Abstract
We have synthesized novel pyrazolo-quinoline analogues (P1–10) in an effort to create newer antitubercular drugs against the rising bacterial resistance. NMR, IR and ESI-MS spectra were utilized to characterize the synthesised compounds. The antitubercular activity of the target compounds was evaluated against Mycobacterium tuberculosis. Six derivatives (P1–6) displayed very significant activity at 1.6 µg/mL concentration and were found to be more active than pyrazinamide standard. Thus, as per the drug susceptibility results the MIC value could be considered between 1.6 and 0.8 µg/mL. In addition, all the synthesised compounds were subjected to molecular docking studies against specific protein, Enoyl acyl carrier protein reductase (InhA) in complex with N-(4-methylbenzoyl)-4-benzylpiperidine, PDB ID: 2NSD. Among all the compounds the most effective compounds were found an Autodock score of 11.6 and 11.2 against 2NSD, respectively. Further, Zebrafish larvae have been used to test the teratogenicity of the synthesised compounds. There were no indications of abnormalities with (P2), (P4), (P5), (P6), and (P10) at 0.5 µM.
Supplementary Information
The online version contains supplementary material available at 10.1134/S1068162023010053.
Keywords: molecular docking, antitubercular, pyrazole, quinoline, zebrafish larvae
INTRODUCTION
The bacteria that cause Tuberculosis (TB) most frequently infect the lungs. TB is majorly transmitted through the air. It only takes a few germs for someone to become sick through inhalation. A lifetime risk of contracting TB is between 5 and 10% for those who have been exposed to the TB bacteria [1]. A person’s chance of getting sick is increased if they have a weakened immune system, diabetes, malnutrition, or HIV and to those who use tobacco. Following COVID-19 (behind HIV/AIDS), TB is the second infectious killer in the world and the 13th largest cause of death overall [2]. Antimycobacterial drug resistance in Mycobacterium tuberculosis strains is a growing issue on a global scale. Though, several medications are being readily available the significant increase in TB drug resistance (MDR-TB) has made it incredibly challenging to treat the illness [3]. Also, one of the biggest problems with TB control is that the current medications don’t work well or don’t work at all for people with HIV and who has XDR-TB [4].
Therefore, the scientists are continually developing new safe and effective anti-TB drugs and searching for new anti-TB pharmacophores.
As one of the essential building blocks for numerous therapeutic compounds, the quinoline core has attracted a lot of interest from both chemists and biologists in this field. In this class bedaquiline has been given the FDA’s approval as a new anti-tubercular drug [5, 6]. An oral medication called mefloquine, a quinoline derivative is being used to treat and prevent malaria. It is interesting to note that several of its analogues have been reported to have excellent antibacterial and anti-tubercular properties [7–10]. The WHO recommended ciprofloxacin and moxifloxacin, two quinolone-based antibiotics used to treat a variety of bacterial infections also as second-line anti-TB medications because they exhibit strong antitubercular action as well. Inhibiting the topoisomerase enzyme, which breaks down replicating DNA, is another advantage of these quinolone drugs. We have chosen to establish new bioactive compounds in this context using the quinoline skeleton.
Additionally, it is well known that pyrazole containing derivatives have a wide spectrum of therapeutic importance, including effects against cancer [11], inflammation [12], bacteria [13], viruses [14], and tuberculosis [15–17]. One of the virtual study demonstrated that pyrazole[1,5-a] pyrimidines are strongly binding to enoyl-ACP reductase (InhA) and were found potent in anti-tubercular agents [18]. The hybrid analogues of pyrazole-quinoline bearing fluorine possess strong binding affinity towards InhA, CYP121 and TMPK [19]. One of the essential enzymes, InhA, is required for M. tuberculosis to produce mycolic acids by catalysing the reduction of long-chain trans-2-enoyl-ACP. Mycolic acids are one of the main components of the mycobacterial cell wall, and are unable to biosynthesized in absence of InhA activity. These findings boosted our interest in looking for pyrazole-substituted possible pharmacologically active leads to target the InhA.
A unique hybrid molecule that is often more bioactive and efficacious than the parent drugs is created when the pharmacophoric units were combined from several bioactive compounds [20–22]. Additionally, this strategy was effective in generating new leads with better selectivity profiling, therapeutic efficacies, and less adverse effects. We have successfully synthesized a new set of hexahydro-5H-pyrazolo [3,4-b] quinoline-5-one derivatives in response to these findings and in search of potential antitubercular medications. The synthesised target compounds (P1–10) were evaluated for their antitubercular efficacy against the M. tuberculosis H37Rv strain. Further, we had undergone a docking study to examine their binding affinity at the active site of InhA. In this process the Zebrafish larvae, was employed to evaluate the teratogenicity effect of all synthesised compounds.
RESULTS AND DISCUSSION
Chemistry
Three steps were executed to make a series of 9‑(furan-2-carbonyl)-3,7,7-trimethyl substituted phenyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b] quinoline-5-one derivatives (P1–10) (Scheme 1). In the first step, dimedone and 3-amino-5-methyl pyrazole were reacted to produce 5-dimethyl-3-(5-methyl-2H-pyrazol-3-yl-amino) cyclohex-2-en-1-one (I). The compound (I) was reacted with different substituted aromatic aldehydes in the subsequent step under reflux in DMF for 2–3 h to produce 3,7,7- trimethyl substituted phenyl- 2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b] quinoline-5-ones (I1–10) as intermediates. The final step was reacting the intermediates (I1–10) with furoyl chloride to produce 9-(furan-2-carbonyl)-3,7,7-trimethyl substituted phenyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b] quinoline-5-one derivatives (P1–10) in quantifiable yields. Following elemental analysis, 1HNMR, IR, and ESI-MS spectra all of the derivatives were characterised.
 |
Scheme 1 . Reagents and conditions: (a) DMF, reflux for 3–4 h, (b) DMF, conc. HCl, reflux for 2–3 h, (c) ethanol, TEA, reflux for 5–6 h.
The presence of the NH signal as a singlet at 12.07 ppm, the pyrazole-NH signal as a singlet at 8.91 ppm, the singlet at 2.2 ppm due to the –CH3 protons of the pyrazole ring, and the singlet at 5.98 ppm corresponding to the –CH of Pyrazole in the 1H NMR spectrum served as proof for the formation of 5,5‑dimethyl-3-(5-methyl-2H-pyrazol-3-yl-amino) cyclohex-2-en-1-one (I). The elucidation of the synthesis of the intermediate compounds (I1–10) was accomplished by the utilisation of spectral and elemental analysis. For instance, a new multiplet between 7.0 and 6.95 in the 1H NMR spectrum of the (I1) molecule was caused by aromatic protons, clearly indicating smooth cyclization. The lack of signal from the pyrazole –CH proton may be taken as supporting evidence for structure (I1). Similar to this, spectral and elemental analysis provided proof of the synthesis of the title compounds (P1–10). In the 1H NMR spectrum of (P1), for instance, the signal of –NH at around 11.70 ppm was missing, and the formation of the final product is supported by the emergence of additional signals as singlets at about 8.19–8.00 ppm caused by protons of the furan ring. Additionally, the structures of compounds (P1–10) and their total proton counts are very well matched.
Anti-Tubercular Activity
Using the MABA, all the target compounds (P1–10) were tested against the M. tuberculosis H37Rv strain. Positive drug standards i.e., isoniazid, ethambutol, pyrazinamide, rifampicin, and streptomycin were used in the study. Table 1 displays the effects of the target and standard compounds. The test results screening reflected that all of the tested analogues (P1–10) were possessed excellent activity. The compounds (P1–6) were shown to be more effective than pyrazinamide (3.2 µg/mL) and to be very powerful inhibitors of M. tuberculosis growth at a concentration of 1.6 µg/mL. As per the results, the Minimum Inhibitory Concentration (MIC) values were found between 1.6 and 0.8 µg/mL. Compound (P7) stopped the growth of M. tuberculosis at a concentration of 3.2 µg/mL. Compound (P7) and (P8) found effective at 6.25 µg/mL. The significant anti-tubercular activity was due to the presence of heteroaryl group pyrazole linked to the quinoline ring. The fact that compounds (P1–6) was found promising in exhibiting excellent anti-tubercular action against the TB strain. It could be explained by the following way that the unsubstituted derivative prone to have potent biological activity greater than pyrazinamide. Presence of electron donating groups like methyl and methoxy (P1) and (P4) at 4th position and electron withdrawing groups like chlorine (P5) at 2nd position revealed potent activity to that of pyrazinamide. Surprisingly, the compounds (P8), (P9) and (P10) with electron withdrawing groups like nitro, flouro and chloro at 4th position showed moderate activity. It is indicated that the presence of electron withdrawing group at 4th position diminishing the activity. Besides, the electron withdrawing group (fluorine) at 3rd position in other analogues (P3) showed equipotent activity to the derivatives which are bearing electron donating group at 4th position (P1) and (P4). Interestingly, the analogues (P7) bearing bromine at 3rd position failed to show potent biological activity.
Table 1. .
Antitubercular activity results of the synthesized compounds
| S. no. | Sample | 100 µg/mL |
50 µg/mL |
25 µg/mL |
12.5 µg/mL |
6.25 µg/mL |
3.12 µg/mL |
1.6 µg/mL |
|---|---|---|---|---|---|---|---|---|
| 1 | P1 | S | S | S | S | S | S | S |
| 2 | P2 | S | S | S | S | S | S | S |
| 3 | P3 | S | S | S | S | S | S | S |
| 4 | P4 | S | S | S | S | S | S | S |
| 5 | P5 | S | S | S | S | S | S | S |
| 6 | P6 | S | S | S | S | S | S | S |
| 7 | P7 | S | S | S | S | S | R | R |
| 8 | P8 | S | S | S | S | S | R | R |
| 9 | P9 | S | S | S | S | S | S | R |
| 10 | P10 | S | S | S | S | R | R | R |
| 11 | Isoniazid | S | S | S | S | S | S | S |
| 12 | Ethambutol | S | S | S | S | S | S | S |
| 13 | Pyrazinamide | S | S | S | S | S | S | R |
| 14 | Rifampicin | S | S | S | S | S | S | S |
| 15 | Streptomycin | S | S | S | S | S | S | S |
S = Sensitive, R = Resistant
Molecular Docking Studies
Molecular docking studies were done on the title compounds (P1–10) against a certain enzyme, InhA in complex with N-(4-methylbenzoyl)-4-benzylpiperidine, PDB ID: 2NSD, with a resolution of 1.90. Autodoc 4.2 software was used to do studies on docking. For all of the compounds (P1–10) inside the active site, the energy of intermolecular contacts (in kcal/mol) and hydrogen bonding interactions between amino acid residues and functional groups of compounds are shown in Table 2.
Table 2.
Docked energies and amino acid residues of binding interactions of ligands and 2NSD
| S. no. | Ligand | Docked energy with 2NSD, kcal/mol | Interaction with amino acids |
|---|---|---|---|
| 1 | P1 | –9.1 | LEU217, LEU218, ARG225 |
| 2 | P2 | –8.2 | LEU217, LEU218, ARG225 |
| 3 | P3 | –11.6 | LEU217, LEU218, ARG225 |
| 4 | P4 | –8.8 | LEU217, ARG225 |
| 5 | P5 | –8.3 | LEU217, LEU218, ARG225 |
| 6 | P6 | –11.2 | LEU217, LEU218, ARG225 |
| 7 | P7 | –8.2 | ARG225 |
| 8 | P8 | –9.2 | LEU217, LEU218, ARG225 |
| 9 | P9 | –9.1 | LEU217, LEU218, ARG225 |
| 10 | P10 | –10.2 | LEU217, LEU218, ARG225 |
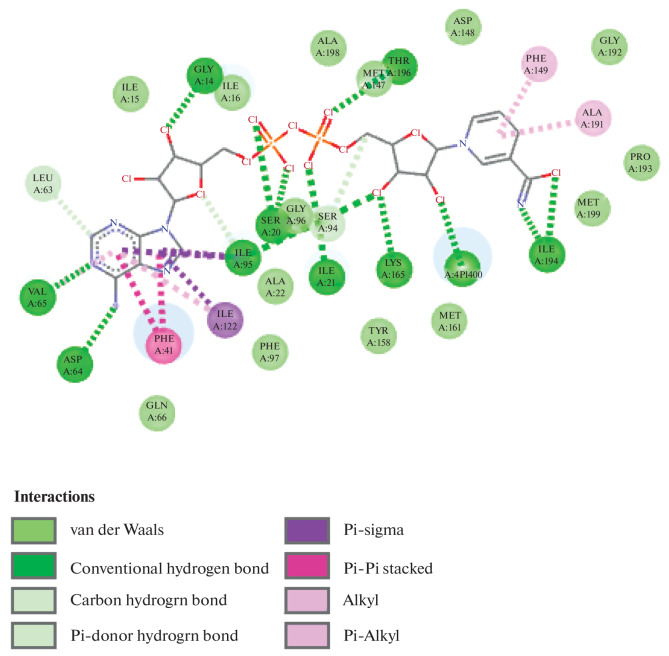
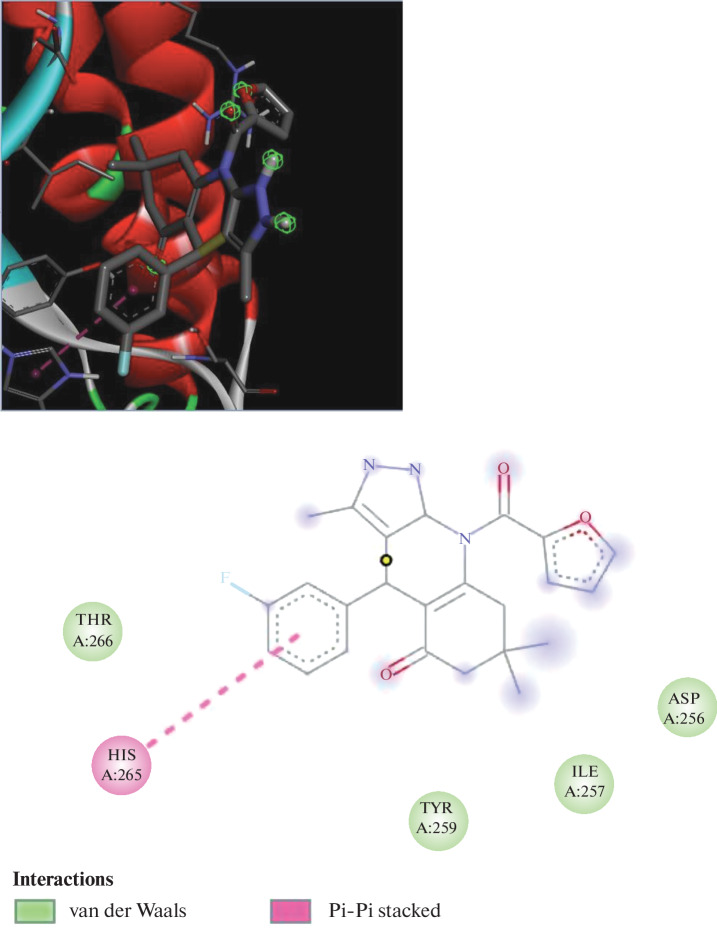
The docking data showed that every molecule was energetically advantageous in terms of Autodock score. By establishing hydrogen bonds with ASN1640 and ASP1644, the ligand docking investigations resulted in a docking score of –8.23 (Fig. 1). The most effective compounds, (P3) and (P6), had an Autodock score of 11.6 and 11.2 against the protein 2NSD, respectively shown in Fig. 2. When compared to the active compounds, the less active compounds had higher dock scores. According to these findings, less active compounds need more energy than highly active molecules in order to achieve good binding interactions with receptor enzymes. The most active substance, (P3), displayed a linkage with the amino acid residues LEU217, LEU218 by pi–pi bonding with the protein 2NSD and demonstrated a ketonic linkage with the amino acid ARG225 through hydrogen bonding. Therefore, greater Mycobacterium tuberculosis enoyl reductase inhibitory activity is anticipated. These binding interactions demonstrate the significance of the ketone group for favourable binding contact.
Fig. 1.
Ligand co-crystal gives a score of –8.23.
Fig. 2.
Docking poses of P3 derivative with protein PDB ID-2NSD.
Teratogenicity Studies
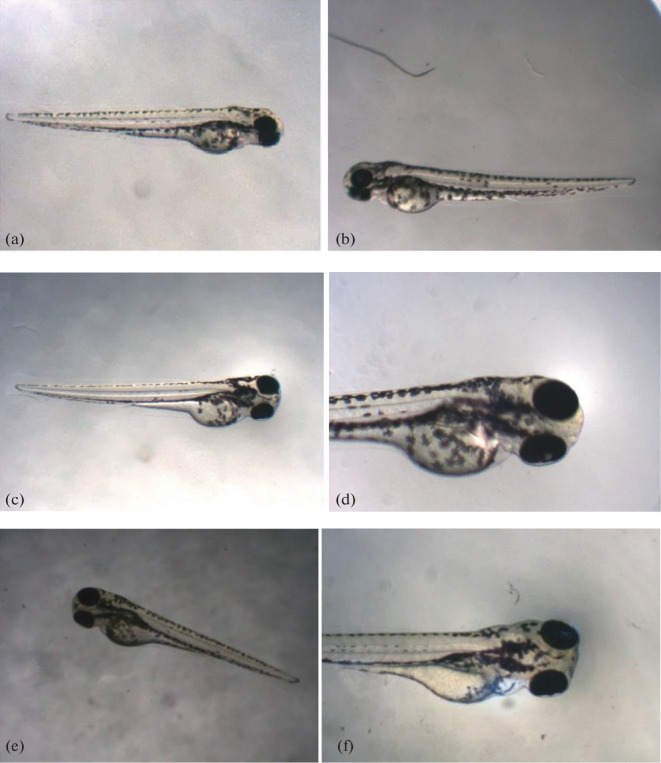
Zebrafish larvae were used to test the teratogenicity of the synthesised compounds (P1–10). Out of the 10 compounds, four were significantly teratogenic for zebrafish larvae and compound (P8) caused fish mortality, according to the increase in yolk sac area and other morphological defects. The safety of (P2), (P4), (P5), (P6), and (P10) at 0.5 µM was demonstrated without any anomalies shown in (Fig. 3).
Fig. 3.
(a) P2 (0.5 µM), (b) P4 (0.5 µM), (c) P5 (0. 5 µM), (d) P6 (0.5 µM), (e) P10 (0.5 µM), (f) P8 (0.5 µM) (Fish with increase in morphological abnormality (Increase in yolk sac area).
EXPERIMENTAL
S.D. Fine Chemicals Limited in Mumbai and Avra Chemicals Pvt Limited in New Delhi provided the materials for the reaction. We used thin layer chromatography (TLC), UV illumination (256 nm), and an iodine chamber with E. Merck 0.25-mm silica gel plates to assess reaction completeness. After recrystallization with aqueous ethanol, the purity of the synthesised derivatives was assessed using a single spot in TLC. The uncorrected melting points were calculated using an ANALAB digital melting point instrument. All 1H NMR and 13C NMR spectra were obtained using DMSO-d6 as the solvent, with TMS serving as the internal standard. Shimadzu FT-IR spectrophotometer and discs containing 1 percent KBr were used to record FTIR spectra. Using a mass spectrometer from the Agilent 1100 series, the mass spectra were recorded.
Synthesis of 5,5-dimethyl-3-(5-methyl-2H-pyrazol-3-yl-amino) cyclohex-2-en-1-one (I). 3 mmol of 3‑amino-5-methyl pyrazole and 3 mmol of dimedone were dissolved in 2 mL of DMF and refluxed for 1.5 h. The obtained mixture was filtered out after cooling and dilution with 7 mL of propan-2-ol.
Synthesis of intermediates (I1–10). A mixture of 1 mmol of compound I and 1 mmol of various aromatic aldehydes in 1 mL of Dimethyl formamide containing a catalytic amount of concentrated hydrochloric acid was heated for 2–3 h under reflux. The precipitate was filtered off and recrystallised from DMF-MeOH (1 : 2).
Synthesis of final compounds (P1–10). In a clean, dry round-bottom flask, 0.5 mL of triethylamine was added dropwise to a mixture of compounds (I1–10) (0.001 mol) and 2-Furoyl chloride (0.001 mol) dissolved in ethanol. The mixture was then heated for 5 to 6 h, the separated solid was filtered and recrystallised with aqueous ethanol, and dried to get the final products (P1–10).
5,5-Dimethyl-3-(5-methyl-2H-pyrazol-3-yl-amino)- cyclohex-2-en-1-one (I). Yield 65%, mp 251–253°C, IR spectrum, ν, cm–1: 3429 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3232.29 NH stretching, 3042.8 aromatic CH stretching, 2956.78 and 2840 CH stretching of CH3 groups symmetric and asymmetric, 1590.65 C=N, 1508.47 C=C ring stretching, 1477.78 and 1368.10 C–H bending of CH3 groups, 1278.47 C–N stretching, 1154.87 C–O stretching. 1H NMR spectrum, δ, ppm: 12.07 s (1H, NH), 8.91 s (1H, NH), 5.98 s (1H), 5.72 s (1H), 2.4 s (2H, CH2), 2.2 s (3H, CH3), 2.0 s (2H, CH2), 1.0 s (6H, 2(CH3)2). 13C NMR spectrum, δ, ppm: 195.9, 158.05, 148.7, 138.7, 99.3, 95.3, 50.1, 41.8, 39.9, 28.1, 10.5. Mass (EI): 219 [M]+, 218[M – 1]+. Found %: C 65.73; H 7.75; N 19.16; O 4.68. C12 H17N3O. Calculated, %: C 65.75; H 7.78; N 19.18; O 4.69.
3,7,7-Trimethyl-4-(P-tolyl)-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (I1). Yield 67%, mp > 300°C, IR spectrum, ν, cm–1: 3445.83 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3234.81 NH hydrogen bonded, 3040 aromatic CH stretching, 2961.97–2860, 2924.92–2860 C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1588.48 C=N stretching, 1588.48, 1542.74, 1502.82, C=C ring stretching, 1431.93, 1354.08 C–H bending of CH3 and CH2 groups, 1286.98 C–N, 1253.29 C–O, 855.12 1,4 di substituted benzene. 1H NMR spectrum, δ, ppm: 11.70 s (1H, NH), 9.64 s (1H, NH of pyrazole ring), 6.95–7.01 m (4H aromatic), 4.87 s (1H, CH), 3.79 s (3H, CH3 of 4-methyl group), 2.18 s (3H CH3 of pyrazole ring) 2.47 d and 2.08 d (2H each of CH2), 0.93 s and 0.99 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 197.2, 155.1, 138.3, 137.8, 133.2, 129.9, 124.6, 111.0, 102.0, 51.2, 43.7, 37.8, 33.6, 28.1, 12.0. Mass (EI): 362 [M]+, 363 [M + 1]+. Found %: C 63.83; H 5.75; N 15.85; O 4.99. C19H24N4O3. Calculated, %: C 64.85; H 5.95; N 15.98; O 4.98.
3,7,7-Trimethyl-4-phenyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b] quinoline-5-one (I2). Yield 70%, mp 296–298°C, IR spectrum, ν, cm–1: 3435.25 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3238.86 NH hydrogen bonded, 3024.59 aromatic CH stretching, 2961.26–2860, 2911.12–2860 C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1629.34 C=N stretching, 1629.34, 1546.16, 1586.09, 1503.33 C=C ring stretching, 1449.87,1330.78 C–H bending of CH3 and CH2 groups, 1254 C–N, 1212 C–O, 725 mono substituted benzene. 1H NMR spectrum, δ, ppm: 11.70 s (1H, NH), 9.65 s (1H, NH of pyrazole ring), 7.01–7.18 m (5H aromatic), 4.91 s (1H CH), 2.37–2.43 d (2H of CH2) 2.09–2.13 d (1H of CH2) and 1.42–1.96 d (1H of CH2) 1.89 s (3H 3CH3 of pyrazole ring) 0.94 s and 1.00 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 192.7, 152.7, 148.4, 146.2, 134.8, 127.7, 127.5, 125.1, 106.9, 103.9, 50.4, 41.00, 31.9, 28.8, 9.39. Mass (EI): 307 [M]+, 308 [M + 1]+. Found %: C 74.36; H 6.86; N 13.73; O 4.56 C19H21N3O. Calculated, %: C 75.12; H 6.88; N 13.76; O 4.58.
4-(3-Florophenyl)-3,7,7,-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (I3). Yield 63%, mp > 300°C, IR spectrum, ν, cm–1: 3459.28 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3245.29 NH hydrogen bonded, 3070.75 aromatic CH stretching, 2962.53–2860, 2912–2860 C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1629.34 C=N stretching, 1611.27, 1546.27, 1586.09, 1503.33 C=C ring stretching, 1489, 1433.41, 1350 C–H bending of CH3 and CH2 groups, 1253.29 C–N, 1208.54 C–O, 771.27 substituted benzene. 1H NMR spectrum, δ, ppm: 11.79 s (1H, NH), 9.75 s (1H, NH of pyrazole ring), 7.24–6.84 dd (4H aromatic), 4.96 s (1H CH), 2.44–2.42 d (2H of CH2) 2.13–2.09 d (1H of CH2) 1.90 s (3H 3CH3 of pyrazole ring) 0.93 s and 1.00 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 195.6, 161.8, 155.2, 138.7, 136.5, 125.8, 117.2, 110.1, 102.0, 52.0, 42.8, 35.6, 32.8, 27.6, 13.0. Mass (EI): 325 [M]+, 324 [M – 1]+. Found %: C 70.15; H 6.28; F 5.23; N 12.95; O 4.63. C19H20FN3O. Calculated, %: C 70.19; H 6.30; F 5.28; N 12.98; O 4.60.
4-(4-Methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (I4). Yield 61%, mp 286–287°C IR spectrum, ν, cm–1: 3435.96 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3239.80 NH hydrogen bonded, 3067 aromatic CH stretching, 2955.00–2918.79, 2820 C–H stretching of CH3, CH2 and OCH3 groups both asymmetric and symmetric, 1595 C=N stretching, 1595, 1586.37, 1546, C=C ring stretching, 1426.53, 1413.08, 1379.87 C–H bending of CH3 and CH2 groups, 1261.75 C–N, 1238.51 C–O, 812 1,4 di substituted benzene. 1H NMR spectrum, δ, ppm: 11.71 s (1H, NH), 9.64 s (1H, NH of pyrazole ring), 7.01–7.03 d (2H aromatic), 6.73–6.71 d (2H aromatic), 4.87 s (1H CH), 3.66 s (3H OCH3) 2.42–2.35 d (2H of CH2) 2.12–2.08 d (2H of CH2) 1.91 s (3H 3CH3 of pyrazole ring) 0.93 s and 0.99 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 196.8, 159.6, 156.0, 137.9, 137.8, 133.2, 129.2, 114.2, 110.6, 56.8, 54.1, 43.6, 33.9, 28.2, 12.9 Mass (EI): 337 [M]+, 338 [M + 1]+. Found %: C 71.72; H 5.98; N 12.48; O 9.56. C20H23N3O2. Calculated, %: C 70.23; H 5.96; N 12.51; O 9.58.
4-(2-Chloroyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (I5). Yield 64%, mp > 300°C, IR spectrum, ν, cm–1: 3432.45 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3261.63 NH hydrogen bonded, 3065.25 aromatic CH stretching, 2966.56–2918.59, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1625.50 C=N stretching, 1625.50, 1550.94, 1593.72, 1503.89 C=C ring stretching, 1430.69, 1373.04, C–H bending of CH3 and CH2 groups, 1259.10 C–N, 1212.03 C–O, 748.44 1,2 di substituted benzene. 1H NMR spectrum, δ, ppm: 11.77 s (1H, NH), 9.76 s (1H, NH of pyrazole ring), 7.28–7.03 m (4H aromatic), 5.34 s (1H CH), 2.50–2.45 d (2H of CH2) 2.13–2.09 d (2H of CH2) 1.88 s (3H CH3 of pyrazole ring) 1.00 s and 0.95 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 197.5, 155.6, 142.3, 138.9, 136.6, 126.1, 125.8, 110.3, 102.2, 53.3, 41.6, 27.8, 13.0 Mass (EI): 341 [M]+, 340 [M – 1]+. Found %: C 66.86; H 5.88; Cl 10.53; N 12.25; O 4.59 C19H20ClN3O. Calculated, %: C 65.26; H 5.89; Cl 10.68; N 12.26, O 4.63.
4-(3,4-Dimethoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (I6). Yield 71%, mp > 300°C, IR spectrum, ν, cm–1: 3440 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3255 NH hydrogen bonded, 3063 aromatic CH stretching, 2954.44 and 2865.01, 2925.77 and 2865.01 C–H stretching of CH3, CH2 and OCH3 groups both asymmetric and symmetric, 1589.94 C=N stretching, 1589.94, 1546.43, 1502.94, 1473.13 C=C ring stretching, 1426.53, 1413.08, 1348 C–H bending of CH3 and OCH3 groups, 1285 C–N, 1261 C–O, 862.35 1,2, tri substituted ring. 1H NMR spectrum, δ, ppm: 11.71 s (1H, NH), 9.63 s (1H, NH of pyrazole ring), 6.76–6.59 m (3H aromatic), 4.88 s (1H CH), 3.65 s (6H (OCH3)2) 2.50–2.44 d (2H of CH2) 2.15–2.11 d (2H of CH2) 1.94 s (3H CH3 of pyrazole ring) 1.01s and 0.97 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 197.5, 155.2, 145.8, 137.8, 135.6, 122.8, 111.8, 110.2, 104.0, 56.2, 51.2, 42.8, 38.1, 32.7, 27.8, 14.2 Mass (EI): 367 [M]+, 368 [M + 1]+. Found %: C 68.64; H 6.86; N 11.44; O 13.06 C21H25N3O3. Calculated, %: C 69.62; H 6.89; N 12.21; O 12.85.
4-(3-Bromophenyl)-3,7,7,-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (I7). Yield 69%, mp > 300°C, IR spectrum, ν, cm–1: 3456.32 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3248.25 NH hydrogen bonded, 3071.35 aromatic CH stretching, 2965.53–2860, 2911–2865 C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1632.29 C=N stretching, 1615.38, 1548.12, 1542.36, 1502.21 C=C ring stretching, 1486, 1438.41, 1358 C–H bending of CH3 and CH2 groups, 1263.29 C–N, 1209.54 C–O, 776.28 substituted benzene. 1H NMR spectrum, δ, ppm: 11.81 s (1H, NH), 9.77 s (1H, NH of pyrazole ring), 7.26–7.12 dd (4H aromatic), 4.93 s (1H CH), 2.88–2.73 d (2H of CH2) 2.14–2.10 d (1H of CH2) 1.90 s (3H CH3 of pyrazole ring) 1.00 s and 0.93 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 197.5, 158.2, 138.6, 136.5, 132.8, 128.5, 123.2, 111.2, 103.5, 51.2, 42.8, 37.1, 27.8, 13.9. Mass (EI): 386 [M]+, 387 [M + 1]+. Found %: C 59.08; H 5.24; Br 20.69; N 10.68; O 04.12 C19H20BrN3O. Calculated, %: C 59.07; H 5.28; Br 20.55; N 11.02; O 04.11.
9-(Furan-2-carbonyl)-3,7,7-trimethyl-4-(p-tolyl)-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (P1). Yield 65%, mp 162–163C, IR spectrum, ν, cm–1: 3387.25 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3134.87 NH hydrogen bonded, 3020 aromatic CH stretching, 2962.33–2929.95, 2870.99 C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1680 carbonyl peak of N–C=O, 1638.93 C=N stretching, 1638.93, 1582.35, 1538, 1510.51 C=C ring stretching, 1459.78, 1430.32, 1359.34 C–H bending of CH3 and CH2 groups, 1283.33 C–N, 1250 C–O, 1054.36 C–O–C, 828.54 1,4 di substituted benzene. 1H NMR spectrum, δ, ppm: 9.44 s (1H, NH of pyrazole ring), 8.19–8.00 s (2H of furan ring), 7.18–7.13 m (4H aromatic), 6.82 s (1H of furan ring), 4.97 s (1H CH), 2.73–2.69 d (1H of CH2), 2.62–2.57 d (1H of CH2) 2.43 s (3H, CH3 of 4-methyl group), 2.21–2.17 d (1H of CH2), 2.04–2.00 d (1H of CH2), 1.97 s (3H CH3 of pyrazole ring) 1.07 s and 0.95 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 194.3, 155.6, 151.5, 150.5, 149.2, 143.8, 139.7, 135.2, 128.2, 125.6, 124.4, 112.8, 109.9, 105.3, 50.3, 40.4, 40.1, 34.5, 32.05, 28.7, 26.8, 14.6, 12.3. Mass (EI): 415 [M]+, 416 [M + 1]+. Found %: C 72.26; H 6.12; N 10.18; O 11.68. C25 H25N3O3. Calculated, %: C 71.96; H 6.05; N 10.65; O 11.59.
9-(Furan-2-carbonyl)-3,7,7-trimethyl-4-phenyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]quinoline-5-one (P2). Yield 69%, mp 158–160°C, IR spectrum, ν, cm–1: 3429.83 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3237.44 NH hydrogen bonded, 3060 aromatic CH stretching, 2960.80–2930, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1680 carbonyl peak of N–C=O, 1629.80 C=N stretching, 1629.80, 1556, 1548, 1503 C=C ring stretching, 1450, 1352, C–H bending of CH3 and CH2 groups, 1257.87 C–N, 1212.13 C–O, 1054.50 C–O–C, 724.52 monosubstituted benzene. 1H NMR spectrum, δ, ppm: 9.80 s (1H, NH of pyrazole ring), 8.17 s (1H of furan ring), 7.25–7.17 m (5H aromatic), 7.11–6.80 m (2H of furan ring), 4.92 s (1H CH), 2.45–2.38 d (2H of CH2), 2.16–1.93 dd (2H of CH2), 1.89 s (3H CH3 of pyrazole ring) 1.01 s and 0.94 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 192.7, 152.9, 148.1, 145.7, 135.4, 127.9, 127.6, 125.2, 107.1, 103.9, 50.2, 40.9, 39.9, 38.7, 28.8, 26.8, 9.39. Mass (EI): 401 [M]+, 402 [M + 1]+. Found %: C 71.89; H 5.82; N 10.62; O 11.96. C24H23N3O3. Calculated, %: C 71.95; H 5.88; N 10.65; O 11.91.
4-(3-Fluorophenyl)-9-(furan-2-carbonyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]- quinoline-5-one (P3). Yield 72%, mp 168–170°C, IR spectrum, ν, cm–1: 3436.15 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3246.09 NH hydrogen bonded, 3072.43 aromatic CH stretching, 2962.37–2930, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1680 carbonyl peak of N–C=O, 1629.88 C=N stretching, 1589, 1545, 1511, 1500 C=C ring stretching, 1453, 1363, C–H bending of CH3 and CH2 groups, 1259 C–N, 1208 C–O, 1056 C–O–C, 852 substituted benzene, 771 monosubstituted ring. 1H NMR spectrum, δ, ppm: 9.72 s (1H, NH of pyrazole ring), 8.17–8.05 s (2H of furan ring), 7.19–7.09 m (4H aromatic), 6.89 s (1H of furan ring), 4.98 s (1H CH), 2.49–2.36 d (2H of CH2), 2.17–2.09 dd (2H of CH2), 1.88 s (3H CH3 of pyrazole ring) 1.00 s and 0.96 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 197.8, 155.8, 154.2, 148.1, 143.9, 144.8, 138.8, 137.2, 130.9, 125.7, 117.0, 115.4, 111.6, 111.2, 102.0, 52.1, 44.1, 37.8, 31.8, 28.1, 12.9. Mass (EI): 419 [M]+, 420 [M + 1]+. Found %: C 67.75; H 5.42; F 4.62; N 10.52; O 11.22. C24H22FN3O3. Calculated, %: C 68.72; H 5.46; N 10.58; O 11.28.
9-(Furan-2-carbonyl)-4-(4-methoxyphenyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]- quinoline-5-one (P4). Yield 72%, mp 168–170°C, IR spectrum, ν, cm–1: 3382.57 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3140 NH hydrogen bonded, 3020 aromatic CH stretching, 2950.26, 2920, 2850 C–H stretching of CH3 and CH2 and OCH3 groups both asymmetric and symmetric, 1682.70 carbonyl peak of N–C=O, 1636.50 C=N stretching, 1636.50, 1569.80, 1545.53, 1517 C=C ring stretching, 1452.36, 1400.22, 1350.52 C–H bending of CH3 and CH2 and OCH3 groups, 1251.55 C–N, 1230.11 C–O, 1027.37 C–O–C, 826.36 1,4 di substituted ring. 1H NMR spectrum, δ, ppm: 9.89 s (1H, NH of pyrazole ring), 7.45 m (3H of furan ring), 7.15–7.09 m (4H aromatic), 6.5 s (1H CH), 3.90 s (3H OCH3) 2.83–2.56 d (2H of CH2), 2.25 s (3H 3CH3 of pyrazole ring) 2.15–2.07 d (2H of CH2) 1.00 s and 0.99 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 197.2, 155.9, 154.2, 148.1, 144.9, 144.2, 138.9, 135.6, 133.7, 130.0, 114.8, 113.9, 111.8, 111.4, 104.2, 56.7, 51.2, 37.4, 31.8, 28.1, 12.8. Mass (EI): 431 [M]+, 432[M + 1]+. Found %: C 68.56; H 5.92; N 8.99; O 13.90. C25H25N3O4. Calculated, %: C 68.59; H 5.94; N 8.98; O 13.96.
4-(2-Chlorophenyl)-9-(furan-2-carbonyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]- quinoline-5-one (P5). Yield 60%, mp 178–180°C, IR spectrum, ν, cm–1: 3407.84 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3121.24 NH hydrogen bonded, 3068.38 aromatic CH stretching, 2959.43–2938, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1670.88 carbonyl peak of N–C=O, 1639.98 C=N stretching, 1587, 1548, 1515, 1502 C=C ring stretching, 1450.02, 1359.81, C–H bending of CH3 and CH2 groups, 1243.84 C–N, 1150.77 C–O, 1030.70 C–O–C, 827.97 substituted benzene, 784.81 monosubstituted ring. 1H NMR spectrum, δ, ppm: 9.96 s (1H, NH of pyrazole ring), 8.18–7.91 m (2H of furan ring), 7.34–7.14 m (4H aromatic), 6.80 d (1H of furan ring), 5.40 s (1H CH), 2.73–2.39 d (2H of CH2), 2.19–1.96 dd (2H of CH2), 1.89 s (3H CH3 of pyrazole ring) 1.02 s and 0.95 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 196.2, 153.9, 152.9, 143.8, 142.1, 139.2, 130.9, 127.6, 126.9, 126.2, 116.4, 113.1, 111.8, 104.0, 52.3, 31.8, 26.4, 12.8. Mass (EI): 435 [M]+, 434 [M – 1]+. Found %: C 66.14; H 5.08; Cl 8.14; N 9.59; O 11.04 C24H22ClN3O. Calculated, %: C 66.18; H 5.09; Cl 8.16; N 9.60, O 11.06.
4-(3,4-Dimrthoxyphenyl)-9-(furan-2-carbonyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]- quinoline-5-one (P6). Yield 66%, mp 148–150°C, IR spectrum, ν, cm–1: 3748.85 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3417.09 NH hydrogen bonded, 3066 aromatic CH stretching, 2957.80 and 2948.05, 2928.76 and 2869.01 C–H stretching of CH3, CH2 and OCH3 groups both asymmetric and symmetric, 1680.99 carbonyl peak of N–C=O, 1638.91 C=N stretching, 1590.16, 1588, 1546, 1511, 1501 C=C ring stretching, 1468.78, 1420.42, 1381.76 C–H bending of CH3 and OCH3 groups, 1262.58 C–N, 1138.57 C–O, 1027.22 C–O–C, 824.58 1,2, tri substituted ring. 1H NMR spectrum, δ, ppm: 9.86 s (1H, NH of pyrazole ring), 9.18–7.91 m (1H of furan ring), 7.34–7.14 m (3H aromatic), 7.09–6.53 m (2H of furan ring), 5.11 s (1H CH), 3.94 s (6H (OCH3)2) 2.69–2.54 d (2H of CH2) 2.18–2.09 d (2H of CH2) 2.01 s (3H CH3 of pyrazole ring) 1.01 s and 0.95 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 194.8, 155.1, 154.8, 148.6, 146.5, 143.8, 137.9, 121.3, 115.8, 112.9, 111.8, 102.0, 57.1, 49.9, 42.9, 31.8, 26.9, 12.8. Mass (EI): 461 [M]+, 462 [M + 1]+. Found %: C 68.67; H 5.90; N 9.18; O 17.01 C26H27N3O5. Calculated, %: C 68.66; H 5.90; N 9.18; O 17.01.
4-(3-Bromophenyl)-9-(furan-2-carbonyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b] quinoline-5-one (P7). Yield 65%, mp 182–184°C, IR spectrum, ν, cm–1: 3412.86 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3122.56 NH hydrogen bonded, 3067.56 aromatic CH stretching, 2961.23–2944, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1680.12 carbonyl peak of N–C=O, 1641.23 C=N stretching, 1591, 1545, 1514, 1501 C=C ring stretching, 1456.23, 1356.23, C–H bending of CH3 and CH2 groups, 1256.35 C–N, 1146.56 C–O, 1029.86 C–O–C, 826.53 substituted benzene, 765.23 monosubstituted ring. 1H NMR spectrum, δ, ppm: 9.86 s (1H, NH of pyrazole ring), 8.19–7.86 m (2H of furan ring), 7.44–7.15 m (4H aromatic), 6.78 d (1H of furan ring), 5.42 s (1H CH), 2.68–2.42 d (2H of CH2), 2.18–1.94 dd (2H of CH2), 1.86 s (3H CH3 of pyrazole ring) 1.00 s and 0.96 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 195.6, 155.9, 151.6, 142.7, 141.1, 138.6, 130.5, 127.5, 126.8, 126.4, 115.8, 113.2, 111.2, 103.9, 52.8, 31.6, 25.9, 13.00. Mass (EI): 480 [M]+, 481 [M + 1]+. Found %: C 62.03; H 4.66; Br 16.68; N 8.81; O 9.96 C24H22BrN3O3. Calculated, %: C 62.05; H 4.68; Br 16.69; N 8.86; O 9.93.
4-(4-Fluorophenyl)-9-(furan-2-carbonyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H pyrazolo[3,4-b]- quinoline-5-one (P8). Yield 74%, mp 162–164°C, IR spectrum, ν, cm–1: 3446.01 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3248.23 NH hydrogen bonded, 3076.59 aromatic CH stretching, 2959.18–2936, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1682.53 carbonyl peak of N–C=O, 1631.52 C=N stretching, 1581, 1546, 1510, 1501 C=C ring stretching, 1459, 1362, C–H bending of CH3 and CH2 groups, 1258 C–N, 1210 C–O, 1049 C–O–C, 862 substituted benzene, 769 monosubstituted ring. 1H NMR spectrum, δ, ppm: 9.88 s (1H, NH of pyrazole ring), 8.18–8.07 s (2H of furan ring), 7.19–7.08 m (4H aromatic), 6.79 s (1H of furan ring), 4.89 s (1H CH), 2.56–2.46 d (2H of CH2), 2.18–2.08 dd (2H of CH2), 1.85 s (3H CH3 of pyrazole ring) 1.06 s and 0.95 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 196.7, 154.9, 154.6, 146.5, 143.5, 141.5, 137.9, 137.4, 131.6, 124.5, 116.5, 114.8, 111.5, 111.4, 102.5, 53.2, 46.5, 38.5, 31.9, 27.9, 12.7. Mass (EI): 419 [M]+, 420 [M + 1] +. Found %: C 67.76; H 5.41; F 4.60; N 10.54; O 11.21. C24H22FN3O3. Calculated, %: C 68.76; H 5.44; N 10.54; O 11.25.
9-(Furan-2-carbonyl)-3,7,7-trimethyl-4-(4-nitrophenyl)-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]- quinoline-5-one (P9). Yield 68%, mp 170–172°C, IR spectrum, ν, cm–1: 3386.56 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3135.65 NH hydrogen bonded, 3026 aromatic CH stretching, 2969.53–2915.56, 2871.45 C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1684 carbonyl peak of N–C=O, 1648.96 C=N stretching, 1641.26, 1586.56, 1541, 1514.52 C=C ring stretching, 1456.69, 1432.23, 1361.26 C–H bending of CH3 and CH2 groups, 1284.56 C–N, 1256 C–O, 1056.56 C–O–C, 823.56 1,4 di substituted benzene. 1H NMR spectrum, δ, ppm: 9.62 s (1H, NH of pyrazole ring), 8.20–8.08 s (2H of furan ring), 7.16–7.11 m (4H aromatic), 6.78 s (1H of furan ring), 4.65 s (1H CH), 2.86–2.59 d (1H of CH2), 2.62–2.59 d (1H of CH2), 2.20–2.15 d (1H of CH2), 2.06–2.02 d (1H of CH2), 1.98 s (3H CH3 of pyrazole ring) 1.05 s and 0.94 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 195.6, 154.2, 153.5, 151.6, 148.5, 144.9, 138.9, 136.5, 129.6, 126.5, 123.9, 112.9, 108.6, 104.3, 51.4, 41.5, 40.8, 33.9, 34.06, 27.6, 26.5, 14.5, 12.1. Mass (EI): 446 [M]+, 447 [M + 1]+. Found %: C 64.58; H 4.96; N 12.56; O 17.94. C24H22N4O5. Calculated, %: C 64.59; H 4.98; N 12.61; O 17.96.
4-(4-Chlorophenyl)-9-(furan-2-carbonyl)-3,7,7-trimethyl-2,4,6,7,8,9-hexahydro-5H-pyrazolo[3,4-b]- quinoline-5-one (P10). Yield 74%, mp 183–185°C, IR spectrum, ν, cm–1: 3415.26 broad peak of hydrogen bonded OH formed due to tautomerism of cyclohexanone ring. 3120.56 NH hydrogen bonded, 3065.56 aromatic CH stretching, 2956.56–2936, C–H stretching of CH3 and CH2 groups both asymmetric and symmetric, 1680.51 carbonyl peak of N–C=O, 1640.52 C=N stretching, 1584, 1539, 1514, 1501 C=C ring stretching, 1449.65, 1356.52, C–H bending of CH3 and CH2 groups, 1241.26 C–N, 1146.56 C–O, 1032.65 C–O–C, 826.56 substituted benzene, 756.59 monosubstituted ring. 1H NMR spectrum, δ, ppm: 9.98 s (1H, NH of pyrazole ring), 8.20–7.96 m (2H of furan ring), 7.46–7.24 m (4H aromatic), 6.79 d (1H of furan ring), 5.39 s (1H CH), 2.69–2.42 d (2H of CH2), 2.18–1.94 dd (2H of CH2), 1.88 s (3H CH3 of pyrazole ring) 1.01 s and 0.97 s (3H each, (CH3)2). 13C NMR spectrum, δ, ppm: 194.8, 154.6, 151.6, 143.6, 141.2, 138.6, 131.2, 128.6, 127.8, 125.4, 117.8, 114.2, 111.9, 104.2, 53.1, 32.6, 26.5, 12.9. Mass (EI): 435 [M]+, 434 [M – 1]+. Found %: C 66.18; H 5.07; Cl 8.13; N 9.58; O 11.02 C24H22ClN3O. Calculated, %: C 66.19; H 5.06; Cl 8.14; N 9.62, O 11.04.
Antitubercular Activity
Using the MABA technique, all the produced compounds (P1–10) were evaluated against M. tuberculosis H37Rv [23, 24]. To prevent medium from drying out during incubation, 200 µL of sterile deionized water was injected into the outside wells of a sterile 96‑well plate. The 96-well plate with diluted compounds received 100 µL of Middle Brook 7H9 broth. Final samples were between 100 and 0.2 µg/mL. Plates with coatings and parafilm seals were incubated for five days at 37°C. The following step involved incubating 25 µL of freshly made 1 : 1 Alamar blue reagent and 10% Tween 80 for 24 h. Pink indicated bacterial growth, while blue indicated no bacterial growth. The lowest drug concentration necessary to prevent the colour transition from blue to pink is known as the MIC.
Molecular Docking
All synthesised derivatives underwent rigid receptor docking, and their postures were visually analysed. After downloading the X-ray crystal structure of InhA with N-(4-methylbenzoyl)-4-benzylpiperidine, PDB ID: 2NSD (Resolution: 1.90), a protein was made [25]. Enzyme preparation utilised Autodoc 4.2 (adding polar hydrogens, adding AD4 type atoms, removing water molecules, and heteroatoms). The binding site was identified using previously known ligand interaction information. During stimulation runs, each ligand was flexible while active site amino acid residues were rigid. Default parameter values were employed.
Teratogenicity Assay
Zebrafish larvae were used to test the teratogenicity of these final compounds, and results were obtained.
Animals. Adult zebrafish were kept in an aquatic housing tank that was rectangular in shape and were fed regular fish food [26]. A 14-h light/dark cycle was employed to keep the fish in their tanks, and the water was kept at room temperature.
Embryo collection. At 6:00 p.m., zebrafish males and females were fed and housed in a breeding tank in a 2 : 3 ratio. The following morning, the embryos were removed, rinsed three times with E3 medium, and incubated at 25–28°C in E3 media. The control group was given 1mL of vehicle, whereas the test group was given ten different test compounds six hours after fertilization. The following medication dosages (n = 10) were evaluated up until day 5: 50, 10, 5, 3, 1, 0.5, 0.25, and 0.01 µM. Five days after fertilization, the larvae were anaesthetized with tricaine methane sulfonate and examined under a microscope. We took photos and compared any morphological deviations to the control group.
CONCLUSIONS
We generated a library of novel quinoline-pyrazole hybrid compounds. The designed analogues were synthesised, characterized by spectral methods and assessed the anti-tubercular potency. Among the synthesised compounds, (P1–6) has exhibited significant anti-tubercular activity. The MIC value found between 1.6 and 0.8 µg/mL. The findings of the docking tests indicated the near closeness of the active moieties to the active regions of the target enzyme. The teratogenicity studies for these compounds were conducted using zebrafish larvae; four out of the ten compounds were highly teratogenic. For instance, fish exposed to the (P8) compound died and morphological abnormalities included an increase in the size of the yolk sac. (P2), (P4), (P5), (P6), and (P10) were demonstrated to be safer at 0.5 µM without exhibiting any abnormalities. Compounds with electron-donating groups like –CH3 and –OCH3 at 4th position and electron-withdrawing groups like flouro and chloro groups at 3rd and 2nd position, respectively were promising lead molecules for prospective therapeutic research. Overall, the findings point to the molecular hybridization technique’s potential value in the creation of novel hybrid antitubercular drugs based on quinoline and pyrazole analogues for lead optimization.
Supplementary Information
ACKNOWLEDGMENTS
The authors are thankful to Koneru Lakshmaiah Educational Foundation, Vaddeswaram, Andhra Pradesh and G. Pulla Reddy college of Pharmacy, Hyderabad, Telangana.
COMPLIANCE WITH ETHICAL STANDARDS
The authors declare that they have no conflicts of interest.
No author of this article conducted any experiments involving humans or animals, and none of the authors conducted any studies that are included in this article.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2021, 2021.
- 2.Jain R., Vaitilingam B., Nayyar A., Palde P.B. Bioorg. Med. Chem. Lett. 2003;13:1051–1054. doi: 10.1016/j.bmc.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 3.Nayak N., Ramprasad J., Dalimba U. J. Fluor. Chem. 2016;183:59–68. doi: 10.1016/j.ejmech.2015.03.024. [DOI] [Google Scholar]
- 4.Corbett E.L., Watt C.J., Walker N., Maher D., Williams B.G., Raviglione M.C., Dye C. Arch. Int. Med. 2003;163:1009–1021. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 5.Mahajan., R., Int. J. App. Basic Med. Res., 2013, vol. 3, pp. 1–3. 10.4103/ijabmr.ijabmr_7_18
- 6.Diacon A.H., Donald P.R., Pym A., Grobusch M., Patientia R.F., Mahanyele R., Bantubani N., Narasimooloo R., De Marez T., Van Heeswijk R., Lounis N. Antimicrob. Agents Chemother. 2012;56:3271–3276. doi: 10.1128/aac.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu C.X., Zhao X., Wang L., Yang Z.C. Microb. Pathog. 2022;165:105507. doi: 10.1038/cdd.2012.115. [DOI] [PubMed] [Google Scholar]
- 8.Eswaran S., Adhikari A.V., Chowdhury I.H., Pal N.K., Thomas K.D. Eur. J. Med. Chem. 2010;45:3374–3383. doi: 10.1016/j.ejmech.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 9.Satyanarayana N., Sathish K., Nagaraju S., Pawar R., Faizan M., Arumugavel M., Shirisha T., Kashinath D. New J. Chem. 2022;46:1637–1642. doi: 10.1039/D1NJ00132A. [DOI] [Google Scholar]
- 10.Katariya K.D., Shah S.R., Reddy D. Bioorg. Chem. 2020;94:103406. doi: 10.1016/j.bioorg.2019.103406. [DOI] [PubMed] [Google Scholar]
- 11.Ran F., Liu Y., Chen X., Zhuo H., Xu C., Li Y., Duan X., Zhao G. Bioorg. Chem. 2021;112:104968. doi: 10.1016/j.bioorg.2021.104968. [DOI] [PubMed] [Google Scholar]
- 12.Masih A., Agnihotri A.K., Srivastava J.K., Pandey N., Bhat H.R., Singh U. J. Biochem. Mol. Toxicol. 2021;35:e22656. doi: 10.1016/j.ijcard.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Verma R., Verma S.K., Rakesh K.P., Girish Y.R., Ashrafizadeh M., Kumar K.S.S., Rangappa K.S. Eur. J. Med. Chem. 2021;212:113134. doi: 10.1016/j.ejmech.2020.113134. [DOI] [PubMed] [Google Scholar]
- 14.Wu Z., Yang W., Hou S., Xie D., Yang J., Liu L., Yang S. Pestic. Biochem. Physiol. 2021;173:104771. doi: 10.1016/j.pestbp.2021.104771. [DOI] [PubMed] [Google Scholar]
- 15.Xu Z., Gao C., Ren Q.C., Song X.F., Feng L.S., Lv Z.S. Eur. J. Med. Chem. 2017;139:429–440. doi: 10.1016/j.ejmech.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 16.Jadhav S.B., Fatema S., Sanap G., Farooqui M. J. Heterocycl. Chem. 2018;55:1634–1644. doi: 10.1002/jhet.3198. [DOI] [Google Scholar]
- 17.Naim M.J., Alam O., Nawaz F., Alam M.J., Alam P. J. Pharm. Bioallied Sci. 2016;8:2. doi: 10.4103/0975-7406.171694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Modi P., Patel S., Chabria M.T. J. Biomol. Struct. Dyn. 2019;37:1736–1749. doi: 10.1016/j.jmgm.2018.02.017. [DOI] [PubMed] [Google Scholar]
- 19.Nayak N., Ramprasad J., Dalimba U.K. J. Fluor. Chem. 2016;183:59–68. doi: 10.1016/j.jfluchem.2016.01.011. [DOI] [Google Scholar]
- 20.Lipson V.V., Shirobokova M.G., Shishkin O.V., Shishkina S.V. Russ. J. Org. Chem. 2006;42:1015–1021. doi: 10.1134/s1070428006070153. [DOI] [Google Scholar]
- 21.Murlykina M.V., Morozova A.D., Zviagin I.M., Sakhno Y.I., Desenko S.M. Front. Chem. 2018;6:527. doi: 10.3389/fchem.2018.00527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Quiroga J., Insuasty B., Hormaza A., Saitz C., Jullian C. J. Heterocycl. Chem. 1998;35:575–578. doi: 10.1002/JHET.5570350313. [DOI] [Google Scholar]
- 23.Lourenco M.C., de Souza M.V., Pinheiro A.C., Ferreira M.D.L., Gonçalves R.S., Nogueira T.C.M., Peralta M.A. Arkivoc. 2007;15:181–191. doi: 10.3998/ark.5550190.0008.f18. [DOI] [Google Scholar]
- 24.Lourenço M.C.S., de Souza M.V.N., Pinheiro A.C., Ferreira M. de L., Gonçalves R.S.B., Nogueira T.C.M., Peralta M.A. Arkivoc. 2007;15:181–191. doi: 10.3998/ark.5550190.0008.f18. [DOI] [Google Scholar]
- 25.Shuler W.G., Smith E.A., Hess S.M., McFadden T., Metz C.R., Van Derveer D.G., Beam C.F. J. Chem. Crystallogr. 2012;42:952–959. doi: 10.1007/s10870-012-0342-5. [DOI] [Google Scholar]
- 26.Panzica-Kelly J.M., Zhang C.X., Danberry T.L., Flood A., DeLan J.W., Brannen K.C., Augustine-Rauch K.A. Reprod. Toxicol. 2010;89:382–395. doi: 10.1002/bdrb.20260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.