Abstract
Aims
Despite general awareness that screening for atrial fibrillation (AF) could reduce health hazards, large-scale implementation is lagging behind technological developments. As the successful implementation of a screening programme remains challenging, this study aims to identify facilitating and inhibiting factors from healthcare providers’ perspectives.
Methods and results
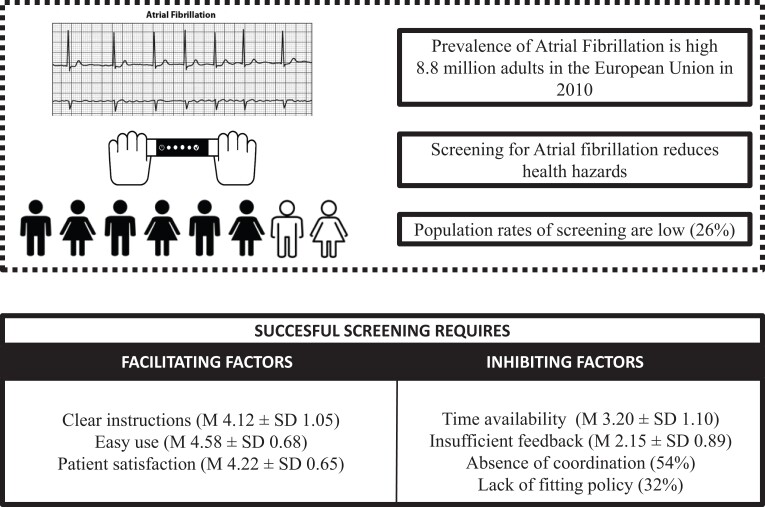
A mixed-methods approach was used to gather data among practice nurses in primary care in the southern region of the Netherlands to evaluate the implementation of an ongoing single-lead electrocardiogram (ECG)-based AF screening programme. Potential facilitating and inhibiting factors were evaluated using online questionnaires (N = 74/75%) and 14 (of 24) semi-structured in-depth interviews (58.3%). All analyses were performed using SPSS 26.0. In total, 16 682 screenings were performed on an eligible population of 64 000, and 100 new AF cases were detected. Facilitating factors included ‘receiving clear instructions’ (mean ± SD; 4.12 ± 1.05), ‘easy use of the ECG-based device’ (4.58 ± 0.68), and ‘patient satisfaction’ (4.22 ± 0.65). Inhibiting factors were ‘time availability’ (3.20 ± 1.10), ‘insufficient feedback to the practice nurse’ (2.15 ± 0.89), ‘absence of coordination’ (54%), and the ‘lack of fitting policy’ (32%).
Conclusion
Large-scale regional implementation of an AF screening programme in primary care resulted in a low participation of all eligible patients. Based on the perceived barriers by healthcare providers, future AF screening programmes should create preconditions to fit the intervention into daily routines, appointing an overall project lead and a General Practitioner (GP) as a coordinator within every GP practice.
Keywords: Atrial fibrillation, Screening programme, Single-lead-handheld ECG-based screening device, Evaluation
Graphical Abstract
Graphical Abstract.
Introduction
Prevalence rates of atrial fibrillation (AF) are sharply increasing due to the ageing population. In 2010, 8.8 million adults in the European Union were estimated to suffer from AF, and this number is expected to more than double by the year 2060.1–3 Accordingly, clinical outcomes (e.g. stroke, systemic embolism and all-cause mortality) are extensive, and healthcare costs will further increase in the upcoming years.4–7 In a recent multicentre randomized controlled trial called STROKESTOP, screening for AF showed a small net benefit compared with standard of care, indicating that screening is safe and beneficial in older populations.8 In contrast, a comparable randomized controlled trial in four centres in Denmark (LOOP-trial) showed no significant reduction in the risk of stroke or systemic arterial embolism.9 Whereas other studies found AF screening to detect undiagnosed patients,6 improve clinical outcomes and decrease overall costs,5,6 the success of current AF screening programmes varies.10 For instance, previous AF screening programmes in the Netherlands have indicated that the diagnostic yield largely depends on the context of screening.8,11–14 The D2AF study has highlighted that the number of newly detected AF in cardiovascular risk management (CVRM) programmes does not substantially increase with the implementation of extensive screening methods.12 Moreover, the participation rate of eligible individuals is often substantially lower than intended.15
With the growing number of technological solutions, AF screening is expected to be easier and more widely available in the near future.10 In recent years, devices such as mobile phones, wrist-worn wearables, and single-lead handheld electrocardiogram (ECG)-based screening devices have been proven to accurately detect AF,5,16–18 showing that technology is no longer a limiting factor in screening interventions. Despite rapid technological advances, studies on opportunistic AF screening in primary care and community screening are scarce.12,19 Frequently used screening methods include systematic and opportunistic screening strategies. Opportunistic screening20 mandates that a healthcare professional check explicitly for AF during routine consultations in the entire population, while systematic screening20 is based on specific criteria such as age. Currently, it is unclear which AF screening strategy should be applied in clinical practice.21,22 Moreover, clear guidance on large-scale implementation of technology-assisted AF screening programmes is lacking. Whereas the European Heart Rhythm Association provided practical guidelines on using digital devices to detect and manage arrhythmias,18 advice on how to implement the most appropriate screening strategy was not provided. In prior research on the implementation of health interventions, various generic factors that can positively or negatively affect an implementation process were found such as the complexity and clarity of the intervention or innovation, user knowledge about the intervention, self-efficacy, and organizational elements such as staff turnover or financial resources and legislation.23 Screening interventions in other medical fields (e.g. cancer) revealed inhibiting factors for implementation such as unfavourable attitudes from the healthcare provider and limited resources.24,25 Specifically for AF screening programmes, more research is needed on implementation strategies to determine how to integrate optimal diagnostic methods in daily work routines.26
This study aims to assess facilitating and inhibiting factors among healthcare providers directly involved in the screening process by evaluating the implementation of a large-scale opportunistic AF screening programme in a unique real-world primary care setting. In particular, we focused on the healthcare provider’s perspective, as the healthcare professionals’ opinion (e.g. knowledge, attitude and time) is critical to identify barriers and facilitators for implementation.
Methods
Population and design
In order to answer the research question, this study evaluates a large regional AF screening programme. To this end, a distinction is made between the participation in the screening programme and the evaluation of the screening programme. The screening programme refers to the AF screening programme, and the evaluation of the screening programme refers to the cross-sectional study with the healthcare providers (practice nurses).
Screening programme
A total of 85 GP practices (39.9% of the total GP practices in the southeast of the Netherlands with approximately 800 000 residents) received an ECG-based screening device. Inclusion criteria were sufficient time for AF screening during the diabetes mellitus (DM) and CVRM programmes, the willingness of the practice nurse to receive training and register data, and the willingness of GPs to take charge of the diagnostic process when necessary. In these practices, a total of 90 000 patients participated in the DM and CVRM programmes, with one to four visits per patient per year. Further instructions were to only include elderly patients (65 years and older) and exclude patients with AF. Practice nurses used an ECG-based screening device (MyDiagnostick) to assess high-risk cardiac patients from the DM and CVRM programmes for undiagnosed AF between August 2018 and December 2020. In a prior study, the MyDiagnostick showed a 100% sensitivity and a 96% specificity for detecting AF and was described as easy to use and suitable for opportunistic AF screening.27
Evaluation of the implementation of the screening programme
The present study evaluates facilitating and inhibiting factors of implementing a screening intervention for AF patients in primary care using a mixed-methods approach consisting of online questionnaires and semi-structured in-depth interviews. Practice nurses who participated in the AF screening programme were approached to participate in an online questionnaire and subsequently asked to participate in a telephone-based semi-structured in-depth interview.
Procedure
Training practice nurses
Prior to the screening programme, practice nurses received instructions from project coordinators who visited the GP practices. The instructions contained information on using the MyDiagnostick (e.g. registration process by sending e-mail and processing information). Subsequently, multiple training moments were planned to inform practice nurses, GPs and other stakeholders with detailed procedural instructions.
Screening routine
Practice nurses were instructed to ask patients to hold the ECG-based screening device for 60 seconds. If the device detected AF (i.e. displayed by a red light), a second reading was performed. If a second red light was displayed, validation with a 12-leads ECG was performed and assessed by a cardiologist. In addition, practice nurses determined the Congestive heart failure or left ventricular dysfunction Hypertension, Age ≥75 (doubled), Diabetes, Stroke, (doubled)-Vascular disease, Age 65-74, Sex category (female) (CHA2DS2-VASc) and Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/alcohol concomitantly (HAS-BLED) risk score, kidney function, and medication intake, supervised by the GP, based on the patient’s medical record. This information was sent to the cardiologist for confirmation and policy regarding anticoagulation or referral to the hospital. In addition, the cardiologist provided advice on the anticoagulation policy and on further diagnostics and treatment, such as whether or not they should be referred to a cardiologist.
Evaluation of facilitators and barriers of the screening programme
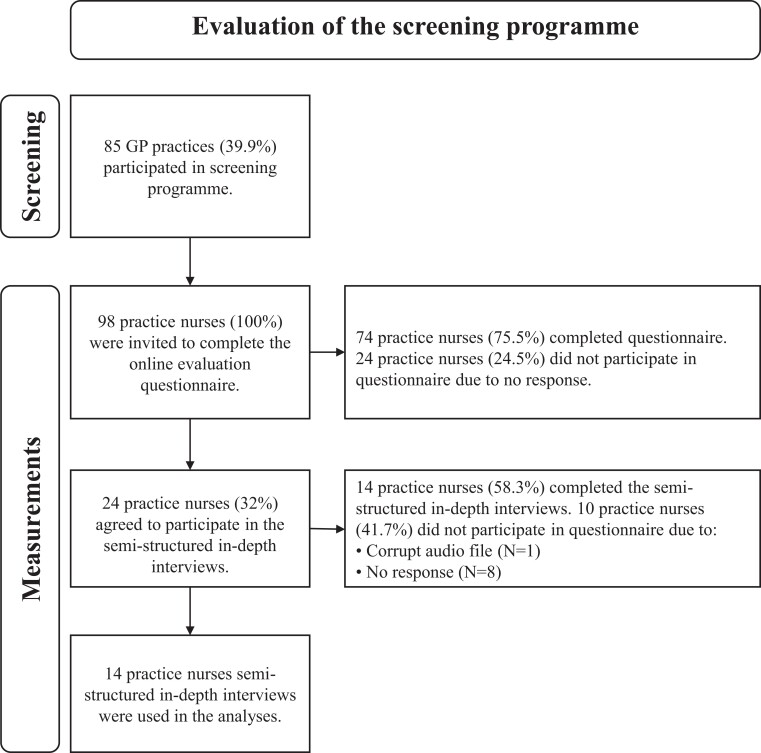
A total of 98 practice nurses were invited, and all provided informed consent and approved of their answers being used for research purposes. Facilitators and inhibitors to implementing the ECG-based screening programme were evaluated by gathering data from practice nurses via online questionnaires and semi-structured in-depth interviews. Practice nurses were invited to participate in the online questionnaires in February 2021 via e-mail. The participating practice nurses received two reminders (biweekly) via e-mail to complete the online questionnaire before March 2021. Furthermore, in the final question of the questionnaire, practice nurses were asked whether they were willing to participate in a semi-structured in-depth interview. Practice nurses who agreed to participate received an invite in April 2021. The interviews were conducted within 2 weeks via phone and (voice) recorded for data analysis and subsequently anonymized.
Measurements
Online questionnaire
The online questionnaire is based on the validated Measurement Instrument for Determinants of Innovations (MIDI).20 The questions were based on a five-point Likert scale (1: ‘totally disagree’ to 5: ‘totally agree’) and subdivided into four domains. The first domain, Innovation (Cronbach alpha = 0.77), referring to the ECG-based screening device, assessed seven different determinants (e.g. procedural clarity and correctness based on factual knowledge). Secondly, the User (Cronbach alpha = 0.78) refers to the practice nurse and contains ten determinants (e.g. personal benefits of the intervention and personal drawbacks). Thirdly, the Organization (Cronbach alpha = 0.71) refers to the GP practice and contains 11 determinants (e.g. staff capacity, availability and feedback to the user). The last domain, consisting of only one question, was the Socio-political context which refers to compliance with the GP practice’s legislation and regulations. In the present study, the MIDI was adapted to fit the purpose of the present study, which is common when using the MIDI23,28 (e.g. some questions have binary or three answer options). A full version of the online questionnaire is provided in Supplementary material online, Appendix 1.
Semi-structured in-depth interviews
The semi-structured in-depth interviews were also adapted from the MIDI questionnaire based on the online questionnaire responses. During the telephone interviews, practice nurses were asked to briefly describe how and by whom the ECG-based screening device was used in their GP practice and how many times a week. In addition, concerning the domain of Innovation, they were asked to indicate the advantages and disadvantages of using the ECG-based screening device. An example question for the domain User was ‘Do you think that the ECG-based screening device fits well within the current guidelines and protocols for patients within the CVRM or DM consultation hours?’. For the domains Organization and Socio-political context, practice nurses were asked whether they felt the GP practices supported the use of the ECG-based screening device and whether the AF screening programme fit well within their GP practices policy.
Statistical analysis
The online questionnaire was analysed per domain using basic descriptive statistics (i.e. percentage, means and standard deviation) with IBM SPSS, version 26.0.29 Mean and standard deviation was displayed for every determinant per domain (mean ± SD), using a scale from 1–5 with higher scores indicating higher satisfaction. In addition, questions phrased from negative to positive were recoded, and for the binary questions, the percentage of ‘yes’ responses will be presented. Finally, the internal reliability of the determinants was assessed with Cronbach’s alpha coefficient.
Audio recordings of the semi-structured in-depth interviews were analysed, summarized and stored by an independent researcher. The transcripts were coded deductively, and the researchers reached a consensus on the chosen broad themes.30 The insights obtained from the semi-structured in-depth interviews were used to support the interpretation of the quantitative data.
Results
From the approximated 64 000 patients participating in the DM and CVRM programmes who were eligible for AF screening, the ECG-based device was held 16 682 times between August 2018 and December 2020, and for 245 (1.5%), there was an indication of AF. After validation with a 12-leads ECG, AF was confirmed in 100 patients (0.6%). As detailed in the flowchart in Figure 1, 98 practice nurses were invited to complete the online questionnaire to evaluate the implementation of the screening programme. A total of 74 (76%) practice nurses completed the online questionnaire, and 24 practice nurses agreed to participate in a semi-structured in-depth interview; 15 completed the interviews (62.5%). Due to a corrupt audio recording 14 interviews were available for analysis.
Figure 1.
Flowchart detailing the GP practices and practice nurses that participated in the screening and the evaluation of the implementation of the screening.
Facilitators and barriers per domain
Innovation
The results of the questionnaires are displayed in Table 1. Regarding the domain innovation, practice nurses reported the instructions about the intervention to be clear (mean ± SD; 4.12 ± 1.05). Furthermore, all practice nurses in the semi-structured in-depth interviews (n = 14) reported an overall positive experience with the instructions. In addition, the practice nurses found the ECG-based screening device uncomplicated (4.58 ± 0.68) and relevant to patients (4.09 ± 0.85). However, the visibility of outcomes was rated lower (3.19 ± 1.12) due to the fact that no additional information is displayed on the device except a red or green light.
Table 1.
Means (M or %) and standard deviations (SD) of the online questionnaire per domain
| N = 74 | |||
|---|---|---|---|
| No. | Innovation | M | SD |
| 1. | Procedural clarity of the use of the ECG-based screening device | 4.12 | 1.05 |
| 2. | Correctness based on factual knowledge | 3.85 | 0.84 |
| 3. | Completeness of supplied information | 4.03 | 0.95 |
| 4. | Complexity of usea | 4.58 | 0.68 |
| 5. | Congruence with GP practice policy | 3.91 | 0.92 |
| 6. | Visibility of outcomes | 3.19 | 1.12 |
| 7. | Relevance for the patient | 4.09 | 0.85 |
| User | |||
| 8A. | Personal benefit of using the ECG-based screening device | 3.68 | 0.98 |
| 8B. | Personal drawbacks of using the ECG-based screening deviceb | 3.20 | 1.10 |
| 9A. | Outcome expectation: Importance on possible detection of AF | 3.68 | 1.04 |
| 9B. | Outcome expectation: Likelihood of possible detection of AF | 3.73 | 0.67 |
| 10. | Job perception | 3.68 | 0.81 |
| 11. | Patient satisfaction of using the ECG-based screening device | 4.22 | 0.65 |
| 12. | Client (patients cooperation in using ECG-based screening device) | 4.45 | 0.58 |
| 13. | Social support (sufficient help from my colleagues) | 4.08 | 0.64 |
| 14. | Descriptive normc | 1.86 | 0.87 |
| 15A. | Subjective norm: Normative beliefs (expectations from colleagues on the use of ECG-based screening device in the GP practice) | 3.58 | 0.70 |
| 15B. | Subjective norm: Motivation to comply (caring about the opinion of others) | 3.31 | 0.72 |
| 16. | Self-efficacy expectation about the ability to use the ECG-based screening device to possibly detect AF | 3.81 | 0.84 |
| 17. | Sufficient knowledge about how to use the ECG-based screening device | 4.28 | 0.61 |
| 18. | Awareness of content of innovation | 3.65 | 0.58 |
| Organization | |||
| 19. | Formal ratification by managemente | 32% | 0.47 |
| 20. | Replacement when staff leave | 2.70 | 0.98 |
| 21. | Staff capacity | 3.55 | 0.94 |
| 22. | Financial resources | 3.47 | 0.69 |
| 23. | Time available to explore the innovation | 3.68 | 0.83 |
| 24. | Availability of material resources and facilities | 3.93 | 0.69 |
| 25. | Coordinatore | 46% | 0.50 |
| 26. | Unrest in the organizatione | 58% | 0.50 |
| 27. | Information available about use of innovation | 3.91 | 0.62 |
| 28. | Feedback to user about innovation process | 2.15 | 0.89 |
| Socio-political context | |||
| 29. | Legislation and regulations | 3.76 | 0.57 |
A higher mean score indicates that a healthcare professional perceives this determinant less as a barrier to implementing (ranging from 1 to 5).
Determinant 4 is scored inversely for readability (low score is an indicator of high complexity).
Determinant 8B is scored inversely for readability (low score is an indicator of little perceived disadvantage).
Determinant 14 has 3 answer options: (i) Hardly any colleague; (ii) Half; (iii) Almost all colleagues.
Determinant 18 has 4 answer options: (i) I do not know the MyDiagnostick; (ii) I know the MyDiagnostick but have not received any instruction yet; (iii) I know the MyDiagnostick and have read the instruction superficially; (iv) I am familiar with the MyDiagnostick and have read the instruction completely and thoroughly.
Determinant 19, 25 and 26 are yes/no questions. The percentage with the answer ‘Yes’ is displayed.
User
Highly rated determinants within the domain User were satisfaction (4.22 ± 0.65), patient cooperation (4.45 ± 0.58), and use and knowledge (4.28 ± 0.61). Yet, half of the practice nurses (44.6%) lacked colleagues who used the ECG-based screening device as intended. In addition, practice nurses reported personal drawbacks from using the ECG-based screening device during consultations (3.20 ± 1.10), such as time available to integrate the intervention into their daily work routine, which was confirmed in the interviews. In addition, the interviewees (n = 5) experienced difficulty with the time it took to register a patient (35.7%). For example, in the interviews, a practice nurse stated, ‘The MyDiagnostick does not take more time than pulse palpating, but the procedure afterwards [Is time consuming]’.
Organization and socio-political context
In 27 (31.7%) GP practices, agreements about the use of the device were made in strategic plans, work plans or otherwise. In about half (45.8%) of the participating GP practices, a coordinator was appointed for the use of the ECG-based screening device. The majority of the GP practices (57.6%) experienced difficulties during the screening due to changing circumstances such as cutbacks, staff changes or the simultaneous deployment of other innovations in their organization. Furthermore, most practices (44,6%) had only one practice nurse trained in the ECG-based screening device, so there was no substitute available in their absence (2.70 ± 0.98), nor were there regular internal meetings about the intervention’s progress. In addition, practice nurses were generally dissatisfied with the lack of feedback about the screening process (2.15 ± 0.89), which was confirmed in the semi-structured in-depth interviews by 35.7% of the interviewees (n = 5). Legislation and regulations were not perceived as an important barrier (3.76 ± 0.57).
Discussion
Despite a well-organized screening programme and relatively high satisfaction, the number of people screened was low in this real-world study in a typical primary care environment. According to healthcare providers, factors, such as the usability of the ECG-based device, sufficient time to explore the intervention, receiving regular feedback, and a clear project leader, should be included in implementing such screening programmes.
According to the practice nurses, several inhibiting factors for implementation were related to the organization of the GP practice. Firstly, the amount of time to explore the intervention during their workday was not sufficient to integrate the intervention into their daily work routines, which is, according to a prior study,31 essential for a successful screening programme. Secondly, similar to what was found in a prior study,32 there was no replacement in case of the absence of a practice nurse, which resulted in a significantly lower percentage of people screened than initially planned.
Other inhibiting factors reported include a lack of feedback regarding the screening programme and a lack of a clear project lead to report to or address for questions. A clear leader who coordinates the screening process prompts higher levels of engagement and successful implementation5 and can motivate the practice nurses and GPs by using persuasive technologies, which are tools for motivating behaviour change such as competition, comparison, and cooperation.33 Another factor that inhibited the screening was that leadership in the GP practices was lacking. Most practice nurses indicated that they feel unsupported by the GP and rarely had staff meetings. Thus, the lack of leadership in the GP practice calls for the GP to take over the coordination of future screening programmes. In addition, practice nurses had different expectations due to the lack of information provided to them and lack of consequences in case they did not follow the intervention guidelines. The GP did not replace personnel in case of absence, which stagnated the intervention in case of absence. Future screening programmes should therefore adapt internal procedures in case of absent personnel.
Another factor that may have negatively influenced implementation is the users’ expectation. In general, the expectation of the practice nurses about the amount of newfound AF did not match with the actual amount of detected AF, resulting in disappointment (cognitive dissonance). Practice nurses should have been better informed, as this could have minimized the gap between their expectations and reality, as suggested in a different study34 on the expectation-confirmation model (ECM). The ECM is considered one of the notable theories that explain users’ post-adoption behaviour. It is based on the expectation-confirmation theory, which reflects the academic validity of relationships among users’ intention to repurchase, satisfaction, perceived performance, and expectations.34 Thus, the EMC should be incorporated in the implementation strategy. An additional strategy that may be applied to increase motivation levels, performance goals, and perceived abilities is the integration of motivational elements such as peer competition within the GP practice.35 Findings from a prior study20 indicated that motivation is an essential facilitator for successfully implementing a screening programme in primary care. The competition gives a sense of accomplishment, comparison allows for subtle and empowering peer pressure, and cooperation provides opportunities for mutual support, group encouragement, and reinforcement and offers opportunities to collaborate, make, and interact with friends.33 However, as intrinsic motivation is not always self-evident, it is often necessary to receive encouragement from others.20 The GPs within each GP practice should have motivated the practice nurses (to increase the feeling of involvement). In addition, an essential element of motivating people to join and care about a project or intervention is to include them in the process and disclose and discuss a clear target goal with them.36 Throughout the screening programme, practice nurses indicated that they did not to understand or know the overall goal, did not prioritize the intervention and did not see the value of using the innovation over regular pulse palpation, which suggests a need for increasing their involvement.36 Therefore, it is advisable to include practice nurses in disclosing and discussing the intervention purpose.
Limitations
This study suffered from some limitations. First, this study may have been subject to selection bias since practice nurses who participated in the semi-structured in-depth interviews (N = 14) could be considered early adopters. Early adopters often have favourable perceptions of a new intervention.37 Additionally, the in-depth interviews may contain socially desirable answers due to participants’ reluctance to criticize the intervention. However, this limitation is not expected to have a significant impact due to the anonymization of the results. In fact, the anonymization may even have resulted in a more negative attitude.38 Secondly, our study found that most GP practices had only one practice nurse trained. However, we did not have information on whether these practices had only one practice nurse available, or whether one nurse among multiple nurses was trained to use the single-lead ECG device. Resultingly, we could not distinguish the differences in facilitators and barriers between practices with only one and practices with multiple nurses. Thirdly, during the study, some of the screened patients visited the GP practice multiple times and were, therefore, screened for AF multiple times. As a result, we are not able to determine exactly how many patients were screened. Therefore, only the amount of times the ECG-based screening device was held can be confirmed. As the primary focus of this study was the evaluation of the implementation of the screening programme, we believe this will not have impacted the results. Finally, the time between the screening programme and the evaluation interviews was 1 year due to COVID-19 (patients were not allowed to come to the GP office), which could have caused participants to forget details when answering the online questionnaire.25
Conclusion
Future screening programmes may be more successful if more time is provided for them during the workday and if the organizational policy is adapted to fit the intervention. Furthermore, implementation may benefit from appointing a clear project lead who can provide regular feedback about the screening programme, monitor the screening process, and motivate the practice nurses and GPs. In addition, GP leadership in GP practices is essential to support practice nurses by having staff meetings, providing time to explore and implement the intervention and replacing absent personnel.
Supplementary Material
Acknowledgements
We would like to thank the general practitioners, practice nurses and diagnostic centre ‘Diagnostiek voor U’ for their active participation and execution of the screenings programme.
Contributor Information
Luc J H J Theunissen, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands; Máxima Medical Centre, De Run 4600, 5504DB, Veldhoven, The Netherlands; Department of Electrical Engineering, Technical University, 5612 AZ, Eindhoven, The Netherlands.
Reyan B E M Abdalrahim, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands; Department of Electrical Engineering, Technical University, 5612 AZ, Eindhoven, The Netherlands.
Lukas R C Dekker, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands; Department of Electrical Engineering, Technical University, 5612 AZ, Eindhoven, The Netherlands; Catharina hospital, Michelangelolaan 2, 5623 EJ, Eindhoven, The Netherlands.
Eric J M Thijssen, Máxima Medical Centre, De Run 4600, 5504DB, Veldhoven, The Netherlands.
Sylvie F A M S de Jong, Elkerliek hospital, Wesselmanlaan 25, 5707 HA, Helmond, The Netherlands.
Peter E Polak, St. Anna hospital, Bogardeind 2, 5664 EH, Geldrop, The Netherlands.
Pepijn H van de Voort, Catharina hospital, Michelangelolaan 2, 5623 EJ, Eindhoven, The Netherlands.
Geert Smits, GP Organization PoZoB, Bolwerk 10-14, 5509 MH, Veldhoven, The Netherlands.
Karin Scheele, GP Organization PoZoB, Bolwerk 10-14, 5509 MH, Veldhoven, The Netherlands.
Annelies Lucas, Diagnostics for You, Boschdijk 1119, 5626 AG, Eindhoven, The Netherlands.
Dennis P A van Veghel, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands; Catharina hospital, Michelangelolaan 2, 5623 EJ, Eindhoven, The Netherlands.
Henricus-Paul Cremers, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands.
Jeroen A A van de Pol, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands; Department of Electrical Engineering, Technical University, 5612 AZ, Eindhoven, The Netherlands.
Hareld M C Kemps, Netherlands Heart Network, De Run 4600, 5504 DB, Veldhoven, The Netherlands; Máxima Medical Centre, De Run 4600, 5504DB, Veldhoven, The Netherlands; Department of Industrial Design, Eindhoven University of Technology, 5612 AZ, Eindhoven, The Netherlands.
Supplementary material
Supplementary material is available at European Heart Journal – Digital Health
Funding
Funding was received by pharmaceutical companies Bayer and Boehringer Ingelheim regarding the single-lead ECG-based device (MyDiagnostick). No other funding was received for this study.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Chan PH, Wong CK, Pun L, Wong YF, Wong MM, Chu DW, Siu CW. Diagnostic performance of an automatic blood pressure measurement device, microlife WatchBP home A, for atrial fibrillation screening in a real-world primary care setting. BMJ Open 2017;7:e013685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Himmelreich JCL, Lucassen WAM, Harskamp RE. CHARGE-AF in a national routine primary care electronic health records database in the Netherlands: validation for 5-year risk of atrial fibrillation and implications for patient selection in atrial fibrillation screening. Open Heart 2021;8:e001459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lippi G, Sanchis-Gomar F, Cervellin G. Global epidemiology of atrial fibrillation: an increasing epidemic and public health challenge. Int J Stroke 2021;16:217–221. [DOI] [PubMed] [Google Scholar]
- 4. Krijthe BP, Kunst A, Benjamin EJ, Lip GY, Franco OH, Hofman A, Witteman JCM, Stricker BH, Heeringa J. Projections on the number of individuals with atrial fibrillation in the European Union, from 2000 to 2060. Eur Heart J 2013;34:2746–2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Freedman B, Camm J, Calkins H, Healey JS, Rosenqvist M, Wang J, Wijeratne T. Screening for atrial fibrillation: a report of the AF-SCREEN international collaboration. Circulation 2017;135:1851–1867. [DOI] [PubMed] [Google Scholar]
- 6. Jacobs MS, Kaasenbrood F, Postma MJ, van Hulst M, Tieleman RG. Cost-effectiveness of screening for atrial fibrillation in primary care with a handheld, single-lead electrocardiogram device in the Netherlands. Europace 2018;20:12–18. [DOI] [PubMed] [Google Scholar]
- 7. Wodchis WP, Bhatia RS, Leblanc K, Meshkat N, Morra D. A review of the cost of atrial fibrillation. Value Health 2012;15:240–248. [DOI] [PubMed] [Google Scholar]
- 8. Svennberg E, Friberg L, Frykman V, Al-Khalili F, Engdahl J, Rosenqvist M. Clinical outcomes in systematic screening for atrial fibrillation (STROKESTOP): a multicentre, parallel group, unmasked, randomised controlled trial. Lancet 2021;398:1498–506. [DOI] [PubMed] [Google Scholar]
- 9. Svendsen JH, Diederichsen SZ, Højberg S, Krieger DW, Graff C, Kronborg C, Olesen MS, Nielsen JB, Holst AG, Brandes A, Haugan KJ, Køber L. Implantable loop recorder detection of atrial fibrillation to prevent stroke (The LOOP Study): a randomised controlled trial. Lancet 2021;398:1507–1516. [DOI] [PubMed] [Google Scholar]
- 10. Welton NJ, McAleenan A, Thom HH, Davies P, Hollingworth W, Higgins JP, Okoli G, Sterne JA, Feder G, Eaton D, Hingorani A, Fawsitt C, Lobban T, Bryden P, Richards A, Sofat R. Screening strategies for atrial fibrillation: a systematic review and cost-effectiveness analysis. Health Technol Assess 2017;21:1–236. [DOI] [PubMed] [Google Scholar]
- 11. Lowres N, Neubeck L, Redfern J, Freedman SB. Screening to identify unknown atrial fibrillation: a systematic review. Thromb Haemost 2013;110:213–222. [DOI] [PubMed] [Google Scholar]
- 12. Uittenbogaart SB, Verbiest-van Gurp N, Lucassen WAM, Winkens B, Nielen M, Erkens PMG, Knottnerus JA, van Weert HCPM, Stoffers HEJH. Opportunistic screening versus usual care for detection of atrial fibrillation in primary care: cluster randomised controlled trial. BMJ 2020;370:m3208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rivezzi F, Vio R, Bilato C, Pagliani L, Pasquetoo G, Saccà S, Verlato R, Migliore F, Iliceto S, Bossone V, Bertaglia E. Screening of unknown atrial fibrillation through handheld device in the elderly. J Geriatr Cardiol 2020;17:495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zwart LAR, Jansen RWMM, Ruiter JH, Germans T, Simsek S, Hemels MEW. Opportunistic screening for atrial fibrillation with a single lead device in geriatric patients. J Geriatr Cardiol 2020;17:143–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Acharya A, Sounderajah V, Ashrafian H, Darzi A, Judah G. A systematic review of interventions to improve breast cancer screening health behaviours. Prev Med 2021;153:106828. [DOI] [PubMed] [Google Scholar]
- 16. Giebel GD, Gissel C. Accuracy of mHealth devices for atrial fibrillation screening: systematic review. JMIR Mhealth Uhealth 2019;7:e13641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang CM, Veiga C, Rodriguez-Andina JJ, Farina J, Iniguez A, Yin S. Using PPG signals and wearable devices for atrial fibrillation screening. IEEE Trans Ind Electron 2019;66:8832–8842. [Google Scholar]
- 18. Svennberg E, Tjong F, Goette A, Akoum N, Di Biaise L, Bordachar P, Boriana G, Burri H, Conte G, Deharo JC, Deneke T, Drossart I, Duncker D, Han JK, Heidbuchel H, Jais P, de Oliviera Figueiredo MJ, Linz D, Lip GYH, Malaczynska-Rajpold K, Márques M, Ploem C, Soejima K, Stiles MK, Wierda E, Vernooy K, Leclercq C, Meyer C, Pisani C, Pak HN, Gupta D, Pürerfellner H, Crijns HJGM, Chavez EA, Willems S, Waldmann V, Dekker L, Wan E, Kavoor P, Turagam MK, Sinner M. How to use digital devices to detect and manage arrhythmias: an EHRA practical guide. Europace 2022;24:979–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ashburner JM, Lee PR, Rivet CM, Barr Vermilya H, Lubitz SA, Zai AH. The implementation and acceptability of a mobile application for screening for atrial fibrillation at home. Telemed J E Health 2021;27:1305–1310. [DOI] [PubMed] [Google Scholar]
- 20. Orchard J, Li JL, Gallagher R, Freedman B, Lowres N, Neubeck L. Uptake of a primary care atrial fibrillation screening program (AF-SMART): a realist evaluation of implementation in metropolitan and rural general practice. Bmc Fam Pract 2019;20:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fitzmaurice DA, Hobbs FDR, Jowett S, Mant J, Murray ET, Holder R, Raftery JP, Bryan S, Davies M, Lip GYH, Allan TF. Screening versus routine practice in detection of atrial fibrillation in patients aged 65 or over: cluster randomised controlled trial. BMJ 2007;335:383–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moran PS, Teljeur C, Ryan M, Smith SM. Systematic screening for the detection of atrial fibrillation. Cochrane Db Syst Rev 2016;6:CD009586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleuren MAH, Paulussen TGWM, Van Dommelen P, Van Buuren S. Towards a measurement instrument for determinants of innovations. Int J Qual Health C 2014;26:501–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goossens J, De Roose M, Van Hecke A, Goemaes R, Verhaeghe S, Beeckman D. Barriers and facilitators to the provision of preconception care by healthcare providers: a systematic review. Int J Nurs Stud 2018;87:113–130. [DOI] [PubMed] [Google Scholar]
- 25. Wang GX, Baggett TP, Pandharipande PV, Park ER, Percac-Lima S, Shepard JAO, Fintelmann FJ, Flores EJ. Barriers to lung cancer screening engagement from the patient and provider perspective. Radiology 2019;290:278–287. [DOI] [PubMed] [Google Scholar]
- 26. Benjamin EJ, Go AS, Desvigne-Nickens P, Anderson CD, Casadei B, Chen LY, Crijns HJGM, Freedman B, Hills MT, Healey JS, Kamel H, Kim DY, Link MS, Lopes RD, Lubitz SA, McManus DD, Noseworthy PA, Perez MV, Piccini JP, Schnabel RB, Singer DE, Tieleman RG, Turakhia MP, van Gelder IC, Cooper LS, Al-Khatib SM. Research priorities in atrial fibrillation screening: a report from a national heart, lung, and blood institute virtual workshop. Circulation 2021;143:372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tieleman RG, Plantinga Y, Rinkes D, Bartels GL, Posma JL, Cator R, Hofman C, Houben RP. Validation and clinical use of a novel diagnostic device for screening of atrial fibrillation. Europace 2014;16:1291–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Wit LM, van Uden-Kraan CF, Lissenberg-Witte BI, Melissant HC, Fleuren MAH, Cuijpers P, Verdonck-de Leeuw IM. Adoption and implementation of a web-based self-management application “Oncokompas” in routine cancer care: a national pilot study. Support Care Cancer 2019;27:2911–2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Resino DA. Descriptive data analysis with IBM SPSS statistics. Rev Complut Educ 2018;29:313–314. [Google Scholar]
- 30. Wu R, Rossos P, Quan S, Reeves S, Lo V, Wong B, Cheung M, Morra D. An evaluation of the use of smartphones to communicate between clinicians: a mixed-methods study. J Med Internet Res 2011;13:e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sieverink F, Kelders S, Braakman-Jansen A, van Gemert-Pijnen J. Evaluating the implementation of a personal health record for chronic primary and secondary care: a mixed methods approach. Bmc Med Inform Decis 2019;19:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Taggar JS, Coleman T, Lewis S, Jones M. Screening for atrial fibrillation—a cross-sectional survey of healthcare professionals in primary care. Plos One 2016;11:e0152086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Orji R, Oyibo K, Lomotey RK, Orji FA. Socially-driven persuasive health intervention design: competition, social comparison, and cooperation. Health Inform J 2019;25:1451–1484. [DOI] [PubMed] [Google Scholar]
- 34. Park E. User acceptance of smart wearable devices: an expectation-confirmation model approach. Telemat Inform 2020;47:101318. [Google Scholar]
- 35. Chen CH, Law V, Chen WY. The effects of peer competition-based science learning game on secondary students’ performance, achievement goals, and perceived ability. Interact Learn Envir 2018;26:235–244. [Google Scholar]
- 36. Barmentloo LM, Dontje ML, Koopman MY, Olij BF, Oudshoorn C, Mackenbach JP, Polinder S, Erasmus V. Barriers and facilitators for screening older adults on fall risk in a hospital setting: perspectives from patients and healthcare professionals. Int J Environ Res Public Health 2020;17:1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Makam AN, Lanham HJ, Batchelor K, Moran B, Howell-Stampley T, Kirk L, Cherukuri M, Samal L, Santini N, Leykum LK, Halm EA. The good, the bad and the early adopters: providers’ attitudes about a common, commercial EHR. J Eval Clin Pract 2014;20:36–42. [DOI] [PubMed] [Google Scholar]
- 38. Clark-Gordon CV, Bowman ND, Goodboy AK, Wright A. Anonymity and online self-disclosure: a meta-analysis. Commun Rep 2019;32:98–111. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.