Abstract
Elevated concentrations of interleukin-1 (IL-1) were found in tissue surrounding biomaterials infected with Staphylococcus epidermidis. To determine the role of IL-1 in biomaterial-associated infection (BAI), IL-1 receptor type I-deficient (IL-1R−/−) and wild-type mice received subcutaneous implants of silicon elastomer (SE) or polyvinylpyrrolidone-grafted SE (SEpvp), combined with an injection of 106 CFU of S. epidermidis or sterile saline. Neither mouse strain was susceptible to BAI around SE. IL-1R−/− mice with SEpvp implants had a no abscess formation and a reduced susceptibility to persistent S. epidermidis infection. The normal foreign body response, characterized by giant-cell formation and encapsulation, was delayed around SEpvp in wild-type mice but not in IL-1R−/− mice. This coincided with enhanced local IL-4 production in IL-1R−/− mice. These data suggest that inhibition of local IL-1 activity may be beneficial for the outcome of BAI.
A variety of biomaterials, such as prosthetic heart valves, artificial joints, and catheters, are used with increasing frequency in diagnostic and therapeutic procedures in modern medicine. The use of such biomaterials has undoubtedly led to medical progress for the benefit of many patients. On the other hand, implantation of an initially sterile biomaterial may quickly be complicated by infection. The majority of biomaterial-associated infections (BAI) (40 to 75%) are caused by the relatively nonpathogenic coagulase-negative staphylococci, especially Staphylococcus epidermidis (5, 50).
The pathogenesis of BAI is still poorly understood. It is assumed that the adherence of bacteria to the implanted biomaterial is the initial step in the pathogenesis of BAI (5, 50). To prevent BAI, novel materials with modified surfaces or with coatings of an antimicrobial agent(s) to reduce bacterial adherence are being developed (4, 8, 36, 46). Adherence, however, is not the only important factor in the pathogenesis. Alterations in the host response in the vicinity of the implanted biomaterial have frequently been suggested to play a role in pathogenesis (9, 32, 54, 56, 57).
Implanted biomaterials always induce an inflammatory reaction, but the intensity may vary, depending on the chemical composition of the material. Exposure of human monocytes or granulocytes to biomaterials in vitro induced the production of various cytokines (10, 12, 16, 18, 52). It was assumed that increased cytokine production might be associated with bioincompatibility reactions, such as enhanced leukocyte activation and enhanced susceptibility to infection. We previously studied the infection and tissue responses around two clinically used biomaterials, polyvinylpyrrolidone-grafted silicon elastomer (SEpvp) and conventional silicon elastomer (SE), in mice. We observed that subcutaneous implantation of sterile SEpvp catheter segments was associated with an earlier and more sustained increase in interleukin-1β (IL-1β) levels in the surrounding tissue than implantation of sterile SE catheter segments (7, 9). Injection of S. epidermidis along the subcutaneously implanted SEpvp segments caused an even more pronounced and protracted local IL-1β production, associated with subcutaneous abscesses and with persistent infection for up to 60 days.
IL-1α and IL-1β are potent proinflammatory cytokines that have been implicated as being important mediators in the pathogenesis of a variety of immunological and infectious diseases (20). IL-1 exerts biological effects by an interaction with the type I IL-1 receptor (IL-1R). At present, the role of IL-1 in the pathogenesis of BAI due to S. epidermidis is unknown. As we found an association between increased IL-1β production and BAI (9), we evaluated the susceptibility of IL-1R gene-deficient (IL-1R−/−) mice to BAI caused by S. epidermidis.
(Part of these data were presented at the 39th Interscience Conference on Antimicrobial Agents and Chemotherapy, 26 to 29 September 1999, San Francisco, Calif. [J. J. Boelens, S. A. Zaat, J. L. A. N. Murk, J. J. Weening, K. P. Dingemans, T. van der Poll, and J. Dankert, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1916, 1999].)
MATERIALS AND METHODS
Animals.
In total, 80 specific-pathogen-free IL-1R−/− mice, backcrossed six times to the C57BL/6 background, and C57BL/6 wild-type mice, 6 to 8 weeks old and weighing 15 to 20 g, were used. IL-1R−/− mice (24) were kindly provided by Immunex Co. (Seattle, Wash.) and bred in our animal facility. Wild-type C57BL/6 mice were from Harlan, Horst, The Netherlands. IL-1R−/− and wild-type mice were matched by age, weight, and sex, housed in individual cages in a pathogen-free environment, and provided with sterile food and water. The Academic Medical Center, University of Amsterdam, Amsterdam, The Netherlands, approved the animal experiments.
Catheter segments.
Catheters of conventional SE and SEpvp (Bioglide), with a diameter of 2.5 mm and a wall thickness of 0.6 mm were obtained from Medtronic PS Medical, Goleta, Calif. One-centimeter-long segments of SE and SEpvp, cut from either catheter under aseptic conditions in a laminar flow cabinet, were stored in sterile petri dishes.
Bacterial strain.
The clinical isolate S. epidermidis strain RP62a (ATCC 35984) was used. This strain is capable of producing slime, determined as described by Christensen et al. (13–15). MICs (in micrograms per milliliter) for strain RP62a, determined by the standard E test, were as follows: rifampin, <0.016; teicoplanin, 0.19; gentamicin, 256; minocyclin, <0.016; and vancomycin, 1.5. Antibiotic susceptibilities were used to confirm the identity of bacteria cultured from catheter segments and tissue homogenates.
Preparation of the bacterial inoculum.
An inoculum of 106 CFU of S. epidermidis RP62a was used. We previously demonstrated that in mice injected with this inoculum along subcutaneously implanted SEpvp, macroscopic abscesses and persistent infection for up to at least 60 days were induced, while around SE neither abscess formation nor infection was observed (6, 9). Two milliliters of an overnight culture of strain RP62a in Trypticase soy broth (Difco, Detroit, Mich.) was inoculated into 100 ml of fresh Trypticase soy broth. After incubation at 37°C for 5 h, 50 ml of this culture was centrifuged at 2,200 × g for 10 min, and the pelleted bacteria were washed twice with 50 ml of pyrogen-free isotonic saline. After the final centrifugation, the pellet was resuspended in pyrogen-free isotonic saline and the optical density at 620 nm was measured. The inoculum of 106 CFU was prepared by diluting the suspension with pyrogen-free isotonic saline, based on an established relationship between bacterial concentration and optical density at 620 nm.
Subcutaneous catheter implantation and administration of the bacterial inoculum.
Mice were anesthetized intraperitoneally with an FFM mix (1 ml of Hypnorm [fentanylcitrate {Fluanisone}], 1 ml of Midazalam, and 2 ml of distilled water) (0.07 ml 10 g of body weight) and placed in a laminar flow cabinet. The backs of the mice were shaved and prepared with 2% (wt/vol) chlorhexidine. On each side an incision (0.3 cm) was made 0.5 cm lateral to the spine. Subsequently, 1-cm-long SE or SEpvp catheter segments were inserted subcutaneously. IL-1R−/− and wild-type mice received either two SE or two SEpvp segments. The incisions were closed with a single 0/6 vicryl stitch. Mice with implanted SE or SEpvp segments were injected with the inoculum subcutaneously along the inserted segments in a 25-μl volume using a repetitive pipette (Stepper model 4001-025; Tridak Division, Brookfield, Conn.). Four IL-1R−/− mice and four wild-type mice received a subcutaneous injection of saline only, on both sides of the spine (saline controls).
Sample collection.
Mice in the saline control group were sacrificed and evaluated at 14 days after injection. After 2, 5, or 14 days, six mice of each of the experimental groups were anesthetized with an FFM mix administered intraperitoneally and subsequently sacrificed by cardiac puncture. Sacrificed mice were placed in a laminar flow cabinet and fixed on a sterile polypropylene plate. A dorsal midline incision was made from the cervical to the lumbal area. Subsequently, the skin and subcutaneous tissue were separated from the muscle fascia up to the flank on both sides. The implantation sites were inspected for purulence, and standardized biopsies (diameter, 12 mm) were taken from the implantation sites using a specially developed tissue sampler as described previously (6). Each single biopsy included skin, subcutaneous tissue, and the inserted segment.
The right-side biopsy from each mouse was placed in 10% buffered formaldehyde (pH 7.3) for histological examination. From the left-side biopsy, the catheter segment was separated from the tissue, washed twice with phosphate-buffered saline (PBS) (8.1 mM Na2HPO4, 1.5 mM KH2PO4, 140 mM NaCl, pH 7.2) and placed in a sterile tube containing 1 ml of PBS. The tissue sample was placed in a tube and weighed, and a volume of pyrogen-free isotonic saline corresponding to four times the weight was added. The weight of the tissue samples varied between 125 and 160 mg.
Histological examination.
After fixation in formaldehyde, the biopsies were embedded in plastic (methylmethacrylaat-butylmethacrylaat) (Merck Schuchart, Hohenbrunn, Germany), sectioned, and stained with hematoxylin-eosin. Slides were examined for five histological features characteristic for tissue reactions after implantation of a foreign body (2). These were (i) infiltration of inflammatory cells, i.e., polymorphonuclear or mononuclear cells; (ii) presence of purulence, i.e., deposition of leukocytes with necrotic burdens; (iii) foreign-body giant-cell formation; (iv) fibrosis, characterized by inflammatory cells, fibroblasts, and newly formed collagen; and (v) encapsulation of the foreign body. Sections were scored independently by three of us (J. J. Boelens, J. L. Murk, and J. J. Weening), using a scale of 0 to 3, indicative of the grade of appearance of each feature: a feature was scored 0 when it was not observed and 3 when it was maximally present. At the time of judgement, none of the investigators knew which sample originated from which mouse. The scales were based on inspection of slides previously obtained from tissue samples taken from subcutaneously inserted SE or SEpvp segments in mice which received bacterial inocula ranging from 0 CFU (saline injection) to 1010 CFU of S. epidermidis RP62a along the inserts (6).
Quantitative culture of SE and SEpvp catheter segments.
The tubes containing SE and SEpvp segments in 1 ml of PBS were sonicated for 30 s in a water bath sonicator (Bransonic B-2200 E4; 47, kHz, 205 W) to dislodge adherent bacteria. The number of viable S. epidermidis cells was assessed by quantitative culture of serial 10-fold dilutions of this PBS sonicate. Six aliquots of 10 μl of each dilution were spotted on a blood agar plate which was incubated overnight at 37°C. The remaining suspension was stored at 4°C. When no growth was observed on the blood agar plates, the stored suspension was centrifuged (2,200 × g, 10 min) and the pellet was resuspended in 100 μl of PBS and plated on blood agar. In addition, the sonicated segments were cultured in 80 ml of modified thioglycolate broth, consisting of 3% (wt/vol) thioglycolate containing 0.03% (wt/vol) polyanetholesulfonic acid, 1 M NaOH, and 0.5% Tween 80, for 72 h at 37°C. For statistical purposes, it was assumed that 1 CFU per 1-cm catheter segment had been present when no growth occurred on the blood agar plates and the catheter segment incubated in the broth was culture positive. The number of adherent S. epidermidis RP62a cells is expressed as number of CFU per catheter segment (1 cm).
Quantitative culture of the homogenates.
Tubes containing the tissue sample in pyrogen-free isotonic saline were homogenized on ice with a tissue homogenizer (Tissue Tearer model 985-370; Biospec Products, Bartlesville, Okla.). After each homogenization, the homogenizer was carefully cleaned, disinfected by subsequently washing in 4% (wt/vol) sodium hypochloride–70% alcohol, and rinsed with pyrogen-free isotonic saline. Seventy microliters of the homogenates was serially 10-fold diluted, and six aliquots of 10 μl of undiluted homogenate and of each dilution were spotted on a blood agar plate, which was incubated overnight at 37°C. In addition, 50 μl of the homogenate was cultured in 80 ml of thioglycolate broth for 72 h at 37°C. For statistical purposes it was assumed that 1 CFU per 50 μl had been present when no growth occurred on the blood agar plates and the homogenate aliquot incubated in the broth was culture positive. The number of persisting S. epidermidis RP62a cells is expressed as number of CFU per gram.
Cytokine assays of the homogenates.
The total homogenates, reduced by 120 μl for the quantitative culture, were diluted in 1 volume of lysis buffer (25) containing 1% Triton X-100, 150 mM NaCl, 30 mM Tris, 2 mM CaCl2, 2 mM MgCl2, 0.2% aprotin, and 0.2% leupeptin (pH 7.40) and incubated on ice for 1 h. Subsequently, the lysed homogenates were centrifuged at 130,000 × g for 15 min at 4°C to remove cell debris. The cell-free supernatants were frozen at −80°C, thawed, centrifuged at 5,000 × g to remove macroaggregates, and stored in aliquots of 35 μl at −80°C until use. Levels of IL-1β (R&D Systems, Minneapolis, Minn.), IL-1α (R&D Systems), and IL-4 (R&D Systems) were measured with commercially available enzyme-linked immunosorbent assay kits and expressed as picograms per milliliter of homogenate.
Statistical analysis.
All values are expressed as mean ± standard error. Two-sample comparisons were made by analysis of variance (ANOVA), and comparisons between cytokine levels of groups over time were made by ANOVA in a general linear model-general factorial. The significance of differences between the frequencies of categorical variables was determined using the χ2 test. A P value of <0.05 was considered significant.
RESULTS
Development of biomaterial-associated infection.
In the saline control mice, no abscess formation was seen. In wild-type mice after injection of 106 CFU of S. epidermidis, abscess formation was observed around SEpvp at all time points tested but not around SE segments. In contrast, in IL-1R−/− mice abscesses were found neither around SE segments nor around SEpvp segments at any time point after inoculation with S. epidermidis (Fig. 1A).
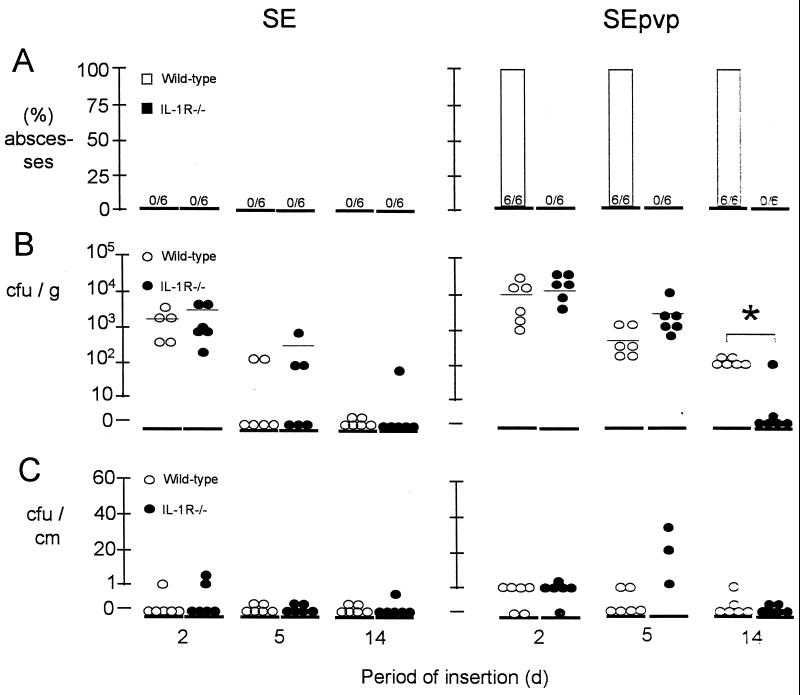
FIG. 1.
(A) Frequency of subcutaneous abscesses along the inserted catheter segments in either wild-type mice of IL-1R−/− mice. (B) Number of CFU of S. epidermidis RP62a cultured from the insertion site homogenates. (C) Number of adherent CFU of S. epidermidis RP62a cultured from subcutaneous implantation of SE and SEpvp after various periods of implantation and challenge with 106 CFU of S. epidermidis RP62a. There were six mice per group per time point tested.
In wild-type mice, at 2 days significantly more colonies were cultured from tissue surrounding SEpvp catheter segments (14,000 ± 12,000 CFU/g) than from tissue surrounding SE segments (1,800 ± 1,400 CFU/g) (P < 0.01) Fig. 1B. Although the numbers of CFU decreased at 5 and 14 days in all tissues (12 of 12) surrounding the SEpvp segments, these tissues remained culture positive. Tissues surrounding SE were culture negative at 5 and 14 days (Fig. 1B). Like in wild-type mice, in IL-1R−/− mice significantly more colonies were cultured from tissues surrounding SEpvp (22,000 ± 12,000 CFU/g) than from tissues surrounding SE (8,000 ± 9,000 CFU/g) (P < 0.05) at 2 days, and at 5 days all tissue homogenates surrounding SEpvp were positive, while all tissue homogenates surrounding SE were negative. At 14 days, however, five out of six tissue homogenates from SEpvp implantation sites were culture negative in IL-1R−/− mice. Thus, significantly fewer (P < 0.05) tissues were culture positive in IL-1R−/− mice than in wild-type mice around SEpvp at 14 days. The cultured S. epidermidis had the same antibiogram as strain RP62a. No colonies were cultured from the tissue samples of mice injected with saline.
SEpvp segments implanted in wild-type mice were significantly more often culture positive for S. epidermidis RP62a than SE segments (7 of 18 versus 1 of 18; P = 0.01) (Fig. 1C). In IL-1R−/− mice similar results were obtained (8 of 18 versus 3 of 18; P = 0.02). The cultured S. epidermidis had the same antibiogram as strain RP62a. In wild-type mice, tissues surrounding SE and SEpvp (7 of 18 and 18 of 18, respectively) were significantly more often culture positive than the corresponding segments (1 of 18 and 7 of 18, respectively) (P < 0.05). Similarly, in IL-1R−/− mice, tissues surrounding SE and SEpvp (10 of 18 and 13 of 18, respectively) were significantly more often culture positive than the corresponding segments (3 of 18 and 8 of 18, respectively) (P < 0.05). This indicates persistence of S. epidermidis in tissue rather than on the catheter surface.
Hence, using an inoculum of 106 CFU of S. epidermidis, in IL-1R−/− mice neither abscess formation nor persistent infection associated with SEpvp was induced, while wild-type mice were highly susceptible to SEpvp-associated abscess formation and infection. Bacteria persisted in the tissue rather than being adherent on the catheter segment.
Histological examination of the inflammatory and foreign-body reaction.
Five histological features characteristic for the tissue inflammation and foreign-body response following the implantation of a foreign body were scored independently by three investigators who were blinded for the origin of the samples. The mean scores for these histological features at each time point are shown in Fig. 2. Variation of scores between investigators was always maximally 0.5 scale unit.
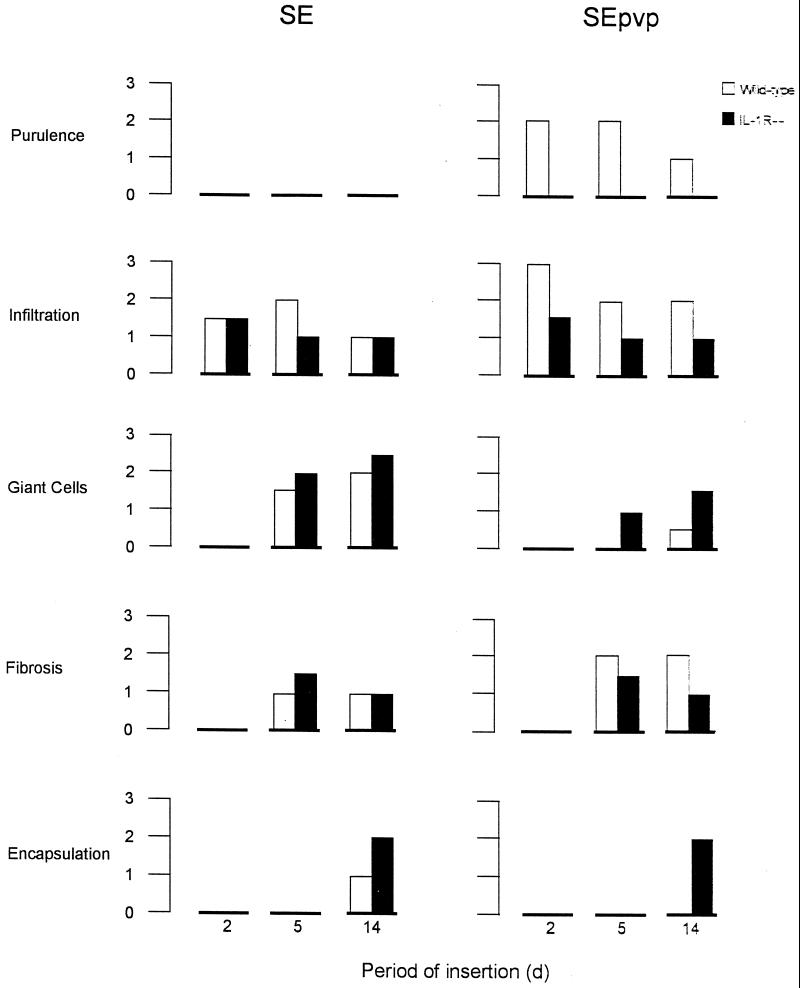
FIG. 2.
Mean scores of infiltration, foreign-body giant cells, fibrosis, and encapsulation, four features characteristic for the implantation of a foreign body, at various time points after subcutaneous implantation of SE or SEpvp and challenge with 106 CFU of S. epidermidis RP62a in either wild-type mice or IL-1R−/− mice. There were six mice per group tested at each time point.
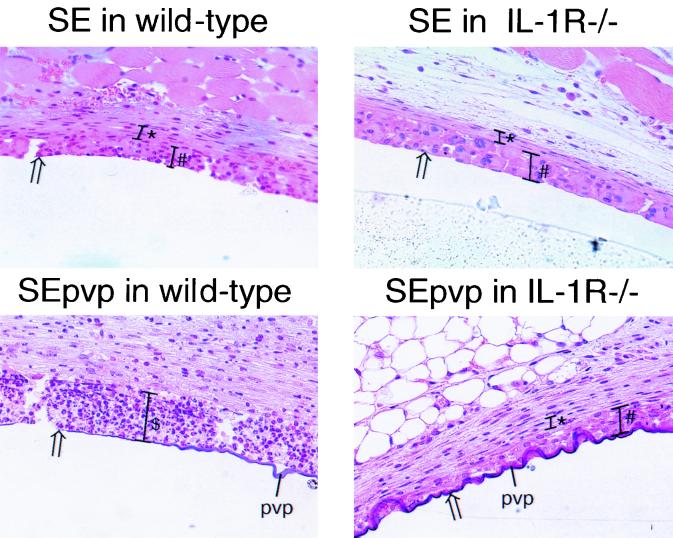
The foreign-body response around SE implanted in IL-1R−/− mice and wild-type mice showed only small differences (Fig. 2 and 3). In contrast, around SEpvp, the foreign-body response was dramatically different in wild-type and IL-1R−/− mice (Fig. 2 and 3). In wild-type mice, a prolonged and intensified inflammatory status was observed around SEpvp, characterized by the presence of purulence and a very strong infiltration up to 14 days, by strong fibrosis, and by delayed giant-cell formation and delayed encapsulation of the foreign body. In contrast, around SEpvp implanted in IL-1R−/− mice, no purulence was present, and a mild inflammation, weak fibrosis, and a normal development of the foreign-body reaction were observed, characterized by a layer containing giant cells bordering the implant at 5 days and the presence of a fibrous capsule at 14 days (Fig. 2 and 3).
FIG. 3.
Gross sections of subcutaneously implanted catheter segments in wild-type and IL-1R−/− mice challenged with S. epidermidis stained with hematoxylineosin. $, abscess formation; #, giant-cell formation; ∗, thickness of the capsule; arrows, catheter-tissue interface.
Hence, around SEpvp implanted in IL-1R−/− mice challenged with S. epidermidis, a normal foreign-body reaction, similar to that around SE implanted in wild-type mice challenged with S. epidermidis, was observed.
Induction of IL-1β, IL-1α, and IL-4.
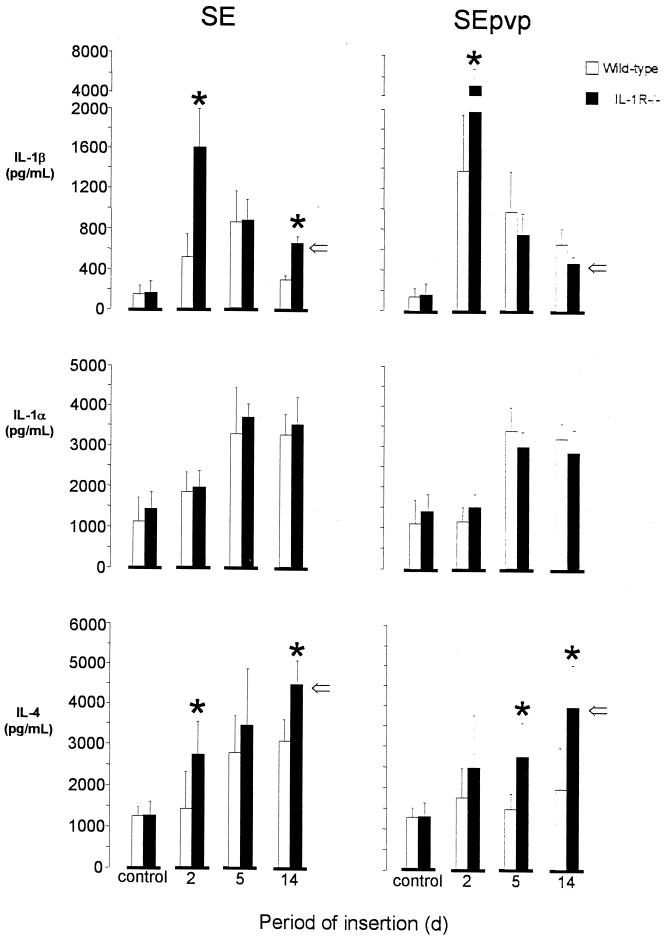
IL-1β was detectable at low levels in saline control wild-type and IL-1R−/− mice (Fig. 4). In wild-type mice injected with S. epidermidis, significantly higher IL-1β levels were found in tissue surrounding SEpvp segments than in tissue surrounding SE (P < 0.01 for the profile of IL-1β over time). In IL-1R−/− mice at 2 days, significantly higher IL-1β levels were found in tissue surrounding the implanted SE (1,624 ± 529 versus 545 ± 211 pg/ml; P = 0.002) and in tissue surrounding SEpvp (5,069 ± 2,478 versus 1,418 ± 763 pg/ml; P = 0.007) than in wild-type mice. At later time points no differences in IL-1β levels between wild-type and IL-1R−/− mice were detected around SEpvp segments. In tissues surrounding SE in wild-type and IL-1R−/− mice, IL-1β levels were equal at 5 days but again were higher in IL-1R−/− mice at 14 days (P < 0.01).
FIG. 4.
Mean (± standard error) implantation site concentrations of IL-1β, IL-1α, and IL-4 at 2, 5, and 14 days after subcutaneous implantation of SE or SEpvp in wild-type of IL-1R−/− mice challenged with 106 CFU S. epidermidis RP62a. There were six mice per group tested at each time point. ∗, P < 0.05 by ANOVA, ⇒, P < 0.05 for over-time levels by general linear model-ANOVA.
IL-1α was detectable in saline control wild-type and IL-1R−/− mice (Fig. 4). In mice inoculated with S. epidermidis, IL-1α levels around the implanted SE and SEpvp segments increased up to 5 days and remained elevated up to 14 days. No differences were found between IL-1α levels in tissues surrounding SE and SEpvp or in tissues from wild-type and IL-1R−/− mice.
IL-4 was detectable in saline control wild-type and IL-1R−/− mice (Fig. 4). In wild-type mice injected with S. epidermidis, IL-4 levels were increased in tissue around SE after 5 days, whereas in tissue surrounding SEpvp no increase was found. The profile of IL-4 in tissue surrounding SE over time was significantly higher (P = 0.025) (Fig. 4) than that in tissue surrounding SEpvp. In tissues surrounding either SE or SEpvp segments from IL-1R−/−, mice significantly higher levels of IL-4 were detected than in wild-type mice (P < 0.01 for both) (Fig. 4).
DISCUSSION
Recently, we found sustained IL-1β production in murine tissues surrounding subcutaneously implanted biomaterials infected with S. epidermidis (9). In the present study, we sought to determine the role of this locally produced IL-1 in the pathogenesis of BAI. We demonstrated that IL-1R−/− mice had no abscess formation and were less susceptible to persistent S. epidermidis infection associated with SEpvp catheters than wild-type mice. The foreign-body reaction around SEpvp was delayed in wild-type mice but not in IL-1R−/− mice. These data suggest that IL-1 plays a detrimental role in this experimental model for BAI.
The IL-1 family consists of two agonist ligands, the potent proinflammatory cytokines IL-1α and IL-1β, and an antagonist ligand, IL-1R antagonist. IL-1α and IL-1β induce cellular effects by binding to the type I IL-1R. The type II IL-1R has no signaling properties but acts as a decoy receptor (3, 20, 51). Hence, mice deficient in IL-1R type I are incapable of responding to either IL-1α or IL-1β (24). The IL-1 family regulates a number of immunological, physiological, and pathophysiological responses. IL-1 has an important role in the host response against bacterial infections (19, 20). However, IL-1 action is tightly regulated, and there seems to be a delicate balance between the beneficial local activity of IL-1 and potentially harmful excessive local or systemic IL-1 activity. Indeed, blockage of IL-1 activity resulted in increased susceptibility to infection (23, 26, 34, 53). On the other hand, protracted and/or systemic production of IL-1 is involved in a variety of inflammatory diseases, such as rheumatic or septic arthritis, pancreatitis, septic shock, and acute respiratory distress syndrome (19, 20, 27, 40). Blocking of IL-1 activity by administration of IL-1R antagonist reduced tissue damage in experimental arthritis (41, 42, 49, 55) and pancreatitis (43–45) and reduced mortality in experimental septic shock models (1, 22, 38). Additionally, IL-1 is assumed to have a role in bioincompatibility (9, 10, 12, 16, 18). In the present study, the enhanced and protracted inflammatory response due to the combined effect of SEpvp and S. epidermidis observed in wild-type mice was strongly attenuated in IL-1R−/− mice. The latter mice did not show abscess formation and were not susceptible to persistent S. epidermidis infection associated with SEpvp. As shown in previous work by us, tissues around SEpvp implanted in wild-type mice remained positive for up to 60 days (7, 9), while five out of six tissues around SEpvp implanted in IL-1R−/− are negative at 14 days. The exaggerated and protracted local IL-1 response around SEpvp in wild-type mice, resulting in abscess formation and delay of the normal foreign-body reaction (2), apparently was beneficial for survival of the relatively avirulent S. epidermidis.
After initial culture positivity (2 days), the SEpvp segments themselves became culture negative after 14 days, in the wild-type mice as well as in the IL-1R−/− mice. However, the tissue around SEpvp in the IL-1R−/− mice remained culture positive. In a previous study similar results were obtained with SEpvp segments to which S. epidermidis (3 × 103 CFU per cm) was added prior to implantation (6), indicating that the persistence in the tissue was not due to the mode of inoculation. Our findings are discordant with the contention that adherence to and growth of bacteria on the biomaterial surface are of decisive importance in the pathogenesis of BAI (5, 50) but suggest that the niche for the persisting S. epidermidis is not the implanted SEpvp segment but rather the surrounding tissue.
The bacterial survival in the present study could have been associated with the abscess (17, 35) along the subcutaneously implanted SEpvp segment rather than with adherence to the catheter surface. Alternatively, exaggerated and protracted local inflammation, as observed in wild-type mice carrying SEpvp segments, may compromise the resolution of an infection by priming for intracellular and extracellular growth of bacteria exceeding the clearance ability of the host. Kanangat et al. (30) reported that high concentrations of lipopolysaccharide, IL-1β, IL-6, and tumor necrosis factor alpha stimulated intracellular growth in monocytes as well as extracellular growth of Staphylococcus aureus, Pseudomonas aeruginosa, and an Acinetobacter sp., which are all important nosocomial pathogens (30). Observation in humans support a role for increased proinflammation in persistent infection: (i) patients suffering from acute respiratory distress syndrome, caused by acute development of diffuse lung inflammation, have persistently elevated levels of proinflammatory cytokines associated with an increased rate of nosocomial infections (27, 40); (ii) patients with long-term exposure to intravascular catheters had exacerbated complications associated with sepsis (37); and (iii) patients who had an extracorporal circulation (11), as well as patients undergoing hemodialysis (28, 48), with enhanced levels of circulating proinflammatory cytokines were highly susceptible.
In tissues surrounding SE and SEpvp, the IL-1β production was significantly higher in IL-1R−/− mice than in wild-type mice. Similarly, in a model of acute pancreatitis (43–45), IL-1R−/− mice had higher IL-1β levels than wild-type mice, suggesting that a negative feedback loop exists between IL-1R and IL-1β production. It should be noted, however, that IL-1α concentrations were not increased in IL-1R−/− mice. Therefore, further studies are warranted to dissect the mechanism involved in elevating tissue IL-1β levels during BAI in IL-1R−/− mice.
The protracted increased IL-1β levels around SEpvp implanted in S. epidermidis-challenged wild-type mice were associated with a reduction in IL-4 production and a delay in foreign body development characterized by the presence of foreign-body giant-cell formation and encapsulation not earlier than at 14 days. Conversely, in the IL-1R−/− mice, increased IL-4 levels and a layer of foreign-body giant cells were present around SEpvp at 5 days, as was the case for S. epidermidis-challenged wild-type mice with SE and for saline-injected wild-type mice with implanted SE or SEpvp segments (data not shown). Apparently, IL-4 is associated with macrophage fusion and the onset of the foreign-body reaction in vivo. This confirms the relevance of similar data obtained by others in vitro (21, 29, 31, 33, 39). In addition, the enhanced (in comparison to in wild-type mice) production of IL-4 around SEpvp in our IL-1R−/− mice despite the high levels of IL-1β is concordant with similar results obtained in a leishmanias infection model (47) and suggests a role for IL-1 in the down-regulation of IL-4 production in vivo in wild-type mice.
Implantation of SEpvp modulated the inflammatory environment in such a way that it primed for bioincompatibility reactions in the presence of S. epidermidis, resulting in enhanced leukocyte activation and persistent infection. Enhancement of the inflammatory response in the presence of bacteria is probably not restricted to SEpvp. Exposure of human monocytes or polymorphonuclear cells to biomaterials in vitro induced the production of IL-1β, tumor necrosis factor alpha, and IL-6 (10, 12, 16, 52). Differences in inducing properties between various biomaterials became apparent only in the presence of lipopolysaccharide, a major cell wall component of gram-negative bacteria (10, 12, 16, 52). This implies that in a material-dependent way, the presence of bacteria or bacterial components can modulate the inflammatory tissue reaction in response to the implanted biomaterial. For biocompatibility a delicately balanced immune response is required. This balance apparently is strictly regulated, and when it is disturbed, an inappropriately strong reaction and pathology will follow. Our in vivo experiments showed that IL-1β might be an important player in this bioincompatibility.
In conclusion, BAI in the SEpvp model was associated with an exaggerated and protracted local IL-1α and IL-1β response. Blocking of IL-1 effects resulted in a reduced susceptibility to BAI and a normal foreign-body reaction, suggesting a role for high IL-1 levels in compromising the host by stimulating bacterial persistence and in inhibiting IL-4 production. Our results suggest an important role for the host response to various biomaterials in the pathogenesis of BAI, rather than for the extent of adherence of bacteria to biomaterial. Local inhibition of IL-1 activity may be of benefit to the host as an adjunctive therapy for infections associated with biomaterials.
ACKNOWLEDGMENT
T. van der Poll is a fellow of the Royal Netherlands Academy of Arts and Sciences.
REFERENCES
- 1.Aiura K, Gelfand J A, Wakabayashi G, Callahan M, Burke J F, Thomson J, Dinarello C A. Interleukin-1 receptor antagonist blocks Staphylococcal induced shock in rabbits. Cytokine. 1991;3:498. [Google Scholar]
- 2.Anderson J M. Inflammatory response to implants. Trans Am Soc Artif Intern Organs. 1988;34:101–107. doi: 10.1097/00002480-198804000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Arend W, Malyak M, Guthridge C, Gabay C. Interleukin-1 receptor antagonist: role in biology. Annu Rev Immunol. 1998;16:27–55. doi: 10.1146/annurev.immunol.16.1.27. [DOI] [PubMed] [Google Scholar]
- 4.Bach A, Bohrer H, Motsch J, Martin E, Geiss H K, Sonntag H G. Prevention of catheter-related infections by antiseptic bonding. J Surg Res. 1993;55:640–646. doi: 10.1006/jsre.1993.1197. [DOI] [PubMed] [Google Scholar]
- 5.Bisno A, Waldvogel F. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C.: American Society for Microbiology; 1994. [Google Scholar]
- 6.Boelens J, Zaat S A, Meeldijk J, Dankert J. Subcutaneous abscess formation around catheters induced by viable and non-viable staphylococcus epidermidis as well as by small amounts of bacterial cell wall components. J Biomed Mater Res. 2000;50:546–556. doi: 10.1002/(sici)1097-4636(20000615)50:4<546::aid-jbm10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 7.Boelens J J, Dankert J, Murk J L, Weening J J, van der Poll T, Dingemans K P, Koole L, Laman J D, Zaat S A. Biomaterial-associated persistence of Staphylococcus epidermidis in pericatheter macrophages. J Infect Dis. 2000;181:1337–1349. doi: 10.1086/315369. [DOI] [PubMed] [Google Scholar]
- 8.Boelens J J, Tan W F, Dankert J, Zaat S A J. Antibacterial activity of antibiotic-soaked polyvinylpyrrolidone-grafted silicon elastomer hydrocephalus shunts. J Antimicrob Chemother. 2000;45:221–224. doi: 10.1093/jac/45.2.221. [DOI] [PubMed] [Google Scholar]
- 9.Boelens J J, Zaat S A J, Murk J L, Weening J J, van der Poll T, Dankert J. Enhanced susceptibility to subcutaneous abscess formation and persistent infection around catheters is associated with sustained interleukin-1β levels. Infect Immun. 2000;68:1692–1695. doi: 10.1128/iai.68.3.1692-1695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonfield T L, Anderson J M. Functional versus quantitative comparison of IL-1 beta from monocytes/macrophages on biomedical polymers. J Biomed Mater Res. 1993;27:1195–1199. doi: 10.1002/jbm.820270910. [DOI] [PubMed] [Google Scholar]
- 11.Cameron D. Initiation of white cell activation during cardiopulmonary bypass: cytokines and receptors. J Cardiovasc Pharmacol. 1996;27(Suppl.):S1–S5. doi: 10.1097/00005344-199600001-00004. [DOI] [PubMed] [Google Scholar]
- 12.Cardona M A, Simmons R L, Kaplan S S. TNF and IL-1 generation by human monocytes in response to biomaterials. J Biomed Mater Res. 1992;26:851–859. doi: 10.1002/jbm.820260703. [DOI] [PubMed] [Google Scholar]
- 13.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983;40:407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Christensen G D, Simpson W A, Younger J J, Baddour L M, Barrett F F, Melton D M, Beachey E H. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. J Clin Microbiol. 1985;22:996–1006. doi: 10.1128/jcm.22.6.996-1006.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.DeFife K M, Yun J K, Azeez A, Stack S, Ishihara K, Nakabayashi N, Colton E, Anderson J M. Adhesion and cytokine production by monocytes on poly(2-methacryloyloxyethyl phosphorylcholine-co-alkyl methacrylate)-coated polymers. J Biomed Mater Res. 1995;29:431–439. doi: 10.1002/jbm.820290403. [DOI] [PubMed] [Google Scholar]
- 17.Deighton M A, Borland R, Capstick J A. Virulence of Staphylococcus epidermidis in a mouse model: significance of extracellular slime. Epidemiol Infect. 1996;117:267–280. doi: 10.1017/s0950268800001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dinarello C A. Cytokines and biocompatibility. Blood Purification. 1990;8:208–213. doi: 10.1159/000169968. [DOI] [PubMed] [Google Scholar]
- 19.Dinarello C A. Role of interleukine-1 in infectious diseases. Immunol Rev. 1992;127:119–146. doi: 10.1111/j.1600-065x.1992.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 20.Dinarello C A. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 21.Dugast C, Gaudin A, Toujas L. Generation of multinucleated giant cells by culture of monocyte-derived macrophages with IL-4. J Leukoc Biol. 1997;61:517–521. doi: 10.1002/jlb.61.4.517. [DOI] [PubMed] [Google Scholar]
- 22.Everaerdt B, Brouckaert P, Fiers W. Recombinant IL-1 receptor antagonist protects against TNF-induced lethality in mice. J Immunol. 1994;152:5041–5049. [PubMed] [Google Scholar]
- 23.Garcia M, Dinarello C A. The interleukin-1 receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniae sepsis in newborn rats. Infect Immun. 1993;61:926–932. doi: 10.1128/iai.61.3.926-932.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glaccum M, Stocking K, Charrier K, Smith J, Willis C, Maliszewski C, Livingstone D J, Peschon J J, Morrissey P J. Phenotypical and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159:3364–3371. [PubMed] [Google Scholar]
- 25.Greenberger M, Streiter R, Goodman R, Standiford T. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumoniae. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 26.Havell E, Moldawer L, Helfgott D, Kilian P, Sehgal P. Type I IL-1 receptor blockade exacerbates murine listeriosis. J Immunol. 1992;148:1486–1492. [PubMed] [Google Scholar]
- 27.Headley A, Tolley E, Meduri G. Infections and the inflammatory response in acute respiratory distress syndrome. Chest. 1997;111:1306–1321. doi: 10.1378/chest.111.5.1306. [DOI] [PubMed] [Google Scholar]
- 28.Herbelin A, Urena P, Ngugen T, Zingraff J, Descamps-Latscha B. Elevated circulating levels of interleukin-6 in patients with chronic renal failure. Kidney Int. 1991;39:954–960. doi: 10.1038/ki.1991.120. [DOI] [PubMed] [Google Scholar]
- 29.Jenney C, DeFife K, Colton E, Anderson J M. Human monocyte/macrophage adhesion, macrophage motility, and IL-4-induced foreign body giant cell formation on silane-modified surfaces in vitro. J Biomed Mater Res. 1998;41:171–184. doi: 10.1002/(sici)1097-4636(199808)41:2<171::aid-jbm1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Kanangat S, Meduri G, Tolley E, Patterson D, Meduri C, Pak C, Griffin J P, Bronze M S, Schaberg D R. Effects of cytokines and endotoxin on the intracellular growth of bacteria. Infect Immun. 1999;67:2834–2840. doi: 10.1128/iai.67.6.2834-2840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao W J, Zhao Q H, Hiltner A, Anderson J M. Theoretical analysis of in vivo macrophage adhesion and foreign body giant cell formation on polydimethylsiloxane, low density polyethylene, and polyetherurethanes. J Biomed Mater Res. 1994;28:73–79. doi: 10.1002/jbm.820280110. [DOI] [PubMed] [Google Scholar]
- 32.Kaplan S S, Heine R P, Simmons R L. Definsins impair phagocytic killing by neutrophils in biomaterial-related infection. Infect Immun. 1999;67:1640–1645. doi: 10.1128/iai.67.4.1640-1645.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazazi F, Chang J, Lopez A, Vadas M, Cunningham A. Interleukin-4 and human immune deficiency virus stimulate LFA-1-ICAM-1-mediated aggregation of monocytes and subsequent giant cell formation. J Gen Virol. 1994;75:2795–2802. doi: 10.1099/0022-1317-75-10-2795. [DOI] [PubMed] [Google Scholar]
- 34.Kozak W, Zheng H, Conn C, Soszynski D, van der Ploeg L, Kluger M. Thermal and behavioral effects of lipopolysaccharide and influenza in interleukin-1beta-deficient mice. Am J Physiol. 1995;269:R969–R977. doi: 10.1152/ajpregu.1995.269.5.R969. [DOI] [PubMed] [Google Scholar]
- 35.Long J, Kapral F. Host response to coagulase-negative staphylococci in abscesses induced within mice. J Med Microbiol. 1993;39:191–195. doi: 10.1099/00222615-39-3-191. [DOI] [PubMed] [Google Scholar]
- 36.Maki D G, Stolz S M, Wheeler S, Mermel L A. Prevention of central venous catheter-related bloodstream infection by use of an antiseptic-impregnated catheter. A randomized, controlled trial. Ann Intern Med. 1997;127:257–266. doi: 10.7326/0003-4819-127-4-199708150-00001. [DOI] [PubMed] [Google Scholar]
- 37.Martin L, Varv T, Davis P, Munger B, Lynch J, Spangler S, Remick D G. Intravascular plastic catheters. How they potentiate tumar necrosis factor release and exacerbate complications associated with sepsis. Arch Surg. 1991;126:1087–1093. doi: 10.1001/archsurg.1991.01410330041005. [DOI] [PubMed] [Google Scholar]
- 38.McIntyre K, Stepan G, Kolinsky D, Benjamin W, Plocinski J, Kaffka K, Campen R A, Chizzonite R, Kilian P L. Interleukin-1 receptor antagonist blocks acute inflammatory response to IL-1 and other agents. J Exp Med. 1991;173:931–939. doi: 10.1084/jem.173.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNally A K, Anderson J M. Interleukin-4 induces foreign body giant cells from human monocytes/macrophages. Am J Pathol. 1995;147:1487–1499. [PMC free article] [PubMed] [Google Scholar]
- 40.Meduri G. The role of the host defense response in the progression and outcome of ARDS: pathophysiological correlations and response to glucocorticoid treatment. Eur Respir. 1996;9:2650–2670. doi: 10.1183/09031936.96.09122650. [DOI] [PubMed] [Google Scholar]
- 41.Miller L, Isa S, Vannier E, Georgilis K, Steer A, Dinarello C A. Live Borrelia burgdorferi preferentially activate interleukin-1 beta gene expression and protein synthesis over the interleukin-1 receptor antagonist. J Clin Invest. 1992;90:906–912. doi: 10.1172/JCI115966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller L, Lynch E A, Isa S, Logan J, Dinarello C A. Balance of synovial fluid IL-1beta and IL-1 receptor antagonist and recovery from Lyme arthritis. Lancet. 1993;341:146–148. doi: 10.1016/0140-6736(93)90006-3. [DOI] [PubMed] [Google Scholar]
- 43.Norman J, Fink G, Sexton C, Carter G. Transgenic animals demonstrate a role for the IL-1 receptor in regulating IL-1beta gene expression at steady-state and during the systemic stress induced by acute pancreatitis. J Surg Res. 1995;63:231–236. doi: 10.1006/jsre.1996.0253. [DOI] [PubMed] [Google Scholar]
- 44.Norman J, Franz M, Messina J, Fabri P, Gower W, Carey L. Decreased mortality of severe acute pancreatitis after proximal cytokine blockage. Ann Surg. 1995;221:625–631. doi: 10.1097/00000658-199506000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Norman J, Franz M, Messina J, Riker A, Fabri P, Rosemurgy A, Gower G R. Interleukin-1 receptor antagonist decreases severity of acute pancreatitis. Surgery. 1995;117:648–655. doi: 10.1016/s0039-6060(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 46.Raad I, Darouiche R, Dupuis J, Abi-Said D, Gabrielli A, Hachem R, Wall M, Harris R, Jones J, Buzaid A, Robertson C, Shenaq S, Curling P, Burke T, Ericsson C. Central venous catheters coated with minocycline and prevention of catheter-related colonization and bloodstream infections. A randomized, double-blind trial. Ann Intern Med. 1997;127:267–274. doi: 10.7326/0003-4819-127-4-199708150-00002. [DOI] [PubMed] [Google Scholar]
- 47.Satoskar A, Okano M, Connaughton S, Raisanen-Sololwski A, David J, Labow M. Enhanced Th2-like responses in IL-1 type 1 receptor-deficient mice. Eur J Immunol. 1998;28:2066–2074. doi: 10.1002/(SICI)1521-4141(199807)28:07<2066::AID-IMMU2066>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 48.Schindler R, Lonnemann G, Shaldon S, Koch K, Dinarello C A. Transcription, not synthesis of interleukin-1 and tumor necrosis factor by complement. Kidney Int. 1990;37:85–93. doi: 10.1038/ki.1990.12. [DOI] [PubMed] [Google Scholar]
- 49.Schwab J, Anderle S, Brown R, Dalldorf F, Thomson J. Pro- and anti-inflammatory roles of interleukin-1 in recurrence of bacterial cell-wall induced arthritis in rats. Infect Immun. 1991;59:4436–4442. doi: 10.1128/iai.59.12.4436-4442.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seifert H, Jansen B, Farr B. Catheter-related infections. 1st ed. New York, N.Y: Marcel Dekker Press; 1997. [Google Scholar]
- 51.Sims J, Giri J, Dowere S. The two interleukin-1 receptors play different roles in IL-1 action. Clin Immunol Immunopathol. 1994;72:9–14. doi: 10.1006/clin.1994.1100. [DOI] [PubMed] [Google Scholar]
- 52.Swartbol P, Truedsson L, Parsson H, Norgren L. Tumor necrosis factor-alpha and interleukin-6 release from white blood cells induced by different graft materials in vitro are affected by pentoxifylline and iloprost. J Biomed Mater Res. 1997;36:400–406. doi: 10.1002/(sici)1097-4636(19970905)36:3<400::aid-jbm15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 53.van der Meer J, Barza M, Wolff S, Dinarello C A. A low dose of recombinant interleukin 1 protects granulocytopenic mice from lethal gram-negative infection. Proc Natl Acad Sci USA. 1988;85:1620–1623. doi: 10.1073/pnas.85.5.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaudaux P, Grau G, Huggler E, Schumacher-Perdreau F, Fiedler F, Waldvogel F A, Lew D P. Contribution of tumor necrosis factor to host defense against staphylococci in a guinea pig model of foreign body infections. J Infect Dis. 1992;166:58–64. doi: 10.1093/infdis/166.1.58. [DOI] [PubMed] [Google Scholar]
- 55.Wooley P, Whalen J, Chapman D, Berger A, Aspar D, Richard K, Staite N D. The effect of an interleukin-1 receptor antagonist protein on Type II cologen and antigen-induced arthritis in mice. Arthritis Rheum. 1993;36:1305–1314. doi: 10.1002/art.1780360915. [DOI] [PubMed] [Google Scholar]
- 56.Zimmerli W, Waldvogel F, Vaudaux P, Nydegger H E. Pathogenesis of foreign body infection: description and characteristics of an animal study. J Infect Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]
- 57.Zimmerli W, Waldvogel F. Pathogenesis of foreign body infection. J Clin Investig. 1984;73:1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]