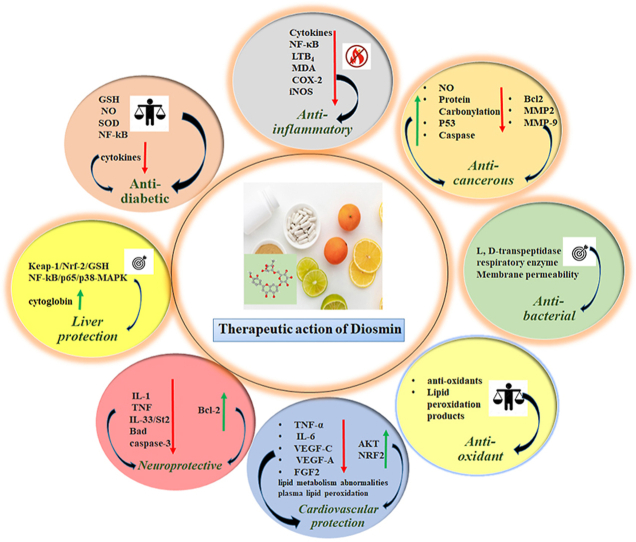
Abstract
Plant-derived flavonoids have been the focus of research for many years mainly in the last decade owing to their therapeutic properties. So far, about 4000 flavonoids have been identified from plants and diosmin (a flavone glycoside) is one of them. Online databases, previous studies, and reviews have been used to gather information on anti-oxidant, immunomodulatory, anti-cancer, anti-parasitic, and anti-microbialproperties of diosmin. Effects of diosmin in combination with other flavonoids have been reviewed thoroughly and its administrative routes are also summarized. Additionally, we studied the effect of diosmin on critical protein networks. It exhibits therapeutic effects in diabetes and its associated complications such as neuropathy and dyslipidemia. Combination of diosmin with hesperidin is found to be very effective in the treatment of chronic venous insufficiency and haemorrhoids. Diosmin is an exquisite therapeutic agent alone as well as in combination with other flavonoids.
Keywords: Diosmin, Herbal drug, Target pathways, Immunomodulation, Anti-oxidant, Anti-Diabetic, chemoprevention, haemorrhoids
Graphical abstract
Highlights
-
•
Diosmin act as antioxidant and anti-inflammatory drug.
-
•
Diosmin shows anti-diabetic properties.
-
•
Diosmin treats chronic venous insufficiency.
1. Introduction
Diosmin (diosmetin 7-O-rutinoside) is a natural flavone glycoside possessing a molecular weight of 608.549 g/mol. IUPAC named it 5 hydroxy-2-(3-hydroxy-4-methoxyphenyl)-7-[(2S,3R,4S,5S,6R)-3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one. Apart from IUPAC name, it is also called as venosmine (Teucrium gnaphalodes) (Barberán et al., 1985). It is commonly occurs in citrus plants belonging to the rutaceae family such as tangerine (Citrus reticulata) (Drahansky et al., 2016) and is obtained by oxidation of hesperidin, a corresponding flavanone glycoside. Diosmin has a double bond between two carbon atoms in the C-ring, which makes it different from hesperidin, present in the pericarp of various citrus fruits. In the year 1925, diosmin was extracted from ScrophularianodosaL. and was first made available as a therapeutic agent in 1969. It is a light yellow or greyish-yellow powder hygroscopic in nature (Bogucka –Kocka et al., 2019), (Srinivasan and Pari, 2012). Ivashev et al. isolated diosmin from theplants belonging to the genus Vicia and Hyssopus officinalis or Teucrium gnaphalodes (Lamiaceae). Representatives of genus Vicia and Hyssopus officinalis have almost 2% diosmin which is sufficient for industrial production for it air dried plant material is first defatted with chloroform, subsequently treated with aqueous ethanol or water (and afterwards with 96% ethanol) and then extracted with the help of dimethyl sulfoxide. The obtained extracts are combined and added into ten times amount of water to obtain crystalline precipitate of diosmin after 48 h (Ivashev et al., 1995). Pharmacokinetic investigations have shown that the enzymes of intestinal microflora rapidly hydrolyse diosmin into glycone; diosmetin, which is readily absorbed and distributed throughout the body. Approximately, next 26–42h diosmin is broken down to phenolic acids or their glycine-conjugated derivatives which are excreted through urine. Diosmin and diosmetin that are not taken up, is excreted in the faces (Srinivasan and Pari, 2012). Diosmin is poorly soluble in water which limits its absorption through the gastro-intestinal tract when administered orally. Formation of inclusion complexes of diosmin with cyclodextrins viz. β-cyclodextrin (β-CD) and 2-hydroxypropyl-β-cyclodextrin (HPβ-CD) enhances its solubility (Ai et al., 2014).
1.1. Anti-oxidant property
Oxidative stress has been reported as a conducive factor in the development of various ailments including myocardial ischemia, cerebral ischemia–reperfusion injury, neuronal cell injury, hypoxia, diabetes, and cancer (Maheshwari et al., 2006). Diosmin possess several therapeutic properties due to its anti-oxidanactivity. Treatment of diabetic rats, induced by streptozotocin nicotinamide (STZ-NA) with diosmin shows ameliorative effects. Diabetic rats show decline in the activities of anti-oxidant enzymes; glutathione-S-transferase (GST), glutathione peroxidase (GPx), superoxide dismutase (SOD) and catalase (CAT) and levels of low molecular weight antioxidants viz. vitamin C, vitamin E and reduced glutathione (GSH) were found to be low whereas the markers of lipid peroxidation (LPO) were found to be elevated in the liver and kidney tissues, compared to normal control rats. Oral administration of diosmin (100 mg/kg/day) for 45 days exhibited improvement in the glycemic and anti-oxidant status of diabetic rats. Lipid peroxidation was also found to be reduced upon treatment with diosmin (Srinivasan and Pari, 2012). It also presents anti-hypertensive property in deoxycorticosterone acetate (DOCA)-salt induced rats.The levels of non-enzymatic and enzymatic antioxidants were found to be decreased whereas the lipid peroxidation products (thiobarbituric acid reactive substances, lipid hydroperoxides and conjugated dienes) were found to be substantially increased in blood plasma and tissues such as liver, kidney, heart and aorta with DOCA which was restored on diosmin treatment. A dose of 50 mg/kg/body weight was found to be most effective. These observations were further confirmed by histopathological studies of kidney and heart (Silambarasan and Raja, 2012). Diosmin also exhibits hepatoprotective effect against ferrous sulfate-induced liver injury in adult male albino rats. Excess iron induces oxidative stress, lipid peroxidation, inflammation, and tissue necrosis. Elevation in ALT, AST, ALP, GGT, LDH activity and bilirubin levels indicates hepatocyte membrane damage. Diosmin treatment significantly normalized these parameters. Diosmin serves as a good hepatoprotective agent as it preserves membrane integrity, relieves oxidative stress and corrects dyslipidemia. The hepato protective potential of diosmin is prominently exerted by its antioxidant and anti-inflammatory activity (Abdel-reheim et al., 2017). Pre-treatment with diosmin reduces oxidative stress in rat heart after ischemia/reperfusion. Reperfusion of ischemic tissues generates oxidative stress which in turn leads to cellular damage (ischemia–reperfusion injury). The hearts of control rats (no diosmin pre-treatment) showed decrease in the activities of enzymatic antioxidants (viz. SOD, CAT and GPx) and GSH levels when subjected to ischemia/reperfusion whereas the levels of lipid peroxidation products were found to be elevated. Oral administration of diosmin (50 and 100 mg/kg) for 7 days was found to normalize these parameters i.e. the activities of enzymatic antioxidants and GSH levels were found to be elevated and a reduction in levels of lipid peroxidation products was observed (Senthamizhselvan et al., 2014). Ischemia-reperfusion injury also leads to retinal edema and tissue damage resulting in loss of vision. In male Wistar rats, diosmin was observed to impede the retinal edema by protecting the blood-retinal barrier and reducing vascular permeability. These protective effects of diosmin might be due to its ability to modulate VEGF/PEDF ratio (Tong et al., 2013). The levels of malondialdehyde (MDA) and the activities of total-superoxide dismutase (T-SOD), glutathione peroxidase (GSH-Px), and catalase (CAT) in the retinal tissues were also normalized after diosmin treatment, that were altered on ischemic–reperfusion injury (Tong et al., 2012). Diosmin also protects against cerebral ischemia–reperfusion injury by activating JAK2/STAT3 signalling pathway in male CD-1 mice (Liu et al., 2014). S5682 (Daflon 500 mg), a purified flavonoid fraction containing diosmin and hesperidin in 9:1 ratio respectively, demonstrate anti-oxidative property in human polymorphonuclear neutrophils (PMNs) which were stimulated in vitro by phorbolmyristate acetate(PMA) (Cypriani et al., 1993). The free-radical scavenging effect of diosmin also confers protection against myocardial infarction (Queenthy and John, 2013).
1.2. Anti-inflammatory property
Many diseases including arthritis, allergy, asthma, autoimmune diseases, atherosclerosis, diabetes, and cancer are the result of inflammation. Inflammation can be characterized by the increased levels of selective biomarkers known as inflammatory markers. Most common inflammatory markers include:
-
●
Cells of the immune system: neutrophils, basophils, eosinophils, platelets, macrophages etc.
-
●
Cell surface receptors and adhesion molecules: selectins (L-selectin, P-selectin and E-selectin) and integrins.
-
●
Soluble mediators: cytokines (IL-1, IL-2, IL-6, TNF-α, TGF-β and IFN-γ), chemokines, NF-κB and acute phase proteins (complement factors, C-reactive protein and the coagulation factor fibrinogen) (Roggen et al., 2014).
Diosmin has been found to alleviate these markers in many studies owing to its anti-inflammatory property. In lung injury induced by lipopolysaccharide (LPS) treatment, pro-inflammatory cytokines (IL-2, IL-6, IL-17 and TNF-α) and NF-κB were found to be elevated. Pre-treatment with diosmin (50 and 100 mg/kg) in male adult Balb/c mice for 7 days showed a significant reduction in the levels of these markers in LPS induced lung injury (Imam et al., 2015). Similar meticulous observations were reported by Islam et al. on Swiss albino mice. They highlighted that pre-treatment with diosmin (100 and 200 mg/kg body weight) for 14 days exerted protective effect against benzo(a)pyrene mediated oxidative stress and lung damage owing to its anti-oxidant and anti-inflammatory properties (Islam et al., 2020). In trinitro benzene sulfonic acid (TNBS) induced rat colitis, diosmin was found to inhibit LTB4 (eicosanoid) and colonic MDA production (Crespo et al., 1999). Studies show that diosmin administered orally at doses 10 and 20 mg/kg b.wt for 9 weeks reduces the levels of COX-2 and iNOS (inflammatory markers) in chemically induced (diethylnitrosamine (DEN) and promoted by 2-acetylaminofluorene (2-AAF) hepatocarcinogenesis in female Wistar rats (Tahir et al., 2013). In addition to this, Orally administered diosmin (25 and 50 mg/kg), was found to modulate the levels of tumour necrosis factor-α (TNF-α) and cyclooxygenase-2 (COX-II) in acetic acid induced (rectal administration of 1 ml acetic acid (4% v/v) ulcerative colitis in male Swiss albino rats. Animals showed significant dose dependent reduction in TNF-α and COX-II levels upon diosmin treatment (Shalkami et al., 2017).
1.3. Anti-cancer property
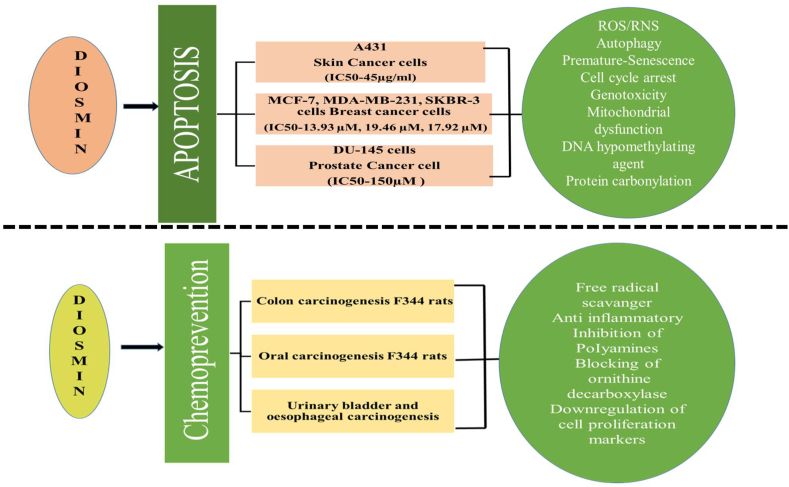
Recent studies have shown that diosmin exerts dose dependent pro-apoptotic effects on various animal cancers including breast, prostrate, colon, oral and urinary bladder. Diosmin has been shown to promote cytostatic autophagy and premature senescence in MCF-7 cells at 5 and 10μM concentrations and induces apoptosis at 20 μM by inducing cytotoxic autophagy along with nitosative stress. Effects of diosmin were also observed on other breast cancer cell lines, MDA-MB-231 and SKBR-3 cells, but MCF-7 was found to be the most responsive. At lower doses, diosmin induced G2/M cell cycle arrest, enhanced levels of p53, p21 and p27, elevated SA-β-gal activity, oxidative stress and DNA damage in MCF-7 cells, all of which are associated with senescence. Apoptosis may be caused by increase in the levels on nitric oxide, ROS, total superoxide and protein carbonylation (Lewinska et al., 2017). Studies on androgen independent prostate cancer cell line, DU145, have confirmed pro-apoptotic activity of diosmin. Geno and cytotoxicity of three flavonoid glycosides (diosmin, naringin and hesperidin) were investigated in DU145 prostate cancer cell line. Maximum genotoxicity was induced by diosmin. These flavonoids were able to induce intracellular redox imbalance or oxidative stress in DU145 cells by impairing the mitochondrial membrane potential resulting in apoptotic cell death. Diosmin caused significant increase in total ROS production. Moreover, treatment with diosmin increased the levels of double stranded nicks in DNA and formation of micronuclei (genotoxicity) (Lewinska et al., 2015). Kuntz et al. observed anti-proliferative potential of diosmin in human colon cancer cell lines (EC50 value: 76.5 ± 6.5 μM for HT- 29 cells and 112.2 ± 6.9 μM for Caco-2 cells) (Kuntz et al., 1999). Diosmin has also been found to inhibit P-glycoprotein mediated efflux of drugs in Caco-2 cells (Hye et al., 2007). Tanaka et al., observed chemopreventive effect of diosmin on azoxymethane induced colon carcinogenesis in male F344 rats. Oral administration of diosmin significantly decreased colon carcinogenesis which might be due to inhibition of ornithine decarboxylase (ODC), a rate determining enzyme in polyamine biosynthesis (Tanaka et al., 1997a). Inhibition of ODC causes cell apoptosis induced by DNA damage (Pendeville et al., 2001). ODC levels have been found to increase in various tissues upon exposure to carcinogens. The colonic mucosa of azoxymethane treated rats show increased ODC activity (Tanaka et al., 1997a). Buddhan et al. studied dose dependent cytotoxic activity of diosmin against A431 skin cancer cells. It induced apoptosis in A431 cells at IC50 of 45 μg/ml, generated excessive ROS. Diosmin treatment also resulted in DNA fragmentation, upregulation of p53, caspase 3 and caspase 9 genes and downregulation of Bcl-2, matrix metalloproteinases-2 and MMP-9 genes in A431 cells. (Rajamanickam and Shanmugam, 2017). Diosmin has been found to inhibit oral carcinogenesis induced by 4-nitroquinoline 1-oxide (4-NQO) in male F344 rats through the inhibition of both ornithine decarboxylase (ODC) and DT-diaphorase activity. 1000 ppm diosmin was provided through dietary mode, starting at 6 weeks of age until 1week after the stop of the carcinogen exposure (Tanaka et al., 1997b). Diosmin is found to be more effective than diosmentin (aglycone form diosmin), in inhibiting oral carcinogenesis (Browning et al., 2005). Oral administration of 1000 ppm diosmin is also effective in inhibiting urinary-bladder carcinogenesis induced by N-butyl-N-(4-hydroxybutyl) nitrosamine in male ICR mice. Reduction of cell proliferation activity was considered as the possible mechanism of diosmin for exerting its chemopreventive effects (Yang et al., 1997). A similar result of diosmin was observed in oesophageal carcinogenesis initiated by N-methyl-N-amylnitrosamine (MNAN) in male Wistar rats (Tanaka et al., 1997c). Chemo therapeutic potential and apoptotic effect of diosmin on different cancer types or cell lines have been summarized in Fig. 1. Anti-metastatic activity of diosmin was also observed on metastatic pulmonary melanoma (B16F10). Both micro and macroscopic studies have shown that diosmin greatly reduces the number of metastatic nodules, implant percentage and invasion index (Martínez et al., 2005; Martinez Conesa et al., 2005). Diosmin works in synergy with IFN-α, in the treatment of metastatic pulmonary melanoma (Álvarez et al., 2009). Diosmin was found to inhibit HA22T cell proliferation (human hepatocellular carcinoma) in nude mice model by inhibiting PI3K–Akt–MDM2 signaling pathway and inducing cell cycle arrest at G2/M phase through p53 activation (Dung et al., 2012) (see Fig. 1).
Fig. 1.
Chemopreventive effects of diosmin on different cancer types and cell lines.
1.4. Anti-diabetic property
Diosmin has been known to exhibit therapeutic effects on diabetes and complications related to it. Diabetes mellitus is a chronic disease characterized by abnormal carbohydrate, protein and fat metabolism and is caused by lack and/or reduced insulin activity. Diosmin has potential anti-hyperglycemic activity as shown by many studies. Pari et al. administred diosmin (25, 50, 100 mg/kg b.w) for 45 days in streptozotocin-nicotinamide (STZ-NA) induced diabetes in male albino wistar rats. They observed improvement in glycemic condition. Diosmin was found to lower the plasma glucose levels in a dose dependent manner. Moreover, oral uptake of diosmin (100 mg/kg b.w) significantly decreased glycosylated haemoglobin and increased haemoglobin and plasma insulin. It also upregulate the key hepatic enzymes, namely, hexokinase and glucose-6-phosphate dehydrogenase and down regulates the glucose-6-phosphatase and fructose-1,6-bisphosphatase in diabetic rats. An increase in body weight of diabetic rats was also reported (Pari and Srinivasan, 2010).
Diosmin was also found to ameliorate abnormalities in lipid metabolism associated with diabetes. Hypercholesterolemia, accumulation of lipids in hepatic tissues, altered plasma lipid and lipoprotein profile are hallmarks of metabolic dyslipidemia associated with type 2 diabetes (Farmer, 2008; Shepherd, 2005). Diosmin treatment effectively normalized the altered levels of plasma lipids, tissue lipids (cholesterol, TGs, FFAs and PLs) and plasma lipoproteins (LDL, VLDL) (Srinivasan and Pari, 2013). Diosmin also reverses the changes in glycoprotein profile associated with type 2 diabetes. In STZ-NA induced diabetic rats the level of plasma glycoproteins were found to increase significantly. In liver and kidney of diabetic rats, the level of hexose, hexosamine and fucose were significantly increased whereas the level of sialic acid was significantly decreased. Diosmin was found to reverse these changes in glycoprotein profile upon oral administration (Leelavinothan Pari Subramani Srinivasan Mohammed Saddiq, 2010). Diosmin nanoparticles have shown more efficacy against diabetes and associated atherosclerosis as it improves water solubility of polymeric matrix and therefore its bioavailability (Om et al., 2020).Neuropathy is one of the most common complications associated with diabetes mellitus affecting more than half of patients. It occurs due to prolonged untreated and uncontrolled hyperglycemia and is characterized by pain, tingling and numbness in the peripheries and slow nerve conduction. Hyperglycemia induced oxidative stress leads to the accumulation of polyols and advanced glycation end products, as well as impairment of (Na+/K+)-ATPase activity and endothelial function. Neurons undergo apoptosis due to oxidative damage. Type 2 diabetes induced in male Sprague-Dawley rats by streptozotocin and high fat diet, on diosmin treatment (50 and 100 mg/kg, p.o.) for 4 weeks showed restriction in development of early diabetic neuropathy. It restored the altered levels of GSH, NO and SOD activity, thereby relieving oxidative stress (Jo et al., 2014). Significant normalization of NF-kB, which plays a pivotal role in the pathogenesis of diabetic neuropathy and other inflammatory diseases were observed on diosmin treatment in alloxan-induced diabetic Wistar rats (Ahmed et al., 2016). In male swiss mice, diosmin was found to relieve neuropathic pain induced by chronic constriction injury (CCI). Intraperitoneal administration of diosmin (1 or 10 mg/kg) was found to relieve CCI-induced mechanical as well as thermal hyperalgesia. Diosmin exposure also suppress spinal cord cytokines (Il-1β and Il-33/St2) and glial cells activation (Bertozzi et al., 2017).Beside this diosmin also act as protective agent in renal stone formation by reducing the pH of urine thus preventing urolithiasis (Vinoth Prabhu et al., 2016).
1.5. Anti-microbial property
Any substance that is capable of destroying bacteria or inhibiting their growth or their ability to reproduce is considered as an anti-bacterial agent. Bacteria have developed resistance against most of the antibiotics. In order to identify new and effective anti-bacterial agents, plant products are being explored. Plant based bioactive components known as phytochemicals are effective against various organisms including fungi, yeasts, bacteria, insects and nematodes.They are able to attenuate peptidoglycan synthesis, damage microbial membrane structures, impairs bacterial membrane surface hydrophobicity and also modulate quorum-sensing (QS) (Monte et al., 2014). Sahu et al. prepared silver nanoparticles (AgNPs) of diosmin to investigate its anti-bacterial property by disc diffusion method against Escherichia coli, Pseudomonasputidaand Staphylococcus aureus. Hexagonal AgNPs of about 5–40 nm in size were found to be mildly anti-bacterial. The size of zone of inhibition produced by diosmin against E. coli, P. putida and S. aureus were 6, 6 and 7 mm respectively. The possible mechanisms of anti-bacterial action were reported to be formation of pits on bacterial cell wall, disruption of cell membrane permeability, inhibition of transduction, inhibition of respiratory enzymes due to free radical formation, and inactivation of various enzymes having thiol group (Sahu et al., 2016). Combination of diosmin with amoxicillin-clavulanic acid (AMC) shows mycobactericidal activity against Mycobacterium marinum. In-vitro validation came from the fact that the survival of M. marinum infected Drosophila melanogaster fly model increased by ∼60% upon treatment with a combination of AMC and diosmin. Further, its antimicrobial activity was confirmed against Mtb H37Ra and MDR clinical isolate. The AMC-diosmin combination was found to target L, D-transpeptidase (Ldt) enzymes involved in Mtb cell wall biosynthesis and induced cellular leakage in M. marinum cells (Pushkaran et al., 2019). Hyssopus officinalis, a source of diosmin, possesses anti-leishmanial activity particularly against Leishmania major species. Diosmin is present in the leaves, stems, sepals and roots of this plant (Hikal and Ahl, 2017). Role of diosmin from H. officinalis can be investigated in future for the cure of infectious diseases like malaria, dengue, Chikungunya, SARS-CoV-2 and Leishmaniasis.
1.6. Combinational therapy
Diosmin (phlebotropic agent) is a veno-active drug administered orally for the treatment of chronic venous insufficiency (CVI) (Russo et al., 2018; Ramelet et al., 2005). In CVI, veins have trouble sending blood from limbs back to the heart as a result of which blood gets pooled in the veins of legs. Reflux of the venous valves is the most common cause of CVI (Christopoulos et al., 1988). Diosmin in combination with hesperidin (MPFF) has been found more effective in relieving venous symptoms than diosmin alone (Ramelet et al., 2005) but a recent study has showed similar efficacies of both preparations (Steinbruch et al., 2020). The most common symptoms of CVI include leg ache, sensation of heaviness or tension, nocturnal cramps, sensation of swelling, restless legs, and itching (Katsenis, 2005). Daflon treatment for 2 months improves sensation of burning, heaviness, weakness and functional discomfort. It also improves blood velocity in the skin microcirculation. Since daflon does not address the initiating factor of CVI, it is not considered as a cure, rather it is considered to improve symptoms associated with the disease (Frick, 2000). In patients suffering from CVI, diosmin as Micronized Purified Flavanoid Fraction (MPFF) have been found to act on venous tone, lymphatic drainage and microcirculation. It improves venous tone by impeding the breakdown of norepinephrine (noradrenaline) by COMT (catechol-O-methyltransferase) thereby prolonging the noradrenergic activity. It decreases the diameter of lymphatic vessels and intra-lymphatic pressure whereas increase the number of functional lymphatics, lymphatic flow, capillary haematocrit and red cell velocity. It also protects microvascular permeability by inhibiting adhesion of leukocytes, their intra-tissue migration and the release of leukocyte (L-selectin) and endothelial (ICAM-1, VCAM-1) adhesion molecules (Inflammatory mediators) (Ramelet, 1016).Daflon treatment for four weeks (four tablets per day, in two divided doses) also shows improvement in the symptoms associated with haemorrhoids (pain, heaviness, bleeding, pruritus and anal discharge) (Meshikhes, 2004). Daflon has also been reported to exhibit anti-oxidative property (Cypriani et al., 1993). Combination of diosmin with amoxicillin-clavulanic acid (AMC) has been found to possess mycobactericidal activity against Mycobacterium marinum (Pushkaran et al., 2019).
Finally, we summarize our review work where diosmin actively plays critical roles in controling well-known signaling components and pathways.Table 2 shows a number of proteins which are known to interact with diosmin. These proteins play important role in inflammatory processes, cancer pathways, antidiabetic, antioxidant, and antibacterial targets.
Tabele 2.
Targeted proteins:In the papers discussed so far, diosmin has been found to target following proteins.
| Therapeutic property | Proteins targeted by diosmin | Effect | References |
|---|---|---|---|
| Anti-oxidant property | Superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) | upregulated | (Srinivasan and Pari, 2012), (Senthamizhselvan et al., 2014), (Tong et al., 2012) |
| iNOS | Downregulated | Abdel-reheim et al. (2017) | |
| VEGF | Down | Tong et al. (2013) | |
| PEDF | Up | ||
| JAK2 and STAT3 (JAK2/STAT3 signalling pathway) | Activates | Liu et al. (2014) | |
| Anti-inflammatory property | Cytokines (IL-2, IL-6, IL-17 and TNF-α) and NF-κB | Down | Imam et al. (2015) |
| COX-2 and iNOS | Down | Tahir et al. (2013) | |
| TNF-α and COX-2 | Down | Shalkami et al. (2017) | |
| Chemotherapeutic properties | p53, p21,p27 and Senescence-associated beta-galactosidase(SA-β-gal) | Up | Lewinska et al. (2017) |
| Ornithine decarboxylase (ODC) | inhibit | Tanaka et al. (1997a) | |
| p53, caspase 3, caspase 9 Bcl-2, matrix metalloproteinase-2 and metalloproteinase-9 |
Up | Rajamanickam and Shanmugam (2017) | |
| Down | |||
| Ornithine decarboxylase (ODC) and DT-diaphorase | inhibit | Tanaka et al. (1997b) | |
| Phosphoinositide 3-kinase, protein kinase B (Akt), mouse double minute 2 homolog (PI3K–Akt–MDM2 signaling pathway) and p53 |
inhibit | Dung et al. (2012) | |
| Anti-diabetic property | hexokinase and glucose-6-phosphate dehydrogenase | inhibit | Pari and Srinivasan (2010) |
| Superoxide dismutase (SOD) | Up | Jo et al. (2014) | |
| NF-kB | Down | Ahmed et al. (2016) | |
| Cyclic guanosine monophosphate (cGMP), protein kinase G (NO/cGMP/PKG/KATP signalling pathway) | Activate | (Bertozzi et al., 2017) | |
| Il-1β and Il-33/St2 | Inhibit | ||
| Anti-bacterial property | Respiratory enzymes | Inhibit | (Sahu et al., 2016) |
| enzymes having thiol group | Inactivate | ||
| L, D-transpeptidase (Ldt) enzymes | Inhibit | (Pushkaran et al., 2019) |
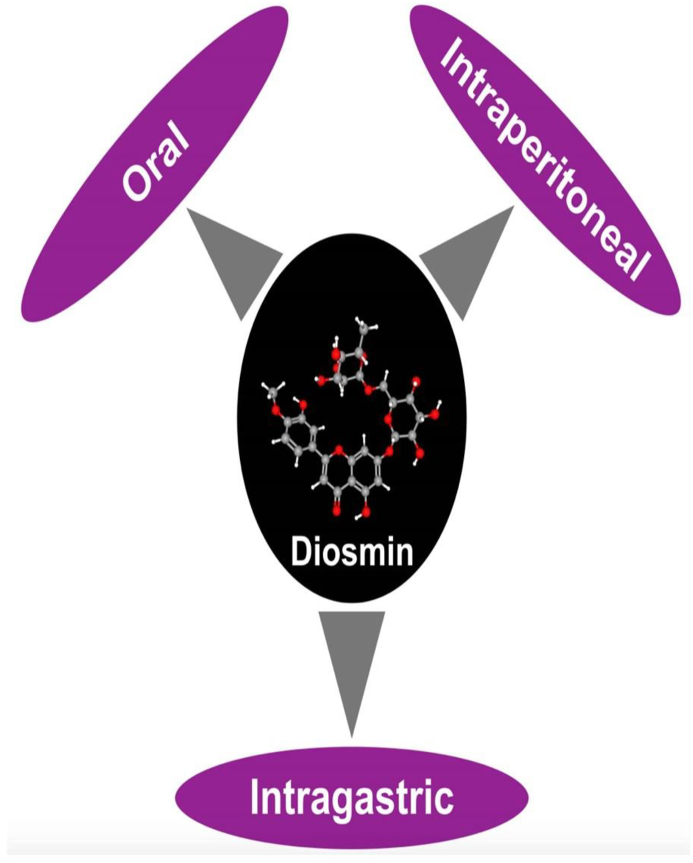
1.7. Administrative routes
In the papers discussed so far, diosmin has been administered through following routes, Oral,intra gastric and intra-peritonial as described in Table 1 and Fig. 2.
Table 1.
Administrative routes of diosmin.
| Route | Dose | Period | Animal model | Property studied | Reference |
|---|---|---|---|---|---|
| oral | 100 mg/kg/day | 45 days | Male albino wistar rats | Anti-oxidant | Srinivasan and Pari (2012) |
| oral | 25, 50 and 100 mg/kg body weight | 6 weeks | Male albino wistar rats | Anti-oxidant | Silambarasan and Raja (2012) |
| oral | 20 mg/kg/day | 10 days | Adult male albino rats | Anti-oxidant (Hepatoprotective effect) | (Abdel-reheim et al., 2017) |
| oral | 50 and 100 mg/kg | 7 days | Male albino wistar rats | Anti-oxidant | (Senthamizhselvan et al., 2014) |
| oral | 50 or 100 mg/kg | 6 days | Male CD-1 mice | Anti-oxidant (I/R injury) | (Liu et al., 2014) |
| oral | 100 and 200 mg/kg | 14 days | Swiss Albino mice | Anti-inflammatory and anti-oxidant | (Islam et al., 2020) |
| oral | 10 and 20 mg/kg | 9 weeks | Female Wistar rats | Anti-inflammatory | (Tahir et al., 2013) |
| oral | 25 and 50 mg/kg | 7 days | Male Swiss albino mice | Anti-inflammatory | (Shalkami et al., 2017) |
| oral | 900 or 1000 ppm | 5 or 28 weeks | Male F344 rats | Chemopreventive (colon carcinogenesis) | (Tanaka et al., 1997a) |
| oral | 1000 ppm | _ | Male F344 rats | Chemopreventive (oral carcinogenesis) | (Tanaka et al., 1997b) |
| oral | 1000 ppm | 8 or 24 weeks | Male ICR mice | Chemopreventive (urinary-bladder carcinogenesis) | Yang et al. (1997) |
| oral | 900 or 1000 ppm | _ | Male Wistar rats | Chemopreventive (oesophageal carcinogenesis) | (Tanaka et al., 1997c) |
| oral | 25, 50, 100 mg/kg b.w | 45 days | Male albino Wistar strain rats | Anti-diabetic | (Pari and Srinivasan, 2010) |
| oral | 100 mg/kg b. w | 45days | Male albino Wistar rats | Anti-diabetic | (Of et al., 2010) |
| oral | 50 and 100 mg/kg | 4 weeks | Male Sprague-Dawley rats | Diabetic-neuropathy | (Jo et al., 2014) |
| oral | 50 mg and 100 mg/kg b.w. | 28 days | Wistar rats | Diabetic-neuropathy | (Ahmed et al., 2016) |
| intra-gastric | 100 mg/kg | _ | Male Wistar rats | Anti-oxidant (I/R injury) | (Tong et al., 2013) |
| intra-gastric | 100 mg/kg | _ | Male Wistar rats | Anti-oxidant (I/R injury) | (Tong et al., 2012) |
| intra-gastric | 100 mg/kg b.w. | 45 days | Male Wistar rats | Anti-diabetic (Antihyperlipidemic effect) | (Srinivasan and Pari, 2013) |
| intra-peritoneal | 1 or 10 mg/kg was | _ | Male Swiss mice | antinociceptive effect | (Bertozzi et al., 2017) |
| oral | 10 mg or 20 mg/kg o | Sprague Dawley rats | anti-urolithiatic | (Vinoth Prabhu et al., 2016) |
Fig. 2.
Administrative routes of diosmin.
Future prospective:Diosmin, a phytocompound with anti-oxidant, anti-inflammatory and anti-microbial activities holds promising therapeutic potential but like other plant based secondary metabolitesthere are several challanges in using it as a therapeutic drug candidate including its solubility, stability, and bio-availability. Russo et al., tested and compared the bioavailability of two diosmin formulations by oral administration to healthy volunteers (62). The study indicated that methods, that favor the bioconversion of diosmin to its aglycone form, raised its plasma concentration and thus increase clinical efficacy of oral administration. Artificial intelligence tools like QSAR, ADMET, molecular-simulations, molecular-docking, pharmacokinetics, and pharmacodynamics studies in future would further strengthen information on this secondary metabolite as a novel therapeutic drug candidate.
Changes in lifestyle have increased incidences of metabolic disorders that include insulin resistance, type 2 diabetes, cardiovascular complications etc. Oxidative stress along with chronic inflammation accelerates these disorders, diosmin being an antioxidant and anti-inflammatory agent; might prove beneficial with respect to current marketed treatment regimen. Alternatively, it could also be used in the treatment of SARS-CoV-2, as it possibly may decrease the viral load by lowering oxidative stress but further experiments with animal models and clinical trials are required to establish diosmin as strong therapeutic molecule in COVID-19 treatment.
2. Conclusion
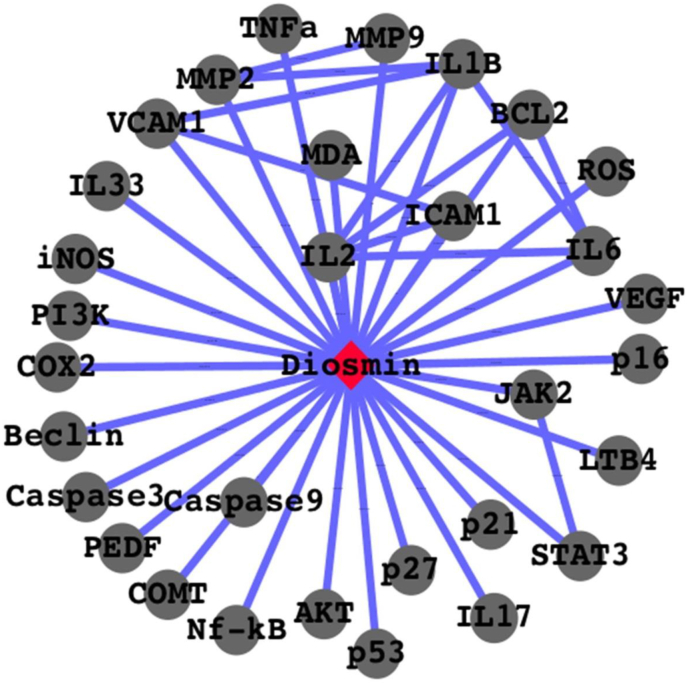
Diosmin, an active flavone glycoside obtained from citrus plants, possesses anti-oxidant, anti-cancer, anti-diabetic and mild anti-bacterial properties. It serves as an excellent therapeutic agent for a number of diseases by the virtue of its biological properties. It relieves oxidative stress by modulating the activities of specific protein markers and induces apoptotic cell death in several cancer cell lines by targeting the key signalling cascade. It also reduces the levels of several inflammation markers which accounts for its anti-inflammatory activities. It also ameliorates the complications associated with diabetes viz. neuropathy and dyslipidemia. Diosmin has been observed to exert most of these effects via interacting with different molecules, both directly and indirectly. Fig. 3 shows some of the direct targets of diosmin based on previous works and FunCoup 2.0 network database. This network was drawn using cytoscape (AlexeyenkoE., 2009; Okawa et al., 2015; Mustafa et al., 2021).shows probable interaction of diosmin with key signalling molecules such as caspase3, NF-kB, Beclin, VEGF, PEDF, JAK2, STAT3, PI3K, iNOS, MMP2, Akt, LTB4, p27, IL-17, COX2, IL-6 etc.Combination of diosmin with other flavonoids, particularly hesperidin, have found to be very effective in the treatment of chronic venous insufficiency and haemorrhoids, thus setting a good example of drug synergism.Thus diosmin treatment looks promising in the treatment of different kinds of cancers, diabetes and diseases associated with oxidative stress and inflammation. Besides serving as an anti-hyperglycemic agent, diosmin also ameliorates complications associated with it. Its ability to regulate VEGF/PEDF ratio can be explored to elucidate its role as a pro-angiogenic or anti-angiogenic factor. Its combination with other flavonoids or phytochemicals need to be explored in future.
Fig. 3.
Protein-protein Interactions appears in diosmin network analysis.
CRediT authorship contribution statement
Saad Mustafa: Conceptualization, Writing – original draft, Methodology, Data curation, Software, Writing – review & editing, Investigation, Visualization. Mahmood Akbar: Writing – review & editing, Writing – original draft, Data curation, Investigation. Mohammad Aasif Khan: Writing – review & editing. Kumari Sunita: Data curation, Software. Shabana Parveen: Data curation, Software, Writing – review & editing. Jogendra Singh Pawar: Writing – review & editing. Sheersh Massey: Data curation, Software. Nupur Rani Agarwal: Visualization, Writing – review & editing. Syed Akhtar Husain: Writing – review & editing, Supervision.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Saad Mustafa, Email: saadmustafa24@gmail.com.
Syed Akhtar Husain, Email: akhtarhusain2000@yahoo.com, shusain@jmi.ac.in.
References
- M.A. Abdel-reheim, B. Anwar, S. Messiha, A.A. Abo-saif, Hepatoprotective Effect of Diosmin on Iron-induced Liver Damage, (n.d.). 10.3923/ijp.2017.529.540. [DOI]
- Ahmed S., Mundhe N., Borgohain M., Chowdhury L., Kwatra M., Bolshette N., Ahmed A., Lahkar M. Diosmin modulates the NF-kB signal transduction pathways and downregulation of various oxidative stress markers in alloxan-induced diabetic nephropathy. Inflammation. 2016;39 doi: 10.1007/s10753-016-0413-4. [DOI] [PubMed] [Google Scholar]
- Ai F., Ma Y., Wang J., Li Y. Preparation, physicochemical characterization and In-Vitro dissolution studies of Diosmin-Cyclodextrin inclusion complexes, Iran. J. Pharm. Res. 2014;13:1115–1123. doi: 10.22037/ijpr.2014.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexeyenko A., E L.L. SonnhammerGlobal networks of functional coupling in eukaryotes from comprehensive data integration. Genome Res. 2009;19:1107–1116. doi: 10.1101/gr.087528.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Álvarez N., Vicente V., Martínez C. Synergistic effect of diosmin and interferon-α on metastatic pulmonary melanoma. Cancer Biother. Radiopharm. 2009;24:347–352. doi: 10.1089/cbr.2008.0565. [DOI] [PubMed] [Google Scholar]
- Barberán F.A.T., Gil M.I., Tomás F., Ferreres F., Arques A. Flavonoid aglycones and glycosides from Teucrium gnaphalodes. J. Nat. Prod. 1985;48:859–860. [Google Scholar]
- Bertozzi M.M., Rossaneis A.C., Fattori V., Longhi-Balbinot D.T., Freitas A., Cunha F.Q., Alves-Filho J.C., Cunha T.M., Casagrande R., Verri W.A. Diosmin reduces chronic constriction injury-induced neuropathic pain in mice. Chem. Biol. Interact. 2017;273:180–189. doi: 10.1016/j.cbi.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Bogucka –Kocka A., Woźniak M., Feldo M., Kocki J., Szewczyk K. Diosmin – isolation techniques, determination in plant material and pharmaceutical formulations, and clinical use. Nat. Prod. Commun. 2019;8 doi: 10.1177/1934578x1300800435. 1934578X1300800. [DOI] [PubMed] [Google Scholar]
- Browning A.M., Walle U.K., Walle T. Flavonoid glycosides inhibit oral cancer cell proliferation - role of cellular uptake and hydrolysis to the aglycones. J. Pharm. Pharmacol. 2005;57:1037–1041. doi: 10.1211/0022357056514. [DOI] [PubMed] [Google Scholar]
- Christopoulos D., Nicolaides A.N., Szendro G. Venous reflux: quantification and correlation with the clinical severity of chronic venous disease. Br. J. Surg. 1988;75:352–356. doi: 10.1002/bjs.1800750419. [DOI] [PubMed] [Google Scholar]
- Crespo M.E., Gálvez J., Cruz T., Ocete M.A., Zarzuelo A. Anti-inflammatory activity of diosmin and hesperidin in rat colitis induced by TNBS. Planta Med. 1999;65:651–653. doi: 10.1055/s-2006-960838. [DOI] [PubMed] [Google Scholar]
- Cypriani B., Limasset B., Carrie M.L., Le Doucen C., Roussie M., De Paulet A., Damon M. Antioxidant activity of micronized diosmin on oxygen species from stimulated human neutrophils. Biochem. Pharmacol. 1993;45:1531–1535. doi: 10.1016/0006-2952(93)90056-3. [DOI] [PubMed] [Google Scholar]
- Drahansky M., Paridah M., Moradbak A., Mohamed A., abdulwahab taiwo Owolabi F., Asniza M., Abdul Khalid S.H. 2016. We are IntechOpen , the World ’ S Leading Publisher of Open Access Books Built by Scientists , for Scientists TOP 1 % p. 13. Intech. i. [DOI] [Google Scholar]
- Dung T.D., Day C.H., Binh T.V., Lin C.H., Hsu H.H., Su C.C., Lin Y.M., Tsai F.J., Kuo W.W., Chen L.M., Huang C.Y. PP2A mediates diosmin p53 activation to block HA22T cell proliferation and tumor growth in xenografted nude mice through PI3K-Akt-MDM2 signaling suppression. Food Chem. Toxicol. 2012;50:1802–1810. doi: 10.1016/j.fct.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Farmer J.A. 2008. Diabetic Dyslipidemia and Atherosclerosis : Evidence from Clinical Trials. [DOI] [PubMed] [Google Scholar]
- Frick R.W. Three treatments for chronic venous insufficiency: escin, hydroxyethylrutoside, and Daflon. Angiology. 2000;51:197–205. doi: 10.1177/000331970005100303. [DOI] [PubMed] [Google Scholar]
- Hikal W.M., Ahl H.A.H.S. Anti-leishmanial activity of Hyssopus officinalis. Review. 2017;3:10–15. [Google Scholar]
- Hye H.Y., Lee M., Hye J.C., Sang K.L., Kim D.H. Effects of diosmin, a flavonoid glycoside in citrus fruits, on P-glycoprotein-mediated drug efflux in human intestinal caco-2 cells. J. Agric. Food Chem. 2007;55:7620–7625. doi: 10.1021/jf070893f. [DOI] [PubMed] [Google Scholar]
- Imam F., Al-Harbi N.O., Al-Harbi M.M., Ansari M.A., Zoheir K.M.A., Iqbal M., Anwer M.K., Al Hoshani A.R., Attia S.M., Ahmad S.F. Diosmin downregulates the expression of T cell receptors, pro-inflammatory cytokines and NF-κB activation against LPS-induced acute lung injury in mice. Pharmacol. Res. 2015;102:1–11. doi: 10.1016/j.phrs.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Islam J., Shree A., Afzal S.M., Vafa A., Sultana S. Protective effect of Diosmin against benzo(a)pyrene-induced lung injury in Swiss Albino Mice. Environ. Toxicol. 2020;35:747–757. doi: 10.1002/tox.22909. [DOI] [PubMed] [Google Scholar]
- Ivashev M.N., Andreeva O.A., Bandyukova V.A., et al. Isolation of diosmin from plants of the genusVicia andHyssop us officinalis and its influence on blood coagulation. Pharm. Chem. J. 1995;29:707–709. doi: 10.1007/BF02219532. [DOI] [Google Scholar]
- Jo O., Sbut F., Sur O., Xuwkhu O.H.V., Grvh W.K.H., Lpsuryhphqw G., Revhuyhg Z.D.V., Wkhupdo L.Q., 1SPUFDUJWF FGGFDU PG EJPTNJO BHBJOTU EJBCFUJD J. Integr. Med. 2014;12:35–41. doi: 10.1016/S2095-4964(14)60001-7. [DOI] [PubMed] [Google Scholar]
- Katsenis K. Micronized purified flavonoid fraction (MPFF)∗: a review of its pharmacological effects, therapeutic efficacy and benefits in the management of chronic venous insufficiency. Curr. Vasc. Pharmacol. 2005;3:1–9. doi: 10.2174/1570161052773870. [DOI] [PubMed] [Google Scholar]
- Kuntz S., Wenzel U., Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur. J. Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. http://www.ncbi.nlm.nih.gov/pubmed/10443335 [DOI] [PubMed] [Google Scholar]
- Leelavinothan Pari Subramani Srinivasan Mohammed Saddiq Preventive effect of diosmin, a bioflavonoid, on glycoprotein changes in streptozotocin-nicotinamide-induced type 2 diabetic rats. Int. J. Pharm. Sci. Res. 2010;1:89–95. http://www.ijpsr.com/V1I10/12 Vol. 1 (10), IJPSR,2010 Paper 6.pdf [Google Scholar]
- Lewinska A., Siwak J., Rzeszutek I., Wnuk M. Diosmin induces genotoxicity and apoptosis in DU145 prostate cancer cell line. Toxicol. Vitro. 2015;29:417–425. doi: 10.1016/j.tiv.2014.12.005. [DOI] [PubMed] [Google Scholar]
- Lewinska A., Adamczyk-Grochala J., Kwasniewicz E., Deregowska A., Wnuk M. Diosmin-induced senescence, apoptosis and autophagy in breast cancer cells of different p53 status and ERK activity. Toxicol. Lett. 2017;265:117–130. doi: 10.1016/j.toxlet.2016.11.018. [DOI] [PubMed] [Google Scholar]
- Liu X., Zhang X., Zhang J., Kang N., Zhang N., Wang H., Xue J., Yu J., Yang Y., Cui H., Cui L., Wang L., Wang X. Diosmin protects against cerebral ischemia/reperfusion injury through activating JAK2/STAT3 signal pathway. MICE. 2014;268:318–327. doi: 10.1016/j.neuroscience.2014.03.032. [DOI] [PubMed] [Google Scholar]
- Maheshwari R.K., Singh A.K., Gaddipati J., Srimal R.C. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78:2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Martinez Conesa C., Vicente Ortega V., Yanez Gascon M.J., Alcaraz Banos M., Canteras Jordana M., Benavente-Garcia O., Castillo J. Treatment of metastatic melanoma B16F10 by the flavonoids tangeretin, rutin, and diosmin. J. Agric. Food Chem. 2005;53:6791–6797. doi: 10.1021/jf058050g. [DOI] [PubMed] [Google Scholar]
- Martínez C., Vicente Ortega V., Yáñez J., Alcaraz M., Castellas M.T., Canteras M., Benavente-García O., Castillo J. The effect of the flavonoid diosmin, grape seed extract and red wine on the pulmonary metastatic B16F10 melanoma. Histol. Histopathol. 2005;20:1121–1129. doi: 10.14670/HH-20.1121. [DOI] [PubMed] [Google Scholar]
- Meshikhes A.W.N. Daflon for haemorrhoids: a prospective, multi-centre observational study. Surgeon. 2004;2:335–338. doi: 10.1016/S1479-666X(04)80032-5. [DOI] [PubMed] [Google Scholar]
- Monte J., Abreu A., Borges A., Simões L., Simões M. Antimicrobial activity of selected phytochemicals against Escherichia coli and Staphylococcus aureus and their biofilms. Pathogens. 2014;3:473–498. doi: 10.3390/pathogens3020473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa S., Pawar J.S., Ghosh I. Fucoidan induces ROS-dependent epigenetic modulation in cervical cancer HeLa cell. Int. J. Biol. Macromol. 2021;181:180–192. doi: 10.1016/j.ijbiomac.2021.03.110. [DOI] [PubMed] [Google Scholar]
- A. Of, W. Membrane, B.Y.T. Elements, 张建 1 ,杨庆山 1 ,谭锋 2 (1., 27 (2010) 1048–1051.
- Okawa S., Angarica V.E., Lemischka I., Moore K. Nature Publishing Group; 2015. A. del Sol A differential network analysis approach for lineagespeci; pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Om H., El-Naggar M.E., El-Banna M., Fouda M.M.G., Othman S.I., Allam A.A., Morsy O.M. Combating atherosclerosis with targeted Diosmin nanoparticles-treated experimental diabetes. Invest. N. Drugs. 2020 doi: 10.1007/s10637-020-00905-6. [DOI] [PubMed] [Google Scholar]
- Pari L., Srinivasan S. Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed. Pharmacother. 2010;64:477–481. doi: 10.1016/j.biopha.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Pendeville H., Carpino N., Marine J.C., Takahashi Y., Muller M., Martial J.A., Cleveland J.L. The ornithine decarboxylase gene is essential for cell survival during early murine development. Mol. Cell Biol. 2001;21:6549–6558. doi: 10.1128/MCB.21.19.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pushkaran A.C., Vinod V., Vanuopadath M., Nair S.S., Nair S.V., Vasudevan A.K., Biswas R., Mohan C.G. Combination of repurposed drug diosmin with amoxicillin-clavulanic acid causes synergistic inhibition of mycobacterial growth. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-43201-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queenthy S.S., John B. Diosmin exhibits anti-hyperlipidemic effects in isoproterenol induced myocardial infarcted rats. Eur. J. Pharmacol. 2013;718:213–218. doi: 10.1016/j.ejphar.2013.08.031. [DOI] [PubMed] [Google Scholar]
- Rajamanickam B., Shanmugam M. Vol. 13. 2017. pp. 471–476. (Diosmin reduces cell viability of A431 skin cancer cells through apoptotic induction). [DOI] [PubMed] [Google Scholar]
- A. Ramelet, CHAPTER 14 - Venoactive Drugs, Fifth Edit, Elsevier Inc., n.d. 10.1016/B978-0-323-07367-7.00020-0. [DOI]
- Ramelet A.A., Boisseau M.R., Allegra C., Nicolaides A., Jaeger K., Carpentier P., Cappelli R., Forconi S. Veno-active drugs in the management of chronic venous disease. Clin. Hemorheol. Microcirc. 2005;33:309–319. [PubMed] [Google Scholar]
- Roggen E.L., Corsini E., van Loveren H., Luebke R. Immunotoxicity testing, toxicogenomics-based cell. Model. 2014;598:57–65. doi: 10.1016/B978-0-12-397862-2.00004-8. [DOI] [Google Scholar]
- Russo R., Chandradhara D., De Tommasi N. Comparative bioavailability of two diosmin formulations after oral administration to Healthy volunteers. Molecules. 2018;23(9):2174. doi: 10.3390/molecules23092174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu N., Soni D., Chandrashekhar B., Satpute D.B., Saravanadevi S., Sarangi B.K., Pandey R.A. Synthesis of silver nanoparticles using flavonoids: hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016;6:173–181. doi: 10.1007/s40089-016-0184-9. [DOI] [Google Scholar]
- Senthamizhselvan O., Manivannan J., Silambarasan T., Raja B. Diosmin pretreatment improves cardiac function and suppresses oxidative stress in rat heart after ischemia/reperfusion. Eur. J. Pharmacol. 2014;736:131–137. doi: 10.1016/j.ejphar.2014.04.026. [DOI] [PubMed] [Google Scholar]
- Shalkami A.S., Hassan M.I.A., Bakr A.G. 2017. Anti-apoptotic Activity of Diosmin in Acetic Acid-Induced Ulcerative Colitis. [DOI] [PubMed] [Google Scholar]
- Shepherd J. Vol. 6. 2005. pp. 15–19. (Does statin monotherapy address the multiple lipid abnormalities in type 2 diabetes). [DOI] [PubMed] [Google Scholar]
- Silambarasan T., Raja B. Diosmin, a bioflavonoid reverses alterations in blood pressure, nitric oxide, lipid peroxides and antioxidant status in DOCA-salt induced hypertensive rats. Eur. J. Pharmacol. 2012;679:81–89. doi: 10.1016/j.ejphar.2011.12.040. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Pari L. Ameliorative effect of diosmin, a citrus flavonoid against streptozotocin-nicotinamide generated oxidative stress induced diabetic rats. Chem. Biol. Interact. 2012;195:43–51. doi: 10.1016/j.cbi.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Pari L. Antihyperlipidemic effect of diosmin : a citrus flavonoid on lipid metabolism in experimental diabetic rats. J. Funct.Foods. 2013;5:484–492. doi: 10.1016/j.jff.2012.12.004. [DOI] [Google Scholar]
- Steinbruch M., Nunes C., Gama R., Kaufman R., Gama G., Suchmacher Neto M., Nigri R., Cytrynbaum N., Brauer Oliveira L., Bertaina I., Verrière F., Geller M. Is nonmicronized diosmin 600 mg as effective as micronized diosmin 900 mg plus hesperidin 100 mg on chronic venous disease symptoms? Results of a noninferiority study. Int. J. Vasc. Med. 2020;2020 doi: 10.1155/2020/4237204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahir M., Rehman M.U., Lateef A., Khan A.Q., Khan R., Qamar W., Hamiza O.O., Ali F., Hasan S.K., Sultana S. Diosmin abrogates chemically induced hepatocarcinogenesis via alleviation of oxidative stress , hyperproliferative and inflammatory markers in murine model. Toxicol. Lett. 2013;220:205–218. doi: 10.1016/j.toxlet.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Makita H., Kawabata K., Mori H., Kakumoto M., Satoh K., Hara A., Sumida T., Tanaka T., Ogawa H. Chemoprevention of azoxymethane-induced rat colon carcinogenesis by the naturally occurring flavonoids, diosmin and hesperidin. Carcinogenesis. 1997;18:957–965. doi: 10.1093/carcin/18.5.957. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Makita H., Ohnishi M., Mori H., Satoh K., Hara A., Sumida T., Fukutani K., Tanaka T., Ogawa H. Chemoprevention of 4-nitroquinoline 1-oxide-induced oral carcinogenesis in rats by flavonoids diosmin and hesperidin, each alone and in combination. Cancer Res. 1997;57:246–252. [PubMed] [Google Scholar]
- Tanaka T., Makita H., Kawabata K., Mori H., Kakumoto M., Satoh K., Hara A., Sumida T., Fukutani K., Tanaka T., Ogawa H. Modulation of N-methyl-N-amylnitrosamine-induced rat oesophageal tumourigenesis by dietary feeding of diosmin and hesperidin, both alone and in combination. Carcinogenesis. 1997;18:761–769. doi: 10.1093/carcin/18.4.761. [DOI] [PubMed] [Google Scholar]
- Tong N., Zhang Z., Gong Y., Yin L., Wu X. Diosmin protects rat retina from ischemia/reperfusion injury. J. Ocul. Pharmacol. Therapeut. 2012;28:459–466. doi: 10.1089/jop.2011.0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong N., Zhang Z., Zhang W., Qiu Y., Gong Y., Yin L., Qiu Q., Wu X. Diosmin alleviates retinal edema by protecting the blood-retinal barrier and reducing retinal vascular permeability during ischemia/reperfusion injury. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinoth Prabhu V., Sathyamurthy D., Ramasamy Anand, Das Saibal, Anuradha Medepalli, Pachiappan Sudhakar. Evaluation of protective effects of diosmin (a citrus flavonoid) in chemical-induced urolithiasis in experimental rats. Pharmaceut. Biol. 2016;54(9) doi: 10.3109/13880209.2015.1107105. [DOI] [PubMed] [Google Scholar]
- Yang M., Tanaka T., Hirose Y., Deguchi T., Mori H., Kawada Y. Chemopreventive effects of diosmin and hesperidin on N-butyl-N-(4-hydroxybutyl)nitrosamine-induced urinary-bladder carcinogenesis in male ICR mice. Int. J. Cancer. 1997;73:719–724. doi: 10.1002/(sici)1097-0215(19971127)73:5<719::aid-ijc18>3.3.co;2-v. [DOI] [PubMed] [Google Scholar]