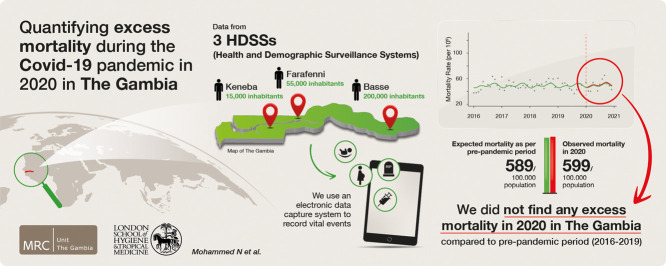
Abstract
Objectives
Estimates for COVID-19-related excess mortality for African populations using local data are needed to design and implement effective control policies.
Methods
We applied time-series analysis using data from three health and demographic surveillance systems in The Gambia (Basse, Farafenni, and Keneba) to examine pandemic-related excess mortality during 2020, when the first SARS-CoV-2 wave was observed, compared to the pre-pandemic period (2016-2019).
Results
Across the three sites, average mortality during the pre-pandemic period and the total deaths during 2020 were 1512 and 1634, respectively (Basse: 1099 vs 1179, Farafenni: 316 vs 351, Keneba: 98 vs 104). The overall annual crude mortality rates per 100,000 (95% CI) were 589 (559, 619) and 599 (571, 629) for the pre-pandemic and 2020 periods, respectively. The adjusted excess mortality rate was 8.8 (-34.3, 67.6) per 100,000 person-month with the adjusted rate ratio (aRR) = 1.01 (0.94,1.11). The age-stratified analysis showed excess mortality in Basse for infants (aRR = 1.22 [1.04, 1.46]) and in Farafenni for the 65+ years age group (aRR = 1.19 [1, 1.44]).
Conclusion
We did not find significant excess overall mortality in 2020 in The Gambia. However, some age groups may have been at risk of excess death. Public health response in countries with weak health systems needs to consider vulnerable age groups and the potential for collateral damage.
Keywords: Excess mortality, Health and demographic surveillance systems, COVID-19, Africa, The Gambia
Graphical abstract
Introduction
In January 2020, COVID-19 was declared a Public Health Emergency of International Concern. At the time, 7818 cases and 170 deaths were reported in 19 countries, mainly in Asia [1]. Almost a year later, COVID-19 spread across the world and caused about 278 million cases and 5.4 million deaths. Despite the extent of the pandemic, Africa, which accounts for approximately 16% of the world population, has been relatively spared by the pandemic as it accounted only for 3% of cases (7 million) and deaths (0.16 million) [2]. This could be partly explained by the predilection of COVID-19 to cause severe disease in the elderly and Africa's relatively young population, where children and young adults less than 25 years of age represent more than 60% of the total population [3,4].
Deaths associated with the COVID-19 pandemic have been significantly underestimated across the world at the start of the epidemic. Even with robust surveillance, accounted fatalities do not include the so-called ‘indirect’ deaths due to pandemic-related changes in healthcare systems and in individual health-seeking behaviors but are not caused by infection with SARS-CoV-2. The sum of direct (viral infections) and indirect deaths would reflect the whole pandemic-related mortality. One approach to measuring the impact of the pandemic on overall mortality is to calculate what has been termed ‘excess mortality.’ This can be defined as an unusual mortality increase during a specific period in a given population above and beyond what we would have expected to see under ‘normal’ conditions [5,6]. Such estimates are calculated by comparing the expected number of deaths due to all causes (based on mortality rates before the pandemic) vs the observed number of deaths or, in the absence of robust empirical data, estimated deaths by mathematical models [7]. In high-income countries, estimates suggest that the true death toll related to the pandemic may have been at least 2-4-fold higher than official reports [8], with some variations according to the approach used for the estimations. Indeed, by January 2022, the estimated toll of directly-related COVID-19 deaths was about 5.5 million, whereas different models estimated the COVID-19-related deaths at 18.2 million (95% CI 17.1, 19.6) [7], 12.6 million (95% CI 9.1, 18.6), [8] and 19.4 million (95% CI 12, 22.4) [8].
Estimating the direct and indirect effects of COVID-19-related mortality in Sub-Saharan Africa (SSA) entails additional challenges. On the one hand, poor surveillance in most SSAs countries would underestimate the true burden, as shown by seroprevalence surveys, that suggest transmission has been as high, if not higher, than in other continents [9]. On the other hand, the lack of death registration in most SSA countries is an additional difficulty in estimating the overall excess of deaths. Therefore, estimates are mainly based on assumptions used for building predictive mathematical models whose results rely on the availability and quality of data.
Here we used data from three regionally distinct health and demographic surveillance systems (HDSS) covering approximately 10% of The Gambian population to calculate excess mortality in 2020, the year of the first COVID-19 epidemic wave.
Methods
The Gambia and the country's response to the COVID-19 pandemic
The Gambia is a small West African country bordering Senegal, except for its coast on the Atlantic Ocean. In 2020, the total estimated population was about 2.42 million, with median age of 17.8 years and life expectancy of 63.3 years [10]. The top eight causes of mortality include lower respiratory infection, ischemic heart disease, neonatal disorders, HIV/AIDS, stroke, tuberculosis, malaria, and diarrheal diseases, [11] similar to reports from the Farafenni HDSS data [12].
Shortly after the first COVID-19 case was diagnosed on March 17, 2020, the country closed its international land, sea, and air borders. On March 27, 2020, a state of emergency was declared, which included the closing of schools, non-essential shops, places of worship, and many workplaces. Initial SARS-CoV-2 testing by polymerase chain reaction aimed at identifying imported cases and tracing and isolating contacts. As the epidemic progressed, the Ministry of Health established testing facilities at strategic, densely populated locations. Demand for testing services was low, and attempts to raise awareness had limited impact, which may have led to the intense transmission, albeit mild, later in 2020, as described elsewhere [13].
A rapid increase in cases only started in July 2020, and by December 31, 2020, 3797 cases and 124 deaths had been reported [14].
HDSS platforms
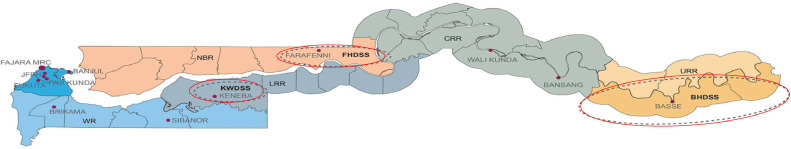
The Medical Research Council Unit, The Gambia at the London School of Hygiene & Tropical Medicine (MRCG at LSHTM), is running three HDSS platforms—namely Basse [15], Farafenni [12] and West Kiang (Keneba) [16]—in largely rural communities and covering a population of more than 250,000 individuals (Figure 1 ). All households in the HDSS area are visited at least once every 4 months. Deaths, births, migrations, pregnancies, marriages, and vaccination records are updated during these visits. Individuals are defined as a resident if they are found to be living in the HDSS geographic area on two consecutive visits. Data collection in 2020 was affected by the COVID-19 pandemic in all three sites, resulting in only two rounds of enumeration instead of the usual three per year.
Figure 1.
Map of The Gambia with the three HDSS sites.
Data and statistical analysis
Data were extracted from the three HDSSs for the period between January 1, 2016, and December 31, 2020, to allow for a complete reporting of deaths during the observation period. Data extracted included date of birth, date of death, site of residence, and population count. The years 2016-2019 were considered pre-pandemic, while 2020 was the pandemic period. Mid-year population was calculated by counting all individuals who were registered as a resident in each HDSS database on July 1st of each year. Age in completed years was categorized into four groups (<1, 1-17, 18-64, and 65+) for stratified analysis. Mortality counts were aggregated by month to create the time-series data.
We calculated average annual mortality before the pandemic and subtracted it from the mortality count in 2020 to obtain the ‘crude’ number of excess deaths estimate during 2020. Excess mortality for each month in 2020 was assessed using P-scores [5], the percentage difference between the reported and projected number of deaths, calculated as:
where the expected number of deaths is approximated by the average mortality count at each month m over the pre-pandemic period.
For our primary analysis, we used an interrupted time-series approach [17] to compare mortality rates between the pre-pandemic period (2016-2019) vs 2020. We adjusted for seasonality using harmonics (sine/cosine pairs). Furthermore, in a sensitivity analysis, we explored adjusting for seasonality using dummy variables for calendar month and adding a time trend. We used Akaike Information Criteria (AIC) [18] to compare best fitting models (lowest AIC).
Let Yt ∼ Poisson(µt) be the monthly death count with Poisson distribution; then our time-series model can be given as:
where µt is the expected number of monthly deaths; t is time(months) since the start of the study; X1t is the dummy variable for pre/during pandemic period; X2t is t (scaled) for time trend assessment; β0 is the offset to account for population size (which is the natural logarithm of the population size); β1 and β2 are the log(rate ratios [RRs]) corresponding to X1 and X2 respectively; f ( s ) represent adjustment for seasonality either using harmonics (sine/cosine pairs) or dummy variables for calendar months. Excess mortality was calculated as (RR-1)*E, where E is the expected (counterfactual) mortality in the absence of the pandemic. Analyses were repeated, stratifying by age group for all HDSS sites together and for each HDSS site. To account for overdispersion, we assumed negative binomial distribution for the monthly mortality count data for all models fitted in this study. R/RStudio [19] and Stata [20] were used to perform all data analyses.
Ethical approval
Data collection as part of the three HDSS sites have ethical approval from The Gambian Government/MRCG at the LSHTM Joint Ethics committee.
Results
Between 2016 and 2019, the total average annual population across the three HDSS sites was 256,843 individuals. During these 4 years, we detected 6049 deaths: 4395 in Basse, 1263 in Farafenni, and 391 in Keneba. The average number of deaths per year was 1512, ranging from 1438 in 2016 to 1606 in 2018. In 2020, the average population was 272,620, and 1634 deaths were recorded (See details in Table 1 ).
Table 1.
Observed mortality counts by year and health and demographic surveillance system site.
| Site | Year |
|||||
|---|---|---|---|---|---|---|
| 2016 | 2017 | 2018 | 2019 | 2020 | Total (2016-2020) | |
| Basse | 1014 | 1127 | 1173 | 1081 | 1179 | 5574 |
| Farafenni | 299 | 309 | 336 | 319 | 351 | 1614 |
| Keneba | 125 | 87 | 97 | 82 | 104 | 495 |
| Total | 1438 | 1523 | 1606 | 1482 | 1634 | 7683 |
Crude excess mortality
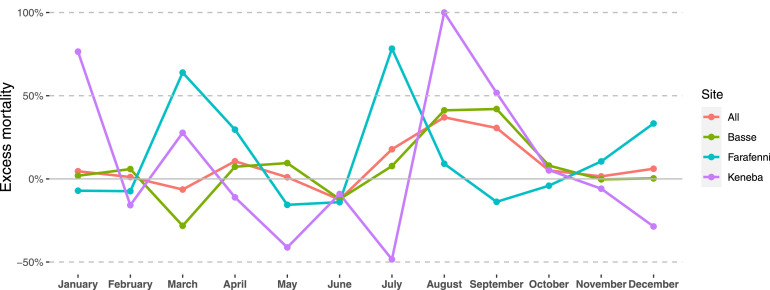
The overall annual crude mortality rates (95% CI) were 589 (559, 619) and 599 (571, 629) per 100,000 for the pre-pandemic and 2020 periods, respectively. This corresponds to an excess mortality rate of 11.1 (-31, 52) per 100,000 population (RR = 1.02 [0.95,1.09]). The point estimates widely varied between the HDSS sites, but in none of them was the excess of deaths significant (Table 2 ). By age groups, again, there was no significant excess mortality for any comparison, although the point estimates differed between age groups (Table 2). For example, RR (95% CIs) over all sites were 1.11 (0.91, 1.35), 0.93 (0.77, 1.12), 1.00 (0.89, 1.12), and 1.08 (0.95, 1.22) among infants (<1 year of age), 1-17 years, 18-64 years, and 65+ years age groups respectively. Peaks in excess deaths occurred at slightly different calendar periods in the different HDSS sites; in July 2020 for Farafenni (P-score approximately 78%), in August 2020 for Keneba (P-score approximately 100%), and in August/September 2020 for Basse (P-score approximately 42%) (Figure 2 ).
Table 2.
Summary of mortality and population counts with crude rate (per 100,000) estimates for excess deaths.
| Age group | Site | Average annual deaths 2016-19 | Average annual population 2016-19 | Deaths2020 | Population2020 | Crude rate2016-19 (95% CI) | Crude rate 2020 (95% CI) | Crude rate difference (95% CI) | Crude rateratio (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| All | All | 1512.25 | 256,842.8 | 1634 | 272,620 | 589 (559, 619) | 599 (571, 629) | 11 (-31, 52) | 1.02 (.95, 1.09) |
| Basse | 1098.75 | 186,229 | 1179 | 198,373 | 590 (556, 626) | 594 (561, 629) | 4 (-44, 53) | 1.01 (.93, 1.09) | |
| Farafenni | 315.75 | 56,014.5 | 351 | 59,944 | 564 (504, 630) | 586 (526, 650) | 21 (-66, 109) | 1.04 (.89, 1.21) | |
| Keneba | 97.75 | 14,599.25 | 104 | 14,303 | 671 (545, 818) | 727 (594, 881) | 56 (-137, 249) | 1.08 (.81, 1.44) | |
| <1 | All | 192 | 8271.5 | 231 | 8988 | 2321 (2004, 2674) | 2570 (2249, 2924) | 249 (-218, 715) | 1.11 (.91, 1.35) |
| Basse | 132.25 | 5972 | 178 | 6560 | 2210 (1849, 2621) | 2713 (2329, 3143) | 503 (-46, 1052) | 1.23 (.97, 1.55) | |
| Farafenni | 43 | 1877.5 | 44 | 1995 | 2290 (1657, 3085) | 2206 (1603, 2961) | -85 (-1030, 860) | .96 (.62, 1.5) | |
| Keneba | 16.75 | 422 | 9 | 433 | 4028 (2347, 6450) | 2079 (950, 3946) | -1950 (-4297, 398) | .52 (.2, 1.22) | |
| 1-17 | All | 233.75 | 130,667.8 | 227 | 136,808 | 179 (157, 204) | 166 (145, 189) | -13 (-45, 18) | .93 (.77, 1.12) |
| Basse | 180.5 | 95,575.75 | 176 | 100,170 | 189 (163, 219) | 176 (151, 204) | -14 (-52, 24) | .93 (.75, 1.15) | |
| Farafenni | 43.5 | 27,450.75 | 40 | 29,293 | 160 (116, 215) | 137 (98, 186) | -24 (-87, 40) | .85 (.54, 1.34) | |
| Keneba | 9.75 | 7641.25 | 11 | 7345 | 131 (63, 241) | 150 (75, 268) | 19 (-101, 139) | 1.14 (.44, 3.01) | |
| 18-64 | All | 582.25 | 109,423.8 | 631 | 118,291 | 532 (490, 577) | 533 (493, 577) | 2 (-58, 62) | 1 (.89, 1.12) |
| Basse | 442.25 | 79,102.5 | 483 | 86,227 | 559 (508, 613) | 560 (511, 612) | 1 (-71, 74) | 1 (.88, 1.14) | |
| Farafenni | 114.75 | 24,617.75 | 120 | 26,441 | 467 (386, 561) | 454 (376, 543) | -13 (-131, 105) | .97 (.75, 1.27) | |
| Keneba | 25.25 | 5703.5 | 28 | 5623 | 438 (284, 647) | 498 (331, 720) | 60 (-192, 312) | 1.14 (.64, 2.03) | |
| 65+ | All | 504.25 | 8443.5 | 545 | 8463 | 5969 (5459, 6514) | 6440 (5910, 7004) | 471 (-280, 1222) | 1.08 (.95, 1.22) |
| Basse | 343.75 | 5553.75 | 342 | 5356 | 6194 (5557, 6884) | 6385 (5726, 7099) | 191 (-750, 1133) | 1.03 (.88, 1.2) | |
| Farafenni | 114.5 | 2059.75 | 147 | 2206 | 5583 (4610, 6702) | 6664 (5630, 7832) | 1080 (-403, 2564) | 1.19 (.93, 1.54) | |
| Keneba | 46 | 830 | 56 | 901 | 5542 (4058, 7392) | 6215 (4695, 8071) | 673 (-1610, 2957) | 1.12 (.75, 1.69) |
Figure 2.
Excess mortality (%) by site for each month in 2020 compared to the period 2016-2019 (P-scores).
Adjusted excess mortality estimates
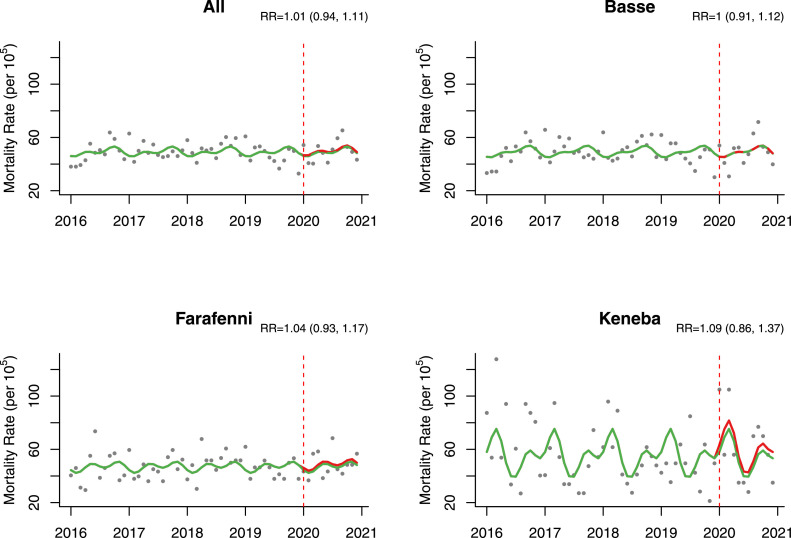
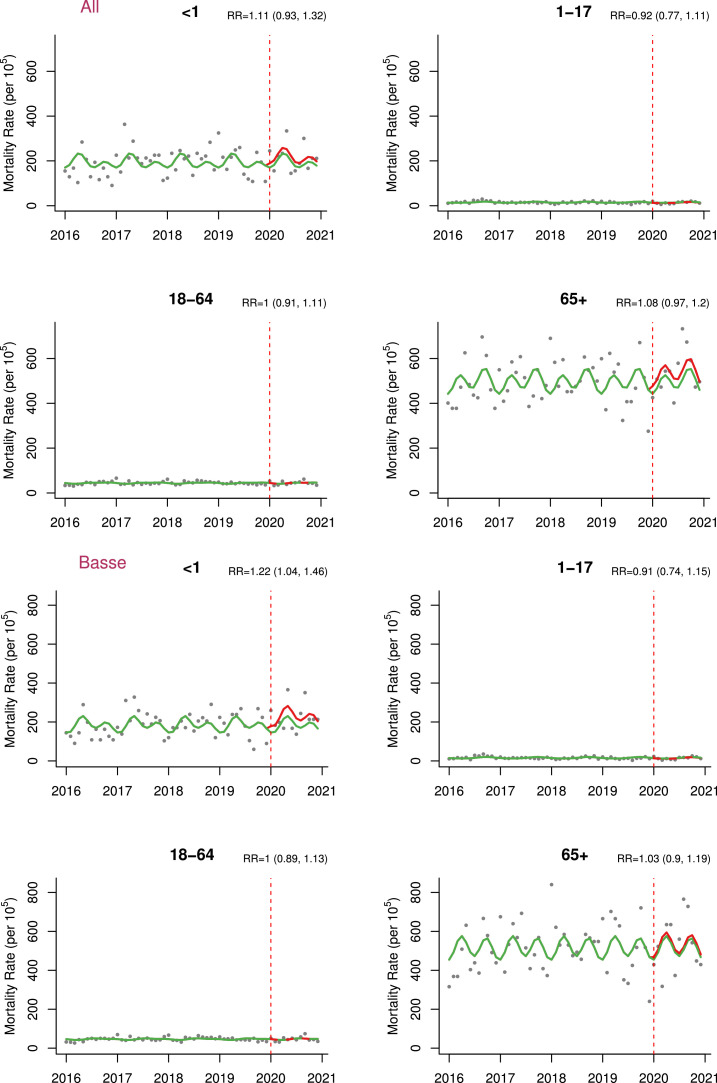
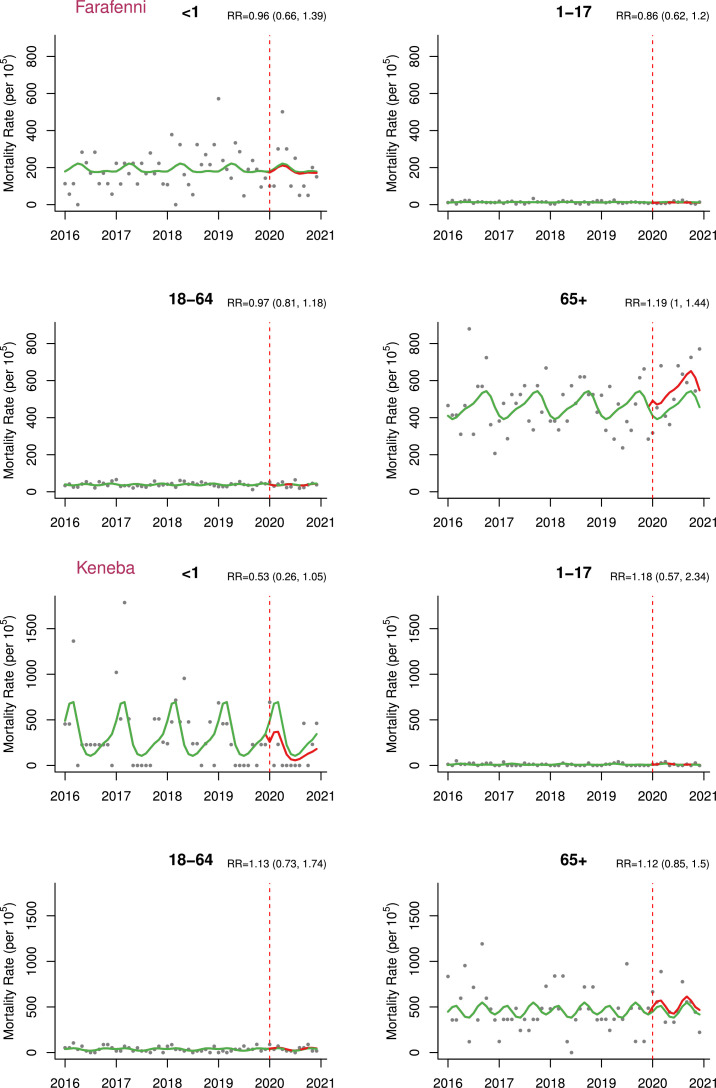
In the time-series analysis adjusted for seasonality, we did not find significant difference in mortality rates during 2020 compared to the pre-pandemic period (adjusted excess mortality = 8.8 [-34.3,67.6] per 100,000 person-month and adjusted RR [aRR] = 1.01 [0.94,1.11]) (Table 3 , Figure 3 ). However, excess mortality was found among infants in the Basse HDSS (adjusted excess mortality = 492.4 [65.5, 1213.4] per 100,000 person-month and aRR = 1.22 [1.04,1.46]) and the Farafenni HDSS for older adults (adjusted excess mortality = 1083.4 [22.7, 2932.9] per 100,000 person-month and aRR = 1.19 [1.00,1.44]) (Table 3, Figure 4 ). Results for all models compared in the sensitivity analyses with corresponding AIC values can be found in Supplementary Appendix (Table S1).
Table 3.
Estimated excess deaths and rate ratios based on modelsa adjusting for seasonality.
| Age group | Site | Excess deaths (95% CI) | Excess mortality rate per 100,000 (95% CI) | Rate ratio (95% CI) |
|---|---|---|---|---|
| All | All | 23.9 (-93.4, 184.2) | 8.8 (-34.3, 67.6) | 1.01 (0.94, 1.11) |
| Basse | 2.2 (-99.9, 151.1) | 1.1 (-50.4, 76.2) | 1 (0.91, 1.12) | |
| Farafenni | 12.4 (-21.3, 63) | 20.7 (-35.5, 105.1) | 1.04 (0.93, 1.17) | |
| Keneba | 8.6 (-10.6, 43.6) | 60.1 (-74.1, 304.8) | 1.09 (0.86, 1.37) | |
| <1 | All | 23.2 (-11.9, 78.9) | 258.1 (-132.4, 877.8) | 1.11 (0.93, 1.32) |
| Basse | 32.3 (4.3, 79.6) | 492.4 (65.5, 1213.4) | 1.22 (1.04, 1.46) | |
| Farafenni | -1.7 (-11, 24.9) | -85.2 (-551.4, 1248.1) | 0.96 (0.66, 1.39) | |
| Keneba | -8 (-7.8, 1.3) | -1847.6 (-1801.4, 300.2) | 0.53 (0.26, 1.05) | |
| 1-17 | All | -20.1 (-47.2, 30.7) | -14.7 (-34.5, 22.4) | 0.92 (0.77, 1.11) |
| Basse | -16.9 (-40.7, 35) | -16.9 (-40.6, 34.9) | 0.91 (0.74, 1.15) | |
| Farafenni | -6.7 (-12.9, 12.8) | -22.9 (-44, 43.7) | 0.86 (0.62, 1.2) | |
| Keneba | 1.7 (-2.1, 24.2) | 23.1 (-28.6, 329.5) | 1.18 (0.57, 2.34) | |
| 18-64 | All | 0.4 (-51.1, 73.4) | 0.3 (-43.2, 62.1) | 1 (0.91, 1.11) |
| Basse | -0.7 (-48.1, 71.4) | -0.8 (-55.8, 82.8) | 1 (0.89, 1.13) | |
| Farafenni | -3.7 (-19.9, 27.5) | -14 (-75.3, 104) | 0.97 (0.81, 1.18) | |
| Keneba | 3.3 (-4.5, 27.7) | 58.7 (-80, 492.6) | 1.13 (0.73, 1.74) | |
| 65+ | All | 40.3 (-15.3, 113.9) | 476.2 (-180.8, 1345.9) | 1.08 (0.97, 1.2) |
| Basse | 9.6 (-29.4, 71.3) | 179.2 (-548.9, 1331.2) | 1.03 (0.9, 1.19) | |
| Farafenni | 23.9 (0.5, 64.7) | 1083.4 (22.7, 2932.9) | 1.19 (1, 1.44) | |
| Keneba | 5.7 (-5.7, 33.6) | 632.6 (-632.6, 3729.2) | 1.12 (0.85, 1.5) |
Assuming negative binomial distribution for over-dispersed mortality count data and harmonics (sine/cosine pairs) to account for seasonality.
Figure 3.
Mortality rate estimates per 100,000 person-month pre-pandemic (2016-2019) and during pandemic (2020 only) by health and demographic surveillance system site with corresponding RR (95% CIs).
Note: Green - estimates in the absence of pandemic/counterfactual; Red - pandemic period estimates; models here adjusted for seasonality using harmonics.
RR, rate ratio.
Figure 4.
Age-stratified excess mortality per 100,000 person-month in 2020 by health and demographic surveillance system site (All, Basse, Farafenni, Keneba).
Note: Green - estimates in the absence of pandemic/counterfactual; Red - pandemic period estimates; models here adjusted for seasonality using harmonics.
RR, rate ratio.
Discussion
According to our time-series analysis of the HDSS data that covers about 10% of the Gambian population, we did not find significant overall excess mortality associated with the COVID-19 pandemic in 2020 when the first wave occurred. This result is consistent with the official number of 124 COVID-19 deaths reported by the end of 2020 [14], and the recently released World Health Organization model that estimates 41 (-6, 94) excess deaths per 100,000 for The Gambia in 2020 [21]. However, other modeling approaches provide different results, with a country's estimated excess mortality ranging between 2154 and 6340 [7,22,23] at a time when the number of reported COVID-19-related deaths was 343 (January 2022). Those other models probably overestimated COVID-19 excess mortality as they are based on data generated in high-income countries and directly applied to other regions. However, we estimated some excess mortality in specific age groups (infants and individuals older than 65 years) in some geographical areas.
Our result (not detecting excess mortality in 2020 in The Gambia) is also in agreement with a study conducted in rural Kenya that showed no excess deaths after the first two waves of COVID-19 [24]. Results from The Gambia and Kenya have been produced by analyzing HDSS data. This is also generally in agreement with the observation that the SSA region may have had a similar level of COVID-19 infections to the rest of the world but fewer deaths [25]. A limitation of this analysis may be the under-representation of urban areas in these HDSS because urban areas are generally the first to be exposed to the virus due to more frequent international contact. However, it has been shown that the infection can spread from urban to rural areas, resulting in a similar burden, even if the epidemic affects rural areas later than urban areas [26,27]. The first SARS-CoV-2 wave in The Gambia probably started from infected individuals who traveled from Senegal because international flights were suspended and Gambian borders are extremely porous [28]. Infected individuals may have entered from Senegal through Farafenni, which is on the trans-Gambian road linking Dakar, the capital, with Ziguinchor, in southern Senegal [29], as also suggested by the genomic analysis [30]. Indeed, the P-scores of excess mortality peaked earlier in Farafenni than in the other two HDSS sites.
Infant mortality in Basse was more than 20% higher in 2020 than before the pandemic, and this could be attributed to the disruption of healthcare provision caused by the pandemic. Indeed, access to health facilities and vaccination clinics at the time of borders closure and lockdown was lower than in the previous 5 years [31]. For example, the total number of infants attending outpatient clinics decreased by 35% in 2020 compared to 2019 [32]. In Farafenni, mortality in the oldest age group (65+ years) increased by 19% compared to pre-pandemic years. Risk of complications and death by COVID-19 is higher in the older age groups, particularly if there are co-morbidities, whose frequency increases with age. For example, in a systematic review and meta-analysis study, COVID-19 infection fatality ratio has been reported to increase progressively with age to 1.4% at age 65, 4.6% at age 75, and 15% at age 85 [33]. Such excess mortality may either be due to the direct effect of the infection and/or by the disruption in health care provision or reluctance to seek health care in health facilities. Nevertheless, it is unclear why this is observed only in Farafenni and not in the other HDSS because the age distribution and prevalence of co-morbidities do not vary across The Gambia [32,34]. Therefore, some specific factors related to the population in the Farafenni region, provision of or access to health care, may have affected the oldest age group in Farafenni. Other potential explanations may include lower data completeness for the oldest age group in other HDSSs or a lower incidence of SARS-CoV-2 according to both age groups and HDSSs. However, a difference in data completeness between HDSS sites is unlikely because the methodologies, control checks, team, and management are more or less similar across sites. Overall, future public health responses to pandemics may need to consider such indirect effects, particularly for vulnerable groups such as young children and the oldest age groups.
Additional limitations of our analysis should be considered. The HDSSs are large databases, but the quality of the data tends to be lower than in active study cohorts or clinical trials. In addition, it is key for the analysis of time trends that case ascertainment is collected homogeneously over the years. In 2020, the HDSS team was able to complete only two rounds of visits, rather than the usual three, and may have missed some deaths, particularly among infants. This is despite our latest data extraction conducted in February 2022 in an attempt to allow for more updates and data cleaning from the household visits. This may have compromised the completeness of the data and limited our ability to determine excess mortality, particularly in this youngest age group.
In conclusion, we did not find significant overall excess of mortality at the time of the COVID-19 epidemic in 2020 in The Gambia. There was probably some excess of deaths in infants and older adults in some geographical areas, suggesting indirect collateral damage may have had some role. There is a need to understand why the overall effect of the pandemic in The Gambia in 2020, as well as in other SSA countries, was less severe than predicted. In addition, public health response to this and other pandemics needs to consider the potential for indirect collateral damage, particularly in countries with weak health systems. Mortality surveillance to understand the effect of subsequent pandemic waves in The Gambia should continue.
Declaration of competing interest
The authors have no competing interests to declare.
Funding
The United Kingdom Medical Research Council (MRC) has provided core financial support for the maintenance of health and demographic surveillance systems. This study was supported and funded by United Kingdom Research and Innovation (grant number MC_PC_20028).
Acknowledgments
We would like to acknowledge those staff who contributed to the health and demographic surveillance system (HDSS) platforms, including office and field staff, over the years. We also want to thank all the residents of the HDSS areas.
Author contributions
NIM and AR contributed to study conception and design, analysis of data, interpretation of results, and drafting and editing of the paper. DJ contributed to the analysis of data and interpretation of results. UDA, EU, GM, and AP contributed to study conception and design, data interpretation, drafting, and editing of the paper. MJ contributed to data acquisition, interpretation of the results, drafting, and editing of the paper. JH contributed to interpretation of the results, drafting, and editing of the paper. EE contributed to data acquisition, drafting, and editing of the paper. BLD, MS, LJ, SG, AKFS, BS, and PG contributed to data acquisition, management, and curation. All authors read and approved the final draft.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2022.12.017.
Appendix. Supplementary materials
References
- 1.World Health Organization. Novel Coronavirus(2019-nCoV) Situation Reports-10, https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200130-sitrep-10-ncov.pdf?sfvrsn=d0b2e480_2; 2020, [accessed 13 April 2020].
- 2.World Health Organization. Weekly epidemiological update on COVID-19 - 28 December 2021, https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—28-december-2021; 2021 [accessed 16 October 2022].
- 3.Martinez-Alvarez M, Jarde A, Usuf E, Brotherton H, Bittaye M, Samateh AL, et al. COVID-19 pandemic in west Africa. Lancet Glob Health. 2020;8:e631–e632. doi: 10.1016/S2214-109X(20)30123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Nations World Population Prospects 2022, https://population.un.org/wpp/default.aspx?aspxerrorpath=/wpp/; 2021 [accessed 21 September 2021].
- 5.Our World in Data. Excess mortality during the Coronavirus pandemic (COVID-19), https://ourworldindata.org/excess-mortality-covid; 2021 [accessed 16 October 2021].
- 6.Eurostat Statistics Explained. Glossary: Excess mortality, https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Glossary:Excess_mortality; 2022 [accessed 28 August 2022].
- 7.COVID-19 Excess Mortality Collaborators Estimating excess mortality due to the COVID-19 pandemic: a systematic analysis of COVID-19-related mortality, 2020–2021. Lancet. 2022;399:1513–1536. doi: 10.1016/S0140-6736(21)02796-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adam D. The pandemic's true death toll: millions more than official counts. Nature. 2022;601:312–315. doi: 10.1038/d41586-022-00104-8. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Over two-thirds of Africans exposed to virus which causes COVID-19: WHO study, https://www.afro.who.int/news/over-two-thirds-africans-exposed-virus-which-causes-covid-19-who-study; 2022 [accessed 10 June 2022].
- 10.Worldometer. Gambia population (2022), https://www.worldometers.info/world-population/gambia-population/; 2022 [accessed 14 April 2022].
- 11.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jasseh M, Gomez P, Greenwood BM, Howie SRC, Scott S, Snell PC, et al. Health & demographic surveillance system profile: Farafenni health and demographic surveillance system in the Gambia. Int J Epidemiol. 2015;44:837–847. doi: 10.1093/ije/dyv049. [DOI] [PubMed] [Google Scholar]
- 13.Abatan B, Agboghoroma O, Akemoke F, Antonio M, Awokola B, Bittaye M, et al. Intense and mild first epidemic wave of coronavirus disease, The Gambia. Emerg Infect Dis. 2021;27:2064–2072. doi: 10.3201/eid2708.204954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worldometer. Gambia COVID - coronavirus statistics, https://www.worldometers.info/coronavirus/country/gambia/; 2022 [accessed 27 June 2022].
- 15.Mackenzie GA, Plumb ID, Sambou S, Saha D, Uchendu U, Akinsola B, et al. Monitoring the introduction of pneumococcal conjugate vaccines into West Africa: design and implementation of a population-based surveillance system. PLoS Med. 2012;9 doi: 10.1371/journal.pmed.1001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hennig BJ, Unger SA, Dondeh BL, Hassan J, Hawkesworth S, Jarjou L, et al. Cohort Profile: the Kiang West Longitudinal Population Study (KWLPS)-a platform for integrated research and health care provision in rural Gambia. Int J Epidemiol. 2017;46:e13. doi: 10.1093/ije/dyv206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002;27:299–309. doi: 10.1046/j.1365-2710.2002.00430.x. [DOI] [PubMed] [Google Scholar]
- 18.Akaike H. Selected papers of hirotugu akaike. Springer; Berlin: 1998. Information theory and an extension of the maximum likelihood principle; pp. 199–213. [DOI] [Google Scholar]
- 19.RStudio Team . 2020. RStudio: integrated development environment for R.http://www.rstudio.com/ Boston. [Google Scholar]
- 20.StataCorp . StataCorp LLC; College Station: 2019. Stata statistical software.https://www.stata.com/ Release 16. [Google Scholar]
- 21.World Health Organization. Global excess deaths associated with COVID-19 (modelled estimates), https://www.who.int/data/sets/global-excess-deaths-associated-with-covid-19-modelled-estimates; 2022 [accessed 27 June 2022].
- 22.Our World in Data. Estimated cumulative excess deaths during COVID, Gambia, https://ourworldindata.org/grapher/excess-deaths-cumulative-economist-single-entity?country=∼GMB; 2022 [accessed 11 August 2022].
- 23.IHME. COVID-19, https://covid19.healthdata.org/gambia?view=cumulative-deaths&tab=trend; 2022 [accessed 28 April 2022].
- 24.Otiende M, Nyaguara A, Bottomley C, Walumbe D, Mochamah G, Amadi D, et al. Impact of the COVID-19 epidemic on mortality in rural coastal Kenya. medRxiv. 07 April 2022. https://www.medrxiv.org/content/10.1101/2022.04.06.22273516v1 [accessed 27 June 2022].
- 25.Cabore JW, Karamagi HC, Kipruto HK, Mungatu JK, Asamani JA, Droti B, et al. COVID-19 in the 47 countries of the WHO African region: a modelling analysis of past trends and future patterns. Lancet Glob Health. 2022;10:e1099–e1114. doi: 10.1016/S2214-109X(22)00233-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paul R, Arif AA, Adeyemi O, Ghosh S, Han D. Progression of COVID-19 from urban to rural areas in the United States: a spatiotemporal analysis of prevalence rates. J Rural Health. 2020;36:591–601. doi: 10.1111/jrh.12486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cuadros DF, Branscum AJ, Mukandavire Z, Miller FD, MacKinnon N. Dynamics of the COVID-19 epidemic in urban and rural areas in the United States. Ann Epidemiol. 2021;59:16–20. doi: 10.1016/j.annepidem.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jallow R. Challenges of the Gambia's COVID-19 response and policy recommendations. Cities Heal. 2020 doi: 10.1080/23748834.2020.1833596. [DOI] [Google Scholar]
- 29.Verfassungsblog. The Use of Emergency Powers in Response to COVID-19 in The Gambia, https://verfassungsblog.de/the-use-of-emergency-powers-in-response-to-covid-19-in-the-gambia/; 2022 [accessed 28 August 2022].
- 30.Kanteh A, Jallow HS, Manneh J, Sanyang B, Kujabi MA, Ndure SL, et al. Genomic epidemiology of SARS-CoV-2 infections in the Gambia, March 2020 to Jan 2022. medRxiv. 09 September 2022. https://www.medrxiv.org/content/10.1101/2022.09.07.22278739v1 [accessed 21 September 2022].
- 31.Department of Strategic Policy and Delivery, Office of The President State House, The Republic of The Gambia. Social, economic and health impacts of Covid-19, https://www.mofea.gm/downloads-file/policy-note-2-social-economic-health-impact-of-cov; 2020 [accessed 27 June 2022].
- 32.Ministry of Health, The Republic of The Gambia. 2020 Service statistics, https://www.moh.gov.gm/wp-content/uploads/2021/10/Final-Service-statistics-Report-2020-13-AUG-2021.pdf; 2020 [accessed 27 June 2022].
- 33.Levin AT, Hanage WP, Owusu-Boaitey N, Cochran KB, Walsh SP, Meyerowitz-Katz G. Assessing the age specificity of infection fatality rates for COVID-19: systematic review, meta-analysis, and public policy implications. Eur J Epidemiol. 2020;35:1123–1138. doi: 10.1007/s10654-020-00698-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The Gambia Bureu of Statistics. Distribution of Population by Gender, Broad Age-groups and Area of Residence, https://www.gbosdata.org/topics/population-and-demography/distribution-of-population-by-gender-broad-agegrou; 2022 [accessed 27 June 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.