 min duration or more. The authors expect that there will be a gradual increase in the risk of ischaemic events that are preventable by oral anticoagulation as the arrhythmia duration, and by inference the arrhythmia burden, increases,10 a concept that was also presented in the most recent AFNET/EHRA consensus statement.12
It is therefore worth considering that screening pathways should address large cohorts or populations with rather short (14–28 days) but continuous monitoring periods, emphasizing night time monitoring, rather than unselective screening of smaller populations over a long time. Digital recruitment and consenting processes as demonstrated in Smart in OAC—AFNET 9 can help include large populations even during a pandemic. The recently published eBRAVE-AF screening trial invited 67.488 German private healthcare policyholders aged 50 years and over of whom 5551 (8.2%) with a median age of 65 years were digitally enrolled.54 The study compared the use of a smartphone camera PPG-based intermittent screening application to usual care in a cross-over design and could show increased yields of newly diagnosed AF; additionally, the median age was older than the digitally enrolling Apple or Huawei Heart studies. Smart in OAC—AFNET 9 targeted and enrolled an even older population following and openly advertised invitations independent of their insurer.
Apart from age, pre-selection of participants did not contribute to the increased screening yield in Smart in OAC—AFNET 9. Both age and self-reported estimated CHA2DS2-VASc-Score were comparable to or even lower than in similar studies29–31,33,34 and most participants were recruited via targeted public media communications and not from hospital patient pools. The ability of night-time recordings may however have increased the yield.
Limitations. While the communication around the study and the options for participation were designed to enable inclusive participation, we cannot exclude some selection of participants that may have influenced the observed atrial arrhythmia detection rate, based on access to a personal smartphone and wireless internet access. Our study targeted the older European population, and the participants were therefore mostly white. Observations may differ in other ethnicities. The remote study design relied on self-reporting of pre-existing medical conditions like known AF, hypertension, or diabetes as well as demographic data by participants. This may have contributed to comparably low reported rates of concomitant medical conditions in this population and also in the screen-positive AA group. Self-reported numbers in this study for hypertension and diabetes were similar to those observed in STROKESTOP.5
The design of our study included subsequent Holter ECG event recorder assessment in participants with positive PPG AA to ensure that participants would be reassured or receive a diagnosis of AF and subsequent treatment as required. Due to the transient nature of paroxysmal atrial arrhythmias and the lack of a simultaneous PPG signal analysis together with the ECG (as the PPG analysis stopped after a positive screen), the assessment in this study does not provide valid information on diagnostic accuracy. We still report results of Holter ECGs as these can be expected in clinical practice if PPG is used for screening. Performing Holter-ECGs on negatively screened participants was limited by operational difficulties as some participants without relevant findings were less keen to undergo further tests and staff of centres were less motivated to provide access to the Holter-ECGs to negatively screened participants during COVID-19 waves. In the future, this could be partially overcome by a central distribution system.
Adverse events directly associated with the PPG recording were minor skin reactions to the wristband and were only reported during the summer months (see Supplementary material online, Table S1). A changeable cotton wristband was offered to replace the standard silicone wristband and participants were able to use any personal wristband of their choice if it could be attached to the PPG unit.
Data on the cost effectiveness of the tested screening system have been published.55,56 The results of Smart in OAC—AFNET 9 will be an important component of a planned health economic (HE) analysis which is beyond the scope of this report.
Conclusions
A fully digital, wearable based PPG screening identifies atrial arrhythmias in 5% of an openly invited population of older adults of 65 years or above without previously known AF or anticoagulation therapy. Advertising targeting older populations and remote technical support when needed enable broad participation and adequate monitoring durations. The majority of atrial arrhythmias were detected a few weeks after the initiation of screening.
SMART in OAC—AFNET 9 results provide robust information on the prevalence of PPG-detected atrial arrhythmias in older adults. The study provides data on different methods to reach out to such populations to offer arrhythmia screening and on patient characteristics with PPG-detected arrhythmias. The study thus generates robust information for the planning of an outcome trial.
Author contributions
A.Z., E.G., L.F., R.B.S., T.H., P.K., and U.S. planned the study. R.B.S., P.K., L.F., A.Z.-.H., D.H., E.C., D.C., and K.J. designed the protocol. K.J., E.C., A.Z.-H., L.F., R.B.S., D.C., and E.G. designed lay-friendly information and prepared ethics applications. R.B.S., S.J.W., A.Z.-H., D.D., and E.G. contributed to participant recruitment and further diagnostics via study centres. A.Z. and E.V. were responsible for statistical analysis. J.O., K.J., and L.F. prepared the literature meta-analysis. E.V., J.O., and L.F. prepared result figures and tables. L.F., P.K., and E.V. wrote the manuscript. All authors made critical comments on the manuscript. L.F., E.V., J.O., P.K., and K.J. revised the study in response to reviewer comments.
Supplementary Material
Acknowledgements
We thank study participants, all colleagues at study sites and at AFNET for their contribution to the study, especially Anke Heiermann and Simone Neumann.
Contributor Information
L Fabritz, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, Martinistr. 52, 20251 Hamburg, Germany; DZHK German Center for Cardiovascular Research, partner site Hamburg/Luebeck/Kiel, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston Wolfson Drive, B15 2TT Birmingham, UK; Atrial Fibrillation NETwork (AFNET), Mendelstr 11, 48149 Münster, Germany.
D L Connolly, Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston Wolfson Drive, B15 2TT Birmingham, UK; Department of Cardiology and R&D, Birmingham City Hospital, Sandwell and West Birmingham Trust, Dudley Road, B18 7QH Birmingham, UK.
E Czarnecki, Atrial Fibrillation NETwork (AFNET), Mendelstr 11, 48149 Münster, Germany.
D Dudek, Jagiellonian University Medical College, Center for Digital Medicine and Robotics, Ul. Kopernika 7E, 33-332 Kraków, Poland; Maria Cecilia Hospital, Via Corriera, 1, 48033 Cotignola RA, Italy.
E Guasch, Institut Clínic Cardio-Vascular, Hospital Clínic, University of Barcelona, Carrer de Villaroel, 170, 08036 Barcelona, CA, Spain, Spain; IDIBAPS, Rosselló 149-153, 08036 Barcelona, CA, Spain; CIBERCV, Monforte de Lemos 3-5, Pabellon 11, Planta 0, 28029 Madrid, Spain.
D Haase, Atrial Fibrillation NETwork (AFNET), Mendelstr 11, 48149 Münster, Germany.
T Huebner, Preventicus GmbH, Ernst-Abbe-Straße 15, 07743 Jena, Germany.
A Zlahoda-Huzior, Department of Measurement and Electronics, AGH University of Science and Technology, Al. Mickiewicza 30, 30-059 Kraków, Poland.
K Jolly, Institute of Applied Health Research, University of Birmingham, Edgbaston, B15 2TT Birmingham, UK.
P Kirchhof, Department of Cardiology, University Heart and Vascular Center Hamburg, Martinistr. 52, 20251 Hamburg, Germany; DZHK German Center for Cardiovascular Research, partner site Hamburg/Luebeck/Kiel, Germany; Institute of Cardiovascular Sciences, University of Birmingham, Edgbaston Wolfson Drive, B15 2TT Birmingham, UK; Atrial Fibrillation NETwork (AFNET), Mendelstr 11, 48149 Münster, Germany.
J Obergassel, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, Martinistr. 52, 20251 Hamburg, Germany; DZHK German Center for Cardiovascular Research, partner site Hamburg/Luebeck/Kiel, Germany.
U Schotten, Atrial Fibrillation NETwork (AFNET), Mendelstr 11, 48149 Münster, Germany; Department of Physiology, Cardiovascular Research Institute Maastricht, Maastricht University Medical Center +, Debyelaan 25, 6229 HX, Maastricht, The Netherlands.
E Vettorazzi, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Christoph-Probst-Weg 1, 20246 Hamburg, Germany.
S J Winkelmann, University Center of Cardiovascular Science, University Heart and Vascular Center Hamburg, University Medical Center Hamburg-Eppendorf, Martinistr. 52, 20251 Hamburg, Germany; Department of Cardiology, University Heart and Vascular Center Hamburg, Martinistr. 52, 20251 Hamburg, Germany.
A Zapf, Institute of Medical Biometry and Epidemiology, University Medical Center Hamburg-Eppendorf, Christoph-Probst-Weg 1, 20246 Hamburg, Germany.
R B Schnabel, Department of Cardiology, University Heart and Vascular Center Hamburg, Martinistr. 52, 20251 Hamburg, Germany; DZHK German Center for Cardiovascular Research, partner site Hamburg/Luebeck/Kiel, Germany; Atrial Fibrillation NETwork (AFNET), Mendelstr 11, 48149 Münster, Germany.
Lead author biography
 Prof Dr Larissa Fabritz studied medicine in Heidelberg, Münster, Lille, Concepcion, and Zurich, including a research year in Washington, DC, USA. Larissa trained at University Hospital Münster, Germany, with habilitation (D.S.) and specialist registration. In 2011, Larissa accepted a tenured clinical academic position at the University of Birmingham, UK, and was promoted to Chair in Cardiovascular Sciences there. She holds a Chair in Inherited Cardiac Conditions at the Center of Cardiovascular Sciences, UKE Hamburg, Germany. Larissa published on arrhythmias and cardiomyopathies. As a member of ESC working groups, EHRA, DZHK, and AFNET steering committee, she contributes to European research consortia and ESC guidelines.
Prof Dr Larissa Fabritz studied medicine in Heidelberg, Münster, Lille, Concepcion, and Zurich, including a research year in Washington, DC, USA. Larissa trained at University Hospital Münster, Germany, with habilitation (D.S.) and specialist registration. In 2011, Larissa accepted a tenured clinical academic position at the University of Birmingham, UK, and was promoted to Chair in Cardiovascular Sciences there. She holds a Chair in Inherited Cardiac Conditions at the Center of Cardiovascular Sciences, UKE Hamburg, Germany. Larissa published on arrhythmias and cardiomyopathies. As a member of ESC working groups, EHRA, DZHK, and AFNET steering committee, she contributes to European research consortia and ESC guidelines.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health online.
Funding
The design of this investigator-initiated trial was developed by the authors and AFNET. Sponsor of the trial is AFNET (https://www.kompetenznetz-vorhofflimmern.de). Financial support was provided from Daiichi Sankyo Europe to AFNET. Preventicus provided the cloud based analytic service Preventicus Heartbeats for analysing the PPG data and screen for AA; plus the comprehensive IT backbone. L.F., P.K., E.G., and U.S. received funding from EU Horizon 2020 CATCH ME (grant agreement number 633196), and MAESTRIA (grant agreement number 965286). L.F. and P.K. have received funding from Accelerator Award by the British Heart Foundation AA/18/2/34218. L.F. is further part-funded by National Institute for Health and Care Research (NIHR) award 1002898 (APRAISE-AS) at University of Birmingham, AFFECT-EU grant agreement number 847770 and German Center for Cardiovascular Research (DZHK). U.S. received funding from the Dutch Heart Foundation (CVON2014–09, RACE V Reappraisal of Atrial Fibrillation: Interaction between hyperCoagulability, Electrical remodeling, and Vascular Destabilisation in the Progression of AF). R.B.S. has received funding from the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation programme (grant agreement number 648131), from the European Union’s Horizon 2020 research and innovation programme [grant agreement number 847770 (AFFECT-EU)] and German Center for Cardiovascular Research (DZHK e.V.) (81Z1710103), German Ministry of Research and Education (BMBF 01ZX1408A) and ERACoSysMed3 (031L0239). P.K. was partially supported by European Union BigData@Heart (grant agreement EU IMI 116074), AFFECT-AF (grant agreement number 847770), British Heart Foundation (PG/17/30/32961; PG/20/22/35093), German Centre for Cardiovascular Research supported by the German Ministry of Education and Research (DZHK), and Leducq Foundation. K.J. is part-funded by the National Institute for Health and Care Research (NIHR) Applied Research Collaboration (ARC) West Midlands and Medical Research Council. The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
Data availability
Data will be made available by AFNET on reasonable request.
References
- 1. US Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Epling JW Jr, Kubik M, Li L, Ogedegbe G, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB. Screening for atrial fibrillation: US preventive services task force recommendation statement. JAMA 2022;327:360–367. [DOI] [PubMed] [Google Scholar]
- 2. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbuchel H, Hindricks G, Kautzner J, Kuck KH, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns H, Breithardt G, EAST-AFNET 4 Trial Investigators . Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med 2020;383:1305–316. [DOI] [PubMed] [Google Scholar]
- 3. Grond M, Jauss M, Hamann G, Stark E, Veltkamp R, Nabavi D, Horn M, Weimar C, Kohrmann M, Wachter R, Rosin L, Kirchhof P. Improved detection of silent atrial fibrillation using 72-hour Holter ECG in patients with ischemic stroke: a prospective multicenter cohort study. Stroke 2013;44:3357–3364. [DOI] [PubMed] [Google Scholar]
- 4. Haeusler KG, Kirchhof P, Kunze C, Tutuncu S, Fiessler C, Malsch C, Olma MC, Jawad-Ul-Qamar M, Kramer M, Wachter R, Michalski D, Kraft A, Rizos T, Groschel K, Thomalla G, Nabavi DG, Rother J, Laufs U, Veltkamp R, Heuschmann PU, Endres M, Mon DI. Systematic monitoring for detection of atrial fibrillation in patients with acute ischaemic stroke (MonDAFIS): a randomised, open-label, multicentre study. Lancet Neurol 2021;20:426–436. [DOI] [PubMed] [Google Scholar]
- 5. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–1506. [DOI] [PubMed] [Google Scholar]
- 6. Svendsen JH, Diederichsen SZ, Hojberg S, Krieger DW, Graff C, Kronborg C, Olesen MS, Nielsen JB, Holst AG, Brandes A, Haugan KJ, Kober L. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet 2021;398:1507–1516. [DOI] [PubMed] [Google Scholar]
- 7. Svennberg E, Tjong F, Goette A, Akoum N, Di Biase L, Bordachar P, Boriani G, Burri H, Conte G, Deharo JC, Deneke T, Drossart I, Duncker D, Han JK, Heidbuchel H, Jais P, de Oliviera Figueiredo MJ, Linz D, Lip GYH, Malaczynska-Rajpold K, Marquez M, Ploem C, Soejima K, Stiles MK, Wierda E, Vernooy K, Leclercq C, Meyer C, Pisani C, Pak HN, Gupta D, Purerfellner H, Crijns H, Chavez EA, Willems S, Waldmann V, Dekker L, Wan E, Kavoor P, Turagam MK, Sinner M. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PubMed] [Google Scholar]
- 8. Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ, ASSERT-II Investigator . Subclinical atrial fibrillation in older patients. Circulation 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 9. Mahajan R, Perera T, Elliott AD, Twomey DJ, Kumar S, Munwar DA, Khokhar KB, Thiyagarajah A, Middeldorp ME, Nalliah CJ, Hendriks JML, Kalman JM, Lau DH, Sanders P. Subclinical device-detected atrial fibrillation and stroke risk: a systematic review and meta-analysis. Eur Heart J 2018;39:1407–1415. [DOI] [PubMed] [Google Scholar]
- 10. Bertaglia E, Blank B, Blomstrom-Lundqvist C, Brandes A, Cabanelas N, Dan GA, Dichtl W, Goette A, de Groot JR, Lubinski A, Marijon E, Merkely B, Mont L, Piorkowski C, Sarkozy A, Sulke N, Vardas P, Velchev V, Wichterle D, Kirchhof P. Atrial high-rate episodes: prevalence, stroke risk, implications for management, and clinical gaps in evidence. Europace 2019;21:1459–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van Gelder IC, Healey JS, Crijns HJ, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, Rienstra M, Connolly SJ. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 12. Schnabel RB, Marinelli EA, Arbelo E, Boriani G, Boveda S, Buckley CM, Camm AJ, Casadei B, Chua W, Dagres N, de Melis M, Desteghe L, Diederichsen SZ, Duncker D, Eckardt L, Eisert C, Engler D, Fabritz L, Freedman B, Gillet L, Goette A, Guasch E, Svendsen JH, Hatem SN, Haeusler KG, Healey JS, Heidbuchel H, Hindricks G, Hobbs FDR, Hubner T, Kotecha D, Krekler M, Leclercq C, Lewalter T, Lin H, Linz D, Lip GYH, Lochen ML, Lucassen W, Malaczynska-Rajpold K, Massberg S, Merino JL, Meyer R, Mont L, Myers MC, Neubeck L, Niiranen T, Oeff M, Oldgren J, Potpara TS, Psaroudakis G, Purerfellner H, Ravens U, Rienstra M, Rivard L, Scherr D, Schotten U, Shah D, Sinner MF, Smolnik R, Steinbeck G, Steven D, Svennberg E, Thomas D, True Hills M, van Gelder IC, Vardar B, Pala E, Wakili R, Wegscheider K, Wieloch M, Willems S, Witt H, Ziegler A, Daniel Zink M, Kirchhof P. Early diagnosis and better rhythm management to improve outcomes in patients with atrial fibrillation: the 8th AFNET/EHRA consensus conference. Europace 2022:euac062. doi: 10.1093/europace/euac062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, Mairesse GH, Mabo P, Camm AJ, Healey JS. Rationale and design of the Apixaban for the reduction of thrombo-embolism in patients with device-detected sub-clinical atrial fibrillation (ARTESiA) trial. Am Heart J 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 14. Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC, Goette A, Huening A, Lip GYH, Simantirakis E, Vardas P. Probing oral anticoagulation in patients with atrial high rate episodes: rationale and design of the non-vitamin K antagonist oral anticoagulants in patients with atrial high rate episodes (NOAH-AFNET 6) trial. Am Heart J 2017;190:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perez MV, Mahaffey KW, Hedlin H, Rumsfeld JS, Garcia A, Ferris T, Balasubramanian V, Russo AM, Rajmane A, Cheung L, Hung G, Lee J, Kowey P, Talati N, Nag D, Gummidipundi SE, Beatty A, Hills MT, Desai S, Granger CB, Desai M, Turakhia MP. Apple heart study I. Large-scale assessment of a smartwatch to identify atrial fibrillation. N Engl J Med 2019;381:1909–1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Guo Y, Wang H, Zhang H, Liu T, Liang Z, Xia Y, Yan L, Xing Y, Shi H, Li S, Liu Y, Liu F, Feng M, Chen Y, Lip GYH, MAFA II Investigators . Mobile photoplethysmographic technology to detect atrial fibrillation. J Am Coll Cardiol 2019;74:2365–2375. [DOI] [PubMed] [Google Scholar]
- 17. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Albert CM, Anderson CS, Antoniou S, Benjamin EJ, Boriani G, Brachmann J, Brandes A, Chao TF, Conen D, Engdahl J, Fauchier L, Fitzmaurice DA, Friberg L, Gersh BJ, Gladstone DJ, Glotzer TV, Gwynne K, Hankey GJ, Harbison J, Hillis GS, Hills MT, Kamel H, Kirchhof P, Kowey PR, Krieger D, Lee VWY, Levin LA, Lip GYH, Lobban T, Lowres N, Mairesse GH, Martinez C, Neubeck L, Orchard J, Piccini JP, Poppe K, Potpara TS, Puererfellner H, Rienstra M, Sandhu RK, Schnabel RB, Siu CW, Steinhubl S, Svendsen JH, Svennberg E, Themistoclakis S, Tieleman RG, Turakhia MP, Tveit A, Uittenbogaart SB, Van Gelder IC, Verma A, Wachter R, Yan BP, AF-Screen Collaborators . Screening for atrial fibrillation: A report of the AF-SCREEN international collaboration. Circulation 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 18. Koenig N, Seeck A, Eckstein J, Mainka A, Huebner T, Voss A, Weber S. Validation of a new heart rate measurement algorithm for fingertip recording of video signals with smartphones. Telemed J E Health 2016;22:631–636. [DOI] [PubMed] [Google Scholar]
- 19. Dorr M, Nohturfft V, Brasier N, Bosshard E, Djurdjevic A, Gross S, Raichle CJ, Rhinisperger M, Stockli R, Eckstein J. The WATCH AF trial: SmartWATCHes for detection of atrial fibrillation. JACC Clin Electrophysiol 2019;5:199–208. [DOI] [PubMed] [Google Scholar]
- 20. Lubitz SA, Faranesh AZ, Atlas SJ, McManus DD, Singer DE, Pagoto S, Pantelopoulos A, Foulkes AS. Rationale and design of a large population study to validate software for the assessment of atrial fibrillation from data acquired by a consumer tracker or smartwatch: the fitbit heart study. Am Heart J 2021;238:16–26. [DOI] [PubMed] [Google Scholar]
- 21. Fabritz L, Connolly D, Czarnecki E, Dudek D, Zlahodaa-Huzior A, Guasch E, Haase D, Huebner T, Jolly K, Kirchhof P, Schotten U, Zapf A, Schnabel RB. Remote design of a smartphone and wearable detected atrial arrhythmia in older adults case finding study: smart in OAC—AFNET 9. Front Cardiovasc Med 2022:9:839202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 23. Reiffel JA, Verma A, Kowey PR, Halperin JL, Gersh BJ, Wachter R, Pouliot E, Ziegler PD, Investigators RA. Incidence of previously undiagnosed atrial fibrillation using insertable cardiac monitors in a high-risk population: the REVEAL AF study. JAMA Cardiol 2017;2:1120–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kirchhof P, Bax J, Blomstrom-Lundquist C, Calkins H, Camm AJ, Cappato R, Cosio F, Crijns H, Diener HC, Goette A, Israel CW, Kuck KH, Lip GY, Nattel S, Page RL, Ravens U, Schotten U, Steinbeck G, Vardas P, Waldo A, Wegscheider K, Willems S, Breithardt G. Early and comprehensive management of atrial fibrillation: executive summary of the proceedings from the 2nd AFNET-EHRA consensus conference ‘research perspectives in AF’. Eur Heart J 2009;30:2969–2977c. [DOI] [PubMed] [Google Scholar]
- 25. Perales CRL, Van Spall HGC, Maeda S, Jimenez A, Latcu DG, Milman A, Kirakoya-Samadoulougou F, Mamas MA, Muser D, Casado Arroyo R. Mobile health applications for the detection of atrial fibrillation: a systematic review. Europace 2021;23:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Varma N, Cygankiewicz I, Turakhia M, Heidbuchel H, Hu Y, Chen LY, Couderc JP, Cronin EM, Estep JD, Grieten L, Lane DA, Mehra R, Page A, Passman R, Piccini J, Piotrowicz E, Piotrowicz R, Platonov PG, Ribeiro AL, Rich RE, Russo AM, Slotwiner D, Steinberg JS, Svennberg E. 2021 ISHNE/HRS/EHRA/APHRS collaborative statement on mHealth in arrhythmia management: digital medical tools for heart rhythm professionals: from the international society for Holter and noninvasive electrocardiology/heart rhythm society/European heart rhythm association/Asia pacific heart rhythm society. J Arrhythm 2021;37:271–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ding EY, Marcus GM, McManus DD. Emerging technologies for identifying atrial fibrillation. Circ Res 2020;127:128–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kahwati LC, Asher GN, Kadro ZO, Keen S, Ali R, Coker-Schwimmer E, Jonas DE. Screening for atrial fibrillation: updated evidence report and systematic review for the US preventive services task force. JAMA 2022;327:368–383. [DOI] [PubMed] [Google Scholar]
- 29. Langer A, Healey JS, Quinn FR, Honos G, Nault I, Tan M, Camara D, Newman DM, Godin R, Program AA. Detection of atrial fibrillation in asymptomatic at-risk individuals. Int J Cardiol 2021;334:55–57. [DOI] [PubMed] [Google Scholar]
- 30. Gladstone DJ, Wachter R, Schmalstieg-Bahr K, Quinn FR, Hummers E, Ivers N, Marsden T, Thornton A, Djuric A, Suerbaum J, von Grunhagen D, McIntyre WF, Benz AP, Wong JA, Merali F, Henein S, Nichol C, Connolly SJ, Healey JS, Investigators SA, Coordinators . Screening for atrial fibrillation in the older population: a randomized clinical trial. JAMA Cardiol 2021;6:558–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gudmundsdottir KK, Fredriksson T, Svennberg E, Al-Khalili F, Friberg L, Frykman V, Hijazi Z, Rosenqvist M, Engdahl J. Stepwise mass screening for atrial fibrillation using N-terminal B-type natriuretic peptide: the STROKESTOP II study. Europace 2020;22:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rooney MR, Soliman EZ, Lutsey PL, Norby FL, Loehr LR, Mosley TH, Zhang M, Gottesman RF, Coresh J, Folsom AR, Alonso A, Chen LY. Prevalence and characteristics of subclinical atrial fibrillation in a community-dwelling elderly population: the ARIC study. Circ Arrhythm Electrophysiol 2019;12:e007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Steinhubl SR, Waalen J, Edwards AM, Ariniello LM, Mehta RR, Ebner GS, Carter C, Baca-Motes K, Felicione E, Sarich T, Topol EJ. Effect of a home-based wearable continuous ECG monitoring patch on detection of undiagnosed atrial fibrillation: the mSToPS randomized clinical trial. JAMA 2018;320:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heckbert SR, Austin TR, Jensen PN, Floyd JS, Psaty BM, Soliman EZ, Kronmal RA. Yield and consistency of arrhythmia detection with patch electrocardiographic monitoring: the multi-ethnic study of atherosclerosis. J Electrocardiol 2018;51:997–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berge T, Brynildsen J, Larssen HKN, Onarheim S, Jenssen GR, Ihle-Hansen H, Christophersen IE, Myrstad M, Rosjo H, Smith P, Tveit A. Systematic screening for atrial fibrillation in a 65-year-old population with risk factors for stroke: data from the Akershus Cardiac Examination 1950 study. Europace 2018;20:f299–f305. [DOI] [PubMed] [Google Scholar]
- 36. Ghazal F, Theobald H, Rosenqvist M, Al-Khalili F. Validity of daily self-pulse palpation for atrial fibrillation screening in patients 65 years and older: a cross-sectional study. PLoS Med 2020;17:e1003063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Halcox JPJ, Wareham K, Cardew A, Gilmore M, Barry JP, Phillips C, Gravenor MB. Assessment of remote heart rhythm sampling using the AliveCor heart monitor to screen for atrial fibrillation: the REHEARSE-AF study. Circulation 2017;136:1784–1794. [DOI] [PubMed] [Google Scholar]
- 38. Svennberg E, Engdahl J, Al-Khalili F, Friberg L, Frykman V, Rosenqvist M. Mass screening for untreated atrial fibrillation: the STROKESTOP study. Circulation 2015;131:2176–2184. [DOI] [PubMed] [Google Scholar]
- 39. Poulsen PB, Hemmingsen U, Melgaard TA, Elleby HB, Wedell-Wedellsborg D, Dybro L, Lund IM, Dixen U, Frost L. Feasibility of screening for atrial fibrillation in a domiciliary setting: opportunistic one-time screening at preventive home visits in municipalities. Scand Cardiovasc J 2022;56:243–246. [DOI] [PubMed] [Google Scholar]
- 40. Senoo K, Yukawa A, Ohkura T, Shoji K, Takigami M, Iwakoshi H, Nishimura T, Nakata M, Teramukai S, Matoba S. Screening for untreated atrial fibrillation in the elderly population: a community-based study. PLoS ONE 2022;17:e0269506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen Y, Huang QF, Sheng CS, Zhang W, Shao S, Wang D, Cheng YB, Wang Y, Guo QH, Zhang DY, Li Y, Lowres N, Freedman B, Wang JG. Detection rate and treatment gap for atrial fibrillation identified through screening in community health centers in China (AF-CATCH): a prospective multicenter study. PLoS Med 2020;17:e1003146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zaprutko T, Zaprutko J, Baszko A, Sawicka D, Szalek A, Dymecka M, Telec W, Kopciuch D, Ratajczak P, Michalak M, Rafal D, Szyszka A, Nowakowska E. Feasibility of atrial fibrillation screening with Mobile health technologies at pharmacies. J Cardiovasc Pharmacol Ther 2020;25:142–151. [DOI] [PubMed] [Google Scholar]
- 43. Orchard J, Li J, Freedman B, Webster R, Salkeld G, Hespe C, Gallagher R, Patel A, Kamel B, Neubeck L, Lowres N. Atrial fibrillation screen, management, and guideline-recommended therapy in the rural primary care setting: a cross-sectional study and cost-effectiveness analysis of eHealth tools to support all stages of screening. J Am Heart Assoc 2020;9:e017080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gwynn J, Gwynne K, Rodrigues R, Thompson S, Bolton G, Dimitropoulos Y, Dulvari N, Finlayson H, Hamilton S, Lawrence M, MacNiven R, Neubeck L, Rambaldini B, Taylor K, Wright D, Freedman B. Atrial fibrillation in indigenous Australians: a multisite screening study using a single-lead ECG device in aboriginal primary health settings. Heart Lung Circ 2021;30:267–274. [DOI] [PubMed] [Google Scholar]
- 45. Sun W, Freedman B, Martinez C, Wallenhorst C, Yan BP. Atrial fibrillation detected by single time-point handheld electrocardiogram screening and the risk of ischemic stroke. Thromb Haemost 2022;122:286–294. [DOI] [PubMed] [Google Scholar]
- 46. Nicole Verbiest-van Gurp, Uittenbogaart SB, Lucassen WAM, Erkens PMG, Knottnerus JA, Winkens B, Stoffers H, van Weert H. Detection of atrial fibrillation in primary care with radial pulse palpation, electronic blood pressure measurement and handheld single-lead electrocardiography: a diagnostic accuracy study. BMJ Open 2022;12:e059172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jatau AI, Bereznicki LR, Wimmer BC, Bezabhe WM, Peterson GM. Improving knowledge and early detection of atrial fibrillation through a community-based opportunistic screening program: what's your beat? Int J Environ Res Public Health 2022;19:6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Denas G, Battaggia A, Fusello M, Franco-Novelletto B, Cancian M, Scalisi A, Pengo V. General population screening for atrial fibrillation with an automated rhythm-detection blood pressure device. Int J Cardiol 2021;322:265–270. [DOI] [PubMed] [Google Scholar]
- 49. Zhang W, Chen Y, Miao CY, Huang QF, Sheng CS, Shao S, Wang D, Xu SK, Lei L, Zhang D, Chen YL, Hu LX, Xia JH, Ye XF, Cheng YB, Wang Y, Guo QH, Li Y, Lowres N, Freedman B, Wang JG, Coordinators A-CI . Quarterly versus annual ECG screening for atrial fibrillation in older Chinese individuals (AF-CATCH): a prospective, randomised controlled trial. Lancet Healthy Longevity 2021;2:E470–E478. [DOI] [PubMed] [Google Scholar]
- 50. Lubitz SA, Atlas SJ, Ashburner JM, Lipsanopoulos ATT, Borowsky LH, Guan W, Khurshid S, Ellinor PT, Chang Y, McManus DD, Singer DE. Screening for atrial fibrillation in older adults at primary care visits: VITAL-AF randomized controlled trial. Circulation 2022;145:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watanabe E, Takahashi N, Aronson R, Ohsawa A, Ishibashi Y, Murakawa Y, SCAN-AF Investigators . Systematic screening for atrial fibrillation in patients at moderate-to-high risk of stroke- potential to increase the atrial fibrillation detection rate (SCAN-AF). Circ J 2022;86:1245–1251. [DOI] [PubMed] [Google Scholar]
- 52. Philippsen TJ, Christensen LS, Hansen MG, Dahl JS, Brandes A. Detection of subclinical atrial fibrillation in high-risk patients using an insertable cardiac monitor. JACC Clin Electrophysiol 2017;3:1557–1564. [DOI] [PubMed] [Google Scholar]
- 53. Deguchi Y, Amino M, Adachi K, Matsuzaki A, Iwata O, Yoshioka K, Watanabe E, Tanabe T. Circadian distribution of paroxysmal atrial fibrillation in patients with and without structural heart disease in untreated state. Ann Noninvasive Electrocardiol 2009;14:280–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rizas KD, Freyer L, Sappler N, von Stulpnagel L, Spielbichler P, Krasniqi A, Schreinlechner M, Wenner FN, Theurl F, Behroz A, Eiffener E, Klemm MP, Schneidewind A, Zens M, Dolejsi T, Mansmann U, Massberg S, Bauer A. Smartphone-based screening for atrial fibrillation: a pragmatic randomized clinical trial. Nat Med 202228:1823–1830. [DOI] [PubMed] [Google Scholar]
- 55. Wahler S, Birkemeyer R, Alexopoulos D, Siudak Z, Muller A, von der Schulenburg JM. Cost-effectiveness of a photopethysmographic procedure for screening for atrial fibrillation in 6 European countries. Health Econ Rev 2022;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Birkemeyer R, Muller A, Wahler S, von der Schulenburg JM. A cost-effectiveness analysis model of preventicus atrial fibrillation screening from the point of view of statutory health insurance in Germany. Health Econ Rev 2020;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available by AFNET on reasonable request.