Abstract
Introduction
The Japanese Respiratory Society (JRS) pneumonia guidelines recommend simple predictive rules, the A-DROP scoring system, for assessment of the severity of community-acquired pneumonia (CAP) and nursing and healthcare-associated pneumonia (NHCAP). We evaluated whether the A-DROP system can be adapted for assessment of the severity of coronavirus disease 2019 (COVID-19) pneumonia.
Methods
Data from 1141 patients with COVID-19 pneumonia were analyzed, comprising 502 patients observed in the 1st to 3rd wave period, 338 patients in the 4th wave and 301 patients in the 5th wave in Japan.
Results
The mortality rate and mechanical ventilation rate were 0% and 1.4% in patients classified with mild disease (A-DROP score, 0 point), 3.2% and 46.7% in those with moderate disease (1 or 2 points), 20.8% and 78.3% with severe disease (3 points), and 55.0% and 100% with extremely severe disease (4 or 5 points), indicating an increase in the mortality and mechanical ventilation rates in accordance with severity (Cochran–Armitage trend test; p = <0.001). This significant relationship between the severity in the A-DROP scoring system and either the mortality rate or mechanical ventilation rate was observed in patients with COVID-19 CAP and NHCAP. In each of the five COVID-19 waves, the same significant relationship was observed.
Conclusions
The mortality rate and mechanical ventilation rate in patients with COVID-19 pneumonia increased depending on severity classified according to the A-DROP scoring system. Our results suggest that the A-DROP scoring system can be adapted for the assessment of severity of COVID-19 CAP and NHCAP.
Keywords: Community-acquired pneumonia, Nursing and healthcare-associated pneumonia, Pneumonia severity, A-DROP, SARS-CoV-2, COVID-19
List of abbreviations
- COVID-19
Coronavirus disease 2019
- CAP
Community-acquired pneumonia
- JRS
Japanese Respiratory Society
- NHCAP
Nursing and healthcare-associated pneumonia
- PSI
Pneumonia Severity Index
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
1. Introduction
The Pneumonia Severity Index (PSI), which defines five risk classes according to 20 clinical and laboratory variables, appears to be an excellent predictor of mortality in patients with community-acquired pneumonia (CAP) [1]. Unfortunately, the PSI may not be practical for routine application in busy hospital emergency departments or primary care settings because of its complicated requirement for the computation of a score based on 20 variables. Thus, the Japanese Respiratory Society (JRS) pneumonia guidelines developed a simple predictive rule, the A-DROP scoring system, for assessment of the severity of pneumonia [2]. Several studies have demonstrated that the A-DROP scoring system is useful for predicting mortality in patients with CAP [[3], [4], [5], [6], [7], [8], [9]]. Nursing and healthcare-associated pneumonia (NHCAP) is also categorized as a type of community-onset pneumonia but is distinct from CAP [10]. The A-DROP scoring system is also useful for predicting mortality in patients with NHCAP [[11], [12], [13]]. In addition, Kohno et al. demonstrated that the mechanical ventilation rate in patients with CAP with acute respiratory failure increased depending on the severity classified according to the A-DROP scoring system [7]. The JRS pneumonia guidelines updated in 2017 recommend the A-DROP scoring system for assessment of the severity of CAP and NHCAP on the basis of systematic reviews and meta-analyses [14].
Since 2020, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has become the most important pathogen in CAP and NHCAP [15]. The purpose of the present study was to evaluate whether the A-DROP system can be adapted for assessment of the severity of COVID-19 pneumonia including CAP and NHCAP. We investigated changes in usefulness of the A-DROP system in each of the COVID-19 waves with the progression of anti-SARS-CoV-2 vaccination and the development of anti-SARS-CoV-2 drugs.
2. Methods
2.1. Study population
The present study was conducted at five institutions (Kansai Medical University Hospital, Kansai Medical University Medical Center, Kansai Medical University Kori Hospital, Kansai Medical University Kuzuha Hospital, and Kansai Medical University Temmabashi General Clinic) between February 2020 and December 2021. We enrolled adult patients diagnosed with community-onset pneumonia, CAP and NHCAP, defined according to the JRS guidelines [2,10]. COVID-19 was diagnosed using a positive reverse transcription polymerase chain reaction test from sputum or nasopharyngeal swab specimens in accordance with the protocol recommended by the National Institute of Infectious Diseases, Japan. Cases of pneumonia mixed with other microorganisms were excluded from the study. During the study period, the 1st to 3rd COVID-19 wave occurred with conventional strains, the 4th wave occurred with lineage B.1.1.7 (Alpha variant), and the 5th wave occurred with lineage B.1.617.2 (Delta variant). Informed consent was obtained from all patients, and the study protocol was approved by the Ethics Committee of Kansai Medical University (approval number 2020319).
2.2. Pneumonia severity score
The severity of pneumonia was evaluated using predictive rules via the A-DROP system proposed by the JRS guidelines: age over 70 years in men and over 75 years in women, dehydration, respiratory failure, orientation disturbance, and low blood pressure [2]. Patients were stratified into four severity classes: 0 points = mild, 1 or 2 points = moderate, 3 points = severe, and 4 or 5 points = extremely severe. A-DROP is a modified version of CURB-65 proposed by the British Thoracic Society [16]. The 30-day mortality rate and rate of requirement for mechanical ventilation during 30 days after onset of symptoms in patients with CAP and NHCAP were examined by using the A-DROP scoring system. Previous studies evaluated the utility of A-DRP system for CAP demonstrated that the area under the receiver operating characteristic (ROC) curve for 28- or 30-day mortality were 0.81–0.88 for A-DROP, 0.80–0.88 for CURB-65 and 0.81–0.89 for PSI [[3], [4], [5], [6], [7], [8]].
2.3. Severity classification of COVID-19
Severity classification of COVID-19 according to the criteria of Ministry of Health, Labour and Welfare is follows: Mild: oxygen saturation level at room air of 96% or more, and no pneumonia shadow was observed; Moderate Ⅰ: oxygen saturation level at room air of 94% or 95%, and pneumonia shadow was observed; Moderate Ⅱ: oxygen saturation level at room air of 93% or less, and need the oxygen therapy; Sever: requirement for the intensive care unit admission or mechanical ventilation.
2.4. Statistical analysis
Statistical analysis was performed using Stat View version 5.0. (SAS Institute Inc, Cary, NC, USA). Discrete variables are expressed as counts (percentages) and continuous variables as medians and interquartile ranges. Frequencies were compared using Fisher's exact test. Between-group comparisons of normally distributed data were performed using Student's t-test. Skewed data were compared using the Mann–Whitney U test. Mortality rate and mechanical ventilation rate, which were categorized by the severity classification of the A-DROP scoring system, were tested using the Cochran–Armitage trend test [17]. The discrimination capability of pneumonia severity indices was evaluated by ROC curve and area under the ROC curve (AUC) were assessed as appropriate. A p value less than 0.05 was considered statistically significant.
3. Results
3.1. Patient characteristics
Data from a total of 1141 patients with COVID-19 pneumonia were analyzed, 502 patients observed in the 1st to 3rd wave period, 338 patients (Alpha variant) observed in the 4th wave, and 301 patients (Delta variant) observed in the 5th wave (Table 1 ). Of the 502 conventional strain group, 403 were inpatients including hotel recuperation and 99 were outpatients. Of the 338 Alpha variant group, 273 were inpatients including hotel recuperation and 65 were outpatients. Of the 301 Delta variant group, 226 were inpatients including hotel recuperation and 75 were outpatients.
Table 1.
Underlying conditions and clinical findings in patients with COVID-19 pneumonia observed in each wave at first examination.
| Variables | 1st to 3rd wave | 4th wave (Alpha variant) | 5th wave (Delta variant) |
|---|---|---|---|
| No. of patients | 502 | 338 | 301 |
| Median age (IQR), years | 65 (47–76) | 64 (51–74) | 50 (39–58) |
| No. of males/females | 312/190 | 229/109 | 169/132 |
| No. (%) of patients with comorbid illnesses | |||
| Diabetes mellitus | 104 (20.7) | 67 (19.8) | 45 (15.0) |
| Chronic lung disease | 57 (11.4) | 43 (12.7) | 28 (9.3) |
| Chronic heart disease | 41 (8.2) | 24 (7.1) | 12 (4.0) |
| Chronic renal disease | 32 (6.4) | 24 (7.1) | 5 (1.7) |
| Cerebrovascular disease | 33 (6.6) | 22 (6.5) | 11 (3.7) |
| Neoplastic disease | 31 (6.2) | 14 (4.1) | 11 (3.7) |
| Chronic liver disease | 15 (3.0) | 9 (2.7) | 10 (3.3) |
| Autoimmune disease | 15 (3.0) | 7 (2.1) | 5 (1.7) |
| No. (%) of patients with the following clinical signs and symptoms | |||
| Fever (≥37.0 °C) | 423 (84.3) | 298 (88.2) | 272 (90.4) |
| Cough | 253 (50.4) | 209 (61.8) | 225 (74.8) |
| Fatigue | 170 (33.9) | 112 (33.1) | 124 (41.2) |
| Shortness of breath | 139 (27.7) | 112 (33.1) | 87 (28.9) |
| Sore throat | 97 (19.3) | 68 (20.1) | 79 (26.2) |
| Loss of taste | 66 (13.1) | 55 (16.3) | 71 (23.6) |
| Anosmia | 54 (10.8) | 49 (14.5) | 71 (23.6) |
| Headache | 54 (10.8) | 33 (9.8) | 37 (12.3) |
| Diarrhea | 51 (10.2) | 27 (8.0) | 8 (2.7) |
| Sputum production | 49 (9.8) | 54 (16.0) | 55 (18.3) |
| Runny nose | 36 (7.2) | 24 (7.1) | 11 (3.7) |
| Joint pain | 28 (5.6) | 14 (4.1) | 20 (6.6) |
| Chest pain | 18 (3.6) | 5 (1.5) | 6 (2.0) |
| Nausea or vomiting | 17 (3.4) | 14 (4.1) | 11 (3.7) |
| Muscle ache | 17 (3.4) | 4 (1.2) | 11 (3.3) |
| Abdominal pain | 6 (1.2) | 1 (0.3) | 6 (2.0) |
| No. (%) of patients with pneumonia type | |||
| Community-acquired pneumonia | 422 (84.1) | 262 (77.5) | 274 (91.0) |
| Nursing and healthcare-associated pneumonia | 80 (15.9) | 76 (22.5) | 27 (9.0) |
| No. (%) of patients with each pneumonia severity score* | |||
| Mild (0 point) | 197 (39.2) | 57 (16.9) | 162 (53.8) |
| Moderate (1 or 2 points) | 239 (47.6) | 222 (65.7) | 124 (41.2) |
| Severe (3 points) | 52 (10.4) | 56 (16.6) | 12 (4.0) |
| Extremely severe (4 or 5 points) | 14 (2.8) | 3 (0.9) | 3 (1.0) |
| No. (%) of patients with COVID-19 severity score according to the criteria of Ministry of Health, Labour and Welfare | |||
| Moderate Ⅰ | 230 (45.8) | 112 (33.1) | 214 (71.1) |
| Moderate ⅠⅠ | 105 (20.9) | 58 (17.2) | 33 (11.0) |
| Severe | 167 (33.3) | 168 (49.7) | 54 (17.9) |
Continuous values are presented as medians and interquartile ranges (IQRs) and categorical/binary values as counts and percentages.
The median age of patients in the 1st to 3rd waves and in 4th wave were 65 years and 64 years old, respectively, but patients in the 5th wave were significantly younger at 50 years old (p < 0.001) (Table 1). The prevalence of patients with NHCAP and the pneumonia severity was significantly lower in the 5th wave than in the 1st to 4th waves.
3.2. Prediction of 30-day mortality in community-onset pneumonia
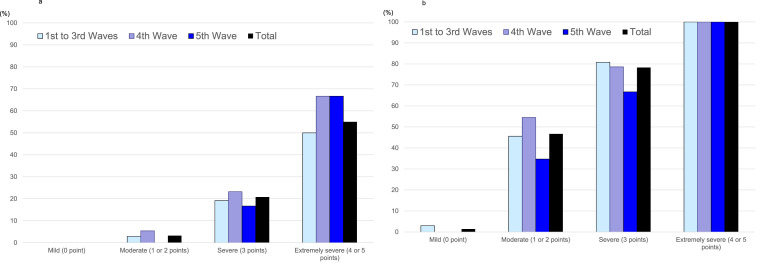
Fig. 1a shows the 30-day mortality rate in patients with COVID-19 CAP and NHCAP according to the severity classification of A-DROP. In the 1st to 3rd waves, the mortality rate was 0% in patients classified with mild disease, 2.9% with moderate disease, 19.2% with severe disease, and 50% with extremely severe disease. In the 4th wave (Alpha variant), the mortality rate was 0% in patients classified with mild disease, 5.4% with moderate disease, 23.2% with severe disease, and 66.7% with extremely severe disease. In the 5th wave (Delta variant), the mortality rate was 0% in patients classified with mild disease, 0% with moderate disease, 16.7% with severe disease, and 66.7% with extremely severe disease. Across all five waves, the mortality rate was 0% in patients classified with mild disease, 3.2% with moderate disease, 20.8% with severe disease, and 55.0% with extremely severe disease, indicating an increase in mortality in accordance with severity (Cochran–Armitage trend test; p = <0.001). The AUC for 30-day mortality was 0.853 in the 1st to 3rd waves, 0.872 in 4th wave and 0.831 in the 5th wave.
Fig. 1.
a. Mortality rate according to the severity classification using A-DROP in patients with COVID-19 pneumonia among the five waves. b. Mechanical ventilation rate according to the severity classification using A-DROP in patients with COVID-19 pneumonia among the five waves.
3.3. Prediction of requirement for mechanical ventilation in community-onset pneumonia
Fig. 1b shows the rate of requirement for mechanical ventilation in patients with COVID-19 CAP and NHCAP according to the severity classification of A-DROP. In the 1st to 3rd waves, the rate of mechanical ventilation was 3.0% in patients classified with mild disease, 45.6% with moderate disease, 80.8% with severe disease, and 100% with extremely severe disease. In the 4th wave (Alpha variant), the rate of mechanical ventilation was 0% in patients with mild disease, 54.5% with moderate disease, 78.6% with severe disease, and 100% with extremely severe disease. In the 5th wave (Delta variant), the rate of mechanical ventilation was 0% in patients with mild disease, 34.7% with moderate disease, 66.7% with severe disease, and 100% with extremely severe disease. Across all five waves, the rate of mechanical ventilation was 1.4% in patients with mild disease, 46.7% with moderate disease, 78.3% with severe disease, and 100% with extremely severe disease, indicating an increase in the requirement for mechanical ventilation in accordance with severity (Cochran–Armitage trend test; p = <0.001).
3.4. Prediction of 30-day mortality and requirement for mechanical ventilation in CAP
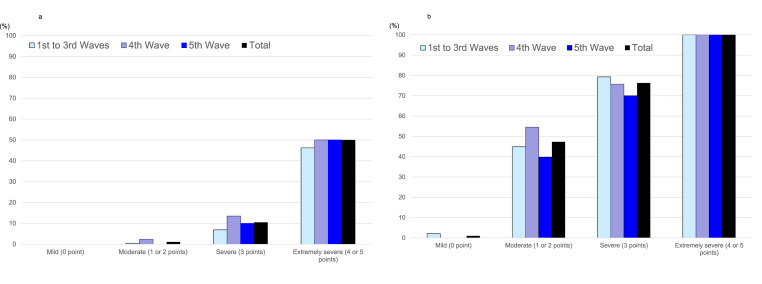
The 30-day mortality rate and mechanical ventilation rate in patients with COVID-19 CAP increased depending on the severity classified according to the A-DROP scoring system in each wave (Cochran–Armitage trend test; p = <0.001) (Fig. 2 a and b).
Fig. 2.
a. Mortality rate according to the severity classification using A-DROP in patients with community-acquired COVID-19 pneumonia among the five waves. b. Mechanical ventilation rate according to the severity classification using A-DROP in patients with community-acquired COVID-19 pneumonia among the five waves.
3.5. Prediction of 30-day mortality and requirement for mechanical ventilation in NHCAP
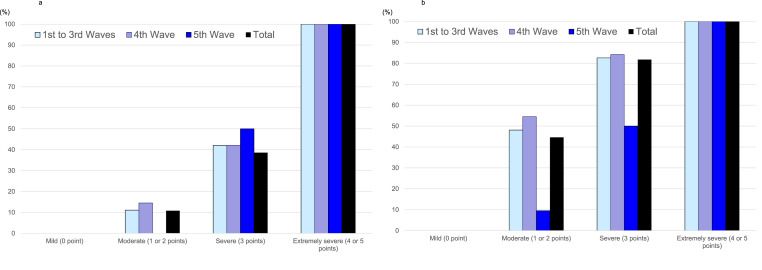
The 30-day mortality rate and mechanical ventilation rate in patients with COVID-19 NHCAP increased depending on the severity classified according to the A-DROP scoring system (Cochran–Armitage trend test; p = <0.001) (Fig. 3 a and b).
Fig. 3.
a. Mortality rate according to the severity classification using A-DROP in patients with nursing and healthcare-associated COVID-19 pneumonia among the five waves. b. Mechanical ventilation rate according to the severity classification using A-DROP in patients with nursing and healthcare-associated COVID-19 pneumonia among the five waves.
3.6. Changes of 30-day mortality in each wave
The mortality rates in patients with CAP among the five waves were identical in each pneumonia severity group. The mortality rate in the NHCAP group with moderate severity disease was almost unchanged between the 1st to 3rd waves (11.1%) and the 4th wave (14.5%) but decreased in the 5th wave (0%) compared with the 1st to 4th waves.
3.7. Changes of requirement for mechanical ventilation in each wave
The requirement for mechanical ventilation rate in the CAP group with moderate severity disease increased in the 4th wave (54.5%) compared with the 1st to 3rd waves (44.9%) (p = 0.088) but decreased significantly in the 5th wave (39.8%, p = 0.024). The requirement for mechanical ventilation in the NHCAP group with moderate to severe disease was almost unchanged between the 1st to 3rd waves (48.1%) and the 4th wave (54.5%) but decreased significantly in the 5th wave (9.5%) compared with the 1st to 4th waves (p = <0.001).
3.8. SARS-CoV-2 vaccine effect
Sixty-five patients (23.7%) in the 5th wave had been vaccinated (BNT162b2 or mRNA-1273) against SARS-CoV-2, of which 32 patients had received one dose and 33 patients had received two doses. The 30-day mortality rate and mechanical ventilation rate in vaccinated patients with two dose (0% and 9.1%, respectively) were lower than those of unvaccinated patients (1.3% and 18.6%, respectively), but these differences were not significant.
4. Discussion
Several studies have been evaluated the utility of severity scores including A-DROP system for COVID-19 pneumonia [[18], [19], [20]]. Fan et al. demonstrated the A-DROP presented the highest discrimination (AUC 0.87), following by CURB-65 (AUC 0.85), and PSI (AUC 0.85) in predicting in-hospital death [18]. Subsequently, Ucan et al. also demonstrated the AUCs for the mortality prediction in COVID-19 were 0.875, 0.873, and 0.859 for A-DROP, PSI, and CURB-65, respectively [19]. Kibar Akilli et al. demonstrated the PSI presented the highest discrimination (AUC 0.971), following by A-DROP (AUC 0.929), and CURB-65 (AUC 0.859) in predicting in-hospital death [20]. Our study evaluated the utility of A-DROP system for COVID-19 pneumonia in different wave (1st wave to 5th wave) and different pneumonia types (CAP and NHCAP).
Between the 1st wave and 5th wave in Japan, anti-SARS-CoV-2 drugs, immune regulators/immunosuppressive drugs and neutralizing antibody drugs were approved as therapeutic drugs against COVID-19 (Table 2 ). Remdesivir [21,22] and dexamethasone [23] were available in the 2nd wave, baricitinib [24] was available in the 3rd wave, anti-SARS-CoV-2 vaccination [[25], [26], [27]] for the elderly was started in the 4th wave, and casirivimab-imdevimab [28] and sotrovimab [29] were available in the 5th wave. Although the therapeutic drugs against COVID-19 were available, the mortality rate and severity were still high in the 4th wave compared with the 1st to 3rd waves in Osaka [30]. As vaccination against SARS-CoV-2 progressed, infection in elderly people has reduced markedly (Table 1) [31]. In addition to vaccination, neutralizing antibody therapy reduced the mortality rate and severity rate in the 5th wave [30]. Our results demonstrated that there was a significant relationship between the severity measured by the A-DROP scoring system and either the mortality rate or mechanical ventilation rate in patients with COVID-19 pneumonia in each wave despite the different treatments, pneumonia types and prevention strategies.
Table 2.
Summary of approved therapeutic drugs and vaccine against COVID-19 between the 1st wave and 7th wave in Japan.
| Wave | Period | Approved drugs and vaccine |
|---|---|---|
| 1st wave | January 2020 to May 2020 | |
| 2nd wave | June 2020 to October 2020 | Remdesivir Dexamethasone |
| 3rd wave | November 2020 to March 2021 | Baricitinib |
| 4th wave (Alpha variant) | April 2021 to June 2021 | Anti-SARS-CoV-2 vaccine BNT162b2 mRNA-1273 ChAdOx1 nCoV-19 |
| 5th wave (Delta variant) | July 2021 to December 2021 | Casirivimab-imdevimab Sotrovimab |
| 6th wave (Omicron subvariant BA.1 and BA.2) | January 2022 to June 2022 | Molnupiravir Nirmatrelvir/ritonavir Tocilizumab |
| 7th wave (Omicron subvariant BA.5) | July 2022 to November 2022 | Tixagevimab/cilgavimab Ensitrelvir |
In previous CAP studies assessing the A-DROP system for predicting 28- or 30-day mortality, the mortality rate was 0% in patients classified with mild disease, 2.5–5.6% in those classified with moderate disease, 9.9–16.1% in those classified with severe disease, and 19.6–34.0% in those classified with extremely severe disease [[3], [4], [5],7]. In our COVID-19 CAP analysis, the mortality rate was 0% in patients classified with mild disease, 0–2.4% in those classified with moderate disease, 6.9–10.0% in those classified with severe disease, and 46.2–50.0% in those classified with extremely severe disease. The mortality rates were very similar between COVID-19 CAP and non-COVID-19 CAP except for extremely severe CAP [[3], [4], [5],7].
Our study had several limitations. In our study, we did not evaluate PSI for the prediction of mortality and requirement for mechanical ventilation because many studies have demonstrated the good correlation between A-DROP and PSI [[18], [19], [20]]. We also did not evaluate CURB-65 because many studies have also demonstrated the good correlation between A-DROP and CURB-65 [[18], [19], [20]]. In addition, the sample size of the group with extremely severe pneumonia in our study was small. In the same way, we could not evaluate the vaccine efficacy because the sample size of the group with vaccination in our study was small.
In conclusion, the present study demonstrated that the mortality rate and mechanical ventilation rate in patients with COVID-19 pneumonia increased depending on the severity classified according to the A-DROP scoring system. The results of this study suggest that the A-DROP scoring system can be adapted for the assessment of severity of COVID-19 CAP and NHCAP.
Funding
No funding was received.
Availability of data and materials
The data will not be shared because of participant confidentiality.
Author’s contributions
All the authors conceived the study, participated in its design and coordination, and collected and managed the data, including quality control. NM and YN drafted the manuscript, and all authors contributed substantially to its revision. All the authors read and approved the final manuscript.
Ethical approval and consent to participate
The study protocol was approved by the Ethics Committee at Kansai Medical University and all participating facilities. Informed consent was obtained from all individual participants in the study.
Consent for publication
Not applicable.
Declaration of competing interest
The authors declare that they have no competing interests.
References
- 1.Fine M.J., Auble T.E., Yealy D.M., Hanusa B.H., Weissfeld L.A., Singer D.E., et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 2.Committee for the Japanese Respiratory Society guidelines for the management of respiratory infections. Guidelines for the management of community acquired pneumonia in adults, revised edition. Respirology. 2006;11(Suppl 3):S79–S133. doi: 10.1111/j.1440-1843.2006.00937_1.x. [DOI] [PubMed] [Google Scholar]
- 3.Shindo Y., Sato S., Maruyama E., Ohashi T., Ogawa M., Imaizumi K., et al. Comparison of severity scoring systems A-DROP and CURB-65 for community-acquired pneumonia. Respirology. 2008;13:731–735. doi: 10.1111/j.1440-1843.2008.01329.x. [DOI] [PubMed] [Google Scholar]
- 4.Usui K., Tanaka Y., Noda H., Ishihara T. Comparison of three prediction rules for prognosis in community acquired pneumonia: pneumonia Severity Index (PSI), CURB-65, and A-DROP. Nihon Kokyuki Gakkai Zasshi. 2009;47:781–785. [PubMed] [Google Scholar]
- 5.Kohno S., Seki M., Watanabe A., CAP Study Group Evaluation of an assessment system for the JRS 2005: A-DROP for the management of CAP in adults. Intern Med. 2011;50:1183–1191. doi: 10.2169/internalmedicine.50.4651. [DOI] [PubMed] [Google Scholar]
- 6.Kasamatsu Y., Yamaguchi T., Kawaguchi T., Tanaka N., Oka H., Nakamura T., et al. Usefulness of a semi-quantitative procalcitonin test and the A-DROP Japanese prognostic scale for predicting mortality among adults hospitalized with community-acquired pneumonia. Respirology. 2012;17:330–336. doi: 10.1111/j.1440-1843.2011.02101.x. [DOI] [PubMed] [Google Scholar]
- 7.Kohno S., Seki M., Takehara K., Yamada Y., Kubo K., Ishizaka A., et al. Prediction of requirement for mechanical ventilation in community-acquired pneumonia with acute respiratory failure: a multicenter prospective study. Respiration. 2013;85:27–35. doi: 10.1159/000335466. [DOI] [PubMed] [Google Scholar]
- 8.Ugajin M., Yamaki K., Hirasawa N., Yagi T. Predictive values of semi-quantitative procalcitonin test and common biomarkers for the clinical outcomes of community-acquired pneumonia. Respir Care. 2014;59:564–573. doi: 10.4187/respcare.02807. [DOI] [PubMed] [Google Scholar]
- 9.Fukuyama H., Ishida T., Tachibana H., Nakagawa H., Iwasaku M., Saigusa M., et al. Validation of scoring systems for predicting severe community-acquired pneumonia. Intern Med. 2011;50:1917–1922. doi: 10.2169/internalmedicine.50.5279. [DOI] [PubMed] [Google Scholar]
- 10.Kohno S., Imamura Y., Shindo Y., et al. Clinical practice guidelines for nursing- and healthcare-associated pneumonia (NHCAP) [complete translation] Respir Investig. 2014;51:103–126. doi: 10.1016/j.resinv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Ito A., Ishida T., Tokumasu H., Yamazaki A., Washio Y. Evaluation of pneumonia severity scoring systems in nursing and healthcare-associated pneumonia for predicting prognosis: a prospective, cohort study. J Infect Chemother. 2020;26:372–378. doi: 10.1016/j.jiac.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Koizumi T., Tsukada H., Ito K., Shibata S., Hokari S., Tetsuka T., et al. A-DROP system for prognostication of NHCAP inpatients. J Infect Chemother. 2017;23:727–733. doi: 10.1016/j.jiac.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 13.Oshitani Y., Nagai H., Matsui H., Aoshima M. Reevaluation of the Japanese guideline for healthcare-associated pneumonia in a medium-size community hospital in Japan. J Infect Chemother. 2013;19:579–587. doi: 10.1007/s10156-012-0517-1. [DOI] [PubMed] [Google Scholar]
- 14.Committee for The Japanese Respiratory Society guidelines for the management of pneumonia in adults . The Japanese Respiratory Society; 2017. The JRS guidelines for the management of pneumonia in adults. [in Japanese)] [Google Scholar]
- 15.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim W.S., Van der Eerden M.M., Laing R., Boersma W.G., Karalus N., et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cochran W.G. Some methods for strengthening the common chi-square tests. Biometrics. 1954;10:417–451. [Google Scholar]
- 18.Fan G., Tu C., Zhou F., Liu Z., Wang Y., Song B., et al. Comparison of severity scores for COVID-19 patients with pneumonia: a retrospective study. Eur Respir J. 2020;56 doi: 10.1183/13993003.02113-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ucan E.S., Ozgen Alpaydin A., Ozuygur S.S., Ercan S., Unal B., Sayiner A.A., et al. DEU COVID Study Group Pneumonia severity indices predict prognosis in coronavirus disease-2019. Respir Med Res. 2021;79 doi: 10.1016/j.resmer.2021.100826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kibar Akilli I., Bilge M., Uslu Guz A., Korkusuz R., Canbolat Unlu E., Kart Yasar K.J. Comparison of pneumonia severity indices, qCSI, 4C-Mortality Score and qSOFA in predicting mortality in hospitalized patients with COVID-19 Pneumonia. Pers Med. 2022;12:801. doi: 10.3390/jpm12050801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., et al. Remdesivir for the treatment of Covid-19 - final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gottlieb R.L., Vaca C.E., Paredes R., Mera J., Webb B.J., Perez G., et al. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–315. doi: 10.1056/NEJMoa2116846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horby P., Lim W.S., Emberson J.R., Mafham M., Bell J.L., Linsell L., et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marconi V.C., Ramanan A.V., de Bono S., Kartman C.E., Krishnan V., Liao R., et al. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;12:1407–1418. doi: 10.1016/S2213-2600(21)00331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Voysey M., Costa Clemens S.A., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2020;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinreich D.M., Sivapalasingam S., Norton T., Ali S., Gao H., Bhore R., et al. REGN-COV2, a neutralizing antibody Cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. doi: 10.1056/NEJMoa2035002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta A., Gonzalez-Rojas Y., Juarez E., Crespo Casal M., Moya J., Falci D.R., et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. doi: 10.1056/NEJMoa2107934. [DOI] [PubMed] [Google Scholar]
- 30.Summary of infection and medical condition from first wave to fifth wave. https://www.pref.osaka.lg.jp/attach/38215/00410045/1-2_1~5kansen1021.pdf
- 31.Ministry of Health, Labour and Welfare. 50th advisory board of countermeasures for COVID-19 infection. https://www.mhlw.go.jp/content/10900000/000826597.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be shared because of participant confidentiality.